Abstract
The in vivo convulsant activities in rats of five representative fluoroquinolones (FQs), norfloxacin, enoxacin, sparfloxacin, fleroxacin, and pefloxacin, were compared. The experimental approach allowed distinction between the drugs’ ability to reach the pharmacological receptors at the level of the central nervous system (pharmacokinetic contribution) and their ability to interact with these receptors (pharmacodynamic contribution). The presence of a methyl group on the piperazine moiety decreased the pharmacodynamic contribution to the convulsant activity by severalfold, and the ratios of concentrations of the FQs in cerebrospinal fluid (CSF) to concentrations of unbound FQs in plasma varied from about 5 to 75% as a function of lipophilicity. Interestingly, FQs with the highest intrinsic convulsant activities had the lowest levels of diffusion in CSF and vice versa. This in vivo approach provides information complementary to that of in vitro experiments and should be recommended for early preclinical assessment of a new FQ’s epileptogenic risk.
Fluoroquinolones (FQs) are frequently used as antimicrobial agents in therapeutics. Because they can spread into the central nervous system (CNS), they have been proposed as alternatives in the treatment of CNS infections (16, 23, 30). Although severe neurological disorders are relatively rare, various side effects, including headache, confusion, hallucination, anxiety, nervousness, nightmares, and seizures, have been reported for patients receiving FQs (4, 6, 8, 17, 33). Convulsive seizures have mostly been reported for high-risk patients such as people with a history of epilepsy (6) and patients who are cotreated with nonsteroidal anti-inflammatory drugs such as fenbufen (4, 5, 33). Although the exact mechanism by which FQs exhibit epileptogenic activity is not yet clear, it has been admitted for a long time that CNS excitation may result from inhibition of γ-aminobutyric acid A (GABAA) binding to its receptors (13). As a consequence, the epileptogenic activities of FQs have most often been assessed from in vitro GABA binding experiments (1, 32, 34). However, FQs alone have no or only a weak affinity for GABAA receptors (3, 12, 15, 19, 34), and in order to observe significant binding to these receptors, biphenyl acetic acid (BPAA) the active metabolite of fenbufen, is usually added to FQs (1, 2, 32, 34). The key concern is that, under these conditions, GABA binding experiments may not be relevant for predicting the convulsant activities of FQs administered alone. More appropriate in vitro approaches have been proposed recently, in particular that using the Xenopus oocyte translation system of exogenous messenger RNA (24) and that of an optimized hippocampus slice model (31). Although these approaches are very useful in characterizing the epileptogenic activities of FQs, they do not take into consideration the drugs’ ability to reach receptors at the CNS level. This ability may vary considerably from one compound to another, due in particular to differences in lipophilicities (18, 21, 22) or differences in affinities for active transport systems, including glycoprotein P (20) at the blood-brain barrier level (27) or at the blood-cerebrospinal fluid (CSF) barrier (26). We have recently proposed an in vivo methodology that requires no mechanistic assumption for the assessment of FQs’ convulsant activities (9). This approach had initially been developed by Danhof and Levy (7) and was subsequently used with several drugs, including compounds that induce convulsions, such as pentylenetetrazol (28) and theophylline (29), to investigate the kinetics of drug action in disease states. This approach consists of administering a drug intravenously to rats until the animals exhibit maximal seizures and measuring the drug’s concentration within the biophase (i.e., in the CSF) at the onset of activity. By doing so, one can differentiate between the pharmacokinetic contribution (the ability to reach receptors) and the pharmacodynamic contribution (the affinity for these receptors) to the in vivo convulsant activities of FQs. This new approach was validated with two representative FQs, pefloxacin and norfloxacin (9), and subsequently submitted to an interspecies comparison (10). It has also been used to characterize the convulsant interaction between pefloxacin and theophylline (25). This methodology is now used to compare the epileptogenic potentials of five representative FQs, two with one methyl (pefloxacin and fleroxacin), one with two methyls (sparfloxacin), and two without a methyl (norfloxacin and enoxacin) on the piperazine moiety.
(This study was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997.)
Pefloxacin was available as a methane sulfonate solution (Bellon Rhône-Poulenc Rorer Laboratories) at a concentration of 80 mg of pefloxacin base per ml (or 240 mmol/liter). Norfloxacin and enoxacin were purchased from Sigma (Saint-Quentin Fallavier, France). Fleroxacin and sparfloxacin were kindly supplied as powders by Roche (Basle, Switzerland) and Rhône Poulenc Rorer (Anthony, France), respectively. Male Sprague-Dawley rats (Depres Breeding Laboratories, St. Doulchard, France) were used in this study. Animals were housed in the animal breeding facilities of our laboratory (authorization no. 0028). Their body weights ranged from 220 to 295 g and averaged 255 ± 23 g (mean ± standard deviation [SD]). The animals were housed in wire cages in a 12-h light–12-h dark cycle for 1 week to allow them adjust to the new environment and to overcome possible stress incurred during transit. They had free access to food (Extralabo M20; Pietrement Laboratories, Ste. Colombe, France) and water. One day before the experiment, rats had a cannula implanted in the left jugular vein under anesthesia with 60 mg of sodium pentotal (Sanofi Laboratories) per kg of body weight. After this surgery animals were housed individually in plastic cages and food was withdrawn 12 h before the experiment, but the animals had free access to water until drug infusion. Pefloxacin solution was adjusted to pH 11.0 by addition of a concentrated NaOH solution. Other FQs were dissolved in a minimum volume of 1 N NaOH, to which an equal volume of phosphate buffer (pH 7.4) was then added, and the pH was subsequently adjusted to 11.0 with 1 N HCl. The final FQ concentration was adjusted to 240 mM by addition of a 5% glucose solution. The actual concentration was then determined by high-performance liquid chromatography (HPLC) and was used for dose calculations. Drug administration was conducted between 2:00 and 6:00 p.m. The day after surgery, the jugular vein cannula was connected to a motor-driven syringe pump (model SE200B; Vial Inc.) containing the FQ solution for infusion at a rate of 960 μmol/h. Animals were kept under a heat lamp to maintain their body temperature. The infusion was stopped when the animals exhibited maximal seizures. Immediately thereafter, rats were anesthetized with an intramuscular injection of 12.5 mg of ketamine (Ketalar, 50 mg/ml; Parke Davis Laboratories) and 5 mg of xylazine hydrochloride (Rompin; Bayer Laboratories), unless they had died following maximal seizures. CSF and blood samples were collected in this order and within 3 to 5 min, as previously described (9). Blood was immediately centrifuged, and plasma was divided in two fractions, one of which was frozen at −20°C until assayed and the other of which was ultrafiltered with a centrifree system (model CF50A; Amicon, Epernon, France). We determined FQ concentrations in CSF, plasma, and plasma ultrafiltrate (UF) by HPLC, using a previously described methodology (9) with the following adjustments. Pipemidic acid was used as an internal standard (IS) for pefloxacin, fleroxacin, and norfloxacin assay, and pefloxacin was used for enoxacin analysis. No IS was used for sparfloxacin determinations. CSF and ultrafiltrate samples were injected directly after dilution into a mixture of 0.1 M citrate buffer (pH 3) plus an appropriate concentration of IS. Plasma samples were diluted by addition of a 1.7% (vol/vol) perchloric acid–IS mixture. Subsequently, the mixture was centrifuged and 20 μl of the supernatant was injected onto the column. Separation was performed with a Kromasil 100 C18 column (5 μm; 150 by 3 mm [inside diameter]). The mobile phase consisted of 0.1 M aqueous citric acid solution containing 13% (vol/vol) acetonitrile and 10 mM tetra butyl ammonium perchlorate, and the flow rate was 0.8 ml/min. The chromatographic system consisted of a model L 6000 pump (Merck) and a model 717 autosampler (Waters) connected to a model 470 fluorimetric detector (Waters). Excitation and emission wavelengths were, respectively, 280 and 445 nm for pefloxacin and norfloxacin, 287 and 440 nm for fleroxacin, and 268 and 400 nm for enoxacin. A Waters model 484 spectrophotometric detector was used for UV detection at 364 nm of sparfloxacin. Chromatographic data were recorded and processed with a Waters model 746 integrator. The limits of quantification in plasma were 1.30 μmol/liter for sparfloxacin and on the order of 0.15 μmol/liter for the other FQs. They were equal to 0.50 μmol/liter for sparfloxacin and 0.10 μmol/liter for the other FQs in UF and CSF. The percentage of error and the intraday coefficient of variation at the limits of quantification were, respectively, less than ±20 and 10%. Synaptic plasma membranes were prepared from the brains of Sprague-Dawley rats as previously described (1) with minor modifications. Brain cortices were homogenized with a potter mixer (Eurostar digital IKA; Labotechnik) in 10 volumes of ice-cold 0.32 M sucrose. The homogenate was centrifuged at 1,000 × g for 10 min, and the supernatant was centrifuged at 2,000 × g for 20 min. The resultant crude membrane pellet was suspended in 50 volumes of 50 mM Tris hydrochloride buffer (pH 7.1) by dispersion (Top-mix 11118; Bioblock) and was centrifuged at 48,000 × g for 20 min. The pellet was suspended in 20 volumes of a 0.05% Triton X-100 solution, incubated for 30 min at 37°C, and washed three times in 20 volumes of the buffer. The final suspension (2.5 mg of protein per ml) was kept frozen at −80°C for at the most 60 days before the binding assay. FQs were dissolved separately in 0.1 N NaOH, except for pefloxacin, which was provided as a salt and dissolved in water. The standard binding assay preparation (1 ml), which contained 100 μl of the membrane suspension, 100 μl of a methanolic solution of BPAA (10−4 M), 200 μl of [3H]muscimol (10 nM; specific activity, 8.1 Ci/mmol; Amersham), 100 μl of the FQ solution or 100 μl of a blank solution, and 500 μl of a 50 mM Tris hydrochloride buffer (pH 7.1), was incubated at 4°C for 30 min. The preparations were then quickly diluted by adding 4 ml of ice-cold buffer and were filtered through glass fiber filters (GF/C; Whatman). The filters were washed twice with 4 ml of the buffer and placed in vials containing 7.5 ml of counting scintillant (Pico-fluor 15; Packard), and cells were counted in a liquid scintillation counter (Tri-carb 2050CA; Packard). Specific binding was defined as the difference between the levels of binding observed in the presence and in the absence of a large excess (1 mM) of unlabeled GABA (Sigma Chemical Co.). Results are ratios of the levels of specific binding in the presence of FQs to those in their absence and are expressed as percentages (SB%). The inhibition data were submitted to nonlinear least-squares regression analysis by using WinNonlin. The following equation, in which Imax represents the maximum inhibitory effect, IC50 represents the concentration of an FQ (in moles per liter) producing 50% of the Imax, and γ represents the slope of the concentration-effect relationship, was fitted to the unweighted concentration data (C): SB% = 100 − [Imax · Cγ/(IC50 + Cγ)]. The Akaike information criterion and the F test were used to discriminate between the ordinary (γ = 1) and the sigmoid inhibitory (γ ≠ 1) models (14). The partition coefficients were determined as previously described (22) with slight modifications. FQs were dissolved at a concentration of 5 μg/ml in an aqueous solution of 0.1 N phosphate buffer (pH 7.4) saturated with n-octanol (Fluka Chemika). Two-milliliter aliquots of these solutions with individual FQs were added to an identical volume of n-octanol saturated with 0.1 N phosphate buffer. The mixture was continuously shaken at room temperature for 1 h, protected from light by wrapping the vials in aluminum foil, and subsequently centrifuged for 10 min at 1,000 × g. The FQ concentrations in the aqueous phase, before and after mixing with n-octanol, were assayed by HPLC as previously described. The apparent partition coefficient at pH 7.4 was calculated by dividing the concentration in the n-octanol phase (assessed by the difference in the aqueous phases before and after the partition) by those in the aqueous phases from six replicates and was expressed after decimal log (log D) transformation.
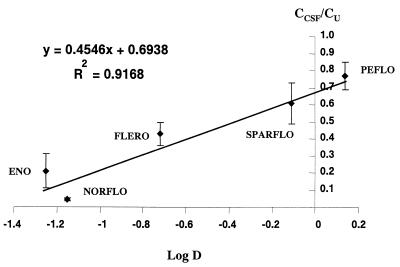
Maximum seizures were obtained after infusion times equal to 29.8 ± 2.9 min (norfloxacin), 42.3 ± 7.7 min (enoxacin), 17.2 ± 2.2 min (sparfloxacin), 19.0 ± 1.5 min (fleroxacin), and 27.7 ± 3.2 min (pefloxacin), with corresponding volumes of solution ranging between 1.1 ± 0.1 ml for sparfloxacin and 2.8 ± 0.5 ml for enoxacin. Most FQs produced generalized tonico-clonic convulsions; however, the tonic phase, which was very well characterized with some compounds, such as pefloxacin, was not always obvious and even absent on most occasions with other FQs, such as norfloxacin. Doses, total concentrations of FQs in plasma (Cp), concentrations of unbound FQs in plasma (Cu) and concentrations of the various FQs in CSF (CCSF), measured at the onset of maximum seizures, are presented on Fig. 1. The highest convulsant dose, observed for enoxacin (2,414 ± 301 μmol/kg), was about 2.5 higher on average than the lowest dose, observed for sparfloxacin (982 ± 129 μmol/kg), and the highest CCSF, observed for pefloxacin (316 ± 37 μmol/l), was more than fivefold higher than the lowest CCSF, observed for norfloxacin (59 ± 13 μmol/liter). Results of in vitro binding experiments demonstrated that FQs without a methyl (norfloxacin and enoxacin) had much greater affinities for the GABAA receptor sites in the presence of BPAA than those with one methyl (pefloxacin and fleroxacin) or two methyls (sparfloxacin) on the piperazine moiety, which is apparent from the IC50s reported in Table 1. Interestingly, γ values were always significantly less than unity, with only one exception, that for fleroxacin. The plot of CCSF/Cu ratios versus corresponding log D values shows a linear relationship between the two parameters, except possibly with norfloxacin (Fig. 2).
FIG. 1.
Doses (a), Cp (b), Cu (c), CCSF (d) of FQs at the onset of maximum seizures in rats. Each point and vertical bar represent means ± SDs (n = 5 to 8). NORFLO, norfloxacin; ENO, enoxacin; SPARFLO, sparfloxacin; FLERO, fleroxacin; PEFLO, pefloxacin.
TABLE 1.
Parameters characteristic of the affinities of FQs for GABAA receptors (IC50 and γ values), diffusion in CSF (CCSF/Cu ratios), whole-body distribution (dose/Cu ratios) at the onset of maximal seizures, and lipophilicity (log D values)
| FQ | IC50 (mol/liter) | γ value | Mean CCSF/Cu ratio ± SD | Mean dose/Cu ratio ± SD (liter/kg) | Mean log D value ± SD |
|---|---|---|---|---|---|
| Norfloxacin | 2.1 × 10−8 | 0.63 | 0.044 ± 0.010 | 1.67 ± 0.10 | −1.15 ± 0.06 |
| Enoxacin | 4.7 × 10−8 | 0.66 | 0.21 ± 0.10 | 3.35 ± 1.36 | −1.25 ± 0.04 |
| Sparfloxacin | 1.1 × 10−4 | 0.74 | 0.61 ± 0.12 | 4.67 ± 1.05 | −0.11 ± 0.07 |
| Fleroxacin | 1.4 × 10−4 | 1a | 0.43 ± 0.07 | 2.15 ± 0.37 | −0.72 ± 0.18 |
| Pefloxacin | 1.1 × 10−4 | 0.61 | 0.77 ± 0.08 | 3.43 ± 0.56 | 0.14 ± 0.05 |
Not significantly different from unity.
FIG. 2.
Relationship between CCSF/Cu ratios and log D values. Each point and vertical bar represent means ± SDs (n = 5 to 8). The solid line was obtained by linear regression analysis. ENO, enoxacin; NORFLO, norfloxacin; FLERO, fleroxacin; SPARFLO, sparfloxacin; PEFLO, pefloxacin.
The experimental approach initially used and validated with pefloxacin and norfloxacin to investigate their convulsant activities in vivo (9) was extended to a larger number of representative FQs. In order to detect structure-activity relationships, these compounds were selected because of their differences in physical properties (lipophilicity) and chemical structures (the nature of the heterocycle, the presence or absence of a methyl group at position 4 of the piperazine moiety, and also the presence of two methyls at the 3,5 position), which are presumably responsible for differences in convulsant activities. The main advantages of the approach used in this study, over those of other strategies, are that the convulsant effects of FQs alone are investigated in vivo and that it is possible to gather information on both the pharmacokinetic and pharmacodynamic contributions to the overall effect. However several potential problems, including some theoretical limits previously mentioned, essentially acute tolerance development, and the presence of metabolites in the biophase at the onset of effect, may compromise the applicability of this approach (9). From a theoretical standpoint, racemate drugs such as ofloxacin are not good candidates for this type of study, as well as for traditional in vitro binding investigations, since they correspond to a mixture of two compounds with possibly different characteristics. Practical problems may also be encountered with the in vivo approach, because in order to provoke seizures, FQs must be infused at relatively high concentrations. This is easily achievable when salts are commercially available (pefloxacin) or can be easily prepared (norfloxacin) (9). However, our attempts to make salts of FQs other than norfloxacin were unsuccessful, and because of the very low solubilities of FQs at physiological pH, basic solutions (pH 11) had to be infused during this study. A quick experiment was conducted in order to verify that this relatively high pH value did not introduce any bias in the results. We observed that, at least with pefloxacin and norfloxacin, data obtained with a solution of a salt at pH 5.5, as in the initial study (9), or a solution of the base at pH 11, as in the present study, were not or were only slightly different (data not shown) and were within the range of usual interoccasion variability (9). However, this nonphysiological pH may have been responsible for the tolerability problems observed in a preliminary experiment with ciprofloxacin, which for that reason could not be included in the main study. The contribution of the pharmacodynamics of FQs to their overall convulsant activities, previously defined as intrinsic convulsant activities (9), can be assessed from their CCSFs at the onset of activity. However, this assumes that CCSF and concentrations in the brain extracellular fluid are the same for all FQs or at least that the ratios of the two concentrations are identical for the various FQs compared. This may not always be true, although CSF is part of the biophase. These results are interesting to compare with the results of the in vitro binding experiments in the presence of BPAA. In general agreement with previously published data (9, 10), the CCSF of norfloxacin was 5.4-fold less than that of pefloxacin, meaning that the intrinsic convulsant activity of norfloxacin was on average 5.4-fold greater than that of pefloxacin. By comparison, the corresponding IC50 varied within a 104 order of magnitude (from 2.1 × 10−8 for norfloxacin to 1.1 × 10−4 for pefloxacin). Such an important difference observed in vitro seems to have no real meaning and to be essentially useless for in vivo extrapolation. Similarly, a difference by a factor of 2 between the IC50s of norfloxacin and enoxacin is probably insignificant whereas the same relative difference between the corresponding CCSF would reflect a real difference. However, in agreement with previously published data (1), it is possible to conclude from our in vitro binding experiments that the two compounds without a methyl group on the piperazine moiety (norfloxacin and enoxacin) demonstrate a much greater affinity for GABAA receptors in the presence of BPAA than the compounds bearing one methyl group (fleroxacin and pefloxacin) or two methyl groups (sparfloxacin) on the piperazine moiety. The protective role of the methyl group is best assessed from a comparison of pefloxacin and norfloxacin data, as was previously done (9), since this methyl group status is the only structural difference between these two compounds. Because the CCSFs of these two compounds are at the two extremes, it can be concluded that the methyl group on the piperazine moiety is of primary importance for determining the convulsant activities of FQs. However, a comparison of CCSFs shows a difference between norfloxacin and enoxacin, which would be difficult to assess from binding experiments for reasons previously discussed. This difference shows that the 4-oxo-naphtyridine heterocycle of enoxacin tends to reduce the convulsant activity of norfloxacin, which possesses the same chemical structure as enoxacin except for a quinolone ring. A major discrepancy between the in vitro and the in vivo results was observed with sparfloxacin. This compound with two methyls on its piperazine moiety has a low affinity for GABAA receptors in the presence of BPAA; in particular, its affinity is much lower than that of enoxacin. However, CCSFs and therefore the intrinsic convulsant activities in vivo of these two compounds were virtually identical. Furthermore, because of a greater CSF permeability, the convulsant dose of sparfloxacin was less than that of enoxacin (Fig. 2). Discrepancies between in vitro and in vivo data may have several origins. One possible explanation is that GABAA is not solely responsible for the convulsant activities of FQs but that other mediators such as glutamate and adenosine may also be involved (11, 12, 31). It is also possible that the relative contribution of each type of mediator is not the same for all FQs, which possibly explains the differences observed in the types of seizures. Another possible explanation for the discrepancies between in vitro and in vivo results is the presence of BPAA, which does not necessarily exacerbate GABAA affinity in similar ways for all the various FQs. Such distortion can be suspected from data in the literature suggesting that the estimated IC50s for norfloxacin and enoxacin were identical in the presence but not in the absence of BPAA (1). The contribution of the pharmacokinetics of FQs to their convulsant activities can be characterized by two ratios. The CCSF/Cu ratio, which has no units, reflects the ability of drugs to diffuse into the CSF. As previously discussed (10), this ratio must be interpreted carefully because it may depend upon the duration of infusion, which varied from 17.2 ± 2.2 to 42.3 ± 7.7 min, on average, for sparfloxacin and enoxacin, respectively. However, we have previously shown that the CCSF/Cu ratio of the most lipophilic FQ, pefloxacin, varied only from 0.74 ± 0.06 to 0.83 ± 0.07 (not significant) when the duration of infusion increased from 12.7 ± 1.4 to 61.0 ± 6.7 min (9). The effect of duration of infusion was much more pronounced in relative terms, and results were statistically different, with the more hydrophilic norfloxacin, with CCSF/Cu ratios ranging from 0.038 ± 0.008 to 0.063 ± 0.025 when the duration of infusion was increased from 12.9 ± 2.3 to 69.4 ± 8.9 min. However, the intercompound differences are likely to be much more important than the effect of duration of infusion. For that reason the relationship between CCSF/Cu ratios and log D values was analyzed. The CCSF/Cu ratios estimated for the five tested FQs ranged from 4.4% for norfloxacin to 77% for pefloxacin and were linearly related to their log D values, that is, to their lipophilicity, with the possible exception of that for norfloxacin (Fig. 2). It can therefore be concluded that, although the very low CCSF/Cu ratios observed with the most hydrophilic FQs suggest that some active efflux transport system may exist at the choroid plexus (26) or/and blood-brain barrier (27), lipophilicity remains the major factor controlling the net diffusion of FQs into CSF (18). However, more thorough investigations of the diffusion of these FQs into CSF are necessary to confirm this hypothesis. In particular, attention should be paid to the nonlinear diffusion of the more hydrophilic FQs into CSF (21). Considering that infusion times were short compared to the elimination half-lives of FQs, the second ratio, dose/Cu, essentially reflects drug distribution in the whole body at the onset of activity. However, for most FQs the distribution process was probably not completed by the end of infusion and therefore dose/Cu ratios must also be interpreted very carefully. However, it is interesting that, unlike CCSF/Cu ratios, dose/Cu ratios do not vary much between FQs (Table 1), suggesting that FQs vary much more in their ability to diffuse within CSF than in their distribution characteristics in general. It is also interesting that FQs with the highest intrinsic convulsant activities have limited diffusion within the CSF and that those with greater diffusion have reduced convulsant activities. It is therefore possible to conclude that, at least for the five compounds tested in this study, the contributions of pharmacokinetics and pharmacodynamics to convulsant activity have offsetting effects, which explains why CCSFs vary by more than 5-fold between compounds although convulsant doses vary in only a 2.5-fold range (Fig. 1).
In conclusion, the estimation of the CCSFs of various FQs at the onset of maximal seizures provided sufficient information to detect structural characteristics responsible for the increased convulsant activities of FQs, which could not always be assessed by the traditional approach with GABAA binding experiments in the presence of BPAA. Therefore, this in vitro approach may not be a reliable indicator of the convulsant risk associated with FQ administration alone. The in vivo approach has also shown that both central diffusion and affinity for the receptors responsible for the epileptogenic activities of FQs vary considerably from one compound to another and therefore that each of these two factors must be considered for prediction of convulsant activity in vivo. Interestingly, among the five FQs tested, compounds with extensive CSF diffusion presented relatively limited intrinsic convulsant activity and those with reduced CSF diffusion presented greater pharmacodynamic activity. When applicable, the in vivo approach used in this study seems to be very valuable in assessing the convulsant activities of new FQs at the early stage of their preclinical development and should be recommended to provide complementary data to those obtained by various appropriate in vitro approaches (24, 31).
Acknowledgments
We acknowledge Roche and Rhône Poulenc Rorer for supplying fleroxacin and sparfloxacin.
REFERENCES
- 1.Akahane K, Sekiguchi M, Une T, Osada Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with γ-aminobutyric acid receptor sites. Antimicrob Agents Chemother. 1989;33:1704–1708. doi: 10.1128/aac.33.10.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akahane K, Tsutomi Y, Kimura Y, Kitano Y. Levofloxacin, an optical isomer of ofloxacin, has attenuated epileptogenic activity in mice and inhibitory potency in GABA receptor binding. Chemotherapy. 1994;40:412–417. doi: 10.1159/000239301. [DOI] [PubMed] [Google Scholar]
- 3.Akaike N, Shirasaki T, Yakushiji T. Quinolones and fenbufen interact with GABAA receptors in dissociated hippocampal cells of the rat. J Neurophysiol. 1991;66:497–504. doi: 10.1152/jn.1991.66.2.497. [DOI] [PubMed] [Google Scholar]
- 4.Anastasio G D, Menscer D, Little J M. Norfloxacin and seizures. Ann Intern Med. 1988;109:169–170. doi: 10.7326/0003-4819-109-2-169. [DOI] [PubMed] [Google Scholar]
- 5.Arcieri G, Griffith E, Gruenwaldt G, Heyd A, O’Brien B, Backer N, August R. Ciprofloxacin: an update on clinical experience. Am J Med. 1987;82(Suppl. 4A):381–386. [PubMed] [Google Scholar]
- 6.Christ W. Central nervous system toxicity of quinolones: human and animal findings. J Antimicrob Chemother. 1990;26(Suppl. B):219–225. doi: 10.1093/jac/26.suppl_b.219. [DOI] [PubMed] [Google Scholar]
- 7.Danhof M, Levy G. Kinetics of drug action in disease states. I. Effect of infusion rate on phenobarbital concentrations in serum, brain and cerebrospinal fluid of normal rats at onset of loss of righting reflex. J Pharmacol Exp Ther. 1984;229:44–50. [PubMed] [Google Scholar]
- 8.Dellamonica, P., and B. Dunais. 1996. Tolerability of fluoroquinolones: focus on pefloxacin. 11(Suppl.2):36–42.
- 9.Delon A, Huguet F, Courtois P, Vierfond J M, Bouquet S, Couet W. Pharmacokinetic-pharmacodynamic contributions to the convulsant activity of pefloxacin and norfloxacin in rats. J Pharmacol Exp Ther. 1997;280:983–987. [PubMed] [Google Scholar]
- 10.Delon A, Pariat C, Courtois P, Bouquet S, Couet W. A new approach for early assessment of the epileptogenic potential of quinolones. Antimicrob Agents Chemother. 1998;42:2756–2758. doi: 10.1128/aac.42.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sarro G, Nava F, Calapai G, De Sarro A. Effects of some excitatory amino acid antagonists and drugs enhancing γ-aminobutyric acid neurotransmission on pefloxacin-induced seizures in DBA/2 mice. Antimicrob Agents Chemother. 1997;41:427–434. doi: 10.1128/aac.41.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd P R, Davies L P, Watson W E, Nielsen B, Dyer J A, Wong L S, Johnston G A R. Neurochemical studies on quinolone antibiotics: effect on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol. 1989;64:404–411. doi: 10.1111/j.1600-0773.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Domagala J M. Structure-activity and structure-side effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 14.Gabrielson J, Weimer D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 2nd ed. Stockholm, Sweden: Swedish Pharmaceutical Press; 1997. pp. 588–603. [Google Scholar]
- 15.Green M A, Halliwell R F. Selective antagonism of the GABAA receptor by ciprofloxacin and biphenylacetic acid. Br J Pharmacol. 1997;122:584–590. doi: 10.1038/sj.bjp.0701411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasbun R, Quagliarello V J. The quinolones. 2nd ed. New York, N.Y: Academic Press, Inc.; 1998. Use of the quinolones in the treatment of bacterial meningitis; pp. 287–301. [Google Scholar]
- 17.Hooper D C, Wolfson J S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985;28:716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa N, Naora K, Iwamoto K. Comparative study of permeability into rat cerebrospinal fluid of the quinolones: dependency on their lipophilicities. Biol Pharm Bull. 1994;17:152–155. doi: 10.1248/bpb.17.152. [DOI] [PubMed] [Google Scholar]
- 19.Imanishi T, Akahane K, Akaike N. Attenuated inhibition by levofloxacin, 1-isomer of ofloxacin, on GABA response in dissociated rat hippocampal neurons. Neurosci Lett. 1995;193:81–84. doi: 10.1016/0304-3940(95)11670-r. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Yano T, Kumiko T, Inui K. Transport of quinolone antibacterial drugs by human-P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J Pharmacol Exp Ther. 1997;282:955–960. [PubMed] [Google Scholar]
- 21.Jaehde U, Langemeijer M W E, de Boer A G, Breimer D D. Cerebrospinal fluid transport and disposition of the quinolones ciprofloxacin and pefloxacin in rats. J Pharmacol Exp Ther. 1992;263:1140–1146. [PubMed] [Google Scholar]
- 22.Jaehde U, Goto T, de Boer A G, Breimer D D. Blood-brain barrier transport rate of quinolone antibacterials in cerebrovascular endothelial cell cultures. Eur J Pharm Sci. 1993;1:49–55. [Google Scholar]
- 23.Janknegt R. Fluorinated quinolones: a review of their mode of action, antimicrobial activity, pharmacokinetics and clinical efficacy. Pharm Weekbl. 1986;8:1–21. doi: 10.1007/BF01975473. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami J, Yamamoto K, Asanuma A, Yanagisawa K, Sawada Y, Iga T. Inhibitory effect of new quinolones on GABAA receptor-mediated response and its potentiation with felbinac in Xenopus oocytes injected with mouse-brain mRNA: correlation with convulsive potency in vivo. Toxicol Appl Pharmacol. 1997;145:246–254. doi: 10.1006/taap.1997.8137. [DOI] [PubMed] [Google Scholar]
- 25.Levasseur L M, Delon A, Greco W R, Faury P, Bouquet S, Couet W. Development of a new quantitative approach for the isobolographic assessment of the convulsant interaction between pefloxacin and theophylline in rats. Pharm Res. 1998;15:1069–1076. doi: 10.1023/a:1011938429379. [DOI] [PubMed] [Google Scholar]
- 26.Ooie T, Suzuki H, Terasaki T, Sugiyama Y. Characterization of the transport properties of a quinolone antibiotic, fleroxacin, in rat choroid plexus. Pharm Res. 1996;13:523–527. doi: 10.1023/a:1016081618149. [DOI] [PubMed] [Google Scholar]
- 27.Ooie T, Terasaki T, Suzuki H, Sugiyama Y. Kinetic evidence for active efflux transport across the blood-brain barrier of quinolone antibiotics. J Pharmacol Exp Ther. 1997;283:293–304. [PubMed] [Google Scholar]
- 28.Ramzan I M, Levy G. Kinetics of drug action in disease states. XIV. Effect of infusion rate on pentylentetrazol concentrations in serum, brain and cerebrospinal fluid of rats at onset of convulsions. J Pharmacol Exp Ther. 1985;234:624–628. [PubMed] [Google Scholar]
- 29.Ramzan I M, Levy G. Kinetics of drug action in disease states. XVI. Pharmacodynamics of theophylline-induced seizures in rats. J Pharmacol Exp Ther. 1986;236:708–713. [PubMed] [Google Scholar]
- 30.Scheld W M. Quinolone therapy for infections of the central nervous system. Rev Infect Dis. 1989;11(Suppl.5):S1194–S1202. doi: 10.1093/clinids/11.supplement_5.s1194. [DOI] [PubMed] [Google Scholar]
- 31.Schmuck G, Schurmann A, Schlüter G. Determination of the excitatory potencies of quinolones in the central nervous system by an in vitro model. Antimicrob Agents Chemother. 1998;42:1831–1836. doi: 10.1128/aac.42.7.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segev S, Rehavi M, Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the γ-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988;32:1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson K J, Brodie M J. Convulsions related to enoxacin. Lancet. 1985;ii:161. doi: 10.1016/s0140-6736(85)90270-3. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji A, Sato H, Kume Y, Tamai I, Okezaki E, Nagata O, Kato H. Inhibitory effects of quinolone antibacterial agents on γ-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988;32:190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]