Significance
Considerable effort is expended to protect today’s children from lead exposure, but there is little evidence on the harms past lead exposures continue to hold for yesterday’s children, who are victims of what we term legacy lead exposures. We estimate that over 170 million Americans alive today were exposed to high-lead levels in early childhood, several million of whom were exposed to five-plus times the current reference level. Our estimates allow future work to plan for the health needs of these Americans and to inform estimation of the true contributions of lead exposure to population health. We estimate population-level effects on IQ loss and find that lead is responsible for the loss of 824,097,690 IQ points as of 2015.
Keywords: population, lead, cognitive
Abstract
Lead is a developmental neurotoxicant in wide industrial use that was once broadly distributed in the environment. The extent of the US population exposed in early life to high levels of lead is unknown, as are the consequences for population IQ. Serial, cross-sectional blood–lead level (BLL) data from National Health and Nutrition Examination Survey (NHANES), a nationally representative sample of US children aged 1 to 5 (n = 11,616) from 1976 to 1980 to 2015 to 2016 was combined with population estimates from the US Census, the Human Mortality Database, and the United Nations. NHANES and leaded gasoline consumption data were used to estimate BLLs from 1940 to 1975. We estimated the number and proportion of people that fall within seven BLL categories (<4.99; 5 to 0.9.99; 10 to 14.9: 15 to 19.9; 20.24.9; 25 to 29.9; and ≥30 µg/dL), by year and birth cohort, and calculated IQ points lost because of lead exposure. In 2015, over 170 million people (>53%) had BLLs above 5 µg/dL in early life (±2.84 million [80% CI]), over 54 million (>17%) above 15 µg/dL, and over 4.5 million (>1%) above 30 µg/dL (±0.28 million [80% CI]). BLLs greater than 5 µg/dL were nearly universal (>90%) among those born 1951 to 1980, while BLLs were considerably lower than 5 µg/dL among those born since 2001. The average lead-linked loss in cognitive ability was 2.6 IQ points per person as of 2015. This amounted to a total loss of 824,097,690 IQ points, disproportionately endured by those born between 1951 and 1980.
The past isn’t dead, it’s not really even the past.
William Faulkner, Requiem for a Nun
The Flint Water Crisis returned the issue of legacy lead contamination to the public’s attention in the fall of 2015 (1), highlighting that harmful lead exposures are still routine in many communities in the United States and around the world. In developed countries, lead’s historic use in paints, pipes, and gasoline has left numerous waters, soils, airways, and homes enriched with this neurotoxicant—threatening the health and development of today’s children. Less obvious but also important is the threat that lead holds for yesterday’s children, many of whom are victims of what we term legacy lead exposures (2).
During the peak era of leaded gasoline in the United States, which ran from the late 1960s to the early 1980s, the average blood–lead level (BLL) for the general US population was routinely three to five times higher than the current reference value for clinical concern and case management referral (3.5 micrograms of lead per deciliter of blood) (3–5). Consequently, millions of adults alive today were exposed to high levels of lead as children. While these exposures were deemed harmless at the time, animal studies and epidemiological evidence accrued in the intervening years reveal that such exposures likely disrupted healthy development across multiple organ systems (particularly the brain, bone, and cardiovascular systems), resulting in subtle deficits to important outcomes, such as cognitive ability, fine motor skills, and emotional regulation (6), that may influence the trajectory of a person’s life (e.g., their educational attainment, health, wealth, and happiness). These deficits largely persist across time and, in some cases, worsen (7, 8) and are now hypothesized to put individuals at risk for difficult-to-treat chronic and age-related diseases, including cardiovascular disease and dementia (9–11).
Despite our improved understanding of the developmental implications and long-term consequences of early life lead exposure, a full accounting of the extent of these legacy exposures in the United States has yet to occur. Such an accounting is critical for the following: understanding the true costs and benefits of continued lead regulation and exposure abatement; understanding the potential contribution of lead to the burden of disease over the past six decades (and recent improvements in some outcomes, like dementia, associated with lead exposure); and, ultimately, improving cognitive, cardiovascular, and aging outcomes in the current generation of adults exposed to lead as children.
To address this need, we produce overall and cohort-specific population estimates of early life lead exposure (BLLs) for the US population in 2015. Using data from the US Census, lead-in-gas consumption statistics, and a continuous national survey of lead exposure conducted by the US Centers for Disease Control and Prevention from 1976 to 2016 (the National Health and Nutrition Examination Surveys [NHANES] II, III, and IV), we estimate the number of people living in the United States in 2015 that were exposed to various lead levels (e.g., ≥5 µg/dL blood–lead) as young children prior to, during, and after the era of leaded gasoline. To inform decision-making and research in domains likely to be influenced by better knowledge on lead exposure trends, including in economics, medicine, public health, and criminal justice, we generate estimates of population deficits in one outcome domain with a well-established lead dose–response relationship—cognitive ability—to describe the potential population-level consequence of legacy lead exposures. Finally, to inform predictions for social demography and public health into the future, we produce population projections until 2100 under the assumptions that BLLs from 2017 onwards are equivalent to those observed in 2016.
Data and Methods
NHANES is a continuous national survey conducted by the US Centers for Disease Control and Prevention (12). We used 1976 to 1980 to 2015 to 2016 data from NHANES II, III (phases 1 and 2), and IV. Details on NHANES study design, protocol, response rates, and specific data collection methods are provided elsewhere (13–15). NHANES conducts physical examinations that included taking venous blood samples according to a standard protocol. All participants were at least 1 and not yet 5 y old. Each child’s BLL was assigned to one of seven categories: <4.9; 5 to 0.9.9; 10 to 14.9; 15 to 19.9; 20 to 24.9; 25 to 29.9; and ≥30 µg/dL Although the blood–lead reference value for child clinical case management was updated from 5 to 3.5 µg/dL in November of 2021, we set the lowest bin to <4.9 µg/dL, as 5 µg/dL was the reference value at the time of analysis and in the year for which we are generating population estimates, 2015.
NHANES has a complex survey design. For this reason, we adjusted our estimates to account for unequal probability of selection into the sample, oversampling, and nonresponse. The sample sizes are present in Table S1. In order to derive year-specific BLL estimates, several analytical choices were necessary. First, NHANES II (1976 to 1980) was intended to represent one sample spanning 4 y. We choose to partition estimates out by year during this period because BLLs dropped rapidly during this time interval. If we had estimated BLLs over this entire time span, we would have underestimated them in earlier years and overestimated them in later years. Estimates made without this partitioning were not substantially different (SI Appendix, Fig. S1). Second, because NHANES III did not begin until 1988, we calculated the linear change between 1980 and 1988 and interpolated BLLs for 1981 to 1987. Third, BLLs were set to be equal across the NHANES III sampling periods. NHANES III consisted of two phases, 1988 to 1991 and 1992 to 1994, thus BLLs for 1988 to 1991 were equivalent as were those for 1992 to 1994. Fourth, in order to provide estimates for 1995 to 1998, we performed a linear interpolation between 1994 and 1999. Fifth, because NHANES IV consists of several biyearly data collections efforts, we set BLLs from adjacent years from 1999 to 2016 to be equal. For example, BLLs from 1999 are equivalent to those from 2000. Because the rate of decline in BLLs flattened out by 1999 as it approached today’s low levels (Fig. 1), this was a reasonable choice.
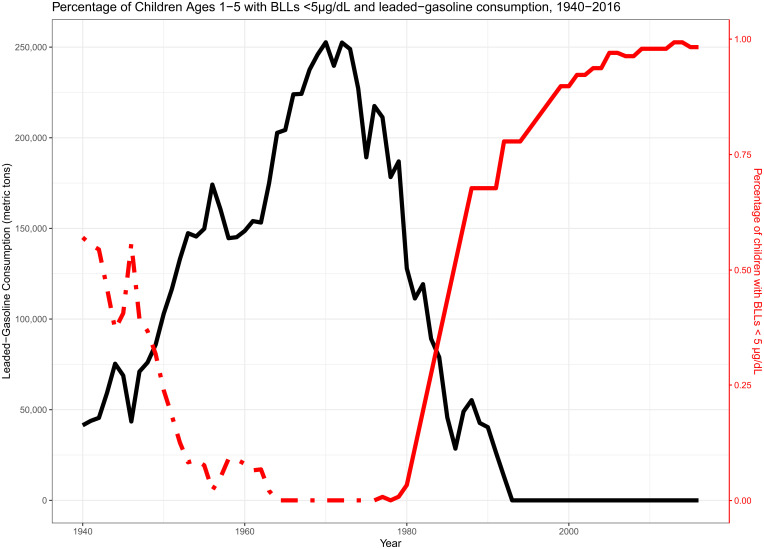
Fig. 1.
Leaded gasoline consumption and the percentage of children with BLLs under 5 µg/dL, 1940 to 2016. Leaded gasoline consumption comes from the Bureau of Mines Minerals Yearbook. The percentage of children aged 1 to 5 with BLLs come from 1976 to 2016 of the NHANES waves 2 to 4 (solid red line), while the dotted line is imputed from regressing childhood BLLs on leaded gasoline consumption.
Analytical Strategy.
The proportion of young children exposed to seven BLL categories was calculated for each year between 1976 and 2016 (n = 11, 616) after adjusting for phase-specific study design characteristics (see Table S1 for year-specific sample sizes). Because accurate and nationally representative BLL estimates do not exist prior to 1976, we predict them as a function of leaded gasoline consumption, the dominant source of lead exposure from the 1940s until the late 1980s (16, 17). Federal estimates of leaded gasoline consumption were obtained from the Bureau of Mines Minerals Yearbook 1933 to 1993 (18). The US Geological Survey archives data from the now defunct US Bureau of Mines which monitored the amount of lead-based gasoline consumed in the United States each year from 1933 to 1993. Leaded gasoline was first sold in 1923 and did not become the dominant source of BLLs until at least 1940. For this reason, our earliest estimates are from 1940. We combined observed BLL estimates from 1976 to 1993 NHANES data with yearly leaded gasoline consumption information. We regressed each of the seven BLL categories on leaded gasoline consumption. For each of these regressions, we estimated a linear model, a quadratic model, and a cubic model. The regression with the best model fit was chosen. Each of the regression equations and model fit are shown in Table S2.
Fig. 1 shows that leaded gasoline consumption closely mirrored the proportion of children with BLLs <5 µg/dL After combining NHANES estimates with leaded gasoline consumption data, BLLs were regressed on leaded gasoline consumption from 1976 to 1993. Year-specific leaded gasoline consumption levels were then included in this regression equation to predict BLL exposure proportions for each year from 1940 to 1975. For instance, the dotted line in Fig. 1 shows the predicted proportion of children that had BLLs <5 µg/dL by year [BLLyear = −7.94 × 10−6 (leadyear) + 1.77 × 10−11 (leadyear) (2) + 0.87; R2 = 0.93]. This procedure was repeated seven times—one for each of the BLL thresholds. (Table S2).
To obtain population estimates from 2015 to 2100, the following procedures were performed. First, we categorized the lead exposure populations into individual cohorts based on ages 0 to 1. For example, we placed the population aged 0 to 1 in 1976 into the 1976 cohort. We repeated this process for the 1940 through 2016 cohorts, the most recent year for the data.
Second, we merged these lead exposure cohorts with population data from the Human Mortality Database (HMD) (19). The HMD contains gold standard, annual estimates of population counts, death counts, and mortality rates based on vital statistics information and census counts. We calculated the raw percentage of each cohort, i, from each time period, t, in each lead exposure category, l, as simply . This provided the initial population in each single-year age group in each cohort under each lead exposure category.
To estimate the fraction of each cohort surviving until it is extinguished, we married our estimates of lead exposure in 2016 with the United Nations’ population projections from World Population Prospects 2019 (20). We aggregated the single-year age groups into 5-y age groups for each lead population estimate to be consistent with the UN projections. We calculated the fraction each age group, a, cohort, i, and lead exposure category, l, of the 2016 estimated population and apply this fraction to the corresponding population between 2020 and 2100 ] to produce the surviving fraction of each cohort until each cohort is extinguished. We assumed future cohorts experience the same BLL exposure as those born in 2016.
Finally, we estimated lead-linked loss in population-level cognitive ability using Lanphear et al.’s (21) benchmark international pooled estimates of IQ deficits by age 5 to 10 y experienced by children from multiple countries with differing levels of lead exposure (21). Likely, population-level IQ loss due to childhood lead exposure was calculated by the following: 1) finding the midpoint of each BLL category; 2) multiplying each midpoint by the BLL-specific IQ loss estimate adopted from Gould (22) binned transformations (22) of Lanphear et al.’s (21) estimates (Table S3); 3) continuing this process for all BLL categories; 4) multiplying this IQ loss number by the population exposed; and 5) summing the results.
Results
Population Estimates in 2015.
Table 1 shows that a history of early life lead exposure was ubiquitous among Americans alive in 2015. Among the 318 million people in the US population, only 131 million (margin of error = 1.4 M; 80% CI) had BLLs below 5 µg/dL, the 2015 threshold for clinical concern and case management (23), when they were children. Conversely, the majority of the population—over 54%—had a BLL above this threshold as children. Furthermore, there were large gradations in the extent of lead exposure among these 54%. For instance, nearly 100 million people (MOE = 1.4 M; 80% CI), or 31% of the population, had childhood BLLs above 10 µg/dL—twice the 2015 reference level and three times the current reference level. Around ∼10 million people alive in 2015 (MOE = 0.4M; 80% CI) had childhood BLLs above 25 ug/dL—five times the level of concern then and seven times the current level.
Table 1.
US population estimates of early life BLLs in 2015*
| Panel A | |||
|---|---|---|---|
| BLL categories (µg/dL) | Population estimates† | Percentage of population | Margin of error (80% confidence) |
| <5 | 130,825,865 | 41.1 | 1,399,699 |
| 5–9.9 | 71,037,022 | 22.3 | 2,471,010 |
| 10–14.9 | 44,986,994 | 14.1 | 1,095,024 |
| 15–19.9 | 28,955,001 | 9.1 | 471,581 |
| 20–24.9 | 15,556,981 | 4.9 | 617,519 |
| 25–29.9 | 5,201,870 | 1.6 | 324,411 |
| ≥30 | 4,724,417 | 1.5 | 257,328 |
| Panel B | |||
| ≥5 | 170,462,286 | 53.5 | 2,842,560 |
| ≥10 | 99,425,264 | 31.2 | 1,405,082 |
| ≥15 | 54,438,270 | 17.1 | 880,442 |
| ≥20 | 25,483,269 | 8.0 | 743,499 |
| ≥25 | 9,926,288 | 3.1 | 414,078 |
*The total population in 2015 = 318,479,402.
†The sum of these population estimates is not equal to the population size due to rounding error.
Estimates of early life BLLs in the US population in 2015. Panel A shows the distribution seven BLL categories. Panel B shows the cumulative BLLs under specified thresholds in 2015. Over 54% of the US population had a BLL above the 5-µg/dL threshold for “safe” exposure as children. Lead estimates exclude everyone born before 1940.
Population Estimates in 2015 by Birth Cohort.
Early childhood lead exposure varied considerably by cohort, as shown in Table 2. The extent of exposure followed an upside down “U” association, in which BLLs were relatively low for cohorts born in the 1940s, increased dramatically for cohorts now middle aged, and decreased dramatically among younger cohorts. Childhood BLLs greater than 5 µg/dL were nearly universal among middle-aged cohorts: 1951 to 1955 (∼90%), 1956 to 1960 (∼99%), 1961 to 1965 (∼97%), 1966 to 1970 (∼100%), 1971 to 1975 (∼100%), and 1976 to 1980 (∼99%). Conversely, childhood BLLs greater than 5 µg/dL were more rare among younger cohorts: 2001 to 2005 (∼6%), 2006 to 2010 (∼3%), and 2011 to 2015 (∼1%). The full extent of these patterns can be seen in Fig. 2 A and C. People born 1951 to 1980 had particularly high childhood BLLs. For instance, ∼78% and 73% of those born between 1966 to 1970 and 1971 to 1975 had childhood BLLs above 15 µg/dL Furthermore, a significant proportion of children in these cohorts (7%) had BLLs above 30 µg/dL
Table 2.
US population estimates of BLLs above the current Centers for Disease Control and Prevention level of concern (>5 µg/dL) in early life by age in 2015
| Birth cohort | Age in 2015 | Population estimates > 5 µg/dL* | Percentage of population > 5 µg/dL* | Margin of error (80% confidence) | Total population |
|---|---|---|---|---|---|
| 2011–2015 | 0–4 | 287,292 | 1.4 | 67,586 | 19,895,276 |
| 2006–2010 | 5–9 | 588,995 | 2.9 | 93,538 | 20,495,848 |
| 2001–2005 | 10–14 | 1,275,797 | 6.2 | 122,263 | 20,634,930 |
| 1996–2000 | 15–19 | 2,752,836 | 13.1 | 266,359 | 21,066,962 |
| 1991–1995 | 20–24 | 5,415,971 | 23.8 | 298,058 | 22,771,013 |
| 1986–1990 | 25–29 | 8,216,431 | 37.0 | 289,680 | 22,180,549 |
| 1981–1985 | 30–34 | 15,639,814 | 72.5 | 246,715 | 21,563,585 |
| 1976–1980 | 35–39 | 19,886,968 | 99.0 | 100,549 | 20,088,551 |
| 1971–1975 | 40–44 | 20,330,987 | 100.0 | 755,208 | 20,330,987 |
| 1966–1970 | 45–49 | 20,792,166 | 100.0 | 775,766 | 20,792,166 |
| 1961–1965 | 50–54 | 21,733,732 | 97.1 | 403,448 | 22,380,634 |
| 1956–1960 | 55–59 | 20,242,589 | 93.7 | 341,126 | 21,595,615 |
| 1951–1955 | 60–64 | 16,813,082 | 89.6 | 301,423 | 18,769,228 |
| 1946–1950 | 65–69 | 9,834,514 | 62.8 | 225,690 | 15,663,276 |
| 1940–1945 | 70–74 | 6,653,362 | 50.9 | 152,093 | 13,061,780 |
*The total population in 2015 was 318,479,402.
Estimates of early life BLLs by birth cohort. Exposure to elevated BLLs follows a “U”-shaped association with relatively low BLLs for cohorts born in the 1940s, increased dramatically for cohorts now middle aged, and decreased dramatically among younger cohorts.
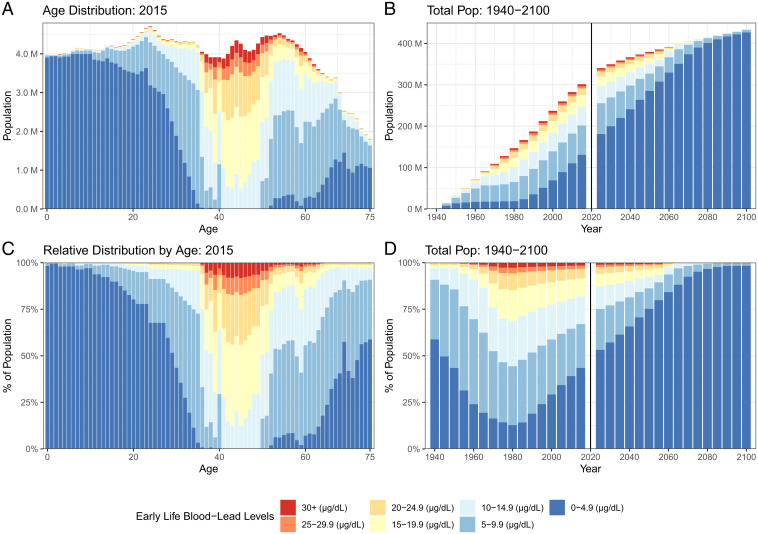
Fig. 2.
Changes in the distribution of childhood BLLs by age (A and C) and over time (B and D). The absolute distribution of BLLs experienced in childhood for US adults in 2015 (A) and the relative distribution (C). The absolute distribution of BLLs (B) and the relative distribution for the entire population over time for the period 1940 to 2100 (D). More than 90% of those born between 1950 and 1980 experienced BLLs in excess of 5 µg/dL, the threshold considered “safe” for children. The legacy of early life lead exposure will stay in the United States for decades to come.
Projections of Population Estimates.
Early childhood lead exposure will remain a hallmark of the US population for the foreseeable future, as shown in Table 3 and Fig. 2 B and D. In 2030, over 43% of the US population will have had BLLs higher than 5 µg/dL in early childhood. A total of 23% of the population will have had BLLs of 10 µg/dL or greater. Fig. 2 B and D also shows that the number of people with legacy lead exposure will decrease dramatically over time (via mortality) until these exposures reflect the most recent and subsequent cohorts.
Table 3.
Projected US population estimates of early life BLLs in 2030*
| Panel A | |||
|---|---|---|---|
| BLL categories (µg/dL) | Population estimates | Percentage of population† | Margin of error (80% confidence) |
| <5 | 199,785,924 | 57.1 | 2,897,704 |
| 5–9.9 | 69,675,603 | 19.9 | 5,178,146 |
| 10–14.9 | 38,501,834 | 11.0 | 2,277,420 |
| 15–19.9 | 22,298,625 | 6.4 | 914,701 |
| 20–24.9 | 11,931,509 | 3.4 | 1,236,306 |
| 25–29.9 | 3,880,403 | 1.1 | 635,120 |
| ≥30 | 3,567,978 | 1.0 | 482,979 |
| Panel B | |||
| ≥5 | 149,855,952 | 42.9 | 5,916,217 |
| ≥10 | 80,180,349 | 22.9 | 2,861,541 |
| ≥15 | 41,678,515 | 11.9 | 1,732,564 |
| ≥20 | 19,379,890 | 5.5 | 1,471,428 |
| ≥25 | 7,448,381 | 2.1 | 797,901 |
*The total projected population in 2030 = 349,641,876.
†The sum of these population estimates is not equal to the population size due to rounding error.
Projections of early life BLLs in 2030. Over 43% of the US population will have had BLLs higher than 5 µg/dL in early childhood and nearly a quarter will have had BLLs of 10 µg/dL or greater. Early childhood lead exposure will remain a hallmark of the US population for the foreseeable future.
Lost Cognitive Ability in the Population.
A total of 824,097,690 million IQ points were lost because of childhood lead exposure among the US population by 2015. This number equates to an average of 2.6 IQ point deficit per person. This average reflects considerable variation by cohort. Estimated lead-linked deficits in cognitive ability were greatest for the 1966 to 1970 cohort (population size ∼20.8 million), which experienced an average deficit of 5.9 IQ points per person. Adjacent cohorts also experienced considerable IQ loss. The 1961 to 1965 cohort experienced a 4.8 IQ point deficit, while the 1971 to 1975 cohort experienced a similar loss of 5.7 IQ points. Because the relative impact of lead exposure on cognitive ability is strongest at lower levels of exposure (i.e., the first units of lead exposure cause the greatest relative harm) (21), we can be reasonably certain that the vast majority of leaded gasoline–exposed cohorts (i.e., those born in the mid-1960s to 1980s) experienced meaningful cognitive loss (>1 IQ point) because of lead exposure. Furthermore, over 7% of the 1966 to 1970 and 1971 to 1975 cohort (which together amounted to nearly 3 million children) had BLLs above 30 µg/dL This exposure corresponded to an average 7.4 IQ point deficit, which is large enough to shift individuals with below average cognitive ability (IQ < 85) into the range of diagnosable intellectual disability (IQ < 70). Projection estimates indicate that IQ loss due to lead exposure will be similar in the near future. By 2030, early childhood lead exposure will have reduced population IQ by 709,054,633 points. This number amounts to an average deficit of 2.03 IQ points per person.
Discussion and Public Health Implications
While current research suggests that millions of today’s adults were exposed to elevated BLLs in early childhood (3), it has not estimated the full scope and effects of decade’s worth of lead exposure for the existing population. We fill this void by quantifying the extent of early childhood exposures in the current population. Our analysis showed considerable variability in the extent of early life BLL exposure, with millions of people having childhood BLLs six or more times higher than the current level of concern, considerable variation in lead exposure across birth cohorts, and considerable, estimated, lead-linked loss in cognitive ability. Furthermore, younger cohorts were exposed to appreciably lower BLLs than other cohorts. Despite this, pronounced exposure to lead in early childhood will remain a hallmark of the US population for the next several decades. Furthermore, even the most recent BLL exposures are historically abnormal. While children today have much lower BLLs than their parents or grandparents did as children, their lead levels are still multiple times higher than their preindustrial ancestors’ levels (24). Additionally, the lead protection gains seen in the United States over the recent past have not been shared internationally: the United Nations Children's Fund estimates that 800 million children worldwide are currently exposed to high levels of lead, largely owing to underregulation of lead-emitting industries in developing countries, particularly battery recycling (25). Moreover, while lead problems in the contemporary United States appear to be dissipating over time in aggregate, thousands of locales across the country have persistent legacy lead problems, many as bad as those seen in Flint during the water crisis (25).
The scope of lead exposure reported here is immense and markedly higher than past work has reported (3, 22, 26). For instance, Boyle et al. (26) recently estimated the number of people exposed to various BLL thresholds during childhood and the resulting loss in grade school–aged IQ points. These authors felt that their results justified concluding that the consequences of lead were best described by phrases such as “astonishingly high” and “alarming loses” and so do we. Their study only covered the years 1999 to 2010, however. We provide such estimates for historical time points when average BLLs were considerably higher (5) and thus find that the size of alarming losses conveyed by Boyle et al. are expanded by several orders of magnitude when we look at older adults’ past exposures. By providing more complete estimates of the number of people exposed to lead in early life, this study makes a considerable step toward understanding the full extent of the damage done to the US population in one specific domain: cognitive ability.
On the individual level, even relatively small deficits in achieved IQ can have a meaningful impact on people’s lives, as cognitive ability, described by IQ, meaningfully predicts a person’s educational and occupational attainment, health, wealth, and happiness (27, 28). Notably, in the multidecade Dunedin Study, Reuben et al. (8) reported a dose–response relationship between childhood lead exposure and mild downward social mobility by midlife. They estimated that 40% of the association was explained by IQ loss.
At the population level, IQ loss estimates are very meaningful. Using smaller estimates of lead exposure and IQ loss than our 824 million lead-linked population IQ point loss, previous studies have reported economic losses to the US economy from lead exposure on the order of between 165 to 319 billion dollars in lost wages (22, 29). The fact that our lead estimates and the IQ loss associated with them are higher underscores the harm done to the US economy more broadly. Such lead loss estimation efforts should be repeated across other domains believed to be influenced by lead exposure (including criminal behavior, personality, psychopathology, social mobility, cardiovascular disease, kidney function, and pathological brain aging). Doing so would generate a more complete understanding of the contributions lead exposures may have made to these important outcomes, the ultimate benefits to society from lead’s removal from gasoline (including, potentially, recent improvements in many of these outcomes) and the costs of ignoring existing lead hazards, including lead’s continued use in aviation fuel in the United States, and its wide underregulated use in commercial and industrial processes internationally (25).
Understanding racial disparities in BLLs across the time—particularly Black/White disparities—are an additional, essential next step for legacy lead-related research. Existing work shows that African Americans have disproportionately higher BLLs at the national level (3). There is no indication that these disparities were smaller in the past. For instance, NHANES identified the average BLL among 3- to 5-y-old children between 1976 to 1980 was 14.9 µg/dL for White children and 20.8 µg/dL for Black children (30). Our own calculations using 1976 to 2015 NHANES data consequently show that most Black adults now under age 45 y experienced considerably higher levels of BLLs in early life than their White counterparts (SI Appendix, Fig. S2), not just those born between 1976 and 1980. Future research should evaluate racial disparities in legacy lead exposure pre-1976. This will inform understanding of lead contributions to health disparities across a multitude of outcomes (e.g., kidney disease, coronary heart disease, and dementia).
Limitations.
While NHANES represents the gold standard for estimating how BLLs have changed over time in the United States, the data derive from several distinct collection efforts. To the extent that the recruitment and sampling procedures led to differing accuracy in providing representative estimates, this study also provided more accurate estimates at some years compared to others. Next, BLLs were predicted rather than observed for the years 1940 to 1975. This meant that several implicit assumptions were made. Most importantly, we made the assumption that the association between leaded gas consumption and observed BLLs from 1976 to 1993 would mirror the association between leaded gas consumption and BLLs from 1940 to 1975. Our prediction of BLLs prior to 1976 did not take into account nongasoline sources of exposure such as urban soils that may have been associated with BLLs in a way that differed between 1940 to 1975 and 1976 to 2016. For instance, it is plausible that the high levels of leaded gasoline consumption in the mid-1970s produced large amounts of lead in urban soils that persisted for decades (31). This limitation, however, is mitigated by a pronounced cross-national body of evidence showing that the magnitude of the increase in BLLs when leaded gasoline consumption increased closely corresponded to the magnitude of the decrease in BLL when leaded gasoline consumption dropped—even when the time intervals corresponding to these changes were staggered across countries (17). Our estimates, based on leaded gasoline consumption, may also have underpredicted high-lead exposures owing to leaded paints and pipes, which tend to aggregate within particular communities (e.g., those with high rates of homes with lead service lines and lead paint in disrepair).
Also, we were unable to gauge the duration of lead exposure in early childhood. Cumulative exposure impedes development in ways that one-time exposures do not (32). Different birth cohorts may have experienced dramatically different levels of exposure across childhood. For example, those born in 1966 likely had consistently high BLLs across childhood while those born in 1980, while being initially exposed to levels similar to their 1966 counterparts, probably experienced marked drops in BLL across childhood. Future work is needed to address this issue.
Next, NHANES samples are not representative with respect to people who immigrated to the United States after 4 y of age. While this is an important limitation, its salience is limited by existing knowledge. First, the data we do have suggests immigrants and nonimmigrants have similar BLLs (33). For instance, 10% of foreign-born children in the United States had BLLs in excess of 10 µg/dL in the 1990s. This is in line with our estimate of ∼9% for the US 1990 cohort. If we were to model foreign-born cohorts, our estimates would likely remain relatively unchanged. Finally, our use of blood–lead assessments instead of bone–lead likely underestimated IQ loss, as BLLs do not fully estimate cumulative exposures (34).
Conclusions
Over half of today’s US population had elevated BLLs in early childhood based on the Center for Disease Control and Prevention’s current threshold for clinical concern. The scope of such widespread exposure, particularly from the late 1950s to the early 1980s, suggests the legacy of lead continues to shape the health and wellbeing of the country in ways we do not yet fully understand. By providing demographic estimates, this study represents an important step toward discerning the full and manifold consequences that lead exposure has placed on the US population. Although these estimates pertain to the United States, the same phenomenon likely occurred in all developed countries (17) and is a current concern in most developing countries (25). Our estimates provide a springboard to understand the global extent that populations were harmed, and continued to be harmed, by legacy lead exposures.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
Data Availability
All data generated or analyzed supported the findings of this research and are included in the supplementary information files.
References
- 1.Zahran S., McElmurry S. P., Sadler R. C., Four phases of the Flint Water Crisis: Evidence from blood lead levels in children. Environ. Res. 157, 160–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuben A., Elliott M., Caspi A., Implications of legacy lead for children’s brain development. Nat. Med. 26, 23–25 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Egan K. B., Cornwell C. R., Courtney J. G., Ettinger A. S., Blood lead levels in U.S. children ages 1-11 Years, 1976-2016. Environ. Health Perspect. 129, 37003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annest J., et al. , Chronological trend in blood lead levels between 1976 and 1980. N. Engl. J. Med. 308, 1373–1377 (1983). [DOI] [PubMed] [Google Scholar]
- 5.Pirkle J. L., et al. , The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 272, 284–291 (1994). [PubMed] [Google Scholar]
- 6.Bellinger D. C., Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 20, 172–177 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Reuben A., et al. , Association of childhood lead exposure with MRI measurements of structural brain integrity in midlife. JAMA 324, 1970–1979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuben A., et al. , Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 317, 1244–1251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bihaqi S. W., Bahmani A., Subaiea G. M., Zawia N. H., Infantile exposure to lead and late-age cognitive decline: Relevance to AD. Alzheimers Dement. 10, 187–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanphear B. P., Rauch S., Auinger P., Allen R. W., Hornung R. W., Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 3, e177–e184 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Reuben A., Childhood lead exposure and adult neurodegenerative disease. J. Alzheimers Dis. 64, 17–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Disease Control and Prevention, National health and nutritional examination survey Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 14 February 2022.
- 13.Center for Disease Control and Prevention, NHANES II (1976-1980). Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/nhanes2/default.aspx. Accessed 6 September 2021.
- 14.Center for Disease Control and Prevention, NHANES III (1988-1994). Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx. Accessed 6 September 2021.
- 15.Center for Disease Control and Prevention, Questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/default.aspx. Accessed 6 September 2021.
- 16.Thomas V. M., The elimination of lead in gasoline. Annu. Rev. Energy Environ. 20, 301–324 (1995). [Google Scholar]
- 17.Thomas V. M., Socolow R. H., Fanelli J. J., Spiro T. G., Effects of reducing lead in gasoline: An analysis of the international experience. Environ. Sci. Technol. 33, 3942–3948 (1999). [Google Scholar]
- 18.US Geological Survey, National Minerals Information Center; Bureau of Minerals and Mines Yearbook (1932-1993). USGS. https://www.usgs.gov/centers/national-minerals-information-center/bureau-mines-minerals-yearbook-1932-1993. Accessed 9 September 2021.
- 19.Database H. M., University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available at www.mortality.org or www.humanmortality.de (data downloaded on 6/1/2021).
- 20.United Nations Database, Department of Economic and Social Affairs, Population Division, World Population Prospects 2019: Methodology of the United Nations Population Estimates and Projections (United Nations, Department of Economic and Social Affairs, Population Division, 2019). [Google Scholar]
- 21.Lanphear B. P., et al. , Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 113, 894–899 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould E., Childhood lead poisoning: Conservative estimates of the social and economic benefits of lead hazard control. Environ. Health Perspect. 117, 1162–1167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulson J. A., Brown M. J.. The CDC blood lead reference value for children: Time for a change. Environ. Health 18, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegal A. R., Smith D. R., Lead levels in preindustrial humans. N. Engl. J. Med. 326, 1293–1294 (1992). [PubMed] [Google Scholar]
- 25.Rees N., Fuller R., The toxic truth: Children’s exposure to lead pollution undermines a generation of future potential. United Nations Children’s Fund. https://www.unicef.org/reports/toxic-truth-childrens-exposure-to-lead-pollution-2020. Accessed 9 September 2020.
- 26.Boyle J., Yeter D., Aschner M., Wheeler D. C., Estimated IQ points and lifetime earnings lost to early childhood blood lead levels in the United States. Sci. Total Environ. 778, 146307 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Kilgour A. H. M., Starr J. M., Whalley L. J., Associations between childhood intelligence (IQ), adult morbidity and mortality. Maturitas 65, 98–105 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Ali A., et al. , The relationship between happiness and intelligent quotient: The contribution of socio-economic and clinical factors. Psychol. Med. 43, 1303–1312 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Grosse S. D., Matte T. D., Schwartz J., Jackson R. J., Economic gains resulting from the reduction in children’s exposure to lead in the United States. Environ. Health Perspect. 110, 563–569 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahaffey K. R., Annest J. L., Roberts J., Murphy R. S., National estimates of blood lead levels: United States, 1976-1980: Association with selected demographic and socioeconomic factors. N. Engl. J. Med. 307, 573–579 (1982). [DOI] [PubMed] [Google Scholar]
- 31.Laidlaw M. A., Filippelli G. M., Resuspension of urban soils as a persistent source of lead poisoning in children: A review and new directions. Appl. Geochem. 23, 2021–2039 (2008). [Google Scholar]
- 32.Schwaba T., et al. , The impact of childhood lead exposure on adult personality: Evidence from the United States, Europe, and a large-scale natural experiment. Proc. Natl. Acad. Sci. U.S.A. 118, e2020104118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC), Elevated blood lead levels among internationally adopted children--United States, 1998. MMWR Morb. Mortal. Wkly. Rep. 49, 97–100 (2000). [PubMed] [Google Scholar]
- 34.Wasserman G. A., et al. , The relationship between blood lead, bone lead and child intelligence. Child Neuropsychol. 9, 22–34 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed supported the findings of this research and are included in the supplementary information files.