Abstract
Reciprocal relations between sleep and adjustment were investigated. Participants included 246 adolescents (M = 15.80 years; 67.5% White, 32.5% Black/African American; 53% female, 47% male) at Time 1 (data collected 2012–2013), 227 at Time 2 (M = 16.78 years) and 215 at Time 3 (M = 17.70 years). Sleep-wake variables were measured with self-reports (sleepiness) and actigraphy (average sleep minutes and efficiency, variability in sleep minutes and efficiency). Adolescents reported on depression and anxiety symptoms, and parents reported on externalizing problems. Greater variability in sleep duration and efficiency as well as sleepiness predicted adjustment problems (range of R2: 36%−60%). Reciprocal relations were supported mostly for sleepiness (range of R2: 16%−32%). Results help understand bidirectional relations between sleep and adjustment.
Keywords: Sleep, adjustment, mental health, reciprocal associations, actigraphy
Poor sleep in community samples of adolescents is common (Gradisar et al., 2011) and associated with adjustment problems (El-Sheikh et al., 2019; Lovato & Gradisar, 2014; Moore et al., 2009). The evidence for relations between sleep and adjustment problems in adolescence is increasing, however gaps remain. In particular, multi-wave examinations of reciprocal relations are relatively rare, though addressing the extent to which sleep predicts adjustment and vice versa (i.e., adjustment as a predictor of sleep) is an important priority for sleep research (El-Sheikh & Buckhalt, 2015). Of the limited existing work to consider bidirectional effects, sleep has mostly been measured with self-reports; objective assessments are needed. Further, sleep is a complex and multifaceted construct, and some sleep-wake parameters highly relevant to adolescence have received little inquiry (e.g., sleep variability; Becker et al., 2017). Building on this literature, we investigated the reciprocal relations between sleep and internalizing and externalizing behaviors in a large community sample of middle to late adolescents using three waves of data with a one-year lag between consecutive waves. Developmentally significant sleep parameters were assessed using subjective and objective measures.
Prior findings based on community samples demonstrate associations between adolescents’ sleep and their adjustment. Using cross-sectional designs, shorter mean sleep duration (e.g., minutes spent asleep), poorer mean sleep quality (e.g., sleep efficiency), inconsistency in sleep schedules, and daytime sleepiness have been associated with depression (Lovato & Gradisar, 2014; Raniti et al., 2017; Shimizu et al., 2020), anxiety (Brown et al., 2018; Moore et al., 2009; Shimizu et al., 2020), and externalizing behaviors (El-Sheikh et al., 2019). Although fewer longitudinal studies have been conducted, these studies also show that various sleep-wake problems predict adjustment problems (e.g., Bustamante et al., 2020). In addition, experimental studies indicate that sleep extension or restriction influences adolescents’ mood (Baum et al., 2014). Thus, multiple research methods converge in finding an association between sleep and mental health in adolescence.
However, important questions remain about relations between adolescents’ sleep and adjustment, including the directionality of associations. Bidirectional pathways are possible. For example, sleep problems may decouple cortical and limbic regions of the brain, which in turn may diminish cognitive control over negative emotions and impulses, and thereby contribute to adjustment problems (Walker & van der Helm, 2009; Yoo et al., 2007). At the same time, emotional and behavioral problems may disrupt sleep (Williamson et al., 2021) because vigilance and sleep are opponent processes (Dahl, 1996). Symptoms of depression and anxiety include increased self-blame, worrying, and thought rumination (Garnefski et al., 2005), all of which may disrupt sleep (McMakin & Alfano, 2015). Similarly, externalizing behaviors often include emotional arousal and dysregulated physiological systems (Ruttle et al., 2011) that may interfere with sleep (Steiger, 2002). Thus, sleep and adjustment may be reciprocally influential.
Empirical investigations have demonstrated reciprocal relations between sleep and adjustment among younger children including preschoolers and middle schoolers (e.g., Quach et al., 2018). Investigations specific to middle and late adolescence are rare, yet important, given the vulnerability for sleep problems during this developmental period. Many adolescents do not obtain sufficient sleep duration (Gradisar et al., 2011) and rates of sleepiness are high (Feinberg, 2013; Gradisar et al., 2011). Further, adolescence is a time of rapid emotional and social change when internalizing symptoms and some externalizing behaviors (e.g., delinquency) rise (Dahl & Gunnar, 2009).
A small but growing literature has examined reciprocal associations among community samples of adolescents. Subjectively reported sleep-wake problems including later bedtime, symptoms of insomnia, and general sleep-related problems have been reciprocally related to more internalizing symptoms (Alvaro et al., 2017; Tochigi et al., 2016) and externalizing behaviors among adolescents (Williamson et al., 2021). In other research with adolescents, self-reported sleep problems predicted greater internalizing symptoms over time, whereas the opposite direction of effect was less evident (Narmandakh et al., 2020; Pieters et al., 2015; Roberts & Duong, 2014; Williamson et al., 2021). Another study, however, found that adjustment problems predicted sleep to a greater degree than the reverse (Hayley et al., 2015). In one of the few exceptions to use actigraphy, bidirectional effects were examined during the transition from middle childhood to early adolescence (ages 8 to 13 years). Shorter actigraphy-derived mean sleep duration and poorer mean sleep quality predicted more internalizing and externalizing behaviors, whereas prediction from adjustment problems to sleep was weaker (Kelly & El-Sheikh, 2014). Among youth transitioning to college, self-reported sleep problems were reciprocally related with increased levels of anxiety over time, and symptoms of depression predicted greater actigraphy-derived sleep onset latency, variability in sleep onset time, and subjective sleep problems (Doane et al., 2015). These studies have advanced our understanding of associations between sleep and adjustment, but inconsistencies limit conclusions about bidirectional effects. In addition, limited work has measured sleep objectively and some sleep parameters that are salient during adolescence (e.g., variability in minutes and quality) have received little inquiry.
We used actigraphy over multiple nights to objectively estimate several sleep parameters. We derived well-established sleep parameters (Meltzer et al., 2012), namely sleep minutes (average number of minutes spent asleep per night) and sleep efficiency (average percentage of minutes spent asleep each night from sleep onset to wake time). We also incorporated actigraphy-derived parameters that are less common but important, including variability in sleep minutes and variability in sleep efficiency over multiple nights. These variables are calculated by quantifying intraindividual daily variation around the mean (Becker et al., 2017). Until recently, variability in sleep parameters have been seldom examined and poorly understood (Bei et al., 2016). However, emerging research suggests that increased sleep variability may more robustly forecast poor developmental outcomes compared to mean composites of sleep parameters (Bei et al., 2016). Investigations of variability in sleep are especially relevant to adolescents. This developmental period is characterized by increased social, academic, and extracurricular demands as well as decreased parental monitoring, which contribute to night-to-night fluctuations in sleep duration and quality (Becker et al., 2017). We also included subjective reports of daytime sleepiness, which taps into sleep need and vulnerability to sleep loss (Feinberg, 2013; Shimizu et al., 2020). Sleepiness confers risk for adjustment problems (Moore et al., 2009), even independent of other sleep parameters including short sleep duration (Feinberg, 2013; Shimizu et al., 2020).
Investigations of adolescents’ sleep variability are rare and mostly limited to variability in sleep duration (Becker et al., 2017). Greater variability in sleep duration has been linked with higher levels of adolescents’ adjustment problems cross-sectionally (Bei, Mander, et al., 2017; Fuligni et al., 2018) and longitudinally (Bustamante et al., 2020). Consistent with recommendations (Bei et al., 2016), mean sleep duration was covaried in some of these studies, indicating that variability in sleep duration conferred unique risk beyond the effect of averaged sleep duration composites (e.g., Bei, Mander, et al., 2017; McHale et al., 2011).
Despite common night-to-night fluctuations in adolescents’ sleep quality (Becker et al., 2017), studies of variability in adolescent sleep quality as related to adjustment are nearly non-existent. However, investigations of other developmental domains and populations provide insight. Among adults, more variability in actigraphy-based fragmentation index (i.e., nocturnal movement) was associated with greater life stress (Mezick et al., 2009). During late childhood and early adolescence, more variability in actigraphy-derived sleep efficiency was related with poorer performance on neurocognitive tasks and working memory (Gruber & Sadeh, 2004). Among 12- to 35-year-olds, those with depression had more variability in actigraphy-derived sleep efficiency than a control group (Robillard et al., 2015). Collectively, these results suggest that variability in adolescents’ sleep may also be reciprocally related to adjustment.
Current Study
We investigated reciprocal relations between sleep and symptoms of depression, anxiety, and externalizing behaviors using three waves of data spanning two years (Time 1 = age 16; Time 2 = age 17; Time 3 = age 18). Actigraphy was used to derive average sleep duration (minutes), variability in sleep duration, average sleep efficiency, and variability in sleep efficiency over seven consecutive nights. Sleepiness was assessed using self-reports. Cross-lagged panel models tested reciprocal relations and whether sleep more robustly predicted adjustment or vice versa. Because internalizing and externalizing behaviors co-occur (Coulombe et al., 2011), we examined all adjustment domains simultaneously to ascertain the unique associations between sleep and each adjustment variable. As part of confirmatory hypothesis testing, we expected bidirectional relations between sleep and adjustment. We had no expectations about whether adjustment would be a more robust predictor of sleep or vice versa due to the inconsistency of available evidence, and these analyses were considered exploratory. Similarly, we had no hypotheses regarding differential effects for either sleep or adjustment parameters and this was treated as exploratory.
We also conducted secondary analyses. Although sleep varies across weeknights in adolescence, fluctuations are particularly evident during transitions to weekends (Becker et al., 2017). For example, it is well-established that adolescents often sleep significantly longer over the weekends (Sun et al., 2019). We conducted novel analyses and explored whether the effects of sleep variability differed when weekend and weeknights were both included versus only weeknights.
Method
Participants
Participants were part of a longitudinal study examining bioregulatory processes, adjustment, and family functioning from middle childhood into adolescence (Family Stress and Youth Development Study). Data for the current study are drawn from Waves 4–6 of the larger investigation; these data were collected with a 1-year interval between time points (2012–2015). For clarity, these waves will be referred to hereafter by the average age of participants (i.e., age 16, 17, 18). At study enrollment, participants were recruited through flyers distributed at local elementary schools in semirural areas and small towns in the southeastern United States. At the initial wave (2005), eligibility to participate required living in a two-parent home with parents who had been living together for at least two years. Exclusion criteria included the youth having a diagnosed sleep disorder (e.g., sleep apnea), as reported by parents. To address attrition resulting from the five-year gap between the third and fourth waves of the study, additional families were recruited into the study at the fourth wave. Families were recruited from the same school systems using the same inclusion/exclusion criteria as those in the original sample.
At age 16, 246 adolescents (M age = 15.79 years, SD = 0.80; 53% female, 47% male; 67% White/European American [EA], 33% Black/African American [AA]) and their parents participated (211 mothers, 171 fathers). The ethnic/racial composition of the sample is reflective from the community from which it was drawn. According to their families’ income-to-needs ratio (US Department of Commerce, 2019), adolescents were from diverse socioeconomic backgrounds (SES); 14% lived below the poverty line (income-to-needs ratio ≤ 1), 29% were near the poverty line (ratio > 1 and ≤ 2), 22% were considered lower middle class (ratio > 2 and ≤ 3), and 35% were middle class (ratio > 3).
About one year later (M time lag between age 16 and age 17 assessments = 367 days; SD = 27 days), 227 adolescents (M age = 16.78 years, SD = 0.77; 55% female, 45% male; 69% EA, 31% AA) and their parents (201 mothers, 168 fathers) participated. Retention from the age 16 assessment was good (n = 222; 90%); an additional five individuals who did not participate at age 16, but participated in W1-W3 of the larger study, were part of the analytical sample at age 17. Comparisons were made to determine whether differences existed on study variables between adolescents who participated at age 16 and age 17 versus those who only participated at age 16; no differences were detected.
About one year after the age 17 assessment (M time lag between age 17 and age 18 assessments = 349 days; SD = 20 days), 215 adolescents (85% retained from age 16 and 93% retained from age 17; M age = 17.70 years, SD = 0.75; 55% female, 45% male; 71% EA, 29% AA) and their parents (190 mothers, 157 fathers) participated at age 18. One individual who was part of the larger study but did not participate at age 16 nor age 17 was part of the analytical sample at age 18. Adolescents who did not participate at age 18 were from lower SES backgrounds compared to those who remained in the study, t(238) = - 2.05, p = .04. No other differences on study variables were noted between those who remained in the study and those who discontinued at age 18. Reasons for attrition included relocation, a busy schedule, and disinterest in continued participation. At the age 18 assessment, 82% of participants were in high school, 13% were attending college, and 5% dropped out of school, graduated, or were missing information on school status. Additionally, 93% were living at home with one or both parents, whereas the remaining 7% of the sample were living at college or elsewhere.
Procedure
The study was approved by the university’s Institutional Review Board. Procedures and measures were identical for each wave. Parents provided written consent and adolescents assented to participate. At each wave, adolescents wore actigraphs at home for seven consecutive nights during the school year excluding holidays. Adolescents also completed a sleep diary nightly, which was used to corroborate actigraphy data. In most cases, adolescents visited the laboratory within a few days after the last night of actigraphy (age 16 M = 3.30 days, SD = 10.13; age 17 M = 2.24 days, SD = 7.81; age 18 M = 0.38 days, SD = 5.38). Adolescents and their parent(s) completed questionnaires online prior to the lab visit or in separate rooms in the lab during the visit.
Measures
Actigraphy Measures of Sleep
Adolescents wore Octagonal Basic Motionlogger actigraphs (Ambulatory Monitoring, Ardsley, NY, USA) on their non-dominant wrists at home while sleeping for up to seven nights. Participants were instructed to place the actigraph on their wrist when they were ready to try to go to sleep and to take it off when they woke up. Additionally, they were instructed to press an event marker button on the actigraph to indicate first attempt to fall asleep and upon awakening in the morning. These event marks appear when coding the actigraphy data using the software that accompanied the actigraph. A sleep diary was used to aid with coding the actigraphy data; nights were excluded when diaries were not available or when diary-reported sleep onset or wake times differed from actigraphy-determined times by more than 30 minutes, which occurred infrequently (5 to 29 cases per night). Participants were called nightly with reminders to wear actigraphs and complete the sleep diaries. Data were scored in Action W2 software using the Sadeh algorithm and zero crossing mode (Sadeh et al., 1994) to derive the number of 1-minute epochs scored as sleep. Sleep onset was defined as the first of three consecutive minutes scored as sleep after reported bedtime; sleep offset was the last of five consecutive minutes scored as sleep prior to wake time. Four indices of sleep were examined in analyses: (a) Average sleep minutes; (b) Intraindividual variability in sleep minutes; (c) Average sleep efficiency; and (d) Intraindividual variability in sleep efficiency. Definitions for sleep minutes and efficiency followed the actigraphy manual (Ambulatory Monitoring). Sleep minutes was calculated as the total number of minutes scored as sleep between actigraphy-determined sleep onset to morning wake time, excluding epochs scored as awake. Sleep efficiency was derived as the percentage of minutes scored as sleep between sleep onset and wake time. For both minutes and efficiency, averages were computed across all available nights at each time point, and variability of sleep across all available nights was computed using the mean-centered coefficient of variance statistic (Snedecor & Cochran, 1967), as done in previous research examining variability in sleep (Becker et al., 2017; Bei et al., 2016). At each wave, average sleep minutes (α = .72–.75 across W1 to W3) and sleep efficiency (α = .92–.94 across W1 to W3) were stable across the seven nights.
Per established guidelines (Meltzer et al., 2012), actigraphy sleep data for those with fewer than five nights were excluded and treated as missing (41 participants at age 16, 66 participants at age 17, and 44 participants at age 18). Reasons for missing data included the use of medication for acute illnesses (e.g., flu) and the exclusion of such nights from analyses, as well as forgetting to wear the actigraph and actigraph malfunction in very few cases. Among those with at least five nights of actigraphy data at age 16, 43% had data for all seven nights, 34% had data for six nights, and 23% had data for five nights (M = 6.20 nights, SD = 0.79; n = 193). At age 17, 24% had data for all seven nights, 31% had data for six nights, and 45% had data for five nights (M = 5.79 nights, SD = 0.81; n = 137). At age 18, 35% had data for all seven nights, 41% had data for six nights, and 24% had data for five nights (M = 6.11 nights, SD = 0.76; n = 157). Missing actigraphy data are typical (Meltzer et al., 2012), and rates of missingness are similar to those in other studies (Bustamante et al., 2020; El-Sheikh et al., 2020).
Sleepiness
Adolescents completed the sleepiness subscale of the School Sleep Habits Survey (Wolfson & Carskadon, 1998), which has demonstrated good reliability and validity (Wolfson & Carskadon, 1998; Wolfson et al., 2003). The sleepiness scale consists of nine items that assess whether participants have struggled to stay awake during various activities over the past two weeks (e.g., “watching T.V.,” “listening to music,” “in a class at school”); because many adolescents were not driving, one item regarding sleepiness while driving was not included. Responses were scored on a four-point scale (1 = did not struggle to stay awake to 4 = both struggled to stay awake and fell asleep) and summed. Internal consistency was good (αs = .71–.74 across ages 16 to 18).
Symptoms of Depression
Adolescents completed the Child Depression Inventory (CDI), which is a reliable and valid measure among adolescents (Kovacs, 1992). The CDI consists of 27 items assessing symptoms of depression over the past two weeks on a three-point scale (e.g., ranging from 0 [“Things will work out for me O.K.”] to 2 [“Nothing will ever work out for me”]). One item concerning suicidal ideation was not administered, and two items relating to sleep disturbances were excluded from analyses. Responses were summed and higher scores reflect greater depression symptoms. Internal consistency was good across study waves (αs = .88–.89). Clinically significant levels of depression (scores ≥ 18, cut-off score of 20 for original 27-item measure adjusted to account for three excluded items) were reported by 6.0%, 11.3%, and 7.2% of the sample at ages 16, 17, and 18, respectively.
Anxiety
Adolescents completed the well-established Revised Children’s Manifest Anxiety Scale (RCMAS-2; Reynolds & Richmond, 2008). The Total Anxiety scale includes 45 items assessing various dimensions of anxiety, including physiological symptoms (e.g., “Often I feel sick in my stomach”), worry (e.g., “I worry a lot of the time”), and social anxiety (e.g., “I fear other people will laugh at me”). Five items pertaining to sleep problems were excluded. Adolescents indicated whether they agreed (coded 1) or disagreed (coded 0) with each statement. Responses were summed, and higher scores reflect greater symptoms of anxiety. The scale had excellent reliability across waves (αs ranged from .92–.93). The RCMAS-2 does not provide a clinical cutoff. In our sample, 4.7% of youth at age 16, 5.9% of youth at age 17, and 4.8% of youth at age 18 scored greater than 2 SDs above the mean.
Externalizing Behaviors
Children are generally better reporters of internalizing symptoms than parents (Angold et al., 1987), whereas parents’ reports of externalizing behaviors are often more reliable than youth reports (Loeber et al., 1990). Thus, mothers and fathers reported on adolescents’ externalizing behaviors using the Personality Inventory for Children (PIC), which is a valid measure of youth adjustment problems through age 18 (Wirt et al., 1990). The externalizing scale assesses impulsivity, delinquency, disruptive behavior, noncompliance, and aggression. Parents rated items as true (coded 1) or false (coded 0) about the adolescent, and responses were summed separately for mothers and fathers. Internal consistency was good across waves for mothers (αs = .87–.93) and fathers (αs = .87–.90). Mothers’ and fathers’ reports were strongly correlated (rs ranged from .65–.87 across waves), and thus were averaged to form an overall composite at each wave. Because the PIC T scores are age and gender corrected, raw scores are more appropriate for longitudinal analyses and thus were utilized. Borderline or clinically significant levels of externalizing behaviors (T scores ≥ 60) were reported by 11.4%, 6.5%, and 6.8% of mothers and 16.4%, 11.3%, and 7.0% of fathers at age 16, 17, and 18, respectively.
Plan of Analysis
Cross-lagged panel models were fit to assess the reciprocal relations between adolescents’ sleep and their socioemotional adjustment using the three waves of data. Five sleep parameters were included: average sleep minutes, variability in sleep minutes, average sleep efficiency, variability in sleep efficiency, and sleepiness. Three adjustment variables were assessed: depression symptoms, anxiety, and externalizing behaviors. Each sleep parameter was examined in a separate model. To better ascertain the unique association that each adjustment variable shared with sleep, all three adjustment variables were examined simultaneously and allowed to covary in the models. In total, five cross-lagged models were fit (one model per each sleep parameter). See Figure 1 for an example. To minimize outlier effects, high-leverage values surpassing 3 SDs from the sample mean were replaced with the value corresponding to ±3 SDs (Tabachnick & Fidell, 2013). In total, 26 values among the actigraphy-derived sleep variables, 10 values among self-reported sleepiness, and 28 values among the adjustment variables were recoded. Skew was assessed using visual observation as well as skewness and kurtosis statistics (±2). No variables were adjusted for skew.
Figure 1.

Reciprocal relations between adolescents’ intraindividual variability in sleep minutes and their adjustment. Unstandardized and standardized coefficients (in parentheses) are provided. Adolescents’ gender, race/ethnicity and socioeconomic status at age 16 and mean sleep minutes at each wave are controlled in analyses. Residual valances among endogenous variables within each wave were allowed to correlate. Model fit: χ2(73) = 201.99, p < .001; χ2/df = 2.77; CFI = .92; RMSEA = .08, p < .001, 95% CI [.07 to .10] Statistically significant lines are solid, whereas nonsignificant lines are dotted.
*p < .05. **p < .01. ***p < .001
In each model, autoregressive effects were estimated for the sleep and adjustment variables. Specifically, each variable at one time point was allowed to predict the same variable at the subsequent wave (e.g., anxiety at age 16 was allowed to predict anxiety at age 17). Controlling for autoregressive effects helps provide information about the stability of each construct over time, reduces bias in parameter estimates, and provides insight into the temporal sequence of relations between study variables (Selig & Little, 2012). In each model, the sleep variable was allowed to predict each of the adjustment variables at the following wave. To estimate reciprocal effects, each adjustment variable was allowed to predict sleep at the subsequent wave. Estimating these paths allowed for the assessment of cross-lagged cascade effects over time (Selig & Little, 2012). The residual variances among exogenous variables within the same wave were allowed to correlate.
Variables known to relate to primary study variables were controlled including adolescents’ sex, race/ethnicity, and family SES at age 16. In initial analyses, number of weekday nights and weekend nights as assessed by actigraphy were considered as covariates, however they did not have an influence and were not retained for parsimony. Significant associations between the control variables and primary study variables were estimated. Following recommendations and towards stringent assessment of study questions, average sleep minutes was controlled at each wave in the model that considered reciprocal relations between variability in sleep minutes and adolescents’ adjustment (Becker et al., 2017). Such covariation is critical to elucidate whether variability in sleep minutes is uniquely related to adjustment beyond the contribution of mean sleep minutes. Similarly, average sleep efficiency at each wave was controlled in the model that considered reciprocal relations between variability in sleep efficiency and adjustment.
The cross-lagged models were fit using Amos 27. Models were considered an acceptable fit if they satisfied at least two of the following criteria: χ2/df < 3, comparative fit index (CFI) > .90, and root mean square error of approximation (RMSEA) ≤ .08 (Browne & Cudeck, 1993). Each fitted model met these criteria. In regard to missing actigraphy data, 78% of adolescents at age 16, 60% at age 17, and 73% at age 18 had valid data (5 or more nights of actigraphy), which were included in analyses. Available data for sleepiness was high and ranged from 96% to 97% across the waves. Similarly, available data across the waves ranged from 95% to 97% for depression symptoms, 95% to 98% for anxiety, and 91% to 94% for externalizing behaviors. Full-information maximum likelihood (FIML) was used to handle missing data (Acock, 2005). The amount of missingness was in the acceptable range for FIML (Enders & Bandalos, 2001). Lastly, we assessed whether missing actigraphy data over the weekend was related to adjustment. Independent samples t-tests were used to determine whether scores on the adjustment variables were related to missing data on the actigraphy-derived weekend sleep variable (0 = missing at least one weekend night; 1 = both weekend nights present). These tests were conducted at both the cross-sectional and longitudinal levels. Results indicated that data were missing completely at random.
As part of secondary analyses, models were refit to investigate reciprocal relations between actigraphy-derived sleep and adjustment while using data solely from weeknights. Each actigraphy variable (i.e., average minutes, variability in minutes, average efficiency, variability in efficiency) was recomputed to include only sleep data from the five weeknights. The sample was identical to that utilized in primary analyses. Findings are reported in the Results.
Results
Descriptive Statistics and Preliminary Analyses
Based on actigraphy, adolescents’ average sleep onset time at age 16 was 11:03pm and morning wake time was 6:28am. Sleep schedules were similar at age 17 (11:08pm; 6:33am) and age 18 (11:36pm; 6:51am). Descriptive statistics of primary variables are included in Table 1. Based on actigraphy, adolescents slept for an average of 6 hours and 51 minutes (SD = 61 minutes) at age 16, 6 hours and 54 minutes (SD = 49) at age 17, and 6 hours and 47 minutes (SD = 57) at age 18; hours refer to actual sleep during the sleep period minus wakings. Average variability in sleep minutes across nights of actigraphy was 24 minutes at age 16, 20 minutes at age 17, and 23 minutes at age 18. The average variability in sleep efficiency across nights of actigraphy was 2.58% at age 16, 1.09% at age 17 and 1.48% at age 18.
Table 1.
Comparison of Means among Observed Study Variables over Time Using Repeated Measures ANOVA
| Variable Name |
Mean (SD) Age 16 |
Mean (SD) Age 17 |
Mean (SD) Age 18 |
F-value |
|---|---|---|---|---|
| 1. Average sleep minutes | 410.73 (60.65) | 413.71 (48.75) | 407.07 (56.52) | .73 |
| 2. Intraindividual variability in sleep minutes | .16 (.07)1 | .14 (.07)3 | .16 (.07) | 4.70* |
| 3. Average sleep efficiency | 90.91 (6.88) | 92.11 (5.76) | 91.92 (6.71) | 1.13 |
| 4. Intraindividual variability sleep efficiency | .051 (.03)1,2 | .038 (.03) | .047 (.04) | 5.81** |
| 5. Sleepiness | 13.71 (3.79) | 13.49 (3.94) | 13.01 (3.74) | 2.10 |
| 6. Depression symptoms | 6.85 (5.61) | 7.05 (6.48) | 6.92 (6.29) | 4.35 |
| 7. Anxiety | 9.18 (7.60) | 8.51 (7.96) | 8.74 (8.17) | 1.61 |
| 8. Externalizing behaviors | 4.07 (4.45)1,2 | 3.46 (3.59) | 3.19 (3.44) | 6.88*** |
Statistically significant difference between means at ages 16 and 17.
Significant difference between ages 16 and 18.
Significant difference between ages 17 and 18.
p < .05;
p < .01;
p < .001.
To help place our secondary analyses involving sleep variability over the full week versus only the weeknights in context, we conducted comparisons between weekday and weekend sleep. Adolescents slept longer on the weekends at age 16 (Mweekday = 395.20, Mweekend = 440.60, t(180) = −7.82, p < .001), age 17 (Mweekday = 404.25, Mweekend = 428.62, t(124) = −3.18, p = .002), and age 18 (Mweekday = 393.49, Mweekend = 416.57, t(151) = −3.53, p < .001). Weekday and weekend sleep efficiency did not significantly differ at age 16 or age 17. Sleep efficiency was higher on weekdays at age 18 (Mweekday = 91.77, Mweekend = 90.92, t(151) = 2.07, p = .04). Further, comparisons were made between sleep variability over the full seven nights of actigraphy versus the five weeknights. Variability in sleep minutes was greater across all seven nights in comparison to only weeknights at ages 16 (Mseven nights = .15, Mweeknights = .12, t(192) = 8.30, p < .001), 17 (Mseven nights = .14, Mweeknights = .10, t(136) = 8.38, p < .001), and 18 (Mseven nights = .16, Mweeknights = .13, t(156) = 6.24, p < .001). Compared to only weeknights, sleep efficiency fluctuated more across all seven nights at ages 17 (Mseven nights = .04, Mweeknights = .03, t(136) = 3.32, p = .001) and 18 (Mseven nights = .05, Mweeknights = .04, t(156) = 2.89, p = .004).
Repeated measures-analysis of variance was used to assess whether the means of primary variables differed across waves (Table 1). Variability in sleep minutes decreased from ages 16 to 17 and increased from ages 17 to 18. Variability in sleep efficiency was greater at age 16 than at ages 17 and 18. Lastly, rates of externalizing behaviors were higher at age 16 in comparison to ages 17 and 18. Bivariate correlations are included in Table 2 and several of the sleep and adjustment variables were associated within and across waves.
Table 2.
Bivariate Correlations among Primary Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Average sleep minutes (age 16) | ||||||||||||||||||||||||
| 2. Variability in sleep minutes (age 16) | −.43*** | |||||||||||||||||||||||
| 3. Average sleep efficiency (age 16) | .60*** | −.19** | ||||||||||||||||||||||
| 4. Variability in sleep efficiency (age 16) | −.53*** | .24** | −.80*** | |||||||||||||||||||||
| 5. Sleepiness (age 16) | −.08 | .17* | .00 | .06 | ||||||||||||||||||||
| 6. Depression symptoms (age 16) | −.14 | .15* | .09 | −.02 | .31*** | |||||||||||||||||||
| 7. Anxiety (age 16) | −.03 | .03 | .05 | −.03 | .30*** | .74*** | ||||||||||||||||||
| 8. Externalizing behaviors (age 16) | −.03 | .12 | .02 | −.01 | .01 | .20** | .07 | |||||||||||||||||
| 9. Average sleep minutes (age 17) | .41*** | −.06 | .20* | −.18 | −.07 | −.01 | .03 | −.03 | ||||||||||||||||
| 10. Variability in sleep minutes (age 17) | −.12 | .32** | .00 | −.01 | −.01 | −.05 | −.13 | .11 | −.22* | |||||||||||||||
| 11. Average sleep efficiency (age 17) | .23* | .02 | .41*** | −.26** | .13 | .21* | .17 | −.01 | .47*** | −.04 | ||||||||||||||
| 12. Variability in sleep efficiency (age 17) | −.17 | −.05 | −.34*** | .24* | .03 | −.15 | −.09 | −.04 | −.43*** | .10 | −.78*** | |||||||||||||
| 13. Sleepiness (age 17) | −.15* | .18* | .03 | .06 | .47*** | .40*** | .39*** | .12 | −.01 | −.06 | .24** | −.17 | ||||||||||||
| 14. Depression symptoms (age 17) | −.12 | .15 | .04 | −.04 | .18** | .63*** | .48*** | .21** | −.11 | .02 | .12 | −.10 | .36*** | |||||||||||
| 15. Anxiety (age 17) | −.05 | .11 | .08 | −.01 | .20** | .60*** | .70*** | .16* | −.01 | −.02 | .13 | −.02 | .40*** | .64*** | ||||||||||
| 16. Externalizing behaviors (age 17) | .06 | .04 | .05 | −.03 | −.03 | .16* | .08 | .75*** | .00 | .13 | −.03 | .03 | .10 | .28*** | .20** | |||||||||
| 17. Average sleep minutes (age 18) | .49*** | −.07 | .34*** | −.30** | −.02 | −.10 | −.11 | −.06 | .63*** | −.11 | .33** | −.19 | −.08 | −.10 | −.09 | .04 | ||||||||
| 18. Variability in sleep minutes (age 18) | −.25** | .18* | −.02 | .05 | −.04 | .06 | .06 | .03 | −.41*** | .25* | −.16 | .20* | .22** | .11 | .15 | .09 | −.37*** | |||||||
| 19. Average sleep efficiency (age 18) | .27** | .01 | .34*** | −.22* | .04 | .07 | .09 | −.05 | .34*** | .08 | .58*** | −.36*** | .18* | .05 | .06 | .03 | .55*** | −.03 | ||||||
| 20. Variability in sleep efficiency (age 18) | −.22* | .08 | −.28** | .18* | −.01 | −.13 | −.16 | .08 | −.24* | −.03 | −.49*** | .31** | −.11 | −.03 | −.04 | .02 | −.43*** | .13 | −.81*** | |||||
| 21. Sleepiness (age 18) | −.11 | .25** | −.01 | .09 | .34*** | .32*** | .33*** | .02 | −.06 | .01 | .14 | −.02 | .51*** | .17* | .22** | −.08 | −.14 | .16* | .01 | .04 | ||||
| 22. Depression symptoms (age 18) | −.20* | .31*** | −.08 | .13 | .19** | .60*** | .48*** | .27*** | .00 | .10 | .10 | .00 | .38*** | .71*** | .57*** | .26*** | −.10 | .16* | .07 | .01 | .27*** | |||
| 23. Anxiety (age 18) | −.17* | .25** | −.07 | .12 | .22** | .53*** | .62*** | .22** | .01 | .03 | .13 | .02 | .38*** | .59*** | .72*** | .21** | −.15 | .16 | .03 | .10 | .35*** | .72*** | ||
| 24. Externalizing behaviors (age 18) | .04 | .08 | −.02 | .07 | .02 | .19** | .09 | .73*** | .09 | .18* | −.01 | .01 | .11 | .26*** | .25*** | .72*** | .03 | .06 | −.02 | .04 | −.05 | .30*** | .23** |
p < .05.
p < .01.
p < .001.
Reciprocal Relations between Adolescents’ Sleep and Socioemotional Adjustment
Average Sleep Minutes and Variability in Sleep Minutes
The model that assessed reciprocal relations between average sleep minutes and adjustment fit the data adequately, χ2(51) = 136.82, p < .001; χ2/df = 2.83; CFI = .94; RMSEA = .08, p = .001, 95% CI [.06 to .10]. Several of the control variables were related to sleep and adjustment. Male status was associated with fewer sleep minutes at age 16 (B = −7.31, β = −.26, p < .001), age 17 (B = −14.85, β = −.15, p = .05), and age 18 (B = −24.01, β = −.21, p = .003). Female status was related to more depression symptoms (B = −.51, β = −.18, p = .005) and anxiety at age 16 (B = −1.01, β = −.27, p < .001). Further, African American status was related to fewer sleep minutes at age 16 (B = −6.26, β = −.24, p = .003) and age 17 (B = −20.86, β = −.19, p = .007) as well as less anxiety at age 17 (B = −1.60, β = −.10, p = .02). Higher SES was associated with fewer externalizing behaviors at age 16 (B = −1.48, β = −.24, p < .001). Higher SES was also related to fewer depression symptoms at age 18 (B = −.70, β = −.15, p < .001) and less anxiety at age 17 (B = −.51, β = −.09, p = .05). In terms of autoregressive effects, relations between average sleep minutes at ages 16 and 17 (B = .34, β = .38, p < .001) and between ages 17 and 18 were significant (B = .69, β = .60, p < .001). Relations between depression symptoms at ages 16 and 17 (B = .65, β = .59, p < .001) and between ages 17 and 18 were significant (B = .58, β = .62, p < .001). Associations between anxiety at ages 16 and 17 (B = .67, β = .65, p < .001) and between ages 17 and 18 were significant (B = .67, β = .67, p < .001). Lastly, the association between externalizing behaviors at ages 16 and 17 was significant (B = .62, β = .76, p < .001) as was the relation between ages 17 and 18 (B = .70, β = .74, p < .001). The examination of bidirectional effects between average sleep minutes and adjustment did not yield any significant relations (models with such non-significant relations are not depicted for brevity).
The model that assessed reciprocal relations between average sleep minutes and adjustment was refit while including sleep data only from weeknights. Compared to findings based on the full week, only one difference emerged. More anxiety at age 17 became a predictor of fewer weeknight sleep minutes at age 18 (B = −1.97, β = −.24, p = .006). There were no other differences.
Next, a model was fit to examine reciprocal relations between variability in sleep minutes and adolescents’ adjustment. The model fit the data adequately, χ2(73) = 201.99, p < .001; χ2/df = 2.77; CFI = .92; RMSEA = .08, p < .001, 95% CI [.07 to .10] (see Figure 1). For covariates, higher SES was related to less variability in sleep minutes at age 16 (B = −.20, β = −.27, p = .003). Average sleep minutes was also controlled at each wave, and average sleep minutes at age 17 was negatively associated with variability in sleep minutes at age 18 (B = −.0005, β = −.31, p < .001); covariates are not shown in the figure. The autoregressive effects across all time points of variability in sleep minutes were significant (Figure 1).
Demonstrative of the role of sleep as a predictor of adjustment, greater variability in sleep minutes at age 16 predicted higher levels of adolescents’ depression symptoms and anxiety at age 17 (Figure 1). In addition, more variability in sleep minutes at age 17 was related to higher levels of depression symptoms, anxiety, and externalizing behaviors at age 18. The opposite direction of effects (adjustment as a predictor of sleep) was evident in relations between externalizing behaviors at age 16 and greater variability in sleep minutes at age 17.
Lastly, the model that examined reciprocal relations between variability in sleep minutes and adjustment was refit while including sleep data only from weeknights. Compared to findings based on the full week, those including only weeknight data indicated that variability in sleep minutes at age 17 no longer predicted symptoms of depression, anxiety, nor externalizing behaviors at age 18. One additional path emerged; more variability in weeknight sleep minutes at age 16 forecasted more externalizing symptoms at age 17 (B = 8.02, β = .13, p = .01).
Average Sleep Efficiency and Variability in Sleep Efficiency
The model that assessed reciprocal associations between adolescents’ average sleep efficiency and adjustment fit the data adequately, χ2(56) = 143.20, p < .001; χ2/df = 2.56; CFI = .94; RMSEA = .08, p < .001, 95% CI [.06 to .09] (model not depicted in figure). Male status was associated with reduced sleep efficiency at age 16 (B = −.69, β = −.21, p = .003). Lower SES was related to greater sleep efficiency at age 16 (B = 1.26, β = .15, p = .03). Regarding autoregressive effects, the association between sleep efficiency at ages 16 and 17 was significant (B = .40, β = .44, p < .001) as was the relation between ages 17 and 18 (B = .68, β = .60, p < .001). The examination of reciprocal relations between average sleep efficiency and adolescents’ adjustment did not yield any significant associations.
The model that assessed reciprocal relations between average sleep efficiency and adjustment was refit while including sleep data only from weeknights. No differences emerged.
The model assessing reciprocal relations between variability in sleep efficiency and adolescents’ adjustment fit the data adequately, χ2(73) = 166.92, p < .001; χ2/df = 2.29; CFI = .94; RMSEA = .07, p = .008, 95% CI [.06 to .09] (Figure 2). Of covariates, African American status (B = .002, β = .13, p = .004) and lower SES (B = −.005, β = −.11, p = .01) were related to more variability in sleep efficiency at age 16. Average sleep efficiency at each wave was controlled, and average sleep efficiency at age 16 was negatively associated with variability in sleep efficiency at age 17 (B = −.002, β = −.44, p < .001). Average sleep efficiency at 17 was negatively related to variability in sleep efficiency at age 18 (B = −.004, β = −.61, p < .001) (control variables not depicted in figure).
Figure 2.
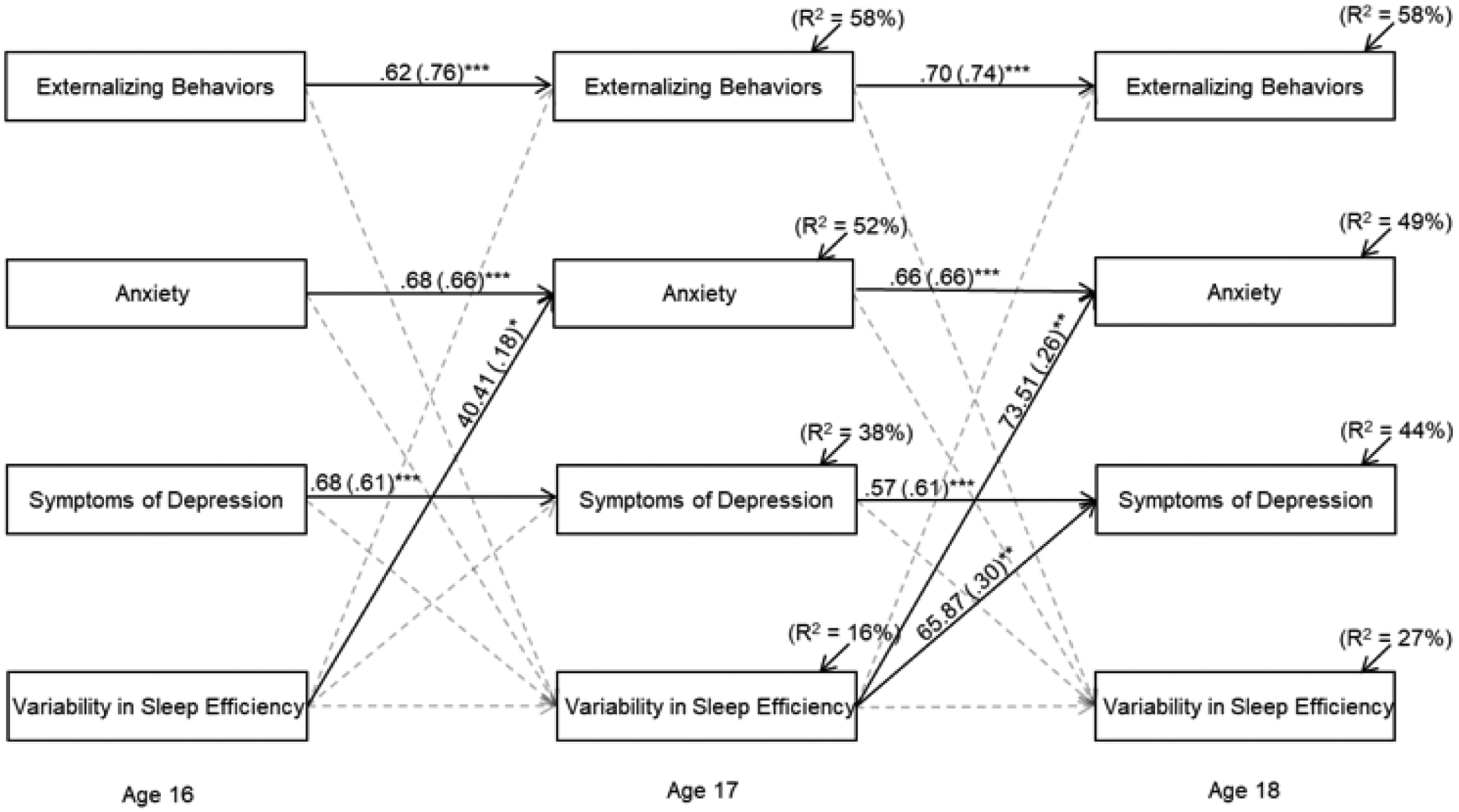
Reciprocal relations between adolescents’ intraindividual variability in sleep efficiency and their adjustment. Unstandardized and standardized coefficients (in parentheses) are provided. Adolescents’ gender race/ethnicity, socioeconomic status at age 16 and mean sleep efficiency at each wave are controlled in analyses. Residual variances among endogenous variables within each wave were allowed to correlate. Model fit: χ2(73) = 166.92, p < .001; χ2/df = 2.29; CFI = .94; RMSEA = .07, p = .008, 95% CI [.06 to .09]. Statistically significant lines are solid, whereas nonsignificant lines are dotted.
*p < .05. **p < .01. ***p < .001.
Illustrating the role of sleep as a predictor of adjustment, greater variability in sleep efficiency at age 16 predicted higher levels of anxiety at age 17 (Figure 2). In addition, more variability in sleep efficiency at age 17 predicted higher levels of depression symptoms and anxiety at age 18. None of the adjustment variables predicted this sleep parameter.
Lastly, the model that examined reciprocal relations between variability in sleep efficiency and adjustment was refit while only including sleep data from weeknights. Compared to findings based on the full week, two differences emerged. Variability in sleep efficiency at age 16 no longer predicted anxiety at age 17, however one additional path became significant; greater variability in weeknight sleep efficiency at age 16 predicted more externalizing behaviors at age 17 (B = 14.38, β = .14, p = .05).
Sleepiness
The model that examined reciprocal relations between sleepiness and adolescents’ adjustment fit the data adequately, χ2(53) = 127.64, p < .001; χ2/df = 2.66; CFI = .95; RMSEA = .07, p = .008, 95% CI [.06 to .09] (Figure 3). Of the control variables, African American status was associated with more sleepiness at age 17 (B = .95, β = .11, p = .05). The autoregressive effects across all time points of sleepiness were significant. More depression symptoms, anxiety, and externalizing behaviors at age 16 predicted higher levels of sleepiness at age 17. Greater sleepiness at age 17 predicted more depression symptoms and anxiety at age 18.
Figure 3.

Reciprocal relations between adolescents’ sleepiness and their adjustment. Unstandardized and standardized coefficients (in parentheses) are provided. Adolescents’ gender race/ethnicity and socioeconomic status at age 16 are controlled in analyses. Residual variances among endogenous variables within each wave were allowed to correlate. Model fit: χ2(53) = 127.64. p< .001; χ2/df = 2.66; CFI = .95; RMSEA = .07, p = .008, 95% CI [.06 to .09], Statistically significant lines are solid, whereas nonsignificant lines are dotted.
*p < .05. **p < .01. ***p < .001.
Discussion
We examined the reciprocal relations between sleep and adjustment in middle to late adolescence. We recruited a relatively large sample, used a three-wave design spanning two years, and assessed multiple sleep parameters. Despite stable autoregressive effects, several sleep-wake parameters, assessed by either actigraphy or self-reports, predicted greater adjustment problems over time. Some reciprocal relations were also detected, particularly for sleepiness. Collectively, findings provide novel insight into the reciprocal relations between adolescents’ sleep and adjustment.
Note that, throughout the Discussion, we focus on findings from primary analyses based on our hypotheses and weeknight and weekend actigraphy data. Secondary analyses that included only weeknights are mentioned later in the Discussion and are discussed only when specified. The results pertaining to the effects of the various sleep parameters on adjustment were mixed. The primary analyses indicated that variability in sleep minutes, but not average sleep minutes, predicted adjustment. Specifically, greater nightly fluctuation in sleep minutes at age 16 predicted more depression and anxiety symptoms at age 17. In addition, greater variability in sleep minutes at age 17 forecasted depression, anxiety and externalizing behaviors one year later. Average sleep minutes was controlled, which helped ascertain the unique effect of sleep variability (Bei et al., 2016). A similar pattern emerged for sleep efficiency: variability in sleep efficiency, but not average sleep efficiency, forecasted adjustment problems. Greater fluctuation in sleep efficiency at age 16 predicted higher levels of anxiety at age 17. Similarly, more variability in sleep efficiency at age 17 forecasted symptoms of depression and anxiety one year later (while controlling for average sleep efficiency). Lastly, more sleepiness at age 17 forecasted higher depression symptoms and anxiety at age 18. Overall, findings provide extensive evidence of longitudinal relations between variability in sleep and sleepiness and adjustment problems across consecutive years in adolescence.
There are plausible explanations why sleep-wake problems may impact adjustment. Sleep problems undermine emotion regulation (Baum et al., 2014), potentially by impairing cognitive control over negative emotions and impulses that contribute to adjustment problems (Walker & van der Helm, 2009; Yoo et al., 2007). In addition, sleep problems are associated with less optimal vagal regulation (El-Sheikh et al., 2013) and higher cortisol levels (Kuhlman et al., 2020), which are related to adjustment problems (Beauchaine, 2015; Figueiredo et al., 2020).
Some past investigations of longitudinal relations between mean sleep minutes and adolescents’ adjustment yielded significant effects. For example, fewer sleep minutes predicted more depression, anxiety, and externalizing behaviors three years later in early adolescence (Kelly & El-Sheikh, 2014). In the present study, mean sleep minutes did not predict mental health. It is not entirely clear why null effects were observed in later adolescence, but plausible explanations exist. Research has shown that mean sleep minutes commonly decreases over time in adolescence (Hysing et al., 2020; Park et al., 2019). However, in our study mean sleep minutes was stable and did not decrease across waves. It is possible that mean sleep minutes remained above levels that confer risk for adjustment problems. This idea is offered as tentative pending additional investigation.
Variability in sleep minutes was robustly associated with adjustment problems. The findings add to a small but growing literature illustrating that variability in sleep minutes is consequential for adjustment above and beyond average sleep minutes (Bei, Mander, et al., 2017; Fuligni et al., 2018). Results of the present study extend this evidence to associations between variability in sleep and adjustment over multiple consecutive years in adolescence. Adolescence is characterized by increased academic demands and social activities as well as reduced parental monitoring, which may contribute to nightly fluctuations in sleep (Becker et al., 2017). Indeed, we found that sleep minutes varied considerably and fluctuated an average of 20 to 24 minutes per night across the waves. Overall, variability in sleep minutes is particularly relevant to adolescence and its continued consideration holds promise for elucidating a key dynamic process in health risk trajectories.
Sleep efficiency is an established sleep parameter and has mostly been calculated by mean compositing multiple nights (Meltzer et al., 2012). Past findings pertaining to longitudinal relations between mean sleep efficiency and adjustment among community samples of adolescents have been mixed, with some illustrating that reduced average sleep efficiency predicted internalizing and externalizing symptoms (e.g., Kelly & El-Sheikh, 2014) and others indicating no associations (Doane et al., 2015). Our results did not provide support for relations between mean sleep efficiency and adjustment.
However, sleep efficiency fluctuates and we found that it varied on average 1.09% to 2.58% per night across the waves. When multiple nights are composited, important variability may be lost, such that those who often sleep poorly may have an average sleep efficiency that reflects adequate sleep quality due to improved sleep on other nights (Bei et al., 2016). Although research is limited, greater variability in sleep quality has served a particularly salient role in the prediction of daytime functioning, including poorer performance on neurocognitive tasks in childhood (Gruber & Sadeh, 2004) and life stress in adulthood (Mezick et al., 2009). Our results indicate that more fluctuation in sleep efficiency forecasts internalizing symptoms over multiple consecutive years in adolescence.
Examinations of variables that mediate relations between sleep variability and adolescents’ adjustment are scarce. However, certain variables warrant attention. It has been suggested that fluctuating sleep/wake patterns may require physiological systems to adapt to changing demands, which if occurs often, could result in wear and tear on the system and this has implications for adjustment (Bei, Seeman, et al., 2017). In addition, variable daily sleep patterns may be associated with circadian misalignment, and this may contribute to negative mood (Bei, Manber, et al., 2017). Inconsistent sleep schedules may also negatively impact diurnal patterns, which has ramifications for health (Bei, Seeman, et al., 2017). Further, in one of the few studies to investigate mechanisms of effects, subjective sleep quality mediated cross-sectional relations between greater variability in actigraphy-derived time in bed and adolescents’ negative mood (Bei, Manber, et al., 2017). Finally, sleep variability may be a byproduct of other variables that may influence adjustment (e.g., life chaos).
As part of secondary analyses, models were refit to explore relations between sleep variability and adjustment using only weekday nights. Several effects remained significant, indicating that more sleep variability during the week is also generally associated with poorer adjustment over time. However, some differences emerged, particularly for variability in sleep minutes. When weekend nights were excluded, variability in sleep minutes at age 17 no longer predicted symptoms of depression, anxiety, and externalizing behaviors at age 18. There are several plausible explanations for differences in results across the two sets of analyses. Sleep duration commonly increases over the weekend (Sun et al., 2019), and in our study, weekend sleep duration was on average 23 to 45 minutes longer compared to weeknights across the waves. Sleep duration thus fluctuated considerably between weekdays and weekends, and this led to increased variability in sleep minutes across the full week in comparison to variability over the weeknights – note that data were collected during the school year reducing variability in sleep during weekdays. When weekend nights are excluded, important variability that may impact development may be lost. Overall, findings highlight the importance of measuring sleep variability over weeknights and weekends.
Our findings provided only limited support for adjustment as a predictor of actigraphy-derived sleep. Externalizing behaviors at age 16 predicted greater variability in sleep minutes at age 17. More support was found for sleepiness. Specifically, depression, anxiety, and externalizing behaviors at age 16 predicted sleepiness at age 17. Internalizing symptoms include worrying and thought rumination (Garnefski et al., 2005), which may increase arousal and jeopardize sleep (McLaughlin & Nolen, 2011; McMakin & Alfano, 2015). Similarly, externalizing behaviors often involve emotional arousal and dysregulated physiological systems (Ruttle et al., 2011) that may disrupt sleep (Steiger, 2002).
Adjustment problems predicted sleepiness to a greater degree than actigraphy-derived sleep. Similar findings in the clinical literature have been reported. For example, youth with anxiety disorders often report daytime sleepiness, yet evidence of short actigraphy-derived duration and poor sleep quality are less evident (McMakin & Alfano, 2015). There are several plausible reasons for these differences. Adjustment problems may require adolescents to exert more energy each day to navigate ordinary daily challenges. Consequently, adolescents with adjustment problems may need more sleep than those with better psychological health and therefore experience more daytime sleepiness (Moore et al., 2009). It is also possible that adjustment problems compromise particular sleep stages (e.g., slow wave sleep, REM sleep) that lead to feelings of sleepiness. Overall, the findings highlight the value of multi-method sleep assessments.
There are ongoing questions as to whether sleep more robustly predicts adolescents’ adjustment or vice versa. Of studies that used community samples, findings have been mixed. Some studies have reported similar levels of support for both directions (Alvaro et al., 2017; Tochigi et al., 2016), whereas others have found that sleep more strongly predicts adjustment (Kelly & El-Sheikh, 2014; Narmandakh et al., 2020; Pieters et al., 2015; Roberts & Duong, 2014) and still others have found that adjustment predicts sleep to a greater degree (Hayley et al., 2015). In our study, actigraphy-derived variability in sleep across the week was a more consistent predictor of adjustment (8 of 12 paths [67%] were significant) than vice versa (1 of 12 paths [8%]). For sleepiness, support for each direction was more similar: 2 of 6 paths (33%) indicated that sleepiness predicted adjustment, and 3 of 6 paths (50%) supported the opposite direction. Across studies there has been inconsistency in sleep assessment methods and parameters, lag times between waves, period of adolescence, and sample demographics (e.g., family income, race/ethnicity). These differences make comparisons difficult and preclude conclusions about which direction is more robust. Continued work is needed to understand the variation in findings.
Among primary analyses, we found considerably more support for sleepiness and sleep variability as predictors of depression symptoms (4 of 6 paths were significant [67%]) and anxiety (5 of 6 paths [83%]) than externalizing behaviors (1 of 6 paths [17%]). Comparisons between internalizing and externalizing outcomes in adolescence have been uncommon, and among the existing studies, findings have been mixed. Some have reported that associations between sleep and internalizing symptoms compared to externalizing behaviors did not differ (Pieters et al., 2015; Shimizu et al., 2020), yet others reported differences similar to our findings (Kelly & El-Sheikh, 2014). In our study, parents reported on externalizing behaviors, which was helpful in reducing possible same-reporter bias. However, parents may not be aware of some of their adolescents’ externalizing behaviors, and this could have impacted the findings. Lastly, adolescents reported on their own sleepiness and internalizing symptoms and same-reporter bias may have influenced the results.
Results of the present study have clinical implications. Sleep-wake problems compromise development; thus, adolescent public health campaigns/initiatives should consider including sleep among other recommended healthy behaviors. Indeed, as part of a randomized controlled trial, adolescents who devoted more time to sleep and received information about sleep hygiene tips slept longer and had fewer symptoms of depression over time (Dewald-Kaufmann et al., 2014). Our results suggest that developing methods to reduce sleep variability may be particularly valuable. It is encouraging that sleep intervention studies have reported some success in reducing fluctuations in adolescents’ sleep (Sousa et al., 2013). Middle to late adolescence may be particularly fitting for such interventions as adolescents increasingly initiate autonomous sleep routines.
The present study includes limitations with corresponding directions for future research. We focused on important parameters of sleep quantity, quality and sleepiness, yet other parameters (e.g., sleep stages) may also be relevant and additional work is warranted. Our study focused on middle to late adolescence; whether findings would be similar in other ages is unknown. A promising future direction includes investigations of later developmental periods as individuals finish high school, move out of the family home, and enter early adulthood. Given the nature of our community sample, results do not necessarily generalize to clinical populations. We recruited families from semi-rural areas and relatively small towns in the southeastern United States, which may limit generalizability to other locales. Reflective of the community, participants were White/European American and Black/African American; other races and ethnicities were not represented, further limiting generalizability. Future studies that examine reciprocal dynamics between sleep and adjustment may want to consider including random intercept cross-lagged models, which may help speak further to within-person effects compared to our results (Berry & Willoughby, 2017). Only two weekend nights were monitored with actigraphy at each wave; additional nights would help further characterize sleep variability over weekends. Lastly, our longitudinal design included a one-year lag between each wave and different patterns may emerge across shorter or longer periods of time. Despite its limitations, the present study employed a rigorous design and provided novel evidence of reciprocal relations between sleep and adjustment.
Acknowledgments:
This research was supported by Grant R01-HD046795 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (PI: Mona El-Sheikh). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank our research laboratory staff, particularly lab coordinator Bridget Wingo, as well as the adolescents and families who participated.
References
- Acock AC (2005). Working with missing values. Journal of Marriage and Family, 67(4), 1012–1028. 10.1111/j.1741-3737.2005.00191.x [DOI] [Google Scholar]
- Alvaro PK, Roberts RM, Harris JK, & Bruni O (2017). The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. Journal of Affective Disorders, 207, 167–174. 10.1016/j.jad.2016.08.032 [DOI] [PubMed] [Google Scholar]
- Angold A, Weissman MM, John K, Merikangas KR, Prusoff BA, Wickramaratne P, Warner V (1987). Parent and child reports of depressive symptoms in children at low and high risk of depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 28(6), 901–915. 10.1111/j.1469-7610.1987.tb00678.x [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, & Beebe DW (2014). Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(2), 180–190. 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 44(5), 875–896. 10.1080/15374416.2015.1038827 [DOI] [PubMed] [Google Scholar]
- Becker SP, Sidol CA, Van Dyk TR, Epstein JN, & Beebe DW (2017). Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Medicine Reviews, 34, 94–121. 10.1016/j.smrv.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, & Manber R (2016). Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124. 10.1016/j.smrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Bei B, Manber R, Allen NB, Trinder J, & Wiley JF (2016). Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep, 40(2). 10.1093/sleep/zsw067 [DOI] [PubMed] [Google Scholar]
- Bei B, Seeman TE, Carroll JE, & Wiley JF (2017). Sleep and physiological dysregulation: A closer look at sleep intraindividual variability. Sleep, 40 (9), 1–10. 10.1093/sleep/zsx109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D, & Willoughby MT (2017). On the practical interpretability of cross-lagged panel models: Rethinking a developmental workhorse. Child Development, 88, 1186–1206. 10.1111/cdev.12660 [DOI] [PubMed] [Google Scholar]
- Brown WJ, Wilkerson AK, Boyd SJ, Dewey D, Mesa F, & Bunnell BE (2018). A review of sleep disturbance in children and adolescents with anxiety. Journal of Sleep Research, 27(3), e12635. 10.1111/jsr.12635 [DOI] [PubMed] [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit. In Bollen KA & Long JS (Eds.), Testing structural equation models (pp. 136–262). Newbury Park, CA: Sage. [Google Scholar]
- Bustamante CM, Rodman AM, Dennison MJ, Flournoy JC, Mair P, & McLaughlin KA (2020). Within-person fluctuations in stressful life events, sleep, and anxiety and depression symptoms during adolescence: A multi-wave prospective study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 61(10), 1116–1125. 10.1111/jcpp.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe JA, Reid GJ, Boyle MH, & Racine Y (2011). Sleep problems, tiredness, and psychological symptoms among healthy adolescents. Journal of Pediatric Psychology, 36(1), 25–35. 10.1093/jpepsy/jsq028 [DOI] [PubMed] [Google Scholar]
- Dahl RE (1996). The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology, 3(1), 44–50. 10.1016/S1071-9091(96)80028-3 [DOI] [PubMed] [Google Scholar]
- Dahl RE, & Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(1), 1–6. 10.1017/S0954579409000017 [DOI] [PubMed] [Google Scholar]
- Dewald-Kaufmann JF, Oort FJ, & Meijer AM (2014). The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: A randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(3), 273–283. 10.1111/jcpp.12157 [DOI] [PubMed] [Google Scholar]
- Doane LD, Gress-smith JL, & Breitenstein RS (2015). Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. Journal of Youth and Adolescence, 44(2), 389–404. 10.1007/s10964-014-0150-7 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, & Buckhalt JA (2015). II. Moving sleep and child development research forward: Priorities and recommendations from the SRCD-sponsored forum on sleep and child development. Monographs of the Society for Research in Child Development, 80(1, Serial No. 316), 15–32. 10.1111/mono.12142 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, & Bagley EJ (2013). Parasympathetic nervous system activity and children’s sleep. Journal of Sleep Research, 22(3), 282–288. 10.1111/jsr.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Saini EK, Gillis BT, & Kelly RJ (2019). Interactions between sleep duration and quality as predictors of adolescents’ adjustment. Sleep Health, 5(2), 180–186. 10.1016/j.sleh.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Shimizu M, Philbrook LE, Erath SA, & Buckhalt JA (2020). Sleep and development in adolescence in the context of socioeconomic disadvantage. Journal of Adolescence, 83, 1–11. 10.1016/j.adolescence.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457. 10.1207/s15328007sem0803_5 [DOI] [Google Scholar]
- Feinberg I (2013). Recommended sleep durations for children and adolescents: The dearth of empirical evidence. Sleep, 36(4), 461–462. 10.5665/sleep.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo P, Ramião E, Azeredo A, Moreira D, Barroso R, & Barbosa F (2020). Relation between basal cortisol and reactivity cortisol with externalizing problems: A systematic review. Physiology & Behavior, 225, 113088. 10.1016/j.physbeh.2020.113088 [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Arruda EH, Krull JL, & Gonzales NA (2018). Adolescent sleep duration, variability, and peak levels of achievement and mental health. Child Development, 89, e18–e28. 10.1111/cdev.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V, & van Etten M (2005). Specificity of relations between adolescents’ cognitive emotion regulation strategies and internalizing and externalizing psychopathology. Journal of Adolescence, 28(5), 619–631. 10.1016/j.adolescence.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Gradisar M, Gardner G, & Dohnt H (2011). Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Medicine, 12(2), 110–118. 10.1016/j.sleep.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Gruber R, & Sadeh A (2004). Sleep and neurobehavioral functioning in boys with attention-deficit/hyperactivity disorder. Sleep, 27, 267–273. 10.1093/sleep/27.2.267 [DOI] [PubMed] [Google Scholar]
- Hayley AC, Skogen JC, Sivertsen B, Wold B, Berk M, Pasco JA, & Overland S (2015). Symptoms of depression and difficulty initiating sleep from early adolescence to early adulthood: A longitudinal study. Sleep, 38(10), 1599–1606. 10.5665/sleep.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysing M, Harvey AG, Bøe T, Heradstveit O, Vedaa Ø, & Sivertsen B (2020). Trajectories of sleep problems from adolescence to adulthood. Linking two population-based studies from Norway. Sleep Medicine, 75, 411–417. 10.1016/j.sleep.2020.08.035 [DOI] [PubMed] [Google Scholar]
- Kelly RJ, & El-Sheikh M (2014). Reciprocal relations between children’s sleep and their adjustment over time. Developmental Psychology, 50(4), 1137–1147. 10.1037/a0034501 [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Kuhlman KR, Chiang JJ, Bower JE, Irwin MR, Seeman TE, McCreath HE, Fuligni AJ (2020). Sleep problems in adolescence are prospectively linked to later depressive symptoms via the cortisol awakening response. Developmental Psychopathology, 32(3), 997–1006. 10.1017/s0954579419000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Green SM, & Lahey BB (1990). Mental health professionals’ perception of the utility of children, mothers, and teachers as informants on childhood psychopathology. Journal of Clinical Child Psychology, 19(2), 136–143. 10.1207/s15374424jccp1902_5 [DOI] [Google Scholar]
- Lovato N, & Gradisar M (2014). A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Medicine Reviews, 18(6), 521–529. 10.1016/j.smrv.2014.03.006 [DOI] [PubMed] [Google Scholar]
- McMakin DL, & Alfano CA (2015). Sleep and anxiety in late childhood and early adolescence. Current Opinion in Psychiatry, 28(6), 483–489. https://doi.org/10.1097%2FYCO.0000000000000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, & Walsh CM (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews, 16(5), 463–475. 10.1016/j.smrv.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, & Reis SE (2009). Intra-individual variability in sleep duration and fragmentation: Associations with stress. Psychoneuroendocrinology, 34, 1346–1354. 10.1016/j.psyneuen.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Ancoli-Israel S, & Redline S (2009). Relationships among sleepiness, sleep time, and psychological functioning in adolescents. Journal of Pediatric Psychology, 34(10), 1175–1183. 10.1093/jpepsy/jsp039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narmandakh A, Roest AM, Jonge P. d., & Oldehinkel AJ (2020). The bidirectional association between sleep problems and anxiety symptoms in adolescents: A TRAILS report. Sleep Medicine, 67, 39–46. 10.1016/j.sleep.2019.10.018 [DOI] [PubMed] [Google Scholar]
- Park H, Chiang JJ, Irwin MR, Bower JE, McCreath H, & Fuligni AJ (2019). Developmental trends in sleep during adolescents’ transition to young adulthood. Sleep Medicine, 60, 202–210. 10.1016/j.sleep.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters S, Burk WJ, Van der Vorst H, Dahl RE, Wiers RW, & Engels RC (2015). Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. Journal of Youth and Adolescence, 44(2), 379–388. 10.1007/s10964-014-0213-9 [DOI] [PubMed] [Google Scholar]
- Quach JL, Nguyen CD, Williams KE, & Sciberras E (2018). Bidirectional associations between child sleep problems and internalizing and externalizing difficulties from preschool to early adolescence. JAMA Pediatrics, 172(2), e174363–e174363. 10.1001/jamapediatrics.2017.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raniti MB, Allen NB, Schwartz O, Waloszek JM, Byrne ML, Woods MJ, Trinder J (2017). Sleep duration and sleep quality: Associations with depressive symptoms across adolescence. Behavioral Sleep Medicine, 15(3), 198–215. 10.1080/15402002.2015.1120198 [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Richmond BO (2008). Revised Children’s Manifest Anxiety Scale (RCMAS-2) (2nd ed.). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Roberts RE, & Duong HT (2014). The prospective association between sleep deprivation and depression among adolescents. Sleep, 37(2), 239–244. 10.5665/sleep.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard R, Hermens DF, Naismith SL, White D, Rogers NL, Ip TK, Mullin SJ, Alvares GA, Guastella AJ, Smith KL, Rong Y, Whitwell B, Southan J, Glozier N, Scott EM, & Hickie IB (2015). Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. Journal of Psychiatry & Neuroscience: JPN, 40(1), 28–37. 10.1503/jpn.130247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, & Schwartzman AE (2011). Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior, 59(1), 123–132. 10.1016/j.yhbeh.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep, 17(3), 201–207. 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- Selig JP, & Little TD (2012). Autoregressive and cross-lagged panel analysis for longitudinal data. In Laursen B, Little TD, & Card NA (Eds.), Handbook of developmental research methods (pp. 265–278). NY: Guilford Press. [Google Scholar]
- Shimizu M, Gillis BT, Buckhalt JA, & El-Sheikh M (2020). Linear and nonlinear associations between sleep and adjustment in adolescence. Behavioral Sleep Medicine, 18(5), 690–704. 10.1080/15402002.2019.1665049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, & Cochran WG (1967). Statistical methods (6th ed.). Ames, Iowa: Iowa State University Press. [Google Scholar]
- Sousa IC, Souza JC, Louzada FM, & Azevedo CVM (2013). Changes in sleep habits and knowledge after an educational sleep program in 12th grade students. Sleep and Biological Rhythms, 11(3), 144–153. 10.1111/sbr.12016 [DOI] [Google Scholar]
- Steiger A (2002). Sleep and the hypothalamo-pituitary-adrencortical system. Sleep Medicine Reviews, 6, 125–138. 10.1053/smrv.2001.0159 [DOI] [PubMed] [Google Scholar]
- Sun W, Ling J, Zhu X, Lee TMC, & Li SX (2019). Associations of weekday-to-weekend sleep differences with academic performance and health-related outcomes in school-age children and youths. Sleep Medicine Reviews, 46, 27–53. 10.1016/j.smrv.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2013). Using Multivariate Statistics: Pearson New International Edition (6th ed.). Upper Saddle River, NJ: Pearson. [Google Scholar]
- Tochigi M, Usami S, Matamura M, Kitagawa Y, Fukushima M, Yonehara H, Sasaki T (2016). Annual longitudinal survey at up to five time points reveals reciprocal effects of bedtime delay and depression/anxiety in adolescents. Sleep Medicine, 17, 81–86. 10.1016/j.sleep.2015.08.024 [DOI] [PubMed] [Google Scholar]
- Walker MP, & van der Helm E (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin, 135(5), 731–748. 10.1037/a0016570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AA, Zendarski N, Lange K, Quach J, Molloy C, Clifford SA, & Mulraney M (2021). Sleep problems, internalizing and externalizing symptoms, and domains of health-related quality of life: Bidirectional associations from early childhood to early adolescence. Sleep, 44(1), 1–11. 10.1093/sleep/zsaa139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, & Carskadon MA (1998). Sleep schedules and daytime functioning in adolescents. Child Development, 69(4), 875–887. 10.1111/j.1467-8624.1998.tb06149.x [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, & Martin JL (2003). Evidence for the validity of a sleep habits survey of adolescents. Sleep, 26(2), 213–216. 10.1093/sleep/26.2.213 [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, & Walker MP (2007). The human emotional brain without sleep—a prefrontal amygdala disconnect. Current Biology, 17(20), R877–R878. 10.1016/j.cub.2007.08 [DOI] [PubMed] [Google Scholar]


