Abstract
We evaluated the pharmacokinetics of amoxicillin-sulbactam (AMX-SUL), a novel drug combination, and its pharmacodynamics against Escherichia coli in 12 volunteers receiving a single oral dose (1,000 mg). Peak serum bactericidal and urine inhibitory activities in most volunteers were observed against E. coli strains for which AMX-SUL MICs were low (2- to 4-mg/liter) (2 strains) and high (≥16-mg/liter) (47 strains), respectively.
The pharmacodynamics, pharmacokinetics, safety, and efficacies of ampicillin-sulbactam and amoxicillin-clavulanic acid have been widely evaluated (3, 5, 6, 11). The resistance of amoxicillin (AMX) to acid hydrolysis increases its bioavailability, compared with ampicillin, when given orally. In addition, sulbactam (SUL) has activity against certain pathogens, such as Acinetobacter spp. (10). Here we assess the pharmacokinetics and pharmacodynamics of a novel combination, AMX-SUL, against Escherichia coli.
Twelve healthy volunteers (six males) (mean age, 34 years; age range, 23 to 45 years; mean weight, 69.9 kg; and weight range, 56 to 90 kg) were included in this study. They had not received any antimicrobial agents in the previous week. After the subjects had fasted for 10 h, blood and urine samples (control, 0 h) were drawn and a tablet containing 500 mg each of AMX and SUL pivaloil-oxymethyl ester (Trifamox IBL; Laboratories Bagó, Buenos Aires, Argentina) was administered to each volunteer. Blood samples were obtained at 1.5, 2, 4, 8, and 12 h after dosing. Urine samples were collected at 0 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 10, and 10 to 12 h after the dosing.
Levels of AMX and SUL in serum and urine were determined by using high-performance liquid chromatography, as described previously (4). A pool of serum including the 0-h samples from all volunteers was used to prepare standards of known drug concentration. The lower limit of detection and intra- and interday variations of drug concentration were 0.4 mg/liter and 3.5 and 4.0% for AMX and 0.8 mg/liter and 4.3 and 4.8% for SUL, respectively.
MICs of AMX and AMX-SUL (ratio, 2:1) (Laboratorios Bagó) were determined with Mueller-Hinton agar (Difco, Detroit, Mich.) following the National Committee for Clinical Laboratory Standards guidelines (8).
Serum bactericidal titers (SBT) against E. coli ATCC 25922 (AMX MIC, 2 mg/liter; AMX-SUL MIC, 2 mg/liter), E. coli ATCC 35218 (AMX MIC, 1,024 mg/liter; AMX-SUL MIC, 8 mg/liter), and an E. coli isolate (Ecc) recovered from urine (AMX MIC, 512 mg/liter; AMX-SUL MIC, 4 mg/liter) were determined by the macrodilution method, following the National Committee for Clinical Laboratory Standards recommendations (9). Briefly, twofold dilutions (1:2 to 1:128) of 0-, 1.5-, 8-, and 12-h serum samples were inoculated with equal volumes of the organism suspension containing roughly 106 CFU/ml in log phase. After a 24-h incubation at 35°C, all samples were subcultured onto blood agar medium for viable cell counts. SBT was determined by the lowest dilution of serum which effected a 99.9% killing of the initial inoculum. Additionally, viable cell counts were performed from peak samples after 8- and 24-h incubations to establish a 24-h time-kill curve as previously described (2).
Filter-sterilized urine samples corresponding to 0 h (growth control) and the 0- to 2-h interval after dosing were diluted 1 to 3 with warmed Mueller-Hinton agar, poured, and left to dry. Plates were inoculated (final inoculum, 104 CFU per spot) with the 3 strains used in the serum assay and 47 additional E. coli strains which had been recovered from the urine of outpatients with urinary tract infections and for which AMX MICs were very high (MIC at which 50% of isolates are inhibited [MIC50], MIC90, and range, >1,024, >1,024, and 1,024 to >1,024 mg/liter, respectively) and AMX-SUL MICs were elevated (MIC50, MIC90, and range, 32, 128, and 16 to 256 mg/liter, respectively). The inhibitory activity of urine was defined as the absence of any growth on the agar after a 24-h incubation at 35°C.
Characterization of β-lactamases was performed by isoelectric focusing on polyacrylamide gels (Pharmacia Biotech, Inc., Piscataway, N.J.), and the β-lactamase activity was subsequently located on the gel by using the iodimetric method, with penicillin and cephaloridine as substrates (7). The pI values were estimated by comparison with those of known β-lactamases. The identity at the family level was established by PCR with specific primers to TEM, SHV, and ampC sequences as described elsewhere (1).
Forty-seven strains, including E. coli ATCC 35218 and E. coli Ecc, harbored a TEM-1-like β-lactamase (pI = 5.4). One isolate had a probable OXA β-lactamase (pI = 7.65), and another isolate showed a TEM-derived enzyme (pI = 5.5) different from TEM-1 and TEM-2. No strains producing more than one detectable β-lactamase were found.
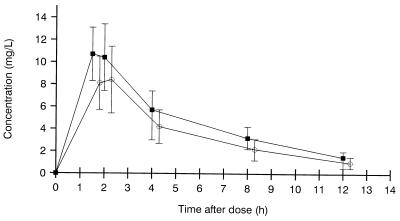
Figure 1 shows the levels of AMX and SUL in serum at different times after dosing. Maximum concentration was reached at 1.5 h (in seven patients) and at 2 h (in five patients) after dosing. The peak concentrations (means ± standard deviations [SD]) (milligrams per liter) of AMX and SUL in serum were 11.3 ± 2.6 and 9.1 ± 2.7, ranging from 7.1 to 16.2 and from 5 to 12.6, respectively. The respective areas under the concentration-time curve were 56.3 and 41.9 mg · h/liter.
FIG. 1.
Levels of AMX (■) and SUL (○) in serum. Values are means ± SDs from 12 volunteers receiving 1,000-mg single oral doses of AMX-SUL. The SUL curve was moved 0.3 h relative to AMX on the graph to avoid overlapping between SD bars.
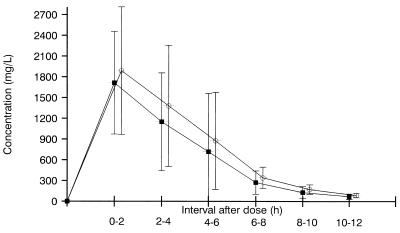
Levels of the drugs in urine are shown in Fig. 2. Maximum concentrations were observed within the first 2 h after dosing in 10 volunteers. Means ± SD for peak values (milligrams per liter) were 1,716 ± 744 (range, 704 to 3,194) and 1,890 ± 922 (range, 557 to 3,150) for AMX and SUL, respectively. Twelve hours after dosing, respective values (milligrams per liter) were 70.9 ± 37.6 (range, 25 to 182) and 87.9 ± 31.8 (range, 20 to 144).
FIG. 2.
Levels of AMX (■) and SUL (○) in urine. Values are means ± SDs from 12 volunteers receiving 1,000-mg single oral doses of AMX-SUL. The SUL curve was moved 0.3 h relative to AMX on the graph to avoid overlapping among SD bars.
The SBT corresponding to the 1.5-h sample against E. coli ATCC 25922 were 1:2 and 1:4 for 8 and 4 volunteers, respectively. SBT against E. coli Ecc were 1:2 for 11 volunteers and <1:2 for the 12th. Titers against E. coli ATCC 35218 were 1:2 and <1:2 for 4 and 8 volunteers, respectively. Serum samples obtained from all volunteers to 0, 8, and 12 h after dosing displayed a titer of <1:2 against all of the three strains.
Time-kill studies with peak-concentration serum samples showed bactericidal activity against E. coli ATCC 25922 and E. coli Ecc, as they revealed mean decreases of roughly 4 log CFU/ml in the number of viable cells from the initial inoculum, after both 8- and 24-h incubations. By contrast, viable cell counts (mean log CFU per milliliter ± SD) for E. coli ATCC 35218 were 7.4 ± 2.8 and 8.9 ± 4.6 after 8- and 24-h incubations, respectively (P < 0.01, compared with the above two strains by Student’s t test). Growth of the three E. coli strains was not affected by any 0-, 8-, or 12-h serum sample.
Urine collected from 10 of 12 volunteers at the 0- to 2-h interval after dosing was able to inhibit all of the 47 isolates for which AMX-SUL MICs were high (i.e., ≥16 mg/liter), as well as the 3 strains for which MICs were low (2 to 8 mg/liter). Urine samples from the remaining two volunteers (who had shown delayed AMX-SUL excretions) were unable to inhibit the two isolates for which the AMX-SUL MICs were highest (i.e., 256 mg/liter).
Like other aminopenicillin–β-lactamase inhibitor combinations, AMX-SUL showed good bioavailability when administered orally. Similar results had previously been reported with a 250-mg dose, reaching peak concentrations of AMX and SUL in serum of 6.2 and 4.5 mg/liter, respectively (4).
Most of the E. coli isolates selected to test the inhibitory activity of urine harbored TEM-1-like β-lactamase, and the AMX-SUL MICs for these isolates were high. Such resistance has been found in 25% of the E. coli strains isolated at our institution during 1997 from young women with cystitis (unpublished data). However, peak concentrations in urine samples from most of the volunteers inhibited these strains. Thus, this study might give the basis of an evaluation of a “urinary breakpoint” for aminopenicillin–β-lactamase inhibitor combinations, since this type of drug may be one of the few options for the treatment of urinary tract infection in pregnant women.
In summary, AMX-SUL displayed properties that make it suitable for clinical trials undertaken to assess its efficacy against some infections, especially those affecting the lower urinary tract.
Informed consent was obtained from the volunteers before enrollment. The study was conducted following the guidelines of the Declaration of Helsinki and received the approval of the Ethics Committee of the Hospital Privado Antártida, Buenos Aires, Argentina.
REFERENCES
- 1.Arlet G, Philippon A. Construction by polymerase chain reaction and intragenic DNA probes for three main types of transferable beta-lactamases (TEM, SHV and CARB) FEMS Microbiol Lett. 1991;82:19–26. doi: 10.1016/0378-1097(91)90414-6. [DOI] [PubMed] [Google Scholar]
- 2.Bantar C, Micucci M, Fernandez Canigia L, Smayevsky J, Bianchini H. Synergy characterization for Enterococcus faecalis strains displaying moderately high-level gentamicin and streptomycin resistance. J Clin Microbiol. 1993;31:1921–1923. doi: 10.1128/jcm.31.7.1921-1923.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campoli-Richards D M, Brogden R N. Sulbactam/ampicillin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1987;33:577–609. doi: 10.2165/00003495-198733060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dall L L, Andreeta H A, Soutric J L, Arenoso H J. Determinación en sangre de amoxicilina y sulbactama luego de una dosis oral única. Cálculo de parámetros farmacocinéticos. Prensa Med Argent. 1990;77:11–14. [Google Scholar]
- 5.Foulds G. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev Infect Dis. 1986;8(Suppl. 5):S503–S511. doi: 10.1093/clinids/8.supplement_5.503. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs M R, Aronoff S C, Johenning S, Shlaes D M, Yamabe S. Comparative activities of the β-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with ampicillin and broad-spectrum penicillins against defined β-lactamase-producing aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1986;29:980–985. doi: 10.1128/aac.29.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labia R, Barthélémy M. L’enzymogramme des beta-lactamases: adaptation en gel de la methode iodometrique. Ann Inst Pasteur Microbiol. 1979;130B:295–304. [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methodology for the serum bactericidal test. Proposed guideline M21-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Retsema J A, English A R, Girard A, et al. Sulbactam/ampicillin: in vitro spectrum potency, and activity in models of acute infections. Rev Infect Dis. 1986;8(Suppl. 5):S528–S534. doi: 10.1093/clinids/8.supplement_5.s528. [DOI] [PubMed] [Google Scholar]
- 11.Slocombe B, Beale A S, Boom R J, Griffin K E, Masters R J, Sutherland R, et al. Antibacterial activity in vitro and in vivo of amoxycillin in the presence of clavulanic acid. In: McGraw-Hill, editor. Progress and perspectives on beta-lactamase inhibition: a review of Augmentin. Post-graduate medicine. New York, N.Y: Custom communications; 1984. pp. 29–49. [Google Scholar]