Abstract
In this observational cross-sectional study, we investigated predictors of orthostatic intolerance (OI) in adults reporting long COVID symptoms. Participants underwent a 3-min active stand (AS) with Finapres® NOVA, followed by a 10-min unmedicated 70° head-up tilt test. Eighty-five participants were included (mean age 46 years, range 25–78; 74% women), of which 56 (66%) reported OI during AS (OIAS). OIAS seemed associated with female sex, more fatigue and depressive symptoms, and greater inability to perform activities of daily living (ADL), as well as a higher heart rate (HR) at the lowest systolic blood pressure (SBP) point before the first minute post-stand (mean HRnadir: 88 vs. 75 bpm, P = 0.004). In a regression model also including age, sex, fatigue, depression, ADL inability, and peak HR after the nadir SBP, HRnadir was the only OIAS predictor (OR = 1.09, 95% CI: 1.01–1.18, P = 0.027). Twenty-two (26%) participants had initial (iOH) and 5 (6%) classical (cOHAS) orthostatic hypotension, but neither correlated with OIAS. Seventy-one participants proceeded to tilt, of which 28 (39%) had OI during tilt (OItilt). Of the 53 who had a 10-min tilt, 7 (13%) had an HR increase >30 bpm without cOHtilt (2 to HR > 120 bpm), but six did not report OItilt. In conclusion, OIAS was associated with a higher initial HR on AS, which after 1 min equalised with the non-OIAS group. Despite these initial orthostatic HR differences, POTS was infrequent (2%). ClinicalTrials.gov Identifier: NCT05027724 (retrospectively registered on August 30, 2021).
Keywords: long COVID, orthostatic intolerance, haemodynamics, tilt table test, postural orthostatic tachycardia syndrome
Introduction
Long COVID or post-COVID-19 syndrome first gained recognition among social support groups and later in scientific and medical communities (Yong, 2021). This condition is not well understood as it affects COVID-19 survivors at all ages and levels of disease severity, with or without pre-existing comorbidities, and regardless of hospitalisation status (Vanichkachorn et al., 2021; Yong, 2021). A common symptom is fatigue, with or without organ-specific symptoms (Jennings et al., 2021; Rogers et al., 2021), which may result in negative impacts on resumption of functional and occupational activities (Yan et al., 2021). A systematic review reported that symptoms of mild COVID-19 may persist after 3 weeks in a third of patients (van Kessel et al., 2021). Another study reported that up to one in four patients with mild COVID-19 were still experiencing symptoms after 1 year (Rank et al., 2021); however, data on the exact prevalence and long-term effects of long COVID are still lacking (Zarei et al., 2021), with an urgent need for research in different populations and settings (Michelen et al., 2021). To aid clinicians and researchers, on October 6, 2021, the World Health Organization (WHO) issued a clinical case definition of post-COVID-19 condition, obtained by a Delphi consensus (WHO, 2021), as follows: “Post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others, which generally have an impact on everyday functioning. Symptoms may be new onset, following initial recovery from an acute COVID-19 episode, or persist from the initial illness. Symptoms may also fluctuate or relapse over time.”
The neurological and cardiovascular overlap in some long COVID symptoms, and in particular the reported occurrence of orthostatic intolerance (OI) (Dani et al., 2021; Paterson et al., 2021; Shah et al., 2021), have raised the hypothesis as to whether some long COVID patients could have measurable autonomic nervous system impairments (Del Rio et al., 2020; Goldstein, 2020; Keyhanian et al., 2020; Barizien et al., 2021; Becker, 2021; Larsen et al., 2021) such as orthostatic hypotension (OH) or postural orthostatic tachycardia syndrome (POTS) (Blitshteyn and Whitelaw, 2021; Johansson et al., 2021; Raj et al., 2021). In this light, we conducted a cross-sectional observational study on a cohort of participants reporting long COVID symptoms to fulfil the following objectives: (1) establish the prevalence of OI, both during an active stand (AS) test and a tilt test; (2) establish the prevalence of OH and POTS in this cohort; and (3) study haemodynamic and non-haemodynamic predictors of OI.
Materials and Methods
Study and Cohort Description
This was a cross-sectional observational study on a participant cohort recruited for the TROPIC (Technology-assisted solutions for the Recognition of Objective Physiological Indicators of post-Coronavirus-19 fatigue) investigation at Trinity College Dublin and St James’s Hospital Dublin, Ireland. The study received full ethical and regulatory approvals. For the reporting, we followed STROBE guidelines (von Elm et al., 2007).
Participants were eligible for inclusion under all the following criteria: (1) age 18 years or older; (2) self-reported history of SARS-CoV-2 infection; (3) experiencing prolonged symptoms such as fatigue; (4) able to mobilise independently (with or without aid); (5) able to transfer independently or with minimal assistance of one person from lying to standing; and (6) able to give informed consent.
Participants were recruited from the following sources in our hospital: (1) falls and syncope unit; (2) geriatric day hospital; (3) post-COVID-19 outpatient clinic; (4) staff who had contracted COVID-19; and (5) participants from earlier post-COVID-19 research who had consented to be contacted for further studies. In addition, we also considered (6) self-referrals. COVID-19 and non-COVID-19 exclusion criteria for enrolment are outlined in the section 1 in Supplementary Material.
Prior to enrolment, participants were provided with a Participant Information Leaflet explaining the aims and procedures of the study. All participants provided explicit, informed, and voluntary consent to partake in the study, were explained the benefits and risks of participating in the research, and had the opportunity to discuss the study and ask questions. Participants were given the opportunity to withdraw from the study at any point and to forego completing components of the assessment protocol as desired.
Procedures
Participants underwent a 3-min AS with Finapres® NOVA, followed by a 10-min unmedicated 70° head-up tilt test. During both, participants had frontal lobe oxygenation monitoring via PortaLite® near-infrared spectroscopy (NIRS). All testing procedures complied with the local hand hygiene, sanitation, personal protective equipment (PPE), and research training protocols. We also considered international best practice recommendations for autonomic testing during the COVID-19 pandemic (Figueroa et al., 2020; Guaraldi et al., 2020; Sinn et al., 2021).
For the active stand, participants underwent a lying-to-standing orthostatic test with non-invasive beat-to-beat blood pressure monitoring using digital photoplethysmography (Finapres® NOVA, Finapres Medical Systems, Amsterdam, Netherlands). The height correction unit was zeroed and implemented as per manufacturer’s specifications. A 5-lead continuous electrocardiogram (ECG) was acquired throughout the test. During the supine rest period, an oscillometric brachial blood pressure measurement was obtained from the non-monitored (right) arm for calibration purposes, once the PhysioCal repetition rate was 70 beats or more (Wesseling, 1996). After at least 5 min of uninterrupted supine rest, a total lying duration of no more than 10 min, and a 10-s countdown, participants were asked to stand, unaided, as quick as possible. The PhysioCal was turned off just before the stand and switched back on at 1-min post-stand. After standing, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were monitored for 3 min. Throughout the recording, participants were asked to remain motionless and in silence, except for reporting any symptoms of concern, with the monitored arm (left) resting extended by the side. Immediately after the stand, and at the end of the test, participants were asked to report whether they had felt any symptoms of dizziness, light-headedness, palpitations, or any other abnormal symptoms.
For the tilt procedure, which was medically supervised and started after a brief non-monitored break following AS, participants were affixed to an electrically motorised tilt table with footboard support and approximately 10 s of travel time between 0° and 70° (Agasan KT-1060/E, AGA Sanitätsartikel GmbH, Löhne, Germany). Throughout the tilt, participants underwent Finapres® NOVA monitoring (with PhysioCal on and continuous ECG monitoring) during an initial period of uninterrupted supine rest of at least 5 min (with a total lying duration of no more than 10 min) and a subsequent head-up tilt to 70° for 10 min or until symptoms developed. An oscillometric brachial blood pressure was also obtained during supine rest. During the head-up tilt phase, participants were asked to report whether they felt any symptoms of dizziness, light-headedness, palpitations, or any other abnormal symptoms, at which point participants were offered to be tilted down. Even without symptoms, if the head-up tilt elicited hypotension (defined as SBP < 90 mmHg), the tilt was aborted in compliance with the pre-specified safety protocol.
For the NIRS-based monitoring of regional cerebral oxygenation of the left frontal lobe during both AS and tilt, we used an optical sensor (PortaLite®, Artinis Medical Systems B.V., Elst, Netherlands), applied approximately 3 cm to the left of the midline of the forehead and 3.5 cm above the bridge of the nose. A close-woven bandage was affixed around the head over the sensor to remove ambient lighting and to exert comfortable pressure for effective contact between the probe and the skin.
Haemodynamic Data Extraction
For SBP, DBP, and HR, values were noted at the various timepoints of AS and head-up tilt from the Finapres® NOVA display screen in accordance with the following standard operating procedure (SOP): baseline values were collected at 60 s prior to AS or head-up tilt, and subsequently at the start of every minute after each procedure. As regards nadir values, for the AS they were noted at the lowest point of SBP following completion of standing and prior to the first-minute post-stand; in the case of the tilt, they were noted at the lowest point of SBP reached between completion of the head-up tilt manoeuvre and prior to the first-minute post-tilt. For the AS, we also modelled the peak HR after the nadir SBP, defined as the maximum of the HR readings obtained at 1, 2, and 3 min. NIRS values were noted following the same SOP from a laptop display connected to the PortaLite® device via OxySoft® software (version 3.2.70), from which we extracted Tissue Saturation Index (TSI) values as the percentage ratio of oxygenated haemoglobin concentration to the total concentration of haemoglobin (Claffey et al., 2020).
Orthostatic Intolerance
For both AS and tilt, OI was defined as self-reported symptoms of dizziness, light-headedness, palpitations, or any other new abnormal symptoms occurring after the orthostatic manoeuvre.
Orthostatic Hypotension Definitions
Initial orthostatic hypotension (iOH) on AS was defined as a difference of >40 mmHg SBP and/or >20 mmHg DBP between baseline and nadir values (Freeman et al., 2011).
Classical orthostatic hypotension on AS (cOHAS) was defined as a difference of ≥20 mmHg SBP and/or ≥10 DBP between each baseline value and its minimum reading between minutes 1, 2, and 3 (Freeman et al., 2011; Brignole et al., 2018). Nadir values were not included in this definition for clear differentiation with iOH and to better reflect cOHAS as normally measured in routine clinical practice with an interval measurement device (Breeuwsma et al., 2018).
Classical orthostatic hypotension on tilt (cOHtilt) was defined as a difference of ≥20 mmHg SBP and/or ≥10 DBP between each baseline value and its minimum reading between nadir and minutes 1, 2, and 3. Nadir was included in this case because iOH is only associated with active rising (Wieling et al., 2007).
Postural Orthostatic Tachycardia Syndrome Definition
We computed the maximum HR between nadir and minutes 1–10 (or the available minutes in case of early tilt termination), to which we subtracted baseline HR. POTS was defined as HR increase >30 bpm or to >120 bpm within 10 min of tilt in the absence of OHtilt and presence of OItilt (Freeman et al., 2011; Brignole et al., 2018).
Other Measures
For the characterisation of the cohort, we collected measures including:
-
•
Demographics: age, sex.
-
•
Anthropometrics: body mass index (BMI) (kg/m2).
-
•
Proportion of third level education (i.e., primary university degree or higher).
-
•
Past medical history including previous or current smoker, hypertension, heart disease (e.g., previous heart attack, angina, congestive heart failure, atrial fibrillation), diabetes mellitus (yes or no).
-
•
Current medications including being on an antihypertensive, beta blocker, antidepressant, or benzodiazepine (yes or no).
-
•
COVID-19 history: date of COVID-19 diagnosis; hospitalisation status (at least 1 overnight stay: yes or no); current symptomatology (from a structured questionnaire including 41 possible symptoms: yes or no for each), and interference with activities of daily living (ADL) (“In the past month, I have had too little energy to do the things I wanted to do”: yes or no).
-
•
The 11-item Chalder Fatigue Scale (CFQ), a self-rating scale developed to measure the severity of physical and mental fatigue (Cella and Chalder, 2010). We employed the Likert scoring system, with an overall scale range from 0 (minimum) to 33 (maximum fatigue).
-
•
The 20-item Center for Epidemiological Studies Depression (CES-D) scale (Radloff, 1977). Scores range from 0 to 60, with higher scores indicating greater depressive symptoms.
-
•
The 22-item Impact of Event Scale—Revised (IES-R) (Creamer et al., 2003), which measured post-traumatic stress disorder (PTSD) symptoms in specific relation to participants’ COVID-19 illness (minimum: 0; maximum: 88).
-
•
Five chair stands time as a measure of functional lower extremity strength (Munoz-Bermejo et al., 2021): time (in seconds) it took a participant to transfer as quick as possible from a seated to a standing position and back to sitting five times.
Statistical Analyses
Statistics were computed with IBM® SPSS® Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp. Descriptives were given with count and percentage (%), mean with standard deviation (SD), median with interquartile range (IQR), and range. Normality of continuous variables was assessed with the one-samples Kolmogorov–Smirnov test. We utilised the SPSS Chart Builder to visualise haemodynamic differences between subgroups via cluster line chart with representation of 95% confidence intervals (CI) around means. To compare characteristics between subgroups, we utilised the non-parametric two-sided Mann–Whitney U test for non-normally distributed continuous variables, and the Chi-square test for dichotomous characteristics. In the latter case, we used the 2-sided Fisher’s exact test when at least one cell had an expected count of <5. In addition, considering the repeated measures nature of the haemodynamic data, we conducted two-way ANOVA tests and calculated the within-subjects’ effects P-values (sphericity-assumed) for the interaction between time and OI groups. Bonferroni was the post hoc test used for pairwise comparisons. To establish independent predictors of dichotomous group membership, we computed logistic regression models, and for each predictor extracted the Odds Ratio (OR) and 95% CI for the OR. Multicollinearity checks were conducted. Statistical significance was defined as P < 0.05.
Ethical Approval
This study received full approval by the St James’s Hospital/Tallaght University Hospital Joint Research Ethics Committee (Submission Number: 104: TROPIC; Approval Date: May 4, 2021) and the St James’s Hospital Research & Innovation Office (Reference: 6566; Approval Date: May 14, 2021). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in the study. All aspects of the study were executed in compliance with the General Data Protection Regulation (GDPR), and Irish regulations including the Health Research Regulations and the Data Protection Act 2018.
Results
Of 92 consecutive participants recruited to the study between May and September 2021, 85 (92.4%) had the AS. Mean age was 46.0 years (SD 10.2, range 25–78), and 63 (74.1%) were women. Overall, fatigue was a very prevalent long COVID symptom in this cohort (93.5%), with other common (>50%) symptoms being shortness of breath (69.6%), sleeping problems (65.2%), ongoing headaches (64.1%), dizziness (63.0%), heart palpitations (60.9%), brain fog (59.8%), muscular pain (54.3%), and chest tightness (53.3%). Table 1 shows additional descriptives of the 85 participants who had the AS. 36.5% had a BMI in the obesity range (≥30 kg/m2), and 1.2% in the underweight range (<18.5 kg/m2). The majority (62.4%) had third level education and 42.4% were current or former smokers. Other than hypertension (17.6%), prevalences of heart disease and diabetes were very low (<5%), and there were no instances of Parkinson’s disease or other known conditions with risk of autonomic impairment. A fifth were on antidepressant medications and less than 20% were on antihypertensives, beta blockers, or benzodiazepines. In terms of COVID-19 history, all but two participants were at least 3 months from the onset of COVID-19, a quarter had been hospitalised, and 81.2% met criteria for WHO clinical case definition of post-COVID-19 condition. Median scores for CFQ, CES-D, and IES-R were 26, 21, and 26, respectively.
TABLE 1.
Clinical characteristics of the overall cohort, as well as comparison between OIAS and non-OIAS subgroups.
| Characteristic | Overall cohort (n = 85) | No OIAS (n = 29) | OIAS (n = 56) | P |
| Mean age, years (SD) | 46.0 (10.2) (range 25–78) | 49.1 (11.9) | 44.5 (9.0) | 0.075a |
| Female sex (%) | 74.1 | 58.6 | 82.1 | 0.019b* |
| Mean BMI, kg/m2 (SD) | 28.3 (5.1) | 27.2 (4.3) | 28.9 (5.4) | 0.148a |
| Mean 5-chair stands time, seconds (SD) | 15.0 (10.4) | 12.8 (5.1) | 16.4 (12.4) | 0.409a |
| Third level education (%) | 62.4 | 53.8 | 69.6 | 0.164b |
| Previous or current smoker (%) | 42.4 | 56.0 | 40.0 | 0.182b |
| History of hypertension (%) | 17.6 | 20.7 | 16.1 | 0.596b |
| History of heart disease (%) | 3.5 | 6.9 | 1.8 | 0.267c |
| History of diabetes (%) | 3.5 | 6.9 | 1.8 | 0.267c |
| On antihypertensive (%) | 16.5 | 24.1 | 12.5 | 0.220c |
| On beta blocker (%) | 15.3 | 13.8 | 16.1 | 1.000c |
| On antidepressant (%) | 20.0 | 17.2 | 21.4 | 0.647b |
| On benzodiazepine (%) | 3.5 | 3.4 | 3.6 | 1.000c |
| Median days post-COVID-19 diagnosis (IQR) | 302.0 (333.0) (range 39–655) | 249.0 (353.5) | 317.5 (297.8) | 0.628a |
| Hospitalised with COVID-19 (%) | 25.9 | 26.9 | 27.8 | 0.936b |
| At least 3 months (>91 days) from the onset of COVID-19 (%) | 97.6 | 96.0 | 98.1 | 0.547c |
| Post-COVID-19 symptoms for at least 2 months (%) | 98.8 | 100 | 98.1 | 1.000c |
| In the past month, I have had too little energy to do the things I wanted to do (%) | 83.5 | 76.0 | 96.3 | 0.011c |
| Median CFQ score (IQR) | 26.0 (8.0) | 24.0 (10.0) | 27.0 (7.8) | 0.042a* |
| Median CES-D score (IQR) | 21.0 (17.0) | 16.0 (16.8) | 24.0 (16.0) | 0.021a* |
| Median IES-R score (IQR) | 25.5 (28.8) | 18.5 (30.3) | 31.0 (28.5) | 0.106a |
| IOH | 25.9 | 33.3 | 34.1 | 0.952b |
| cOHAS | 5.9 | 3.6 | 7.5 | 0.654c |
OIAS, orthostatic intolerance during active stand; SD, standard deviation; BMI, body mass index; IQR, interquartile range; CFQ, Chalder Fatigue Scale; CES-D, Center for Epidemiological Studies Depression scale; IES-R, Impact of Event Scale—Revised; iOH, initial orthostatic hypotension; cOHAS, classical orthostatic hypotension during active stand. aTwo-sided Mann–Whitney U test; bChi-square test; ctwo-sided Fisher’s exact test; *statistically significant (P < 0.05).
During the AS, 56 participants (65.9%) reported OIAS. The frequencies of OIAS symptoms were as follows: “slightly light-headed” (n = 31, 55.4%), “light-headed” (n = 15, 26.8%), “dizzy” (n = 5, 8.9%), “slightly dizzy” (n = 4, 7.1%), and “very light-headed” (n = 1, 1.8%). Two of the 85 participants had an early AS termination due to non-hypotensive/cardiac OIAS symptoms (n = 1 before the first minute, and n = 1 before the third minute). Table 1 shows the comparison between OIAS and non-OIAS subgroups. In a survey, 22 (25.9%) participants fulfilled the criteria for iOH, and 5 (5.9%) for cOHAS, and neither of the two (P = 0.952 and P = 0.654, respectively) were significantly associated with OIAS. OIAS was more likely in women (P = 0.019) and was associated with higher CFQ (P = 0.042) and CES-D (P = 0.021) scores. The presence of OIAS was more likely to be associated with the ADL impairment criterion used for our application of the WHO clinical case definition (P = 0.011), with virtually all participants with OIAS (96.3%) reporting too little energy to do the things they wanted to do in the past month.
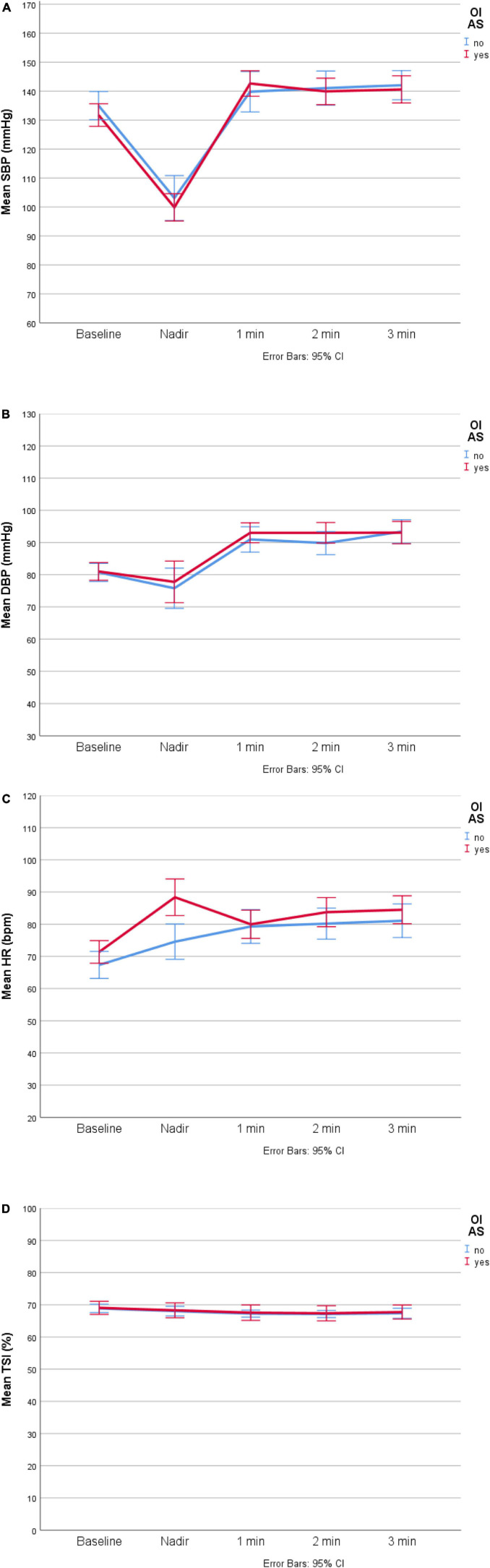
In terms of the haemodynamic comparison between OIAS and non-OIAS subgroups (Table 2), participants reporting OIAS had a higher HR at the lowest SBP point before the first-minute post-stand (mean HRnadir: 88 vs. 75 bpm, P = 0.004; two-way ANOVA: P < 0.001 for the main effect of time, P = 0.006 for the interaction OIAS*time, and P = 0.033 for the Bonferroni-adjusted post hoc analysis). There were no baseline or subsequent HR differences, or any BP or NIRS differences. In the haemodynamic visualisation in Figure 1, participants’ finishing BP levels (at 3 min) seemed higher than at baseline, with 95% CIs around means that clearly did not overlap in the case of DBP (panel B), but without any suggested differences between OIAS and non-OIAS subgroups. On closer inspection, for the overall cohort, there was a statistically significant difference between baseline and 3-min DBP (mean 81.0 vs. 93.1 mmHg, paired-samples t-test P < 0.001).
TABLE 2.
Haemodynamic comparison between OIAS and non-OIAS subgroups.
| No OIAS (initial and final n = 29) | OIAS (initial n = 56) (final n = 54) | P | |
| Mean oscillometric baseline SBP, mmHg (SD) | 131.9 (13.3) (range 103–158) | 131.2 (15.0) (range 106–169) | 0.541a |
| AS: mean baseline SBP, mmHg (SD) | 135.0 (12.8) | 131.7 (14.3) | 0.216a |
| AS: mean nadir SBP, mmHg (SD) | 103.1 (18.9) | 99.9 (16.6) | 0.500a |
| AS: mean SBP at 1 min, mmHg (SD) | 139.8 (18.0) | 142.6 (15.8) | 0.621a |
| AS: mean SBP at 2 min, mmHg (SD) | 141.0 (15.2) | 139.9 (16.7) | 0.570a |
| AS: mean SBP at 3 min, mmHg (SD) | 142.0 (12.8) | 140.6 (17.0) | 0.328a |
| Mean oscillometric baseline DBP, mmHg (SD) | 80.7 (7.9) (range 63–97) | 80.9 (9.7) (range 66–109) | 0.700a |
| AS: mean baseline DBP, mmHg (SD) | 80.7 (7.4) | 81.0 (10.1) | 0.947a |
| AS: mean nadir DBP, mmHg (SD) | 75.8 (13.3) | 77.8 (20.2) | 0.868a |
| AS: mean DBP at 1 min, mmHg (SD) | 91.0 (10.2) | 93.0 (11.1) | 0.419a |
| AS: mean DBP at 2 min, mmHg (SD) | 89.8 (9.3) | 93.0 (11.7) | 0.233a |
| AS: mean DBP at 3 min, mmHg (SD) | 93.4 (9.3) | 93.1 (12.6) | 0.680a |
| AS: mean baseline HR, bpm (SD) | 67.3 (11.0) (range 49–94) | 71.4 (12.9) (range 50–113) | 0.210a |
| AS: mean nadir HR, bpm (SD) | 74.6 (12.3) | 88.4 (19.6) | 0.004a* |
| AS: mean HR at 1 min, bpm (SD) | 79.3 (13.5) | 80.0 (15.8) | 0.948a |
| AS: mean HR at 2 min, bpm (SD) | 80.2 (12.5) | 83.7 (16.6) | 0.451a |
| AS: mean HR at 3 min, bpm (SD) | 81.1 (13.2) | 84.5 (15.6) | 0.352a |
| AS: peak HR after the nadir SBP, bpm (SD) | 83.1 (13.7) | 86.5 (16.2) | 0.434a |
| AS: mean baseline TSI,% (SD) | 68.9 (3.3) (range 61–78) | 69.1 (7.4) (range 32–82) | 0.852a |
| AS: mean nadir TSI,% (SD) | 68.0 (3.8) | 68.3 (8.3) | 0.510a |
| AS: mean TSI at 1 min,% (SD) | 67.3 (2.8) | 67.6 (8.4) | 0.384a |
| AS: mean TSI at 2 min,% (SD) | 67.1 (2.8) | 67.4 (8.3) | 0.490a |
| AS: mean TSI at 3 min,% (SD) | 67.4 (3.1) | 67.8 (6.1) | 0.878a |
AS, active stand; OIAS, orthostatic intolerance during AS; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; bpm, beats per minute; TSI, tissue saturation index. Two of the 85 participants had an early AS termination (n = 1 before the first minute, n = 1 before the third minute), both due to non-hypotensive/cardiac OIAS symptoms. aTwo-sided Mann–Whitney U test; *statistically significant (P < 0.05). Two-way ANOVA P-values for the interaction: SBP: P = 0.359; DPB: P = 0.887; HR: P = 0.006 (see further details in the Section “Results”); NIRS: P = 0.281.
FIGURE 1.
∣ Haemodynamic visualisation of OIAS (n = 56) and non-OIAS (n = 29) groups. (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). (C) Heart rate (HR). (D) Tissue saturation index (TSI). bpm, beats per minute; CI, confidence interval.
In the logistic regression model to investigate predictors of OIAS (Table 3), the only significant predictor after controlling for age, sex, fatigue, depression, ADL inability, and peak HR after the nadir SBP, HRnadir was the only OIAS predictor (OR = 1.09, 95% CI: 1.01–1.18, P = 0.027).
TABLE 3.
Logistic regression model with predictors of OIAS.
| OR | 95% CI for OR |
P | ||
| Lower | Upper | |||
| Age | 0.98 | 0.91 | 1.05 | 0.604 |
| Female sex | 1.97 | 0.32 | 12.06 | 0.463 |
| CFQ score | 0.96 | 0.80 | 1.16 | 0.666 |
| CES-D score | 1.07 | 0.99 | 1.16 | 0.080 |
| In the past month, I have had too little energy to do the things I wanted to do | 4.48 | 0.32 | 62.08 | 0.263 |
| HR at nadir | 1.09 | 1.01 | 1.18 | 0.027 |
| Peak HR after the nadir SBP | 0.97 | 0.89 | 1.06 | 0.495 |
AS, active stand; CFQ, Chalder Fatigue Scale; CES-D, Center for Epidemiological Studies Depression scale; HR, heart rate; SBP, systolic blood pressure; OR, odds ratio; CI, confidence interval.
Of the 85 participants who had the AS, 71 (83.5%) had a tilt table test. In a survey, 14 participants did not have a tilt for reasons including history of recurrent vasovagal syncope (at least two lifetime episodes), a body weight >120 kg (tilt table safety limit) or not consenting. All tilt participants had had the AS test. Of them, 28 (39.4%) had OI during tilt (OItilt). The frequencies of OItilt symptoms were as follows: “slightly light-headed” (n = 10, 35.7%), “light-headed” (n = 8, 28.6%), “slightly dizzy” (n = 3, 10.7%), “dizzy” (n = 2, 7.1%), “very light-headed” (n = 1, 3.6%), “palpitations” (n = 1, 3.6%), “head spinning” (n = 1, 3.6%), “drained” (n = 1, 3.6%), and “weak” 1 (n = 1, 3.6%). No instances of arrhythmia or acute myocardial ischaemia were detected in the continuous ECG trace. As regards OI agreement between AS and tilt, 78.6% (n = 22) of those who had OItilt had previously reported OIAS (P = 0.020). In a survey, 18 of the 71 participants had an early tilt termination due to symptoms (n = 2 before the second minute, n = 3 before the third minute, n = 1 before the fourth minute, n = 2 before the fifth minute, n = 5 before the sixth minute, n = 2 before the eighth minute, and n = 3 before the 10th minute). Of all the early terminations, three did not relate to development of OItilt symptoms. No pre-syncope or syncope occurred in any of the participants. All OItilt symptoms were reported as transient.
Section 2 in the Supplementary Material shows the comparison between OItilt and non-OItilt subgroups. In a survey, 22 participants (33.3% among the 66 with a tilt of at least 3 min) fulfilled criteria for cOHtilt, which was not significantly associated with OItilt (P = 0.916). Of the 53 who had a 10-min tilt, 7 (13%) had an HR increase > 30 bpm without OHtilt (2 to HR > 120 bpm), but six did not report OItilt. POTS was therefore present in n = 1 (1.9%). In the 18 participants whose tilt was terminated early, none of the available data fulfilled POTS criteria. As shown in the Supplementary Material, there were no statistically significant differences between OItilt subgroups across other clinical (section 2) or haemodynamic characteristics (sections 3, 4), and no significant predictors of OItilt in the regression model (section 5).
Discussion
In this study, we investigated predictors of OI in adults reporting long COVID symptoms. OI during active stand (OIAS) was reported by 66% of our sample and seemed associated with female sex, more fatigue and depressive symptoms, and greater inability to perform ADL, as well as a higher heart rate at the lowest systolic blood pressure point before the first-minute post-stand (HRnadir). In a regression model also including age, sex, fatigue, depression, ADL inability, and peak HR after the nadir SBP, HRnadir was the only OIAS predictor. 26% of participants had initial and 6% cOHAS, but neither correlated with OIAS. Of the participants who had a tilt, 39% had OI during tilt, and 33% had cOHtilt; and of the participants who completed a 10-min tilt, only 2% (n = 1) fulfilled POTS criteria.
The HR at the time of nadir SBP after stand seemed more important than the peak HR after the nadir SBP as a predictor of OIAS. In this light, findings might reflect different baroreceptor-related HR responses in participants with OIAS, possibly due to lower efferent vagus nerve activity, and/or higher sympathetic activation. In this regard, it has been described that with incomplete loss of baroreflex afferents, a mild syndrome of orthostatic tachycardia or OI may appear; in some cases, it may primarily reflect interruption of efferent right vagus nerve activity, leading to a loss of parasympathetic input to the sinus node, with a consequent increase in heart rate; and in other cases, mild sympathetic activation may occur with stress and provoke tachycardia disproportionate to the increase in blood pressure (Ketch et al., 2002). Indeed, other authors have described the possibility of depressed vagal tone (Leitzke et al., 2020) with or without baroreceptor dysfunction that may lead to tachycardia and heightened cardiac contractility, vascular resistance, and venous return (Becker, 2020).
While SARS-CoV-2 might be able to affect neurovascular integrity via direct cytotoxic or indirect pro-inflammatory mechanisms (Khosravani, 2021), our results are in the context of a high burden of psychological symptoms, which is in keeping with other reports (Qi et al., 2021; Bucciarelli et al., 2022). Given our recruitment focus, the proportion of fatigue in our cohort was higher than elsewhere (Akbarialiabad et al., 2021; Lopez-Leon et al., 2021; Sanchez-Ramirez et al., 2021; Sandler et al., 2021). For contextualisation to our cohort, previous research showed that a CFQ score of 29 discriminated between chronic fatigue sufferers and controls in 96% of cases (Cella and Chalder, 2010); CES-D scores of 16 or greater can signal risk for clinical depression (Lewinsohn et al., 1997); and an IES-R score of 33 and above is suggestive of PTSD (Creamer et al., 2003). Even though in our regression model the HRnadir finding seemed to eclipse previously significant univariate associations with depression and fatigue/ADL inability, adverse psychological states may influence the behaviour of the autonomic nervous system (Peckerman et al., 2003; De Vos et al., 2017); furthermore, in susceptible individuals, discrepancies between predicted and experienced interoceptive signals may engender anxiety during an acute physiological arousal (such as an active stand), which may manifest as transient tachycardia (Miglis and Muppidi, 2017; Owens et al., 2018).
In our cohort there seemed to be evidence of diastolic orthostatic hypertension, fulfilling on average the criterion of a rise in DBP ≥ 10 mmHg within 3 min following AS (Jordan et al., 2020). The pathological significance of this finding is not clear and merits further investigation; indeed, orthostatic hypertension has been found in healthy subjects but also associated with higher (including hypertension) (Jordan et al., 2020) and lower (Wijkman et al., 2016) cardiovascular risks, with more research still needed to clarify its mechanisms and impacts (Jordan et al., 2020). Interestingly, even though orthostatic hypertension did not seem related to OI in our cohort, it has been described that some patients with chronic OI develop symptoms despite a hypertensive response to standing, suggesting that the symptoms of chronic OI may somehow be elicited by central responses to the inappropriate tachycardia, even in the absence of any actual reduction in perfusion pressure (Narkiewicz and Somers, 1998).
In a previous study where autonomic testing was conducted a median of 119 days following acute COVID-19 infection, 22% of patients fulfilled the criteria for POTS (Shouman et al., 2021), in contrast with 2% in our sample with a median delay to testing of 302 days. While six of our seven tilt participants with HR increase >30 bpm and without OHtilt had chronic symptoms of OI lasting at least 6 months, only one had OItilt during testing. A previous case report showed no improvement in COVID-19-associated POTS symptoms approximately 5.5 months after symptom onset (Miglis et al., 2020); in a case series of 20 patients, it was reported that most (85%) self-reported residual symptoms 6–8 months after COVID-19, although many felt that they had improved (Blitshteyn and Whitelaw, 2021). Three case reports have documented improvement in POTS after COVID-19 infection, with or without pharmacological support (Ishibashi et al., 2021; Ocher et al., 2021; O’Sullivan et al., 2021). To build on the anecdotal evidence, longitudinal studies are required to assess the evolution of post-COVID POTS in the same cohorts.
Our study has several important limitations. Firstly, from a study design perspective, generalisability of the findings cannot be assumed given the non-probabilistic recruitment. The evidence we presented is cross-sectional and observational, hence causation cannot be inferred. In addition, we did not have a sample of controls, which can be beneficial in the study of long COVID (Amin-Chowdhury and Ladhani, 2021). Statistical underpower is likely, given the many instances where the statistic of choice for comparisons was the Fisher’s exact test, and a small sample size that precluded inclusion of a greater number of predictors in the regression models. Whilst we did not conduct an a priori calculation of the sample size, we performed a post hoc power calculation analysis for the main positive finding of the study, namely the difference in nadir HR between the two OIAS groups. According to the power calculator on https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html, given mean HRnadir of 75 for non-OI, 88 for OI, a common standard deviation of 16, 2-sided test, α (type I error) of 0.05, and power of 0.80, the minimum sample size for each OI group should have been 24, which fits our observed sample sizes.
An important consideration is that we admitted to the study participants with self-reported history of SARS-CoV-2 infection and long COVID symptoms, but we did not verify test positivity as part of the study’s inclusion criteria. Some participants were ill during the COVID-19 peaks in March/April 2020 and January/February 2021, when polymerase chain reaction (PCR) tests in Ireland were not available to those who were not highly symptomatic. As PCR verification was not considered during data collection, we cannot retrospectively identify those without a confirmed history of COVID-19. This may bring some bias to the results and interpretation of our data, and further underscores the importance to perform replication work in other cohorts where COVID-19 verification is available.
Another limitation is that our testing protocol did not include other standardised autonomic tests such as heart rate variability with paced breathing or blood pressure response to Valsalva manoeuvre. Respiratory activity was not captured during the AS and tilt. However, in the same clinical environment, Townsend et al. (2021) performed those tests on a different long COVID cohort and reported negative findings. Our study did not have more detailed measures of baroreflex function, or any imaging or biomarker information (e.g., haematological, biochemical, immunological). Some authors have evaluated the behaviour of the neuroautonomic nervous system through specific non-invasive electrocardiographic variables predicting alteration of the system itself (Piccirillo et al., 2016; Piccirillo et al., 2020), or correlated the neuropsychological alterations to those of the neuroautonomic imbalance (Wells et al., 2020); however, our study did not collect this type of information.
For ethical approval reasons, in some cases, our research tilts had to be stopped sooner (e.g., with only mild symptoms) than is often the case for tilts used in clinical practice to look for full symptom reproduction. From a haemodynamic data processing point of view, other studies have extracted the raw data from the Finapres® and performed signal averaging prior to analyses, for example in 5-s bins (van der Velde et al., 2007). While post hoc signal averaging can theoretically reduce the risk of spurious observations due to signal artefacts (Finucane et al., 2019), in this study we followed the direct observation method that is routinely utilised in clinical practice for the contemporaneous clinical assessment of patients.
In conclusion, in this cohort of participants reporting long COVID symptoms, the prevalences of OI during AS and tilt test were 66 and 39%, respectively. The prevalences of initial and classic OH during AS were 26 and 6%, respectively. Classic OH during tilt was present in 33%, and POTS was only present in 2%. OIAS was associated with a higher initial HR on AS, which after 1 min equalised with the non-OIAS group. The burden of psychological symptoms in this cohort was high and findings may be related to interoceptive mechanisms. However, our findings require external replication. More research is required to understand the mechanisms and long-term prognosis of autonomic function in long COVID, to better delineate therapies and estimate the need for services.
Data Availability Statement
The datasets presented in this article are not readily available because of the conditions of the ethical approval. Requests to access the datasets should be directed to RRO, romeroor@tcd.ie.
Ethics Statement
This study involving human participants was reviewed and approved by St James’s Hospital/Tallaght University Hospital Joint Research Ethics Committee (Submission Number: 104: TROPIC; Approval Date: 4 May 2021) and the St James’s Hospital Research and Innovation Office (Reference: 6566; Approval Date: 14 May 2021). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in the study. All aspects of the study were executed in compliance with the General Data Protection Regulation (GDPR), and Irish regulations including the Health Research Regulations and the Data Protection Act 2018. The participants provided their written informed consent to participate in this study.
Author Contributions
RR-O and AM: conceptualisation. RR-O, AM, and LB: methodology. AM, GJ, FX, ED, and RR-O: clinical data collection. RR-O, AM, GJ, and ED: formal analysis and investigation. RR-O: writing—original draft preparation and funding acquisition. AM, GJ, FX, ED, and LB: writing—review and editing. RR-O and LB: resources. ED, LB, and RR-O: supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to our participants for their involvement in the study.
Funding
This study (Technology Assisted Solutions for the Recognition of Objective Physiological Indicators of Post-Coronavirus-19 Fatigue: TROPIC Study) was funded by a grant from Science Foundation Ireland (SFI) under Grant number 20/COV/8493 and supported by SFI Grant number 18/FRL/6188. The funder had no role in the conduct of the research and/or preparation of the article; in study design; in the collection, analysis, and interpretation of data; in writing of the report; or in the decision to submit the manuscript for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.833650/full#supplementary-material
References
- Akbarialiabad H., Taghrir M. H., Abdollahi A., Ghahramani N., Kumar M., Paydar S., et al. (2021). Long COVID, a comprehensive systematic scoping review. Infection 49 1163–1186. 10.1007/s15010-021-01666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Chowdhury Z., Ladhani S. N. (2021). Causation or confounding: why controls are critical for characterizing long COVID. Nat. Med. 27 1129–1130. 10.1038/s41591-021-01402-w [DOI] [PubMed] [Google Scholar]
- Barizien N., Le Guen M., Russel S., Touche P., Huang F., Vallee A. (2021). Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 11:14042. 10.1038/s41598-021-93546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R. C. (2020). Anticipating the long-term cardiovascular effects of COVID-19. J. Thromb. Thrombolysis 50 512–524. 10.1007/s11239-020-02266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R. C. (2021). Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor’s page series. J. Thromb. Thrombolysis 52 692–707. 10.1007/s11239-021-02549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitshteyn S., Whitelaw S. (2021). Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol. Res. 69 205–211. 10.1007/s12026-021-09185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwsma A. C., Hartog L. C., Kamper A. M., Groenier K. H., Bilo H. J. G., Kleefstra N., et al. (2018). Diagnosing orthostatic hypotension with continuous and interval blood pressure measurement devices. J. Hum. Hypertens. 32 831–837. 10.1038/s41371-018-0091-9 [DOI] [PubMed] [Google Scholar]
- Brignole M., Moya A., De Lange F. J., Deharo J. C., Elliott P. M., Fanciulli A., et al. (2018). 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 39 1883–1948. [DOI] [PubMed] [Google Scholar]
- Bucciarelli V., Nasi M., Bianco F., Seferovic J., Ivkovic V., Gallina S., et al. (2022). Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc. Med. 32 12–17. 10.1016/j.tcm.2021.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Chalder T. (2010). Measuring fatigue in clinical and community settings. J. Psychosom. Res. 69 17–22. 10.1016/j.jpsychores.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Claffey P., Perez-Denia L., Rivasi G., Finucane C., Kenny R. A. (2020). Near-infrared spectroscopy in evaluating psychogenic pseudosyncope-a novel diagnostic approach. QJM 113 239–244. 10.1093/qjmed/hcz257 [DOI] [PubMed] [Google Scholar]
- Creamer M., Bell R., Failla S. (2003). Psychometric properties of the impact of event scale - revised. Behav. Res. Ther. 41 1489–1496. 10.1016/j.brat.2003.07.010 [DOI] [PubMed] [Google Scholar]
- Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., et al. (2021). Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin. Med. 21 e63–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A., De Keyser J., De Raedt S. (2017). Role of infarct location and pre-existing depression on cardiac baroreceptor sensitivity in subacute ischemic stroke. Acta Neurol. Belg. 117 655–659. 10.1007/s13760-017-0814-7 [DOI] [PubMed] [Google Scholar]
- Del Rio R., Marcus N. J., Inestrosa N. C. (2020). Potential role of autonomic dysfunction in covid-19 morbidity and mortality. Front. Physiol. 11:561749. 10.3389/fphys.2020.561749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J. J., Cheshire W. P., Claydon V. E., Norcliffe-Kaufmann L., Peltier A., Singer W., et al. (2020). Autonomic function testing in the COVID-19 pandemic: an American Autonomic Society position statement. Clin. Auton. Res. 30 295–297. 10.1007/s10286-020-00702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane C., Van Wijnen V. K., Fan C. W., Soraghan C., Byrne L., Westerhof B. E., et al. (2019). A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin. Auton. Res. 29 427–441. 10.1007/s10286-019-00606-y [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F. B., Benditt D. G., Benarroch E., Biaggioni I., et al. (2011). Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 21 69–72. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S. (2020). The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 30 299–315. 10.1007/s10286-020-00714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi P., Barletta G., Baschieri F., Calandra-Buonaura G., Provini F., Cortelli P. (2020). Testing cardiovascular autonomic function in the COVID-19 era: lessons from bologna’s autonomic unit. Clin. Auton. Res. 30 325–330. 10.1007/s10286-020-00710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y., Yoneyama K., Tsuchida T., Akashi Y. J. (2021). Post-COVID-19 postural orthostatic tachycardia syndrome. Intern. Med. 60:2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings G., Monaghan A., Xue F., Mockler D., Romero-Ortuño R. (2021). A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J. Clin. Med. 10:5913. 10.3390/jcm10245913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Ståhlberg M., Runold M., Nygren-Bonnier M., Nilsson J., Olshansky B., et al. (2021). Long-haul post–COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the swedish experience. JACC Case Rep. 3 573–580. 10.1016/j.jaccas.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J., Ricci F., Hoffmann F., Hamrefors V., Fedorowski A. (2020). Orthostatic hypertension: critical appraisal of an overlooked condition. Hypertension 75 1151–1158. 10.1161/HYPERTENSIONAHA.120.14340 [DOI] [PubMed] [Google Scholar]
- Ketch T., Biaggioni I., Robertson R., Robertson D. (2002). Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation 105 2518–2523. [DOI] [PubMed] [Google Scholar]
- Keyhanian K., Umeton R. P., Mohit B., Davoudi V., Hajighasemi F., Ghasemi M. (2020). SARS-CoV-2 and nervous system: from pathogenesis to clinical manifestation. J. Neuroimmunol. 350 577436. 10.1016/j.jneuroim.2020.577436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H. (2021). The dysfunction is in the details: neurovascular changes in COVID-19. Can. J. Neurol. Sci. 48 1–2. 10.1017/cjn.2020.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N. W., Stiles L. E., Miglis M. G. (2021). Preparing for the long-haul: autonomic complications of COVID-19. Auton. Neurosci. 235:102841. 10.1016/j.autneu.2021.102841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitzke M., Stefanovic D., Meyer J. J., Schimpf S., Schonknecht P. (2020). Autonomic balance determines the severity of COVID-19 courses. Bioelectron. Med. 6:22. 10.1186/s42234-020-00058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. M., Seeley J. R., Roberts R. E., Allen N. B. (1997). Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12 277–287. 10.1037//0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P. A., Cuapio A., et al. (2021). More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 11 16144–16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelen M., Manoharan L., Elkheir N., Cheng V., Dagens A., Hastie C., et al. (2021). Characterising long COVID: a living systematic review. BMJ Glob. Health 6:e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis M. G., Muppidi S. (2017). Is postural tachycardia syndrome in the head or in the heart? And other updates on recent autonomic research. Clin. Auton. Res. 27 145–147. 10.1007/s10286-017-0423-9 [DOI] [PubMed] [Google Scholar]
- Miglis M. G., Prieto T., Shaik R., Muppidi S., Sinn D. I., Jaradeh S. (2020). A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 30 449–451. 10.1007/s10286-020-00727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Bermejo L., Adsuar J. C., Mendoza-Munoz M., Barrios-Fernandez S., Garcia-Gordillo M. A., Perez-Gomez J., et al. (2021). Test-retest reliability of five times sit to stand test (FTSST) in adults: a systematic review and meta-analysis. Biology 10:510. 10.3390/biology10060510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K., Somers V. K. (1998). Chronic orthostatic intolerance: part of a spectrum of dysfunction in orthostatic cardiovascular homeostasis? Circulation 98 2105–2107. 10.1161/01.cir.98.20.2105 [DOI] [PubMed] [Google Scholar]
- Ocher R. A., Padilla E., Hsu J. C., Taub P. R. (2021). Clinical and laboratory improvement in hyperadrenergic postural orthostatic tachycardia syndrome (POTS) after COVID-19 infection. Case Rep. Cardiol. 2021:7809231. 10.1155/2021/7809231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J. S., Lyne A., Vaughan C. J. (2021). COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 14:e243585. 10.1136/bcr-2021-243585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens A. P., Low D. A., Critchley H. D., Mathias C. J. (2018). Emotional orienting during interoceptive threat in orthostatic intolerance: dysautonomic contributions to psychological symptomatology in the postural tachycardia syndrome and vasovagal syncope. Auton. Neurosci. 212 42–47. 10.1016/j.autneu.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Paterson I., Ramanathan K., Aurora R., Bewick D., Chow C. M., Clarke B., et al. (2021). Long COVID-19: a primer for cardiovascular health professionals, on behalf of the ccs rapid response team. Can. J. Cardiol. 37 1260–1262. 10.1016/j.cjca.2021.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckerman A., Lamanca J. J., Qureishi B., Dahl K. A., Golfetti R., Yamamoto Y., et al. (2003). Baroreceptor reflex and integrative stress responses in chronic fatigue syndrome. Psychosom. Med. 65 889–895. 10.1097/01.psy.0000079408.62277.3d [DOI] [PubMed] [Google Scholar]
- Piccirillo G., Moscucci F., Fabietti M., Di Iorio C., Mastropietri F., Sabatino T., et al. (2020). Age, gender and drug therapy influences on Tpeak-tend interval and on electrical risk score. J. Electrocardiol. 59 88–92. 10.1016/j.jelectrocard.2020.01.009 [DOI] [PubMed] [Google Scholar]
- Piccirillo G., Moscucci F., Fiorucci C., Di Iorio C., Mastropietri F., Magri D. (2016). Time- and frequency-domain analysis of beat to beat P-wave duration, PR interval and RR interval can predict asystole as form of syncope during head-up tilt. Physiol. Meas. 37 1910–1924. 10.1088/0967-3334/37/11/1910 [DOI] [PubMed] [Google Scholar]
- Qi T., Hu T., Ge Q. Q., Zhou X. N., Li J. M., Jiang C. L., et al. (2021). COVID-19 pandemic related long-term chronic stress on the prevalence of depression and anxiety in the general population. BMC Psychiatry 21:380. 10.1186/s12888-021-03385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: a self report depression scale for research in the general population. Appl. Psychol. Meas. 1 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raj S. R., Arnold A. C., Barboi A., Claydon V. E., Limberg J. K., Lucci V. M., et al. (2021). Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin. Auton. Res. 31 365–368. 10.1007/s10286-021-00798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank A., Tzortzini A., Kling E., Schmid C., Claus R., Loll E., et al. (2021). One year after mild COVID-19: the majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. J. Clin. Med. 10:3305. 10.3390/jcm10153305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. P., Watson C. J., Badenoch J., Cross B., Butler M., Song J., et al. (2021). Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J. Neurol. Neurosurg. Psychiatry 92 932–941. 10.1136/jnnp-2021-326405 [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramirez D. C., Normand K., Zhaoyun Y., Torres-Castro R. (2021). Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 9:900. 10.3390/biomedicines9080900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler C. X., Wyller V. B. B., Moss-Morris R., Buchwald D., Crawley E., Hautvast J., et al. (2021). Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect. Dis. 8:ofab440. 10.1093/ofid/ofab440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah W., Hillman T., Playford E. D., Hishmeh L. (2021). Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372:n136. 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- Shouman K., Vanichkachorn G., Cheshire W. P., Suarez M. D., Shelly S., Lamotte G. J., et al. (2021). Autonomic dysfunction following COVID-19 infection: an early experience. Clin. Auton. Res. 31 385–394. 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn D. I., Muppidi S., Miglis M. G., Jaradeh S. (2021). Autonomic function test during the COVID-19 pandemic: the Stanford experience. Clin. Auton. Res. 31 127–129. 10.1007/s10286-020-00752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Moloney D., Finucane C., Mccarthy K., Bergin C., Bannan C., et al. (2021). Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One 16:e0247280. 10.1371/journal.pone.0247280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde N., Van Den Meiracker A. H., Stricker B. H., Van Der Cammen T. J. (2007). Measuring orthostatic hypotension with the finometer device: is a blood pressure drop of one heartbeat clinically relevant? Blood Press. Monit. 12 167–171. 10.1097/MBP.0b013e3280b083bd [DOI] [PubMed] [Google Scholar]
- van Kessel S.a.M, Olde Hartman T. C., Lucassen P., Van Jaarsveld C. H. M. (2021). Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam. Pract. 39 159–167. 10.1093/fampra/cmab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanichkachorn G., Newcomb R., Cowl C. T., Murad M. H., Breeher L., Miller S., et al. (2021). Post-COVID-19 syndrome (long haul syndrome): description of a multidisciplinary clinic at mayo clinic and characteristics of the initial patient cohort. Mayo Clin. Proc. 96 1782–1791. 10.1016/j.mayocp.2021.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D. G., Egger M., Pocock S. J., Gotzsche P. C., Vandenbroucke J. P., et al. (2007). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370 1453–1457. [DOI] [PubMed] [Google Scholar]
- Wells R., Malik V., Brooks A. G., Linz D., Elliott A. D., Sanders P., et al. (2020). Cerebral blood flow and cognitive performance in postural tachycardia syndrome: insights from sustained cognitive stress test. J. Am. Heart Assoc. 9:e017861. 10.1161/JAHA.120.017861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling K. H. (1996). Finger arterial pressure measurement with Finapres. Z. Kardiol. 85(Suppl. 3), 38–44. [PubMed] [Google Scholar]
- WHO (2021). A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus: 6 October 2021. Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed 26 October 2021) [Google Scholar]
- Wieling W., Krediet C. T., Van Dijk N., Linzer M., Tschakovsky M. E. (2007). Initial orthostatic hypotension: review of a forgotten condition. Clin. Sci. 112 157–165. 10.1042/CS20060091 [DOI] [PubMed] [Google Scholar]
- Wijkman M., Lanne T., Ostgren C. J., Nystrom F. H. (2016). Diastolic orthostatic hypertension and cardiovascular prognosis in type 2 diabetes: a prospective cohort study. Cardiovasc. Diabetol. 15:83. 10.1186/s12933-016-0399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Yang M., Lai C. L. (2021). Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines 9:966. 10.3390/biomedicines9080966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S. J. (2021). Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect. Dis. 53 737–754. 10.1080/23744235.2021.1924397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Bose D., Nouri-Vaskeh M., Tajiknia V., Zand R., Ghasemi M. (2021). Long-term side effects and lingering symptoms post COVID-19 recovery. Rev. Med. Virol. Online ahead of print., 10.1002/rmv.2289, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because of the conditions of the ethical approval. Requests to access the datasets should be directed to RRO, romeroor@tcd.ie.