Abstract
The role of mast cells in colorectal cancer (CRC) has been an area of intense interest. Mast cell density is closely related to CRC development and prognosis. The identification of mast cell progenitors (MCps) in peripheral blood provides an opportunity to explore the frequency and distribution of mast cells in the circulation and tumour microenvironment of patients with CRC at different disease stages. The aim of the presents study was to investigate the changes of MCps and mast cells in CRC. Flow cytometry was used to measure the circulating frequency of MCps in 37 patients with CRC and 12 healthy control (HC) patients, and the frequency of mast cells in tissue from 15 patients with CRC and 7 patients with haemorrhoids. In the present study, lower levels of circulating MCps in patients with CRC were found, which was significantly related to CRC development. After surgery, the frequency of circulating MCps was significantly increased. However, the frequency of mast cells in tumour tissues was lower than that in adjacent normal tissues and compared with HC tissues and was not associated with CRC progression.
Keywords: colorectal cancer, mast cell progenitors, mast cell
Introduction
Colorectal cancer (CRC) is the third most commonly occurring and the second leading cause of cancer-related mortality worldwide; ~1.8 million new CRC cases and 900,000 deaths are reported annually. In addition, the incidence of CRC at a relatively young age has begun to increase (1). With the deepening understanding of antitumour immunity, it is being shown that immune factors are crucial to outcomes of patients with cancer (2). However, previous studies have mainly focused on the effect of adaptive immunity on CRC (3,4), whereas studies on innate immunity are limited.
Mast cells are the progeny of haematopoietic stem cells, identified as CD4−CD8−CD19−CD14−CD117+FcεRI+ (5); mast cell progenitors (MCps) circulate in the peripheral blood, reach the target organ through chemoattraction to mediators, and then mature and eventually differentiate in peripheral tissues (6). In 2016, Dahlin et al (7) first identified MCps as CD4−CD8−CD19−CD14−CD34hiCD117+FcεRI+ cells. This discovery facilitated the quantification and characterization of this rare cell population in healthy individuals and patients with CRC.
Mast cells, as a group of innate immune cells, are widely distributed in human tissues (8). Mast cells can release large amounts of potent biologically active mediators and serve key roles in angiogenesis, tissue remodelling and immune regulation (9). The effect of mast cell infiltration on the tumour microenvironment has been widely studied (10–14). Previous studies have focused on mast cell density, which was identified by immunohistochemistry and is related to CRC prognosis and local angiogenesis (15–19). However, our understanding of the characteristics of circulating MCps and tumour-infiltrating mast cells in CRC remains inadequate.
In the present study, the MCp phenotype and the changes in MCp distribution in patients with CRC were investigated based on tumour progression.
Materials and methods
Human tissue samples
A total of 37 patients with newly diagnosed CRC were recruited from the First Hospital of Jilin University (Changchun, China) between July 2019 and April 2021. None of the participants received chemotherapy or radiotherapy before surgery. Patients with infectious disease, autoimmune disease or multiple primary cancers were excluded. Tumour staging was based on the Tumour-Node-Metastasis (TNM) cancer staging system of the American Joint Committee for Cancer 2017 (20). All samples were pathologically confirmed to be adenocarcinoma. A total of 15 pairs of fresh intestinal tumour tissue (six from colon and nine from rectum) and adjacent normal tissue (at least 5 cm distant from the tumour site) were collected and separated by a professional surgeon. In addition, 12 age- and sex-matched healthy control (HC) patients were recruited; these patients had no other gastrointestinal disease. A total of seven HC tissue control samples were obtained from these patients who underwent a procedure for the treatment of prolapse and haemorrhoids.
The patients with CRC were divided into two groups according to TNM staging: 22 patients with stage I/II disease were considered early stage, and the other 15 patients with stage III/IV disease were considered advanced stage. All patients provided written informed consent. The present study was approved (approval no. 2018-464) by the Ethics Committee of The First Hospital of Jilin University, and the experimental protocol was established according to the guidelines of The Declaration of Helsinki.
Clinical examination
Clinical data including sex, age, clinical staging, pathological grading and laboratory test results were obtained from hospital records. The level of serum carcinoembryonic antigen (CEA) was detected with an ADVIA Centaur XP immunoassay system (Siemens AG).
Isolation of single cells
A total of 37 preoperative blood samples and 10 postoperative peripheral blood samples were collected. Peripheral blood mononuclear cells were acquired by density gradient centrifugation (800 × g, 30 min, 20°C) using Ficoll-Paque Plus (Amersham; Cytiva).
Fresh intestinal tissue was repeatedly flushed with 0.9% normal saline. Samples were cut into 5-mm2 pieces. The samples were placed in D-Hanks (Hank's Balanced Salt Solution without Ca2+ and Mg2+; Beijing Solarbio Science & Technology Co., Ltd.) containing DL-dithiothreitol, ethylenediaminetetraacetic acid and 2-mercaptoethanol (all from Sigma-Aldrich; Merck KGaA) and incubated for 20 min in a constant temperature water bath at 37°C. Subsequently, the samples were washed with D-Hanks 3–4 times to completely remove any residual reagent. Then, the samples were digested with Hanks buffered saline solution (Beijing Solarbio Science & Technology Co., Ltd.) containing Liberase™ DL Research Grade and DNase I (both from Sigma-Aldrich; Merck KGaA) for 20 min in a constant temperature water bath at 37°C. The filtrate was collected using a cell strainer (Falcon; Thermo Fisher Scientific, Inc.) to separate the tissues and cell suspension, and then the cells were acquired by centrifugation (300 × g, 10 min, 4°C).
Flow cytometric analysis
Isolated cells and the live/dead marker Fixable Viability Stain 780 (BD Biosciences; 1 µl stain for 1 ml of cell suspension at 1–10×106 cells/ml) were mixed and incubated in the dark for 10 min at room temperature. Subsequently, the cells were washed with phosphate-buffered saline (PBS); after centrifugation (336 × g, 5 min, 4°C), the supernatant was discarded. The cells were stained with anti-human CD45-BB515 HI30 (cat. no. 564585), anti-human CD4-APC-H7 RPA-T4 (cat. no. 560158), anti-human CD8-APC-H7 SK1 (cat. no. 560179), anti-human CD14-APC-H7 M5E2 (cat. no. 561384), anti-human CD19-APC-H7 HIB19 (cat. no. 560727), anti-human CD34-APC 581 (cat. no. 555824), anti-human CD117-BV421 104D2 (cat. no. 563856) and anti-human FcεR1α-PE AER-37 (cat. no. 566607) (all 1:100; all from BD Biosciences) antibodies at room temperature for 30 min in the dark. After washing with PBS, cells were examined on a CytoFLEX S flow cytometer (Beckman Coulter, Inc.). The number of CD45+ cells/sample (at least 500,000 events) was analysed using CytExpert software v. 2.0 (Beckman Coulter, Inc.).
Treatment and follow-up
All patients with primary CRC underwent surgery, and peripheral blood samples were collected from 10 patients 7 days after surgery. None of the 10 patients showed symptoms such as fever after surgery, and they did not receive chemotherapy or radiotherapy during this period.
Statistical analyses
The horizontal line in each figure represents the mean and 95% CI of each group. Results in table were presented as the median and minimum-maximum range. Differences between two groups were analysed using independent Student's t-test or a Mann-Whitney U test if appropriate. Statistical differences among the four groups were analysed using the Kruskal-Wallis test with the Dunnett's post hoc test. Receiver operating characteristic (ROC) curve was calculated using SPSS. The Wilcoxon test was used to compare groups before and after treatment. Correlation analysis was performed with the Spearman's rank correlation test. SPSS statistics v. 24.0 (IBM Corp.) was used for all statistical analyses and GraphPad Prism v. 9.3.1 (GraphPad Software, Inc.) was used to generate the graphs. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
To investigate the distribution of circulating MCps in patients with newly diagnosed CRC, a total of 37 patients with CRC and 12 age- and sex-matched HCs were recruited; patient data are presented in Table I. The 37 patients with CRC included 22 patients with early-stage disease and 15 patients with advanced-stage disease. There were no differences in sex, age, or leukocyte levels among the three groups, and no difference in tumour location was observed between the early-stage and advanced-stage patients with CRC. Patients with advanced-stage CRC had higher serum CEA levels compared with those with early-stage CRC (P=0.0128). HC tissue, tumour tissue and adjacent normal tissue from 15 patients with CRC were analysed in this study.
Table I.
Clinicopathological characteristics of subjects.
| Blood | Tissue | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Clinicopathological characteristic | Healthy controls (n=12) | Early CRC (n=22) | Advanced CRC (n=15) | Early CRC (n=5) | Advanced CRC (n=10) |
| Median age, yearsa | 61.5 (55–69) | 64.5 (48–79) | 59 (47–82) | 56 (48–63) | 50.5 (49–75) |
| ≤60 | 6 | 8 | 9 | 4 | 7 |
| >60 | 6 | 14 | 6 | 1 | 3 |
| Sex | |||||
| Male | 8 | 14 | 10 | 3 | 8 |
| Female | 4 | 8 | 5 | 2 | 2 |
| Tumour location | |||||
| Colon | NA | 6 | 6 | 2 | 4 |
| Rectum | NA | 16 | 9 | 3 | 6 |
| TNM stage | |||||
| I/II | NA | 13 | 10 | 3 | 7 |
| III/IV | NA | 9 | 5 | 2 | 3 |
| Serum CEA (ng/ml)a | NA | 2.44 (0.20-38.47) | 5.13 (1.55-113.00)b | 1.11 (0.20-38.47) | 5.00 (1.55-113.00) |
| WBC count (×109 cells/l)a | 5.44 (3.67-8.59) | 5.58 (2.78-12.00) | 5.92 (3.92-10.00) | 4.94 (4.79-5.77) | 5.90 (3.92-10.00) |
Results are presented as the median (minimum-maximum).
P<0.05 vs. early CRC. CRC, colorectal cancer; CEA, carcinoembryonic antigen; WBC, white blood cell; TNM, Tumour-Node-Metastasis.
Frequency of circulating MCps is decreased in patients with CRC and is related to CRC progression
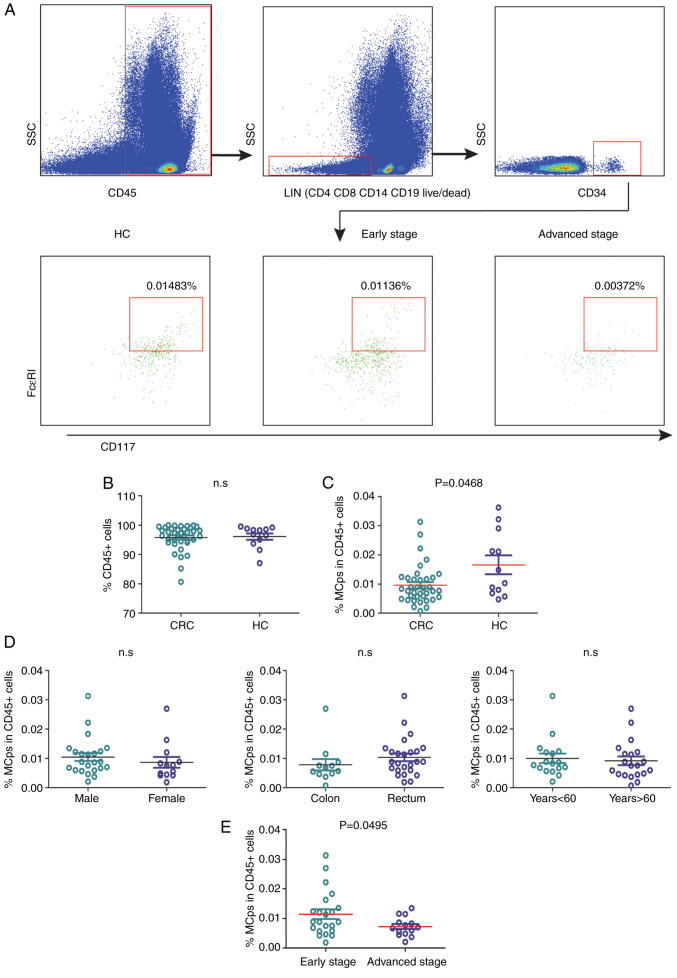
The MCp frequency in patients with CRC and HC patients was analysed by flow cytometry (Fig. 1A). The patients with CRC and HCs had similar percentages of CD45+ leukocytes in the peripheral blood (Fig. 1B). The frequency of MCps in patients with CRC was significantly lower compared with that in HCs (P=0.0468; Fig. 1C). Age, sex and tumour location had no influence on the quantification of MCps in patients with CRC (Fig. 1D). Moreover, the MCp level in patients with advanced-stage disease was lower compared with that in patients with early-stage disease (P=0.0495; Fig. 1E).
Figure 1.
Analysis of peripheral blood mononuclear cells isolated from 37 patients with CRC and 12 age- and sex-matched HCs. (A) The frequency of blood MCps was analysed by flow cytometry. MCps were identified as CD4−CD8−CD19−CD14−CD34hiCD117+FcεRI+ cells. The percentage indicates the frequency of MCps in the CD45+ cell population. (B) The frequency of CD45+ cells in patients with CRC and HCs. (C) The frequency of MCps in the CD45+ cell population in patients with CRC and HCs. In the CRC patient group, the frequency of MCps in the CD45+ cell population was compared based on (D) sex, age and tumour location and (E) early- and advanced-stage CRC. The horizontal line represents the mean of the data. CRC, colorectal cancer; HC, healthy control; MCps, mast cell progenitors; n.s, no significance; SSC, side scatter.
MCp frequency is related to CRC progression
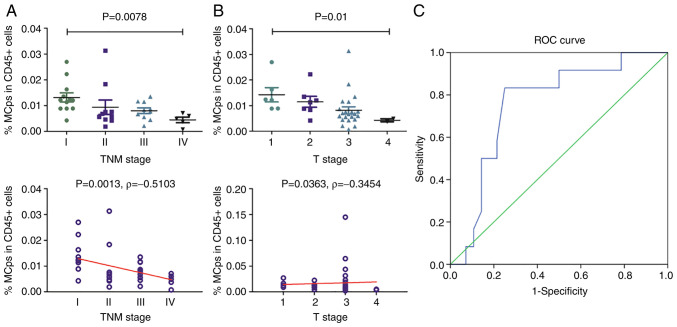
The relationship between MCp frequency and CRC progression was analysed. It was revealed that the frequency of circulating MCps was significantly associated with TNM stage (P=0.0078; Fig. 2A) and the depth of tumour invasion (P=0.01; Fig. 2B).
Figure 2.
Correlation analysis between the frequency of MCps and clinical data in 37 CRC patients. Association and correlation between the MCp frequency and (A) TNM and (B) T stage; Spearman's correlation coefficient was used for correlation analysis. (C) ROC curve among MCps and colorectal cancer stage. TNM staging of 37 CRC patients was evaluated, including 12 patients with TNM I and 25 patients with TNM II/III/IV. MCps, mast cell progenitors; ROC, receiver operating characteristic; TNM, Tumour-Node-Metastasis.
To assess whether MCps can be used for the differentiation of CRC, receiver operating characteristic (ROC) curves were plotted. It was identified that the frequency of circulating MCps has certain reference value for the differentiation of stage I CRC from other three stages of CRC (P=0.011; AUC=0.756; Fig. 2C).
Frequency of circulating MCps in patients with CRC following treatment
The effect of surgical treatment on circulating MCps in patients with CRC was investigated. The preoperative and postoperative (7 days after surgery) frequencies of circulating MCps in 10 patients with CRC were compared. The frequency of circulating MCps after treatment was significantly higher compared with that before treatment (P=0.028; Fig. 3).
Figure 3.
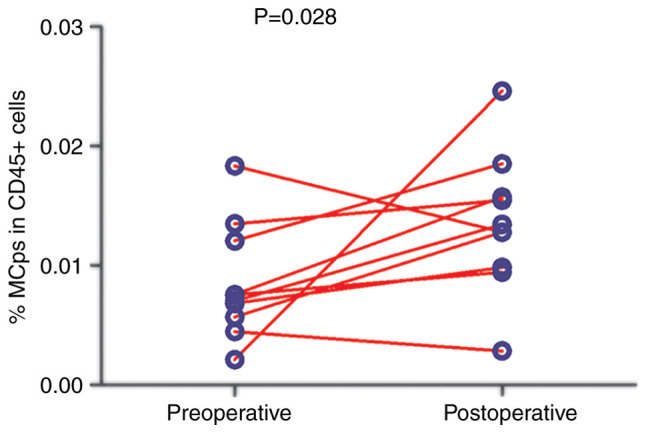
Comparison of the frequency of preoperative and postoperative circulating MCps (n=10). All 10 patients had early-stage CRC. MCps, mast cell progenitors.
Frequency of mast cells infiltrating tumour and adjacent normal tissues from patients with CRC
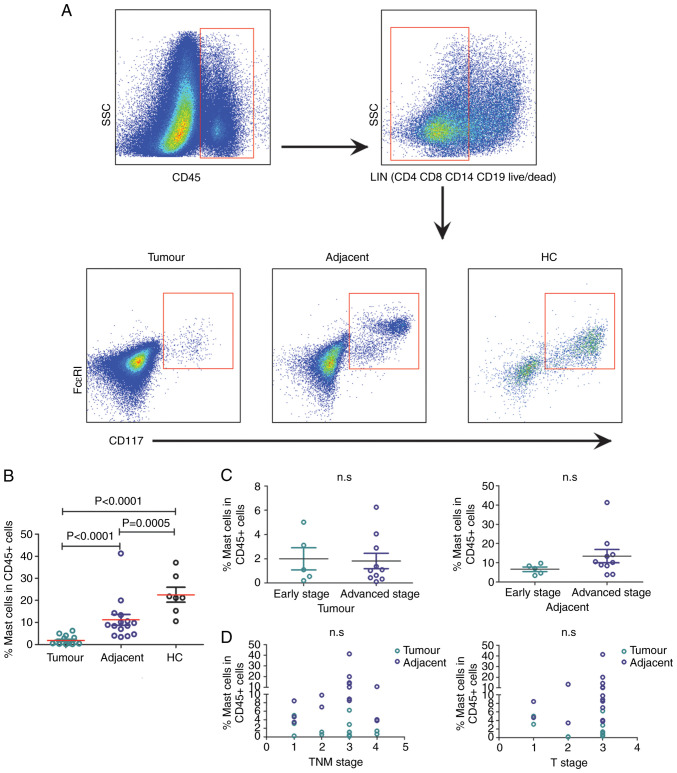
Finally, to explore whether the frequency of mast cells in tissues changed during CRC progression, the percentage of mast cells among the total CD45+ leukocyte population in tumour and adjacent normal tissues from patients with CRC and HC tissue was analysed using flow cytometry (Fig. 4A). Consistent with a previous study (21), the frequency of mast cells infiltrating in tumour tissue was significantly lower compared with that in the adjacent normal tissue and HC tissue (both P<0.0001; Fig. 4B). In addition, the frequency of mast cells in adjacent normal tissue was significantly lower compared with that in HC tissue (P=0.0005; Fig. 4B).
Figure 4.
Analysis of mast cells isolated from 15 pairs of fresh intestinal tumour tissues and adjacent normal tissues, as well as 7 HC tissues. (A) The frequency of tissue-infiltrating mast cells was analysed by flow cytometry. The percentage indicates the frequency of mast cells in the CD45+ cell population. (B) The frequency of mast cells in tumour tissues, adjacent normal tissue and HC tissues. (C) The frequency of mast cells in tumour and adjacent normal tissues from patients with early- and advanced-stage CRC. (D) The frequency of mast cells in tumour and adjacent normal tissues does not correlate with TNM stage or T stage in patients with CRC, as determined by Spearman's correlation coefficient analysis. CRC, colorectal cancer; n.s, no significance; SSC, side scatter; TNM, Tumour-Node-Metastasis.
The frequency of mast cells in adjacent normal tissue did not differ between early-stage and advanced-stage patients (Fig. 4C). Additionally, the frequency of mast cells in tumour and adjacent normal tissue was not associated with T stage or TNM stage (Fig. 4D).
Discussion
By studying the phenotype, distribution and clinical relevance of mast cells at different locations and in patients with different stages of CRC, the present study comprehensively analysed the characteristic changes in MCps and mast cells during CRC progression from multiple perspectives. The data showed that the frequency of circulating MCps was lower in patients with CRC and was associated with TNM stage and T stage. This suggested that a lower MCp level may reflect CRC progression. To the best of our knowledge, this is the first study to systematically analyse the changes of MCps and mast cells in the circulation and tissues of patients with CRC.
Previous studies reported that interaction between CRC cells and mast cells ultimately promotes tumour growth (22,23). Mast cells can be divided into immature circulating MCps and mature mast cells distributed in tissues. MCps are direct precursors of mast cells in the peripheral blood, and previous definitions of mast cell precursors have been unclear (24,25). However, the definition of the circulating MCp phenotype provides a new research direction (6). The present results suggested that age, sex and tumour location do not affect the frequency of MCps in patients with CRC, but patients with CRC do have significantly lower circulating MCp levels compared with HCs. More importantly, patients with advanced-stage CRC were found to have lower MCp levels compared with patients with early-stage CRC. With increasing TNM stage and T stage, the proportion of circulating MCps decreased significantly. It has been reported that a high level of tumour stroma-infiltrating mast cells is an independent unfavourable predictor in patients with muscle invasive bladder cancer and biliary tract cancer (26,27). Hence, it was concluded that the frequency of MCps may be an independent indicator of aggressive CRC features in patients and, in particular, may be used to distinguish early- and advanced-stage patients with CRC.
Radical resection is the preferred treatment for the majority of patients with CRC. A total of 10 patients with CRC were followed up at 7 days following surgery, and it was observed that after surgical treatment, the frequency of MCps increased significantly. These data suggested that the frequency of MCps can be used as an indicator to evaluate the effectiveness of surgery.
Chronic low-grade inflammation is a hallmark of cancer, and mast cells are an important part of this inflammation (28). The role of mast cells in the tissue microenvironment of CRC is an area of research interest. A number of studies have suggested that the density of mast cells is negatively correlated with disease outcomes in CRC (16–18,29), whereas others hold the opposite view (30–32). In addition to the influence of race (33), region (34) and other factors, such as age and sex, we hypothesized that the method for detecting mast cells in tissues may also be one of the reasons for this phenomenon. Mast cells can be divided into tryptase-positive-only (MC T) and tryptase-positive/chymase-positive (MC TC) subtypes depending on the protease they contain. The subtype distribution also varies according to the organ in which they are found (35). It was reported that in human CRC tissues, the MC TC subtype accounts for ~75% of all mast cells. Both mast cell types seem to serve an important role in CRC pathogenesis (31). However, previous studies on tumour mast cells mainly analysed the effect of the MC T subtype on CRC prognosis (21,23). In the present study, total mast cells were defined as CD4−CD8−CD19−CD14−CD117+FcεRI+ (36) to investigate their components and phenotypes in cancer tissues. Flow cytometry was used to analyse the proportion of mature mast cells among CD45+ leukocytes, and it was found that the frequency of mast cells was highest in HC tissues, followed by adjacent normal tissues and then tumour tissues, which was consistent with a previous study (19). The frequency of mast cells in both HC and adjacent normal tissues did not reflect CRC progression.
In the present study, a lower frequency of circulating MCps was observed in patients with CRC, particularly in patients with advanced-stage disease. The frequency of MCps is related to CRC progression, which may reflect aggressive CRC features. Furthermore, according to the present data, the frequency of mast cells in tissue did not reflect the severity of the disease. There are certain limitations to the present study that should be mentioned; for example, MC T and MC TC subtypes were not detected by immunohistochemistry, thus total MC and MC T subtype could not be compared simultaneously. The migration of MCps to the intestine and the presence of mast cells in intestinal tissue after surgery need to be further investigated. The present findings may provide new insights into the role of mast cells in regulating CRC processes.
Acknowledgements
We thank our colleagues at the First Hospital of Jilin University (Changchun, China), they are Mr. Yue Yudong, M.M., for his help in sample collection, Ms. Lin Fangnan, M.M., for discussions and Ms. Liu Jing, M.M., for assistance with FACS.
Funding Statement
The present study was supported by The National Natural Science Foundation of China (grant nos. 30972610, 81273240, 91742107 and 81570002), The National Key Research and Development Program (grant nos. 2017YFC0910000 and 2017YFD0501300) and The Jilin Province Science and Technology Agency (grant nos. JJKH20211164KJ, JJKH20211210KJ, 20200403084SF, JLSWSRCZX2020-009, 20200901025SF, 20190101022JH, 2019J026, 20170622009JC, 2017C021, 2017J039, SXGJXX2017-8, JJKH20180197KJ, DBXM154-2018 and 2018SCZWSZX-015).
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
All authors contributed to the study concept and design as well as the interpretation of the data. PiZ, PeZ and RS wrote the original draft and conducted the experiments. TT acquired the samples and collated the data. YiJ wrote, reviewed and edited the manuscript. AL and XH formally analysed and confirm the authenticity of all the raw data. YaJ provided the resources, and wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Written informed consent was obtained from each participant. The experimental protocol was established according to the guidelines of The Declaration of Helsinki and was approved by the Human Ethics Committee of the First Hospital of Jilin University (Changchun, China; approval no. 2018-464).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Bruni D. Tumor Immunology and tumor evolution: Intertwined histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 3.IJsselsteijn ME, Sanz-Pamplona R, Hermitte F, de Miranda NFCC. Colorectal cancer: A paradigmatic model for cancer immunology and immunotherapy. Mol Aspects Med. 2019;69:123–129. doi: 10.1016/j.mam.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–667. doi: 10.1038/s41577-021-00534-x. [DOI] [PubMed] [Google Scholar]
- 5.Lv Y, Zhao Y, Wang X, Chen N, Mao F, Teng Y, Wang T, Peng L, Zhang J, Cheng P, et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway. J Immunother Cancer. 2019;7:54. doi: 10.1186/s40425-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallgren J, Gurish MF. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol. 2011;716:14–28. doi: 10.1007/978-1-4419-9533-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlin JS, Malinovschi A, Öhrvik H, Sandelin M, Janson C, Alving K, Hallgren J. Lin-CD34hi CD117int/hi FcεRI+ cells in human blood constitute a rare population of mast cell progenitors. Blood. 2016;127:383–391. doi: 10.1182/blood-2015-06-650648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marone G, Galli SJ, Kitamura Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002;23:425–427. doi: 10.1016/S1471-4906(02)02274-3. [DOI] [PubMed] [Google Scholar]
- 9.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komi DEA, Redegeld FA. Role of mast cells in shaping the tumor microenvironment. Clin Rev Allergy Immunol. 2020;58:313–325. doi: 10.1007/s12016-019-08753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata M, Shijubo N, Walls AF, Ichimiya S, Abe S, Sato N. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Arch. 2003;443:565–573. doi: 10.1007/s00428-003-0842-y. [DOI] [PubMed] [Google Scholar]
- 12.Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, Resta L. Tissue remodelling in breast cancer: Human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58:1096–1106. doi: 10.1111/j.1365-2559.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 13.Ranieri G, Ammendola M, Patruno R, Celano G, Zito FA, Montemurro S, Rella A, Di Lecce V, Gadaleta CD, Battista De Sarro G, Ribatti D. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol. 2009;35:115–120. doi: 10.3892/ijo_00000319. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Zhai L, Xue R, Shi J, Zeng Q, Gao C. Mast cell tryptase contributes to pancreatic cancer growth through promoting angiogenesis via activation of angiopoietin-1. Int J Mol Sci. 2016;17:864. doi: 10.3390/ijms17060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammendola M, Sacco R, Sammarco G, Donato G, Montemurro S, Ruggieri E, Patruno R, Marech I, Cariello M, Vacca A, et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: Possible biological-clinical significance. PLoS One. 2014;9:e99512. doi: 10.1371/journal.pone.0099512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265–1275. doi: 10.1111/j.1440-1746.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- 17.Malfettone A, Silvestris N, Saponaro C, Ranieri G, Russo A, Caruso S, Popescu O, Simone G, Paradiso A, Mangia A. High density of tryptase-positive mast cells in human colorectal cancer: A poor prognostic factor related to protease-activated receptor 2 expression. J Cell Mol Med. 2013;17:1025–1037. doi: 10.1111/jcmm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Ichikawa Y, Nakagawa K, Kumamoto T, Mori R, Matsuyama R, Takeda K, Ota M, Tanaka K, Tamura T, Endo I. High infiltration of mast cells positive to tryptase predicts worse outcome following resection of colorectal liver metastases. BMC Cancer. 2015;15:840. doi: 10.1186/s12885-015-1863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acikalin MF, Oner U, Topçu I, Yaşar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37:162–169. doi: 10.1016/j.dld.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Amin MB, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC, editors. AJCC Cancer Staging Manual. 8th edition. Springer; New York, NY: 2017. [DOI] [Google Scholar]
- 21.Xia Q, Wu XJ, Zhou Q, Jing-Zeng , Hou JH, Pan ZZ, Zhang XS. No relationship between the distribution of mast cells and the survival of stage IIIB colon cancer patients. J Transl Med. 2011;9:88. doi: 10.1186/1479-5876-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Blokhuis B, Derks Y, Kumari S, Garssen J, Redegeld F. Human mast cells promote colon cancer growth via bidirectional crosstalk: Studies in 2D and 3D coculture models. Oncoimmunology. 2018;7:e1504729. doi: 10.1080/2162402X.2018.1504729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlin JS, Hallgren J. Mast cell progenitors: Origin, development and migration to tissues. Mol Immunol. 2015;63:9–17. doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Drew E, Huettner CS, Tenen DG, McNagny KM. CD34 expression by mast cells: Of mice and men. Blood. 2005;106:1885–1887. doi: 10.1182/blood-2005-03-1291. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Zhu Y, Xu L, Zhang J, Xie H, Fu H, Zhou Q, Chang Y, Dai B, Xu J. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology. 2018;7:e1474317. doi: 10.1080/2162402X.2018.1474317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo X, Wang J, Suo T, Ni X, Liu H, Shen S, Li M, Wang Y, Liu H, Xu J. Tumor-infiltrating mast cells predict prognosis and gemcitabine-based adjuvant chemotherapeutic benefit in biliary tract cancer patients. BMC Cancer. 2018;18:313. doi: 10.1186/s12885-018-4220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, Granata F. Are mast cells MASTers in cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu G, Wang S, Cheng P. Tumor-infiltrating tryptase(+) mast cells predict unfavorable clinical outcome in solid tumors. Int J Cancer. 2018;142:813–821. doi: 10.1002/ijc.31099. [DOI] [PubMed] [Google Scholar]
- 30.Mehdawi L, Osman J, Topi G, Sjölander A. High tumor mast cell density is associated with longer survival of colon cancer patients. Acta Oncol. 2016;55:1434–1442. doi: 10.1080/0284186X.2016.1198493. [DOI] [PubMed] [Google Scholar]
- 31.Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol. 2005;11:1210–1214. doi: 10.3748/wjg.v11.i8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Curran T, Sun Z, Gerry B, Findlay VJ, Wallace K, Li Z, Paulos C, Ford M, Rubinstein MP, Chung D, Camp ER. Differential immune signatures in the tumor microenvironment are associated with colon cancer racial disparities. Cancer Med. 2021;10:1805–1814. doi: 10.1002/cam4.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu K, Miao L, Chen W, Wu H, Gong Y, Tu X, Gou W, Pan B, Qu C, Wu X, Wang B. Establishment of the reference intervals of lymphocyte subsets for healthy Chinese Han adults and its influencing factors. Ann Transl Med. 2021;9:1495. doi: 10.21037/atm-21-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wernersson S, Pejler G. Mast cell secretory granules: Armed for battle. Nat Rev Immunol. 2014;14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 36.Huber M, Cato ACB, Ainooson GK, Freichel M, Tsvilovskyy V, Jessberger R, Riedlinger E, Sommerhoff CP, Bischoff SC. Regulation of the pleiotropic effects of tissue-resident mast cells. J Allergy Clin Immunol. 2019;144((Suppl 4)):S31–S45. doi: 10.1016/j.jaci.2019.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.