Abstract
Background/Aim: Adenocarcinoma of the pancreas is one of the most aggressive malignant diseases in humans. Characteristics of this tumour type are poor response to radiotherapy and chemotherapeutic agents as well as metastasis in the absence of an organ capsule. The best therapeutic option is surgical removal of the tumour followed by chemotherapy or radiotherapy. Yet, even after surgical R0-resection, the 5-year survival probability is only about 20% because of the high recurrence rate of this tumour and complications due to metastases. Furthermore, recent studies have shown that the perioperative period is a particularly vulnerable phase, during which tumour progression and metastasis may be facilitated. The effects of analgesics administered during the perioperative period are still unknown. The present work investigated the effects of analgesics on pancreatic cancer cell migration in vitro.
Materials and Methods: The migratory potential of pancreatic cancer cells was analysed using a Cell Migration Assay Kit with a Boyden chamber, in which cells migrate through a semi-permeable membrane under different stimuli. Cell concentration was measured by reading fluorescence (Ex/Em=530/590 nm) in a plate reader.
Results: Migration in PANC-1 pancreatic cancer cells was significantly decreased after 24 h stimulation with 100 μM of ropivacaine, 100 nM of sufentanil, 1,000 μM of ropivacaine and 1,000 nM of sufentanil. In the PaTu 8988t cell line, incubation with 10 μM of ropivacaine caused a slight but statistically significant increase in migration, whereas lidocaine, metamizole and paracetamol did not significantly affect migration.
Conclusion: The risk of tumour progression and metastasis seems to be increased during major oncological surgical interventions. The recent advances in the molecular and biological understanding of pathogenesis of pancreatic cancer have not yet significantly improved patient outcome. Therefore, further studies are needed to identify the underlying mechanisms of this aggressive tumour and establish new therapeutic options for the future.
Keywords: Migration, pancreatic cancer, cancer, analgesics, metamizole, paracetamol, sufentanil, ropivacaine, lidocaine
Adenocarcinoma of the pancreas is one of the most aggressive malignant diseases in humans. More than 9 of 10 patients with pancreatic cancer die within 5 years of diagnosis (1,2). This type of tumour is marked by poor response to radiotherapy and chemotherapeutic agents as well as by early lymphogenic, perineural, haematogenic and peritoneal metastasis in the absence of an organ capsule (3-5). Furthermore, pancreatic adenocarcinoma does not present any characteristic early symptoms, and appropriate screening tests are lacking (6). First symptoms are usually caused by the invasion of the tumour into surrounding anatomic structures such as the stomach or colon, but may also be due to distant metastases, for instance in the liver or lungs (7). Thus, at the time of diagnosis, most tumours are already classified as non-curative and have a poor prognosis (8).
The best therapeutic option is surgical removal of the tumour followed by chemotherapy or radiotherapy (9). Yet, even after surgical R0-resection, the 5-year survival probability is only about 20% because of the high recurrence rate of this tumour and complications due to metastases (10,11). Furthermore, recent studies have shown that the perioperative period is a particularly vulnerable phase, in which tumour progression and metastasis may be facilitated (12). The effects of analgesics administered via peridural anaesthesia or as lidocaine infusion during the perioperative phase or for postoperative pain management are still unknown. The aim of this study was to investigate the effects of analgesics on pancreatic cancer cell migration in vitro.
Materials and Methods
Cell lines. The human pancreatic cancer cell lines PaTu 8988t and PANC-1 were obtained from Professor Ellenrieder (Philipps University of Marburg, Germany). PaTu 8988t and PANC-1 cells were maintained in Dulbecco's modified Eagle’s medium (Sigma-Aldrich, St. Gallen, Switzerland) supplemented with 10% foetal calf serum (FCS) (Sigma-Aldrich) and 5% Myco Zap (Lonza Verviers SPRL, Verviers, Belgium). Cells were cultured in humidified CO2 atmosphere (5%) at 37˚C and maintained in monolayer culture. Experiments were done with cells at ~70-80% confluence.
Reagents. Commercially available ropivacaine (Fagron, Barsbüttel, Germany), sufentanil (Sigma-Aldrich) and lidocaine (Sigma-Aldrich, St. Gallen, Switzerland) were used for this study. Metamizole was purchased from Fluka (München, Germany), and paracetamol from Merck (Darmstadt, Germany). Final concentrations were obtained by diluting the drugs in standard growth media. All solutions were prepared freshly prior to use.
Cell migration assay. Cell migratory potentials were evaluated using a cell migration Assay Kit (abcam, Cambridge, UK). The test uses a Boyden chamber in which cells migrate through a semi-permeable membrane under different stimuli. In brief, cells were treated with the appropriate medication (0 μM, 10 μM, 100 μM or 1,000 μM of metamizole; 0 μM, 10 μM, 100 μM or 1,000 μM of paracetamol; 0 μM, 10 μM, 100 μM or 1,000 μM of lidocaine; 0 μM, 10 μM, 100 μM or 1,000 μM of ropivacaine; 0 nM, 10 nM, 100 nM or 1,000 nM of sufentanil or the combination of 0 μM of ropivacaine and 0 nM of sufentanil, 10 μM of ropivacaine and 10 nM of sufentanil, 100 μM of ropivacaine and 100 nM of sufentanil or 1,000 μM of ropivacaine and 1,000 nM of sufentanil) in serum-free medium for 2 h. Afterwards, 200,000 cells of the human pancreatic cancer cell lines PaTu 8988t or PANC-1 were placed into the upper chamber, and a stimulant was pitted into the lower chamber. The chambers were incubated at 37˚C for 4 h. The migrating cells passed through the semi-permeable membrane and migrated into the bottom chamber or adhered to the bottom of the upper chamber. After dismantling, cell migration was directly analysed by reading fluorescence (Ex/Em=530/590 nm) in a plate reader. All tests were done with three wells per treatment group and performed as two independent experiments.
Statistical analysis. Data are presented as mean±SD. The non-parametric Mann Whitney U-test was used for statistical evaluation of the data. p-Values of <0.05 were considered significant. IBM SPSS Statistics (Version 26, IBM, New York, NY, USA) and Excel Version 2013 (Microsoft, Redmond, WA, USA) packages were employed for statistical analysis.
Results
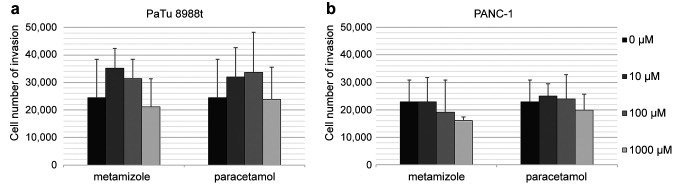
Analysis of migration in pancreatic cancer cells. PaTu 8988t and PANC-1 pancreatic cancer cells were stimulated with 0 μM, 10 μM, 100 μM or 1,000 μM of metamizole or with 0 μM, 10 μM, 100 μM or 1,000 μM of paracetamol (Figure 1a and b). Metamizole and paracetamol did not significantly affect migration.
Figure 1. The effects of metamizole and paracetamol on cell migration in PaTu 8988t (a) and PANC-1 (b) pancreatic cancer cells in vitro. Cell migration was quantified by using a Boyden chamber in which cells migrate through a semi-permeable membrane under different stimuli. *Statistical significance at p<0.05 compared to untreated controls.
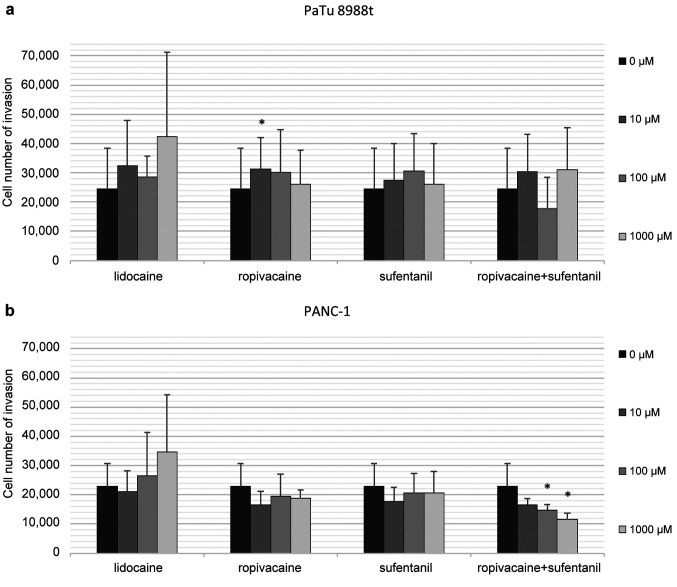
Behaviour of cell migration and analysis of cell concentration. PaTu 8988t pancreatic cancer cells and PANC-1 were stimulated with 0 μM, 10 μM, 100 μM or 1,000 μM of lidocaine, 0 μM, 10 μM, 100 μM or 1,000 μM of ropivacaine and 0 nM, 10 nM, 100 nM or 1,000 nM of sufentanil or the combination of 0 μM of ropivacaine and 0 nM of sufentanil, 10 μM of ropivacaine and 10 nM of sufentanil, 100 μM of ropivacaine and 100 nM of sufentanil or 1,000 μM of ropivacaine and 1,000 nM of sufentanil (Figure 2a and b). The combination of 100 μM of ropivacaine and 100 nM of sufentanil and 1,000 μM of ropivacaine and 1,000 nM of sufentanil had significantly decreased the migration of PANC-1 pancreatic cancer cells after 24 h stimulation (Figure 2b). In a PaTu 8988t cell line (Figure 2a), incubation with 10 μM of ropivacaine caused a slight but statistically significant increase in migration. Lidocaine did not significantly affect migration in either cell line.
Figure 2. The effects of lidocaine, ropivacaine and sufentanil and the combination of ropivacaine and sufentanil on migration in PaTu 8988t (a) and PANC-1 (b) pancreatic cancer cells in vitro. The cell migration rate was detected by using a Boyden chamber in which cells migrate through a semi-permeable membrane under different stimuli. *Statistical significance at p<0.05 compared to untreated controls.
Discussion
At the time of death, up to 80% of patients with adenocarcinoma of the pancreas are found to have metastases of the liver, 60% of the peritoneum and 50-70% of the lungs or pleura (13). In carcinogenesis, metastasis of tumour cells represents the endpoint of a multi-step process (14). Metastases occur when cancer cells become detached from the original tumour, migrate with blood or lymph and re-colonise and multiply in other tissues. Molecular biological analyses have shown the loss or inactivation of cell-cell or cell-matrix adhesion molecules (15) and, in invasive tumours, the simultaneous upregulation of adhesion molecules (16).
Recent studies have indicated that the perioperative period is a particularly vulnerable phase, during which tumour progression and metastasis may be facilitated (12). The effects of analgesics administered via peridural anaesthesia or as lidocaine infusion during the perioperative phase or for postoperative pain management are still unknown.
In the first studies analysing the effects of analgesics on metastasis in tumour cells, the selective COX-2 inhibitor celecoxib was found to reduce the gelatinolytic activity of matrix metalloproteinase 2 and 9 and to decrease the invasion capacity in oral squamous cell carcinoma (17,18). According to Li et al., celecoxib inhibited the proliferation, invasion and migration of PANC-1 pancreatic cancer cells (19). Aspirin inhibited the motility and subsequently the migration and invasion of prostate cancer cells by suppressing the binding of tumour cells to fibronectin and vitronectin (20). Indomethacin reduced the invasion capacity of breast cancer cells, most likely due to changes in the choline-phospholipid and triacylglycerol metabolism (21). In previous studies acetaminophen and metamizole revealed proapoptotic effects in colon cancer and antiproliferative effects in pancreatic cancer cells (22). Through increased expression of the differentiation markers, paracetamol seems to be able to change breast cancer cells into a more benign type marked by reduced tumour growth, low invasion capacity and increased sensitivity to anti-tumour agents (23). In our study, however, metamizole and acetaminophen did not significantly affect the migration of pancreatic cancer cells. Further preclinical and clinical studies are required to decide if these drugs can be safely administered in patients with pancreatic adenocarcinoma.
The effect of regional anaesthesia on tumour progression has also been the focus of many clinical studies in recent years, but the obtained data show somewhat contradictory results. For peridural anaesthesia, the long-acting local anaesthetic ropivacaine is used, a local anaesthetic of the amide type (24). Local anaesthetics act by blocking voltage-gated sodium channels of the neuronal axon. In this process, the local anaesthetic binds to the inside of the inactivated sodium channel, thus preventing the rapid influx of sodium into the cell, which is important for depolarization. The conduction of stimuli in the nerve is inhibited, and pain transmission is stopped (25). Such ion channels are not only found on the axons of peripheral nerves but have also been detected in various tumour entities, such as in cancer cells of the breast, colon and prostate (26). Thereby, increased expression of voltage-gated sodium channels seems to be associated with increased tumour metastasis (27,28). Circulating tumour cells partially bind to vessel walls via microtentacles. Tertacaine, and to a lesser extent also lidocaine, inhibits the spread of these microtentacles, subsequently reducing the metastatic potential of breast cancer cells (29). Meanwhile, reduced expression of voltage-gated sodium channels correlates with decreased cell proliferation and invasiveness, thus inducing apoptosis in astrocytoma cells (30). Piegeler et al. investigated the effects of local anaesthetics on migration in adenocarcinoma of the lung. Incubation with ropivacaine and lidocaine reduced ICAM phosphorylation, which is associated with inhibited cell migration. Ester-type local anaesthetics did not produce such anti-metastatic effects. Moreover, these effects seem to be independent of the function of local anaesthetics that inhibits the sodium channel (31).
To improve the analgesic effect of peridural anaesthesia, ropivacaine is often combined with the opiate sufentanil (32). As a pure antagonist, sufentanil binds to opioid receptors of the nervous system (33) and has been shown to improve the quality of analgesia. Similarly, the addition of opioids to local anaesthetics in peridural anaesthesia leads to a faster onset of the required effect, thus enabling a dose reduction of the individual components (34). This phenomenon can also be observed in the intravenous application of lidocaine in major abdominal surgery compared to single general anaesthesia. The decrease in peri- and postoperative pain levels also significantly reduces the need of anaesthetics and opioid analgesics (35). For opiates, data on the migration, invasion and metastatic potential of tumours have been inconsistent so far. In one study, morphine inhibited the adhesion, migration and invasion of colon cancer cells in vitro and the expression of MMP2 and 9 in breast cancer cells (36), whereas in other in vitro studies, morphine increased the migration and invasion of breast and bladder cancer cells (37,38).
Samples from patients with non-small cell lung cancer showed a 5- to 10-fold increase in μ opioid receptor expression. In animal models, treatment of lung cancer cells with the opioid antagonist methylnaltrexone or inactivation of the μ-receptor resulted in a 65% reduction in lung metastases (39). Interestingly, opioid receptors do not always appear to be involved in mediating the effects of μ-agonists. In some studies, effects also occurred in μ-receptor-negative cells, or the observed effects could not be antagonised by naloxone. There is some evidence that the effects of opioids on tumour cells are also mediated by the nitrite oxidase system (36), the bradykinin 2 receptor (38) or the NET 1 gene (37). A study on breast cancer treatment by Exadaktylos et al. showed that combining general anaesthesia with paravertebral blockade for mastectomy was associated with a significantly better prognosis than general anaesthesia alone. After 36 months, recurrence-free and metastasis-free survival was 94% in the paravertebral group versus 77% in the general anaesthesia group (40). De Oliveira et al. found a reduced risk of recurrence for intraoperative epidural anaesthesia in patients operated on for ovarian cancer (41). In contrast, results from other retrospective studies indicated that epidural anaesthesia had no benefit on overall survival in patients undergoing cytoreductive surgery for ovarian cancer (42,43). A meta-analysis with 14 included studies concluded that there may be a benefit of epidural anaesthesia compared to general anaesthesia in terms of overall survival but not recurrence-free survival (44).
In the present study, migration of PANC-1 pancreatic cells was significantly reduced after 24 h stimulation with 100 μM of ropivacaine and 100 nM of sufentanil as well as with 1,000 μM of ropivacaine and 1,000 nM of sufentanil, which underlines the positive effect of epidural anaesthesia found in preliminary studies. The potential molecular and biological background of this effect remains unclear. Tumour progression and migration is regulated of specific signaling and transcription pathways. Many proteins are involved in the carcinogenic process, which can act as transcription factors or cofactors, and have a significant impact on the regulation of target genes.
Conclusion
Recent advances in the molecular and biological understanding of the pathogenesis of pancreatic cancer (45) have not yet significantly improved patient outcome (46). The risk of tumour progression and metastasis appears to be increased during the perioperative period of major oncological surgical interventions. The perioperative period is particularly associated with the administration of a variety of substances for balanced anaesthesia and postoperative pain management. The extent to which these drugs affect the carcinogenesis of pancreatic cancer needs to be investigated in further studies. The aim is to identify the underlying mechanisms of this aggressive tumour and to establish new therapeutic options for the future.
Conflicts of Interest
The Authors declare that they have no competing interests.
Authors’ Contributions
All Authors have made substantial contributions to the conception, design, analysis and the interpretation of this research article. They have been involved in the critical revision of the manuscript with regard to important intellectual content. All authors have given their final approval for the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We thank Sigrid Bamberger, Regina Lindner, Gabriele Bollwein, Marion Schindler and Ruth Spaeth for technical assistance. We thank Monika Schoell for linguistic support.
References
- 1.Kaatsch P, Spix C, Katalinic A, Hentschel S, Luttmann S, Stegmaier C, Waldeyer- Sauerland M, Waldmann A, Caspritz S, Christ M, Ernst A, Folkerts J, Hansmann J, Klein S. Krebs in Deutschland 2013/2014. Berlin, Robert Koch-Institute, 2017. Available at: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2017/krebs_in_deutschland_2017.pdf?__blob=publicationFile. [Last accessed on February 9, 2022]
- 2.Schmid RM. [Pancreatic cancer] Praxis (Bern 1994) 2006;95(44):1709–1712. doi: 10.1024/1661-8157.95.44.1709. [DOI] [PubMed] [Google Scholar]
- 3.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 5.Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 6.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15(4):462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 7.Böhmig M, Rosewicz S. [Pancreatic carcinoma] Z Gastroenterol. 2004;42(3):261–268. doi: 10.1055/s-2004-812693. [DOI] [PubMed] [Google Scholar]
- 8.Sakorafas GH, Tsiotou AG, Tsiotos GG. Molecular biology of pancreatic cancer; oncogenes, tumour suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev. 2000;26(1):29–52. doi: 10.1053/ctrv.1999.0144. [DOI] [PubMed] [Google Scholar]
- 9.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128(6):1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. Am J Gastroenterol. 2007;102(7):1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 11.Strobel O, Werner J. Langzeitverlauf nach operativer Tumorentfernung und Chemotherapie des duktalen Pankreaskarzinoms. In: Erkrankungen des Pankreas. . In: Beger HG, Büchler MW, Dralle H, Lerch MM, Malfert-Heiner P, Mössner J, Riemann JF, editors. Berlin Heidelberg, Springer. 2013. p. pp. 415. [Google Scholar]
- 12.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110(6):1636–1643. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 13.Lillemoe KD, Melton GB, Cameron JL, Pitt HA, Campbell KA, Talamini MA, Sauter PA, Coleman J, Yeo CJ. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg. 2000;232(3):430–441. doi: 10.1097/00000658-200009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Li WZ, Huo QJ, Wang XY, Xu F. Inhibitive effect of celecoxib on the adhesion and invasion of human tongue squamous carcinoma cells to extracellular matrix via down regulation of MMP-2 expression. Prostaglandins Other Lipid Mediat. 2010;93(3-4):113–119. doi: 10.1016/j.prostaglandins.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Kwak YE, Jeon NK, Kim J, Lee EJ. The cyclooxygenase-2 selective inhibitor celecoxib suppresses proliferation and invasiveness in the human oral squamous carcinoma. Ann NY Acad Sci. 2007;1095:99–112. doi: 10.1196/annals.1397.014. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Gu Z, Xiao Z, Zhou T, Li J, Sun K. Anti-tumor effect and mechanism of cyclooxygenase-2 inhibitor through matrix metalloproteinase 14 pathway in PANC-1 cells. Int J Clin Exp Pathol. 2015;8(2):1737–1742. [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd FP Jr, Slivova V, Valachovicova T, Sliva D. Aspirin inhibits highly invasive prostate cancer cells. Int J Oncol. 2003;23(5):1277–1283. [PubMed] [Google Scholar]
- 21.Ackerstaff E, Gimi B, Artemov D, Bhujwalla ZM. Anti-inflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia. 2007;9(3):222–235. doi: 10.1593/neo.06673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bundscherer AC, Malsy M, Gruber MA, Graf BM, Sinner B. Acetaminophen and metamizole induce apoptosis in HT 29 and SW 480 colon carcinoma cell lines in vitro. Anticancer Res. 2018;38(2):745–751. doi: 10.21873/anticanres.12280. [DOI] [PubMed] [Google Scholar]
- 23.Takehara M, Hoshino T, Namba T, Yamakawa N, Mizushima T. Acetaminophen-induced differentiation of human breast cancer stem cells and inhibition of tumor xenograft growth in mice. Biochem Pharmacol. 2011;81(9):1124–1135. doi: 10.1016/j.bcp.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54(4):587–599. doi: 10.1016/j.cden.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Biscoping J, Bachmann-Mennenga MB. [Local anesthetics from ester to isomer] Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35(5):285–292. doi: 10.1055/s-2000-324. [DOI] [PubMed] [Google Scholar]
- 26.Curatolo M. Regional anesthesia in pain management. Curr Opin Anaesthesiol. 2016;29(5):614–619. doi: 10.1097/ACO.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 27.Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625(1-3):206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin) 2012;6(5):352–361. doi: 10.4161/chan.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon JR, Whipple RA, Balzer EM, Cho EH, Matrone MA, Peckham M, Martin SS. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res Treat. 2011;129(3):691–701. doi: 10.1007/s10549-010-1239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing D, Wang J, Ou S, Wang Y, Qiu B, Ding D, Guo F, Gao Q. Expression of neonatal Nav1.5 in human brain astrocytoma and its effect on proliferation, invasion and apoptosis of astrocytoma cells. Oncol Rep. 2014;31(6):2692–2700. doi: 10.3892/or.2014.3143. [DOI] [PubMed] [Google Scholar]
- 31.Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117(3):548–559. doi: 10.1097/ALN.0b013e3182661977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann-Mennenga B, Veit G, Steinicke B, Biscoping J, Heesen M. Efficacy of sufentanil addition to ropivacaine epidural anaesthesia for Caesarean section. Acta Anaesthesiol Scand. 2005;49(4):532–537. doi: 10.1111/j.1399-6576.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 33.Bujedo BM, Santos SG, Azpiazu AU. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8(3):177–192. doi: 10.5055/jom.2012.0114. [DOI] [PubMed] [Google Scholar]
- 34.Gomar C, Fernandez C. Epidural analgesia-anaesthesia in obstetrics. Eur J Anaesthesiol. 2000;17(9):542–558. doi: 10.1046/j.1365-2346.2000.00733.x. [DOI] [PubMed] [Google Scholar]
- 35.Vigneault L, Turgeon AF, Côté D, Lauzier F, Zarychanski R, Moore L, McIntyre LA, Nicole PC, Fergusson DA. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58(1):22–37. doi: 10.1007/s12630-010-9407-0. [DOI] [PubMed] [Google Scholar]
- 36.Gach K, Szemraj J, Wyrębska A, Janecka A. The influence of opioids on matrix metalloproteinase-2 and -9 secretion and mRNA levels in MCF-7 breast cancer cell line. Mol Biol Rep. 2011;38(2):1231–1236. doi: 10.1007/s11033-010-0222-z. [DOI] [PubMed] [Google Scholar]
- 37.Ecimovic P, Murray D, Doran P, McDonald J, Lambert DG, Buggy DJ. Direct effect of morphine on breast cancer cell function in vitro: role of the NET1 gene. Br J Anaesth. 2011;107(6):916–923. doi: 10.1093/bja/aer259. [DOI] [PubMed] [Google Scholar]
- 38.Vassou D, Notas G, Hatzoglou A, Castanas E, Kampa M. Opioids increase bladder cancer cell migration via bradykinin B2 receptors. Int J Oncol. 2011;39(3):697–707. doi: 10.3892/ijo.2011.1063. [DOI] [PubMed] [Google Scholar]
- 39.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112(3):558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis. Anesthesiology. 2006;105(4):660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira GS Jr, Ahmad S, Schink JC, Singh DK, Fitzgerald PC, McCarthy RJ. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg Anesth Pain Med. 2011;36(3):271–277. doi: 10.1097/AAP.0b013e318217aada. [DOI] [PubMed] [Google Scholar]
- 42.Lacassie HJ, Cartagena J, Brañes J, Assel M, Echevarría GC. The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population. Anesth Analg. 2013;117(3):653–660. doi: 10.1213/ANE.0b013e3182a07046. [DOI] [PubMed] [Google Scholar]
- 43.Capmas P, Billard V, Gouy S, Lhommé C, Pautier P, Morice P, Uzan C. Impact of epidural analgesia on survival in patients undergoing complete cytoreductive surgery for ovarian cancer. Anticancer Res. 2012;32(4):1537–1542. [PubMed] [Google Scholar]
- 44.Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One. 2013;8(2):e56540. doi: 10.1371/journal.pone.0056540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23(Suppl 10):x135–x138. doi: 10.1093/annonc/mds313. [DOI] [PubMed] [Google Scholar]
- 46.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]