Abstract
Objective:
Ecological momentary assessment (EMA) may help with the development of more targeted interventions for caregivers’ depression, yet the use of this method has been limited among cancer caregivers. This study aimed to demonstrate the feasibility of EMA among cancer caregivers and the use of EMA data to understand affective correlates of caregiver depressive symptoms.
Methods:
Caregivers (N=25) completed a depressive symptom assessment (Patient Health Questionnaire-8) and then received eight EMA survey prompts per day for seven days. EMA surveys assessed affect on the orthogonal dimensions of valence and arousal. Participants completed feedback surveys regarding the EMA protocol at the conclusion of the week-long study.
Results:
Of 32 caregivers approached, 25 enrolled and participated (78%), which exceeded the a priori feasibility cutoff of 55%. The prompt completion rate (59%, or 762 of 1,286 issued) did not exceed the a priori cutoff of 65%, although completion was not related to caregivers’ age, employment status, physical health quality of life, caregiving stress, or depressive symptoms or the patients’ care needs (ps>.22). Caregivers’ feedback about their study experience was generally positive. Mixed-effects location scale modeling showed caregivers’ higher depressive symptoms were related to overall higher reported negative affect and lower positive affect, but not to affective variability.
Conclusions:
Findings from this feasibility study refute potential concerns that an EMA design is too burdensome for distressed caregivers. Clinically, findings suggest the potential importance of not only strategies to reduce overall levels of negative affect, but also to increase opportunities for positive affect.
Keywords: Cancer, Oncology, Affect, Caregivers, Depression, Ecological Momentary Assessment, Psycho-Oncology
INTRODUCTION
Novel technology-based interventions hold significant potential to increase psychosocial care accessibility and affordability for family caregivers. In addition, existing psychosocial care could be made more efficient and effective with dynamic assessment of caregivers’ daily experiences, facilitated by technology, by fitting the right intervention to a caregivers’ specific needs.1 More scalable and targeted treatments may be particularly beneficial for addressing depression among cancer caregivers – approximately one in four report clinically significant depressive symptoms,2–5 but interventions have shown minimal success in addressing these symptoms among caregivers.6,7 Prior research provides limited information on the mechanisms related to caregivers’ psychological symptoms, given that cross-sectional and retrospective self-report methods have been most common. Assessing day-to-day features of caregivers’ depressive symptoms is necessary to optimize treatments for caregivers that provide them with the right interventions for their unique needs in the right time and order.
Although caregiving stress theories have sought to describe cancer caregivers’ trajectories of emotional adjustment,8,9 existing studies have not been designed to define reliable day-to-day markers of depressive symptoms among caregivers. Ecological momentary assessment (EMA) is the gold-standard method for studying dynamic phenomena to help guide the development of more optimized and timely technology-delivered interventions.10–12 One potential candidate for a modifiable and proximal marker to target with depression interventions among caregivers is affective variability, or the degree to which an individual’s mood tends to be stable versus fluctuating. In the general population, depression risk has been associated both with affective variability – both high variability, meaning frequent swings in affect intensity and type, and low variability, meaning blunted emotional responsivity.13–16 Among caregivers, who have described their day-to-day emotional experiences as “roller coasters,”17 cross-sectional study findings suggest that more variable affect is associated with lower caregiving burden potentially due to protective effects from upswings of positive affect.18 Identifying proximal targets of clinical disorders common among caregivers using intensive longitudinal data collection strategies is needed to both improve existing caregiver interventions by selecting and ordering intervention components that will most effectively address caregivers’ depression, as well as to develop effective technology-based health interventions that will scale to address the exponential rise in caregiver psychosocial needs.
Of over 5,400 citations returned from a PubMed search in November 2020 with mentions of “ecological momentary assessment” – or related terms “experience sampling,” “ambulatory assessment,” “intensive longitudinal,” or “daily diary” – in the title or abstract, seven reported findings from six unique studies among a sample of cancer caregivers (see Supplementary Table 1 for study citations and overviews). Of these studies, only two issued multiple prompts per day – with a maximum of three per day – and none focused on caregivers’ depressive symptoms. As such, the overarching objective of this study was to establish the feasibility of EMA methodology to study affect and depressive symptoms among cancer caregivers. First, we document the feasibility of conducting an EMA study among active cancer caregivers in terms of recruitment, engagement, and acceptability; we secondarily demonstrate one novel analytic technique enabled by intensive longitudinal data examining the relation between caregivers’ self-reported depressive symptoms and their affective variability.
METHODS
Participants
This study was approved by the Institutional Review Board at the University of Virginia (HSR-IRB #21308). Participants were recruited from two outpatient oncology clinics at the NCI Designated University of Virginia Cancer Center from March to September 2019. Eligible individuals were caregivers who self-reported: (1) providing unpaid care (e.g., practical, medical, or emotional support) to a family member/friend receiving anticancer therapy, (2) being aged ≥18, (3) comfort speaking and reading English, and (4) owning a smartphone (Android or iOS). Only one caregiver per patient was eligible to participate. All individuals provided written informed consent prior to participating.
Procedure
Partnering physicians identified potentially eligible caregivers and provided a warm handoff to JG who completed screening and informed consent. Participants were told that the purpose of the study was to examine cancer caregivers’ day-to-day mood. Upon recruitment, enrolled caregivers completed an online baseline survey. Beginning the day following completion of the baseline survey, participants were administered eight EMA surveys per day through their smartphone for seven consecutive days. This design was selected as it is comparable to EMA designs commonly used to examine mood and depression among adults,19 and a recent study among a college student sample suggests that higher daily sampling frequency with a brief questionnaire may not be associated with higher reported burden or compromised data quality.20 A time-contingent sampling design was used: Qualtrics survey links were delivered by text messages sent randomly within a stratified schedule of eight 1.5-hour windows between 9 AM and 9 PM.a Survey link texts could be delivered between two and 180 minutes apart. Participants were instructed to complete surveys upon receiving the text message; surveys completed more than 1 hour after the prompt was issued were excluded. On the final day of completing surveys, participants were asked to complete an online feedback survey. Participants were compensated $50 for participation, which was not contingent on number of EMA surveys or feedback survey completion.
Measures
Baseline survey.
Baseline surveys collected caregiver sociodemographic and caregiving contextual information. Depressive symptoms experienced during the 2 weeks prior to baseline were self-reported using the Patient Health Questionnaire-8 (PHQ-8).21 Items are rated on a Likert scale from 0 (“Not at all”) to 3 (“Nearly every day”). Summed scores range from 0 to 24, with higher scores indicating higher depressive symptoms and a score of 5 or greater indicative of mild clinically significant depressive symptoms.21,22 The scale demonstrated good reliability in the present sample (Cronbach’s α=.90).
To examine whether caregiving burden factors were associated with EMA survey completion, caregivers also reported their physical health quality of life (QOL; PROMIS Short-form Physical Functioning-423; higher T-scores indicate better QOL; α=.96), caregiving stress (Pearlin Stress Scale – 4-item Caregiving Overload subscale24; higher mean scores indicate greater stress; α=.77), and number of Activities of Daily Living (ADL) and Instrumental ADL (IADL) for which the patient is dependent (of 15 total ADL/IADL assessed).
EMA surveys: Affect.
Affect was assessed based on two intersecting, orthogonal dimensions of valence (displeasure to pleasure) and arousal (deactivated to activated). Twenty emotion words reported by Kuppens and colleagues13 from their model of affective variability were used.14 Participants rated how much they currently felt each emotion item on a scale from 0 (“Do not feel this way at all”) to 6 (“Feel this strongly”). Four discrete categories of emotion were first computed: Negative active affect (NAA) represents the average of responses to nervous, stressed, tense, embarrassed, and upset; negative deactive affect (NDA) averages sluggish, sad, bored, depressed, and disappointed; positive active affect (PAA) averages enthusiastic, happy, proud, excited, and alert; and positive deactive affect (PDA) averages calm, peaceful, satisfied, relaxed, and content. In accordance with Kuppens’ and colleagues’ formulas,13 affect scales were computed as the following: total negative affect represents the difference between NAA and its inverse of PDA; total positive affect represents the difference between PAA and its inverse of NDA; valence represents the sum of PAA and PDA less the sum of NAA and NDA; activation represents the sum of NAA and PAA less the sum of NDA and PDA.
Feedback survey.
Participants reported feedback about the daily EMA surveys on five items adapted from prior work (see Figure 2 for items).25 Responses were recorded using a six-item Likert response format (“Strongly Disagree” to “Strongly Agree”). If a participant rated an item unfavorably (e.g., disagreed with the item “the questions were easy to understand”), an open-ended response item appeared to solicit additional feedback.
Figure 2.
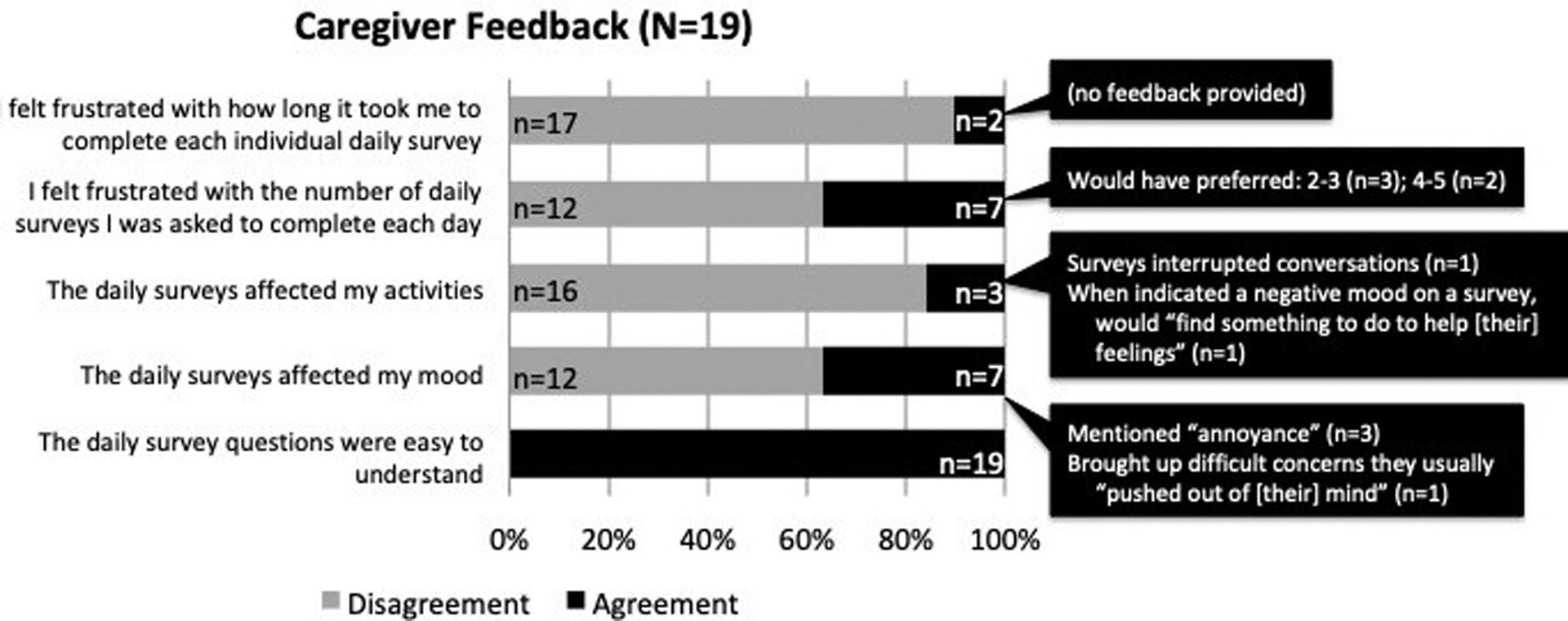
Caregiver feedback
Data Analysis
Descriptive statistics (medians [Mdn] with interquartile ranges or frequencies, as appropriate) for study variables are presented in Table 1. Descriptive analyses were conducted using R software (version 3.6.3). Statistical significance was set at α=.05, two-tailed tests.
Table 1.
Sample descriptives (N=25)
| Participant characteristics | n(%) or Mdn(Range) |
|---|---|
| Age | 54 (27–75) |
| Gender (female) | 17 (68%) |
| Household income* (<$100,000) | 9 (43%) |
| Formally employed (yes) | 13 (52%) |
| Physical health quality of life | 57.00 (33.20–57.00) |
| Patient dependent for 1 or more ADL/IADL* (yes) | 13 (54%) |
| Caregiving stress | 2 [1.50–2.25] |
| Relationship to patient | |
| Spouse | 16 (64%) |
| Adult child | 3 (12%) |
| Other | 6 (24%) |
| Patients’ Outpatient Cancer Clinic | |
| Gastrointestinal | 16 (64%) |
| Head and Neck | 9 (36%) |
| Feasibility Metrics | n(%) or Mdn%[IQR%] |
| Total survey responses** | 762 (59%) |
| Proportion of surveys receiving responses per participant | 61% [39%−75%] |
| Participants responding to ≥65% of surveys | 12 (48%) |
| Mood | Mdn[IQR] |
| Depressive symptoms | 3 [1–7] |
Percentage responding – 4 declined to provide income, 1 declined to report ADL/IADL.
Responses logged within 1 hour from survey issuance.
Note: Mdn = Median; IQR = Interquartile range; PHQ-8 = Patient Health Questionnaire-8; PSS = Pearlin Stress Scale, Caregiving Stress Overload subscale.
Our primary aim was to examine EMA feasibility in terms of recruitment, engagement, and participant feedback. To determine recruitment feasibility, an a priori benchmark of 55% enrollment was set based on national norms for recruitment to studies of cancer caregivers.26 For EMA engagement feasibility, due to a dearth of EMA studies in chronic health populations, an a priori benchmark of 65% prompt completion was set based on national norms for prompt completion in typical EMA studies.27,28 The correlations between survey response completion rate and participants’ age, employment status, physical health quality of life, caregiving stress, and depressive symptoms and patient ADL/IADL dependency were examined. Quantitative and qualitative data from participant feedback surveys were tabulated.
Our secondary aim was to demonstrate an analytic technique, mixed-effects location scale modeling, enabled by the collection of intensive longitudinal data, which we use to examine relations between caregivers’ depressive symptoms and affect variability. Mixed-effects location scale models extend traditional mixed effects models by directly modeling within-subjects variability in terms of subject-level random effects in order to capture and explain individual differences in participants’ variability (stage 1) and the extent to which these individual differences in variability relate to other constructs (stage 2).29 To illustrate, fictional data is presented in Figure 1 for four hypothetical participants: A and B have similar high “location” estimates because they both report high overall mean negative affect over the course of a day relative to participants C and D. However, A and B differ in their affective variability, or “scale”: Participant A has a higher scale estimate as their negative affect reports are more erratic, while participant B has a lower scale estimate as their reports are more consistent. These random effect estimates for each participant’s affective location and scale are extracted from the stage 1 models and used as independent variables in stage 2 to detect associations with an identified outcome variable. These models have been used to understand cancer-related health behaviors like physical activity30 and smoking cessation31 in the general population, but have not yet been used to characterize affective variability among people with a history of cancer or their family caregivers.
Figure 1.
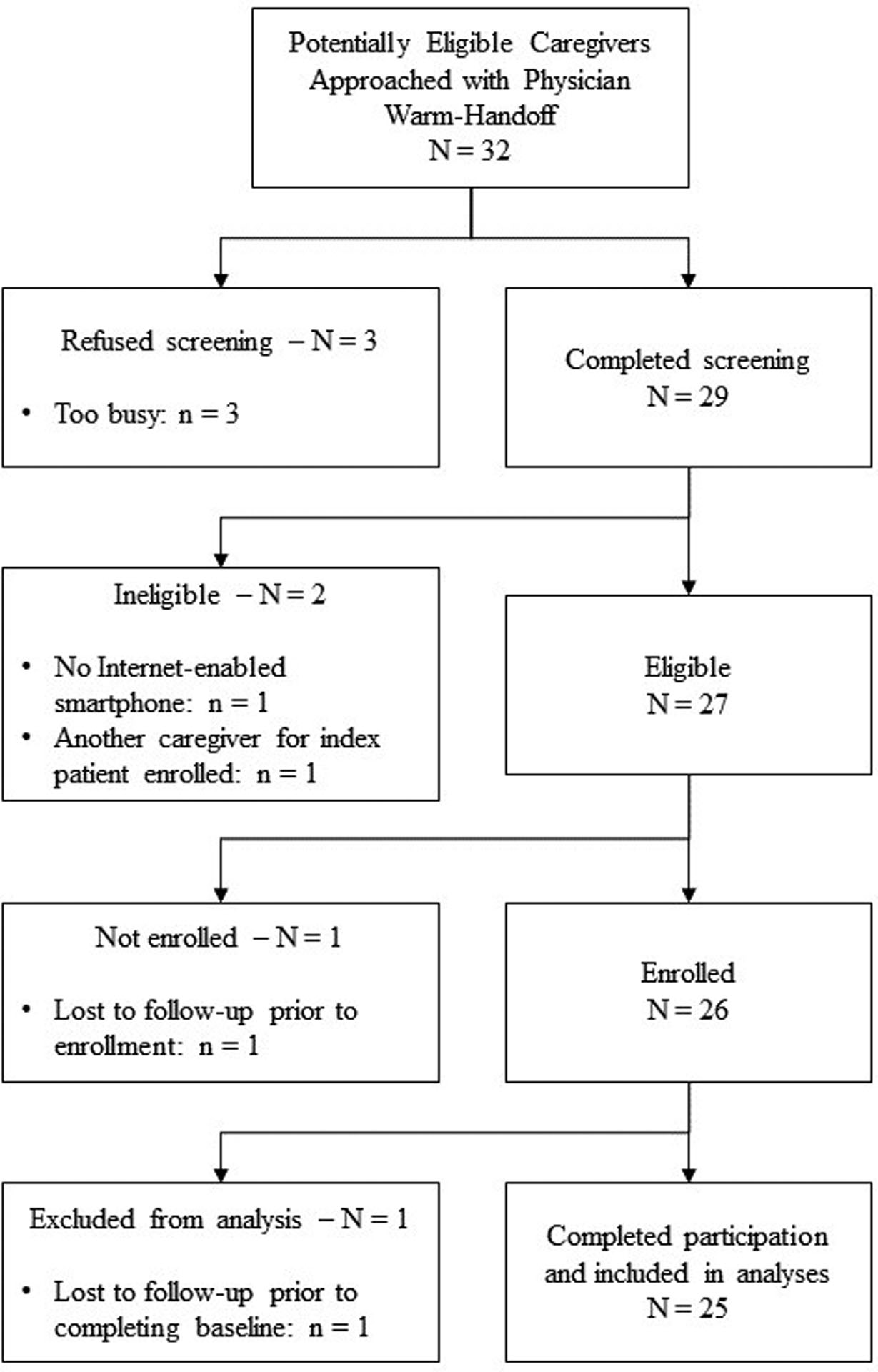
Recruitment and Enrollment Flow Diagram
To test the relation between affective variability and depressive symptoms, separate two-stage mixed-effects location scale models were run for each affective construct (i.e., NAA, NDA, PAA, PDA, total positive affect, total negative affect, activation, and valence). In stage 1, individuals’ own mean NAA (i.e., random intercepts or “location”) and their own variability in NAA (i.e., random within-subject variance or “scale”) were estimated as random subject effects. These random effects from stage 1 were then included as independent variables (main effects of scale and location) and crossed as an interaction effect (scale by location effect) in a regression model of depressive symptoms as outcome in stage 2. Analyses are conducted via MixWILD standalone software.32
RESULTS
Sample Characteristics
Sample characteristics of the 25 participating caregivers are listed in Table 1 and summarized here. Participant ages ranged from 27 to 75 (Mdn=54) and approximately two-thirds were women (n=17), two in five reported a yearly household income under $100,000 (n=9), and half were formally employed (n=13). Approximately half of the caregivers reported their care recipient was dependent for one or more ADL/IADL (n=13), two-thirds were the spouse of the cancer patient (n=16), and caregivers reported on average that they felt “somewhat” overloaded by caregiving (Mdn=2). About two-thirds of the participants were recruited from the outpatient gastrointestinal cancer clinic (n=16; 64%) and the remaining nine participants were recruited from the outpatient head and neck cancer clinic (36%). About half of the participants reported mild depressive symptoms or higher on the PHQ-8 (n=12; 48%).
EMA Feasibility
Recruitment.
As detailed in Figure 1, thirty-two individuals were approached by the research assistant, with three refusing screening (9%, all cited being too busy). Of the 29 individuals screened, two were ineligible (7%; one due to not having an Internet-enabled smartphone, one due to another caregiver for the same patient having already enrolled in the study). Of the 27 individuals screened and eligible, one was lost to follow-up prior to enrollment (4%), but all others enrolled. Of the 26 enrolled participants, one was lost to follow-up prior to completing any assessments (4%) and was excluded from analyses. The 25 participating individuals represent 93% of confirmed eligible individuals and 78% of all individuals approached for recruitment, exceeding our a priori benchmark of 55% for determining recruitment feasibility.
EMA engagement.
Of 1,286 total prompts issued, participants completed 762 surveys (total completion rate=59%), falling short of our a priori benchmark of a 65% prompt completion rate for determining EMA design feasibility. The median percentage of surveys completed by each participant was 61% (e.g., 34 of 56 total prompts). Twelve participants (48%) surpassed our a priori benchmark of completing ≥65% of issued prompts. The range of prompt completion rates was 20% to 98%. Prompt completion rates did not differ between weekend days versus weekdays (t[24]=0.04, p=.97), nor did rates differ by participants’ age (r=.26, p=.22), being employed (t[22.78]=0.15, p=.88), physical health quality of life (r=0.23, p=.28), patient dependency on 1 or more ADL/IADL (t[21.60]=−0.10, p=.92), caregiving stress (r=−.12, p=.57), or depressive symptoms (r=−.05, p=.91).
Participant feedback.
Of the 25 enrolled participants, 19 (76%) completed the final feedback survey. Responders completed more prompts (Mdn=37 prompts) and reported lower depressive symptoms (PHQ-8 Mdn=3) compared to the six non-responders (Mdn=21 prompts, PHQ-8 Mdn=7.5). Quantitative and qualitative data are presented in Figure 2. Feedback was generally positive – for instance, all 19 respondents agreed that the survey questions were easy to understand, 17 of the 19 were satisfied with the time required to complete each survey, and 16 of the 19 indicated surveys didn’t affect their activities. Two-thirds also indicated surveys did not affect their mood and found the number of surveys acceptable; among the remaining one-third, some participants indicated that surveys could be an “annoyance” and some would have preferred fewer prompts per day.
Relation of Depressive Symptoms with Affective Variability
Two-stage mixed effects location-scale models tested whether individuals’ affective means (location) or affective variability (scale) relate to their depressive symptoms. As reported in Table 2, individuals who reported higher average NAA, NDA, total negative affect, and activation relative to the sample (positive location estimates) also tended to endorse higher depressive symptoms; those who reported lower average PDA, total positive affect, and valence relative to the sample (negative location estimates) also tended to endorse higher depressive symptoms. There were no reliable effects detected for individual affective variability (scale estimates) on depressive symptoms. Although there was a trend towards individuals who reported more erratic total positive affect relative to the sample (positive scale estimate) also tending to report higher depressive symptoms (p=.07), the 95% confidence interval included 0, limiting the ability to conclude that a robust relationship exists between these variables. There were also no reliable effects detected between depressive symptoms and the interaction effects of individuals’ affective means with their affective variability.
Table 2.
Linear Regression Estimates from Mixed-Effects Location Scale Modeling Stage 2 Models Testing Effects of Affect on Depressive Symptoms
| Model | Unstandardized Effect (b) | 95% Confidence Interval of b | Standardized Effect (z) | p |
|---|---|---|---|---|
| Negative Active Affect | ||||
| Location | 3.36 | 1.95, 4.76 | 4.68 | <.001 |
| Scale | −0.05 | −1.42, 1.31 | −0.08 | .94 |
| Location × Scale | 0.12 | −1.44, 1.68 | 0.15 | .88 |
| Negative Deactive Affect | ||||
| Location | 3.49 | 2.27, 4.72 | 5.59 | <.001 |
| Scale | −1.14 | −2.60, 0.33 | −1.52 | .13 |
| Location × Scale | 0.14 | −1.43, 1.71 | 0.17 | .86 |
| Total Negative Affect | ||||
| Location | 3.06 | 1.73, 4.39 | 4.52 | <.001 |
| Scale | −0.07 | −1.53, 1.39 | −0.09 | .93 |
| Location × Scale | 0.19 | −1.61, 1.98 | 0.20 | .84 |
| Positive Active Affect | ||||
| Location | −0.59 | −2.50, 1.31 | −0.61 | .54 |
| Scale | 0.88 | −0.95, 2.71 | 0.94 | .35 |
| Location × Scale | −0.56 | −2.57, 1.45 | −0.55 | .59 |
| Positive Deactive Affect | ||||
| Location | −2.32 | −3.87, −0.77 | −2.94 | .003 |
| Scale | −0.45 | −2.35, 1.45 | −0.47 | .64 |
| Location × Scale | 0.49 | −1.53, 2.50 | 0.47 | .64 |
| Total Positive Affect | ||||
| Location | −2.15 | −3.64, −0.65 | −2.81 | .005 |
| Scale | 1.36 | −0.13, 2.85 | 1.78 | .07 |
| Location × Scale | −0.67 | −2.04, 0.71 | −0.95 | .34 |
| Valence | ||||
| Location | −2.85 | −4.26, −1.43 | −3.94 | <.001 |
| Scale | 0.67 | −0.91, 2.25 | 0.83 | .40 |
| Location × Scale | −0.44 | −1.94, 1.07 | −0.57 | .57 |
| Activation | ||||
| Location | 2.41 | 0.61, 4.22 | 2.62 | .009 |
| Scale | 0.53 | −1.23, 2.29 | 0.59 | .56 |
| Location × Scale | −0.41 | −2.01, 2.19 | −0.31 | .76 |
DISCUSSION
Findings from this first study using EMA to understand day-to-day markers of depressive symptoms among cancer caregivers suggests that cancer caregivers are amenable to participating in studies with an EMA design and allays potential concerns that these designs may be too burdensome for this population. Although prompt completion rates in the present study did fall closely below the typical rates in prior EMA research,27,28 engagement was not related to factors including caregivers’ depressive symptoms, subjective caregiving burden, or the patients’ care needs. Findings also support that caregivers’ depressive symptoms relate in expected directions with their overall levels of self-reported day-to-day negative and positive affect, although effects were not detected between caregivers’ depressive symptoms and their affective variability. Taken together, this study demonstrates the capability of EMA designs to feasibly and acceptably study affective markers of depression among cancer caregivers with the potential to guide the development of more scalable, effective, and timely interventions.
Because repeated assessment in the context of daily routines is less biased by retrospection,10–12 EMA methodology is especially beneficial for the study of a psychological disorder such as depression, which by its nature includes cognitive biases affecting the recollection of mood-incongruent events. Feasibility data from this study suggest that the one-week EMA design with baseline and feedback surveys was acceptable to most caregivers. With more than three caregivers enrolled in this study of every four approached, the recruitment rate for this study far exceeds the average recruitment rate of 55% across prior cancer caregiving studies.26 Importantly, engagement in this study, as measured by percentage of survey prompts completed, was not associated with factors including caregivers’ self-reported caregiving burden or depressive symptoms. These findings together refute potential concerns that an EMA design is too burdensome for distressed caregivers.
Our overall engagement of about 60% of prompts completed did not exceed our a priori benchmark of 65%, meaning our recorded engagement was slightly lower than average engagement typical in other EMA studies reported among the general population.27,28 Our engagement was also low compared to a previously published EMA study among cancer caregivers that recorded a 90.5% survey completion rate, though a key difference is that the previously published study examining communication and relationship satisfaction employed a design of twice-daily prompts over 14 consecutive days.33 Although EMA studies invoke a common methodology of repeated longitudinal assessments, there is considerable variation in the frequency of assessments and the duration of the study period, which should be determined based on the research question. In the current study, we used a relatively heavy sampling strategy to understand variations in daily mood – a subjective experience more subject to recall bias than reporting more discrete events like social interactions. Studies that implement more (vs. less) frequent surveys in a short period of time can collect richer data about daily life, but tend to be susceptible to lower EMA completion rates.34
Our engagement was also below that from a study among dementia caregivers with a similar design (10 prompts per day for six days), which reported over 78% of prompts completed by participants.25 A key difference, however, is that the study among dementia caregivers provided one to two phone calls to participants during the EMA period to resolve problems completing assessments, whereas we provided no outreach to participants. Design choices to increase compliance to EMA should be carefully considered in terms of costs versus benefits for each study. Although compliance monitoring is common,27,28 it adds research staff costs; although fewer prompts per day may have been preferred by caregivers, more frequent assessment results in richer data affording a more nuanced understanding of time-varying experiences.35
Datasets generated by an EMA design facilitate nuanced examinations between caregivers’ depressive symptoms and their day-to-day affective patterns. Results from the intra-individual variability modeling technique revealed that, as expected, caregivers who reported higher depressive symptoms tended to report higher overall levels of negative affect and lower overall levels of positive affect across their completed EMA prompts relative to the sample; however, the degree to which a caregivers’ affect was relatively consistent versus erratic was not related to their depressive symptoms. These results differ from prior work that demonstrated affective variability – both too much and too little – relates to depression risk.13–16 While our analytic methods differ from those prior studies, these discrepant findings suggest close examination of how risk factors and mechanisms of depression among non-caregivers compare and contrast to those among caregivers may help to improve caregiver interventions. Future studies should examine the extent to which these states of higher NA and lower PA not only characterize caregivers with concurrently higher depressive symptoms, but also whether these affective characteristics represent individual risk factors for developing depression throughout the course of caregiving.
Clinical Implications
Dynamic, in vivo assessment of caregivers’ psychological distress could provide important insights into matching the right intervention component to a caregiver’s specific needs and to provide that intervention at the most appropriate time. Findings from data analyses suggest the potential importance of not only strategies to reduce overall levels of negative affect, but also to increase opportunities for positive affect – even if only sporadically – to managing depressive symptoms among cancer caregivers. Considering the time demands of caregiving, some caregivers have reported feeling frustrated by, or just being unable to enact, well-meaning directives to “take care of themselves,”36 or feel guilty when they do.37 Directly addressing competing demands while incorporating strategies to feasibly cultivate and enjoy positive experiences may be important to buffer against depressive symptoms among caregivers. The ubiquity of smartphones38 – and particular use of smartphones among caregivers39,40 – suggests this medium may be uniquely suited to deliver interventional “nudges” to increase positive emotions across the day while decreasing negative ones may sum to reduced risk for chronic depression among caregivers over time. Further research to identify modifiable markers of caregiver depressive symptoms will be essential to develop such models of more effective and accessible technology-delivered depression interventions for caregivers.
Study Limitations
This study was not pre-registered. Given the primary aims of this study were related to understanding feasibility in terms of study recruitment, engagement, and acceptability, the sample size was not determined specifically to power analyses examining the effects between depressive symptoms and daily measured constructs. Future research with a larger sample size powered for main hypotheses related to depressive symptoms and daily constructs is warranted. Despite the small sample size, a strength of our sample was the wide range of depressive symptom ratings reported across participants. Related to the primary aim of feasibility, we were less likely to receive feedback surveys from participants who engaged less frequently with the EMA surveys and who reported higher depressive symptoms, despite multiple contacts to request feedback. Findings from our feedback questionnaire must therefore be interpreted with caution.
Conclusions
From this first study to describe the feasibility of EMA to understand day-to-day markers of depressive symptoms among cancer caregivers, findings suggests that this ecologically-valid, repeated assessment methodology is feasible and generally acceptable among caregivers. Moreover, caregiver depressive symptoms and subjective caregiving burden were not related to how many prompts caregivers completed, refuting potential concerns that this design is too burdensome for distressed caregivers. Caregivers’ overall average levels of positive and negative affect were related to their depressive symptoms, which suggests the pertinence of interventional strategies to both reduce general negative affect as well as enhance experiences of positive affect in depression interventions for caregivers. Continued EMA research is warranted to better understand trajectories of caregiver psychological distress and potential intervention targets in order to develop more effective and accessible interventions.
Supplementary Material
Supplementary Table 1. Studies implementing EMA with Cancer Caregivers
Supplementary Figure 1. Illustration of negative affect location and scale: Note. Traditional mixed-effects models assume equivalence in individuals’ within-subjects variability – meaning that all individuals’ variability is assumed to be the same. As depicted in the figure, however, it is a reasonable assumption that affective variability meaningfully differs across participants. Mixed-effects location scale models extend traditional mixed effects models by directly modeling within-subjects variability in terms of subject-level random effects in order to capture and explain individual differences in within-subjects variability. In the first modeling stage, individual participants’ own average affect relative to the sample is computed as a random effect as the “location” estimate. A positive location estimate indicates that the participant has a higher average affect score relative to the sample (e.g., reports higher average negative affect relative to other caregivers sampled; like participants A & B), while a negative location estimate indicates that the participant has a lower average affect score relative to the sample (e.g., reports lower average negative affect relative to other caregivers sampled; like participants C & D). Similarly, individual participants’ own variability in their reported affect across measures relative to the sample is computed as a random effect as the “scale” estimate. A positive scale estimate indicates that the participant reports more erratic affect scores relative to the sample (e.g., reports more erratic levels of negative affect relative to other caregivers sampled; like participants A & D), while a negative scale estimate indicates that the participant reports more consistent affect scores relative to the sample (e.g., reports more consistent levels of affect relative to other caregivers sampled; like participants B & C). These effects from stage 1 models are extracted and imputed as independent variables in the stage 2 models to detect associations with a dependent variable of interest (i.e., depressive symptoms). To account for uncertainty in random effects estimates, the MIXWILD software uses plausible value methodology (Mislevy, 1991, Psychometrika) to impute the random effects 500 times from the estimate posterior distribution – this approach accounts for the uncertainty in the random effect estimates. The stage 2 analyses are repeated for each set of imputed random effect estimates, and then averaged (using Rubin’s rules for multiple imputation) to yield overall regression estimates.
ACKNOWLEDGEMENTS:
We are sincerely grateful for the caregivers who participated in this study, as well as for the recruitment assistance of B.J. Ferrebee Ghamandi.
FUNDING:
This study was funded by ACS grant #IRG 81-001-26. Dr. Shaffer was supported in part by the NIH NCATS Award Numbers UL1TR003015 and KL2TR003016. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICTS OF INTEREST: None.
Of the 25 participants, four were affected by a distribution error in Qualtrics (active August 1–6, 2019), the platform used to deliver survey text messages, resulting in only the first prompt of the day being issued. One participant experienced two affected days, one experienced four affected days, one experienced five affected days, and one experienced six affected days.
DATA AVAILABILITY STATEMENT:
Data available upon reasonable request from the authors.
REFERENCES
- 1.Fisher AJ. Toward a dynamic model of psychological assessment: Implications for personalized care. Journal of consulting and clinical psychology. 2015;83(4):825. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y, Shaffer KM, Carver CS, Cannady RS. Prevalence and predictors of depressive symptoms among cancer caregivers 5 years after the relative’s cancer diagnosis. Journal of Consulting and Clinical Psychology. 2014;82(1):1. [DOI] [PubMed] [Google Scholar]
- 3.Rhee YS, Yun YH, Park S, et al. Depression in family caregivers of cancer patients: The feeling of burden as a predictor of depression. Journal of Clinical Oncology. 2008;26(36):5890–5895. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. The Lancet Oncology. 2013;14(8):721–732. [DOI] [PubMed] [Google Scholar]
- 5.Girgis A, Lambert SD, McElduff P, et al. Some things change, some things stay the same: a longitudinal analysis of cancer caregivers’ unmet supportive care needs. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2013;22(7):1557–1564. [DOI] [PubMed] [Google Scholar]
- 6.Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA: A Cancer Journal for Clinicians. 2010;60(5):317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’toole MS, Zachariae R, Renna ME, Mennin DS, Applebaum A. Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta-analysis. Psycho-oncology. 2017;26(4):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijboer C, Tempelaar R, Sanderman R, Triemstra M, Spruijt RJ, Van Den Bos GA. Cancer and caregiving: the impact on the caregiver’s health. 1998;7(1):3–13. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher BS, Miaskowski C, Given B, Schumacher K. The cancer family caregiving experience: an updated and expanded conceptual model. European Journal of Oncology Nursing. 2012;16(4):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- 11.Gorin AA, Stone AA. Recall biases and cognitive errors in retrospective self-reports: A call for momentary assessments. In: Handbook of Health Psychology. Lawrence Erlbaum; 2001:405–413. [Google Scholar]
- 12.Clark DM, Teasdale JD. Diurnal variation in clinical depression and accessibility of memories of positive and negative experiences. Journal of abnormal psychology. 1982;91(2):87. [DOI] [PubMed] [Google Scholar]
- 13.Kuppens P, Van Mechelen I, Nezlek JB, Dossche D, Timmermans T. Individual differences in core affect variability and their relationship to personality and psychological adjustment. Emotion. 2007;7(2):262. [DOI] [PubMed] [Google Scholar]
- 14.Kuppens P, Oravecz Z, Tuerlinckx F. Feelings change: Accounting for individual differences in the temporal dynamics of affect. Journal of personality and social psychology. 2010;99(6):1042. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM. Stress, stress-related disease, and emotional regulation. Handbook of emotion regulation. Published online 2007:606–615. [Google Scholar]
- 16.Wichers M, Simons CJP, Kramer IMA, et al. Momentary assessment technology as a tool to help patients with depression help themselves. Acta Psychiatrica Scandinavica. 2011;124(4):262–272. doi: 10.1111/j.1600-0447.2011.01749.x [DOI] [PubMed] [Google Scholar]
- 17.Stamataki Z, Ellis JE, Costello J, Fielding J, Burns M, Molassiotis A. Chronicles of informal caregiving in cancer: using ‘The Cancer Family Caregiving Experience’model as an explanatory framework. Supportive care in cancer. 2014;22(2):435–444. [DOI] [PubMed] [Google Scholar]
- 18.Robertson SM, Zarit SH, Duncan LG, Rovine MJ, Femia EE. Family caregivers’ patterns of positive and negative affect. Family Relations. 2007;56(1):12–23. [Google Scholar]
- 19.aan het Rot M, Hogenelst K, Schoevers RA. Mood disorders in everyday life: A systematic review of experience sampling and ecological momentary assessment studies. Clinical psychology review. 2012;32(6):510–523. [DOI] [PubMed] [Google Scholar]
- 20.Eisele G, Vachon H, Lafit G, et al. The effects of sampling frequency and questionnaire length on perceived burden, compliance, and careless responding in experience sampling data in a student population. Assessment. Published online 2020:1073191120957102. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114(1):163–173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General Hospital Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®−29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885–1891. doi: 10.1007/s11136-018-1842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30(5):583–594. [DOI] [PubMed] [Google Scholar]
- 25.van Knippenberg RJM, De Vugt ME, Ponds RW, Myin-Germeys I, van Twillert B, Verhey FRJ. Dealing with daily challenges in dementia (deal-id study): an experience sampling study to assess caregiver functioning in the flow of daily life. International journal of geriatric psychiatry. 2017;32(9):949–958. [DOI] [PubMed] [Google Scholar]
- 26.Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones A, Remmerswaal D, Verveer I, et al. Compliance with ecological momentary assessment protocols in substance users: a meta‐analysis. Addiction. 2019;114(4):609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen CKF, Schneider S, Stone AA, Spruijt-Metz D. Compliance With Mobile Ecological Momentary Assessment Protocols in Children and Adolescents: A Systematic Review and Meta-Analysis. J Med Internet Res. 2017;19(4). doi: 10.2196/jmir.6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedeker D, Mermelstein RJ, Demirtas H. Modeling Between- and Within-Subject Variance in Ecological Momentary Assessment (EMA) Data Using Mixed-Effects Location Scale Models. Stat Med. 2012;31(27). doi: 10.1002/sim.5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunton GF, Atienza AA, Huh J, Castro C, Hedeker D, King AC. Applying mixed-effects location scale modeling to examine within-person variability in physical activity self-efficacy. International Journal of Statistics in Medical Research. 2013;2(2):117–122. [Google Scholar]
- 31.Courvoisier D, Walls TA, Cheval B, Hedeker D. A mixed-effects location scale model for time-to-event data: A smoking behavior application. Addictive Behaviors. 2019;94:42–49. doi: 10.1016/j.addbeh.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 32.Hedeker D, Nordgren R. MIXREGLS: A Program for Mixed-Effects Location Scale Analysis. J Stat Softw. 2013;52(12):1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer SL, Romano JM, Todd M, et al. Links between communication and relationship satisfaction among patients with cancer and their spouses: results of a fourteen-day smartphone-based ecological momentary assessment study. Frontiers in psychology. 2018;9:1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nezlek JB. Diary Methods. Sage; 2012. [Google Scholar]
- 35.Csikszentmihalyi M Handbook of Research Methods for Studying Daily Life. Guilford Press; 2011. [Google Scholar]
- 36.Shaw J, Harrison J, Young J, et al. Coping with newly diagnosed upper gastrointestinal cancer: a longitudinal qualitative study of family caregivers’ role perception and supportive care needs. Supportive Care in Cancer. 2013;21(3):749–756. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor DL. Self-identifying as a caregiver: Exploring the positioning process. Journal of Aging Studies. 2007;21(2):165–174. doi: 10.1016/j.jaging.2006.06.002 [DOI] [Google Scholar]
- 38.Pew Research Center. Internet and Technology: Mobile Factsheet.; 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/
- 39.Fox S, Duggan M, Purcell K. Family Caregivers are Wired for Health. Pew research center. Published online 2013. http://www.pewinternet.org/2013/06/20/family-caregivers-are-wired-for-health/ [Google Scholar]
- 40.Shaffer KM, Chow PI, Cohn WF, Ingersoll KS, Ritterband LM. Informal Caregivers’ Use of Internet-Based Health Resources: An Analysis of the Health Information National Trends Survey. JMIR Aging. Published online In press. doi: 10.2196/11051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Studies implementing EMA with Cancer Caregivers
Supplementary Figure 1. Illustration of negative affect location and scale: Note. Traditional mixed-effects models assume equivalence in individuals’ within-subjects variability – meaning that all individuals’ variability is assumed to be the same. As depicted in the figure, however, it is a reasonable assumption that affective variability meaningfully differs across participants. Mixed-effects location scale models extend traditional mixed effects models by directly modeling within-subjects variability in terms of subject-level random effects in order to capture and explain individual differences in within-subjects variability. In the first modeling stage, individual participants’ own average affect relative to the sample is computed as a random effect as the “location” estimate. A positive location estimate indicates that the participant has a higher average affect score relative to the sample (e.g., reports higher average negative affect relative to other caregivers sampled; like participants A & B), while a negative location estimate indicates that the participant has a lower average affect score relative to the sample (e.g., reports lower average negative affect relative to other caregivers sampled; like participants C & D). Similarly, individual participants’ own variability in their reported affect across measures relative to the sample is computed as a random effect as the “scale” estimate. A positive scale estimate indicates that the participant reports more erratic affect scores relative to the sample (e.g., reports more erratic levels of negative affect relative to other caregivers sampled; like participants A & D), while a negative scale estimate indicates that the participant reports more consistent affect scores relative to the sample (e.g., reports more consistent levels of affect relative to other caregivers sampled; like participants B & C). These effects from stage 1 models are extracted and imputed as independent variables in the stage 2 models to detect associations with a dependent variable of interest (i.e., depressive symptoms). To account for uncertainty in random effects estimates, the MIXWILD software uses plausible value methodology (Mislevy, 1991, Psychometrika) to impute the random effects 500 times from the estimate posterior distribution – this approach accounts for the uncertainty in the random effect estimates. The stage 2 analyses are repeated for each set of imputed random effect estimates, and then averaged (using Rubin’s rules for multiple imputation) to yield overall regression estimates.
Data Availability Statement
Data available upon reasonable request from the authors.


