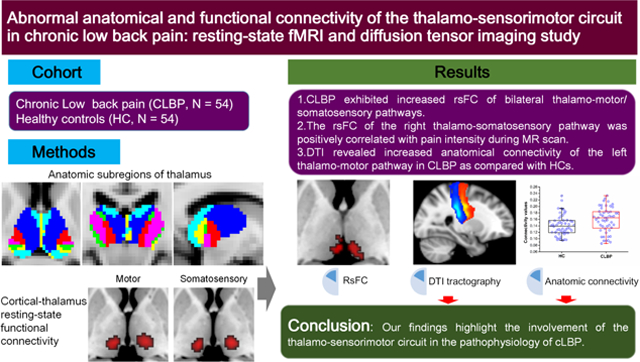
Abstract
Thalamocortical dysfunction is thought to underlie the pathophysiology of chronic pain revealed by electroencephalographic studies. The thalamus serves as a primary relay center to transmit sensory information and motor impulses via dense connections with the somatosensory and motor cortex. In this study, diffusion tensor imaging (probabilistic tractography) and resting-state functional magnetic resonance imaging (functional connectivity) were used to characterize the anatomical and functional integrity of the thalamo-sensorimotor pathway in chronic low back pain (cLBP). Fifty-four patients with cLBP and 54 healthy controls were included. The results suggested significantly increased anatomical connectivity of the left thalamo-motor pathway characterized by probabilistic tractography in patients with cLBP. Moreover, there was significantly increased resting-state functional connectivity (rsFC) of bilateral thalamo-motor/somatosensory pathways in patients with cLBP as compared to healthy controls. We also detected a significant positive correlation between pain intensity during the MRI scan and rsFC of the right thalamo-somatosensory pathway in cLBP. Our findings highlight the involvement of the thalamo-sensorimotor circuit in the pathophysiology of cLBP.
Keywords: chronic low back pain, thalamus, diffusion tensor imaging, probabilistic tractography, thalamo-sensorimotor circuit, resting-state functional connectivity
Graphical Abstract
Introduction
Pain is an unpleasant sensory experience usually accompanied by psychological distress. As a relay in coding sensory and emotional/motivational components of pain via several parallel ascending pathways (Groh et al., 2018), the thalamus transmits sensory information and motor impulses via dense connections with the somatosensory and motor cortex. Accumulating evidence suggests that circuits between the thalamus and cortex (especially the sensorimotor cortex) play an important role in mediating the perception of pain, and functional/anatomical alterations in thalamocortical circuits may be associated with the development and persistence of chronic pain (Alshelh et al., 2016;Bastuji et al., 2016;Groh et al., 2018;Henssen et al., 2019;Tu et al., 2019;Vanneste et al., 2018;Zippo et al., 2016). Thus, dysfunction of the thalamocortical pathway may underlie the pathophysiology of chronic pain (Garcia et al., 2021;Prichep et al., 2018;Schmidt et al., 2012;Tu et al., 2020).
Low back pain (LBP) is a leading cause of disability worldwide and is difficult to effectively treat due to unknown specific nociceptive cause (Hartvigsen et al., 2018). Previous studies have suggested that chronic LBP (cLBP) is associated with thalamic abnormalities and impaired sensorimotor control (Brumagne et al., 2019;Claeys et al., 2015). For instance, investigators have found that cLBP is associated with reduction of gray matter (GM) volume (Apkarian et al., 2004) and larger amplitude of low-frequency fluctuations in the thalamus (Zhang B. et al., 2019) as well as larger thickness (Kong et al., 2013) and volume (Li T. et al., 2018) of the sensorimotor cortex. Using a voxel-by-voxel hub functional connectivity method (Buckner et al., 2009), we found that the degree of intrinsic functional connectivity at the bilateral primary somatosensory cortex (S1) and motor areas (M1) was enhanced when patients with cLBP experienced high pain intensity compared to low pain intensity (Kong et al., 2013). Alterations of the cerebral blood flow in the thalamus have also been associated with pain intensity increase in cLBP (Lee et al., 2019).
Diffusion tensor imaging (DTI) (Basser et al., 1994) and blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) (Fox and Raichle, 2007) are two widely applied imaging modalities to investigate anatomic and functional connections within the brain. DTI can measure the integrity of the white matter (WM) based on quantifying the macroscopic axonal organization in the brain (Mori and Zhang, 2006). It can provide two common measures, fractional anisotropy (FA) and mean diffusivity (MD), to characterize WM integrity (Basser and Pierpaoli, 1996). Resting-state functional connectivity (rsFC) can display the intrinsic functional organization among spatially distributed brain regions by quantifying the temporal correlation of the spontaneous BOLD signal. Both DTI and resting-state fMRI have been used in characterizing thalamocortical connectivity alterations in neuropsychiatric disorders such as chronic low back pain (Tu et al., 2020), schizophrenia (Giraldo-Chica et al., 2018), psychosis (Cho et al., 2016), autism spectrum disorder (Nair et al., 2013), and alterations resulting from their treatments (Zhang Y. et al., 2021), thus demonstrating their potential in elucidating the role of the thalamocortical circuit in these disorders. Previous DTI studies have suggested reduced FA values in thalamic radiation (Ma et al., 2020) and the primary somatosensory cortex (S1) (Kim et al., 2020) in patients with cLBP. Although altered DTI measurments at the thalamus and sensori-motor areas has been reported in cLBP separately, whether there is altered anatomical connectivity between the thalamus and sensori-motor areas in cLBP remains unknown.
Thus, this study aimed to simultaniously examine the anatomical and functional thalamocortical connectivity in non-specific cLBP and its association with pain intensity by combining probabilistic tractography and rsFC analyses. We hypothesized that repeated attacks of cLBP can alter the functional thalamocortical connectivity, which may further change the anatomic connection. Thus, cLBP is associated with both abnormal anatomical and functional connectivity of the thalamocortical pathway, particularly the thalamo-sensorimotor pathways. We chose non-specific cLBP in this study, as it is the most common subtype of LBP (Maher et al., 2017).
Methods
Participants
A total of 54 patients with cLBP aged 18–60 years and 54 demographically-similar healthy controls were included in this study (about half of the data has been used in a recent cLBP study (Tu et al., 2020)). All patients with cLBP and healthy controls were recruited via advertisements across local communities. Patients were included only when their pain duration was longer than three months and their pain intensity was 4 or higher on a 0–10 continuous Visual Analog Scale (VAS) during screening. Both the mean level of pain intensity in the past week and the pain intensity during the MRI scan were recorded. No patients reported that they were receiving regular pharmacological treatment (including opioid treatments) for their back pain, except for over-the-counter painkillers when needed during the experiment. Detailed inclusion and exclusion criteria can be found in the Supplemental materials. All participants gave their written informed consent before the MRI scan. The study was approved by Partners Institutional Review Board of Massachusetts General Hospital (Boston, MA, USA).
Questionnaires
Pain intensity of all patients in the past week was evaluated by VAS before the MRI scan. In addition, we measured pain intensity before and after the resting-state fMRI scan (the average of the two ratings was used as the back pain intensity during the MRI scan). Depression symptoms of the cLBP patients were evaluated with the Beck Depression Inventory-II (BDI) (Beck et al., 1996).
MRI data acquisition
All MR data were acquired at the Martinos Center for Biomedical Imaging using a 3.0 T Siemens scanner equipped with a 32-channel head coil. A T1-weighted 3-dimensional multi-echo magnetization-prepared rapid gradient-echo (MEMPRAGE) sequence (repetition time (TR): 2200 milliseconds (ms), echo time (TE): 1.54 ms, slice thickness: 1mm, 176 axial slices covering the whole brain; matrix=256×256) was used to acquire the high resolution structural images. Resting-state functional MRI data were acquired using a T2-weighted gradient-echo and echo planar imaging sequence (TR/TE = 3000ms/30ms, flip angle: 90°, slice thickness: 3 mm, inter-slice gap: 0.88 mm, 44 slices, and 164 time points). All subjects were required to open their eyes and blink normally during the fMRI scan. DTI data were acquired using echo-planar diffusion-weighted images covering the whole brain (TR = 103000 ms, TE = 85 ms, matrix = 128×128, slice thickness = 2mm (zero gap), 64 slices. Two degrees of diffusion weighting (b = 0 s/mm2 and b = 1000 s/mm2) were used. DTI data were acquired in 61 non-collinear directions.
Regions of interest for thalamus/cortical areas
The regions of interest (ROIs) for the thalamus and all cortical regions were obtained from the “Harvard-Oxford cortical/subcortical structural atlases” (Frazier et al., 2005) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Selected cortical structures were combined to generate six bilateral nonoverlapping ROIs: the prefrontal cortex, motor cortex, somatosensory cortex, posterior parietal cortex, temporal lobe, and occipital lobe, which were used as targets in the probabilistic tractography. Similar segmentation methods have been used in previous studies (Giraldo-Chica et al., 2018;Sheffield et al., 2020;Yao et al., 2020). The thalamus determined from the atlas was used as seed in the probabilistic tractography. In order to eliminate voxels with low grey matter intensity, the cortical ROIs were masked at a threshold of 0.15 using the Laboratory of Neuroimaging probabilistic atlas gray matter tissue map (Shattuck et al., 2008). Detailed information of the specific brain regions contained in the six cortical ROIs can be found in Supplemental Table 1.
DTI probabilistic tractography
A seed-to-target (i.e., thalamus to cortex) probabilistic tractography was performed to characterize the thalamo-cortical anatomical connectivity by probtrackx2 (Behrens et al., 2007) of the Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/FDT) embedded in the FMRIB Software Library (version 5.0.9) (Smith et al., 2004). Tractography was performed only for the ipsilateral hemisphere (i.e. from left thalamus to left cortex) similar to methods used in previous studies(Giraldo-Chica et al., 2018;Nair et al., 2013). From probtrackx2, 12 seed-to-target voxel-wise volumes were generated in which the value of each voxel within the thalamus (seed) represented the sampling numbers from that voxel to the relevant cortical ROI (target). The connectivity of the thalamus-to-each cortical ROI was calculated by dividing the sampling number reaching that region by the total sampling number within the thalamus reaching all cortical ROIs. These values were the total connectivity (in percent) which would be used in the following primary DTI analysis described below.
Primary DTI analysis
Given the important role of the somatosensory and motor cortex in cLBP (Brumagne et al., 2019), we have pre-defined the anatomical connectivity (defined by probabilistic tractography) between the thalamus and motor/somatosensory regions as our primary outcomes. Analysis of covariance (ANCOVA) was performed to compare the difference of the anatomical connectivity (in percent) between the cLBP and healthy controls for above measurements, with age and gender included as covariates. A false discovery rate (FDR) correction was applied to these comparisons. After that, a cluster-wise analysis in FSL software was performed to localize the potential between-group differences in thalamus with a thresholded of P = 0.05 (familywise error (FWE) corrected).
Secondary DTI analysis
We also performed secondary/exploratory DTI analysis to investigate the between-group differences of the 1) FA, MD, and volumes in the thalamo-motor/somatosensory pathways, and 2) the anatomical connectivity (in percent) between thalamus and other cortical areas, i.e., the bilateral prefrontal cortex, posterior parietal cortex, temporal lobe, and occipital lobe. ANCOVA was applied to explore the differences between the cLBP and healthy control groups on these measurements. Age and gender were included as covariates. A threshold of P < 0.05 uncorrected was applied due to the exploratory nature of the secondary analysis (Li G. et al., 2017).
RsFC analysis
Resting-state BOLD-fMRI data were analyzed using CONN toolbox version 18b (http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). We discarded the first four time points to remove the signal instability. Preprocessing included realignment and unwarping of the functional images, slice-timing correction, motion correction, co-registration with structural T1 data, spatial normalization into the standard Montreal Neurological Institute (MNI, Canada) space, and smoothing (full width at half maximum = 6 mm). The default CONN preprocessing pipeline was automatically set up to use a combination of aCompCor (White and cerebrospinal fluid ROIs, 5 components each), motion regression (6 first-order temporal derivatives + 6 motion parameters as regressors), and filtering to reduce noise (band: 0.008–0.09Hz).
A “seed-to-voxel” functional connectivity analysis was conducted with the cortical ROIs as seeds including bilateral prefrontal cortex, motor cortex, somatosensory cortex, posterior parietal cortex, temporal lobe, and occipital lobe. Functional connectivity maps for six cortical ROIs were created for each individual. Briefly, the mean time series from each cortical ROI was computed and used as the reference time course. The Pearson correlation coefficients were computed between each cortical ROI and all other brain voxels to generate correlation maps. To remove the head motion, several regressors were entered, including six motion correction parameters and their first temporal derivatives, gray matter, WM, and cerebrospinal fluid. For group analyses, a Fisher’s r-to-z transformation was done to convert the correlation coefficients to z-values in order to improve normality (Lowe et al., 1998). Finally, we combined the left and right thalamus into one seed mask based on the automated anatomical labeling and IBASPM 71 brain atlas (Collins et al., 1995). For each ROI of the six cortical regions, their connectivity with the thalamus were explored by a small-volume correction analysis implemented in SPM, with a significance level of voxel-wise P < 0.005 and cluster-wise P < 0.05 after FDR correction in the between-group comparisons. In all between-group analyses, age and gender were included as covariates.
Statistical analysis
SPSS 24.0 (SPSS Inc.) was used to conduct the statistical analyses. Between-group differences of age were compared by two sample t-tests. The gender difference between groups was evaluated by chi-squared tests. P-values < 0.05 were considered statistically significant. We also explored the association between the brain connectivity assessments, pain intensities (pain in the past week and during the resting state MRI scan), and depression scores in cLBP group.
Since altered thalamic grey matter density has been reported in chronic back pain (Apkarian et al., 2004), we also extracted the mean thalamic grey matter density (bilateral) for all participants and analyzed the association between thalamic grey matter density and their rsFC. The T1-weighted structural images were processed using voxel-based morphometry within the SPM12 (Ashburner J, 2016). Segmentation was applied to the structural images of each subject and the probability maps of gray matter, white matter, and cerebrospinal fluid were generated. The grey matter density of the thalamus for each subject was extracted by using the gray matter maps and thalamus masks.
Results
Demographic and clinical data
No significant differences were found in age (P = 0.3, two-sample t-test) or gender (P = 0.578, χ2 test). Demographic and clinical data of participants are shown in Table 1. The mean BDI scores of the cLBP patients were 3.5 (Stand Deviation = 7.3), indicating low depressive symptoms in these patients. The mean pain intensity in the past week and during the resting-state MRI scan was 5.6 ±1.5, and 4.0 ± 0.3 respectively in cLBP patients.
Table 1.
Demographic and clinical data.
| Item | HC (N = 54) | CLBP (N = 54) | P value |
|---|---|---|---|
| Age (years) | 30.3 ± 6.7 (23–50)a | 32.3 ± 9.6 (19–53) | 0.3 |
| Gender (male/female) | 22/32 | 22/32 | 0.58 |
| Pain intensity in past week | NA | 5.6 ±1.5 | NA |
| Pain intensity during scan | NA | 4.0 ± 0.3 | NA |
| Pain duration (years) | NA | 7.0 ± 5.3 | NA |
| BDI scores | NA | 3.5 ± 7.3 | NA |
| Mean of total FD (mm) | 0.08 ± 0.045 | 0.092 ± 0.041 | 0.15 |
HC, healthy control; CLBP, chronic low back pain; NA, not available; BDI, Beck Depression Inventory; FD, Framewise displacement.
mean ± standard deviation
Thalamocortical anatomical connectivity
Results from primary DTI analysis:
The thalamus was parceled into 6 subregions corresponding to six cortical ROIs via probabilistic tractography in both cLBP and healthy controls, as shown in Fig. 1. The thalamo-motor and thalamo-somatosensory WM pathways revealed by probabilistic tractography in healthy controls are shown in Fig. 2. Between-group comparisons revealed significantly larger anatomical connectivity (in percent) of the left thalamo-motor pathway in patients with cLBP than in healthy controls (P = 0.036, FDR corrected, Fig. 3A).
Figure 1. Thalamus subregions characterized by DTI.
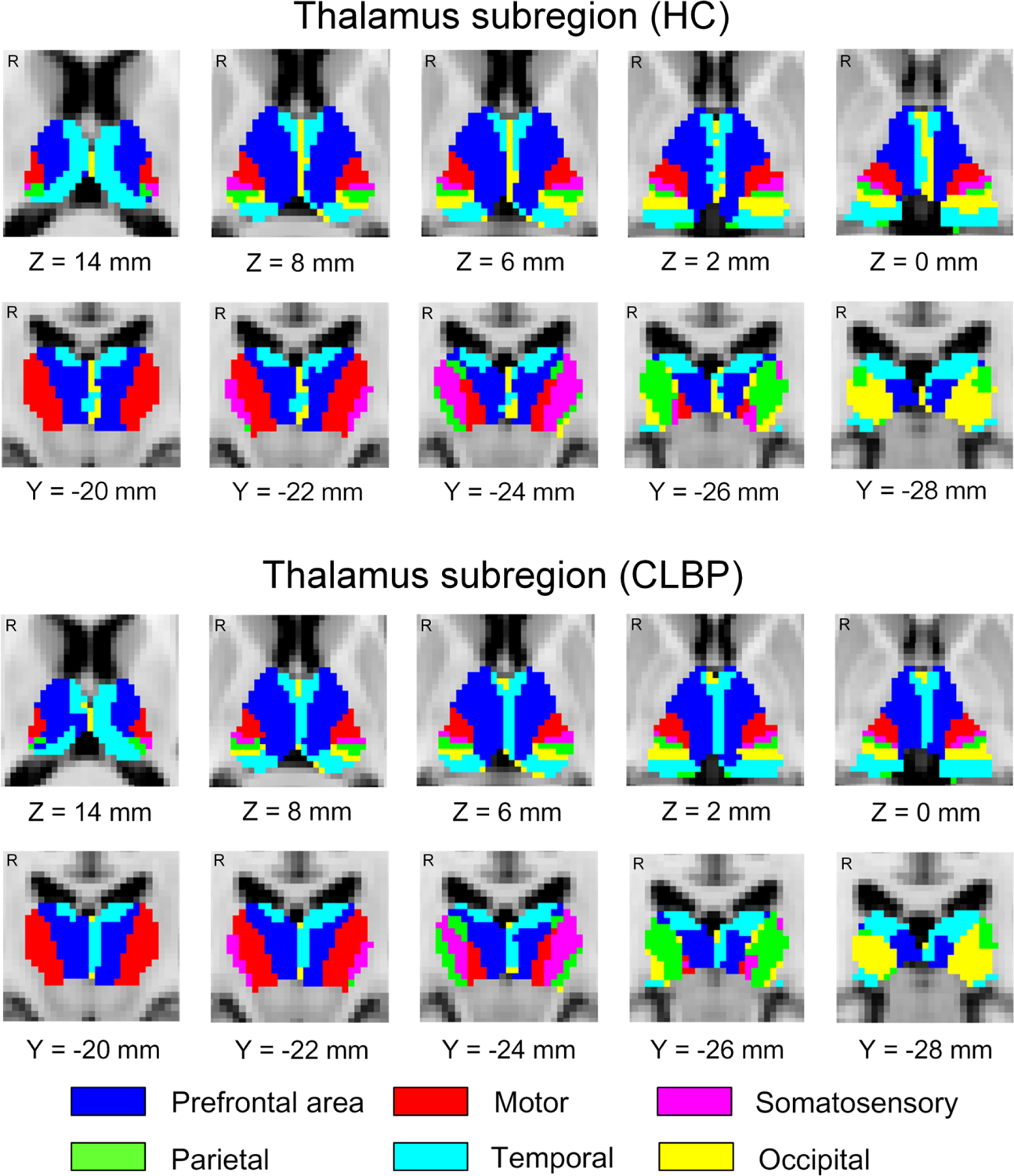
The thalamus subregions in 54 healthy controls (a) and 54 patients with chronic low back pain revealed by probabilistic tractography are shown. Each color represents one thalamic subregion corresponding to its cortical region of interest. HC, healthy controls; CLBP, chronic low back pain; R, right; mm, millimeter. “Z” and “Y” are Montreal Neurological Institute coordinates, representing axial and coronal views, respectively.
Figure 2. Thalamo-motor/somatosensory pathways characterized by DTI.
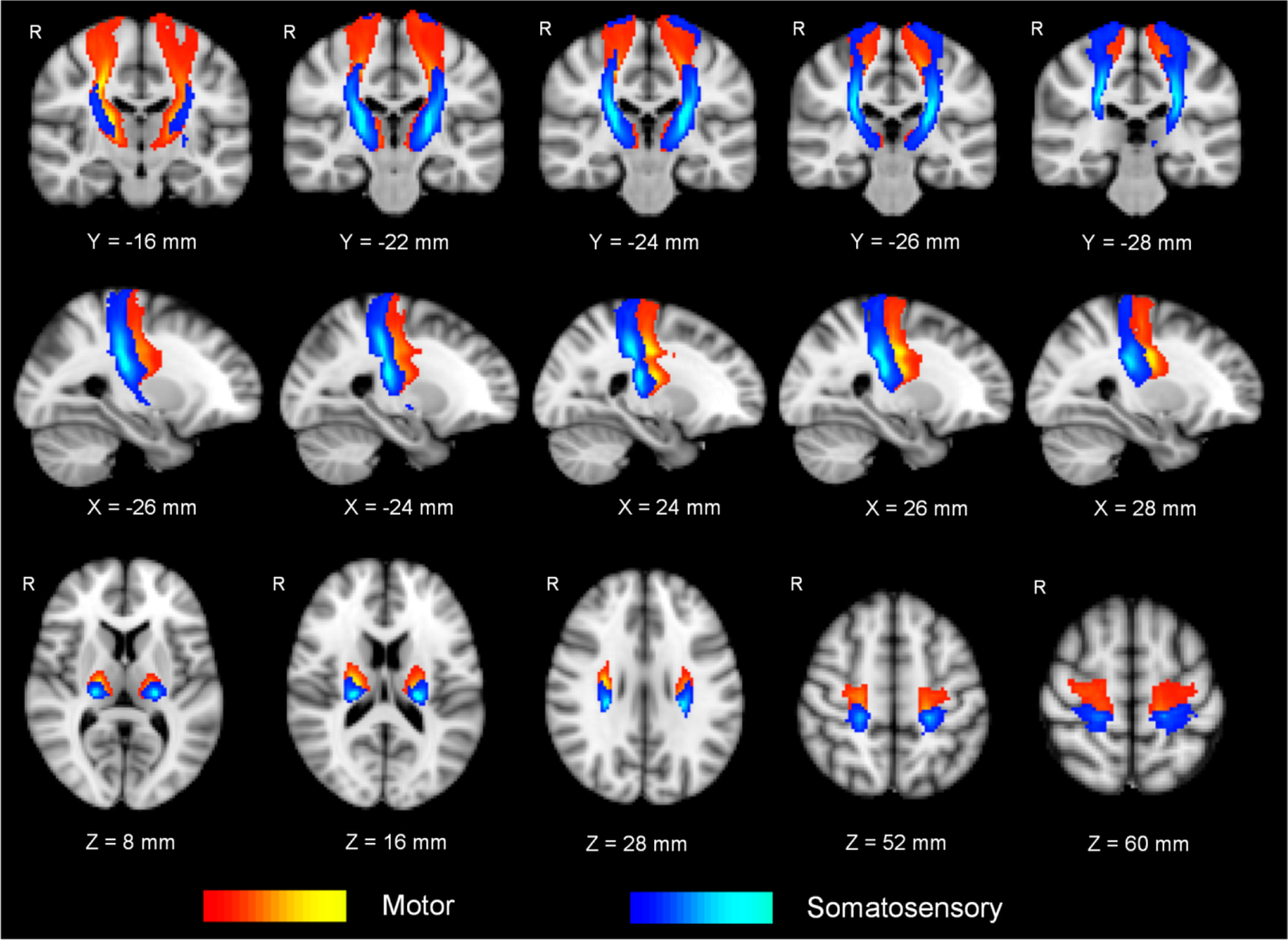
The thalamo-motor and thalamo-somatosensory pathways are group-based maps in 54 healthy controls revealed by probabilistic tractography. The “red-yellow” represents the thalamo-motor pathway and “blue-light blue” represents the thalamo-somatosensory pathway. The bluer and yellower represent the larger sampling numbers. “X”, “Y”, and “Z” are Montreal Neurological Institute coordinates, representing the sagittal, coronal and axial views, respectively. mm, millimeter; R, right.
Figure 3. Anatomical connectivity of the thalamo-motor pathway.
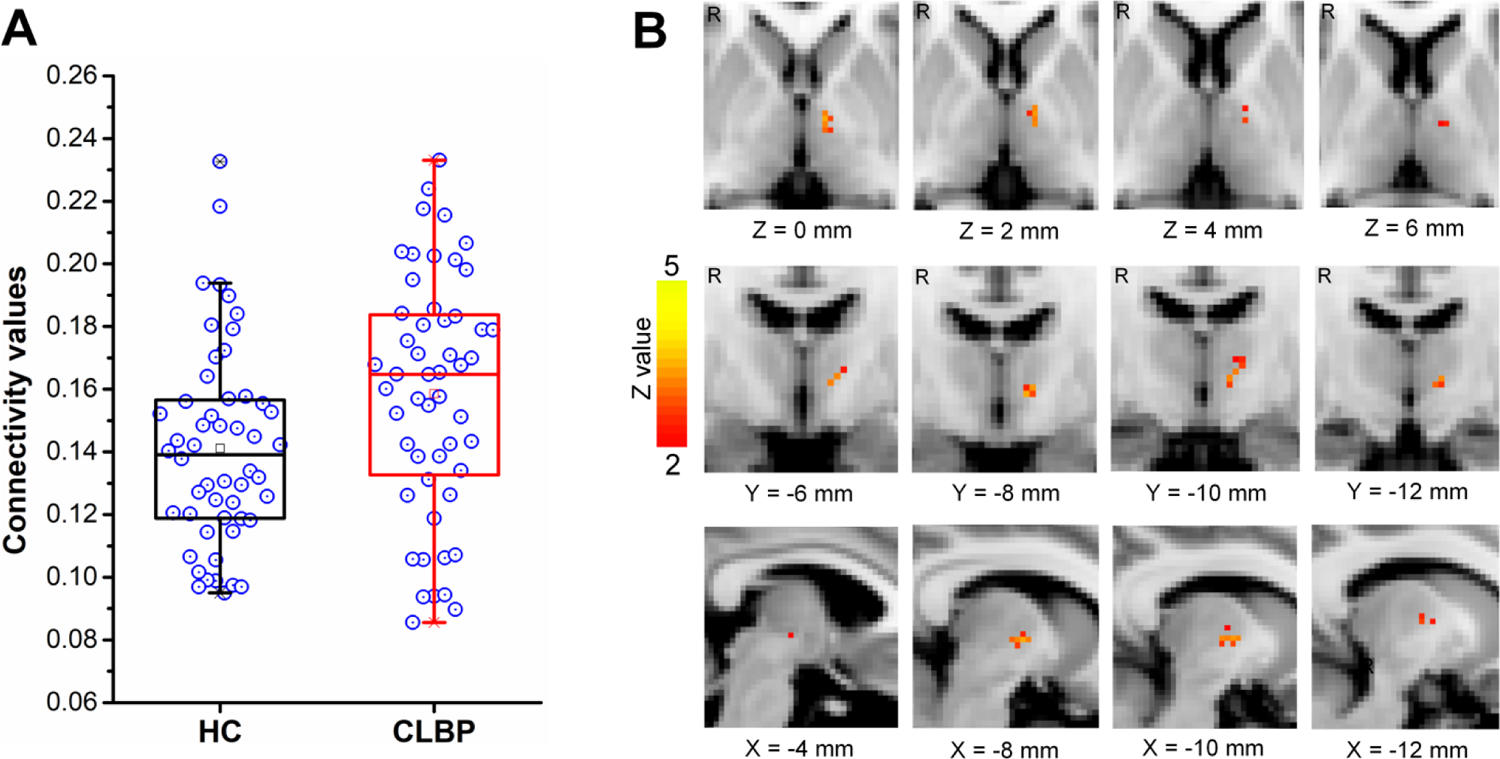
(A) Box-and-whisker plot showing the anatomic connectivity values of the left thalamo-motor pathway in healthy controls and patients with chronic low back pain. The open circles indicate the anatomical connectivity strengths of the left thalamo-motor pathway in both groups. The upper and lower edges of the box mark the 25th and 75th percentiles. The error bars represent the 95% confidence level. (B) Group differences of the anatomical connectivity in the left thalamo-motor pathway revealed by cluster-wised diffusion tensor imaging tractography. HC, healthy controls; CLBP, chronic low back pain; R, right. mm, millimeter; “X”, “Y”, and “Z” are Montreal Neurological Institute (MNI) coordinates, representing sagittal, coronal and axial views, respectively.
Cluster-wise analyses with FSL suggested consistent results by presence of significantly larger connectivity of the left thalamo-motor pathway in patients with cLBP (centered at peak coordinates X = −8, Y = −8, Z = 0, FWE corrected, Fig. 3B) than in healthy controls. The connectivity values of the thalamo motor/somatosensory pathways can be seen in Table 2.
Table 2.
Anatomical connectivity of the thalamo-motor/somatosensory pathway revealed by probabilistic tractography.
| Cortical region | Hemisphere | Group | F | P | |
|---|---|---|---|---|---|
| HC | CLBP | ||||
| Motor cortex | Left | 0.14 ± 0.03 | 0.16± 0.04 | 7.17 | 0.036* |
| Motor cortex | Right | 0.16 ± 0.04 | 0.15 ± 0.03 | 0.56 | 0.608 |
| Somatosensory cortex | Left | 0.09 ± 0.03 | 0.1 ± 0.02 | 0.06 | 0.814 |
| Somatosensory cortex | Right | 0.08 ± 0.03 | 0.07 ± 0.02 | 0.34 | 0.608 |
HC, healthy controls; CLBP, chronic low back pain. The P values were obtained using analysis of covariance after controlling for age and gender (after correcting for the false discovery rate).
Results from secondary DTI analysis:
Patients with cLBP showed significantly larger volumes of bilateral thalamo-somatosensory pathways than healthy controls (left, P = 0.034, right, P = 0.02; Table 3). There were no significant between-group differences for FA and MD values in bilateral thalamo-motor and thalamo-somatosensory pathways (P > 0.05, Table 3). Meanwhile, no significant between-group differences (P > 0.05 for all pathways) were detected for the anatomical connectivity (in percent) of the thalamus and other cortical areas including PFC, parietal, temporal, and occipital cortices (Supplemental Table 2).
Table 3.
Volumes, FA, and MD values of the thalamo-motor/somatosensory fibers.
| Fiber | Volume | P 1 | FA | P 2 | MD | P 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| HC | CLBP | HC | CLBP | HC | CLBP | ||||
| Thal-motor_L | 32855 ± 6155a | 33588 ± 6359 | 0.445 | 0.389 ± 0.025 | 0.388± 0.019 | 0.968 | 0.00082 ± 0.00009 | 0.00081 ± 0.0008 | 0.377 |
| Thal-motor_R | 28243 ± 6255 | 29446 ± 6903 | 0.198 | 0.396 ± 0.025 | 0.394 ± 0.019 | 0.708 | 0.00081 ± 0.00009 | 0.00081 ± 0.00009 | 0.882 |
| Thal-Somato_L | 23907 ± 5127 | 26061± 5619 | 0.034* | 0.383 ± 0.024 | 0.381± 0.021 | 0.814 | 0.00083± 0.00008 | 0.00083± 0.00009 | 0.467 |
| Thal-Somato_R | 19635 ± 5650 | 21912 ± 5334 | 0.02* | 0.392 ± 0.026 | 0.39 ± 0.021 | 0.708 | 0.00083 ± 0.00009 | 0.00083 ± 0.0001 | 0.66 |
HC, healthy controls; CLBP, chronic low back pain;
the unit of the volumes is mm3.
P1, P2, and P3 represent the between-group differences of volumes, FA and MD values of the thalamo-motor/somatosensory fibers respectively, obtained from analysis of variance after correcting for age and gender. FA, fraction anisotropy; MD, mean diffusivity; Thal, thalamus; Somato, somatosensory; L, left; R, right;
P < 0.05.
Thalamocortical resting-state functional connectivity
Patients with cLBP demonstrated significantly enhanced rsFC of bilateral thalamo-motor and bilateral thalamo-somatosensory circuits (P < 0.05 for both, FDR corrected). No clusters survived for the between-group differences of other thalamocortical pathways at the threshold we set. The between-group differences of the rsFC in the thalamo-motor/somatosensory pathways are shown in Table 4 and Fig. 4.
Table 4.
Group differences of the resting-state functional connectivity in thalamo-motor/somatosensory pathways.
| Cortical seed | Side of thalamus | MNI coordinates | Cluster size (number of voxels) | Z Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Motor cortex | Left | −4 | −20 | 16 | 46a | 3.35 |
| Right (Cluster 1) | 8 | −28 | 8 | 13 | 3.71 | |
| Right (Cluster 2) | 4 | −14 | 14 | 21 | 3.46 | |
| Left | −10 | −14 | 16 | 111 | 3.72 | |
| Somatosensory cortex | ||||||
| Right | 8 | −26 | 8 | 36 | 4.06 | |
MNI, Montreal Neurologic Institute.
Voxel size was 2×2×2 mm3.
Figure 4. Resting-state functional connectivity of thalamocortical pathways.
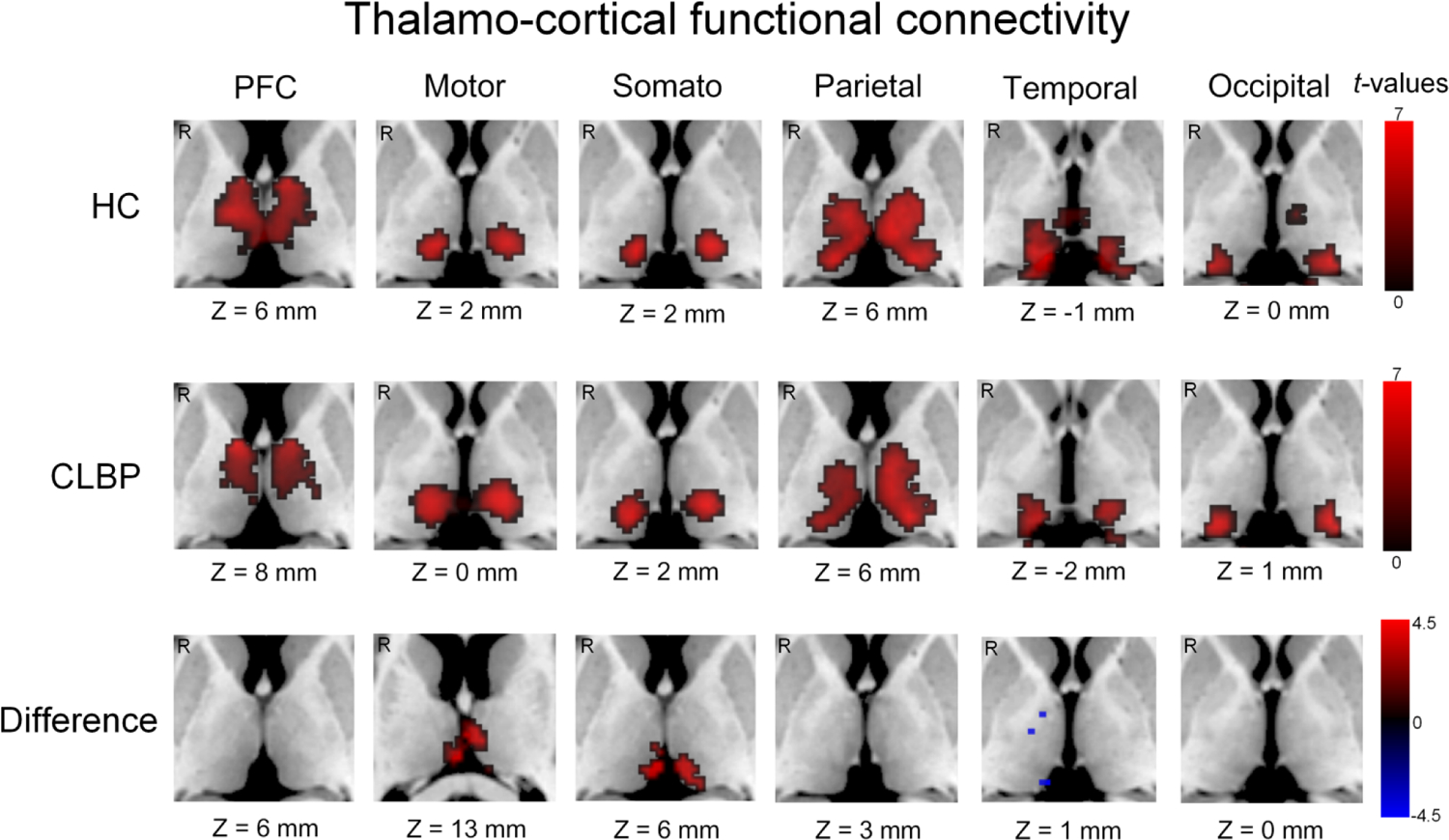
Clusters in the first two rows were displayed only if they were larger than 50 voxels. The third row represents the group difference between patients with chronic low back pain and healthy controls. HC, healthy controls; CLBP, chronic low back pain; PFC, prefrontal cortex; somato, somatosensory cortex; mm, millimeter; R, right. “Z” represents Montreal Neurological Institute (MNI) coordinates, representing axial view.
Association analysis
Association analysis showed a significant positive correlation between pain intensity during the resting-state MRI scan and rsFC of the right thalamo-somatosensory pathway (r = 0.32, P = 0.036, FDR corrected) in patients with cLBP. There was no significant correlation between clinical measures and anatomical connectivity of the thalamo-motor/somatosensory pathway (P > 0.05 for all pathways, FDR corrected). Detailed information of the association analysis can be found in Supplemental Table 3.
Correlation between rsFC and anatomical connectivity of the left thalamo-motor pathway in cLBP was performed. The results suggested no significant correlation (r = 0.109; P = 0.212, FDR corrected). There were also no significant correlation between the thalamic grey matter density and the rsFC of thalamo-motor (r = −0.083, P = 0.55, FDR corrected) as well as thalamo-somatosensory (r = −0.085, P = 0.543, FDR corrected) pathways in cLBP patients.
Discussion
We investigated the alterations of the anatomical and functional connectivity in thalamo-motor/somatosensory circuits in patients with cLBP via resting-state fMRI and DTI. We found that patients with cLBP exhibited 1) significantly increased anatomical connectivity of the left thalamo-motor pathway, and 2) significantly increased rsFC of the thalamo-motor/somatosensory pathways in bilateral hemispheres as compared to healthy controls. The rsFC between the somatosensory cortex and the right thalamus was positively correlated with pain intensity in cLBP patients.
In our study, the thalamus was parceled into 6 subregions by probabilistic tractography. The map of subregions is similar to previous reports using similar methods (Behrens et al., 2003;Nair et al., 2013). The results validate the methodology of our study.
We detected enhanced anatomical connectivity of the left thalamo-motor pathway in cLBP by DTI probabilistic tractography. Motor abnormalities have been repeatedly reported in patients with cLBP in previous studies (Christe et al., 2021;Hodges and Smeets, 2015;Wilke and Buhmann, 2013;Zawadka et al., 2021). The neuroplasticity in the motor system has been suggested as a modulator of spinal motor control in recurrent LBP (Tsao et al., 2011;Tsao et al., 2008;Tsao et al., 2010). Studies have shown loss of discrete cortical organization associated with inputs to back muscles in LBP (Tsao et al., 2011) and reorganization of the motor cortex associated with postural control deficits of trunk muscles in cLBP (Tsao et al., 2008;Tsao et al., 2010). Previous studies also reported reduced gray matter density of the thalamus in patients with cLBP (Apkarian et al., 2004;Ivo et al., 2013). Our findings extended previous findings by elucidating the altered anatomic connectivity between the thalamus and the motor cortex.
We observed thalamo-motor anatomic linkage alterations in the left hemisphere but not in the right hemisphere in cLBP. Hemispheric asymmetry of the thalamus (larger volume in the left side) has been reported in a previous study (Kang et al., 2015). Larger FA and smaller apparent diffusion coefficient (ADC) values were also suggested in the left thalamus in right-handed children (Wilde et al., 2009). Moreover, lateralization of acute nociceptive processing in the thalamus has been reported by an fMRI study; greater activation of the thalamus was found in the hemisphere contralateral to the stimulus (Youell et al., 2004). The thalamic activity can be altered by contralateral neuropathic pain revealed by single photon emission computed tomography (SPECT) (Ushida et al., 2010). Lateralized cLBP has been suggested to have lateralized function of the primary motor cortex and anticipatory postural activation of paravertebral mutifidus muscles (Masse-Alarie et al., 2017). In the present study, the details of cLBP locations were not clearly recorded, which precludes us from doing further analysis. Thus, it is unclear whether alterations in the left hemisphere are due to cLBP side-related differences. Considering the importance of the brain asymmetry and pain, future studies are needed to validate our findings.
We did not detect significant differences in FA or MD values between cLBP and healthy controls. The connectivity values in probabilistic tractography and the FA/MD values are different indices derived from different algorithms. The connectivity values from probabilistic tractography represent the sampling times of each voxel in the thalamus to corresponding cortical areas. FA describes how much molecular displacements vary in space and is related to the presence of oriented structures (Leal, 2019), i.e., the directional preference of diffusion. MD represents the molecular diffusion rate (Soares et al., 2013). Different algorithms and measurements might explain the discrepancy of the results from probabilistic tractography and those of FA/MD analyses.
Altered thalamic grey matter density has been reported in previous studies (Apkarian et al., 2004;Ivo et al., 2013), but our results did not find a significant association between the altered connectivity and grey matter density. We speculate that this may be due to different etiologies of cLBP in these studies. Patients with cLBP in our study are mainly experiencing non-specific pain, while patients with cLBP in previous studies have neuropathic pain (Apkarian et al., 2004). Future studies are needed to distinguish the subtypes of LBP in order to accurately characterize the brain mechanisms of LBP with different etiologies.
We found altered rsFC in bilateral thalamo-motor/somatosensory pathways in cLBP as compared to healthy controls. Previous studies have also suggested reciprocal interactions between motor and sensory cortices. For example, motor outputs are generally determined by sensory inputs, including information of body position and movement and the information from nociceptive brain networks (Brumagne et al., 2004;Flor et al., 1997). Some studies have reported extensive reorganization of the primary somatosensory cortex in chronic back pain patients (Flor et al., 1997). Other studies suggested that the reorganization of neuronal networks of the motor cortex in people with recurrent LBP could be normalized by motor training (Tsao et al., 2010). The alterations in thalamo-motor and thalamo-somatosensory functional connectivity in patients with cLBP, combined with the positive correlation between pain intensity and right thalamo-somatosensory rsFC may suggest abnormal information transmission among these brain regions associated with persistent pain in cLBP patients. This result is also consistent with our explotary analysis results which showed that cLBP is associated with bilateral thalamo-somatosensory volume increase.
In a previous study (Tu et al., 2020) using dorsal medial/ventral medial (DM/VM) nucleus as seed, we found decreased static functional connectivity between the DM/VM nucleus and superior frontal gyrus (SFG), insula as well as postcentral gyrus in cLBP. In this study, using the primary somatosensory cortex (whole postcentral gyrus) as seed, we found increased functional connectivity located mainly in the lateral division of the thalamus in cLBP patients.
Previous studies suggest two ascending pathways through which the thalamus receives nociceptive signals (Groh et al., 2018). One is the lateral thalamocortical pathway coding the sensory discriminative dimensions of pain, and the other is the medial thalamocortical pathway, which mainly codes the emotional component of pain (Groh et al., 2018). Furthermore, studies have shown that the pathway between the DM nucleus and PFC is associated with executive function (De Witte et al., 2011;Giraldo-Chica et al., 2018). Our results, combined with those from our previous study, suggest that cLBP may be associated with alternations in both lateral and medial thalamocortical pathways as indicated by functional connectivity changes.
There were several limitations in our study worth discussing. First, lack of correlation between anatomical and functional connectivity of the thalamo-motor pathway was found in cLBP patients. Previous studies suggest that structural connectivity partially explains functional connectivity (Honey et al., 2009;Jones and Cercignani, 2010;O’Muircheartaigh et al., 2015). Nevertheless, the underlying relationship between structural and functional connectivity remains unclear and further investigation using multi-modality fusion techniques to explore their association is needed. Second, it is inadequate to explain the thalamocortical rsFC changes using a single unidimensional assessment of pain perception such as pain intensity. A future study is needed to explore the association between the brain functional/structural alterations and more comprehensive pain indices. Third, depression has been associated with altered brain functional connectivity (Magioncalda et al., 2020;Wang et al., 2019). We didn’t collect BDI scores in healthy controls. Thus, we were not able to include BDI score or other measures of emotional distress in the data analysis. However, because the average BDI score in our study is quite low (with an average score of 3.5 ± 7.3), less than mild depression (score ⩾ 14) as defined by the BDI manual (Beck et al., 1996), we believe it is unlikely that depressive symptoms may have confounded the results from group comparisons. Future studies are needed to replicate our findings. Fourth, as a cross-sectional study, we could not test if the anatomical connectivity change observed is due to the predisposition or plasticity following functional connectivity alternation. Future longitudinal analysis is needed to test if the anatomical connectivity change existed before the development of chronic pain.
As for the image processing procedures, large cortical seeds containing many functional subregions were used in the functional connectivity analysis, which might preclude us from detecting the differences of the thalamus and cortical subregions (e.g. medial prefrontal cortex or dorsolateral prefrontal cortex) between the two groups. Further studies are needed to detect the specificity of connections between the thalamus and those more narrowly defined brain regions. Also, only the ipsilateral connectivity was considered in the DTI analysis as it is the predominant connectivity in the development of the brain. Further study is needed to detect abnormalities in the hemispheric interaction of the DTI indices in cLBP.
In conclusion, we observed significantly increased thalamo-motor anatomical connectivity and increased bilateral thalamo-motor/somatosensory rsFCs in patients with cLBP than in healthy controls. The increased rsFC of the right thalamo-somatosensory was positively correlated with pain intensity in cLBP. Our findings highlight the involvement of the thalamo-sensorimotor circuit in the pathophysiology of cLBP.
Supplementary Material
Highlights.
Thalamocortical dysrhythmia is thought to underlie the pathophysiology of chronic pain.
CLBP is associated with increased anatomical connectivity of the left thalamo-motor pathway revealed by DTI.
Resting-state functional connectivity between bilateral motor/somatosensory cortex and the thalamus is increased in CLBP patients.
Somatosensory-thalamic resting-state functional connectivity is correlated with back pain intensity.
Abnormal thalamocortical connectivity might indicate disrupted communication between motor/somatosensory cortex and the thalamus in cLBP.
Fundings
This study is supported by R01 AT008563, R33 AT009310, R33AT009341, R34DA046635 (through the NIH HEAL Initiative), and R01AG063975 from NIH.
Abbreviations:
- cLBP
chronic low back pain
- DTI
diffusion tensor imaging
- rsFC
resting-state functional connectivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
JK has a disclosure to report (holding equity in a startup company (MNT) and pending patents to develop new neuromodulation tools), but declares no conflict of interest. All other authors declare no conflicts of interest in any form or kind in relation to this study and its publication.
Data Availability Statement
All of the dataset are in-house dataset and are available from the corresponding author upon reasonable request.
References
- Alshelh Z, Di Pietro F, Youssef AM, Reeves JM, Macey PM, Vickers ER, Peck CC, Murray GM, Henderson LA (2016) Chronic Neuropathic Pain: It’s about the Rhythm. J Neurosci 36:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner JBG, Chen C, Daunizeau J, Flandin G, Friston K, Kiebel S, Kilner J, Litvak V, Moran R, Penny W, SPM12 manual, 2016.
- Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219. [DOI] [PubMed] [Google Scholar]
- Bastuji H, Frot M, Mazza S, Perchet C, Magnin M, Garcia-Larrea L (2016) Thalamic Responses to Nociceptive-Specific Input in Humans: Functional Dichotomies and Thalamo-Cortical Connectivity. Cereb Cortex 26:2663–2676. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996) Beck depression inventory-II. San Antonio 78:490–498. [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Verschueren S (2004) Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett 366:63–66. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Diers M, Danneels L, Moseley GL, Hodges PW (2019) Neuroplasticity of Sensorimotor Control in Low Back Pain. J Orthop Sports Phys Ther 49:402–414. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KI, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, Kim SN, Kwon JS (2016) Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull 42:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe G, Crombez G, Edd S, Opsommer E, Jolles BM, Favre J (2021) Relationship between psychological factors and spinal motor behaviour in low back pain: a systematic review and meta-analysis. Pain 162:672–686. [DOI] [PubMed] [Google Scholar]
- Claeys K, Dankaerts W, Janssens L, Pijnenburg M, Goossens N, Brumagne S (2015) Young individuals with a more ankle-steered proprioceptive control strategy may develop mild non-specific low back pain. J Electromyogr Kinesiol 25:329–338. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC (1995) Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping 3:190–208. [Google Scholar]
- De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Marien P (2011) Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex 47:273–319. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N (1997) Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224:5–8. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J (2005) Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162:1256–1265. [DOI] [PubMed] [Google Scholar]
- Garcia PS, Kreuzer M, Hight D, Sleigh JW (2021) Effects of noxious stimulation on the electroencephalogram during general anaesthesia: a narrative review and approach to analgesic titration. Br J Anaesth 126:445–457. [DOI] [PubMed] [Google Scholar]
- Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND (2018) Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry 83:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Krieger P, Mease RA, Henderson L (2018) Acute and Chronic Pain Processing in the Thalamocortical System of Humans and Animal Models. Neuroscience 387:58–71. [DOI] [PubMed] [Google Scholar]
- Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M, Buchbinder R, Hartvigsen J, Cherkin D, Foster NE, Maher CG, Underwood M, van Tulder M, Anema JR, Chou R, Cohen SP, Menezes Costa L, Croft P, Ferreira M, Ferreira PH, Fritz JM, Genevay S, Gross DP, Hancock MJ, Hoy D, Karppinen J, Koes BW, Kongsted A, Louw Q, Öberg B, Peul WC, Pransky G, Schoene M, Sieper J, Smeets RJ, Turner JA, Woolf A (2018) What low back pain is and why we need to pay attention. The Lancet 391:2356–2367. [DOI] [PubMed] [Google Scholar]
- Henssen D, Dijk J, Knepfle R, Sieffers M, Winter A, Vissers K (2019) Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: A systematic review and meta-analysis. Neuroimage Clin 24:102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Smeets RJ (2015) Interaction between pain, movement, and physical activity: short-term benefits, long-term consequences, and targets for treatment. Clin J Pain 31:97–107. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009) Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivo R, Nicklas A, Dargel J, Sobottke R, Delank KS, Eysel P, Weber B (2013) Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 22:1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Cercignani M (2010) Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820. [DOI] [PubMed] [Google Scholar]
- Kang X, Herron TJ, Ettlinger M, Woods DL (2015) Hemispheric asymmetries in cortical and subcortical anatomy. Laterality 20:658–684. [DOI] [PubMed] [Google Scholar]
- Kim H, Mawla I, Lee J, Gerber J, Walker K, Kim J, Ortiz A, Chan ST, Loggia ML, Wasan AD, Edwards RR, Kong J, Kaptchuk TJ, Gollub RL, Rosen BR, Napadow V (2020) Reduced tactile acuity in chronic low back pain is linked with structural neuroplasticity in primary somatosensory cortex and is modulated by acupuncture therapy. Neuroimage 217:116899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Spaeth RB, Wey HY, Cheetham A, Cook AH, Jensen K, Tan Y, Liu H, Wang D, Loggia ML, Napadow V, Smoller JW, Wasan AD, Gollub RL (2013) S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal PRL (2019) Fraction of anisotropy and apparent diffusion coefficient as diagnostic tools in trigeminal neuralgia. Acta Neurochir (Wien) 161:1403–1405. [DOI] [PubMed] [Google Scholar]
- Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, Chan ST, Gerber J, Schmithorst VJ, Edwards RR, Wasan AD, Berna C, Kong J, Kaptchuk TJ, Gollub RL, Rosen BR, Napadow V (2019) Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain 160:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, Devereaux PJ, Thabane L (2017) An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol 46:746–755. [DOI] [PubMed] [Google Scholar]
- Li T, Zhang S, Kurata J (2018) Suppressed descending pain modulatory and enhanced sensorimotor networks in patients with chronic low back pain. J Anesth 32:831–843. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998) Functional Connectivity in Single and Multislice Echoplanar Imaging Using Resting-State Fluctuations. NeuroImage 7:119–132. [DOI] [PubMed] [Google Scholar]
- Ma J, Wang X, Qiu Q, Zhan H, Wu W (2020) Changes in Empathy in Patients With Chronic Low Back Pain: A Structural-Functional Magnetic Resonance Imaging Study. Front Hum Neurosci 14:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magioncalda P, Martino M, Conio B, Lee HC, Ku HL, Chen CJ, Inglese M, Amore M, Lane TJ, Northoff G (2020) Intrinsic brain activity of subcortical-cortical sensorimotor system and psychomotor alterations in schizophrenia and bipolar disorder: A preliminary study. Schizophr Res 218:157–165. [DOI] [PubMed] [Google Scholar]
- Maher C, Underwood M, Buchbinder R (2017) Non-specific low back pain. Lancet 389:736–747. [DOI] [PubMed] [Google Scholar]
- Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C (2017) The side of chronic low back pain matters: evidence from the primary motor cortex excitability and the postural adjustments of multifidi muscles. Exp Brain Res 235:647–659. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Muller RA (2013) Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136:1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Keller SS, Barker GJ, Richardson MP (2015) White Matter Connectivity of the Thalamus Delineates the Functional Architecture of Competing Thalamocortical Systems. Cereb Cortex 25:4477–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichep LS, Shah J, Merkin H, Hiesiger EM (2018) Exploration of the Pathophysiology of Chronic Pain Using Quantitative EEG Source Localization. Clin EEG Neurosci 49:103–113. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Naranjo JR, Brenneisen C, Gundlach J, Schultz C, Kaube H, Hinterberger T, Jeanmonod D (2012) Pain ratings, psychological functioning and quantitative EEG in a controlled study of chronic back pain patients. PLoS One 7:e31138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW (2008) Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39:1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Huang AS, Rogers BP, Giraldo-Chica M, Landman BA, Blackford JU, Heckers S, Woodward ND (2020) Thalamocortical Anatomical Connectivity in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Danneels LA, Hodges PW (2011) ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine (Phila Pa 1976) 36:1721–1727. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW (2008) Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131:2161–2171. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW (2010) Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain 14:832–839. [DOI] [PubMed] [Google Scholar]
- Tu Y, Fu Z, Mao C, Falahpour M, Gollub RL, Park J, Wilson G, Napadow V, Gerber J, Chan ST, Edwards RR, Kaptchuk TJ, Liu T, Calhoun V, Rosen B, Kong J (2020) Distinct thalamocortical network dynamics are associated with the pathophysiology of chronic low back pain. Nat Commun 11:3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Fu Z, Zeng F, Maleki N, Lan L, Li Z, Park J, Wilson G, Gao Y, Liu M, Calhoun V, Liang F, Kong J (2019) Abnormal thalamocortical network dynamics in migraine. Neurology 92:e2706–e2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushida T, Fukumoto M, Binti C, Ikemoto T, Taniguchi S, Ikeuchi M, Nishihara M, Tani T (2010) Alterations of contralateral thalamic perfusion in neuropathic pain. Open Neuroimag J 4:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Song JJ, De Ridder D (2018) Thalamocortical dysrhythmia detected by machine learning. Nat Commun 9:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lariviere S, Xu Q, Vos de Wael R, Hong SJ, Wang Z, Xu Y, Zhu B, Bernasconi N, Bernasconi A, Zhang B, Zhang Z, Bernhardt BC (2019) Community-informed connectomics of the thalamocortical system in generalized epilepsy. Neurology 93:e1112–e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, Wang ZJ, Hanten G, Li X, Ramos MA, Sabir SH, Vasquez AC, Menefee D, Levin HS (2009) Diffusion tensor imaging of hemispheric asymmetries in the developing brain. J Clin Exp Neuropsychol 31:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke J, Buhmann HW (2013) Quality of fundamental movement patterns in chronic low back pain patients: a quasi-experimental cross-sectional study. Sportverletz Sportschaden 27:219–225. [DOI] [PubMed] [Google Scholar]
- Yao B, Neggers SFW, Kahn RS, Thakkar KN (2020) Altered thalamocortical structural connectivity in persons with schizophrenia and healthy siblings. Neuroimage Clin 28:102370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youell PD, Wise RG, Bentley DE, Dickinson MR, King TA, Tracey I, Jones AK (2004) Lateralisation of nociceptive processing in the human brain: a functional magnetic resonance imaging study. Neuroimage 23:1068–1077. [DOI] [PubMed] [Google Scholar]
- Zawadka M, Smolka J, Skublewska-Paszkowska M, Lukasik E, Zielinski G, Bys A, Gawda P (2021) Altered squat movement pattern in patients with chronic low back pain. Ann Agric Environ Med 28:158–162. [DOI] [PubMed] [Google Scholar]
- Zhang B, Jung M, Tu Y, Gollub R, Lang C, Ortiz A, Park J, Wilson G, Gerber J, Mawla I, Chan ST, Wasan A, Edwards R, Lee J, Napadow V, Kaptchuk T, Rosen B, Kong J (2019) Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth S0007-0912:30143–30146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang Y, Li H, Yan Z, Zhang Y, Liu X, Hou X, Chen W, Tu Y, Hodges S, Chen H, Liu B, Kong J (2021) Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: an fMRI study. Reg Anesth Pain Med 46:145–150. [DOI] [PubMed] [Google Scholar]
- Zippo AG, Valente M, Caramenti GC, Biella GE (2016) The thalamo-cortical complex network correlates of chronic pain. Sci Rep 6:34763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the dataset are in-house dataset and are available from the corresponding author upon reasonable request.


