Abstract
Background
Anxiety disorders are a potentially disabling group of disorders that frequently co‐occur with alcohol use disorders. Comorbid anxiety and alcohol use disorders are associated with poorer outcomes, and are difficult to treat with standard psychosocial interventions. In addition, improved understanding of the biological basis of the conditions has contributed to a growing interest in the use of medications for the treatment of people with both diagnoses.
Objectives
To assess the effects of pharmacotherapy for treating anxiety in people with comorbid alcohol use disorders, specifically: to provide an estimate of the overall effects of medication in improving treatment response and reducing symptom severity in the treatment of anxiety disorders in people with comorbid alcohol use disorders; to determine whether specific medications are more effective and tolerable than other medications in the treatment of particular anxiety disorders; and to identify which factors (clinical, methodological) predict response to pharmacotherapy for anxiety disorders.
Search methods
Review authors searched the specialized registers of The Cochrane Collaboration Depression, Anxiety and Neurosis Review Group (CCDANCTR, to January 2014) and the Cochrane Drugs and Alcohol Group (CDAG, to March 2013) for eligible trials. These registers contain reports of relevant randomized controlled trials (RCT) from: the Cochrane Central Register of Controlled Trials (CENTRAL, all years), MEDLINE (1950 to date), EMBASE (1974 to date) and PsycINFO (1967 to date). Review authors ran complementary searches on EMBASE, PubMed, PsycINFO and the Alcohol and Alcohol Problems Science Database (ETOH) (to August 2013). We located unpublished trials through the National Institutes of Health (NIH) RePORTER service and the World Health Organization (WHO) International Clinical Trials Registry Platform (to August 2013). We screened reference lists of retrieved articles for additional studies.
Selection criteria
All true RCTs of pharmacotherapy for treating anxiety disorders with comorbid alcohol use disorders. Trials assessing drugs administered for the treatment of drinking behaviour, such as naltrexone, disulfiram and acamprosate were not eligible for inclusion in this systematic review.
Data collection and analysis
A systematic review is a standardised evaluation of all research studies that address a particular clinical issue.
Two review authors independently assessed RCTs for inclusion in the review, collated trial data and assessed trial quality. We contacted investigators to obtain missing data. We calculated categorical and continuous treatment effect estimates and their 95% confidence intervals (CI) for treatment using a random‐effects model with effect‐size variability expressed using Chi2 and I2 heterogeneity statistics.
Main results
We included five placebo‐controlled pharmacotherapy RCTs (with 290 participants) in the review. Most of the trials provided little information on how randomization was performed or on whether both participants and study personnel were blinded to the intervention. Two of the three trials reporting superiority of medication compared with placebo on anxiety symptom outcomes were industry funded. We regarded one trial as being at high risk of bias due to selective reporting.
Study participants had Diagnostic and Statistical Manual (DSM) III‐ and DSM IV‐diagnosed alcohol use disorders and post‐traumatic stress disorder (two studies), social anxiety disorder (SAD; two studies) or generalized anxiety disorder (GAD; one study). Four trials assessed the efficacy of the selective serotonin re‐uptake inhibitors (SSRIs: sertraline, paroxetine); one RCT investigated the efficacy of buspirone, a 5‐hydroxytryptamine (5‐HT) partial agonist. Treatment duration lasted between eight and 24 weeks. Overall, 70% of participants included in the review were male.
There was very low quality evidence for an effect of paroxetine on global clinical response to treatment, as assessed by the Clinical Global Impressions ‐ Improvement scale (CGI‐I). Global clinical response was observed in more than twice as many participants with paroxetine than with placebo (57.7% with paroxetine versus 25.8% with placebo; risk ratio (RR) 2.23, 95% CI 1.13 to 4.41; 2 trials, 57 participants). However, there was substantial uncertainty regarding the size of the effect of paroxetine due to the small number of studies providing data on clinically diverse patient samples. The second primary outcome measure was reduction of anxiety symptom severity. Although study investigators reported that buspirone (one trial) was superior to placebo in reducing the severity of anxiety symptoms over 12 weeks, no evidence of efficacy was observed for paroxetine (mean difference (MD) ‐14.70, 95% CI ‐33.00 to 3.60, 2 trials, 44 participants) and sertraline (one trial). Paroxetine appeared to be equally effective in reducing the severity of post‐traumatic stress disorder (PTSD) symptoms as the tricyclic antidepressant desipramine in one RCT. The maximal reduction in anxiety disorder symptom severity was achieved after six weeks with paroxetine (two RCTs) and 12 weeks with buspirone (one RCT), with maintenance of medication efficacy extending to 16 with paroxetine and 24 weeks with buspirone. There was no evidence of an effect for any of the medications tested on abstinence from alcohol use or depression symptoms. There was very low quality evidence that paroxetine was well tolerated, based on drop‐out due to treatment‐emergent adverse effects. Nevertheless, levels of treatment discontinuation were high, with 43.1% of the participants in the studies withdrawing from medication treatment. Certain adverse effects, such as sexual problems, were commonly reported after treatment with paroxetine and sertraline.
Authors' conclusions
The evidence‐base for the effectiveness of medication in treating anxiety disorders and comorbid alcohol use disorders is currently inconclusive. There was a small amount of evidence for the efficacy of medication, but this was limited and of very low quality. The majority of the data for the efficacy and tolerability of medication were for SSRIs; there were insufficient data to establish differences in treatment efficacy between medication classes or patient subgroups. There was a small amount of very low quality evidence that medication was well tolerated. There was no evidence that alcohol use was responsive to medication.
Large, rigorously conducted RCTs would help supplement the small evidence‐base for the efficacy and tolerability of pharmacotherapy for anxiety and comorbid alcohol use disorders. Further research on patient subgroups who may benefit from pharmacological treatment, as well as novel pharmacological interventions, is warranted.
Plain language summary
Medication for treating anxiety disorders in people with alcohol use problems
Who may be interested in this review?
People with anxiety disorders and alcohol use problems, as well as their healthcare providers.
Why is this review important?
People with anxiety disorders often also abuse alcohol or have alcohol dependence. All anxiety disorders involve long‐lasting and excessive fear, and can be classified according to the cause of the fear: generalized anxiety disorder (everyday situations), obsessive‐compulsive disorder (repetitive thoughts and behaviours), panic disorder (panic attacks), post‐traumatic stress disorder (previous traumatic events), social anxiety disorder (negative judgements by others) and specific phobia (specific objects or situations). When people with anxiety disorders abuse or are dependent on alcohol, they may be more disabled and difficult to treat than when they have either condition on its own. Psychotherapy is most often used in treating anxiety disorders in people with alcohol use problems. In psychotherapy people are encouraged to explore their feelings, moods, behaviours, thoughts and reactions to the cause of their anxiety. Psychotherapy does not always work though, so it is important to test whether medications are an effective treatment option.
What questions does this review aim to answer?
We wanted to find out whether medication is effective in treating people with both anxiety disorders and alcohol use problems. For this reason, we systematically searched for randomized controlled trials (RCTs) of medication in treating people with both disorders. RCTs provide a more accurate measure of the effectiveness of medication by making sure that people in the study have an equal chance of being treated with medication or placebo.
Which studies were included in the review?
This review found five RCTs in 290 adults (average age 37.4 years) with anxiety and alcohol use disorders. The evidence is current up to January 2014. Two trials looked at social anxiety disorder, two looked at post‐traumatic stress disorder and one trial looked at generalized anxiety disorder. All of the included trials took place in the USA. Most of the study participants were male (70%), and were classified as having alcohol dependence (79%).
What does the evidence from the review tell us?
It was not possible to tell whether medication was effective in treating people with anxiety and alcohol use disorders. Although more than twice as many people (57.7%) with social anxiety disorder who were treated with paroxetine in two trials showed signs of clinical improvement compared with people receiving placebo (25.8%), the quality of the evidence was very low. One study reported that buspirone reduced anxiety disorder symptoms after 12 weeks of treatment. None of the other studies found reductions in symptoms. Treatment with medication appeared to be acceptable to participants, but again the quality of the evidence showing this was very low. Certain medication side effects, such as sexual problems, were commonly reported after treatment with paroxetine and sertraline. There was no evidence that treatment had an effect on alcohol use.
It was difficult to interpret the findings reported by the studies included in this review. Many participants (43.1% altogether) dropped out of the studies before treatment ended. In addition, outcomes that were reported were either not precise, or appeared to be based on the selective reporting of measures that showed an effect of medication. Funding of two of the studies by drug companies may also have led to reporting of results that favoured the medication.
Summary of findings
Summary of findings 1. Paroxetine compared with placebo for anxiety and comorbid alcohol use disorders.
| Paroxetine compared with placebo for anxiety and comorbid alcohol use disorders | ||||||
| Patient or population: people with anxiety and comorbid alcohol use disorders Settings: Drug and alcohol treatment and community settings in South Carolina, USA Intervention: paroxetine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Paroxetine | |||||
| Treatment response CGI‐I | Study population | RR 2.23 (1.13 to 4.41) | 57 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ | |
| 258 per 1000 | 575 per 1000 (292 to 1000) | |||||
| Moderate | ||||||
| 248 per 1000 | 553 per 1000 (280 to 1000) | |||||
| Symptom severity reduction LSAS | ‐ | The mean symptom severity reduction in the intervention groups was 14.7 lower (33 lower to 3.6 higher) | ‐ | 44 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
| Treatment acceptability | Study population | RR 3.29 (0.14 to 76.33) | 57 (2 studies) | ⊕⊝⊝⊝ verylow1,2,3,4,5 | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Proportion heavy drinking days | Study population | Not estimable | 0 (0) | See comment | No data | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Proportion of days abstinent | ‐ | The mean proportion of days abstinent in the intervention groups was 0.08 higher (0.26 lower to 0.43 higher) | ‐ | 54 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,6 | ‐ |
| Drinks per drinking day | ‐ | The mean drinks per drinking day in the intervention groups was 2.42 lower (4.97 lower to 0.14 higher) | ‐ | 54 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
| Depression symptom reduction | ‐ | The mean depression symptom reduction in the intervention groups was 2.3 lower (7.51 lower to 2.91 higher) | ‐ | 15 (1 study) | ⊕⊝⊝⊝ very low2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI: Clinical Global Impressions scale; CI: confidence interval; LSAS: Liebowitz Social Anxiety Scale; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Book 2008 restricted participation to people who drank to cope with their social anxiety symptoms and who were not seeking treatment for alcohol disorders. In addition, the authors acknowledged that the low levels of drinking observed in their sample may have biased their results with respect to people with social anxiety disorder seeking treatment for alcohol abuse/dependence. Therefore, their findings may not generalize to all people with social anxiety disorder and comorbid alcohol use disorders. 2 The 95% confidence interval around the effect estimate for the outcome was very wide. 3 Too few trials to reliably assess publication bias. 4 Inconsistency or statistical heterogeneity could not be calculated, due to too few events.
5 Drop‐outs due to adverse events only provided a surrogate measure of treatment acceptability. 6 Measure of heterogeneity and consistency (I2 statistic) indicated that variability between effect estimates from individual trials exceeded what would be expected by chance.
Background
Description of the condition
Anxiety disorders are highly prevalent and are associated with high social, personal and economic costs. According to traditional Diagnostic and Statistical Manual (DSM) IV‐TR criteria (APA 2000), this class of disorders includes generalized anxiety disorder (GAD), obsessive‐compulsive disorder (OCD), panic disorder (PD), post‐traumatic stress disorder (PTSD), social anxiety disorder (SAD) and specific phobia (SP). Though differing in specific symptom profiles, the anxiety disorders are characterized by a state of chronic physiological hyperarousal to fear‐inducing contexts (such as those involving social interaction in the case of SAD).
Alcohol use disorders include alcohol dependence and abuse. Anxiety disorders frequently co‐occur with alcohol dependence, defined in the DSM‐IV‐TR as the presence of three of the following criteria any time over a 12‐month period: evidence of tolerance to alcohol; symptoms of withdrawal following termination of alcohol use; consumption of increasing alcoholic beverages in larger amounts or over a longer time span than anticipated; unsuccessful attempts to reduce alcohol intake; the spending of large amounts of time acquiring alcohol; reduction or quitting of social, occupational or recreational activities due to drinking; and continued drinking despite knowledge that this leads to persistent or recurrent physical or psychological problems (APA 2000). Comorbid alcohol abuse is less commonly observed, can only be diagnosed in the absence of alcohol dependence, and is defined as a clinically significant occurrence within a 12‐month timeframe of at least one of the following criteria: a recurrent pattern of alcohol use that: interferes with obligations at work, school or home; places a person in physically hazardous situations; results in legal problems; or causes or exacerbates persistent or recurrent social or interpersonal problems (APA 2000).
The diagnosis of alcohol dependence is approximately two to four times more likely for individuals diagnosed with a range of anxiety disorders (Regier 1990; Kessler 1997; Hasin 2007), while alcohol abuse may be under‐represented relative to individuals without anxiety disorders (Kessler 1997; Boschloo 2013). One nationally representative survey in the US found lifetime prevalence rates of co‐occurring alcohol dependence and anxiety disorders to be as high as 35.8% for men and 60.7% for women (Kessler 1997). In contrast, the European Study of the Epidemiology of Mental Disorders reported a prevalence rate of only 0.1% for comorbidity of these diagnoses over a 12‐month period across six European countries (Alonso 2004). Nevertheless, the co‐occurrence of these disorders has been associated with more severe symptoms, higher rates of relapse and a corresponding increase in the utilization of mental health services (Kessler 1996). There is also some epidemiological evidence of a poorer prognosis and increased rates of relapse for people with anxiety disorders in alcoholism treatment programmes (Driessen 2001; Kushner 2005).
Several theoretical models have articulated the nature of the relationship between alcohol dependence/abuse and anxiety disorders. Those that assign primacy to the anxiety disorders typically ascribe to the notion that alcohol is abused to help cope with anxiety‐provoking situations. Such models include the Tension Reduction Theory (TRT) (Kushner 1990), and the Self‐Medication Hypothesis (SMH) (Khantzian 1985), and may be particularly relevant for anxiety disorders that typically precede onset of dependence, such as SAD and agoraphobia (Kushner 1990; Brady 1993). Indeed, evidence of a causal interaction between anxiety disorder symptoms and alcohol use disorders includes a possible dose‐response relationship between the severity of social phobia symptoms and degree of abusive drinking (Morris 2005), as well as the finding that early treatment of anxiety disorder symptoms reduced subsequent alcohol abuse (Kendall 2004).
Description of the intervention
Certain forms of psychotherapy, such as cognitive behaviour therapy (CBT), have been recommended in clinical practice guidelines as first‐line treatments for anxiety disorders (NICE 2005; CPA 2006). Although a Cochrane review is currently underway to assess the efficacy of psychotherapy for comorbid PTSD and substance use disorders (Roberts 2012), there is reason to believe that the effectiveness of CBT for treating anxiety disorders might be limited by comorbid alcohol dependency. Alcohol consumption likely to impair desensitisation to stressors and modification of maladaptive cognitions, core components of CBT (Morris 2005). In contrast, behavioural programmes designed for treating alcohol dependence may not be effective in reducing anxiety disorder symptoms, as evident in one controlled trial of CBT for comorbid alcoholism and PD (Bowen 2000). As predicted by SMH, increasing rates of relapse following the application of stress‐inducing treatment modalities, such as exposure therapy, might offer one explanation for the failure to detect reduced relapse rates in a number of randomized controlled trials (RCTs) of alcoholism treatment interventions that have incorporated CBT for anxiety disorders (Bowen 2000; Randall 2001a; Schadé 2005a).
The possible limitations of psychotherapy for people with anxiety disorder with comorbid alcohol dependence, and the increasing elucidation of the biological substrates of alcohol dependency and abuse suggest that pharmacotherapy should be considered as a treatment option (Bühler 2011). The selective serotonin re‐uptake inhibitors (SSRIs) are regarded as first‐line medications in treating anxiety disorders (BAP 2014). The evidence for the efficacy of SSRIs in reducing alcohol consumption is less clear, with some evidence that SSRIs may produce favourable outcomes when treating people with less severe alcohol dependence (Pettinati 2000), but may actually worsen drinking outcomes when administered concurrently with CBT for severe alcohol dependence (Kranzler 2006). Conversely, naltrexone, an opioid receptor antagonist with US Food and Drug Administration (FDA) approval for relapse prevention in alcohol dependence, has shown promise in reducing the severity of PTSD symptoms in several open‐label trials (Bills 1993; Lubin 2002). Nevertheless, one large 12‐week RCT of naltrexone and the acetaldehyde dehydrogenase inhibitor, disulfiram, in a treatment‐seeking veteran sample, did not demonstrate that these medications were significantly more effective in reducing PTSD symptoms compared with placebo (Petrakis 2006).
The possibility that alcoholism and anxiety disorders may reinforce one another following onset suggests that treatment strategies that target both forms of psychopathology might be effective. Although this is consistent with the expert consensus view (Stewart 2008; Smith 2012), empirical support for the potential usefulness of combining medication with psychotherapy in the simultaneous treatment of both disorders is mixed (Back 2006; Ciraulo 2013). Reported reductions in social anxiety symptoms in people receiving mirtazapine, specific serotonergic and noradrenergic re‐uptake inhibitor (SNRI) antidepressant, as part of a CBT alcohol detoxification protocol, suggest that medications that target neurotransmitter systems implicated in both anxiety and alcohol use disorders may be beneficial in treating this comorbid patient population (Liappas 2003; Liappas 2005). Support for this conclusion is weakened somewhat by difficulty in disentangling these treatment effects from reductions in withdrawal‐induced anxiety symptoms, and failure to detect similar effects in controlled studies that combine CBT with venlafaxine, another SNRI (Liappas 2005; Ciraulo 2013).
How the intervention might work
Low levels of extracellular serotonin have been documented in human brains after chronic exposure to alcohol (see Mukherjee 2008 for a review). Medications that increase the availability of serotonin in the synapses, either through inhibiting the re‐uptake of serotonin into the pre‐synaptic terminal (as in SSRIs) or through other mechanisms (as in buspirone) would be expected to normalize brain function with associated improvements in anxiety symptoms. In addition, alterations in the functioning of the gamma‐aminobutyric acid (GABA) neurotransmitter system have been identified as fundamental to alcohol response, dependency, vulnerability to alcohol use disorders and pharmacotherapy of these disorders (Krystal 2006). The activation of GABA receptors and inhibition of the sympathetic system (with corresponding inhibition of the noradrenergic pathway) have been implicated in the stress‐reducing effects observed following alcohol consumption. Notably, alcohol dependence and withdrawal is associated with a subsequent reduction in GABA activity (Krystal 2006), potentially explaining how the initial anxiolytic effects of imbibing alcohol gives way to increasing levels of anxiety with alcohol dependence (Kushner 1990). GABAenergic medications that have demonstrated some efficacy in treating anxiety disorders, such as the benzodiazepines, and the anticonvulsant pregabalin (Feltner 2003; Pande 2004; Pohl 2005; Rickels 2005), might be expected to be effective in people with comorbid alcohol disorders. However, benzodiazepines are generally not recommended given the risk of dependency, and the finding of prolonged withdrawal symptoms and increased alcohol use following a randomized controlled detoxification treatment with lorazepam (Malcolm 2002).
Why it is important to do this review
There are a number of shortcomings in the literature on the treatment of anxiety disorders in populations diagnosed with comorbid alcohol dependence. A general paucity of evidence exists for recommendations for treating affective or anxiety disorders that are comorbid with substance use disorders (Watkins 2005). Clinical trials of medication for treating anxiety disorders also frequently exclude people with comorbid alcohol dependency, limiting the generalisability of their findings (Hoertel 2012). The small samples employed by dual‐diagnosis medication trials may also have prevented the detection of significant differences in the efficacy of treatments for anxiety disorders. A systematic review and meta‐analysis, employing the methodology of The Cochrane Collaboration, would help to quantify the extent of these shortcomings, and would extend the narrative reviews conducted to date in this patient population (Schadé 2003; Berenz 2012; Lev‐Ran 2012). Moreover, through the quantitative synthesis of trial data, such a review would help address other questions of interest in the treatment of anxiety disorders with comorbid alcohol dependence or abuse, including the relative efficacy of different drugs in treating particular anxiety disorders; the clinical effectiveness of these same drugs across anxiety disorders; and whether patient characteristics, such as gender, predict response to treatment.
Objectives
To assess the effects of pharmacotherapy for treating anxiety in people with comorbid alcohol use disorders, specifically: to provide an estimate of the overall effects of medication in improving treatment response and reducing symptom severity in the treatment of anxiety disorders in people with comorbid alcohol use disorders; to determine whether particular medications are more effective and tolerable than other medications in the treatment of particular anxiety disorders; to identify which factors (clinical, methodological) predict response to pharmacotherapy for anxiety disorders.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs of pharmacotherapy for treating anxiety disorders with comorbid alcohol use disorders. We only included group‐based treatments if they employed a cluster randomization design. We excluded trials in which the allocation sequence was generated via a quasi‐random procedure (such as determining group allocation by day of the week or alternation). We applied no language restrictions.
We included both trials targeting relapse prevention as well as the treatment of ongoing alcohol dependence or abuse in the review. Alcohol withdrawal may result in a short‐term increase in anxiety symptoms, with evidence that these symptoms stabilize within four to eight weeks of the onset of abstinence (Schuckit 1988; Driessen 2001). Therefore, we did not included relapse prevention studies that diagnosed anxiety disorders with a symptom profile similar to that observed during withdrawal (i.e. GAD, PD) within four weeks after the discontinuation of alcohol consumption in the review.
Types of participants
Age
We imposed no age restrictions.
Diagnosis
We included people diagnosed with alcohol dependence or abuse and an anxiety disorder according to DSM‐III (APA 1980), DSM‐IV (APA 1994), or DSM‐IV‐TR (APA 2000) criteria.
Comorbidities
We used the diagnosis of additional comorbid psychiatric diagnoses (excluding other secondary anxiety disorders) as an exclusion criteria. We revised the original protocol to include participants with major depressive disorder (MDD), given the frequent co‐occurrence of MDD in this comorbid population (see Differences between protocol and review).
The presence of physical disabilities did not qualify as an exclusion criteria, with the exception of traumatic brain injury in RCTs of PTSD (due to difficulties in distinguishing between the symptoms of PTSD and traumatic brain injury).
Setting
We applied no restrictions with regards to country in which the trial took place, the number of centres involved or whether trials were conducted in outpatient or inpatient settings.
Types of interventions
Experimental interventions
All medication interventions in which the drug was administered to treat anxiety disorders were eligible for inclusion in this review. However, we did not include trials in which people were receiving concurrent psychotropic medications or that were limited to the comparison of drugs administered with the purpose of affecting drinking behaviour, such as naltrexone, disulfiram and acamprosate.
As we anticipated that most treatment studies targeting anxiety disorders in people with comorbid alcohol use disorders would employ concurrent behavioural modification programmes to treat alcohol dependence/abuse, the presence of such strategies did not serve as an exclusion criteria.
Control interventions
Control interventions included placebo, standard treatment and other medications.
Types of outcome measures
Primary outcomes
Clinical treatment response. The number of responders versus non‐responders was determined from the Clinical Global Impressions scale ‐ Improvement item (CGI‐I) (or closely related measure), a widely used global outcome measure (Guy 1976). Responders are defined on the CGI‐I as those with a score of 1 = 'very much' or 2 = 'much' improved.
Reduction of symptom severity determined from a variety of validated continuous outcome measures, such as the Liebowitz Social Anxiety Scale (LSAS) (Liebowitz 1987), the Clinician Administered PTSD Scale (CAPS) (Blake 1990), the Hamilton Anxiety scale (HAM‐A) (Hamilton 1959), and the Panic Disorder Severity Scale (PDSS) (Shear 1997).
Acceptability of medication determined by the total proportion of participants who withdrew from RCTs due to treatment‐emergent adverse events, which is a surrogate measure used in the absence of other more direct indicators of acceptability. This quantitative measure of the acceptability of medication was supplemented with a narrative review of the most common drug‐related adverse events for both the included and excluded studies (defined as those occurring in at least 10% of the participants given medication), as well as significant differences in the rate of occurrence of drug‐related adverse events between medication and control groups.
Secondary outcomes
Scores on rating scales for disorders other than the primary anxiety disorder, including:
abstinence and reduction of alcohol use assessed using the component subscales of standardized instruments such as the Timeline Followback scale (TLFB) (Sobell 1992). In trials that did not use such an instrument, we assessed these outcomes in terms of the operational definitions employed by study authors;
reduction of comorbid symptoms of depression assessed using scales such as the Beck Depression Inventory (BDI) (Beck 1961), the Hamilton Depression scale (HAM‐D) (Hamilton 1969), and the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979).
The effectiveness of medication for treating anxiety disorders was assessed with measures of:
quality of life, such as the 36‐item Sort Form (SF‐36) Health Survey (Ware 1992);
functional disability, such as the Sheehan Disability Scale (SDS), which includes subscales to assess work‐, social‐ and family‐related impairment (Sheehan 1996).
Timing of outcome assessment
Where studies assessed outcomes at multiple time points, we collated data from the assessments that occurred up until 12 weeks after initiation of treatment for the assessment of the short‐term effectiveness of the medication. Where available, we combined data from assessments made after three months as part of an analysis of long‐term medication effectiveness.
Selection among multiple measures for the same outcome
Where multiple instruments were employed to assess anxiety disorder symptom severity, we gave preference to gold‐standard clinician‐rated instruments, including the LSAS for Social Anxiety Disorder, CAPS for PTSD, HAM‐A for GAD, and PDSS for PD. Where these were not employed, we used self rating versions of these scales, such as the self rating version of the LSAS (Oakman 2003). Finally, we would have considered other self rating instruments, based on an evaluation of their published psychometric properties. With regards to comorbid depression, a variety of established instruments is currently employed, including the BDI, HAM‐D and MADRS. In this instance, we determined which outcome to include in the meta‐analysis by maximizing the proportion of data from the same scale across trials.
Search methods for identification of studies
Electronic searches
We conducted searches for published studies on the following databases:
The specialized registers of The Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDANCTR, to January 2014) (Appendix 1) and the Cochrane Drugs and Alcohol Group (CDAG, to March 2013) (Appendix 2). These registers contain reports of relevant RCTs from the Cochrane Central Register of Controlled Trials (CENTRAL, all years), MEDLINE (1950 to date), EMBASE (1974 to date) and PsycINFO (1967 to date).
The review authors conducted additional searches (to August 2013) on PubMed (Appendix 3), using the highly sensitive search strategy developed by Robinson and Dickersin (Robinson 2002), as well as EMBASE (Ovid) (Appendix 4), PsycINFO (Ovid) (Appendix 5) and the Alcohol and Alcohol Problems Science Database (etoh.niaaa.nih.gov) (Appendix 6).
We located ongoing trials using the National Institutes of Health (NIH) RePORTER service (August 2013). We accessed additional trials via the search portal of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch/) (August 2013). The WHO database includes the ClinicalTrials.gov, Australian New Zealand Clinical Trials Registry and ISRCTN databases.
We conducted searches in line with the Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards for the conduct and reporting of new Cochrane reviews of interventions (www.editorial-unit.cochrane.org/mecir).
Searching other resources
Reference lists
We scanned the bibliographies of all identified trials for additional studies.
Correspondence
We obtained published and unpublished trials from key researchers, as identified by the frequency with which they were cited in the bibliographies of RCTs and open‐label studies.
Data collection and analysis
Selection of studies
One review author (JI) initially screened the abstracts of RCTs that were potentially eligible for inclusion. Two review authors (DW and TA) independently assessed potential RCTs, based on information included in the main body of the trial report, or it's abstract, in cases in which the article was not accessible.
We listed studies for which additional information was required in order to determine eligibility under Studies awaiting classification, pending the availability of this information. We resolved any disagreements in the independent trial assessment and data collation procedures by discussion with a third review author (JI).
Data extraction and management
We designed spreadsheet forms for recording descriptive information, summary statistics of the outcome measures, the quality scale ratings and associated commentary. We subsequently exported data to Review Manager 5 software (RevMan 2012). Where information was missing, the review authors contacted investigators by email in an attempt to obtain this information. Where this was not successful, we retrieved the data from figures included in the paper, using a data extraction utility (3gdata; www.frantz.fi/software/g3data.php).
Two review authors (DW, TA) independently collated the following information from each trial that satisfied the inclusion criteria.
Description of the trials, including the primary researcher, year of publication and source of funding.
Characteristics of the interventions, including the number of participants randomized to the treatment and control groups, number of total drop‐outs per group as well as the number that dropped out due to adverse effects, dose of medication and period over which it was administered, and drugs used for treating the anxiety disorder and alcohol dependence. We recorded details of any concurrent psychotherapy.
Characteristics of trial methodology, including the diagnostic (e.g. DSM‐IV; APA 1994) and exclusionary criteria employed, the screening instrument used (e.g. Structured Clinical Interview for DSM‐IV (SCID; Spitzer 1996) for both the primary and comorbid diagnoses, presence of comorbid MDD, use of a placebo run‐in, whether a minimal severity criterion was employed, number of centres involved, and period of abstinence in relapse‐prevention trials.
Characteristics of participants, including gender distribution and mean and range of ages, mean length of time since diagnosis of the anxiety disorder and alcohol abuse/dependence, number of participants in the sample with MDD, and baseline severity of the anxiety disorder and alcohol abuse/dependence, as assessed by the trial's primary outcome measure or another commonly employed scale.
Outcome measures employed (primary and secondary), and summary continuous (means and standard deviations (SD)) and dichotomous (number of responders) data. Additional information was also included, such as whether data reflected the intention‐to‐treat (ITT) with last observation carried forward (LOCF) or completer/observed cases (OC) sample, and the minimal period required for inclusion of participants in the LOCF analyses. We also recorded other methods of estimating the outcome for participants who dropped out of the study, such as mixed effects (ME) modelling.
Main planned comparisons
We compared the following interventions:
medication versus placebo;
medication versus standard treatment;
medication versus other medications;
combination of medication and concurrent psychotherapy versus pharmacotherapy alone.
We grouped specific pharmacological interventions according to medication class, according to a pragmatic schema based on mechanism of action and year of introduction recommended by The Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) review group for this purpose. These included:
5‐HT (serotonin) partial agonists;
anticonvulsants;
antipsychotics;
benzodiazepines;
beta‐blockers;
monoamine oxidase inhibitors (MAOIs);
noradrenaline re‐uptake inhibitors (NARIs);
noradrenergic and specific serotonergic antidepressants (NaSSAs);
reversible inhibitors of monoamine oxidase A (RIMAs);
serotonin antagonist and re‐uptake inhibitors (SARIs);
serotonin and noradrenaline re‐uptake inhibitors (SNRIs);
selective serotonin re‐uptake inhibitors (SSRIs);
tricyclic antidepressants (TCAs);
other medications.
Assessment of risk of bias in included studies
Two review authors (DW, TA) independently assessed the methodological quality of the trials using the 'Risk of bias' instrument recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). This tool addresses six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues. We listed a description of pertinent information from the study for each domain, and made a judgement relating to the risk of bias assigned (low risk, unclear risk and high risk). We resolved any disagreements about methodological quality by consultation with a third review author (JI).
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RR) and their 95% confidence intervals (CI) and number needed to treat for an additional beneficial outcome (NNTB) for the dichotomous primary outcome of interest (CGI‐I or related measure). We used RR instead of odds ratio (OR), as ORs are more difficult to interprete than RRs, and when confused with RRs they tend to overestimate the size of the treatment effect. This is especially the case when the occurrence of the outcome of interest is common (as anticipated in this review, with an expected response greater than 20%) (Deeks 2011). We defined NNTB as the inverse of the absolute risk difference due to the active intervention. In this review, it was used to indicate the number of participants who required treatment with medication, relative to control participants, before a single additional participant in the medication group responded to treatment. We calculated the number of people who relapsed in relapse‐prevention studies for categorical measures of treatment response for both the anxiety disorder and alcohol use.
Continuous data
We had planned to calculate mean difference (MD) or standardized mean difference (SMD) estimates and their 95% CIs for continuous summary data.
Unit of analysis issues
Trials with multiple treatment groups
Unit‐of‐analysis bias may be introduced from trials testing the efficacy of medication through comparing the summary statistics for multiple groups against the same control group (Deeks 2011). This is a particular issue for trials comparing different dosages of medication or multiple intervention arms versus a common control group. We found no eligible trials employing these designs for inclusion in the current version of the review. We will minimize potential bias resulting from inclusion of dose comparison studies in future versions of this review by pooling the means and SDs across all of the treatment arms as a function of the number of participants in each arm. We will restrict the pooling of outcome data to those arms that employ at least the minimum dose recommended by clinical guidelines. We will circumvent unit‐of‐analysis bias resulting from the simultaneous comparison of multiple arms from the same trial in future updates of this review by means of a multiple‐treatments meta‐analysis (MTM) (Lumley 2002). An MTM allows the assessment of treatment efficacy through the combination of both direct and indirect comparisons of all interventions on a specific outcome. Potential unit‐of‐analysis bias can be subsequently assessed in a sensitivity analysis in which the results obtained are compared with those from a meta‐analysis restricted to data from direct comparisons of interventions.
Cross‐over trials
We found no RCTs employing cross‐over study designs for inclusion in this review. Please refer to the Differences between protocol and review section for details of how cross‐over trials will be analysed for future versions of this review.
Cluster‐randomized trials
In cluster‐randomized trials, groups of individuals rather than individuals are randomized to different interventions. Cluster‐randomized trials face potential issues because participants within any one cluster often tend to respond in a similar manner, and thus their data can no longer be assumed to be independent of one another. Cluster‐randomized trials also face risk of bias issues including recruitment bias, baseline imbalance, loss of clusters, incorrect analyses and comparability with individually randomized trials. We found no cluster‐randomized trials that were eligible for inclusion in this review. To prevent unit‐of‐analysis errors in future updates of this review, we will divide the effective sample size of each comparison group in trials that did not adjust for clustering by the design effect metric (Higgins 2011b), with the intraclass correlation coefficient (ICC) that is incorporated within the design effect set equivalent to the median ICC from published cluster‐randomized pharmacotherapy RCTs for anxiety disorders.
Dealing with missing data
All analyses of dichotomous data were ITT. We used the total number of participants randomized to the different comparison groups as the denominator in comparisons of treatment response. We included only data from trials that provided information on the original group size (prior to drop‐outs) in the analyses of treatment response. We gave preference within studies to the inclusion of summary statistics for continuous outcome measures derived from ME models, followed by LOCF and OC summary statistics (in that order). This is in line with evidence that ME methods are more robust to bias than LOCF analyses (Verbeke 2000).
Assessment of heterogeneity
We assessed heterogeneity of treatment response and symptom severity visually from the forest plot of RR, to determine whether the differences between the results of trials were greater than would be expected by chance alone. We also assessed heterogeneity by means of the Chi2 test of heterogeneity. We interpreted a P value of less than 0.10 in the Chi2 test as evidence of heterogeneity, given the low power of the Chi2 statistic when the number of trials is small (Deeks 2011). In addition, we used the I2 heterogeneity statistic reported by Review Manager 5 to determine differences in effect size across trials that cannot be explained by chance alone (Higgins 2003; RevMan 2012). We interpreted the magnitude of heterogeneity for the primary outcomes following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), as follows:
0% to 40%: might be important;
30% to 60%: moderate;
50% to 90%: substantial;
75% to 100%: considerable.
In recognition of the possibility of differential effects for different medications, we stratified all of the outcome comparisons by the drug employed.
Assessment of reporting biases
Publication is not necessarily related to study quality and indeed publication may imply certain biases (Dickersin 1992; Song 2000). The review authors planned to inspect a funnel plot of treatment response visually in order to detect small‐trial effects, including those resulting from publication bias. However, this was not feasible in the current version of the review, given the small number of included trials. In future updates, we will undertake this if we identify more than 10 studies.
Data synthesis
We obtained dichotomous and continuous treatment effect estimates from a random‐effects model and expressed results in terms of a mean effect size for each subgroup, as well as by 95% CIs. We stratified comparisons of global treatment response and reduction of anxiety disorder symptom severity by study design (acute treatment interventions (12 weeks or less) and maintenance studies (more than 12 weeks)). We included data from the assessment point closest to 12 weeks in secondary outcome analyses for each trial. We grouped the comparisons by medication class, as listed in the Data extraction and management section of the review. We only combined outcome data for drugs within each of these medication classes in the meta‐analyses on the proviso that the medications were administered for the treatment of the same anxiety disorder, with separate analyses reported for individual drugs within each class otherwise.
Where there was evidence that data were skewed, we planned to obtain individual patient data (where possible) for normalizing the data by means of log transformation techniques. If this proved unsuccessful, we would have excluded those studies providing skewed data from the analysis. For the purposes of this review, the following constituted evidence of skewness: cases in which the difference between the observed mean and the lowest possible value or highest possible value on the scale was less than twice as large as the SD (Deeks 2011), or where data were reported as skewed by the study authors.
Subgroup analysis and investigation of heterogeneity
We had planned several subgroup analyses to assess the degree to which clinical (gender, presence of MDD in sample) and methodological differences (single centre or multicentre trials, whether trials were industry funded, order of anxiety/alcohol interventions) between trials might have systematically influenced differences observed in the primary treatment outcomes (see Differences between protocol and review). However, it was not possible to conduct these subgroup analyses due to an insufficient number of eligible trials.
Sensitivity analysis
We included a series of sensitivity analyses in the original protocol of this review. These included a 'worst‐case/best‐case' test of the assumptions regarding the outcome of drop‐outs, as well as a test of differences resulting from analysing treatment responders rather than non‐responders. We did not conduct these analyses for the reasons provided in the Differences between protocol and review section.
'Summary of findings' tables
Table 1 shows the results for the treatment comparisons on the primary and secondary outcomes of this review. We compiled this table using GRADE Pro 3.6 software. 'Summary of findings' tables present the findings of a review in a transparent and simple tabular format, and provide key information concerning the quality of evidence and the magnitude of effect of the interventions examined (Higgins 2011a).
We classified study quality downgrades as 'serious' (downgrading the quality rating by one level) or 'very serious' (downgrading the quality grade by two levels), and based them on five factors:
limitations in the design and implementation;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
imprecision of results;
high probability of publication bias.
We determined best evidence by the following conclusions:
high quality: further research is very unlikely to change our confidence in the estimate of effect;
moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low quality: we are very uncertain about the estimate.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
Initial searches of CCDAN's specialized trial register retrieved 307 records: 36 studies (from the CCDANCTR‐Studies Register) and an additional 271 untagged/uncoded references (from the CCDANCTR‐References Register) (see Figure 1 for the PRISMA trial selection flowchart). A January 2014 update of the CCDANCTR search yielded 19 unique studies. The specialized register for the Cochrane Drug and Alcohol review groups yielded 79 references. A search of the PubMed (1581 references), EMBASE (2597 references), PsycINFO (723 references) and Alcohol and Alcohol Problems Science Database (AAPSD) (1234 references) databases retrieved a cumulative total of 6135 records, of which 5111 were unique. Records from the NIH RePORTER database totalled 336 and WHO ICTRP database totalled 43 unique results. We retained less than 1% (54/5733) of the records following inspection of their titles and abstracts, and kept eight abstracts from unpublished studies for further assessment. Five trials satisfied all inclusion criteria upon inspection of the full‐text reports (Tollefson 1992; Randall 2001b; Brady 2005; Book 2008; Petrakis 2012). We contacted the lead investigators of all of the included studies (Drs Sudie Back, Sarah Book, Ismene Petrakis, Carrie Randall and Gary Tollefson) via email for additional information. Authors for two of the five studies responded to our queries. Six trials are awaiting classification, pending the availability of additional information and four trials are listed as ongoing.
1.
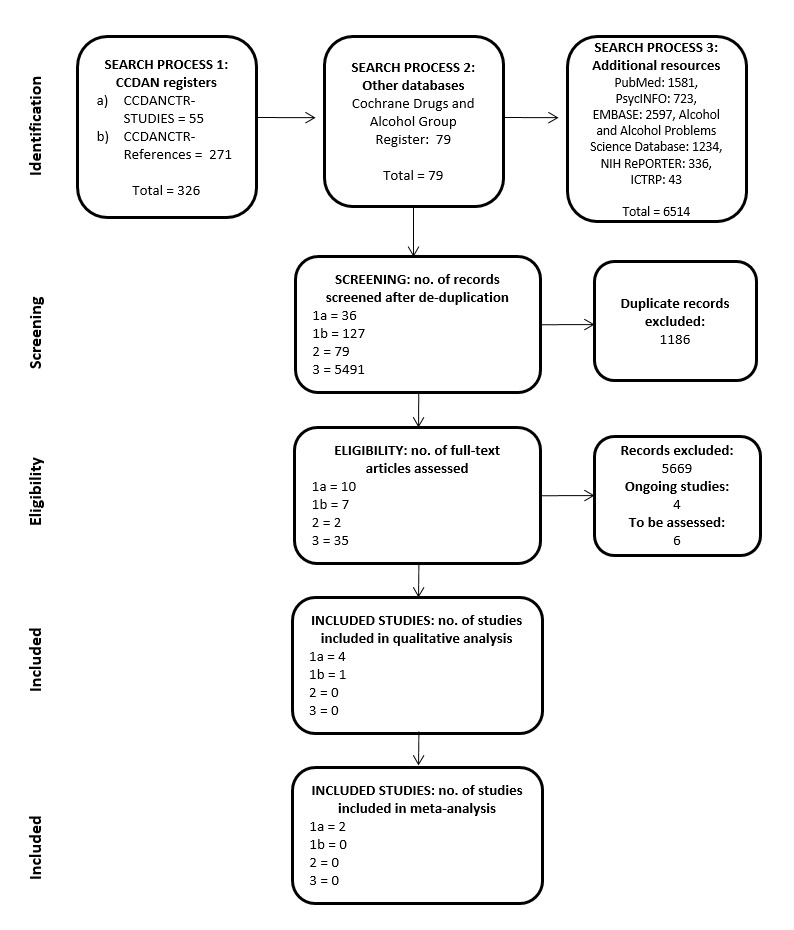
PRISMA flowchart
Included studies
Five RCTs were eligible for inclusion in the review, with a sample size of 290 participants. The Characteristics of included studies table shows a summary of the trials.
Design
Four included studies employed a parallel‐group design in which participants were randomized to monotherapy with either medication or placebo (Tollefson 1992; Randall 2001b; Brady 2005; Book 2008). Petrakis 2012 randomly allocated participants to one of four interventions, in which antidepressants were supplemented with either naltrexone or placebo.
Sample sizes
Study samples were generally small, ranging in size between 15 and 94 (mean 29, SD 23.9).
Setting
All of the included studies were conducted in the US. Petrakis 2012 was the only trial that took place at more than one centre (two centres).
Participants
The review included two RCTs assessing interventions for PTSD (Brady 2005; Petrakis 2012), two evaluating pharmacotherapies for generalized SAD (Randall 2001b; Book 2008), and RCT for GAD (Tollefson 1992). In the trials that distinguished between alcohol use subtype, the majority of the participants were diagnosed with alcohol dependence (79%), and the remainder with alcohol abuse (21%) (Randall 2001b; Brady 2005; Book 2008; Petrakis 2012); Tollefson 1992 did not provide information on diagnostic subtype. Book 2008 restricted study participation to individuals who described themselves as drinking to cope with the SAD symptoms. Four of the included studies diagnosed anxiety disorders and alcohol abuse/dependence according to DSM‐IV criteria (Randall 2001b; Brady 2005; Book 2008; Petrakis 2012), while one study employed DSM‐III criteria (Tollefson 1992). The participants across all included trials were 37.4 years old (SD 6.5) on average, with a majority consisting of males (70%). The samples in both Randall 2001b and Petrakis 2012 consisted of fewer than 20% women.
The interventions for PTSD differed with respect to PTSD subtype and symptom severity; the sample in Brady 2005 was restricted to individuals exposed to civilian traumas with a moderately severe PTSD (CAPS total score 58.9), while the participants in Petrakis 2012 consisted primarily of combat veterans (81/88 participants, 92%) with greater severity of PTSD symptoms (CAPS total score: 70.9). Although both PTSD trials restricted comorbid substance abuse to alcohol dependence, drinking behaviour was more extreme in Petrakis 2012 than Brady 2005 (mean number of drinks on each drinking occasion/day: 23.5 in Petrakis 2012 and six in Brady 2005).
Diagnoses in the included RCTs of current anxiety disorders in addition to those that formed the focus of the interventions were relatively infrequent. Comorbid anxiety disorders in at least 10% of participants included GAD (Book 2008: 8/42 participants) SAD (Brady 2005: 15/92 participants), SP (Brady 2005:12/92 participants) and PD (Brady 2005:15/92 participants; Randall 2001b: 2/15 participants). Petrakis 2012 provided no information regarding comorbid anxiety disorders, although they excluded participants with serious current psychiatric symptoms from the study. Brady 2005 observed high rates of concurrent major depression only in the PTSD interventions, where MDD was present in 48% of the participants and Petrakis 2012 described MDD as being present in a large subsample of participants (personal communication; 1 August 2012). Tollefson 1992 did not permit concurrent Axis I psychiatric diagnoses.
Interventions
Brady 2005 initiated treatment of civilian PTSD with 50 mg/day of sertraline (49 participants) or placebo (45 participants), increasing doses incrementally by 50 mg to a target of 150 mg/day by the third week of the 12‐week intervention. Petrakis 2012 randomly allocated participants diagnosed with PTSD to one of four intervention groups, constructed by combining treatment with the TCA desipramine or the SSRI paroxetine with either naltrexone or placebo. These groups were desipramine/placebo (24 participants), paroxetine/placebo (20 participants), desipramine/naltrexone (22 participants) and paroxetine/naltrexone (22 participants). For the purposes of this review, we compared only the antidepressant and placebo combinations.
Book 2008 and Randall 2001b randomized people with SAD to treatment with 60 mg/day of paroxetine or placebo. In Book 2008, people with generalized SAD randomized to medication (20 participants) and placebo (22 participants) were started at 10 mg/day in the first week, uptitrated to 20 mg/day in week two, 40 mg/day by week three and then maintained on the full dose of 60 mg/day from the fourth week of the 16‐week trial. In Randall 2001b, the dosage was started at 20 mg/day in study participants randomized to medication (six participants) or placebo (nine participants), increased to 40 mg/day by the second week and increased to 60 mg/day, where tolerated, from the third week to the end of the eight‐week study.
Tollefson 1992 administered 15 mg/day of the 5‐HT partial agonist buspirone (26 participants) or placebo (25 participants) at study onset to people with GAD, uptitrated to at least 30 mg/day by week two, to a maximum of 60 mg/day at week three to four, after which the dose was held constant for the remainder of the 24‐week trial.
The interventions included in this review varied in duration from eight to 24 weeks. Two trials included individual CBT targeting alcohol abuse as part of the treatment protocol (Randall 2001b; Brady 2005). These interventions were based on the MATCH (Matching Alcoholism Treatment to Client Heterogeneity) project protocol (Project MATCH 1993). In Randall 2001b, participants were provided with an individual session of motivational interviewing, whereas in Brady 2005, participants were administered weekly one‐hour sessions of CBT. Tollefson 1992 controlled the number of Alcoholics Anonymous meetings (an alcoholics treatment programme) attended by study participants.
Outcomes
Anxiety disorder outcomes
Brady 2005 and Petrakis 2012 assessed PTSD severity using the CAPS. In addition, Brady 2005 employed the Impact of Event Scale (IES) (Horowitz 1979), and the Civilian Mississippi Scales for PTSD (MISS) (Keane 1988). Book 2008 and Randall 2001b assessed SAD symptom severity using a modified version of LSAS, in which participants were instructed to respond to the fear and avoidance items as if they did not have access to alcohol with which to cope. Connor 2000 used the CGI‐I and the Social Phobia Inventory (SPIN) to assess treatment response. Tollefson 1992 assessed the response of GAD symptoms using the HAM‐A (Hamilton 1959), and the CGI‐I. We extracted data on the HAM‐A from a figure included in Tollefson 1992 (Figure 2).
Drinking outcomes
The TLFB was the most commonly employed measure of the effect of medication on drinking in the studies included in this review (Randall 2001b; Brady 2005), or a modified version thereof (Book 2008; Petrakis 2012). The TLFB provides measures of total number of drinks, number of drinks per drinking day, proportion of drinking days and proportion of heavy drinking days. The TLFB was modified in Book 2008 to include items assessing drinking to cope with SAD symptoms. Petrakis 2012 used the Substance Abuse Calendar, a scale derived from the TLFB, to assess drinking behaviour (the mean number of drinks per week, per cent heavy drinking days and drinks per drinking day) as well as other substance use. Another frequently used measure of drinking behaviour included the Addiction Severity Index (ASI; McLellan 1990), which measures impairment across a number of functional domains (including social, legal, medical and psychiatric). Three of the five RCTs administered the ASI (Tollefson 1992; Randall 2001b; Brady 2005). The ASI was the only drinking outcome reported in the trial of GAD (Tollefson 1992).
Excluded studies
The most common reason for excluding studies from the review was that the investigators assessed the effect of pharmacotherapy on anxiety symptoms without diagnosing anxiety disorders according to DSM criteria (Caponi 1985; Loo 1986; Krupitsky 1993; Kranzler 1994; Guardia 2012). In addition, Malcolm 1992 and Ciraulo 2013 required that participants diagnosed with anxiety disorders including GAD and PD be abstinent from alcohol for fewer than four weeks prior to the start of treatment. We excluded the studies by Batki 2011 and Oluwadara 2013 because they allowed the inclusion of participants receiving concurrent psychotropic medication. See Characteristics of excluded studies table for more details.
Ongoing studies
We identified four ongoing studies that may be eligible for inclusion in future versions of this review. One RCT is currently recruiting participants to evaluate the efficacy of 12 weeks of treatment with a fixed dose (16 mg) of the alpha‐1 adrenergic receptor antagonist prazosin relative to placebo in people with PTSD and alcohol dependence (NCT00744055). In addition, one 12‐week placebo‐controlled RCT of 16 mg prazosin (three times daily), in which the interventions will be administered to 150 alcohol‐dependent participants evenly split by presence of a concurrent anxiety disorder, was listed as recruiting participants as of April 2013 (NCT00585780). Dr Batki and colleagues are conducting a second 12‐week double‐blinded placebo‐controlled RCT investigating the efficacy of the anticonvulsant topiramate in people diagnosed with current PTSD and alcohol use disorders who are drinking heavily, in which the maximum dosage of 300 mg/day will be administered to 150 veterans (NCT01749215). We excluded the earlier pilot study as 60% of the participants were receiving concurrent psychotropic medication (Batki 2011). Finally, 50 veterans with PTSD will be randomly assigned in a 3: 1 ratio to 12 weeks of zonisamide or placebo in addition to Enhanced Cognitive Processing Therapy‐C (E‐CPT‐C), with the medication uptitrated to 400 mg/day during the first six weeks and henceforth maintained to the end of the study (NCT01847469). See Characteristics of ongoing studies table for further details.
Studies awaiting classification
Six trials are currently awaiting classification (see Characteristics of studies awaiting classification table). Five of these are unpublished, including a trial in which 180 outpatients meeting DSM‐IV criteria for alcohol dependence or abuse and with a comorbid diagnosis of PD, social phobia or GAD were randomized to 12 weeks of treatment (including a one‐week initial run‐in period and a two‐week taper period) with venlafaxine, CBT or placebo medication with relaxation therapy (NCT00248612), and a small 12‐week comparison of the atypical antipsychotic quetiapine (maximum of 300 to 400 mg/day) with placebo in 20 people with alcohol dependence and a comorbid anxiety disorder (NCT00352469). We have requested additional information from the investigators in one small, 12‐week, placebo‐controlled paroxetine trial in 20 outpatients with comorbid PTSD and substance dependence (NCT00330239). We are also waiting clarification on the number of participants receiving concurrent antidepressant medication in one placebo‐controlled prazosin trial for alcohol dependence and PTSD (NCT01518972). Although the manner in which PTSD (total scores of at least 50 on the CAPS) and alcohol use disorders (diagnosis with alcohol abuse or dependence or the consumption of more than 35 standard drinks per week over the previous four weeks) was defined in male veterans appears to preclude an ongoing study of the anticonvulsant topiramate (maximum dose 400 mg/day) from inclusion in this review, we will defer a final decision until additional information regarding the participants is obtained at study completion (NCT01408641). Finally, data for people who had completed a 21‐day alcohol dependence treatment programme, who were diagnosed with an affective or anxiety (or both) disorder (without comorbid antisocial personality disorder), and who subsequently participated in a six‐month follow‐up RCT of bromocriptine, a dopamine agonist, and nortriptyline, an adrenergic re‐uptake inhibitor, may be included in a future version of this review, pending additional information on the criteria employed in diagnosing anxiety disorders in this published study (Powell 1995).
Risk of bias in included studies
We assessed risk of bias using The Cochrane Collaboration's 'Risk of bias' tool, which assessed bias across multiple domains, including random sequence generation, allocation concealment, blinding (of outcome assessment and participants and personnel), incomplete outcome data, selective reporting and other potential sources of bias (see Figure 2 and Figure 3 for summaries of judgements across studies and domains).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Three of the five included trials provided insufficient information to determine whether they employed adequate randomization procedures (Tollefson 1992; Randall 2001b; Petrakis 2012). The two remaining RCTs employed urn randomization (Brady 2005; Book 2008). Book 2008 grouped random sequences by gender, social anxiety severity and diagnosis with MDD and Brady 2005 grouped random sequences by sex, depressive disorder, trauma type and age of index trauma.
Allocation concealment
Institutional research pharmacists maintained the treatment allocation in both Book 2008 and Randall 2001b. Neither Brady 2005 nor Tollefson 1992 provided information on how allocation was concealed.
Blinding
Blinding of outcome assessment
The extent to which the assessment of study outcomes was blinded was unclear in two of the trials, in which the study design was merely described as "double‐blinded" (Brady 2005; Petrakis 2012). Randall 2001b assessed outcomes separately from side effect evaluation and medication administration, while Book 2008 and Tollefson 1992 described assessors involved in evaluation of outcomes as blinded (in Tollefson 1992, blinding was only described for the primary efficacy outcome).
Blinding of participants and personnel
Both Book 2008 and Randall 2001b used matching capsules for medication and placebo to ensure the blinding of study personnel and participants. Petrakis 2012 described dispensing medication in blister packs that were packaged in separate bottles, labelled as antidepressants in the one container and naltrexone in the other. Email correspondence with the investigators confirmed that this procedure was employed to blind participants to whether they were receiving desipramine or paroxetine (labelled antidepressant) and naltrexone or placebo (labelled naltrexone). Tollefson 1992and Brady 2005 provided insufficient information to determine whether both parties were blinded.
Incomplete outcome data
A large proportion of participants withdrew prematurely from the trials (mean 43.1%; see Effects of interventions), although attrition rates were relatively low in Book 2008. Drop‐out rates were significantly higher in the paroxetine (55%) than the desipramine (35%) groups in Petrakis 2012, although potential bias resulting from differential attrition in this trial may have been ameliorated somewhat by employing a mixed‐effects regression approach to model missing data. Likewise, a substantially larger proportion of study withdrawals in the placebo (84%) than buspirone (61.5%) treatment arms after 24 weeks in Tollefson 1992 may have introduced bias into the outcomes reported for this trial. None of the studies compared study drop‐outs with those who remained in the study, making it difficult to determine the extent to which attrition bias may have affected the study results.
Selective reporting
Evidence of incomplete reporting of study outcomes was evident for Tollefson 1992, in which it appears as if they only report those outcomes amongst the many that were assessed that yielded statistically significant group differences. Protocols were not available for most of the included studies (Brady 2005; Randall 2001b; Tollefson 1992), making determination of reporting bias difficult.
Other potential sources of bias
A greater proportion of participants in Tollefson 1992 had previously been exposed to benzodiazepines in the buspirone than the placebo arms. Although the authors asserted that this may have blunted the response to buspirone, they based this conclusion on post‐hoc findings from one small controlled trial (Schweizer 1986), and it was not sufficient to mask the observation of a significant medication effect in reducing the severity of GAD symptoms.
Effects of interventions
See: Table 1
It was not possible to conduct all planned comparisons, as, with exception of the comparison of desipramine and paroxetine (Petrakis 2012), the RCTs included in this review only provided data on the efficacy and acceptability of medication compared with placebo.
Comparison 1: medication versus placebo
One RCT compared the 5‐HT partial agonist buspirone versus placebo (51 participants diagnosed with GAD) (Tollefson 1992). Three studies provided data on outcomes for the comparison of SSRIs versus placebo (Randall 2001b; Brady 2005; Book 2008). Two trials compared paroxetine versus placebo (57 participants; Table 1) and one trial compared sertraline versus placebo (94 participants).
Comparison 1.1: 5‐HT partial agonists versus placebo
1.1.1: buspirone versus placebo
1.1.1.1: anxiety disorder treatment response
There were no data on treatment responders on the CGI‐I (Tollefson 1992).
1.1.1.2: reduction of anxiety disorder symptom severity
The trial investigators reported that treatment with buspirone resulted in an advantage for medication at the 12‐week assessment on the HAM‐A (t = ‐2.6, degrees of freedom (df) = 40, P value < 0.01). Extraction of data from a bar chart in Tollefson 1992 depicting treatment response on the HAM‐A (Figure 2 in Tollefson 1992) revealed that buspirone reduced the score on the HAM‐A in 42 people with GAD by a mean of 5 points at week 12 relative to placebo. A significant divergence between the buspirone and placebo arms in the response of GAD symptoms to treatment emerged at the 12‐week assessment, with the size of the effect remaining stable for the following 12 weeks of the study.
1.1.1.3: acceptability of treatment
There were insufficient data on the number of people who withdrew due to treatment‐emergent adverse effects for meta‐analysis (Analysis 1.3). Three participants being treated with 24 weeks of buspirone withdrew from treatment compared with one person receiving placebo (reasons for withdrawal not provided) (Tollefson 1992). The most frequently occurring drug‐related adverse event in response to 24 weeks of buspirone was dizziness, reported by 9/26 (35%) participants receiving medication compared with 4/25 (16%) participants receiving placebo (Tollefson 1992).
1.3. Analysis.

Comparison 1: Medication versus placebo, Outcome 3: Treatment acceptability
1.1.1.4: abstinence and reduction of alcohol use
There were no data on drinking outcomes for the single trial of buspirone (Tollefson 1992).
1.1.1.5: reduction of comorbid symptoms of depression
Tollefson 1992 reported a reduction in symptoms of depression (as assessed on the HAM‐D) only at the week 12 assessment of the 24‐week treatment intervention (t = ‐2.08, df = 40, P value = 0.05).
1.1.1.6: quality of life
We found no data to determine the effects of buspirone on quality of life.
1.1.1.7: functional disability
We found no data to determine the effects of buspirone on functional disability associated with the anxiety disorder. Buspirone had a greater adverse effect than placebo on clinician rating of "functioning" assessed using the ASI, though this was not regarded by the investigators as problematic.
Comparison 1.2: selective serotonin re‐uptake inhibitors versus placebo
1.2.1: paroxetine versus placebo
1.2.1.1: anxiety disorder treatment response
There was very low quality evidence for an effect of paroxetine on treatment response in people diagnosed with SAD, as assessed on the CGI‐I (RR 2.23, 95% CI 1.13 to 4.41, 2 trials, 57 participants; Analysis 1.1) (Randall 2001b; Book 2008). Low variability was observed for effect estimates across trials and is of unclear clinical importance (heterogeneity: I2 = 0%, Chi2 = 0.25, df = 1, P value = 0.62). More than twice as many participants responded to short‐term treatment with paroxetine than placebo (57.7% with paroxetine versus 25.8% with placebo). This translates to an NNTB of 4, indicating that four participants would have to be treated with paroxetine for one additional treatment responder, relative to the control condition.
1.1. Analysis.

Comparison 1: Medication versus placebo, Outcome 1: Treatment response
1.2.1.2: reduction of anxiety disorder symptom severity
There was a lack of evidence for the efficacy of 12 weeks of treatment with paroxetine in decreasing the severity of SAD symptoms (MD ‐14.70, 95% CI ‐33.00 to 3.60, 2 trials, 44 participants; Analysis 1.2). The quality of the evidence on this outcome was very low and between‐trial heterogeneity on this outcome of unclear clinical importance (I2 = 0, Chi2 = 0.03, P value = 0.87). Longer‐term treatment over 16 weeks was reported in Book 2008 as resulting in a mean reduction in total LSAS scores of 53% (SD = 29.52%) in the paroxetine group versus 32% (SD = 29.08%) in the placebo group (t = 2.34, df = 40, P value < 0.05). Separating the treatment effects into an early (six weeks or less) and later phase (seven to 16 weeks), the investigators observed the largest reduction in symptoms with administration of a maximum of 60 mg/day of paroxetine by six weeks of treatment, with a levelling off of the treatment effect for the remaining 10 weeks of the trial. Using a similar 20 to 60 mg/day flexible dosing regimen, Randall 2001b also reported a maximal medication effect after six weeks of the intervention, after which treatment response stabilized for the remainder of the study. The mean reduction at study endpoint in the total LSAS score reported by Randall 2001b was 44% for paroxetine and 14% for placebo.
1.2. Analysis.

Comparison 1: Medication versus placebo, Outcome 2: Symptom severity reduction
1.2.1.3: acceptability of treatment
There was very low quality evidence of differences in the acceptability of paroxetine and placebo interventions based on drop‐out rates due to adverse events (RR 3.29, 95% CI 0.14 to 76.33, 2 trials, 57 participants; Analysis 1.3). Two of the six participants who were administered with paroxetine for eight weeks experienced fatigue, with somnolence, nausea, abnormal ejaculation, headache and dry mouth reported by one person each (Randall 2001b). Sixteen weeks of treatment with paroxetine was associated with a greater frequency of adverse events in Book 2008 for three of 32 items from an adverse effects checklist; 11/20 (55%) participants on paroxetine experienced anorgasmia/delayed ejaculation compared with 4/22 (18%) participants on placebo (P value = 0.01). The equivalent figures for tremor were 45% with paroxetine versus 14% with placebo (P value = 0.03) and 35% with paroxetine versus 5% with control for myoclonus (P value = 0.01).
1.2.1.4: abstinence and reduction of alcohol use
Evidence that data for the proportion of heavy drinking days was not normally distributed in either Book 2008 or Randall 2001b mitigated against conducting a meta‐analysis of this outcome; for instance, the SD of patient data on this outcome was almost twice as large as the mean in the medication intervention in Randall 2001b (mean 0.214, SD 0.391). Very low quality evidence for the proportion of days during the trial in which participants were abstinent was inconclusive with respect to the efficacy of short‐term treatment with paroxetine relative to placebo (MD 0.08, 95% CI ‐0.26 to 0.43, 2 trials, 54 participants; Analysis 1.4). There was substantial variability for effect estimates (I2 = 68%; Chi2 = 3.11, P value = 0.08), although statistically significant reductions on medication were not observed for either Book 2008 or Randall 2001b. Although the number of drinks consumed on a drinking day was numerically smaller in the paroxetine and placebo groups (4.73 with paroxetine versus 7.36 with placebo), and was observed to a similar extent in both trials providing data on this outcome (I2 = 0%, Chi2 = 0.52, P value = 0.47), there was no evidence that paroxetine reduced the number of drinks consumed (MD ‐2.42, 95% CI ‐4.97 to 0.14, 2 trials, 64 participants; Analysis 1.5). In Book 2008, treatment with paroxetine was associated with a reduction in the proportion of drinking events motivated by the need to cope with social anxiety relative to placebo by the 16‐week assessment. This was evident for modified TLFB measures of the proportion of participants who reported avoiding social situations if they could not drink during or prior to the event (25% with paroxetine versus 45% with placebo, P value = 0.006), as well as the percentage of people who reported drinking before social events to feel more comfortable (35% with paroxetine versus 68% with placebo, P value = 0.014) (Thomas 2008).
1.4. Analysis.

Comparison 1: Medication versus placebo, Outcome 4: Proportion of days abstinent
1.5. Analysis.

Comparison 1: Medication versus placebo, Outcome 5: Drinks per drinking day
1.2.1.5: reduction of comorbid symptoms of depression
There were insufficient data on the effect of acute treatment with paroxetine on depression for meta‐analysis.
1.2.1.6: quality of life
There were no data to determine the effects of paroxetine on quality of life.
1.2.1.7: functional disability
There were no data to determine the effects of paroxetine on functional disability.
1.2.2: sertraline versus placebo
1.2.2.1: anxiety disorder treatment response
There were no data from the trial of sertraline included in this review to determine the effects of this sertraline on treatment response (Brady 2005).
1.2.2.2: reduction of anxiety disorder symptom severity
Brady 2005 reported marginal superiority of sertraline to placebo in reducing the severity of PTSD symptoms on the CAPS total score, though this effect was not statistically significant (F = 2.68, df = 2,68, P value = 0.08).
1.2.2.3: acceptability of treatment
The trial reported no drop‐outs due to adverse events for either the sertraline or placebo groups. Sexual dysfunction, headache, dizziness, insomnia, nervousness and drowsiness were reported as the most commonly occurring adverse events in the sertraline arm, though exact frequencies were not provided (Brady 2005).
1.2.2.4: abstinence and reduction of alcohol use
There were no differences over the course of the treatment for sertraline versus placebo on percentage of drinking days (23.0% with sertraline versus 20.4% with placebo), number of drinks consumed per day (mean (SD): 2.0 (2.9) with sertraline versus 1.4 (1.9) with placebo), number of drinks consumed per drinking day (mean (SD): 6.8 (6.5) with sertraline versus 6.3 (7.8) with placebo) and number of heavy drinking days (mean (SD): 10.4 (2.3) with sertraline versus 8.9 (2.5) with placebo) (Brady 2005).
1.2.2.5: reduction of comorbid symptoms of depression
Brady 2005 reported no evidence for the reduction of depression symptoms, as assessed by the BDI, after 12 weeks of treatment with sertraline.
1.2.2.6: quality of life
We found no data to determine the effects of sertraline on quality of life.
1.2.2.7: functional disability
We found no data to determine the effects of sertraline on functional disability.
Comparison 2: medication versus standard treatment
We found no RCTs comparing treatment of anxiety and comorbid alcohol use disorders with treatment 'as usual'.
Comparison 3: medication versus other medications
We found one study that compared paroxetine plus placebo with desipramine plus placebo (Petrakis 2012).
3.1: paroxetine plus placebo versus desipramine plus placebo
3.1.1: anxiety disorder treatment response
We found no data on treatment responders on the CGI‐I for the trial comparing paroxetine plus placebo with desipramine plus placebo (Petrakis 2012).
3.1.2: reduction of anxiety disorder symptom severity
An analysis across all four arms of Petrakis 2012 revealed no difference between paroxetine and desipramine on reductions in the CAPS total score over time, as reported in the trial report (F = 1.25, df = 6108.8, P value > 0.05; Analysis 2.1).
2.1. Analysis.

Comparison 2: Medication versus other medications, Outcome 1: Symptom severity reduction
3.1.3: acceptability of treatment
We found insufficient data on the acceptability of treatment with paroxetine and desipramine (in combination with placebo) for 12 weeks for meta‐analysis (Analysis 2.2). In the paroxetine plus placebo arm, 1/20 participants discontinued treatment after having a seizure and 1/20 participants was hospitalized due to severe anxiety (Petrakis 2012). Petrakis 2012 reported that significantly more participants treated with desipramine reported gastrointestinal symptoms than participants treated with paroxetine (with concurrent placebo or 50 mg/day of naltrexone). The ability of this study to detect group differences in drug‐related adverse effects may have been compromised by the investigators' decision to only report events that were significantly more common at a Bonferroni corrected statistical threshold of alpha = 0.007 (to control for comparisons for seven adverse effect symptom groups; gastrointestinal, emotional, cold and flu symptoms, skin, sexual, neurological and cardiac).
2.2. Analysis.

Comparison 2: Medication versus other medications, Outcome 2: Treatment acceptability
3.1.4: abstinence and reduction of alcohol use
Fifty‐one per cent of participants in Petrakis 2012 remained abstinent throughout the study. Comparison of the paroxetine plus placebo and desipramine plus placebo arms revealed greater reductions on drinking outcomes for the desipramine plus placebo group, including endpoint assessments for mean number of drinking days (Analysis 2.3), drinks per drinking day (Analysis 2.4) and proportion of heavy drinking days (Analysis 2.5). This is consistent with the finding that the administration of desipramine with either placebo or naltrexone resulted in greater reductions in the proportion of heavy drinking days (F = 7.22, df = 1,84, P value < 0.01) and the mean number of drinks per drinking day (F = 5.04, df = 1,84, P value < 0.05) than the equivalent comparison groups for paroxetine.
2.3. Analysis.

Comparison 2: Medication versus other medications, Outcome 3: Mean number of drinking days
2.4. Analysis.

Comparison 2: Medication versus other medications, Outcome 4: Drinks per drinking day
2.5. Analysis.

Comparison 2: Medication versus other medications, Outcome 5: Proportion heavy drinking days
3.1.5: reduction of comorbid symptoms of depression
There were insufficient data on the effects of paroxetine and desipramine (in combination with placebo) on symptoms of depression for meta‐analysis (Analysis 2.6).
2.6. Analysis.

Comparison 2: Medication versus other medications, Outcome 6: Reduction in depression symptoms
3.1.6: quality of life
We found no data to determine the effects of medication versus other medications on quality of life.
3.1.7: functional disability
We found no data to determine the effects of medication versus other medication on functional disability.
Comparison 4: combination of medication plus concurrent psychotherapy versus pharmacotherapy alone
We identified no eligible RCTs that compared treatment of anxiety and comorbid alcohol use disorders with medication plus concurrent psychotherapy versus pharmacotherapy alone.
Subgroup analyses
It was not possible to conduct any of the planned subgroup analyses (see Differences between protocol and review), due to the small number of trials included in this review.
Sensitivity analyses
It was not possible to conduct any of the planned sensitivity analyses (see Differences between protocol and review), due to the small number of trials included in this review.
Reporting bias
We planned to inspect a funnel plot of treatment response in order to detect small‐trial effects, including those resulting from publication bias. However, this was not feasible in the current version of the review, given the small number of included trials.
Discussion
Summary of main results
Evidence collated as part of this review to determine the efficacy of medication in treating anxiety disorder symptoms in people with comorbid alcohol use disorders was inconclusive. Although the majority of data on treatment efficacy in this review were from serotonergic drugs, we rated evidence on this outcome as being of very low quality. This was primarily due to the small number of studies providing data on a clinically diverse population. Despite imprecise estimates of the effect of the SSRI paroxetine, the quantitative synthesis of data from RCTs included in this review provided preliminary support for the efficacy of this medication in improving clinical response in people with SAD (see Table 1). More than twice as many people with SAD responded to paroxetine (57.7%) than placebo (25.8%) after a mean of 12 weeks of treatment. This difference in treatment response was equivalent to an additional four people who would have to be treated with medication for one additional treatment responder, relative to the control condition and corresponding imprecise estimates of effect.
With the exception of one trial of buspirone (Tollefson 1992), there was no evidence that the severity of anxiety disorders symptoms were reduced after acute (12 weeks or less) treatment with medication. Moreover, caution should be exercised in interpreting the finding from Tollefson 1992 of a significant reduction of GAD symptoms following 12 weeks of treatment with buspirone, given concerns regarding possible reporting bias (see Quality of the evidence). A narrative review of changes in SAD symptom severity on treatment over time suggested that paroxetine may have to be administered for at least six weeks in doses of up to 60 mg/day to achieve maximal reductions in SAD symptoms.
We found few effects of pharmacotherapy on drinking outcomes in this review. Lack of evidence for the effectiveness of the SSRIs in reducing drinking was consistent with the observation that the majority of rigorously designed studies of the efficacy of SSRIs in treating alcohol abuse without comorbidities has been negative (Torrens 2005). Instead, there were preliminary indications from Petrakis 2012 that TCAs may be more effective for this purpose, while potentially possessing similar efficacy in treating anxiety disorder symptoms.
Despite a high overall rate of attrition in the included studies, we found no evidence of an increased number of treatment withdrawals due to drug‐related adverse events. This suggests that pharmacotherapy and interventions with serotonergic drugs in particular may be an acceptable treatment option for anxiety and comorbid alcohol use disorders. Although this finding was based on very low quality evidence with respect to paroxetine, the only medication for which sufficient data were available for meta‐analysis for this outcome, this was in agreement with previous reports that SSRIs are well tolerated in the treatment of the major anxiety disorders (Koen 2011). Reports of high frequencies of sexual dysfunction in response to treatment with paroxetine and sertraline is also consistent with the adverse effects profile for the SSRIs (Brady 2005; Book 2008).
Overall completeness and applicability of evidence
There was a paucity of evidence regarding the clinical effectiveness of medication. The trials included in this review were restricted to the SSRIs paroxetine and sertraline, the TCA desipramine, and the partial serotonin agonist buspirone. Furthermore, we identified no rigorously conducted trials of medication treatment for comorbid alcohol misuse and PD or OCD for this review.
The SSRIs have generally been recommended as first‐line drugs in meta‐analyses and systematic reviews of pharmacotherapy for anxiety disorders (Stein 2009). Although this review provided some evidence that these drugs may also be effective in people with concurrent alcohol dependence or abuse, there were few direct comparisons of the efficacy of different drugs in this population. The observation in Petrakis 2012 that the TCA desipramine and SSRI paroxetine were of equivalent efficacy in reducing PTSD symptom severity after 12 weeks should be considered preliminary, given the potential bias introduced through the substantially higher drop‐out rate for the paroxetine (55%) than desipramine (35%) treatment arm in this study. In addition, the lack of a placebo‐only arm in this RCT leaves open the possibility that the effects on anxiety symptoms attributed to medication reflect general effects of study participation.
It is not clear to what extent the results of this review are applicable to patients typically seen in the clinic. The majority of the studies included in this review reported restricting inclusion to people without other substance use disorders (Tollefson 1992; Randall 2001b; Brady 2005; Book 2008), despite evidence for a high degree of comorbidity between alcohol and drug use disorders in community samples (Hasin 2007), and that concurrent drug use problems increase the utilisation of mental health services (Cohen 2007). Book 2008 restricted participation in a 16‐week trial of paroxetine to people who drank to cope with their social anxiety symptoms and who were not seeking treatment for alcohol disorders. Therefore, reported reductions in drinking to cope with social anxiety symptoms in this trial may not generalize to all participants receiving medication for comorbid SAD and alcohol use disorders. Finally, close to three‐quarters of the participants from the RCTs included in this review were male, despite large‐scale, nationally representative community surveys in the USA reporting a greater 12‐month and lifetime prevalence of anxiety disorders in women than men (Vesga‐López 2008; McLean 2011). The preponderance of men in this review may reflect the nature of recruitment sources utilized (veterans in Petrakis 2012), the possibility of a stronger association of anxiety disorders with alcohol use disorders in men (Vesga‐López 2008; Dawson 2012), and evidence that men with alcohol use disorders may more readily receive specialized mental health/substance use treatment than women (Booth 2000; Dawson 2012; Alvanzo 2014). The observation that men and women metabolize alcohol differently (Kwo 1998), that anxiety disorders precede alcohol dependence significantly more frequently in women than men (Kessler 1997), and that men have different expectancies regarding the effects of alcohol than women (Morris 2005), further limits the strength of the conclusions of this review with regards to the efficacy of medication in treating anxiety disorders in women with concurrent alcohol use disorders.
All of the trials included in this review applied either DSM‐III or DSM‐IV diagnostic criteria in screening participants to determine study eligibility. It is not yet apparent how findings from these trials apply to people diagnosed using DSM‐V criteria. Post‐hoc observations in Brady 2005 and Pettinati 2000 suggested that sertraline may also be associated in less severe late‐onset alcoholism with greater reductions in drinking following pharmacotherapy, while this medication may result in heavier drinking in more severe early‐onset alcoholics. Therefore, the treatment implications of the observation that lack of convergence between diagnoses made using DSM‐IV and DSM‐V criteria are most evident at the less severe end of the alcohol use disorder spectrum may need to be considered (Dawson 2013).
We were unable to retrieve sufficient data to address the majority of the outcomes of interest in this review, despite a comprehensive search of the literature. None of the included studies assessed the effect of medication on quality of life and functional disability, despite documented impairments in these domains for both alcoholism and anxiety disorders (Ugochukwu 2013; Baxter 2014), and evidence that pharmacotherapy may improve quality of life in placebo‐controlled trials of anxiety disorder (Hofmann 2013). Moreover, failure to detect effects of medication on alcohol use in people with comorbid anxiety disorders in this review may reflect the fact that the effects of medication on alcohol use are likely to be complex and multifactorial, and that current measures of drinking do not adequately capture this complexity.
The trials included in this review were restricted to a few drugs with relatively circumscribed, primarily serotonergic, mechanisms of action. RCTs of other drugs that target multiple neurotransmitter systems and for which there is some evidence from controlled trials for effectiveness in treating anxiety disorders did not meet the inclusion criteria for this review (e.g. mirtazapine, Liappas 2003; venlafaxine, Ciraulo 2013). Although the TCA desipramine demonstrated equivalent efficacy in treating PTSD than the SSRI paroxetine in Petrakis 2012, and was actually more effective in reducing the severity of drinking behaviour, the overdose potential of TCAs suggest it should not be prescribed as a first‐line drug in people with a history of substance misuse (Shah 2001). In contrast, preliminary evidence from case studies of a possible association between treatment with SSRIs and heavier drinking in certain people (Brookwell 2014), and increases in drinking in early‐onset alcohol‐dependent people treated with 12 weeks of sertraline relative to controls (Kranzler 2012), highlights the importance of identifying drugs with extra‐serotonergic mechanisms of action that are effective in treating this comorbid population of people.
Emerging drugs that target the GABA and glutaminergic systems, such as the N‐methyl‐D‐aspartate (NMDA) antagonists memantine and ketamine, as well as the anticonvulsant topiramate, hold promise in treating comorbid anxiety and alcohol use disorders (Sofuoglu 2014). GABA dysregulation has been documented in alcoholism (Krystal 2006), and initial findings from one RCT of PTSD suggest it plays a role in anxiety disorders as well (Tucker 2007). Indeed, RCTs of topiramate for the treatment of PTSD and alcohol dependence or abuse are currently underway (NCT01408641; NCT01749215). Although the GABA analogue pregabalin has demonstrated some success in RCTs of anxiety disorders, including GAD (Feltner 2003; Pohl 2005; Rickels 2005), and SAD (Pande 2004), case reports of abuse of pregabalin among people with a history of substance abuse indicates that additional characterization of its safety profile in people with comorbid anxiety and alcohol use disorders is warranted (Schwan 2010; Gahr 2013; Papazisis 2013). Other extra‐serotonergic agents being trialed in this population include the alpha‐adrenergic blocker prazosin (NCT00585780; NCT00744055), and the sulphonamide anticonvulsant zonisamide (NCT01847469). It is of some concern that we were unable to identify ongoing RCTs in other anxiety disorders besides PTSD, despite the scarcity of rigorous trials evaluating the efficacy and tolerability of medication for these conditions in people with alcohol use disorders.
Quality of the evidence
Considerable uncertainty regarding estimates of the effects of medication over the short‐term is reflected in the rating of evidence for all outcomes in this review as being of very low quality. Although RCTs represent the gold‐standard study design for clinical trials, we downgraded ratings of quality for particular outcomes for a variety of reasons, including the imprecision of effect estimates (see Table 1). In the absence of published study protocols, selective reporting of outcomes may also have biased conclusions regarding treatment efficacy for some of the included RCTs. For instance, one of the two trials assessing the effect of medication in participants treated for longer than 12 weeks may have been susceptible to this form of bias (Tollefson 1992), undermining the finding that medication is effective over the long term in treating comorbid anxiety and alcohol use disorders.
High attrition rates represent a general cause for concern in this review, with 43.1% of participants across all eligible studies withdrawing prior to study endpoint. Apart from the difficulty of ruling out the possibility that reasons for drop‐out vary as a function of the intervention, and hence may lead to biased outcomes, sample attrition would have further compromised the ability of the small studies included in this review to detect effects of medication on alcohol use and anxiety disorder symptoms. Failure to detect an effect of medication on any of the drinking outcomes employed in this review may reflect the low power of these studies, even when we combined their data in a meta‐analysis. For instance, the small sample employed in Randall 2001b (15 participants) may partially account for the lack of evidence of group differences in drinking outcomes in this trial, despite moderate effect sizes (ranging from 0.54 to 0.66), as well as a statistically significant treatment response for drinking on the CGI‐I after eight weeks (Chi2 = 2.78, df = 1, P value = 0.05).
Treatment withdrawal rates were particularly large in Petrakis 2012 and Tollefson 1992. The greater efficacy of desipramine compared with paroxetine after 12 weeks in treating alcohol dependence in Petrakis 2012 could explain the higher drop‐out rate in the paroxetine (55%) than the desipramine (35%) group, particularly given the relatively severe alcohol dependence presented at baseline in this trial. This is consistent with the notion that the likelihood of retention in intervention programmes for anxiety disorders depends to a large extent on successful treatment of comorbid alcohol use disorders. This interpretation is supported by the observation that the drop‐out rate in the paroxetine arm of Petrakis 2012 was almost a third larger than the mean drop‐out rate (39%) for three RCTs of paroxetine in largely civilian PTSD samples (Marshall 2001; Tucker 2001; Marshall 2007), and that it was comparable with withdrawal rates observed in other pharmacotherapy trials for male alcoholics with comorbid psychiatric disorders (Powell 1995). The large overall withdrawal rate after 24 weeks of treatment with buspirone in Tollefson 1992 (16/26 (61.5%) participants) raises questions regarding the suitability of this medication for maintenance treatment, particularly given the possibility that people in this study may have been aware that they were receiving the active drug. Although the adequacy of study blinding is a general concern in this study, as none of the included studies assessed the degree to which participants were able to guess which arm they had been assigned to at study endpoint, Kranzler and colleagues reported that between two‐thirds and three‐quarters of the alcohol‐dependent participants in a placebo‐controlled double‐blind study of buspirone were able to guess their group assignment at the end of the study (Kranzler 1994).
Potential biases in the review process
One complication of the limited number of studies included in this review was that the presence of possible sources of bias could not be tested formally, by means of planned subgroup analyses. This may have been particularly problematic with regard to the decision to not exclude studies containing participants diagnosed with MDD (see Differences between protocol and review). Although this change to the protocol was regarded as warranted by the observation that MDD is frequently comorbid with both anxiety disorders and substance use disorders, the classification of all of the drugs assessed in this review as antidepressants suggests that any evidence of their efficacy in treatment anxiety and comorbid alcohol use disorders may have arisen from their effectiveness in treating symptoms of depression. This concern is somewhat ameliorated by the null findings of the majority of studies that tested the intervention on depression symptoms (Randall 2001bBrady 2005;Petrakis 2012). The only exception was the finding of a significant reduction in depression symptoms on the HAM‐D at 12 weeks in the only trial to exclude people with a DSM‐III diagnosis of MDD (Tollefson 1992).
Insufficient data for conducting planned tests of publication bias, in which studies that reported positive results for the active intervention were more likely to be published, represented another potential source of bias in this review (Hopewell 2009). Moreover, both RCTs that were funded by pharmaceutical companies reported efficacy of medication in treating anxiety disorders (Tollefson 1992; Randall 2001b). Reports that pharmacotherapy trials that reported positive effects for medication were more likely to be industry funded (Als‐Nielsen 2003; Baker 2003) raises the possibility that these trials may have been biased, further complicating the interpretation of data supporting the efficacy of medication in industry‐funded trials in this review.
In addition, the decision to include studies in which participants were diagnosed with alcohol abuse rather than dependence may potentially have reduced the effect of treatment, given the relatively poor reliability of the diagnosis of alcohol abuse compared with dependence (Hasin 2003), and a stronger association between dependence and anxiety disorders (Kessler 1997). The effect of this decision was again likely to have been minimal, though, as people diagnosed with alcohol abuse formed a small proportion of the total sample in two of the three studies that did not restrict trial participation to alcohol‐dependent participants (21% in Book 2008 and 7% in Randall 2001b, with no data available for Tollefson 1992).
Additional potential sources of bias in this review included the assessment of the effect of medication on alcohol use using the TLFB, a subject‐rated measure of alcohol use that may be susceptible to multiple forms of bias, such as recall and social desirability bias. Bias may also have been introduced through pre‐screening of the electronic database search results by a single review author (JI) as part of the trial identification process, prior to the independent application of the full set of inclusion criteria. Finally, the decision to classify the medications by medication class in a post‐hoc fashion, while in keeping with the recommended grouping, based on mechanism of action, of these drugs by the CCDAN review group, could potentially have introduced bias in interpretation of these results.
Agreements and disagreements with other studies or reviews
This review identified few rigorously designed RCTs assessing the effectiveness of medication in people with comorbid anxiety and alcohol use disorders, despite employing a systematic and comprehensive search of the literature. Therefore, we concur with the authors of qualitative reviews that there is an urgent need for additional controlled pharmacotherapy trials in this population (Schadé 2003; Berenz 2012; Lev‐Ran 2012). Moreover, we were able to confirm that RCTs do not demonstrate robust effects of serotonergic medications on the frequency and quantity of drinking in the eligible RCTs.
Preliminary evidence presented in this review for the efficacy of serotonergic agents in treating anxiety disorders may underestimate the effect of these drugs for interventions that target subgroups based on drinking history and individual differences in the metabolism of serotonin. For instance, one randomized double‐blind trial of 12 weeks of sertraline treatment (200 mg/day) for alcohol dependence reported beneficial effects that persisted over a three‐month follow‐up period only for late‐onset alcoholics with the 'LL' variant of the 5HTTLPR serotonin transporter gene (Kranzler 2012). Furthermore, findings from one placebo‐controlled RCT of the serotonin‐3 antagonist ondansetron suggested that serotonergic drugs might be more effective at reducing mood symptoms (including anxiety) in early‐onset rather than late‐onset alcoholism (Johnson 2003). Taken together, these findings imply that serotonergic medications such as the SSRIs and buspirone may be most effective in treating both anxiety and alcohol use disorders in people with less severe early‐onset alcohol dependence, and that efficacy of this treatment may be influenced by genetic factors.
Authors' conclusions
Implications for practice.
The evidence‐base for the effectiveness of medication in treating anxiety disorders and comorbid alcohol use disorders is currently inconclusive. Evidence for a response to pharmacotherapy (namely selective serotonin re‐uptake inhibitors (SSRIs)) was limited by the paucity of rigorous studies, contributing to very low quality estimates of outcome. There was also very low quality evidence that medication in these comorbid participants was well tolerated, with equivalent proportions of participants withdrawing prior to study endpoint in the medication and comparison groups. High overall rates of attrition were observed in some of the randomized controlled trials (RCTs) included in the review. Accordingly, although there was little evidence that medication has an impact on alcohol use (with the possible exception of the tricyclic antidepressant (TCA) desipramine), successful retention in treatment may be facilitated by targeting alcohol use as part of combined interventions.
Implications for research.
Controlled studies of the efficacy and tolerability of pharmacotherapy for anxiety disorders and comorbid alcohol use disorders are remarkably sparse, given the recognition that anxiety disorders may play a major role in the pathogenesis, early onset and continuation of alcohol dependence. Future multicentre RCTs could help identify patient subgroups that respond preferentially to treatment with serotonergic drugs, based on clinical and demographics factors (moderate versus severe alcoholics, men versus women), as well as genetic factors; isolate promising pharmacological interventions with novel mechanisms of action; and assess the efficacy of candidate drugs with established anxiolytic potential, such as pregabalin, topiramate, trazodone and venlafaxine in treating this patient population. The relative timing of the onset of anxiety disorders and alcohol dependence may have treatment implications and would also warrant additional investigation. Finally, studies targeting the predictors of treatment withdrawal, including the influence of alcohol use on attrition and the response of alcohol use to treatment, would be valuable in attempts to improve adherence in this difficult‐to‐treat population.
What's new
| Date | Event | Description |
|---|---|---|
| 26 November 2020 | Amended | A typo has been corrected. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 12 November 2020 | Amended | Clarification message from the Co‐ordinating Editor added to the Declarations of interest statement about the review’s compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
Acknowledgements
We would like to acknowledge searches conducted on behalf of this review by Sarah Dawson, the Trials Search Co‐ordinator of the Cochrane Depression, Anxiety and Neurosis group; as well as Sarah's counterparts at the Cochrane Drugs and Alcohol group, Simona Vecchi and Susanna Mitrova. We are grateful to Drs Sudie Back, Carrie Randall, Ismene Petrakis and Elizabeth Ralevski for responding to email requests for additional trial data. We also appreciate the responses to queries regarding trial methodology by Drs John Roache, Steven Batki, David Pennington and Josep Serecigni.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer: The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. The Cochrane Collaboration Depression, Anxiety and Neurosis Group's Specialized Register (CCDANCTR)
The Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK, a references register and a studies based register. The CCDANCTR‐References Register contains over 35,000 reports of randomized controlled trials in depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), EMBASE (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization's trials portal (ICTRP), ClinicalTrials.gov, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies can be found on the Group's website.
CCDANCTR‐Studies was searched (to 16 January 2014) using the following strategy: Condition = (anxiety or panic or phobic or obsessive or compulsive or "post‐traumatic") AND Condition or Co‐morbidity = (alcohol*)
CCDANCTR‐References was searched (to 16 January 2014) using a more sensitive set of terms to find additional untagged/uncoded reports of RCTs: ((anxi* or *phobi* or PTSD or post‐trauma* or posttrauma or "post trauma*" or "combat disorder" or panic or OCD or obsess* or compulsi* or GAD or "stress disorder" or "stress reaction” or "acute stress" or neurosis or neuroses or neurotic or psychoneuro*) AND ((alcoholi* or “alcohol use*") or (alcohol and (addict* or comorbid* or co‐morbid* or co‐occur* or depend* or concurren* or secondary or misus* or abus* or problem*)))):ti,ab,kw,ky,emt,mh,mc
Key to field tags: ti: title; ab: abstract; kw: keywords; ky: additional keywords; emt: EMTREE headings; MH: MeSH Headings; MC: MeSH check words
Appendix 2. The Cochrane Drugs and Alcohol Group's Specialized Register (CDAG)
Search conducted in November 2011 (records current until to November 2011): Search strategy: anxiety AND alcohol*
Updated search conducted in March 2013 (records current to March 2013): Search strategy: (alcohol* OR drink*) searched in title, abstract, keywords, intervention, diagnosis AND (anxiety OR anxious OR gad OR phobic OR phobia* OR agoraphobi* OR claustraphobi* OR panic OR (obsess* AND compulsi*) OR ocd OR post‐trauma* OR posttrauma* OR ptsd OR 'stress disorder' OR neurosis OR neuroses OR neurotic OR psychoneuro*) in all fields AND date >=2011
Appendix 3. PubMed
Search conducted in August 2013 (records from 1966 to August 2013) Search strategy: 1. Alcohol‐Related Disorders [mesh] 2. alcohol* [tiab] 3. dependen* or disorder* or drink* or misuse or abuse* or consumption [tiab] 4. #2 AND #3 5. alcoholism [mesh] 6. exp drinking behaviour 7. #1 or #4 or #5 or #6 8. Anxiety* [tiab] 9. Anxiety Disorders [mesh] 10. #8 OR #9 11. randomized controlled trial [pt] 12. controlled clinical trial [pt] 13. random* [tiab] 14. placebo [tiab] 15. drug therapy [mesh] 16. trial [tiab] 17. groups [tiab] 18. #11 or #12 or #13 or #14 or #15 or #16 or #17 19. Animals[mesh] NOT humans [mesh] 20. #18 NOT #19 21. #4 AND #10 AND #20
Appendix 4. EMBASE
Search conducted in August 2013 (records from 1966 to August 2013) Search strategy: 'anxiety disorder'/exp OR 'anxiety'/exp OR anxiety:ab,ti OR anxious:ab,ti OR gad:ab,ti OR phobic:ab,ti OR phobia*:ab,ti OR agoraphobi*:ab,ti OR claustraphobi*:ab,ti OR panic:ab,ti OR obsess*:ab,ti OR compulsi*:ab,ti OR ocd:ab,ti OR (post NEAR/1 trauma*):ab,ti OR posttrauma*:ab,ti OR ptsd:ab,ti OR 'stress disorder' OR neurosis:ab,ti OR neuroses:ab,ti OR neurotic:ab,ti OR psychoneuro*:ab,ti AND ('alcoholism'/exp OR alcoholi*:ab,ti OR ('drinking behavior'/exp OR 'alcohol'/de OR alcohol AND ('comorbidity'/exp OR addict* OR comorbid* OR depend* OR concurren* OR secondary OR misus* OR abus* OR problem OR (co NEXT/1 (morbid* OR occur*)):ab,ti))) AND ('crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR 'randomized controlled trial'/exp) AND [humans]/lim AND [embase]/lim AND [1‐11‐2011]/sd
Appendix 5. PsycINFO
Search conducted in August 2013 (records from 1970 to August 2013) Search strategy: (random* or controlled) and anxiety and alcohol
Appendix 6. Alcohol and Alcohol Problems Science database (ETOH)
Search conducted in August 2013 (records from 1977 to 2003) Search strategy: "anxiety disorder*"/"posttraumatic stress disorder"/PTSD/"specific phobia"/"simple phobia"/"social phobia"/"social anxiety disorder"/"panic disorder"/"generalized anxiety disorder"/"obsessive compulsive disorder"/OCD
Data and analyses
Comparison 1. Medication versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment response | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.13, 4.41] |
| 1.1.1 SSRI: paroxetine vs. placebo | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.13, 4.41] |
| 1.2 Symptom severity reduction | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐14.70 [‐33.00, 3.60] |
| 1.2.1 SSRI: paroxetine vs. placebo | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐14.70 [‐33.00, 3.60] |
| 1.3 Treatment acceptability | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 SSRI: paroxetine vs. placebo | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [0.14, 76.33] |
| 1.3.2 SSRI: sertraline vs. placebo | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.3 5‐HT partial agonist: buspirone vs. placebo | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.32, 25.92] |
| 1.4 Proportion of days abstinent | 2 | 54 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.26, 0.43] |
| 1.4.1 SSRI: paroxetine vs. placebo | 2 | 54 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.26, 0.43] |
| 1.5 Drinks per drinking day | 2 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐4.97, 0.14] |
| 1.5.1 SSRI: paroxetine vs. placebo | 2 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐4.97, 0.14] |
Comparison 2. Medication versus other medications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Symptom severity reduction | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐4.80 [‐19.41, 9.81] |
| 2.1.1 Paroxetine + placebo vs. desipramine + placebo | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐4.80 [‐19.41, 9.81] |
| 2.2 Treatment acceptability | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 Paroxetine + placebo vs. desipramine + placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Mean number of drinking days | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 Paroxetine + placebo vs. desipramine + placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Drinks per drinking day | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 Paroxetine + placebo vs. desipramine + placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Proportion heavy drinking days | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.1 Paroxetine + placebo vs. desipramine + placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6 Reduction in depression symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6.1 Paroxetine + placebo vs. desipramine + placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Book 2008.
| Study characteristics | ||
| Methods | DESIGN Description: randomized, placebo‐controlled flexible‐dose study with end‐of‐study taper. 1 optional individual motivational enhancement therapy session provided BLINDING Participants: yes Assessors: yes Administrators: yes ALLOCATION CONCEALMENT Method: group assignment maintained by investigational pharmacist RANDOMIZATION Method: urn randomization by gender, SAD severity and presence of comorbid MDD | |
| Participants | SAMPLE Description: 42 DSM‐IV SAD and alcohol dependence (79%) or abuse (21%), 48% female, mean age: 29 years, baseline severity on LSAS: 90. Mean of 6 drinks on each drinking occasion in past month, with at least 15 standard drinks in last 30 days. Comorbid psychopathology over 10%: GAD (8/42) SCREENING Primary diagnosis: SCID Comorbidity: SCID | |
| Interventions | Interventions: paroxetine 60 mg/day (10 mg/day week 1, 20 mg/day week 2, 40 mg/day week 3, 60 mg/day week 4) versus placebo x 16 weeks | |
| Outcomes | Primary outcomes: LSAS‐SR (modified), TLFB (drinks per drinking day, days abstinent, drinks per week, proportion heavy drinking days), modified to include items assessing drinking to cope with SAD Secondary outcomes: LSAS subscales, CGI‐I for SAD, SPIN, Drinking to Cope survey Data estimation: mixed‐effects modelling for primary outcomes. LOCF for SPIN and LSAS subscales | |
| Notes | INDUSTRY SUPPORT Industry funded: no Medication provided by industry: yes Any of the authors work for industry: no ADDITIONAL INFORMATION Drop‐out rates: 7/20 (35%) participants in paroxetine and 8/22 (36.5%) in placebo groups stopped taking medications by study endpoint | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized to either paroxetine or matching capsule placebo, using a computerized urn randomization program" ... "Urn randomization variables were gender, social anxiety severity (baseline LSAS total score ≤ 76 vs. > 76), and the presence of co‐occurring major depressive disorder as determined by the SCID" |
| Allocation concealment (selection bias) | Low risk | Quote: "group assignment was maintained by an investigational pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paroxetine and placebo were provided in matching capsules Quote: "All individuals involved in direct care or evaluation of study subjects, or who were involved in study supervision, were blind to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All individuals involved in direct care or evaluation of study subjects...were blind to group assignment." "Data analyses were conducted maintaining coded group assignment (group A vs. group B), and the blind was broken when analyses were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rates were statistically equivalent at different timepoints, and statistical analyses of primary outcomes employed all available data Quote: "All but four participants provided week 16 (end of trial) data, for a 90% research data completion rate" The proportion of dropouts or those that continued on treatment till study endpoint was not statistically different between groups |
| Selective reporting (reporting bias) | Unclear risk | It was not possible to determine whether all outcomes were reported, as the protocol for the study (clinicaltrials.gov/ct2/show/record/NCT00246441) did not contain information on specific outcomes |
| Other bias | Unclear risk | Insufficient information to determine whether additional source of bias exists |
Brady 2005.
| Study characteristics | ||
| Methods | DESIGN Description: randomized, placebo‐controlled double‐blind, parallel‐group fixed‐dose design with 1‐week placebo run‐in and end‐of‐study 4‐day medication taper. Participants received 1 hour weekly of individual CBT targeted at alcohol dependence and based on the Project MATCH treatment protocol BLINDING Participants: unclear Assessors: unclear Administrators: unclear ALLOCATION CONCEALMENT Method: unclear RANDOMIZATION Method: urn randomization by sex, depressive disorder status, trauma type, age at index trauma | |
| Participants | SAMPLE Description: 94 DSM‐IV civilian PTSD and alcohol dependence, 45.7% female, mean age: 36.6 years, baseline PTSD severity on CAPS: 58.9, mean drinks per day at baseline (prior 90 days): 12.9; 48/94 with additional depression/dysthymia SCREENING Primary diagnosis: SCID, CAPS Comorbidity: SCID | |
| Interventions | Description: sertraline 150 mg/day (50 mg/day for first 2 days, 100 mg/day for next 2 days, 150 mg/day from day 5, in 50 mg tablets) versus placebo x 12 weeks | |
| Outcomes | Outcomes: CAPS, IES, MISS, TLFB (% drinking days, % heavy drinking days, mean number of drinks per day, mean number of drinks per drinking day), ASI, HAM‐D, OCDS Data estimation: mixed‐effects ANOVA or ANCOVA modelling for continuous variables, Chi2 analysis for categorical outcomes | |
| Notes | INDUSTRY SUPPORT Industry funded: no Medication provided by industry: yes Any of the authors work for industry: no ADDITIONAL INFORMATION Drop‐out rates: 18/49 (36.7%) participants in sertraline and 15/45 (31.1%) participants in placebo groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "urn randomization was used to ensure equal representation in each group by sex, depressive disorder, trauma type, and age of index trauma" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation was provided in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial described as "double‐blinded". It is unclear from the report who exactly was blinded Quote: "each week, participants received a 10‐day supply of medications, included blinded study medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment provided in report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information on differences at baseline between drop‐outs and treatment completers not provided |
| Selective reporting (reporting bias) | Unclear risk | It was not possible to determine whether all outcomes were reported, as no protocol for the study was available |
| Other bias | Unclear risk | Quote: "that assessments of alcohol use was based on self report and thus may be biased" |
Petrakis 2012.
| Study characteristics | ||
| Methods | DESIGN Description: randomized, double‐blind, parallel‐group fixed‐dose design, 2‐week titration for antidepressants. All participants also received clinical management Enhancement therapy BLINDING Participants: unclear Assessors: unclear Administrators: unclear ALLOCATION CONCEALMENT Method: unclear RANDOMIZATION Method: unclear | |
| Participants | SAMPLE Description: 88 DSM‐IV PTSD and alcohol dependence, mean age: 47.1 years, 8.9% female, 75% Caucasian, mean number of standard drinks on drinking day: 23.5, abstinent ≥ 2 days and ≤ 29 days before treatment SCREENING Primary diagnosis: SCID | |
| Interventions | Description: 4 interventions: desipramine 200 mg/day (attained after 2 weeks, starting at 25 mg/day), paroxetine 40 mg/day (starting at 10 mg/day), naltrexone 50 mg/day (25 mg/day on first day) and placebo, administered in the following combinations: desipramine plus placebo, paroxetine plus placebo, desipramine plus naltrexone, paroxetine plus naltrexone x 12 weeks | |
| Outcomes | Primary outcomes: CAPS, SAC (mean number of drinks per week, % heavy drinking days, drinks per drinking day) Secondary outcomes: OCDS, Systematic Assessment of Treatment Emergent Events Data estimation: mixed‐effects modelling | |
| Notes | INDUSTRY SUPPORT Industry funded: no Medication provided by industry: unclear Any of the authors work for industry: no ADDITIONAL INFORMATION Drop‐out rates: 16/46 (35%) participants in desipramine and 23/42 (55%) in paroxetine groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Authors responded to email request for additional information by stating that this procedure was followed as "We wanted to make sure that one bottle contained one of two antidepressants (desipramine or paroxetine), and the other bottle contained either naltrexone or placebo. The labeling of the bottles was done arbitrarily by the pharmacy with this objective in mind" (Elizabeth Ralevski, 30 August 2013) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Medication was dispensed in blister packs, and the study was described as "double‐blinded", although no information was provided to identify which parties were blinded Quote: "study medications were dispensed in blister packs (during the 2‐week titration period for antidepressants) and packaged in separate bottles (after titration for 10 weeks), so subjects received two bottles, one labeled 'naltrexone/antidepressant study medication' and the other 'naltrexone study medication' " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as "double‐blinded" without information identifying which parties were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The drop‐out rate was significantly higher in the paroxetine than desipramine groups, with no comparison of differences in demographic/clinical profile of drop‐outs in the different treatment arms |
| Selective reporting (reporting bias) | Unclear risk | It was not possible to determine whether all outcomes were reported, as the protocol for the study (clinicaltrials.gov/ct2/show/NCT00338962) did not contain information on specific outcomes |
| Other bias | Unclear risk | The numbers reported for study withdrawal from the individual treatment arms did not correspond exactly between the study flow chart (Figure 1) and the text. |
Randall 2001b.
| Study characteristics | ||
| Methods | DESIGN Description: randomized, placebo‐controlled double‐blind, parallel‐group flexible‐dose design. Participants received a single individual session of motivation enhancement therapy targeted towards their alcohol abuse based on MATCH treatment manual BLINDING Participants: yes, medication and placebo tablets visually "matched" Assessors: yes, LSAS administrator blind to adverse effects Administrators: partially, clinician blind to LSAS and SPIN but not CGI‐I ALLOCATION CONCEALMENT Method: institutional research pharmacy dispensed medications RANDOMIZATION Method: order pre‐determined by pharmaceutical company | |
| Participants | SAMPLE Description: 15 DSM‐IV SAD with concurrent alcohol dependence (14 participants)/abuse (1 participant), 13.3% female, mean age: 35.8 years, LSAS total at baseline: 75.8 SCREENING Primary diagnosis: MINI ‐ Plus, SCID, ≥ 15 standard drinks in past 30 days Comorbidity: SCID | |
| Interventions | Paroxetine 20 mg/day (week 1), 40 mg/day (week 2), 60 mg/day (week 3+) versus placebo x 8 weeks. Single motivational therapy session for alcoholism also offered | |
| Outcomes | Primary outcomes: LSAS, CGI‐I for SAD, SPIN, TLFB (total drinks, drinks per drinking day, % days abstinent, % days drinking), CGI‐I for social phobia and drinking Secondary outcomes: BDI, ASI, ADS Data estimation: LOCF | |
| Notes | INDUSTRY SUPPORT Industry funded: yes Medication provided by industry: yes Any of the authors work for industry: no ADDITIONAL INFORMATION Drop‐out rates: 1/16 (16.7%) participants in paroxetine and 1/9 (11.1%) participants in placebo groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized according to a predetermined order prepared by the pharmaceutical company" |
| Allocation concealment (selection bias) | Low risk | Quote: "the institutional research pharmacy maintained the blind and dispensed all study medications" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded, as medication and placebo tablets visually "matched". Clinicians were also blinded to LSAS outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | LSAS assessment kept separate from administration of medication and adverse effects assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "..two participants received the first week's medication but failed to attend a medication management session, and one participant was determined by group consensus to be cognitively impaired and to have suspect data. These three subjects were excluded from all data analysis without breaking the study blind" The reasons for why the participants failed to attend the medication management session or which group they were assigned to was not clear Quote: "Five of the 6 patients in the paroxetine group and 8 of the 9 patients in the placebo group completed all 8 weeks of the trial" No information was provided on the causes of study withdrawal |
| Selective reporting (reporting bias) | Unclear risk | It was not possible to determine whether all outcomes were reported, as no protocol for the study was available |
| Other bias | Unclear risk | It is not possible to rule out other sources of bias, based on the information contained in the report. The trial was industry funded. Quote: "This work was supported by an investigator‐initiated award from SmithKline Beecham (to J.R.D.), who also supplied the drug and matched placebo" |
Tollefson 1992.
| Study characteristics | ||
| Methods | DESIGN Description: randomized, double‐blind, parallel‐group flexible‐dose design with a 1‐week placebo run‐in BLINDING Participants: unclear Assessors: yes Administrators: unclear ALLOCATION CONCEALMENT Method: unclear RANDOMIZATION Method: unclear | |
| Participants | SAMPLE Description: 51 DSM‐III GAD with concurrent alcohol dependence/abuse referred from chemical dependence treatment programmes, 27.5% female, mean age: 38.4 years, baseline HAM‐A score: approximately 25 (from figure 2 in article) SCREENING Primary diagnosis: SCID, HAM‐A score > 18, HAM‐D score < 18, abstinence from alcohol consumption for at least 30 and not more than 90 days Comorbidity: SCID | |
| Interventions | Buspirone 15 mg/day (week 1), ≥ 30 mg/day (week 2) to maximum 60 mg/day (3‐4 week, after which held constant) versus placebo x 24 weeks | |
| Outcomes | Primary outcomes: HAM‐A treatment response (≥ 30% reduction on HAM‐A total score and score < 18 = responders; ≥ 30% reduction on HAM‐A total score only = partial responders; otherwise classified as non‐responders), CGI Secondary outcomes: ASI, HAM‐D Data estimation: LOCF from 4 weeks for efficacy analyses | |
| Notes | INDUSTRY SUPPORT Industry funded: yes Medication provided by industry: unclear Any of the authors work for industry: no ADDITIONAL INFORMATION Drop‐out rates: 16/26 (61.5%) participants in buspirone and 21/25 (84%) in placebo groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomised to either buspirone or placebo" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation is provided in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as "double‐blinded", but no information provided on who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "anxiolytic efficacy was determined by blinded HAM‐A score reduction" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the proportion of participants who dropped at by 4 weeks was similar between groups (4/26 for buspirone and 5/25 for placebo), there were more drop‐outs by study endpoint in the placebo (21/25 participants) than buspirone group (16/26 participants), with significantly more people in the placebo group dropping out due to lack of efficacy or worsening of symptoms accounting for this difference. Unfortunately, while the authors confirm in the study report that there was no difference in demographics or ratings on the Cloninger personality scale between all 51 randomized participants and the participants who completed at least 4 weeks of treatment, they did not report a similar analysis for study endpoint |
| Selective reporting (reporting bias) | High risk | Only reported examples of items on questionnaires such as the ASI that were significantly different between groups, without indicating how many comparisons were conducted altogether |
| Other bias | Unclear risk | Prior benzodiazepine exposure was greater in the buspirone than the placebo group, which may predict a less robust response to buspirone. The trial was industry funded Quote: "the authors wish to acknowledge..[ ]..a concept grant from Mead‐Johnson Pharmaceuticals |
ADS: Alcoholism Dependency Scale ; ANCOVA: analysis of covariance; ANOVA: analysis of variance; ASI: Addiction Severity Index; BDI: Beck Depression Inventory; CAPS: Clinician Administered PTSD Scale; CBT: cognitive behaviour therapy; CGI: Clinical Global Impressions scale; DSM: Diagnostic and Statistical Manual; GAD: generalized anxiety disorder; HAM‐A: Hamilton Anxiety scale; HAM‐D: Hamilton Depression scale; IES: Impact of Event Scale; LSAS: Liebowitz Social Anxiety Scale (Clinician administered); LSAS‐SR: Liebowitz Social Anxiety (Self Report); MATCH: Matching Alcoholism Treatment to Client Heterogeneity; MDD: major depressive disorder; MINI: Mini International Neuropsychiatric Interview; MISS: Civilian Mississippi Scales for PTSD; OCDS: Obsessive Compulsive Drinking Scale; PTSD: post‐traumatic stress disorder; SAC: The Substance Abuse Calendar; SAD: social anxiety disorder; SCID: Structured Clinical Interview for DSM‐IV; SPIN: Social Phobia Inventory; TLFB: Timeline Followback scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batki 2011 | Participants received concurrent psychotropic medication and study did not screen out comorbid diagnoses other than major depressive disorder and secondary anxiety disorders |
| Caponi 1985 | Participants not diagnosed with anxiety disorders according to DSM‐III+ criteria |
| Ciraulo 2013 | Intervention was anxiety disorders including generalized anxiety disorder and panic disorder initiated after 5 days of abstinence attained, potentially confounding withdrawal effects with the anxiety disorders |
| Guardia 2012 | No screening for anxiety disorder (investigators use score of ≥ 7 in the General Health Questionnaire‐28 scale to screen for comorbid psychiatric symptoms) |
| Kranzler 1994 | Participants not diagnosed with anxiety disorders according to DSM‐III+ criteria and required to be abstinent for a minimum of 1 week only |
| Krupitsky 1993 | Anxiety determined using Spielberger's tests and Taylor's Anxiety Scale of the Minnesota Multiphasic Personality Inventory |
| Krupitsky 2013 | Participants not formally diagnosed with anxiety disorders according to DSM criteria |
| Liappas 2003 | Participants not diagnosed with social anxiety disorder according to DSM criteria. Treatment was also not assigned randomly, but consecutively |
| Loo 1986 | Participants were eligible for inclusion if diagnosed with "anxious states" |
| Malcolm 1992 | Participants were required to only have been abstinent from alcohol for 2 weeks prior to initiation of study medication |
| Oluwadara 2013 | Excluded as certain concurrent psychotropic medications were allowed; serious head injury was also allowed |
| Schadé 2005b | Participants receiving treatment for anxiety disorders were given choice of initiating treatment with an antidepressant (fluvoxamine). Pharmacotherapy was only administered to 22/47 participants |
DSM: Diagnostic and Statistical Manual.
Characteristics of studies awaiting classification [ordered by study ID]
NCT00248612.
| Methods | Participants were randomized in a double‐blind method (participant, carer, investigator and outcomes assessor all described as blinded) to 12 weeks of treatment with venlafaxine, CBT or placebo after participating in outpatient treatment for alcoholism. Study begins with 1‐week placebo run‐in, and ends with a 2‐week taper. Outcome assessment took place at study endpoint and 3, 6, 9 and 12 months post‐study |
| Participants | 180 English‐speaking adults, aged 18‐65 years (inclusive), with DSM‐IV diagnoses of alcohol dependence or abuse, and comorbid panic disorder, social phobia or generalized anxiety disorder |
| Interventions | Venlafaxine and CBT versus relaxation training and placebo (no dosage information provided) |
| Outcomes | Primary outcomes described as "drinking status over the course of treatment and during the treatment follow‐up", with secondary outcome including treatment completion, remission rates, "anxiety‐disorder free rates", abstinence rates and drinking frequency |
| Notes |
NCT00330239.
| Methods | Double‐blind, placebo‐controlled randomized assignment to 12 weeks of treatment with paroxetine |
| Participants | Adults aged 18‐65 years with DSM‐IV criteria for PTSD (chronic subtype, based on CAPS‐1) and substance dependence disorder (last 3 months, excluding caffeine and nicotine) |
| Interventions | Paroxetine (Paxil CR) (12.5‐50 mg/day) versus placebo (medication will be initiated at 12.5 mg and increased every 3 days as tolerated to the terminal dose in the double‐blind phase) |
| Outcomes | Primary outcome measures: CAPS; Clinical Global Impressions Scale |
| Notes | Contacted authors on 17 February 2010 for information on inclusion of study, but after a few preliminary responses (from Drs Brady and Sonne), no additional responses received (GlaxoSmithKline sponsored study) |
NCT00352469.
| Methods | Double‐blind, placebo‐controlled randomized assignment to 12 weeks of treatment with quetiapine |
| Participants | 20 participants, aged 19‐65 years (inclusive), with DSM‐IV diagnosis of alcohol dependence and a comorbid anxiety disorder |
| Interventions | Quetiapine (50 mg on days 1‐2, 150 mg on days 3‐4 and 300‐400 mg on days 5‐42) versus placebo |
| Outcomes | Primary outcome: reduction in alcohol use and increase in duration of sobriety, measured by the Timeline Followback method and breathalyser test Secondary outcomes: Pennsylvania Craving Scale, HAM‐A |
| Notes |
NCT01408641.
| Methods | Double‐blind (participant, carer, investigator, outcomes assessor), placebo‐controlled randomized assignment to 14 weeks of treatment with topiramate |
| Participants | 30 veterans, aged 18‐65 years (inclusive), with DSM‐IV diagnosis of current PTSD and alcohol use disorders |
| Interventions | Topiramate uptitrated over 6 weeks to 400 mg or highest tolerated dose versus placebo in matching capsules |
| Outcomes | Primary outcome: number of days of heavy drinking Secondary outcomes: number of days abstinent, amount of PTSD symptoms, number of memory/cognitive complaints |
| Notes |
NCT01518972.
| Methods | Randomized double‐blind placebo‐controlled clinical trial |
| Participants | 60 participants (25% women) with current primary DSM‐IV diagnosis of alcohol dependence and PTSD |
| Interventions | Prazosin titrated per study protocol vs. matched placebo for 6 weeks; with concomitant medical management based on the procedures of the COMBINE Study (Combining Medications and Behavioral Interventions for Alcoholism) |
| Outcomes | Primary outcomes: alcohol use during the 12‐week medication phase of the study and reports of craving during the same time period; PTSD symptom severity (whether reductions in PTSD mediate the effect of prazosin) |
| Notes | The entry for the study lists 1 of the exclusion criteria as a "psychiatric disorder requiring any medication other than anti‐depressants". Contacte Dr. Simpson on 12 February 2014 to obtain clarification on what proportion of her current intake of participants were receiving antidepressants while receiving the intervention (if any) |
Powell 1995.
| Methods | Participants in each of 3 diagnostic groups were randomized to bromocriptine or nortriptyline treatment arms, with participants in each arm subsequently re‐randomized to receive either active medication or placebo. The diagnostic groups were: "pure" alcohol dependence, alcohol dependence with comorbid anxiety and affective disorders, and alcohol dependence and antisocial personality disorder with or without comorbid Axis I psychiatric disorders |
| Participants | 216 male inpatient veterans with DSM‐III‐R diagnosis of alcohol dependence, mean age: 41.3 years (standard deviation 9.2). Participants diagnosed with comorbid anxiety and affective disorders, as well as antisocial personality disorder were also included |
| Interventions | Bromocriptine (3 x daily doses of 2.5 mg/day at study onset, increased to 5 mg/day by months 4‐6) versus nortriptyline (25‐75 mg/day at bedtime) x 6 months. Placebo dosing was matched to the respective medication arms (1 capsule increased to 2 capsules by months 4‐6, 3 times daily, for bromocriptine and placebo capsules at bedtime for nortriptyline) |
| Outcomes | Alcohol Severity Scale, Severity of Alcohol Dependence Questionnaire (SADQ), visual analogue alcohol craving scale, Beck Depression Inventory, Beck Anxiety Inventory, Symptom Check List‐90 and the Global Assessment Scale |
| Notes | It is unclear whether anxiety disorders were diagnosed according to DSM criteria |
CAPS: Clinician Administered; CBT: cognitive behaviour therapy; DSM: Diagnostic and Statistical Manual; HAM‐A: Hamilton Anxiety scale; PTSD; post‐traumatic stress disorder.
Characteristics of ongoing studies [ordered by study ID]
NCT00585780.
| Study name | Effect of Prazosin on Alcohol Craving; Stress Dysregulation and Alcohol Relapse |
| Methods | Randomized, placebo‐controlled, double‐blind trial of 12 weeks of treatment with prazosin |
| Participants | 75 participants with anxiety dependence and comorbid anxiety disorders (subtypes not specified) |
| Interventions | The alpha1‐adrenergic antagonist prazosin (16 mg/day) versus placebo |
| Outcomes | Describe the short‐term (12‐week) and follow‐up assessment of the effect of prazosin versus placebo on primary alcohol use outcomes and secondary outcomes including alcohol craving, negative mood symptoms, smoking and sleep |
| Starting date | September 2012 |
| Contact information | Prof. Rajita Sinha, rajita.sinha@yale.edu |
| Notes |
NCT00744055.
| Study name | Prazosin for Treatment of Patients With Alcohol Dependence (AD) and Post Traumatic Stress Disorder (PTSD) |
| Methods | Randomized, placebo‐controlled, double‐blind (participant, carer and investigator were blinded) trial of 12 weeks of treatment with prazosin |
| Participants | 120 (projected ) DSM‐IV PTSD and current comorbid alcohol dependence, heavy drinking episode within the last 14 days, aged 21‐65 years (inclusive) |
| Interventions | Alpha1‐adrenergic antagonist prazosin (16 mg/day) versus placebo |
| Outcomes | Information not provided |
| Starting date | January 2009 |
| Contact information | Elizabeth Ralevski, Ph.D. (+1)203‐932‐5711 ext. 4282, elizabeth.ralevski@yale.edu |
| Notes |
NCT01749215.
| Study name | A Controlled Trial of Topiramate Treatment for Alcohol Dependence in Veterans with PTSD |
| Methods | Double‐blind (participant, investigator), placebo‐controlled randomized assignment to 12 weeks of treatment with topiramate |
| Participants | 150 veterans with PTSD, aged 18‐65 years. Level of drinking must meet criteria for "at‐risk " or "heavy" drinking by NIAAA threshold, and participants "must express a desire to reduce alcohol consumption with the possible long‐term goal of abstinence" |
| Interventions | Topirimate vs. placebo, both up to 300 mg/day |
| Outcomes | Primary outcome: reduction in alcohol use assessed using the TLFB Secondary outcomes: reduction in PTSD severity on the PTSD Checklist (PCL). Other secondary outcomes include the effect of topiramate on impulsivity, risk‐taking and decision‐making, assessed using the Balloon Analogue Risk Task (BART) and Delay Discounting (DD) task |
| Starting date | February 2013 |
| Contact information | Steven L. Batki, M.D. (+1)415‐221‐4810 ext. 3671, steven.batki@ucsf.edu; Brooke A. Lasher, B.A. (+1)415‐221‐4810 ext. 4954, brooke.lasher@va.gov |
| Notes |
NCT01847469.
| Study name | Zonisamide in Addition to E‐CPT‐C for Veterans With PTSD and Comorbid Alcohol Dependence |
| Methods | Double‐blind, placebo‐controlled randomized assignment to 6 weeks' titration and 6 weeks' maintenance of treatment with zonisamide. Participants will receive E‐CPT‐C therapy for the 12 weeks of treatment. Randomization will be done using 3: 1 ratio and will be performed by the research pharmacy using a random assignment in blocks of 4, with 3 assigned to active medication and 1 to placebo |
| Participants | 50 veterans with PTSD, aged 18‐65 (inclusive), with DSM‐IV diagnosis of current PTSD and alcohol use disorders |
| Interventions | Zonisamide (400 mg) or placebo |
| Outcomes | Primary outcomes: CAPS; TLFB assessment |
| Starting date | June 2013 |
| Contact information | Elizabeth Ralevski, Ph.D. (+1)203‐932‐5711 ext. 4282, elizabeth.ralevski@yale.edu; Diana Limoncelli, B.A. (+1)203‐932‐5711 ext. 5217, diana.limoncelli@yale.edu |
| Notes |
CAPS: Clinician Administered PTSD Scale; DSM: Diagnostic and Statistical Manual; PTSD: post‐traumatic stress disorder; TLFB: Timeline Followback.
Differences between protocol and review
The protocol for this review included the description of a strategy to identify ongoing trials through browsing records listed on the clinicaltrials.gov website, under the categories of "anxiety disorders", "alcohol‐related disorders" and "alcoholism". We subsequently regarded this search as unnecessary given the inclusion of records from the clinicaltrials.gov database in the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/trialsearch/), another electronic register that was searched for ongoing trials for this review. We omitted the planned search of the metaRegister of Controlled Trials database (mRCT) (www.controlled-trials.com) as searches using the NIH RePORTER and WHO ICRP databases was regarded as adequate for identifying unpublished studies. In addition, we excluded the Cochrane Central Register of Controlled Trials (CENTRAL) from the search strategy described in the original protocol, as results from a search of CENTRAL are included on a quarterly basis in the specialized registers for the CCDAN and CDAG review groups.
The original protocol restricted inclusion to trials in which participants were diagnosed with alcohol dependence rather than alcohol abuse, given consistent findings of the greater reliability of the diagnosis of alcohol dependency (Hasin 2003), and a stronger relationship between dependence and anxiety disorders (Kessler 1997). We decided to omit this requirement, given that it would have excluded a number of otherwise eligible studies that did not explicitly indicate that they excluded participants with alcohol abuse from their sample. Accordingly, we changed the title of the protocol from "Pharmacotherapy for anxiety disorders and comorbid alcohol dependency" to "Pharmacotherapy for anxiety and comorbid alcohol use disorders". In addition, we revised the original protocol to include participants with major depressive disorder (MDD), given the frequent co‐occurrence of MDD in this comorbid population.
The protocol included a planned sensitivity analysis to determine whether treatment response varies as a function of the use of treatment response versus non‐response as an outcome statistic. This comparison may be necessary in the light of evidence that treatment response may result in less consistent outcome statistics than non‐response (Deeks 2002) when the control group event rate is higher than 50%. We did not perform this analysis, as the proportion of responders on the Clinical Global Impressions (CGI‐I) in the control group (26%) in the only meta‐analysis of global clinical response conducted as part of this review was lower than 50%.
We also planned a 'worst‐case/best‐case' analysis as part of the protocol to determine whether the exclusion of participants who were lost to follow‐up (LTF) influenced the findings of treatment efficacy (Deeks 2011). In this analysis, all the missing data for the treatment group are recorded as non‐responders in the worst‐case scenario, whereas in the best case, all missing data in the control group are treated as non‐responders. Should the conclusions regarding treatment efficacy not differ between these two comparisons, it can be assumed that missing data in trial reports do not have a significant influence on outcome. We did not conduct this analysis since the trials that provided data on the CGI‐I did so for their entire samples.
The protocol included eligibility criteria for studies employing cross‐over designs, although none was found for the current review. Cross‐over trials will only be included in the meta‐analytic component of future versions of this review when it is possible to extract medication and placebo/comparator data from the first treatment period, or when the inclusion of data from both treatment periods is justified through a wash‐out period of sufficient duration as to minimize the risk of carry‐over effects. An adequate wash‐out period is defined in accordance with clinical practice as at least two weeks for all drugs, with the exception of fluoxetine, for which a minimum wash‐out period of four weeks will be required, given the long plasma half‐life of this drug. For trials in which we regard the wash‐out period as adequate, we will include data from both periods only when it is possible to determine the SE of the MD in response between groups (Elbourne 2002). We will obtain the summary statistics required to derive the SE of interest from the trial report. For trials for which this information is missing, we will impute the summary statistics through averaging the relevant statistic from other included cross‐over trials with comparable control conditions. In cases in which the wash‐out period is of an insufficient duration, or in which the small number of cross‐over trials does not justify the separate analysis of the summary statistics, we will combine only treatment and placebo/comparator data from the first treatment period with the data from parallel randomized controlled trials (RCTs).
In the protocol, we stated that the primary outcome comparisons would be stratified by medication class, in recognition of the possibility of differential effects for different medications. Individual drugs were to be classified as selective serotonin re‐uptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), reversible monoamine oxidase inhibitors (RIMAs), benzodiazepines or "other medication". Instead of employing this categorical schema, we added a list of medication classes consistent with a schema based on medication class and year of introduction recommended by the CCDAN review group to the section on Data extraction and management. We only combined outcome data for drugs within each of these medication classes in the meta‐analysis on the proviso that they were administered for the treatment of the same anxiety disorder, given that the particular anxiety disorder treated may have implications for the relative time of onset of comorbid alcohol use disorders, as well as the prognosis when treating the anxiety disorder.
We did not conduct planned analyses of potential clinical and methodological moderators of treatment response due to an insufficient number of trials that satisfied inclusion criteria for this review. Future updates of this review will group trials according to the following clinical sources of heterogeneity (number of trials permitting):
gender of participants. Primary outcomes will be compared between trials that consist predominantly of men or women (defined arbitrarily as constituting greater than 70% of the total sample);
whether the sample included people diagnosed with major depression. Such an analysis might assist in determining the extent to which the efficacy of a medication in treating post‐traumatic stress disorder (PTSD) is independent of its ability to reduce symptoms of depression, an important consideration given the classification of many of these medications as antidepressants.
In addition, we will use the following criteria in future versions of this review to assess the extent of methodological sources of heterogeneity:
the involvement of participants from a single centre or multiple centres. Single centre trials are more likely to be associated with lower sample size but less variability in clinician ratings;
whether trials were industry funded. In general, published trials that are sponsored by pharmaceutical companies appear more likely to report positive findings than trials that are not supported by for‐profit companies (Als‐Nielsen 2003; Baker 2003);
the relative order of implementing treatment for anxiety disorders and alcohol dependence. We will conduct comparisons between treatment effects for trials that employ treatment for the anxiety disorder first, alcohol dependence first, or that implement treatment for both disorders simultaneously;
whether psychotherapy was implemented concurrently with pharmacotherapy.
Contributions of authors
Jonathan Ipser wrote the initial draft of the protocol, provided feedback on the methodological components of the review, analysed the data and contributed towards the writing of the review.
Don Wilson helped coordinate the protocol and provided subject expertise in the writing of its background section.
Don Wilson and Taiwo Akindipe were responsible for study selection and data extraction, contributed towards the discussion and conclusion sections of the review, and prepared the abstract and plain language summary.
Carli Sager co‐ordinated the initial database search for eligible studies and made final changes in response to reviewer comments.
Dan Stein conceived the idea of the review, and provided feedback on drafts of the protocol and review.
Jonathan Ipser stands as guarantor of the review.
Sources of support
Internal sources
University of Cape Town, Cape Town, South Africa
External sources
-
MRC Research Unit on Anxiety and Stress Disorders, South Africa
Partial funding for this review was provided by the MRC.
Declarations of interest
Potential conflicts of interest for individual review authors:
Jonathan Ipser has no known conflicts of interest.
Don Wilson is employed by the University of Cape Town and the Western Cape Provincial government. As part of his duties, he occasionally conducts clinical trials, some of which are funded by pharmaceutical companies (including GlaxoSmithKline and Organon). He has presented on behalf of Sanofi‐Synthélabo and Eli Lilly in the past. He has previously participated in industry‐sponsored trials in mood disorders. From 2011‐2014, Dr. Wilson received no research grants or consultancy honoraria. Dr. Wilson was involved in a Servier‐sponsored trial of agomelatine for depression, which ended in 2014, for which his department/faculty received reimbursement. He also gave lectures at meetings sponsored by Sanofi‐Synthélabo and Eli Lilly.
Taiwo Akindipe has no known conflicts of interest.
Carli Sager is currently contracted to Parexel International and sub contracted to MSD and Eli Lilly. Carli Sager had no known conflicts of interest while retrieving studies for inclusion in this review.
Dan Stein received research grants or consultancy honoraria (or both) from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier and Sun from 2011‐2014.
Clarification statement added from the Co‐ordinating Editor on 12 November 2020: This review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with theCochrane conflict of interest policy, which includes the relevant parts of theCochrane Commercial Sponsorship Policy. It will be updated within 12 months. The update will have a majority of authors and lead author free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Book 2008 {published data only}
- Book SW, Thomas SE, Randall PK, Randall C. Paroxetine reduces social anxiety in individuals with a co-occurring alcohol use disorder. Journal of Anxiety Disorders 2008;22(2):310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Book SW, Randall CL. A complex relationship between co-occurring social anxiety and alcohol use disorders: what effect does treating social anxiety have on drinking? Alcoholism, Clinical and Experimental Research 2008;32(1):77-84. [DOI] [PubMed] [Google Scholar]
Brady 2005 {published data only}
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. Journal of Nervous and Mental Diseases 2006;194(9):690-6. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcoholism, Clinical and Experimental Research 2005;29(3):395-401. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC, Killeen T, Simpson K, Randall C, Back SE, et al. Sertraline in comorbid PTSD/alcohol dependence. Proceedings of the 65th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2003 Jun 14-19; Florida 2003.
- Labbate LA, Sonne SC, Randal CL, Anton RF, Brady KT. Does comorbid anxiety or depression affect clinical outcomes in patients with post-traumatic stress disorder and alcohol use disorders? Comprehensive Psychiatry 2004;45(4):304-10. [DOI] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. American Journal on Addictions 2003;12(5):412-23. [PubMed] [Google Scholar]
Petrakis 2012 {published data only}
- Petrakis I, Jane JS, O'Brien E, Keegan K, Ralevski E, Desai N. Effect of depression on outcomes in a clinical trail evaluating antidepressants +/- naltrexone for treatment of alcohol dependence and PTSD. Alcoholism, Clinical and Experimental Research 2013;37:19A. [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, et al. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology 2012;37(4):996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Randall 2001b {published data only}
- Randall CL, Johnson MR, Thevos AK, Sonne SC, Thomas SE, Willard SL, et al. Paroxetine for social anxiety and alcohol use in dual-diagnosed patients. Depression and Anxiety 2001;14(4):255-62. [DOI] [PubMed] [Google Scholar]
Tollefson 1992 {published data only}
- Tollefson GD, Lancaster SP, Montague-Clouse J. The association of buspirone and its metabolite 1-pyrimidinylpiperazine in theremission of comorbid anxiety with depressive features and alcohol dependency. Psychopharmacology Bulletin 1991;27(2):163-70. [PubMed] [Google Scholar]
- Tollefson GD, Montague-Clouse J, Lancaster SP. Buspirone in comorbid alcohol dependency and generalized anxiety disorders. Drug Therapeutics 1990;20 Suppl:35-51. [Google Scholar]
- Tollefson GD, Montague-Clouse J, Tollefson SL. Treatment of comorbid generalized anxiety in a recently detoxified alcoholic population with a selective serotonergic drug (buspirone). Journal of Clinical Psychopharmacology 1992;12(1):19-26. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Batki 2011 {published data only}
- Batki SL, Lasher BA, Heinz AJ, Herbst E, Metzler T, Prathikanti S, et al. Risk-taking, impulsivity, memory and executive functioning as outcome predictors in a trial of topiramate for alcohol dependence in veterans with PTSD. Alcoholism, Clinical and Experimental Research 2012;36:226A. [Google Scholar]
- Batki SL, Lasher BA, Herbst E, Prathikanti S, Metzler T, Tarasovsky G, et al. Topiramate treatment of alcohol dependence in veterans with PTSD: baseline alcohol use and PTSD severity. American Journal on Addictions 2011;21(4):382. [Google Scholar]
- Batki SL, Lasher BA, Metzler T, Heinz AJ, Herbst E, Waldrop A, et al. Predictors of outcome in a pilot controlled trial of topiramate for alcohol dependence in veterans with PTSD. Alcoholism, Clinical and Experimental Research 2013:17A. [Google Scholar]
- Kalapatapu RK, Delucchi KL, Lasher, BA, Vinogradov S, Batki SL. Alcohol use biomarkers predicting cognitive performance: a secondary analysis in veterans with alcohol dependence and posttraumatic stress disorder. Military Medicine 2013;178(9):974-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos G-M, Lasher BA, Heinz AJ, Kalapatapu R, Herbst E, Metzler T, et al. Urinary ethyl glucuronide and ethyl sulfate testing to evaluate the efficacy of topiramate treatment of alcohol dependence in veterans with PTSD. Alcoholism, Clinical and Experimental Research 2013;37:19A. [Google Scholar]
Caponi 1985 {published data only}
- Caponi A, Realini R, Macciocchi A. A double-blind clinical trial of camazepam vs bromazepam in the treatment of anxio-depressive syndrome in alcoholic patients [Studio doppio cieco camazepam versus bromazepam nel trattamento delle sindromi ansioso-depressive in soggetti etillisti]. Progresso Medico 1985;41(12):731-7. [Google Scholar]
Ciraulo 2013 {published data only}
- Ciraulo DA, Barlow DH, Gulliver SB, Farchione T, Morissette SB, Kamholz BW, et al. The effects of venlafaxine and cognitive behavioral therapy alone and combined in the treatment of co-morbid alcohol use-anxiety disorders. Behaviour Research and Therapy 2013;51(11):729-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guardia 2012 {published data only}
- Guardia J. Clinical trial with ziprasidone for the treatment of psychiatric pathology, not being a severe mental disorder, associated to alcohol dependence. clinicaltrials.gov/ct2/show/NCT00197951 (accessed February 2014).
Kranzler 1994 {published data only}
- Kranzler HR, Burleson JA, Del Boca FK, Babor TF, Korner P, Brown J, et al. Buspirone treatment of anxious alcoholics. A placebo-controlled trial. Archives of General Psychiatry 1994;51(9):720-31. [DOI] [PubMed] [Google Scholar]
Krupitsky 1993 {published data only}
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grinenko AJ, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug and Alcohol Dependence 1993;33(2):157-63. [DOI] [PubMed] [Google Scholar]
Krupitsky 2013 {published data only}
- Krupitsky E, Yerish S, Rybakova K, Kiselev A. Single blind placebo controlled randomized clinical trial of trazodon for alcoholism comorbid with affective disorders. Alcoholism, Clinical and Experimental Research 2013;37:18A. [Google Scholar]
Liappas 2003 {published data only}
- Liappas J, Paparrigopoulos T, Tzavellas E, Christodoulou G. Alcohol detoxification and social anxiety symptoms: a preliminary study of the impact of mirtazapine administration. Journal of Affective Disorders 2003;76(1-3):279-84. [DOI] [PubMed] [Google Scholar]
Loo 1986 {published data only}
- Loo H, Guelfi JD, Malka R, Brion S, Cottin M, Gailledreau J, et al. Anxiolytic efficacy and tolerance of tetrabamate in anxious patients abusing alcohol. Multicenter double-blind versus placebo study [Efficacité anxiolytique et tolérance du tétrabamate chez des patients anxieux abusant de l'alcool. Etude multicentrique en double insu contre placebo]. Encephale 1986;12(5):291-7. [PubMed] [Google Scholar]
Malcolm 1992 {published data only}
- Malcolm R, Anton RF, Randall CL, Johnston A, Brady K, Thevos A. A placebo-controlled trial of buspirone in anxious inpatient alcoholics. Alcoholism, Clinical and Experimental Research 1992;16(6):1007-13. [DOI] [PubMed] [Google Scholar]
- Moak DH, Anton RF, Malcolm R, Randall CL, Brady K. Alcoholic subjects with anxiety disorder: characteristics of completers and noncompleters in a pharmacologic study. American Journal on Addictions 1993;2(1):39-47. [Google Scholar]
Oluwadara 2013 {published data only}
- Oluwadara BO, Roache JD, Guajardo J, Raj JJ. Correlation pattern of alcohol use and PTSD symptoms among combat veterans in treatment for comorbid PTSD and alcohol use disorder. Alcoholism, Clinical and Experimental Research 2013;37:331A. [Google Scholar]
- Roache JD, Guajardo J, Raj JJ. Preliminary examination of alcoholism subtypes in PTSD and alcohol use dual diagnosis. Alcoholism, Clinical and Experimental Research 2013;37(Suppl 2):331A. [Google Scholar]
Schadé 2005b {published data only}
- Schadé A, Marquenie LA, Balkom AJ, Koeter MM, den Brink W, Dyck R. The effectiveness of anxiety treatment on alcohol-dependent patients with a comorbid phobic disorder: a randomised controlled trial [Effectiviteit van een toegevoegde behandeling voor angstklachten bij alcoholafhankelijke patienten met een fobische stoornis]. Tijdschrift Voor Psychiatrie 2008;50(3):137-48. [PubMed] [Google Scholar]
- Schadé A, Marquenie LA, Balkom AJ, Koeter MW, Beurs E, den Brink W, et al. The effectiveness of anxiety treatment on alcohol-dependent patients with a comorbid phobic disorder: a randomized controlled trial. Alcoholism, Clinical and Experimental Research 2005;29(5):794-800. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00248612 {unpublished data only}
- Farchione TJ. CBT and venlafaxine treatments for anxiety in alcoholism. clinicaltrials.gov/ct2/show/NCT00248612 (accessed August 2013).
NCT00330239 {published data only}
- Sonne SC, Brady KT. Paroxetine treatment in outpatients with comorbid PTSD and substance dependence. clinicaltrials.gov/ct2/show/NCT00330239 (accessed February 2014).
NCT00352469 {published data only}
- Sattar P. 12 week prospective double blind placebo controlled randomized trial of Seroquel SR for alcohol dependence and comorbid anxiety. clinicaltrials.gov/ct2/show/NCT00352469 (accessed August 2013).
NCT01408641 {published data only}
- Fischer B. A 14-week randomized, placebo-controlled study of topiramate for alcohol use disorders in veterans with posttraumatic stress disorder. clinicaltrials.gov/ct2/show/NCT01408641 (accessed February 2014).
NCT01518972 {published data only}
- Simpson T. A placebo-controlled trial of prazosin in individuals with co-occurring alcohol dependence and PTSD. clinicaltrials.gov/ct2/show/NCT01518972 (accessed February 2014).
Powell 1995 {published data only}
- Powell BJ, Campbell JL, Landon JF, Liskow BI, Thomas HM, Nickel EJ, et al. A double-blind, placebo-controlled study of nortriptyline and bromocriptine in male alcoholics subtyped by comorbid psychiatric disorders. Alcoholism, Clinical and Experimental Research 1995;19(2):462-8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00585780 {published data only}
- Sinha R. Prazosin to reduce stress-induced alcohol/drug craving and relapse. clinicaltrials.gov/ct2/show/NCT00585780 (accessed August 2013).
NCT00744055 {published data only}
- Ralevski E. The use of prazosin for treatment of patients with alcohol dependence (AD) and post traumatic stress disorder (PTSD). clinicaltrials.gov/ct2/show/NCT00744055 (accessed August 2013).
NCT01749215 {published data only}
- Batki, SL. A controlled trial of topiramate treatment for alcohol dependence in veterans with PTSD. clinicaltrials.gov/ct2/show/NCT01749215 (accessed February 2014).
NCT01847469 {published data only}
- Petrakis I. Zonisamide in addition to enhanced cognitive processing therapy-C (E-CPT-C) for veterans with PTSD and comorbid alcohol dependence. clinicaltrials.gov/show/NCT01847469 (accessed February 2014).
Additional references
Alonso 2004
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al, ESEMeD/MHEDEA 2000 Investigators, European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. 12-Month comorbidity patterns and associated factors in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica Supplementum 2004;420:28-37. [DOI] [PubMed] [Google Scholar]
Als‐Nielsen 2003
- Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? Journal of the American Medical Association 2003;290:921-8. [DOI] [PubMed] [Google Scholar]
Alvanzo 2014
- Alvanzo AAH, Storr CL, Mojtabai R, Green KM, Pacek RL, La Flair LN, et al. Gender and race/ethnicity differences for initiation of alcohol-related service use among persons with alcohol dependence. Drug and Alcohol Dependence 2014;140:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). 4th revised edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Back 2006
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. Journal of Nervous and Mental Disease 2006;194(9):690-6. [DOI] [PubMed] [Google Scholar]
Baker 2003
- Baker CB, Johnsrud MT, Crismon ML, Rosenheck RA, Woods SW. Quantitative analysis of sponsorship bias in economic studies of antidepressants. British Journal of Psychiatry 2003;183:498-506. [DOI] [PubMed] [Google Scholar]
BAP 2014
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 2014;28:403-39. [DOI] [PubMed] [Google Scholar]
Baxter 2014
- Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychological Medicine 2014;22:1-12. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
Berenz 2012
- Berenz EC, Coffey SF. Treatment of co-occurring posttraumatic stress disorder and substance use disorders. Current Psychiatry Reports 2012;14(5):469-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bills 1993
- Bills LJ, Kreisler K. Treatment of flashbacks with naltrexone. American Journal of Psychiatry 1993;150(9):1430. [DOI] [PubMed] [Google Scholar]
Blake 1990
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behavior Therapy 1990;21:187-8. [Google Scholar]
Booth 2000
- Booth BM, Kirchner J, Fortney J, Ross R, Rost K. Rural at-risk drinkers: correlates and one-year use of alcoholism treatment services. Journal of Studies on Alcohol 2000;61:267-77. [DOI] [PubMed] [Google Scholar]
Boschloo 2013
- Boschloo L, Vogelzangs N, den Brink W, Smit JH, Veltman DJ, Beekman ATF, et al. Depressive and anxiety disorders predicting first incidence of alcohol use disorders: results of the Netherlands Study of Depression and Anxiety (NESDA). Journal of Clinical Psychiatry 2013;74:1233-40. [DOI] [PubMed] [Google Scholar]
Bowen 2000
- Bowen RC, D'Arcy C, Keegan D, Senthilselvan A. A controlled trial of cognitive behavioral treatment of panic in alcoholic inpatients with comorbid panic disorder. Addictive Behaviors 2000;25(4):593-7. [DOI] [PubMed] [Google Scholar]
Brady 1993
- Brady KT, Lydiard RB. The association of alcoholism and anxiety. Psychiatric Quarterly 1993;64(2):135-49. [DOI] [PubMed] [Google Scholar]
Brookwell 2014
- Brookwell L, Hogan C, Healy D, Mangin D. Ninety-three cases of alcohol dependence following SSRI treatment. International Journal of Risk & Safety in Medicine 2014;26:99-107. [DOI] [PubMed] [Google Scholar]
Bühler 2011
- Bühler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcoholism, Clinical and Experimental Research 2011;35:1771-93. [DOI] [PubMed] [Google Scholar]
Cohen 2007
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence 2007;86:214-21. [DOI] [PubMed] [Google Scholar]
Connor 2000
- Connor KM, Davidson JR, Churchill LE, Sherwood A, Foa E, Weisler RH. Psychometric properties of the Social Phobia Inventory (SPIN). New self-rating scale. British Journal of Psychiatry 2000;176:379-86. [DOI] [PubMed] [Google Scholar]
CPA 2006
- Canadian Psychiatric Association. Clinical practice guidelines. Management of anxiety disorders. Canadian Journal of Psychiatry 2006;51(8 Suppl 2):9S-91S. [PubMed] [Google Scholar]
Dawson 2012
- Dawson DA, Goldstein RB, Grant BF. Factors associated with first utilization of different types of care for alcohol problems. Journal of Studies on Alcohol and Drugs 2012;73:647-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2013
- Dawson DA, Goldstein RB, Grant BF. Differences in the profiles of DSM-IV and DSM-5 alcohol use disorders: implications for clinicians. Alcoholism, Clinical and Experimental Research 2013;37 Suppl 1:E305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575-600. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Dickersin 1992
- Dickersin K, Min YI, Meinert CL. Factors influencing the publication of research results: follow-up of applications submitted to two substantial review boards. JAMA 1992;267(3):374-8. [PubMed] [Google Scholar]
Driessen 2001
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol and Alcoholism 2001;36(3):249-55. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Feltner 2003
- Feltner DE, Crockatt JG, Dubovsky SJ, Cohn CK, Shrivastava RK, Targum SD, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multi-center study of pregabalin in patients with generalized anxiety disorder. Journal of Clinical Psychopharmacology 2003;23(3):240-9. [DOI] [PubMed] [Google Scholar]
Gahr 2013
- Gahr M, Franke B, Freudenmann RW, Kölle MA, Schönfeldt-Lecuona C. Concerns about pregabalin: further experience with its potential of causing addictive behaviors. Journal of Addiction Medicine 2013;7:147-9. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU assessment manual for psychopharmacology. Washington, DC: U.S. National Institute of Health, 1976. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50-5. [DOI] [PubMed] [Google Scholar]
Hamilton 1969
- Hamilton M. Standardised assessment and recording of depressive symptoms. Psychiatria, Neurologia, Neurochirurgia 1969;72:201-5. [PubMed] [Google Scholar]
Hasin 2003
- Hasin D. Classification of alcohol use disorders. Alcohol Research & Health 2003;27(1):5-17. [PMC free article] [PubMed] [Google Scholar]
Hasin 2007
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry 2007;64(7):830-42. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JTP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(6):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoertel 2012
- Hoertel N, Le Strat Y, Blanco C, Lavaud P, Dubertret C. Generalizability of clinical trial results for generalized anxiety disorder to community samples. Depression and Anxiety 2012;29:614-20. [DOI] [PubMed] [Google Scholar]
Hofmann 2013
- Hofmann SG, Wu JQ, Boettcher H, Sturm J. Effect of pharmacotherapy for anxiety disorders on quality of life: a meta-analysis. Quality of Life Research 2013;23(4):1141-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horowitz 1979
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine 1979;41(3):209-18. [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson BA, Ait-Daoud N, Ma JZ, Wang Y. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcoholism, Clinical and Experimental Research 2003;27(11):1773-9. [DOI] [PubMed] [Google Scholar]
Keane 1988
- Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. Journal of Consulting and Clinical Psychology 1988;56(1):85-90. [DOI] [PubMed] [Google Scholar]
Kendall 2004
- Kendall PC, Safford S, Flannery-Schroeder E, Webb A. Child anxiety treatment: outcomes in adolescence and impact on substance use and depression at 7.4-year follow-up. Journal of Consulting and Clinical Psychology 2004;72(2):276-87. [DOI] [PubMed] [Google Scholar]
Kessler 1996
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. American Journal of Orthopsychiatry 1996;66(1):17-31. [DOI] [PubMed] [Google Scholar]
Kessler 1997
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry 1997;54(4):313-21. [DOI] [PubMed] [Google Scholar]
Khantzian 1985
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry 1985;142(11):1259-64. [DOI] [PubMed] [Google Scholar]
Koen 2011
- Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues in Clinical Neuroscience 2011;13(4):423-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kranzler 2006
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcoholism, Clinical and Experimental Research 2006;20(9):1534-41. [DOI] [PubMed] [Google Scholar]
Kranzler 2012
- Kranzler HR, Armeli S, Tennen H. Post-treatment outcomes in a double-blind, randomized trial of sertraline for alcohol dependence. Alcoholism, Clinical and Experimental Research 2012;36(4):739-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2006
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Archives of General Psychiatry 2006;63(9):957-68. [DOI] [PubMed] [Google Scholar]
Kushner 1990
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. American Journal of Psychiatry 1990;147(6):685-95. [DOI] [PubMed] [Google Scholar]
Kushner 2005
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcoholism, Clinical and Experimental Research 2005;29(8):1432-43. [DOI] [PubMed] [Google Scholar]
Kwo 1998
- Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology 1998;115:1552-7. [DOI] [PubMed] [Google Scholar]
Lev‐Ran 2012
- Lev-Ran S, Balchand K, Lefebvre L, Araki KF, Le Foll B. Pharmacotherapy of alcohol use disorders and concurrent psychiatric disorders: a review. Canadian Journal of Psychiatry 2012;57(6):342-9. [DOI] [PubMed] [Google Scholar]
Liappas 2005
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Progress in Neuro-psychopharmacology & Biological Psychiatry 2005;29:55-60. [DOI] [PubMed] [Google Scholar]
Liebowitz 1987
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry 1987;22:141-73. [DOI] [PubMed] [Google Scholar]
Lubin 2002
- Lubin G, Weizman A, Shmushkevitz M, Valevski A. Short-term treatment of post-traumatic stress disorder with naltrexone: an open-label preliminary study. Human Psychopharmacology 2002;17(4):181-5. [DOI] [PubMed] [Google Scholar]
Lumley 2002
- Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in Medicine 2002;21(16):2313-24. [DOI] [PubMed] [Google Scholar]
Malcolm 2002
- Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, Ballenger JC. The effects of carbamazepine and lorazepam on single versus multiple previous alcohol withdrawals in an outpatient randomized trial. Journal of General Internal Medicine 2002;17(5):349-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2001
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. American Journal of Psychiatry 2001;158(12):1982-8. [DOI] [PubMed] [Google Scholar]
Marshall 2007
- Marshall RD, Lewis-Fernandez R, Blanco C, Simpson HB, Lin SH, Vermes D, et al. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depression and Anxiety 2007;24(2):77-84. [DOI] [PubMed] [Google Scholar]
McLean 2011
- McLean CP, Asnaani A, Litz BT, Hofmann SGJ. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research 2011;45:1027-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLellan 1990
- McLellan AT, Parikh G, Bragg A, Cacciola J, Fureman B, Incmikoski R. Addiction Severity Index: Administration Manual. 5th edition. Philadelphia: Pennsylvania Veterans' Administration Center for Studies of Addiction, 1990. [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Morris 2005
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clinical Psychology Review 2005;25(6):734-60. [DOI] [PubMed] [Google Scholar]
Mukherjee 2008
- Mukherjee S, Das SK, Vaidyanathan K, Vasudevan, DM. Consequences of alcohol consumption on neurotransmitters - an overview. Current Neurovascular Research 2008;5(4):266-72. [DOI] [PubMed] [Google Scholar]
NICE 2005
- National Institute for Health and Care Excellence Clinical Guideline 31. Obsessive-compulsive disorder: core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder, 2005. www.nice.org.uk/guidance/cg031 (accessed 30 December 2014). [PubMed]
Oakman 2003
- Oakman J, Van Ameringen M, Mancini C, Farvolden P. A confirmatory factor analysis of a self-report version of the Liebowitz Social Anxiety Scale. Journal of Clinical Psychology 2003;59:149-61. [DOI] [PubMed] [Google Scholar]
Pande 2004
- Pande AC, Feltner DE, Jefferson JW, Davidson JRT, Pollack M, Stein MB, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder. Journal of Clinical Psychopharmacology 2004;24(2):141-9. [DOI] [PubMed] [Google Scholar]
Papazisis 2013
- Papazisis G, Garyfallos G, Sardeli C, Kouvelas D. Pregabalin abuse after past substance-seeking behavior. International Journal of Clinical Pharmacology and Therapeutics 2013;51:441-2. [DOI] [PubMed] [Google Scholar]
Petrakis 2006
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biological Psychiatry 2006;60(7):777-83. [DOI] [PubMed] [Google Scholar]
Pettinati 2000
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism, Clinical and Experimental Research 2000;24(7):1041-9. [PubMed] [Google Scholar]
Pohl 2005
- Pohl RB, Feltner DE, Fieve RR, Pande AC. Efficacy of pregabalin in the treatment of generalized anxiety disorder: double-blind, placebo-controlled comparison of BID versus TID dosing. Journal of Clinical Psychopharmacology 2005;25(2):151-8. [DOI] [PubMed] [Google Scholar]
Project MATCH 1993
- Project MATCH . Project MATCH (Matching Alcoholism Treatment to Client Heterogeneity): rationale and methods for a multisite clinical trial matching patients to alcoholism treatment. Alcoholism, Clinical and Experimental Research 1993;17(6):1130-45. [DOI] [PubMed] [Google Scholar]
Randall 2001a
- Randall CL, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: a first step toward developing effective treatments. Alcoholism, Clinical and Experimental Research 2001;25(2):210-20. [PubMed] [Google Scholar]
Regier 1990
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8. [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rickels 2005
- Rickels K, Pollack MH, Feltner DE, Lydiard RB, Zimbroff DL, Bielski RJ, et al. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Archives of General Psychiatry 2005;62(9):1022-30. [DOI] [PubMed] [Google Scholar]
Roberts 2012
- Roberts NP, Roberts PA, Bisson JI. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD010204. [DOI: 10.1002/14651858.CD010204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150-3. [DOI] [PubMed] [Google Scholar]
Schadé 2003
- Schadé A, Marquenie LA, Van Balkom AJ, De Beurs E, Van Dyck R, Van Den Brink W. Do comorbid anxiety disorders in alcohol-dependent patients need specific treatment to prevent relapse? Alcohol and Alcoholism 2003;38(3):255-62. [DOI] [PubMed] [Google Scholar]
Schadé 2005a
- Schadé A, Marquenie LA, Balkom AJ, Koeter MW, Beurs E, den Brink W, et al. The effectiveness of anxiety treatment on alcohol-dependent patients with a comorbid phobic disorder: a randomized controlled trial. Alcoholism, Clinical and Experimental Research 2005;29(5):794-800. [DOI] [PubMed] [Google Scholar]
Schuckit 1988
- Schuckit MA, Monteiro MG. Alcoholism, anxiety and depression. British Journal of Addiction 1988;83(12):1373-80. [DOI] [PubMed] [Google Scholar]
Schwan 2010
- Schwan S, Sundström A, Stjernberg E, Hallberg E, Hallberg P. A signal for an abuse liability for pregabalin - results from the Swedish spontaneous adverse drug reaction reporting system. European Journal of Clinical Pharmacology 2010;66:947-53. [DOI] [PubMed] [Google Scholar]
Schweizer 1986
- Schweizer E, Rickels K, Lucki I. Resistance to the anti-anxiety effect of buspirone in patients with a history of benzodiazepine use. New England Journal of Medicine 1986;314:719-20. [DOI] [PubMed] [Google Scholar]
Shah 2001
- Shah R, Uren Z, Baker A, Majeed A. Deaths from antidepressants in England and Wales 1993-1997: analysis of a new national database. Psychological Medicine 2001;31:1203-10. [DOI] [PubMed] [Google Scholar]
Shear 1997
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative Panic Disorder Severity Scale. American Journal of Psychiatry 1997;154:1571-5. [DOI] [PubMed] [Google Scholar]
Sheehan 1996
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. International Clinical Psychopharmacology 1996;11 Suppl 3:89-95. [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith JP, Randall CL. Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol Research 2012;34:414-31. [PMC free article] [PubMed] [Google Scholar]
Sobell 1992
- Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors(s). Measuring Alcohol Consumption: Psychological and Biological Methods. Totowa: Humana Press, 1992:41-72. [Google Scholar]
Sofuoglu 2014
- Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addictive Behaviors 2014;39:428-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2000
- Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technology Assessment 2000;4(10):1-115. [PubMed] [Google Scholar]
Spitzer 1996
- Spitzer R, Williams J, Gibbons M. Instructional Manual for the Structured Clinical Interview for DSM-IV. New York: New York State Psychiatric Institute, 1996. [Google Scholar]
Stein 2009
- Stein DJ, Ipser J, McAnda N. Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectrums 2009;14(1 Suppl 1):25-30. [PubMed] [Google Scholar]
Stewart 2008
- Stewart SH, Conrod PJ. Anxiety disorder and substance use disorder co-morbidity: common themes and future directions. In: Anxiety and Substance Use Disorders: the Vicious Cycle of Comorbidity. Series in Anxiety and Related Disorders. New York: Springer, 2008:239-57. [Google Scholar]
Thomas 2008
- Thomas SE, Randall PK, Book SW, Randall CL. A complex relationship between co-occurring social anxiety and alcohol use disorders: what effect does treating social anxiety have on drinking? Alcoholism, Clinical and Experimental Research 2008;32(1):77-84. [DOI] [PubMed] [Google Scholar]
Torrens 2005
- Torrens M, Fonseca F, Mateu G, Farré M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug and Alcohol Dependence 2005;78(1):1-22. [DOI] [PubMed] [Google Scholar]
Tucker 2001
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. Journal of Clinical Psychiatry 2001;62(11):860-8. [DOI] [PubMed] [Google Scholar]
Tucker 2007
- Tucker P, Trautman RP, Wyatt DB, Thompson J, Wu SC, Capece JA, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2007;68:201-20. [DOI] [PubMed] [Google Scholar]
Ugochukwu 2013
- Ugochukwu C, Bagot KS, Delaloye S, Pi S, Vien L, Garvey T, et al. The importance of quality of life in patients with alcohol abuse and dependence. Harvard Review of Psychiatry 2013;21:1-17. [DOI] [PubMed] [Google Scholar]
Verbeke 2000
- Verbeke M, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer, 2000. [Google Scholar]
Vesga‐López 2008
- Vesga-López O, Schneier FR, Wang S, Heimberg RG, Liu SM, Hasin DS, et al. Gender differences in generalized anxiety disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Journal of Clinical Psychiatry 2008;69:1606-16. [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Watkins 2005
- Watkins KE, Hunter SB, Burnam MA, Pincus HA, Nicholson G. Review of treatment recommendations for persons with a co-occurring affective or anxiety and substance use disorder. Psychiatric Services 2005;56(8):913-26. [DOI] [PubMed] [Google Scholar]


