Abstract
SARS-CoV-2 infection poses increased risks of poor outcomes during pregnancy, including preterm birth and stillbirth. There is also developing concern over the effects of SARS-CoV-2 infection on the placenta, and these effects seem to vary between different viral variants. Despite these risks, many pregnant individuals have been reluctant to be vaccinated against the virus owing to safety concerns. We now have extensive data confirming the safety and effectiveness of COVID-19 vaccination during pregnancy, although it will also be necessary to determine the effectiveness of these vaccines specifically against newly emerging viral variants, including Omicron. In this Progress article, I cover recent developments in our understanding of the risks of SARS-CoV-2 infection in pregnancy, and how vaccination can reduce these.
Subject terms: Vaccines, SARS-CoV-2, Reproductive biology
In this Progress article, Male summarizes our current understanding of the risks associated with SARS-CoV-2 infection in pregnancy. Importantly, the article highlights the now substantial body of evidence supporting the safety and efficacy of COVID-19 vaccination in pregnancy.
Introduction
Viruses that cause pneumonia, including severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), have long been known to be of particular concern during pregnancy1. So as the world entered the SARS-CoV-2 pandemic in early 2020, clinicians and scientists working in obstetrics knew that their patients were likely to be at increased risk. Initially, lockdowns and a tendency towards risk avoidance masked some of the increased risks associated with SARS-CoV-2 infection in pregnancy2,3, but with the passing of time the risks have become clearer. Although pregnant people were excluded from the first trials of COVID-19 vaccines, the pressing need to protect this group meant that the vaccines were rolled out to them in advance of the completion of clinical trials, and we now have extensive real-world data confirming the safety and effectiveness of the vaccines during pregnancy. In this Progress article, I cover recent developments in our understanding of the risks of SARS-CoV-2 infection that are specific to pregnancy, and how vaccination can safely reduce these.
SARS-CoV-2 infection in pregnancy
Obstetric outcomes following SARS-CoV-2 infection
Pregnancy is associated with increased disease severity in those infected with SARS-CoV-2: a meta-analysis of 92 studies comparing outcomes for pregnant patients with COVID-19 with age and sex-matched non-pregnant patients with COVID-19 found that pregnancy increases the risk of needing intensive care (OR 2.13, 95% confidence interval (CI) 1.54–2.95), invasive ventilation (OR 2.59, CI 2.28–2.94) and extracorporeal membrane oxygenation (OR 2.02, CI 1.22–3.34), although the risk of all-cause mortality was not increased (OR 0.96, CI 0.79–1.18)4. A more recent meta-analysis of 111 studies, which compared outcomes for pregnant patients infected with SARS-CoV-2 with those who were not infected, found that infection significantly increased the odds of premature delivery (OR 1.48, 95% CI 1.22–1.8), pre-eclampsia (OR 1.6, CI 1.2–2.1), stillbirth (OR 2.36, CI 1.24–4.46), neonatal mortality (OR 3.35, CI 1.07–10.5) and maternal mortality (OR 3.08, CI 1.5–6.3)5.
Since the publication of these meta-analyses, further large studies have also found increased risks of maternal morbidity and mortality6, preterm birth (PTB) and perinatal death associated with SARS-CoV-2 infection in pregnancy7,8. There is also evidence that both maternal9 and neonatal10 outcomes were worse during the Delta wave of the SARS-CoV-2 pandemic than in preceding periods.
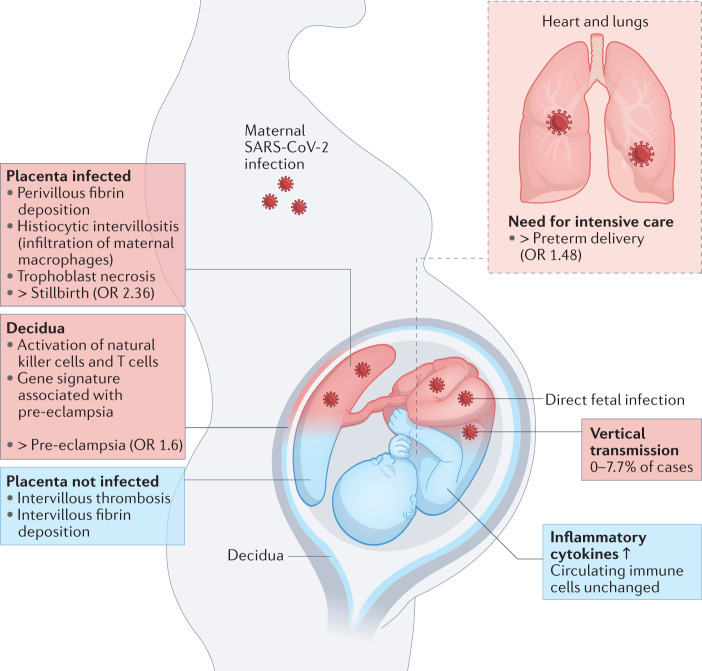
The increased risk of PTB associated with SARS-CoV-2 infection seems to be driven largely by iatrogenic PTBs, with doctors opting to deliver the infant to try to save the critically ill patient11. The increased risk of stillbirth and pre-eclampsia are more likely to be associated with inflammatory changes affecting the placenta (Fig. 1).
Fig. 1. Direct versus indirect effects of SARS-CoV-2 infection on the fetus and placenta.
Maternal SARS-CoV-2 infection can impact pregnancy in numerous ways. The need for intensive care associated with severe disease can necessitate delivering the infant, causing an increased rate of preterm delivery. Placental infection can be associated with SARS-CoV-2 placentitis, which is associated with an increased risk of stillbirth. Even in the absence of placental infection, inflammatory changes are observed in the decidua and placenta, and these may be linked to the increased risk of pre-eclampsia associated with SARS-CoV-2 infection in pregnancy. SARS-CoV-2 can also be vertically transmitted to infect the fetus, although this is uncommon. Blue indicates indirect outcomes on the fetus and placenta associated with maternal infection with SARS-CoV-2, whereas red indicates outcomes associated with direct fetal infection.
SARS-CoV-2 and the placenta
The placenta expresses the cellular receptors for SARS-CoV-2, namely angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2)12–15, and some patients with COVID-19 do become viraemic16, meaning there is the potential for SARS-CoV-2 infection of the placenta. However, SARS-CoV-2 viraemia in pregnancy seems to be uncommon12, and there is little placental co-expression of ACE2 and TMPRSS2, which is required for the canonical route of virus entry into the cell12–14. Moreover, placental expression of ACE2 declines over the course of pregnancy15.
In addition to the general defences the placenta has against viral infection17, these factors might be expected to protect the placenta from infection with SARS-CoV-2. Indeed, placental infection seems to be uncommon. However, SARS-CoV-2-associated coagulation and inflammation occur even in the absence of placental infection, most commonly manifesting as intervillous thrombosis and fibrin deposition12,15,18–20. The mucosal lining of the uterus is a maternal tissue into which the placenta implants and, in pregnancy, is called the decidua. Examination of the decidua in pregnancies affected by SARS-CoV-2 demonstrated local activation of maternal natural killer cells and T cells, including the expression of gene signatures associated with pre-eclampsia15,21.
A more severe inflammatory syndrome occurs when the placenta does become infected; namely, SARS-CoV-2 placentitis. This is characterized by histiocytic intervillositis, perivillous fibrin deposition and trophoblast necrosis, and is emerging as a risk factor for fetal distress or demise22–26. A series of 68 cases of SARS-CoV-2 placentitis associated with either stillbirth or neonatal death found that the causes of death were likely to be fetal hypoxic-ischaemic injury resulting from severe placental damage, rather than fetal infection with SARS-CoV-2. Indeed, placental infection with SARS-CoV-2 does not necessarily equate with fetal infection; in this case series, infection of the fetus was only confirmed in 2 of the 68 cases26.
SARS-CoV-2 and the fetus
Numerous studies have reported SARS-CoV-2 infection in infants born to infected individuals. The largest of these have examined infection by nasopharyngeal swab, finding the rate at which infants test positive for SARS-CoV-2 as between 0.9 and 2.8%27–30. However, infants who test positive in this way have not necessarily been infected in utero, as they may have been infected by horizontal transmission shortly after birth.
Numerous smaller studies have examined umbilical cord blood to more accurately identify those neonates infected by vertical transmission. Although the fetus begins producing both IgG and IgM between 12 and 20 weeks of gestation, maternal IgG can cross the placenta so only the presence of IgM signals fetal exposure to antigen. In pregnancies affected by SARS-CoV-2 infection, detection of Spike-specific IgM in cord blood has been reported in between 0 and 7.7% of cases21,27,31. A systematic review of studies examining the presence of the viral genome in cord blood found it in 2.9% of cases27, although since then a larger case series of 64 deliveries was unable to detect the viral genome in the umbilical cord blood of any infant12.
Increased levels of inflammatory cytokines have been observed in the cord blood of neonates, even in the absence of placental infection21,32. It is unclear whether these cytokines were produced locally by the fetus or reflect maternal cytokines that have crossed the placenta33. However, the findings that immune cells in cord blood show higher cytokine production if the pregnancy was affected by SARS-CoV-2 infection and that IL-8 concentrations are generally higher in cord blood than that in maternal blood suggest that at least some of these cytokines may be produced by the neonate32.
New variants, new outcomes?
One important caveat to much of the preceding data is that they were collected in earlier waves of the pandemic, in which the predominant variants of SARS-CoV-2 were different from those we face now. Of particular concern is that reports of SARS-CoV-2 placentitis were rare in the first wave, caused by the original strain of SARS-CoV-2, but became increasingly common in the Alpha and Delta variant waves of the pandemic24,25. This demonstrates that we cannot necessarily assume that obstetric outcomes will be the same in the current wave, or in future ones, as they have been previously. At the time of writing, we do not have solid data on how the Omicron wave has affected pregnant people.
Vaccine safety in pregnancy
Benefits of vaccination in pregnancy
Vaccination in pregnancy to prevent maternal morbidity and mortality, or to confer passive immunity to the infant, has a long and successful history34. As early as 1879, it was noticed that infants born to individuals who received the smallpox vaccine during pregnancy were themselves protected, and similar observations were made for pertussis and tetanus vaccination in the middle of the twentieth century. Similar to SARS-CoV-2 infection, infection with influenza virus in pregnancy is associated with increased maternal morbidity and, as a result, influenza vaccination in pregnancy has been recommended in the United States since 1997, although it was not until 2005 that clinical trials formally demonstrated its benefits. In the United Kingdom, influenza and pertussis vaccination have been routinely offered in pregnancy since 2010 and 2012, respectively.
Safety of COVID-19 vaccination in pregnancy
The increased potential for severe consequences following SARS-CoV-2 infection in pregnancy makes COVID-19 vaccination of this population particularly attractive. However, pregnant patients naturally want to know whether vaccination is safe for them and their infants. Although we await clinical trial data in this population, pregnant people have been vaccinated against COVID-19 since December 2020, and we now have safety data from more than 185,000 individuals vaccinated during pregnancy (Table 1).
Table 1.
Epidemiological studies on the safety of COVID-19 vaccination in pregnancy
| Study | Number of participants vaccinated in pregnancy | Country | Approach | Outcomes examined | Impact of COVID-19 vaccination | Ref. |
|---|---|---|---|---|---|---|
| v-safe pregnancy registry | 5,096 | United States | Registry | Stillbirth, preterm birth (PTB), small for gestational age (SGA), neonatal death, congenital abnormalities | None detected | 38 |
| PTB, SGA, neonatal intensive care unit (NICU) admission, neonatal death, congenital abnormalities | None detected | 42 | ||||
| Miscarriage | None detected | 43 | ||||
| BORN Ontario | 64,234 | Canada | Registry | PTB, stillbirth, SGA | None detected | 44 |
| Stock et al., 2022 | 18,399 | Scotland | Registry | PTB, perinatal death | None detected | 8 |
| Bookstein-Peretz et al., 2021 | 390 | Israel | Registry | Miscarriage, PTB, SGA, NICU admission | None detected | 41 |
| Norwegian National Health Registries | 1,003 | Norway | Case–control | Miscarriage | None detected | 47 |
| Vaccine Safety Datalink | 31,080 | USA | Case–control | Stillbirth | None detected | 45 |
| Miscarriage | None detected | 46 | ||||
| Cohort | PTB, SGA | None detected | 48 | |||
| Wainstock et al., 2021 | 913 | Israel | Cohort | PTB, pre-eclampsia, SGA | None detected | 49 |
| Blakeway et al., 2021 | 140 | England | Cohort | PTB, stillbirth, SGA, NICU admission, congenital abnormalities | None detected | 51 |
| Maccabi Healthcare Services | 24,288 | Israel | Cohort | Miscarriage, PTB, stillbirth, pre-eclampsia, SGA, SARS-CoV-2 infection | Reduced risk of SARS-CoV-2 infection | 52 |
| Cohort | PTB, SGA, congenital abnormalities, death and hospitalization of infants up to 6 months old | None detected | 50 | |||
| Theiler et al., 2021 | 140 | United States | Cohort | PTB, stillbirth, pre-eclampsia, SGA, NICU admission, SARS-CoV-2 infection | Reduced risk of SARS-CoV-2 infection | 53 |
| UK Health Security Agency | 58,165 | United Kingdom | Cohort | PTB, stillbirth, SGA | None detected | 54 |
Results from the 12 studies summarized show no increased risk of any poor obstetric outcome associated with COVID-19 vaccination. The total number of participants included in these studies is 185,309. This has been calculated as the sum of all participants, except for those in Blakeway et al.51 and Stock et al.8, who are also included in the UK Health Security Agency data and would otherwise be counted twice.
Because the first countries to offer the COVID-19 vaccine in pregnancy, namely the United States and Israel, were using the mRNA vaccines BNT162b2 (Pfizer) and mRNA-1273 (Moderna), the first data available were on these vaccines. As a result, when other countries later made the vaccines available in pregnancy, many preferentially offered mRNA vaccines to this group. Because of this, mRNA-based COVID-19 vaccines have been most widely used in pregnancy and, therefore, the majority of safety data come from these vaccines.
A key finding with regards to safety is that IgM is not detected in umbilical cord blood following vaccination in pregnancy31,35,36. This indicates that the vaccine has not elicited an immune response in the fetus, suggesting that it has not crossed the placental barrier. In line with this, one study that looked for SARS-CoV-2 Spike mRNA or protein in placenta and cord blood following vaccination was unable to detect it36. COVID-19 vaccination in pregnancy is also not associated with pathological changes to the placenta37. These findings indicate that a direct effect of vaccination on fetal development is unlikely. However, local and systemic immune reactions to COVID-19 vaccination do occur in pregnant people, at roughly the same rate at which they occur in the general population38–41. Therefore, it is important to consider the possibility that the immune response to COVID-19 vaccination could affect the placenta or fetus and undertake epidemiological studies to determine whether it could be associated with any poor obstetric outcome. Such studies have taken one of three broad approaches: registry studies, case–control studies and cohort studies (Table 1).
Registry studies
Registry studies recruit participants at the time of vaccination, determine the outcomes of their pregnancies and compare the rates at which adverse events occur in the registry population relative to those seen either in the general pregnant population or during pregnancy historically. The first such study used the v-safe pregnancy registry of the US Centers for Disease Control and Prevention (CDC). Among 713 people vaccinated in pregnancy who had given birth by 30 March 2021, the rates of adverse events were the same as have been reported historically38, and a follow-up study looking at 1,613 vaccinated people who had given birth by September 2021 continued to find a normal rate of adverse events42. Focusing only on those vaccinated before 20 weeks, there was also no increased risk of miscarriage following vaccination43.
The Better Outcomes Registry and Network (BORN) comprises 64,234 people vaccinated against COVID-19 during pregnancy in Ontario, Canada. Of the 31,343 individuals who had given birth by 31 October 2021, the rate of stillbirth, PTB or infants being born small for their gestational age was not increased, compared with either historical data or the background rate44. A study of 18,399 people vaccinated against COVID-19 during pregnancy in Scotland found no increased risk of PTB or neonatal mortality, compared with the general pregnant population8. An early registry study in Israel examined only 390 people vaccinated with BNT162b2 during pregnancy, but also found no increased risk of miscarriage, PTB or infants being born small for their gestational age41.
Case–control studies
Case–control studies identify individuals who experience a predefined adverse event and determine whether those people are more likely to have experienced a particular exposure than those who did not experience the event. Two such studies have been done in the US Vaccine Safety Datalink system, which includes 31,080 people vaccinated during pregnancy. One of these studies found no indication that COVID-19 vaccination is linked to stillbirth45; the second found that people who experienced a miscarriage were no more likely to have been vaccinated in the preceding 28 days than those who did not miscarry46. A similar study carried out in Norway found that, among 18,447 pregnancies, those that ended in miscarriage were no more likely to have received a COVID-19 vaccine in either the preceding 3-week or 5-week period than those that continued47.
Cohort studies
Cohort studies examine the outcomes for those who are vaccinated against COVID-19 in pregnancy, compared with the outcomes of a contemporary cohort of unvaccinated people. Because the participants in these studies have not been randomized to vaccination, there are systematic differences between those who chose to receive a COVID-19 vaccine and those who declined, although the majority of studies attempt to control for these variables, either with multivariate analysis48–50 or by identifying pairs matched for potential confounders51,52. Among seven cohort studies there was no increased risk of miscarriage52, pre-eclampsia49,52,53 or any adverse outcome at the time of birth48–54 associated with COVID-19 vaccination in pregnancy. One study that followed up infants after birth, to an average of 134 days, also found no increased risk of death or hospitalization in the first months of life in infants born following vaccination during pregnancy50.
Although each of these approaches to addressing the question of COVID-19 vaccine safety in pregnancy has its own weakness, the findings from each approach lend weight to the others. Together with the sheer number of participants in these studies, this gives us confidence that COVID-19 vaccination is not associated with adverse pregnancy outcomes.
Vaccine efficacy
Effectiveness of COVID-19 vaccination in pregnancy
Although it is untrue to say that the immune system is weakened in pregnancy, it does differ from the non-pregnant state, with a shift away from cell-mediated immune responses. This is demonstrated by the long-standing observation that cell-mediated autoimmune diseases tend to go into remission during pregnancy, whereas antibody-mediated diseases can flare55. It is therefore not unreasonable to ask whether COVID-19 vaccination is as effective at preventing disease during pregnancy as it is in the broader population.
Early attempts to answer this question looked at vaccine immunogenicity in pregnant participants, compared with age and sex-matched non-pregnant controls. Two reports found that the titres of anti-Spike, anti-RBD (receptor binding domain of the Spike protein) and SARS-CoV-2 neutralizing antibodies were the same in the two groups39,56. Importantly, both of these studies found higher virus-specific antibody titres associated with COVID-19 vaccination compared with SARS-CoV-2 infection, underlining the benefits of vaccination even in those who have already been infected. A third study, using systems serology, also found that overall titres of antibodies did not differ between the groups but further revealed that after only one dose of vaccine, antibody effector functions were induced with delayed kinetics in the pregnant group compared with the non-pregnant group: following the second dose, there was no significant difference between the groups57. Comparing vaccine responses across trimesters using this approach, the same team also found a subtle reduction in antibody effector functions following vaccination in the second trimester, compared with vaccination in the first or third trimesters, which might reflect trimester-specific immune alterations that are known to be associated with a somewhat more quiescent state in the second trimester58. Comparing T cell responses, it was shown that Spike-induced production of IFNγ by total and central memory CD4+ and CD8+ T cells following vaccination did not differ between pregnant and non-pregnant groups56.
More recently, two cohort studies from Israel have enabled an estimate of COVID-19 vaccine effectiveness during pregnancy, finding it to be roughly the same as in the general population52,59. UK surveillance data have not been used to model vaccine effectiveness, but nevertheless point to COVID-19 vaccination being effective in pregnancy, particularly against severe disease60. In Scotland, 68% of the pregnant population was unvaccinated by October 2021, but unvaccinated individuals accounted for 77.4% of all SARS-CoV-2 infections, 90.9% of COVID-19 hospitalizations and 98% of intensive care unit admissions among pregnant people; furthermore, all perinatal deaths following SARS-CoV-2 infection in pregnancy occurred in unvaccinated individuals8. These data sets were collected largely during the period when the Alpha variant was dominant, but with some contribution from the Delta wave. As the Omicron variant becomes prominent, it will be important to determine the effectiveness of vaccination specifically against this strain, how this varies following a booster dose and the extent to which protection wanes over time. It will also be necessary to determine the extent to which vaccination provides protection specifically against COVID-19-associated pregnancy complications.
Protection of infants by maternal COVID-19 vaccination
As expected, maternal IgG raised by vaccination during pregnancy crosses the placenta and is present in umbilical cord blood at birth31,35,36,39,56,58,61–63, remaining detectable in the blood of more than half of infants at 6 months61. Transplacental transfer of IgG following vaccination against tetanus and pertussis provides infants with protection against these diseases, and by analogy, COVID-19 vaccination in pregnancy may provide infants with some protection against COVID-19. Early estimates suggest that vaccination after 20 weeks of pregnancy is 80% effective (95% CI, 55–91%) and vaccination before 20 weeks is 32% effective (95% CI, 43–68%) at preventing hospitalization of infants younger than 6 months old with COVID-19 (ref.64).
This increased protection of infants following vaccination later in pregnancy is in line with findings that maximum cord blood antibody titres are achieved when vaccination occurs in the late second to early third trimester62,63. This is likely to reflect higher maternal IgG titres at the time of birth when vaccination has occurred more recently, as the efficiency of Spike-specific IgG transfer across the placenta is highest following vaccination in the first trimester58. However, it is important to be clear that the main benefit of COVID-19 vaccination in pregnancy is the reduction in risk of disease during pregnancy. Therefore, the timing of vaccination should seek to optimize protection during pregnancy, rather than that of the infant after birth.
Notably, the titres of anti-Spike antibodies seen in cord blood are lower following SARS-CoV-2 infection in pregnancy compared with COVID-19 vaccination in pregnancy56,61. This is likely to be a result of both lower maternal IgG titres being elicited by infection compared with vaccination56,61 and also because the transport of SARS-CoV-2-specific antibodies is compromised following SARS-CoV-2 infection in the third trimester12,65.
Conclusion
SARS-CoV-2 infection poses significant risks to pregnant people and their infants, but COVID-19 vaccination is safe in pregnancy. This underlies the recommendation that pregnant people receive the COVID-19 vaccine, which is now being made by public health bodies all over the world66,67. Despite these recommendations, in many countries uptake of the COVID-19 vaccine in pregnancy remains low, so it is essential that we continue to communicate this message to those who are making the decision about COVID-19 vaccination, for themselves and their infants. Although it is understandable that this group might feel cautious, the task has been made more difficult by the proliferation of misinformation about the safety of COVID-19 vaccination in pregnancy. Strong public health messaging is needed, but more importantly we must ensure that midwives and obstetricians are adequately equipped to counsel their patients on the benefits of COVID-19 vaccination.
Competing interests
The author declared no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Mascio D, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty AB, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni E, et al. Symptoms and syndromes associated with SARS-CoV-2 infection and severity in pregnant women from two community cohorts. Sci. Rep. 2021;11:6928. doi: 10.1038/s41598-021-86452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allotey J, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchand G, et al. Review and meta-analysis of COVID maternal and neonatal clinical features and pregnancy outcomes to June 3rd 2021. AJOG Glob. Rep. 2021;3:100049. doi: 10.1016/j.xagr.2021.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz TD, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piekos SN, et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit. Health. 2022;4:e95–e104. doi: 10.1016/S2589-7500(21)00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock SJ, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022 doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strid P, et al. COVID-19 severity among women of reproductive age with symptomatic laboratory-confirmed SARS-CoV-2 by pregnancy status — United States, Jan 1, 2020–Sep 30, 2021. ResearchSquare. 2021 doi: 10.21203/rs.3.rs-1090075/v1. [DOI] [Google Scholar]
- 10.The National Perinatal Epidemiology Unit. Key information on COVID-19 in pregnancy. NPEUwww.npeu.ox.ac.uk/assets/downloads/npeu-news/MBRRACE-UK_Rapid_COVID_19_DEC_2021_-__Infographic_v13.pdf (2021).
- 11.Vousden N, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS ONE. 2021;16:e0251123. doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edlow AG, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 2020;3:e2030455. doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pique-Regi R, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2021;9:e58716. doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashary N, et al. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020;8:783. doi: 10.3389/fcell.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu-Culligan A, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal–fetal interface. medRxiv. 2021;2:591–610. doi: 10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fajnzylber J, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Ann. Rev. Virol. 2014;1:133–146. doi: 10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- 18.Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020;46:151530. doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhu M, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smithgall MC, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77:994–999. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Flores V, et al. Maternal–fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022;13:320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins JC, Torous VF, Roberts DJ. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch. Pathol. Lab. Med. 2021;145:1341–1349. doi: 10.5858/arpa.2021-0246-SA. [DOI] [PubMed] [Google Scholar]
- 23.Linehan L, et al. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shook LL, et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J. Infect. Dis. 2022;225:754–758. doi: 10.1093/infdis/jiac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald B, et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 Alpha. Arch. Pathol. Lab. Med. 2022 doi: 10.5858/arpa.2021-0586-SA. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DA, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch. Pathol. Lab. Med. 2022 doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 27.Kotlyar AM, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224:35–53. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodworth KR, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy — SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherman VJ, et al. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): first report from the Pregnancy Coronavirus Outcomes Registry (PRIORITY) study. Clin. Infect. Dis. 2021;73:e2810–e2813. doi: 10.1093/cid/ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman M, et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325:2076–2086. doi: 10.1001/jama.2021.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beharier O, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Invest. 2021;131:e150319. doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gee S, et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021;22:1490–1502. doi: 10.1038/s41590-021-01049-2. [DOI] [PubMed] [Google Scholar]
- 33.Dahlgren J, et al. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 34.Mackin DW, Walker SP. The historical aspects of vaccination in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021;76:13–22. doi: 10.1016/j.bpobgyn.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prahl M, et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy. medRxiv. 2021 doi: 10.1101/2021.12.09.21267423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanes ED, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet. Gynecol. 2021;138:281–283. doi: 10.1097/AOG.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimabukuro TT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray KJ, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021;225:303. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadali RAK, et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021;225:458–460. doi: 10.1016/j.ajog.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bookstein Peretz S, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021;58:450–456. doi: 10.1002/uog.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson, C. K. COVID-19 vaccine safety in pregnancy: updates from the v-safe COVID-19 Vaccine Pregnancy Registry. Centers for Disease Control and Preventionhttps://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/09-COVID-Olson-508.pdf (2021).
- 43.Zauche LH, et al. Receipt of mRNA COVDID-19 vaccines and risk of spontaneous abortion. N. Engl. J. Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BORN Ontario. COVID-19 vaccination during pregnancy in Ontario. BORN Ontariohttps://www.bornontario.ca/en/whats-happening/covid-19-vaccination-during-pregnancy-in-ontario.aspx (2021).
- 45.Kharbanda E. O. Safety of COVID-19 vaccination in pregnancy, interim data from the Vaccine Safety Datalink. Centers for Disease Controlhttps://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/10-COVID-Kharbanda-508.pdf (2021).
- 46.Kharbanda EO, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnus MC, et al. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N. Engl. J. Med. 2021;385:2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipkind HS, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR. 2022;71:26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldshtein I, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blakeway H, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am. J. Obstet. Gynecol. 2021;10:S0002–S9378. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldshtein I, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theiler RN, et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM. 2021;3:100467. doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.UK Health Security Agency. COVID-19 vaccine surveillance report. Week 4. UK Health Security Agencyhttps://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf (2022).
- 55.Buyon JP. The effects of pregnancy on autoimmune diseases. J. Leukoc. Biol. 1998;6:281–287. doi: 10.1002/jlb.63.3.281. [DOI] [PubMed] [Google Scholar]
- 56.Collier AY, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atyeo C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021;13:eabi8631. doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atyeo C, et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. medRxiv. 2021 doi: 10.1101/2021.11.12.21266273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dagan N, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 60.Vousden N, et al. Impact of SARS-CoV-2 variant on the severity of maternal infection and perinatal outcomes: data from the UK Obstetric Surveillance System national cohort. medRxiv. 2021 doi: 10.1101/2021.07.22.21261000. [DOI] [Google Scholar]
- 61.Shook LL, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rottenstreich A, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin. Microbiol. Infect. 2021;3:S1198–S1743. doi: 10.1016/j.cmi.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang YJ, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet. Gynecol. 2021;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 64.Halasa NB, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months — 17 states, July 2021–January 2022. MMWR. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atyeo C, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.UK Health Security Agency. Pregnant women urged to come forward for COVID-19 vaccination. GOV.UKhttps://www.gov.uk/government/news/pregnant-women-urged-to-come-forward-for-covid-19-vaccination (2021).
- 67.Centers for Disease Control and Prevention. COVID-19 vaccine monitoring systems for pregnant people. CDChttps://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/monitoring-pregnant-people.html (2021).