Abstract
Frankincense is a hard gelatinous resin exuded by Boswellia serrata. It contains a complex array of components, of which acetyl-11-keto-beta-boswellic acid (AKBA), a pentacyclic triterpenoid of the resin class, is the main active component. AKBA has a variety of physiological actions, including anti-infection, anti-tumor, and antioxidant effects. The use of AKBA for the treatment of mental diseases has been documented as early as ancient Greece. Recent studies have found that AKBA has anti-aging and other neurological effects, suggesting its potential for the treatment of neurological diseases. This review focuses on nervous system-related diseases, summarizes the functions and mechanisms of AKBA in promoting nerve repair and regeneration after injury, protecting against ischemic brain injury and aging, inhibiting neuroinflammation, ameliorating memory deficits, and alleviating neurotoxicity, as well as having anti-glioma effects and relieving brain edema. The mechanisms by which AKBA functions in different diseases and the relationships between dosage and biological effects are discussed in depth with the aim of increasing understanding of AKBA and guiding its use for the treatment of nervous system diseases.
Keywords: 3-O-Acetyl-11-keto-β-boswellic acid, Nervous system diseases, Biological activity, Therapeutic mechanism, Application potential
Introduction
Plants have long been used for the treatment of human disease and are important sources for drug development (Koehn & Carter, 2005; Liu et al., 2018). Frankincense is a hard gelatinous resin exuded from the olive plant Boswellia serrata (Niphadkar & Rathod, 2017). The chemical constituents of frankincense are complex and diverse, and are mainly divided into three categories: resin, gum, and volatile oil. In China, frankincense is often used in traditional Chinese medicine (Hou et al., 2014); as it is able to activate blood circulation to relieve pain, soothe tendons, and promote detumescence, it is commonly used to treat bruises, rheumatism, rheumatoid arthritis, and osteoarthritis. The pentacyclic triterpenoid compound 3-acetyl-11-keto-β-boswellic acid (AKBA), is the main active component of frankincense (Weber et al., 2006). Currently, several animal and clinical trials have confirmed that AKBA from frankincense extract possesses a variety of pharmacological activities, including anti-inflammatory (Wang et al., 2018), anti-infection (Raja et al., 2011a, 2011b), anti-tumor (Gerbeth et al., 2011), antioxidant (Han et al., 2019), and anti-aging (Bishnoi et al., 2005) actions, as well as having biological activity in the nervous system (Hamidpour et al., 2013). AKBA has been shown to modulate multiple signaling pathways, including the NF-κB (Park et al., 2011; Xiong et al., 2019), Nrf2/HO-1 (Minj et al., 2021a, 2021b), and ERK pathways (Jiang et al., 2018). AKBA can also inhibit 5-lipoxygenase (5-LOX) (Gilbert et al., 2020), leukocyte esterase (Rall et al., 1996) and TNF-α (Al-Dhubiab et al., 2020), which are key enzymes in leukotriene synthesis. In terms of anti-infection, AKBA provides resistance to bacterial infection by inhibiting biofilm formation (Raja et al., 2011a, 2011b) and has been found to be effective against the SARS-CoV-2 virus through its ability to bind functional proteins of the virus (Caliebe et al., 2021). In vitro experiments have shown that AKBA is also active against Leishmania donovani (Greve et al., 2021). AKBA plays an active role in breast cancer (Bini Araba et al., 2021; Schmiech et al., 2021), non-small-cell lung cancer (Lv et al., 2021), and gastric cancer (Sun et al., 2020) by inducing apoptosis. Additionally, AKBA attenuates oxidative stress through the TGF-β1/Smad3 pathway (Shang et al., 2016). In terms of the nervous system, frankincense has been used for the treatment of mental illnesses since the time of ancient Greece (Laios et al., 2019). The anti-5-LOX activity of AKBA has been found to be beneficial in the treatment of age-related neurodegenerative diseases (Qu et al., 2000). AKBA can also inhibit glioma progression by inhibiting autophagy (Li et al., 2020), cell cycle arrest (Li et al., 2018), or the inhibition of factors regulating cell death (Conti et al., 2018).
Although current research has confirmed the diverse biological functions of frankincense, there are few comprehensive reviews on AKBA. AKBA clearly has complex mechanisms of action, and we believe that AKBA has great potential for the treatment of neurological diseases. Here, we have analyzed various studies and summarize recent research on the biological activities of AKBA in the nervous system, to extend our understanding of the compound and its potential application for the treatment of nervous system diseases.
Actions of AKBA in the Nervous System
Promotion of Nerve Injury Repair and Nerve Regeneration
Schwann cells, the primary glial cells of the peripheral nervous system, contribute to the microenvironment for peripheral nerve regeneration through their secretion of neurotrophic factors, adhesion molecules, and extracellular matrix components (Cattin et al., 2015; Han et al., 2017). Axonal damage results in the activation of extracellular regulated protein kinase (ERK) signaling pathways in Schwann cells both at the injury site and distal to it, which, in turn, promote nerve regeneration by the production of neurotrophic factors (Webber & Zochodne, 2010). In vitro experiments have shown that ERK pathway activation-induced dedifferentiation of Schwann cells in myelinated nerve fibers (Harrisingh et al., 2004), with the dedifferentiated Schwann cells transforming to myelin-associated progenitor-like cells that are able to differentiate and grow, resulting in regeneration of the nerve (Clements et al., 2017; Lopez-Verrilli et al., 2013). Jiang et al. developed a sciatic nerve-crush injury model in rats, followed by the intraperitoneal injection of 1.5, 3, and 6 mg/kg AKBA every three days. The results showed that AKBA increased the expression of pERK1/2 in Schwann cells and promoted nerve repair. In addition, AKBA regulated the proliferation and myelination of Schwann cells by increasing ERK phosphorylation, which ultimately promoted the sciatic nerve repair (Jiang et al., 2020).
Neuroprotective Effects in Ischemic Brain Injury
Oxidative and cytotoxic injury plays an important role in the pathogenesis of cerebral ischemia, and targeting these processes may have potential for treating cerebral ischemia. Transcription factor erythroid-derived nuclear factor-related factor-2 (Nrf2) acts as a free radical scavenger to maintain redox homeostasis. Upregulation of Nrf2 and HO-1 expression protects the brain from injury caused by middle cerebral artery occlusion and middle cerebral artery occlusion (MCAO) resulting from ischemia reperfusion. Thus, Nrf2/HO-1 may be a key target for the treatment of cerebral ischemia (Yang et al., 2009).
In a cerebral ischemia model constructed using primary neuronal glucose and oxygen deprivation (OGD), AKBA increased the expression of Nrf2 and HO-1 and protected against OGD-induced oxidative damage. In a rat MCAO model of cerebral ischemia simulated by reperfusion, AKBA significantly reduced the cerebral infarction area and the numbers of apoptotic nerve cells and increased the National Institute of Health stroke scale’s scores. All these results indicate that AKBA plays a neuroprotective role in ischemic brain injury by regulating Nrf2/HO-1 (Ding et al., 2014).
The pathogenesis of ischemic brain injury also involves pathways associated with oxidative stress. AKBA-loaded O-carboxymethyl chitosan nanoparticles (AKBA-NP) have been shown to exert effective antioxidant and oxidative effects by increasing Nrf2/HO-1 and reducing the expression of NF-κB and 5-LOX compared with AKBA administration alone, indicating that AKBA-NPs are an effective drug delivery system in the treatment of cerebral ischemia (Ding et al., 2016). As a 5-LOX inhibitor, AKBA combined with cyclooxygenase-2 (COX-2) inhibitors was found to alleviate kainate-induced excitatory neurotoxicity as well as oxidative brain injury through antioxidant activity (Bishnoi et al., 2007).
Mitigation of Brain Aging
COX-2 and 5-LOX are two enzymes involved in the oxidation of arachidonic acid. In the central nervous system, their expression increases during aging, and have been associated with Alzheimer’s disease, an age-related disease (Fujimi et al., 2007; Qu et al., 2000). Administration of 100 mg/kg of AKBA and 2.42 mg/kg of the COX-2 inhibitor Nimesulide for 15 days was found to reverse age-induced memory loss in mice.
The combination of AKBA and Nimesuride also reduced oxidative damage caused by aging. Although AKBA alone could reduce oxidative damage, the effect was more remarkable when it was administered together with Nimesuride. These findings indicate that AKBA could mitigate the effects of aging and reduce the development of age-related brain diseases (Bishnoi et al., 2005). Moreover, in vitro experiments have shown that AKBA has proangiogenic properties (Bertocchi et al., 2018), suggesting that AKBA may have potential in counteracting capillary damage leading to the functional impairment of the aging brain (Wang et al., 2004).
Inhibitory Effects of Neurological Inflammation
Neurological inflammation is involved in the pathogenesis of neurodegenerative diseases, which can cause dysfunction of cognitive and behavioral (Sayed & El Sayed, 2016). Studies have shown that 5-LOX is a key enzyme involved in the biosynthesis of the inflammatory mediator leukotriene Gilbert et al. found that inhibition of 5-LOX by AKBA reduced inflammation (Gilbert et al., 2020). AKBA was found to inhibit the expression of inflammatory cytokines, such as TNF-α, IL-1, IL-2, IL-6, INF-γ, ICAM-1, and C3aR. AKBA also suppressed activation of the complement system by blocking the transformation of C3 to C3a and C3b (Ahmad et al., 2019; Ammon, 2016). Lipopolysaccharide (LPS) is a potent pro-inflammatory factor that causes cognitive dysfunction. Sayed et al. demonstrated the anti-inflammatory and neuroprotective effects of AKBA in LPS-mediated neuroinflammation model in mice using. Y-Maze experiments to measure the effects on behavior. The authors found that after seven days of AKBA (5 mg/kg) administration to LPS (0.8 mg/kg)-treated mice the time spent by the mice in the novel arm of the Y-Maze increased. This was associated with inhibition of the NF-κB pro-inflammatory pathway through the degradation of IκB-α, reversing the behavioral disorders of the mice induced by LPS-mediated neuroinflammation (Sayed et al., 2018; Syrovets et al., 2005).
Amelioration of Memory Impairment
The hippocampus is a sensitive area of the brain involved in learning and memory functions (Lisman et al., 2017). Studies have shown that rats fed with frankincense during pregnancy produced offspring with more dendritic branches in the pyramidal neurons in the CA3 region of the hippocampus and better learning and memory abilities (Hamidpour et al., 2013), indicating that frankincense intervention during pregnancy could improve the memory and intelligence of the offspring. Frankincense has also been found to improve learning and, especially, memory in the elderly (Hosseini et al., 2010). It has been demonstrated that frankincense is able to improve memory by up-regulating the expression of brain derived neurotrophic factor (BDNF) in the hippocampus (Khalaj-Kondori et al., 2016; Yuan et al., 2010). Experiments by Gunasekaran et al. using scopolamine-induced dementia rat models, the animals were treated with AKBA (5, 10, and 15 mg/kg, ip) and donepezil (2.5 mg/kg, ip). The results suggested that AKBA significantly reversed scopolamine-induced memory impairment, and, furthermore, that AKBA reduced acetylcholinesterase activity without affecting the GABA- and glutamate-mediated neuronal excitability. These findings suggest that AKBA could alleviate dementia through anti-cholinesterase activity and preservation of cholinergic function (Gunasekaran et al., 2021a, 2021b).
Additionally, AKBA improved the memory impairment caused by the inflammatory injury of the nervous system after LPS administration. In a study (Marefati et al., 2020), 40 male rats were divided into four groups: control (DMSO + saline), LPS (1 mg/kg), LPS + AKBA (5 mg/kg) and LPS + AKBA (10 mg/kg) to conduct Morris water maze and passive avoidance response tests. Compared with the LPS-treated rats, elapsed time and traveled distance in the target quarter of the Morris water maze were found to be prolonged in the AKBA-treated group. The passive avoidance response test found that the AKBA-treated rats spent more time in the light rooms with less time in the dark compartments than LPS-treated rats, and that movement from the bright compartment to the dark chamber was significantly decreased. In conclusion, compared with the LPS group, the results of Morris Water Maze test and passive avoidance response test showed that the memory functions of the AKBA-treated had improved significantly (Marefati et al., 2020).
Alleviation of Neurotoxicity
Excessive amounts of glutamic acid (Glu), a neurotransmitter in the central nervous system, have been shown to lead to neuronal dysfunction and degeneration (Lau & Tymianski, 2010). Rajabian et al. showed that AKBA played a protective role in Glu-induced neuronal injury. PC12 and N2a neuronal cells were pretreated with AKBA (2.5–10 µM) before inducing injury by excess Glu administration. The results showed increased cell death in both cell lines after incubation with Glu for 24 h, while AKBA pretreatment increased the cell viability (Rajabian et al., 2020). AKBA has also been found to reverse glutamate abnormalities caused by chronic unpredictable mild stress through influencing the central hypothalamic–pituitary–adrenal axis (Gunasekaran et al., 2021a, 2021b). AKBA was also able to alleviate the toxic effects of Glu, namely, increased levels of intracellular reactive oxygen species (ROS) (Cattin et al.), and lipid peroxidation, by increasing superoxide dismutase activity and reducing oxidative DNA damage (Rajabian et al., 2016).
Up-regulation of the 5-LOX and COX enzymes which catalyze the incorporation of arachidonic acid into prostaglandins and leukotrienes renders neurons more susceptible to degeneration in the central nervous system (Bishnoi et al., 2007; Tuncer & Banerjee, 2015). Andis Klegeris et al. developed in vitro testing assays to detect neurotoxicity in microglia and other mononuclear macrophages. As an inhibitor of 5-LOX, AKBA may be considered a possible neuroprotectant that reduces toxicity to the microglia/macrophages (Klegeris & McGeer, 2002).
Anti-glioma Effects
Recent studies have focused on the anti-tumor actions of AKBA (Pillai et al., 2021). Glioma is one of the most common primary malignancies of the central nervous system, accounting for 12% to 15% of all types of brain tumors (Li et al., 2018, 2020). Animal experiments demonstrated that AKBA (100 mg/kg) could improve the metabolism of mice with glioblastoma in the U87-MG glioma orthotopic model. Western blotting has shown that AKBA reduced the expression of ATG5, p62, LC3B, p-ERK/ERK, and p53 but increased the phosphorylation of mTOR in mice with glioblastoma. These results suggested that the anti-glioblastoma effect of AKBA could be realized by treating metabolic dysfunction in tumor cells and inhibiting autophagy. Consequently, AKBA can inhibit the glioma growth by modulation of the ERK signaling pathway and p53 protein to inhibit autophagy (Li et al., 2020). Abnormal activation of the NF-κB signaling pathway has been shown to lead to glioma, but not glioblastoma, progression (Puliyappadamba et al., 2014). Therapeutic effects of AKBA (10 μM, 20 μM, 30 μM, 40 μM) in combination with radiation have been observed in glioma (GBM subcutaneous tumor model), indicating that AKBA has potential anti-tumor effects and also enhances the effects of radiation. An ectopic glioblastoma model was also used to estimate the effects of combining AKBA and radiation, observing that the combined therapy had a stronger inhibitory effect than AKBA or radiation alone (Conti et al., 2018). Moreover, AKBA inhibited the proliferation, migration, invasion, and colony formation in U251 and U87-MG glioblastoma cells through the release of lactic dehydrogenase and reducing DNA synthesis. These results are consistent with the proposed mechanism that AKBA inhibits glioblastoma proliferation by arresting the cell cycle in the G2/M phase, suggesting that AKBA might have potential application as a chemotherapeutic treatment for glioblastoma (Li et al., 2018).
Remission of Brain Edema
In 2002, frankincense extract was classified by the European Medicines Agency as an “orphan drug” for the treatment of brain edema resulting from brain tumors (Gerbeth et al., 2013). The anti-inflammatory function of AKBA is known to play a positive role in the treatment of brain edema (Di Pierro et al., 2019). Peritumoral edema is one of the main causes of neuro-related diseases in patients with brain tumors, and dexamethasone is the drug of choice for reducing peritumoral edema associated with primary and secondary brain tumors (Gerbeth et al., 2011). However, there are many side effects, and the hormonal medicine has limited curative effect (Uomoto & Brockway, 1992). Patients with a brain tumor who have received radiation therapy usually suffer from the clinical symptom of brain edema; although dexamethasone is widely used for this symptom, it has numerous side effects and limited therapeutic effect. A randomized cohort study by Simon et al. published in Cancer randomly divided 44 patients with malignant brain tumors into several groups: control (radiotherapy + placebo), radiotherapy + AKBA (BS 4200 mg/die). The area of brain edema was measured immediately and the results showed reduction in the area of more than 75% in 60% of patients treated with AKBA combined with radiotherapy, which was significantly superior to the placebo control group (Kirste et al., 2011).
Another randomized controlled trial published on J Pharm Biomed Anal conducted a double-blind clinical trial on 14 patients with high dose of 4200 mg BS per day and 13 patients with placebo. After monitoring the boswellic acid levels in the serum with HPLC–MS, the results suggested that boswellic acids are promising treatments for peritumoral edema (Gerbeth et al., 2011). Twenty patients with glioblastoma multiforme were treated with surgery, radiotherapy and chemotherapy, and temozolomide. Patients were treated with 4500 mg/die Monoselect AKBA™ while receiving radiation therapy, and brain edema was assessed at 4, 12, 22, and 34 weeks after surgery. Monoselect AKBA™ complementary therapy significantly reduced the radiochemotherapy-induced cerebral edema (Di Pierro et al., 2019).
Discussion
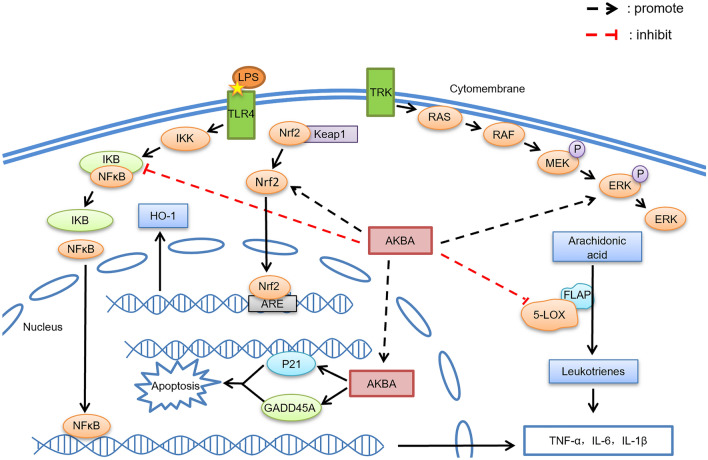
Figure 1 summarizes the main signaling pathways influenced by AKBA, indicating its likely mechanisms of action. Moreover, we have summarized and collated the roles and related mechanisms of AKBA in different diseases (Table 1).
Fig. 1.
The major central nervous system signaling pathways influenced by AKBA. Inhibition of 5-LOX to prevent the formation of leukotrienes. 2. Inhibition of the NF-κB signaling pathway. 3. Promotion of ERK pathway phosphorylation. 4. Cell cycle arrest at G2/M to induce cell apoptosis. 5. Upregulation of the expression of Nrf2 and HO-1
Table 1.
Action mechanisms of AKBA in various nervous system diseases
| The disease/symptom | The effect of AKBA | Action mechanism | The dose of AKBA |
|---|---|---|---|
| Alzheimer's disease | Anti-inflammation | Inhibits 5-LOX to slow down aging; | 100 mg/kg Bishnoi et al. (2005) |
| Inhibits the NF-κB inflammatory signaling pathway | 100 mg/kg Wei et al. (2020) | ||
| Inhibit oxidative damage | Upregulate the expression of Nrf2 and HO-1 | 5 mg/kg Wei et al. (2020) | |
| Reduce the neurotoxicity | Play a role in the neuron injury induced by glutamate | 10 μM Rajabian et al. (2016) | |
| Cerebral ischemia | Anti-inflammation | Inhibits 5- LOX; | 10 mg/kg Ding et al. (2016) |
| Inhibits the NF-κB inflammatory signaling pathway | 10 mg/kg Ding et al. (2016) | ||
| Inhibit oxidative damage | Upregulate the expression of Nrf2 and HO-1 | 20 mg/kg Ding et al. (2014) | |
| Reduce the neurotoxicity | Play a role in the neuron injury induced by glutamate | 10 μM Rajabian et al. (2016) | |
| Neurotoxicity and oxidative damage | Anti-neurotoxicity | Inhibits 5-LOX | 100 mg/kg Bishnoi and et al. (2007) |
| Glioma | Anti-tumor | Inhibits the NF-κB inflammatory signaling pathway | 30-50 μM Conti et al. (2018) |
| Glioblastoma | Inhibit glioblastoma proliferation | Arrest the cell cycle in G2/M | 100 mg/kg Li et al. (2018) |
| Dysfunction of cerebral endothelial cells after glycosyl-oxygen stripping | Anti-inflammation | Reduce inflammation factors and protein expression level | 20 μM Ahmad et al., (2019) |
| Behavioral disorders caused by inflammation of the nervous system | Anti-inflammation | Inhibits the NF-κB inflammatory signaling pathway | 5 mg/kg Sayed et al. (2018) |
| Improve learning and memory disorders | Adjust inflammatory responses | Establish a balance between inflammatory and pro-inflammatory factors | 5 or 10 mg/kg Marefati et al. (2020) |
| Nerve injury repair and nerve regeneration | Promote Schwann cell proliferation and myelination | Enhance the phosphorylation level of the ERK signaling pathway | 10 mg/kg Jiang et al. (2020) |
| Amyotrophic lateral sclerosis | Inhibit oxidative damage | Upregulate the expression of Nrf2 and HO-1 | 100 mg/kg Minj and et al. (2021a, 2021b) |
The application of AKBA in vitro experiments is in units of mg/kg; in vivo experiments is in units of μM
Although AKBA is known to reduce oxidative damage, this action appears to require different doses in different diseases; this may be related to varying degrees of cell damage in each disease. For example, in vitro experiments in rats with Alzheimer’s disease, cerebral ischemia, and amyotrophic lateral sclerosis, the doses of AKBA were 5 mg/kg, 20 mg/kg, and 100 mg/kg, respectively. Therefore, we speculate that the reduction in oxidative damage by AKBA is dose-dependent, suggesting that this should be further investigated and verified.
Coincidentally, in terms of different diseases different doses of AKBA also appear to be important in the inhibition of inflammation, for example, in the in vitro trials of behavioral disorders caused by inflammation of the nervous system, cerebral ischemia, and Alzheimer’s disease, the doses of AKBA used were 5 mg/kg, 10 mg/kg, and 100 mg/kg, respectively, with all doses having similar effects.
However, it is possible that AKBA may have similar overall effects resulting from different mechanisms. For example, in Alzheimer’s disease, AKBA can both inhibit 5-LOX to slow down aging and inhibit the NF-κB signaling pathway at doses of 100 mg/kg, with similar effects seen in cerebral ischemic diseases. This suggests that AKBA has similar dosage effects in targeting specific diseases even if the pathway mechanisms are different.
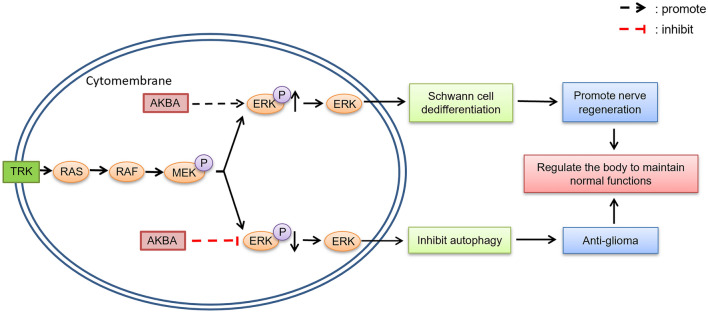
ERK1/2 activity and its duration of action are critical for cell functioning (Subramaniam & Unsicker, 2010). The activation of the ERK pathway can both promote the growth of gliomas (Jin et al., 2019), inhibit osteoclast formation (Shi et al., 2021) and play an important role in the regeneration of peripheral nerves (Hausott & Klimaschewski, 2019). AKBA has been found to play an active role in repairing nerve injury and anti-glioma action by regulating the expression of the p-ERK protein. However, the specific mechanism of action and the reasons for the different effects need further investigation. Comparing the role of AKBA in the of different treatment diseases revealed that AKBA increased the expression of pERK1/2 in Schwann cells and thus promoted nerve injury repair (Jiang et al., 2018), while AKBA reduced the expression of phosphorylated p-ERK/ERK protein in its anti-tumor actions (Li et al., 2020) and inhibited bone loss (Shi et al., 2021). Here, we propose the hypothesis that this could have been due to different modes of action of AKBA in different microenvironments, producing different effects on ERK; however, the results of its action are to regulate homeostasis to maintain the homeostasis of normal function and life activities (Fig. 2).
Fig. 2.
Different roles of AKBA in the ERK signaling pathway
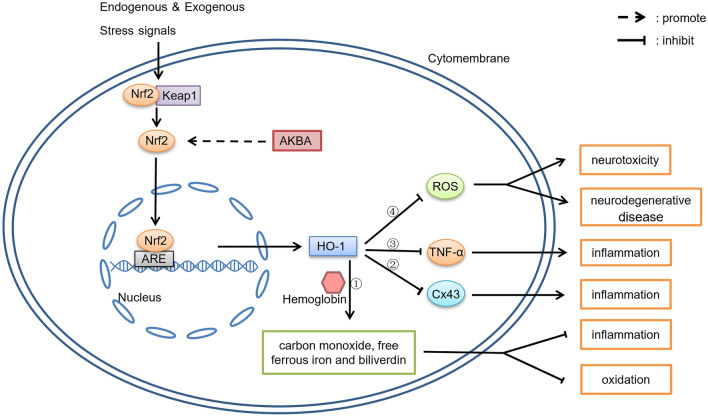
The regulatory action of AKBA on the Nrf2/HO-1 signaling pathway is summarized in Fig. 3. We hypothesize that AKBA inhibits the inflammatory signaling pathway by means of the Nrf2/HO-1 axis.
Fig. 3.
The regulatory action of AKBA on the Nrf2/HO-1 signaling pathway. 1. HO-1 catalyzes the degradation of hemoglobin to carbon monoxide, free ferrous iron and biliverdin, subsequently degrading biliverdin to bilirubin (Fig. 3.①); these products are considered to have an important role in anti-oxidation and anti-inflammation. 2. HO-1 inhibits the production of Cx43 (Fig. 3.②) and TNF-α (Fig. 3.③) to reduce the inflammatory response. 3. ROS is one of the major leading factors in the development and progression of many cerebrovascular and neurodegenerative diseases, HO-1 inhibits ROS (Fig. 3.④)
The Nrf2/HO-1 axis plays a major role in reducing inflammation (Ahmed et al., 2017). HO-1 is a limiting enzyme that catalyzes the degradation of hemoglobin to carbon monoxide, free ferrous iron, and biliverdin, and degrades biliverdin to bilirubin (Fig. 3.①) (Ahmed et al., 2017; Naito et al., 2014). These products are considered to have important antioxidant and anti-inflammatory actions (Abraham & Kappas, 2008). In addition, HO-1 can also inhibit the production of Cx43 (Fig. 3.②) (Zhou et al., 2020) and TNF-α (Fig. 3.③) (Yu et al., 2009) to inhibit the inflammatory response. Excess ROS levels contribute significantly to the development and progression of many cerebrovascular and neurodegenerative diseases (Fig. 3.④) (Sivandzade et al., 2019). Nrf2/HO-1 has been found to reduce ROS levels and thus inhibit apoptosis (Su et al., 2019). Therefore, AKBA can suppress the negative effects produced by ROS by activating Nrf2/HO-1.
Neuroinflammation is considered to be an important component of the pathogenesis of neurodegenerative and psychiatric disorders (Arioz et al., 2019). In diseases such as Alzheimer’s disease, cerebral ischemia, and amyotrophic lateral sclerosis, we find that AKBA can play a role in reducing oxidative damage, preventing demyelination, and promoting remyelination by up-regulating the expression of Nrf2 and HO-1 (Minj et al., 2021a, 2021b).
Since Nrf2/HO-1 inhibits inflammation while tumors promote inflammation and coupled with the activation of Nrf2/HO-1 by AKBA, we propose that AKBA can inhibit the tumor-induced inflammatory response by activating the Nrf2/HO-1 pathway (Rojo de la Vega et al., 2018). However, there is no specific evidence to confirm this conjecture. It does, however, offer a direction to explore the relationship between AKBA and Nrf2 in reducing oxidative damage, preventing demyelination, and promoting remyelination.
In a mouse model of oxygen-induced retinopathy characterized by pathological retinal angiogenesis, Matteo Lulli et al. found that AKBA had an anti-angiogenic effect and could inhibit vascular endothelial growth factor (VEGF) expression and VEGF-2 phosphorylation by inhibiting STAT3 in a dose-dependent manner, with the greatest effect at a dose of 10 mg/kg (Lulli et al., 2015). An additional study using injury to porcine aortic endothelial cells found that AKBA could promote angiogenesis at low concentrations (3.8 ng/ml) (Bertocchi et al., 2018). From this, we speculate that the promotion and inhibition of angiogenesis by AKBA is largely related to the type of lesion in the blood vessels. When the lesion includes hypervascularity, then high concentrations of AKBA have an anti-angiogenic effect, while in lesions characterized by blood vessel reduction due to damage, low concentrations of AKBA have a pro-angiogenic effect.
The hypoxic environments of tumors can lead to increased angiogenesis (Rojo de la Vega et al., 2018). Stable knockout of the Nrf2 gene has been found to reduce HIF-1α protein levels, thereby reducing the expression of VEGF and angiopoietin and, in time, inhibiting the continuous generation of blood vessels in tumors (Ji et al., 2014; Kim et al., 2011). These findings suggest that it would be fruitful to explore whether exogenous administration of low concentrations of AKBA after knockdown of the Nrf2 gene can increase vascular endothelial production to antagonize the reduction of vascular endothelium induced by knockdown of the Nrf2 gene.
Frankincense is a traditional medicine that has been used since ancient times in many countries, such as China, India, and the Middle East. As a main active ingredient in frankincense, AKBA is known to have multiple actions, including anti-inflammatory, anti-infection, and anti-tumorigenic effects. In recent years, along with the discovery of the effect of AKBA on the amelioration in cognitive deficits (Wei et al., 2020) and the inhibition of neuronal apoptosis, the biological activity and application potential of AKBA in nervous system diseases has attracted attention. In this review, we summarized the research findings of AKBA in nervous system diseases. AKBA was found to play a critical role in the promotion of nerve regeneration, reduction of brain aging, inhibition of inflammation, improvement of memory, alleviation of neurotoxicity, suppression of gliomas, and the treatment of brain edema. AKBA in combination with a COX-2 inhibitor could enhance resistance to brain aging and the repair of oxidative stress-induced neuronal injuries. In addition, the data in a comparative toxicogenomic database indicated that there was significant evidence to confirm the therapeutic effect of AKBA in memory disorders (Bishnoi et al., 2005; Niu et al., 2007; Tanaka et al., 2011), seizures (Bishnoi et al., 2007; Zhang et al., 2006), and in movement disorders (Bishnoi et al., 2005). Thus, it is apparent that AKBA has great potential as a small molecule drug in the treatment of nervous system diseases in the future although further studies are required to elucidate its mechanism of action in detail.
Acknowledgements
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation (ZJNSF) (LY20H250001), the Key Laboratory of Aging and Cancer Biology of Zhejiang Province and its open project (2020E10016), the National Natural Science Foundation of China (NSFC) (81601428), and the Research Start-Up Funds of Hangzhou Normal University (2019QDL017; 2019QDL008).
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuqing Gong, Xinyi Jiang and Suibi Yang have contributed equally.
Contributor Information
Yong Fang, Email: yong.fang@ems.hrbmu.edu.cn.
Jing Wu, Email: wu.jing@hznu.edu.cn.
References
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological Reviews. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Khan SA, Kindelin A, Mohseni T, Bhatia K, Hoda MN, et al. Acetyl-11-keto-β-boswellic acid (AKBA) attenuates oxidative stress, inflammation, complement activation and cell death in brain endothelial cells following OGD/reperfusion. Neuromolecular Medicine. 2019;21:505–516. doi: 10.1007/s12017-019-08569-z. [DOI] [PubMed] [Google Scholar]
- Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica Et Biophysica Acta, Molecular Basis of Disease. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Al-Dhubiab BE, Patel SS, Morsy MA, Duvva H, Nair AB, Deb PK, et al. The beneficial effect of Boswellic acid on bone metabolism and possible mechanisms of action in experimental osteoporosis. Nutrients. 2020;12:3186. doi: 10.3390/nu12103186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon HP. Boswellic acids and their role in chronic inflammatory diseases. Advances in Experimental Medicine and Biology. 2016;928:291–327. doi: 10.1007/978-3-319-41334-1_13. [DOI] [PubMed] [Google Scholar]
- Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Frontiers in Immunology. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchi M, Isani G, Medici F, Andreani G, Tubon Usca I, Roncada P, et al. Anti-inflammatory activity of boswellia serrata extracts: An in vitro study on porcine aortic endothelial cells. Oxidative Medicine and Cellular Longevity. 2018;2018:2504305. doi: 10.1155/2018/2504305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini Araba A, Ur Rehman N, Al-Araimi A, Al-Hashmi S, Al-Shidhani S, Csuk R, et al. New derivatives of 11-keto-β-boswellic acid (KBA) induce apoptosis in breast and prostate cancers cells. Natural Product Research. 2021;35:707–716. doi: 10.1080/14786419.2019.1593165. [DOI] [PubMed] [Google Scholar]
- Bishnoi M, Patil CS, Kumar A, Kulkarni SK. Protective effects of nimesulide (COX Inhibitor), AKBA (5-LOX Inhibitor), and their combination in aging-associated abnormalities in mice. Methods and Findings in Experimental and Clinical Pharmacology. 2005;27:465–470. doi: 10.1358/mf.2005.27.7.920929. [DOI] [PubMed] [Google Scholar]
- Bishnoi M, Patil CS, Kumar A, Kulkarni SK. Co-administration of acetyl-11-keto-beta-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology. 2007;79:34–41. doi: 10.1159/000097627. [DOI] [PubMed] [Google Scholar]
- Caliebe RH, Scior T, Ammon HPT. Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: Bioinformatic studies. Archiv Der Pharmazie. 2021;354:e2100160. doi: 10.1002/ardp.202100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, et al. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, Ashraf A, et al. The wound microenvironment reprograms schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron. 2017;96:98–114.e117. doi: 10.1016/j.neuron.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S, Vexler A, Edry-Botzer L, Kalich-Philosoph L, Corn BW, Shtraus N, et al. Combined acetyl-11-keto-β-boswellic acid and radiation treatment inhibited glioblastoma tumor cells. PLoS ONE. 2018;13:e0198627. doi: 10.1371/journal.pone.0198627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F, Simonetti G, Petruzzi A, Bertuccioli A, Botta L, Bruzzone MG, et al. A novel lecithin-based delivery form of Boswellic acids as complementary treatment of radiochemotherapy-induced cerebral edema in patients with glioblastoma multiforme: A longitudinal pilot experience. Journal of Neurosurgical Sciences. 2019;63:286–291. doi: 10.23736/s0390-5616.19.04662-9. [DOI] [PubMed] [Google Scholar]
- Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, et al. Neuroprotection by acetyl-11-keto-β-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Science and Reports. 2014;4:7002. doi: 10.1038/srep07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Qiao Y, Wang M, Zhang H, Li L, Zhang Y, et al. Enhanced neuroprotection of Acetyl-11-Keto-β-Boswellic Acid (AKBA)-loaded O-carboxymethyl chitosan nanoparticles through antioxidant and anti-inflammatory pathways. Molecular Neurobiology. 2016;53:3842–3853. doi: 10.1007/s12035-015-9333-9. [DOI] [PubMed] [Google Scholar]
- Fujimi K, Noda K, Sasaki K, Wakisaka Y, Tanizaki Y, Iida M, et al. Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer's disease: The Hisayama Study. Dementia and Geriatric Cognitive Disorders. 2007;23:423–431. doi: 10.1159/000101957. [DOI] [PubMed] [Google Scholar]
- Gerbeth K, Hüsch J, Fricker G, Werz O, Schubert-Zsilavecz M, Abdel-Tawab M. In vitro metabolism, permeation, and brain availability of six major boswellic acids from Boswellia serrata gum resins. Fitoterapia. 2013;84:99–106. doi: 10.1016/j.fitote.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Gerbeth K, Meins J, Kirste S, Momm F, Schubert-Zsilavecz M, Abdel-Tawab M. Determination of major boswellic acids in plasma by high-pressure liquid chromatography/mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2011;56:998–1005. doi: 10.1016/j.jpba.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Gilbert NC, Gerstmeier J, Schexnaydre EE, Börner F, Garscha U, Neau DB, et al. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nature Chemical Biology. 2020;16:783–790. doi: 10.1038/s41589-020-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve HL, Kaiser M, Mäser P, Schmidt TJ. Boswellic acids show in vitro activity against Leishmania donovani. Molecules. 2021;26:3651. doi: 10.3390/molecules26123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran V, Augustine A, Avarachan J, Khayum A, Ramasamy A. 3-O-Acetyl-11-keto-β-boswellic acid ameliorates chronic unpredictable mild stress induced HPA axis dysregulation in relation with glutamate/GABA aberration in depressive rats. Clinical and Experimental Pharmacology and Physiology. 2021 doi: 10.1111/1440-1681.13567. [DOI] [PubMed] [Google Scholar]
- Gunasekaran V, Avarachan J, Augustine A, Khayum A. 3-O-Acetyl-11-keto-β-boswellic acid ameliorates acquired, consolidated and recognitive memory deficits through the regulation of hippocampal PPAR γ, MMP9 and MMP2 genes in dementia model. Heliyon. 2021;7:e08523. doi: 10.1016/j.heliyon.2021.e08523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M. Frankincense (rǔ xiāng; boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. Journal of Traditional & Complementary Medicine. 2013;3:221–226. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhao JY, Wang WT, Li ZW, He AP, Song XY. Cdc42 promotes schwann cell proliferation and migration through Wnt/β-catenin and p38 MAPK signaling pathway after sciatic nerve injury. Neurochemical Research. 2017;42:1317–1324. doi: 10.1007/s11064-017-2175-2. [DOI] [PubMed] [Google Scholar]
- Han L, Xia Q, Zhang L, Zhang X, Li X, Zhang S, et al. Induction of developmental toxicity and cardiotoxicity in zebrafish embryos/larvae by acetyl-11-keto-β-boswellic acid (AKBA) through oxidative stress. Drug and Chemical Toxicology. 2019 doi: 10.1080/01480545.2019.1663865. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO Journal. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B, Klimaschewski L. Promotion of peripheral nerve regeneration by stimulation of the Extracellular Signal-Regulated Kinase (ERK) pathway. Anatomical Record. 2019;302:1261–1267. doi: 10.1002/ar.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini M, Hadjzadeh MA, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Archives of Pharmacal Research. 2010;33:463–468. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- Hou Q, He WJ, Hao HJ, Han QW, Chen L, Dong L, et al. The four-herb Chinese medicine ANBP enhances wound healing and inhibits scar formation via bidirectional regulation of transformation growth factor pathway. PLoS ONE. 2014;9:e112274. doi: 10.1371/journal.pone.0112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Wang H, Zhu J, Zhu L, Pan H, Li W, et al. Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. International Journal of Cancer. 2014;135:574–584. doi: 10.1002/ijc.28699. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang Y, Zhang B, Fei X, Guo X, Jia Y, et al. Acetyl-11-keto-β-boswellic acid regulates the repair of rat sciatic nerve injury by promoting the proliferation of Schwann cells. Life Sciences. 2020;254:116887. doi: 10.1016/j.lfs.2019.116887. [DOI] [PubMed] [Google Scholar]
- Jiang XW, Zhang BQ, Qiao L, Liu L, Wang XW, Yu WH. Acetyl-11-keto-β-boswellic acid extracted from Boswellia serrata promotes Schwann cell proliferation and sciatic nerve function recovery. Neural Regeneration Research. 2018;13:484–491. doi: 10.4103/1673-5374.228732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Li D, Yang T, Liu F, Kong J, Zhou Y. PTPN1 promotes the progression of glioma by activating the MAPK/ERK and PI3K/AKT pathways and is associated with poor patient survival. Oncology Reports. 2019;42:717–725. doi: 10.3892/or.2019.7180. [DOI] [PubMed] [Google Scholar]
- Khalaj-Kondori M, Sadeghi F, Hosseinpourfeizi MA, Shaikhzadeh-Hesari F, Nakhlband A, Rahmati-Yamchi M. Boswellia serrata gum resin aqueous extract upregulatesBDNF but not CREB expression in adult male rat hippocampus. Turkish Journal of Medical Sciences. 2016;46:1573–1578. doi: 10.3906/sag-1503-43. [DOI] [PubMed] [Google Scholar]
- Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D, Lee YM, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Cancer Research. 2011;71:2260–2275. doi: 10.1158/0008-5472.Can-10-3007. [DOI] [PubMed] [Google Scholar]
- Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, et al. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. 2011;117:3788–3795. doi: 10.1002/cncr.25945. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiology of Aging. 2002;23:787–794. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. Rediscovering natural products as a source of new drugs. Discovery Medicine. 2005;5:159–164. [PubMed] [Google Scholar]
- Laios K, Lytsikas-Sarlis P, Manes K, Kontaxaki MI, Karamanou M, Androutsos G. Drugs for mental illnesses in ancient greek medicine. Psychiatriki. 2019;30:58–65. doi: 10.22365/jpsych.2019.301.58. [DOI] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Archiv—European Journal of Physiology. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Li W, Liu J, Fu W, Zheng X, Ren L, Liu S, et al. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. Journal of Experimental & Clinical Cancer Research. 2018;37:132. doi: 10.1186/s13046-018-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ren L, Zheng X, Liu J, Wang J, Ji T, et al. 3-O-Acetyl-11-keto- β -boswellic acid ameliorated aberrant metabolic landscape and inhibited autophagy in glioblastoma. Acta Pharmaceutica Sinica B. 2020;10:301–312. doi: 10.1016/j.apsb.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nature Neuroscience. 2017;20:1434–1447. doi: 10.1038/nn.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhu G, Fan Y, Du Y, Lan M, Xu Y, et al. Natural products research in China From 2015 to 2016. Frontiers in Chemistry. 2018;6:45. doi: 10.3389/fchem.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- Lulli M, Cammalleri M, Fornaciari I, Casini G, Dal Monte M. Acetyl-11-keto-β-boswellic acid reduces retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Experimental Eye Research. 2015;135:67–80. doi: 10.1016/j.exer.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Lv M, Zhuang X, Zhang Q, Cheng Y, Wu D, Wang X, et al. Acetyl-11-keto-β-boswellic acid enhances the cisplatin sensitivity of non-small cell lung cancer cells through cell cycle arrest, apoptosis induction, and autophagy suppression via p21-dependent signaling pathway. Cell Biology and Toxicology. 2021;37:209–228. doi: 10.1007/s10565-020-09541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marefati N, Beheshti F, Memarpour S, Bayat R, Naser Shafei M, Sadeghnia HR, et al. The effects of acetyl-11-keto-β-boswellic acid on brain cytokines and memory impairment induced by lipopolysaccharide in rats. Cytokine. 2020;131:155107. doi: 10.1016/j.cyto.2020.155107. [DOI] [PubMed] [Google Scholar]
- Minj E, Upadhayay S, Mehan S. Nrf2/HO-1 signaling activator acetyl-11-keto-beta Boswellic Acid (AKBA)-mediated neuroprotection in methyl mercury-induced experimental model of ALS. Neurochemical Research. 2021;46:2867–2884. doi: 10.1007/s11064-021-03366-2. [DOI] [PubMed] [Google Scholar]
- Minj E, Upadhayay S, Mehan S. Nrf2/HO-1 signaling activator acetyl-11-keto-beta Boswellic acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-induced experimental model of ALS. Neurochemical Research. 2021 doi: 10.1007/s11064-021-03366-2. [DOI] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Archives of Biochemistry and Biophysics. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Niphadkar SS, Rathod VK. Adsorption kinetics, isotherm, and thermodynamics studies of acetyl-11-keto-β-boswellic acids (AKBA) from Boswellia serrata extract using macroporous resin. Preparative Biochemistry & Biotechnology. 2017;47:804–812. doi: 10.1080/10826068.2017.1342263. [DOI] [PubMed] [Google Scholar]
- Niu Q, Yang Y, Zhang Q, Niu P, He S, Di Gioacchino M, et al. The relationship between Bcl-gene expression and learning and memory impairment in chronic aluminum-exposed rats. Neurotoxicity Research. 2007;12:163–169. doi: 10.1007/bf03033913. [DOI] [PubMed] [Google Scholar]
- Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. PLoS ONE. 2011;6:e26943. doi: 10.1371/journal.pone.0026943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pillai P, Pooleri GK, Nair SV. Role of testosterone levels on the combinatorial effect of Boswellia serrata extract and enzalutamide on androgen dependent LNCaP cells and in patient derived cells. Integrative Cancer Therapies. 2021;20:1534735421996824. doi: 10.1177/1534735421996824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliyappadamba VT, Hatanpaa KJ, Chakraborty S, Habib AA. The role of NF-κB in the pathogenesis of glioma. Molecular and Cellular Oncology. 2014;1:e963478. doi: 10.4161/23723548.2014.963478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurobiology of Aging. 2000;21:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Raja AF, Ali F, Khan IA, Shawl AS, Arora DS. Acetyl-11-keto-β-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Research Notes. 2011;4:406. doi: 10.1186/1756-0500-4-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja AF, Ali F, Khan IA, Shawl AS, Arora DS, Shah BA, et al. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiology. 2011;11:54. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabian A, Boroushaki MT, Hayatdavoudi P, Sadeghnia HR. Boswellia serrata protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells. DNA and Cell Biology. 2016;35:666–679. doi: 10.1089/dna.2016.3332. [DOI] [PubMed] [Google Scholar]
- Rajabian A, Sadeghnia HR, Hosseini A, Mousavi SH, Boroushaki MT. 3-Acetyl-11-keto-β-boswellic acid attenuated oxidative glutamate toxicity in neuron-like cell lines by apoptosis inhibition. Journal of Cellular Biochemistry. 2020;121:1778–1789. doi: 10.1002/jcb.29413. [DOI] [PubMed] [Google Scholar]
- Rall B, Ammon HP, Safayhi H. Boswellic acids and protease activities. Phytomedicine. 1996;3:75–76. doi: 10.1016/s0944-7113(96)80015-8. [DOI] [PubMed] [Google Scholar]
- Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed AS, El Sayed NS. Co-administration of 3-acetyl-11-Keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic pathways. Journal of Molecular Neuroscience. 2016;59:58–67. doi: 10.1007/s12031-016-0734-7. [DOI] [PubMed] [Google Scholar]
- Sayed AS, Gomaa IEO, Bader M, El Sayed N. Role of 3-acetyl-11-Keto-beta-boswellic acid in counteracting LPS-induced neuroinflammation via modulation of miRNA-155. Molecular Neurobiology. 2018;55:5798–5808. doi: 10.1007/s12035-017-0801-2. [DOI] [PubMed] [Google Scholar]
- Schmiech M, Ulrich J, Lang SJ, Büchele B, Paetz C, St-Gelais A, et al. 11 Keto-α-Boswellic acid, a novel triterpenoid from Boswellia spp. with chemotaxonomic potential and antitumor activity against triple-negative breast cancer cells. Molecules. 2021;26:366. doi: 10.3390/molecules26020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang P, Liu W, Liu T, Zhang Y, Mu F, Zhu Z, et al. Acetyl-11-Keto-β-Boswellic acid attenuates prooxidant and profibrotic mechanisms involving transforming growth factor-β1, and improves vascular remodeling in spontaneously hypertensive rats. Science and Reports. 2016;6:39809. doi: 10.1038/srep39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gu Y, Wang Y, Bai J, Xiong L, Tao Y, et al. Inhibitory effect of acetyl-11-keto-β-boswellic acid on titanium particle-induced bone loss by abrogating osteoclast formation and downregulating the ERK signaling pathway. International Immunopharmacology. 2021;94:107459. doi: 10.1016/j.intimp.2021.107459. [DOI] [PubMed] [Google Scholar]
- Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biology. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R, Su W, Jiao Q. NGF protects neuroblastoma cells against β-amyloid-induced apoptosis via the Nrf2/HO-1 pathway. FEBS Open Bio. 2019;9:2063–2071. doi: 10.1002/2211-5463.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS Journal. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- Sun MX, He XP, Huang PY, Qi Q, Sun WH, Liu GS, et al. Acetyl-11-keto-β-boswellic acid inhibits proliferation and induces apoptosis of gastric cancer cells through the phosphatase and tensin homolog /Akt/ cyclooxygenase-2 signaling pathway. World Journal of Gastroenterology. 2020;26:5822–5835. doi: 10.3748/wjg.v26.i38.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovets T, Büchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. The Journal of Immunology. 2005;174:498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kondo H, Kanda K, Ashino T, Nakamachi T, Sekikawa K, et al. Involvement of interleukin-1 in lipopolysaccaride-induced microglial activation and learning and memory deficits. Journal of Neuroscience Research. 2011;89:506–514. doi: 10.1002/jnr.22582. [DOI] [PubMed] [Google Scholar]
- Tuncer S, Banerjee S. Eicosanoid pathway in colorectal cancer: Recent updates. World Journal of Gastroenterology. 2015;21:11748–11766. doi: 10.3748/wjg.v21.i41.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uomoto JM, Brockway JA. Anger management training for brain injured patients and their family members. Archives of Physical Medicine and Rehabilitation. 1992;73:674–679. [PubMed] [Google Scholar]
- Wang LN, Xu D, Gui QP, Zhu MW, Zhang HH, Hu YZ. Morphological and quantatitive capillary changes in aging human brain. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:104–107. [PubMed] [Google Scholar]
- Wang MX, Zhao JX, Meng YJ, Di TT, Xu XL, Xie XJ, et al. Acetyl-11-keto-β-boswellic acid inhibits the secretion of cytokines by dendritic cells via the TLR7/8 pathway in an imiquimod-induced psoriasis mouse model and in vitro. Life Sciences. 2018;207:90–104. doi: 10.1016/j.lfs.2018.05.044. [DOI] [PubMed] [Google Scholar]
- Webber C, Zochodne D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Experimental Neurology. 2010;223:51–59. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Weber CC, Reising K, Müller WE, Schubert-Zsilavecz M, Abdel-Tawab M. Modulation of Pgp function by boswellic acids. Planta Medica. 2006;72:507–513. doi: 10.1055/s-2006-931536. [DOI] [PubMed] [Google Scholar]
- Wei C, Fan J, Sun X, Yao J, Guo Y, Zhou B, et al. Acetyl-11-keto-β-boswellic acid ameliorates cognitive deficits and reduces amyloid-β levels in APPswe/PS1dE9 mice through antioxidant and anti-inflammatory pathways. Free Radical Biology & Medicine. 2020;150:96–108. doi: 10.1016/j.freeradbiomed.2020.02.022. [DOI] [PubMed] [Google Scholar]
- Xiong L, Liu Y, Zhu F, Lin J, Wen D, Wang Z, et al. Acetyl-11-keto-β-boswellic acid attenuates titanium particle-induced osteogenic inhibition via activation of the GSK-3β/β-catenin signaling pathway. Theranostics. 2019;9:7140–7155. doi: 10.7150/thno.35988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Research. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Yu XH, Liu J, Zhang Y, Wang XM. Effect of alpha-zearalanol upon the expression of HO-1 gene and the cytosolic free calcium level in tumor necrosis factor alpha-stimulated human endothelial cell. Zhonghua Yi Xue Za Zhi. 2009;89:3280–3284. [PubMed] [Google Scholar]
- Yuan XY, Li YH, Qi ZH, Peng MY, Wan Z, Wang GP, et al. Effect of acetyl-11-keto-β-boswellic acid on proliferation, apoptosis and cell cycle of human acute myeloid leukemia cell line HL-60. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18:1440–1444. [PubMed] [Google Scholar]
- Zhang LR, Li XT, Tang WL, Wang YM, Cheng NN, Chen BY. Changes in brain interleukin-1beta following the coadministration of norfloxacin with biphenylacetic acid in rats. European Journal of Pharmacology. 2006;543:21–26. doi: 10.1016/j.ejphar.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ao L, Yan Y, Li C, Li W, Ye A, et al. Levo-corydalmine attenuates vincristine-induced neuropathic pain in mice by upregulating the Nrf2/HO-1/CO pathway to inhibit connexin 43 expression. Neurotherapeutics. 2020;17:340–355. doi: 10.1007/s13311-019-00784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]