Abstract
Background/Aim: Natural skin whiteners have been investigated for centuries. The development of preparations that safely achieve whitening of hyper-pigmented skin lesions is a challenge for the cosmetics industry. Furthermore, promoting rapid wound healing and minimizing inflammation in injured skin are key to prevent from abnormal pigmentation in scar tissue. Natural products, including the fungus Tremella fuciformis (TF), are attracting attention as potential sources of lead compounds for these applications.
Materials and Methods: We investigated the in vitro effects of TF on melanogenesis in murine B16F10 cells. Melanin and tyrosinase levels were measured after treatment with TF. Wound healing in human keratinocytes (HaCaT) and fibroblasts (Detroit 551) was also determined via cell migration assay prior to TF exposure.
Results: TF significantly decreased melanin content and tyrosinase expression in a concentration-dependent manner in B16F10 cells. Furthermore, TF promoted wound healing in human HaCaT keratinocytes and Detroit 551 fibroblasts.
Conclusion: TF proved effectively on inhibiting melanogenesis and promoting wound healing in vitro, demonstrating its potential as a novel skin-whitening agent. However, further clinical studies of safety and efficacy are required.
Keywords: Tremella fuciformis (TF), melanogenesis, melanin, tyrosinase, wound healing
Many people equate a light complexion to youth and beauty (1). Although a bronze tan is being increasingly recognized as a desirable trait in some Western countries, there is a greater overall interest in skin whitening, especially in Eastern countries. The search for safe and effective natural skin whiteners has persisted for centuries (2).
Melanin, a group of natural pigments found in most organisms, is produced by epidermal melanocytes. It is a major determinant of skin color and protects against ultraviolet (UV) irradiation (3). Melanogenesis is a multistage chemical process, involving tyrosinase and tyrosinase-related proteins, and the oxidation and polymerization of the amino acid-tyrosine (4). Tyrosinase, the rate-limiting enzyme in melanogenesis, catalyzes two critical steps in melanin production (5). Numerous studies have reported that the inhibition of melanogenesis prevents skin tanning and hyperpigmentation (6). In addition, the management of cutaneous injury and scarring has long been a challenge for plastic surgeons and dermatologists. An inflammatory response is induced by cutaneous injuries (1,7). Melanocytes and their production by melanogenesis are influenced by important cellular mediators in various ways. Increased inflammation in skin lesions results in increased and prolonged activation of melanogenesis, which may lead to uncontrolled melanocyte proliferation and melanoma (7,8). Furthermore, skin injury with disruption to normal melanogenesis causes dyspigmentation (9). Consequently, substantial research effort is directed toward promoting rapid wound healing and minimizing inflammation in injured skin to prevent pigmentation abnormalities of the resulting scar tissue (10,11).
Mercury has historically been used as a key ingredient in skin-lightening products. However, the health hazards of mercury resulted in its elimination from skin-lightening products in many countries in recent decades (12-14). The use of other hazardous chemicals, such as hydroquinone in skin-lightening products, have also raised public concern about their dangers to health and emphasizes the importance of government regulations (15,16). Therefore, the development of preparations that can safely achieve whitening and bleaching of hyper-pigmented lesions is a major challenge for the cosmetics industry. Natural products are attracting considerable attention as potential sources of lead compounds and drug candidates (17-20). The plant sources of such natural products are generally also established herbal medicines and dietary foods (21).
The fungus, Tremella fuciformis (TF) (Figure 1A), commonly known as white auricularia, snow fungus, snow ear, white jelly mushroom, and silver ear fungus, occurs widely, especially in tropical areas (22,23). It can be found growing on the dead branches of broadleaf trees; however, TF is commercially cultivated and popularly used in cuisine and herbal medicine (24,25). TF is commonly preserved as drying mushroom (Figure 1B) and processed into powder for medicinal use (Figure 1C). TF is rich in proteins, polysaccharides, and dietary fiber but low in energy and lipid content (26,27). Various bioactivities have been attributed to TF, including immunomodulation, anti-oxidation, anti-hyperglycemia, anti-hypercholesterolemia, anti-tumor, anti-aging, and helping with memory impairment (28). However, to the best of our knowledge, no study has been reported for the effects of TF on skin lightening and wound healing in vitro. The goal of this study was to investigate the in vitro effects of TF on the B16F10 murine melanoma cell line as well as human HaCaT keratinocytes and Detroit 551 fibroblasts.
Figure 1. Gross view (A) and dry preserved T. fuciformis (TF) (B). A typical powder for medicinal use (C).
Materials and Methods
Chemicals. Dried and preserved TF was pulverized as previously described (29). Dulbecco’s modified Eagle’s medium (DMEM), Minimum essential medium (MEM), fetal bovine serum (FBS), trypsin-EDTA, L-glutamine, penicillin G, and streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The primary antibodies (against tyrosinase and β-actin) and anti-rabbit immunoglobulin IgG HRP-linked secondary antibodies were purchased from GeneTex International Corporation (Hsinchu, Taiwan, ROC). All other chemicals were purchased from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany).
Cell culture. B16F10 (a murine melanoma cell line from a C57BL/6J mouse) and Detroit 551 (a human fibroblast cell line) were purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan, ROC). HaCaT, a human keratinocyte cell line, was obtained from CLS Cell Lines Service GmbH (Eppelheim, Germany). B16F10 and HaCaT cells were individually cultured in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin (100 Units/ml penicillin G and 100 μg/ml streptomycin), and 2 mM L-glutamine in a humidified atmosphere at 37˚C in 5% CO2. Detroit 551 cells were cultured at 37˚C in 75 cm2 culture flasks with 10% FBS, 90% MEM, 100 Units/ml penicillin G, and 100 μg/ml streptomycin in a humidified 5% CO2 atmosphere.
Morphology and cell viability assays. B16F10 cells were seeded in a 96-well plate at an initial density of 1×104 cells/100 μl. The cells were incubated at 37˚C with or without different concentrations of TF (50, 100, 200, and 300 μg/ml) for 24 h. Cell images were then photographed via a phase-contrast microscope at ×200 magnification (Leica Microsystems GmbH, Wetzlar, Germany). After that, addition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (0.5 mg/ml) was added to each well before further incubation at 37˚C for 4 h. Subsequently, the culture medium was removed, and the formazan crystals were dissolved with 100 μl dimethyl sulfoxide (DMSO) in isopropanol. Absorbance was measured spectrophotometrically at 570 nm via SpectraMax iD3 multimode microplate reader (Molecular Devices Ltd., San Jose, CA, USA). The cell survival ratio was expressed as a percentage of the control, as previously described (30).
Melanin measurement. B16F10 cells (1×104 cells/100 μl) were placed in a 96-well cell culture plate, and allowed to attach overnight at 37˚C. The cells were exposed to different concentrations of TF (50, 100, 200, and 300 μg/ml) for 48 h at 37˚C, and then incubated for an additional 24 h in the presence or absence of 0.5 μM α-melanocyte stimulating hormone (α-MSH) (Sigma-Aldrich). The cells were subsequently washed twice with PBS, and lysed for 1 h at 90˚C in 1 M NaOH containing 10% DMSO. The total melanin in each cell suspension was determined by measuring the absorbance at 405 nm using a spectrophotometric multi-plate reader (SpectraMax iD3 multimode microplate reader, Molecular Devices Ltd.). The melanin content of the TF-treated cells was expressed as a percentage of the untreated cells. The total melanin content was determined according to a previously described method (31), with slight modifications.
Western blot analysis. B16F10 cells (5×106 cells per 75T flask) were incubated at 37˚C with TF at different concentrations (100, 200, and 300 μg/ml) for 24 h before exposure to 0.5 μM α-MSH for an additional 24 h. Cell samples were lysed in Trident RIPA Lysis Buffer (GeneTex). Protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of the protein sample (40 μg) were prepared and loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels (32,33). Proteins were then transferred to an Immobilon-P polyvinylidene difluoride transfer membrane (Merck KGaA, Darmstadt, Germany) prior to blocking with 5% skim milk for 1 h at room temperature. The membrane was subsequently incubated overnight at 4˚C with primary antibodies against tyrosinase and β-actin at a dilution of 1:1,000. Membranes were then incubated for 1 h at 25˚C with an anti-rabbit IgG horseradish peroxidase (HRP)-linked secondary antibody at a dilution of 1:10,000. Blot visualization was performed using the Immobilon Western Chemiluminescent HRP Substrate (Merck KGaA), and all bands of immunoblots were normalized to the densitometric value of β-actin. The bands were quantified by densitometry using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MA, USA) (34,35).
Dynamic wound healing assay. HaCaT cells (1×104 cells/well) into a 96-well plate overnight were scratched using Incucyte 96-Well Woundmaker Tool (Essen BioScience, Ann Arbor, MI, USA) and then treated with or without 100 and 200 μg/ml TF in serum-free DMEM. The cell migration images and wound width were recorded over 12 h with data collection every 30 min and monitored using Incucyte S3 Live-Cell Analysis System and Incucyte Scratch Wound Analysis Software Module (Essen BioScience), as previously described (36).
Cell migration assay. Detroit 551 cells were transferred to a 6-well tissue culture plate for 24 h, and the cells were grown up to 90% confluence. Subsequently, each well was scratched with a micropipette tip to create a denuded zone of constant width (1 mm). The cells were then cultured in serum-free MEM and incubated at 37˚C with different concentrations of TF (100, and 200 μg/ml) for 24 h. The cells and the denuded zones were photographed under phase-contrast microscopy (×100), as previously described (34,37).
Statistical analysis. All data are presented as the mean±standard deviation of three separate experiments. One-way analysis of variance followed by Dunnett’s test was conducted to analyze the differences between groups and multiple comparisons (SPSS software version 26.0, Chicago, IL, USA). The statistical significance was set at p<0.05 or p<0.001.
Results
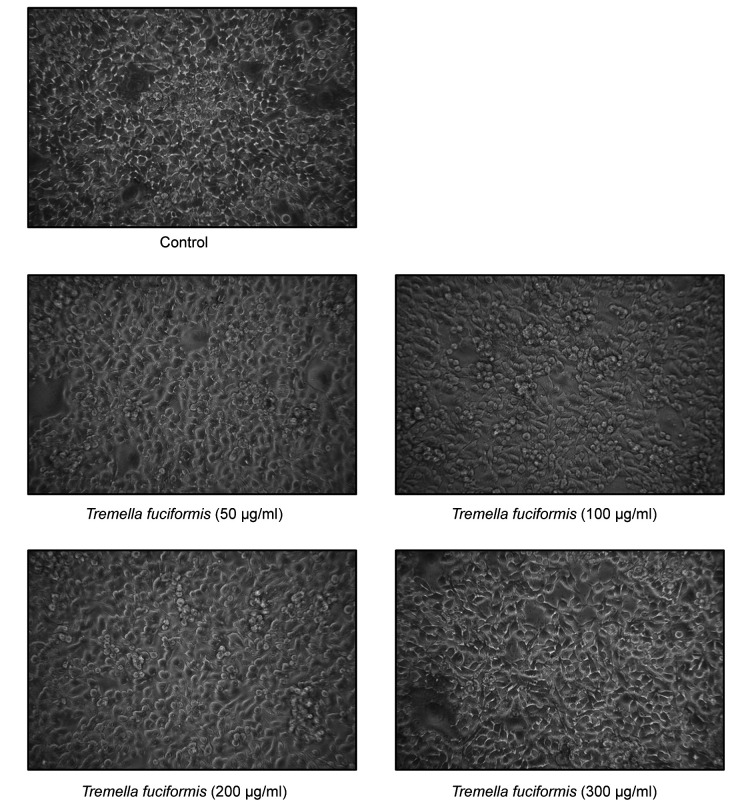
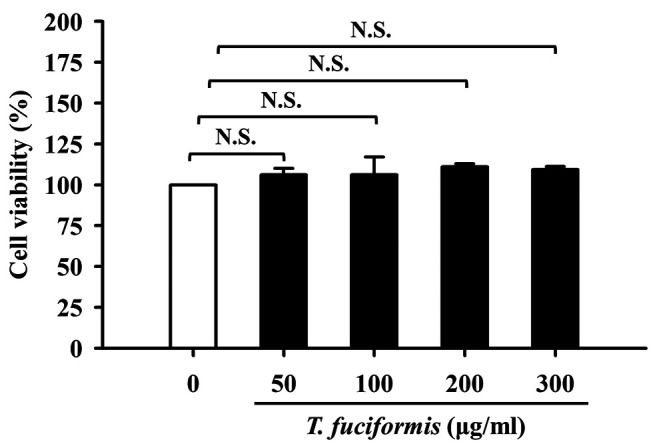
In vitro proliferation of murine melanoma B16F10 cells was unaffected by TF concentrations of up to 300 μg/ml. The cells were treated with various concentrations of TF (50, 100, 200, and 300 μg/ml) and analyzed using the MTT cell viability assay. TF treatment did not induce any changes in cell morphology (Figure 2), and no significant effect of the number of viable B16F10 cells was found when compared with untreated cells (Figure 3). Therefore, treatment with TF at the highest tested concentration of 300 μg/ml was suitable for subsequent evaluation in the melanin synthesis and tyrosinase activity.
Figure 2. Effects of different concentrations of T. fuciformis (TF) (50, 100, 200, and 300 μg/ml) on B16F10 murine melanoma cell morphology. Cell images were obtained via a phase-contrast microscope at ×200 magnification.
Figure 3. Effects of different concentrations (50, 100, 200, and 300 μg/ml) of T. fuciformis (TF) on B16F10 murine melanoma cell viability. The results shown are the averages of triplicate experiments±standard deviation. N.S.: Not significant.
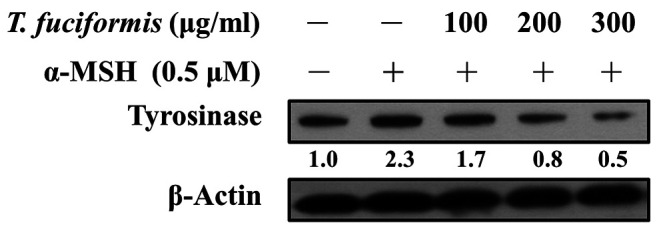
TF decreased melanin content and tyrosinase levels in B16F10 cells. Melanin production is a multistage chemical process involving tyrosinase and tyrosinase-related proteins (5,38). We measured the melanin content of B16F10 cells (Figure 4), which exhibited a concentration-dependent decreasing trend in response to TF treatment. Significant reductions in melanin content were observed in the 100, 200, and 300 μg/ml of TF treatment groups compared with the α-MSH-treated group. Tyrosinase is the rate-limiting enzyme in melanogenesis and catalyzes two critical steps (39). Therefore, we further evaluated the tyrosinase levels in B16F10 cells after TF treatment at 100, 200, and 300 μg/ml, showing that tyrosinase expression was decreased in a concentration-dependent manner (Figure 5).
Figure 4. Effects of different concentrations of T. fuciformis (TF) on melanin content in B16F10 murine melanoma cells. The cells were exposed to TF (50, 100, 200, and 300 μg/ml) for 48 h, followed by 24 h incubation with or without 0.5 μM α-melanocyte-stimulating hormone (α-MSH). The results shown are the averages of triplicate experiments±standard deviation. #p<0.05 vs. the α-MSH-untreated control group. ***p<0.001 vs. α-MSH-treated control.
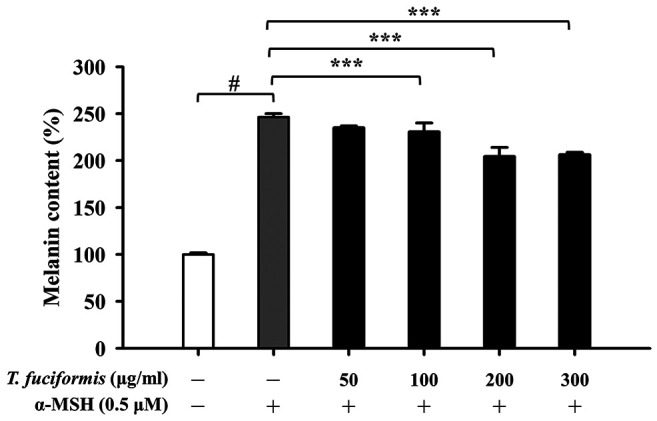
Figure 5. Effects of different concentrations of T. fuciformis (TF) on tyrosinase expression in B16F10 murine melanoma cells. The cells were exposed to TF (100, 200, and 300 μg/ml) for 48 h, followed by 24 h incubation with or without 0.5 μM α-melanocyte-stimulating hormone (α-MSH). Tyrosinase protein levels were detected via western blot. β-Actin was to ensure equal loading.
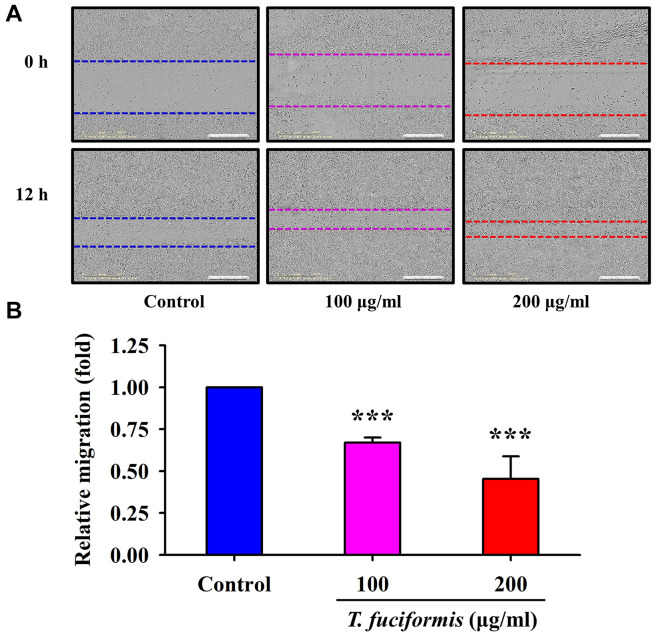
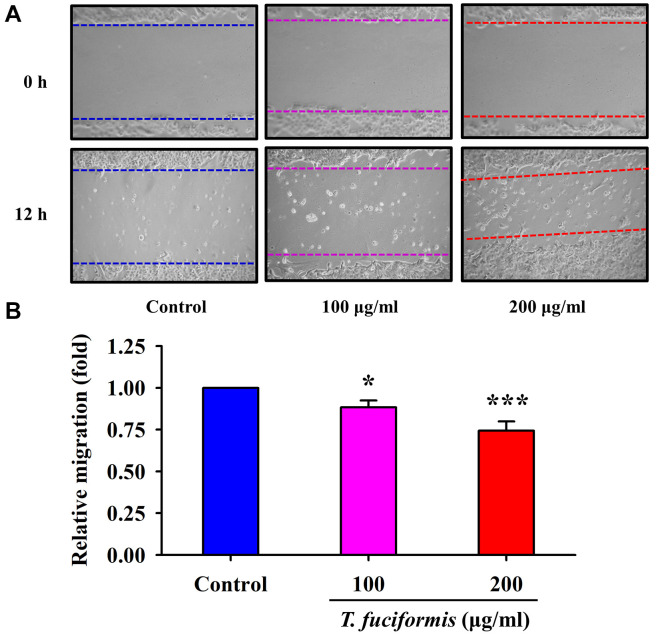
TF promoted cell motility in human keratinocytes (HaCaT) and human skin fibroblasts (Detroit 551). Previous studies have reported that rapid wound healing prevents from abnormalities of pigmentation and hyperpigmentation (40). Thus, we treated human keratinocyte HaCaT cells and human fibroblast Detroit 551 cells with different concentrations of TF (100 and 200 μg/ml) to evaluate cell migration using a wound-healing assay. The results for HaCaT and Detroit 551 cells (Figure 6 and Figure 7, respectively), revealed that the edge distances in the TF treatment groups were significantly shorter than that in the control group. Furthermore, the dynamic observation via Incucyte S3 Live-Cell Analysis System also showed that the wound was closing progressively after incubation with or without TF (100 and 200 μg/ml) in HaCaT cells (supplementary video, available at: https://youtu.be/4uYg3wg7l0g). Therefore, TF promoted both HaCaT and Detroit 551 cell migration in a concentration-dependent manner.
Figure 6. Effects of different concentrations of T. fuciformis (TF) (100, and 200 μg/ml) on wound healing in human keratinocytes (HaCaT cell line). The cells were photographed (A), and cell migration (B) was quantified. The results shown are the averages of triplicate experiments±standard deviation. ***p<0.001 vs. the control group.
Figure 7. Effects of different concentrations of T. fuciformis (TF) (100, and 200 μg/ml) on cell migration in human skin fibroblasts (Detroit 551 cells). The cells were photographed (A), and cell migration (B) was quantified. The results shown are the averages of triplicate experiments±standard deviation. *p<0.05 and ***p<0.001 vs. the control group.
Discussion
Natural skin whitening has been explored for centuries because of its cultural associations with the youth and beauty (41). Mercury-containing skin lighteners were once widely used; however, their popularity declined as following their association with health hazards (12). Natural compounds are receiving significant attention as potential skin-whitening agents. To the best of our knowledge, this is the first study to report on the effects of TF on skin complexion. TF effectively reduced melanin production in B16F10 cells and promoted wound healing in human HaCaT keratinocytes and Detroit 551 fibroblasts.
Melanin is produced by epidermal melanocytes, which are the main determinants of skin color (42). Interestingly, skin color and pigmentation are not determined by the number of melanocytes within the epidermis and dermis, but rather by the activity of melanocytes (43). Staricco et al. (44,45) reported that there was no significant difference in the number of melanocytes between black and white-skinned individuals. Within a particular individual, the highest numbers of melanocytes typically occur in the head, neck, limbs, and genitalia, and the lowest numbers on the chest and abdomen (44,45). Iozumi et al. (46) subsequently demonstrated that tyrosinase levels and activity determine pigmentation in cultured human melanocytes. The rate-limiting enzyme, tyrosinase, catalyzes two critical steps in melanin production, namely the hydroxylation of L-tyrosine to form L-dihydroxyphenylalanine (L-DOPA), and the oxidation of L-DOPA into the corresponding dopaquinone (47). The inhibition of melanogenesis has been reported in many studies to prevent skin tanning and hyperpigmentation (6). B16F10, a mouse melanoma cell line, is known to have stable melanin production, and that is an excellent cellular model for evaluating melanogenic effects (48,49). TF treatment neither induced any changes in cell morphology (Figure 2) nor significantly affected B16F10 cell viability (Figure 3). Furthermore, melanin content (Figure 4) and tyrosinase expression (Figure 5) in B16F10 cells were decreased in a concentration-dependent manner as a result of treatment with TF.
Skin injury disrupts normal melanogenesis, resulting in dyspigmentation, which has long been a challenge for plastic surgeons and dermatologists (50). Wound healing following skin injury is involved in three main stages: inflammation, proliferation, and remodeling (51). Excess inflammation plays a major role in impaired wound healing and the etiology of scarring. Furthermore, increased inflammation results in increased and prolonged activation of melanogenesis, leading to uncontrolled melanocyte proliferation, dyspigmentation, and melanoma (7,8). Recent studies indicated that TF can modulate the body’s immune functions by regulating immune cells and molecules and their activities without significant side effects (52). Shi et al. (53) reported that TF modulated CD4+ T cell proliferation and polarization in mice with Pseudomonas aeruginosa-infected, full-thickness burn injuries, resulting in reduced levels of IL-10. The use of TF may effectively enhance immune status (54). Furthermore, TF has been shown to possess antioxidant properties and may act as a potential therapeutic agent for oxidative-stress-associated skin diseases and aging (55). Shen et al. (56) reported that TF suppressed hydrogen peroxide-triggered injury in human skin fibroblasts via up-regulation of SIRT1, while Wen et al. (57) reported that TF scavenged 87% and 80% of superoxide and hydroxyl radicals, respectively, in a rat model of UV-induced skin damage.
In the proliferation stage of wound healing, greater numbers of keratinocytes and fibroblasts proliferate and migrate to wound margins, thereby promoting wound formation (58). A previous study reported that TF pretreatment reduced oxidative stress and cell apoptosis in hydrogen peroxide-treated skin fibroblasts. Moreover, it was also shown that TF inhibited p16, p21, p53, and caspase-3 expression, and activated extracellular signal-regulated kinase and Akt serine/threonine kinase 1 (59). To the best of our knowledge, no study has discussed the effects of TF on wound healing. Herein, we showed that TF significantly promoted wound healing in human HaCaT keratinocytes (Figure 6) and human Detroit 551 fibroblasts (Figure 7). Promoting rapid wound healing and minimizing the inflammatory process in injured skin are key factors in preventing pigmentation abnormalities of the resulting scar (60).
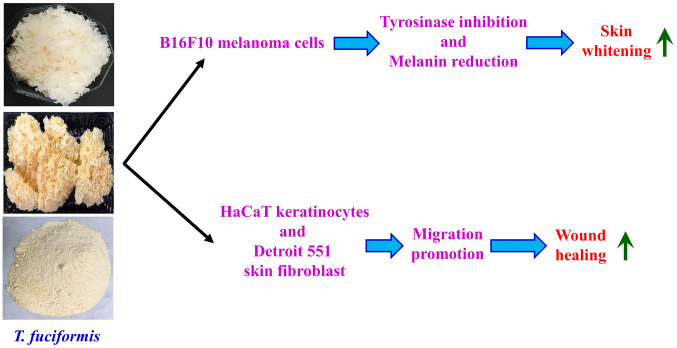
In summary, we are the first to report the effects of TF on melanogenesis and the promotion of wound healing (Figure 8). TF significantly reduced melanin production and tyrosinase protein levels in B16F10 cells; TF also effectively promoted the migration of human keratinocytes and fibroblasts. Our data suggest that TF may prove useful as a novel skin-whitening candidate in the future. Further clinical studies are required to assess the safety and efficacy of TF in the near future.
Figure 8. Summarized in vitro effects of Tremella fuciformis (TF) on skin whitening and wound healing in the present study.
Funding
The Authors are grateful for the financial support provided by the China Medical University Hospital (grant no. DMR-106-179), Taipei Veterans General Hospital (grant no. V110B-038), and Chung-Jen Junior College of Nursing, Health Sciences and Management (grant no. 110-002).
Conflicts of Interest
The Authors declare that they have no competing interests in relation to this study.
Authors’ Contributions
Conceptualization and study design: JHC, JSY and YJC. Cell migration, cell viability, western blotting and melanin content detection: JHC and FJT. Wound healing assay and acquisition of data: JHC and THL. Statistical analysis of all data and interpretation of results: JHC, FJT, THL, JSY, and YJC confirm the authenticity of all the raw data. All Authors read and approved the final manuscript.
Acknowledgements
The Authors would like to thank Mr. Chang-Wei Li (AllBio Science, Inc., Taichung, Taiwan) for his excellent technical support. The Authors would also like to acknowledge the work of Mr. Kai-Hsiang Chang and Mr. Chin-Chen Lin (Tekon Scientific Corporation, Taipei, Taiwan) for their assistance and equipment support on this study. We also thank the Office of Research and Development, China Medical University (Taiwan, ROC) for providing Medical Research Core Facilities to perform the experiments and data analysis.
References
- 1.Eassa HA, Eltokhy MA, Fayyaz HA, Khalifa MKA, Shawky S, Helal NA, Eassa HA, Youssef SF, Latz IK, Nounou MI. Current topical strategies for skin-aging and inflammaging treatment: science versus fiction. J Cosmet Sci. 2020;71(5):321–350. [PubMed] [Google Scholar]
- 2.Yap WN. Tocotrienol-rich fraction attenuates UV-induced inflammaging: A bench to bedside study. J Cosmet Dermatol. 2018;17(3):555–565. doi: 10.1111/jocd.12421. [DOI] [PubMed] [Google Scholar]
- 3.Hollis DE, Scheibner A. Ultrastructural changes in epidermal Langerhans cells and melanocytes in response to ultraviolet irradiation, in Australians of Aboriginal and Celtic descent. Br J Dermatol. 1988;119(1):21–31. doi: 10.1111/j.1365-2133.1988.tb07097.x. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Poli A, Di Cosmo A, d’Ischia M. N-Methyl-D-aspartate receptor stimulation activates tyrosinase and promotes melanin synthesis in the ink gland of the cuttlefish Sepia officinalis through the nitric Oxide/cGMP signal transduction pathway. A novel possible role for glutamate as physiologic activator of melanogenesis. J Biol Chem. 2000;275(22):16885–16890. doi: 10.1074/jbc.M909509199. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Lee MS. Biochemistry of melanin synthesis: in vivo effects of MSH on tyrosinase and melanogenesis of pigmentary system. Yale J Biol Med. 1973;46(5):493–499. [PMC free article] [PubMed] [Google Scholar]
- 6.Makino ET, Mehta RC, Banga A, Jain P, Sigler ML, Sonti S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol. 2013;12(3):s16–s20. [PubMed] [Google Scholar]
- 7.Fu C, Chen J, Lu J, Yi L, Tong X, Kang L, Pei S, Ouyang Y, Jiang L, Ding Y, Zhao X, Li S, Yang Y, Huang J, Zeng Q. Roles of inflammation factors in melanogenesis (Review) Mol Med Rep. 2020;21(3):1421–1430. doi: 10.3892/mmr.2020.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prunieras M. Melanocytes, melanogenesis, and inflammation. Int J Dermatol. 1986;25(10):624–628. doi: 10.1111/j.1365-4362.1986.tb04521.x. [DOI] [PubMed] [Google Scholar]
- 9.Dobos G, Trojahn C, D’Alessandro B, Patwardhan S, Canfield D, Blume-Peytavi U, Kottner J. Effects of intrinsic aging and photodamage on skin dyspigmentation: an explorative study. J Biomed Opt. 2016;21(6):66016. doi: 10.1117/1.JBO.21.6.066016. [DOI] [PubMed] [Google Scholar]
- 10.Lee AY. Skin pigmentation abnormalities and their possible relationship with skin aging. Int J Mol Sci. 2021;22(7):3727. doi: 10.3390/ijms22073727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz W. [Abnormalities of pigmentation and skin appendices] Dermatologica. 1952;104(6):449–452. [PubMed] [Google Scholar]
- 12.Dórea JG. Additional comments to “Potential health consequences of applying mercury-containing skin-lightening creams during pregnancy and lactation periods”. Int J Hyg Environ Health. 2016;219(8):920–921. doi: 10.1016/j.ijheh.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Al-Saleh I. Potential health consequences of applying mercury-containing skin-lightening creams during pregnancy and lactation periods. Int J Hyg Environ Health. 2016;219(4-5):468–474. doi: 10.1016/j.ijheh.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Pollock S, Taylor S, Oyerinde O, Nurmohamed S, Dlova N, Sarkar R, Galadari H, Manela-Azulay M, Chung HS, Handog E, Kourosh AS. The dark side of skin lightening: An international collaboration and review of a public health issue affecting dermatology. Int J Womens Dermatol. 2020;7(2):158–164. doi: 10.1016/j.ijwd.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draelos ZD, Deliencourt-Godefroy G, Lopes L. An effective hydroquinone alternative for topical skin lightening. J Cosmet Dermatol. 2020;19(12):3258–3261. doi: 10.1111/jocd.13771. [DOI] [PubMed] [Google Scholar]
- 16.Tai Y, Wang C, Wang Z, Liang Y, Du J, He D, Fan X, Jordt SE, Liu B. Involvement of Transient Receptor Potential Cation Channel Member A1 activation in the irritation and pain response elicited by skin-lightening reagent hydroquinone. Sci Rep. 2017;7(1):7532. doi: 10.1038/s41598-017-07651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijayasinghe YS, Bhansali P, Viola RE, Kamal MA, Poddar NK. Natural products: a rich source of antiviral drug lead candidates for the management of COVID-19. Curr Pharm Des. 2021;27(33):3526–3550. doi: 10.2174/1381612826666201118111151. [DOI] [PubMed] [Google Scholar]
- 18.Ansari N, Khodagholi F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: molecular mechanism aspect. Curr Neuropharmacol. 2013;11(4):414–429. doi: 10.2174/1570159X11311040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YH, Lee HJ, Lee CJ, Park JS. Natural products as sources of novel drug candidates for the pharmacological management of osteoarthritis: a narrative review. Biomol Ther (Seoul) 2019;27(6):503–513. doi: 10.4062/biomolther.2019.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, Vuorela H. Natural products in the process of finding new drug candidates. Curr Med Chem. 2004;11(11):1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- 21.Kolodziej H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo. Phytomedicine. 2007;14 Suppl 6:9–17. doi: 10.1016/j.phymed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Chen A, Ge X, Li S, Zhang T, Xu H. Chain conformation and physicochemical properties of polysaccharide (glucuronoxylomannan) from Fruit Bodies of Tremella fuciformis. Carbohydr Polym. 2020;245:116354. doi: 10.1016/j.carbpol.2020.116354. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Ma F, Li R, Ren G, Yan D, Zhang H, Zhu X, Wu R, Wu J. Degradation of Tremella fuciformis polysaccharide by a combined ultrasound and hydrogen peroxide treatment: Process parameters, structural characteristics, and antioxidant activities. Int J Biol Macromol. 2020;160:979–990. doi: 10.1016/j.ijbiomac.2020.05.216. [DOI] [PubMed] [Google Scholar]
- 24.Fan XZ, Yao F, Yin CM, Shi DF, Gao H. Optimization of fermentation process and its impact on gene transcription of intracellular polysaccharide synthesis in the wood ear medicinal mushroom Auricularia auricula-judae (Agaricomycetes) Int J Med Mushrooms. 2020;22(6):581–592. doi: 10.1615/IntJMedMushrooms.2020035033. [DOI] [PubMed] [Google Scholar]
- 25.Liang CH, Wu CY, Lu PL, Kuo YC, Liang ZC. Biological efficiency and nutritional value of the culinary-medicinal mushroom Auricularia cultivated on a sawdust basal substrate supplement with different proportions of grass plants. Saudi J Biol Sci. 2019;26(2):263–269. doi: 10.1016/j.sjbs.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Li X, Yang Q, Zhang C, Song X, Wang W, Jia L, Zhang J. Antioxidation, anti-hyperlipidaemia and hepatoprotection of polysaccharides from Auricularia auricular residue. Chem Biol Interact. 2021;333:109323. doi: 10.1016/j.cbi.2020.109323. [DOI] [PubMed] [Google Scholar]
- 27.Miao J, Regenstein JM, Qiu J, Zhang J, Zhang X, Li H, Zhang H, Wang Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int J Biol Macromol. 2020;150:102–113. doi: 10.1016/j.ijbiomac.2020.02.054. [DOI] [PubMed] [Google Scholar]
- 28.Wu YJ, Wei ZX, Zhang FM, Linhardt RJ, Sun PL, Zhang AQ. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int J Biol Macromol. 2019;121:1005–1010. doi: 10.1016/j.ijbiomac.2018.10.117. [DOI] [PubMed] [Google Scholar]
- 29.Lin CP, Tsai SY. Differences in the moisture capacity and thermal stability of Tremella fuciformis polysaccharides obtained by various drying processes. Molecules. 2019;24(15):2856. doi: 10.3390/molecules24152856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang JH, Tsai FJ, Hsu YM, Yin MC, Chiu HY, Yang JS. Sensitivity of allyl isothiocyanate to induce apoptosis via ER stress and the mitochondrial pathway upon ROS production in colorectal adenocarcinoma cells. Oncol Rep. 2020;44(4):1415–1424. doi: 10.3892/or.2020.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Chen J, Quan J, Xiang D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered. 2021;12(1):3065–3076. doi: 10.1080/21655979.2021.1941699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu SP, Shibu MA, Tsai FJ, Hsu YM, Tsai CH, Chung JG, Yang JS, Tang CH, Wang S, Li Q, Huang CY. Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1α induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr Metab (Lond) 2020;17:12. doi: 10.1186/s12986-020-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu CC, Chiang JH, Tsai FJ, Hsu YM, Juan YN, Yang JS, Chiu HY. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int J Oncol. 2019;54(4):1271–1281. doi: 10.3892/ijo.2019.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horng CT, Yang JS, Chiang JH, Lu CC, Lee CF, Chiang NN, Chen FA. Inhibitory effects of tetrandrine on epidermal growth factor-induced invasion and migration in HT29 human colorectal adenocarcinoma cells. Mol Med Rep. 2016;13(1):1003–1009. doi: 10.3892/mmr.2015.4635. [DOI] [PubMed] [Google Scholar]
- 35.Lee HP, Wang SW, Wu YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, Li TM, Tang CH. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric Immunol. 2019;30(1):1033–1045. doi: 10.1080/09540105.2019.1660623. [DOI] [Google Scholar]
- 36.Lu CC, Yang JS, Chiu YJ, Tsai FJ, Hsu YM, Yin MC, Juan YN, Ho TJ, Chen HP. Dracorhodin perchlorate enhances wound healing via β-catenin, ERK/p38, and AKT signaling in human HaCaT keratinocytes. Exp Ther Med. 2021;22(2):822. doi: 10.3892/etm.2021.10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CC, Chen KB, Tsai CH, Tsai FJ, Huang CY, Tang CH, Yang JS, Hsu YM, Peng SF, Chung JG. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-ĸB signaling pathways. J Food Biochem. 2019;43(7):e12902. doi: 10.1111/jfbc.12902. [DOI] [PubMed] [Google Scholar]
- 38.Lee TH, Lee MS. Studies on MSH-induced melanogenesis: effect of long-term administration of MSH on the melanin content and tyrosinase activity. Endocrinology. 1971;88(1):155–164. doi: 10.1210/endo-88-1-155. [DOI] [PubMed] [Google Scholar]
- 39.Wilczek A, Mishima Y. Inhibitory effects of melanin monomers, dihydroxyindole-2-carboxylic acid (DHICA) and dihydroxyindole (DHI) on mammalian tyrosinase, with a special reference to the role of DHICA/DHI ratio in melanogenesis. Pigment Cell Res. 1995;8(2):105–112. doi: 10.1111/j.1600-0749.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 40.Hollinger JC, Angra K, Halder RM. Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J Clin Aesthet Dermatol. 2018;11(2):28–37. [PMC free article] [PubMed] [Google Scholar]
- 41.Hermann H. [Therapy of syphilis and certain skin diseases as reflected in the writings of Francisco Hernandez and Francisco Ximenez; a contribution on the history of dermatology in the 16th and 17th centuries] Medizinische. 1956;(14):527–530. [PubMed] [Google Scholar]
- 42.Goldschmidt H, Raymond JZ. Quantitative analysis of skin color from melanin content of superficial skin cells. J Forensic Sci. 1972;17(1):124–131. [PubMed] [Google Scholar]
- 43.Magnin PH, Rothman S. Inhibition of melanin formation by human epidermis. Dermatologica. 1957;115(3):315–320. doi: 10.1159/000256019. [DOI] [PubMed] [Google Scholar]
- 44.Staricco RJ. Qualitative and quantitative data on melanocytes in human epidermis treated with thorium X. J Invest Dermatol. 1957;29(3):185–195. doi: 10.1038/jid.1957.86. [DOI] [PubMed] [Google Scholar]
- 45.Staricco RJ, Pinkus H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol. 1957;28(1):33–45. doi: 10.1038/jid.1957.4. [DOI] [PubMed] [Google Scholar]
- 46.Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J Invest Dermatol. 1993;100(6):806–811. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Moellmann G, Kuklinska E. L-tyrosine, L-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in Bomirski Ab amelanotic melanoma cells. Pigment Cell Res. 1989;2(2):109–116. doi: 10.1111/j.1600-0749.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 48.Prezioso JA, Damodaran KM, Wang N, Bloomer WD. Mechanism(s) regulating inhibition of thymidylate synthase and growth by gamma-L-glutaminyl-4-hydroxy-3-iodobenzene, a novel melanin precursor, in melanogenic melanoma cells. Biochem Pharmacol. 1993;45(2):473–481. doi: 10.1016/0006-2952(93)90085-b. [DOI] [PubMed] [Google Scholar]
- 49.Słominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J Cell Sci. 1988;89 (Pt 3):287–296. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- 50.Cao J, Tyburczy ME, Moss J, Darling TN, Widlund HR, Kwiatkowski DJ. Tuberous sclerosis complex inactivation disrupts melanogenesis via mTORC1 activation. J Clin Invest. 2017;127(1):349–364. doi: 10.1172/JCI84262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaspar K, Kukova G, Bunemann E, Buhren BA, Sonkoly E, Szollosi AG, Muller A, Savinko T, Lauerma AI, Alenius H, Kemeny L, Dieu-Nosjean MC, Stander S, Fischer JW, Ruzicka T, Zlotnik A, Szegedi A, Homey B. The chemokine receptor CCR3 participates in tissue remodeling during atopic skin inflammation. J Dermatol Sci. 2013;71(1):12–21. doi: 10.1016/j.jdermsci.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Ivanov KP. [The conference on hematopoiesis and the physiological functions of the body’s immune system] Fiziol Zh Im I M Sechenova. 1995;81(9):154–156. [PubMed] [Google Scholar]
- 53.Shi LB, Zhang HW, Cui YY. [Implication of different expression of IL-2 mRNA and IL-10 mRNA in CD4(+)CD25(+)T cell induced immune tolerance of liver transplantation in rat] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20(5):257–260. [PubMed] [Google Scholar]
- 54.Guardiola FA, Bahi A, Jiménez-Monreal AM, Martínez-Tomé M, Murcia MA, Esteban MA. Dietary administration effects of fenugreek seeds on skin mucosal antioxidant and immunity status of gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2018;75:357–364. doi: 10.1016/j.fsi.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 55.Giri SS, Sukumaran V, Park SC. Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2019;92:612–620. doi: 10.1016/j.fsi.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 56.Shen T, Duan C, Chen B, Li M, Ruan Y, Xu D, Shi D, Yu D, Li J, Wang C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol Med Rep. 2017;16(2):1340–1346. doi: 10.3892/mmr.2017.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem. 1997;378(11):1247–1257. [PubMed] [Google Scholar]
- 58.Parkinson EK. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br J Cancer. 1985;52(4):479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najafi A, Adutwum E, Yari A, Salehi E, Mikaeili S, Dashtestani F, Abolhassani F, Rashki L, Shiasi S, Asadi E. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm. Cell Tissue Res. 2018;372(1):149–159. doi: 10.1007/s00441-017-2743-4. [DOI] [PubMed] [Google Scholar]
- 60.Cooke JV, Goldring D, Kahn LI. The occurrence of changes resembling the inflammatory in skin injured and incubated after excision. J Exp Med. 1953;97(5):651–662. doi: 10.1084/jem.97.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]