Abstract
In the 1970s, treatment with thyroid extract was superseded by levothyroxine, a synthetic l form of tetraiodothyronine. Since then, no major innovation has emerged for the treatment of hypothyroidism. The biochemical definition of subclinical hypothyroidism is a matter of debate. Indiscriminate screening for hypothyroidism has led to overdiagnosis and treatment initiation at lower serum levels of thyroid-stimulating hormone (TSH) than previously. Adverse health effects have been documented in individuals with hypothyroidism or hyperthyroidism, and these adverse effects can affect health-related quality of life (QOL). Levothyroxine substitution improves, but does not always normalize, QOL, especially for individuals with mild hypothyroidism. However, neither studies combining levothyroxine and liothyronine (the synthetic form of tri-iodothyronine) nor the use of desiccated thyroid extract have shown robust improvements in patient satisfaction. Future studies should focus not only on a better understanding of an individual’s TSH set point (the innate narrow physiological range of serum concentration of TSH in an individual, before the onset of hypothyroidism) and alternative thyroid hormone combinations and formulations, but also on autoimmunity and comorbidities unrelated to hypothyroidism as drivers of patient dissatisfaction. Attention to the long-term health consequences of hypothyroidism, beyond QOL, and the risks of overtreatment is imperative.
Treatment of hypothyroidism with thyroid extract was replaced by synthetic levothyroxine in the 1970s. A decade later, the development of sensitive thyroid-stimulating hormone (TSH) assays led to a reduction in the average daily dose of levothyroxine, following the demonstration that serum TSH was often suppressed, indicating overtreatment1. Patient dissatisfaction with levothyroxine became evident in the 1990s1. Evidence from rodent experiments highlighted that levels of tri-iodothyronine (T3) in tissues could only be reproduced by continuous administration of both liothyronine (the synthetic form of T3) and levothyroxine2. This finding prompted studies to investigate the efficacy of using a combination of levothyroxine and liothyronine. Interpretations of these studies vary3, and guidelines have struggled to translate the evidence into clear clinical messages4,5. In the meantime, indiscriminate screening has led to overdiagnosis of hypothyroidism, and the biochemical threshold for initiating treatment has been lowered6. Treatment of hypothyroidism with a combination of liothyronine and levothyroxine has become more widespread and is promoted by some experts and patient advocates7–10, in the absence of clear evidence of benefit (discussed in this Review). Such treatments might lead to suppression of serum levels of TSH11, which is concerning as evidence from the past few years points to associations of both raised and suppressed serum levels of TSH with increased mortality12,13 and dementia14,15. As we enter the third decade of the twenty-first century, uncertainty about the optimal treatment of hypothyroidism prevails. Much of the debate is driven by considerations of the response to treatment as measured by quality of life (QOL).
This Review aims to interpret the clinical observations of patient dissatisfaction despite good biochemical management in the context of insights from recent studies into the molecular actions of thyroid hormones16 and an understanding of the determinants and utility of patient-reported QOL in patients with both overt and subclinical primary hypothyroidism. It differs from many other excellent reviews on hypothyroidism by including authors who have been involved not only in generating the primary laboratory and clinical evidence, which is the subject of this Review Article, but who also represent a range of views as to the aetiology of impaired QOL in primary hypothyroidism.
Definition of hypothyroidism
The diagnosis of primary hypothyroidism depends on an elevated serum level of TSH, reflecting the high sensitivity of the hypothalamic–pituitary axis to changes in circulating levels of thyroid hormones16. The earliest stage of hypothyroidism occurs when the circulating level of TSH is elevated while thyroid hormone levels are normal. The initial description of subclinical hypothyroidism stated that it is asymptomatic, but also recognized a second stage of mild hypothyroidism associated with nonspecific symptoms, normal tetraiodothyronine (T4) levels and increased TSH levels17. In overt hypothyroidism, thyroid failure progresses to a low serum level of free T4, accompanied usually, but not always, by symptoms18. The term ‘mild hypothyroidism’ has been largely abandoned in favour of a binary classification of subclinical or overt hypothyroidism. However, in 2015, a call was made to revive this category based on a TSH cut-off of 10 mU per litre19, although a quarter of the original group of patients with mild hypothyroidism actually had TSH levels below 10 mU per litre18. In a 2019 review of 20 surveys from across Europe, 4.7% of the population had undiagnosed hypothyroidism, which was subclinical in 4.1% of these individuals20. This finding reflects the high prevalence of the disease and the poor performance of classic symptoms of hypothyroidism in indicating hypothyroid disease21.
Free T4.
T4 that is not bound to protein in the circulation and is therefore available to act on tissues
TSH and thyroid hormone levels are treated as univariate in the standard classification of hypothyroidism; however, a multivariate approach might allow a more accurate diagnosis of euthyroidism22. In studies of euthyroid populations without thyroid disease, a reduction in levels of free T4 and, to a lesser extent, levels of free T3 show stronger associations with adverse outcomes than reduced levels of TSH23. Thus, an elevated TSH level is a valuable test with which to detect primary hypothyroidism but might be less helpful at indicating hypothyroidism within the tissues. Additionally, elevated levels of TSH can be due to causes other than primary hypothyroidism (BOX 1). TSH levels rise with age, so that the upper limit of the reference range might be shifted upwards by 3 mU per litre in those who are 70–89 years old24. Not recognizing this fact can lead to overdiagnosis of subclinical hypothyroidism24,25. Individuals aged over 55 years with TSH levels in the upper tertile of the normal range live longer than individuals with levels in the middle and lower tertiles, suggesting the need for age-specific reference intervals for TSH26. A single measurement of elevated levels of TSH can normalize naturally, especially if TSH levels are below 10 mU per litre, and repeated measurements are required to confirm hypothyroidism27. Obesity and smoking are associated with minor elevations in levels of TSH28, whereas levels of TSH tend to be lower in pregnancy than in non-pregnant women29. Additionally, TSH has a diurnal rhythm (amplitude 0.4 mU per litre, with acrophase at 3 am)30. However, these factors are unlikely to cause difficulty in making a diagnosis of hypothyroidism.
Box 1 |. Causes of an elevated serum levels of TSH.
With normal thyroid hormone levels
Subclinical hypothyroidism
During recovery from a non-thyroidal illness
During recovery from subacute or silent thyroiditis
Drugs (amiodarone, lithium, metoclopramide, domperidone)
Assay interference due to heterophile antibodies or macro-TSH
Mutations in the TSH receptor (partial TSH resistance)
Addison disease (corrected by glucocorticoid replacement)
With abnormal thyroid hormone levels
Overt hypothyroidism (low free T4)
Thyroid hormone resistance syndrome (elevated free T4)
TSH-secreting pituitary adenoma (elevated free T4)
Hypothalamic–pituitary disease (low free T4)
Acrophase.
The time at which a peak in a circadian rhythm occurs
Pathophysiology of hypothyroidism
Thyroid hormone signalling.
The thyroid gland contributes all of the T4 and approximately 20% of the T3 in the circulation; the remaining T3 is produced peripherally, through T4 deiodination3. Although circulating levels of T3 are critical to systemic thyroid hormone action, there is also substantial physiological control that is tissue-specific16,31. In addition, most studies focus on T3 as the key mediator of thyroid hormone signalling, but a 2021 study indicates that T4 itself might be able to trigger biological effects that are distinct from those triggered by T3 (REF.32).
Several tissues express type 1 iodothyronine deiodinase (DIO1) and type 2 iodothyronine deiodinase (DIO2), allowing the tissues to convert T4 to T3 and return T3 to the circulation. Studies using rat models revealed that T3 produced in the liver via DIO1 returns rapidly to the circulation, whereas T3 produced in the brain, pituitary and brown adipose tissue via DIO2 remains in the tissue much longer and can initiate thyroid hormone signalling locally33,34. This is because DIO1 resides in the cellular plasma membrane whereas DIO2 resides in the endoplasmic reticulum, closely associated with the nucleus35. Therefore, thyroid hormone signalling in tissues that express DIO2 is defined by the sum of incoming T3 from the serum (which normally occupies approximately 50% of the thyroid hormone receptors) and T3 produced intracellularly via deiodination. This additional (intracellular) source of T3 can elevate thyroid hormone receptor occupancy up to nearly 100%, thereby enhancing thyroid hormone signalling36. A unique property of DIO2 is its ‘self-destructive’ behaviour triggered by T4, namely, the process of T4 activation to T3 ubiquitinates DIO2, tagging it for destruction in the proteasomal system37. As a result, during hypothyroidism, the fractional activation of T4 to T3 is accelerated, whereas in hyperthyroidism the opposite is seen.
A third deiodinase, DIO3, inactivates both T4 and T3, which terminates thyroid hormone action16. The prevalent paradigm suggests that local thyroid hormone signalling reflects the balance between the activating and inactivating activities of DIO2 and DIO3, respectively16,31. Thus, local control of thyroid hormone signalling is tightly coordinated and the intensity of thyroid hormone signalling can differ among tissues. A key implication of this fact is that optimization of thyroid hormone replacement based on an index, such as serum levels of TSH, that is exclusively secreted by the pituitary gland might be sub-optimal or detrimental for other tissues (such as liver, skeletal muscle, adipose tissue or bone).
Serum levels of TSH as a diagnostic tool.
The medial basal hypothalamus (MBH) and the pituitary gland function as a unit to determine the activity level of the thyroid gland. This unit constantly monitors circulating levels of T4 and T3, leading to adjustments in TSH secretion38. Whereas T3 in serum can be detected directly by the MBH and pituitary cells, T4 in the serum must be converted to T3 before being detected by the MBH–pituitary unit. Detection of T4 is possible because the MBH and pituitary gland express the highest level of DIO2 in the brain, converting T4 to T3 inside the thyrotrophs and tanycytes that are located near the thyrotropin-releasing hormone (TRH)-secreting neurons38 (FIG. 1).
Fig. 1 |. Roles of the DIO1 and DIO2 pathways in the TSH feedback mechanism during treatment with levothyroxine.
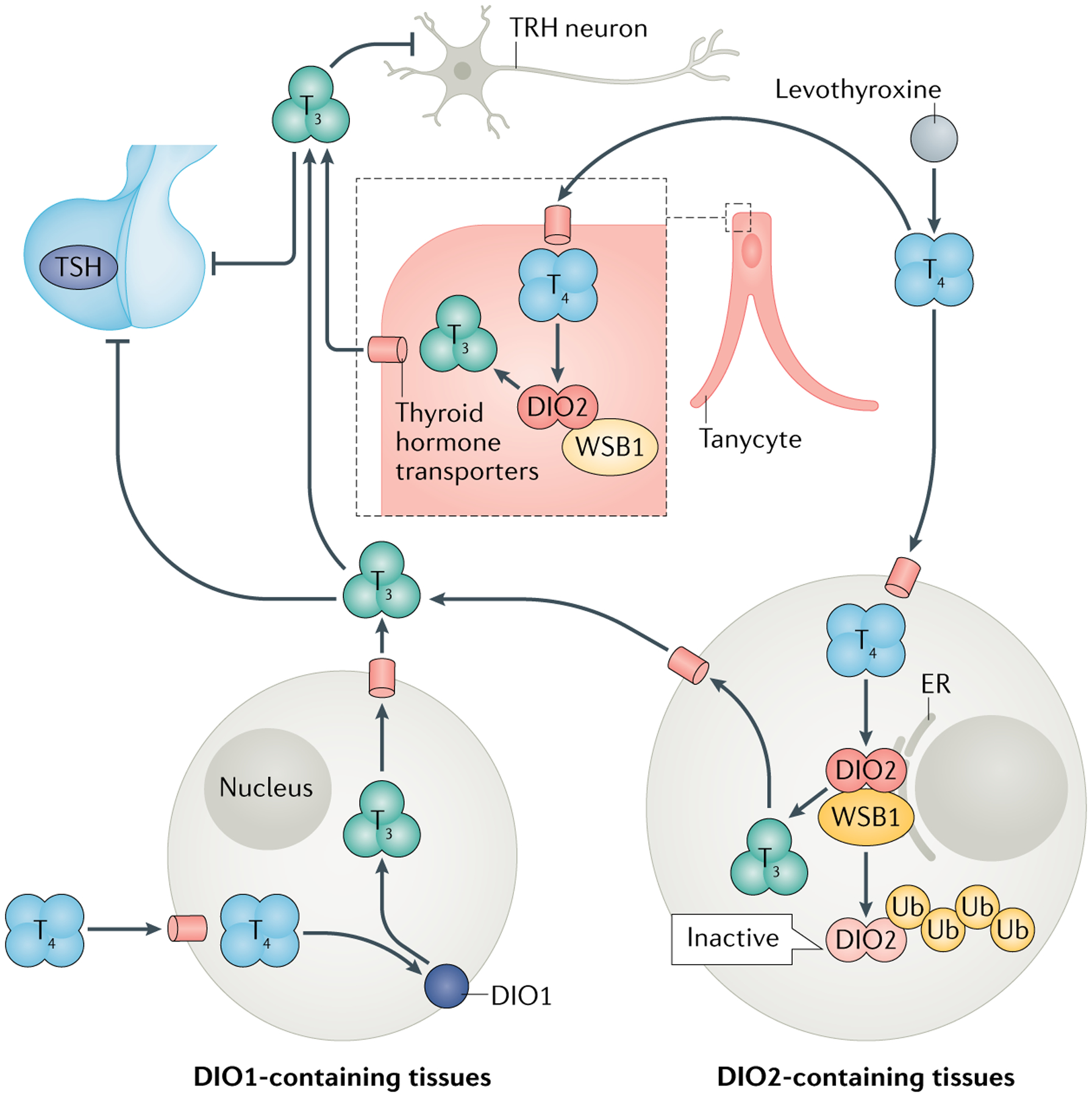
Levothyroxine is converted into triiodothyronine (T3) by the action of type 1 deiodinase (DIO1) and type 2 diodinase (DIO2). T3 produced in DIO1-expressing tissues (such as the liver) rapidly returns to the circulation, whereas T3 produced in DIO2-expressing tissues (such as the brain, pituitary and adipose tissue) remains in the tissue longer and stimulates local thyroid hormone signalling. Conversion of levothyroxine or thyroxine (T4) to T3 triggers the ubiquitination of DIO2 and its destruction by the proteasome. Serum levels of T4 are detected by the medial basal hypothalamus (MBH)–pituitary unit. Tanycytes within the MBH express high levels of DIO2, allowing them to convert T4 into T3, which then suppresses thyroid-stimulating hormone (TSH) secretion from the thyroid and thyrotropin releasing hormone (TRH) secretion from TRH neurons. ER, endoplasmic reticulum; Ub, ubiquitin. WSB1 is a subunit of ubiquitin ligase. FIGURE 1 adapted with permission from REF.128, Mary Ann Liebert Inc.
Thyrotrophs.
Cells in the anterior pituitary gland that secrete TSH
Tanycytes.
Specialized ependymal cells lining the walls of the third ventricle
The MBH–pituitary unit is highly sensitive to changes in levels of T4 and T3. A small drop in the serum levels of T4 immediately reduces T3 signalling in the MBH and pituitary, increasing secretion of TRH and TSH38. The reduction in T3 signalling occurs only because DIO2 is much less sensitive to tagging with ubiquitin in the MBH and pituitary gland than in other tissues, faithfully transducing serum levels of T4. In other words, the levels of T3 in the MBH and pituitary are an accurate reflection of serum levels of T4 (REF.39). Although a drop in serum levels of T3 also stimulates TSH secretion, the homeostatic mechanisms that preserve serum levels of T3 during hypothyroidism minimize its value as a diagnostic tool. The unique regulation of DIO2 in the MBH–pituitary unit makes serum levels of TSH an excellent index of thyroid activity, and an ideal biochemical marker for diagnosis of primary hypothyroidism. Nonetheless, strictly speaking, TSH levels reflect thyroid hormone signalling in the pituitary. Ideally, the identification of other markers that reflect thyroid hormone signalling in other tissues would provide a much more concise picture of global thyroid status.
TSH levels during levothyroxine treatment.
The advent of synthetic levothyroxine and sensitive TSH immunoassays led to the therapeutic goal of hypothyroidism treatment being switched from clinical to biochemical, that is, the normalization of serum levels of TSH. However, this rationale is flawed because the MBH–pituitary unit adjusts its secretion of TSH in response to changes in the levels of both T3 and T4, not T4 alone.
Typically, the dose of levothyroxine given to patients with hypothyroidism is adjusted with progressive elevation in T4 levels at intervals of 4–6 weeks. Studies in rats have shown that, as this is happening, T4 converts to T3 in the MBH and pituitary via the DIO2 pathway, slowing down TSH secretion38,39. T4 is also converted to T3 in all peripheral tissues via the DIO1 and DIO2 pathways, elevating serum levels of T3. However, normalization of T4 levels does not normalize serum levels of TSH because direct thyroid secretion of T3 is missing in patients with hypothyroidism and serum levels of T3 are relatively low when compared with healthy individuals with similar TSH levels. This scenario has been found in rat models as well as in patients treated with levothyroxine. To correct this issue, the physician further increases the dose of levothyroxine. This increased dose slightly elevates serum levels of T4 to a sufficient degree to normalize T3 content in the MBH and pituitary gland, which in turn normalizes TSH levels. However, T4 to T3 conversion is less efficient in the periphery compared with in the MBH–pituitary unit because T4 accelerates DIO2 ubiquitination and degradation39, and DIO1 activity is only fully normalized once T3 levels are fully restored, which limits the increase in circulating levels of T3 (REF.40). Thus, typically a patient treated with levothyroxine might have normal serum levels of TSH, but with T4 levels at, or slightly above, the upper limit of the normal range and serum levels of T3 at, or slightly below, the lower limit of the normal range (FIG. 1).
Studies in patients with hypothyroidism and rat models have shown that, in some tissues, thyroid hormone signalling remains subnormal under the scenario described earlier in this Review (normal TSH, marginally high free T4, marginally low free T3)2,39,41,42. Some patients seem to be more affected than others, which might reflect polymorphisms in genes encoding thyroid hormone transporters and DIO2 (REFS43,44), or inactivating mutations in the gene that encodes DIO1 (REF.45). These compounding factors would exaggerate differences between the MBH–pituitary unit compared with the periphery in their ability to activate T4 to T3. Many of these findings were obtained in levothyroxine-treated rat models, which faithfully reproduce a normalization of serum levels of TSH at a high T4 to T3 ratio16.
Generalized versus local tissue hypothyroidism.
Impaired cognition and feeling tired are among the main concerns of symptomatic patients treated with levothyroxine46,47. This observation suggests that T3 content in the brain is not fully restored by levothyroxine monotherapy. Indeed, the brain responds to subtle changes in local thyroid hormone signalling48, and is unique in that deiodinases play a substantial part in determining thyroid hormone signalling in the brain49. DIO2 is expressed in glial cells50,51 and the paracrine signalling by glial cell-derived T3 activates neuronal gene expression52. It is estimated that more than half of the thyroid hormone receptors in the brain are occupied with T3 produced in glial cells34,49. This scenario sets conditions that maximize the impact of even minor defects in the DIO2 pathway, such as gene polymorphisms. A mouse with glial-specific inactivation of the Dio2 gene exhibited depression–anxiety behaviour53, compatible with a localized reduction in thyroid hormone signalling.
Some patients with hypothyroidism carry the Thr92Ala-DIO2 polymorphism54, which reduces DIO2 activity by around 20%. These patients have improved clinical response to therapy containing liothyronine in some, but not all, studies43. This finding supports the idea of potential hypothyroidism within the brain in patients taking levothyroxine54. Indeed, a subsequent study showed that the association between therapy that contains liothyronine and improved clinical outcomes became even stronger when a polymorphism in the thyroid hormone transporter MCT10 was also considered, although the small sample size was a serious limitation43,55. The fact that this polymorphism is prevalent (the minor allele frequency varies between 17% and 47%)43 and that study outcomes are not readily reproducible across different populations is puzzling. Further studies on molecular signalling of thyroid hormone and tissue-specific responses should clarify the relationship between genetic polymorphisms, treatments for hypothyroidism and QOL.
Unmet patient needs in hypothyroidism
The aim of treatment in primary hypothyroidism is defined as levels of TSH within the reference range, without stipulations regarding symptoms29. We have highlighted the pathophysiological issues that this goal raises. In practice, some patients receiving conventional levothyroxine treatment do not return to the premorbid state when the TSH level is normalized. For example, more patients taking levothyroxine had impaired QOL scores, as judged by questionnaires, compared with controls not taking levothyroxine in a cross-sectional primary care survey46. In addition, treated patients had poorer cognitive function and wellbeing compared with national reference values for these tests47. Furthermore, anxiety and depression scores were higher in women with hypothyroidism taking levothyroxine than in those who were not56. Online surveys have confirmed such dissatisfaction and shown that this was worse in those taking levothyroxine alone or combined with liothyronine compared with those taking desiccated thyroid extract (DTE)57. However, the differences between these groups were small, and overall between 20% and 60% of patients remained dissatisfied regardless of treatment (FIG. 2).
Fig. 2 |. Proportion of dissatisfaction expressed by patients with self-reported hypothyroidism by type of treatment for hypothyroidism.
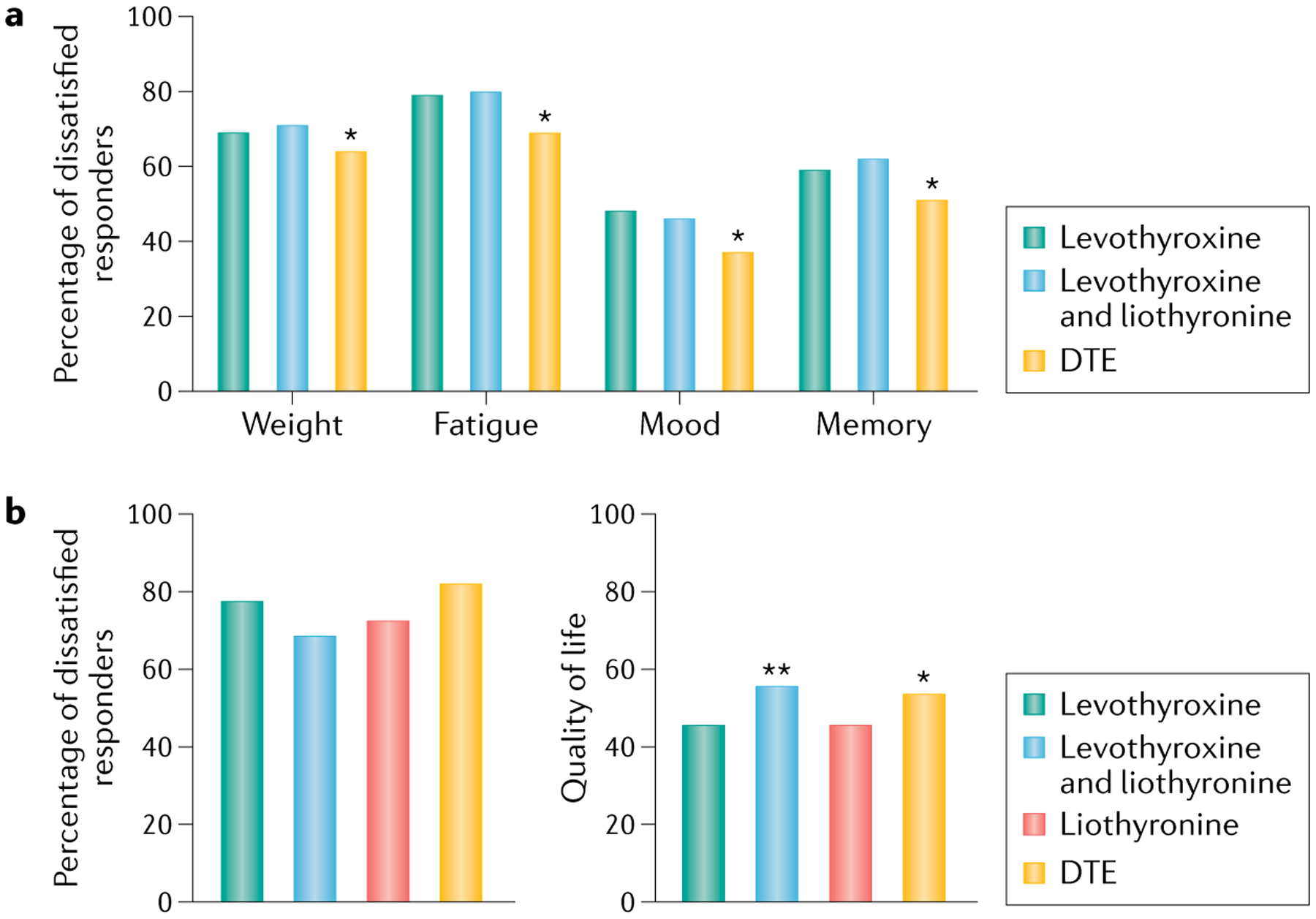
a | Percentage of dissatisfied responders across four categories: weight, fatigue, mood and memory. Responders received one of three treatments: levothyroxine (n = 6,949); levothyroxine and liothyronine combination therapy (n = 978) or desiccated thyroid extract (DTE) (n = 3,239). An asterisk denotes that the values for DTE were statistically significantly different (P < 0.05) to other treatments. b | Percentage of dissatisfied responders (left) receiving either levothyroxine (n = 677), levothyroxine and liothyronine combination therapy (n = 124), liothyronine (n = 45) or DTE (n = 123). Quality of life (QOL) scores (right) for the same patient groups. A score of 100 represents the best possible QOL while a score of 0 represents the worst. Asterisks denote statistically significant differences between levothyroxine compared with levothyroxine and liothyronine combination (**P = 0.001), and levothyroxine compared with DTE (*P = 0.010). These graphs show that the differences between different treatments are minor and that a substantial proportion of patients are dissatisfied and have a suboptimal QOL regardless of type of treatment, which suggests that combination treatment with levothyroxine and liothyronine or DTE often fails to restore health-related QOL. Panel a is derived from data from Peterson et al.57; panel b is derived from data from Mitchell et al.8.
These studies have inherent biases owing to subjectivity, case ascertainment and response rates, which favour inclusion of those who have symptoms and particular disease-related beliefs. The persistent symptoms reported are often nonspecific (for example, tiredness, depression and ‘brain fog’), but might include feeling cold, hoarseness, dry skin, weight gain and constipation; hypothyroid-like symptoms can overlap with the experiences of the euthyroid population18.
Such dissatisfaction demands attention given the large number of people with hypothyroidism and the increasing prescription of levothyroxine, particularly for subclinical hypothyroidism58,59. There are three possible explanations for this patient dissatisfaction, which are not mutually exclusive. The first possibility is that treatment with levothyroxine alone is physiologically unable to achieve adequate T3 levels in the tissues, possibly determined by genetic polymorphisms in deiodinases and hormone transporters. Alternatively, the symptoms could be due to an ongoing autoimmune process rather than hypothyroidism60,61. The final possibility is that the symptoms these patients are experiencing are unrelated to their thyroid disease. In this regard, similar symptoms have been reported in many other disorders, including Addison disease62, and any diagnostic labelling can itself be associated with poorer self-rated health than for individuals without a diagnosis63. Moreover, persistent unexplained physical symptoms are common in primary care64. Given the prevalence of thyroid dysfunction20, it is inevitable that new cases of hypothyroidism will be discovered coincidentally in such patients. The situation is aggravated by inappropriate treatment of equivocal TSH abnormalities.
Patient expectations for the effect of hypothyroidism therapy are increasing, in some cases driven by poor online information or distorted by healthism65; meanwhile, professional certainty over the optimal treatment for hypothyroidism seems to be declining among physicians5.
Tools for QOL determination
QOL is a complex and challenging concept, though it is commonly used and broadly correlates to an individual’s wellbeing66. Health-related QOL (HRQOL) encompasses the parts of QOL that relate to health. HRQOL is multi-dimensional and based on perceptions of health, including physical, social and psychological factors67. HRQOL might be unaffected in individuals who have a disease when they are asymptomatic and unaware of it. In this context, it is interesting that in a large population study, hypothyroidism identified by screening during the study was not associated with perceived poor health, whereas individuals who already had the diagnostic label of hypothyroidism reported poor health despite being treated63. Researchers have developed techniques that conceptualize and measure the multiple domains of HRQOL67. HRQOL is of particular importance when a treatment causes unwanted adverse effects, even while other outcome measures (such as survival) might be favourable. From the perspective of clinical research, HRQOL is an important metric that complements other measures of treatment efficacy. HRQOL is usually measured by patients’ self-rating. The tools can be generic or disease-specific; the latter are more sensitive, while the former allow comparisons across diseases68. The choice of HRQOL tool depends on whether it is intended to inform patients and healthcare professionals about the HRQOL benefit of an intervention or to inform policy makers about the relative value of a treatment.
Three often-used, generic HRQOL questionnaires are EQ-5D, SF-36 and WHOQOL, which have been validated and tested for cross-cultural applicability69–72. A variety of generic questionnaires have been used in studies of patients with hypothyroidism73 (TABLE 1). Single-item scores for HRQOL alone are considered insufficient to demonstrate the relative effectiveness of one intervention compared with another because they are subject to bias and often too crude to detect changes in health. The European Network For Health Technology Assessment recommends that HRQOL questionnaires should be completed by the patients themselves73, given that biases due to the use of proxies have been identified repeatedly74–76. Furthermore, they recommend that HRQOL measures must be valid, reliable, responsive and acceptable77,78.
Table 1 |.
Randomized studies showing altered QOL during treatment of subclinical or mild hypothyroidism
| Condition | QOL measure | Type of study | Effect on QOL with levothyroxine compared with placebo | Number of patients (mean age in years) | Ref. |
|---|---|---|---|---|---|
| Younger participants a | |||||
| Levothyroxine versus placebo for 48 weeks | Billewicz & Zulewski Symptom Score | Randomized, double-blind trial | Improved symptom scores on levothyroxine | 66 (58) | Meier et al. (2001)92 |
| Levothyroxine versus placebo for 26 weeks | Hospital Anxiety and Depression Scale, GHQ30 | Randomized, double-blind trial | Worse anxiety on levothyroxine | 40 (53) | Kong et al. (2002)93 |
| Levothyroxine versus placebo for 52 weeks | BDI, GHQ30, Wechsler Memory Scale-Revised | Randomized, double-blind trial | No change | 69 (62) | Jorde et al. (2006)94 |
| Levothyroxine and placebo (given in a random order) for 12 weeks | ThyDQoL, SF-36 | Randomized blinded crossover trial | Tiredness improved on levothyroxine | 100 (54) | Razvi et al. (2007)95 |
| Levothyroxine versus placebo for 12 weeks | WMS | Randomized, double-blind trial | Improved memory on levothyroxine | 60 (34) | Aghili et al. (2012)100 |
| Levothyroxine versus placebo for 6 months | BDI, Zulewski, SF-36 | Randomized, double-blind trial | Marginal benefits on SF-36 | 71 (50) | Reuters et al. (2012)97 |
| Levothyroxine versus placebo for 12 weeks | BDI | Randomized, double-blind trial | Somatic symptoms statistically significantly improved by levothyroxine versus placebo | 60 (34) | Najafi et al. (2015)98 |
| Older participants a | |||||
| Levothyroxine versus placebo for 40 weeks | CTQ Composite psychometric memory score | Randomized, double-blind trial | Improved memory score on levothyroxine | 37 (68) | Jaeschke et al. (1996)91 |
| Levothyroxine versus placebo for 52 weeks | Mini-Mental State Examination, cognitive function | Randomized, double-blind trial | No change | 94 (74) | Parle et al. (2010)96 |
| Levothyroxine versus placebo for 52 weeks | ThyPRO | Randomized, double-blind trial | No change | 737 (74) | Stott et al. (2017)101 |
| Levothyroxine versus placebo for 52 weeks | ThyPRO, EQ5D | Randomized, double-blind trial | No change | 105 (84) | Mooijaart et al. (2019)99 |
BDI, Beck’s Depression Inventory; CTQ, Chronic Thyroid Questionnaire; GHQ, General Health Questionnaire; WMS, Wechsler Memory Scale.
Younger participants are defined as having a mean age of 50 years; Older participants are defined as having a median age >65 years.
Responsive.
The responsiveness of an instrument determines its ability to detect relevant clinical changes over time
Acceptable.
How acceptable an instrument is determines its ease of use by participants
Several disease-specific tools have been developed for thyroid diseases and used in trials of patients with hypothyroidism (TABLE 1). An extensive review of thyroid-related HRQOL tools68 identified three questionnaires pertaining to hypothyroidism, the Chronic Thyroid Questionnaire79, the Thyroid Symptom Questionnaire (TSQ)46 and the Underactive Thyroid-Dependent Quality of Life Questionnaire80. However, robust validation was lacking for all three (absence of evaluation by qualitative studies, cognitive interviewing, clinical known-groups comparisons, multitrait analyses, differential item functioning and structural equation modelling81). It also became apparent that patients with thyroid conditions and clinicians had different and complementary perspectives on HRQOL82. Whereas patients were primarily concerned about the psychosocial impact on their everyday condition, clinicians were focused on disease characteristics (such as physical symptoms and signs of hypothyroidism)83.
The realization that there was a need for a sensitive and validated HRQOL tool led to the development of Thyroid Patient Related Outcome (ThyPRO)81,82 for patients with benign thyroid diseases. ThyPRO consists of 13 scales (FIG. 3). Four of these pertain to symptoms associated with goitre, hypothyroidism, hyperthyroidism and eye symptoms. The remaining scales cover mental health symptoms, function and wellbeing, and participation and functionality in social situations. Clinical validity and the test–retest reliability of the ThyPRO questionnaire were found to be high82. Prospective evaluation of responsiveness of ThyPRO in a large cohort of patients with hypothyroidism, hyperthyroidism and non-toxic goitre showed that ThyPRO was responsive to treatment across the range of benign thyroid diseases and performed better than SF-36 (REF.81). Psychometric modelling supported the construct validity of ThyPRO; however, some potential problems were identified (the questionnaire was long (85 items) and reported in numerous (13) scales)84,85. These potential shortcomings and the fact that ThyPRO is lengthy led to the development of a shorter 39-item version86, which was found to preserve responsiveness, clinical validity and test–retest reliability. Cross-cultural validity has since been demonstrated for this shorter version87 and the minimal important change was defined in 202188. ThyPRO has been translated into 19 languages89. The short version of ThyPRO86 seems to be the most appropriate tool for studying HRQOL in patients with hypothyroidism (FIG. 3).
Fig. 3 |. Changes in components of QOL before and after treatment of hypothyroidism.
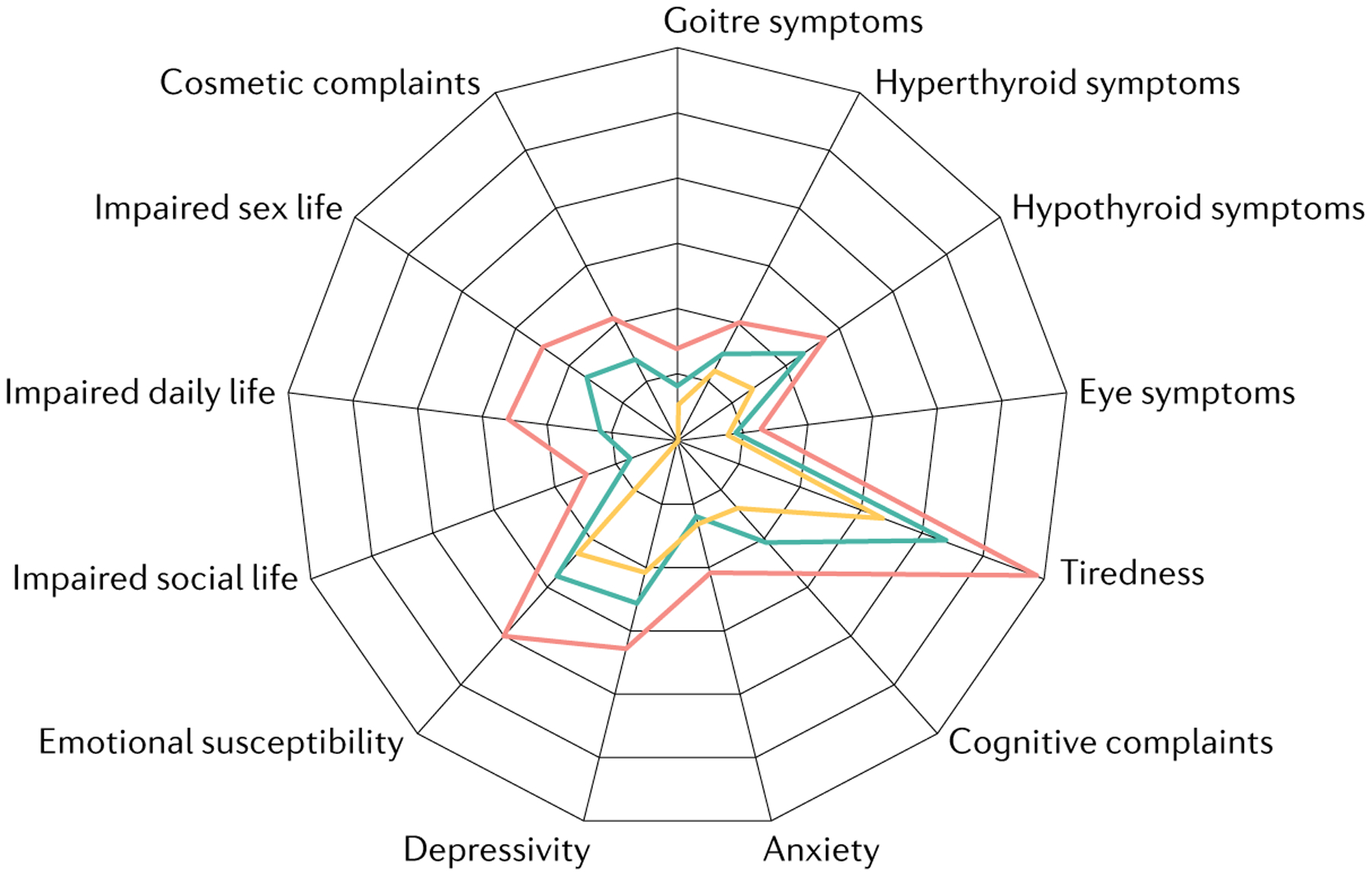
Radar plot showing patient-related outcome using ThyPRO, at baseline (red) and six months after starting levothyroxine treatment for autoimmune hypothyroidism (green), compared with normative data (yellow). FIGURE 3 is adapted from REF.107, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Test–retest reliability.
Stability of the scores obtained from the same person on two or more separate occasions
Minimal important change.
The smallest change in an outcome that is perceived by an individual patient as important
Effect of treating mild hypothyroidism
Impact of levothyroxine treatment on QOL.
Although patients with mild or subclinical hypothyroidism might have more symptoms (such as tiredness and cognitive issues) compatible with hypothyroidism compared with euthyroid patients90, there is very limited evidence to support QOL improvements with levothyroxine replacement in these patients. Overall, 11 studies have randomized patients with subclinical hypothyroidism to receive levothyroxine or placebo and reported subjective outcomes91–100 (TABLE 1). These studies are mostly small and quite heterogeneous, with four studies recruiting mainly older participants (median age >65 years, n = 9,732)96,99,101, whereas the remaining seven studies recruited younger patients (mean age 50 years, n = 466). Most studies examined more than one subjective outcome and the overall conclusion from meta-analyses is that levothyroxine did not improve symptoms, QOL or cognition102,103.
Nevertheless, the two largest studies are notable for their differences in design and outcomes95,101. The ‘TRUST’ study randomized 737 older people (median age 74 years) recruited largely from primary care databases to receive levothyroxine (median dose 50 μg daily) or placebo for a year101. The mean TSH level before treatment was 6.4 mU per litre in the participants randomized to levothyroxine, falling to a mean of 3.6 mU per litre during treatment. Although this study was originally designed to examine vascular endpoints, only three patients died of cardiovascular events, and the primary outcomes became fatigue and QOL, which were measured using ThyPRO. However, because of the recruitment strategy, patients had QOL scores indicating a low symptom burden at the study commencement and these scores were not improved by a small dose of levothyroxine. Further analysis of participants aged 80 years or older from this study and another trial of similar design with mean baseline TSH values of 6.3–6.4 mU per litre (n = 251 in total) also showed no positive effect of levothyroxine treatment on QOL scores99. By contrast, a 2007 study95 performed a crossover study of 100 younger patients (mean age 54 years), with a median TSH of 5.2 mU per litre on placebo and 0.5 mU per litre while taking 100 μg levothyroxine daily. They found a statistically significant reduction in reported tiredness from 89% to 78% during levothyroxine treatment, however, all other QOL indicators, including SF-36, were not significantly changed. Three of the other studies in younger participants (mean ages ranging from 34 to 58 years) also found statistically significant benefits from levothyroxine treatment92,98,100, whereas one study showed more anxiety symptoms in the active treatment arm93.
Although most of the current evidence is derived from these two larger studies95,101, neither study was perfect in design, and the levothyroxine doses and achieved serum concentrations of TSH were quite different. It is unknown whether treatment of subclinical hypothyroidism in elderly participants who have symptoms of hypothyroidism or whose TSH values at the start of the study are clearly above an age-adjusted reference range might still show some symptomatic benefits. In addition, there is little good-quality evidence to inform management of younger people less than approximately 60 years of age with subclinical hypothyroidism. Based on these studies, clinical guidelines do not recommend treating subclinical hypothyroidism in elderly people unless their serum levels of TSH rise to 10 mU per litre or higher104,105. However, the guideline endorsing a cut-off of 20 mU per litre105 is based on a meta-analysis103 in which the mean pooled baseline TSH values of patients included in the respective outcome meta-analyses were in the range of 6.4–7.3 mU per litre, with the upper limit of the 95% confidence intervals being 6.9–8.8 mU per litre106. By contrast, European and North American guidelines endorse a time-limited trial of levothyroxine therapy in symptomatic younger individuals as clinical experience shows that around 40% of these individuals will experience some subjective improvement29,104. Although there are signals of these positive effects in several of the randomized trials in younger participants92,93,98,100, these findings remain to be confirmed in larger, longer duration trials conducted in this age group.
Effect of treating overt hypothyroidism
Impact of no treatment compared with levothyroxine on QOL.
Signs and symptoms of overt hypothyroidism resolve with initiation of levothyroxine therapy18. However, there are few studies examining QOL when patients with hypothyroidism are initiated on levothyroxine therapy and clearly such studies are uncontrolled unless there is a subgroup of untreated patients. A mixed study of 78 patients with overt or subclinical hypothyroidism with a median age of 47 years showed that levothyroxine administration improved 9 of 13 ThyPRO scales and 5 of 8 SF-36 scales, although there were still deficits compared with normative data and only 12 participants had overt hypothyroidism107 (FIG. 3). In another study in which patients with overt hypothyroidism were started on a full replacement dose of 1.6 μg/kg levothyroxine versus a 25 μg dose with incremental increases once every 4 weeks for 24 weeks, clinical symptoms and QOL measured using the RAND-36 questionnaire had improved in both groups by 24 weeks108 (TABLE 2).
Table 2 |.
Studies showing altered QOL during treatment of overt hypothyroidisma
| Overt hypothyroidism | Ref. | ||||
|---|---|---|---|---|---|
| Condition | QOL measure | Type of study | Effect on QOL | Number of patients | |
| Untreated versus treated with levothyroxine: initiation of levothyroxine | ThyPRO, SF-36 | Open label (all untreated to treated) | Improved, based on both ThyPRO and SF-36 | 78 (15% overt hypothyroidism) | Winther et al. (2016)107 |
| Untreated versus treated with levothyroxine: initiation of levothyroxine | Clinical and symptom scores RAND-36 | Open label (all untreated to treated), low dose versus full dose initiation | Improved based on RAND-36 in both low and full dose arms | 50 (median TSH 48–61 mIU per litre) | Roos et al. (2005)108 |
| Untreated versus treated with levothyroxine: withdrawal of levothyroxine | SF-36 | Withdrawal compared with rTSH | Decreased QOL with withdrawal | 228 | Schroeder et al. (2006)109 |
| Untreated versus treated with levothyroxine: withdrawal of levothyroxine | Questionnaire designed by investigators | Withdrawal compared with rTSH | Decreased QOL with withdrawal | 291 | Lee et al. (2010)110 |
| Untreated versus treated with levothyroxine: withdrawal of levothyroxine | FACT-F | Withdrawal compared with rTSH | Decreased QOL with withdrawal | 74 | Taieb et al. (2009)111 |
| Untreated versus treated with levothyroxine: withdrawal of levothyroxine | FACT-F | Withdrawal compared with rTSH | Decreased QOL with withdrawal and duration of withdrawal | 78 | Chow et al. (2006)112 |
| Levothyroxine treated versus control populations (matched controls) | GHQ | Cross-sectional euthyroid patients versus matched controls | Decreased QOL in patients | 1,922 | Saravanan et al. (2002)46 |
| Levothyroxine treated versus control populations (reference values) | Symptom checklist-90 RAND-36 | Cross-sectional euthyroid patients versus reference values | Decreased QOL in patients | 2,509 (141 patients; 2,368 reference individuals) | Wekking et al. (2005)47 |
| Levothyroxine treated versus healthy controls | ThySRQ, ThyDQoL HDS, BDI, SF-36, WBQ-12, SCL-90-R | Cross-sectional euthyroid patients versus healthy controls | Decreased QOL in patients using ThySRQ symptom number and tiredness, BDI, WBQ-12 | 36 | Quinque et al. (2013)113 |
| Patients receiving levothyroxine to target different TSH goals | GHQ-28, SF-36, TSQ | Randomized, blinded, three-period crossover study | No significant effect | 56 | Walsh et al. (2006)115 |
| Patients receiving levothyroxine to target different TSH goals | SF-36, mood profile of states | Randomized, double-blind trial | No significant effect | 138 | Samuels et al. (2018)116 |
| Patients receiving levothyroxine with different TSH values within the normal range | ThySRQ, ThyDQoL HDS, BDI,SF-36, WBQ-12, SCL-90-R | Cross-sectional | QOL (based on ThySRQ) worse with upper normal TSH values | 102 | Quinque et al. (2013)113 |
| Patients receiving levothyroxine, some with iatrogenic hypothyroidism | SF-36 | Cross-sectional | Decreased QOL with undertreatment (both subclinical and overt hypothyroidism) | 2,057 (21.5% subclinical, 4.4% overt) | dos Santos Vigario et al. (2013)117 |
| Patients receiving levothyroxine, some with iatrogenic hypothyroidism | RAND-36 | Cross-sectional | No significant effect | 9,491 (10.3% TSH 4–10 mU per litre, 0.7% TSH >10 mU per litre) | Klaver et al. (2013)118 |
| Patients taking levothyroxine switched from tablet to liquid levothyroxine | ThyTSQ | Open label | Improved QOL with liquid levothyroxine | 418 | Guglielmi et al. (2018)119 |
| Patients taking levothyroxine switched to levothyroxine versus levothyroxine and liothyronine | SF-36, BDI, SCL-90-R | Randomized blinded, two-period crossover study | Improved with levothyroxine and liothyronine | 59 | Nygaard et al. (2009)123 |
| Patients taking levothyroxine switched to levothyroxine versus levothyroxine and liothyronine | Visual analogue scale | Randomized, non-blinded, two-period crossover study | Improved with levothyroxine and liothyronine | 33 | Bunevicius et al. (1999)124 |
| Patients taking levothyroxine switched to levothyroxine versus levothyroxine and liothyronine | GHQ-12, TSQ, HADS | Randomized, double-blind trial | GHQ-12 caseness (but not GHQ Likert) and HADS improved with levothyroxine and liothyronine at 3 months but not at 12 months | 697 | Saravanan et al. (2005)126 |
| Patients taking levothyroxine switched to levothyroxine versus levothyroxine and liothyronine | GHQ-28 | Randomized trial | Reduced anxiety and/or insomnia scores with levothyroxine and liothyronine | 71 | Valizadeh et al. (2009)125 |
| Patients taking levothyroxine switched to levothyroxine and liothyronine | ThyPRO-39 | Open label trial | Improved ThyPRO composite score with levothyroxine and liothyronine | 23 | Michaelsson et al. (2018)146 |
| Patients taking levothyroxine, levothyroxine and liothyronine, liothyronine, or DTE | Scale from 0–100 | Online survey | Improved QOL associated with levothyroxine and liothyronineor DTE | 969 | Mitchell et al. (2021)8 |
| Patients taking levothyroxine, levothyroxine and liothyronine, liothyronine, or DTE | Scale from 0–10 | Online survey | Improved QOL associated with DTE | 12,146 | Peterson et al. (2018)57 |
| Patients taking levothyroxine switched to levothyroxine versus DTE | GHQ-12, TSQ-36, BDI | Randomized, double-blind crossover trial | No statistically significant effect | 70 | Hoang et al. (2013)132 |
| Patients taking levothyroxine switched to levothyroxine monotherapy vs levothyroxine and liothyronine combination vs DTE | TSQ-36, GHQ-12, WMS-IV, BDI | Randomized double-blind crossover trial | No statistically significant effect | 75 | Shakir et al. (2021)122 |
BDI, Beck’s Depression Inventory; DTE, desiccated thyroid extract; FACT-F, Functional Assessment of Chronic illness Therapy-Fatigue; GHQ, General Health Questionnaire; HADS, Hospital Anxiety and Depression questionnaire; SCL, Symptom Check List; ThyDQoL, Underactive Thyroid-Dependent Quality of Life Questionnaire; TSQ, Thyroid Symptom Questionnaire; WBQ, Well-Being Questionnaire; WMS, Wechsler Memory Scale.
Nine randomized controlled trials of combination therapy did not show a benefit of levothyroxine and liothyronine to QOL. One trial did not report QOL parameters147.
However, there are several studies in which participants underwent a reversal of therapy, and levothyroxine was withheld during procedures for diagnosis or treatment of thyroid cancer (TABLE 2). Although these patients developed profound hypothyroidism, it was of relatively abrupt onset and short duration and so the effects on QOL might differ from those occurring with chronic hypothyroidism. For example, in a 2006 study using the SF-36 to assess QOL, withdrawal from levothyroxine led to a decline in all eight domains compared with both withdrawal and recombinant TSH (rTSH) groups at baseline and the rTSH group following rTSH administration109. The SF-36 scores were similar to those reported by patients with heart failure and depression, indicating a substantial decrease in QOL. Similar declines in QOL during levothyroxine withdrawal compared with use of rTSH were seen in other studies using a questionnaire designed by the investigators110 and using the Functional Assessment of Chronic illness Therapy-Fatigue (FACT-F) questionnaire and a visual analogue scale111. In another study, FACT-F scores worsened with increased duration of levothyroxine withdrawal112. In summary, although these studies do not provide randomized control trials of levothyroxine versus placebo for treatment of overt hypothyroidism, the benefits to QOL of treating overt hypothyroidism are undisputed and can be inferred from these ‘withdrawal’ studies.
During levothyroxine treatment.
Patients with hypothyroidism have impaired QOL compared with populations without hypothyroidism, despite achieving normal levels of TSH (TABLE 2). This was shown in a study in which surveys were sent to patients with hypothyroidism undergoing treatment and age-matched and sex-matched control individuals without hypothyroidism. Worse psychological wellbeing measured using the General Health Questionnaire (GHQ) was found in patients with hypothyroidism compared with control individuals46. Another study found impaired wellbeing in treated patients compared with standard reference values47. Similarly, in a study of treated patients versus healthy controls, the TSQ, symptom number and tiredness, Beck’s depression inventory, and Well-Being Questionnaire-12 (WBQ-12) were worse in the treated patients113. QOL in patients with hypothyroidism might be worse in those with higher BMIs114. Two blinded trials that altered the levothyroxine dose in patients receiving monotherapy to achieve different TSH values did not show resultant changes in the QOL of patients with hypo thyroidism115,116 (TABLE 2). Notably, one of these studies found that participants preferred the dose of levothyroxine that they perceived to be higher, irrespective of the actual dose administered116.
By contrast, a cross-sectional study showed worse QOL according to the Hypothyroidism Symptom Rating Questionnaire (ThySRQ) for patients whose TSH values were in the upper part of the normal range compared with those whose TSH values were in the lower part of the normal range113. Undertreatment of patients, regardless of whether the iatrogenic (undertreated) hypothyroidism is subclinical or overt, resulted in decreased QOL as measured by the SF-36 questionnaire117. However, in another study of patients being treated for hypothyroidism, QOL measured using the RAND 36-Item Health Survey did not differ between euthyroid individuals and those with either iatrogenic, subclinical or overt hypothyroidism118. There do not appear to be controlled trials of the effect of levothyroxine formulations on QOL. In one uncontrolled trial of patients taking levothyroxine tablets 30–60 minutes before breakfast who were switched to liquid levothyroxine with breakfast, there was an improvement in the ThyTSQ score119. However, given that both the levothyroxine formulation and the levothyroxine timing were changed simultaneously, it is difficult to determine which contributed to the improved TSQ score.
Levothyroxine and liothyronine combination treatment.
Fifteen randomized, controlled trials have examined the effect of levothyroxine and liothyronine combination therapy compared with levothyroxine monotherapy on various parameters (13 of these have been reviewed in a clinical practice guideline for the treatment of hypothyroidism120)121,122. Fourteen trials examined mood or QOL and ten of these 14 trials did not show improvement in these parameters. Two trials showed improvement in multiple measures123,124, whereas two trials showed improvement in a minority of measures125,126 (TABLE 2). Several trials showed a placebo effect during the levothyroxine therapy arm, as suggested by the improvement of QOL measurements in both treatment groups despite no change in levothyroxine dose120,123. In a 1999 trial124, the improvement was seen in the Profile of Mood Scores and a Visual-Analogue scale assessing mood and physical symptoms. In a 2009 trial123, improvement was seen in the SF-36 questionnaire, the Beck’s depression inventory, and the SCL 90-R scale. In a 2005 trial126, there was improvement in the GHQ scores in both groups at 3 months, with significantly more benefit in the combination therapy group, which was not maintained at 12 months. During a further 2009 trial125, the only improvement in patients receiving combination therapy was a decreased score in the anxiety and/or insomnia subscale of the GHQ-28, without changes in other subscales. By contrast, one substantial study found statistically significant worsening of GHQ-28 scores and increases in anxiety and nausea in the combined levothyroxine and liothyronine group127. Taken together, these studies do not suggest a beneficial effect of levothyroxine and liothyronine therapy for most participants.
Despite the lack of clear benefit of combination therapy, a 2021 consensus statement concluded that future trials that addressed some of the shortcomings of the prior trials could potentially be worthwhile128,129. The shortcomings identified in these published trials included many small studies with inadequate power, short-duration studies, failure to include patients who remained symptomatic while taking levothyroxine therapy, use of once-daily liothyronine administration and failure to study thyroid-specific patient-reported outcomes. With respect to adequate power, there has yet to be a prospective, adequately powered study of the effect of deiodinase polymorphisms or other genetic variants relevant to thyroid hormone homeostasis on patient response to therapy. Future studies that address these critiques, and potentially utilize a sustained-release liothyronine preparation, are anticipated now that the phase I trial of such a preparation has been completed. The results of a pharmacokinetic study of a sustained release T3 preparation (polyzincliothyronine) have been published online130 and a sustained release T4 and T3 combination might be forthcoming.
Desiccated thyroid hormone treatment.
Several surveys and uncontrolled studies suggest that patients prefer DTE treatment to levothyroxine treatment7,8,57,131, probably based on participant self-selection for unblinded treatments, but only two randomized studies have compared DTE to levothyroxine122,132. Using a crossover design, patients received each therapy for 16 weeks, and neurocognitive testing was performed at baseline and at the end of each period132. Despite marked biochemical differences characterizing the two therapies (lower free T4 while taking DTE; higher T3 with DTE), there were no differences in the primary outcomes, including memory, mood and QOL (TABLE 2). The QOL measures included a modified TSQ and the GHQ-12. There was a preference for DTE in 49% of the participants. A second randomized crossover study of 75 people confirmed no QOL benefit from DTE treatment compared with levothyroxine monotherapy or combined levothyroxine and liothyronine treatment122.
Effect of overtreatment on QOL.
The thyrotoxic state can be associated with neuropsychiatric symptoms, including euphoria133. Large doses of liothyronine have been used in euthyroid patients with depression, with apparently some efficacy134. Thyroid hormone ingestion in excess can, at least for some patients, improve HRQOL135. The long-term repercussions for morbidity and mortality of such overtreatment, well documented for levothyroxine12,13, remain unclarified for levothyroxine and liothyronine combination treatment.
Thyrotoxic.
A metabolic state characterized by elevated serum levels of tri-iodothyronine
Horizon scanning.
The fact that the commonest cause of hypothyroidism is autoimmune and that its clinical phase is preceded by subclinical disease defined by the presence of autoantibodies3 suggests potential interventions aimed at arresting or reversing the autoimmune attack. Emerging insights into the regulation of the autoimmune response136 might lead to therapies other than hormonal substitution, with potentially complete restoration or prevention of impairment of HRQOL. Regenerative medicine might also be able to offer novel solutions to patients with established hypothyroidism of other aetiologies137.
Conclusions
The appropriate threshold of serum levels of TSH at which to initiate levothyroxine treatment is still debated and has been falling over the past 20 years6; meanwhile, hypothyroidism is diagnosed in some individuals who are biochemically euthyroid, many of whom receive thyroid hormone therapy9. These trends in diagnosis and treatment are accompanied (and in many cases are driven), by high expectations by patients that their QOL8 will be improved by thyroid hormone treatment. Against this backdrop, clinicians are faced with a substantial minority of patients for whom levothyroxine treatment has failed to improve their QOL49.
The principal use of QOL instruments is in clinical research. Thyroid-specific QOL should be quantified with instruments able to measure relevant patient-related outcomes for hypothyroidism68. The most robust of these instruments is the ThyPRO, which has high content validity and test–retest reliability81,82. It responds to changes in benign thyroid disease phenotype and has cross-cultural validity; also, a minimally important change for hypothyroid symptoms has been defined88. Despite this, large well defined populations are needed to demonstrate statistically significant differences in QOL in relation to medical intervention128,129.
Content validity.
Extent to which the items tested are representative of the entire domain the test seeks to measure
The underlying causes of impaired QOL in patients with hypothyroidism are unclear, but might be classifiable into three broad categories. These are an inability of levothyroxine alone to achieve adequate T3 levels in the tissues128, inflammation caused by underlying autoimmunity138, or other physical139 and psychosocial co-morbidities140–142. An extensive body of evidence, summarized in systematic reviews and a meta-analysis conducted within the past five years, has shown that levo thyroxine and liothyronine combination therapy and DTE treatments were no different to levothyroxine alone in terms of QOL, neurocognitive function and somatic symptoms120,132. The latter two hypotheses on the cause of poor QOL have been studied less rigorously.
In line with the multifactorial aetiology of hypothyroidism143, the aetiology of poor QOL is also most probably multifactorial. Therefore, all the previously described causes of suboptimal QOL need further exploration in well controlled prospective trials including aetiologically better defined (and, ideally, genetically characterized) individuals with hypothyroidism144. While investigating a variety of thyroid hormone combinations and formulations145 and non-pharmacological interventions, we should also be reminded that persistent thyroid dysfunction is a health hazard5 for individuals with hypothyroidism. Over-zealous treatment is also a health hazard, as the duration of decreased TSH in treated individuals has a greater impact on mortality than duration of elevated levels of TSH10. The measurement of serum levels of TSH for diagnosis of primary hypothyroidism is the most reliable diagnostic test and the normal range for TSH at present remains the most appropriate target for patients treated with levothyroxine13.
Key points.
Epidemiological data suggest that the prevalence of (typically mild) hypothyroidism is increasing, partly owing to increased screening, which has led to a lower threshold for initiating treatment with levothyroxine.
Approximately 10–15% of individuals with hypothyroidism treated with levothyroxine experience persistent symptoms and dissatisfaction with therapy (that might or might not be due to their hypothyroidism), which can lead to overtreatment.
Health-related quality of life (QOL) is a complementary measure to morbidity and mortality; it should be measured with a validated thyroid-specific instrument for patient-related outcomes.
Poor QOL has been attributed to failure to achieve adequate T3 levels in tissues, polymorphisms in deiodinase and hormone transporter genes and/or symptoms unrelated to hypothyroidism such as autoimmune disease.
There is little evidence of durable QOL improvements with levothyroxine and liothyronine combination therapy, or from therapy with desiccated thyroid hormone, from a multitude of randomized controlled trials and meta-analyses.
Future research should investigate non-thyroidal causes of impaired QOL in patients with hypothyroidism as, at present, overtreatment for hypothyroidism constitutes a greater threat to health than undertreatment.
Acknowledgements
J.J. has received support from NIDCR grant R01DE025822 and NCATS grant UL1TR001409. A.C.B. has received support from NIH grants DK58538 and DK65055.
Footnotes
Competing interests
S.H.P. has received speaker fees from Quidel, Sanofi and Berlin Chemie, and consulting fees from Apitope. P.P. has received consulting fees from IBSA Institut Biochimique and speaker fees from Berlin Chemie. L.H. has received consulting fees from IBSA Institut Biochimique and speaker fees from Berlin Chemie. A.C.B. is a consultant for AbbVie, Allergan, Synthonics, Sention, and Thyron. J.J. and A.P.W. declare no competing interests.
References
- 1.McAninch EA & Bianco AC The history and future of treatment of hypothyroidism. Ann. Intern. Med 164, 50–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F & Morreale de Escobar G Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J. Clin. Invest 96, 2828–2838 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaker L, Bianco AC, Jonklaas J & Peeters RP Hypothyroidism. Lancet 390, 1550–1562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okosieme O et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin. Endocrinol 84, 799–808 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Perros P European thyroid association guidelines on l-T4 + l-T3 combination for hypothyroidism: a weary step in the right direction. Eur. Thyroid J 1, 51–54 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PN et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Int. Med 174, 32–39 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Toloza FJK et al. Patient experiences and perceptions associated with the use of desiccated thyroid extract. Medicina 56, 161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell AL, Hegedus L, Zarkovic M, Hickey JL & Perros P Patient satisfaction and quality of life in hypothyroidism: an online survey by the British Thyroid Foundation. Clin. Endocrinol 94, 513–520 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Negro R et al. Use of thyroid hormones in hypothyroid and euthyroid patients; the 2019 Italian Survey. Eur. Thyroid. J 9, 25–31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonklaas J, Tefera E & Shara N Short-term time trends in prescribing therapy for hypothyroidism: results of a survey of American Thyroid Association members. Front. Endocrinol 10, 31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.la Cour JL et al. Risk of over- and under- treatment with levothyroxine in primary care in Copenhagen, Denmark. Eur. J. Endocrinol 185, 673–679 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, Brix TH & Hegedus L Over- and undertreatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 28, 566–574 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Perros P, Nirantharakumar K & Hegedus L Recent evidence sets therapeutic targets for levothyroxine-treated patients with primary hypothyroidism based on risk of death. Eur. J. Endocrinol 184, C1–C3 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Thvilum M et al. Increased risk of dementia in hypothyroidism: a Danish nationwide register-based study. Clin. Endocrinol 94, 1017–1024 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Folkestad L, Brandt F, Lillevang-Johansen M, Heiberg Brix T & Hegedüs L Graves’ disease and toxic nodular goiter, aggravated by duration of hyperthyroidism, are associated with Alzheimer’s and vascular dementia: a registry-based long-term follow-up of two large cohorts. Thyroid 30, 672–680 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Bianco AC et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr. Rev 40, 1000–1047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evered DC, Ormston BJ, Smith PA, Hall R & Bird T Grades of hypothyroidism. Br. Med. J 1, 657–662 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zulewski H, Muller B, Exer P, Miserez AR & Staub JJ Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J. Clin. Endocrinol. Metab 82, 771–776 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Wiersinga WM Guidance in subclinical hyperthyroidism and subclinical hypothyroidism: are we making progress? Eur. Thyroid. J 4, 143–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendes D, Alves C, Silverio N & Batel Marques F Prevalence of undiagnosed hypothyroidism in Europe: a systematic review and meta-analysis. Eur. Thyroid. J 8, 130–143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carle A et al. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur. J. Endocrinol 171, 593–602 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Hoermann R, Larisch R, Dietrich JW & Midgley JE Derivation of a multivariate reference range for pituitary thyrotropin and thyroid hormones: diagnostic efficiency compared with conventional single-reference method. Eur. J. Endocrinol 174, 735–743 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald SP, Bean NG, Falhammar H & Tuke J Clinical parameters are more likely to be associated with thyroid hormone levels than with thyrotropin levels: a systematic review and meta-analysis. Thyroid 30, 1695–1709 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surks MI & Hollowell JG Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J. Clin. Endocrinol. Metab 92, 4575–4582 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Vadiveloo T, Donnan PT, Murphy MJ & Leese GP Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J. Clin. Endocrinol. Metab 98, 1147–1153 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Bano A et al. Association of thyroid function with life expectancy with and without cardiovascular disease: the Rotterdam study. JAMA Int. Med 177, 1650–1657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somwaru LL, Rariy CM, Arnold AM & Cappola AR The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J. Clin. Endocrinol. Metab 97, 1962–1969 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Moura Souza A & Sichieri R Association between serum TSH concentration within the normal range and adiposity. Eur. J. Endocrinol 165, 11–15 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Garber JR et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 22, 1200–1235 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Russell W et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab 93, 2300–2306 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Gereben B et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev 29, 898–938 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galton VA, Martinez ME, Dragon JA, St Germain DL & Hernandez A The intrinsic activity of thyroxine is critical for survival and growth and regulates gene expression in neonatal liver. Thyroid 31, 528–541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianco AC, Salvatore D, Gereben B, Berry MJ & Larsen PR Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev 23, 38–89 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Larsen PR, Silva JE & Kaplan MM Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr. Rev 2, 87–102 (1981). [DOI] [PubMed] [Google Scholar]
- 35.Baqui MM, Gereben B, Harney JW, Larsen PR & Bianco AC Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 141, 4309–4312 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Bianco AC & Silva JE Cold exposure rapidly induces virtual saturation of brown adipose tissue nuclear T3 receptors. Am. J. Physiol 255, E496–E503 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Gereben B, Goncalves C, Harney JW, Larsen PR & Bianco AC Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol. Endocrinol 14, 1697–1708 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Larsen PR Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N. Engl. J. Med 306, 23–32 (1982). [DOI] [PubMed] [Google Scholar]
- 39.Werneck de Castro JP et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J. Clin. Invest 125, 769–781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gereben B, McAninch EA, Ribeiro MO & Bianco AC Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol 11, 642–652 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridgway EC et al. Therapy of primary hypothyroidism with l-triiodothyronine: discordant cardiac and pituitary responses. Clin. Endocrinol 13, 479–488 (1980). [DOI] [PubMed] [Google Scholar]
- 42.Escobar-Morreale HF, Rey F, Obregon MJ & Escobar GM Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137, 2490–2502 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Bianco AC & Kim BS Pathophysiological relevance of deiodinase polymorphism. Curr. Opin. Endocrinol. Diabetes Obes 25, 341–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verloop H, Dekkers OM, Peeters RP, Schoones JW & Smit JW Genetics in endocrinology: genetic variation in deiodinases: a systematic review of potential clinical effects in humans. Eur. J. Endocrinol 171, R123–R135 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Franca MM et al. Human type 1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid 31, 202–207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saravanan P et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol 57, 577–585 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Wekking EM et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol 153, 747–753 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Marcelino CP et al. Temporal pole responds to subtle changes in local thyroid hormone signaling. J. Endocr. Soc 4, bvaa136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crantz FR, Silva JE & Larsen PR An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology 110, 367–375 (1982). [DOI] [PubMed] [Google Scholar]
- 50.Tu HM et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138, 3359–3368 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Guadano-Ferraz A, Obregon MJ, St Germain DL & Bernal J The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc. Natl Acad. Sci. USA 94, 10391–10396 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freitas BC et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Invest 120, 2206–2217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bocco BM et al. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-like behavior in male mice. Endocrinology 157, 3682–3695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panicker V et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J. Clin. Endocrinol. Metab 94, 1623–1629 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Carle A, Faber J, Steffensen R, Laurberg P & Nygaard B Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment — data using a blind, randomized, clinical study. Eur. Thyroid. J 6, 143–151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panicker V et al. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the HUNT study. Clin. Endocrinol 71, 574–580 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Peterson SJ et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 28, 707–721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonklaas J & DeSale S The ages and TSH values of patients being prescribed levothyroxine. Ther. Adv. Endocrinol. Metab 11, 2042018820937896 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM & Brito JP Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 5, 246–248 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Watt T et al. Is thyroid autoimmunity per se a determinant of quality of life in patients with autoimmune hypothyroidism? Eur. Thyroid. J 1, 186–192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guldvog I et al. Thyroidectomy versus medical management for euthyroid patients with Hashimoto disease and persisting symptoms: a randomized trial. Ann. Int. Med 170, 453–464 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Oksnes M et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J. Clin. Endocrinol. Metab 97, 568–576 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Jorgensen P, Langhammer A, Krokstad S & Forsmo S Diagnostic labelling influences self-rated health. A prospective cohort study: the HUNT Study, Norway. Fam. Pract 32, 492–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamahewa K, Buszewicz M, Walters K, Marston L & Nazareth I Persistent unexplained physical symptoms: a prospective longitudinal cohort study in UK primary care. Br. J. Gen. Pract 69, e246–e253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenhalgh T & Wessely S ‘Health for me’: a sociocultural analysis of healthism in the middle classes. Br. Med. Bull 69, 197–213 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Measuring healthy days. CDCP https://www.cdc.gov/hrqol/pdfs/mhd.pdf (2000). [Google Scholar]
- 67.Bakas T et al. Systematic review of health-related quality of life models. Health Qual. Life Outcomes 10, 134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watt T et al. Quality of life in patients with benign thyroid disorders. A review. Eur. J. Endocrinol 154, 501–510 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Gao F et al. The Singaporean English and Chinese versions of the EQ-5D achieved measurement equivalence in cancer patients. J. Clin. Epidemiol 62, 206–213 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Cheung YB, Machin D, Fong KY, Thio ST & Thumboo J Discriminative ability of the Short-Form 36 health survey: a tale of two versions. Qual. Life Res 14, 555–559 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Ware JE Jr, Keller SD, Gandek B, Brazier JE & Sullivan M Evaluating translations of health status questionnaires. Methods from the IQOLA project. International Quality of Life Assessment. Int. J. Technol. Assess. Health Care 11, 525–551 (1995). [DOI] [PubMed] [Google Scholar]
- 72.Skevington SM & Epton T How will the sustainable development goals deliver changes in well-being? A systematic review and meta-analysis to investigate whether WHOQOL-BREF scores respond to change. BMJ Glob. Health 3, e000609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.eunethta. Endpoints used for relative effectiveness assessment of pharmaceuticals: health-related quality of life and utility measures. European Network for Health Technology Assessment https://www.eunethta.eu/wp-content/uploads/2013/01/Health-related-quality-of-life.pdf (2013). [Google Scholar]
- 74.Fitzpatrick R, Davey C, Buxton MJ & Jones DR Evaluating patient-based outcome measures for use in clinical trials. Health Technol. Assess 2, 1–74 (1998). [PubMed] [Google Scholar]
- 75.Oczkowski C & O’Donnell M Reliability of proxy respondents for patients with stroke: a systematic review. J. Stroke Cerebrovasc. Dis 19, 410–416 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Williams LS et al. How valid are family proxy assessments of stroke patients’ health-related quality of life? Stroke 37, 2081–2085 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Guyatt GH, Feeny DH & Patrick DL Measuring health-related quality of life. Ann. Intern. Med 118, 622–629 (1993). [DOI] [PubMed] [Google Scholar]
- 78.Frei A, Svarin A, Steurer-Stey C & Puhan MA Self-efficacy instruments for patients with chronic diseases suffer from methodological limitations — a systematic review. Health Qual. Life Outcomes 7, 86 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaeschke R, Guyatt G, Cook D, Harper S & Gerstein HC Spectrum of quality of life impairment in hypothyroidism. Qual. Life Res 3, 323–327 (1994). [DOI] [PubMed] [Google Scholar]
- 80.McMillan CV, Bradley C, Woodcock A, Razvi S & Weaver JU Design of new questionnaires to measure quality of life and treatment satisfaction in hypothyroidism. Thyroid 14, 916–925 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Watt T et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J. Clin. Endocrinol. Metab 99, 3708–3717 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Watt T et al. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur. J. Endocrinol 162, 161–167 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Watt T et al. Which domains of thyroid-related quality of life are most relevant? Patients and clinicians provide complementary perspectives. Thyroid 17, 647–654 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Watt T et al. Confirmatory factor analysis of the thyroid-related quality of life questionnaire ThyPRO. Health Qual. Life Outcomes 12, 126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watt T et al. Few items in the thyroid-related quality of life instrument ThyPRO exhibited differential item functioning. Qual. Life Res 23, 327–338 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Watt T et al. Development of a short version of the thyroid-related patient-reported outcome ThyPRO. Thyroid 25, 1069–1079 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Watt T et al. Cross-cultural validity of the thyroid-specific quality-of-life patient-reported outcome measure, ThyPRO. Qual. Life Res 24, 769–780 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Nordqvist SF et al. Determining minimal important change for the thyroid-related quality of life questionnaire ThyPRO. Endocr. Connect 10, 316–324 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watt T et al. Thyroid-specific patient reported outcome (ThyPRO). ePROVIDE https://eprovide.mapi-trust.org/instruments/thyroid-specific-patient-reported-outcome#member_access_content (2021). [Google Scholar]
- 90.Canaris GJ, Manowitz NR, Mayor G & Ridgway EC The Colorado thyroid disease prevalence study. Arch. Intern. Med 160, 526–534 (2000). [DOI] [PubMed] [Google Scholar]
- 91.Jaeschke R et al. Does treatment with l-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J. Gen. Intern. Med 11, 744–749 (1996). [DOI] [PubMed] [Google Scholar]
- 92.Meier C et al. TSH-controlled l-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study). J. Clin. Endocrinol. Metab 86, 4860–4866 (2001). [DOI] [PubMed] [Google Scholar]
- 93.Kong WM et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am. J. Med 112, 348–354 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Jorde R et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J. Clin. Endocrinol. Metab 91, 145–153 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Razvi S et al. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J. Clin. Endocrinol. Metab 92, 1715–1723 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Parle J et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J. Clin. Endocrinol. Metab 95, 3623–3632 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Reuters VS et al. Effects of subclinical hypothyroidism treatment on psychiatric symptoms, muscular complaints, and quality of life. Arq. Bras. Endocrinol. Metabol 56, 128–136 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Najafi L et al. Depressive symptoms in patients with subclinical hypothyroidism — the effect of treatment with levothyroxine: a double-blind randomized clinical trial. Endocr. Res 40, 121–126 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Mooijaart SP et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. J. Am. Med. Assoc 322, 1977–1986 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aghili R et al. Changes of subtests of Wechsler Memory Scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch. Med. Sci 8, 1096–1101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stott DJ, Rodondi N, Bauer DC & Group TS Thyroid hormone therapy for older adults with subclinical hypothyroidism. N. Engl. J. Med 377, e20 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Villar HC, Saconato H, Valente O & Atallah AN Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst. Rev 2007, CD003419 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feller M et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. J. Am. Med. Assoc 320, 1349–1359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pearce SH et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur. Thyroid. J 2, 215–228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bekkering GE et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. Br. Med. J 365, l2006 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Sawka AM et al. Patient context and thyrotropin levels are important when considering treatment of subclinical hypothyroidism. Thyroid 29, 1359–1363 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Winther KH et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS ONE 11, e0156925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP & Berghout A The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch. Intern. Med 165, 1714–1720 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Schroeder PR et al. A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J. Clin. Endocrinol. Metab 91, 878–884 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Lee J et al. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid 20, 173–179 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Taieb D et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin. Endocrinol 71, 115–123 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Chow SM et al. Health-related quality-of-life study in patients with carcinoma of the thyroid after thyroxine withdrawal for whole body scanning. Laryngoscope 116, 2060–2066 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Quinque EM, Villringer A, Kratzsch J & Karger S Patient-reported outcomes in adequately treated hypothyroidism — insights from the German versions of ThyDQoL, ThySRQ and ThyTSQ. Health Qual. Life Outcomes 11, 68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelderman-Bolk N, Visser TJ, Tijssen JP & Berghout A Quality of life in patients with primary hypothyroidism related to BMI. Eur. J. Endocrinol 173, 507–515 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Walsh JP et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J. Clin. Endocrinol. Metab 91, 2624–2630 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Samuels MH, Kolobova I, Niederhausen M, Janowsky JS & Schuff KG Effects of altering levothyroxine (l-T4) doses on quality of life, mood, and cognition in l-T4 treated subjects. J. Clin. Endocrinol. Metab 103, 1997–2008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.dos Santos Vigário P et al. Inadequate levothyroxine replacement for primary hypothyroidism is associated with poor health-related quality of life — a Brazilian multicentre study. Endocrine 44, 434–440 (2013). [DOI] [PubMed] [Google Scholar]
- 118.Klaver EI et al. Thyroid hormone status and health-related quality of life in the LifeLines Cohort Study. Thyroid 23, 1066–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guglielmi R et al. Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr. Metab. Immune Disord. Drug. Targets 18, 235–240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jonklaas J et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid 24, 1670–1751 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaminski J, Miasaki FY, Paz-Filho G, Graf H & Carvalho GA Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch. Endocrinol. Metab 60, 562–572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shakir MKM et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J. Clin. Endocrinol. Metab 106, e4400–e4413 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nygaard B, Jensen EW, Kvetny J, Jarlov A & Faber J Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur. J. Endocrinol 161, 895–902 (2009). [DOI] [PubMed] [Google Scholar]
- 124.Bunevicius R, Kazanavicius G, Zalinkevicius R & Prange AJ Jr Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N. Engl. J. Med 340, 424–429 (1999). [DOI] [PubMed] [Google Scholar]
- 125.Valizadeh M et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr. Res 34, 80–89 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Saravanan P, Simmons DJ, Greenwood R, Peters TJ & Dayan CM Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J. Clin. Endocrinol. Metab 90, 805–812 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Walsh JP et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J. Clin. Endocrinol. Metab 88, 4543–4550 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Jonklaas J et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid 31, 156–182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jonklaas J et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Eur. Thyroid. J 10, 10–38 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dumitrescu AM et al. Extended absorption of liothyronine from poly-zinc-liothyronine (PZL): results from a phase 1, double-blind, randomized, and controlled study in humans. Thyroid 10.1089/thy.2021.0304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pepper GM & Casanova-Romero PY Letter to the Editor. Endocr. Pract 24, 230 (2018). [DOI] [PubMed] [Google Scholar]
- 132.Hoang TD, Olsen CH, Mai VQ, Clyde PW & Shakir MK Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab 98, 1982–1990 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Bunevicius R & Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs 20, 897–909 (2006). [DOI] [PubMed] [Google Scholar]
- 134.Kelly T An examination of myth: a favorable cardiovascular risk–benefit analysis of high-dose thyroid for affective disorders. J. Affect. Disord 177, 49–58 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Lewis DH, Kumar J, Goulden P & Barnes DJ Improvements in quality of life in hypothyroid patients taking Armour thyroid. Endocr. Abstr 15, P359 (2008). [Google Scholar]
- 136.Weetman AP An update on the pathogenesis of Hashimoto’s thyroiditis. J. Endocrinol. Invest 44, 883–890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Posabella A et al. Derivation of thyroid follicular cells from pluripotent stem cells: insights from development and implications for regenerative medicine. Front. Endocrinol 12, 666565 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bullmore E The art of medicine: inflamed depression. Lancet 392, 1189–1190 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Thvilum M et al. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. a nationwide register study. PLoS ONE 8, e75789 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thvilum M et al. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 24, 802–808 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Thvilum M, Brandt F, Brix TH & Hegedus L Hypothyroidism is a predictor of disability pension and loss of labor market income: a Danish register-based study. J. Clin. Endocrinol. Metab 99, 3129–3135 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Heiberg Brix T, Ferlov-Schwensen C, Thvilum M & Hegedüs L Death by unnatural causes, mainly suicide, is increased in patients with Hashimoto’s thyroiditis. A nationwide Danish register study. Endocrine 65, 616–622 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Brix TH & Hegedus L Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin. Endocrinol 76, 457–464 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Perros P et al. The enigma of persistent symptoms in hypothyroid patients treated with levothyroxine: a narrative review. Clin. Endocrinol 10.1111/cen.14473 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Nagy EV, Perros P, Papini E, Katko M & Hegedus L New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid 31, 193–201 (2021). [DOI] [PubMed] [Google Scholar]
- 146.Michaelsson LF et al. Levothyroxine/Liothyronine combination therapy and quality of life: is it all about weight loss? Eur. Thyroid. J 7, 243–250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fadeyev VV, Morgunova TB, Melnichenko GA & Dedov II Combined therapy with l-thyroxine and l-triiodothyronine compared to l-thyroxine alone in the treatment of primary hypothyroidism. Hormones 9, 245–252 (2010). [DOI] [PubMed] [Google Scholar]


