Abstract
Extracellular vesicles (EVs) in plants have emerged as key players in cell-to-cell communication and cross-kingdom RNAi between plants and pathogens by facilitating the exchange of RNA, proteins, and other molecules. In addition to their role in intercellular communication, plant EVs also show promise as potential therapeutics and indicators of plant health. However, plant EVs exhibit significant heterogeneity regarding their protein markers, size, and biogenesis pathways that strongly influence their composition and functionality. While mammalian EVs can be generally classified as exosomes that are derived from multivesicular bodies (MVBs), microvesicles that are shed from the plasma membrane, or as apoptotic bodies that originate from cells undergoing apoptosis, plant EVs remain poorly studied in comparison. At least three subclasses of EVs have been identified in Arabidopsis leaves to date including Tetraspanin-positive exosomes derived from MVBs, Penetration 1 (PEN1)-positive EVs, and EVs derived from exocyst-positive organelle (EXPO). Differences in plant starting material and isolation techniques though have resulted in different purities, quality and compositions of the resulting EVs, complicating efforts to better understand the role of these EVs in plants. We performed a comparative analysis on commonly used plant EV isolation methods and have identified an effective protocol for extracting clean apoplastic washing fluid (AWF) and isolating high quality intact and pure EVs of Arabidopsis thaliana. These EVs can then be used for various applications or to assess the cargos and functionality of these EVs in plants. Furthermore, this process can be easily adopted to other plant species of interest.
Keywords: Arabidopsis, extracellular vesicles, apoplastic washing fluid, exosomes
INTRODUCTION:
Plant extracellular vesicles (EVs) are small, lipid bilayer-enclosed vesicles that are released into the extracellular space and contain RNA, proteins, lipids, and other molecules that have been shown to facilitate cell-to-cell communication and cross-kingdom RNAi between plants and microbes (Columbo et al., 2014; Cai et al., 2021). Similar to mammalian EVs, plant EVs can be classified into multiple sub-classes based on their biogenesis pathways resulting in extreme diversity in protein markers, vesicle size, and potential function (Rutter and Innes, 2017; Cai et al., 2018; Wang et al., 2010; He, Hamby, and Jin 2021). As such, many different techniques including polymer-based precipitation, size exclusion chromatography, and ultracentrifugation have been used to isolate EVs from biological fluids with varying advantages and disadvantages (Brennan et al., 2020; Liangsupree et al., 2021). However, differential ultracentrifugation remains the most popular and widely used method for collecting EVs, especially small EVs like exosomes (Thery et al., 2006; Liangsupree et al., 2021). To date, EVs have been isolated from the apoplastic washing fluid (AWF) of several plant tissues ranging from Arabidopsis leaves (Rutter and Innes, 2017; Cai et al., 2018), to Nicotiana benthamia leaves (Movahed et al., 2019), sunflower seeds (Regente et al., 2009), and olive pollen grains (Prado et al., 2014), demonstrating their ubiquitous nature across plant species. Differences in centrifugation speeds and how the apoplastic washing fluid (AWF) is collected though have led to a lack of standardization for plant EV isolation, impeding efforts to further characterize and investigate the biological functions of these EVs. In this protocol, we describe a recommended method for the isolation of EVs from the AWF of Arabidopsis thaliana, with an emphasis on the collection of exosome-like EVs, by using differential ultracentrifugation, density gradient fractionation and immuno-isolation (Huang et al., 2021). We first detail collection of clean AWF from Arabidopsis leaves and the use of differential ultracentrifugation with a final speed of 100,000xg for isolation of EVs (Fig. 1). Density gradient fractionation using sucrose gradients is then described to further separate the EVs based upon their flotation density. Immuno-isolation, which is the most advanced EV purification method and enables collection of a specific subclass of EVs through antibodies targeting specific protein markers, is also described using an Arabidopsis tetraspanin-8 (TET8) antibody. Through this process, pure and intact EVs can be collected and used to address fundamental questions regarding their functions in plants or for various applications like drug delivery.
Figure 1.
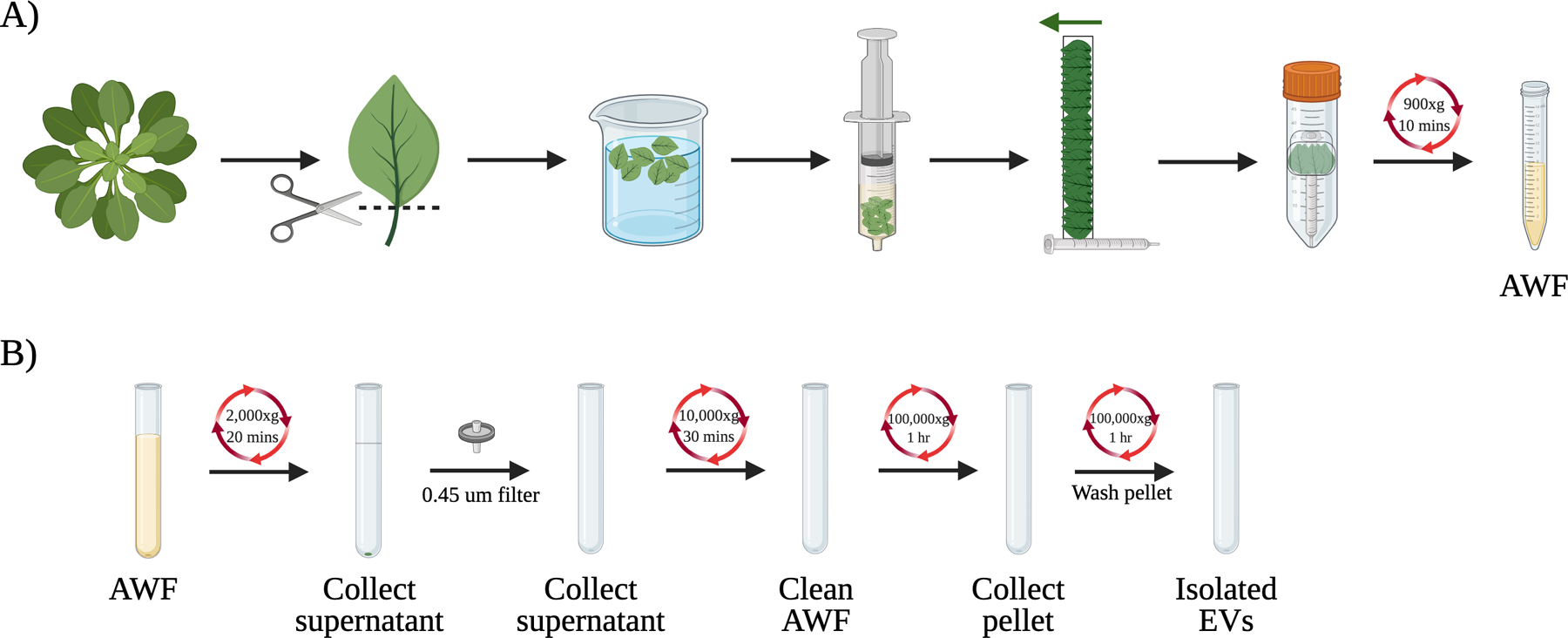
Overview of the EV isolation process from Arabidopsis. (A) Initial steps to isolate apoplastic washing fluid (AWF) from the detached leaves of Arabidopsis. Rosette leaves were collected from Arabidopsis and the leaf blades were isolated using a razor blade. The leaves were placed into a syringe and carefully vacuum infiltrated with infiltration buffer before being arranged on a piece of clear tape and wrapped around a syringe. The taped leaves were then placed into a 50 mL conical tube and the AWF was collected after centrifugation at 900xg. (B) EVs are collected from AWF using differential ultracentrifugation. Cellular debris and large vesicles are removed through 2,000xg, 0.45 μm filtration, and 10,000xg steps to produce the clean AWF. The clean AWF is then centrifuged at 100,000xg to obtain the isolated EVs.
BASIC PROTOCOL 1:
Isolation of EVs from the apoplastic washing fluid of Arabidopsis thaliana
In this protocol, we describe a recommended process for collecting the AWF from Arabidopsis leaves and isolating EVs using differential ultracentrifugation. The AWF is first extracted using an established infiltration-centrifugation method (Wang et al., 2005; Hatsugai et al., 2009; O’Leary et al., 2014). EVs are then isolated from the AWF using differential ultracentrifugation with initial, low speed steps (2,000xg and 10,000xg) to remove cellular debris and large vesicles, and finally, high speed centrifugation at 100,000xg to pellet the small exosome-like EVs.
Materials:
Arabidopsis thaliana plants
Milli-Q H2O
Infiltration buffer (see recipe in Reagents and Solutions)
Razor blade/scissors
Paper towels
200 mL syringe (BD Biosciences with Luer-Lok)
Parafilm
Clear tape
1 mL syringe (BD Biosciences with Luer-Lok)
50 mL conical tubes (Falcon brand)
Refrigerated centrifuge
15 mL conical tubes (Falcon brand)
0.45 μm Nylon filter (VWR brand)
Ultracentrifuge tubes (Beckman Coulter)
Ultracentrifuge (Beckman Coulter XPN-100)
Isolation of apoplastic washing fluid (AWF) using infiltration-centrifugation
-
1
Isolate the distal (blade) zone of the leaves by cutting each leaf at the base of the blade to remove the petiole and place cut leaves in a container of Milli-Q water until all leaves have been harvested (Fig. 2A).
Usually at least 100 four-week old Arabidopsis thaliana plants for each genotype/treatment of interest are required for EV isolation. Plants should be grown under short day conditions (8 hrs light/16 hrs dark). Do not collect leaves that are cut or damaged. It is important to perform the following steps (1–9) as quickly as possible to reduce the effect of wounding on the leaves or to work as a team with other people to reduce the processing time.
-
2
Wash the leaves 3 times with Milli-Q water and place on a paper towel to remove excess water.
-
3
Fill a 200 mL needleless syringe with leaves to the 50 mL mark and lightly tap the syringe on the benchtop to settle the leaves before adding 90 mLs of infiltration buffer.
Do not pack the leaves into the syringe as this may damage them and lead to contamination of the AWF.
-
4
Gently push the syringe plunger in until all air has been removed from the syringe, leaving only leaves and infiltration buffer. Seal the syringe opening by pressing a piece of 4x folded parafilm onto the opening to create an airtight seal.
-
5
Infiltrate the leaves by pulling the syringe plunger towards yourself as far as possible without breaking the vacuum seal, pausing 5 seconds in the fully extended position, and then slowly allowing the plunger to be pulled back into the syringe. Repeat this step 2–3 times as needed until all leaves are infiltrated with the infiltration buffer.
In this step, you are fighting against the vacuum so it will take some strength to pull the plunger towards yourself. It is best to tightly hold the syringe with one hand and pull the plunger towards yourself with the other. When the leaves are infiltrated with buffer, they become darker in color and translucent.
-
6
When all leaves are infiltrated in the syringe, gently pull out the plunger and pour out the excess infiltration buffer without losing any leaves. Tap the syringe onto a paper towel to collect the infiltrated leaves.
-
7
Repeat steps 3–6 until all leaves have infiltrated and gently pat leaves dry with a paper towel to remove any residual infiltration buffer on the leaves.
-
8
Place a ~12 inch piece of scotch tape sticky side up and wrap one end around an empty, needleless 1mL syringe right below the larger end of the syringe. Carefully layer ~50 infiltrated leaves in multiple layers onto the sticky side of the tape with the base of their blades aligned towards the bottom of the syringe and the leaf tip towards the top of the syringe for collection (Fig. 2B).
Not all the leaves need to be touching the tape if it is secured properly so multiple layers of leaves on one piece of tape will help protect the leaves from damage. It is very important to align the leaves in the same direction with the base of blade facing one end and towards the bottom of the collecting tube so that the apoplastic washing fluid is easily collected by centrifugation.
-
9
Secure the leaves to the syringe by wrapping the tape bound leaves around the syringe. Tape across the top of the bundle twice with additional scotch tape to firmly secure the leaves and place the wrapped syringe into a 50 mL collecting Falcon tube with the cut end of the leaf blades and the bottom of the syringe facing the bottom of the collecting Falcon tube (Fig 2C).
Do not tape the leaves too tightly though or leaf damage may occur and result in broken cells contaminating the apoplast washing fluid.
-
10
Centrifuge the sample at 4°C for 10 mins at 900xg to collect the apoplast washing fluid (AWF). The resulting AWF should be clear and transparent. Place the AWF in a new 15 mL conical tube or tubes as needed and store on ice. Repeat steps 8–10 until the AWF of all the infiltrated leaves have been collected.
A fixed rotor attachment should be used for this step to prevent damage to the leaves, which would result in contamination of the AWF by cells and cellular components. As such, the AWF should not be green or light green in color, which would indicate contamination by cells and the presence of chloroplasts/chlorophyll. This contamination should be avoided or minimized by properly following the above steps. If the sample is too contaminated, it will be best to just restart to avoid inaccurate results.
Figure 2.
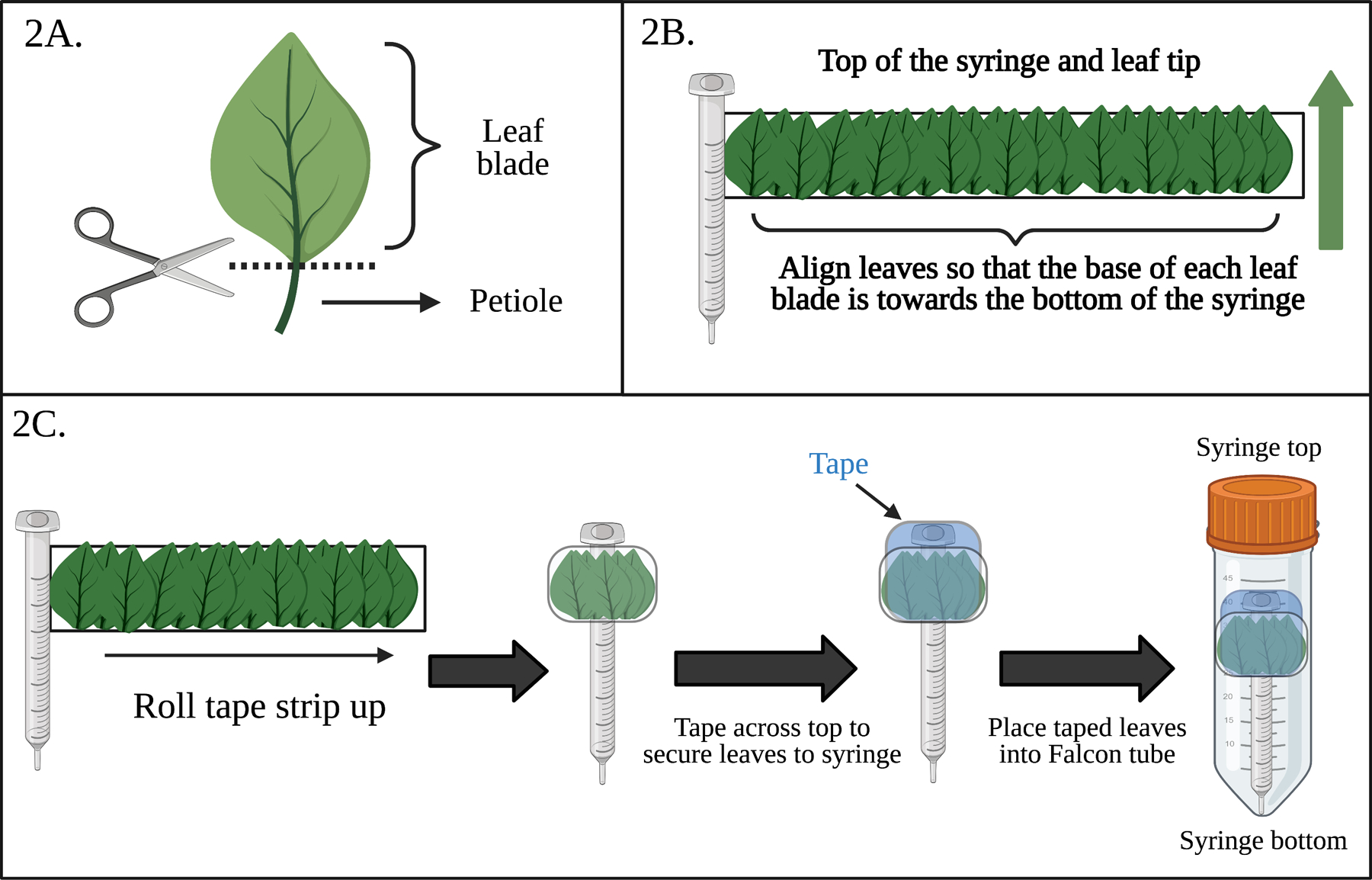
Diagrams of critical steps for EV isolation from Arabidopsis. (A) Diagram of where A. thaliana leaves should be cut to isolate the leaf blade and completely remove the petiole. (B) Diagram of how infiltrated leaves should be arranged on the tape strip. All leaves are oriented in the same way such that the leaf tips are at the top of the syringe and the base of the leaf blade is at the bottom of the syringe. The green arrow indicates the direction of the leaves on the tape. (C) Flowchart of how the taped leaves should be wrapped around the syringe, taped at the top, and the placed into 50 mL Falcon tubes for centrifugation.
Isolation of EVs from AWF using differential centrifugation
-
11
Centrifuge the AWF at 4°C for 20 mins at 2,000xg using a swing bucket attachment to remove large cellular debris. Discard the pellet and syringe filter the supernatant using a 0.45 μm Nylon filter to remove large vesicles.
-
12
Transfer the supernatant to new centrifuge tubes and centrifuge at 10,000xg for 30 mins at 4°C to further remove cellular debris and large vesicles and to collect the cleaned AWF.
-
13
Place the cleaned AWF in 17 mL ultracentrifuge tubes and centrifuge for 1 hr at 100,000xg at 4°C. Wash the pellet by resuspending the pellet with ~ 16 mLs of infiltration buffer and centrifuge again at 100,000xg for 1 hr at 4°C.
Spinning at 100,000xg promotes sedimentation of small vesicles like exosomes compared to lower speeds and washing helps to removes non-EV proteins by resuspending them in the buffer.
-
14
Resuspend the pellet in infiltration buffer (10–50 μL) and use immediately or store at 4°C (short-term storage) or −80°C (long-term storage, not recommended) for further experiments.
The pellet will not be visible so it is important to gently scrape the bottom of the tube with your pipet tip as you resuspend the pellet to assist in the resuspension process. Be confident though that the EVs are there in the pellet and adjust the volume of infiltration buffer used for resuspension as needed to concentrate the EVs.
BASIC PROTOCOL 2:
Density gradient fractionation of EVs using sucrose gradients
While Basic Protocol 1 results in the collection of relatively pure and intact EVs from the AWF of Arabidopsis, it may be necessary to further purify the sample for certain applications as ultracentrifugation results in the sedimentation of a mixture of heterogenous classes of vesicles from the AWF. As such, high speed density gradient fractionation is a traditional technique for separating vesicle populations based upon their equilibrium density and flotation speed. Here, we describe the use of sucrose gradients for the isolation of specific EV subpopulations based on their densities. Iodixanol (OptiPrep) gradients can also be used in place of sucrose gradients.
Materials:
Sucrose
Infiltration buffer (See Reagents and Solutions)
Isolated EVs from Basic Protocol 1
Scale
15 or 50 mL conical tubes (Falcon brand)
Waterbath at 60°C
Ultracentrifuge tubes (Beckman Coulter)
Ultracentrifuge (Beckman Coulter XPN-100)
-
Prepare 10–90% sucrose stocks (w/v) with compositions of 10%, 16%, 22%, 28%, 34%, 40%, 46%, 52%, 58%, 64%, 70%, and 90% in conical tubes using infiltration buffer.
Preparation of the 70% and 90% sucrose stocks requires heating at 60°C to solubilize all the sucrose.
-
Prepare a discontinuous gradient by carefully layering 1 mL of each solution in a 15 mL ultracentrifuge tube. The most concentrated stocks should be at the bottom so the 90% stock should be added first. Do not add the 10% sucrose layer yet.
In order to avoid disrupting the different sucrose layers, slowly pipet the 1 mL of each solution onto the top of the previous layer without the pipet tip penetrating into the previous layer.
Mix 100 μL of EVs in infiltration buffer with the 1 mL of 10% sucrose and then pipet this final layer on the top of the discontinuous gradient.
Centrifuge the samples using an ultracentrifuge with a swinging bucket rotor for 16 hrs at 100,000xg and 4°C.
Collect 6 fractions of 2 mL each for every sample by carefully pipetting from the top and transfer the collected fractions to new 17 mL ultracentrifuge tubes.
Resuspend the collected fractions in ~ 16 mLs of infiltration buffer and centrifuge for 1 hr at 100,000xg and 4°C to obtain a pellet for each fraction.
-
Remove the supernatant, resuspend each pellet in 20 μL infiltration buffer, and use immediately or store at 4°C (short-term storage) or −20°C (long-term storage) for further experiments.
Similar to Basic Protocol 1, the pellets will not be visible after ultracentrifugation, so it is important to gently scrape the bottom of the tube with your pipet tip as you resuspend the pellet to assist in the resuspension process. Be confident though that the EVs are there in the pellet and adjust the volume of infiltration buffer used for resuspension as needed to concentrate the EVs. Resuspension of the pellet in 10 μL of infiltration buffer can be used to generate more concentrated samples.
BASIC PROTOCOL 3:
Immuno-isolation of EVs using Arabidopsis tetraspanin 8 (TET8) antibody
Basic Protocol 2 enables separation and purification of EVs based on their density; however, to separate a specific subclass of EVs, immuno-isolation is one of the most powerful and advanced methods. This method isolates a specific subclass of EVs using beads coated with an antibody that recognizes the specific protein marker exposed on EV membranes (such as exosome marker CD63 in mammalian cells and TET8 in Arabidopsis). Use of this method can further prevent cytoplasmic protein or RNA from contaminating isolated EVs.
Materials:
Arabidopsis TET8 antibody (Lab made) (He et al., 2021)
Rabbit immunoglobulin G (Thermo Fisher)
Protein A beads (Roche)
Bovine serum albumin (Sigma)
Immunoprecipitation buffer (See Reagents and Solutions)
Isolated EVs from Basic Protocol 1
Rotator
200 μL and 1.7 ml tubes (Eppendorf)
Microcentrifuge (Eppendorf)
Wash 50 μL of protein A beads three times with immunoprecipitation buffer using a 1.7 mL tube.
Combine protein A beads with 5 μL of either TET8 antibody or Rabbit immunoglobulin G as a negative control and incubate in 500 μL of immunoprecipitation buffer for 3 hrs at 4 °C.
Wash tagged beads three times with immunoprecipitation buffer and collect the washed beads by centrifuging at 300xg for 5 mins at 4 °C.
-
Resuspend isolated plant EVs in 100 μL of immunoprecipitation buffer and add to the antibody-coupled beads in a 200 μL tube.
200 μL tubes are used to increase the binding efficiency between EVs and antibody-coupled beads.
Incubate the EVs and beads together overnight at 4 °C using a rotator.
-
Wash the bead-bound EVs three times with immunoprecipitation buffer and collect the bead-bound EVs by centrifuging at 300xg for 5 mins at 4 °C.
Bead-bound EVs can then be directly used for RNA extraction or to detect proteins.
REAGENTS AND SOLUTIONS:
Infiltration buffer (pH 6.0)
20 mM MES hydrate
2 mM calcium chloride
0.1 M sodium chloride
900 mL Milli-Q water
Adjust pH to 6.0 with NaOH
Bring up to 1 liter with Milli-Q water
Store at room temperature for up to 6 months
Immunoprecipitation buffer (pH 7.5)
20 mM MES hydrate
2 mM calcium chloride
0.1 M sodium chloride
0.3% Bovine serum albumin
Adjust pH to 7.5 with NaOH
Bring up to 1 liter with Milli-Q water
Use immediately
COMMENTARY:
Background Information:
EVs were first observed in plants in the 1960s through transmission electron microscopy images of carrot cells (Halperin 1967). However, efforts focused on understanding these lipid bilayer-enclosed vesicles and their functions in plants have only recently progressed compared to the field of mammalian EVs. Following the example of mammalian EVs, ultracentrifugation-based isolation methods were developed that utilized low initial speeds to remove cellular debris and organelles and then high speeds to pellet plant EVs based upon their density (Théry et al., 2006). Although ultracentrifugation enables the sedimentation of intact EVs and removal of most cellular contaminants, the diversity of EVs with regards to their intracellular origin, molecular composition, and heterogeneity often requires the use of multiple isolation methods in tandem. Commonly, techniques such as density gradient fractionation or immunoaffinity capture are utilized to enable further purification of specific subclasses of EVs by separating them based on floatation density or the presence of EV-specific markers (Konoshenko et al., 2018). While density gradient fractionation can provide fairly pure EV samples, it can be challenging to distinguish between EVs with similar densities. However, the use of antibodies specific to EV surface markers, such as the plant exosome marker protein TET8, can enable purification of individual subclasses of EVs in plants if markers are known and available, making it a powerful technique for EV isolation (Liangsupree et al., 2021). In addition to considerations regarding which isolation method is ideal, plant EVs are unique from mammalian EVs in that collection of the AWF must also occur. Since EVs are primarily located in the apoplastic fluid of the extracellular space of plant cells, nanovesicles derived from damaged plant tissue or extracted plant juices that contain intracellular vesicles and vesicles formed from the fusion of damaged cellular membranes represent impure populations that should not be considered as EVs (Pinedo et al., 2021). As such, isolation of the AWF from detached plant leaves using infiltration-centrifugation has been shown to be an effective and superior method for collecting clean AWF and purer EVs (Huang et al., 2021). Investigations utilizing these plant EVs have thus far enabled insights into the role of EVs in plant-microbe interactions like cross-kingdom communication, in defense response, and in growth and development (Cai et al., 2018; Roth et al., 2019; Regente et al., 2017). There is also growing interest in the potential application of these plant EVs in areas such as drug delivery or as biomarkers, which requires relatively pure and homogenous EV populations. Therefore, by standardizing a proper EV isolation technique across plant species, questions regarding plant EV biogenesis, their cargo, characterization of various EV subclasses, and their functional roles in plant immunity and signaling can continue to be addressed.
Critical Parameters and Troubleshooting:
There are several critical parameters and steps that should be considered in order to successfully isolate pure and intact EVs that can be used for characterization by nanoparticle tracking analysis and transmission electron microscopy or for Western blotting and other assays to investigate the composition and contents of the EVs. Most importantly, this protocol should be performed as quickly as possible to minimize the impact of wounding on the leaves, which would result in poor quality or contaminated EVs. As such, although this protocol can be performed by a single user, we recommend teamwork when processing the large number of leaves.
Collection of AWF from Arabidopsis leaves:
It is critical that the apoplastic washing fluid is carefully collected to avoid contamination with other cellular factors. As such, we recommend using detached leaves, rather than the whole aerial plant, to avoid collection of cytoplasmic molecules like chlorophyll. Additionally, it is important to wash the leaves after detachment to remove any cytoplasmic contaminants from damaged cells and ensure that the infiltrated leaves are all oriented with the cut site towards the bottom of the 50 mL conical tube to avoid cell damage during centrifugation. If the AWF is carefully collected, it should be quite clear in color. A slight or obvious yellow or greenish color indicates potential contamination by broken cells and the presence of chlorophyll.
Amount of starting material:
The yield of EVs is heavily dependent upon the amount of starting material used to perform the isolation. While we recommend at least 100 plants per genotype/treatment, this amount is only enough to perform one assay such as a Western blot or one characterization such as confocal microscopy or nanoparticle tracking analysis. As such, the amount of starting material should be scaled to reflect the number of assays and characterizations planned for the isolated EVs. The concentration can also be adjusted by altering the volume of infiltration buffer used to resuspend the pellet.
Time Considerations:
For isolation of EVs from AWF, it can take up to 5 hrs depending on how many plants are being used. The most time-consuming step is cutting each leaf and infiltrating them with the infiltration buffer. Separating the EVs using density gradient fractionation requires 17 hrs total of ultracentrifugation and an additional 1 hr to make the sucrose gradients and add the EVs. Immuno-isolation using antibodies requires a 3 hr and an overnight incubation, but the actual time at the bench is less than 1 hr. It is best to use EVs the same day after isolation or as quickly as possible if the purpose is to characterize the morphology using nanoparticle tracking analysis (NTA) NTA or transmission electron microscopy (TEM). If needed, storage at 4°C appears to be fine for 1–2 days without significant aggregation or other effects on the morphology. If EVs are to be used for western blotting or other assays interrogating the composition or contents of EVs where the morphology is not of importance, it is fine to store EVs at −20°C.
Understanding Results:
If these protocols are correctly performed, the user will have collected pure and intact EVs from Arabidopsis. To assess the purity and intactness of collected EVs, we recommend the use of techniques such as TEM to look at the morphology and Western blotting for cellular contaminants such as Rubisco. Additionally, the yield of EVs from 72 4-week old plants after resuspension of the pellet in 50 μL was approximately 1.91 ×108 particles/mL as determined by NTA. As a benchmark, the user can also refer to Huang et al., 2021 for what the AWF should look like after centrifugation at 900xg and for an example of transmission electron microscopy images of the EVs. The user can also use nanoparticle tracking analysis to confirm the presence of EVs and their size distribution in a sample before use in other assays. An average size of ~90–100 nm has been observed for EVs collected following Basic Protocol 1.
ACKNOWLEDGEMENTS:
Work in the H.J. laboratory was supported by grants from the National Institutes of Health (R35 GM136379), the National Science Foundation (IOS2017314), the United States Department of Agriculture National Institute of Food and Agriculture (2021–67013-34258 and. 2019–70016-29067), the Australian Research Council Industrial Transformation Research Hub (IH190100022), as well as the CIFAR Fungal Kingdom fellowship to H.J. The authors would also like to acknowledge BioRender.com for their assistance in figure creation.
Footnotes
Basic Protocol 1: Isolation of EVs from the apoplastic fluid of Arabidopsis thaliana
Basic Protocol 2: Density gradient fractionation of EVs
Basic Protocol 3: Immuno-isolation of EVs using Arabidopsis tetraspanin 8 (TET8) antibody
CONFLICT OF INTEREST STATEMENT:
None of the co-authors have a conflict of interest to declare.
Contributor Information
Angela Chen, Department of Microbiology and Plant Pathology, University of California Riverside, Riverside, CA.
Baoye He, Department of Microbiology and Plant Pathology, University of California Riverside, Riverside, CA.
Hailing Jin, Department of Microbiology and Plant Pathology, University of California Riverside, Riverside, CA.
DATA AVAILABILITY STATEMENT:
Data sharing not applicable – no new data generated
LITERATURE CITED:
- Brennan K, Martin K, FitzGerald SP, O’Sullivan J, Wu Y, Blanco A, Richardson C, & Mc Gee MM (2020). A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Scientific reports, 10(1), 1039. doi: 10.1038/s41598-020-57497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang SD, & Jin H (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science, 360, 1126–1129. doi: 10.1126/science.aar4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, He B, Wang S, Fletcher S, Niu D, Mitter N, Birch PRJ, & Jin H (2021). Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annual Review of Plant Biology, 72, 497–524. doi: 10.1146/annurev-arplant-081720-010616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, & Théry C (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology, 30, 255–289. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Halperin W & Jensen WA (1967). Ultrastructural Changes during Growth and Embryogenesis in Carrot Cell Cultures. Journal of Ultrastructure Research, 18(3–4), 428–443. doi: 10.1016/S0022-5320(67)80128-X [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, & Hara-Nishimura I. (2009). A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes & Development, 23(21), 2496–2506. doi: 10.1101/gad.1825209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Cai Q, Qiao L, Huang C-Y, Wang S, Miao W, Ha T, Wang Y, & Jin H. (2021). RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nature Plants, 7, 342–351. doi: 10.1038/s41477-021-00863-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hamby R, & Jin H. (2021). Plant extracellular vesicles: Trojan horses of cross-kingdom warfare. FASEB BioAdvances, 3(9), 657–664. doi: 10.1096/fba.2021-00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang S, Cai Q, & Jin H. (2021). Effective methods for isolation and purification of extracellular vesicles from plants. Journal of Integrative Plant Biology, doi: 10.1111/jipb.13181 [DOI] [PMC free article] [PubMed]
- Konoshenko MY, Lekchnov EA, Vlassov AV, & Laktionov PP (2018). Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Research International, 2018, 8545347. doi: 10.1155/2018/8545347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangsupree T, Multia E, & Riekkola M-L. (2021). Modern isolation and separation techniques for extracellular vesicles. Journal of Chromatography A, 1636, 461773. doi: 10.1016/j.chroma.2020.461773 [DOI] [PubMed] [Google Scholar]
- Movahed N, Cabanillas DG, Wan J, Vali H, Laliberte JF, & Zheng HQ (2019). Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves. Plant Physiology, 180 (3), 1375–1388. doi: 10.1104/pp.19.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary BM, Rico A, McCraw S, Fones HN, & Preston GM (2014). The infiltration-centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example. Journal of Visualized Experiments: JoVE, (94), 52113. doi: 10.3791/52113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinedo M, de la Canal L, & de Marcos Lousa C (2021). A call for Rigor and standardization in plant extracellular vesicle research. Journal of Extracellular Vesicles, 10(6), e12048. doi: 10.1002/jev2.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado N, Alche JD, Casado-Vela J, Mas S, Villalba M, Rodriguez R, & Batanero E (2014). Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Molecular Plant, 7(3), 573–577. doi: 10.1093/mp/sst153 [DOI] [PubMed] [Google Scholar]
- Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, & de la Canal L (2009). Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS letters, 583(20), 3363–3366. doi: 10.1016/j.febslet.2009.09.041 [DOI] [PubMed] [Google Scholar]
- Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, & de la Canal L. (2017). Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. Journal of Experimental Botany, 68, 5485–95. doi: 10.1093/jxb/erx355 [DOI] [PubMed] [Google Scholar]
- Roth R, Hillmer S, Funaya C, Chiapello M, Schumacher K, Lo Presti L, Kahmann R, & Paszkowski U (2019). Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nature Plants, 5, 204–211 doi: 10.1038/s41477-019-0365-4 [DOI] [PubMed] [Google Scholar]
- Rutter BD, & Innes RW (2017). Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiology, 173(1), 728–741. doi: 10.1104/pp.16.01253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, & Clayton A (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology, Chapter 3 doi: 10.1002/0471143030.cb0322s30 [DOI] [PubMed]
- Wang D, Weaver ND, Kesarwani M, & Dong X (2005). Induction of protein secretory pathway is required for systemic acquired resistance. Science (New York, N.Y.), 308(5724), 1036–1040. doi: 10.1126/science.1108791 [DOI] [PubMed] [Google Scholar]
- Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, Wang X, Robinson DG, & Jiang L (2010). EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. The Plant Cell, 22(12), 4009–4030. doi: 10.1105/tpc.110.080697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated


