Abstract
Increased nitrogen (N) deposition threatens global biodiversity, but its effects in arid urban ecosystems are not well studied. In addition to altered N availability, urban environments also experience increases in other pollutants, decreased population connectivity, and altered biotic interactions, which can further impact biodiversity. In deserts, annual plant communities make up most of the plant diversity, support wildlife, and contribute to nutrient cycling and ecosystem processes. Functional tradeoffs allowing coexistence of a diversity of annual plant species are well established, but maintenance of diversity in urban conditions and with increased availability of limiting nutrients has not been explored. We conducted a 13-year N and phosphorus (P) addition experiment in Sonoran Desert preserves in and around Phoenix, AZ, to test how nutrient availability interacts with growing season precipitation, urban location, and microhabitat to affect winter annual plant diversity. Using structural equation modeling and generalized linear mixed modeling, we found that annual plant taxonomic diversity was significantly reduced in N-enriched and urban plots. Water availability in both current and previous growing seasons impacted annual plant diversity, with significant interaction effects showing increased diversity in wetter years and greater responsiveness of the community to water following a wet year. However, there were no significant interactions between N enrichment and water availability, urban location, or microhabitat. Lowered diversity in urban preserves may be partly attributable to increased urban N deposition. Changes in biodiversity of showy species like annual wildflowers in urban preserves can have important implications for connections between urban residents and nature, and reduced diversity and community restructuring with N enrichment represents a challenge for future preservation of aridland biodiversity.
Keywords: Desert annual plants, fertilization, CAP LTER, nitrogen, biodiversity, plant community composition, species richness, Arizona
Introduction
Anthropogenic activity has significantly increased global nitrogen (N) deposition and availability, which alters ecosystem structure and function (Sala et al. 2000, Bobbink et al. 2010, Pardo et al. 2011, Ackerman et al. 2019). High N availability can reduce diversity and change the species composition of primary producers with potentially long-lasting effects (Elser et al. 2007, Pardo et al. 2011, Field et al. 2014, Harpole et al. 2016, Payne et al. 2017). Urban activities, including fossil fuel burning, contribute to increased N deposition, and ecosystems within or close to cities tend to experience higher N availability than do relatively distant ecosystems (Fenn et al. 2003b, Galloway et al. 2008, Bettez and Groffman 2013). N deposition rates are expected to rise as cities grow, leading to ever greater effects on urban and surrounding regions (Fenn et al. 2003a, Liu et al. 2013, Ackerman et al. 2019). Rapid urban growth is expected in arid and semi-arid regions worldwide (Seto et al. 2011, United Nations 2014); thus, improved understanding is needed of the effects of increased N availability on biodiversity and ecological functioning in dryland ecosystems.
Most research to date on the effects of elevated N availability on terrestrial ecosystem function has focused on grasslands and forests, with comparatively little work in arid and semi-arid ecosystems (Bobbink et al. 2010, Pardo et al. 2011). However, arid and semi-arid ecosystems make up a large fraction of the global land area (about 30%, Gamo et al. 2013) and function differently than wetter (more mesic) ecosystems in fundamental ways. In mesic systems, addition of the common limiting nutrients—N and phosphorus (P) —typically increases net primary production (NPP) while decreasing plant species diversity (Elser et al. 2007, LeBauer and Treseder 2008, Bobbink et al. 2010, Fay et al. 2015). In deserts, however, both water and nutrients limit NPP, and lack of water can diminish the effects of increased nutrient availability (Noy-Meir 1973, Hooper and Johnson 1999, Snyman 2002, Rao and Allen 2010, Yahdjian et al. 2011, Ladwig et al. 2012, Sponseller et al. 2012). These interactions between the effects of water and nutrients on NPP in arid and semi-arid systems are also likely to affect the relationships among nutrient availability, species composition, and diversity.
Desert ecosystems are inherently patchy when compared to mesic ecosystems, with a high degree of temporal and spatial heterogeneity in water availability and soil resources (Noy-Meir 1973). Rainfall is infrequent, often spatially localized, and highly variable from year to year. Sparsely distributed, long-lived shrubs and trees create “islands of fertility” under their canopies with more organic material, increased N concentrations, and greater soil moisture relative to interplant spaces (Schlesinger et al. 1996, Schade and Hobbie 2005). Given this spatial and temporal variability, desert plant communities are strongly influenced by facilitative effects, whereby shrubs and trees buffer temporal variability for other species in close proximity (Holzapfel and Mahall 1999, Butterfield and Callaway 2013, Mclntire and Fajardo 2014). Thus, small-scale patchiness in deserts may lead to landscape-level plant responses to increased nutrient availability in deserts that are not observed in more mesic settings.
In addition to the variability inherent in desert ecosystems, plant communities located in aridland cities are subject to physical, chemical, and biological stressors that arise from urbanization. For example, urban greenspaces are more fragmented and experience greater air, water, noise, and light pollution than do non-urban preserves (Grimm et al. 2008, McDonald et al. 2009). Urban plant communities also tend to have fewer native plant species and more non-native species than non-urban counterparts (Walker et al. 2009). Greater atmospheric deposition, and thus nutrient availability, in urban spaces may interact with desert landscape patchiness to result in different plant community responses than are observed in more natural spaces. For example, dominance of non-native species in urban communities could lead to different community-level responses to variable climatic conditions.
Responses to short- and long-term alterations to aridland environments, such as chronic nutrient enrichment and other urban influences, can be explored in the dynamic and diverse winter annual plant community. Annual plants are an important component of aridland plant communities and make up about 50% of all plant species diversity in the Sonoran Desert (Venable et al. 1993). These species have a rapid life cycle, which enables communities to quickly respond to yearly variations in environmental conditions (Mulroy and Rundel 1977). Desert annual plants are an important resource for pollinators and species like the threatened Agassiz’s desert tortoise (Gopherus agassizii) (Jennings and Berry 2015) and can account for up to half of desert primary production in wet years (Hadley and Szarek 1981). Additionally, some annual plant species were historically cultivated as an important cold-season food source for the Hohokam people (Bohrer 1991) and are a charismatic feature of the desert for contemporary visitors (Ryan 2011).
Much is known about how desert annual plant species respond to variability in precipitation and temperature (Venable et al. 1993, Pake and Venable 1995, Gremer et al. 2012, Huxman et al. 2013). Functional tradeoffs between resource acquisition and stress tolerance can explain long-term community coexistence among species under highly variable conditions, as species with differing strategies thrive under different sets of environmental conditions (Angert et al. 2007, Kimball et al. 2011, Gremer et al. 2013, Ge et al. 2019). Long-term monitoring of Sonoran Desert winter annuals has shown the impacts of climate change on coexistence and competitive interactions, with increased abundance of cold-tolerant species (due to altered germination timing) and species with more demographically consistent populations (i.e., less dependent on yearly conditions for germination) over time (Kimball et al. 2010, Huxman et al. 2013).
Following the principles of the leaf economics spectrum (Reich 2014), increased nutrient availability can alter a plant’s physiological tradeoffs associated with water use, nutrient acquisition, and growth, resulting in shifts in functional or phylogenetic composition. However, the co-occurring effects of chronic nutrient enrichment and climate variability have not been evaluated in these communities.
Annual plants rely entirely on the seed bank for continuity from year to year, and therefore exhibit bet-hedging strategies whereby only a small fraction of seeds germinate in any given year (Adondakis and Venable 2004, Venable 2007, Gremer and Venable 2014, Gremer et al. 2016). However, environmental conditions outside of the immediate germination and growth period can influence a given year’s emergent community. For example, previous year and preceding summer conditions can affect germination response of winter annuals (Adondakis and Venable 2004, Bowers 2005), and favorable conditions for growth in a given year are likely to result in greater seed set (Pake and Venable 1995). Annual plant survival and reproduction may also depend on proximity to shrubs and their resource islands, suggesting additional complex dynamics between previous conditions, current conditions, and microsite characteristics (Pake and Venable 1995, Holzapfel and Mahall 1999). Interactions between these various drivers of annual plant composition and chronic nutrient enrichment have not been well explored, particularly over long time periods capturing both spatial and temporal variation in environmental conditions.
We conducted a 13-year nutrient fertilization experiment in Sonoran Desert preserves across a precipitation gradient within and around metropolitan Phoenix, Arizona, to ask how nutrient enrichment, climate, microhabitat, and location in the urban environment interact to shape Sonoran Desert winter annual plant communities. We hypothesized that (1) annual plants are primarily limited by water, which will result in negligible effects of nutrient addition in dry years; (2) nutrient enrichment changes the nutrient acquisition and water use efficiency physiological tradeoffs of annual plant communities, leading to changes in diversity and composition; (3) urban preserves support lower annual plant diversity due to higher ambient levels of atmospheric N deposition; and (4) shrubs buffer resource variability for annual plants through facilitative interactions, resulting in higher annual plant diversity below shrub canopies in lower-resource years and treatments. We tested these hypotheses by measuring annual plant community composition in 15 sites during eight years of the 13-year nutrient enrichment experiment. Because the desert preserve sites occur along a precipitation gradient as well as within and outside the urban matrix (with differing rates of N deposition), we predicted that both urban and N-fertilized communities would have reduced diversity compared to non-urban unfertilized communities. We also predicted that diversity would increase along the precipitation gradient during wet years. Additionally, we predicted higher annual plant diversity in spaces under shrubs, especially in dry years.
Methods
Site description
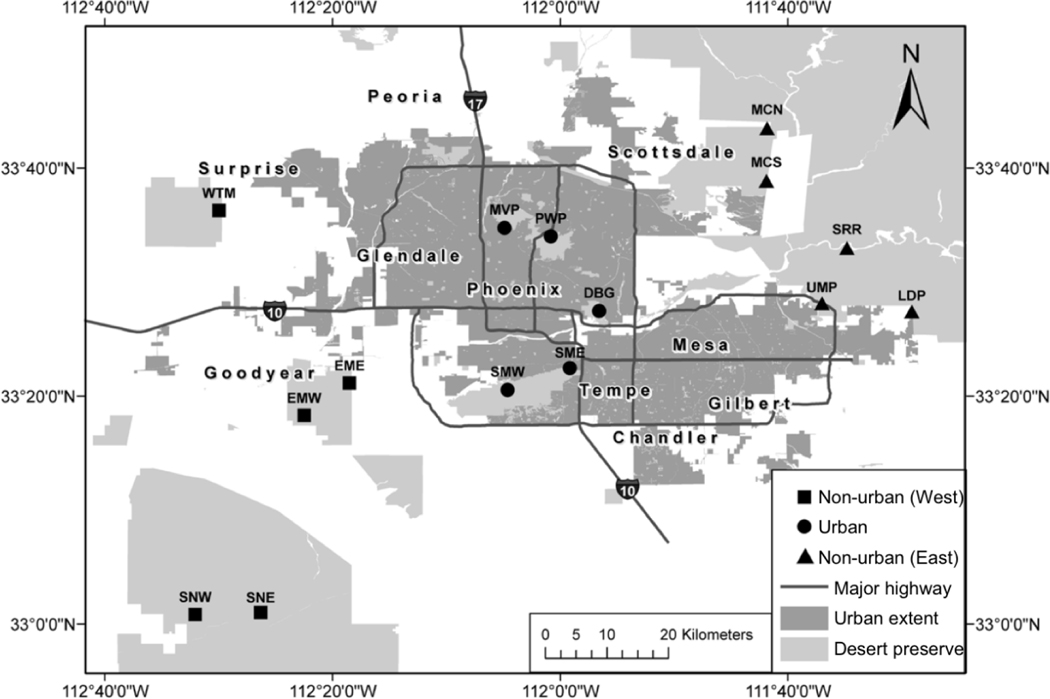
We established 15 sites in native Sonoran Desert preserves within the Central Arizona-Phoenix Long Term Ecological Research (CAP LTER) study area, in and around the Phoenix metropolitan area (Fig. 1, Table 1). Sites were grouped by region, with five sites in the west valley, five in the east valley, and five in the metropolitan area (for additional site description, see Hall et al. 2011, Cook et al. 2018). Winter precipitation varied predictably across regions, with increasing rainfall from west to east (Table 1). Measured N deposition in the urban sites (7.2 ± 0.4 kg N ha−1 y−1) was elevated compared to the surrounding preserves to the east and west (6.1 ± 0.3 kg N ha−1 y−1), and was lower than expected from measurements in other cities (Cook et al. 2018). We compare annual plant communities and environmental variables in the five desert preserves in the metropolitan area (hereafter “urban”) to the 10 desert preserves outside the city (hereafter “non-urban”).
Figure 1.
Map of study sites in and around the Phoenix metropolitan area, adapted from Hall et al. (2011). All sites are located in Sonoran Desert preserves.
Table 1.
Experimental sites.
| Region | Site Code1 | Site Name | Site Elevation (m) | Average Precipitation (mm)2 |
|---|---|---|---|---|
|
| ||||
| Urban | DBG | Desert Botanical Garden | 396 | 78 |
| Urban | MVP | Mountain View Park (North Mountain) | 397 | 71 |
| Urban | PWP | Piestewa Peak (Phoenix Mountain Preserve) | 456 | 76 |
| Urban | SME | South Mountain Park, East | 372 | 62 |
| Urban | SMW | South Mountain Park, West | 458 | 59 |
| Non-urban (East) | LDP | Lost Dutchman State Park | 620 | 132 |
| Non-urban (East) | MCN | McDowell Mountain Regional Park, North | 476 | 115 |
| Non-urban (East) | MCS | McDowell Mountain Regional Park, South | 539 | 102 |
| Non-urban (East) | SRR | Salt River Recreation Area | 434 | 120 |
| Non-urban (East) | UMP | Usery Mountain Regional Park | 592 | 95 |
| Non-urban (West) | EME | Estrella Mountain Regional Park, East | 331 | 55 |
| Non-urban (West) | EMW | Estrella Mountain Regional Park, West | 382 | 53 |
| Non-urban (West) | SNE | Sonoran Desert National Monument, East | 492 | 52 |
| Non-urban (West) | SNW | Sonoran Desert National Monument, West | 375 | 55 |
| Non-urban (West) | WTM | White Tank Mountain Regional Park | 454 | 73 |
For additional site characteristics, see Hall et al. (2011) and Cook et al. (2018).
Mean winter growing season precipitation (October - March) for the period 2006 to 2018
Nutrient enrichment treatments
At each of the 15 sites, we established four nutrient-addition treatment plots: N-fertilized, P-fertilized, N + P-fertilized, and unfertilized/control. Plots were 20 m × 20 m squares in order to capture landscape patchiness and create large fertilized areas, and plots at a site were at least 5 m apart. Nutrient treatments within a site were randomly assigned, and plots were located with consideration of topography to avoid runoff from fertilized to unfertilized plots. Each plot contained at least five individuals of two common shrubs, Larrea tridentata (DC.) Coville and Ambrosia deltoidea (Torr.) W.W. Payne or Ambrosia dumosa (A. Gray) W.W. Payne, and excluded leguminous trees.
Nutrient treatments were added as hand-broadcast solids twice annually, once between December and February and once between June and August to follow the first winter and summer rains, respectively. Fertilization began in December of 2005. Phosphorus-enriched plots received triple superphosphate at 120 kg P ha−1 yr−1 from 2006–2008, reduced to 60 kg P ha−1 yr−1 in 2009 and 12 kg P ha−1 yr−1 from 2010–2018. Phosphorus fertilization was initially in excess to increase the probability of P reaching deep shrub roots and was decreased over time. Nitrogen in the form of ammonium nitrate (NH4NO3) was initially added at twice the hypothesized rate of N deposition in urban centers (Fenn et al. 2003b) and then decreased after ten years, with 60 kg N ha−1 yr−1 from 2006–2015, 45 kg N ha−1 yr−1 in 2016, and 30 kg N ha−1 yr−1 in 2017 and 2018. N + P-fertilized plots received both amendments at the rates given above.
Annual plant composition
We measured winter annual plant community composition at estimated peak biomass, which was between February and March depending on the timing of winter rainfall. Peak biomass was in February in all years except 2013, when it occurred in March. Yearly measurements began in 2008 and continued through 2018, with the exceptions of 2011, 2012, and 2014, years with relatively low winter precipitation. Low precipitation conditions were captured in 2018.
In each 20 m × 20 m treatment plot, we established four permanent 1 × 1 m quadrats for plant community composition measurements. Quadrats were located in different microhabitats: two under Larrea tridentata shrub canopies and two in the open spaces between shrubs (Facelli and Temby 2002, Schade and Hobbie 2005). All annual species within each quadrat were identified to the lowest possible taxon (<1% not identifiable to genus; Appendix S1: Table S1). Species nomenclature follows The Plant List (2013). Species nativity to Arizona was determined from the USDA Plants database (www.plants.usda.gov). Genera were defined as native if only native species were observed, non-native if only non-native species were observed, and mixed if both native and non-native species were observed or if genus-level identifications may have been either native or non-native species (Appendix S1: Table S1).
The abundance of each taxon was estimated as the total fraction of the 1 m × 1 m quadrat covered by individuals of that taxon. For all years after 2008, overlapping species were counted separately, so the total cover of all species in a plot may be greater than 100%. In 2008, abundance was estimated using a different methodology. For this reason, diversity calculations using abundance omit data from 2008.
We established four additional permanent 1 m × 1 m quadrats within the 20 m × 20 m plots for measurement of annual aboveground net primary productivity (ANPP) in the same years as community composition sampling. As with community composition, two quadrats were located under shrubs and two between shrubs. We clipped all aboveground living plant material at the soil surface from 0.25 m2 subplots in the corners of permanent 1 m × 1 m quadrats. The subplot clipped rotated each year, such that there were at least 3 years between clipping the same subplot. Collected plant material was dried at 60°C for 48 hours then weighed to get an estimate of ANPP. Measured ANPP in the replicate quadrats was averaged to give one estimate (g m−2) for each combination of site, nutrient treatment, and microhabitat per year.
Some irregularities occurred across our eight years of annual plant sampling when permanent plots could not be located or accessed in a particular year. ANPP was not sampled at SNE, SNW, DBG, MVP, MCN, or UMP in 2018; at UMP, SRR, MCS, or MVP in 2015; or at three DBG plots and one SRR plot in 2013, resulting in a total of 876 ANPP samples for all years. Additionally, plots at MVP were sampled for ANPP but not community composition in 2008. We therefore analyzed a total of 952 samples for community composition (15 sites × 4 treatments × 2 microhabitats × 8 years - 1 site × 4 treatments × 2 microhabitats × 1 year; Grimm et al. 2019).
Diversity metrics
All analyses were performed in R version 3.6.1 (R Core Team 2019). We quantified annual plant diversity using species richness, Shannon diversity, and phylogenetic diversity as metrics. For all diversity metrics, species lists from the two replicate community composition subplots were combined and measured cover was averaged by species. The most commonly observed genus, Pectocarya, contained three species (P. recurvata, P. heterocarpa, and P. platycarpa), but was reduced to genus and treated as a single species for the purpose of these analyses due to difficulty identifying to species when fruits were immature. Shannon diversity was calculated using community percent cover data, omitting data from 2008. We calculated species richness using the function specnumber and Shannon diversity using the function diversity from R package vegan (version 2.5.3, Oksanen et al. 2018).
Phylogenetic diversity can give additional insight into the composition of communities relative to more simplistic measures such as species richness by describing the evolutionary history and relatedness of communities (Webb et al. 2002, Cavender-Bares et al. 2009). Additionally, in the absence of physiological trait measurements, phylogenetic diversity can act as a proxy for functional diversity (Webb et al. 2002, Cavender-Bares et al. 2009). To determine phylogenetic distances between taxa, we used the angiosperm phylogeny defined by Smith and Brown (2018) and constructed from GenBank and Open Tree of Life taxa with a backbone provided by Magallón et al. (2015). This tree was chosen in place of the commonly used phylogeny from Zanne et al. (Zanne et al. 2014) because it included all genera recorded in this study. We considered phylogenetic relationships at the genus rather than species level because congeners were sometimes cryptic and difficult to distinguish in all years (e.g., Pectocarya recurvata, P. heterocarpa, and P. platycarpa). Multiple species were observed for 16 genera (out of 78 total), with more than two distinct species observed for only Cryptantha (5 species) and Pectocarya (3 species; Appendix S1: Table S1). Comparisons at the genus rather than species level may result in higher measured phylogenetic diversity if many congenerics are present; however, most diversity in this community is captured at the genus level as there were few recorded congenerics for nearly all genera. Using congeneric.merge from the R package pez (version 1.1–1, Pearse et al. 2015), we merged all species into the tree, reduced the entire tree to genus, and trimmed it to include only genera recorded in this study (Appendix S1: Fig. S1).
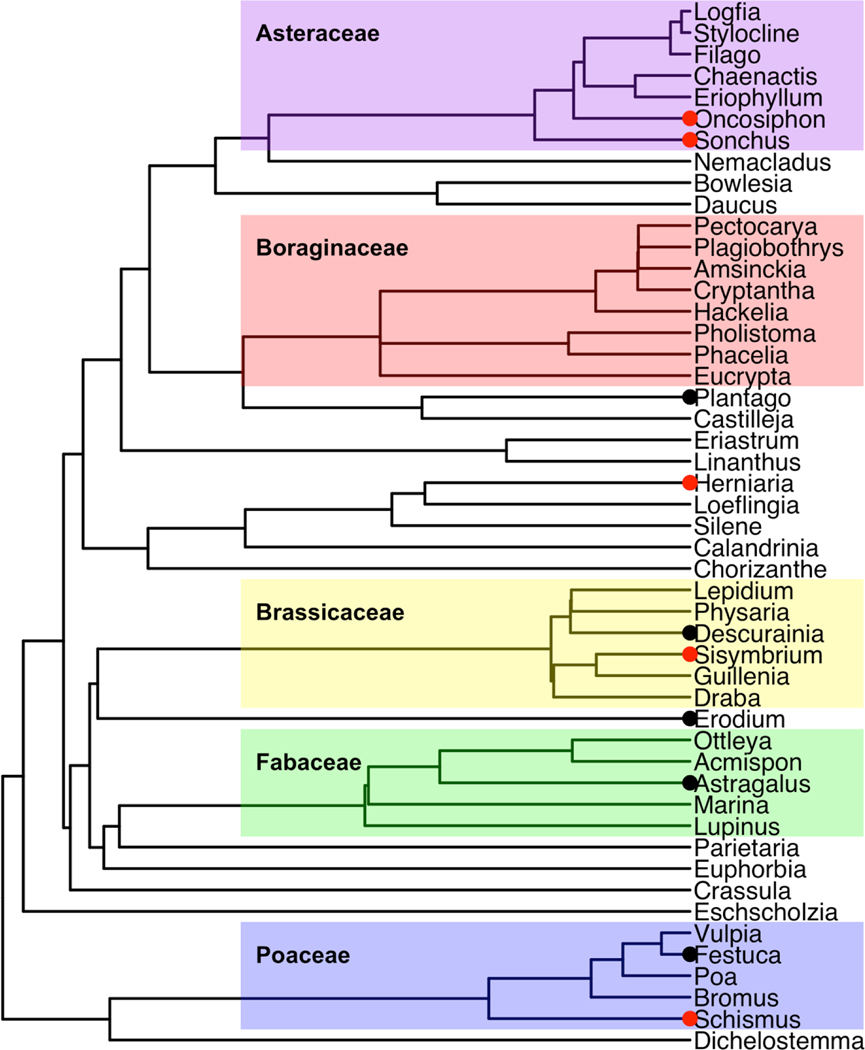
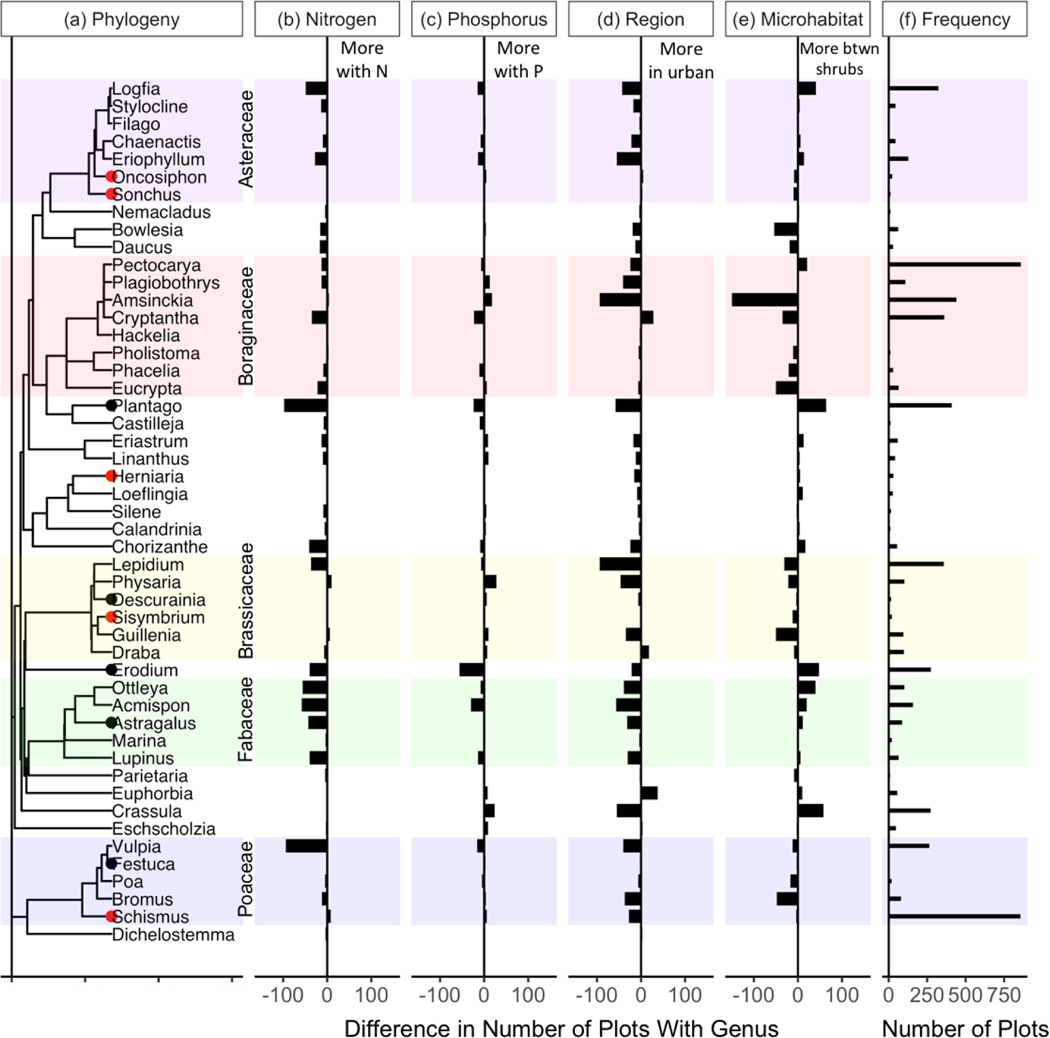
Phylogenetic trees were visualized using R package ggtree (version 1.16.6, Yu et al. 2017). For the purpose of visualizations, genera observed in less than 0.5% of samples (<5 observations across all 952 community composition plots, 29 genera out of 78 total) were removed (Fig. 2, Appendix S1: Fig. S1, Appendix S1: Table S1). Trees are labeled by family to show groupings of potential functional importance. All observed genera were included in calculated diversity metrics.
Figure 2.
Phylogenetic tree including all genera observed in at least 0.5% of all samples in this study (see Appendix S1: Fig. S1 for complete tree). Tree is adapted from the ALLMB tree defined by Smith and Brown (2018). Colors represent family groupings for families with at least five genera observed. Red points show genera with only non-native species observed and black points show genera with some native and some non-native species. Genera with no points include only native species in this community.
We considered phylogenetic diversity using mean pairwise distance (MPD), a measure of the average evolutionary distance from a taxon in a sample to its closest relative in the sample. We chose this metric to represent divergence or relatedness of communities, and to complement our species richness metric rather than using a more highly correlated metric such as Faith’s phylogenetic distance (Tucker et al. 2017). Our MPD measurements were standardized against a null model where tip labels in the phylogeny were shuffled to give a standardized effect size (SES) of mean pairwise distance (hereafter “SES MPD”), or the relatedness of a community compared to a random community drawn from the phylogeny. Samples with no plants or only one genus observed could not be included in calculations of phylogenetic diversity (74 of 952 samples were therefore excluded). SES MPD was calculated in R using the ses.mpd function from package picante (version 1.7, Kembel et al. 2010).
Water availability
We collected climate data for the winter growing season, defined as beginning in October when annual plants first germinate (Pake and Venable 1995, Venable and Pake 1999), and ending at peak biomass, when annual plant community composition was sampled each year.
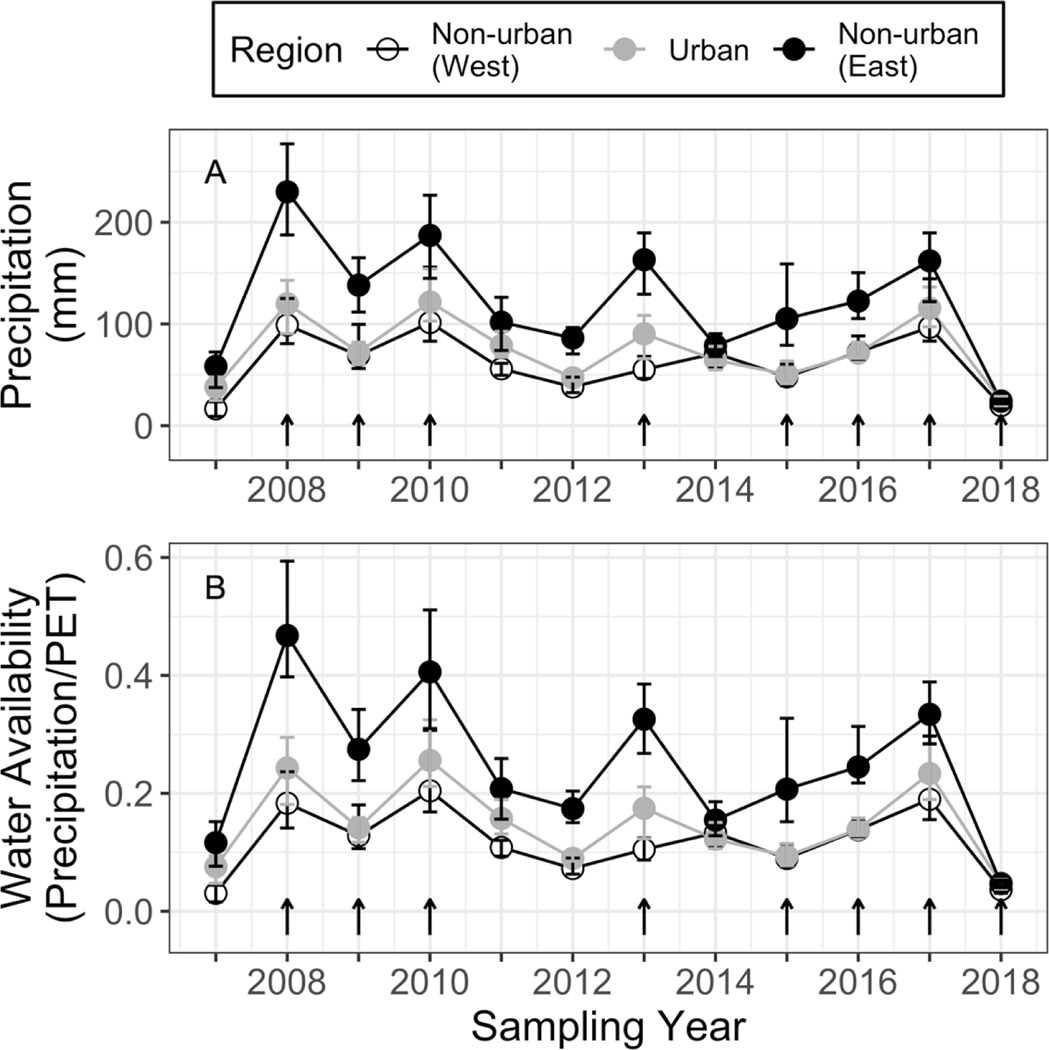
To represent the overall climatic conditions in each year and site, we used a simple aridity index defined as total precipitation (mm) divided by potential evapotranspiration (PET; mm) (UNEP 1992). This aridity index (hereafter “water availability”) estimates the amount of water inputs relative to PET as estimated by temperature, such that higher values indicate wetter conditions and lower values indicate drier conditions (Fig. 3). In addition to the current growing season water availability, we considered water availability in the previous growing season, defined as October through the end of March of the year before annual plant sampling, as a potentially important antecedent condition that may affect current season annual plant growth.
Figure 3.
Precipitation (A) and water availability (B) recorded during the winter annual plant growing season (October - March). Points show the mean value for the five experimental sites within each region, and error bars show the range for a given region and year. Arrows above the x axis indicate years in which the annual plant community was sampled.
We gathered precipitation data from the Flood Control District of Maricopa County (FCDMC) (http://www.fcd.maricopa.gov/) and temperature data (used to calculate PET) from both FCDMC and the National Climate Data Center (NCDC) (https://www.ncdc.noaa.gov/cdo-web/). Each of our 15 study sites was matched with the nearest 3–5 precipitation stations and 1–2 temperature stations from these sources (Appendix S1: Table S2). Precipitation stations were located within 10 km ground distance and 150 m elevation distance from each site if possible, while temperature stations were within 20 km ground distance and 100 m elevation distance from each site. For precipitation stations, a small ground distance was considered more important than a small elevation difference due to typically patchy rainfall patterns in the region, while for temperature stations elevation was considered more important to avoid temperature gradients with altitude. Where no data were available for a given site and day from stations within these distances, more distant stations were used (Appendix S1: Table S3). We averaged daily values of rainfall, maximum temperature, and minimum temperature where data from multiple stations were available for a given site and day. For two sites (DBG and LDP), micrometeorological stations maintained by CAP LTER were located on site beginning in 2010 (Grimm et al. 2017). When and where available, these data were used in place of data from FCDMC or NCDC sensors. Comparison of CAP measurements and estimates from FCDMC/NCDC sensors for the two sites where both were available showed good agreement (Appendix S1: Fig. S2).
To calculate PET, we input monthly average minimum and maximum temperatures to the hargreaves function in R package SPEI (version 1.7, Beguería and Vicente-Serrano 2017), using site latitudes to estimate radiation. Hargreaves PET has been shown to perform well in arid and semi-arid environments (Samani and Pessarakli 1986, Hargreaves and Allen 2003) and requires only temperature data. We then summed precipitation and predicted Hargreaves PET over the entire growing season and divided precipitation by PET to get our calculated relative water availability index (Fig. 3).
Diversity and community composition analysis
We used structural equation modeling (SEM) and generalized linear mixed modeling to evaluate the effects of nutrient addition (N and P), water availability, urban location (urban/non-urban), and microhabitat (under or between shrubs) on diversity of Sonoran Desert winter annual plants. With SEM, we explored how our various predictors were related to multiple response variables through direct and indirect pathways. With mixed modeling, we investigated interaction effects between predictors, taking into account the nested, repeated measures experimental design. We also considered changes in community composition with these predictors using ordination, PERMANOVA, and similarity percentage (SIMPER) analyses.
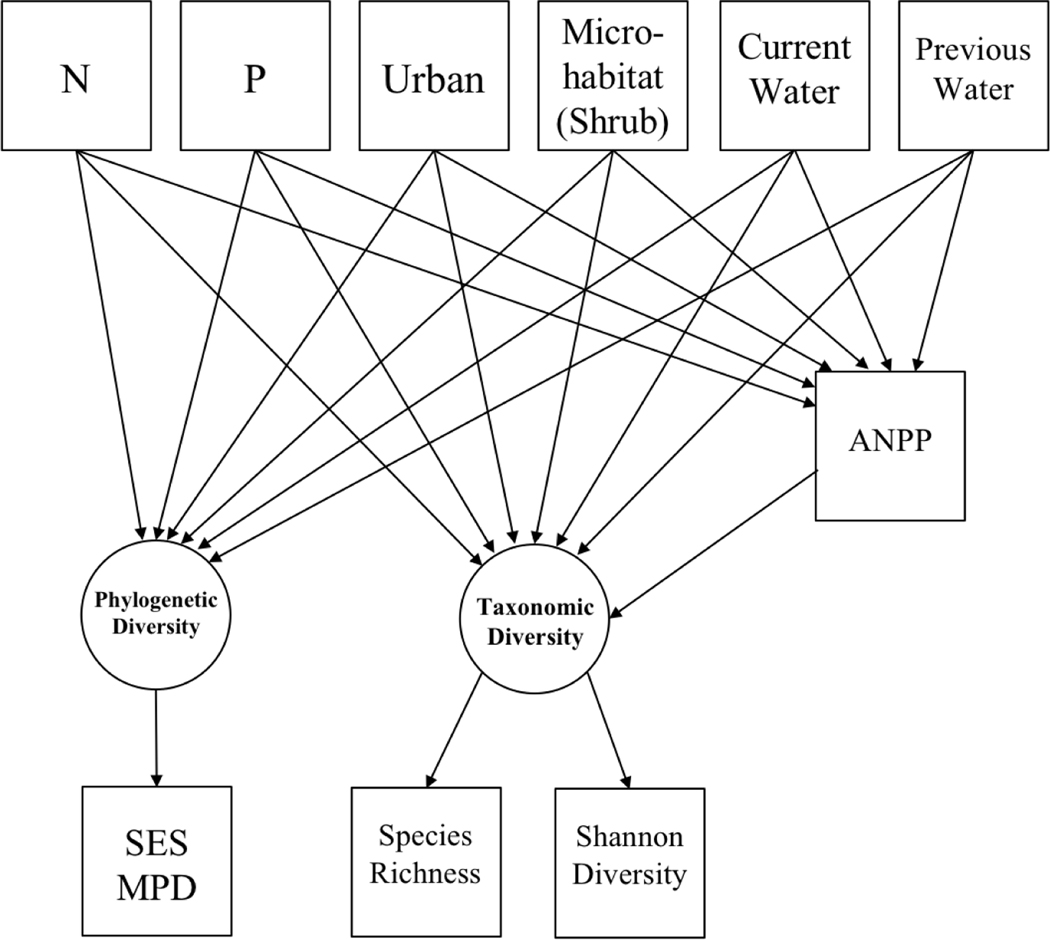
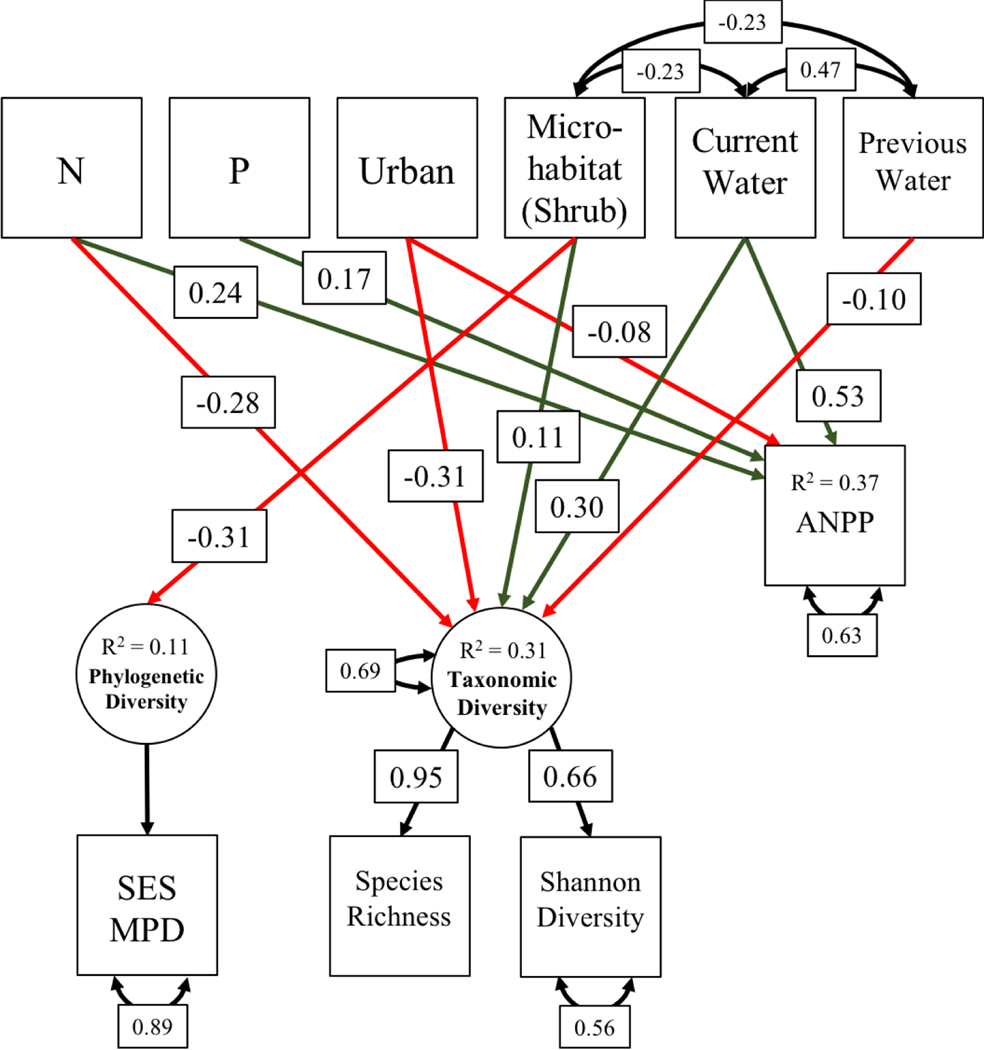
We used SEM to compare the effects of our predictors on annual plant taxonomic diversity (as defined by species richness and Shannon diversity) and phylogenetic diversity (defined by SES MPD). We also tested for indirect effects of our predictors on taxonomic diversity via changes in ANPP (Fig. 4), as expected if diversity is reduced through increased light or other resource competition (Hautier et al. 2009). The SEM was fit using R package lavaan (version 0.6–5, Rosseel 2012) and plotted using package semPlot (version 1.1, Epskamp 2019). A total of 704 samples had complete data (omitted 248 samples missing SES MPD, Shannon diversity, and/or ANPP, including all 2008 samples) and were used to fit the SEM. The latent variable “taxonomic diversity” was defined by species richness and Shannon diversity, with the loading for species richness set to 1. The SEM fit with Tucker-Lewis Index (TLI) = 0.83, Comparative Fit Index (CFI) = 0.96, Standardized Root Mean Square Residual (SRMR) = 0.03, and Root Mean Square Error of Approximation (RMSEA) = 0.09. While the commonly used RMSEA metric indicates only an acceptable model fit, it is known to be overly strict in cases with few degrees of freedom such as this model with eight degrees of freedom (Kenny et al. 2015). TLI also does not indicate a good fit (<0.95), but CFI and SRMR do support the model fit (>0.95 and <0.08 respectively) (Taasoobshirazi and Wang 2016), so we accepted the model as a reasonable representation of the data.
Figure 4.
Theoretical path diagram of annual plant diversity as shaped directly by nutrient availability, water availability, microhabitat, and urban or non-urban location, and indirectly by these predictors via change in ANPP. The latent variable Phylogenetic Diversity is defined entirely by the measured variable standardized effect size of mean phylogenetic distance (SES MPD). The loading for species richness onto the latent variable Taxonomic Diversity was fixed to one. N = nitrogen addition, P = phosphorus addition, Urban = sample in an urban site, Microhabitat (Shrub) = sample located under a shrub, Current Water = current growing season water availability, Previous Water = previous growing season water availability, ANPP = aboveground net primary productivity, SES MPD = standardized effect size of mean pairwise distance.
We further explored drivers of taxonomic diversity using generalized linear mixed models, considering interaction effects between predictors and including random intercepts by site and plot. We chose to use species richness as the response variable for these models, as SEM results showed that our predictors were able to explain most variation in taxonomic diversity as defined primarily by species richness. Fixed factors included in the global model were N addition (no addition as the base level), P addition (no addition as the base level), urban region (non-urban as the base level), microhabitat (location between shrubs as the base level), growing season water availability, and previous growing season water availability. From SEM analysis, all predictors except P addition were significantly related to taxonomic diversity. Therefore, we included pairwise interactions between all main effects except P. As current and previous year water availability were the only numeric predictors included and were on the same scale, they were not standardized. To accommodate our nested design, we included a random intercept for site. We accounted for repeated measures over time within the same plots by including a random intercept for plot nested within site. Models were fit with the glmer function in the lme4 package (version 1.1.19, Bates et al. 2015) using a Poisson distribution to account for count data. The overdispersion factor for the global model was 1.1 as measured using the function dispersion_glmer in package blmeco (version 1.4, Korner-Nievergelt et al. 2015), so we assume no overdispersion. All possible models were created from the global model using the dredge function in the MuMIn package (version 1.42.1, Barton 2018). We selected the model with the lowest AlCc (Appendix S1: Table S4). Models within 2 AlCc added one non-significant predictor or interaction to the model with the lowest AlCc; thus, we interpret the top model only. The top model included significant pairwise interactions between current growing season water availability, previous growing season water availability, and urban location. We therefore tested for a significant three-way interaction between these predictors, comparing AIC values for the top model with and without a three-way interaction term.
In addition to modeling diversity, we evaluated the relationships between annual plant community composition and nutrient treatment, urban location, microhabitat, and water availability using functions in the R package vegan (version 2.5.3, Oksanen et al. 2018). We calculated the genus-level community dissimilarity matrix using presence/absence of each genus with the binary Bray-Curtis dissimilarity index using the function vegdist. We then used the function metaMDS to create a nonmetric multidimensional scaling (NMDS) plot with three dimensions to visualize differences in community composition by our variables of interest. We used a scree plot to identify that three dimensions were needed to obtain a stress score below 0.2. To determine statistical significance of changes in community composition, we fitted environmental vectors for current and previous season water availability to the NMDS using function envfit, with fit significance determined by permutation tests with 999 permutations stratified by site. We tested for effects of urban location, nutrient treatment, and microhabitat on community composition using PERMANOVA, followed by a similarity percentage (SIMPER) analysis to identify discriminating genera between group levels (e.g., urban/non-urban, N/no N). These analyses were run with the adonis and simper functions, respectively. For PERMANOVA, permutations were restricted by site to account for the nested design, and 999 permutations were run. Significant SIMPER differences in genera by urban location, nutrient treatment, and microhabitat were determined using permutation tests with 100 permutations. We also checked for differences in within-group variance using function betadisper to test PERMANOVA assumptions of homogeneity of multivariate dispersions.
Results
Both structural equation modeling and mixed modeling approaches showed negative impacts of N addition and urban location on annual plant taxonomic diversity (Fig. 5, Table 2). Current and previous season water availability were significant predictors of taxonomic diversity with complex interaction effects. The effect of microhabitat on taxonomic diversity was significant and positive (i.e., greater diversity under shrubs; Fig. 5, Table 2). While nutrients, urban location, and water directly impacted productivity, there was no evidence of indirect effects of nutrients, water, microhabitat, or urban location on taxonomic diversity via productivity (Appendix S1: Table S5). Only microhabitat significantly affected phylogenetic diversity. P did not affect either taxonomic or phylogenetic diversity. Overall community composition shifted significantly in response to N, P, water, microhabitat, and urban location. However, water availability had the largest effect and other variables explained relatively little variance (PERMANOVA partial R2 < 0.05 for N, P, microhabitat and urban location). In the following sections, we investigate these relationships with respect to our hypotheses.
Figure 5.
Structural equation model showing impacts of nutrient enrichment, urban location, microhabitat, and water availability on taxonomic and phylogenetic diversity of annual plants. Green paths indicate positive relationships; red lines indicate negative relationships. Only statistically significant (p < 0.05) paths are shown, and values in boxes on paths give standardized model regression coefficients (see Appendix S1: Table S5 for non-standardized coefficients). The theorized path between productivity and taxonomic diversity was not statistically significant and so does not appear in the diagram. Double-headed arrows on individual boxes show residual variation in response variables. Double-headed arrows between boxes show correlation between predictors. N = nitrogen addition, P = phosphorus addition, Urban = urban location, Microhabitat (Shrub) = sample located under a shrub, Current Water = current growing season water availability, Previous Water = previous growing season water availability, ANPP = aboveground net primary productivity, SES MPD = standardized effect size of mean pairwise distance.
Table 2.
Generalized linear mixed model of annual plant species richness, with site and plot as random factors. Only predictors included in the final model are shown.
| Predictor | Estimate | Standard Error | z value | p value |
|---|---|---|---|---|
|
| ||||
| Current season water availability | −1.1 | 0.3 | −3.62 | 0.0003 |
| Microhabitat | 0.06 | 0.02 | 2.21 | 0.03 |
| N | −0.26 | 0.03 | −10.30 | <0.0001 |
| Previous season water availability | −5.5 | 0.5 | −10.77 | <0.0001 |
| Urban | −0.9 | 0.3 | −2.63 | 0.009 |
| Current season water availability × Previous season water availability | 10 | 1 | 7.96 | <0.0001 |
| Current season water availability × Urban | −1 | 1 | −0.62 | 0.53 |
| Previous season water availability × Urban | 0 | 2 | −0.04 | 0.97 |
| Current season water availability × Previous season water availability × Urban | 16 | 8 | 2.17 | 0.03 |
Effects of nutrients and water
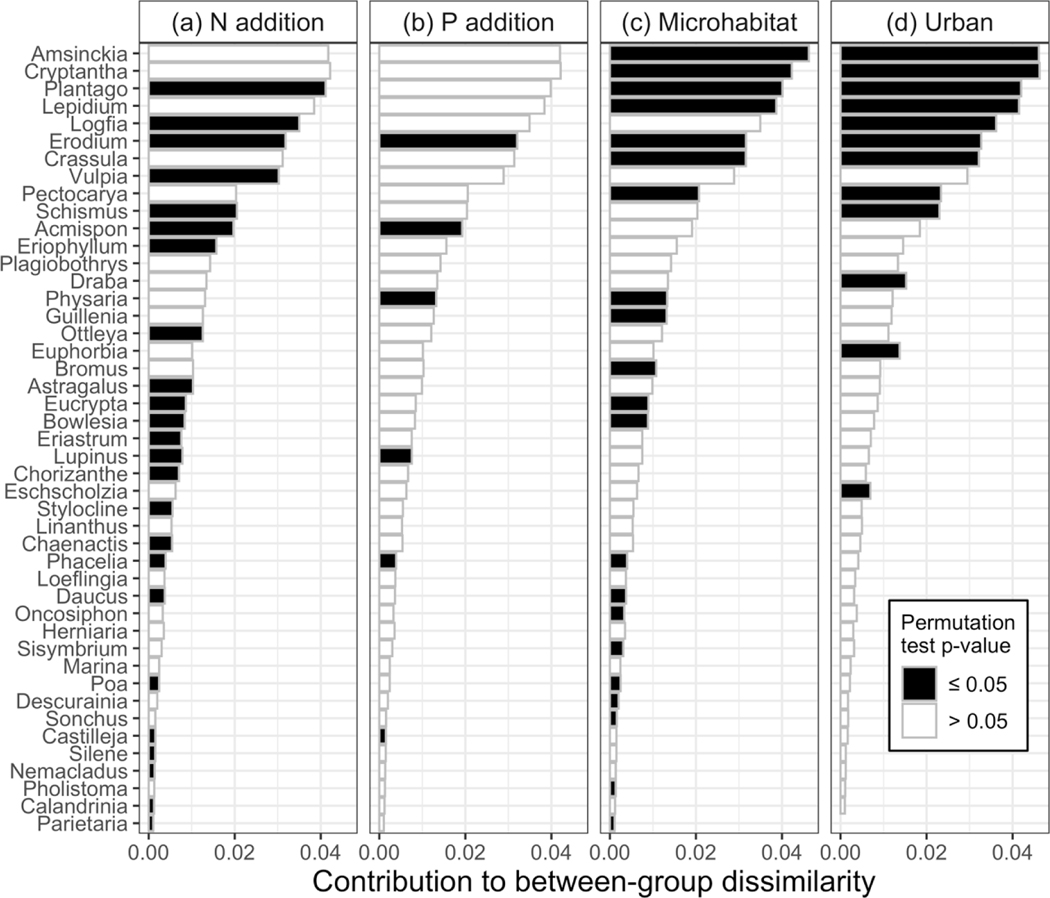
N enrichment significantly reduced annual plant taxonomic diversity but not phylogenetic diversity, while P enrichment was not significantly related to either taxonomic or phylogenetic diversity (Fig. 5). No significant interaction was found between N addition and other environmental variables. The lack of significant interactions suggests that N addition suppressed annual plant diversity in both wet and dry conditions (Fig. 6), regardless of urban location or microhabitat. Community composition was significantly different with both N enrichment (PERMANOVA, partial R2 = 0.018, p = 0.001; Appendix S1: Fig. S3) and P enrichment (PERMANOVA, partial R2 = 0.004, p = 0.001; Appendix S1: Fig. S4) when accounting for differences by site, although the differences were small. Nearly all genera were less common in N-enriched plots (Fig. 7b), with Plantago, Logfia, Erodium, and Vulpia having the largest significant contributions to community differentiation by N enrichment (Fig. 8a). All Fabaceae genera were less commonly found in N-fertilized plots (Fig. 7b). Additionally, N-enriched plots were more similar in composition to one another than were plots without N (betadisper, p = 0.02). Genus responses to P enrichment were more varied but minimal for most taxa (Fig. 7c).
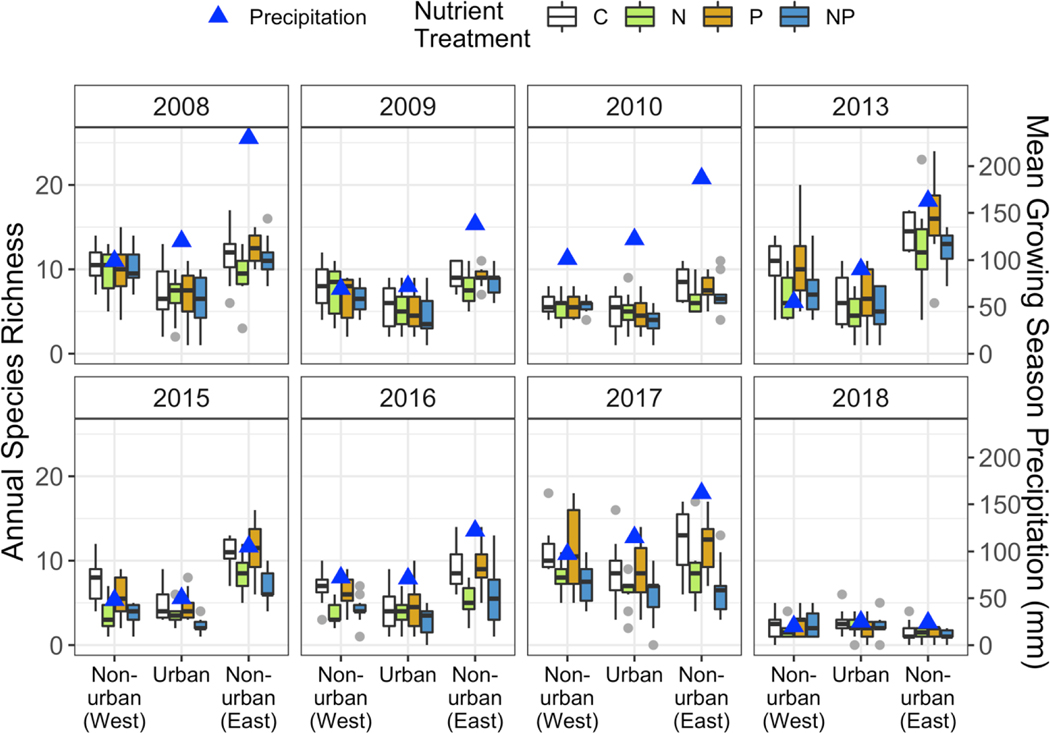
Figure 6.
Annual plant species richness by nutrient enrichment treatment, region, and year. Boxes show species richness with grey points indicating outliers, and blue triangles show the average growing season precipitation for a given region and year. C = control/no nutrient addition, N = nitrogen addition, P = phosphorus addition, NP = nitrogen and phosphorus addition.
Figure 7.
Difference in number of plots containing each genus by (b, c) N or P enrichment, (d) urban or non-urban location, and (e) microhabitat (under or between shrubs), out of 952 total plots. Note that there were twice as many non-urban as urban samples, so the number of non-urban plots containing each genus was divided by two. Panel (f) shows the total number of plots containing each genus. Plot background shading shows family groupings. Dots on genus names indicate that the genus contained all non-native species (red dot) or some non-native species (black dot).
Figure 8.
Contribution of genera to community dissimilarity between plots (a, b) with and without N or P enrichment, (c) under or between shrubs, and (d) in urban or non-urban locations (from SIMPER analysis). Black bars show species that contributed significantly to community dissimilarity at the p ≤ 0.05 level while hollow bars were not statistically significant.
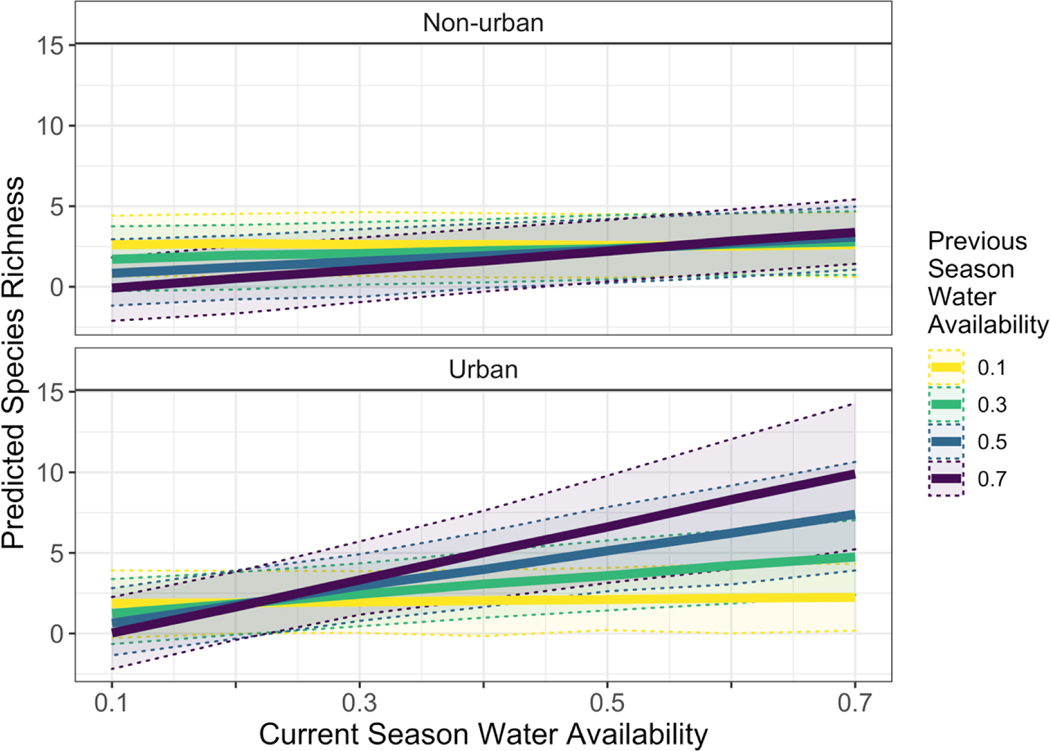
While SEM showed a positive effect of current season water availability and a negative effect of previous season water availability on annual plant taxonomic diversity, mixed modeling of species richness included a significant three-way interaction among urban location, current growing season water availability, and previous growing season water availability, suggesting more complex relationships (Table 2). Annual plant species richness increased with current season water availability more following a wetter year than after a dry year, with little effect of current season water availability on species richness following a dry year (Fig. 9). Species richness responded more positively to both current and previous season water availability in urban than in non-urban sites. A broader range of water availability conditions was measured in non-urban sites (current season 0.04 – 0.79; previous season 0.05 – 0.59) than in urban sites (current season 0.05 – 0.42; previous season 0.08 – 0.30), and thus, predictions for urban environments outside the measured range should be interpreted with caution. Additionally, due to dry conditions in March, water availability (precipitation/PET) for a given year was often lower when considered as the previous year’s water availability (Oct-Mar) rather than the current season water availability (Oct-Feb, except 2013).
Figure 9.
Model-predicted annual plant species richness showing interactions between current season water availability, previous season water availability, and urban location. Predicted values are shown only to illustrate the modeled interaction terms and are not forecasts. Predictions were generated using the top generalized linear mixed model with a hypothetical dataset containing pairwise combinations of previous and current season water availability across the observed range in urban and non-urban locations. All other variables were held constant, with no N and P addition, microhabitat under shrubs, and random intercept for the control plot at site UMP.
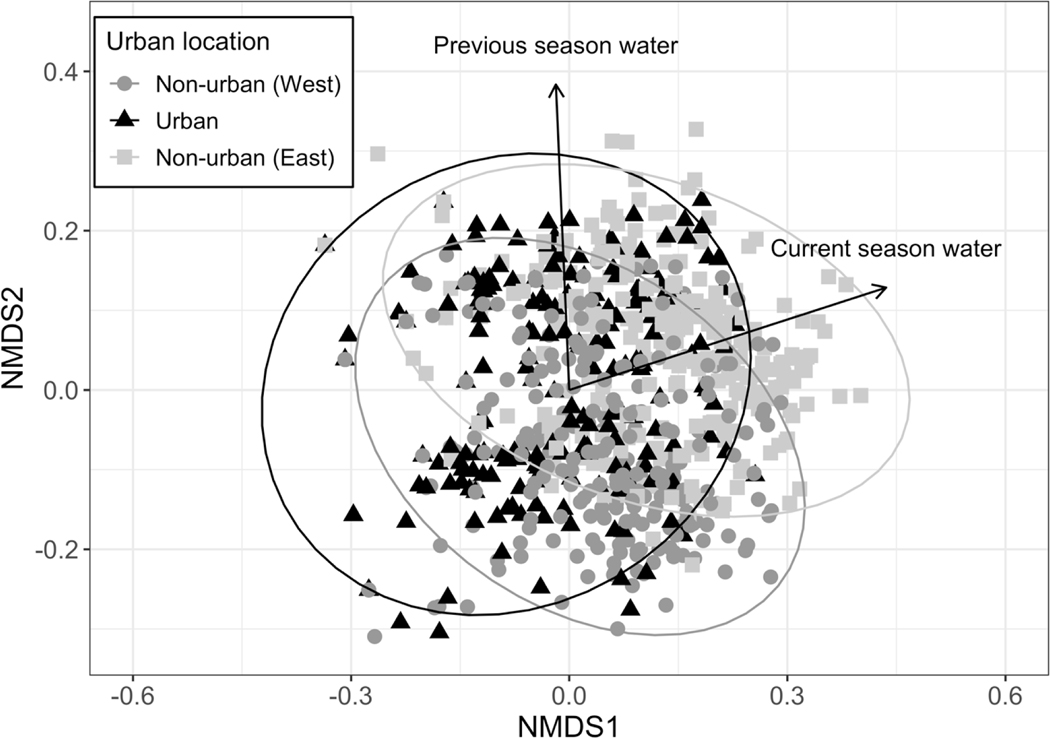
Based on NMDS analysis, both current and previous growing season water availability significantly distinguished community composition across samples (Fig. 10; current water R2 = 0.207, p = 0.001; previous water R2 = 0.147, p = 0.001). However, phylogenetic diversity did not vary significantly with water availability (Fig. 5).
Figure 10.
NMDS of all plots based on presence/absence of genera. NMDS stress = 0.14. Environmental vectors are significant as determined by permutation tests, with current water R2 = 0.207, p = 0.001 and previous water R2 = 0.147, p = 0.001. Ellipses show 95% confidence intervals for each region.
Effects of urban location
Urban sites had significantly lower taxonomic diversity than did non-urban sites, and species richness was more responsive to changes in water availability in urban than in non-urban sites (Fig. 5, Fig. 9, Table 2). Community composition differed between urban and non-urban locations when accounting for site (PERMANOVA, partial R2 = 0.047, p = 0.001; Fig. 10). There was more variation in community composition in non-urban than in urban sites (betadisper, p = 0.03). However, when comparing the three regions, there was no significant difference between urban and either non-urban West or non-urban East sites (betadisper; urbanWest p = 0.30, urban-East p = 0.26). Differentiation between urban and non-urban sites was most strongly driven by eight taxa that were less common in urban sites (Amsinkia, Plantago, Lepidium, Logfia, Erodium, Crassula, Pectocarya, and Schismus) and three that were more common in urban sites (Cryptantha, Draba, and Euphorbia; Fig. 7, Fig. 8). At the species level, Cryptantha exhibited varied responses to urban location, with C. maritima more commonly found in urban sites, C. angustifolia more commonly found in non-urban sites, and the most frequently observed species, C. decipiens, nearly evenly distributed between urban and non-urban sites. Few congenerics were observed for genera other than Cryptantha, and no other highly differentiating genus contained species with different responses to urban location. As observed with N addition, most genera were less common in urban sites. The non-native or partially non-native genera Plantago, Schismus, and Erodium all significantly differentiated urban and non-urban sites, but were all more common in non-urban locations (Fig. 7, Fig. 8).
Effects of microhabitat
Taxonomic diversity was higher beneath shrubs while phylogenetic diversity was reduced (i.e., more taxonomically diverse but phylogenetically clustered communities under shrubs; Fig. 5, Table 2). Microhabitat was the only significant predictor of phylogenetic diversity. Community composition differed significantly by microhabitat (PERMANOVA, partial R2 = 0.024, p = 0.001) when accounting for site, though the effect was very small (Appendix S1: Fig. S5). SIMPER analysis identified Amsinckia as the top genus contributing to differences by microhabitat, followed by Cryptantha, Plantago, and Lepidium (Fig. 8). Amsinckia and Cryptantha, both members of the Boraginaceae family, were more commonly found under shrubs (Fig. 7). Most grasses (family Poaceae) and mustards (family Brassicaceae, including Lepidium) were also more common under shrubs, while members of Fabaceae, Asteraceae, and several other families that were less diverse in this community (including Plantago, family Plantaginaceae) were more often found between shrubs (Fig. 7).
Discussion
Maintenance of native biodiversity in the context of rapid urbanization and changing climate patterns is a major challenge for conservation. With our long-term experimental approach, we found that annual plant communities were less diverse in Sonoran Desert preserves located in the urban core and in experimentally N-enriched plots compared to unenriched and non-urban locations. Diversity was impacted by water availability in both the current and previous growing season, with a greater impact of current season water availability following a wet previous growing season. The decline in diversity with N addition was not moderated by water availability or microhabitat. Our use of multiple statistical techniques and community diversity metrics allows us to more fully elucidate these interactions and describe their effects. Our findings suggest that arid and semi-arid annual plant biodiversity is likely to decline with increased N deposition, despite water limitation and the marked spatial and temporal resource patchiness that characterizes desert environments.
Effects of water and nutrient addition on annual plant communities
Experimental N addition had uniformly negative effects on annual plant taxonomic diversity. Meanwhile, P addition had no effect on annual plant diversity. Our observed N addition effect matches previous findings in more mesic systems showing declines in plant diversity with increased N (Pardo et al. 2011, Harpole et al. 2016, Payne et al. 2017). However, contrary to our predictions, the effects of N on diversity were not moderated by water availability or microhabitat as expected if water served as the primary driver followed by N. Rather, we found support for co-regulation of winter annual plant composition and diversity by water and N.
Diversity loss resulting from greater biomass of dominant plant species and increased competition for light is a typical mechanism for declining diversity with fertilization (Hautier et al. 2009, Borer et al. 2014). Yet, we found no relationship between productivity and diversity that would support this interpretation in our study. Previous research at these sites also showed no relationship between annual plant ANPP and diversity following herbivore exclusion (Davis et al. 2015). Co-limitation rather than sequential limitation by water and N further suggests a different mechanism for diversity loss following N enrichment.
One possible explanation for reduced diversity with N enrichment is a competitive advantage for those species with nutrient-intensive growth strategies. Nitrogen allocation is fundamental to the physiological tradeoff between relative growth rate (RGR) and water use efficiency (WUE), which is a key tradeoff in desert annual plant communities (Angert et al. 2007, Gremer et al. 2013, Huxman et al. 2013). Long-term diversity is maintained in these communities through coexistence of species with diverse strategies along the RGR-WUE tradeoff (Huxman et al. 2008, Gremer et al. 2013). Thus, the consistent promotion of high growth rate species under higher nutrient conditions may limit the overall diversity of the community, as documented in more mesic systems (Isbell et al. 2013, Avolio et al. 2014). We hypothesize that N-enriched environments reduce the competitive advantage of stress-tolerant low RGR species while promoting resource-intensive, high RGR species, thus reducing overall diversity in this community. Future research measuring physiological traits of annuals under N fertilization and urban influences would yield additional insight into how coexistence patterns are affected by N availability.
Our findings illustrate a complex relationship between water availability and annual plant diversity that is likely mediated through the seed bank. The interacting effects of current water availability and water availability in the previous growing season on annual plant diversity may be due to increased seed production in wet years. Therefore, we see greater responsiveness of the community to water following wet than following dry years. In this community, germination, growth, and reproduction of some species are more responsive to variation in environmental conditions, such as timing of precipitation and temperature variability, while other species have more consistent germination and growth patterns across years (Venable 2007, Angert et al. 2010, Huxman et al. 2013). Annual plant seeds have variable, low germination rates and may lie dormant in the seed bank for long periods of time (Adondakis and Venable 2004), but most of the viable seeds in a particular year are likely to have been produced in the previous year (Moriuchi et al. 2000). Thus, although the seed bank is able to preserve diversity over the long term, the majority of annual plants in a given year are likely to result from seeds in the previous year and are thereby influenced by previous year growing conditions. Differences in responsiveness of species to yearly conditions could accumulate over time. For example, multiple wet years produce a rich seed bank of species ready to respond to further wet conditions, whereas dry years produce dominant species’ seeds that may not be as responsive to wet conditions. Although we show a relationship between the previous year’s environmental conditions and the current year’s annual plant responses, long-term shifts in climate, including rainfall patterns, could lead to longer-term changes in the seed bank and the resulting annual plant community (Huxman et al. 2013). The interaction among current season water availability, previous season water availability, and urban location may reflect this type of long-term difference in the responsiveness of urban and non-urban preserves to water availability. While we present here two possible mechanisms for changes in annual plant diversity in response to nutrient addition and water availability (changes in RGR-WUE tradeoff strategies and alterations in the seed bank over time), further research is needed to explore and confirm these mechanisms.
One limitation to this study is the consideration of both water availability and annual plant community responses at the yearly time scale. Previous work has shown that winter annual plant species respond differently to the timing of rainfall and temperature conditions within the winter growing season, and that species dominance may change over the course of the season (Kimball et al. 2012, Huxman et al. 2013). Repeated sampling during the growing season to determine community responses to individual rainfall events and temperature changes in urban and N-enriched conditions would complement and extend the present analysis by adding insight into intra-annual variability and temporal as well as spatial heterogeneity.
Diversity in urban deserts
Annual plant diversity was lower and community composition was somewhat altered in urban Sonoran Desert preserves within the city compared to non-urban preserves at the outer edges of the city. N deposition is relatively low in these sites compared to other cities (Cook et al. 2018) and is at the lower end of estimated critical loads for more mesic systems (Simkin et al. 2016), but falls within the expected range of critical loads for North American deserts (Fenn et al. 2010, Pardo et al. 2011). However, given the relatively small difference in N deposition between the urban and non-urban sites included in this study, other urban conditions such as increased ozone pollution, park use by people and pets, land use legacies, and population isolation may be more likely causes of reduced plant diversity in urban preserves. Previous research shows that changes in food web structure (e.g., increased herbivory due to loss of predators) in urban preserves would not result in the observed decrease in annual plant diversity (Davis et al. 2015). In our study, urban locations experienced reductions in annual plant diversity with further experimental N addition, indicating that continued losses of diversity are likely if N deposition increases. Although urban open space parks can be managed to preserve intact soils and woody plant structure, ephemeral desert plant communities with rapid life cycles may be difficult to maintain when the negative impacts of nearby air pollution cross preserve boundaries. Nonetheless, urban preserves provide valuable access to natural landscapes for city residents and can provide refugia for native wildlife (Cox et al. 2018, Threlfall and Kendal 2018). Maintaining diverse plant communities—even in these relatively disturbed spaces—is a desirable goal for city managers and residents.
While previous work has found increased non-native plant diversity and abundance in both urban and N-enriched habitats (Brooks 2003, Walker et al. 2009, Rao and Allen 2010, Dolan et al. 2011), we did not find evidence of more non-natives in urban preserves or N-enriched plots compared to non-urban preserves and unenriched plots. Rather, it appears that native species were lost in urban locations and with N addition, but were not replaced by non-natives, leading to some of the observed declines in overall diversity but relatively small changes in composition. Other work in the Sonoran Desert has shown increased non-native annual forb and grass frequency over a longer time period (1983 – 2005) at a single site near Tucson (Bowers et al. 2006); in our sites, an increase in non-native species is not likely due to elevated N deposition or other urban influences. All of the preserves in our study as well as the site studied by Bowers et al. (2006) have experienced some disturbance related to road construction, recreation, and/or scientific research and thus may all share some conditions allowing for non-native expansion, even for those preserves we designate as non-urban. Non-native annual plant spread in this community may be more related to low-level disturbance than to highly urban conditions.
Landscape patchiness and the effects of microhabitat
Annual plant diversity was higher under shrubs, which we would expect from the existence of fertile resource islands that accumulate under their canopies (Schlesinger and Pilmanis 1998, Facelli and Temby 2002). However, species under and between shrubs had similar responses to water availability, nutrient enrichment, and urban conditions, unlike the differential responses under and between shrubs observed in other studies (Pake and Venable 1995, Brooks 2003). The benefits of facilitation by shrubs for winter annual plants in this community may be relatively small compared to the range of responses induced by interannual climatic variability, and thus unlikely to buffer the effects of increased variability with climate change and elevated N deposition in urban areas. Additionally, shrub resource islands may have within-year temporal effects on annual plant community composition by altering germination and senescence timing (Kimball et al. 2011), which our sampling approach would not have captured. Further sampling to evaluate how annual plant communities in different microhabitats change over the course of the growing season could yield additional insights into how urban and N-enrichment effects alter annual plant community composition.
In addition to differences in taxonomic diversity, we found greater phylogenetic clustering in communities under shrubs. Closely-related groups of taxa may share functional traits allowing them to benefit from conditions beneath shrub canopies (Aguilera et al. 1999, Facelli and Temby 2002), although some important traits may vary even within closely-related groups (Huxman et al. 2008). The balance between facilitation and competition is dependent on compatibility between plant functional traits, and so this more closely related group of taxa beneath shrub canopies likely represents those with functional traits that best align with conditions provided by shrubs (Butterfield and Callaway 2013).
Implications for desert conservation
How might we expect desert annual plant communities to change in the future? With the combined impacts of climate change and urban growth, annual plant communities are likely to decrease in overall diversity and experience shifts in community composition (Kimball et al. 2010, Huxman et al. 2013). Increased N deposition is a concern for maintaining long-term diversity in this ephemeral community, especially in urban preserves where admiration and enjoyment of these attractive and short-lived plants may help build an appreciation of the desert for residents of arid cities. Depletion of annual plant diversity may result in muted responses of the community to certain environmental conditions, potentially leading to even greater interannual variability in the emergent community as plant strategies adapted to some conditions become less common (Huxman et al. 2013, Bharath et al. 2020). Changes in diversity of annual plants can have important impacts on showy wildflower displays appreciated (and monetized) by people, as well as floral and herbaceous resources for desert pollinators and other wildlife (Ryan 2011, Jennings and Berry 2015). As urban populations increasingly experience a loss of connection with nature (Soga and Gaston 2016), diversity of showy species like wildflowers in accessible urban preserves may be particularly influential for building positive attitudes toward the environment. Multi-year experiments such as this one show responses to a range of environmental conditions and help predict how communities will change in the future. Our finding that annual diversity and community composition are strongly influenced by consecutive years of water availability suggests that considering only the conditions of a single growing season will not be sufficient to understand and predict community outcomes. Increased variability and multi-year drought may have compounding effects on annual plant diversity, with attendant outcomes for people and wildlife.
Supplementary Material
Acknowledgements
We thank Sally Wittlinger, Stevan Earl, and Quincy Stewart for their contributions in the collection and management of this long-term dataset. This material is based upon work supported by the National Science Foundation under grant number DEB-1832016, Central Arizona-Phoenix Long-Term Ecological Research Program (CAP LTER). The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Data Availability
Data are available from the Central Arizona-Phoenix Long-Term Ecological Research Program (CAP LTER) under Grimm et al. (2019): https://sustainability.asu.edu/caplter/data/data-catalog/view/knb-lter-cap.632/
Literature Cited
- Ackerman D, Millet DB, and Chen X. 2019. Global estimates of inorganic nitrogen deposition across four decades. Global Biogeochemical Cycles 33:100–107. [Google Scholar]
- Adondakis S, and Venable DL 2004. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 85:2582–2590. [Google Scholar]
- Aguilera LE, Gutiérrez JR, and Meserve PL 1999. Variation in soil micro-organisms and nutrients underneath and outside the canopy of Adesmia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average rainfall. Journal of Arid Environments 42:61–70. [Google Scholar]
- Angert AL, Horst JL, Huxman TE, and Venable DL 2010. Phenotypic plasticity and precipitation response in Sonoran Desert winter annuals. American Journal of Botany 97:405–411. [DOI] [PubMed] [Google Scholar]
- Angert AL, Huxman TE, Barron-Gafford GA, Gerst KL, and Venable DL 2007. Linking growth strategies to long-term population dynamics in a guild of desert annuals. Journal of Ecology 95:321–331. [Google Scholar]
- Avolio ML, Koerner SE, La Pierre KJ, Wilcox KR, Wilson GWTT, Smith MD, and Collins SL 2014. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. Journal of Ecology 102:1649–1660. [Google Scholar]
- Bartoń K. 2018. MuMIn: multi-model inference. [Google Scholar]
- Bates D, Mächler M, Bolker BM, and Walker SC 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Beguería S, and Vicente-Serrano SM 2017. SPEI: calculation of the standardised precipitation-evapotranspiration index. [Google Scholar]
- Bettez ND, and Groffman PM 2013. Nitrogen deposition in and near an urban ecosystem. Environmental Science & Technology 47:6047–6051. [DOI] [PubMed] [Google Scholar]
- Bharath S, Borer ET, Biederman LA, Blumenthal DM, Fay PA, Gherardi LA, Knops JMH, Leakey ADB, Yahdjian L, and Seabloom EW 2020. Nutrient addition increases grassland sensitivity to droughts. Ecology 101:e02981. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J-W, Fenn M, Gilliam F, Nordin A, Pardo L, and De Vries W. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications 20:30–59. [DOI] [PubMed] [Google Scholar]
- Bohrer VL 1991. Recently recognized cultivated and encouraged plants among the Hohokam. Kiva 56:227–235. [Google Scholar]
- Borer ET, Seabloom EW, Gruner DS, Harpole WS, Hillebrand H, Lind EM, Adler PB, Alberti J, Anderson TM, Bakker JD, Biederman L, Blumenthal D, Brown CS, Brudvig LA, Buckley YM, Cadotte M, Chu C, Cleland EE, Crawley MJ, Daleo P, Damschen EI, Davies KF, DeCrappeo NM, Du G, Firn J, Hautier Y, Heckman RW, Hector A, HilleRisLambers J, Iribarne O, Klein JA, Knops JMH, La Pierre KJ, Leakey ADB, Li W, MacDougall AS, McCulley RL, Melbourne BA, Mitchell CE, Moore JL, Mortensen B, O’Halloran LR, Orrock JL, Pascual J, Prober SM, Pyke DA, Risch AC, Schuetz M, Smith MD, Stevens CJ, Sullivan LL, Williams RJ, Wragg PD, Wright JP, and Yang LH 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–520. [DOI] [PubMed] [Google Scholar]
- Bowers JE 2005. El Niño and displays of spring-flowering annuals in the Mojave and Sonoran deserts. The Journal of the Torrey Botanical Society 132:38–49. [Google Scholar]
- Bowers JE, Bean TM, and Turner RM 2006. Two decades of change in distribution of exotic plants at the Desert Laboratory, Tucson, Arizona. Madroño 53:252–263. [Google Scholar]
- Brooks ML 2003. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. Journal of Applied Ecology 40:344–353. [Google Scholar]
- Butterfield BJ, and Callaway RM 2013. A functional comparative approach to facilitation and its context dependence. Functional Ecology 27:907–917. [Google Scholar]
- Cavender-Bares J, Kozak KH, Fine PVA, and Kembel SW 2009. The merging of community ecology and phylogenetic biology. Ecology Letters 12:693–715. [DOI] [PubMed] [Google Scholar]
- Cook EM, Sponseller R, Grimm NB, and Hall SJ 2018. Mixed method approach to assess atmospheric nitrogen deposition in arid and semi-arid ecosystems. Environmental Pollution 239:617–630. [DOI] [PubMed] [Google Scholar]
- Cox DTC, Shanahan DF, Hudson HL, Fuller RA, and Gaston KJ 2018. The impact of urbanisation on nature dose and the implications for human health. Landscape and Urban Planning 179:72–80. [Google Scholar]
- Davis MK, Cook EM, Collins SL, and Hall SJ 2015. Top-down vs. bottom-up regulation of herbaceous primary production and composition in an arid, urbanizing ecosystem. Journal of Arid Environments 116:103–114. [Google Scholar]
- Dolan RW, Moore ME, and Stephens JD 2011. Documenting effects of urbanization on flora using herbarium records. Journal of Ecology 99:1055–1062. [Google Scholar]
- Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, and Smith JE 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10:1135–1142. [DOI] [PubMed] [Google Scholar]
- Epskamp S. 2019. semPlot: path diagrams and visual analysis of various SEM packages’ output. [Google Scholar]
- Facelli JM, and Temby AM 2002. Multiple effects of shrubs on annual plant communities in arid lands of South Australia. Austral Ecology 27:422–432. [Google Scholar]
- Fay PA, Prober SM, Harpole WS, Knops JMH, Bakker JD, Borer ET, Lind EM, MacDougall AS, Seabloom EW, Wragg PD, Adler PB, Blumenthal DM, Buckley YM, Chu C, Cleland EE, Collins SL, Davies KF, Du G, Feng X, Firn J, Gruner DS, Hagenah N, Hautier Y, Heckman RW, Jin VL, Kirkman KP, Klein J, Ladwig LM, Li Q, McCulley RL, Melbourne BA, Mitchell CE, Moore JL, Morgan JW, Risch AC, Schütz M, Stevens CJ, Wedin DA, and Yang LH 2015. Grassland productivity limited by multiple nutrients. Nature Plants 1:15080. [DOI] [PubMed] [Google Scholar]
- Fenn ME, Allen EB, Weiss SB, Jovan S, Geiser LH, Tonnesen GS, Johnson RF, Rao LE, Gimeno BS, Yuan F, Meixner T, and Bytnerowicz A. 2010. Nitrogen critical loads and management alternatives for N-impacted ecosystems in California. Journal of Environmental Management 91:2404–2423. [DOI] [PubMed] [Google Scholar]
- Fenn ME, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L, Bowman WD, Sickman JO, Meixner T, Johnson DW, and Neitlich P. 2003a. Ecological effects of nitrogen deposition in the western United States. BioScience 53:404–420. [Google Scholar]
- Fenn ME, Haeuber R, Tonnesen GS, Baron JS, Grossman-Clarke S, Hope D, Jaffe DA, Copeland S, Geiser L, Rueth HM, and Sickman JO 2003b. Nitrogen emissions, deposition, and monitoring in the western United States. BioScience 53:391–403. [Google Scholar]
- Field CD, Dise NB, Payne RJ, Britton AJ, Emmett BA, Helliwell RC, Hughes S, Jones L, Lees S, Leake JR, Leith ID, Phoenix GK, Power SA, Sheppard LJ, Southon GE, Stevens CJ, and Caporn SJM 2014. The role of nitrogen deposition in widespread plant community change across semi-natural habitats. Ecosystems 17:864–877. [Google Scholar]
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, and Sutton MA 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. [DOI] [PubMed] [Google Scholar]
- Gamo M, Shinoda M, and Maeda T. 2013. Classification of arid lands, including soil degradation and irrigated areas, based on vegetation and aridity indices. International Journal of Remote Sensing 34:6701–6722. [Google Scholar]
- Ge X-YM, Scholl JP, Basinger U, Huxman TE, and Venable DL 2019. Functional trait trade-off and species abundance: insights from a multi-decadal study. Ecology Letters 22:583–592. [DOI] [PubMed] [Google Scholar]
- Gremer JR, Kimball S, Angert AL, Venable DL, and Huxman TE 2012. Variation in photosynthetic response to temperature in a guild of winter annual plants. Ecology 93:2693–2704. [DOI] [PubMed] [Google Scholar]
- Gremer JR, Kimball S, Keck KR, Huxman TE, Angert AL, and Venable DL 2013. Water-use efficiency and relative growth rate mediate competitive interactions in Sonoran Desert winter annual plants. American Journal of Botany 100:2009–2015. [DOI] [PubMed] [Google Scholar]
- Gremer JR, Kimball S, and Venable DL 2016. Within- and among-year germination in Sonoran Desert winter annuals: bet hedging and predictive germination in a variable environment. Ecology Letters 19:1209–1218. [DOI] [PubMed] [Google Scholar]
- Gremer JR, and Venable DL 2014. Bet hedging in desert winter annual plants: optimal germination strategies in a variable environment. Ecology Letters 17:380–387. [DOI] [PubMed] [Google Scholar]
- Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, and Briggs JM 2008. Global change and the ecology of cities. Science 319:756–760. [DOI] [PubMed] [Google Scholar]
- Grimm NB, Hall SJ, Kaye JP, Allen J, and Childers DL 2017. CAP weather stations at Papago Park and Lost Dutchman State Park in the greater Phoenix metropolitan area, ongoing since 2010. https://sustainability.asu.edu/caplter/data/data-catalog/view/knb-lter-cap.636/. [Google Scholar]
- Grimm NB, Hall SJ, Kaye JP, Allen J, and Childers DL 2019. Desert fertilization experiment: investigation of Sonoran Desert ecosystem response to atmospheric deposition and experimental nutrient addition, ongoing since 2006. https://sustainability.asu.edu/caplter/data/data-catalog/view/knb-lter-cap.632/. [Google Scholar]
- Hadley NF, and Szarek SR 1981. Productivity of desert ecosystems. BioScience 31:747–753. [Google Scholar]
- Hall SJ, Sponseller RA, Grimm NB, Huber D, Kaye JP, Clark C, and Collins SL 2011. Ecosystem response to nutrient enrichment across an urban airshed in the Sonoran Desert. Ecological Applications 21:640–660. [DOI] [PubMed] [Google Scholar]
- Hargreaves GH, and Allen RG 2003. History and evaluation of Hargreaves evapotranspiration equation. Journal of Irrigation and Drainage Engineering 129:53–63. [Google Scholar]
- Harpole WS, Sullivan LL, Lind EM, Firn J, Adler PB, Borer ET, Chase J, Fay PA, Hautier Y, Hillebrand H, MacDougall AS, Seabloom EW, Williams R, Bakker JD, Cadotte MW, Chaneton EJ, Chu C, Cleland EE, D’Antonio C, Davies KF, Gruner DS, Hagenah N, Kirkman K, Knops JMH, La Pierre KJ, McCulley RL, Moore JL, Morgan JW, Prober SM, Risch AC, Schuetz M, Stevens CJ, and Wragg PD 2016. Addition of multiple limiting resources reduces grassland diversity. Nature 537:93–96. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Niklaus PA, and Hector A. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324:636–638. [DOI] [PubMed] [Google Scholar]
- Holzapfel C, and Mahall BE 1999. Bidirectional facilitation and interference between shrubs and annuals in the Mojave Desert. Ecology 80:1747–1761. [Google Scholar]
- Hooper DU, and Johnson L. 1999. Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293. [Google Scholar]
- Huxman TE, Barron-Gafford G, Gerst KL, Angert AL, Tyler AP, and Venable DL 2008. Photosynthetic resource-use efficiency and demographic variability in desert winter annual plants. Ecology 89:1554–1563. [DOI] [PubMed] [Google Scholar]
- Huxman TE, Kimball S, Angert AL, Gremer JR, Barron-Gafford GA, and Venable DL 2013. Understanding past, contemporary, and future dynamics of plants, populations, and communities using Sonoran Desert winter annuals. American Journal of Botany 100:1369–1380. [DOI] [PubMed] [Google Scholar]
- Isbell F, Reich PB, Tilman D, Hobbie SE, Polasky S, and Binder S. 2013. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proceedings of the National Academy of Sciences 110:11911–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings WB, and Berry KH 2015. Desert tortoises (Gopherus agassizii) are selective herbivores that track the flowering phenology of their preferred food plants. PLoS ONE 10:e0116716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, and Webb CO 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kaniskan B, and McCoach DB 2015. The performance of RMSEA in models with small degrees of freedom. Sociological Methods & Research 44:486–507. [Google Scholar]
- Kimball S, Angert AL, Huxman TE, and Venable DL 2010. Contemporary climate change in the Sonoran Desert favors cold-adapted species. Global Change Biology 16:1555–1565. [Google Scholar]
- Kimball S, Angert AL, Huxman TE, and Venable DL 2011. Differences in the timing of germination and reproduction relate to growth physiology and population dynamics of Sonoran Desert winter annuals. American Journal of Botany 98:1773–1781. [DOI] [PubMed] [Google Scholar]
- Kimball S, Gremer JR, Angert AL, Huxman TE, and Venable DL 2012. Fitness and physiology in a variable environment. Oecologia 169:319–329. [DOI] [PubMed] [Google Scholar]
- Korner-Nievergelt F, Roth T, von Felten S, Guelat J, Almasi B, and Korner-Nievergelt P. 2015. blmeco: data files and functions accompanying the book “Bayesian data analysis in ecology using linear models with R, BUGS and Stan.” [Google Scholar]
- Ladwig LM, Collins SL, Swann AL, Xia Y, Allen MF, and Allen EB 2012. Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia 169:177–185. [DOI] [PubMed] [Google Scholar]
- LeBauer DS, and Treseder KK 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Han W, Tang A, Shen J, Cui Z, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, and Zhang F. 2013. Enhanced nitrogen deposition over China. Nature 494:459–462. [DOI] [PubMed] [Google Scholar]
- Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, and Hernández-Hernández T. 2015. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist 207:437–453. [DOI] [PubMed] [Google Scholar]
- McDonald RI, Forman RTT, Kareiva P, Neugarten R, Salzer D, and Fisher J. 2009. Urban effects, distance, and protected areas in an urbanizing world. Landscape and Urban Planning 93:63–75. [Google Scholar]
- Mclntire EJB, and Fajardo A. 2014. Facilitation as a ubiquitous driver of biodiversity. New Phytologist 201:403–416. [DOI] [PubMed] [Google Scholar]
- Moriuchi KS, Venable DL, Pake CE, and Lange T. 2000. Direct measurement of the seed bank age structure of a Sonoran Desert annual plant. Ecology 81:1133–1138. [Google Scholar]
- Mulroy TW, and Rundel PW 1977. Annual plants: adaptations to desert environments. BioScience 27:109–114. [Google Scholar]
- Noy-Meir I. 1973. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics 4:25–51. [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, and Wagner H. 2018. vegan: community ecology package. [Google Scholar]
- Pake CE, and Venable DL 1995. Is coexistence of Sonoran Desert annuals mediated by temporal variability in reproductive success? Ecology 76:246–261. [Google Scholar]
- Pardo LH, Fenn ME, Goodale CL, Geiser LH, Driscoll CT, Allen EB, Baron JS, Bobbink R, Bowman WD, Clark CM, Emmett B, Gilliam FS, Greaver TL, Hall SJ, Lilleskov EA, Liu L, Lynch JA, Nadelhoffer KJ, Perakis SS, Robin-Abbott MJ, Stoddard JL, Weathers KC, and Dennis RL 2011. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecological Applications 21:3049–3082. [Google Scholar]
- Payne RJ, Dise NB, Field CD, Dore AJ, Caporn SJM, and Stevens CJ 2017.Nitrogen deposition and plant biodiversity: past, present, and future. Frontiers in Ecology and the Environment 15:431–436. [Google Scholar]
- Pearse WD, Cadotte MW, Cavender-Bares J, Ives AR, Tucker C, Walker SC, and Helmus MR 2015. pez: phylogenetics for the environmental sciences. Bioinformatics 31:2888–2890. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rao LE, and Allen EB 2010. Combined effects of precipitation and nitrogen deposition on native and invasive winter annual production in California deserts. Oecologia 162:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB 2014. The world-wide “fast-slow” plant economics spectrum: a traits manifesto. Journal of Ecology 102:275–301. [Google Scholar]
- Rosseel Y. 2012. lavaan: An R package for structural equation modeling. Journal of Statistical Software 48:1–36. [Google Scholar]
- Ryan J. 2011. Anthoethnography: emerging research into the culture of flora, aesthetic experience of plants, and the wildflower tourism of the future. New Scholar 1:28–40. [Google Scholar]
- Sala OE, Chapin FSI, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Skykes MT, Walker BH, Walker M, and Wall DH 2000. Global biodiversity scenarios for the year 2100. Science 287:1770–1774. [DOI] [PubMed] [Google Scholar]
- Samani ZA, and Pessarakli M. 1986. Estimating potential crop evapotranspiration with minimum data in Arizona. Transactions of the ASAE 29:522–524. [Google Scholar]
- Schade JD, and Hobbie SE 2005. Spatial and temporal variation in islands of fertility in the Sonoran Desert. Biogeochemistry 73:541–553. [Google Scholar]
- Schlesinger WH, and Pilmanis AM 1998. Plant-soil interactions in deserts. Biogeochemistry 42:169–187. [Google Scholar]
- Schlesinger WH, Raikes JA, Hartley AE, and Cross AF 1996. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–374. [Google Scholar]
- Seto KC, Fragkias M, Güneralp B, and Reilly MK 2011. A meta-analysis of global urban land expansion. PLoS ONE 6:e23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin SM, Allen EB, Bowman WD, Clark CM, Belnap J, Brooks ML, Cade BS, Collins SL, Geiser LH, Gilliam FS, Jovan SE, Pardo LH, Schulz BK, Stevens CJ, Suding KN, Throop HL, and Waller DM 2016. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proceedings of the National Academy of Sciences of the United States of America 113:4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, and Brown JW 2018. Constructing a broadly inclusive seed plant phylogeny. American Journal of Botany 105:302–314. [DOI] [PubMed] [Google Scholar]
- Snyman HA 2002. Short-term response of rangeland botanical composition and productivity to fertilization (N and P) in a semi-arid climate of South Africa. Journal of Arid Environments 50:167–183. [Google Scholar]
- Soga M, and Gaston KJ 2016. Extinction of experience: the loss of human-nature interactions. Frontiers in Ecology and the Environment 14:94–101. [Google Scholar]
- Sponseller RA, Hall SJ, Huber DP, Grimm NB, Kaye JP, Clark CM, and Collins SL 2012. Variation in monsoon precipitation drives spatial and temporal patterns of Larrea tridentata growth in the Sonoran Desert. Functional Ecology 26:750–758. [Google Scholar]
- Taasoobshirazi G, and Wang S. 2016. The performance of the SRMR, RMSEA, CFI, AND TLI: an examination of sample size, path size, and degrees of freedom. Journal of Applied Quantitative Methods 11:31–41. [Google Scholar]
- The Plant List. 2013. The Plant List. Version 1.1. http://www.theplantlist.org/.
- Threlfall CG, and Kendal D. 2018. The distinct ecological and social roles that wild spaces play in urban ecosystems. Urban Forestry & Urban Greening 29:348–356. [Google Scholar]
- Tucker CM, Cadotte MW, Carvalho SB, Davies TJ, Ferrier S, Fritz SA, Grenyer R, Helmus MR, Jin LS, Mooers AO, Pavoine S, Purschke O, Redding DW, Rosauer DF, Winter M, and Mazel F. 2017. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biological Reviews 92:698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP. 1992. World Atlas of Desertification. Edward Arnold, New York, New York, USA. [Google Scholar]
- United Nations. 2014. World Urbanization Prospects: The 2014 Revision.
- Venable DL 2007. Bet hedging in a guild of desert annuals. Ecology 88:1086–1090. [DOI] [PubMed] [Google Scholar]
- Venable DL, and Pake CE 1999. Population ecology of Sonoran Desert annual plants. Pages 115–142 in Robichaux RH, editor. Ecology of Sonoran Desert Plants and Plant Communities. University of Arizona Press. [Google Scholar]
- Venable DL, Pake CE, and Caprio AC 1993. Diversity and coexistence of Sonoran Desert winter annuals. Plant Species Biology 8:207–216. [Google Scholar]
- Walker JS, Grimm NB, Briggs JM, Gries C, and Dugan L. 2009. Effects of urbanization on plant species diversity in central Arizona. Frontiers in Ecology and the Environment 7:465–470. [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, and Donoghue MJ 2002. Phylogenies and community ecology. Annual Review of Ecology and Systematics 33:475–505. [Google Scholar]
- Yahdjian L, Gherardi L, and Sala OE 2011. Nitrogen limitation in arid-subhumid ecosystems: a meta-analysis of fertilization studies. Journal of Arid Environments 75:675–680. [Google Scholar]
- Yu G, Smith D, Zhu H, Guan Y, and Lam TT-Y 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution 8:28–36. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB, Royer DL, Soltis DE, Stevens PF, Westoby M, Wright IJ, Aarssen L, Bertin RI, Calaminus A, Govaerts R, Hemmings F, Leishman MR, Oleksyn J, Soltis PS, Swenson NG, Warman L, and Beaulieu JM 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506:89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.