Abstract
The black soldier fly larvae (BSFL) may serve as a promising tool in the animals feed production industry. The input organic wastes may be contaminated by insecticides that affect both the insect’s mass rearing, and the animals feed process. Therefore, in the current study the assessment of oxidative stress parameters of the black soldier fly (BSF) were investigated to quantify the deleterious effect of malathion-contaminated kitchen waste (1:1 vegetable: fruit waste) container on the insect. The different developmental stages of insect (adult and larva) were exposed to different concentrations (0, 0.005, 0.01, 0.015, and 0.02 mg/mL) of malathion. The results showed that the mean value of the reactive oxygen species (ROS), which included hydrogen peroxide (H2O2) and superoxide anion radicals (O2•-) concentrations were lower in larval stage than in adults, in all treated groups (0, 0.005, 0.01, 0.015, and 0.02 mg/mL malathion concentration). Also, the protein carbonyls amount and lipid peroxides levels were decreased in the 0.02 mg/mL Malathion compared to the control values. However, the cluster analysis revealed slight dissimilar patterns for control insects and the highest malathion concentration (0.02 mg/ml). These stage-related differences could occur from the different growth dynamic functions of larvae and adults. The larvae were distinguished by robust growth, and significant oxygen consumption. The results verified that oxidative stress parameters, especially protein carbonyls and α, α-diphenyl-β-picrylhydrazyl (DPPH) were promising, cheap, quick and cost-effective applications for determining the macromolecules damage, and antioxidant ability of H. illucens enclosed with malathion exposure. These findings described that malathion application induces macromolecules damage mediated through oxidative stress injury.
Subject terms: Biochemistry, Biological techniques, Biotechnology, Immunology, Environmental sciences
Introduction
The organic waste contamination poses a grave threat on the environment1. This required the contaminants and pollutants to be assessed; to protect the environment and the living organism's health2. Food processing could be affected by environmental pollutants. Briefly, the whole life cycle of food industry, from cradle to grave stages, may lead to maximizing the hazards of organic waste recycling3. Moreover, the food industry cradle stage, which include crop production, may include the pesticides contamination. The pesticides are used to avoid the negative impact of different pests on crop productivity4. Nowadays, the production and consumption of pesticides have been applied for agricultural and non-agricultural practices, until they reached more than two million tons’ consumption/year5. The pesticide fate, transport, and dispersion may have a harmful effect on the natural ecosystems including biodiversity loss; impact on non-target species; or even adverse effect on air, soil, and water quality6. Therefore, the pesticide consumption was considered as an interfering agent to the environment quality and various vital processes such as photosynthesis, biosynthesis reactions and microbes' molecular composition7. organophosphate (OP) insecticides were famous due to their ability to accumulate with low toxicity and persistence rate8. The action mechanism of OP insecticides depends on the degradation and interaction processes. The degradation process includes breaking down of malathion into malaxon. Then, it interacts with the active site of acetylcholinesterase leading to inhibition of acetylcholine hydrolysis and paralysis. In addition, the toxicity of pesticides, especially malathion, depended on oxygen free radicals' induction9,10.
The grave stage of the food industry was concerned with the recycling of organic wastes which may contaminated with pesticides11. Insects are widespread and are characterized by their sensitivity to environmental changes and their ability to be used in the biomonitoring and bioremediation programs12,13. In addition, insect’s oxidative stress parameters could be used to quantify the pollutants effect, like malathion exposure level, on the living organisms. Nowadays, BSF was considered as a biotechnology tool, due to its ecofriendly behavior; it was known for its capability to ensure the circular economy concept14, and its ability to solve the contamination problems especially those dealt with agricultural and organic waste management13,15,16.
Generally, the oxidative stress of the contaminated ecosystem could occur internally and externally, as a result of imbalance status between ROS and antioxidants17,18. The ROS included O2•-, H2O2, singlet oxygen, peroxyl radical, nitric oxide and hydroxyl radical (•OH)19. When the ROS levels exceeded the antioxidant’s levels, they led to macromolecules damage in the form of protein carbonyls, enzyme inactivation, lipid peroxides, and genotoxicity13,20,21. Antioxidants included non-enzymatic antioxidants (such as reduced glutathione (GSH), α-tocopherol, ascorbic acid, and β-carotene), and enzymatic antioxidants (such as superoxide dismutase (SOD), catalase (CAT), peroxidase (Px), polyphenol oxidase (PPO), ascorbate peroxidase (APOx), and acetylcholine esterase (AChE))22. In addition, the antioxidant non-enzymatic activity can be detected by DPPH assay, in which antioxidants can inhibit the lipid oxidation. So, the scavenging rate of DPPH radical can determine free-radical scavenging capacity 23. The potential usage of oxidative stress parameters, to assess the impacts of malathion on insect bioreactor, especially BSF fed on organic waste, wasn’t studied before24.
This work aimed to assess the impact of malathion-contaminated organic waste (fruits and vegetables) on the oxidative stress parameters of BSF. We measured ROS (H2O2 and O2•-) concentration, macromolecules damage (protein carbonyls and lipid peroxides), enzymatic antioxidant response (SOD, CAT, and PPO) and non-enzymatic antioxidants (DPPH and GSH) in the midgut homogenates of 5th larval instar (MHL) and male adult (MHA) of BSF, which were exposed to different malathion concentration (0, 0.005, 0.01, 0.015, and 0.02 mg/ mL).
Results
The concentration of ROS
The concentration of H2O2 and O2•- in MHL and MHA, which were exposed to different malathion concentration; were shown in Fig. 1. The ANOVA test, Tukey's-b, Post Hoc test showed that the results of H2O2 included SS = 1878.6 and 735.7, MS = 469.6 and 183.9, F = 1075.6 and 46.9, df = 4; and p value < 0.001 in larval and adult stages, respectively. Also, the results of O2•-, in both larval and adult stages included SS = 10.13 and 6.35, MS = 2.5 and 1.6, F = 13.5 and 9.4, df = 4; and p value < 0.001, respectively. The mean value of both H2O2 and O2•- were lower in larvae than adults at all experimental concentration of malathion. The H2O2 concentration has direct correlation with malathion concentration at the larval stage of H. illucens (Fig. 1A); however, the highest H2O2 concentration was recorded at the malathion concentration 0.005 and 0.015 mg/ml in the adult males and larva, respectively (Fig. 1A). The O2•- production rate showed non-significant elevations/ depressions in malathion treated groups compared to control groups, in both larval and adult insects (Fig. 1B).
Figure 1.
Reactive oxygen species (ROS) concentration, that expressed as hydrogen peroxide (H2O2) concentration (A), and superoxide anion (O2•-) concentration (B). The data were expressed as mean ± SE. The concentrations were obtained from gut homogenates of 5th instar larvae and males of Hermetia illucens, which their food containers were exposure to different concentrations of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/ml). Mean values marked with the different lowercase letters were significantly different among malathion concentration assessment (ANOVA test Tukey's-b, Post Hoc test, p < 0.05).
Oxidative damage assay
The mean protein carbonyls amount recorded higher values in treated larvae and adults than in control, except for the highest treated group (0.02 mg/ml group) (Table 1). It was elevated about 27.7% in the 0.015 mg/ml MHL group and 44.1% in the 0.005 mg/ ml MHA, compared to the control groups. However, the protein carbonyls amount showed almost the same values as the control groups in both 0.02 mg/ml MHL and MHA groups.
Table 1.
The macromolecules oxidative damage in form of amount of protein carbonyls (OD/ mg protein), and concentration of lipid peroxides (mM cumene hydroperoxide/ mg protein). The protein carbonyls and lipid peroxides concentrations were obtained from gut homogenates of 5th instar larvae and males of Hermetia illucens, which their food containers were exposure to different concentration of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/ml).
| Malathion concentration (mg/ml) | Protein carbonyls amount (OD/mg protein) | Lipid peroxidation concentration (mM cumene hydroperoxide/ mg protein) | ||
|---|---|---|---|---|
| Larvae (MHL) | Adult (MHA) | Larvae (MHL) | Adult (MHA) | |
| 0 | 36.56 ± 2.4a | 43.8 ± 0.1a | 11.47 ± 0.3 | 34.4 ± 0.5a |
| 0.005 | 38.52 ± 1.6ab | 62.7 ± 2.5 | 34.11 ± 1.4ab | 38.4 ± 1.9ac |
| 0.01 | 42.64 ± 0.8bc | 48.3 ± 1.6ab | 31.07 ± 0.8a | 22.35 ± 0.4b |
| 0.015 | 46.56 ± 0.3c | 48.9 ± 0.3b | 35.68 ± 1.5b | 39.21 ± 1.1c |
| 0.02 | 36.17 ± 1.9a | 43.6 ± 1.2a | 22.74 ± 1.4 | 26.1 ± 1.55b |
The data were expressed as mean ± SE.
Mean values marked with the different lowercase letters were significantly different among malathion concentration assessment (ANOVA test Tukey's-b, Post Hoc test, p < 0.05).
The lipid peroxidation concentration reached its highest value, for both MHL and MHA, at 0.015 mg/ml malathion concentration. It was increased by 3.11-fold, and 1.14-fold, respectively, compared to the control values (Table 1). Meanwhile, the macromolecules oxidative damage, in form of Lipid peroxidation concentration, was decreased in MHL and MHA 0.02 mg/ ml treated groups, where it recorded lower value than control group in MHA.
Enzymatic antioxidant response
In the control groups of H. illucens, the antioxidant enzymatic response, expressed as SOD and CAT, was significantly lower in the larval stage than in adult stage (Fig. 2A and B). Both SOD and CAT antioxidant activity were significantly (p < 0.05) higher in MHL and MHA 0.02 mg/ml malathion groups than the control groups (Fig. 2A and B). Yet, there was a significant (p < 0.05) decrease in PPO activity at 0.02 mg/ml malathion concentration than control groups at larval and adult stages (Fig. 2C).
Figure 2.
Activity of antioxidant enzymes in form of: (A) superoxide dismutase (SOD), (B) catalase (CAT), and (C) polyphenol oxidase (PPO) (OD/ mg protein/ min). The enzymes activity was expressed as mean ± SE values. The enzymatic antioxidant activity obtained from gut homogenates of 5th instar larvae and males of Hermetia illucens which their food containers were exposure different concentration of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/ml). Mean values marked with the different lowercase letters were significantly different among malathion concentration assessment (ANOVA test Tukey's-b, Post Hoc test, p < 0.05).
Non-enzymatic antioxidant response
The values of non-enzymatic antioxidant responses were represented in Fig. 3A and B. While DPPH recorded significantly higher value at 0.005 and 0.02 mg/ml malathion treated groups than the control group. In addition to the DPPH concentration of the control adult was significantly higher than all the treated adult groups (Fig. 3A). On the contrary, GSH concentration elevated significantly in all the treated larval groups, and in 0.01, and 0.02 mg/ml malathion adult groups compared to the control groups of H. illucens (Fig. 3B).
Figure 3.
Activity of non-enzymatic response in form of the concentration of anti-radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) (percentage of inhibition) (A), and antioxidant glutathione reduced (GSH) (µg/ mg protein) (B). The non-enzymatic antioxidants concentration was expressed, in a box-plot graph, as median, min., max., P25, and p75 values. The non- enzymatic antioxidant concentration obtained from gut homogenates of 5th instar larvae and males of Hermetia illucens, which their food containers were exposure different concentration of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/ml). Mean values marked with the different lowercase letters were significantly different among malathion concentration assessment (ANOVA test Tukey's-b, Post Hoc test, p < 0.05).
Relation and interaction assessment
Pearson′s correlation analysis between malathion concentration and oxidative stress assays (ROS concentration, macromolecules damage, enzymatic and non-enzymatic antioxidant assays), revealed the most significant relationship in both larvae and adult stages (Table 2). In larval stage, there was a correlation at p < 0.001 between malathion concentration and H2O2, PPO and GSH. Also, the strong correlation at p < 0.05 level was observed between malathion concentration (0–0.02 mg/ml) and SOD or CAT. Yet, there was no significant correlation between malathion concentration at adult stage, except for SOD at p < 0.001, and PPO, DPPH and GSH at p < 0.05 level (Table 2). However, the GEE interaction analysis between malathion concentration, developmental stage, interaction and intercept showed a significant influence between these factors in all oxidative stress parameters except in case of developmental stage effect on the levels of DPPH (Table 3).
Table 2.
Pearson′s correlation coefficient to detect the effect of malathion different concentration (0, 0.005, 0.01, 0.015, and 0.02 mg/ml) on reactive oxygen species concentration (ROS), (in form of hydrogen peroxide (H2O2), superoxide anion radical (O2•-)), macromolecules damage (in form of protein carbonyls amount and lipid peroxides concentration), enzymatic antioxidant response (inform of superoxide dismutase (SOD), catalase (CAT), and polyphenol oxidase (PPO)), and non-enzymatic antioxidant response (inform of anti-radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and antioxidant glutathione reduced (GSH)) in the gut homogenates of 5th instar larvae and males of Hermetia illucens.
| Category | Assessment | stage | r | Equation | R2 | Type |
|---|---|---|---|---|---|---|
| ROS | H2O2 | Larvae | 0.91 | Y = 1234.6 X | -0.8 | Linear equation for prediction |
| Adult | − 0.17 | Y = 3056.2 X | − 15.1 | |||
| O2•- | Larvae | − 0.06 | Y = 1152.8 X | − 75.3 | ||
| Adult | 0.36 | Y = 1398.2 X | − 118.9 | |||
| Macromolecules damage | Protein carbonyls | Larvae | 0.22 | Y = 2721.4 X | − 23.4 | |
| Adult | − 0.27 | Y = 3204.7 X | − 17.1 | |||
| Lipid peroxides | Larvae | 0.37 | Y = 1962.2 X | − 1.8 | ||
| Adult | − 0.31 | Y = 2036.6 X | − 8.3 | |||
| Enzymatic antioxidants | SOD | Larvae | 0.57* | Y = 429.2 X | − 0.7 | |
| Adult | 0.92** | Y = 1531.4 X | − 0.01 | |||
| CAT | Larvae | 0.58* | Y = 407.3 X | 0.3 | ||
| Adult | 0.11 | Y = 1239.9 X | − 7.2 | |||
| PPO | Larvae | − 0.91** | Y = 996.1 X | − 3.5 | ||
| Adult | − 0.52* | Y = 1332.2 X | − 11.1 | |||
| Non-enzymatic antioxidants | DPPH | Larvae | 0.35 | Y = 3739.8 X | − 2.9 | |
| Adult | − 0.51* | Y = 3372.9 X | − 16.3 | |||
| GSH | Larvae | 0.82** | Y = 2648.3 X | − 0.4 | ||
| Adult | 0.54* | Y = 3917.6 X | − 5.3 |
* significant at p < 0.05; ** significant at p < 0.001.
Table 3.
Generalized Estimating Equation to analyze the malathion concentration (0, 0.005, 0.01, 0.015, and 0.002 mg/ml), insect developmental stage (5th larval instar and adult males), combined effect of malathion concentration with insect developmental stage and finally intercept on reactive oxygen species concentration (ROS), (inform of hydrogen peroxide (H2O2), superoxide anion radical (O2•-)), macromolecules damage (inform of protein carbonyls amount and lipid peroxides concentration), enzymatic antioxidant response (inform of superoxide dismutase (SOD), catalase (CAT), and polyphenol oxidase (PPO)), and finally non-enzymatic antioxidant response (inform of anti-radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and antioxidant glutathione reduced (GSH)) in the gut homogenates of 5th instar larvae and adult males of Hermetia illucens.
| Category | Source | Chi-square | df | p-value | QIC* |
|---|---|---|---|---|---|
| Concentration effect | |||||
| ROS | H2O2 | 629.9 | 4 | < 0.000 | 63.5 |
| O2•- | 95.3 | 4 | < 0.000 | 23.5 | |
| Macromolecules damage | Protein carbonyls | 128.8 | 4 | < 0.000 | 167.1 |
| Lipid peroxides | 434.0 | 4 | < 0.000 | 117.5 | |
| Enzymatic antioxidants | SOD | 5535.3 | 4 | < 0.000 | 24.4 |
| CAT | 1001.3 | 4 | < 0.000 | 132.2 | |
| PPO | 2401.3 | 4 | < 0.000 | 30.3 | |
| Non-enzymatic antioxidants | DPPH | 122.67 | 4 | < 0.000 | 500.3 |
| GSH | 405.6 | 4 | < 0.000 | 210.3 | |
| Developmental stage effect | |||||
| ROS | H2O2 | 6471.9 | 1 | < 0.000 | 63.5 |
| O2•- | 564.1 | 1 | < 0.000 | 23.5 | |
| Macromolecules damage | Protein carbonyls | 134.9 | 1 | < 0.000 | 167.1 |
| Lipid peroxides | 59.9 | 1 | < 0.000 | 117.5 | |
| Enzymatic antioxidants | SOD | 8777 | 1 | < 0.000 | 24.4 |
| CAT | 393.8 | 1 | < 0.000 | 132.2 | |
| PPO | 20.1 | 1 | < 0.000 | 30.3 | |
| Non-enzymatic antioxidants | DPPH | 0.81 | 1 | > 0.05 | 500.3 |
| GSH | 530.1 | 1 | < 0.000 | 210.3 | |
| Concentration × developmental stage effect | |||||
| ROS | H2O2 | 5157.2 | 4 | < 0.000 | 63.5 |
| O2•- | 92.6 | 4 | < 0.000 | 23.5 | |
| Macromolecules damage | Protein carbonyls | 84.8 | 4 | < 0.000 | 167.1 |
| Lipid peroxides | 865.6 | 4 | < 0.000 | 117.5 | |
| Enzymatic antioxidants | SOD | 2871.2 | 4 | < 0.000 | 24.4 |
| CAT | 1940.3 | 4 | < 0.000 | 132.2 | |
| PPO | 1703.5 | 4 | < 0.000 | 30.3 | |
| Non-enzymatic antioxidants | DPPH | 522.3 | 4 | < 0.000 | 500.3 |
| GSH | 254.5 | 4 | < 0.000 | 210.3 | |
| Intercept effect | |||||
| ROS | H2O2 | 17,370.3 | 1 | < 0.000 | 63.5 |
| O2•- | 88,702.9 | 1 | < 0.000 | 23.5 | |
| Macromolecules damage | Protein carbonyls | 12,279.8 | 1 | < 0.000 | 167.1 |
| Lipid peroxides | 8068.8 | 1 | < 0.000 | 117.5 | |
| Enzymatic antioxidants | SOD | 29,937.1 | 1 | < 0.000 | 24.4 |
| CAT | 1025.1 | 1 | < 0.000 | 132.2 | |
| PPO | 38,556.1 | 1 | < 0.000 | 30.3 | |
| Non-enzymatic antioxidants | DPPH | 5268.4 | 1 | < 0.000 | 500.3 |
| GSH | 9106.6 | 1 | < 0.000 | 210.3 | |
* Quasi Like hood under Independence Model Criterion.
The Dendrogram of the cluster analysis, using Ward′s Method, revealed slightly dissimilar patterns for control insect groups and 0.02 mg/ml malathion concentration. The clustering oxidative stress assessment and antioxidant response of both larval and adult stages were shown in Fig. 4a–d. The level of oxidative stress assessment was highly similar in larval stage at 0, 0.005, and 0.01 mg/ml malathion concentration (Fig. 4a) however, the cluster of ROS and macromolecules damage occurred in adult stage at 0, 0.005, 0.01, and 0.015 mg/ml malathion (Fig. 4b). The 0, 0.005, and 0.01 mg/ml malathion concentration created a separate cluster in antioxidant response system of larval H. illucens (Fig. 4c), though, in adult stage 0, 0.005, and 0.015 mg/ml malathion formed a separate cluster in antioxidant enzymatic and non-enzymatic response in gut homogenates of H. illucens (Fig. 4d).
Figure 4.
Dendrogram of the cluster analysis (using Ward′s Method) applied for oxidative stress assessment (in form of cluster the reactive oxygen species concentration (ROS), and macromolecules damage) in the gut homogenate of 5th larval instar (A), and male adult stage (B) of Hermetia illucens. The cluster analysis which applied for antioxidant response system (inform of enzymatic antioxidant response and non-enzymatic antioxidant response) in the gut homogenate of 5th larval instar (C), and male adult stage (D) of Hermetia illucens, which their food containers were exposure to different concentration of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/mL).
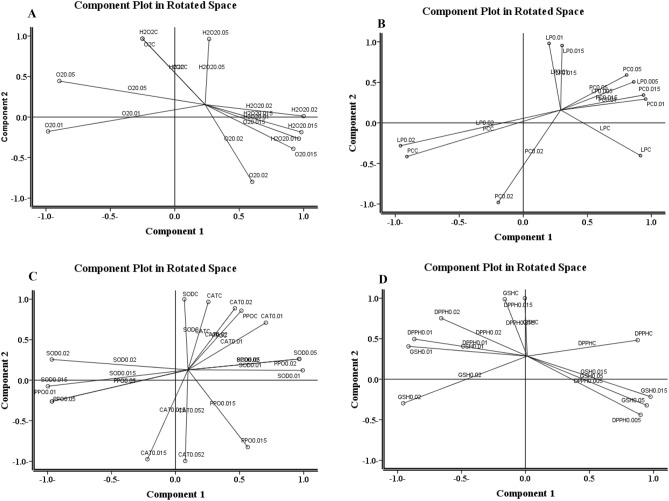
Environmental computing of the effect of different malathion concentration (0, 0.005, 0.01, 0.015, and 0.02 mg/ml) and developmental stage (5th larval instar and adult male) were shown in Fig. 5a–d. These effects were assessed in the form of principal component analysis (PCA). The PCA was done through variance co-variance matrix analysis with two different components. Also, the eigen value tended to be dependent on 2 variables which were classified into first component and second component. The 1st component is different malathion concentration and the 2nd one is developmental stage. The normalization rotation method revealed that the O2•- production rate, in groups treated with malathion concentration from 0 to 0.01 mg/ml, had a great ROS levels in larval and adult stages (Fig. 5a). Meanwhile, the macromolecules damage, in the form of protein carbonyls and lipid peroxide, was detected at concentration 0 or 0.02 mg/ ml malathion (Fig. 5b). The variance–covariance analysis showed a high variability of enzymatic antioxidant system along malathion concentration (Fig. 5c). However, the first component of PCA tended to be localized centric and reflected between non-enzymatic response and malathion concentration (Fig. 5d).
Figure 5.
Environmental computing of different malathion concentration (0, 0.005, 0.01, 0.015, and 0.02 mg/ml) and developmental stage effect (5th larval instar, and adult male) in form of principal component analysis (PCA). The PCA was analyzed the two different component, which applied for reactive oxygen species concentration (ROS), (inform of hydrogen peroxide (H2O2), superoxide anion radical (O2•-)) (A), macromolecules damage (inform of protein carbonyls amount and lipid peroxides concentration) (B), enzymatic antioxidant response (inform of superoxide dismutase (SOD), catalase (CAT), and polyphenol oxidase (PPO)) (C), and finally non-enzymatic antioxidant response (inform of anti-radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and antioxidant glutathione reduced (GSH)) (D) in the gut homogenates of Hermetia illucens.
Discussion
Many studies focused on the role of H. illucens in organic waste recycling process, and the insect’s valuable products, such as chitin, lipids, and proteins, which could be used on various industrial applications25–32. The modern scientific researches focused on using BSF larvae, that fed on organic waste, as animal feed. These wastes might contain different kinds and concentrations of pesticides which affect both the insect bioreactor and the feeding animals. The new stress problems such as the pesticides application or the mechanisms beyond the accumulation process were considered as one of the most interesting research points which focused on the phenomena of hormesis, adaptation, and mitigation33. Moreover, the lethargic effect of humans on the environmental components led to a dangerous and even lethal, backlash from ecosystem especially living organisms34. Several of the previous studies approved the assessment of oxidative stress in the biomonitoring of environmental pollution35–37. Exposure to the pesticides may raise the oxidative stress status directly by increasing the reactive oxygen species (ROS) over generation rate of the non-enzymatic and enzymatic antioxidants in the cells36,38. Maintaining the normal levels of oxidants in the cells is of utmost priority to avoid the negative actions of oxidative stress products, such as protein carbonyls and lipid peroxides levels on the living organisms’ health36,39. Organophosphates could initiate ROS production and oxidation products in the cells40,41. malathion may indirectly increase the production of ROS inside the cells through disrupting the respiratory metabolism42, It also contains the P-S bond (“thion”) that may convert to P-O bond (“oxon”), by the action of a microsomal system of enzymes named mixed-function oxidases (MFO), and cytochrome P450 (CYP450)43. The oxon compounds are highly toxic and can initiate oxidative damage to the living cells. malathion has a toxic effect on pupa, male and female of the peach fruit fly, Bactrocera zonata, with a higher resistance ratio in the field population than in laboratory insect population44. In the current study, a slight increase in the H2O2 and O2•- concentration was observed in the MHA than MHL by using all malathion concentration course (0–0.02 mg/ml) (Fig. 1). This explained the oxidants accumulation possibility38 or the antioxidants levels’ depletion in the adult stage than in the immature stage36. However, the H2O2 concentration in 120th generation old of Spodoptera exigua didn't change after the exposure to 44 μg/g of dry weight Cd45. The efficiency of the elimination process of oxidants and oxidative products may be impaired in the stressful conditions. This phenomenon occurred in this study where, the ROS, as O2•- production rate, of the MHA increased than MHL (Fig. 1b). Also, H. illucens can normalize the concentration of H2O2 and O2•- especially in MHA. However, when the MHL was exposed to high concentration of malathion (0.02 mg/ml), the concentration of H2O2 increased significantly than control values (Fig. 1a). Similarly, the toxicity of OP compounds induced some oxidative stresses, like elevation in the protein carbonyls amount or lipid peroxides level in some living organisms46,47, and caused physiological and pathological changes in tissues48. The LC50 value of methidathion pesticide could affect the malondialdehyde level and antioxidant enzyme activities in the gut tissues of Lymantria dispar (Lepidoptera) larvae49. Similarly, our results recorded the highest concentration of protein carbonyls amount at 0.015 mg/ml malathion in the MHL, and the highest lipid peroxides concentration in MHL, and MHA at the same malathion concentration (Table 1). Yet, there was no significant difference between the highest concentration of malathion (0.02 mg/ml) and control (0 mg/ml) in both MHL and MHA (Table 1). This may be due to the action of enzymatic and non-enzymatic response36. The current study detected fluctuations in the concentration of protein carbonyls throughout the malathion concentration-course (Table 1), and this may reflect on the fluctuating homeostatic mechanism balance between protein degradation and production of protein carbonyls36,50. Lipid peroxidation can disrupt the membrane of the polyunsaturated phospholipids bilayer structure and function51. Also, products of lipid peroxidation are capable of disrupting conformations of many cellular proteins, including enzymes, by forming cross links with these proteins, inactivating their functions52. Lipid peroxidation is considered as a chain reaction; it produces lipid radical, lipid peroxyl radical, and then lipid hydroperoxide. This reaction can be stopped by termination reactions, such as the recombination of lipid peroxyl radicals and by a reaction with glutathione catalyzed by peroxidase53. Therefore, the pattern of fluctuation of lipid peroxides in H. illucens, formed post treatment with different concentration of Malathion, may be due to unbalanced levels of lipid peroxides production and their repairing mechanisms that may include antioxidant enzymes.
Generally cited that, malathion is considered as neurotoxic component that inhibits the neuronal cholinesterase enzyme activity. Also, the peroxidative effects of OP were studied on the activities of antioxidant enzymes, and on lipid peroxidation in-vitro54, and in-vivo studies55. malathion treatment resulted in the elevation of lipid peroxidation concentration which was considered as an indicator of oxidative stress induction56. The results showed that the malathion applications led to elevation in the activities of the key antioxidant enzymes, SOD and CAT, over the constitutive levels except for different cases of malathion concentration (Fig. 2a and b). The observed elevation seemed to occur in concomitance with the oxidative damages of the macromolecules; and may be in response to the formation of ROS as a consequence to the treated stressor36. SOD and CAT have a primary role in the oxidative stress defense through ROS elimination53. However, the significant depletion of PPO activity, which catalyzes the oxidation of phenolic compounds to quinones, occurred in both MHL and MHA treatments along concentration course of malathion (Fig. 2c). malathion treatment could acidify the medium and inhibit the PPO activity57. The DPPH results of MHL showed a significant increase in the 0.005 and 0.015 mg/ml malathion concentration treatment, compared to control values. Similarly, the malathion applications caused a serious risk to Saccharomyces cerevisiae (fungus). This in-vitro study showed that a flavonoid compound called naringin can inhibit some enzymes and can detoxify the DPPH radical57. Glutathione (GSH) acts as a redox factor to balance the redox state of the cell58. Reduced GSH is a chief cellular thiol element in the antioxidative system. Additionally, GSH and DPPH have a significant ROS scavenging role. The chemical stressors can increase the glutathione concentration in animals59. However, the one generation of Spodoptera exigua (Lepidoptera) which was exposed to Cd, didn't show elevation in the GSH concentration60–62. Our results showed that, the increase in GSH concentration was significant in both MHL and MHA, especially, at 0.015, 0.01 and 0.02 mg/ml malathion concentration, respectively (Fig. 3b).
The interaction analysis, obtained from the computation of generalized estimating equation (GEE), revealed that the different concentration of malathion (0, 0.005, 0.01, 0.015, 0.02 mg/ml), the different developmental stage (MHL and MHA), and the interaction of these terms significantly influenced the physiological endpoints we measured (H2O2,O2•-, protein carbonyls, lipid peroxides, SOD, CAT, PPO, DPPH, and GSH) (p value < 0.05), except for DPPH in the developmental stage effect (p value > 0.05) (Table 3).
It has been concluded that the malathion exposure of insect food container can induce oxidative stress in the larval and adult male stages of H. illucens. The levels of ROS, macromolecules damage, enzymatic and non-enzymatic response in BSF to different malathion concentration may be used as a possible mechanism of malathion toxicity. The biochemical analysis of insects could be used as a novel strategy for assess the risk of pesticides accumulation on food container of recyclers of organic waste especially insects. Briefly, the tested hypothesis in this research has proved the ability of using oxidative stress parameters as bioindicator of malathion impact on the organic waste recycler, BSF. Meanwhile, malathion has uniform prooxidant properties in BSF, unlike the other phenolic compounds related to insecticides. The upcoming work will further investigate the fate of malathion in BSF, will answer a question about the role of the insect in reducing the toxicity and severity of malathion, and will shed more light on its role in bioremediation.
Materials and methods
Insects rearing and Malathion application
A colony of H. illucens was supplied from Al Qalyobia governorate and was reared under laboratory conditions for several generations, in the Department of Entomology, Faculty of Science, Cairo University. The experiments were made at summertime 2020, where the rearing conditions were (14:10 L:D; 34˚ ± 2; 60% RH) for adults and (0:24 L:D; 34˚ ± 2; 75% RH) for larvae. The insects were kept in mesh cages 30*30*40 cm3 for adults (100 adults/ cage), and 20*20*10 cm3 for larvae (200 larvae/ cage). Larvae were supplied daily with kitchen waste, 1:1 vegetable: fruit waste, (1000 larvae/ one kg kitchen waste) from household located at Giza Governorate, while adults were hydrated with water and sugar24.
The malathion application was performed by immersing the insect food container with different malathion concentrations (0, 0.005, 0.01, 0.015, or 0.02 mg/ml) for 24 h. Simultaneously, control insects were treated with immersing food container with distilled water. The levels of oxidative stress parameters of control insects were taken 100% levels. The range of low-level insecticide contamination of malathion were applied. Insects were divided into 2 groups: 5th larval instar, and male adult. The adult experiment specimens were taken into account the male’s not females insects in order to avoid the compounding effects of ovarian development in the female insects. The sex of adult specimens was differentiating according to the insect morphological characters; where the males are characterized by a rounded genital apparatus. Each group was divided into 5 sub-groups of 250 individuals, which were exposed to malathion (0, 0.005, 0.01, 0.015, or 0.02 mg/ml) for 24 h post application. For each sub-group, 50 insects were dissected, after 24 h’ malathion application, to isolate gut tissues.
About 7.5 gm gut tissues, of each experimental sub-group, were homogenate in 7.5 ml ice-cold phosphate buffer (50 mM; pH 7.0 contained, 1 ml of 0.1% Triton X-100, 1 ml of 0.05 mM CaCl2); and were centrifuged at 2000 × g for 10 min at 4 °C. The clear sample were stored at − 20 °C until use for further analysis. Each experiment was replicated three times.
The concentration of ROS
The concentration of H2O2 was determined spectrophotometrically according to the method of Junglee et al.63. Briefly, using one step extraction-colorimetric procedure in which, homogenization step using PBS, pH = 7.0 mixed with 0.25 ml Trichloroacetic acid (TCA) (0.1% (w:v)), 0.5 ml KI (1 M), then the 1 ml samples were centrifuged at 12,000 × g for 15 min at 4 °C, and finally, the absorbance was measured at 240 nm. For the superoxide anion radical (O2•-) production rate of samples was determined using colorimetric analysis according to the method of Chen and Li64. The reaction mixture contains 0.25 mL epinephrine (1 mM), 0.25 mL NADPH (1 mM), 0.5 mL sodium phosphate buffer (PBS) (50 mM; pH 7.0), and 1 mL of the samples. The level of superoxide anion radical was determined by the rate of conversion of epinephrine to adrenochrome with 1 mM NADPH as substrate. The absorbance difference (A485–A575) was recorded.
Oxidative damage assay
Protein carbonyls amount assay was performed according to procedure from Levine et al.65. After homogenization and centrifugation steps, a mixture of 800 µL sample, and 200 µl 2, 4-dinitrophenyl hydrazine (DNPH) (10 mM) was incubated for 30 min at room temperature, then precipitated with 1 ml TCA (1%). The pellet was washed four times with 1 ml absolute ethanol/ethyl acetate (1:1) mixture and dissolved in 1 mL of PBS (50 mM; pH 7.0), before being measured at 366 nm.
The lipid peroxides concentration was measured according to Hermes-Lima et al.66. After homogenization and centrifugation step, a mixture of 200 µl sample, 400 µl FeSO4 (1 mM), 200 µl H2SO4 (0.25 M), and 200 µl xylenol orange (1 mM) were added, then absorbance measured at 580 nm. The mixture was incubated in the dark for 3 h at room temperature, and finally the absorbance re-measured at 580 nm after adding of 10 µl cumene hydroperoxides (0.05 mM) (as an internal standard). The change in absorbance due to addition of internal standard was calculated.
Antioxidant enzymatic response
SOD activity was measured based on the procedure described by Misra and Fridovich67. The reaction mixture was as follows: 0.4 ml of a sodium carbonate buffer (200 mM; pH 10.0), 35 µl of EDTA (10 mM), 87 µl of the sample and 0.5 ml of freshly prepared epinephrine (15 mM). The absorbance was measured at 480 nm.
The activity of CAT was assessed in compliance with the method of Aebi68. The reaction mixture contained 0.9 ml of a potassium phosphate buffer (50 mM, pH 7.0), 60 µl of the sample and 40 µl of freshly prepared H2O2 (10 mM). The change in absorbance was measured at 240 nm over a period of 0.5 min. The method of Kumar and Khan69 was used to assess the PPO activity in a reaction mixture containing 0.9 ml of a potassium phosphate buffer (50 mM, pH 7.0), 0.25 ml of 0.1 M catechol and 0.25 mL sample. The reaction formed purpurogallin which was measured at 495 nm.
Antioxidant non-enzymatic response
DPPH antioxidant activity was determined according to Blois70, by adding 0.5 ml DPPH (o.5 M) to 0.5 ml sample and incubated for 20 min before measuring absorbance at 525 nm. DPPH assay was based on the scavenging capability measurement. The nitrogen atom contains an odd electron which is reduced by delivering a hydrogen atom from antioxidants to hydrazine. The procedure of Allen et al.71 was adapted for determining GSH concentration. Briefly, the reaction mixture, containing 150 μl sample, 800 μl PBS (50 mM; pH 8.0), and 50 μl 5, 5´-Diothio bis-2-nitrobenzoic acid (2 mM), then incubated at 25 °C for 20 min. The absorbance of the reaction mixture was 412 nm. The GSH content was determined from a GSH standard curve. The total protein concentration of samples was determined spectrophotometrically according to the method of Bradford72. Briefly, 0.9 mL of the Coomassie brilliant blue (0.5 mM) were mixed with 0.1 mL sample and incubated at room temperature for 2 min. The OD of the protein sample was measured at 595 nm.
Statistical analysis, relation, and interaction assessment
Statistical analysis was performed using IBM SPSS Statistics for Windows (Version 17.0. Armonk, NY: IBM Corp.). A parametric test was carried out using ANOVA test Tukey's-b, Post Hoc test for assessment the malathion concentration effect and T-test, for insect developmental stage. Correlations between malathion concentration and the experimental assays of oxidative stress parameters, including, ROS, macromolecules damage, enzymatic antioxidant response and non-enzymatic antioxidant response, were performed based on Pearson’s regression analysis using linear regression models. Hierarchical Cluster Analysis (HACA) based on agglomerative statistics using Ward′s Method was calculated for oxidative stress parameters. The goal of HACA is to find possible clusters or groups among the observational units, based on level of similarities and differences12. Generalized Estimating Equation (GEE) was used to examine the effect of malathion concentration, insect developmental stage, combined effect of concentration and developmental stage, and finally intercept on the parameters of oxidative stress. The principal component analysis (PCA) was performed the possible assessment of different malathion concentration and developmental stage on the oxidative stress parameters of BSF.
Ethical approval and consent to participate
This article does not contain any studies with human participants or animals that require ethical approval.
Acknowledgements
The authors would like to thank Dr. Mahitab Ezzat El Daly, English Editor, for her guidance in the manuscript English proofreading. This study was done at the Entomology Department, Faculty of Science, Cairo University, Egypt, with the assistance of the team members (Aya hamdy, Nada Yasser, Mohamed Yasser, Aya Aboelhassan, Mohamed Ashraf, Dyaa Bassiony, Abdallah Nagah, Hamed Ashry, Sohila Mohamed, Muhamed Saeed, Naira Essam, Habiba Mohamed, Omnia Mahmoud, and Mariam Hazem) of the project entitled " recycling of organic wastes using ecofriendly-biological technology " which supported and funded by Cairo University, Egypt.
Abbreviations
- ROS
Reactive oxygen species
- H2O2
Hydrogen peroxide
- O2•-
Superoxide anion radicals
- OP
Organophosphate pesticides
- DPPH
α, α-Diphenyl-β-picrylhydrazyl
- GSH
Reduced glutathione
- SOD
Superoxide dismutase
- CAT
Catalase
- Px
Peroxidase
- PPO
Polyphenol oxidase
- APOx
Ascorbate peroxidase
- AChE
Acetylcholine esterase
- MHL
The midgut homogenates of 5th larval instar of Hermetia illucens
- MHA
The midgut homogenates of male adult of Hermetia illucens
- TCA
Trichloroacetic acid
- DNPH
2, 4-Dinitrophenyl hydrazine
- PBS
Sodium phosphate buffer
- HACA
Hierarchical Cluster Analysis
- PCA
Principle component analysis
- GEE
Generalized Estimating Equation
Author contributions
G.M.E.-B: conceptualization, supervision, writing (original draft preparation), writing (review and editing) and visualization; E.A.A.: conceptualization, investigation, methodology, resources, writing (original draft preparation), visualization, and funding acquisition.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was supported by the Cairo University project fund entitled " recycling of organic wastes using ecofriendly-biological technology ".
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferronato N, Torretta V. Waste mismanagement in developing countries: a review of global issues. Int. J. Environ. Res. Public Health. 2019;16(6):1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Shafy HI, Mansour MS. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018;27(4):1275–1290. [Google Scholar]
- 3.Roy P, Nei D, Orikasa T, Xu Q, Okadome H, Nakamura N, Shiina T. A review of life cycle assessment (LCA) on some food products. J. Food Eng. 2009;90(1):1–10. [Google Scholar]
- 4.Prakasam A, Sethupathy S, Lalitha S. Plasma and RBCs antioxidant status in occupational male pesticide sprayers. Clin. Chim. Acta. 2001;310(2):107–112. doi: 10.1016/s0009-8981(01)00487-9. [DOI] [PubMed] [Google Scholar]
- 5.Abhilash PC, Singh N. Pesticide use and application: an Indian scenario. J. Hazard. Mater. 2009;165(1–3):1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel D. Amounts of pesticides reaching target pests: environmental impacts and ethics. J. Agric. Environ. Ethics. 1995;8(1):17–29. [Google Scholar]
- 7.DeLorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: a review. Environ. Toxicol. Chem. Int. J. 2001;20(1):84–98. doi: 10.1897/1551-5028(2001)020<0084:toptam>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Tchounwou PB, Patlolla AK, Yedjou CG, Moore PD. Environmental exposure and health effects associated with Malathion toxicity. Toxic. Hazard Agrochem. 2015;51:2145–2149. [Google Scholar]
- 9.Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol. Lett. 1999;105(3):197–205. doi: 10.1016/s0378-4274(98)00399-3. [DOI] [PubMed] [Google Scholar]
- 10.Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sabzevari O. Biochemical evidence for free radicalinduced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003;22(4):205–211. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- 11.Saer A, Lansing S, Davitt NH, Graves RE. Life cycle assessment of a food waste composting system: environmental impact hotspots. J. Clean. Prod. 2013;52:234–244. [Google Scholar]
- 12.Azam I, Afsheen S, Zia A, Javed M, Saeed R, Sarwar MK, Munir B. Evaluating insects as bioindicators of heavy metal contamination and accumulation near industrial area of Gujrat, Pakistan. BioMed. Res. Int. 2015 doi: 10.1155/2015/942751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelfattah EA, Augustyniak M, Yousef HA. Stage-, sex-and tissue-related changes in H2 O2, glutathione concentration, and glutathione-dependent enzymes activity in Aiolopus thalassinus (Orthoptera: Acrididae) from heavy metal polluted areas. Ecotoxicology. 2021;30(3):478–491. doi: 10.1007/s10646-021-02354-0. [DOI] [PubMed] [Google Scholar]
- 14.Cohn Z, Latty T, Abbas A. Understanding dietary carbohydrates in black soldier fly larvae treatment of organic waste in the circular economy. Waste Manag. 2022;137:9–19. doi: 10.1016/j.wasman.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Rochow VB, Chakravorty J. Notes on entomophagy and entomotherapy generally and information on the situation in India in particular. Appl. Entomol. Zool. 2013;48(2):105–112. [Google Scholar]
- 16.Ravi HK, Degrou A, Costil J, Trespeuch C, Chemat F, Vian MA. Larvae mediated valorization of industrial, agriculture and food wastes: biorefinery concept through bioconversion, processes, procedures, and products. Processes. 2020;8(7):857. [Google Scholar]
- 17.Dos-Anjos NA, Schulze T, Brack W, Val AL, Schirmer K, Scholz S. Identification and evaluation of cyp1a transcript expression in fish as molecular biomarker for petroleum contamination in tropical freshwater ecosystems. Aquat. Toxicol. 2011;103(1):46–52. doi: 10.1016/j.aquatox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115(2):81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert DL. Fifty years of radical ideas. Ann. New York Acad. Sci. 2000;899(1):1–14. doi: 10.1111/j.1749-6632.2000.tb06172.x. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelfattah EA, Augustyniak M, Yousef HA. Biomonitoring of genotoxicity of industrial fertilizer pollutants in Aiolopus thalassinus (Orthoptera: Acrididae) using alkaline comet assay. Chemosphere. 2017;182:762–770. doi: 10.1016/j.chemosphere.2017.05.082. [DOI] [PubMed] [Google Scholar]
- 22.Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011;101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Zheng CD, Li G, Li HQ, Xu XJ, Gao JM, Zhang AL. DPPH-scavenging activities and structure-activity relationships of phenolic compounds. Nat. Prod. Commun. 2010;5(11):1934578X1000501112. [PubMed] [Google Scholar]
- 24.Barragan-Fonseca KB, Dicke M, van Loon JJ. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects Food Feed. 2017;3(2):105–120. [Google Scholar]
- 25.Hahn T, Tafi E, Paul A, Salvia R, Falabella P, Zibek S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020;95(11):2775–2795. [Google Scholar]
- 26.Mouithys-Mickalad A, Schmitt E, Dalim M, Franck T, Tome NM, van Spankeren M, et al. Black soldier fly (Hermetia illucens) larvae protein derivatives: potential to promote animal health. Animals. 2020;10(6):941. doi: 10.3390/ani10060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scala A, Cammack JA, Salvia R, Scieuzo C, Franco A, Bufo SA, et al. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.)(Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-76571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelfattah, E. A., & Lim, J. W. Biotechnology application of organic waste management using black soldier fly, Hermetia illucens. African J. Biol. Sci., 17(1), 171–187 (2021).
- 29.Abdelfattah EA, Abd El-Monem DH, Mahmoud AH, Aboelhassan AM, Ibrahim NY, Abdallah DB, Abdelhamid AN, Hussein MY, Fawzy MA, Abdelhamid HA. The various vulnerable products and services from organic waste management using Black soldier fly. Hermetia illucens. Zool. Entomol. Lett. 2021;1(1):44–56. [Google Scholar]
- 30.Franco A, Scieuzo C, Salvia R, Petrone AM, Tafi E, Moretta A, et al. Lipids from Hermetia illucens, an Innovative and sustainable source. Sustainability. 2021;13(18):10198. [Google Scholar]
- 31.Liu C, Wang C, Yao H, Chapman SJ. Pretreatment is an important method for increasing the conversion efficiency of rice straw by black soldier fly larvae based on the function of gut microorganisms. Sci. Total Environ. 2021;762:144118. doi: 10.1016/j.scitotenv.2020.144118. [DOI] [PubMed] [Google Scholar]
- 32.Triunfo M, Tafi E, Guarnieri A, Scieuzo C, Hahn T, Zibek S, et al. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics. 2021;8(2):40. [Google Scholar]
- 33.Zhang L, Yan W, Xie Z, Cai G, Mi W, Xu W. Bioaccumulation and changes of trace metals over the last two decades in marine organisms from Guangdong coastal regions, South China. J. Environ. Sci. 2020;98:103–108. doi: 10.1016/j.jes.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Abdelfattah, E. A., & Renault, D. Effect of different doses of the catecholamine epinephrine on antioxidant responses of larvae of the flesh fly Sarcophaga dux. Environ. Sci. Pollut. Res., 17(1), 1–8. (2021). [DOI] [PubMed]
- 35.Abdelfattah EA, Dorrah MA. L-dopa and ferrous iron increase dna strand-breaks in the desert locust Schistocerca gregaria (Orthoptera: Acridade) Efflatounia. 2015;15:1–7. [Google Scholar]
- 36.Renault D, Dorrah MA, Mohamed AA, Abdelfattah EA, Bassal TT. Assessment of oxidative stress and activities of antioxidant enzymes depicts the negative systemic effect of iron-containing fertilizers and plant phenolic compounds in the desert locust. Environ. Sci. Pollut. Res. 2016;23(21):21989–22000. doi: 10.1007/s11356-016-7391-9. [DOI] [PubMed] [Google Scholar]
- 37.Abdelfattah, E. A. Effect of different concentration and application time of vitamin B12 on antioxidant response of Physiophora alceae. African J. Biol. Sci. 17(1), 189–203. (2021).
- 38.Yousef HA, Abdelfattah EA, Augustyniak M. Antioxidant enzyme activity in responses to environmentally induced oxidative stress in the 5th instar nymphs of Aiolopus thalassinus (Orthoptera: Acrididae) Environ. Sci. Pollut. Res. 2019;26(4):3823–3833. doi: 10.1007/s11356-018-3756-6. [DOI] [PubMed] [Google Scholar]
- 39.Abdelfattah EA. Biomolecules oxidation and antioxidant enzymes response as a result of injection of oxidative stressor into 5th instar of Schistocerca gregaria (Orthoptera, Acrididae) Entomol. Ornithol. Herpetol. 2016;5(181):2161–2983. [Google Scholar]
- 40.Altuntas I, Delibas N, Doguc DK, Ozmen S, Gultekin F. Role of reactive oxygen species in organophosphate insecticide phosalone toxicity in erythrocytes in vitro. Toxicol. Vitro. 2003;17(2):153–157. doi: 10.1016/s0887-2333(02)00133-9. [DOI] [PubMed] [Google Scholar]
- 41.Aksoy L, Alper Y. The effects of royal jelly on oxidative stress and toxicity in tissues induced by malathion, an organophosphate insecticide. J. Hell. Vet. Med. Soc. 2019;70(2):1517–1524. [Google Scholar]
- 42.Büyükgüzel K. Malathion-induced oxidative stress in a parasitoid wasp: effect on adult emergence, longevity, fecundity, and oxidative and antioxidative response of Pimpla turionellae (Hymenoptera: Ichneumonidae) J. Econ. Entomol. 2006;99(4):1225–1234. [PubMed] [Google Scholar]
- 43.Giri S, Prasad SB, Giri A, Sharma GD. Genotoxic effects of malathion: an organophosphorus insecticide, using three mammalian bioassays in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002;514(1–2):223–231. doi: 10.1016/s1383-5718(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 44.Bakr RF, Refaei BM, Radwan EM, El-Heneady AA. Toxicological and Biochemical Effects of Malathion and Spinosad on the Peach Fruit Fly, Bacterorcera zonata (saunders) Egyp. Acad. J. Biol. Sci. F Toxicol. Pest Control. 2016;8(1):7–19. [Google Scholar]
- 45.Augustyniak M, Płachetka-Bożek A, Kafel A, Babczyńska A, Tarnawska M, Janiak A, Zawisza-Raszka A. Phenotypic plasticity, epigenetic or genetic modifications in relation to the duration of Cd-exposure within a microevolution time range in the beet armyworm. PLoS ONE. 2016;11(12):e0167371. doi: 10.1371/journal.pone.0167371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gultekin F, Ozturk M, Akdogan M. The effects of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro) Arch. Toxicol. 2000;74:533–538. doi: 10.1007/s002040000167. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Liu J, Zhang R, Zhang J, Guo Y, Ma E. Biochemical effects of acute phoxim administration on antioxidant system and acethylcholinesterase in Oxya chinensis (Thunberg) (Orthoptera: Acrididae) Pestic. Biochem. Physiol. 2011;100:23–26. [Google Scholar]
- 48.Uzun FG, Kalender S, Durak D, Demir F, Kalender Y. Malathion-induced testicular toxicity in male rats and the protective effect of vitamins C and E. Food Chem. Toxicol. 2009;47(8):1903–1908. doi: 10.1016/j.fct.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Dubovskiy IM, Martemyanow VV, Vorontsova YL, Rantala MJ, Gryzanova EV, Glupov VV. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae) Comp. Biochem. Physiol. Part C. 2008;148:1–5. doi: 10.1016/j.cbpc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Costa V, Quintanilha A, Moradas-Ferreira P. Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. IUBMB Life. 2007;59(4–5):293–298. doi: 10.1080/15216540701225958. [DOI] [PubMed] [Google Scholar]
- 51.Girotti AW. Mechanisms of lipid peroxidation. J. Free Radic. Biol. Med. 1985;1:87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 52.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu WH. Nitric oxide synthase in motor neurons after axotomy. J. Histochem. Cytochem. 1994;42:451–457. doi: 10.1177/42.4.7510317. [DOI] [PubMed] [Google Scholar]
- 54.Altuntas I, Kilinc I, Orhan H, Demirel R, Koylu H, Delibas N. The effects of diazinon on lipid peroxidation and antioxidant enzymes in erythrocytes in vitro. Hum. Exp. Toxicol. 2004;23(1):9–13. doi: 10.1191/0960327104ht408oa. [DOI] [PubMed] [Google Scholar]
- 55.John S, Kale M, Rathore N, Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 2001;12(9):500–504. doi: 10.1016/s0955-2863(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 56.Fortunato JJ, Agostinho FR, Réus GZ, Petronilho FC, Dal-Pizzol F, Quevedo J. Lipid peroxidative damage on malathion exposure in rats. Neurotox. Res. 2006;9(1):23–28. doi: 10.1007/BF03033304. [DOI] [PubMed] [Google Scholar]
- 57.Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. 2018;44(1):36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 58.Zhou P, Smith NL, Lee CY. Potential purification and some properties of Monroe apple peel polyphenol oxidase. J. Agric. Food Chem. 1993;41(4):532–536. [Google Scholar]
- 59.Gerçek E, Zengin H, Erişir FE, Yılmaz Ö. Biochemical changes and antioxidant capacity of naringin and naringenin against malathion toxicity in Saccharomyces cerevisiae. Comp. Biochem. Physiol. Part C Toxicol. Pharm. 2021;241:108969. doi: 10.1016/j.cbpc.2020.108969. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Wang M, Jiang R, Zheng L, Chen W. Evaluation of joint toxicity of heavy metals and herbicide mixtures in soils to earthworms (Eisenia fetida) J. Environ. Sci. 2020;94:137–146. doi: 10.1016/j.jes.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 61.Jozefczak M, Remans T, Vangronsveld J, Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012;13(3):3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kafel A, Zawisza-Raszka A, Szulińska E. Effects of multigenerational cadmium exposure of insects (Spodoptera exigua larvae) on anti-oxidant response in haemolymph and developmental parameters. Environ. Pollut. 2012;162:8–14. doi: 10.1016/j.envpol.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 63.Junglee S, Urban L, Sallanon H, Lopez-Lauri F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014;5:730–736. [Google Scholar]
- 64.Chen WP, Li PH. Chilling-induced Ca2+ overload enhances production of active oxygen species in maize (Zea mays L.) cultured cells: the effect of abscisic acid treatment. Plant Cell Environ. 2001;24(8):791–800. [Google Scholar]
- 65.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Method Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 66.Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe (III) xylenol orange complex formation. Free Rad. Bio Med. 1995;19(3):271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- 67.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 68.Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 69.Kumar KB, Khan PA. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Int. J. Exp. Biol. 1982;20(5):412–416. [PubMed] [Google Scholar]
- 70.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 71.Allen RG, Farmer KJ, Newton RK, Sohal RS. Effects of paraquat administration on longevity, oxygen consumption, lipid peroxidation, superoxide dismutase, catalase, glutathione reductase, inorganic peroxides and glutathione in the adult housefly. Comp. Biochem. Phys. Comp. Pharm. 1984;78(2):283–288. doi: 10.1016/0742-8413(84)90084-7. [DOI] [PubMed] [Google Scholar]
- 72.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.