Key Points
Question
What is the effect size of manual therapy (MT) to increase maximum interincisal opening after head and neck radiotherapy?
Findings
In this case series of 49 disease-free survivors of head and neck cancer who had radiation-associated trismus, clinically meaningful improvement in oral opening was observed following MT at a mean (SD) of 4.1 (1.9) mm (0.45 effect size) after a single MT session and 6.4 (4.8) mm (0.7 effect) after serial MT sessions. Manual therapy was well tolerated by all patients; only mild soreness was reported by 3 patients.
Meaning
In this study, all patients showed an immediate response to treatment with MT following the first MT session, suggesting that MT may be considered as a frontline treatment to improve oral opening quickly; improved oral opening was observed across all patient subtypes, including groups that are typically considered treatment-refractory or at risk for developing trismus (ie, >5 years posttreatment, coexisting morbidities, advanced disease, and/or aggressive oncology treatment).
Abstract
Importance
Trismus is highly prevalent in head and neck cancer (HNC) survivorship. Current standards for trismus treatment include various stretch-based exercise protocols as a primary and single treatment modality with limited evidence regarding the role of manual therapy (MT) for this indication.
Objective
To assess the effect size and associations of response to MT to increase oral opening in the setting of radiation-associated trismus.
Design, Setting, and Participants
This retrospective case series was conducted at the University of Texas MD Anderson Cancer Center between 2016 and March 2020 (before COVID-19 interruption) and included 49 disease-free survivors of HNC who were referred for treatment of radiation-associated trismus.
Intervention
Intraoral MT (including or excluding external head and neck) targeting the muscles of mastication.
Main Outcomes and Measures
Maximum interincisal opening (MIO) before and after the initial MT session compared with serial MT sessions. Covariates were examined to determine the association with response to MT for trismus.
Results
A total of 49 survivors of HNC (13 women [27%]; 24 [49%] 64 years or younger; 25 [51%] 65 years or older; mean [range] of 6.6 [0-33] years postradiotherapy were included, 9 [18.4%] of whom underwent a single MT session; 40 [81.6%] who underwent multiple sessions [mean, 6; median (range), 3 (2-48)]). The MIO improved after a single session by a mean (SD) of 4.1 (1.9) mm (0.45 effect size) and after serial MT sessions by a mean (SD) of 6.4 (4.8) mm with an effect size of 0.7. No covariates were found to be clinically meaningfully associated with MIO improvement following MT.
Conclusions and Relevance
The findings of this case series study suggest that MT improved MIO with a medium to large effect size in survivors of HNC with radiation-associated trismus. The results suggest that the largest increase in oral opening was achieved after the initial treatment and although gains were more modest, oral opening continued to improve with serial treatment. Covariates were not associated with MT response, suggesting that patients with clinical features often considered treatment refractory (eg, advanced disease, multiple lines of oncology treatment, ≥5 years posttreatment) may benefit from treatment with MT. Manual therapy may be a beneficial frontline or adjuvant treatment when combined with traditional stretching therapy. A clinically meaningful increase in oral opening has the potential to improve swallow function, speech, pain, and quality of life.
This case series study examines the effect size and associations of response to manual therapy to increase oral opening in the setting of radiation-associated trismus.
Introduction
Trismus, defined as the inability to fully open the mouth, is a common oral morbidity associated with head and neck cancer (HNC) therapy. After xerostomia, trismus was reported as the second most burdensome morbidity for survivors of HNC.1 Prevalence rates vary depending on the study method and criteria used, ranging from 5% to 42%.2,3 Radiation-related trismus is often attributed to fibrosis, scar tissue formation, nerve damage, muscle atrophy, or a combination thereof, thus restricting the muscles of mastication.4 Associated deficits have physical, psychosocial, and safety implications, affecting function such as swallowing (difficulty with food insertion, biting, chewing, oral transfer), communication (voice, resonance, and speech), and oral access (dental restoration/examination, oral intubation, hygiene, emesis). A recent survey of survivors of oropharyngeal cancer found decreased quality of life in patients reporting trismus, as well as a negative association between diet and trismus severity.5 These deficits are also associated with increased social isolation and, in extreme circumstances, an increased risk of suicide.5,6,7
Human papillomavirus–positive HNC is associated with a high probability of long-term cure, which has generated a growing subset of survivors who live years with long-term effects of treatment.8,9,10 To our knowledge, there is limited well-structured research surrounding optimal intervention for the effect of radiation-associated trismus. A recent systematic review highlighted variability in treatment programs, including stretching devices, techniques, duration, and repetition. Most therapeutic exercise programs for trismus are reactive and demonstrate some improvement, but none restored maximum interincisal opening (MIO) to a normative range.11 Current trismus treatment protocols do not consider or adapt treatment based on soft tissue presentation (eg, muscle hypertonicity, scar tissue, lymphedema). Soft tissue consideration is critical to determine the effect of local and regional pliability or tensegrity (soft tissue tension and integrity across the entire structure/region) to determine optimal trismus treatment.
Manual therapy (MT) is an umbrella term for soft tissue mobilization techniques that uses physical and movement-related manipulations of the body. Manual therapy encompasses various techniques, including manual lymphatic drainage, myofascial release, massage, and passive and active stretching. These techniques apply variable amounts of pressure and stretch connective tissue and joints to restore fluid transport and range of motion.12 Manual therapy techniques improve circulation, reduce local ischemia, stimulate proprioception, decrease muscle spasms and adhesions, and improve MIO in patients with temporomandibular joint disorders.13,14,15 MT is generally considered safe but rare serious adverse events (SAE) can include damage to hard or soft tissue requiring surgery as a result of MT. More common adverse events (AEs) include discomfort, headache, inflammation, redness, pain, bleeding, lightheadedness, fainting sensation, general fatigue, soreness, and/or fatigue in the area of massage. Very little is published in the literature regarding MT for trismus in the HNC population. Although the etiology surrounding radiation-associated trismus differs from that of other forms of temporomandibular joint disorders, the theory of MT and its application specifically targeting MIO, may be effective in treating the HNC population.12,16 To date, Stubblefield et al17 published a retrospective cohort study on the efficacy of device driven therapies with MT; however, MT details were not provided. Dijsktra et al18 also reported on a retrospective case series examining exercise and various physical therapy techniques. Nedeljak et al19 highlighted the MT knowledge gap in their recent review of MT interventions in the HNC population. This study aims to demonstrate that MT can be considered either as a frontline or adjuvant treatment for radiation-induced trismus. The purpose of this study was to estimate effect size of MT to increase MIO in the setting of radiation-associated trismus, specifically after a single session and serial sessions, and explore factors associated with response to MT in the treatment of radiation-associated trismus.
Methods
Study Population and Design
This single-institution retrospective case series examined adult, disease-free (at the time of trismus referral) survivors of HNC who received MT at the University of Texas MD Anderson Cancer Center (Houston) between 2016 and March 1, 2020, for radiation-associated trismus after receiving curative-intent radiation therapy with or without neoadjuvant therapy. Survivors who did not meet these criteria or did not have pre-post measurements were excluded from this study. The electronic health record (EHR) of 49 survivors who met these eligibility criteria were analyzed. This analysis was approved by the institutional review board of the University of Texas, and a waiver of informed consent was obtained because of the use of retrospective data.
Study Measures
The MIO was measured by the treating clinician at the beginning and end of MT at each session using a Therabite ruler between the maxillary and mandibular central incisors or corresponding location on the gums. The final treatment measurement at therapy discharge following a series of manual therapy sessions was defined as after serial MT sessions.
Severity Grading
To our knowledge, there is no universally accepted, validated trismus grading scale for survivors of HNC. Trismus severity was graded according to a previously published (but not psychometrically validated) grading scale based on MIO.20 Severe trismus was defined as 15 mm or less interincisally, moderate trismus as 16 mm to 25 mm, 25 mm to 35mm as mild trismus, and greater than 35 mm as a normal opening. Severity grading was classified at baseline, post MT session 1, and after serial MT sessions.
Therapy Description
Each patient participated in MT that targeted masticatory muscles for the purposes of improving oral opening, ranging from 15 minutes to 60 minutes per visit. Manual therapy included intraoral soft tissue and mandibular joint mobilization, including any combination of internal/external passive stretching, active assistance stretching, active stretching, myofascial release, manual lymphatic drainage, massage, and strengthening against manual resistance of the mouth, face, and/or neck (eAppendix in the Supplement). No stretching devices were used during MT sessions.
Home Exercise Program
Forty-three of 49 patients (88%) received a home exercise program (HEP) (Table). The HEPs were not recommended if not clinically indicated or if the patient declined training. The HEP components were based on personalized individual patient need and the most commonly included internal/external MT, followed by stretching (passive and/or active of the face, mouth, and cervical regions), while fewer patients received device-assisted stretching exercises. Patients previously instructed on and prescribed a passive range of motion (PROM) device were instructed to continue use as a component of their HEP as tolerated in addition to MT. Home exercise program compliance was also patient-reported and recorded from the EHR as full (total compliance with set/repetitions as prescribed), partial (≥1 times per day, less than full prescription), or noncompliant (less than once per day or no compliance) for the patients who received an HEP following MT session 1 and returned for follow-up MT (40 [81.6%]).
Table. Patient Demographic Characteristics, Clinical History, and Response to Treatment With MT for Radiation-Associated Trismus.
| Characteristic | No./total No. (%) | MIOa | ||||
|---|---|---|---|---|---|---|
| Pretreatment MIO, mm (SD) | After single MT session, mm (SD) | Change after single MT session, mm (95% CI) | After serial MT sessions, mm (SD) [No./total No.] | Change after serial MT sessions, mm (95% CI) | ||
| No. | 49 | 49 | 49 | 49 | 40 | 40 |
| All patients | 49/49 (100) | 19 (9) | 24 (9) | 4 (4-5) | 25 (9) [40/40] | 6 (5-8) |
| Sex | ||||||
| Men | 36/49 (73) | 19 (9) | 23 (9) | 4 (4-5) | 24 (9) [30/40] | 6 (4-7) |
| Women | 13/49 (27) | 21 (10) | 24 (11) | 3 (2-5) | 28 (9) [10/40] | 8 (3-13) |
| Age, mean, y | ||||||
| ≤64 | 24/49 (49) | 21 (9) | 24 (9) | 4 (3-5) | 24 (9) [20/40] | 5 (3-7) |
| ≥65 | 25/49 (51) | 18 (9) | 23 (9) | 4 (4-5) | 26 (9) [20/40] | 7 (5-10) |
| HNC site | ||||||
| Oral cavity | 13/49 (27) | 20 (11) | 24 (11) | 4 (2-5) | 26 (9) [11/40] | 4 (1-8) |
| Oropharynx | 24/49 (49) | 20 (8) | 24 (8) | 4 (4-5) | 25 (9) [18/40] | 7 (4-9) |
| Other | 12/49 (24) | 18 (10) | 22 (10) | 4 (3-6) | 24 (9) [11/40] | 7 (6-9) |
| Tumor stage | ||||||
| 1 | 10/49 (20) | 23 (10) | 27 (9) | 4 (3-5) | 27 (10) [9/40] | 5 (3-7) |
| 2 | 10/49 (20) | 21 (6) | 25 (6) | 5 (3-6) | 28 (6) [10/40] | 7 (2-12) |
| 3 | 8/49 (16) | 20 (7) | 25 (7) | 5 (3-7) | 28 (5) [5/40] | 9 (5-14) |
| 4 | 16/49 (33) | 15 (11) | 19 (11) | 4 (3-4) | 20 (9) [13/40] | 6 (3-9) |
| T0/unknown | 5/49 (10) | 23 (9) | 28 (8) | 4 (2-6) | 27 (11) [3/40] | 4 (-.5-9) |
| Treatment combination | ||||||
| CRT | 23/49 (47) | 20 (9) | 25 (8) | 5 (4-5) | 26 (8) [17/40] | 7 (5-8) |
| Radiation alone | 6/49 (12) | 22 (8) | 26 (9) | 4 (2-6) | 25 (7) [5/40] | 6 (4-8) |
| Surgery + PORT/concurrent | 20/49 (41) | 18 (10) | 22 (10) | 4 (3-4) | 24 (10) [18/40] | 6 (3-8) |
| Time postradiation | ||||||
| ≤ 12 mo | 14/49 (29) | 21 (11) | 25 (11) | 4 (3-5) | 26 (10) [11/40] | 7 (2-12) |
| >12 mo-≤2 y | 9/49 (18) | 21 (10) | 24 (10) | 3 (2-4) | 28 (8) [7/40] | 4 (−2 to 10) |
| >2-≤5 y | 5/49 (10) | 19 (9) | 25 (6) | 5 (2-9) | 29 (7) [5/40] | 10 (5-14) |
| ≥5 y | 21/49 (43) | 18 (8) | 22 (9) | 4 (4-5) | 22 (7) [17/40] | 6 (5-7) |
| Recurrenceb | ||||||
| No | 27/49 (55) | 20 (9) | 24 (9) | 4 (3-5) | 26 (9) [21/40] | 7 (5-9) |
| Yes | 22/49 (45) | 18 (10) | 23 (10) | 4 (3-5) | 24 (9) [19/40] | 6 (3-8) |
| Osteoradionecrosisc | ||||||
| No | 32/49 (65) | 22 (9) | 26 (9) | 4 (4-5) | 27 (8) [26/40] | 7 (5-9) |
| Yes | 17/49 (35) | 15 (8) | 19 (8) | 4 (3-4) | 22 (9) [14/40] | 6 (2-9) |
| Adult comorbidity evaluation 27 score | ||||||
| 0 (none) | 6/49 | 25 (7) | 29 (7) | 4 (2-6) | 31 (6) [3/40] | 5 (−10 to 22) |
| 1 (mild) | 18/49 | 20 (10) | 24 (10) | 4 (1-3) | 28 (9) [15/40] | 8 (6-11) |
| 2 (moderate) | 6/49 | 12 (7) | 17 (8) | 4 (2-6) | 17 (9) [5/40] | 5 (1-10) |
| 3 (severe) | 19/49 | 19 (9) | 23 (9) | 4 (3-5) | 24 (9) [17/40] | 5 (3-8) |
| HEP after first session of MT | ||||||
| Prescribed | 43/49 (88) | NA | NA | NA | 25 (9) [28/40] | 6 (5-8) |
| Full compliance | 18/43 (42) | NA | NA | NA | 25 (9) [18/28] | 6 (4-9) |
| Partial compliance | 8/43 (19) | NA | NA | NA | 23 (6) [8/28] | 7 (3-11) |
| Noncompliant | 2/43 (5) | NA | NA | NA | 34 (4) [2/28] | 8 (−5-21) |
| Unknown | 12/43 (28) | NA | NA | NA | 24 (10) [15/28] | 5 (2-9) |
| Not prescribedd | 6/49 (12) | NA | NA | NA | 24 (10) [12/40] | 5 (3-9) |
Abbreviations: CRT, induction chemotherapy and/or concurrent chemoradiation; HEP, home exercise program; HNC, head and neck cancer site: other, nasopharynx, hypopharynx, salivary gland, unknown; MIO, maximum interincisal opening; MT, manual therapy; PORT, postoperative radiation therapy.
All data rounded to the nearest whole number.
Patients who received treatment for recurrent disease before.
History of treatment for osteoradionecrosis.
HEP not clinically indicated or patient declined training.
Adverse Events
Patients were monitored for adverse events (AEs) before, throughout, and at the end of each session. Adverse events were recorded in the EHR.
Statistical Analysis
To assess the change in oral opening after MT in the setting of radiation-associated trismus, MIO before and after initial treatment and after serial MT measurements were compared using paired sample t tests. Effect size was calculated and compared with thresholds detected using distributional methods, including the minimal clinically important difference (MCID, 0.5 baseline MIO SD). With 49 patients, the study had a 90% power to detect an effect size of 0.7 with a 2-sided α of .05. For the primary end point of change in MIO, we had a 90% power to detect the observed mean (SD) improvement in MIO of 4.1 (1.9) mm after a single session of MT; there was a mean (SD) improvement of 6.4 (4.8) mm after serial MT with a 2-sided α of .05 (40 [81.6%]).
Descriptive statistics of baseline characteristics were tabulated and summarized along with 95% CIs of MIO at baseline. To determine an association between candidate risk factors and MIO change posttreatment, an independent 2-sample t test with unequal variance assumption and 1-way analysis of variance were conducted based on the number of levels within each factor. If significant differences were found in the 1-way analysis of variance, we used the Bonferroni correction procedure for multiple pairwise comparisons to control for the increase in type 1 errors. Nonparametric testing included the Kruskal-Wallis test for multiple group comparison when normality was not met. A significance level of .05 was used for all statistical inference. All statistics were calculated using Stata, version 16 (StataCorp).
Secondary Analysis
To explore factors associated with response to MT in treating trismus, trismus risk factors commonly reported in the literature were selected.5,6,11 Potential factors included tumor classification (T stage), treatment combination, site of HNC, time posttreatment, history of osteoradionecrosis (ORN) and/or recurrent HNC, baseline MIO, and adult comorbidity evaluation 27 (ACE-27) scores. The validated ACE-27 instrument was used to grade the overall comorbidity burden in this cohort. The overall ACE-27 score is calculated as none (0), mild (1), moderate (2), or severe (3) and is determined by the highest scored ailment.21
Results
The EHRs of 49 survivors who met eligibility criteria were reviewed. Nine patients (18.4%) received a single MT session without serial sessions, as the patients transitioned out of the survivorship clinic and were unable to commute to the institution or were lost to follow-up; 40 of 49 patients (81.6%) completed multiple MT sessions (median, 3; range, 2-48 treatments). This case series demonstrated heterogeneity among the population referred for radiation-associated trismus; demographic trends and clinical characteristics in this series are represented in the Table. The mean age at the time of treatment with 1 session of MT was 63 years (range, 34-80 years), 36 patients (73%) were men, and 24 (49%) had a history of primary tumors located in the oropharynx, followed by the oral cavity (13 [26.5%]) or other location (12 [24.5%]). Other primary tumor sites included the nasopharynx, hypopharynx, and salivary gland. All primary tumor stages (0/unknown, 1, 2, 3, 4) were represented in this population. All patients were treated with radiation therapy; 23 of 49 (47%) were treated with concurrent chemoradiation. Recurrent disease, defined as patients who received treatment for recurrent disease before treatment with MT (but were disease-free during the first session of MT), was identified in 22 of 49 patients (45%); primary tumor staging was used to stratify patients with recurrent disease. History of treatment for ORN was identified in 17 of 49 patients (35%). In surgical cases, the muscles of mastication were manipulated according to operative notes in 19 of 24 patients (79%). Tumor invasion into the muscle(s) of mastication was confirmed in 10 of 40 patients (25%); however, for 9 patients (18%), this information was unavailable.
MT Response
The MIO improved following a single MT session at a mean (SD) of 4.1 (1.9) mm (95% CI, 4.0-5.0 mm) (mean [SD] MIO before MT, 19.4 [9.2] mm; mean [SD] after MT,23.6 [9.2] mm). The mean (SD) improvement after serial MT sessions (pre-MT to last serial MT) was 6.4 (4.8) mm (95% CI, 5.0-8.0 mm) (mean [SD] MIO before, 19.4 [9.1] mm; after, 25.5 [9.3] mm). By covariate, MIO changes following single and serial MT sessions are detailed in the Table.
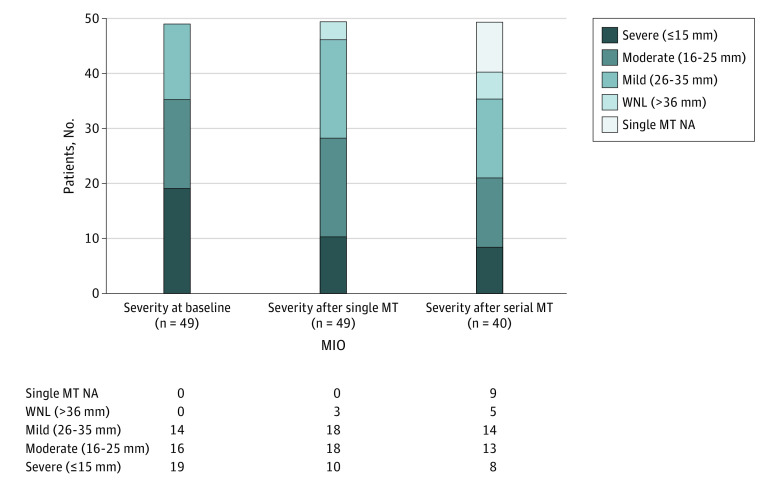
All patients tolerated MT sessions well without severe AEs; mild soreness was reported by 3 patients (6.1%). Figure 1 shows the change in MIO from pre-MT to after single and serial sessions of MT for the entire cohort. Figure 2 illustrates the total change in MIO among all 49 patients. Forty-six patients (94%) had an improved MIO following therapy with from pretreatment to the final visit; 3 of 49 (6%) experienced decreased MIO following serial MT sessions. However, all 3 of these patients were later found to have recurrent disease. After a single MT session, the mean (SD) MIO increase of 4.1 (1.9) mm represented a 0.45 effect size; an MCID of 4.6 mm was surpassed after serial MT with a mean (SD) MIO increase to 6.4 (4.8) mm, representing a 0.7 effect size. Sixteen of 49 patients (33%) achieved MCID after a single MT session with a mean (SD) improvement of 6.4 (1.2) mm; 29 of 40 patients (73%) achieved MCID after serial MT sessions with a mean (SD) improvement of 8.4 (3.7) mm.
Figure 1. Changes in Oral Opening Following Manual Therapy (MT).
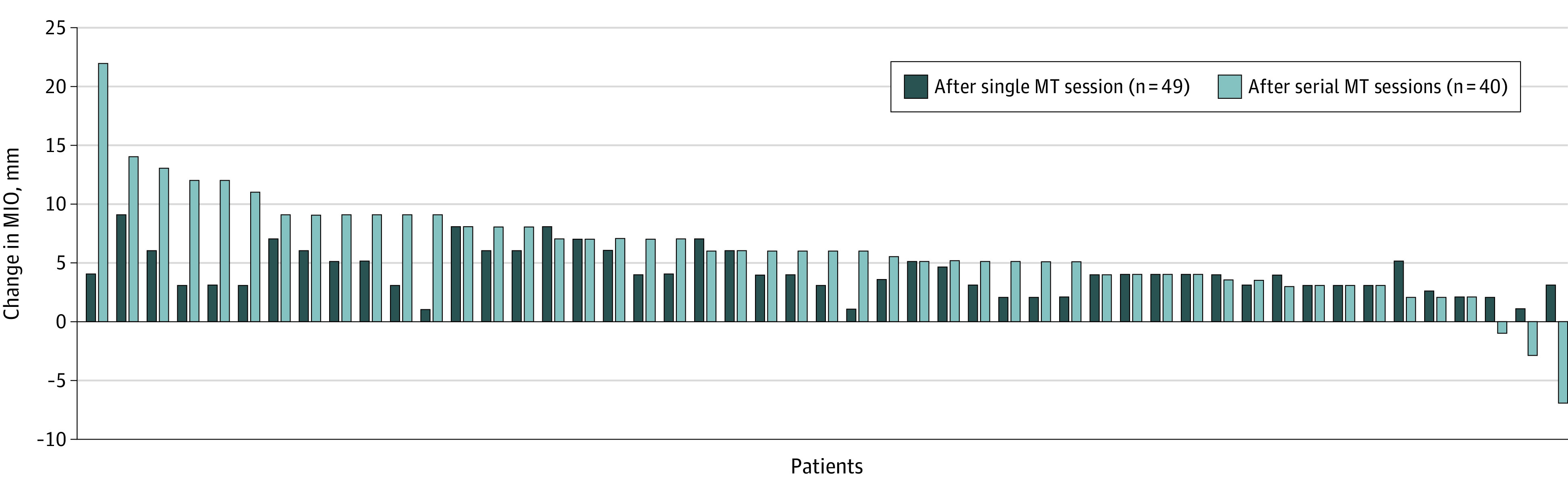
MIO indicates maximum interincisal opening.
Figure 2. Maximum Interincisal Opening (MIO) Across Time and Changes Following Manual Therapy (MT).
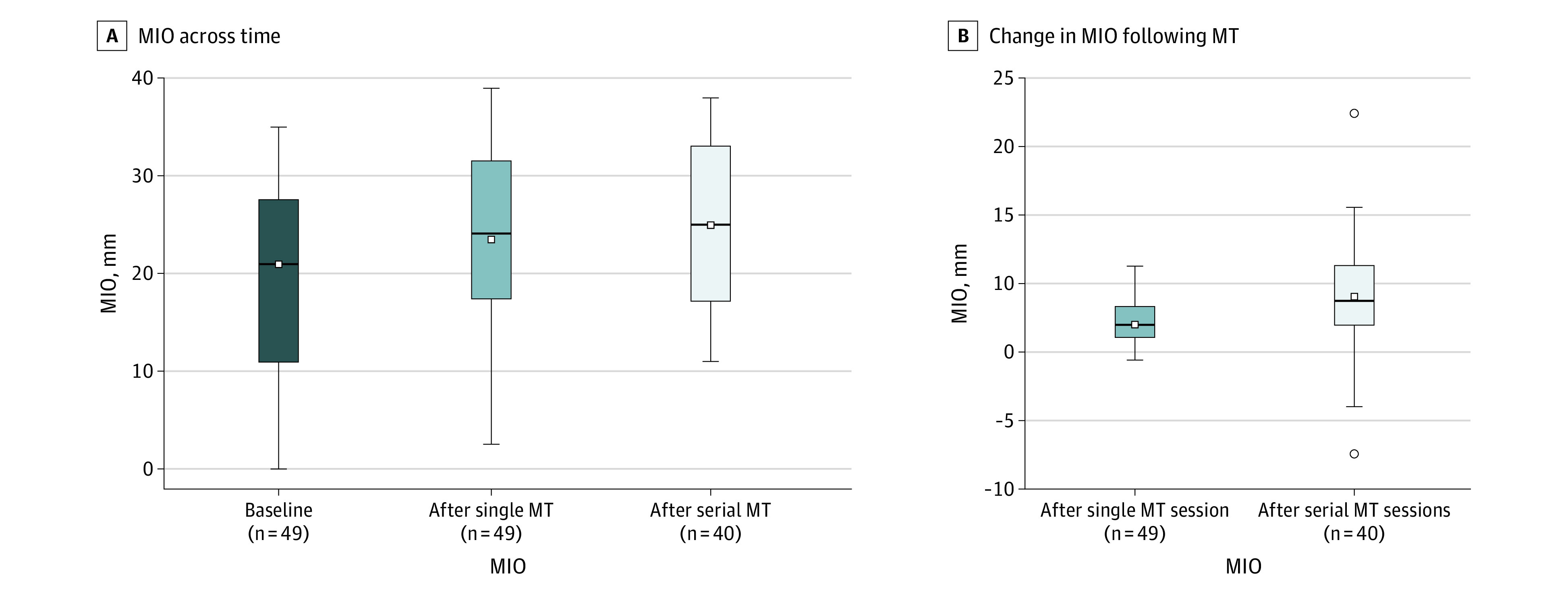
The boxes indicate quartiles; the whiskers, minimum and maximum MIO; the small white squares, mean MIO; the horizontal lines, median MIO; and the small white circles, outliers.
Severity Grading
Trismus severity classifications (mild, moderate, severe) over time are illustrated in Figure 3. Before MT, the largest proportion of patients (19 of 49 [39%]) were found to have severe trismus, and none had MIO in a normal range. After a single session of MT, 9 of the 19 patients (47.4%) initially in the severe category improved 1 or more severity grades. After a single MT session, trismus rates improved to 18 participants (37%) being categorized as mild, 18 (37%) as moderate, and 3 (6%) as normal (>35 mm). Following serial MT, 19 of 40 patients (48%) had mild trismus or a normal opening (>26 mm).
Figure 3. Maximum Interincisal Opening (MIO) Severity.
NA indicates not applicable; WNL, within normal limit.
Home Exercise Program
A PROM device was prescribed 6 weeks or earlier before the first session of MT for 7 of 49 patients (14%). Sixteen of 49 (33%) were prescribed a PROM device greater than 6 weeks before the first session of MT, and 26 of 49 patients (53%) had no previous PROM prescription. In addition to clinician-provided MT, 43 of 49 patients (88%) were prescribed a HEP. The HEP recommendations included self-administered MT among 40 of 43 patients (93%), active range of motion facial and/or neck stretching for 23 of 43 (53%), and a PROM device for 13 of 43 (33%). Home exercise program compliance was recorded for 28 of 40 patients (70%) who returned for serial MT, as reported in the Table (compliance recorded in serial MT subset). Of the 40 patients, 18 (45%) had full compliance, 8 (20%) partial compliance, 2 (5%) noncompliance, and 12 (30%) with an unknown level of compliance. Twelve of 40 patients (30%) did not have compliance recorded because of missing EHR data and recurrent disease.
Covariates
Clinically meaningful associations were not detected between MIO change and tumor stage, treatment combination, site of HNC, time posttreatment, treatment for recurrent disease, baseline MIO, and ACE-27 scores. The Table displays the 95% CIs for baseline MIO, posttreatment MIO after the first session, and MIO after serial MT, as well as change in MIO after the first and last treatments stratified by covariates. History of ORN was associated with MIO at baseline (15 mm; 95% CI, 11-19); however, MIO change following MT did not differ by ORN status. Of the 3 patients with negative MIO scores during serial MT visits, all 3 had a history of treatment for ORN, as well as active recurrence diagnosed after the first session of MT.
Discussion
Clinical response to MT as a frontline or adjuvant treatment for radiation-associated trismus is unknown. This case series, mostly comprising patients with moderate to severe radiation-associated trismus, found clinically meaningful improvement in MIO with a moderate 0.45 effect size after a single session of MT and a large 0.7 effect size exceeding MCID after serial MT sessions. These results aligned with a recent randomized clinical trial by Rodriguez-Sanz et al,22 who reported that the arm that received MT and exercise for chronic neck pain and restricted upper cervical rotation experienced improvement in all patient perception and functional variables compared with the exercise group alone.
Most published intervention data for radiation-associated trismus focus on exercise therapy rather than MT. A systematic review revealed that MIO outcomes with PROM devices/exercise therapy alone varied substantially, ranging from −1.9 to 13.6. The authors concluded that PROM stretching was beneficial but no exercise technique was superior, and no conclusion could be drawn because of variability.11 The effect sizes associated with MT in the present case series also compare favorably with those reported in similar studies examining trismus exercise for this indication. Tang et al23 completed a randomized clinical trial of trismus exercise (without MT) with a cohort with similar demographic characteristics as the present study (sample size, 43; time postradiation, >4.5 years; treatment duration, 3 months, 3 times daily, and 15 repetitions of passive and active exercises with a reported effect size of −0.28 [intervention group] and −1.2 [control group]). Kamstra et al24 reported an effect size of 0.84 in the study’s cohort of 69 patients 15 months after treatment with radiation using the Therabite ruler 4 to 5 times a day with variable repetitions during a 6-month period. It is not ideal to compare the data in this series to previously reported outcomes because of differences in treatment technique, duration, and follow-up timeline. A randomized clinical trial would offer controlled comparison of these techniques.
The improvements in MIO associated with MT session 1 were largest and achieved with MT alone without an oral stretching device; thus, the MIO change represented an immediate therapeutic effect estimate of effect size achievable with MT alone. Covariates in this case series, while clinically common prognosticators, were not meaningfully associated with response to MT (as measured by MIO improvement), suggesting chronic and previously assumed refractory radiation-induced trismus can benefit from MT. Despite the lack of an association between covariates and MIO improvement, the symptom and functional burden of radiation-associated trismus (including increased symptom burden associated with recurrent disease) warrants early intervention.11
Future Directions
This publication highlights the need for a universally accepted, validated trismus grading scale to improve consistency in measuring and reporting outcomes. Future prospective studies and randomized clinical trials are necessary to systematically examine the efficacy of MT and potential integration with conventional trismus therapy. Future research is also needed to investigate functional and quality of life outcomes associated with MT intervention.
Strengths and Limitations
A strength of this study is the confidence in the immediate therapeutic effect estimate derived from response to a single MT session in a well-defined clinical population (ie, improvement in MIO without device-driven stretching intervention). Several inherent weaknesses exist in this case series. Patient-reported outcomes were not available as measures of quality of life and trismus burden. Clinicians assessing MIO were not masked to the intervention, and a bias in measurement is possible. Variable follow-up of patients in the routine clinical environment limited knowledge of durability of MIO gains. Attributable to the design of all retrospective case series, no control group data were available to determine causality or decrease the threat of bias. Retrospective findings provide an inferior level of evidence, as they inherently do not include a priori selection of study variables and outcomes mandated by a prospective study design. In addition to nonstandard application of HEPs, concurrent therapies could confound the serial MT results.
Institutional quality control measures, such as advanced clinician training, templated EHR case history before treatment with MT, consistent procedural standards for MIO measurements, and templated clinical interview before and after treatment with MT to identify AEs/severe AEs were used to mitigate inherent limitations in a retrospective case series to assess a behavioral therapy like MT. Lack of a control group is a critical limitation to this initial work. Advantages of this clinical case series design include observational data collection in the patients’ real-world environment, which may improve generalizability.
Trismus can have various etiologies and other confounders in addition to radiation-fibrosis (eg, pain, lymphedema, scar tissue, muscle adhesions). By addressing these soft tissue pathologies with MT as indicated, we hypothesize that a synergist effect is likely when MT is added as a therapeutic component to programs of conventional stretching exercise for trismus. Lymphedema and fibrosis may affect oral opening, and by concurrently addressing soft tissue changes, it may optimize treatment and provide branching logic guidelines for therapeutic intervention of trismus. To date, to our knowledge, there are no treatments to slow or reverse the progression of fibrosis, but supportive treatments, such as MT, have the potential to improve and maintain function and quality of life.25
Conclusions
In this case series study, following treatment with MT, oral opening increased an average of 4.1 mm (95% CI, 4-5) after a single session and 6.4 mm (95% CI, 5-8) after serial MT sessions in patients with radiation-associated trismus. The largest increase in oral opening was achieved after the initial treatment; however, oral opening may continue to improve with serial treatment. Risk factor analyses suggest that even patients who have been traditionally considered treatment-refractory and are 5 years or longer posttreatment with coexisting morbidities, advanced disease, and/or received aggressive oncology treatment have the potential to benefit from treatment with MT. Partial and full compliance with an HEP likely improves MIO gains. Increased oral opening has the potential to improve swallow function, speech, pain, and quality of life; however, more research is needed to determine effect of oral opening and these functional outcomes. Based on these findings, MT may potentially be considered as a frontline treatment modality to improve oral opening quickly as a noninvasive method or adjuvant treatment to improve trismus outcomes.
eAppendix.
References
- 1.Kamstra JI, Jager-Wittenaar H, Dijkstra PU, et al. Oral symptoms and functional outcome related to oral and oropharyngeal cancer. Support Care Cancer. 2011;19(9):1327-1333. doi: 10.1007/s00520-010-0952-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337-342. doi: 10.1016/j.ijom.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 3.Dijkstra PU. Dynasplint for the management of trismus after treatment of upper aerodigestive tract cancer: a retrospective study. Ear Nose Throat J. 2012;91(7):E35. [PubMed] [Google Scholar]
- 4.Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT; Trismus Section, Oral Care Study Group, Multinational Association for Supportive Care in Cancer/International Society of Oral Oncology . A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18(8):1033-1038. doi: 10.1007/s00520-010-0847-4 [DOI] [PubMed] [Google Scholar]
- 5.Cardoso RC, Kamal M, Zaveri J, et al. Self-reported trismus: prevalence, severity and impact on quality of life in oropharyngeal cancer survivorship: a cross-sectional survey report from a comprehensive cancer center. Support Care Cancer. 2021;29(4):1825-1835. doi: 10.1007/s00520-020-05630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a Maxillofacial Oncology clinic. Oral Oncol. 2008;44(5):430-438. doi: 10.1016/j.oraloncology.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 7.Osazuwa-Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer. 2018;124(20):4072-4079. doi: 10.1002/cncr.31675 [DOI] [PubMed] [Google Scholar]
- 8.Pezzuto F, Buonaguro L, Caponigro F, et al. Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology. 2015;89(3):125-136. doi: 10.1159/000381717 [DOI] [PubMed] [Google Scholar]
- 9.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50(5):380-386. doi: 10.1016/j.oraloncology.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403. doi: 10.1016/j.oraloncology.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 11.Kamstra JI, van Leeuwen M, Roodenburg JLN, Dijkstra PU. Exercise therapy for trismus secondary to head and neck cancer: A systematic review. Head Neck. 2017;39(11):2352-2362. doi: 10.1002/hed.24859 [DOI] [PubMed] [Google Scholar]
- 12.Jones MA. Clinical reasoning in manual therapy. Phys Ther. 1992;72(12):875-884. doi: 10.1093/ptj/72.12.875 [DOI] [PubMed] [Google Scholar]
- 13.Calixtre LB, Moreira RF, Franchini GH, Alburquerque-Sendín F, Oliveira AB. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trials. J Oral Rehabil. 2015;42(11):847-861. doi: 10.1111/joor.12321 [DOI] [PubMed] [Google Scholar]
- 14.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14(5):531-538. doi: 10.1016/j.math.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armijo-Olivo S, Pitance L, Singh V, Neto F, Thie N, Michelotti A. Effectiveness of manual therapy and therapeutic exercise for temporomandibular disorders: systematic review and meta-analysis. Phys Ther. 2016;96(1):9-25. doi: 10.2522/ptj.20140548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Threlkeld AJ. The effects of manual therapy on connective tissue. Phys Ther. 1992;72(12):893-902. doi: 10.1093/ptj/72.12.893 [DOI] [PubMed] [Google Scholar]
- 17.Stubblefield MD, Manfield L, Riedel ER. A preliminary report on the efficacy of a dynamic jaw opening device (dynasplint trismus system) as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil. 2010;91(8):1278-1282. doi: 10.1016/j.apmr.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Dijkstra PU, Sterken MW, Pater R, Spijkervet FK, Roodenburg JL. Exercise therapy for trismus in head and neck cancer. Oral Oncol. 2007;43(4):389-394. doi: 10.1016/j.oraloncology.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Nedeljak J, Armijo-Olivo S, Hernandez IA, Nayar S, McNeely ML. A scoping review of physiotherapeutic interventions for trismus in head and neck cancer: where is the manual therapy? Physiotherapy Canada. Published online June 15, 2021. doi: 10.3138/ptc-2020-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owosho AA, Pedreira Ramalho LM, Rosenberg HI, et al. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT). J Craniomaxillofac Surg. 2016;44(9):1408-1413. doi: 10.1016/j.jcms.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. doi: 10.1001/jama.291.20.2441 [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Sanz J, Malo-Urriés M, Corral-de-Toro J, et al. Does the addition of manual therapy approach to a cervical exercise program improve clinical outcomes for patients with chronic neck pain in short- and mid-term? a randomized controlled trial. Int J Environ Res Public Health. 2020;17(18):E6601. doi: 10.3390/ijerph17186601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Shen Q, Wang Y, Lu K, Wang Y, Peng Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther Onkol. 2011;187(1):39-44. doi: 10.1007/s00066-010-2151-0 [DOI] [PubMed] [Google Scholar]
- 24.Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer. 2013;21(4):951-957. doi: 10.1007/s00520-012-1610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubblefield MD. Clinical evaluation and management of radiation fibrosis syndrome. Phys Med Rehabil Clin N Am. 2017;28(1):89-100. doi: 10.1016/j.pmr.2016.08.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.