Abstract
Background.
Few data on intracranial group A Streptococcus (GAS) infection in children are available. Here, we describe the demographic, clinical, and diagnostic characteristics of 91 children with intracranial GAS infection.
Methods.
Cases of intracranial GAS infection in persons ≤18 years of age reported between 1997 and 2014 were identified by the Centers for Disease Control and Prevention’s population- and laboratory-based Active Bacterial Core surveillance (ABCs) system. Medical charts were abstracted using a active, standardized case report form. All available isolates were emm typed. US census data were used to calculate rates.
Results.
ABCs identified 2596 children with invasive GAS infection over an 18-year period; 91 (3.5%) had an intracranial infection. Intracranial infections were most frequent during the winter months and among children aged <1 year. The average annual incidence was 0.07 cases per 100 000 children. For 83 patients for whom information for further classification was available, the principal clinical presentations included meningitis (35 [42%]), intracranial infection after otitis media, mastoiditis, or sinusitis (34 [41%]), and ventriculoperitoneal shunt infection (14 [17%]). Seven (8%) of these infections progressed to streptococcal toxic shock syndrome. The overall case fatality rate was 15%. GAS emm types 1 (31% of available isolates) and 12 (13% of available isolates) were most common.
Conclusions.
Pediatric intracranial (GAS) infections are uncommon but often severe. Risk factors for intracranial GAS infection include the presence of a ventriculoperitoneal shunt and contiguous infections in the middle ear or sinuses.
Keywords: group A Streptococcus, mastoiditis, meningitis, sinusitis, ventriculoperitoneal shunt
Group A Streptococcus (GAS) frequently causes superficial skin infections and pharyngitis and, less commonly, severe invasive infections, including necrotizing fasciitis, pneumonia, sepsis, and streptococcal toxic shock syndrome (STSS). Although rare, intracranial GAS infections are associated with a higher case fatality rate (CFR) than are nonintracranial GAS infections and can result in neurological sequelae, such as hearing loss, hydrocephalus, developmental delays, and motor deficits [1, 2]. Earlier reports have suggested that intracranial infections secondary to GAS generally arise from contiguous spread from a parameningeal focus such as otitis media (OM), mastoiditis, or sinusitis [3], but few reviews have been conducted recently, and they have usually included only a small number of cases [2, 4–7].
We reviewed all intracranial infections caused by GAS among US children aged ≤18 years using the Active Bacterial Core surveillance (ABCs) system. We present here a systematic assessment of the demographics, clinical syndromes, comorbid conditions, diagnostic imaging modalities used (and their findings), emm types, treatment, and outcomes of children with intracranial GAS infection in the United States.
MATERIALS AND METHODS
ABCs System
ABCs is an active, population- and laboratory-based system that monitors invasive bacterial disease caused by pathogens of public health importance in 10 geographically distinct sites in the United States [8–10]. Funded by the Centers for Disease Control and Prevention’s Emerging Infections Program, ABCs personnel routinely contact all participating clinical microbiology laboratories that serve residents in the ABCs catchment areas to identify cases. Chart reviews are conducted for each case to obtain demographic information, disease presentation, and underlying medical conditions.
ABCs defines a case of invasive GAS infection as a case in which GAS is isolated from a normally sterile site (eg, blood, cerebrospinal fluid [CSF]) or from a wound culture associated with necrotizing fasciitis or STSS in a resident of an ABCs area. All available GAS isolates are collected for further characterization. emm typing (identification of the gene encoding the cell surface M protein, a major virulence factor) is performed at the Centers for Disease Control and Prevention’s Streptococcus laboratory as described previously [11].
For the purposes of this study, we defined an intracranial or central nervous system (CNS) GAS case as an ABCs case in which GAS was isolated from (1) CSF in any patient or (2) any sterile site in a patient with signs and symptoms specific to intracranial infection (ie, an epidural, cerebral, subdural, or cochlear infection in a patient from whom isolates for culture were obtained in the operating room) or a diagnosis of meningitis was recorded in the medical record. We included all ABCs case-patients aged ≤18 years with GAS who were identified between 1997 and 2014.
We collected additional data on clinical presentation, laboratory findings, and antibiotic therapy using a supplemental standardized questionnaire. Using available data, we then assigned each case-patient to 1 of 3 categories, (1) children with primary infection (meningitis in a child who did not have a ventriculoperitoneal [VP] shunt or ear, nose, and throat [ENT]-related symptoms), (2) children with a VP shunt infection, or (3) children without a VP shunt who had signs or symptoms of an ENT-related syndrome such as OM, mastoiditis, or sinusitis (eg, ear infection, ear/sinus pain/swelling) or those for whom mastoiditis or sinusitis was noted in their imaging results.
ABCs GAS surveillance has included the following areas: California (3-county San Francisco Bay area since 1997), Colorado (5-county Denver area since 2001), Connecticut (statewide since 1997), Georgia (20-county Atlanta area from 1997 to 1999 and 2004 to 2014 and statewide in 2000–2003), Maryland (6-county Baltimore area since 1997), Minnesota (statewide since 1997), New Mexico (statewide since 2004), New York (7-county Rochester area since 1997 and 15-county Rochester and Albany areas since 1999), Oregon (3-county Portland area since 1997), and Tennessee (5 urban counties in 1997–1999, 11 counties in 2000–2009, and 20 counties since 2010). When calculating incidence rates to analyze for trends over time, we restricted our analysis to cases detected between 2004 and 2014 and included only cases from GAS surveillance areas that had remained constant for that period (ie, excluding the additional counties in Tennessee that began surveillance in 2010). In 2014, a total of 8.1 million children aged ≤18 years lived in the surveillance areas. Regional and national post–US census population estimates for each year were used as the total populations.
This study was part of public health surveillance and thus exempt from institutional review board (IRB) requirements in most states; however, whenever necessary, the study was approved by the local IRB.
RESULTS
Demographic Characteristics and Disease Rates
Between 1997 and 2014, a total of 2596 cases of invasive GAS infection among persons aged ≤18 years were identified by ABCs; 91 (3.5%) of these cases met our definition of intracranial disease. The incidence of all invasive GAS infections remained relatively stable from 2004 to 2014, ranging from 1.4 to 2.3 cases per 100 000 children ≤18 years old annually (Figure 1). Intracranial GAS accounted for 1.9% to 5.2% of invasive GAS infections each year for an average annual incidence of intracranial GAS infection (during 2004–2014) of 0.07 per 100 000 children (range, 0.03 per 100 000 in 2012 to 0.10 per 100 000 in 2008, 2012, and 2014). The majority of intracranial infections (73%) occurred between November and April (Figure 2), which is consistent with the known seasonality of overall invasive GAS in the United States. Twenty (22%) children were <1 year of age, and 14 (15%) were ≤3 months of age.
Figure 1.
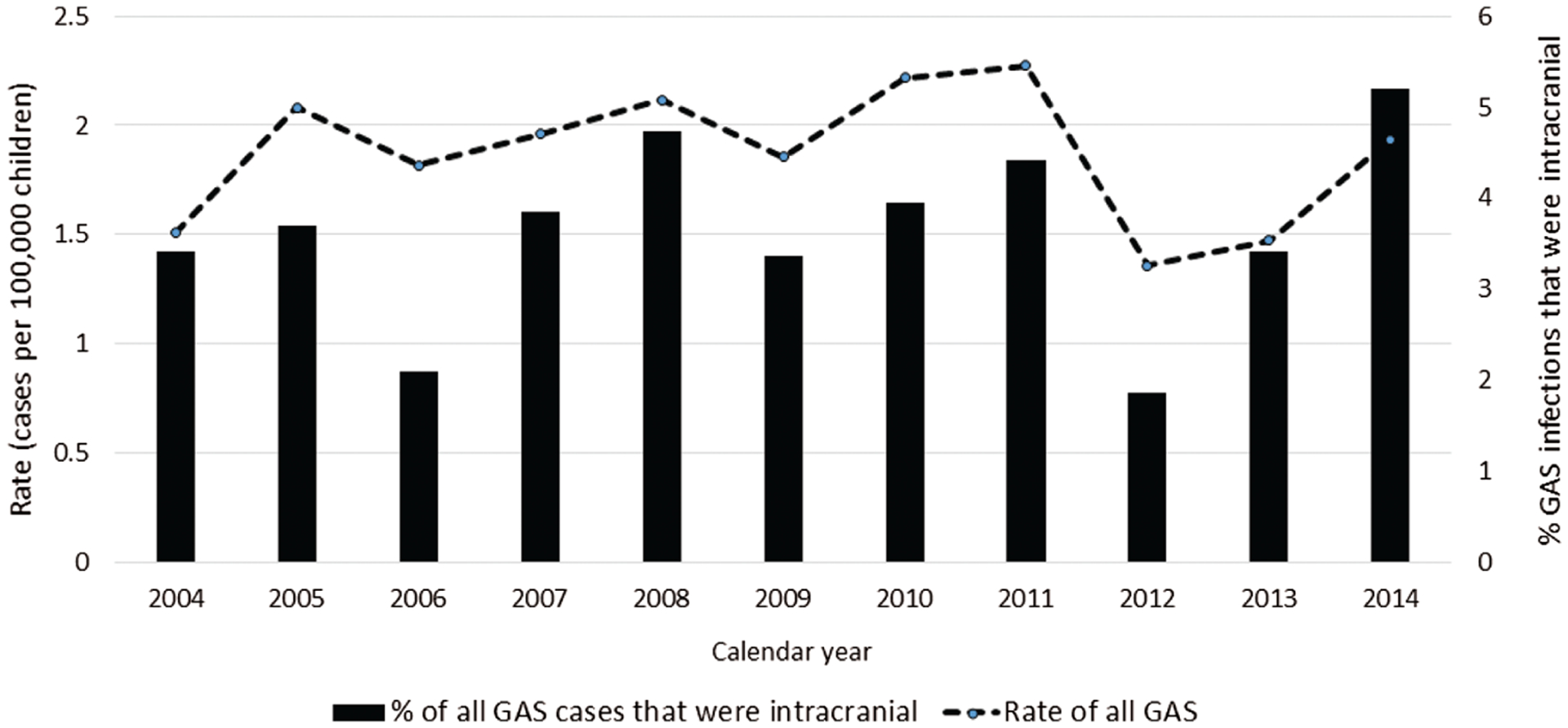
Rates (cases per 100 000) of invasive group A Streptococcus (GAS) infection among children aged ≤18 years and percentages of those GAS infections that were intracranial, according to calendar year—Active Bacterial Core surveillance, 2004–2014.
Figure 2.
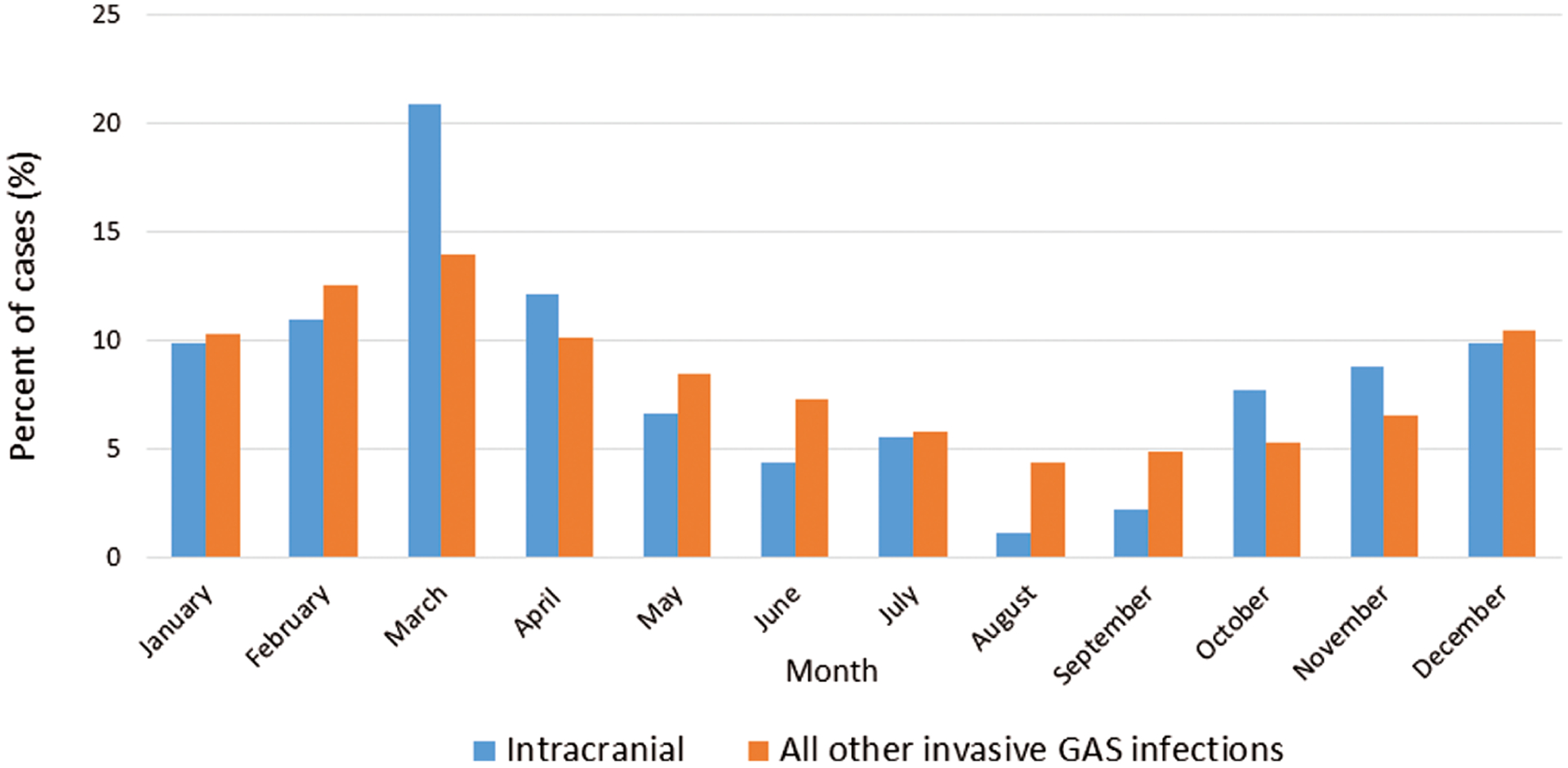
Percentages of invasive group A Streptococcus (GAS) cases reported, according to month, for intracranial versus nonintracranial infections among children aged ≤18 years—Active Bacterial Core surveillance, 1997–2014
Laboratory Results
GAS was identified by blood culture alone for 18 (20%) patients, CSF culture alone for 37 (41%) patients, and both blood and CSF for 21 (23%) patients. In addition, GAS was isolated from both blood and an epidural abscess (1 patient), an epidural abscess alone (3 patients), and a cerebral abscess, not further specified (5 patients). GAS was isolated also from each of the following sites alone from 1 patient each: VP shunt tip, mastoid tissue, subdural abscess, cochlear abscess, temporal muscle abscess, and prevertebral space. emm sequence typing was available for 70 (77%) of the patients; emm types 1 (n = 22 [31%]) and 12 (n = 9 [17%]) were the most common (Table 1). The distribution of emm types was similar to that for children with other non-CNS GAS infections; emm types 1 and 12 accounted for 28% and 12% of the infections, respectively [10, 12, 13].
Table 1.
Principal Syndromes Among Intracranial GAS Infectionsa
| Principal Intracranial Syndrome (No. of Cases) | Other Syndrome(s) (n) | Age (Range [Median]) | Antibiotics Administered (n) | No. (%) of Deathsb | emm Types (n)c |
|---|---|---|---|---|---|
| Meningitis (35) | STSS (4); septic arthritis (1); septic shock (1) | 4 days-14 years (2.5 years) | Third-generation cephalosporins (21); vancomycin (19); ampicillin (7) | 6 (17) | 1 (10), 12 (4), 89 (3), 4 (2), 28 (2), 118 (2), 9, 11, 49, 59, 77, 87, 122, unknown (5) |
| ENT infection (34a) (acute otitis media, sinusitis, mastoiditis) | STSS (2); septic arthritis (2); septic shock (1) | 46 days-15 years (8.6 years) | Third-generation cephalosporins (28); vancomycin (26) | 3 (9) | 1 (9), 6 (4), 5 (2), 12 (2), 3, 4, 18, 59, 75, 118, unknown (11) |
| VP shunt infection (14) | STSS (1) | 30 days-10 years (4.6 years) | Third-generation cephalosporins (9); vancomycin (13) | 0 (0) | 12 (3), 28 (2), 1, 2, 3, 11, 44, 75, 86, unknown (2) |
Abbreviations: ENT, ear, nose, and throat; GAS, group A Streptococcus; STSS, streptococcal toxic shock syndrome; VP, ventriculoperitoneal.
Of a total of 83 patients, which includes children for whom presenting symptoms were available and 2 children for whom presenting symptoms were not available but who had mastoiditis noted in the chart. A supplemental questionnaire was not completed for 8 children; therefore, a determination of principal intracranial syndrome could not be made.
Five children who died could not be classified because no information on their symptoms was available.
For emm types without a number in parentheses, only 1 isolate with that emm type was found
Presenting Symptoms, Principal Clinical Syndromes, and Treatment
Data on presenting symptoms were available for 81 (89%) children with intracranial infection. The most common symptoms were fever (78%), headache (49%), neck pain (32%), abdominal pain (27%), weakness (22%), ear pain/infection (22%), sore throat (21%), seizures (20%), and photophobia (16%).
A supplemental questionnaire was completed in 83 (92%) of the cases. On the basis of data available in the medical chart, of the 3 primary CNS syndromes among the case-patients, we found 35 (42%) cases of meningitis, 34 (41%) cases of an ENT-related syndrome (OM, mastoiditis, or sinusitis, including 2 cases without data on primary presentation but with “mastoiditis” noted in the chart), and 14 (17%) cases of VP shunt infection (Table 1). Children with an ENT-related syndrome were generally older than those who presented with meningitis or VP shunt infection (P < .001, Kruskal-Wallis test).
Of the 81 children for whom data on clinical course were available, 72 (89%) received antibiotics. Of the 9 children for whom we could not confirm receipt of antibiotics, 3 died before reaching the hospital and 1 was diagnosed initially as having a viral illness and died before the correct diagnosis was made. Antibiotic treatment status was unknown for the remaining 5 children. Among the children who received antibiotics, the most commonly administered antibiotics were vancomycin and third-generation cephalosporins (n = 58 [81%] for each); 50 (69%) children received both antibiotics. Twenty-nine (40%) children received a β-lactam antibiotic (eg, penicillin, ampicillin, or piperacillin-tazobactam). Three children who received antibiotics died; 1 received penicillin, vancomycin, and ceftriaxone, 1 received vancomycin and ceftriaxone, and 1 received vancomycin and rifampin. Susceptibility data for isolates from 2 of these 3 children were available, and both of them were susceptible to vancomycin and penicillin.
GAS Risk Factors and Outcome
Known risk factors for intracranial GAS infection were identified for 23 (25%) patients. The presence of a VP shunt for the treatment of hydrocephalus was the most common risk factor identified (n = 14 [15%]); 2 patients with a VP shunt also had spina bifida. The length of time between the date of the last documented VP shunt manipulation to the onset of intracranial infection (based on date of first positive GAS culture result) ranged from 6 days to nearly 8 years (median, 14 months). GAS was isolated from the CSF of all 14 patients with a VP shunt infection; positive blood culture results were also found for 2 of these patients.
Seven (8%) patients had a history of trauma before their intracranial GAS infection, 4 with blunt trauma (2 to the head), 1 with penetrating trauma of the arm, and 2 with both blunt and penetrating trauma to the head after a motor vehicle crash. In these patients who suffered trauma, the time from injury to the first positive culture result ranged from 1 to 7 days. Varicella, a commonly reported risk factor for GAS infection, was not reported in any of the involved patients. One 3-month-old boy developed emm59 GAS meningitis 38 days after GAS sepsis with the same emm type and survived both infections; we found no evidence that the infant was immunocompromised. A 2-year-old boy with symptoms of OM developed a cochlear abscess 7 months after placement of a cochlear implant. An 11-year-old boy with a CSF leak from a congenital malformation had mastoiditis and survived.
Of the 91 patients, 14 (15%) died; this CFR is higher than that among children with nonintracranial invasive GAS infection reported to ABCs (3%). Among the patients whose disease we were able to categorize, the CFR was highest among those with meningitis (17%) and those with an ENT-related syndrome (9%) (Table 1). One child with sinusitis died as a result of cerebral herniation caused by acute purulent meningitis. None of the 14 children with a VP shunt infection died. We were unable to classify the disease according to primary CNS presentation in 5 children who died because no information on their symptoms was available. One patient for whom no evidence of hydrocephalus was found before GAS infection developed increased intracranial pressure as a consequence of the infection; the patient underwent subsequent CSF drainage through the placement of a VP shunt and survived.
Patients Aged <1 Year
The highest rate of intracranial GAS infection occurred among children aged <1 year (0.31 cases per 100 000 population vs an average rate of 0.06 cases per 100 000 population in children aged 1–18 years). Of these 20 patients aged <1 year, 13 had meningitis, 4 had a VP shunt infection, and 1 had OM with intracranial extension; the disease in 2 of these children could not be classified. Of these 20 patients, 4 (20%) died. One neonate developed a large right middle cerebral artery infarction with extensive gyral edema but survived.
Imaging Results
Among the 81 patients with an intracranial GAS infection for whom supplemental clinical data were available, 58 (72%) underwent either head computed tomography or magnetic resonance imaging. The results of 55 of these scans were available for review (16 [46%] patients with primary infection, 26 [76%] with ENT-related symptoms, and 13 [93%] with a VP shunt infection). Among the patients with ENT-related symptoms, imaging revealed an intracranial abscess (4 patients), epidural empyema (2 patients), or subdural empyema (5 patients). Fewer intracranial abnormalities were noted in patients with primary infection and in those with a VP shunt infection. Three patients with a primary infection had an abscess noted in their chart, and 2 had a subdural effusion.
DISCUSSION
In a large population-based surveillance system, we found that rates of intracranial GAS infection were relatively low compared to those of other nonintracranial invasive GAS infections and were more common in the first year of life. These infections, although rare, were associated with substantial mortality rates, especially in those aged <1 year. The most common risk factor for an intracranial infection was the presence of a VP shunt, which indicates that providers should consider GAS to be a possible infectious pathogen in children with a VP shunt.
In the literature, the overall incidence of invasive GAS infection in children has varied substantially according to time and location but is relatively rare. In Canada in the early 1990s, the incidence was found to be 1.9 cases per 100 000 children aged <5 years. A review in Finland found an incidence of 0.93 cases per 100 000 children aged <15 years between 1996 and 2000, and the incidence increased to 1.80 cases in 2001–2005 and 2.50 in 2006–2010 [14]. In the United States from 1995 to 1999, the incidence of invasive GAS infection was 6.3 cases per 100 000 children aged <2 years [8].
As noted in our study and previously reported in the literature, intracranial GAS infection is uncommon; 1 small US study in 2001–2014 reported that GAS infection accounted for only 2.2% of meningitis cases (vs meningitis caused by Streptococcus pneumoniae [33%], Neisseria meningitidis [29%], and group B Streptococcus [18%]) [5]. Our 15% CFR was approximately half the 27% rate reported for a series of adults with GAS meningitis [15], possibly because of the fewer underlying comorbidities in children. It should be noted that in our analysis, all 4 children who were confirmed to have not received antibiotics died. These children died before the correct diagnosis was made (3 before they reached the hospital and 1 after being incorrectly diagnosed with a viral illness). If we exclude these 4 deaths, our CFR rate decreases to 11%, which speaks to the need for rapid diagnosis of and appropriate treatment for intracranial GAS infection.
The pathogenesis of intracranial GAS infection is multifactorial; it can arise secondary to bacteremia, parameningeal infection, pharyngitis, or head trauma [16, 17]. GAS meningitis in adults is typically associated with neurosurgical conditions or upper respiratory tract infection [6, 18, 19]. Neurosurgery, skull fracture, CSF leakage, and upper respiratory tract infection have been found to predispose children to GAS meningitis [19, 20]. Skin lesions, impetigo, varicella, and erysipelas have been reported also to be associated with GAS meningitis [2, 20–23]. Although these conditions were not found often in our patients, such conditions might not have been noted in the medical records and therefore were not detected. As identified in our study, underlying medical conditions that predispose patients to intracranial GAS infection are all anatomic in origin (eg, VP shunt, trauma) rather than systemic (eg, nephrotic syndrome, human immunodeficiency virus infection), which is in contrast to the results of studies of adults and children with GAS meningitis in developing countries [7, 24].
Many cases of intracranial GAS infection in the literature and in our study arose secondary to intracranial extension of OM, mastoiditis, or sinusitis. Others have reported that OM caused by GAS seems to be particularly locally aggressive when compared with S pneumoniae or Haemophilus influenzae OM [25]. Because the distribution of emm types among patients with intracranial GAS infection mirrored the distribution of other invasive GAS infections identified by ABCs, it is unlikely that any specific emm type carries a predilection for the CNS.
Our report has summarized a large population-based study that identified multiple cases of intracranial GAS infection associated with VP shunts. In previous publications, Staphylococcus aureus and coagulase-negative Staphylococcus species accounted for the vast majority of VP shunt infections, whereas less commonly noted are Enterococcus species and Streptococcus viridans; these studies reported no cases of GAS infection [26–34]. A recent case report described a 7-year-old boy who presented with fever, a scalp wound infection, and a VP shunt infection caused by GAS [35]. The mechanism of infection was hypothesized to be autoinoculation from a pharyngeal source. The original portal of entrance for GAS among the VP shunt infections in our study remains unclear. However, the seeding of VP shunts after bacteremia in children has been well documented [36–38]. Because the median duration from the last shunt manipulation to the culture date was more than 1 year, for most of these infections, manipulation seems unlikely to have been the direct cause for VP shunt infection, which supports the role of transient GAS bacteremia in the seeding of the shunt.
In summary, intracranial GAS infection should be suspected in all children with a parameningeal or intracranial infection and among young children who have a VP shunt or have experienced a recent trauma. Rapid recognition of these infections and prompt treatment should lead to improved outcomes.
Acknowledgment.
We appreciate the contributions of Jens C. Krause, MD.
Financial support.
Support for ABCs is provided by the Emerging Infections Program of the Centers for Disease Control and Prevention (Atlanta, GA).
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Quesnel S, Nguyen M, Pierrot S, et al. Acute mastoiditis in children: a retrospective study of 188 patients. Int J Pediatr Otorhinolaryngol 2010; 74:1388–92. [DOI] [PubMed] [Google Scholar]
- 2.de Almeida Torres RS, Fedalto LE, de Almeida Torres RF, et al. Group A Streptococcus meningitis in children. Pediatr Infect Dis J 2013; 32:110–4. [DOI] [PubMed] [Google Scholar]
- 3.Baraldés MA, Domingo P, Mauri A, et al. Group A streptococcal meningitis in the antibiotic era. Eur J Clin Microbiol Infect Dis 1999; 18:572–8. [DOI] [PubMed] [Google Scholar]
- 4.Levy C, Bidet P, Bonacorsi S, Cohen R; Bacterial Meningitis Study Group. Group A streptococcal meningitis in children: French surveillance network from 2001 to 2012. Pediatr Infect Dis J 2013; 32:1041–2. [DOI] [PubMed] [Google Scholar]
- 5.Nigrovic LE, Kuppermann N, Malley R; Bacterial Meningitis Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Children with bacterial meningitis presenting to the emergency department during the pneumococcal conjugate vaccine era. Acad Emerg Med 2008; 15:522–8. [DOI] [PubMed] [Google Scholar]
- 6.Sommer R, Rohner P, Garbino J, et al. Group A beta-hemolytic Streptococcus meningitis: clinical and microbiological features of nine cases. Clin Infect Dis 1999; 29:929–31. [DOI] [PubMed] [Google Scholar]
- 7.Seale AC, Davies MR, Anampiu K, et al. Invasive group A Streptococcus infection among children, rural Kenya. Emerg Infect Dis 2016; 22:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group A Streptococcus disease in the United States, 1995–1999. Clin Infect Dis 2002; 35:268–76. [DOI] [PubMed] [Google Scholar]
- 9.Shepard CW, Rosenstein NE, Fischer M; Active Bacterial Core Surveillance Team. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr Infect Dis J 2003; 22:418–22. [DOI] [PubMed] [Google Scholar]
- 10.Nelson GE, Pondo T, Toews KA, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Streptococci group A subtyping request form: Blast 2.0 server. Available at: http://www2a.cdc.gov/ncidod/biotech/strepblast.asp. Accessed November 8, 2018.
- 12.Wajima T, Murayama SY, Sunaoshi K, et al. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and noninvasive disease. J Med Microbiol 2008; 57:1383–8. [DOI] [PubMed] [Google Scholar]
- 13.Shulman ST, Tanz RR, Dale JB, et al. ; North American Streptococcal Pharyngitis Surveillance Group. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin Infect Dis 2009; 49:78–84. [DOI] [PubMed] [Google Scholar]
- 14.Tapiainen T, Launonen S, Renko M, et al. Invasive group A streptococcal infections in children: a nationwide survey in Finland. Pediatr Infect Dis J 2016; 35:123–8. [DOI] [PubMed] [Google Scholar]
- 15.van de Beek D, de Gans J, Spanjaard L, Sela S, Vermeulen M, Dankert J. Group A streptococcal meningitis in adults: report of 41 cases and a review of the literature. Clin Infect Dis 2002; 34:e32–6. [DOI] [PubMed] [Google Scholar]
- 16.Mathur P, Arora NK, Kapil A, Das BK. Streptococcus pyogenes meningitis. Indian J Pediatr 2004; 71:423–6. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell TJ. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol 2003; 1:219–30. [DOI] [PubMed] [Google Scholar]
- 18.Pettersen G, Ovetchkine P, Tapiero B. Group A streptococcal meningitis in a pediatric patient following cochlear implantation: report of the first case and review of the literature. J Clin Microbiol 2005; 43:5816–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson NW, Copeland B, Bastian JF. Posttraumatic meningitis in adolescents and children. Pediatr Neurosurg 1990; 16:17–20. [DOI] [PubMed] [Google Scholar]
- 20.Steppberger K, Adams I, Deutscher J, et al. Meningitis in a girl with recurrent otitis media caused by Streptococcus pyogenes—otitis media has to be treated appropriately. Infection 2001; 29:286–8. [DOI] [PubMed] [Google Scholar]
- 21.Brandt CM, Kitz R, Lütticken R, Brade V. Streptococcus pyogenes meningitis complicating varicella in a 3-month-old child. Scand J Infect Dis 2003; 35:876–8. [DOI] [PubMed] [Google Scholar]
- 22.Gradon JD, Chopnick EK, Lutwick LI, et al. Group A streptococcal meningitis complicating varicella. Pediatr Infect Dis J 1991; 10:786–7. [PubMed] [Google Scholar]
- 23.Walsh M, Chodock R, Quinn C, Peglow S. Group A beta-hemolytic streptococcal meningitis associated with uncomplicated varicella. Am J Emerg Med 1994; 12:602–3. [DOI] [PubMed] [Google Scholar]
- 24.Asnis DS, Knez T. Group A streptococcal meningitis. Arch Intern Med 1998; 158:810, 3–4. [DOI] [PubMed] [Google Scholar]
- 25.Segal N, Givon-Lavi N, Leibovitz E, et al. Acute otitis media caused by Streptococcus pyogenes in children. Clin Infect Dis 2005; 41:35–41. [DOI] [PubMed] [Google Scholar]
- 26.Conen A, Walti LN, Merlo A, et al. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis 2008; 47:73–82. [DOI] [PubMed] [Google Scholar]
- 27.Bui CJ, Tubbs RS, Pate G, et al. Infections of pediatric cerebrospinal fluid shunts related to fundoplication and gastrostomy. J Neurosurg 2007; 107:365–7. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 2001; 94:195–201. [DOI] [PubMed] [Google Scholar]
- 29.Turgut M, Alabaz D, Erbey F, et al. Cerebrospinal fluid shunt infections in children. Pediatr Neurosurg 2005; 41:131–6. [DOI] [PubMed] [Google Scholar]
- 30.McGirt MJ, Zaas A, Fuchs HE, et al. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 2003; 36:858–62. [DOI] [PubMed] [Google Scholar]
- 31.Bruinsma N, Stobberingh EE, Herpers MJ, et al. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect 2000; 6:202–6. [DOI] [PubMed] [Google Scholar]
- 32.Celebi S, Hacimustafaoglu M, Ozdemir O, Ozakin C. Nosocomial Gram-positive bacterial infections in children: results of a 7 year study. Pediatr Int 2007; 49:875–82. [DOI] [PubMed] [Google Scholar]
- 33.Reddy GK, Bollam P, Caldito G. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: long-term single institution experience. World Neurosurg 2012; 78:155–63. [DOI] [PubMed] [Google Scholar]
- 34.Prusseit J, Simon M, von der Brelie C, et al. Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children. Pediatr Neurosurg 2009; 45:325–36. [DOI] [PubMed] [Google Scholar]
- 35.Patel C, Chaudhuri NR, Gaur S. Group A Streptococcus ventriculoperitoneal shunt infection in a child. Pediatr Infect Dis J 2012; 31:660. [DOI] [PubMed] [Google Scholar]
- 36.Patriarca PA, Lauer BA. Ventriculoperitoneal shunt-associated infection due to Haemophilus influenzae. Pediatrics 1980; 65:1007–9. [PubMed] [Google Scholar]
- 37.Lerman SJ. Haemophilus influenzae infections of cerebrospinal fluid shunts. Report of two cases. J Neurosurg 1981; 54:261–3. [DOI] [PubMed] [Google Scholar]
- 38.Brandstetter Y, Melzer-Lange M, Chusid MJ. Haemophilus influenzae B meningitis in a patient with a ventriculoperitoneal shunt and meningomyelocele. Wis Med J 1990; 89:461–3. [PubMed] [Google Scholar]


