Abstract
Objective
To evaluate antithrombotic (AT) use in individuals with atrial fibrillation (AF) and at high risk of stroke (CHA2DS2-VASc score ≥2) and investigate whether pre-existing AT use may improve COVID-19 outcomes.
Methods
Individuals with AF and CHA2DS2-VASc score ≥2 on 1 January 2020 were identified using electronic health records for 56 million people in England and were followed up until 1 May 2021. Factors associated with pre-existing AT use were analysed using logistic regression. Differences in COVID-19-related hospitalisation and death were analysed using logistic and Cox regression in individuals with pre-existing AT use versus no AT use, anticoagulants (AC) versus antiplatelets (AP), and direct oral anticoagulants (DOACs) versus warfarin.
Results
From 972 971 individuals with AF (age 79 (±9.3), female 46.2%) and CHA2DS2-VASc score ≥2, 88.0% (n=856 336) had pre-existing AT use, 3.8% (n=37 418) had a COVID-19 hospitalisation and 2.2% (n=21 116) died, followed up to 1 May 2021. Factors associated with no AT use included comorbidities that may contraindicate AT use (liver disease and history of falls) and demographics (socioeconomic status and ethnicity). Pre-existing AT use was associated with lower odds of death (OR=0.92, 95% CI 0.87 to 0.96), but higher odds of hospitalisation (OR=1.20, 95% CI 1.15 to 1.26). AC versus AP was associated with lower odds of death (OR=0.93, 95% CI 0.87 to 0.98) and higher hospitalisation (OR=1.17, 95% CI 1.11 to 1.24). For DOACs versus warfarin, lower odds were observed for hospitalisation (OR=0.86, 95% CI 0.82 to 0.89) but not for death (OR=1.00, 95% CI 0.95 to 1.05).
Conclusions
Pre-existing AT use may be associated with lower odds of COVID-19 death and, while not evidence of causality, provides further incentive to improve AT coverage for eligible individuals with AF.
Keywords: atrial fibrillation, COVID-19, epidemiology, electronic health records, drug monitoring
Introduction
Atrial fibrillation (AF) is a disturbance of heart rhythm affecting 37.5 million people globally1 and significantly increases the risk of stroke.2 Anticoagulants (AC), a subtype of antithrombotics (AT), reduce the risk of stroke3 and are recommended for individuals with AF and at high risk of stroke (CHA2DS2-VASc score ≥2, the National Institute for Health and Care Excellence (NICE) threshold).4 5 Despite improvements in AC uptake, previous evaluations suggest that up to one-third of individuals with AF and CHA2DS2-VASc score ≥2 in the UK may not be on AC,6 with around 15% on no type of AT.6 Hypotheses for this suboptimal medication centre around clinical overestimation of bleeding and fall risk in elderly patients,6 7 but the potential drivers of AT use remain underexplored at the population scale.
COVID-19 has presented another risk factor for individuals with AF, who are at increased risk of poor outcomes if they become infected.8 Observational evidence from Germany (n=6637) suggests that pre-existing AC use, but not antiplatelets (AP—another subtype of AT), may reduce mortality in individuals hospitalised with COVID-19.9 However, evidence is discordant, with a US study (n=3772) observing no difference in mortality in groups on AC or AP.10 In the UK, a larger study (n=70 464 of 372 746) explored AC and AC subtypes (warfarin vs direct oral anticoagulants (DOACs)) in individuals with AF and observed that AC was associated with lower COVID-19-specific mortality.11 This observational evidence is promising, but it does not compare all subtypes of AT and only covered the period up to 28 September 2020.
This study, therefore, set out to conduct the largest scale evaluation of AT use in individuals with AF to date in routinely updated, linked, population-scale electronic health record (EHR) data for 56 million people in England.12 Using this statistical power, this study investigated what factors are associated with pre-existing AT use and whether pre-existing AT use (across subtypes) is associated with COVID-19-related hospitalisation and death.
Methods
Study design and data sources
We conducted a cohort analysis using the newly established National Health Service (NHS) Digital Trusted Research Environment for England, which provides secure, remote access to linked, person-level EHR data for over 56 million people.12 Available data sources cover primary care, secondary care, pharmacy dispensing, death registrations and COVID-19 tests and vaccines. We used the General Practice Extraction Service Extract for Pandemic Planning and Research (GDPPR) for demographic and diagnostic data (eg, a diagnosis of AF) and the NHS Business Service Authority Dispensed Medicines (BSADM) for medication exposure data (eg, pre-existing AT use) as this is the most accurate available representation of the medication an individual takes. Hospital Episode Statistics (HES), COVID-19 Hospitalisations in England Surveillance System, Secondary Uses Service, and the Office for National Statistics (ONS) Civil Registration of Deaths were used for COVID-19 hospitalisation and death. Public Health England’s Second Generation Surveillance System was used to identify COVID-19 test results, and the COVID-19 vaccination events data set was used for COVID-19 vaccine status.
Study populations
Individuals were included in the study if registered with a general practice (GP) in England (at least one record in the GDPPR data set with a valid person pseudo-identifier), ≥18 years old and alive on 1 January 2020, had available data on sex, ethnicity and GP location (based on the most recent, available data across primary care (GDPPR), secondary care (HES) and death registrations (ONS)), and had a diagnosis of AF (coded in GDPPR) with a CHA2DS2-VASc score ≥2 (calculated from the sum of components13 coded in GDPPR).
Individuals with contraindications to subtypes of AT (eg, DOACs in mitral stenosis, prosthetic mechanical valves, antiphospholipid antibody syndrome) were included as they are still eligible for other AT subtypes (eg, AP, warfarin).
To investigate exposure to pre-existing AT use on COVID-19-related hospitalisation and death, the inclusion criteria of a recorded COVID-19 event were applied. A COVID-19 event was defined as any positive test (PCR or lateral flow), a coded diagnosis in primary or secondary care, or a COVID-19 diagnosis on a death certificate (see Thygesen et al 14 and CALIBER15 for further details and phenotyping algorithms).
All phenotyping algorithms used are available on GitHub (https://github.com/BHFDSC/CCU020/tree/main/england/phenotypes) and online supplemental figure 1 provides a flow chart of individuals excluded at each stage.
heartjnl-2021-320325supp001.pdf (6.2MB, pdf)
Study variables
Medication exposure
An individual was defined as taking a particular medication if they had one or more dispensed prescription (coded in the NHS BSADM) in the previous 6 months. We purposefully defined a liberal threshold to support evaluation of AT usage up to May 2021 that may have included unusual buying patterns (eg, bulk buying) caused by the pandemic.
Mutually exclusive medication categories were constructed for AC only, AP only, AP and AC, and no AT. Apixaban, rivaroxaban, dabigatran and edoxaban were collectively categorised as DOACs for comparison with warfarin. For analysis, three mutually exclusive medication categories were tested (any AT vs no AT, AC only vs AP only, DOACs vs warfarin).
Outcomes
We defined two COVID-19 outcomes: COVID-19-related hospitalisation and COVID-19 death. COVID-19 hospitalisation included any hospital admission with a recorded COVID-19 diagnosis in any position (eg, not the primary diagnosis). COVID-19 death included individuals with a COVID-19 diagnosis on their death certificate in any position, a registered death within 28 days of their first recorded COVID-19 event or a discharge destination denoting death after a COVID-19 hospitalisation. Follow-up for COVID-19 outcomes ended on 1 May 2021, with the final follow-up date as either the date of the outcome of interest (eg, COVID-19 death) or the study end date (1 May 2021).
Covariates
Covariates were preselected based on potential associations with pre-existing AT use6 or COVID-19 outcomes and included demographics (age, sex, ethnicity, geographical location, socioeconomic status, as measured by the Index of Multiple Deprivation decile), comorbidities that increase risk of stroke and bleeding (congestive heart failure, hypertension, stroke, vascular disease, diabetes, uncontrolled hypertension, renal disease, liver disease, prior major bleeding, hazardous alcohol use, history of fall, body mass index (BMI), smoking status) and other medications (antihypertensives, lipid-regulating drugs, proton pump inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, other immunosuppressants and COVID-19 vaccination status, defined as at least one vaccine recorded in the COVID-19 vaccination events data set prior to the individual’s COVID-19 event).
The same covariates (excluding COVID-19 vaccination status) were used as independent variables to test associations with pre-existing AT use (for any AT vs no AT, AP only vs AC only, DOACs vs warfarin) and to calculate a propensity score for use as an additional covariate in the COVID-19 outcome analysis (as demonstrated in Elze et al 16).
Statistical analysis
Descriptive statistics were used to summarise the study population characteristics and were stratified by medication category. Pairwise Pearson’s correlation coefficients were used to check for potential collinearities between covariates. Multivariable logistic regression was used to test associations with pre-existing AT use and calculate the propensity score.
Multivariable logistic regression and Cox regression were used to test differences between exposure groups (any AT vs no AT, AC only vs AP only, DOACs vs warfarin) for COVID-19-related hospitalisation and death. An additional post-hoc analysis compared dabigatran (a thrombin inhibitor) against factor Xa inhibitors (apixaban, edoxaban, rivaroxaban). Logistic and Cox regression methods were selected to evaluate potential differences between event-based (logistic regression) and time-to-event-based (Cox regression) analysis. All covariates including the propensity score were included in both methods (as demonstrated in Elze et al 16). For variables with incomplete data (BMI: 9.3% missing), individual values were imputed with the cohort mean.
Two sensitivity analyses were conducted. First, to evaluate the potential impact of different time periods, analysis was repeated for 1 January 2020–1 December 2020, prior to the introduction of vaccines and the 29 December 2020 cases peak of the second wave.17 Second, to validate the potential effect on COVID-19-specific outcomes, analysis was repeated with COVID-19 hospitalisation and death defined exclusively as the primary recorded diagnosis (coded first on hospital record or death certificate).
Primary results are reported from the multivariable logistic regression models covering the full time period (1 January 2020–1 May 2021), with the other analyses reviewed for concordance.
Data preparation was performed using Python V.3.7 and Spark SQL (V.2.4.5) on Databricks Runtime V.6.4 for Machine Learning, with analysis performed using R V.4.0.3. All codes for data preparation and analysis are available on GitHub (https://github.com/BHFDSC/CCU020/tree/main/england/code), with full results available at the following microsite: https://alexhandy1.shinyapps.io/at-evaluation-results/.
Patient and public involvement
The UK National Institute for Health Research-British Heart Foundation (BHF) Cardiovascular Partnership lay panel comprising individuals affected by cardiovascular disease reviewed and approved this project.
Results
Evaluation of AT use
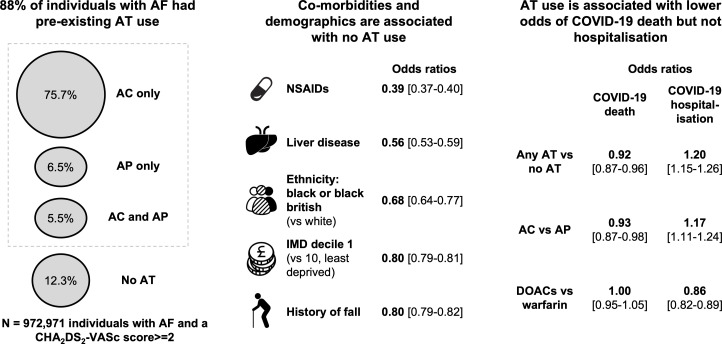
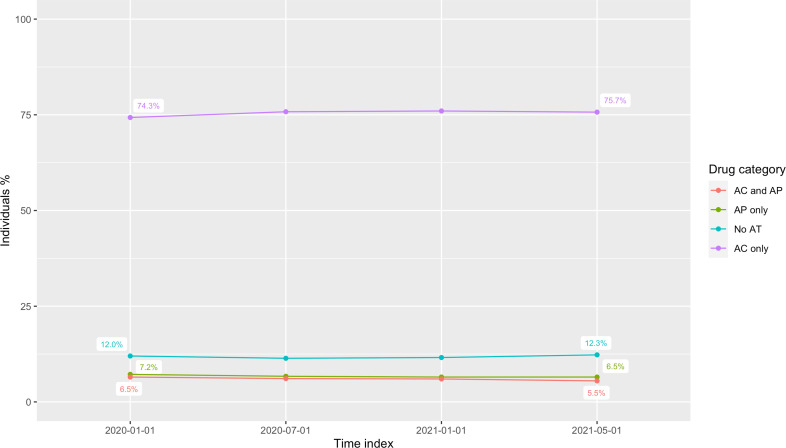
From a total of 55 903 113 individuals registered with a GP practice in England, 972 971 (1.7%) had a diagnosis of AF and a CHA2DS2-VASc score ≥2 on 1 January 2020 and 88.0% (n=856 336) had pre-existing AT use, with 74.3% (n=722 737) on AC only (see figure 1 for key study findings). The demographic and clinical characteristics of this cohort are summarised in tables 1–3. By May 2021, the proportion of individuals on any AT had fallen to 87.7%, but only AC had increased to 75.7% (see figure 2). For individuals on any AT, warfarin prescriptions fell from 24.8% in January 2020 to 17.1% in May 2021, while DOACs rose from 60.3% to 69.5% (see online supplemental figure 2).
Figure 1.
Visual overview of key study findings. AC, anticoagulants; AF, atrial fibrillation; AP, antiplatelets; AT, antithrombotics; DOACs, direct oral anticoagulants; IMD, Index of Multiple Deprivation; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 1.
Study population demographic characteristics by antithrombotic medication category
| Total n (%) |
Any AT n (%) |
AC only n (%) |
AP only n (%) |
AC and AP n (%) |
No AT n (%) |
|
| Individuals | 972 971 (100) | 856 336 (88) | 722 737 (74.3) | 70 498 (7.2) | 63 101 (6.5) | 116 635 (12) |
| Age, mean years (±SD) | 79 (±9.3) | 79 (±9) | 79 (±8.9) | 79 (±10) | 78 (±8.9) | 78 (±11) |
| 65–74 | 229 464 (23.6) | 198 956 (23.2) | 166 943 (23.1) | 16 018 (22.7) | 15 995 (25.3) | 30 508 (26.2) |
| ≥75 | 686 578 (70.6) | 610 497 (71.3) | 518 205 (71.7) | 49 702 (70.5) | 42 590 (67.5) | 76 081 (65.2) |
| Female | 449 279 (46.2) | 387 184 (45.2) | 338 477 (46.8) | 28 622 (40.6) | 20 085 (31.8) | 62 095 (53.2) |
| Ethnicity | ||||||
| White | 932 571 (95.8) | 822 292 (96) | 696 757 (96.4) | 66 237 (94) | 59 298 (94) | 110 279 (94.6) |
| Asian or Asian British | 20 557 (2.1) | 17 699 (2.1) | 12 797 (1.8) | 2536 (3.6) | 2366 (3.7) | 2858 (2.5) |
| Black or black British | 9418 (1) | 7658 (0.9) | 6200 (0.9) | 862 (1.2) | 596 (0.9) | 1760 (1.5) |
| Mixed | 3194 (0.3) | 2636 (0.3) | 2115 (0.3) | 274 (0.4) | 247 (0.4) | 558 (0.5) |
| Other ethnic groups | 7231 (0.7) | 6051 (0.7) | 4868 (0.7) | 589 (0.8) | 594 (0.9) | 1180 (1) |
| Geographical locations | ||||||
| South East | 172 714 (17.8) | 150 276 (17.5) | 127 207 (17.6) | 11 566 (16.4) | 11 503 (18.2) | 22 438 (19.2) |
| North West | 143 391 (14.7) | 127 860 (14.9) | 106 990 (14.8) | 10 705 (15.2) | 10 165 (16.1) | 15 531 (13.3) |
| East of England | 104 591 (10.7) | 92 676 (10.8) | 78 194 (10.8) | 7408 (10.5) | 7074 (11.2) | 11 915 (10.2) |
| South West | 108 250 (11.1) | 94 816 (11.1) | 80 009 (11.1) | 7863 (11.2) | 6944 (11) | 13 434 (11.5) |
| Yorkshire and the Humber | 108 285 (11.1) | 96 113 (11.2) | 81 386 (11.3) | 8405 (11.9) | 6322 (10) | 12 172 (10.4) |
| West Midlands | 111 062 (11.4) | 97 555 (11.4) | 83 383 (11.5) | 7836 (11.1) | 6336 (10) | 13 507 (11.6) |
| East Midlands | 83 786 (8.6) | 74 596 (8.7) | 63 978 (8.9) | 5773 (8.2) | 4845 (7.7) | 9190 (7.9) |
| London | 95 746 (9.8) | 81 824 (9.6) | 66 815 (9.2) | 7495 (10.6) | 7514 (11.9) | 13 922 (11.9) |
| North East | 45 146 (4.6) | 40 620 (4.7) | 34 775 (4.8) | 3447 (4.9) | 2398 (3.8) | 4526 (3.9) |
| IMD deciles | ||||||
| 1 (most deprived) | 78 061 (8) | 68 894 (8) | 56 583 (7.8) | 6490 (9.2) | 5821 (9.2) | 9167 (7.9) |
| 10 (least deprived) | 106 436 (10.9) | 93 984 (11) | 80 764 (11.2) | 6795 (9.6) | 6425 (10.2) | 12 452 (10.7) |
Percentages should be interpreted vertically for all variables, for example, proportion within category for variable, except for the first row showing percentage of individuals across AT medication categories.
AC, anticoagulants; AP, antiplatelets; AT, antithrombotics; IMD, Index of Multiple Deprivation.
Table 2.
Study population comorbidities that increase the risk of stroke and bleeding by antithrombotic medication category
| Total n (%) |
Any AT n (%) |
AC only n (%) |
AP only n (%) |
AC and AP n (%) |
No AT n (%) |
|
| CHA2DS2-VASc score components | ||||||
| Vascular disease | 169 797 (17.5) | 159 892 (18.7) | 103 946 (14.4) | 23 815 (33.8) | 32 131 (50.9) | 9905 (8.5) |
| Stroke/TIA/thromboembolism | 196 899 (20.2) | 183 140 (21.4) | 150 588 (20.8) | 16 611 (23.6) | 15 941 (25.3) | 13 759 (11.8) |
| Congestive heart failure | 247 562 (25.4) | 228 877 (26.7) | 192 023 (26.6) | 15 038 (21.3) | 21 816 (34.6) | 18 685 (16) |
| Diabetes | 268 437 (27.6) | 242 060 (28.3) | 197 216 (27.3) | 21 602 (30.6) | 23 242 (36.8) | 26 377 (22.6) |
| Hypertension | 675 676 (69.4) | 600 623 (70.1) | 505 514 (69.9) | 49 678 (70.5) | 45 431 (72) | 75 053 (64.3) |
| CHA2DS2-VASc score, mean (±SD) | 3.9 (±1.4) | 4 (±1.4) | 3.9 (±1.4) | 4.1 (±1.5) | 4.4 (±1.5) | 3.4 (±1.3) |
| 2 | 172 172 (17.7) | 138 750 (16.2) | 120 969 (16.7) | 10 912 (15.5) | 6869 (10.9) | 33 422 (28.7) |
| 3 | 245 979 (25.3) | 213 057 (24.9) | 184 241 (25.5) | 16 289 (23.1) | 12 527 (19.9) | 32 922 (28.2) |
| 4 | 252 047 (25.9) | 224 255 (26.2) | 190 707 (26.4) | 17 875 (25.4) | 15 673 (24.8) | 27 792 (23.8) |
| 5 | 162 318 (16.7) | 149 105 (17.4) | 122 356 (16.9) | 12 995 (18.4) | 13 754 (21.8) | 13 213 (11.3) |
| ≥6 | 140 455 (14.4) | 131 169 (15.3) | 104 464 (14.5) | 12 427 (17.6) | 14 278 (22.6) | 9286 (8) |
| HAS-BLED score components | ||||||
| Renal disease | 315 940 (32.5) | 284 379 (33.2) | 237 965 (32.9) | 24 423 (34.6) | 21 991 (34.9) | 31 561 (27.1) |
| Liver disease | 8462 (0.9) | 6707 (0.8) | 5440 (0.8) | 788 (1.1) | 479 (0.8) | 1755 (1.5) |
| Stroke | 196 493 (20.2) | 182 756 (21.3) | 150 232 (20.8) | 16 606 (23.6) | 15 918 (25.2) | 13 737 (11.8) |
| Major bleeding event | 335 289 (34.5) | 293 096 (34.2) | 240 703 (33.3) | 27 431 (38.9) | 24 962 (39.6) | 42 193 (36.2) |
| Harmful alcohol use | 28 970 (3) | 25 572 (3) | 21 162 (2.9) | 2274 (3.2) | 2136 (3.4) | 3398 (2.9) |
| Uncontrolled hypertension | 66 576 (6.8) | 58 873 (6.9) | 48 444 (6.7) | 5395 (7.7) | 5034 (8) | 7703 (6.6) |
| History of fall | 119 738 (12.3) | 103 615 (12.1) | 85 718 (11.9) | 10 717 (15.2) | 7180 (11.4) | 16 123 (13.8) |
| BMI, mean (±SD) | 28.7 (±6) | 28.8 (±6) | 28.8 (±6.1) | 28.1 (±5.6) | 29 (±5.8) | 27.9 (±5.9) |
| Smoking status (ever smoker) | 638 775 (65.7) | 566 860 (66.2) | 472 208 (65.3) | 48 567 (68.9) | 46 085 (73) | 71 915 (61.7) |
Percentages should be interpreted vertically for all variables, for example, proportion within category for variable.
HAS-BLED score component bleeding medications excluded as it is measured within exposures and labile international normalized ratio excluded as it could not be accurately extracted from data sets.
AC, anticoagulants; AP, antiplatelets; AT, antithrombotics; BMI, body mass index; TIA, transient ischaemic attack.
Table 3.
Study population characteristics of COVID-19 outcomes and other medications by antithrombotic medication category
| Total n (%) |
Any AT n (%) |
AC only n (%) |
AP only n (%) |
AC and AP n (%) |
No AT n (%) |
|
| COVID-19 outcomes | ||||||
| Any COVID-19 event | 77 364 (8) | 67 087 (7.8) | 54 756 (7.6) | 6743 (9.6) | 5588 (8.9) | 10 277 (8.8) |
| COVID-19 hospitalisation | 37 418 (3.8) | 33 150 (3.9) | 26 887 (3.7) | 3201 (4.5) | 3062 (4.9) | 4268 (3.7) |
| COVID-19 hospitalisation (primary diagnosis) | 27 011 (2.8) | 23 919 (2.8) | 19 375 (2.7) | 2319 (3.3) | 2225 (3.5) | 3092 (2.7) |
| COVID-19 death | 21 116 (2.2) | 18 173 (2.1) | 14 553 (2) | 2055 (2.9) | 1565 (2.5) | 2943 (2.5) |
| COVID-19 death (primary diagnosis) | 15 297 (1.6) | 13 158 (1.5) | 10 522 (1.5) | 1508 (2.1) | 1128 (1.8) | 2139 (1.8) |
| Other medications | ||||||
| Antihypertensives | 540 678 (55.6) | 498 113 (58.2) | 412 077 (57) | 40 375 (57.3) | 45 661 (72.4) | 42 565 (36.5) |
| Lipid-regulating drugs | 589 568 (60.6) | 547 521 (63.9) | 441 736 (61.1) | 51 120 (72.5) | 54 665 (86.6) | 42 047 (36.1) |
| Proton pump inhibitors | 409 429 (42.1) | 369 461 (43.1) | 286 984 (39.7) | 39 180 (55.6) | 43 297 (68.6) | 39 968 (34.3) |
| NSAIDs | 19 448 (2) | 14 608 (1.7) | 11 101 (1.5) | 2317 (3.3) | 1190 (1.9) | 4840 (4.1) |
| Corticosteroids | 80 347 (8.3) | 71 706 (8.4) | 59 511 (8.2) | 5929 (8.4) | 6266 (9.9) | 8641 (7.4) |
| Other immunosuppressants | 13 216 (1.4) | 11 690 (1.4) | 9498 (1.3) | 1152 (1.6) | 1040 (1.6) | 1526 (1.3) |
| COVID-19 vaccine prior to COVID-19 event | 9463 (1) | 8248 (1) | 6799 (0.9) | 824 (1.2) | 625 (1) | 1215 (1) |
Percentages should be interpreted vertically for all variables, for example, proportion within category for variable.
Pre-existing medication use was determined as ≥1 dispensed prescription in the 6 months prior to the cohort start date (1 January 2020).
AC, anticoagulants; AP, antiplatelets; AT, antithrombotics; NSAIDs, non-steroidal anti-inflammatory drugs.
Figure 2.
Individual antithrombotic prescriptions by drug category, January 2020–May 2021. AC, anticoagulants; AP, antiplatelets; AT, antithrombotics.
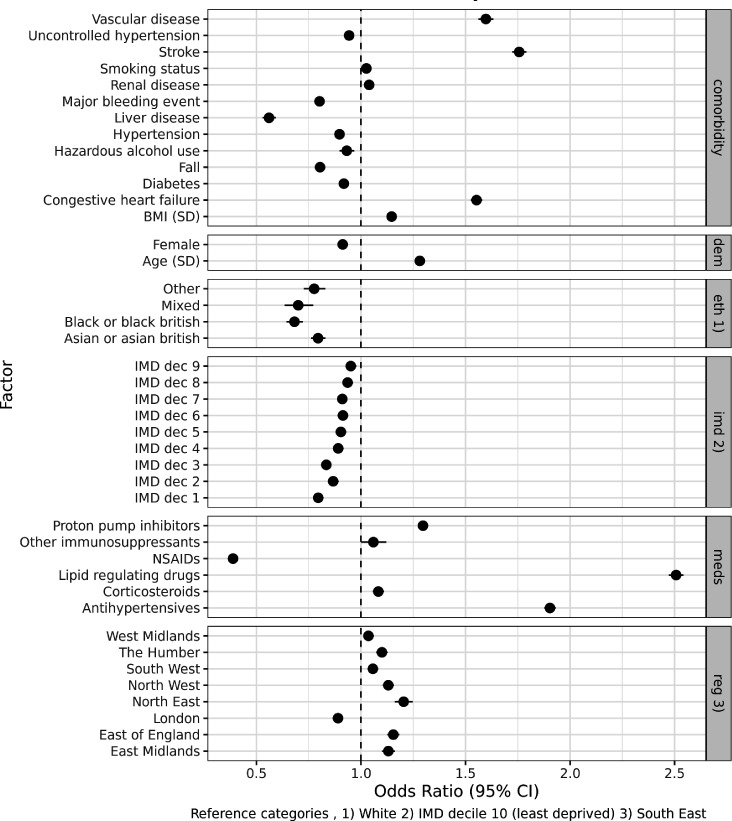
The factors associated with pre-existing AT use versus no AT are shown in figure 3. Lipid-regulating drugs (OR=2.50, 95% CI 2.47 to 2.54) and antihypertensives (OR=1.90, 95% CI 1.88 to 1.93) were associated with the highest odds of pre-existing AT use, followed by comorbidities in the CHA2DS2-VASc score (stroke: OR=1.76, 95% CI 1.72 to 1.79; vascular disease: OR=1.60, 95% CI 1.56 to 1.63). In contrast, NSAIDs (OR=0.39, 95% CI 0.37 to 0.40), liver disease (OR=0.56, 95% CI 0.53 to 0.59) and history of falls (OR=0.80, 95% CI 0.79 to 0.82) were associated with reduced odds.
Figure 3.
Factors associated with antithrombotics versus no antithrombotics (1 January 2020), using multivariable logistic regression. BMI, body mass index; IMD, Index of Multiple Deprivation; NSAIDs, non-steroidal anti-inflammatory drugs.
Differences were also observed across demographics, ethnicity, socioeconomic status and geographical location, with women (OR=0.91, 95% CI 0.90 to 0.92) and individuals from ethnic minorities and lower socioeconomic positions associated with lower odds of AT use (eg, ethnicity of black or black British vs white; OR=0.68, 95% CI 0.64 to 0.72).
In other AT subtypes (AC vs AP and DOACs vs warfarin), the results were broadly consistent (see online supplemental figures 3 and 4), with the primary exception of vascular disease which was associated with reduced odds of AC versus AP (OR=0.37, 95% CI 0.36 to 0.38).
AT use and COVID-19 outcomes
From 972 971 individuals who had a diagnosis of AF and a CHA2DS2-VASc score ≥2 on 1 January 2020, 8% (n=77 364) had a recorded COVID-19 event, 3.8% (n=37 418) had a COVID-19-related hospitalisation and 2.2% (n=21 116) died when followed up to 1 May 2021. The characteristics of individuals with a recorded COVID-19 event are summarised in online supplemental tables 1–3. Mean age (81) and comorbidities (mean CHA2DS2-VASc score 4.2) were both marginally higher compared with the full cohort. The proportion of individuals with pre-existing AT use was also marginally lower at 86.7%, but otherwise demographic and clinical characteristics were consistent.
Pre-existing AT use was associated with lower odds of COVID-19 death (OR=0.92, 95% CI 0.87 to 0.96), but higher odds of COVID-19 hospitalisation (OR=1.20, 95% CI 1.15 to 1.26) (see figure 4). The same pattern was observed for AC versus AP (COVID-19 death: OR=0.93, 95% CI 0.87 to 0.98; COVID-19 hospitalisation: OR=1.17, 95% CI 1.11 to 1.24), but not for DOACs versus warfarin (COVID-19 death: OR=1.00, 95% CI 0.95 to 1.05; COVID-19 hospitalisation: OR=0.86, 95% CI 0.82 to 0.89). Dabigatran was associated with lower odds of COVID-19 death (OR=0.80, 95% CI 0.71 to 0.91) and hospitalisation (OR=0.88, 95% CI 0.79 to 0.98) compared with factor Xa inhibitors (see online supplemental figure 5).
Figure 4.
Comparison of AT medication exposures on COVID-19 outcomes (followed up to 1 May 2021) using propensity score adjusted multivariable logistic regression. AC, anticoagulants; AP, antiplatelets; AT, antithrombotics; DOACs, direct oral anticoagulants.
These results were all directionally consistent across Cox regression analysis and the sensitivity analyses (see online supplemental figures 6–8).
Full results are available on the following microsite: https://alexhandy1.shinyapps.io/at-evaluation-results/.
Discussion
Main findings
In 972 971 individuals with AF and a CHA2DS2-VASc score ≥2, we observed 88.0% (n=856 336) with pre-existing AT use, which was associated with lower odds of COVID-19 death (OR=0.92, 95% CI 0.87 to 0.96). Although this association may not be causal, it provides further incentive to improve AT coverage for eligible individuals with AF.
Of the AF cohort analysed, 8% (n=77 364) had a recorded COVID-19 event, of which 3.8% (n=37 418) had a COVID-19-related hospitalisation and 2.2% (n=21 116) died. A marginally lower risk of COVID-19 death was observed for those with pre-existing AT use, which directionally aligns with the most comparable previous studies.9 11 AT use was, however, associated with higher odds of COVID-19 hospitalisation. This observation remained consistent when including only hospitalisations and deaths where COVID-19 was the first coded diagnosis. Higher observed risk of hospitalisation could reflect increased health-seeking behaviour (both patient-driven or by a clinician) of those with pre-existing AT use or may indicate that any risk reduction associated with AT use only materialises in the most serious cases. The same pattern was observed in AC versus AP and supports the findings of Fröhlich et al 9 that AC may be associated with lower risk of death than AP. For DOACs versus warfarin, no difference was observed between groups for COVID-19 death, but DOACs were associated with marginally reduced odds of COVID-19 hospitalisation. Our analysis did not directly investigate the previously reported observation that vitamin K depletion through warfarin is harmful,18 but more generally our findings suggest that it is unlikely that warfarin is associated with more severe COVID-19 outcomes compared with DOACs.11
Although these associations across AT subtypes do not prove causality, they provide further incentive to improve AT coverage for individuals with AF that are already at high risk of stroke. Previous evaluations in the UK have estimated that around 15% of these individuals do not take any AT and around 17% take AP only rather than the recommended AC.3 6 Our evaluation found around 12% on no AT and around 7% on AP only, which suggests national-level guidance19 and primary care incentives such as the Quality and Outcomes Framework20 continue to have a positive impact. Nonetheless, one in five individuals remain on a suboptimal medication regimen. Shifts from warfarin to DOACs observed in this study and others21 were recommended by COVID-19 guidance22 and demonstrate the potential impact of rapidly disseminated medications policy using population-scale EHR data.
Identifying which factors are associated with AT use is key to further lowering the proportion of individuals on suboptimal medication. NSAIDs displayed the strongest association with no AT use and likely reflects the association between NSAIDs and increased risk of major bleeding in individuals with AF.23 For comorbidities, liver disease had the strongest association with no AT use, which is also supported by clinical evidence.24 However, recent evidence suggests25 26 more personalised risk calculations for bleeding and stroke may enable more individuals with liver disease to benefit from AT. History of falls was the comorbidity with the second strongest association with no AT use, suggesting it remains a key factor in AT medicating decisions and may be overweighted as a proxy for bleeding risk.7 27 In the UK, NICE guidance was recently updated4 to explicitly address this issue and it will be important to track the impact of this in future evaluations. On demographics, lower odds of AT use were observed in women, but this is likely influenced by using NICE’s primary threshold for the CHA2DS2-VASc score of 2 for both sexes. The CHA2DS2-VASc score allocates 1 point to women and 0 for men, resulting in a larger proportion of comparatively healthy women (eg, 12% and 25% of women in the cohort have vascular disease and diabetes vs 21% and 33%, respectively, in men). However, demographic differences in AT use across ethnicity and socioeconomic status mirror systematic healthcare inequalities that have been reported previously.28 29 Targeted outreach to these groups will be key to improving AT use further.
Strength and limitations
Routinely updated, linked, population-scale EHR data sets provide the statistical power to robustly analyse targeted subgroups and control for a wide range of potential confounders. The prevalence of individuals with AF and CHA2DS2-VASc score ≥2 in our cohort is similar to that observed in the Quality and Outcomes Framework,20 which provides an external validation for our data set. All code is open-source and an updated nationwide evaluation can be rapidly created for future time points.
The study does have limitations. First, the reported associations do not demonstrate causality and residual confounding is unlikely to have been fully eliminated. For example, in-hospital treatment regimens were not analysed so differences in COVID-19 outcomes due to additional targeted anticoagulation regimens30 or other medications cannot be accounted for in our analysis. While we attempted to mitigate confounding through careful cohort selection, covariates and propensity score adjustment, our study design does not control for all potential factors associated with the initiation of AT use which may influence COVID-19 outcomes. Second, our decision (supported by Elze et al 16) to include all covariates and the propensity score for the COVID-19 analysis could theoretically lead to overfitting; however, Elze et al’s16 own analysis demonstrates limited differences between methods. Lastly, exposure to AT medication was defined as one or more dispensed prescriptions (recorded in NHS BSADM) in the previous 6 months. Other studies have used different time periods and prescription frequency counts9 11 and adherence was not measured. We purposefully defined a liberal threshold to support evaluation of AT usage up to May 2021 that may have included unusual buying patterns (eg, bulk buying) caused by the pandemic. The trade-off is that for the COVID-19 outcome analyses it increases the probability of including a minority of ‘exposed’ individuals who had ceased regular, pre-existing AT medication.
Conclusions
Pre-existing AT use may be associated with lower odds of COVID-19 death and, while not evidence of causality, provides further incentive to improve AT coverage for eligible individuals with AF.
Key messages.
What is already known on this subject?
Recent observational studies have shown that individuals routinely taking anticoagulants experienced less severe COVID-19 outcomes.
These correlations are inconsistent across studies and have not compared all major subtypes of antithrombotics in one study.
What might this study add?
Using routinely updated, linked electronic health record data for 56 million people in England, we were able to analyse antithrombotic use and their subtypes while controlling for a wide range of potential confounders.
We identified 972 971 individuals with atrial fibrillation and a high risk of stroke (measured as CHA2DS2-VASc score ≥2) and observed 88.0% (n=856 336) with pre-existing antithrombotic use, which was associated with lower odds of COVID-19 death (OR=0.92, 95% CI 0.87 to 0.96).
How might this impact on clinical practice?
These findings can help shape global antithrombotic medication policy and provide population-scale, observational analysis results alongside gold standard randomised control trials to help assess whether a potential beneficial effect of pre-existing antithrombotic use on COVID-19 death alters risk to benefit assessments in antithrombotic prescribing decisions.
Acknowledgments
This work is carried out with the support of the BHF Data Science Centre led by HDR UK. This study makes use of de-identified data held in NHS Digital’s TRE for England and made available via the BHF Data Science Centre’s CVD-COVID-UK Consortium. This work uses data provided by patients and collected by the NHS as part of their care and support. We would also like to acknowledge all data providers who make health relevant data available for research.
Footnotes
Twitter: @amibanerjee1, @BHFDataScience, @HDR_UK, @tomlincr, @MK_statistics, @ReechaSofat
Contributors: All authors drafted and reviewed the manuscript. AH led the design and implementation of the analysis and is the guarantor. CD, CT, JHT, MAM, MK, RT, SH, SD and VW supported the design and quality assurance of the data preparation and analysis code. AB, AMW, CLMS, DB, RS, RD and SD supported the overall study design and provided clinical expertise.
CS is the Director of the BHF Data Science Centre and coordinated approvals for and access to data within NHS Digital’s TRE for England for CVD-COVID-UK.
Members of the wider CVD-COVID-UK Consortium (https://www.hdruk.ac.uk/wp-content/uploads/2021/09/210909-CVD-COVID-UK-Consortium-Members.pdf) also provided comments on drafts of the protocol and manuscript.
Funding: The British Heart Foundation Data Science Centre (grant no: SP/19/3/34678, awarded to Health Data Research (HDR) UK) funded the co-development (with NHS Digital) of the Trusted Research Environment, provision of linked data sets, data access, user software licences, computational usage, and data management and wrangling support, with additional contributions from the HDR UK dData and cConnectivity component of the UK Governments’ cChief sScientific aAdviser’s nNational cCore sStudies programme to coordinate national COVID-19 priority research. Consortium partner organisations funded the time of contributing data analysts, biostatisticians, epidemiologists and clinicians.AH is supported by research funding from the HDR UK Text Analytics Implementation Project. AB is supported by research funding from the National Institute for Health Research (NIHR), British Medical Association, AstraZeneca, and UK Research and Innovation. AMW is supported by the BHF-Turing Cardiovascular Data Science award (BCDSA\100005) and by core funding from UK MRC (MR/L003120/1), BHF (RG/13/13/30194; RG/18/13/33946) and NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). CT is supported by a UCL UKRI Centre for Doctoral Training in AI-enabled Healthcare studentship (EP/S021612/1), MRC Clinical Top-Up and a studentship from the NIHR Biomedical Research Centre at University College London Hospitals NHS Trust. DB holds a UK Research and Innovation (UKRI) Fellowship as part of Health Data Research UK (HDRUK; MR/S00310X/1). MAM is supported by research funding from AstraZeneca. MK is funded by the British Heart Foundation (grant: FS/18/5/33319). RD is supported by (1) NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, London, UK; (2) Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust; (3) the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking (grant agreement no: 116074; this Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and is chaired by DE Grobbee and SD Anker, partnering with 20 academic and industry partners and ESC); (4) the National Institute for Health Research University College London Hospitals Biomedical Research Centre; (5) the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London; (6) the UK Research and Innovation London Medical Imaging & Artificial Intelligence Centre for Value Based Healthcare; and (7) the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. SD is supported by: (1) Health Data Research UK London, which receives its funding from HDR UK funded by the UK MRC, EPSRC, ESRC, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh government), Public Health Agency (Northern Ireland), BHF, and Wellcome Trust; (2) The NIHR Biomedical Research Centre at University College London Hospital NHS Trust; (3) The Alan Turing Institute (EP/N510129/1); (4) The British Heart Foundation Accelerator Award (ref AA/18/6/24223); (5) The BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement (ref 116074); (6) The British Heart Foundation Data Science Centre (ref SP/19/3/34678); (7) The UKRI/NIHR funded Multimorbidity Mechanism and Therapeutics Research Collaborative (MR/V033867/1); (8) The Longitudinal Health and Wellbeing COVID-19 National Core Study, which was established by the UK Chief Scientific Officer in October 2020 and funded by UK Research and Innovation (grant references MC_PC_20030 and MC_PC_20059), (9) The Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation(grant reference MC_PC_20058), and (10) The CONVALESCENCE study of long COVID, which is funded by NIHR/UKRI.AB, AMW, RD and SD are part of the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking (grant agreement no: 116074).
Disclaimer: The views expressed are those of the authors and not necessarily those of the organisations listed. The funders of this work played no role in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The de-identified data used in this study are available via the CVD-COVID-UK Consortium, coordinated by BHF Data Science Centre, for accredited researchers working on approved projects in NHS Digital’s TRE for England, but as restrictions apply they are not publicly available. The authors and colleagues across the CVD-COVID-UK Consortium have invested considerable time and energy in developing the data resource used here and are keen to ensure that it is used widely to maximise its value. For enquiries about data access, please see www.healthdatagateway.org/dataset/7e5f0247-f033-4f98-aed3-3d7422b9dc6d or email bhfdsc@hdruk.ac.uk.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Data access approval was granted to the CVD-COVID-UK Consortium through the NHS Digital Online Data Access Request Service (ref: DARS-NIC-381078-Y9C5K). The BHF Data Science Centre's Approvals and Oversight Board deemed that this project (proposal CCU020 Evaluation of antithrombotic use and COVID-19 outcomes) fell within the scope of the consortium’s ethical and regulatory approvals. Analyses were conducted by an approved researcher (AH) via secure remote access to the Trusted Research Environment. Only summarised, aggregate results were exported, following manual review by the NHS Digital ‘safe outputs’ escrow service, to ensure no output placed in the public domain contains information that may be used to identify an individual (see reference 12). The North East-Newcastle and North Tyneside 2 research ethics committee provided ethical approval for the CVD-COVID-UK research programme (REC no: 20/NE/0161).
References
- 1. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–21. 10.1177/1747493019897870 [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 2014;45:2599–605. 10.1161/STROKEAHA.114.006070 [DOI] [PubMed] [Google Scholar]
- 3. Cowan JC, Wu J, Hall M, et al. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J 2018;39:2975–83. 10.1093/eurheartj/ehy411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NICE . Recommendations | atrial fibrillation: diagnosis and management | guidance | NICE, 2021. Available: https://www.nice.org.uk/guidance/ng196/chapter/Recommendations#stroke-prevention [Accessed 30 Jun 2021].
- 5. Proietti M, Lane DA, Boriani G, et al. Stroke prevention, evaluation of bleeding risk, and anticoagulant treatment management in atrial fibrillation contemporary international guidelines. Can J Cardiol 2019;35:619–33. 10.1016/j.cjca.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 6. Lacoin L, Lumley M, Ridha E, et al. Evolving landscape of stroke prevention in atrial fibrillation within the UK between 2012 and 2016: a cross-sectional analysis study using CPRD. BMJ Open 2017;7:e015363. 10.1136/bmjopen-2016-015363 [DOI] [Google Scholar]
- 7. Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017;84:35–40. 10.3949/ccjm.84a.16016 [DOI] [PubMed] [Google Scholar]
- 8. Yang H, Liang X, Xu J, et al. Meta-Analysis of atrial fibrillation in patients with COVID-19. Am J Cardiol 2021;144:152–6. 10.1016/j.amjcard.2021.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fröhlich GM, Jeschke E, Eichler U, et al. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol 2021;110:1041–50. 10.1007/s00392-020-01783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tremblay D, van Gerwen M, Alsen M, et al. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood 2020;136:144–7. 10.1182/blood.2020006941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong AY, Tomlinson L. Association between oral anticoagulants and COVID-19 related outcomes: two cohort studies. medRxiv 2021. [Google Scholar]
- 12. Wood A, Denholm R, Hollings S, et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ 2021;373:n826. 10.1136/bmj.n826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjerring Olesen J, Torp-Pedersen C, Lock Hansen M. The value of the CHA 2 DS 2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS 2 score 0-1: a nationwide cohort study. Thromb Haemost 2012;107. [Google Scholar]
- 14. Thygesen JH, Tomlinson C, Hollings S. Understanding COVID-19 trajectories from a nationwide linked electronic health record cohort of 56 million people: phenotypes, severity, waves & vaccination. medRxiv 2021. 10.1101/2021.11.08.21265312 [DOI] [Google Scholar]
- 15. Denaxas S, Gonzalez-Izquierdo A, Direk K, et al. UK phenomics platform for developing and validating electronic health record phenotypes: caliber. J Am Med Inform Assoc 2019;26:1545–59. 10.1093/jamia/ocz105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–57. 10.1016/j.jacc.2016.10.060 [DOI] [PubMed] [Google Scholar]
- 17. Cases in the UK | coronavirus in the UK. Available: https://coronavirus.data.gov.uk/details/cases [Accessed 30 Jun 2021].
- 18. Dofferhoff ASM, Piscaer I, Schurgers LJ. Reduced vitamin K status as a potentially modifiable risk factor of severe coronavirus disease 2019. Clin Infect Dis 2020. [Google Scholar]
- 19. NICE . Atrial fibrillation: management | guidance | NICE, 2014. Available: https://www.nice.org.uk/guidance/cg180 [Accessed 22 Jun 2021].
- 20. NHS Digital . QOF 2019-20 | NHS digital. Available: https://qof.digital.nhs.uk/ [Accessed 30 Jun 2021].
- 21. Curtis HJ, MacKenna B, Walker AJ. OpenSAFELY: impact of national guidance on switching from warfarin to direct oral anticoagulants (DOACs) in early phase of COVID-19 pandemic in England. medRxiv 2020:2020.12.03.20243535. [Google Scholar]
- 22. NHS England . Specialty guides for patient management during the coronavirus pandemic - Clinical guide for the management of anticoagulant services during the coronavirus pandemic, 2020. Available: https://www.england.nhs.uk/coronavirus/publication/specialty-guides/%0Ahttps://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C0077-Specialty-guide_Anticoagulant-services-and-coronavirus-v1-31-March.pdf [Accessed 30 Jun 2021].
- 23. Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal anti-inflammatory drugs and oral anticoagulants in the ARISTOTLE trial. Circulation 2020;141:10–20. 10.1161/CIRCULATIONAHA.119.041296 [DOI] [PubMed] [Google Scholar]
- 24. Søgaard KK, Horváth-Puhó E, Grønbaek H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol 2009;104:96–101. 10.1038/ajg.2008.34 [DOI] [PubMed] [Google Scholar]
- 25. Qamar A, Vaduganathan M, Greenberger NJ, et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol 2018;71:2162–75. 10.1016/j.jacc.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 26. Lee S-R, Lee H-J, Choi E-K, et al. Direct oral anticoagulants in patients with atrial fibrillation and liver disease. J Am Coll Cardiol 2019;73:3295–308. 10.1016/j.jacc.2019.04.052 [DOI] [PubMed] [Google Scholar]
- 27. Donzé J, Clair C, Hug B, et al. Risk of falls and major bleeds in patients on oral anticoagulation therapy. Am J Med 2012;125:773–8. 10.1016/j.amjmed.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 28. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marmot M, Bell R. Fair society, healthy lives. Public Health 2012;126 Suppl 1:S4. 10.1016/j.puhe.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 30. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–68. 10.1093/eurheartj/ehaa500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2021-320325supp001.pdf (6.2MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The de-identified data used in this study are available via the CVD-COVID-UK Consortium, coordinated by BHF Data Science Centre, for accredited researchers working on approved projects in NHS Digital’s TRE for England, but as restrictions apply they are not publicly available. The authors and colleagues across the CVD-COVID-UK Consortium have invested considerable time and energy in developing the data resource used here and are keen to ensure that it is used widely to maximise its value. For enquiries about data access, please see www.healthdatagateway.org/dataset/7e5f0247-f033-4f98-aed3-3d7422b9dc6d or email bhfdsc@hdruk.ac.uk.