GENERAL CONSIDERATIONS
Obstructive sleep apnea (OSA) is a common condition with major neurocognitive and cardiovascular sequelae.1 The disease is characterized by repetitive collapse of the pharyngeal airway during sleep yielding repetitive hypoxemia and hypercapnia with associated catecholamine surges. This breathing pattern leads to sleep fragmentation and autonomic changes that predispose to associated disease consequences.2 Although commonly thought to be purely a result of upper airway crowding (frequently attributed to obesity), obstructive apneas do not occur during wakefulness.3 In addition, there is considerable overlap in airway collapsibility (as measured by passive critical closing pressure or Pcrit technique) between patients with OSA and controls such that clearly other mechanisms are involved.4 Recent studies have emphasized the variability in OSA both from the standpoint of mechanisms underlying disease (ie, endotypes) as well as from sequelae of disease (ie, phenotypes).5,6 As a result of this variability, OSA is being considered a disease amenable to precision medicine from the standpoint of diagnosis, treatment, as well as prognostication.7
PATHOGENESIS AND ENDOTYPES
Anatomic compromise at the level of the pharyngeal airway is necessary, although not always sufficient, for the development of OSA.8,9 Based on imaging as well as assessment of mechanics, patients with OSA are at risk of pharyngeal collapse as compared with matched controls.10 The patency of the pharyngeal airway is maintained during wakefulness ostensibly through protective pharyngeal reflexes that increase pharyngeal dilator muscle activity and prevent upper airway collapse.11 As one example, the genioglossus muscle has phasic activity (bursts with each inspiration) and is state-dependent (has higher activity during wakefulness than during sleep) and has been extensively studied in this context.12 The decrease in activity of the genioglossus muscle (and other upper airway dilators) at the onset of sleep may lead to pharyngeal collapse in those who are anatomically predisposed.13
Control of breathing may also be important in OSA pathogenesis.14–16 Loop gain is an engineering term used to define the stability or instability in a feedback control system. A system with high loop gain is one which is prone to instability, whereas a system with low loop gain is intrinsically stable. A high loop gain system may develop oscillations with minor perturbations, whereas a system with low loop gain is one that will remain stable even with marked perturbations. Fluctuations in output from the respiratory central pattern generator in the brainstem lead to oscillations in activity of both the diaphragm and the upper airway dilator muscles. Thus, patients with high loop gain (and an anatomic predisposition) may develop upper airway collapse and obstruction when output to the upper airway dilator muscles is at its nadir.17
The ability to tolerate reductions in ventilation—the respiratory arousal threshold—is often variable between people and has also received recent attention.18–20 Some patients have a low arousal threshold (wake up easily), whereas others have a high arousal threshold (sleep soundly).21 Evidence suggests that most patients with OSA have some periods of stable breathing that occur spontaneously and are thought to result from activation of upper airway dilator muscles by endogenous stimuli (eg, CO2, negative intrapharyngeal pressure).22,23 In patients with a high arousal threshold, the accumulation of these respiratory stimuli can lead to activation of pharyngeal dilator muscles and thus periods of stable breathing. In contrast, patients with a low arousal threshold may have repetitive apneas. In this setting recurrent arousals from sleep reduce the period for sufficient accumulation of respiratory stimuli such that pharyngeal protective reflexes are not engaged.
Although the abovementioned factors are reasonably well established, recent emphasis has been placed on individual variability in each of these traits (endotypes). Some patients have primarily an anatomic problem predisposing them to pharyngeal collapse, whereas others have dysfunction in the upper airway dilator muscles, and still others have unstable ventilatory control (high loop gain) predisposing to pharyngeal collapse. It is not uncommon for patients to have multiple abnormalities that may be underlying OSA.24,25
Most of the concepts described earlier treat the upper airway as a collapsible tube with uniform collapsibility (ie, Starling resistor). One of the features of the Starling resistor is flow limitation such that increasing respiratory effort will not lead to increased flow. However, in practice a variety of flow patterns are observed across patients, which suggest that upper airway behavior is more complex.26 Moreover, Azarbarzin and colleagues27 have recently suggested that the different flow pattern “signatures” can be used to predict the variable sites of airway collapse. At least, in theory, 2 patients with the same collapsibility (as measured by passive Pcrit) might have very different anatomy and may respond differently to the same therapy aimed at improving anatomy, such as an oral appliance.
The personalized medicine approach to treating OSA involves recognition of this individual variability. Different interventions are available to influence each of these traits and may be effective strategies to treat OSA at least in select individuals and especially in those patients intolerant of continuous positive airway pressure (CPAP). For example, uvulopalatopharyngoplasty may be a useful strategy for patients who have OSA as a result of anatomic compromise at the level of the velopharynx.28,29 Similarly, implantable hypoglossal nerve stimulators are effective in a subset of patients with OSA, although there are substantial barriers to this modality including cost, the need for multiple invasive procedures, and the inability to accurately predict responders.30–32 On the other hand, surgical interventions may ineffective in patients who primarily have an issue with unstable ventilatory control.33 Another example is the use of oxygen or acetazolamide, which are 2 strategies to lower loop gain and have been shown to be effective in a subset of patients with OSA.34 Similarly, nonmyorelaxant hypnotic agents (eg, trazodone or eszopiclone) have been shown to increase the arousal threshold and may be useful in reducing sleep-disordered breathing events in select patients with a low arousal threshold predisposing to OSA.35,36 In contrast, hypnotics may be ineffective or even deleterious in patients who have other underlying OSA mechanisms. Combination interventions have also been tried with some success to treat OSA in patients with multiple endotypes.25
DETERMINING THE OBSTRUCTIVE SLEEP APNEA ENDOTYPE
The challenge of determining the mechanisms underlying OSA has been the subject of extensive investigation.37 Traditional assessment of the OSA endotype required multiple overnight experiments conducted by an MD or PhD to assess patients’ physiology and to determine the magnitude of the variables (eg, pharyngeal collapsibility, loop gain, arousal threshold). However, recent emphasis has been placed on making these techniques more accessible to clinicians such that pathophysiologic traits could be estimated without the need for complex experiments. Several approaches have been used. First, Sands and colleagues38 have recently used signal processing techniques to analyze standard polysomnography to make estimates of the various endotypes underlying OSA.39 Orr and colleagues40 have extended some of these findings to allow the assessment of some traits using home sleep testing. Further work will be required to validate these techniques and to determine their clinical utility. Second, Edwards and colleagues20 have developed a regression equation to determine the arousal threshold using variables derived from standard polysomnography. For example, using the apnea-hypopnea index (AHI), the nadir saturation, and the presence of hypopneas and apneas, the investigators were able to define greater than 60% of the variance in the arousal threshold. Such efforts may allow the clinician to classify patients without the need for sophisticated physiologic testing that may be burdensome and limit willingness to participate in the diagnostic process. Third, technological innovations are allowing acquisition of data using wearable technologies or using blood biomarkers to help to draw conclusions. For example, technologies are being developed to assess sleep stages and oxygen levels in individuals, which may allow the assessment of pathophysiologic traits. Shin and colleagues have also reported in abstract form the ability to predict the arousal threshold using serum biomarkers such as high-sensitivity C-reactive protein and fasting blood glucose. Ongoing metabolomics research may further these efforts as well.41 More work is required, but the realization that OSA is more than just an anatomic disease has opened the door to a personalized medicine approach to sleep apnea treatment.
OBSTRUCTIVE SLEEP APNEA PHENOTYPES
Although endotypes have been extensively studied, the variable OSA phenotypes have received less attention. Phenotypes have been described using various techniques. For example, Ye and colleagues6 performed cluster analyses on large cohorts and found that patients with OSA have varying symptoms. Some have daytime sleepiness, others have difficulty sleeping at night, and there are those who remain relatively asymptomatic. These observations have been extended to other cohorts that have also demonstrated that symptoms change with CPAP treatment according to the original cluster.6,42,43 Zinchuk and colleagues44 also used cluster analysis and were able to risk stratify patients with OSA more effectively based on polysomnographic variables than with the AHI alone. The realization that not all patients with OSA are at risk of the same complications has several implications. First, further mechanistic research is imperative to determine why some patients with OSA get some complications, whereas others seem relatively protected. Secondly, with respect to clinical trials, a “one size fits all” strategy of trying to prevent a partiular OSA consequence (eg, cardiovascular disease) by giving all patients with OSA nasal CPAP may be ineffective if only a subset is at risk. Finally, these findings also have clinical implications because interventions may be tailored to reduce the risk of a particular complication in a patient with a specific predisposition.
SUMMARY: WHY ENDOPHENOTYPES MATTER
There are several reasons why understanding OSA mechanisms on an individualized basis may facilitate a personalized medicine approach to therapy:
Endotype predicts phenotype. As stated, the mechanisms underlying OSA are highly variable as are the disease manifestations. In theory, OSA from a particular underlying mechanism may have different disease consequences than OSA caused by a different mechanism. As one example, OSA in the elderly seems to be more a function of upper airway anatomy and less an issue with loop gain as compared with younger patients with OSA.45–47 The authors and other investigators have hypothesized that the mechanisms underlying OSA in the elderly may explain the lack of observed complications in these patients in some large epidemiologic cohorts.48,49 On the other hand, younger patients with OSA tend to have higher loop gain, which may increase risk of cardiovascular disease, as large, negative pleural pressure excursions can adversely affect cardiac loading conditions and ventricular wall stress.
Endotype determines response to therapy. Nasal CPAP therapy is highly efficacious but not always well tolerated. Stanchina and colleagues50 observed that patients with OSA with high loop gain might be at risk of developing treatment-emergent central apneas on therapy (ie, complex OSA) and thus may not tolerate CPAP as well as other patients with OSA.51 Such findings have implications for individual patient management, because patients likely to have an inadequate response to CPAP would ideally be identified a priori such that other interventions could be considered. Another example, which the authors and other investigators have recently observed, is that patients with unstable ventilatory control (high loop gain) are more likely to fail upper airway surgery with OSA as compared with matched individuals with low loop gain.33,52 These findings may be helpful to stratify which patients are likely to benefit (or not) from a particular surgical intervention.
Diseases associated with OSA may predispose based on specific endotypes. Several studies have suggested that patients with posttraumatic stress disorder are at risk of OSA although the mechanisms are unclear.53,54 Based on available data, the authors have speculated that such individuals may have a low arousal threshold and thus may be at risk of developing OSA on this basis. Similarly, certain patients with neuromuscular disease are known to be at risk of OSA, perhaps due to impairment in upper airway dilator muscle function.
Endotype may define individualized interventional strategies. The use of oxygen and/or hypnotics is likely to be beneficial for some patients with OSA but not for others, depending on underlying mechanisms.
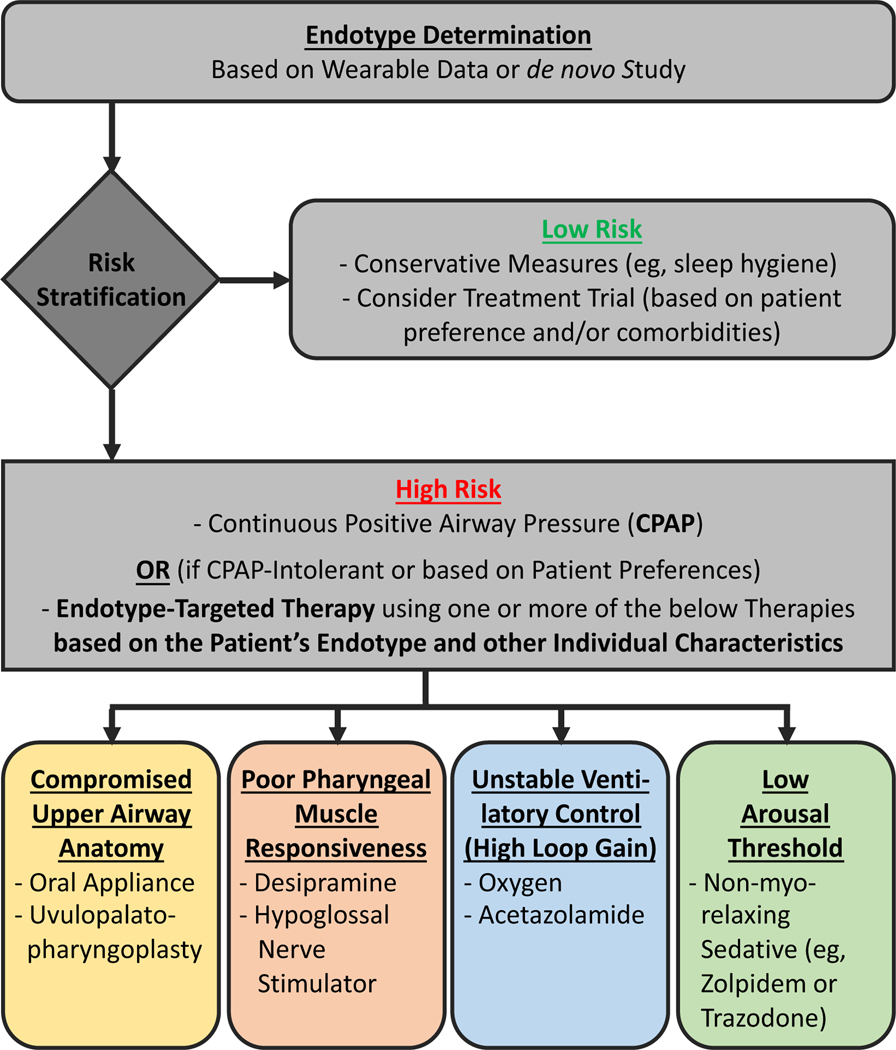
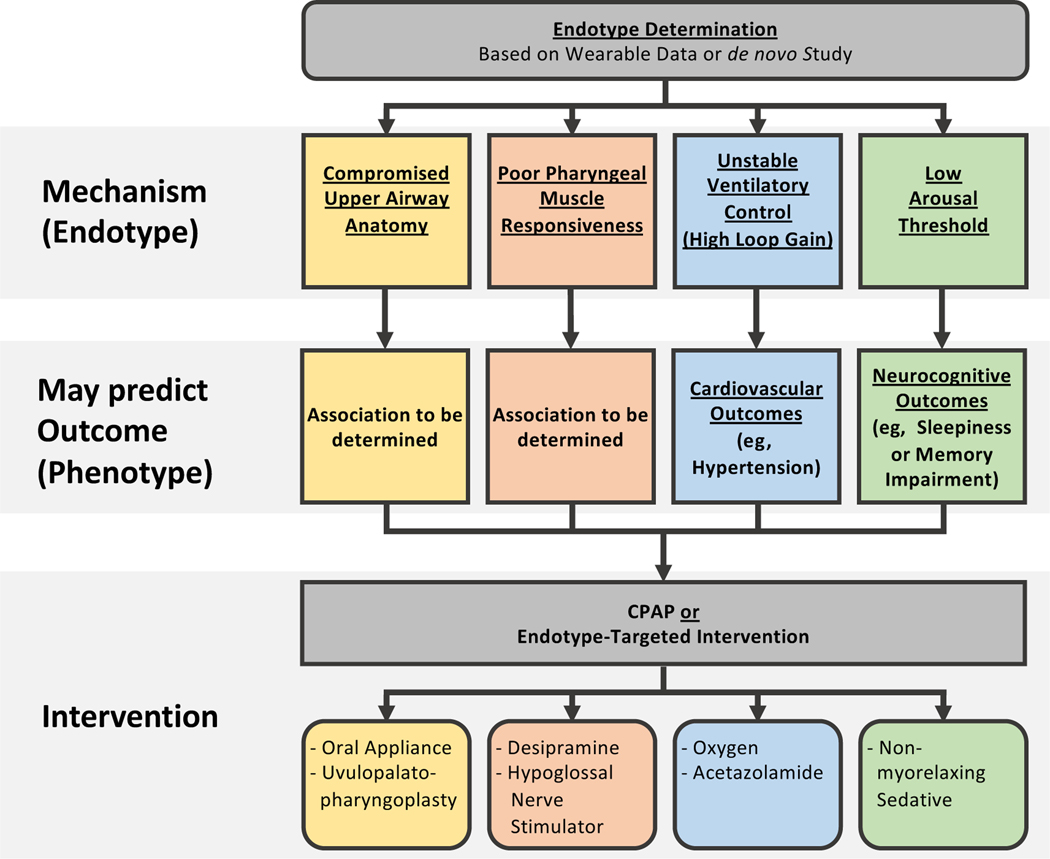
Based on the conceptual framework discussed earlier, the authors believe that OSA endophenotypes have essential implications for advancing OSA management. They may soon be used in the clinic to deliver a precision medicine-style approach to patient care (Fig. 1), and ultimately they may drive the future success of OSA clinical trials such that efforts will focus on high-risk patients who are most likely to benefit from a particular intervention (Fig. 2).55
Fig. 1.
Personalized medicine approach in clinical practice based on endotypes. Determination of an individual’s endotype could be used for risk stratification to help decide whether (aggressive) treatment is indicated. In highrisk patients who are CPAP intolerant, this information could further be used to tailor therapy toward the individual’s underlying sleep apnea causes. Note that in a given individual sleep apnea may potentially be due to multiple traits, thus requiring combination therapy; for example, a patient may have sleep apnea due to both high loop gain and low arousal threshold and may thus require both oxygen and a sedative in order to achieve stable breathing at night.
Fig. 2.
Personalized medicine approach in clinical trial design based on endotypes. As discussed in the text, the authors speculate that an individual’s sleep apnea endotype may predict which adverse health outcomes this person is at risk for; it certainly is predictive of which interventions other than CPAP may be efficacious. In this para-digm, a trial seeking to improve hypertension would maximize its power (ie, chance of success) by enrolling primarily patients with OSA due to high loop gain using CPAP (or potentially oxygen) as the intervention. Note that in a given individual sleep apnea may be due to more than one trait, thus increasing the risk of several adverse health outcomes; for example, a patient with OSA from high loop gain and low arousal threshold may be at risk of both hypertension and impaired memory and thus be a good candidate for trials focusing on either outcome.
SUMMARY AND FUTURE DIRECTIONS
New insights into OSA have opened the door to personalized medicine whereby individual patient characteristics can help guide management. However, many questions remain unanswered:
How should endotype be best determined in the clinic and should these assessments change management? Ultimately the answer to this question will likely involve sophisticated analyses of physiologic signals (eg, from polysomnogram, home sleep testing, or wearable technology) combined with plasma biomarkers.
Should patients with OSA known to be at risk of various disease complications be treated in an individualized manner, for example, should an otherwise healthy patient with OSA predicted to be at high risk of OSA-induced cardiovascular complications receive statin therapy and aggressive lifestyle modification? Such decisions may be guided by underlying OSA mechanisms and by risk stratification using novel techniques.
How should new biomarkers be best discovered in the OSA field? Advances have been made using exosomes, microRNAs and metabolomics/lipidomics that have allowed discovery of new potential causal pathways to disease.56,57 Recent insights into the microbiome have added the possibility that OSA affects gut bacteria, which in turn alter the associated metabolites important in the pathogenesis of atherosclerosis.58
How can positive airway pressure (PAP) adherence be best optimized using new technology? Recent publications have shown potential benefits to patient engagement and interactive educational devices.59,60 Our clinical experience suggests that ultimately different patients will respond to varying strategies, for example, intensive support versus real-time patient feedback versus monetary incentives or other approaches.61,62
How will wearable technology affect OSA diagnosis and treatment in the future? Technological advances may well allow OSA diagnosis and some sleep assessment to occur on an ongoing basis. Such data may be helpful diagnostically and may motivate behavioral changes such as improving sleep hygiene, and increasing PAP use. As the capability of wearable devices improve, the 3 pillars of health (diet, exercise, and sleep) could be managed more comprehensively. Big Data approaches derived from wearable devices will allow more robust conclusions over time.
OSA is an exciting field due to the tremendous progress that has been made in our understanding over recent years. The future looks bright because patients are yet to fully realize the benefits from fresh insights into sleep science and recent advancements in technology that are ongoing. OSA endophenotypes, an expanded array of OSA biomarkers, and a new wearable technology are 3 key elements that are currently leaping bench to bedside and are likely to have a significant impact on the substantial burden of disease-facing society.
KEY POINTS.
The mechanisms underlying obstructive sleep apnea (OSA) are highly variable given that any single factor explains only a portion of the variance in OSA occurrence.
The apnea-hypopnea index (AHI) is an imperfect metric of disease severity, because various complications are predicted by different sleep-disordered breathing variables, for example, 4% desaturation predicts hypertension, whereas 2% desaturation predicts insulin resistance. Similarly, arousal frequency predicts memory consolidation, and the duration of oxyhemoglobin saturation less than 90% predicts platelet aggregation. Thus, the optimal AHI definition may vary with the outcome of interest.
Not all patients with OSA are at risk of the same complications. Cluster analyses and clinical trials suggest that some patients with OSA are at risk of cardiovascular complications, whereas others may be at risk of neurocognitive sequelae, and still others may remain asymptomatic.
Although continuous positive airway pressure (CPAP) remains the treatment of choice for OSA, personalized medicine approaches are being used to optimize adherence to therapy. Examples include wearable technology with real-time patient feedback, which may help engage motivated patients and encourage CPAP use.
Different interventions are now being tried to treat OSA based on endotypes (mechanisms) underlying the disease. Oxygen or acetazolamide may be effective for patients with unstable ventilatory control (high loop gain), whereas palatal surgery may be effective for patients with primarily an anatomic problem at the level of the velopharynx.
Acknowledgments
The authors did not receive any funding for this work.
Footnotes
Disclosure Statement: The authors do not have any relationship with a commercial company that has direct financial interest in the subject matter or materials discussed in this article or with any company making a competing product.
REFERENCES
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383(9918): 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005;142(3):187–97. [DOI] [PubMed] [Google Scholar]
- 3.Welch K, Foster G, Ritter C, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep 2002;25:532–42. [PubMed] [Google Scholar]
- 4.Gleadhill I, Schwartz A, Wise R, et al. Upper airway collabsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991; 143:1300–3. [DOI] [PubMed] [Google Scholar]
- 5.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44(6):1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pack AI. Application of personalized, predictive, preventative, and participatory (P4) medicine to obstructive sleep apnea. A roadmap for improving care? Ann Am Thorac Soc 2016;13(9):1456–67. [DOI] [PubMed] [Google Scholar]
- 8.Haponik E, Smith P, Bohlman M, et al. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis 1983;127:221–6. [DOI] [PubMed] [Google Scholar]
- 9.Schwab R, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168:522–30. [DOI] [PubMed] [Google Scholar]
- 10.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol (1985) 1997;82(4):1319–26. [DOI] [PubMed] [Google Scholar]
- 11.Mezzanotte WS, Tangel DJ, White DP. Waking genio-glossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms). J Clin Invest 1992;89:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 1996;19(10):827–53. [DOI] [PubMed] [Google Scholar]
- 13.Tangel DJ, Mezzanotte WS, White DP. The influence of NREM sleep on the activity of the palatoglossus and levator palatinin muscles in normal men. J Appl Physiol (1985) 1995;78:689–95. [DOI] [PubMed] [Google Scholar]
- 14.Khoo M Determinants of ventilatory instability and variability. Respir Physiol 2000;122:167–82. [DOI] [PubMed] [Google Scholar]
- 15.Younes M, Ostrowski M, Thompson W, et al. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001;163(5): 1181–90. [DOI] [PubMed] [Google Scholar]
- 16.Sands SA, Mebrate Y, Edwards BA, et al. Resonance as the mechanism of daytime periodic breathing in patients with heart failure. Am J Respir Crit Care Med 2017;195(2):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudgel DW, Devadatta P, Quadri M, et al. Mechanism of sleep-induced periodic breathing in convalescing stroke patients and healthy elderly subjects. Chest 1993;104(5):1503–10. [DOI] [PubMed] [Google Scholar]
- 18.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997; 20(8):654–75. [DOI] [PubMed] [Google Scholar]
- 19.Younes M Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169(5):623–33. [DOI] [PubMed] [Google Scholar]
- 20.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2014;190(11):1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gleeson K, Zwillich CW, WHite DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 1990;142:295–300. [DOI] [PubMed] [Google Scholar]
- 22.Stanchina M, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical loading during NREM sleep. Am J Respir Crit Care Med 2002;165:945–9. [DOI] [PubMed] [Google Scholar]
- 23.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007;62(10):861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards BA, Sands SA, Owens RL, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep 2016;39(11):1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry SA, Joosten SA, Sands SA, et al. Response to a combination of oxygen and a hypnotic as treatment for obstructive sleep apnoea is predicted by a patient’s therapeutic CPAP requirement. Respirology 2017;22(6):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens RL, Edwards BA, Sands SA, et al. The classical Starling resistor model often does not predict inspiratory airflow patterns in the human upper airway. J Appl Physiol (1985) 2014;116(8):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azarbarzin A, Sands SA, Marques M, et al. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J 2018;51(2) [pii:1701419]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg 2004;130(6):659–65. [DOI] [PubMed] [Google Scholar]
- 29.Kezirian EJ. Nonresponders to pharyngeal surgery for obstructive sleep apnea: insights from druginduced sleep endoscopy. Laryngoscope 2011; 121(6):1320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strollo PJ Jr, Malhotra A. Stimulating therapy for obstructive sleep apnoea. Thorax 2016;71(10): 879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upperairway stimulation for obstructive sleep apnea. N Engl J Med 2014;370(2):139–49. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra A Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):170–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Ye J, Han D, et al. Physiology-based modeling may predict surgical treatment outcome for obstructive sleep apnea. J Clin Sleep Med 2017;13(9): 1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep 2013;36(2):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120(12):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc 2015; 12(5):758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114(7):911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sands SA, Edwards BA, Terrill PI, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018;197(9):1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J 2014;45(2): 408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr JE, Sands SA, Edwards BA, et al. Measuring loop gain via home sleep testing in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018;197(10):1353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montesi SB, Bajwa EK, Malhotra A. Biomarkers of sleep apnea. Chest 2012;142(1):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keenan BT, Kim J, Singh B, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep 2018;41(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pien GW, Ye L, Keenan BT, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort. Sleep 2018;41(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinchuk AV, Jeon S, Koo BB, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax 2018; 73(5):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med 2010;31(5):618–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep 2014; 37(7):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi M, Namba K, Tsuiki S, et al. Clinical characteristics in two subgroups of obstructive sleep apnea syndrome in the elderly: comparison between cases with elderly and middle-age onset. Chest 2010;137(6):1310–5. [DOI] [PubMed] [Google Scholar]
- 48.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleepdisordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors [see comments]. Sleep 1995;18(3):149–57. [DOI] [PubMed] [Google Scholar]
- 50.Stanchina M, Robinson K, Corrao W, et al. Clinical use of loop gain measures to determine continuous positive airway pressure efficacy in patients with complex sleep apnea. A pilot study. Ann Am Thorac Soc 2015;12(9):1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pepin JD, Woehrle H, Liu D, et al. Adherence to positive airway therapy after switching from CPAP to ASV: a Big data analysis. J Clin Sleep Med 2018; 14(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joosten SA, Leong P, Landry SA, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep 2017; 40(7). [DOI] [PubMed] [Google Scholar]
- 53.Orr JE, Smales C, Alexander TH, et al. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among veterans. J Clin Sleep Med 2017;13(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med Rev 2000;4(2):183–200. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra A, Morrell MJ, Eastwood PR. Update in respiratory sleep disorders: epilogue to a modern review series. Respirology 2018;23(1):16–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebkuchen A, Carvalho VM, Venturini G, et al. Metabolomic and lipidomic profile in men with obstructive sleep apnoea: implications for diagnosis and biomarkers of cardiovascular risk. Sci Rep 2018; 8(1):11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharjee R, Khalyfa A, Khalyfa AA, et al. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: a proof of concept study. J Clin Sleep Med 2018;14(5): 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue J, Zhou D, Poulsen O, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine-oxide. Am J Respir Cell Mol Biol 2017;57(5):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malhotra A, Crocker ME, Willes L, et al. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018;153(4):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5(2): 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoy CJ, Venelle M, Douglas NJ. Can CPAP use be improved? Am J Respir Crit Care Med 1997;155: 304A. [Google Scholar]
- 62.Hoy CJ, Vennelle M, Kingshott RN, et al. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med 1999; 159(4 Pt 1):1096–100. [DOI] [PubMed] [Google Scholar]