Abstract
Escherichia coli (E. coli) is a Gram-negative bacterium that belongs to the family Enterobacteriaceae. While E. coli can stay as an innocuous resident in the digestive tract, it can cause a group of symptoms ranging from diarrhea to live threatening complications. Due to the increased rate of antibiotic resistance worldwide, the development of an effective vaccine against E. coli pathotypes is a major health priority. In this study, a reverse vaccinology approach along with immunoinformatics has been applied for the detection of potential antigens to develop an effective vaccine. Based on our screening of 5,155 proteins, we identified lipopolysaccharide assembly protein (LptD) and outer membrane protein assembly factor (BamA) as vaccine candidates for the current study. The conservancy of these proteins in the main E. coli pathotypes was assessed through BLASTp to make sure that the designed vaccine will be protective against major E. coli pathotypes. The multitope vaccine was constructed using cytotoxic T lymphocyte (CTL), helper T lymphocyte (HTL), and B cell lymphocyte (BCL) epitopes with suitable linkers and adjuvant. Following that, it was analyzed computationally where it was found to be antigenic, soluble, stable, and non-allergen. Additionally, the adopted docking study, as well as all-atom molecular dynamics simulation, illustrated the promising predicted affinity and free binding energy of this constructed vaccine against the human Toll-like receptor-4 (hTLR-4) dimeric state. In this regard, wet lab studies are required to prove the efficacy of the potential vaccine construct that demonstrated promising results through computational validation.
Keywords: Escherichia coli, reverse vaccinology, immunoinformatics, epitope mapping, multitope vaccine
Introduction
According to its pathogenicity in humans, E. coli can be classified into an extraintestinal infection-causing E. coli (primarily uropathogenic E. coli, UPEC, and neonatal meningitis E. coli, NMEC) and strains that cause enteric infections (divided into 6 pathotypes; enteropathogenic E. coli [EPEC], enterohemorrhagic E. coli [EHEC], enteroaggregative E. coli [EAEC], enteroinvasive E. coli [EIEC], enterotoxigenic E. coli [ETEC] and diffusely adherent E. coli [DAEC]). Additionally, two further pathotypes have emerged recently; adherent invasive E. coli [AIEC] that is predicted to be associated with Crohn's disease and does not produce any toxins, while the other pathotype, Shiga toxin-producing enteroaggregative E. coli [STEAEC], causes food poisoning due to the action of Shiga toxin and was reported to be the causative agent for the 2011 Germany E. coli outbreak (1). People can be easily infected with E. coli by swallowing a small amount of it in water, vegetables, or meat where the fecal-oral route is the major way of transmission (2). Another common way of categorizing E. coli is by serotype through detection of surface antigens O and H. Currently, more than 190 serogroups have been identified (3).
Currently, pathogenic E. coli is a major public health concern because of possessing a low infectious dose and simple transmission through food and water (4). Excessive usage of different classes of antibiotics, especially in cases that do not require this form of treatment, contributed largely to the appearance of antibiotic resistance in bacterial strains that were previously sensitive even for the traditional classes of antibiotics (5). Antibiotic resistance of E. coli is extensively studied for two major reasons; firstly, it represents the most common infective Gram-negative pathogen for humans (6), secondly, resistant E. coli can affect other bacteria in the gastrointestinal tract through transferring antibiotics resistance determinants (7). The resistance rates are demonstrating continuous inclement throughout the last few years (8). An alternative solution to fight against this pathogen is designing an effective vaccine against its common pathotypes. While this solution was adopted by many research groups around the world, there is no officially approved vaccine against pathogenic E. coli in the market (9).
Many trials have been performed to generate an effective vaccine for various E. coli pathotypes. Regarding intestinal E. coli, Shiga toxin-based vaccine (10), attenuated bacteria-based vaccine (11), and polysaccharide-based vaccine (12) have been proposed for EHEC while autotransporter-based vaccine (13) and adhesion based vaccine (14) were proposed for ETEC. Moving to extraintestinal E. coli, capsular-based vaccine (15) and iron scavenger receptors-based vaccine (16) were designed. The previous approaches for designing an effective vaccine are based mainly on studying the virulence properties for each pathotype of E. coli and as we mentioned above none of these vaccines have been approved by FDA yet. In the current study, we planned to merge two advanced approaches, reverse vaccinology and immunoinformatics for a vaccine design where these approaches would be applied on conserved protein candidates in various E. coli pathotypes to generate a vaccine with possible cross-reactivity.
Reverse vaccinology is a novel approach for vaccine designing that demonstrated a great development in the last few years as a result of the revolution in sequencing techniques and the data availability in highly organized databases. Additionally, computational tools that can deal with this large amount of data and translate it to valuable outcomes that can be used in applied research also showed great progress (17). This approach for vaccine design has been utilized for a protein-based vaccine design against viral, bacterial, and fungal infectious agents (18–20). Moreover, it was also applied to design an effective vaccine for animals against ETEC, a common pathotype of E. coli that leads to post-weaning diarrhea in pigs (21). Recent studies showed that multitope vaccines had superiority over single-component ones regarding their effectiveness (22). In the current study, we aim to investigate the whole proteome of E. coli and select potential protein candidates through the reverse vaccinology approach. Following that, immunogenic epitopes from these candidates would be mapped and gathered to initiate a multitope vaccine that would be assessed computationally for its, physicochemical, chemical, and immunological characteristics to be nominated as a potential vaccine candidate against pathogenic E. coli.
Materials and Methods
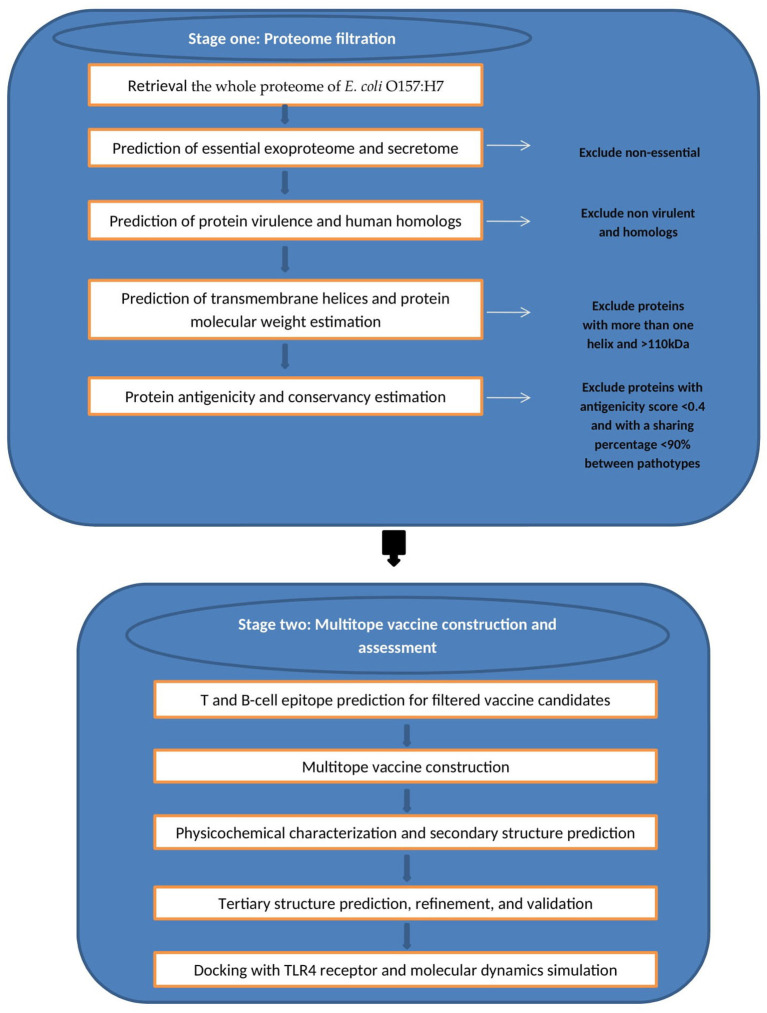
We divided the current study into two main stages (Figure 1) wherein the first stage we handled the proteome of E. coli with successive filtration steps of reverse vaccinology approach to nominate protein candidates for vaccine design while in the second stage we constructed, analyzed, and assessed the multiepitope vaccine that was created based on B and T cell epitopes of protein candidates from the first stage.
Figure 1.
Graphical representation of the methodology employed for the multi-epitope vaccine design against E. coli.
Data Retrieval
The amino acid sequences of the whole proteome of E. coli O157:H7 str. Sakai was retrieved with Genbank assembly accession No: GCF_000008865.2. This strain was selected as it represents the reference pathogenic E. coli strain on NCBI.
Prediction of Essential Exoproteome and Secretome
Living organisms cannot survive without their essential genes (23). Therefore, the first filtration step was detecting these genes using Geptop 2.0, where a list of essential proteins was detected out of 5,155 proteins (the complete proteome of the studied strain) (24). The default essentiality score cutoff (0.24) was used for this process. Following that, PSORTb v3.0.2 online server (25) was employed to predict the subcellular localization of the filtered genes. The overall result of this filtration phase was a list of exoproteome and secretome essential proteins.
Prediction of Virulence Protein
Essential exoproteome and secretome were applied to virulenpred (26) to estimate the virulence potential.
Determination of Human Homologs
Protein list from the above stage was analyzed against human proteome on NCBI using BLASTp to check for human homologs, and those with ≥35% identity were removed from our list.
Prediction of Transmembrane Helices and Protein Molecular Weight Estimation
The presence of transmembrane helices and proteins' molecular weight estimation were detected through TMHMM (27), and Expasy tool (28), respectively. Those with molecular weight <110 kDa and had ≤1 transmembrane helix were selected for antigenicity assessment in the next step (29).
Protein Antigenicity Analysis
Protein Antigenicity was detected by Vaxijen V 2.0 (30), where proteins with antigenicity score more than 0.4 were considered antigenic.
Analysis of Protein Conserved Identity With Various E. coli Pathotypes
Recommended proteins from previous steps were assessed for their conservation among 10 E. coli strains where BLASTp analysis was performed for each protein against a representative strain of each E. coli pathotype to confirm that the designed vaccine will have the ability to act against any pathogenic E. coli which is a major aim of this study. Proteins with a sharing percentage <90% were eliminated and considered not conserved.
T-Cell and B-Cell Epitope Prediction
After completing the whole reverse vaccinology filtration steps, successful candidates were transferred to the epitope mapping stage where the filtered proteins were submitted to SignalP-5.0 Server to anticipate the location of signal peptides. Moving to the next step, the mature polypeptides were mapped for B and T cell epitopes through the online server of the Immune Epitope Database (IEBD) (31). The first epitopes to be predicted were CTLs, which were anticipated against HLA allele reference set that provided more than 97% in terms of population coverage (32). Secondly, HTLs were also predicted against HLA reference set to cover more than 99% in terms of population coverage (33). Moreover, HTL epitopes were analyzed for their ability to induce γ-INF vs. other cytokines through the webserver (http://crdd.osdd.net/raghava/ifnepitope/), IL-4 induction ability was estimated through the webserver (https://webs.iiitd.edu.in/raghava/il4pred/), while IL-10 induction ability was assessed through the webserver (https://webs.iiitd.edu.in/raghava/il10pred/). For both HTLs and CTLs, the affinity of the filtered peptides toward respective alleles was assessed where the peptide 3D structure was estimated via PEP FOLD 3 webserver (34); concurrently, the 3D structure of HLA-B*44:03 (PDB ID 4JQX) and HLA-DRB1*04:01 (PDB ID 5JLZ) was retrieved from the protein data bank to act as receptors for MHC-I and MHC-II epitopes, respectively, and the docking study was performed through AutoDock Vina (35). The final assessment for HTLs and CTLs was the conservancy prediction where the web-based tool from IEDB analysis resources was employed for this process (36). The last set of epitopes, BCLs were finally estimated through IEBD. After prediction, several characteristics for every single epitope were predicted to nominate the best candidates that would be used for the multitope construct. These characteristics were the population coverage, conservancy profile, antigenicity score, allergenicity, and toxicity probabilities; where IEDB analysis tools, Vaxijen, AllerTOP, and ToxinPred were employed, respectively, for these assessments.
Multitope Vaccine Construction
In order to construct a potential multitope vaccine, the best six candidates of CTL, HTL, and BCL epitopes from the step of epitope mapping were linked through GGGS, GPGPG, and KK amino acid linkers, respectively, where these linkers were incorporated to provide in vivo separation of the assembled epitopes (37). Apart from the epitopes, PADRE sequence and β defensin adjuvant were added to finalize the designed vaccine construct. This potential vaccine construct was assessed for its immunogenicity score, allergenicity, and toxicity probabilities through the same servers that were employed previously for single epitopes estimation.
Physicochemical Features, Protein Solubility Assessment, and Secondary Structure Prediction
ProtParam, a tool available on Expasy server (28) was employed to assess the physicochemical properties of the designed potential vaccine. The propensity upon overexpression in E. coli and the protein secondary structure of the multitope construct were anticipated via SOLpro server (38) and PSIPRED 4.0 webserver (39) respectively.
Tertiary Structure Prediction, Refinement, and Validation
It is essential to predict the potential vaccine 3D structure to be able to assess its binding with a toll-like receptor. The current study utilized 3Dpro webserver (40) for this purpose. Computational estimation of a protein tertiary structure is performed through a molecule bending and twisting to create a structure with the least possible energy and maximum state of stability where the employed server runs this process via an analysis of the structural similarity between the protein sequence under prediction and the data available on PDB. Following tertiary structure prediction, GalaxyRefine server (41) was employed to perform refinement for the generated structure in terms of stability and protein-energy, and the enhanced structures vs. the original one were assessed by Ramachandran plot analysis (42) and ProSA (43).
Conformational B-Cell Epitope Prediction
The conformational B-cell epitopes were analyzed for the multitope design after 3D structure prediction and refinement. The ElliPro Server (http://tools.iedb.org/ellipro), which is a reliable tool for detection of B cell epitopes against a specific antigen, was employed for this prediction (44).
Disulfide Engineering of the Designed Vaccine
Prior to initiating a docking study for the constructed potential vaccine, it was recommended to enhance the stability of the 3D format of the designed construct. Disulfide bonds were proved to enhance protein geometric conformation and consequently elevate its stability. Disulfide by Design 2.0 (45) was assigned for this process.
Docking of Designed Vaccine With hTLR-4
The current study employed molecular docking as a prediction tool for assessment of preferred orientation of the ligand, the current study vaccine construct, to its corresponding receptor and estimate the binding affinity (46). Inflammations triggered by E. coli are involved mainly with TLR-4 (47). Hence, the hTLR-4 (PDB id: 4G8A) was chosen as a receptor for the potential epitope-based constructed vaccine, the ligand, and ClusPro 2.0 server (48) was utilized to run this docking study. This server predicts the best docking models by performing billions of conformations, clustering of the 1,000 lowest energy structures generated and removing steric clashes. The 2 PDB files were uploaded to ClusPro server and the docking process was performed using default parameters.
Dihedral Coordinate-Based Normal-Mode Analyses
The iMODS server was employed to investigate and analyze the collective flexibility as well as motion functions of the constructed epitope vaccine in relation to the bound hTLR-4 protein target since the latter server possesses the advantage of being fast and efficient (http://www.imods.chaconlab.org/) (49). This server can predict several values such as eigenvalues which reflected a harder deformation when this value is high (50). The atoms and residues of both the bound hTLR-4 protein and epitope vaccine ligand were continuously indexed where atoms of number range 1–9,567 and 9,568–15,225 were, respectively, assigned for the epitope-bound hTLR-4 (the initial 27–627 amino acids) and epitope vaccine itself (the following 1–380 amino acids).
Molecular Dynamics Simulations
The molecular dynamics computational approach was applied to describe the epitope/hTRL-4 molecular behaviors in addition to measuring the stability of such protein-protein complex (51). The hTLR-4 can exist in a monomeric state as well as m-shaped dimeric architecture where the latter is prior to the initiation of downstream signal transduction (52, 53). Additionally, reported crystalline hTLR-4 showed a significant N-glycosylation profile with several oligosaccharide moieties being linked to their surface at conserved residues (54, 55). In these regards, four docked hTLR-4/epitope vaccine complexes were investigated for the impact of glycosylation and/or hTLR-4 oligomerization state on the binding affinity as well as the thermodynamic stability of the constructed epitope vaccine. Simulated complexes were dimeric or monomeric hTLR-4 states being either glycosylated or not (Glycosylated dimer = GlyDim; Sole dimer = SolDim; Glycosylated monomer = GlyMon, Sole monomer = SolMon). Each of the latter adopted models were individually subjected to 100 ns explicit molecular dynamics runs using GROMACS-2019 (http://www.gromacs.org/) (56) and under CHARMM36m forcefield for the protein simulations (57–60). Under periodic boundary conditions, the TIP3P water model 3D-box was used to solvate the investigated protein-protein complex model with 10 Å marginal distances (61). The standard ionization state of both protein amino acids was assigned under physiological pH 7.4, whereas the whole constructed system charge was via sufficient chloride and potassium ion numbers introduced through Monte-Carlo ion-placement method (62). The atomic counts of the four constructed models were 438642, 424612, 242818, and 237898 atoms for GlyDim; SolDim; GlyMon, and SolMon, respectively.
The constructed system was minimized throughout 5 ps using a steepest-descent algorithm (63), and subsequently subjected to double-staged equilibration for 100 ps/stage under a constant number of particles, Volume, and Temperature (NVT; 303.15 K, Berendsen temperature coupling regulation) and a constant number of particles, Pressure, and Temperature (NPT; 303.15 K and 1 atm. Pressure, Parrinello-Rahman barostat regulation) ensembles for the first and second stage, respectively. Throughout both minimization and equilibration stages, the original protein foldings were preserved and all heavy atoms were restrained at 1,000 kJ/mol.nm2 force constant. Finally, the minimized/equilibrated systems were produced through 100 ns explicit molecular dynamics runs under NPT ensemble using Particle-Mesh Ewald algorithm for long-range electrostatic interactions computation. Linear constraint LINCS method modeled the covalent bond lengths at 2 fs integration time step sizes (64). van der Waals and Coulomb's non-bounded interactions were truncated at 10 Å via Verlet cut-off schemes (65). The MD simulations were performed using Aziz® Supercomputer (King Abdulaziz University's High-Performance Computing Center), via 5 nodes with 24 CPUs/node and 8 MPI processes/node. Adopted nodes run CentOS-6.4 with dual Intel® E5-2695v2 (24 cores/node; i.e. 2.4 GHz 12 Cores) offering 96 GB Memory/node. The MD simulation of the dimer hTLR-4 states took ~228 and 218 h for the glycosylated and non-glycosylated proteins, respectively. While as the monomeric states took nearly 132 and 128 h for the glycosylated and non-glycosylated proteins, respectively.
The trajectory-oriented analytical parameters; root-mean-square deviation (RMSD), RMS-fluctuation (RMSF), radius of gyration (Rg), and solvent-accessible surface area (SASA) were computed through molecular dynamics trajectory analysis using GROMACS built-in analytical scripts. The free binding energies, as well as residue-wise energy contribution between hTLR-4 protein target and epitope vaccine ligand, were estimated via Molecular Mechanics/Poisson-Boltzmann Surface Area (MM/PBSA) using GROMACS/g_mmpbsa scripts (66). The SASA-only model of the free-binding energy (ΔGTotal = ΔGMolecularMechanics + ΔGPolar + ΔGApolar) was used across the whole 100 ns molecular dynamics runs. Important MM/PBSA parameters for polar/solvation calculations were set at 1.40 Å solvent probe radius, 80 pdie solvent dielectric constants, 1 vdie standard vacuume, and 2 pdie solute dielectric constants. Regarding only-SASA non-polar solvation; 1.40 Å SASA solvent probe radius, 0.0227 kJ/mol.Å2 solvent surface tension, and 3.8493 kJ/mol offset constant, were used. Finally, parameters for the continuum-integral-based model were set as 1.25 Å solvent probe radius, 200 quadrature points/Å2, and 0.0334 Å3 bulk solvent density. Representing ligand-protein conformations at specific timeframes was done via Schrödinger-Pymol V.2.0.6 graphical package.
Immune Simulation of the Designed Vaccine
The C-ImmSim server (67) was employed to predict the stimulated immune response against the designed vaccine through a computational approach. We followed the technique of prime—booster—booster for this investigation and that was achieved by injecting the designed vaccine three times with 4 weeks intervals. This approach was applied to obtain a long-lasting immune response.
Results
The main output of the filtration steps that have been applied in the current study to define potential candidates are shown in Figure 2.
Figure 2.
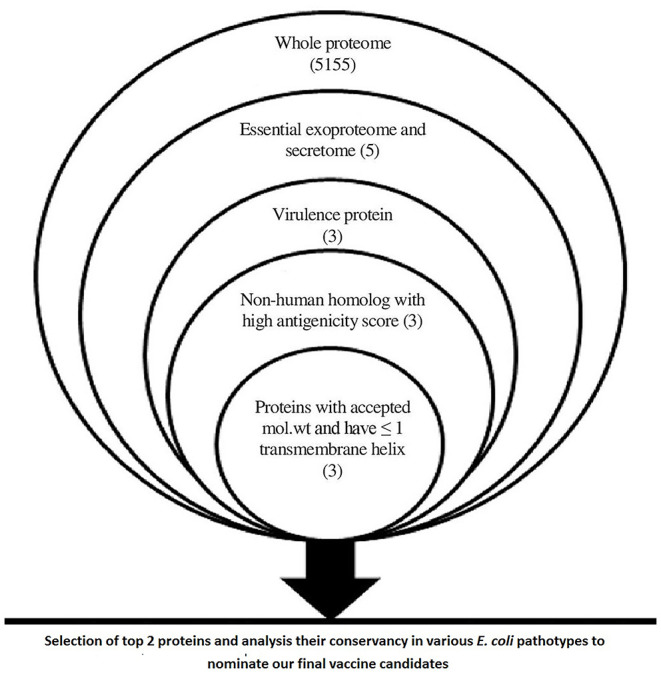
A chart summarizing applied filtration steps for the nomination of potential vaccine candidates by applying in silico reverse vaccinology technique.
Proteome Analysis for Selection of Vaccine Candidates
Data within Figure 2 demonstrates the results of filtration steps of E. coli O157:H7 str. Sakai proteome where three proteins (lipopolysaccharide assembly protein LptD, outer membrane protein assembly factor BamA, and lipopolysaccharide assembly protein LptE) matched all the requirements. We selected the top 2 proteins that passed all the filtration steps and had the highest antigenicity score to obtain an accepted length of multiepitope vaccine after connecting the best-predicted T and B cell epitopes from these 2 proteins so we nominated LptD and BamA as our final vaccine candidates, characteristics of these candidates are shown in Supplementary Table 1.
Conservation Analysis of Nominated Vaccine Candidates Among E. coli Pathotypes
Conservation analysis of our nominated vaccine candidates showed that these proteins were found with high conservation among various E. coli pathotypes (Supplementary Table 2) which confirmed their ability to protect against different E. coli infections, therefore we designed the multitope vaccine based on these 2 proteins.
T Cell Epitopes
In order to select the best T cell epitopes for constructing the multitope vaccine, the top 100 generated peptides from IEBD per each protein candidate were estimated for their antigenicity score, allergenicity, and toxicity probabilities, and the top 10 peptides, which demonstrated the lowest percentile rank, the highest binding affinity and antigenicity score more than 0.4 were grouped in Table 1 (for MHC-I peptides) and Table 2 (for MHC-II peptides). The binding affinity of the selected candidates of CTLs and HTLs was assessed through a docking study where the generated docked complexes for CTLs are shown in Supplementary Figure 1 and the docked complexes for HTLs are shown in Supplementary Figure 2. The binding scores of all complexes are shown in Supplementary Table 3. These scores ranged between −7.6 and −8.5 for CTLs, and from −7.1 to −8.1 for HTLs. The receptors of this docking study were deposited in the protein data bank with docked peptides that were firstly removed before running the current docking analysis then docked again separately to their respective receptor to act as a control for our docking study. The binding score for MHC-I control was −7.5 and that of MHC-II was −7.6, By comparing the binding energy score for our candidate list and the controls we can confirm that these candidates are promising to be selected for the construction of the multitope vaccine. Another significant factor that was considered in the selection of the epitopes for constructing the multitope vaccine was the population coverage. The IEDB population coverage analysis tool was employed for this process. The whole list of predicted epitopes for both protein candidates showed the epitopes arranged in descending way based on their binding affinity to the different alleles therefore we selected the top 10%, that would represent the epitopes with high binding affinity to respective alleles, and collected these alleles per each epitope to analyze the population coverage for single epitopes (Supplementary Table 4) then we analyzed the population coverage for the combined CTLs, combined HTLs and the multitope vaccine (Supplementary Table 5).
Table 1.
Top-ranked T-cell epitopes (MHC-I peptides) of BamA and LptD proteins.
| Epitope | Protein | Antigenicity | Allergenicity | Toxicity | Immunogenicity | Conservancy (%) |
|---|---|---|---|---|---|---|
| KTDDFTFNY | BamA | 1.74 | Non | Non | 0.2750 | 100 |
| FKTDDFTFNY | BamA | 1.54 | Non | Non | 0.3020 | 100 |
| MSAGIALQW | BamA | 1.41 | Non | Non | 0.1361 | 100 |
| NVDAGNRFY | BamA | 0.41 | Allergen | Non | 0.1855 | 100 |
| AELSVTNPY | BamA | 0.97 | Non | Non | −0.1052 | 100 |
| AEIQQINIV | BamA | 0.78 | Non | Non | 0.0114 | 100 |
| YANSVRTSF | BamA | 0.53 | Allergen | Non | −0.1435 | 100 |
| RMSAGIALQW | BamA | 1.5 | Allergen | Non | 0.1080 | 100 |
| QADDADLSDY | BamA | 0.82 | Non | Non | −0.0478 | 100 |
| YSDPSNIRM | BamA | 0.82 | Non | Non | −0.0285 | 100 |
| RTGDDNITW | LptD | 1.7 | Allergen | Non | 0.1838 | 100 |
| FSEQNTSSY | LptD | 0.43 | Non | Non | −0.2901 | 100 |
| KLDESVNRV | LptD | 0.79 | Non | Non | 0.0104 | 100 |
| RIYGQAVHF | LptD | 0.66 | Non | Non | 0.0106 | 100 |
| SPEYIQATL | LptD | 0.49 | Allergen | Non | 0.1050 | 100 |
| ATSNSSIEY | LptD | 1.13 | Allergen | Non | −0.2062 | 100 |
| KVGPVSIFY | LptD | 0.71 | Non | Non | 0.0650 | 100 |
| TLEPRAQYLY | LptD | 1.04 | Non | Non | −0.0088 | 100 |
| IYDDAAVERF | LptD | 0.5 | Allergen | Non | 0.2593 | 100 |
| KQADSMLGV | LptD | 0.8 | Allergen | Non | −0.2809 | 100 |
Table 2.
Top-ranked T-cell epitopes (MHC-II peptides) of BamA and LptD proteins.
| Epitope | Protein | Antigenicity | Allergenicity | Toxicity | INF-γ | IL-4 | IL-10 | Conservancy (%) |
|---|---|---|---|---|---|---|---|---|
| DPSNIRMSAGIALQW | BamA | 1.26 | Non-allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| PSNIRMSAGIALQWM | BamA | 1.22 | Non-allergenic | Non-toxic | Positive | Non-inducer | Non-inducer | 100 |
| SNIRMSAGIALQWMS | BamA | 1.03 | Non-allergenic | Non-toxic | Positive | Non-inducer | Non-inducer | 100 |
| KLAGDLETLRSYYLD | BamA | 0.66 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| QKLAGDLETLRSYYL | BamA | 0.62 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| NIRMSAGIALQWMSP | BamA | 1.25 | Non-allergenic | Non-toxic | Positive | Non-inducer | Non-inducer | 100 |
| QRVAVGAALLSMPVR | BamA | 0.51 | Non-allergenic | Non-toxic | Positive | Non-inducer | Non-inducer | 100 |
| DYTNKSYGTDVTLGF | BamA | 0.79 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| NKSYGTDVTLGFPIN | BamA | 0.99 | Non-allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| TNKSYGTDVTLGFPI | BamA | 1.07 | Non-allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| GPVSIFYSPYLQLPV | LptD | 0.68 | Non-allergenic | Non-toxic | Positive | Non-inducer | Non-inducer | 100 |
| VQLNYRYASPEYIQA | LptD | 1.11 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| VGPVSIFYSPYLQLP | LptD | 0.69 | Allergenic | Non-toxic | Positive | Inducer | Inducer | 100 |
| VSIFYSPYLQLPVGD | LptD | 0.45 | Allergenic | Non-toxic | Positive | Inducer | Inducer | 100 |
| LNYRYASPEYIQATL | LptD | 0.88 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| KVGPVSIFYSPYLQL | LptD | 0.88 | Non-allergenic | Non-toxic | Positive | Inducer | Inducer | 100 |
| NYRYASPEYIQATLP | LptD | 0.66 | Allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| YLPYYWNIAPNMDAT | LptD | 1.76 | Allergenic | Non-toxic | Positive | Inducer | Inducer | 100 |
| AKYTTTNYFEFYLPY | LptD | 1.13 | Non-allergenic | Non-toxic | Positive | Inducer | Non-inducer | 100 |
| SSIEYRRDEDRLVQL | LptD | 0.81 | Non-allergenic | Non-toxic | Positive | Non-inducer | Inducer | 100 |
B-Cell Epitope Identification
Bepipred Linear Epitope Prediction 2.0 was used as a prediction method. It identified 30 and 21 B-cell epitopes for BamA and LptD proteins, respectively. Peptides with a length between 9:18 amino acids were analyzed for their antigenicity and peptides with antigenicity score >0.4 were tested for their allergenicity and toxicity (Table 3).
Table 3.
Predicted B-cell epitopes of BamA and LptD proteins.
| BamA | LptD | ||||||
|---|---|---|---|---|---|---|---|
| Epitope | Antigenicity | Allerge-nicity | Toxicity | Epitope | Antigenicity | Allerge-nicity | Toxicity |
| PVRTGDTVNDEDIS | 1.24 | Allergen | Non | KEAPGQPEPV | 0.96 | Non | Non |
| ASGVRVGESLDRT | 0.97 | Non | Non | DKVYEDEHPNDDSS | 0.41 | Non | Non |
| IRFEGNDTSKDAV | 1.14 | Non | Non | PSYFNDFDNKYGSSTDGY | 1.22 | Non | Non |
| TDTQRVPGSP | 0.49 | Non | Non | QVFSEQNTSSYS | 0.42 | Non | Non |
| FQADDADLSDYTNK | 0.56 | Non | Non | QTNLDWYNSRNTTKLDES | 0.75 | Non | Non |
Construction of Multitope Vaccine
From Tables 1–3, six epitopes per each table (three from each protein candidate) were chosen based to constitute the basis of the multitope vaccine (graphical representation for the constructive components is shown in Supplementary Figure 3). Moreover, β-defensin and PADRE peptide were also incorporated to finalize a potential vaccine sequence of 380 amino acids in length and its sequence was as the following:
“EAAAKGIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKKEAAAKAKFVAAWTLKAAAGGGSKTDDFTFNYGGGSAELSVTNPYGGGSAEIQQINIVGGGSFSEQNTSSYGGGSRIYGQAVHFGGGSTLEPRAQYLYGPGPGDPSNIRMSAGIALQWGPGPGQRVAVGAALLSMPVRGPGPGTNKSYGTDVTLGFPIGPGPGKVGPVSIFYSPYLQLGPGPGAKYTTTNYFEFYLPYGPGPGSSIEYRRDEDRLVQLKKASGVRVGESLDRTKKIRFEGNDTSKDAVKKTDTQRVPGSPKKKEAPGQPEPVKKPSYFNDFDNKYGSSTDGYKKQTNLDWYNSRNTTKLDESKKAKFVAAWTLKAAAGGGS”.
This construct was assessed to be non-allergen with an antigenicity score of 1.07 (estimated by VaxiJen v2.0) and 0.959 (estimated by ANTIGENpro).
Physicochemical Properties Assessment and Secondary Structure Prediction
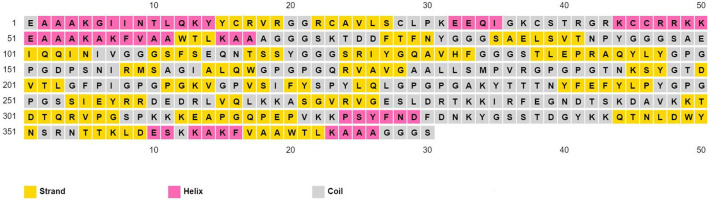
The physicochemical properties of the predicted vaccine construct were detected by using the ProtParam server and demonstrated in Supplementary Table 6. The designed vaccine had a SOLpro SVM score of 0.95; therefore it was predicted to be soluble as SOLpro values > 0.5 are considered as soluble. Vaccine secondary structure prediction demonstrated the presence of 14.2% helix, 37.9% strand, and 47.9% coil structure (Figure 3).
Figure 3.
Secondary structure prediction of designed multitope vaccine using PESIPRED server.
Tertiary Structure Prediction, Refinement, and Validation
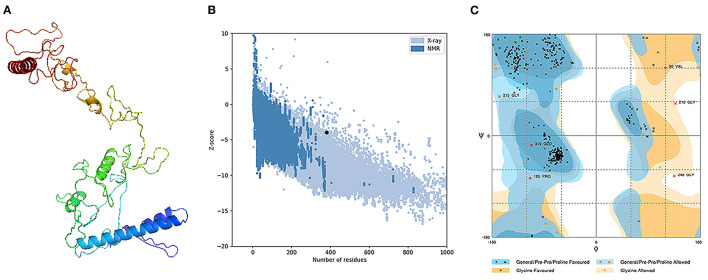
The assessment of the primary 3D structure, through Ramachandran plot analysis and ProSA online server, demonstrated that 87.6, 6.3, and 6.1% of residues were located in favored, allowed, and outlier regions, and the Z-score −3.45, respectively. While these values may be considered acceptable for a predicted 3D structure, we continued with refinement for better structure creation. Protein refinement occurred through the help of GalaxyRefine web server and the best model, regarding scores improvement, Figure 4A demonstrated the following scores' enhancement, the Z-score enhanced from −3.45 to −3.9 (Figure 4B) while the Ramachandran plot analysis scores became 96%, 2.4%, and 1.6% for residues in favored, allowed, and outlier regions, respectively (Figure 4C).
Figure 4.
Structural analysis of the predicted 3D structure of the vaccine. (A) The three-dimensional structure of vaccine obtained after molecular refinements; (B) ProSA-web evaluation of the vaccine structure; (C) Ramachandran plot analysis of the protein structure after molecular refinements.
Conformational B-Cell Epitope Prediction
The tertiary structure and folding of the designed vaccine may generate new conformational B-cell epitopes and for this purpose, we used ElliPro server conformational. In the current assessment, the server predicts 9 new epitopes and their scores were between 0.514 and 0.84 (Supplementary Table 7). The predicted 3D models of the generated epitopes are shown in Supplementary Figure 4.
Vaccine Disulfide Engineering
Usage of DbD2 server for disulfide bond assign demonstrated that 26 pairs of amino acids are eligible to make disulfide bonds while in terms of other parameters such as energy and Chi3 value, this number reduced to only 2 pairs. Therefore, 4 mutations were performed at the residues pairs of SER89-ARG187 and PRO324-PHE330. The followed values of energy and Chi3 to recommend disulfide engineering were below 2.2 and from −87 to +97, respectively.
Molecular Docking of the Vaccine With TLR4
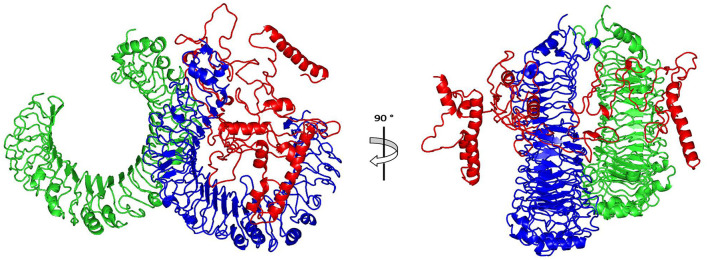
The ClusPro 2.0, which was employed for the docking study, generated 30 models and the model number 0.00 (Figure 5) exhibited the lowest binding energy score of −1420.9 kcal/mol which implicate a good affinity and stability of the constructed complex.
Figure 5.
Docked complex of vaccine constructs with human TLR4; vaccine constructs in red color and TLR4 receptor in green and blue colors.
Dihedral Coordinate-Based Normal-Mode Analyses
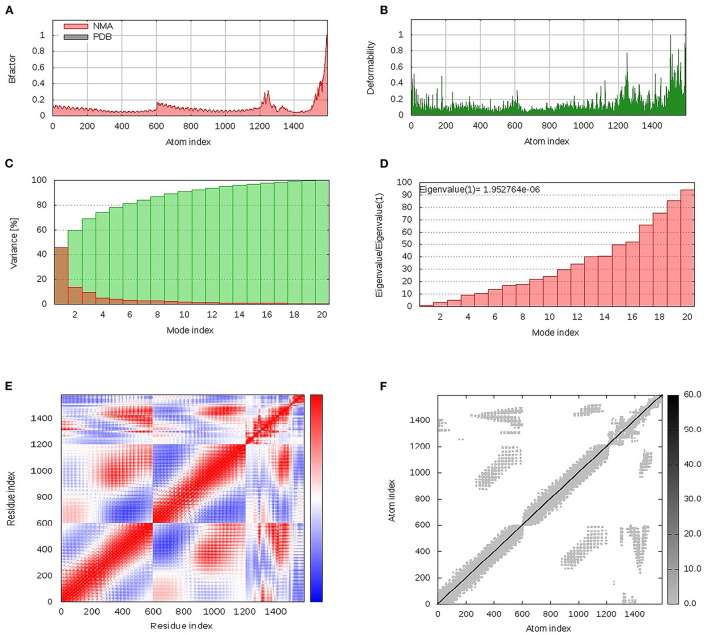
Within the iMOS server finding analysis, B-factor correlates with the relative magnitude of atom displacement around conformational equilibria. Values were significantly higher for the epitope vaccine ligand (atom index; 9,568–15,225), particularly at its respective carboxy terminus, in relation to that of the hTLR-4 target protein (atom index; 1–9,567) (Figure 6A). The B-factor results were recapitulated via the complex deformability index presented in Figure 6B where each vaccine residue, particularly at the carboxy end, showed individual distortions being higher than those of the hTLR-4 target protein. The estimated eigenvalue, which represents the motion stiffness of the complex, was 1.95e−06, where being in inverse order in relation to variance predicting the significantly higher mobility of the vaccine as compared to the hTLR-2 complex across collective functional motions (Figures 6C,D). The iMOS provided the covariance matrix illustrating the coupled residue pairs demonstrating anti-correlated (blue color), correlated (red color), or uncorrelated (white color) motions. The hTLR-4 depicted lower predicted correlated residue-pair motions than did the epitope vaccine ligand, however, the latter protein showed less anti-correlated motions (Figure 6E). Finally, the obtained elastic-network model explains the differential flexibility patterns among both investigated proteins (Figure 6F). Represented in different colors, the elastic-network model describes the atom pairs linked via springs relying on the stiffness degree between them. Stiffer strings were correlated to dark gray colors. The target hTLR-4 protein showed continuous dark-gray bands along the normal distribution of stiffer string, while the residues of the epitope vaccine illustrated non-continuous gray bands around the same immobility normal string, particularly for those residues near the carboxy terminus.
Figure 6.
Dihedral coordinate-based normal-mode analyses of multitope vaccine-hTLR-4 complex; ligand-receptor interaction was assessed throughout comparative (A) B-factor indices, (B) deformabilities, (C) variance, (D) eigenvalues, (E) covariance of residue indices, and (F) elastic network analysis.
Molecular Dynamics Simulation Analyses
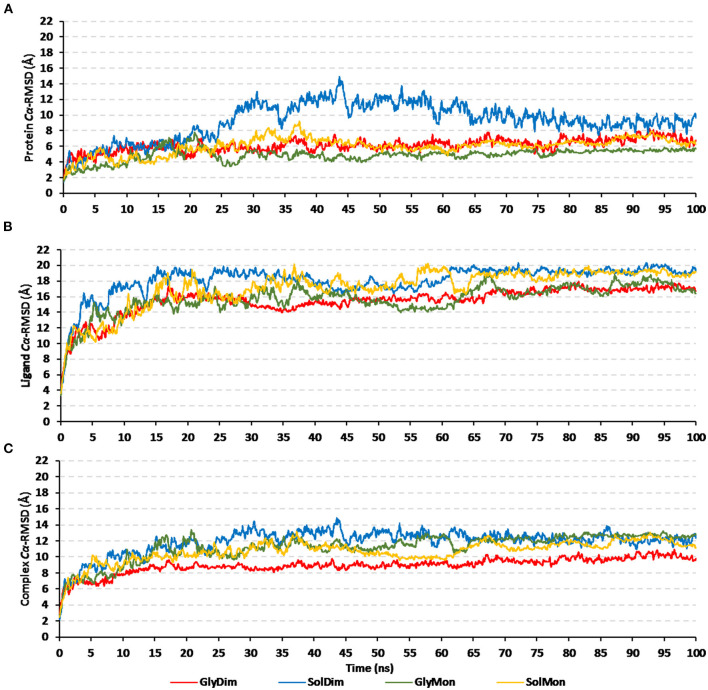
Throughout the 100 ns molecular dynamics runs, each simulated protein depicted thermodynamic behavior being typical through molecular dynamics simulations (Figure 7). The monitored proteins' RMSD deviations, in relation to corresponding alpha-carbon (Cα-RMSD) of the reference protein, showed an initial increase over the initial frames owing to the release of constraints at the beginning of the simulation stage. Steady RMSD tones were depicted for almost all protein models beyond the first 30 ns and till the end of the molecular dynamics runs (i.e., for >75 ns). The RMSD trajectories for the epitope vaccine and respective bound hTLR-4 were around two-fold differences the thing that ensured sufficient protein convergence as well as significant ligand accommodation at the target pocket. The latter findings ensured the adequacy of the 100 ns MD simulation timeline to grasp sufficient thermodynamic information within efficient computational cost and without the need for more time extension. Regarding comparative Cα-RMSDs analysis between the hTLR-4 target proteins and their corresponding in-bound epitope vaccine ligands, higher RMSD trajectories were illustrated for the ligand proteins (Figures 7A,B). The latter was obvious since the obtained average Cα-RMSD values across the protein's trajectory plateau and till MD simulation end courses were 8.58 ± 0.72 and 15.92 ± 0.32 Å for the hTLR-4 and vaccine ligands, respectively. Concerning the comparative RMSD analysis for the oligomeric hTLR-4 states, steadier and lower Cα-RMSD fluctuation were depicted for the dimeric state as compared to those of the monomer ones. On similar bases, significantly lower and more steady Cα-RMSD tones were depicted within models where the hTLR-4 target proteins were covalently bound to higher oligosaccharides. Except for limited fluctuations, the monitored Cα-RMSDs of the combined hTLR-4/epitope proteins showed rapid equilibration plateaus (11.23 ± 0.11 Å), beyond 25 ns and till the end of the simulations runs (Figure 7C). Notably, the least fluctuating complex Cα-RMSD trajectories (9.30 ± 0.57 Å) were seen for the dimeric N-glycosylated hTLR-4/vaccine system.
Figure 7.
RMSD trajectory analysis for the four investigated epitope vaccine ligand-bound hTLR-4 target models across 100 ns explicit molecular dynamics simulation runs. Time-evolution of (A) Proteins' Cα-RMSD, (B) Ligands' Cα-RMSD, and (C) Complexes' Cα-RMSD, along molecular dynamics timeframes (ns).
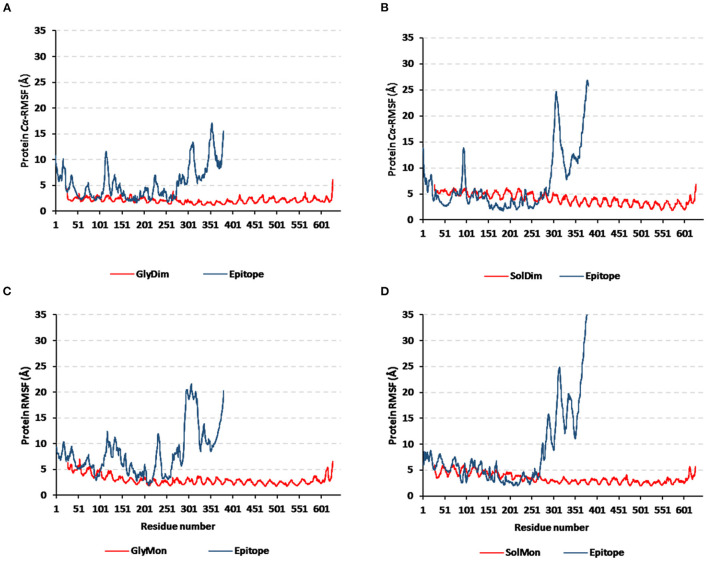
Monitoring the Cα-RMSF tones across the whole simulated trajectories (100 ns) provided interesting information regarding the residue-wise fluctuation pattern of each corresponding simulated protein. Interestingly, typical well-behaved molecular simulation profiles were depicted for each simulated protein where terminal residues and their vicinal ranges showed higher mobility patterns (high Cα-RMSF) as compared to those at the core regions (Figure 8). As a general observation, the epitope vaccine ligands showed much higher residue-wise fluctuation profiles as compared to their corresponding in-bound hTLR-4 target proteins. The latter observation was most recognized for the vaccines' respective carboxy end amino acids as well as their vicinal residues (high residue sequence numbering; from 301 to 380) in relation to those of the N-terminus. Notably, the depicted vaccine-oriented fluctuation trends were more associated with monomeric/non-N-glycosylated hTLR-4 state (Cα-RMSF up to 35 Å) as compared to other oligomeric models. On similar bases, the lowest RMSF-based mobility trends were assigned to the N-glycosylated dimeric hTLR-4 model as compared to any other simulated model.
Figure 8.
Protein's residue-wise fluctuation patterns. Monitored Cα-RMSF tones across the whole 100 ns molecular dynamics runs for the investigated hTLR-4 bound epitope vaccine within different oligomeric/saccharide-linked states. (A) glycosylated dimeric, (B) non-glycosylated dimeric, (C) glycosylated monomeric, and (D) non-glycosylated monomeric states, along the sequence residue numbers (bound TLR-4 protein target = 27–627; epitope vaccine = 1–380 residue ranges).
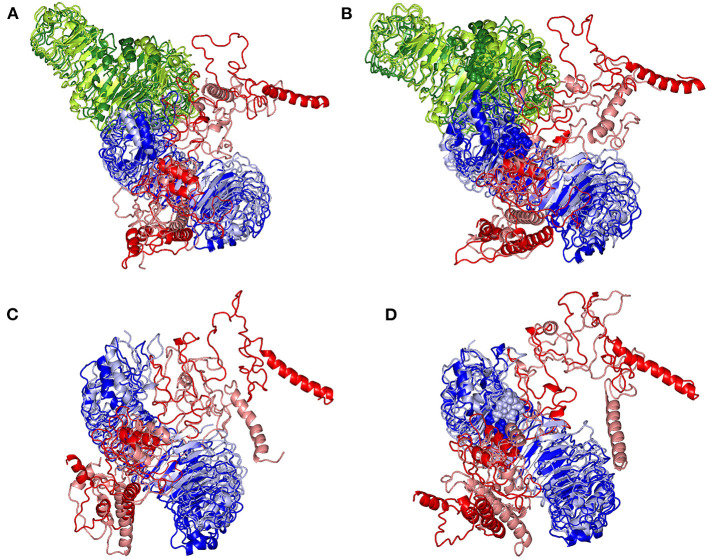
Subsequent analysis of the key alterations within the conformations of both simulated epitope vaccine and hTLR-4 protein was proceeded throughout examining each simulated model at the initial and last molecular dynamics timeframes. Extracted frame lines at 0 and 100 ns were subjected to 1 × 10−3 Kcal/mol.A2 gradient minimization using MOE-Molecular Operating Environment software. The RMSDs of the overlaid conformations were 6.645, 9.855, 5.488, and 7.784 Å for the glycosylated dimeric, non-glycosylated dimeric, glycosylated monomeric, and non-glycosylated monomeric complexes. Notably, all simulated models illustrated stable binding states for the epitope vaccine at the hTLR-4 binding site (Figure 9). Limited conformational changes were assigned for the hTLR-4 target protein across the four simulated models. On the contrarily, the simulated epitope vaccines depicted significant conformational changes causing them to adopt a more compacted conformation/orientation at the hTLR-4 binding site. Within the four simulated models, the epitope vaccine showed more profound movement for its carboxy end and vicinal regions as compared to that of its N-terminus, the thing that allowed proximity of the vaccine C-terminus toward the hTLR-4 lateral side and near the hTLR-4 1:1 homodimerization interface. The latter dynamic behavior was most recognized at the monomeric hTLR-4 states as compared to the dimeric ones, as well as at the non-glycosylated hTLR-4 state in relation to that bounded to the higher oligosaccharides. Notably, applying both Rg and SASA analysis for the epitope vaccine within the four simulated models showed comparable findings (Supplementary Figure 5). Both analytical parameters showed high values at the beginning of the MD simulation runs, yet as the simulation proceeded, the epitope vaccine attained lower as well as much steady plateaued trajectories till the end of the MD timeframes. It is worth mentioning that higher Rg fluctuations tones (around 15-65 ns), as well as late equilibration plateau (not before 70 ns), were achieved for the non-glucosylated/monomeric states as compared to the glycosylated/dimeric ones (Supplementary Figure 5A). Similar findings were also illustrated with SASA analysis where at non-glucosylated/monomeric states, the epitope vaccine exhibited higher fluctuations around 40 ns and till the end of the MD runs (Supplementary Figure 5B).
Figure 9.
Conformational changes for the simulated hTLR-4/epitope vaccine complexes. Overlaid snapshots of the 3D-cartoon representation of the; (A) glycosylated dimeric, (B) non-glycosylated dimeric, (C) glycosylated monomeric, and (D) non-glycosylated monomeric states at 0 ns and 100 ns of the molecular dynamics runs. The hTLR-4 target proteins (blue/green) and epitope vaccine ligands (red) are colored in respective to the initial or last extracted frames (dark and light colors are for 0 ns and 100 ns extracted frames, respectively). Moieties of N-glycosylation are presented as spheres and colored in respective to their linked hTLR-4 protomer and extracted frame.
The designed epitope vaccine across the simulated models showed significant free binding energies toward the hTLR-4 binding sites being estimated as kJ/mol ± SD (Table 4). The highest negative values of binding-free energies were assigned to the dimeric states of hTLR-4 rather than their respective monomeric ones. Additionally, the glycosyl-bound target proteins depicted a higher affinity toward the anchored epitope vaccine than those being non-glycosylated. Dissecting the obtained total free binding energies ΔGTotal binding showed a preferential energy contribution for the electrostatic non-bonded interactions over the van der Waal potentials. Across the four simulated models, the comparable pattern of energy term contributions was depicted depending on the glycosylation status of the target hTLR-4 protein. Differentially higher electrostatic and lower van der Waal values were depicted for the glycosylated target protein states over the monomeric ones. Regarding the solvation energy term contributions, both non-polar and polar solvation energies were of higher negative and positive values, respectively, for the non-glycosylated target proteins over their respective glycosylated forms. The non-polar solvation energy of interaction was monitored across the 100 ns of MD simulation using the SASA-only model calculation for the individual hTLR-4, epitope vaccine, as well as their respective combination at each oligomeric/glucosylic state (Supplementary Figure 6). Almost steady solvation energy terms were depicted for the four simulated proteins across the whole MD simulation run. On the other hand, the ligand's solvation energy of interaction showed a significant drop to lower values starting from around 40 ns and till the end of the MD runs achieving the average energy plateau for more than half the simulation timeframes. Steadier solvation energy tones were assigned for the dimeric/glycosylated targets rather than those of the lower simpler states. Notably, the complex solvation energy patterns were significantly impacted via the ligand's values and in turn its respective dynamic behavior rather with those of the simulated hTLR-4 proteins.
Table 4.
Free binding energies (ΔGTotal binding) and individual energy terms regarding the designed multitope vaccine at hTLR-4 target protein binding site.
| Energy (kJ/mol ±SD) | Ligand-receptor complex | |||
|---|---|---|---|---|
| Glycosylated dimer | Non-glycosylated dimer | Glycosylated monomer | Non-glycosylated monomer | |
| ΔGvan der Waal | −732.671 ± 72.004 | −780.821 ± 82.502 | −782.349 ± 115.500 | −932.685 ± 23.538 |
| ΔGElectrostatic | −7954.091 ± 79.714 | −7798.818 ± 11.828 | −7845.389 ± 86.071 | −7710.021 ± 97.634 |
| ΔGSolvation; Polar | 1529.300 ± 11.244 | 1650.603 ± 18.770 | 1497.129 ± 39.595 | 1743.513 ± 12.268 |
| ΔGSolvation; SASA | −98.206 ± 13.476 | −106.847 ± 9.345 | −97.202 ± 16.784 | −111.119 ± 24.432 |
| ΔGTotal binding | −7255.668 ± 73.478 | −7035.883 ± 38.444 | −7227.811 ± 20.760 | −7010.312 ± 11.335 |
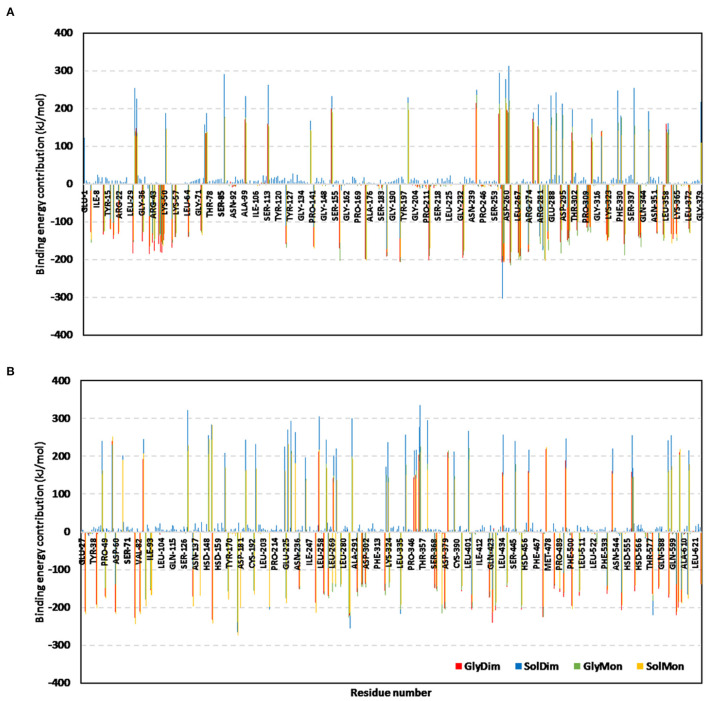
The ΔGTotal binding was further decomposed identifying the residue-wise energy contribution for both the vaccine and hTLR-4 where the more negative is the better (Figure 10 and Supplementary Figure 7). Findings within the latter figure showed higher positive-valued energy contributions for the vaccines in bound to non-glycosylated hTLR-4 states, while the vaccines of glycosylated forms depicted significant negative-valued energy contributions with a wider range of contributing residues. Similar residue-wise energy contribution patterns were assigned for the hTLR-4 residues.
Figure 10.
Residue-wise binding-free energy decomposition for the simulated epitope vaccine-hTLR-4 complexes. The energy contributions in kJ/mol within the total free binding energies for the residues comprising the simulated (A) epitope vaccine; (B) hTLR-4 target proteins. Energy contributions are represented against the residue number.
Immune Simulation of the Designed Vaccine
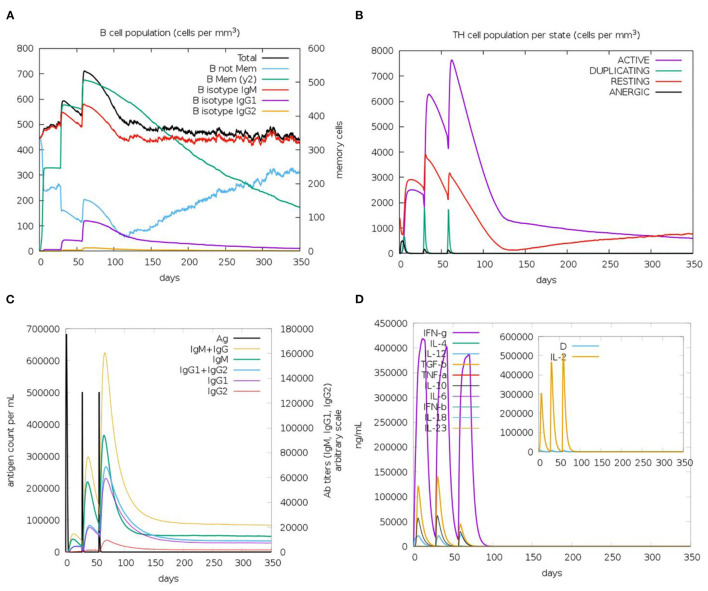
The immune response regarding antibody titer, cytokines level, B and T cells population is shown in Figure 11. The current study potential vaccine was estimated to stimulate a high level of IgM+IgG which increases with consecutive doses of the vaccine. Regarding cytokines level, several classes were stimulated and INF-γ exhibited the highest level of induced cytokine. Moreover, the count of stimulated T and B cells demonstrated an increase with the doses of the vaccine, and the highest level was obtained as a result of the second booster dose of the vaccine.
Figure 11.
The predicted immune response after designed vaccine injection. (A,B) shows the population of B and T cells, respectively, while (C,D) demonstrate antibody and cytokine count in a response to vaccine administration.
Discussion
Many microorganisms face difficulty in cultivation or attenuation leading to undesirable immune response, proving that the classical approaches for vaccine development against these pathogens require a technical revolution (68). Therefore, the last few years witnessed a large turn in the employed approaches for vaccine development where the multi-omics approaches stepped forward and preceded the traditional ones (69). Recent studies that utilized bioinformatics and structural biology tools for the generation of epitope-based vaccines, that included the antigenic parts only and demonstrated a promising ability for fighting against pathogenic microorganisms, represented a large percentage of the whole studies directed to the vaccine development (70).
The development of an effective vaccine against the several pathotypes of E. coli has faced many obstacles. The complex nature of this bacteria and its genetic plasticity hinders the trials for vaccine development. Additionally, lack of broadly applicable testing to assess disease burden, particularly in remote areas where incidence may be quite high besides the occurrence of several bacterial pathotypes are another barriers (71). The current study tried to address these difficulties by analyzing the complete genome of E. coli to find common conserved vaccine candidates that cover different pathotypes then assess the characteristics of this potential vaccine computationally before moving to the future phase of wet lab validation. In our analysis, the reverse vaccinology approach was employed to generate a shortlist of potential vaccine candidates after analysis of E. coli complete proteome then immunoinformatics computational tools were applied for designing a multitpe vaccine based on filtered vaccine candidates. Only two outer membrane proteins (LptD and BamA) were chosen after applying the filtration steps on 5,155 proteins. The same approach has been reported to be successful in protein filtration and potential vaccine candidature with many pathogens, for instance; Klebsiella Pneumoniae (72), Staphylococcus aureus (19), Mycobacterium tuberculosis (73), Shigella flexneri (74), Pseudomonas aeruginosa (75), Moraxella catarrhalis (76), and Nipah virus (18). In addition to that, E. coli was one of the main pathogens that were analyzed for designing a vaccine through a bioinformatics approach in many previous studies. We can divide these studies into two main categories, firstly, studies that just predicted single B and T cell epitopes of protein candidates of certain pathotypes of E. coli such as Khan and Kumar (77) and Mehla and Ramana (78). Secondly, more advanced studies that designed and validated a multitope vaccine through a computational approach such as (79), where the vaccine was designed based on Intimin, Stx, Lt, and Cfa proteins and directed against ETEC and EHEC, Another study (80), the vaccine was designed based on IutA and FimH proteins and directed against UPEC, a third study (81), the vaccine was designed based on the bacterial type-3 secretion system and directed against extraintestinal pathogenic E. coli. In the current study, we followed the approach of the second group of studies but at the same time, we introduced two unique points that greatly affect the design and the potential application. First, of all, unlike mentioned studies that selected their vaccine candidates based on literature mentioned virulent proteins of specific E. coli pathotype, we nominated the vaccine candidates of the current study after a complete filtration of a reference pathogenic E. coli strain where the filtration steps represented a basic portion of the study to select proteins that are antigenic, virulent, with a low similarity percentage with human proteins, essential, and conserved throughout various pathotypes of E. coli. Consequently, we came up with two novel vaccine candidates (BamA and LptD), and therefore the designed multitope vaccine based on these proteins would have different sequences and characteristics from previously discussed ones. The criteria of protein conservation moved us to the second unique point in the current study which is cross-reactivity. As mentioned, previous trials targeted only specific E. coli pathotype in their vaccine design while in this study we targeted essential proteins with a high percentage of conservation in basic E. coli pathotypes thus the predicted vaccine would have a potential activity against different E. coli pathotypes. Based on what mentioned above, we can choose the steps of common conserved protein candidates selection for several E. coli pathotypes and defining the prominent epitopes of these candidates as the most important steps that shaped the novelty of the current potential vaccine construct.
As mentioned above, the process of vaccine design through the immunoinformatics approach has been applied against several pathogens with a common methodology. On the other hand, with the continuous development in computational tools, a recent study (82) proposed a multitope vaccine against COVID-19 through the integration of a deep learning approach for prediction and design. In that study, DeepVacPred was employed to conduct multiple predictions in a time-saving manner. The current study followed the former methodology, especially that the designed vaccines by this common methodology showed promising results during the in-vivo validation (80, 83) and as mentioned above the main focus of this study was the development of a potential vaccine that covers most E. coli pathotypes and deeply investigate the characteristics of this construct more than developing novel prediction methodologies.
In the current study, several online servers were used to identify potential vaccine candidates against E. coli pathotypes where the nominated proteins were found to be highly conserved in the majority of E. coli pathotypes. In addition to that, they were outer membrane proteins, essential for bacterial survival, had a high antigenicity score, and were non-homologous to human proteins to ensure their safety in clinical trials. Bacterial outer membrane proteins play an important role in molecule transporting, membrane integrity maintenance, in addition to pathogenesis (84). Moreover, their easy accession to the host immune system gives them the advantage to be highly potential candidates for vaccine development (85). Lipopolysaccharide (LPS) is a major structural component in most Gram-negative bacteria and it is essential for bacterial growth, LptD is one of eight proteins involved in the proper assembly of LPS after its biosynthesis in E. coli (86). Regarding BamA, it belongs to the Omp85 family, which is characterized as a major antigenic and immunogenic protein expressed by most Gram-negative pathogenic bacteria (87).
Vaccine construction using mapped epitopes is a sophisticated strategy to trigger an immune response against infectious agents (88). On the other hand, reliance on peptide vaccines for human usage has faced some limitations as single peptide epitopes were found to be not strong enough to stimulate a suitable and prolonged immune response and exhibited low immunogenicity in comparison with live attenuated vaccines. Additionally, there are some questions about the stability of these peptides to induce the immune response before getting lysed by human proteolytic enzymes (89). To overcome these limitations, the current study proposed vaccine was constructed based on a combination of peptides with suitable linkers where those peptides were filtered through several criteria, and only conserved, highly antigenic, non-allergen, and non-toxic epitopes were selected to design the multitope vaccine. multi-epitope vaccines are considered a better choice than monovalent ones as they can stimulate efficient humoral as well as cellular immune responses (90). In addition to these epitopes, other components were added to the final vaccine construct to improve the immune response for this potential vaccine. β-defensin adjuvant was incorporated to create a deposit of the antigenic compound at the site of the vaccine that is steadily released over time, elongating the robust immune response and overcoming one of the main peptide vaccine limitations (91). Moreover, suitable linkers were used to join the selected epitopes from each candidate protein where they provide effective separation between the epitopes (92). Firstly, EAAAK was employed to improve the bi-functional catalytic activity, give stiffness in addition to enhancing fusion protein stability (93). The second linker, GPGPG, was selected for its ability to induce HTL immune response and the ability to break the junctional immunogenicity, resulting in individual epitopes' restoration of immunogenicity (94). The final linker, KK, was employed because of its ability to bring the pH value close to the physiological range (95). In addition to the epitopes, linkers, and adjuvant, the PADRE sequence was also added as it has been revealed that this sequence could minimize the polymorphism of HLA molecules in the population (96). Analyzing the results of the multitope vaccine assessment showed that, the current potential vaccine construct is antigenic with an antigenicity score of 1.07 which was more than the corresponding scores of the designed multitope vaccines against severe acute respiratory syndrome coronavirus 2 (97), Marburg virus (94), and Leptospira (98). Regarding the population coverage, the world coverage showed 100% for the constructed vaccine and that was similar to the corresponding coverage reported in (99). For the binding affinity of the single CTLs to the representative alleles, the scores ranged between −7.6 and −8.4, which was smaller than the range reported in (100) but at the same time was large enough when compared to the control binding score of the current study. Moving to the binding energy of the whole multitope vaccine, we reported here a score of −1420.9 kcal/mol, which was smaller than corresponding scores of vaccines predicted with a similar approach (18, 69, 76), implying that a strong binding with TLR would occur. Collectively, The final construct exhibited promising physicochemical, immunological, and chemical characteristics when assessed computationally where molecular dynamics simulation studies were adopted to give a close view of the behavior of this potential vaccine with the receptors of the immune system.
Finally, the designed epitope vaccine showed significant confinement and stability within the hTLR-4 binding site throughout the conducted 100 ns explicit molecular dynamic runs. With preferentiality for the dimeric glycosylated target protein, the designed vaccine exhibited steady conventional thermodynamic behavior with Cα-RMSDs leveling up for more than 70 ns. The adopted Cα-RMSD analytical tool allows the estimation of molecular deviation from the designated original/reference structure, the thing that can be used for ensuring ligand-target stability/confinement as well as the validity of the MD protocol (101). Obtaining Cα-RMSD at low values as well as being rapidly equilibrated has been correlated with the strong affinity of the designed vaccine ligand against target protein as well as the successful convergence of the simulated models requiring no further molecular dynamics simulation runs (102). This Cα-RMSD-based vaccine-hTLR-4 stability was comparable to the stability of several reported proteinaceous multitope vaccines targeting different microorganisms TLRs (103, 104). Regarding the obtained residue-wise fluctuation analysis, the higher Cα-RMSF values of the epitope vaccine as compared to hTLR-4 can be reasonably correlated to their differential tertiary structure folding and/or packing. Generally, the Cα-RMSF flexibility analysis tool estimates the averaged deviations of protein's residues in relation to their reference positions, the thing that would provide a valuable evaluation of protein's residues regarding their respective dynamic behavior being represented through flexibility and fluctuation (105). In these regards, the incorporation of long α-helices with flexible β-loop connections within the vaccine's designed structures would rationalize the initial relaxation and significant convergence into more stable compacted conformations. On the other hand, hTLR-4 exhibited densely packed shoe-like architecture with plenty of highly ordered parallel β-sheets. This differential inherited flexibility was also highlighted through the 3D-conformational analysis between initial and last frames as well as the adopted dihedral coordinate-based normal mode analysis. Having non-uniform stiffness/immobility profiles, as well as high B-factor, deformability, and mobility indices, conferred the profound flexibility being assigned for the vaccine (50). The assigned high immobility profiles for the epitope vaccine were most recognized through its carboxy-terminal amino acids and vicinal residues as being clearly demonstrated through the conformational analysis as well as RMSF, Rg, and SASA findings.
It is worth mentioning that the high RMSF flexibility of the 301–380 residue range was highly reasoned since these residues started the MD simulation being apart from the hTLR-4 interface and then ended being near the hTLR-4 lateral side. Nevertheless, the epitope vaccine rapidly attained a more stable compacted conformation/orientation in relation to the bound hTLR-4 as the MD runs proceeded. The latter more profoundly stable conformation/orientation of the vaccine was mostly related to the movement of this residue range toward hTLR-4 lateral side representing the dimerization interface. This was confirmed through the Rg and SASA analysis where values significantly dropped as the MD simulation proceeded reaching to lower steady trajectories for more than half of the MD simulation timeframes. Generally, Rg accounts for the global stability of either ligand/protein ternary structures, where such stability parameter is defined as the mass-weighted RMSD for atom groups in relation to their respective common center of mass (106). Thus, the depicted dynamic behavior of simulated vaccine to exhbit low Rgs maintaining a plateau around an average value conferred significant stability/compactness at the hTLR-4 binding. Notably, the Rg finding further highlighted the preferential vaccine anchoring at the dimeric and/or glycosylated hTLR-4 states since the monomeric and/or non-glycosylated ones achieved higher fluctuations and late Rg equilibration trajectories (not before 70ns) suggesting non-optimal compactness and intermolecular binding around these timeframes. Findings from the ligand's SASA analysis came in good agreement with the above preferential complex stability since the simulated vaccine showed steadier SASA tone along the average equilibration plateau around 40 ns and till the simulation end. Since SASA is a quantitative measurement about the extent of protein/solvent interaction correlating for molecular surface area assessable to solvent, thus, low SASA tones imply relative structural shrinkage under the impact of the solvent surface charges yielding more compact and stable conformations (107). The latter findings were also consistent with the vaccine's non-polar solvation energy (only SASA-model) across the MD simulation runs. It is worth mentioning that all above epitope-hTLR-4 flexibility patterns were also similarly depicted within several reported studies investigating the potential binding affinity of peptide-based vaccines toward microbial TLRs (103, 104).
The presented study further highlights the impact of hTLR-4 oligomerization as well as oligosaccharide states on vaccine binding. Depicting lower Cα-RMSD and RMSF values with more steady tones at glycosylated dimeric model raised the suggestion that N-glycosylation and hTLR-4 dimerization were beneficial for vaccine anchoring at the target binding site. Accumulated evidence has illustrated the importance of TLR ectodomains' N-glycosylation for orchestrating the localization and signaling capacity (108). Additionally, N-linked glycosylation (sialylation) of hTLR-4 and its coreceptor, MD-2, enhances the lipoprotein-driven nuclear factor kappa-B activation, cytokine expressions, and, as well as regulates hTLR-2 and hTLR-3 signaling pathway (109–111). Furthermore, sialylated residues are important for mediating the association between hTLR-4 and MD-2, enhancing hTLR-4 dimerization, as well as the assembly of complete TLRs signaling complexes (108, 112). Thus, having the importance in enhancing ligand anchoring at TLRs' binding sites as well as facilitating TLRs dimerization it was highly reasoned why highly stabilized/steady thermodynamic behaviors, as well as less fluctuating/, mobilized residues were assigned for both glycosylated dimeric hTLR-4/vaccine complex. The MM/PBSA-driven free binding energy calculations also highlighted the higher affinity of the simulated vaccine toward the glycosylated hTLR-4 in relation to those being non-glycosylated. Depicting higher negative total free binding energies as well as more pronounced Coloumb's electrostatic energy contributions were highlighted for the more favored vaccine anchoring/affinity toward the glycosylated target proteins. Binding to the N-glycosylation chains was also found satisfactory to counterbalance the predicted electrostatic penalties and solvation energies during epitope vaccine ligand binding since lower polar solvation energy terms (ΔGSolvation) were depicted at the glycosylated models. This was also confirmed through monitoring the non-polar solvation energy via the only SASA-model across the MD simulation runs.
Generally, solvation energy terms represent significant repulsive forces against the ligand-binding since binding is a solvent displacement process. It was depicted that these large repulsive forces were mediated majorly by the hTLR-4 residues rather than by the vaccine amino acids as being depicted within the residue-wise energy contributions the thing that could be related to the high ordered water molecules at the hydrophobic surface of the TLR-2 ligand-binding site. Thus, the presence of N-linked glycosylation chains would minimize such repulsive penalties against the vaccine binding. On the other hand, the total non-polar interactions (ΔGvan der Waal plus ΔGSASA) were shown to be higher at the non-glycosylated models conferring their respective larger surface area as well as higher hydrophobic potentialities toward vaccine binding. The latter was rationalized since accumulated evidence has considered the general TLRs binding site to be extended and more hydrophobic in nature (52–55, 113–115). Based on the above evidence, it was satisfactory that the designed epitope vaccine depicted significant binding affinity toward the hTLR-4 binding pocket with higher preferentiality toward the glycosylated dimeric state.
Conclusion
The current study aimed to demonstrate the role of modern approaches for vaccine development as a potential solution to fight resistant pathogens. Here, we reported two proteins namely BamA and LptD, after the filtration of the whole proteome of E. coli reference strain, to act as a base for multitope vaccine construct against E. coli pathotypes. The multitope construct included top-ranked epitopes of the filtered proteins in addition to beta-defensin and PADRE peptide. The molecular modeling simulation studies illustrated relevant affinity/binding of the designed epitope vaccine toward the hTLR-4 binding pocket, yet with higher preferentiality toward the glycosylated dimeric state. Finally, The predicted physicochemical and immunological characteristics of the constructed vaccine nominated it as a potential solution against several E. coli pathotypes and recommended its movement to wet lab validation.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
MS, MB, MSA, MA, and KD: conceptualization, methodology, and original draft preparation. SA, EF, SE, AL, and RE: writing—review and editing. MS and MA: supervision and project administration. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Taif University Researchers Supporting Project number (TURSP-2020/202), Taif University, Taif, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our great appreciation to the Deanship of Scientific Research, Taif University, Taif, Kingdom of Saudi Arabia for funding our project through Taif University Researchers Supporting Project number (TURSP-2020/202). The simulations in this work were performed at King Abdulaziz University's High-Performance Computing Center (Aziz Supercomputer) (http://hpc.kau.edu.sa), the authors, therefore, acknowledge with thanks the center for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.829467/full#supplementary-material
Predicted positions of MHC-I peptides (yellow color) in the 3D structure of HLA-B*44:03 receptor (red color), structures (A–F) are for epitopes number 1, 2, 3, 4, 5, and 6, respectively, from Supplementary Table 3.
Predicted positions of MHC-II peptides (yellow color) in the 3D structure of HLA-DRB1*04:01 receptor (red color), structures (A–F) are for epitopes number 1, 2, 3, 4, 5, and 6, respectively, from Supplementary Table 3.
Graphical map for the designed multitope vaccine.
The 3D model of the 9 predicted conformational B-cell epitopes in the refined final vaccine construct where the yellow residues represent the epitopes and the gray ones are for the rest of the predicted vaccine. Letters from A to I represents the predicted epitopes in Supplementary Table 7 from 1 to 9, respectively.
Global stability of the epitope vaccine at the four simulated models across 100 ns explicit molecular dynamics simulation runs. Time-evolution of (A) Rg, (B) SASA, along molecular dynamics timeframes (ns).
Non-polar solvation energy terms using the SASA-only model calculation for the four investigated epitope vaccine ligand-bound hTLR-4 target models across 100 ns explicit molecular dynamics simulation runs. Time-evolution of (A) Proteins, (B) Ligands, and (C) Complexes, along molecular dynamics timeframes (ns).
Residue-wise binding-free energy decomposition for the simulated epitope vaccine-hTLR-4 complexes. 3D-representation (Cartoon) for the regions implying favored binding interactions on the initial/reference vaccine/hTLR-4 complex. Glycosylated dimeric, non-glycosylated dimeric, glycosylated monomeric, and non-glycosylated monomeric states are at upper-left, upper-right, lower-left, and lower right quadrant, respectively (Only one protomer is shown for clarity). Regions of the proteins are in spectrum colors from dark blue (negative-valued ΔG kJ/mol conferring highly favored attractive binding forces) down to dark red (positive-valued ΔG kJ/mol conferring unfavored high repulsive binding forces).
Characteristics of Potential Vaccine Candidates for E. coli O157:H7 str. Sakai.
Protein conservancy of the selected candidates among E. coli pathotypes.
Selected epitopes' binding energies with representative MHC-I and MHC-II alleles.
Reacting alleles and population coverage of filtered CLTs and HTLs.
Population coverage percentage of the multitope vaccine and its constructing peptides (CTLs and HTLs).
Predicted physicochemical properties of the chimeric vaccine.
The predicted conformational B cell epitopes from the refined vaccine 3D construct.
References
- 1.Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. (2012) 3:71–87. 10.4161/gmic.19182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould D. Causes, prevention and treatment of Escherichia coli infections. Nurs Stand. (2010) 24:50–6. 10.7748/ns.24.31.50.s46 [DOI] [PubMed] [Google Scholar]
- 3.Fratamico PM, DebRoy C, Liu Y, Needleman DS, Baranzoni GM, Feng P. Advances in molecular serotyping and subtyping of Escherichia coli. Front Microbiol. (2016) 7:644. 10.3389/fmicb.2016.00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suardana IW, Artama WT, Widiasih DA, Ngurah IG, Mahardika K. Genetic Diversity of Escherichia coli O157:H7 strains using random amplified polymorphic DNA (RAPD). Int Res J Microbiol. (2013) 55:671–4. [Google Scholar]
- 5.Puzari M, Sharma M, Chetia P. Emergence of antibiotic resistant Shigella species: A matter of concern. J Infect Public Health. (2018) 11:451–4. 10.1016/j.jiph.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 6.Yang SC, Lin CH, Aljuffali IA, Fang JY. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch Microbiol. (2017) 199:811–25. 10.1007/s00203-017-1393-y [DOI] [PubMed] [Google Scholar]
- 7.Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop São Paulo. (2014) 56:341–6. 10.1590/S0036-46652014000400012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collignon P. Resistant Escherichia coli—we are what we eat. Clin Infect Dis. (2009) 49:202–4. 10.1086/599831 [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois AL, Wierzba TF, Walker RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. (2016) 34:2880–6. 10.1016/j.vaccine.2016.02.076 [DOI] [PubMed] [Google Scholar]
- 10.Mejías MP, Hiriart Y, Lauché C, Fernández-Brando RJ, Pardo R, Bruballa A, et al. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Sci Rep. (2016) 6:24913. 10.1038/srep24913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira Oliveira A, Almeida Cardoso S. Bruno dos Reis Almeida F, Licursi de Oliveira L, Pitondo-Silva A, Gomes Soares S, Seixas Hanna E. Oral immunization with attenuated Salmonella vaccine expressing Escherichia coli O157: H7 intimin gamma triggers both systemic and mucosal humoral immunity in mice. Microbiol Immunol. (2012) 56:513–22. 10.1111/j.1348-0421.2012.00477.x [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A, Li J, Shiloach Y, Bobbins JB, Szu SC. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2-5-year-old children. J Infect Dis. (2006) 193:515–21. 10.1086/499821 [DOI] [PubMed] [Google Scholar]
- 13.Harris JA, Roy K, Woo-Rasberry V, Hamilton DJ, Kansal R, Qadri F, et al. Directed evaluation of enterotoxigenic Escherichia coli autotransporter proteins as putative vaccine candidates. PLoS Negl Trop Dis. (2011) 5:e1428. 10.1371/journal.pntd.0001428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Iqbal J, Gómez-Duarte OG. Murine immunization with CS21 pili or LngA major subunit of enterotoxigenic Escherichia coli (ETEC) elicits systemic and mucosal immune responses and inhibits ETEC gut colonization. Vet Microbiol. (2017) 202:90–100. 10.1016/j.vetmic.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis. (2017) 17:528–37. 10.1016/S1473-3099(17)30108-1 [DOI] [PubMed] [Google Scholar]
- 16.Habibi M, AsadiKaram MR, Bouzari S. Evaluation of prevalence, immunogenicity and efficacy of FyuA iron receptor in uropathogenic Escherichia coli isolates as a vaccine target against urinary tract infection. Microb Pathog. (2017) 110:477–83. 10.1016/j.micpath.2017.07.037 [DOI] [PubMed] [Google Scholar]
- 17.Rappuoli R, Bottomley MJ, D'Oro U, Finco O. De Gregorio E. Reverse vaccinology 20: Human immunology instructs vaccine antigen design. J Exp Med. (2016) 213:469–81. 10.1084/jem.20151960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soltan MA, Eldeen MA, Elbassiouny N, Mohamed I, El-damasy DA, Fayad E, Ali OAA, Raafat N, Eid RA, Al-karmalawy AA. Proteome based approach defines candidates for designing a multitope vaccine against the nipah virus. Int J Mol Sci. (2021) 22:9330. 10.3390/ijms22179330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltan MA, Magdy D, Solyman SM, Hanora A. Design of staphylococcus aureus new vaccine candidates with B and T cell epitope mapping, reverse vaccinology, and immunoinformatics. Omi A J Integr Biol. (2020) 24:195–204. 10.1089/omi.2019.0183 [DOI] [PubMed] [Google Scholar]
- 20.Soltan MA, Eldeen MA, Elbassiouny N, Kamel HL, Abdelraheem KM, El-Gayyed HA, et al. In silico designing of a multitope vaccine against rhizopus microsporus with potential activity against other mucormycosis causing fungi. Cells. (2021) 10:3014. 10.3390/cells10113014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu T, Seo H, Moxley RA, Zhang W. Mapping the neutralizing epitopes of F18 fimbrial adhesin subunit FedF of enterotoxigenic Escherichia coli (ETEC). Vet Microbiol. (2019) 230:171–7. 10.1016/j.vetmic.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho TF, Haddad JPA, Paixão TA, Santos RL. Meta-analysis and advancement of brucellosis vaccinology. PLoS ONE. (2016) 11:e0166582. 10.1371/journal.pone.0166582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Carranza E, Barajas H, Alcaraz LD, Servín-González L, Ponce-Soto GY, Soberón-Chávez G. Variability of bacterial essential genes among closely related bacteria: the case of Escherichia coli. Front Microbiol. (2018) 9:1059. 10.3389/fmicb.2018.01059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen QF, Liu S, Dong C, Guo HX, Gao YZ, Guo FB. Geptop 20: an updated, more precise, and faster Geptop server for identification of prokaryotic essential genes. Front Microbiol. (2019) 10:1236. 10.3389/fmicb.2019.01236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 30: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. (2010) 26:1608–15. 10.1093/bioinformatics/btq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg A, Gupta D. VirulentPred: A SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinform. (2008) 9:62. 10.1186/1471-2105-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. (2001) 305:567–80. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 28.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. (1999) 112:531–52. 10.1385/1-59259-584-7:531 [DOI] [PubMed] [Google Scholar]
- 29.Vishnu US, Sankarasubramanian J, Gunasekaran P, Rajendhran J. Novel vaccine candidates against brucella melitensis identified through reverse vaccinology approach. Omi A J Integr Biol. (2015) 19:722–9. 10.1089/omi.2015.0105 [DOI] [PubMed] [Google Scholar]
- 30.Doytchinova IA, Flower DR. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. (2007) 8:4. 10.1186/1471-2105-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleri W, Paul S, Dhanda SK, Mahajan S, Xu X, Peters B, et al. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front Immunol. (2017) 8:278. 10.3389/fimmu.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiskopf D, Angelo MA, De Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci USA. (2013) 110:E2046–53. 10.1073/pnas.1305227110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. (2011) 63:325–35. 10.1007/s00251-011-0513-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y, Maupetit J, Derreumaux P, Tufféry P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J Chem Theory Comput. (2014) 10:4745–58. 10.1021/ct500592m [DOI] [PubMed] [Google Scholar]
- 35.Oleg T, Arthur JO. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. (2012) 31:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bui HH, Sidney J, Li W, Fusseder N, Sette A. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinform. (2007) 8:361. 10.1186/1471-2105-8-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajighahramani N, Nezafat N, Eslami M, Negahdaripour M, Rahmatabadi SS, Ghasemi Y. Immunoinformatics analysis and in silico designing of a novel multi-epitope peptide vaccine against Staphylococcus aureus. Infect Genet Evol. (2017) 48:83–94. 10.1016/j.meegid.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 38.Magnan CN, Randall A, Baldi P. SOLpro: Accurate sequence-based prediction of protein solubility. Bioinformatics. (2009) 25:2200–7. 10.1093/bioinformatics/btp386 [DOI] [PubMed] [Google Scholar]
- 39.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. (1999) 292:195–202. 10.1006/jmbi.1999.3091 [DOI] [PubMed] [Google Scholar]
- 40.Cheng J, Randall AZ, Sweredoski MJ, Baldi P. SCRATCH: a protein structure and structural feature prediction server. Nucleic Acids Res. (2005) 33:W72–6. 10.1093/nar/gki396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heo L, Park H, Seok C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. (2013) 41:W384–8. 10.1093/nar/gkt458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan M, Hasan R, Hazrat Ali M, Fazle Rabbee M, Al-Hakim B, Mohammad Rejwan H, et al. In-silico characterization and homology modeling of catechol 1,2 dioxygenase involved in processing of catechol-an intermediate of aromatic compound degradation pathway. Glob J Sci Front Res. (2015) [Google Scholar]
- 43.Wiederstein M, Sippl MJ. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. (2007) 35:W407–10. 10.1093/nar/gkm290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponomarenko J, Bui HH Li W, Fusseder N, Bourne PE, Sette A, Peters B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. (2008) 9:514. 10.1186/1471-2105-9-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig DB. Dombkowski AA. Disulfide by Design 20: A web-based tool for disulfide engineering in proteins. BMC Bioinform. (2013) 14:346. 10.1186/1471-2105-14-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solanki V, Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci Rep. (2018) 8:9044. 10.1038/s41598-018-26689-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finamore A, Roselli M, Imbinto A, Seeboth J, Oswald IP, Mengheri E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal caco-2 cells and pig explants. PLoS ONE. (2014) 9:e94891. 10.1371/journal.pone.0094891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, et al. The ClusPro web server for protein-protein docking. Nat Protoc. (2017) 12:255–78. 10.1038/nprot.2016.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awan FM, Obaid A, Ikram A, Janjua HA. Mutation-structure-function relationship based integrated strategy reveals the potential impact of deleterious missense mutations in autophagy related proteins on hepatocellular carcinoma (HCC): a comprehensive informatics approach. Int J Mol Sci. (2017) 18:139. 10.3390/ijms18010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res. (2014) 42:W271–6. 10.1093/nar/gku339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey RK, Verma P, Sharma D, Bhatt TK, Sundar S, Prajapati VK. High-throughput virtual screening and quantum mechanics approach to develop imipramine analogues as leads against trypanothione reductase of leishmania. Biomed Pharmacother. (2016) 83:141–52. 10.1016/j.biopha.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 52.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. (2009) 388:621–5. 10.1016/j.bbrc.2009.08.062 [DOI] [PubMed] [Google Scholar]
- 53.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. (2008) 29:182–91. 10.1016/j.immuni.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 54.Ohto U, Yamakawa N, Akashi-Takamura S, Miyake K, Shimizu T. Structural analyses of human toll-like receptor 4 polymorphisms D299G and T399I. J Biol Chem. (2012) 287:40611–7. 10.1074/jbc.M112.404608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. (2009) 458:1191–5. 10.1038/nature07830 [DOI] [PubMed] [Google Scholar]
- 56.Páll S, Abraham MJ, Kutzner C, Hess B, Lindahl E. Tackling exascale software challenges in molecular dynamics simulations with GROMACS. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). [Google Scholar]
- 57.Al-Karmalawy AA, Dahab MA, Metwaly AM, Elhady SS, Elkaeed EB, Eissa IH, et al. Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Front Chem. (2021) 9:661230. 10.3389/fchem.2021.661230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmaaty AA, Darwish KM, Khattab M, Elhady SS, Salah M, Hamed MIA, et al. In a search for potential drug candidates for combating COVID-19: computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J Biomol Struct Dyn. (2021) 30:1–28. 10.1080/07391102.2021.1918256 [DOI] [PubMed] [Google Scholar]
- 59.Helal MA, Shouman S, Abdelwaly A, Elmehrath AO, Essawy M, Sayed SM, et al. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J Biomol Struct Dyn. (2020) 16:1–11. 10.1080/07391102.2020.1822208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, De Groot BL, et al. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat Methods. (2016) 14:71–3. 10.1038/nmeth.4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saleh AH, Abdelwaly A, Darwish KM, Eissa AAHM, Chittiboyina A, Helal MA. Deciphering the molecular basis of the kappa opioid receptor selectivity: a molecular dynamics study. J Mol Graph Model. (2021) 106:107940. 10.1016/j.jmgm.2021.107940 [DOI] [PubMed] [Google Scholar]
- 62.Ross GA, Rustenburg AS, Grinaway PB, Fass J, Chodera JD. Biomolecular Simulations under Realistic Macroscopic Salt Conditions. J Phys Chem B. (2018) 122:5466–86. 10.1021/acs.jpcb.7b11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaki AA, Ashour A, Elhady SS, Darwish KM, Al-Karmalawy AA. Calendulaglycoside A showing potential activity against SARS-CoV-2 main protease: molecular docking, molecular dynamics, and SAR studies. J Tradit Complement Med. (2021) (Online ahead of print) 10.1016/j.jtcme.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: a linear constraint solver for molecular simulations. J Comput Chem. (1997) 18:1463–72. [DOI] [Google Scholar]
- 65.Páll S, Hess B. A flexible algorithm for calculating pair interactions on SIMD architectures. Comput Phys Commun. (2013) 184:2641–50. 10.1016/j.cpc.2013.06.003 [DOI] [Google Scholar]
- 66.Kumari R, Kumar R, Consortium OSDD, Lynn A. g _ mmpbsa—A GROMACS tool for MM-PBSA and its optimization for high-throughput binding energy calculations. J Chem Inf Model. (2014) 54:1951–62. 10.1021/ci500020m [DOI] [PubMed] [Google Scholar]
- 67.Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: The use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE. (2010) 5:e9862. 10.1371/journal.pone.0009862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. (2009) 10:837–52. 10.2217/pgs.09.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasan M, Azim KF, Begum A, Khan NA, Shammi TS, Imran AS, et al. Vaccinomics strategy for developing a unique multi-epitope monovalent vaccine against Marburg marburgvirus. Infect Genet Evol. (2019) 70:140–57. 10.1016/j.meegid.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 70.Jiang P, Cai Y, Chen J, Ye X, Mao S, Zhu S, et al. Evaluation of tandem Chlamydia trachomatis MOMP multi-epitopes vaccine in BALB/c mice model. Vaccine. (2017) 35:3096–103. 10.1016/j.vaccine.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 71.Fleckenstein JM. Confronting challenges to enterotoxigenic Escherichia coli vaccine development. Front Trop Dis. (2021) 20. 10.3389/fitd.2021.709907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dar HA, Zaheer T, Shehroz M, Ullah N, Naz K, Muhammad SA, et al. Immunoinformatics-aided design and evaluation of a potential multi-epitope vaccine against klebsiella pneumoniae. Vaccines. (2019) 7:88. 10.3390/vaccines7030088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan MK, Zaman S, Chakraborty S, Chakravorty R, Alam MM, Bhuiyan TR, et al. In silico predicted mycobacterial epitope elicits in vitro T-cell responses. Mol Immunol. (2014) 61:16–22. 10.1016/j.molimm.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 74.Leow CY, Kazi A, Ismail CMKH, Chuah C, Lim BH, Leow CH, et al. Reverse vaccinology approach for the identification and characterization of outer membrane proteins of shigella flexneri as potential cellular-and antibody-dependent vaccine candidates. Clin Exp Vaccine Res. (2020) 9:15–25. 10.7774/cevr.2020.9.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solanki V, Tiwari M, Tiwari V. Prioritization of potential vaccine targets using comparative proteomics and designing of the chimeric multi-epitope vaccine against Pseudomonas aeruginosa. Sci Rep. (2019) 9:5240. 10.1038/s41598-019-41496-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soltan MA, Elbassiouny N, Gamal H, Elkaeed EB, Eid RA, Eldeen MA, et al. In Silico Prediction of a Multitope Vaccine against Moraxella catarrhalis: Reverse Vaccinology and Immunoinformatics. Vaccines. (2021) 9:669. 10.3390/vaccines9060669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan F, Kumar A. An integrative docking and simulation-based approach towards the development of epitope-based vaccine against enterotoxigenic Escherichia coli. Netw Model Anal Heal Informatics Bioinforma. (2021) 10:11. 10.1007/s13721-021-00287-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehla K, Ramana J. Identification of epitope-based peptide vaccine candidates against enterotoxigenic Escherichia coli: a comparative genomics and immunoinformatics approach. Mol Biosyst. (2016) 12:890–901. 10.1039/C5MB00745C [DOI] [PubMed] [Google Scholar]
- 79.Jeshvaghani FS, Rahjerdi AK, Amani J, Rad I, Jafari M, Salmanian AH. Designing and structure evaluation of multi-epitope vaccine against ETEC and EHEC, an in silico approach. Protein Pept Lett. (2015) 23:33–42. 10.2174/0929866522666151026122116 [DOI] [PubMed] [Google Scholar]
- 80.Hasanzadeh S, Habibi M, Shokrgozar MA, Ahangari Cohan R, Ahmadi K, Asadi Karam MR, et al. In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli. Sci Rep. (2020) 10:16258. 10.1038/s41598-020-73179-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wieser A, Magistro G, Nörenberg D, Hoffmann C, Schubert S. First multi-epitope subunit vaccine against extraintestinal pathogenic Escherichia coli delivered by a bacterial type-3 secretion system (T3SS). Int J Med Microbiol. (2012) 302:10–8. 10.1016/j.ijmm.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 82.Yang Z, Bogdan P, Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. (2021) 11:3238. 10.1038/s41598-021-81749-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muhammad SA, Zafar S, Rizvi SZ, Imran I, Munir F, Jamshed MB, et al. Experimental analysis of T cell epitopes for designing liver cancer vaccine predicted by system-level immunoinformatics approach. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G1055–69. 10.1152/ajpgi.00068.2020 [DOI] [PubMed] [Google Scholar]
- 84.Lin J, Huang S, Zhang Q. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect. (2002) 4:325–31. 10.1016/S1286-4579(02)01545-9 [DOI] [PubMed] [Google Scholar]
- 85.Rizwan M, Naz A, Ahmad J, Naz K, Obaid A, Parveen T, et al. VacSol: a high throughput in silico pipeline to predict potential therapeutic targets in prokaryotic pathogens using subtractive reverse vaccinology. BMC Bioinform. (2017) 18:106. 10.1186/s12859-017-1540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol. (2016) 14:337–45. 10.1038/nrmicro.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su YC, Wan KL, Mohamed R, Nathan S. Immunization with the recombinant Burkholderia pseudomallei outer membrane protein Omp85 induces protective immunity in mice. Vaccine. (2010) 28:5005–11. 10.1016/j.vaccine.2010.05.022 [DOI] [PubMed] [Google Scholar]
- 88.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, et al. Proof of principle for epitope-focused vaccine design. Nature. (2014) 507:201–6. 10.1038/nature12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. (2016) 7:842–54. 10.1039/C5SC03892H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. (2011) 411:206–15. 10.1016/j.virol.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta A, Rosato AJ, Cui F. Vaccine candidate designed against carcinoembryonic antigen-related cell adhesion molecules using immunoinformatics tools. J Biomol Struct Dyn. (2021) 10.1080/07391102.2020.1797539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Sun W, Guo J, Zhao G, Sun S, Yu H, et al. In silico design of a DNA-based HIV-1 multi-epitope vaccine for Chinese populations. Hum Vaccines Immunother. (2015) 39:6084–98. 10.1080/21645515.2015.1012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen X, Zaro JL, Shen WC. Fusion protein linkers: Property, design and functionality. Adv Drug Deliv Rev. (2013) 65:1357–69. 10.1016/j.addr.2012.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sami SA, Marma KKS, Mahmud S, Khan MAN, Albogami S, El-Shehawi AM, et al. Designing of a multi-epitope vaccine against the structural proteins of marburg virus exploiting the immunoinformatics approach. ACS Omega. (2021) 6:32043–71. 10.1021/acsomega.1c04817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu Y, Sun X, Li B, Huang J, Zhan B, Zhu X. Vaccination with a paramyosin-based multi-epitope vaccine elicits significant protective immunity against Trichinella spiralis infection in mice. Front Microbiol. (2017) 8:1475. 10.3389/fmicb.2017.01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghaffari-Nazari H, Tavakkol-Afshari J, Jaafari MR, Tahaghoghi-Hajghorbani S, Masoumi E, Jalali SA. Improving multi-epitope long peptide vaccine potency by using a strategy that enhances CD4+ T Help in BALB/c mice. PLoS ONE. (2015) 10:e0142563. 10.1371/journal.pone.0142563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obaidullah AJ, Alanazi MM, Alsaif NA, Albassam H, Almehizia AA, Alqahtani AM, et al. Bin. Immunoinformatics-guided design of a multi-epitope vaccine based on the structural proteins of severe acute respiratory syndrome coronavirus 2. RSC Adv. (2021) 11:18103–21. 10.1039/D1RA02885E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar P, Lata S, Shankar UN, Akif M. Immunoinformatics-based designing of a multi-Epitope chimeric vaccine from multi-domain outer surface antigens of leptospira. Front Immunol. (2021) 12:735373. 10.3389/fimmu.2021.735373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahmud S, Rafi MO, Paul GK, Promi MM, Shimu MSS, Biswas S, Bin, Dhama K, Alyami SA, Moni MA, et al. Designing a multi-epitope vaccine candidate to combat MERS-CoV by employing an immunoinformatics approach. Sci Rep. (2021) 11:15431. 10.1038/s41598-021-92176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rakib A, Sami SA, Mimi NJ, Chowdhury MM, Eva TA, Nainu F, et al. Immunoinformatics-guided design of an epitope-based vaccine against severe acute respiratory syndrome coronavirus 2 spike glycoprotein. Comput Biol Med. (2020) 124:103967. 10.1016/j.compbiomed.2020.103967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arnittali M, Rissanou AN, Harmandaris V. Structure of biomolecules through molecular dynamics simulations. Procedia Comput Sci. (2019) 156:69–78. 10.1016/j.procs.2019.08.18130525930 [DOI] [Google Scholar]
- 102.Liu K, Watanabe E, Kokubo H. Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations. J Comput Aided Mol Des. (2017) 31:201–11. 10.1007/s10822-016-0005-2 [DOI] [PubMed] [Google Scholar]
- 103.Chauhan V, Rungta T, Goyal K, Singh MP. Designing a multi-epitope based vaccine to combat Kaposi Sarcoma utilizing immunoinformatics approach. Sci Rep. (2019) 9:2517. 10.1038/s41598-019-39299-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khatoon N, Pandey RK, Prajapati VK. Exploring Leishmania secretory proteins to design B and T cell multi-epitope subunit vaccine using immunoinformatics approach. Sci Rep. (2017) 7:8285. 10.1038/s41598-017-08842-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benson NC, Daggett V. A comparison of multiscale methods for the analysis of molecular dynamics simulations. J Phys Chem B. (2012) 116:8722–31. 10.1021/jp302103t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Likić VA, Gooley PR, Speed TP, Strehler EE. A statistical approach to the interpretation of molecular dynamics simulations of calmodulin equilibrium dynamics. Protein Sci. (2005) 14:2955–63. 10.1110/ps.051681605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pirolli D, Sciandra F, Bozzi M, Giardina B, Brancaccio A, De Rosa MC. Insights from molecular dynamics simulations: Structural basis for the V567D mutation-induced instability of zebrafish alpha-dystroglycan and comparison with the murine model. PLoS ONE. (2014) 9:e103866. 10.1371/journal.pone.0103866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrao R, Li J, Bergamin E, Wu H. Structural insights into the assembly of large oligomeric signalosomes in the toll-like receptor-interleukin-1 receptor superfamily. Sci Signal. (2012) 5:re3. 10.1126/scisignal.2003124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, et al. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for toll-like receptor activation and cellular signaling. J Biol Chem. (2011) 286:36532–49. 10.1074/jbc.M111.237578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng C, Stamatos NM, Dragan AI, Medvedev A, Whitford M, Zhang L, et al. Sialyl residues modulate LPS-mediated signaling through the toll-like receptor 4 complex. PLoS ONE. (2012) 7:e32359. 10.1371/journal.pone.0032359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohnishi T, Muroi M, Tanamoto K. N-Linked Glycosylations at Asn 26 and Asn 114 of Human MD-2 Are Required for Toll-Like Receptor 4-Mediated Activation of NF-κB by Lipopolysaccharide. J Immunol. (2001) 167:3354–9. 10.4049/jimmunol.167.6.3354 [DOI] [PubMed] [Google Scholar]
- 112.Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, et al. Neu1 desialylation of sialyl α-2,3-linked β-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. (2010) 22:314–24. 10.1016/j.cellsig.2009.09.038 [DOI] [PubMed] [Google Scholar]
- 113.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. (2007) 130:1071–82. 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 114.Ho MK, Se CO Ki JL, Kasamatsu J, Jin YH, Beom SP, Lee H, et al. Structural diversity of the hagfish variable lymphocyte receptors. J Biol Chem. (2007) 282:6726–32. 10.1074/jbc.M608471200 [DOI] [PubMed] [Google Scholar]
- 115.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. (2006) 24:497–518. 10.1146/annurev.immunol.24.021605.090542 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted positions of MHC-I peptides (yellow color) in the 3D structure of HLA-B*44:03 receptor (red color), structures (A–F) are for epitopes number 1, 2, 3, 4, 5, and 6, respectively, from Supplementary Table 3.
Predicted positions of MHC-II peptides (yellow color) in the 3D structure of HLA-DRB1*04:01 receptor (red color), structures (A–F) are for epitopes number 1, 2, 3, 4, 5, and 6, respectively, from Supplementary Table 3.
Graphical map for the designed multitope vaccine.
The 3D model of the 9 predicted conformational B-cell epitopes in the refined final vaccine construct where the yellow residues represent the epitopes and the gray ones are for the rest of the predicted vaccine. Letters from A to I represents the predicted epitopes in Supplementary Table 7 from 1 to 9, respectively.
Global stability of the epitope vaccine at the four simulated models across 100 ns explicit molecular dynamics simulation runs. Time-evolution of (A) Rg, (B) SASA, along molecular dynamics timeframes (ns).
Non-polar solvation energy terms using the SASA-only model calculation for the four investigated epitope vaccine ligand-bound hTLR-4 target models across 100 ns explicit molecular dynamics simulation runs. Time-evolution of (A) Proteins, (B) Ligands, and (C) Complexes, along molecular dynamics timeframes (ns).
Residue-wise binding-free energy decomposition for the simulated epitope vaccine-hTLR-4 complexes. 3D-representation (Cartoon) for the regions implying favored binding interactions on the initial/reference vaccine/hTLR-4 complex. Glycosylated dimeric, non-glycosylated dimeric, glycosylated monomeric, and non-glycosylated monomeric states are at upper-left, upper-right, lower-left, and lower right quadrant, respectively (Only one protomer is shown for clarity). Regions of the proteins are in spectrum colors from dark blue (negative-valued ΔG kJ/mol conferring highly favored attractive binding forces) down to dark red (positive-valued ΔG kJ/mol conferring unfavored high repulsive binding forces).
Characteristics of Potential Vaccine Candidates for E. coli O157:H7 str. Sakai.
Protein conservancy of the selected candidates among E. coli pathotypes.
Selected epitopes' binding energies with representative MHC-I and MHC-II alleles.
Reacting alleles and population coverage of filtered CLTs and HTLs.
Population coverage percentage of the multitope vaccine and its constructing peptides (CTLs and HTLs).
Predicted physicochemical properties of the chimeric vaccine.
The predicted conformational B cell epitopes from the refined vaccine 3D construct.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.