Abstract
Background.
Breast arterial calcification (BAC), a common incidental finding in mammography, has been shown to be associated with angiographic coronary artery disease and cardiovascular disease (CVD) outcomes. We aimed to: 1) Examine the association of BAC presence and quantity with hard atherosclerotic CVD (ASCVD) and global CVD; 2) Ascertain model calibration, discrimination and reclassification of ASCVD risk; 3) Assess the joint effect of BAC presence and 10-year Pooled Cohorts Equations (PCE) risk on ASCVD.
Methods:
Cohort study in a large health plan in Northern California, USA, of 5,059 women aged 60–79 years recruited after attending mammography screening between 10/2012 and 2/2015. BAC status (presence versus absence) and quantity (calcium mass mg) was determined using digital mammograms. Pre-specified endpoints were incident hard atherosclerotic CVD and a composite of global CVD.
Results:
Twenty-six percent of women had BAC > 0 mg. After a mean (SD) follow-up of 6.5 (1.6) years, we ascertained 155 (3.0%) ASCVD events and 427 (8.4%) global CVD events. In Cox regression adjusted for traditional CVD risk factors, BAC presence was associated with a 1.51 (95% CI, 1.08–2.11; p=0.02) increased hazard of ASCVD and a 1.23 (95% CI, 1.002–1.52; p=0.04) increased hazard of global CVD. While there was no evidence of dose-response association with ASCVD, a threshold effect was found for global CVD at very high BAC burden (95th percentile when BAC present). BAC status provided additional risk stratification of the PCE risk. We noted improvements in model calibration and reclassification of ASCVD: the overall net reclassification improvement (NRI) was 0.12 (95% CI, 0.03–0.14; p=0.01) and the bias-corrected clinical-NRI was 0.11 (95% CI, 0.01–0.22; p=0.04) after adding BAC status.
Conclusions:
Our results indicate that BAC has potential utility for primary CVD prevention and therefore support the notion that BAC ought to be considered a risk-enhancing factor for ASCVD among postmenopausal women.
Keywords: Breast arterial calcification, cardiovascular disease, women’s health, cohort study
Subject Terms: Epidemiology, Lifestyle, Prevention: Cardiovascular disease, Primary Prevention, Women, Sex, Gender
BACKGROUND
Cardiovascular disease (CVD), including coronary heart disease (CHD), and cancer are the top two causes of death among women in the US.1 Among asymptomatic women, the first manifestation of underlying CHD is often unexpected acute myocardial infarction or sudden death. Furthermore, although women tend to have lower burden of obstructive coronary artery disease (CAD) on angiography, they tend to have worse prognosis after myocardial infarction compared with men.2 Thus, further investigation of sex-specific CVD risk markers is imperative.
The American Cancer Society (ACS) recommends that women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years, women aged 45 to 54 years should be screened annually and women 55 years and older should transition to biennial screening or have the opportunity to continue screening annually.3 In the US, attendance to screening mammography is 68% among women 50 and older and 71% among women 50 to 64 and in managed care is 88.4% overall.4 Breast arterial calcification (BAC) is commonly seen in mammograms and is currently not considered a clinically-actionable incidental finding. However, there is mounting evidence that BAC correlates with angiographically-defined CAD5–8 and portends increased risk of CVD outcomes.9–13 Several studies also support associations of BAC with subclinical CVD including carotid intimal media thickness14 and coronary artery calcification (CAC).7, 15, 16 However, prior literature on BAC has important limitations and, to this date, BAC is not mentioned in CVD primary prevention guidelines.17 In particular, many earlier studies used outdated film/screen systems which relied on assessment of presence versus. absence of BAC (i.e., no quantification of BAC) and were based (with two exceptions10, 12) on small sample sizes. Furthermore, none of the studies focused on establishing the role of BAC and BAC quantity in independent prediction, calibration, discrimination and reclassification of CVD risk.
To shed light on the potential role of BAC assessment on next-generation primary prevention CVD risk stratification, we aimed to: 1) Examine the prospective association of BAC presence and quantity with hard atherosclerotic CVD (ASCVD) and global CVD; 2) Ascertain improvement in model calibration, discrimination and reclassification of ASCVD risk after considering BAC and BAC quantity; and 3) Assess the joint effect of BAC presence and 10-year estimated Pooled Cohorts Equation ASCVD risk on ASCVD, which has the potential to inform therapeutic choices and intensity of treatment.
METHODS.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Cohort Description
MINERVA (MultIethNic study of brEast aRterial calcium gradation and cardioVAscular disease) is a large, racially and ethnically diverse cohort of postmenopausal women. Details of recruitment, study procedures and baseline characteristics are published elsewhere.18 In brief, eligible participants were female active members of Kaiser Permanente of Northern California (KPNC) between the ages of 60 and 79 when they attended regular mammography screening at one of nine KPNC facilities (Oakland, Richmond, Pleasanton, Antioch, Walnut Creek, San Francisco, Santa Clara, Campbell and Mountain View) between 10/24/2012 and 2/13/2015. Women attending mammography for diagnostic purposes were not eligible. Those with a prior history of myocardial infarction, coronary revascularization, stroke, heart failure, peripheral vascular disease, breast cancer, mastectomy or breast implants, Alzheimer’s disease/dementia, chronic dialysis/renal transplant or not having an assigned primary care provider were also excluded. A total of 201,830 women underwent screening mammography at the study centers, and 46,112 met eligibility criteria. Of those, 5,145 women with available digital, uncompressed mammograms were recruited. Of those, 86 had one or more missing covariates of interest, resulting in a final sample of 5,059. We retained (using dummy variables) 83 participants with missing data on age at menarche and 1,845 participants with missing data on breast feeding. For the analysis of global CVD, an additional 24 women were excluded due to prevalent CVD conditions not previously detected at recruitment and that were part of global CVD. The study was approved by the Institutional Review Boards of the participating institutions and all participants signed an informed consent.
Study Procedures
BAC Assessment
All images were acquired using full-field digital mammography units (Senographe 2000D, General Electric Medical Systems, Milwaukee, WI or Selenia Hologic, Hologic Inc., Malborough, MA). Standard full-field digital mammograms were acquired from mediolateral oblique (MLO) and craniocaudal (CC) projections. A new, but rigorously validated densitometry method was used to estimate a continuous BAC mass (in milligrams [mg]) score using raw (uncompressed) digital mammograms prospectively acquired and transmitted to the BAC Reading Center at UC Irvine Department of Radiological Sciences.19 Intra- and inter-machine variability has been addressed before.20 Examples of severe, moderate and light BAC burden are provided in Figure 1. BAC appears as high-attenuation parallel lines in arterial vessel walls.
Figure 1.
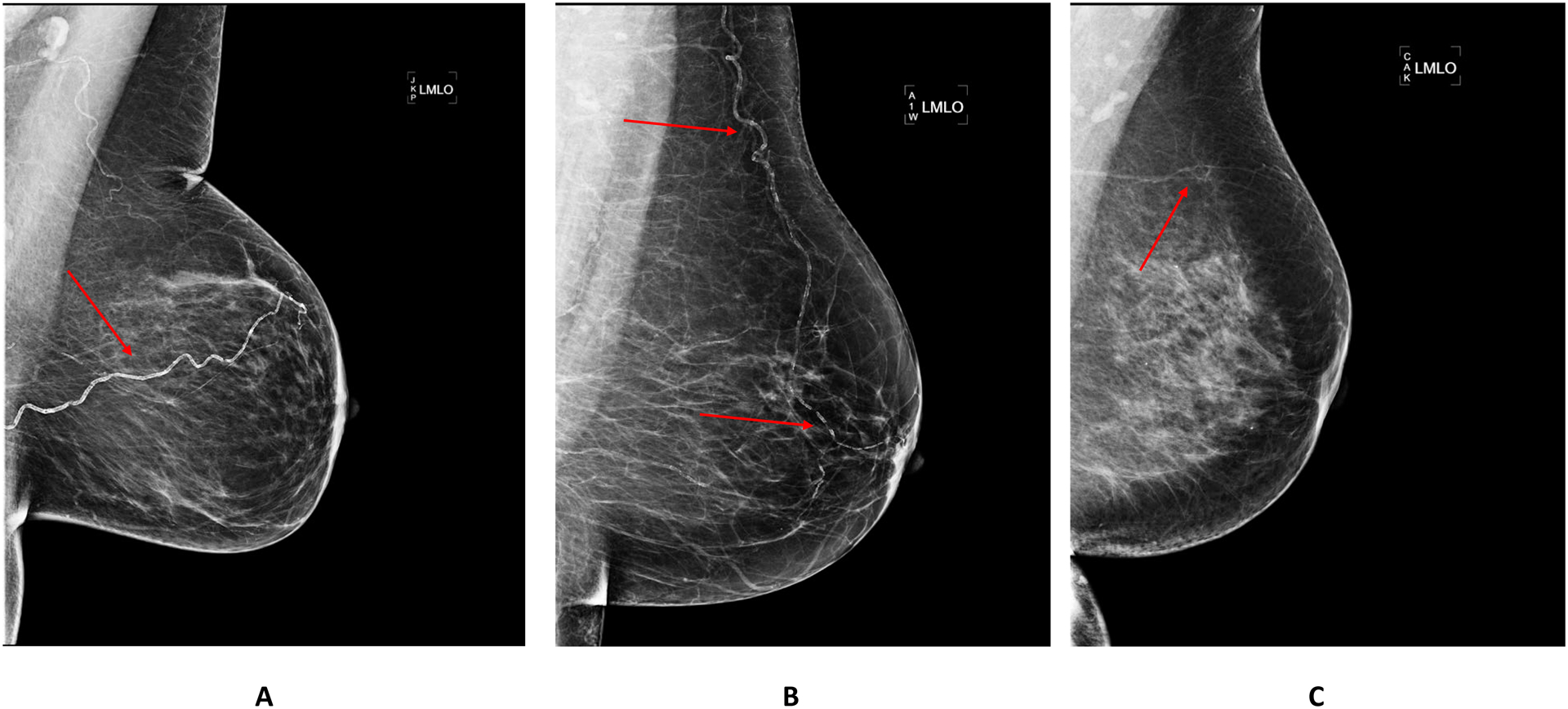
Examples of Mammographic Images with Severe (A), Moderate (B) and Light (C) BAC. BAC: breast arterial calcification.
Cardiovascular Outcomes.
Incident ASCVD (including acute myocardial infarction, ischemic stroke or CVD death) and a composite of global CVD (including ischemic heart disease, cerebrovascular disease, heart failure, cardiomyopathy, deep vein thrombosis\pulmonary embolism, cardiac arrest, peripheral arterial disease, retinal vascular occlusion and CVD death) were ascertained through December 31, 2020 using standard validated ICD-9, ICD-10, CPT4 procedure codes and underlying cause of death (see Table S1). To establish the validity of the event ascertainment using codes and underlying cause of death, a physician investigator (C.I.) adjudicated a 20% random sample of global CVD events (n=85) by examining the electronic health records. For ASCVD, the positive predictive value (PPV) was 97% (32/33) and for global CVD the PPV was 93% (79/85). PPV for individual CVD outcomes are provided in Table S2. The mean (SD) length of follow-up was 6.5 (1.6 years).
Covariate Assessment
Age, ethnicity, education attainment, smoking, reproductive history (menarche, age at menopause, menopausal hormone therapy, number of live births, breast feeding) and parental history of premature CHD were ascertained with a questionnaire self-administered during the clinic visit (n=4,400) or administered by phone for those not attending clinic visit (n=659). Clinic visits or phone interviews took place, on average, 3.2 months (SD=3.0) after the screening mammography. Details of clinic procedures and laboratory methods can be found elsewhere.18 Glycemic status was defined as normoglycemia, prediabetes and diabetes diagnosis or treatment. Hypertension was defined as self-report of hypertension and/or self-report of treatment for hypertension and/or SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg.
Statistical Methods
We first assessed the distribution of demographic, behavioral and clinical factors according to BAC absence (BAC=0 mg) versus presence (BAC>0 mg). Differences in continuous variables were tested using the t-test and differences in distribution of categorical variables using the Chi-Square test. Age-adjusted rates per 1,000 person-years of ASCVD, global CVD and its individual components according to BAC presence versus. absence were estimated using Poisson regression with right-censoring at death (n=63) or termination of health plan membership (n=726). We used the Kaplan-Meier method to compare survival curves across BAC groups for both ASCVD and global CVD. To assess the association of BAC quantity with outcomes, we considered BAC categorized as 0 mg (referent group) and then tertiles among those with BAC>0. Hazard ratios and 95% confidence intervals of BAC presence and quantity with ASCVD and global CVD were generated using Cox proportional hazards models with both minimal adjustment (Model 1: age, ethnicity, education level) and further adjustment for smoking status, glycemic status, LDL-cholesterol, HDL-cholesterol and hypertension in Model 2. No violations of the proportionality assumption were detected for BAC as absence versus presence or for the four-level categorical BAC variable (all p values for Schoenfeld residuals test > 0.67).
We ran Cox models with restricted cubic splines (with four knots at the 5th, 35th, 65th, and 95th percentiles of BAC) to examine possible nonlinear associations of continuous BAC with ASCVD and global CVD adjusting for Model 2 covariates. We also parametrized BAC as a categorical variable with three levels using more extreme cut-points (80th, 90th and 95th percentile of BAC score when BAC present, corresponding to 15.6 mg, 31.8 mg and 52.3 mg, respectively). We estimated hazards of ASCVD according to joint categories of BAC presence versus. absence and 3 levels of PCE risk (< 5%, 5 to 20% and >20%) with BAC=0 mg and <5% PCE risk as reference and adjustment for education level. In addition, we formally tested the interaction between categorical PCE risk and BAC presence versus absence first unadjusted and then adjusting for education level. Sensitivity analyses restricting to women not on cholesterol lowering therapy was performed.
We examined whether risk estimates derived from models using the Pooled Cohorts Equation (PCE) risk and BAC have better performance than risk estimates derived from models using PCE risk solely. We estimated 5-year cumulative incidence using a Cox model that included PCE and BAC, where BAC was dichotomous (0, >0 mg) or 4-category BAC variables (using tertiles of BAC when BAC present) and compared these risks to those estimated using PCE alone. We examined calibration visually through calibration plots and analytically using the Greenwood-D’Agostino-Nam test for calibration.21, 22 We assessed discrimination using the Harrell C-index at a time horizon of 5-years to align with the predicted 5-year cumulative incidence used in the analyses.23 We then estimated the overall category-based net reclassification index (NRI) to assess the extent to which adding BAC to the risk models moves risk upward among ASCVD cases and downward among non-cases, and the bias-corrected clinical NRI (cNRI) among those with borderline or intermediate risk using the standard PCE categories of <5, 5 to <7.5, 7.5 to 20 and ≥ 20 percent, respectively.24, 25 Significance level was set at p<0.05.
RESULTS
BAC > 0 mg was present in 26.5 percent of the cohort. Compared to women with no BAC, those with BAC were older, more likely to be white or Hispanic, less likely to be black or Asian and less likely to had pursued graduate studies or a professional degree (Table 1). Women with BAC had non-clinically significant higher HDL-C, a higher prevalence of prediabetes and diabetes, higher systolic blood pressure and higher prevalence of hypertension. Having any level of BAC was also associated with higher parity. Whereas 5.5% of women in the BAC=0 mg group were in the high PCE risk group (i.e., ≥20%), 12.1% of women in the BAC>0 mg group were in the high PCE risk group (i.e., ≥20%)
Table 1.
Baseline Cohort Characteristics by BAC Absence/Presence Status (n=5,059).
| BAC = 0 mg n = 3,721 (73.5%) |
BAC > 0 mg n = 1,338 (26.5%) |
p‡ | |
|---|---|---|---|
| Age (years) | 65.2 ± 4.2 | 67.1 ±4.8 | <.0001 |
| Ethnicity | <.0001 | ||
| White | 1,898 (51.0%) | 778 (58.2%) | |
| Black | 587 (15.8%) | 173 (12.9%) | |
| Hispanic/Latina | 431 (11.6%) | 188 (14.1%) | |
| Asian | 746 (20.1%) | 183 (13.7%) | |
| Other or Unknown race | 59 (1.6%) | 15 (1.2%) | |
| Educational attainment | 0.003 | ||
| Less than completed high school or GED | 133 (3.6%) | 62 (4.6%) | |
| Completed high school or GED | 665 (17.9%) | 280 (20.9%) | |
| At least some college/completed college | 1,828 (49.1%) | 658 (49.2%) | |
| Graduate school or professional degree | 1,095 (29.4%) | 338 (25.3%) | |
| Smoking status | 0.76 | ||
| Never | 2,338 (62.8%) | 834 (62.3%) | |
| Former | 1,234 (33.2%) | 455 (34.0%) | |
| Current | 149 (4.0%) | 49 (3.7%) | |
| BMI (Kg/m2) | 27.7 ± 6.1 | 27.8 ±6.0 | 0.57 |
| Total cholesterol (mg/dL) | 207 ± 37 | 207 ± 39 | 0.78 |
| LDL-C (mg/dL) | 121 ± 32 | 120 ± 33 | 0.40 |
| HDL-C (mg/dL) | 65 ± 16 | 66 ± 17 | 0.02 |
| Glycemic status* | 0.02 | ||
| Normoglycemia | 1,921 (51.6%) | 637 (47.6%) | |
| Prediabetes | 1,347 (36.2%) | 509 (38.0%) | |
| Diabetes diagnosis or treatment | 453 (12.2%) | 192 (14.4%) | |
| Systolic blood pressure (mmHg) | 123 ± 16 | 124 ± 15 | 0.01 |
| Diastolic blood pressure (mmHg) | 69 ± 11 | 68 ± 10 | 0.16 |
| On antihypertensive medication | 0.0002 | ||
| No | 2,311 (62.1%) | 753 (56.3%) | |
| Yes | 1,410 (37.9%) | 585 (43.7%) | |
| Hypertension† | 0.0009 | ||
| No | 1,680 (45.2%) | 534 (39.9%) | |
| Yes | 2,041 (54.9%) | 804 (60.1%) | |
| On cholesterol lowering drugs | 0.11 | ||
| No | 1,016 (27.3%) | 335 (25.0%) | |
| Yes | 2,705 (72.7%) | 1003 (75.0%) | |
| Menarche (years) | 0.15 | ||
| < 12 | 735 (19.8%) | 298 (22.3%) | |
| 12–13 | 1,948 (52.4%) | 685 (51.2%) | |
| ≥ 14 | 974 (26.2%) | 336 (25.2%) | |
| Missing | 64 (1.7%) | 19 (1.4%) | |
| Early menopause | 0.46 | ||
| No | 3,106 (83.5%) | 1,105 (82.6%) | |
| Yes | 615 (16.5%) | 233 (17.4%) | |
| Menopausal hormone therapy | 0.06 | ||
| No | 3,289 (88.4%) | 1,208 (90.3%) | |
| Yes | 425 (11.4%) | 128 (9.6%) | |
| Missing | 7 (0.2%) | 2 (0.2%) | |
| History of breast feeding‡ | 0.25 | ||
| No | 523 (14.1%) | 248 (18.5%) | |
| Yes | 1,711 (46.0%) | 732 (54.7%) | |
| Missing | 1,487 (40.0%) | 358 (26.8%) | |
| Number of live births | <.0001 | ||
| 0 | 1,650 (44.3%) | 448 (33.5%) | |
| 1–2 | 1,404 (37.8%) | 461 (34.5%) | |
| ≥ 3 | 667 (17.9%) | 429 (32.1%) | |
| Parental history of premature CAD | 0.18 | ||
| No | 3,521 (94.6%) | 1,253 (93.7%) | |
| Yes | 200 (5.4%) | 85 (6.4%) | |
| Untransformed BAC score (mg) | 0 | 11.6 ± 23.2 | <.0001 |
| Log (BAC+1) | 0.0 ± 0.0 | 1.66 ± 1.3 | <.0001 |
| Pooled Cohorts 10-year ASCVD Risk | <.0001 | ||
| < 5% | 1,343 (36.1%) | 343 (25.6%) | |
| 5% to < 7.5% | 852 (22.9%) | 272 (20.3%) | |
| 7.5 to < 20% | 1,323 (35.6%) | 561 (41.9%) | |
| ≥ 20% | 203 (5.5%) | 162 (12.1%) |
Normoglycemia: no self-report and HbA1c ≤ 5.7% and no self-report of treatment and fasting glucose < 100; prediabetes: no self-report and HbA1c > 5.7 but ≤ 6.5% and no self-report of treatment and fasting glucose >= 100 and < 126; diabetes diagnosis or treatment: self-report or HbA1c > 6.5% or fasting glucose >= 126 or self-report of treatment
self-report of diagnosis of hypertension or self-report of treatment for hypertension or SBP > 140 mmHg or DBP > 90 mmHg; ‡ ANOVA or Chi-square
p-value calculated after excluding those with missing values.
BAC: breast arterial calcification; GED: general education diploma; BMI: body mass index; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CAD: coronary artery disease; ASCVD: atherosclerotic cardiovascular disease.
After a mean (SD) follow-up of 6.5 (1.6) years, 155 ASCVD events (3.0%) and 427 global CVD events (8.4%) were ascertained. All age-adjusted rates per 1,000 person-years were consistently higher among women with BAC than those without BAC, and statistically significantly so (p≤ 0.04) for ischemic stroke, CVD death, hard ASCVD, cerebrovascular disease, cardiomyopathy, deep venous thrombosis/pulmonary embolism, peripheral arterial disease, retinal vascular occlusion and global CVD (Table 2). Women with BAC showed worse ASCVD and global CVD survival when considering presence versus absence (both p<0.001). Whereas there was no clear separation of survival curves for tertiles of BAC when BAC was present in the case of ASCVD, there was a suggestion of separation of tertiles 2 and 3 versus 1 for global CVD (Figure S1).
Table 2.
Number of Events and Age-adjusted rates of Individual CVD Outcomes in the ASCVD Cohort and the Global CVD cohort.
| Outcomes in the Hard ASCVD cohort (n=5,059; 155 events) |
BAC = 0 mg n=3,721 (73.5%) |
BAC > 0 mg n=1,338 (26.5%) |
p | ||
|---|---|---|---|---|---|
| Num events | Age-adjusted rate per 1,000 person-years |
Num events | Age-adjusted rate per 1,000 person-years |
||
| Acute myocardial infarction | 33 | 1.31 | 18 | 1.75 | 0.09 |
| Ischemic stroke | 50 | 2.00 | 27 | 2.74 | 0.04 |
| CVD death | 15 | 0.58 | 18 | 1.65 | 0.0003 |
| Any of the above | 95 | 3.81 | 60 | 5.96 | 0.0002 |
| Outcomes in the Global CVD cohort (n=5,035; 427 events) |
BAC = 0 mg n=3,704 (73.6%) |
BAC > 0 mg n=1,331 (26.4%) |
p | ||
| Ischemic heart disease (acute myocardial infarction + coronary angioplasty/stent/bypass graft surgery) | 44 | 1.78 | 20 | 2.01 | 0.25 |
| Cerebrovascular disease (ischemic stroke + hemorrhagic stroke) | 71 | 2.84 | 36 | 3.54 | 0.048 |
| Heart failure | 66 | 2.47 | 33 | 2.77 | 0.08 |
| Cardiomyopathy | 5 | 0.20 | 6 | 0.61 | 0.03 |
| Deep vein thrombosis\pulmonary embolism | 24 | 0.88 | 16 | 1.29 | 0.04 |
| Cardiac arrest | 2 | 0.08 | 3 | 0.30 | 0.09 |
| Peripheral arterial disease | 95 | 3.89 | 46 | 4.82 | 0.04 |
| Retinal vascular occlusion | 2 | 0.07 | 4 | 0.31 | 0.04 |
| CVD Death | 15 | 0.58 | 17 | 1.56 | 0.0006 |
| Any of the above | 281 | 11.45 | 146 | 14.63 | <.0001 |
BAC: breast arterial calcification; CVD: cardiovascular disease; ASCVD: atherosclerotic cardiovascular disease.
In Model 1, BAC presence was associated with 1.58 (95% CI, 1.13–2.20; p=0.007) increased hazard of ASCVD, and adjustment for traditional CVD risk factors attenuated the strength of association only slightly (Table 3). In the models using BAC tertiles among those with any BAC we observed significant associations with ASCVD for tertile 1 and 2, but not for tertile 3. In Model 1, BAC presence was associated with 1.28 (95% CI, 1.04–1.57; p=0.02) increased hazard of global CVD, and adjustment for traditional risk factors did not appreciably diminish the strength of association (Table 3). In the model using BAC tertiles we did not observe a dose-response association with global CVD either and the hazard ratio was only significant for tertile 2. Additional threshold models using more extreme cut-points for BAC (80th, 90th and 95th percentile of BAC when BAC was present) are shown in Table S3. Whereas for ASCVD a dose-response pattern was not observed (the confidence intervals overlapped), a dose-response pattern (the confidence intervals did not overlap) was present for global CVD when considering the 95th percentile threshold.
Table 3.
Hazard of ASCVD and Global CVD Associated with BAC Presence and Gradation.
| Model 1 HR* (95% CI) |
P | Model 2 HR† (95% CI) |
p | |
|---|---|---|---|---|
| Hard ASCVD (n=5,059; 155 events) | ||||
| BAC Presence vs. Absence Model | ||||
| BAC > 0 mg vs. BAC = 0 mg | 1.58 (1.13–2.20) | 0.007 | 1.51 (1.08–2.11) | 0.02 |
| BAC Gradation Model | ||||
| Tertile 1 when BAC is present vs. BAC = 0 mg | 1.80 (1.31–2.88) | 0.01 | 1.74 (1.09–2.77) | 0.02 |
| Tertile 2 when BAC is present vs. BAC = 0 mg | 1.65 (1.03–2.66) | 0.04 | 1.60 (0.99–2.58) | 0.05 |
| Tertile 3 when BAC is present vs. BAC = 0 mg | 1.27 (0.75–2.17) | 0.37 | 1.24 (0.73–2.11) | 0.42 |
| Global CVD (n=5,035; 427 events) | ||||
| BAC Presence vs. Absence Model | ||||
| BAC > 0 mg vs. BAC = 0 mg | 1.28 (1.04–1.57) | 0.02 | 1.23 (1.002–1.52) | 0.048 |
| BAC Gradation Model | ||||
| Tertile 1 when BAC is present vs. BAC = 0 mg | 1.20 (0.87–1.65) | 0.27 | 1.16 (0.84–1.60) | 0.37 |
| Tertile 2 when BAC is present vs. BAC = 0 mg | 1.41 (1.05–1.90) | 0.02 | 1.38 (1.02–1.85) | 0.03 |
| Tertile 3 when BAC is present vs. BAC = 0 mg | 1.22 (0.90–1.67) | 0.20 | 1.21 (0.89–1.65) | 0.23 |
age, race, education level
+ glycemic status, smoking, LDL-C and hypertension
BAC: breast arterial calcification; LDL: low-density lipoprotein; CVD: cardiovascular disease; ASCVD: atherosclerotic cardiovascular disease; HR: hazard ratio.
The restricted cubic spline Cox regression model indicated no departure from a linear relationship of BAC with ASCVD or with global CVD risk (Figure 2). Results of the analysis of joint categories of 10-year Pooled Cohorts Equations and presence/absence of BAC are shown in Table 4. Relative to low (<5%) risk women with no BAC, and after adjustment for education level, low risk women with BAC were at 2.28 (95% CI, 1.00–5.21; p=0.05) increased hazard of hard ASCVD. Women in the borderline to intermediate PCE risk group (5 to < 20%) were at 2.94 (95% CI, 1.69–5.14; p=0.0001) increased hazard of hard ASCVD if they had no BAC, and at 4.19 (95% CI, 2.31–7.59; p<0.0001) increased hazard of hard ASCVD if they had any BAC. High risk women (>20%) were at 3.62 (95% CI, 1.53–8.56; p=0.003) increased hazard of hard ASCVD if they had no BAC, and at 5.98 (95% CI, 2.74–13.03; p<0.0001) increased hazard of ASCVD if they had any BAC. The interaction between categorical PCE by BAC presence versus absence was not statistically significant in the unadjusted model (p=0.37) or the model adjusted for education level (p=0.36). We are also providing in Table S4 results of the joint analysis of the PCE and BAC using alternative cut-points at 5%/7.5%/10% for both ASCVD and global CVD. For ASCVD, BAC provides risk enhancement in all PCE groups except 5 to 7.5%. For global CVD, BAC provides risk enhancement in all groups except < 5%.
Figure 2.
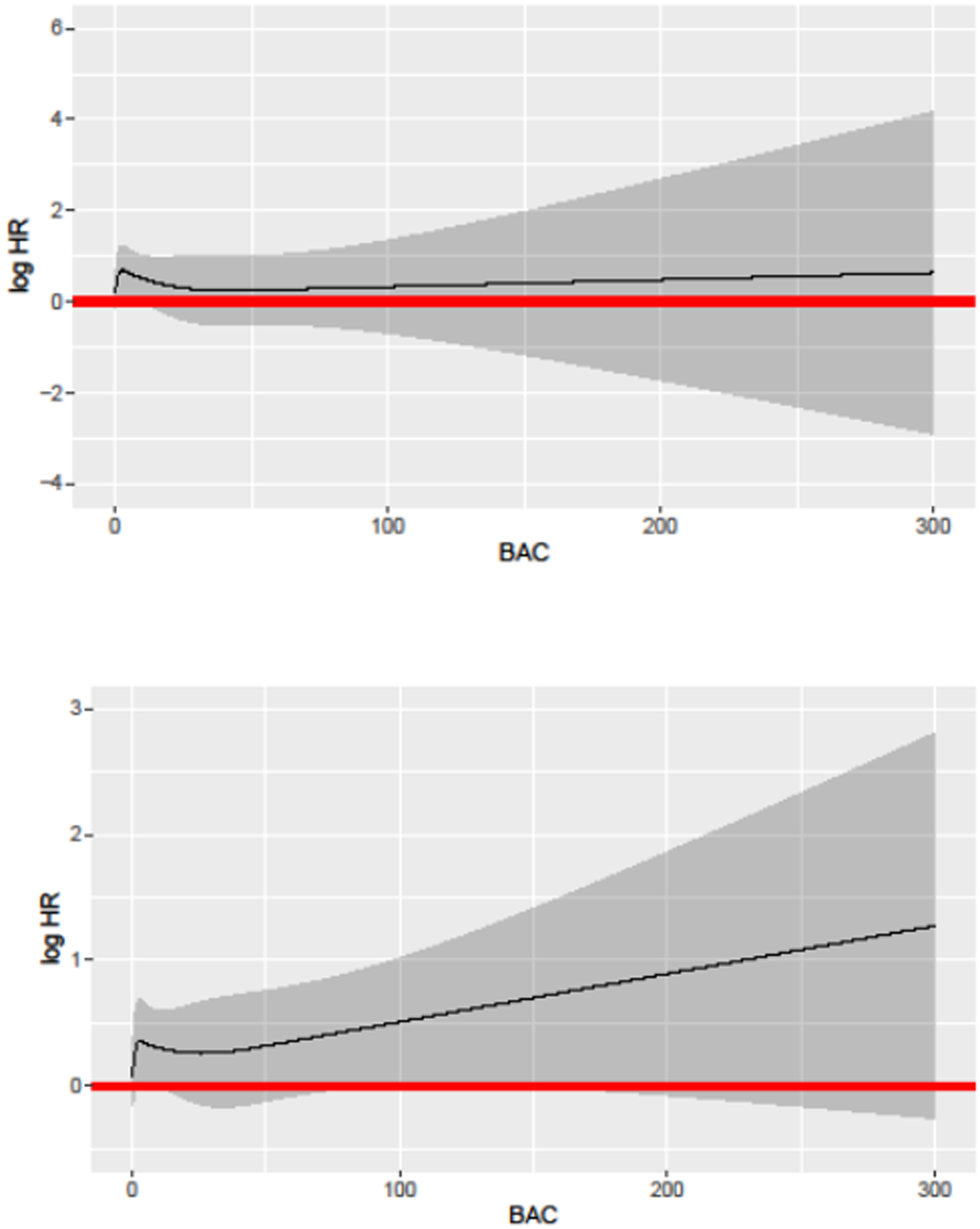
Restricted Cubic Splines Model for ASCVD (Top Panel) and Global CVD (Bottom Panel). ASCVD: atherosclerotic cardiovascular disease; CVD: cardiovascular disease.
Table 4.
ASCVD Risk by Joint Categories of 10-year Pooled Cohort Equations and BAC (n=5,059).
| Joint Categories of 10-year Pooled Cohort Equations Risk and Presence vs. Absence of BAC | Number of Women in Category | Number of events | Age-adjusted rate per 1,000 person-years | Unadjusted HR (95% CI) | p | Adjusted* HR (95% CI) |
p |
|---|---|---|---|---|---|---|---|
| < 5% with BAC = 0 mg | 1343 | 15 | 1.72 | 1.00 | 1.00 | ||
| < 5% with BAC > 0 mg | 343 | 9 | 3.93 | 2.28 (1.00–5.21) | 0.05 | 2.28 (1.00–5.21) | 0.05 |
| 5 to < 20% with BAC = 0 mg | 2175 | 72 | 5.07 | 2.93 (1.68–5.11) | 0.0002 | 2.94 (1.69–5.14) | 0.0001 |
| 5 to < 20% with BAC > 0 mg | 833 | 40 | 7.22 | 4.15 (2.29–7.51) | <.0001 | 4.19 (2.31–7.59) | <.0001 |
| ≥ 20% with BAC = 0 mg | 203 | 8 | 6.23 | 3.57 (1.51–8.42) | 0.004 | 3.62 (1.53–8.56) | 0.003 |
| ≥ 20% with BAC > 0 mg | 162 | 11 | 10.37 | 5.90 (2.71–12.84) | <.0001 | 5.98 (2.74–13.03) | <.0001 |
+ education level
P-values for interaction between PCE categorical (0 if < 5%; 1 if 5 to 20%; 2 if ≥20%) * BAC absence versus presence = 0.37 in unadjusted model, =0.36 in model adjusted for education level.
BAC: breast arterial calcification; ASCVD: atherosclerotic cardiovascular disease; HR: hazard ratio.
Addition of BAC as presence versus absence resulted in a well-calibrated model (p=0.51) (Table S5). On the other hand, models considering a 4-level BAC variable (tertiles when BAC > 0 mg) did not improve model calibration (p=0.05). The C-index (measure of discrimination) for the model containing only PCE risk was 63.4 and it increased (not statistically significantly) to 64.3 after adding BAC presence versus absence (p=0.28), and to 64.0 (p=0.27) after adding the 4-level BAC variable using tertiles. The overall category-based net reclassification improvement (NRI) was 0.12 (95% CI, 0.03–0.22; p=0.01) after adding BAC presence versus absence and 0.07 (95% CI, 0.00–0.14; p=0.06) after adding the 4-level BAC variable using tertiles. The bias-corrected clinical NRI was 0.11 (95% CI, 0.01–0.22; p=0.04) after adding BAC presence versus absence and 0.07 (95% CI, 0.00–0.15; p=0.07) after adding the 4-level BAC variable using tertiles. The reclassification tables are provided in Table S6. The gains in reclassification were mostly driven by up-risking of women with BAC.
We performed sensitivity analyses among women not on cholesterol lowering therapy (Table S7). Whereas the association with ASCVD was maintained (although it lost statistical significance; p=0.11), the association with global CVD became weaker and non-significant (p=0.80). To gain further insight into potential clinical utility of BAC, we stratified the cohort into groups according to receipt of cholesterol lowering drugs, BAC status and PCE risk groups. Of the women with BAC present (n=1,338), 335 (25 percent) were not taking cholesterol lowering drugs. Among those 335 women, 196 (58.5 percent) were in borderline or intermediate PCE risk groups (Table S8).
DISCUSSION
Our main findings in a large cohort of postmenopausal women who were 60 to 79 years old at the time of the screening mammogram are that presence of BAC (after 6.5 years of follow-up) was statistically significantly associated with 1.51 increased hazard of incident ASCVD and with 1.23 increased hazard of incident global CVD in multivariate models that adjusted for traditional CVD risk factors. The strength of associations reported here is commensurate to what has been reported in the prior BAC literature for CHD and CVD death by cohort studies.10, 12, 26 There was no clear dose-response association of BAC with either ASCVD or global CVD when BAC was modeled using tertiles. It is plausible that 6 years of follow-up is not enough time and does not provide enough number of events (in equal size groups among women with BAC>0) for the dose-response association to emerge. It is important to keep in mind, however, that our data shows a threshold effect for global CVD at very high burden of calcification (above the 90th percentile of BAC) that would also need to be verified with extended follow-up period.
The prevalence of BAC was 26.5%, which is in the range (10–50%) reported by prior studies. Our results are consistent with earlier reports of associations of BAC presence with older age, white and Hispanic ethnicities, low educational attainment, diabetes, high parity and hypertension.10, 14, 19, 26 Unlike several studies that found an inverse association between BAC presence and smoking,10, 11, 26 there was no association between BAC and smoking in our cohort. Mechanisms proposed in the literature include effects of smoking on weight and estrogen metabolism and selective survival of smokers without BAC after the age of 50. However, the latter explanation appears unlikely in MINERVA because we recruited women who reported current smoking in the age range 60 to 79. Thus a satisfactory explanation remains elusive. Also, and as noted by others5, 6, 14, 15, 27, 28, no relationship was seen between BAC presence and LDL cholesterol level.
We also found that BAC adds prognostic information at every level of the PCE risk. This implies that women with BAC at borderline or intermediate ASCVD risk may be candidates for more aggressive treatment, and women with BAC already at high risk may be candidates for intensification of therapy. At this point is not known whether statin treatment would actually lead to progression of BAC, as is the case with CAC, since statins promote coronary atheroma calcification and plaque stabilization.29 In our cohort, 25 percent of women with BAC were not on cholesterol lowering drugs, and of those, 58 percent were in intermediate or borderline PCE risk groups. This particular segment of the population uniquely identified by BAC would therefore benefit from initiation of a risk discussion to adjust preventive options.
We noted a poor performance of the PCE in our cohort (C=0.63) and very little improvement in discrimination after adding BAC variables. The PCE were derived in historical cohorts that don’t reflect contemporary treatment patterns like we see in MINERVA, where 74 percent of the cohort is on cholesterol lowering medication.
Our results also demonstrate that inclusion of BAC presence versus absence resulted in a well-calibrated model and led to a clinically meaningful and statistically significant reclassification overall (NRI=0.12) and among women in borderline or intermediate PCE risk groups (cNRI=0.11). However, it was also noted that inclusion of a 4-level BAC variable resulted in lower NRI estimates (overall and clinical NRI both=0.07 and p≥0.06). We recognize however that the NRI remains a controversial index of prediction improvement.30
CAC and BAC and two vascular calcification phenotypes with different pathophysiology and etiology: whereas CAC represents intimal calcium deposits related to the atherosclerotic process (and is closely related to smoking and hyperlipidemia), BAC represents medial calcium deposits leading to vascular stiffness and is related more closely to diabetes and hypertension.31–33 The arterial supply to the breast is primarily derived from branches of the internal thoracic (mammary) artery, intercostal arteries, and the lateral thoracic artery. Arterial branches of the internal and lateral thoracic arteries branch out deep into the breast parenchyma. BAC is almost exclusive for arteries, as venous calcifications in the breast are rare. Because of size (small to medium) and biology, breast arteries are comparable to epicardial or microvascular circulation and distinct from large vessels such as the coronaries or the aorta.
It should be pointed out there is no current standard for reporting gradation of BAC. Our approach was an objective novel densitometry method that generates a BAC continuous calcium score, analogous to the coronary calcium score. This method is not clinically available yet, but could be included in next-generation mammography equipment in the same way abdominal aortic calcification is visualized by DXA systems.34 Our group has made advances in machine learning that are bringing the densitometry method for BAC assessment closer to clinical translation.35 Other groups have proposed a “0” to “12” point BAC gradation Likert-type scale incorporating number of vessels involved, the longest length of vessel involvement and the density of calcium as visually ascertained by the radiologist.5–7, 16 This is, however, a time-consuming approach that places heavy burden on the reader and does not quantitatively measure calcium mass.
From the patient’s perspective, it is important to cite the work of Margolis et al.,36 who demonstrated that 96 percent of the 678 surveyed women wanted to be informed about BAC found at mammography. These data, together with our findings, supports the widespread adoption of BAC reporting on mammography and incorporation of BAC in primary prevention guidelines as a novel risk enhancing factor.
Strengths of the MINERVA cohort include: the large size, deep rigorous phenotyping of risk factors, lifestyle and reproductive factors, ethnic diversity, availability of 6.5 years of follow up, and objective quantitative assessment of BAC quantity using contemporary digital mammography. We recognize several limitations. First, our findings may not be generalizable to uninsured populations or to women younger than 60. Second, we were unable to examine individual CVD outcomes such as myocardial infarction, stroke or heart failure due to limited statistical power at this time. MINERVA was designed to examine the two composite outcomes (ASCVD and global CVD) presented in this report. Third, we did not have computed tomography assessment of coronary artery calcium, so we were unable to perform head-to-head comparison of these two vascular phenotypes. Newallo et al. reported that, on 204 African-American women (mean age 52 years), BAC presence has a sensitivity of 67% and a specificity of 85% for CAC >100.7 Fourth, the densitometry method for assessment of BAC quantity has only been validated by our group, so external validation is warranted. Finally, we did not assess the number of calcified vascular lesions in the breast, thus we were unable to examine the importance of localized versus diffuse BAC.
In conclusion, our analysis in a large, ethnically diverse cohort demonstrates that presence of BAC in mammograms is independently related to incident ASCVD and to global CVD, and therefore adds to the body of evidence that assessment and reporting of BAC status has potential utility to change clinical practice and impact primary CVD prevention for women. Further research in large cohorts with longer follow-up period is needed to better delineate the dose-response association between BAC burden and CVD outcomes and to establish the value of BAC in women before age 60.
Supplementary Material
CLINICAL PERSPECTIVE.
This observational study in a large, ethnically diverse cohort of insured women between the ages of 60 and 79 years demonstrates that presence of breast arterial calcification (BAC) in contemporary digital mammograms is independently associated with incident atherosclerotic cardiovascular disease (ASCVD) and global CVD, and therefore adds to the body of evidence that assessment and reporting of BAC status has potential utility to change clinical practice and impact primary CVD prevention for women. Further research is warranted to better delineate the dose-response association between BAC burden and CVD outcomes and to establish the value of BAC in women before age 60. Our findings therefore support the adoption of assessment and reporting of BAC on mammograms and the incorporation of BAC in ASCVD primary prevention guidelines in women over age 60 as a novel risk enhancing factor.
Funding:
MINERVA is an investigator-initiated cohort study funded by the National Heart, Lung, and Blood Institute (RO1HL106-043; multiple PIs Iribarren and Molloi).
Non-standard Abbreviations
- ACS
American Cancer Society
- BAC
Breast arterial calcification
- CAC
Coronary artery calcification
- CAD
coronary artery disease
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- DXA
Dual Energy X-Ray Absorptiometry
- ASCVD
Atherosclerotic cardiovascular disease
- CI
Confidence Interval
- PCE
American Heart Association / American College of Cardiology Pooled Cohorts Equation
- NRI
Net reclassification improvement
- cNRI
Clinical net reclassification improvement
- MINERVA
MultIethNic study of brEast aRterial calcium gradation and cardioVAscular disease
- KPNC
Kaiser Permanente of Northern California
- MLO
Mediolateral oblique
- CC
craniocaudal
- ICD-9
International classification of diseases 9th revision
- ICD-10
International classification of diseases 10th revision
- CPT4
Current procedure terminology, 4th edition
- PPV
Positive predictive value
- SD
Standard deviation
- UC
University of California
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
Footnotes
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN et al. American Heart Association Council on Epidemiology and Prevention Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, Walter LC, Church TR, Flowers CR, LaMonte SJ. American Cancer S. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314:1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenkrantz AB, Fleming M, Duszak R Jr. Variation in Screening Mammography Rates Among Medicare Advantage Plans. J Am Coll Radiol. 2017;14:1013–1019. [DOI] [PubMed] [Google Scholar]
- 5.Kelly BS, Scanl OE, Heneghan H, Redmond CE, Healy GM, Mc Dermott E, Heffernan EJ, Prichard R, Mc Nally S. Breast Arterial Calcification on screening mammography can predict significant Coronary Artery Disease in women. Clin Imaging. 2018;49:48–53. [DOI] [PubMed] [Google Scholar]
- 6.Mostafavi L, Marfori W, Arellano C, Tognolini A, Speier W, Adibi A, Ruehm SG. Prevalence of coronary artery disease evaluated by coronary CT angiography in women with mammographically detected breast arterial calcifications. PLoS One. 2015;10:e0122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newallo D, Meinel FG, Schoepf UJ, Baumann S, De Cecco CN, Leddy RJ, Vliegenthart R, Mollmann H, Hamm CW, Morris PB et al. Mammographic detection of breast arterial calcification as an independent predictor of coronary atherosclerotic disease in a single ethnic cohort of African American women. Atherosclerosis. 2015;242:218–21. [DOI] [PubMed] [Google Scholar]
- 8.Ruzicic D, Dobric M, Vukovic M, Hrncic D, Dordevic S, Ruzicic M, Aleksandric S, Dordevic-Dikic A, Beleslin B. The correlation of SYNTAX score by coronary angiography with breast arterial calcification by digital mammography. Clin Radiol. 2018;73:454–459. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira JA, Pompei LM, Fernandes CE, Azevedo LH, Peixoto S. Breast arterial calcification is a predictive factor of cardiovascular disease in Brazilian postmenopausal women. Climacteric. 2009;12:439–44. [DOI] [PubMed] [Google Scholar]
- 10.Iribarren C, Go AS, Tolstykh I, Sidney S, Johnston SC, Spring DB. Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health (Larchmt). 2004;13:381–9; discussion 390–2. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka M, Warren R, Luben R, Camus J, Denton E, Sala E, Day N, Khaw KT. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? AJR Am J Roentgenol. 2006;187:73–80. [DOI] [PubMed] [Google Scholar]
- 12.Kemmeren JM, van Noord PA, Beijerinck D, Fracheboud J, Banga JD, van der Graaf Y. Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: The DOM Project. Doorlopend Onderzoek Morbiditeit en Mortaliteit. Am J Epidemiol. 1998;147:333–41. [DOI] [PubMed] [Google Scholar]
- 13.Schnatz PF, Marakovits KA, O’Sullivan DM. The association of breast arterial calcification and coronary heart disease. Obstet Gynecol. 2011;117:233–41. [DOI] [PubMed] [Google Scholar]
- 14.Sedighi N, Radmard AR, Radmehr A, Hashemi P, Hajizadeh A, Taheri AP. Breast arterial calcification and risk of carotid atherosclerosis: Focusing on the preferentially affected layer of the vessel wall. Eur J Radiol. 2011;79 250–6. [DOI] [PubMed] [Google Scholar]
- 15.Chadashvili T, Litmanovich D, Hall F, Slanetz PJ. Do breast arterial calcifications on mammography predict elevated risk of coronary artery disease? European journal of radiology. 2016;85:1121–4. [DOI] [PubMed] [Google Scholar]
- 16.Moradi M, Adibi A, Abedi M. Relationship between breast arterial calcification on mammography with CT Calcium scoring and coronary CT angiography results. Adv Biomed Res. 2014;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iribarren C, Sanchez G, Husson G, Levine-Hall T, Quesenberry C, Sam DL, Maier J, Chaudhary RS, Patel M, Sadeghi B et al. MultIethNic Study of BrEast ARterial Calcium Gradation and CardioVAscular Disease: cohort recruitment and baseline characteristics. Annals of epidemiology. 2018;28:41–47 e12. [DOI] [PubMed] [Google Scholar]
- 19.Molloi S, Xu T, Ducote J, Iribarren C. Quantification of breast arterial calcification using full field digital mammography. Med Phys. 2008;35:1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molloi S, Mehraien T, Iribarren C, Smith C, Ducote JL, Feig SA. Reproducibility of breast arterial calcium mass quantification using digital mammography. Acad Radiol. 2009;16:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med. 2015;34:1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44. [DOI] [PubMed] [Google Scholar]
- 24.Paynter NP, Cook NR. A bias-corrected net reclassification improvement for clinical subgroups. Med Decis Making. 2013;33:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Noord PA, Beijerinck D, Kemmeren JM, van der Graaf Y. Mammograms may convey more than breast cancer risk: breast arterial calcification and arterio-sclerotic related diseases in women of the DOM cohort. Eur J Cancer Prev. 1996;5:483–7. [PubMed] [Google Scholar]
- 27.Matsumura ME, Maksimik C, Martinez MW, Weiss M, Newcomb J, Harris K, Rossi MA. Breast artery calcium noted on screening mammography is predictive of high risk coronary calcium in asymptomatic women: a case control study. Vasa. 2013;42:429–33. [DOI] [PubMed] [Google Scholar]
- 28.Topal U, Kaderli A, Topal NB, Ozdemir B, Yesilbursa D, Cordan J, Ediz B, Aydinlar A. Relationship between the arterial calcification detected in mammography and coronary artery disease. Eur J Radiol. 2007;63:391–5. [DOI] [PubMed] [Google Scholar]
- 29.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 30.Pepe MS, Fan J, Feng Z, Gerds T, Hilden J. The Net Reclassification Index (NRI): a Misleading Measure of Prediction Improvement Even with Independent Test Data Sets. Stat Biosci. 2015;7:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmonds ME, Morrison N, Laws JW, Watkins PJ. Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed). 1982;284:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Greenberg JS, Javitt MC. Breast calcifications due to Monckeberg medial calcific sclerosis. Radiographics. 1999;19:1401–3. [DOI] [PubMed] [Google Scholar]
- 33.Sakata N, Noma A, Yamamoto Y, Okamoto K, Meng J, Takebayashi S, Nagai R, Horiuchi S. Modification of elastin by pentosidine is associated with the calcification of aortic media in patients with end-stage renal disease. Nephrol Dial Transplant. 2003;18:1601–9. [DOI] [PubMed] [Google Scholar]
- 34.Schousboe JT, Lewis JR, Kiel DP. Abdominal aortic calcification on dual-energy X-ray absorptiometry: Methods of assessment and clinical significance. Bone. 2017;104:91–100. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ding H, Bidgoli FA, Zhou B, Iribarren C, Molloi S, Baldi P. Detecting Cardiovascular Disease from Mammograms With Deep Learning. IEEE Trans Med Imaging. 2017;36:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolies LR, Yip R, Hwang E, Oudsema RH, Subramaniam VR, Hecht H, Narula J. Breast Arterial Calcification in the Mammogram Report: The Patient Perspective. AJR Am J Roentgenol. 2019;212:209–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


