Abstract
Background:
With aging, the human atrium invariably develops amyloid composed of atrial and B-type natriuretic peptides (ANP, BNP). Preamyloid oligomers (PAOs) are the primary cytotoxic species in amyloidosis, and they accumulate in the atrium during human hypertension and a murine hypertensive model of AF susceptibility. We tested the hypothesis that PAOs derived from natriuretic peptides cause cytotoxic and electrophysiologic effects in atrial cells that promote arrhythmia susceptibility, and that oligomer formation is enhanced for a mutant (mut) form of ANP linked to familial atrial fibrillation (AF).
Methods:
Oligomerization was assessed by Western blot analysis. Bioenergic profiling was performed using the Seahorse platform. Mitochondrial dynamics were investigated with immunostaining, and gene expression quantitated using RT quantitative PCR. Action potentials and ionic currents were recorded using patch clamp methods, and intracellular calcium measured using Fura-2.
Results:
Oligomer formation was markedly accelerated for mutANP compared to wild-type (WT) ANP. Oligomers derived from ANP, BNP, and mutANP suppressed mitochondrial function in atrial HL-1 cardiomyocytes, associated with increased superoxide generation and reduced biogenesis, while monomers had no effects. In hypertensive mice, atrial cardiomyocytes displayed reduced action potential duration (APD) and maximal dV/dT of Phase 0 (Vmax), with an elevated resting membrane potential (RMP), compared to normotensive mice. Similar changes were observed when atrial cells were exposed to oligomers. MutANP monomers produced similar electrophysiologic effects as mutANP oligomers, likely due to accelerated oligomer formation, while ANP and BNP monomers did not. Oligomers decreased Na+ current, inward rectifier K+ current, and L-type Ca++ current, while increasing sustained and transient outward K+ currents, to account for these effects.
Conclusions:
These findings provide compelling evidence that natriuretic peptide oligomers are novel mediators of atrial arrhythmia susceptibility. Moreover, the accelerated oligomerization by mutANP supports a role for these mediators in the pathophysiology of this mutation in AF.
Keywords: atrial fibrillation, preamyloid oligomers, atrial natriuretic peptide, B-type natriuretic peptide, electrophysiology, mitochondrial function
Journal Subject Terms: Pathophysiology, Ion channels/membrane transport
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia worldwide, causing substantial morbidity and mortality. Unfortunately, contemporary therapy using pharmacologic and/or catheter-based approaches remains ineffective for many patients. A major factor preventing development of effective treatment is continued limited insight into the molecular mechanisms that promote AF susceptibility. Thus, unraveling causative mediators and pathways is critical to identify novel therapeutic targets.
The most important risk factor for AF is age. Increasingly, aging-related, degenerative diseases are linked to proteotoxicity, or cellular dysfunction caused by aberrant protein aggregation, as a causative mechanism.1 In Alzheimer’s disease, misfolded amyloid β1-42 monomers initially aggregate to form small diffusible oligomers in a time- and concentration-dependent manner. These preamyloid oligomers (PAOs) have been identified to be the primary cytotoxic species causing acute neurotoxicity and disease progression, rather than mature amyloid fibrils.2
In the heart, proteotoxicity is now recognized to promote ventricular dysfunction and cardiomyopathy.3, 4 In addition, amyloidosis predictably develops in the human atrium with aging, exceeding 90% in the ninth decade of life, and its presence is linked to AF.5 In this senile form of amyloid, natriuretic peptides have been implicated: atrial deposits are uniformly immunoreactive for ANP, while BNP has also been detected.5, 6 Interestingly, a frameshift mutation in NPPA which encodes ANP has been linked to early-onset, familial AF.7 The mutation eliminates the normal stop codon and generates ANP containing an anomalous C-terminus of 12 additional residues (mutant ANP, or mutANP). AF susceptibility occurs in the absence of atrial structural remodeling, and electrophysiologic mechanisms have been proposed to explain the association of this mutation with AF.7-9 For Alzheimer’s, amyloid β1-42 mutations that disrupt native protein structure promote enhanced monomer aggregation to cause inherited, early-onset disease.10 Based on several lines of evidence, we hypothesized that a similar mechanism, i.e., enhanced oligomer formation, is operative for mutANP to promote AF susceptibility. Oligomer toxicity has been primarily studied in neurologic diseases, and a fundamental feature is prominent interaction of oligomers with biologic membranes.11 PAOs can directly modulate the function of plasma membrane proteins or promote internalization. They can also disrupt membrane integrity to alter intracellular calcium homeostasis and mitochondrial function, as well as generation of reactive oxygen species (ROS). In addition, PAOs derived from different proteins share a common structural epitope, forming the basis for common pathophysiologic mechanisms for oligomers generated in different organs.12
We have previously identified PAOs composed of ANP in the atria of patients undergoing cardiac surgery, where their presence was linked to hypertension.13 Subsequently, we investigated a mouse model of ang II-mediated hypertension causing AF susceptibility. In hypertensive mice, we observed that AF development was associated with diffuse accumulation of natriuretic peptide oligomers in extra- and intra-cellular regions in both atria, and that oligomer formation was accelerated by oxidative stress.14 Based on these data, we hypothesized that natriuretic peptide oligomers serve as proarrhythmic mediators in the atria.
In the present study, we report that oligomerization of mutant ANP was markedly accelerated compared to the WT protein. In addition, WT and mutant natriuretic peptide oligomers caused mitochondrial dysfunction and superoxide production, as well as electrophysiologic effects in atrial cells that closely resembled hypertension-mediated electrical remodeling in murine atrium. These findings establish natriuretic peptide oligomers as viable proteotoxic mediators of arrhythmia susceptibility during hypertension, and they support a role for PAOs in the cellular pathophysiology of mutANP in AF susceptibility.
METHODS
An expanded version of the Methods outlined below is available in the Data Supplement. The data that support the findings of this study are available from the corresponding author upon request.
Oligomer generation
ANP, mutANP, and BNP peptides were synthesized by RS Synthesis, with oligomer formation assayed by Western analysis14 using an anti-α-ANP antibody.
Animals
Animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services. Hypertensive C57Bl/6J male mice were generated using angiotensin (ang) II as described14 and cardiomyocytes were isolated.
Cytotoxicity and bioenergetic profiling
Atrial cell ATP levels were measured to assay cytotoxicity, and oxygen consumption rate (OCR) was assayed using the Seahorse XF Cell Mito Stress Test to determine bioenergetic parameters, following exposure to natriuretic peptide monomers or oligomers.
Immunostaining, real-time quantitative PCR (RT-qPCR), reactive oxygen species (ROS) detection
Mitochondrial abundance was assayed by immunostaining with an antibody directed against the translocase of the outer mitochondrial membrane 20 (TOMM20). Expression of peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1α) and nuclear respiratory factor 1 (NRF1) was quantitated using RT-qPCR. Superoxide production was determined using dihydroethidium.
Electrophysiologic recordings and intracellular calcium measurements
Action potentials were recorded from single atrial mouse and HL-1 cells using current clamp, while ionic currents were recorded using voltage clamp protocols.15 Cytosolic Ca++ was optically measured in atrial cardiomyocytes loaded with Fura-2 AM.
Statistics
Results are presented as mean ± SEM. Data were analyzed as defined in the Figure Legends using the Wilcoxon signed rank test, Mann-Whitney U test, or one-way ANOVA with Dunnett’s post hoc test as appropriate.
RESULTS
Cytotoxic oligomer formation is enhanced for mutANP
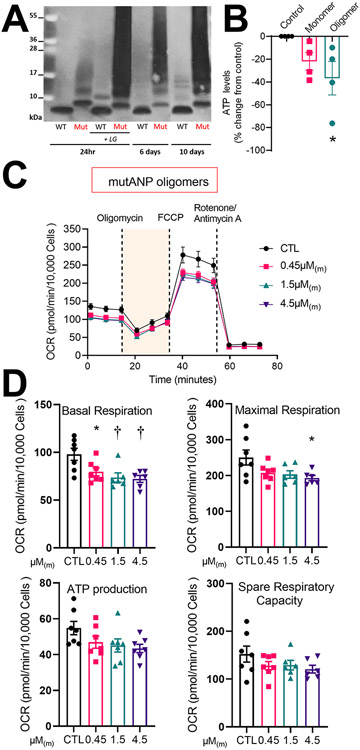
Analogous to Aβ1-42 mutations that cause early-onset Alzheimer’s disease, we hypothesized that the disordered structure of mutANP would cause it to misfold more readily than WT ANP and increase oligomerization. Purified peptides were incubated for variable periods of time (1-10d) in the absence or presence of 15-E2- isolevuglandins (IsoLG), a reactive lipid oxidative stress mediator that promotes PAO oligomerization, followed by Western blotting. Oligomer formation was markedly accelerated for mutANP, as indicated by the appearance of higher molecular weight bands, compared to WT ANP in a time- and concentration-dependent manner (Figure 1A, Supplemental Figure I). This process was further enhanced in the presence of IsoLG, as previously shown for oligomerization of ANP, BNP, and Aβ1-42.14, 16 As demonstrated for WT natriuretic peptide oligomers,14 24hr exposure to mutANP oligomers suppressed ATP production in atrial HL-1 cells (Figure 1B). Taken together, these results demonstrate that mutANP promotes the accelerated formation of cytotoxic oligomers.
Figure 1. Oligomerization of mutANP is markedly accelerated to generate cytotoxic oligomers that promote mitochondrial dysfunction in atrial cardiomyocytes.
A. ANP and mutANP peptides (10μM) were oligomerized at RT for indicated times followed by Western blot analysis. In parallel, peptides were co-incubated with 15-E2-IsoLG to promote oligomerization as positive controls. B. Cellular ATP levels were measured following incubation with mutANP monomer or oligomers (30μM monomers incubated at RT for 24hr and diluted to a concentration equivalent to 0.45μM monomers, or 0.45μM(m)). N=4 independent experiments; *P<0.05 vs. control, one-way ANOVA followed by Dunnet’s post hoc test. C. Seahorse bioenergetic profiling is illustrated for the mitochondrial stress test using atrial HL-1 cells cultured in the absence (control or CTL) or presence of mutANP oligomers (diluted to 0.45, 1.5, or 4.5μM(m)) for 24hr. Oxygen consumption rate (OCR) was analyzed following sequential injection of respiratory chain inhibitors at indicated time points. D. Bioenergetic parameters were measured following incubation without (CTL) or with mutANP oligomers (OCR values corrected for the number of Hoechst positive nuclei). N=6-7 independent experiments; *P<0.05 and †P<0.01 vs. control, one-way ANOVA with Dunnett’s post-hoc test.
Natriuretic peptide oligomers alter mitochondrial function and biogenesis in atrial myocytes
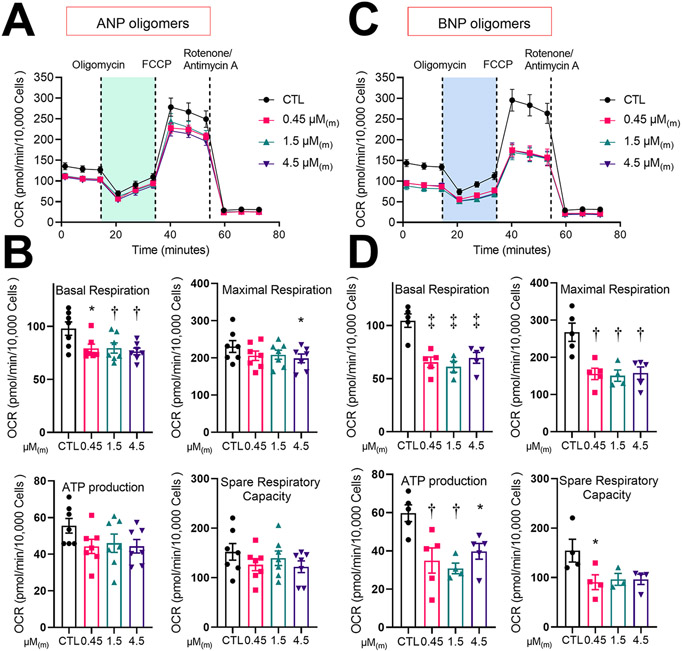
To investigate the effects of natriuretic peptide oligomers on mitochondrial function, atrial HL-1 cardiomyocytes were treated for 24hr with increasing amounts of oligomers followed by bioenergetic profiling using the Seahorse XF cell Mito Stress Test, with oxygen consumption rate (OCR) as an indicator of mitochondrial function. mutANP oligomers caused significant reduction in both basal and maximal respiration, with non-significant trends to lower spare respiratory capacity (difference between basal and maximally stimulated OCR) and ATP production (Figure 1C and 1D). ANP oligomers had similar effects at equivalent concentrations (Figure 2A and 2B), while BNP oligomers were the most potent, with a significant reduction in all 4 parameters (Figure 2C and 2D). On the other hand, freshly prepared monomers from all 3 peptides had no effect on mitochondrial function (Supplemental Figure II). Thus, natriuretic peptide oligomers cause mitochondrial dysfunction in atrial myocytes that does not occurs with monomers.
Figure 2. Oligomers derived from BNP and ANP also disrupt mitochondrial function.
Bioenergetic profiling results are displayed for atrial cells cultured in the presence or absence of ANP (panels A and B) or BNP (panels C and D) oligomers for 24hr, using the same format as in Figure 1C and 1D. N=6-7 (panel B) or 3-5 (panel D) independent experiments; *P<0.05, †P<0.01, and ‡P<0.001 vs. control, one-way ANOVA with Dunnett’s post-hoc test.
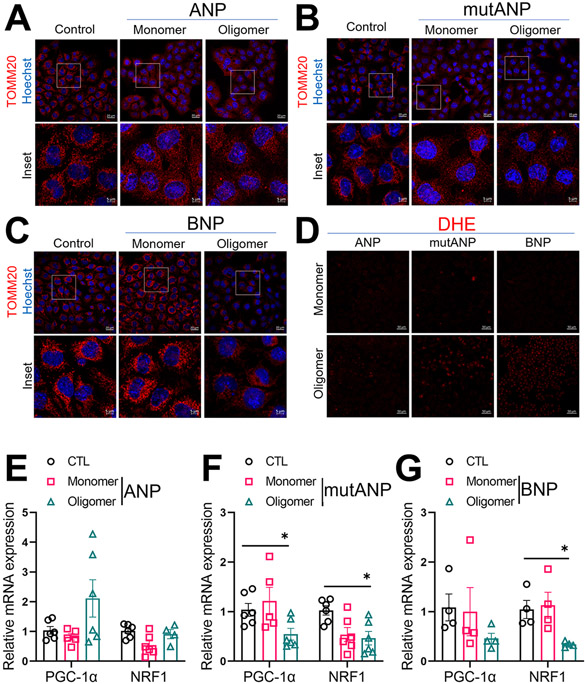
To investigate the role of mitochondrial biogenesis in these effects, we performed immunostaining for TOMM20 following chronic exposure (24hr) as an indicator of mitochondrial abundance. TOMM20 immunoreactivity was similar between untreated control atrial HL-1 cells and those exposed to monomers of ANP, mutANP and BNP (Figure 3A-3C), and ANP oligomers (Figure 3A). However, exposure to mutANP or BNP oligomers reduced immunoreactivity, indicating a reduction in mitochondrial number. Given that oligomers derived from other amyloid-forming proteins can induce ROS formation, we measured intracellular superoxide (O2•−) levels with DHE staining using live cell confocal microscopy in cells exposed to monomers and oligomers (24hr). While DHE staining was comparable between control cells and those treated with monomers or ANP oligomers, there was demonstrable increase in O2•− generation in the presence of either mutANP or BNP oligomers (Figure 3D).
Figure 3. Changes in mitochondrial dynamics, ROS generation and mitochondrial biogenesis following treatment with natriuretic peptide monomers or oligomers.
Treatment of atrial HL-1 cardiomyocytes with mutANP (B) and BNP oligomers (C) but not ANP oligomers (A) caused a substantial reduction in TOMM20 immunostaining (boxed region in upper panel [scale bar, 20μm] shown in inset in lower panel [scale bar, 5μm]). D. Exposure of cardiomyocytes to mutANP and BNP oligomers caused intracellular O2•− generation (scale bar, 50μm). E-G. mRNA levels are illustrated for genes encoding PGC-1α and NRF1 (normalized for control [CTL] values) following atrial cell treatment with monomers or oligomers prepared from ANP (E), mutANP (F) and BNP (D). N=4-6 biological replicates for each treatment (3 technical replicates each); *P<0.05 vs. control, Mann-Whitney test.
Because peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1α) plays a central role in cellular energetics, we analyzed mRNA levels of PGC-1α and nuclear respiratory factor 1 (NRF1), a transcription factor activated by PGC-1α responsible for transcription of key mitochondrial enzymes, following chronic treatment.17 Both natriuretic peptide monomers as well as ANP oligomers had no effect on levels of PGC-1α and NRF1 (Figure 3E-3G). However, expression for both genes was significantly downregulated in the presence of mutANP oligomers, with a significant decline in NRF1 with BNP oligomers. These results indicate that mutANP and BNP oligomers reduced mitochondrial abundance, at least in part by suppressing PGC-1α and NRF1 expression.
Hypertension-related atrial electrical remodeling and oligomers cause similar electrophysiologic effects
As noted above, we previously found that murine hypertension was associated with development of atrial natriuretic peptide oligomers coincident with AF susceptibility.14 Given that PAOs are recognized to alter the function of plasma membrane proteins,2, 11 we hypothesized that these oligomers modulate atrial electrophysiology to promote arrhythmogenesis. To test this hypothesis, action potentials were recorded in single atrial cardiomyocytes obtained from sham (normotensive) and hypertensive mice (Supplemental Figure III). With hypertension, action potential duration was significantly shorter (APD90 [at 90% repolarization]: 34.5±1.6 vs 65.6±1.5ms, n=9, 13; P<0.05; Table 1), with a reduction in the maximal dV/dT of Phase 0 (Vmax; 22.6±0.3 vs 24.4±0.2 mV/ms; P<0.05) and a rise of the resting membrane potential (RMP) to more positive values (−66.8±0.6 vs −69.7±0.9mV; P<0.05). Abbreviated repolarization and depressed Vmax also occur during atrial tachycardia remodeling18 and they are conditions that favor reentry, while myocyte depolarization enhances ectopic activity that can initiate it. Thus, hypertension-mediated electrical remodeling promotes AF susceptibility.
Table 1:
Atrial action potentials from normotensive and hypertensive mice
| RMP mV |
APD90 ms |
APD50 ms |
Vmax mV/ms |
|
|---|---|---|---|---|
| Sham | −69.7±0.9 | 65.6±1.5 | 23.8±1.5 | 24.4±0.2 |
| AngII | −66.8±0.6* | 34.5±1.6† | 20.0±0.8* | 22.6±0.3† |
P<0.05
P<0.01; data obtained 3 sham (n=9) and 3 hypertensive (n=13) mice
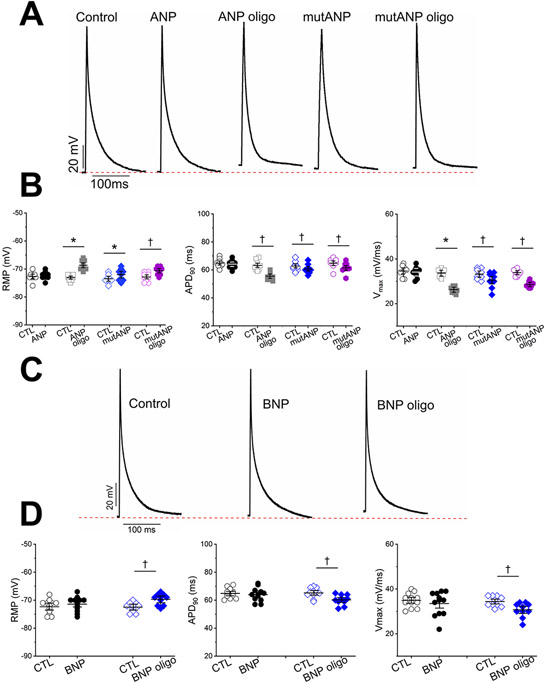
We then sought to compare these results to the direct electrophysiologic effects of oligomers on normal mouse atrial cardiomyocytes. With acute extracellular (bath) application, oligomers derived from ANP, BNP and mutANP significantly shortened APD90 (Figure 4A-4D, Table 2), accompanied by a reduction in Vmax and a significant rise in RMP. Similar findings were also observed using atrial HL-1 cells with both acute extracellular and intracellular (pipette) administration (Supplemental Tables I and II). These results demonstrate proarrhythmic electrophysiologic effects of natriuretic peptide oligomers in 2 different atrial cell preparations that are remarkably similar to hypertension-mediated atrial electrical remodeling in vivo.
Figure 4. Oligomers, and mutANP monomers, cause proarrhythmic electrophysiologic effects, while monomers do not.
A. Mouse atrial action potentials are shown for a control cell and following acute bath exposure to monomers or oligomers (oligo) of WT and mutANP. Dashed line represents resting membrane potential (RMP) for the control cell. B. Experimental data are displayed for RMP, action potential duration at 90% repolarization (APD90), and maximal dV/dT of Phase 0 (Vmax) for control (CTL) and post-peptide exposure (500nM; ANP monomers and oligomers n=9, 9; mutANP monomers and oligomers n=8, 9). *P<0.05 and †P<0.01 vs. control), Paired Samples Wilcoxon signed rank test or Mann-Whitney test. C. and D. Similar data are shown following exposure to BNP monomers or oligomers (500nM) using the same format as panels A and B (BNP monomers and oligomers n=12, 10). *P<0.05 and †P<0.01 vs. control, Paired Samples Wilcoxon signed rank test.
Table 2.
Natriuretic peptides: Effects on mouse atrial myocyte action potentials
| RMP mV |
APD90 ms |
APD50 ms |
Vmax mV/ms |
|
|---|---|---|---|---|
| Control | −72.7±0.5 | 64.9±4.9 | 22.8±2.3 | 34.6±0.8 |
| ANP | −72.3±0.5 | 64.1±4.9 | 22.1±2.4 | 34.1±0.8 |
| Control | −73.1±0.4 | 63.2±3.5 | 23.0±2.2 | 33.7±0.6 |
| ANP oligo | −68.7±0.6* | 45.4±1.8† | 16.7±1.3† | 25.9±0.4* |
| Control | −73.0±0.6 | 62.8±3.7 | 23.9±0.4 | 33.3±0.8 |
| mutANP | −72.0±0.8* | 60.6±3.8† | 20.8±0.3† | 30.5±1.3† |
| Control | −72.9±0.5 | 64.7±3.0 | 24.2±0.2 | 33.9±0.5 |
| mutANP oligo | −70.7±0.5† | 61.4±2.8† | 20.7±0.3† | 28.6±0.4† |
| Control | −72.3±1.8 | 64.8±1.0 | 23.0±0.8 | 34.5±1.2 |
| BNP | −71.4±1.3 | 63.9±1.4 | 22.1±1.4 | 33.5±1.3 |
| Control | −72.5±0.5 | 65.2±3.0 | 23.7±0.3 | 34.4±0.7 |
| BNP oligo | −69.7±0.6† | 60.2±3.0† | 19.7±0.3† | 30.7±1.0† |
oligo represents oligomer
P<0.05
P<0.01; n=8-12
Initial peptide concentration = 500 nM
Electrophysiologic effects of mutANP monomers resemble those of oligomers
Unlike oligomers, monomers of Aβ1-42 are not toxic to neurons. Similarly, we found that ANP and BNP monomers had no effect on either mitochondrial respiration (Supplemental Figure II) or action potentials in atrial mouse or HL-1 cardiomyocytes (Figure 4, Table 2) during chronic or acute exposure, respectively. In contrast, direct exposure to mutANP monomers caused significant shortening of APD, suppression of Vmax, and elevation in RMP resembling the effects of oligomers (Figure 4A-4B, Table 2). However, there were disparate effects on cellular bioenergetics: although ATP production and mitochondrial function trended downwards in the presence of mutANP monomers, these changes were not significant (Figure 1B, Supplemental Figure II).
Natriuretic peptide oligomers and mutANP monomers modulate atrial ionic currents
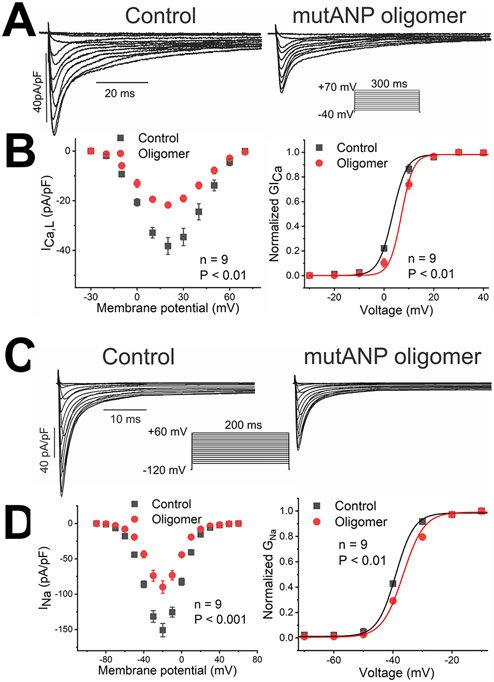
Additional experiments were performed to investigate the ionic basis of the action potential changes caused by natriuretic peptide oligomers and mutANP monomers. In mouse atrial cardiomyocytes, major components of repolarization include the transient outward current ITo and rapidly activating, sustained K+ current, or Isus, encoded by KCNA5.19 With acute exposure, both oligomers and mutANP monomers significantly increased the amplitude of ITo, with mutANP oligomers having the greatest effect (+31%; Figure 5A and Table 3). Similar effects were also observed to enhance Isus (by up to 40%; +19% for mutANP oligomers; Figure 5C and Table 3). These changes were accompanied by small but consistent shifts in the voltage dependence of channel activation for both currents to more negative potentials (Figure 5B and 5D; Supplemental Table III). A hallmark of atrial tachycardia and AF-related remodeling is a reduction in ICa,L.18 Most preparations tested suppressed ICa,L (by up to 43%), although BNP oligomers had the opposite effect (+19% increase; Figure 6A and Table 3), with variable changes in channel gating (Supplemental Table III). For Na+ current, all preparations reduced both peak and late INa (up to 35% and 31%, respectively), with a shift in channel activation to more positive potentials (Figure 6D and Table 3, Supplemental Table III). Thus, an increase in repolarizing K+ currents and reduced ICa,L would promote APD shortening, while decreased INa accounts for the suppression observed in Vmax.
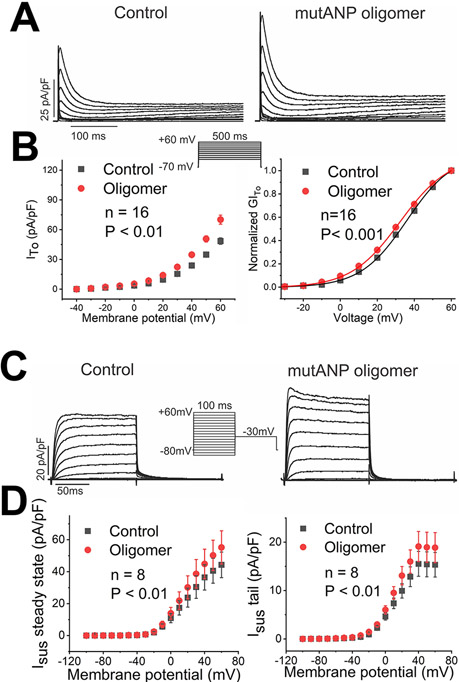
Figure 5. Transient outward (ITo) and rapidly activating, sustained (Isus) currents are increased by mutANP oligomers.
A. K+ currents elicited are displayed under control conditions and following exposure to mutANP oligomers (500nM; voltage clamp protocol shown in inset). B. Summary data are illustrated for the current-voltage relationship on the left and conductance-voltage plot on the right (n=16; P values shown on the Figure). C. Similar data are displayed for Isus currents, with current-voltage curves in panel D for steady-state (left) and tail (right) currents (n=8).
Table 3:
Natriuretic peptides: Significant effects on ionic currents
| INa§ | Late-INa§ | ICa,L‡ | IK1§ | IKv1.5∥ | ITo§ | |
|---|---|---|---|---|---|---|
| pA/pF | pA/pF | pA/pF | pA/pF | pA/pF | pA/pF | |
| Control | −72.3±4.9 | 0.27±0.01 | −47.0±5.4 | −76.0±9.5 | 4.3±0.7 | 42.5±2.9 |
| ANP oligo | −47.1±3.1* | 0.22±0.01* | −31.7±3.8* | −45.9±5.9* | 7.1±0.7* | 47.1±3.1* |
| Control | −59.0±4.7 | 0.24±0.004 | −28.9±3.9 | −96.4±15 | 12.3±1.5 | 46.5±3.2 |
| mutANP | −42.7±3.2* | 0.20±0.002† | −18.1±2.5† | −58.2±9.0* | 13.3±1.7* | 54.3±3.5* |
| Control | −59.6±6.0 | 0.23±0.004 | −59.3±7.5 | −88.4±7.8 | 15.3±2.5 | 48.5±3.2 |
| mutANP oligo | −39.2±4.4* | 0.17±0.004† | −34.5±3.0† | −54.8±5.8* | 18.9±3.1* | 70.1±4.6* |
| Control | −50.5±3.0 | 0.32±0.004 | −38.0±2.2 | −101.7±6.6 | 17.3±2.6 | 43.8±1.6 |
| BNP oligomer | −36.5±2.6* | 0.22±0.003† | −46.7±3.3* | −80.5±6.8* | 22.2±3.5* | 51.5±1.9* |
oligo represents oligomer
P<0.05
P<0.01
mouse atrial cells (n=3 mice for each group; n=7-15)
HL-1 cells (n=7-16)
Ltk− cells (n=7-13)
Figure 6. Oligomers derived from mutANP suppress L-type Ca++ and Na+ currents, with rightward shifts in the voltage dependence of channel activation.
Using a format similar to Figure 5, data are illustrated for the effects of mutANP oligomers on ICa,L (A and B) and INa (C and D).
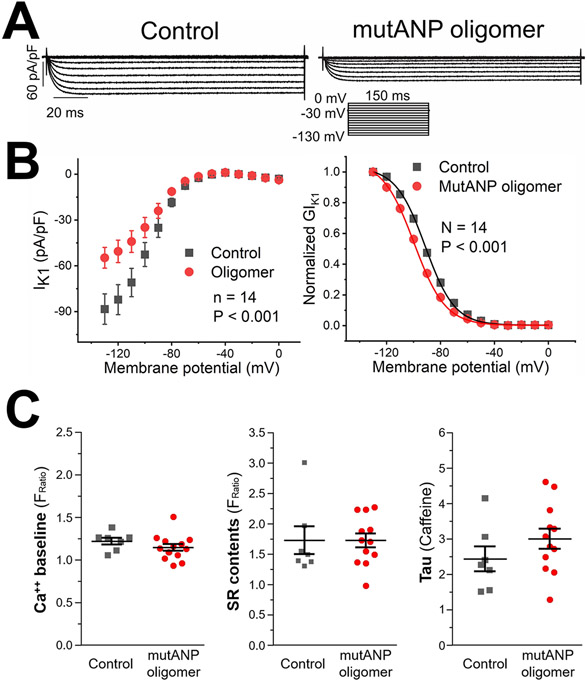
The cardiomyocyte resting membrane potential is primarily maintained by the inward rectifier IK1. Not surprisingly, all preparations examined reduced IK1 (up to 40%; Figure 7A and Table 3) to account for elevation of this parameter. This effect was accompanied by consistent shifts in channel activation to more negative potentials (Figure 7B; Supplemental Table III).
Figure 7. MutANP oligomers reduce inward rectifier currents, with no effect on intracellular Ca++ concentration or SR content.
A. Data are shown for the effects of mutANP oligomers on IK1. B. In atrial HL-1 cardiomyocytes, extracellular exposure to mutANP oligomers (1μM) did not alter baseline Ca++ concentration, SR content, or the Ca++ transient decay rate (tau) after caffeine administration, when compared to control cells (n=13, 7 cells, respectively).
Natriuretic peptide oligomers do not alter intracellular Ca++ concentration
In neurons, exposure to Aβ1-42 oligomers can increase intracellular Ca++ from both internal and external stores.20 To test the hypothesis that natriuretic peptide oligomers could have a similar effect in atrial cells, atrial HL-1 cardiomyocytes were incubated with mutANP oligomers, followed by loading with Fura-2 to assay intracellular Ca++ levels. Compared to untreated control cells, there was no effect of mutANP oligomers on baseline Ca++ concentration, total SR Ca++ content, or Na+-Ca++ exchanger function following either acute or chronic (24hr) exposure (Figure 7C).
DISCUSSION
Our results provide compelling evidence that natriuretic peptide oligomers are novel mediators of AF susceptibility that promote proarrhythmic metabolic and electrophysiologic effects in atrial cells (Figure 8). We have previously shown that oligomer burden in human atrium is linked to hypertension,13 and that natriuretic peptide oligomers develop in the atria of hypertensive mice coincident with AF vulnerability.14 An important driver of PAO production is oxidative stress, specifically highly-reactive IsoLGs that are invariably generated with increased ROS. For mutANP linked to familial AF, oligomer formation is markedly accelerated, and this is further enhanced by IsoLGs. Importantly, our findings demonstrate that the effects of oligomers on atrial cell electrophysiology mimic the electrical remodeling that occurs in a murine model of hypertension-mediated AF susceptibility. In addition, PAOs cause mitochondrial dysfunction, with a reduction in ATP levels and mitochondrial biogenesis, as well as increase in ROS, which also increase arrhythmia susceptibility. As in the brain, we hypothesize that the acute electrophysiologic effects result from direct interaction of oligomers with ion channels to modify function, while the metabolic and transcriptional changes related to mitochondrial function and abundance are mediated by internalized oligomers that develop with chronic exposure.
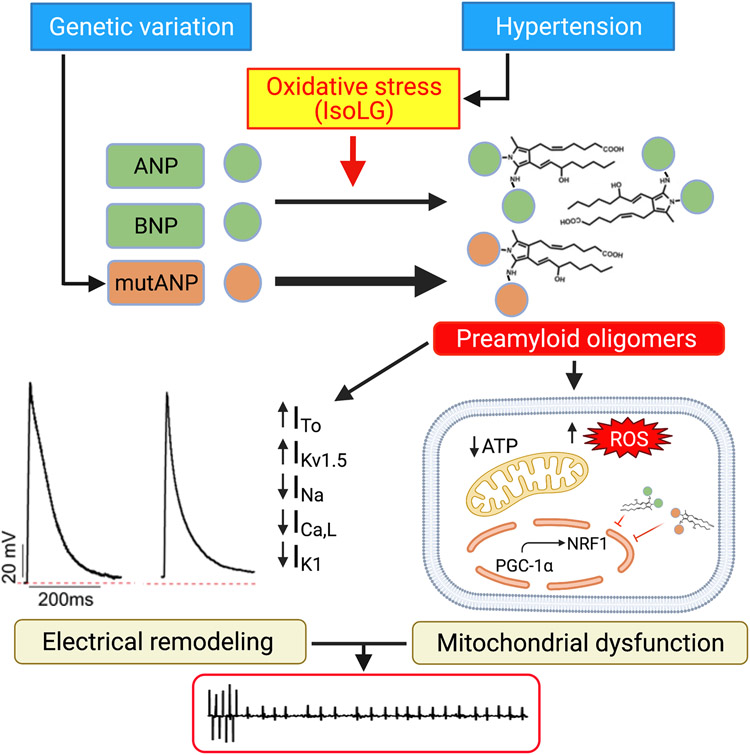
Figure 8. Mechanism(s) of increased AF susceptibility mediated by natriuretic peptide oligomers.
Hypertension-related oxidative stress and mutANP accelerate PAO formation, leading to proarrhythmic electrical remodeling and mitochondrial dysfunction. Modulation of ion channel function underlies action potential changes, while reduced mitochondrial biogenesis/ATP production and increased ROS further promote the AF substrate. Created with BioRender.com.
The major arrhythmogenic mechanisms that cause AF are reentry and ectopic atrial activity. Focal firing in the setting of a vulnerable substrate is required for AF initiation, and the elevation in RMP that we observed would increase automaticity. Initiation of reentry is favored by abbreviated refractoriness and slowed conduction. Thus, the reduction in APD, Vmax, and INa that occur during both hypertension and oligomer exposure create a proarrhythmic atrial substrate to increase AF susceptibility in vivo. Our findings are consistent with prior studies in rat models of hypertension, where reduced refractoriness and SCN5A expression, as well as heterogeneous conduction, have been demonstrated.21, 22 Nonetheless, there are conflicting results for atrial electrophysiologic remodeling seen in other hypertension models, likely caused by differences in methods (e.g., dose and/or duration of ang II infusion), method of generating hypertension, and concomitant structural remodeling.
Our data demonstrated that mutANP linked to familial AF misfolds much more readily than WT ANP, accelerating aggregation and PAO formation. Analogous to mutations causing early-onset Alzheimer’s disease, it is highly likely that this enhanced oligomer generation for mutANP increases its pathogenicity in humans. Recently, the NPPA mutation under study was modeled in mice by expression of human WT or mutANP.9 As for affected patients, mice generating mutANP were more susceptible to AF than mice expressing WT human ANP. Notably, atrial myocytes from mutANP mice demonstrated electrophysiologic findings very similar to our results, with reduced APD and Vmax, depolarized RMP, suppression of Na+ and L-type Ca++ currents, and an increase in sustained K+ currents compared to those from WT ANP mice. Transcriptional remodeling was observed, and these effects were attributed to altered ion channel expression. However, the electrophysiologic effects of mutANP that we observed in our experiments were rapid in onset consistent with a direct action of mutANP on ion channel function. Such actions are consistent with the known properties of PAOs.
In addition to electrophysiologic abnormalities, natriuretic peptide oligomers altered myocyte bioenergetics by disrupting mitochondrial function, an effect consistent with findings in hypertension.23 Prior studies have shown that mitochondrial function is altered in the atria of animal models and patients with AF,24, 25 and there is increasing evidence that this abnormality is linked to AF pathogenesis. Multiple AF risk factors such as hypertension, obesity, and diabetes mellitus are associated with oxidative stress and inflammation, processes that promote mitochondrial dysfunction, leading to reduced ATP production and further increases in ROS. These effects alter multiple cellular processes, in particular ion channel homeostasis and excitation-contraction coupling. Recent studies support the concept that mitochondrial dysfunction drives detrimental electrical, contractile, and structural remodeling that contribute to the AF substrate. For example, inhibitors of the sodium-glucose cotransporter-2 or dipeptidyl peptidase-4 not only improved mitochondrial dysfunction in animal models of diabetes, but they also reduced AF vulnerability.26, 27
Oligomers originating from different amyloid-forming proteins display common pathophysiologic mechanisms, and therefore, it is not surprising that numerous parallels exist between the toxicity of PAOs derived from natriuretic peptides and those formed by Aβ. Mitochondrial abnormalities are present in the brains of Alzheimer’s patients, and Aβ oligomers depress both mitochondrial function and biogenesis.28 PAOs have been shown to interact with lipid membranes in a nonspecific manner to increase permeability, causing cellular depolarization.20 They also associate with multiple neuronal membrane proteins including ion channels and receptors, causing dysfunction of synapses and respiratory chain components.20, 29 Based on our results, natriuretic peptide oligomers appear to have similar pleiomorphic effects.
An intriguing aspect of our results is the finding that the electrophysiologic effects of mutANP monomers were basically identical to those of mutANP oligomers, although mutant ANP monomers did not alter cardiomyocyte bioenergetics. Although Aβ1-42 monomers are not harmful to neurons, synthesized protein released into the extracellular space can quickly begin to misfold and aggregate. Recently, it was discovered that extracellular monomers preferentially migrate to and bind the plasma membrane in a concentration-dependent manner.30 Lipid-Aβ interactions at the plasma membrane accelerate peptide aggregation and the endocytosis of resulting oligomers, which can promote metabolic inhibition. In our experiments, we hypothesize that like Aβ1-42 monomers, mutANP monomers oligomerized rapidly at the cardiomyocyte plasma membrane to alter ion channel function and atrial electrophysiology. However, it appears most likely that the subsequent oligomer internalization was not sufficiently rapid to cause significant mitochondrial dysfunction during the time course of our experiments.
Regarding limitations, we cannot rule out the possibility that natriuretic peptide oligomers simply develop in association with hypertension-mediated electrical remodeling, rather than as cause and effect. However, the murine model demonstrates that AF susceptibility develops coincident with the appearance of oligomers and electrical remodeling in the atria, while oligomers cause essentially identical electrophysiologic effects in 2 different atrial cell preparations – findings that provide a compelling argument for their contribution to the pathophysiology. In addition, we cannot eliminate the possibility that the electrophysiologic effects of mutANP monomers are indeed mediated by the monomeric species rather than oligomers. Indeed, we conducted the monomer experiments immediately or within a few hours following reconstitution of the synthesized peptide. However, we find it highly improbable that the non-oligomerized protein would cause effects identical to those observed with 3 different species of oligomers.
In conclusion, our findings demonstrate that oligomers formed from WT and mutant natriuretic peptides are viable proarrhythmic mediators, causing detrimental metabolic and electrophysiologic effects in the atrium that promote AF susceptibility. Moreover, accelerated oligomerization by mutANP supports a pathophysiologic role for these mediators in human AF. These results add to the mounting evidence that proteotoxicity is a major pathologic mechanism causing cardiac disease in humans.
Supplementary Material
What is Known:
Amyloid formation invariably occurs in the aging human atrium, composed of atrial and B-type natriuretic peptides (ANP, BNP).
Preamyloid oligomers (PAOs) are the cytotoxic species in amyloidosis, and they accumulate in the atrium during hypertension in humans and in a murine hypertensive model of atrial fibrillation (AF) susceptibility.
Whether oligomers have properties that could promote AF susceptibility is not known.
What the Study Adds:
Natriuretic peptide oligomers caused detrimental metabolic effects in atrial cells, as well as proarrhythmic electrophysiologic changes resembling those of experimental hypertension, while monomers did not.
Oligomer formation was accelerated for an ANP mutation linked to familial AF. For mutant ANP, both monomers and oligomers were electrophysiologically active.
These studies establish natriuretic peptide oligomers as proarrhythmic mediators in the atrium. In addition, accelerated oligomerization by mutant ANP supports a role for these mediators in the pathophysiology of the mutation.
ACKNOWLEDGEMENTS
Experiments were performed using the Vanderbilt High-throughput Screening (HTS) Core Facility. The HTS Core receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485). The Agilent Seahorse XFe96 Analyzer is housed and managed within the Vanderbilt HTS Core Facility and was purchased through NIH Shared Instrumentation Grant 1S10OD018015.
SOURCES OF FUNDING
This work was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health [HL096844 and HL133127]; the American Heart Association, Southeast Affiliate [2160035] and National Center [18SFRN34230125 (K.T.M. is the Basic Project PI)]; and the National Center for Advancing Translational Sciences of the National Institute of Health [UL1 TR000445]. Confocal microscopy and image analysis were performed through the Vanderbilt Cell Imaging Shared Resource (also supported by the NIH [CA68485, DK20593, DK58404, DK59637 and EY08126]).
ABBREVIATIONS
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- PAO
preamyloid oligomer
- AF
atrial fibrillation
- mutANP
mutant ANP
- WT
wild-type
- APD
action potential duration
- RMP
resting membrane potential
- Aβ
amyloid β
- ROS
reactive oxygen species
- IsoLG
isolevuglandin
- OCR
oxygen consumption rate
- AM
acetoxymethyl
- NT
normal Tyrode’s
- SR
sarcoplasmic reticulum
- TEA
tetraethylammonium
- ang II
angiotensin II
- Vmax
maximal dV/dT of Phase 0
- TOMM20
translocase of the outer mitochondrial membrane 20
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1
- NRF1
nuclear respiratory factor 1
Footnotes
DISCLOSURES
There are no disclosures for any of the authors.
REFERENCES
- 1.Willis MS and Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med. 2013;368:455–464. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero-Munoz MJ, Castillo-Carranza DL and Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88:468–478. [DOI] [PubMed] [Google Scholar]
- 3.McLendon PM and Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res. 2015;116:1863–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evangelisti A, Butler H and Del Monte F. The heart of the Alzheimer's: A mindful view of heart disease. Front Physiol. 2020;11:625974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A and Goette A. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–2097. [DOI] [PubMed] [Google Scholar]
- 6.Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Martin SS, Rapezzi C, Bacchi Reggiani ML and Marinelli G. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J. 2004;25:1237–1241. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de AM, Burnett JC Jr. and Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua R, MacLeod SL, Polina I, Moghtadaei M, Jansen HJ, Bogachev O, O'Blenes SB, Sapp JL, Legare JF and Rose RA. Effects of wild-type and mutant forms of atrial natriuretic peptide on atrial electrophysiology and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2015;8:1240–1254. [DOI] [PubMed] [Google Scholar]
- 9.Menon A, Hong L, Savio-Galimberti E, Sridhar A, Youn SW, Zhang M, Kor K, Blair M, Kupershmidt S and Darbar D. Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation. J Mol Cell Cardiol. 2019;132:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomiyama T and Shimada H. APP Osaka mutation in familial Alzheimer's disease-Its discovery, phenotypes, and mechanism of recessive inheritance. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Garcia M, Fusco G and De Simone A. Membrane interactions and toxicity by misfolded protein oligomers. Frontiers in Cell and Developmental Biology. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glabe CG and Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. [DOI] [PubMed] [Google Scholar]
- 13.Sidorova TN, Mace LC, Wells KS, Yermalitskaya LV, Su PF, Shyr Y, Atkinson JB, Fogo AB, Prinsen JK, Byrne JG, et al. Hypertension is associated with preamyloid oligomers in human atrium: a missing link in atrial pathophysiology? J Am Heart Assoc. 2014;3:e001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol-Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, et al. Highly-reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci. 2020;5:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Shen W, Rottman JN, Wikswo JP and Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38:299–308. [DOI] [PubMed] [Google Scholar]
- 16.Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ and Oates JA. Prostaglandin H2 (PGH2) accelerates formation of amyloid β1-42 oligomers. J Neurochem. 2002;82:1003–1006. [DOI] [PubMed] [Google Scholar]
- 17.Rowe GC, Jiang A and Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijman J, Voigt N, Nattel S and Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 19.Brouillette J, Clark RB, Giles WR and Fiset C. Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol. 2004;559:777–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasumoto T, Takamura Y, Tsuji M, Watanabe-Nakayama T, Imamura K, Inoue H, Nakamura S, Inoue T, Kimura A, Yano S, et al. High molecular weight amyloid β(1-42) oligomers induce neurotoxicity via plasma membrane damage. FASEB J. 2019;33:9220–9234. [DOI] [PubMed] [Google Scholar]
- 21.Xia PP, Li LJ, Qi RD, Shi JJ, Ju WZ and Chen ML. Electrical and histological remodeling of the pulmonary vein in 2K1C hypertensive rats: Indication of initiation and maintenance of atrial fibrillation. Anatol J Cardiol. 2018;19:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau DH, Shipp NJ, Kelly DJ, Thanigaimani S, Neo M, Kuklik P, Lim HS, Zhang Y, Drury K, Wong CX, et al. Atrial arrhythmia in ageing spontaneously hypertensive rats: unraveling the substrate in hypertension and ageing. PLoS One. 2013;8:e72416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayeva M and Ardehali H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr Hypertens Rep. 2010;12:426–432. [DOI] [PubMed] [Google Scholar]
- 24.Jeganathan J, Saraf R, Mahmood F, Pal A, Bhasin MK, Huang T, Mittel A, Knio Z, Simons R, Khabbaz K, et al. Mitochondrial dysfunction in atrial tissue of patients developing postoperative atrial fibrillation. Ann Thorac Surg. 2017;104:1547–1555. [DOI] [PubMed] [Google Scholar]
- 25.Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX and Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang Z, Zhao Y, Jiang N, Qiu J, Yang Y, Li J, Liang X, Wang X, Tse G, et al. Alogliptin, a dipeptidyl peptidase-4 inhibitor, alleviates atrial remodeling and improves mitochondrial function and biogenesis in diabetic rabbits. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G and Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer's disease. J Alzheimers Dis. 2018;62:1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayed R and Lasagna-Reeves CA. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33 Suppl 1:S67–S78. [DOI] [PubMed] [Google Scholar]
- 30.Jin S, Kedia N, Illes-Toth E, Haralampiev I, Prisner S, Herrmann A, Wanker EE and Bieschke J. Amyloid-β(1-42) aggregation initiates its cellular uptake and cytotoxicity. J Biol Chem. 2016;291:19590–19606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarnath V, Amarnath K, Masterson TS, Davies S and Roberts LJ. A simplified synthesis of the diasteromers of levuglandin E2. Synthetic Communications. 2005;35:397–408. [Google Scholar]
- 32.Sidorova TN, Yermalitskaya LV, Mace LC, Wells KS, Boutaud O, Prinsen JK, Davies SS, Roberts LJ, Dikalov SI, Glabe CG et al. Reactive γ-ketoaldehydes promote protein misfolding and preamyloid oligomer formation in rapidly-activated atrial cells. J Mol Cell Cardiol. 2015;79:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A and Izzo NJ. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy of Sciences. 1998;95:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White SM, Constantin PE and Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–9. [DOI] [PubMed] [Google Scholar]
- 35.Mace LC, Yermalitskaya LV, Yi Y, Yang Z, Morgan AM and Murray KT. Transcriptional remodeling of rapidly stimulated HL-1 atrial myocytes exhibits concordance with human atrial fibrillation. J Mol Cell Cardiol. 2009;47:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyders DJ, Tamkun MM and Bennett PB. A rapidly activating and slowly inactivating potassium channel cloned from human heart: Functional analysis after stable mammalian cell culture expression. Journal of General Physiology. 1993;101:513–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.