Abstract
A single 400-mg oral or intravenous (i.v.) dose of moxifloxacin was given to each of eight healthy male volunteers, and 6 weeks later the dose was administered by the other route. The concentrations of the drug in plasma, cantharidin-induced inflammatory fluid, and urine were measured over the subsequent 24 h. The mean maximum concentrations observed in plasma were 4.98 μg/ml after oral dosing and 5.09 μg/ml after i.v. dosing. The mean maximum concentrations attained in the inflammatory fluid were 2.62 and 3.23 μg/ml, respectively. The mean elimination half-lives from plasma were 8.32 and 8.17 h, respectively. The overall penetration into the inflammatory fluid was 103.4 and 104.2%. Over 24 h 15% of the drug was recovered in the urine when administered by either route.
Moxifloxacin (Bay 12-8039) is a methoxy fluoroquinolone which shows enhanced activity against gram-positive bacterial pathogens in particular Streptococcus pneumoniae strains, including those resistant to penicillin (2, 8). Preliminary information suggests that moxifloxacin has an elimination half-life (t1/2) appropriate to once-daily dosing (5, 6). In this crossover study, the pharmacokinetics and penetration of moxifloxacin into an inflammatory exudate (7) were examined following a single 400-mg dose given by the oral or intravenous (i.v.) route.
Eight healthy male volunteers between the ages of 26 and 41 years (mean age, 33.5 years; mean height, 175 cm; mean weight, 72 kg) were enrolled. They had no history of serious illness, atopy, alcohol or drug abuse, or an acute illness in the 14 days prior to the start of the study. They had not received any prescribed or over-the-counter medication in the 14 days prior to the first dose of moxifloxacin.
Approval for this study was granted by the Hospital Ethical Committee of City Hospital Trust and all volunteers gave written informed consent. All volunteers underwent a full history and examination, including echocardiogram (ECG), and were shown to have normal hematological and biochemical profiles and normal urinalysis.
Each volunteer received 400 mg of oral or i.v. moxifloxacin (administered over 1 h) in a random order and 6 weeks later (to allow blister healing) received the agent by the other route.
On the evening prior to the study 0.2% cantharidin-impregnated plasters (2 by 1 cm) were attached to the subjects’ forearms to induce blister formation. Plasma samples were obtained from the contralateral arm following insertion of an i.v. catheter kept patent by flushing with 0.9% saline (Antigen Pharmaceuticals, Rosecrea, Ireland).
The dose was given to the fasting subjects by either route with 200 ml of water. The subjects then fasted for a further 4 h except for water ad libitum. About 10 ml of venous blood was collected predose and then at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h postdose.
Between 50 and 100 μl of inflammatory fluid was aspirated with a fine needle prior to administration of the dose and then at 1, 2, 3, 4, 6, 8, 12, and 24 h. The blister was resealed with a plastic spray dressing (Opsite, Smith & Nephew, Hill, England). Urine was collected 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdose. At 30 h postdose the routine hematological and biochemical tests were repeated. Prior to the study a cross-validation procedure was undertaken by Bayer AG (Leverkusen, Germany). Spiked samples were assayed by the microbiological procedure described below and a high-performance liquid chromatography method (5). Linear regression analysis comparison of the two methods gave a correlation coefficient r of 0.988.
Drug analysis.
All samples were analyzed within 1 h of collection. Concentrations of moxifloxacin in plasma, inflammatory fluid, and urine were measured by a microbiological assay. Assay plates containing Iso-Sensitest agar (Oxoid, Basingstoke, England) were flooded with a suspension of Escherichia coli 4004 (Bayer AG) in order to give nonconfluent growth. The calibrator range was 0.04 to 1.5 μg/ml. Internal controls and quality assurance samples were prepared in human plasma (Bradsure Biologicals, Market Harborough, England) or in 70% human plasma (to simulate the protein content of the blister fluid) and phosphate buffer (pH 7) for the assay of moxifloxacin in plasma inflammatory exudate and urine, respectively.
The lower limit of detection was 0.02 μg/ml. The coefficient of variation within the assays was 8.0%. Quality control samples were included; the mean + 2 standard deviations for these samples was 14.8%.
Standard noncompartmental analysis was used to determine the pharmacokinetic parameters. The maximum concentration of moxifloxacin in plasma (Cmax), the time to Cmax (Tmax), the area under the plasma or skin blister fluid curve up to the last measurable concentration (AUClast), the area under the plasma or skin blister fluid curve extrapolated to infinity (AUC0–∞), and the t1/2 in plasma or skin blister fluid were calculated by the noncompartmental model 200 of PCNONLIN (version 4.2a; Scientific Consulting Inc., Apex, N.C.).
The mean concentration found in plasma and inflammatory fluid are shown in Fig. 1 and 2; the derived pharmacokinetic parameters are summarized in Table 1. The data was collected for seven of the eight volunteers because one volunteer had an adverse event that required withdrawal from the study (see below).
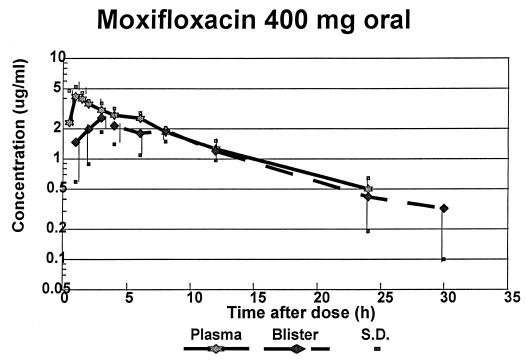
FIG. 1.
Drug concentrations in plasma and blister fluid following oral administration of 400 mg of moxifloxacin.
FIG. 2.
Drug concentrations in plasma and blister fluid following i.v. administration of 400 mg of moxifloxacin.
TABLE 1.
Pharmacokinetic parameters of moxifloxacin in seven healthy volunteers following a single 400-mg dose
| Administration route and value | Pharmacokinetics in plasma
|
Pharmacokinetics in blister fluid
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUClast (μg · h/ml) | AUC0–∞ (μg · h/ml) | Urine elimination (% of dose) | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUClast (μg · h/ml) | AUC0–∞ (μg · h/ml) | Penetration (%) | |
| Oral | ||||||||||||
| Mean | 4.98 | 1.0 | 8.32 | 39.0 | 45.49 | 15.10 | 2.62 | 3.86 | 10.0 | 32.55 | 40.32 | 83.5 |
| SD | 1.01 | 0.91 | 1.70 | 2.16 | 4.68 | 3.61 | 0.88 | 2.34 | 4.44 | 5.95 | 10.04 | 14.9 |
| Minimum | 3.88 | 0.5 | 7.05 | 36.42 | 39.41 | 10.2 | 1.41 | 2.0 | 5.46 | 24.3 | 28.91 | 60.5 |
| Maximum | 6.76 | 3.0 | 10.9 | 42.84 | 50.58 | 19.9 | 3.84 | 8.0 | 17.47 | 41.15 | 54.80 | 103.4 |
| Intravenous | ||||||||||||
| Mean | 5.09 | 8.17 | 39.13 | 45.34 | 15.2 | 3.23 | 2.43 | 9.54 | 36.33 | 42.74 | 93.7 | |
| SD | 1.11 | 1.58 | 5.80 | 8.0 | 3.40 | 0.43 | 1.13 | 1.33 | 3.9 | 5.31 | 8.3 | |
| Minimum | 3.88 | 6.65 | 28.89 | 32.17 | 8.1 | 2.72 | 1.0 | 7.70 | 30.11 | 36015 | 81.3 | |
| Maximum | 6.80 | 11.26 | 45.23 | 54.26 | 20.0 | 3.82 | 4.0 | 11.54 | 43.28 | 49.94 | 104.2 | |
The mean Cmaxs of moxifloxacin were remarkably similar, being 4.98 and 5.09 μg/ml following oral and i.v. administration, respectively. The time at which the Cmax occurred was 1 h after oral administration and at the end of the infusion following i.v. administration. The mean terminal t1/2s from plasma were also similar, being 8.32 and 8.17 h after oral and i.v. dosing, respectively. The AUClast and AUC0–∞ for both routes of administration were remarkably similar; hence, the extent of bioavailability of the oral formulation is approximately 100%.
Moxifloxacin penetrated into the inflammatory fluid rapidly, with the mean Tmax following oral administration being 3.86 h and that following i.v. administration being 2.4 h. Individual variation was greater after the former mode of administration. The mean Cmax following the oral dose was 2.62 μg/ml, and that following the i.v. dose was 3.23 μg/ml, but the ranges were similar.
The t1/2s for moxifloxacin from the inflammatory exudate were slightly greater than those from plasma, being a mean of 10.0 and 9.54 h following oral and i.v. administration, respectively. The percentage penetration of moxifloxacin into inflammatory fluid, calculated by comparing the AUC0–∞ for measurements taken in the inflammatory exudate with that for measurements taken in plasma, were 103.4% after oral administration and 104.2% after i.v. administration, and the ranges of these data were similar. The degree of penetration was greater than that which we reported for trovafloxacin (64% after oral administration) (7) and similar to that of ciprofloxacin (102.8%) (1). This may be related to the higher protein binding of trovafloxacin (87.9%) (7) in comparison to that of moxifloxacin (48%) (5).
The mean urinary elimination of the drug in the 48 h following the oral dose was 15.1%, remarkably similar to that after i.v. administration, 15.2%. The mean total clearance was 147.8 ml/min after oral dosing and 151.5 ml/min after i.v. dosing, with mean renal clearances of 22.3 and 23.0 ml/min, respectively. The mean urinary concentrations following the oral and i.v. doses, respectively, were 55.1 and 127 μg/ml at 8 h, 69.4 and 71.5 μg/ml at 12 h, and 38.1 and 38.2 μg/ml at 24 h. Statistical analysis (by paired t test) of the individual Cmax, t1/2, and AUC values for plasma revealed no significant differences (P > 0.05) between oral and i.v. administration. The Cmax in blister fluid was lower than that in plasma (P < 0.01). The t1/2 in blister fluid was significantly greater than that in plasma (P = 0.004).
One volunteer, scheduled to receive his first dose by the I.V. route experienced severe phlebitis at the site of i.v. administration following 30 ml of drug infusion. At the same time, a maculopapular rash developed over the volunteer’s trunk and arms. Vital signs showed a tachycardia but no bronchospasms. An ECG was immediately performed; the corrected cardiac interval was not prolonged; 100 mg of hydrocortisone was administered i.v., and the phlebitis and rash disappeared within 35 min. This volunteer who had a hypersensitivity reaction typical of those reported for this group of drugs (4) was withdrawn from the study and hence did not receive the second dose by the oral route. Five volunteers experienced headaches 4 to 12 h postdose, all resolving spontaneously (for four of five volunteers) or following administration of 1 g of paracetamol (for one of five volunteers). Physical examination revealed no abnormalities attributable to moxifloxacin administration. The biochemical, hematological, and ECG parameters studied revealed no abnormalities.
There is limited published information on the pharmacokinetics of moxifloxacin (5, 6). Our results are in good agreement with these earlier findings in terms of the AUC data. We noted the Cmax to be greater at doses of about 5 μg/ml (when given by either route), while the earlier reports found the value to be 2.5 to 3.0 μg/ml when given by mouth. The two earlier reports also suggested a longer t1/2 from plasma at 11 to 14 h (depending on whether two-compartment or noncompartmental analysis, respectively, was performed). Our data suggest a lower value of ca. 8 h.
Moxifloxacin appears to penetrate rapidly and completely into the inflammatory exudate. The MIC of moxifloxacin at which 90% of the common respiratory tract pathogens (such as S. pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae) are inhibited is ≤0.25 μg/ml. This concentration is exceeded in both plasma and inflammatory fluid for at least 24 h. In addition, an AUC-to-MIC ratio (AUIC) of >125 is deemed to predict efficacy; in selected respiratory tract infections (3), the AUIC of moxifloxacin for these pathogens is ca. 180. Both these facts suggest that moxifloxacin in a dose of 400 mg per day, given either orally or by i.v. administration, should be efficacious in the treatment of respiratory and other infections caused by susceptible pathogens. The high bioavailability and great similarity of pharmacokinetics following either route of administration suggest that a switch from i.v. to oral use should be straightforward.
Acknowledgments
We thank A. Dalhoff of Bayer AG for advice and financial support.
REFERENCES
- 1.Catchpole C, Andrews J M, Woodcock J, Wise R. The comparative pharmacokinetics and tissue penetration of ciprofloxacin 400 mg IV and 750 p.o. J Antimicrob Chemother. 1994;33:103–110. doi: 10.1093/jac/33.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Dalhoff A, Peterson H, Endermann M. In vitro activity of Bay 12-8039, a new methoxyquinlone. Chemotherapy (Basel) 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 3.Forest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahlmann R, Lode H. Safety overview: toxicity, adverse effects and drug interactions. In: Andriole V T, editor. The quinolones. San Diego, Calif: Academic Press; 1988. [Google Scholar]
- 5.Stass H H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a novel 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stass H H, Kubitza D, Schühly U. p. In Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C. 1997. Bay 12-8039, a new 8-methoxy-quinolone: pharmacokinetics (PK), safety (S) and tolerability (T) of single ascending intravenous doses in healthy male volunteers, abstr. F-153. [Google Scholar]
- 7.Wise R, Mortiboy D, Child J, Andrews J M. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:47–49. doi: 10.1128/aac.40.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]