Abstract
Background/Aim: Lung cancer notably contributes to tumor-associated mortality worldwide, and standard chemotherapy is used for lung cancer patients. However, its therapeutic efficacy remains unsatisfactory. This study aimed to evaluate the effects and molecular mechanisms of sorafenib and bufalin combination therapy on lung cancer cells in vitro.
Materials and Methods: NCI-H292 cells were treated with sorafenib, bufalin, and sorafenib in combination with bufalin. Cell viability, ROS production, Ca2+ release, and mitochondrial membrane potential were examined by flow cytometric assay. Annexin V/PI staining and chromatin condensation were examined by the apoptosis assays. Finally the molecular mechanism of apoptosis-associated protein expression was investigated by western blotting.
Results: NCI-H292 cells treated with sorafenib in combination with bufalin showed significantly decreased viability, enhanced cellular apoptosis, and DNA condensation when compared to that with sorafenib or bufalin alone. Moreover, the combination treatment exhibited higher reactive oxygen species (ROS) production and lower mitochondrial membrane potential (ΔΨm). The combined treatment resulted in higher expression of SOD but lower catalase compared to sorafenib treatment alone. Compared to sorafenib or bufalin treatment alone, the combination treatment resulted in lower Bcl-2 expression but higher Bax, Bad, APAF-1, caspase-3, and caspase-9.
Conclusion: Sorafenib in combination with bufalin shows more potent cytotoxic effects and cell apoptosis than sorafenib or bufalin treatment alone in NCI-H292 cells. The combined treatment significantly enhanced apoptotic cell death in NCI-H292 lung cancer cells by activating ROS-, mitochondria-, and caspase-signaling pathways in vitro.
Keywords: Sorafenib, bufalin, combination therapy, lung cancer, apoptosis
Cancer affects human health globally with its incidence being the most severe public issue of the 21st century. The global number of lung cancer deaths remains high yearly (1). Lung cancers are mainly divided into two subtypes, including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). However, the NSCLC subtype occupies about 80-85% of lung cancers (2). Currently, patients undergo surgery, radiation, chemotherapy, and targeted therapy as the most commonly used strategies for lung cancer. Yet clinical outcomes of current therapies remain unsatisfactory: the 5-year survival rate of lung cancer patients is less than 15% (3), and that of patients with metastatic disease is less than 10% (4). Thus, identifying novel anti-cancer drugs to minimize the side-effects or improving the effectiveness of treatments is considered a matter of urgent importance (5).
Sorafenib, a multi-kinase inhibitor for treating advanced liver cancer, is generally well-tolerated with promising efficacy in various malignancies, including lung cancer. Furthermore, it has been shown to be functional against various tumors in preclinical models (6) and has also been presented to exert anti-cancer activity in patients with relapsed NSCLC (7). In particular, ARAF mutation is an oncogenic factor that drives lung adenocarcinoma and is also used as an indicator for sorafenib response (8). In vitro studies have shown that sorafenib inhibited epithelial-to-mesenchymal transition (EMT) mediated by transforming growth factor-β1 in A549 human lung cancer cells (9). However, the development of drug resistance after sorafenib treatment resulted in limited survival benefit with meager response rates (10). Many studies have reported the antitumor effect of sorafenib in preclinical NSCLC models (11,12). Nevertheless, sorafenib treatment is also accompanied by side-effects such as adverse hand-foot skin diseases, hypertension, and diarrhea (13), which result in implementation of low doses, thereby affecting efficacy.
Recently, natural products and their derivatives have shown striking potential for modern drug discovery and development (14). Bufalin, a class of steroids, is purified from the parotid glands and skin of a Chinese toad popularly known as Chansu (Bufo gargarizans) and is used in Chinese traditional medicine (15,16). Bufalin showed antitumor activities in many human cancer cells such as leukemia cells (17), T24 bladder cancer cells (18), MGC803 gastric cancer cells (19), DU145 and PC3 prostate cancer cells (20), glioma cancer cells (21), breast cancer (22), and liver cancer (23). Our previous findings have shown that bufalin caused apoptotic cell death in human nasopharyngeal carcinoma cells (24) and against lung cancer xenograft animals in vivo (25). Furthermore, we also found that bufalin promoted phagocytosis of macrophages in peripheral blood and the peritoneal cavity in leukemia mice in vivo (26).
Due to the adverse side-effects of sorafenib treatment, new therapeutic strategies have focused on combining sorafenib with natural drugs for improving the critical defects of sorafenib treatment. Therefore, the present study investigated the effects of sorafenib combined with bufalin on inducing cell apoptosis in NCI-H292 human lung cancer cells. We found that combination treatment demonstrates a synergistic effect on inducing cell death and causing cell apoptosis via intrinsic pathways in NCI-H292 cells.
Material and Methods
Chemicals and reagents. Sorafenib, bufalin, 4,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI), trypsin-EDTA, the reagents for the detection of ROS, Ca2+, mitochondrial membrane potential (ΔΨm), and RIPA buffer were brought from Sigma-Aldrich Corp. (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine serum (FBS), penicillin, streptomycin, and L-glutamine were brought from Invitrogen Life Technologies (Carlsbad, CA, USA). PVDF membranes were brought from Merck Millipore (Billerica, MA, USA). Primary antibodies against ROS stress- and apoptosis-associated proteins were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and Cell Signaling Technology (Irvine, CA, USA). Secondary antibodies (goat anti-rabbit or mouse IgG) were received from Amersham Pharmacia Biotech, Inc (Piscataway, NJ, USA).
Cell culture. The NCI-H292 human lung cancer cells (non-small cell lung cancer cells) were brought from Food Industry Research and Development Institute (Hsinchu, Taiwan, ROC). Cells were maintained in RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, and 1% antibiotics (100 μg/ml streptomycin and 100 units/ml penicillin) under a humidified atmosphere of 5% CO2 at 37˚C according to the guidelines from the provider (27).
Cell viability and morphology. NCI-H292 cells at a density of 1×105 cells per well were seeded in 12-well plates overnight. Cells were subjected to sorafenib (0, 10, 15, and 20 μM), bufalin (0, 60, 90, and 120 nM), or sorafenib (0, 10, 15, and 20 μM) combined with bufalin (0, 60, 90, and 120 nM) for 48 h. Before the cells being processed, the cell morphology was observed and imaged under a phase-contrast microscope.
After treatment, NCI-H292 cells were individually collected for cell viability detection. The cell pellets were resuspended in PI solution (5 μg/ml) and measured by flow cytometry. In addition, the effects of combination treatment on cell viability were evaluated for calculating the coefficient of drug interaction (CDI) value as cited previously (28).
Determination of cell apoptotic cell death. NCI-H292 (1×105 cells per well) were added in 12-well plates. Following indicated drug treatments (15 μM sorafenib, 90 nM bufalin, or the combination of 15 μM sorafenib with 90 nM bufalin) for 48 h, cells were isolated and harvested. Cells were determined the apoptotic cell death by staining with FITC-conjugated Annexin V/PI solution for 15 min in the dark. After incubation, cells were measured the apoptotic cell number by flow cytometric assays described previously (27).
Chromatin condensation assay. NCI-H292 cells were seeded in 12-well plates at a density of 1×105 cells/well and incubated with 15 μM sorafenib, 90 nM bufalin, or a combination of 15 μM sorafenib with 90 nM bufalin for 48 h. After treatment, cells in each well were fixed with a freshly prepared 4% paraformaldehyde (in PBS) for 15 min and permeabilized their cell membranes by 0.1% Triton X-100 for 5 min. DAPI solution (2 μg/ml) was then incubated with the cells for 10 min, and the nuclear morphology of the cells was observed and photographed with a fluorescence microscope at 100× as described previously (27).
Determination of reactive oxygen species and intracellular Ca2+ production and mitochondrial membrane potential (ΔΨm) levels. NCI-H292 cells (1×105 cells/well) were added in 12-well plates and treated with 15 μM sorafenib, 90 nM bufalin, or the combination of 15 μM sorafenib with 90 nM bufalin for 48 h. After treatment, cells were collected and resuspended in 500 μl of DCFH-DA (10 μM) for evaluating the changes in the levels of reactive oxygen species (ROS; H2O2 content). For determining intracellular Ca2+ levels or analyzing the changes of ΔΨm, cells were harvested and resuspended in 500 μl of Fluo-3/AM (2.5 μg/ml) or DiOC6 (4 μmol/ml), respectively, and measured by flow cytometry as cited previously (27).
Western blotting analysis. NCI-H292 (1×106 cells) cells were seeded in 10-cm dishes and incubated with 15 μM sorafenib, 90 nM bufalin, or the combination treatment (15 μM sorafenib and 90 nM bufalin) for 48 h. After exposure to different treatments, cells were gathered, and cell lysates were obtained by adding ice-cold RIPA buffer containing protease inhibitor (SigmaFAST Protease Inhibitor Cocktail; Sigma-Aldrich). The Bradford reagent (Bio-Rad protein assay kit; Hercules, CA, USA) was used to calculate the protein concentrations (27). A certain amount of total protein (30 μg) was electrophoresed on 8-12% of SDS-polyacrylamide gel and subsequently transferred onto PVDF membranes. The resultant membrane was blocked with 2% BSA for 1 h at room temperature. Subsequently, the membranes were incubated with specific primary antibodies at 4˚C overnight. After treatment with corresponding horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature, protein bands were visualized using the Enhanced Chemiluminescence kit (NEN Life Science Products, Inc, Boston, MA, USA) as described previously (27). The intensity of protein bands was determined and quantified using NIH-ImageJ version 1.47.
Statistical analysis. All experiments were performed three times, and data were presented as the mean±SD. One-way ANOVA analysis was analyzed to compare two experimental groups. For all tests, a p-value<0.05 was considered to be statistically significant.
Results
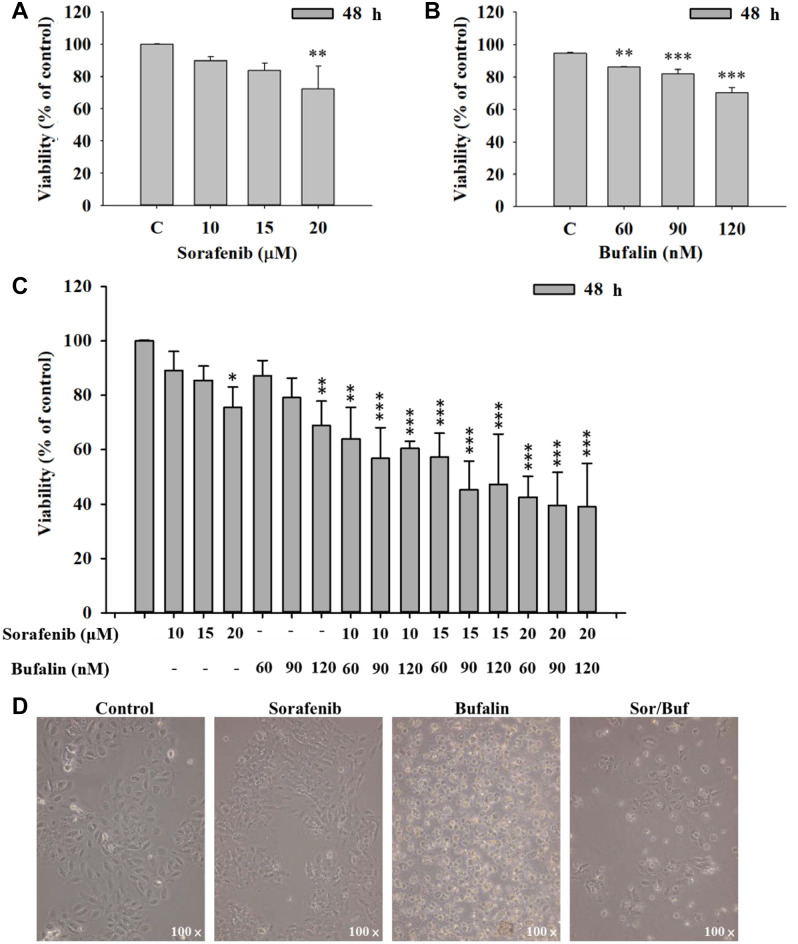
Sorafenib, bufalin, or sorafenib in combination with bufalin affected cell viability and morphology of NCI-H292 cells. NCI-H292 cells were incubated with different concentrations of sorafenib (0, 10, 15, and 20 μM) or bufalin (0, 60, 90, and 120 nM) for 48 h. For cell viability, cells were collected and determined the PI exclusion by flow cytometry. Results revealed that treatment with 20 μM sorafenib or 60-120 nM bufalin significantly decreased the total viable cell number by 33%, or around 12-24%, respectively (Figure 1A and B).
Figure 1. The effects of sorafenib, bufalin, or the combination of sorafenib with bufalin on cell viability and morphology in NCI-H292 cells. Cells (1×105 cells/well) were treated with sorafenib (0, 10, 15, and 20 μM) (A), or bufalin (0, 60, 90, and 120 nM) (B) for 48 h, cells were harvested and the cell viability was measured by flow cytometry. Cells were treated with the combination of sorafenib (10-20 μM) and bufalin (60-120 nM) for 48 h, and total cell viability was measured (C). The cell morphology after cells were treated with 15 μM sorafenib, 90 nM bufalin, and the combination of 15 μM sorafenib and 90 nM bufalin, was observed and photographed using contrast-phase microscopy. Results aere displayed as mean±SD. *p<0.05, **p<0.01, or ***p<0.001, significant difference when compared to controls.
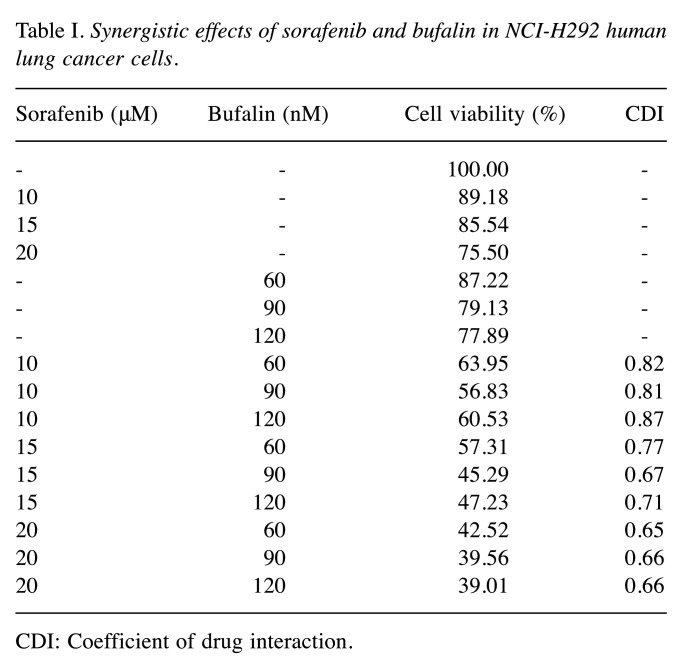
To further determine the effects of combination treatment on cell viability, the cells were incubated with the combination of sorafenib (10-20 μM) and bufalin (60-120 nM) for 48 h, and cell viability was examined. Results revealed that the treatment of sorafenib in combination with bufalin significantly decreased the viability of NCI-H292 cells compared to that of sorafenib or bufalin treatment alone (Figure 1C). Furthermore, after calculating the coefficient of drug interaction (CDI) value, optimal effects on cell viability were displayed in the combination of sorafenib at 15 μM and bufalin at 90 nM due to the lower concentration of sorafenib and optimal CDI value of 0.67 (Table I). Thus, the drug combination at 15 μM sorafenib and 90 nM bufalin was applied for further experiments.
Table I. Synergistic effects of sorafenib and bufalin in NCI-H292 human lung cancer cells.
CDI: Coefficient of drug interaction.
After treatment, cell morphology was examined and snaped under phase-contrast microscopy. Results revealed that cells subjected to the two-drug combination (15 μM of sorafenib and 90 nM of bufalin) exhibited more significant morphological changes than those treated with sorafenib or bufalin alone (Figure 1D). These observations were also consistent with the results of the total cell viability.
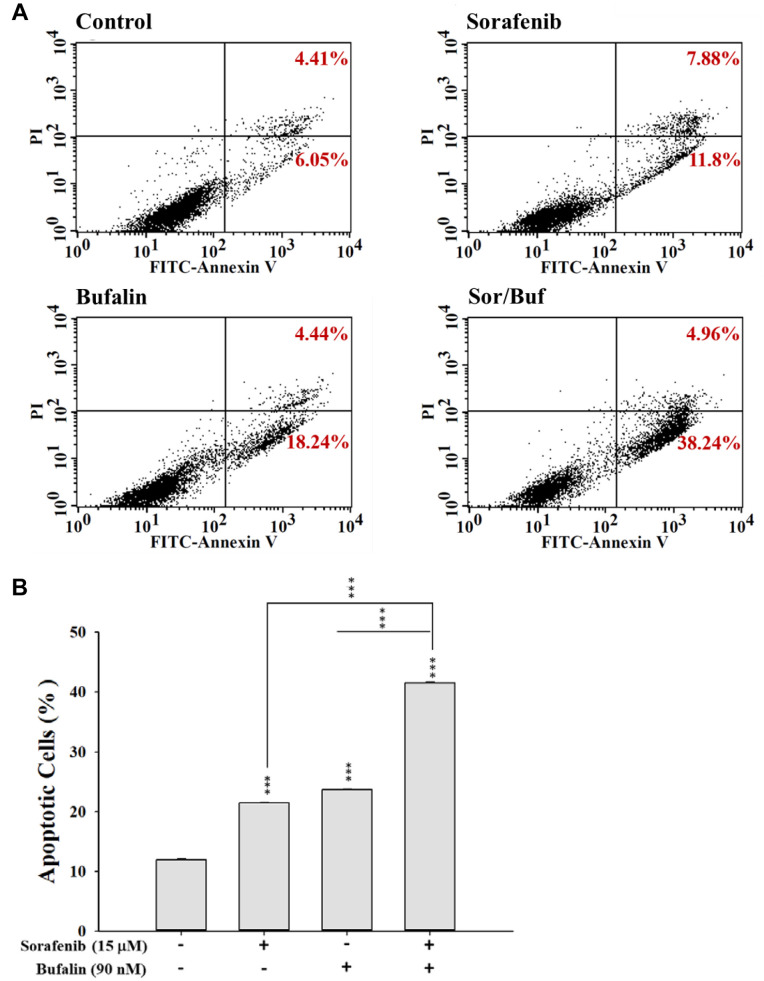
Sorafenib, bufalin, or sorafenib in combination with bufalin affected the apoptosis in NCI-H292 cells. For measuring the apoptotic effects of sorafenib, bufalin, or the combination of sorafenib with bufalin on NCI-H292 cells, cells were stained with Annexin V and PI and assayed by flow cytometry. Firstly, cells were incubated with 15 μM sorafenib, 90 nM bufalin, or 15 μM sorafenib in combination with 90 nM bufalin for 48 h. Subsequently, cells were probed with Annexin V and stained with the PI solution to measure cell death, including earlier or later apoptotic cell death, using flow cytometric assay. Results revealed that treatment of NCI-H292 cells with the two-drug combination for 48 h led to a higher percentage of apoptotic cells than that of sorafenib or bufalin treatment alone (Figure 2A and B). These observations indicated that the two-drug combination showed an enhanced effect on NCI-H292 cells, consistent with the results of cell viability assays.
Figure 2. Sorafenib, bufalin, or their combination treatment affected cell apoptosis in NCI-H292 cells. Cells (1×105 cells/well) were subjected to 15 μM sorafenib, 90 nM bufalin, or combination of 15 μM sorafenib with 90 nM bufalin for 48 h. Cells were treated with Annexin V/PI solution to determine the percentage of apoptosis, including earlier and late apoptotic cell death, by flow cytometric assays (A). The percentage of cell apoptosis was calculated (B). Data represent mean±SD. *p<0.05, **p<0.01, or ***p<0.001, a statistically significant difference between the two groups.
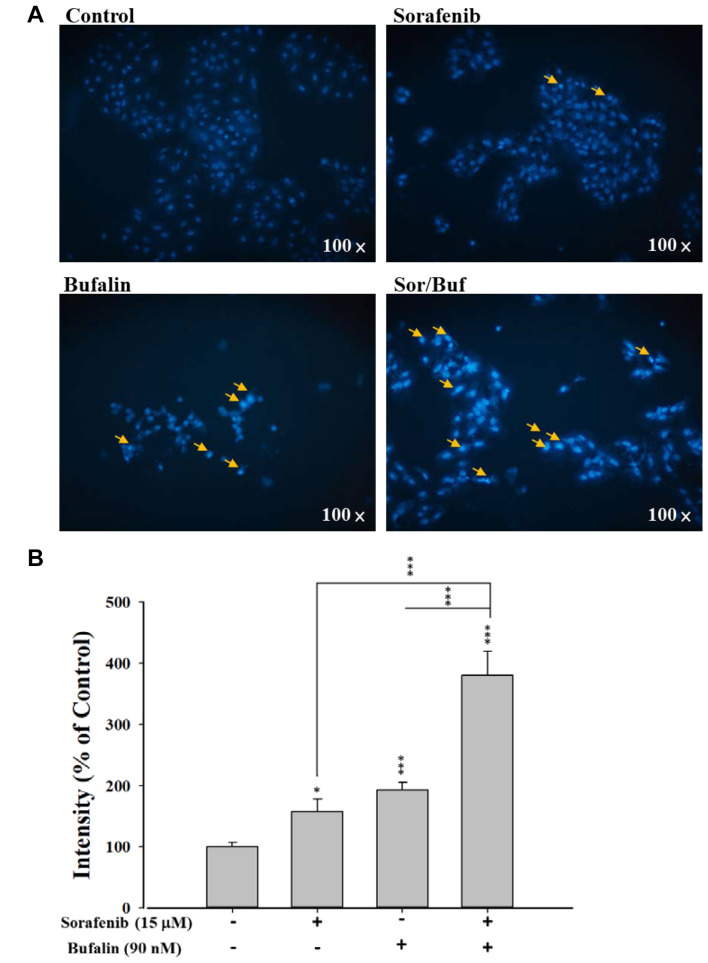
Sorafenib, bufalin, or sorafenib in combination with bufalin affected DNA condensation in NCI-H292 cells. In order to assess the effect of individual and combination drug treatment on the nuclear morphology, DAPI staining was performed. NCI-H292 cells were incubated with 15 μM sorafenib, 90 nM bufalin, or drug combination for 48 h. Then cells were stained with DAPI solution, examined, and photographed (Figure 3A and B). Compared to single-drug treatment (sorafenib or bufalin alone), the two-drug combination treatment results in significant DNA condensation in NCI-H292 cells, as demonstrated by higher fluorescence intensity in the nucleus of the cells (Figure 3A). Calculation of fluorescence intensity further clearly revealed that the two-drug combination treatment presented higher DNA condensation in NCI-H292 cells than sorafenib or bufalin treatment alone (Figure 3B).
Figure 3. Sorafenib, bufalin, or sorafenib combined with bufalin affected DNA condensation in NCI-H292 cells. Cells (1×105 cells/well) were subjected to 15 μM sorafenib, 90 nM bufalin, or the combination of 15 μM sorafenib with 90 nM bufalin for 48 h. After treatment, cells were stained with DAPI solution and then observed and photographed under a fluorescence microscope at 100× (A). The relative intensity of DAPI fluorescence in the nucleus was calculated (B). Data represent mean±SD. *p<0.05, **p<0.01, or ***p<0.001, a statistically significant difference between the two groups.
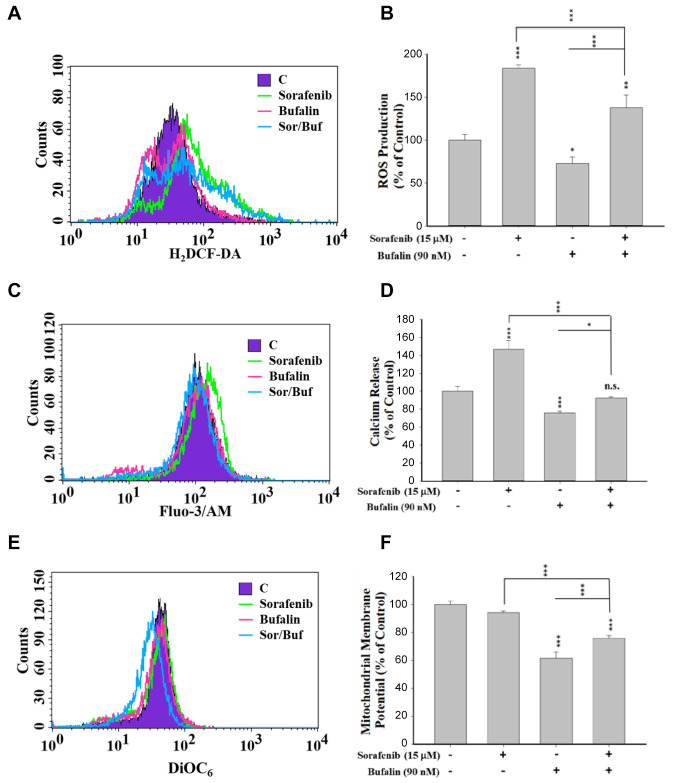
Sorafenib, bufalin, or sorafenib in combination with bufalin affected the production of reactive oxygen species, Ca2+ release, and the levels of mitochondrial membrane potential in NCI-H292 cells. Oxidative and ER stresses and mitochondria membrane potential (MMP; ΔΨm) are involved in various cell apoptosis aspects. For determining the specific mechanism underlying cellular apoptosis, we monitored the changes of ROS, Ca2+, and MMP. NCI-H292 cells were treated with 15 μM sorafenib, 90 nM bufalin, or their combination for 48 h. Then their ROS production, Ca2+ release, and the changes of ΔΨm levels were examined by flow cytometric assays (Figure 4). Data revealed that the combination of sorafenib and bufalin treatment resulted in a higher ROS production (Figure 4A and B), Ca2+ release (Figure 4C and D), and ΔΨm (Figure 4E and F) compared to treatment of bufalin alone at 48 h. Nevertheless, sorafenib and bufalin combination treatment caused a lower level of ROS production (Figure 4A and B), Ca2+ release (Figure 4C and D), and ΔΨm (Figure 4E and F) compared to treatment of sorafenib alone at 48 h. However, combination treatment increased ROS production but decreased the ΔΨm, and did not alter the Ca2+ release at 48 h.
Figure 4. The effects of sorafenib, bufalin, or the combination of sorafenib with bufalin on the production of reactive oxygen species (ROS) and Ca2+ and the levels of mitochondrial membrane potential (ΔΨm) in NCI-H292 cells. Cells (1×105 cells/well) were treated 15 μM sorafenib, 90 nM bufalin, or the combination of 15 μM sorafenib with 90 nM bufalin for 48 h. Then cells were collected and resuspended in DCFH-DA solution for measuring the changes of ROS (H2O2) (A and B), in Fluo-3/AM solution for measuring intracellular Ca2+ level (C and D), and in DiOC6 reagent for measuring ΔΨm levels (E and F) by flow cytometry. Data represent mean±SD. *p<0.05, **p<0.01, or ***p<0.001, a significant difference when compared to sorafenib- or bufalin-treated alone. n.s., No significant difference when compared to sorafenib- or bufalin-treated alone (p>0.05).
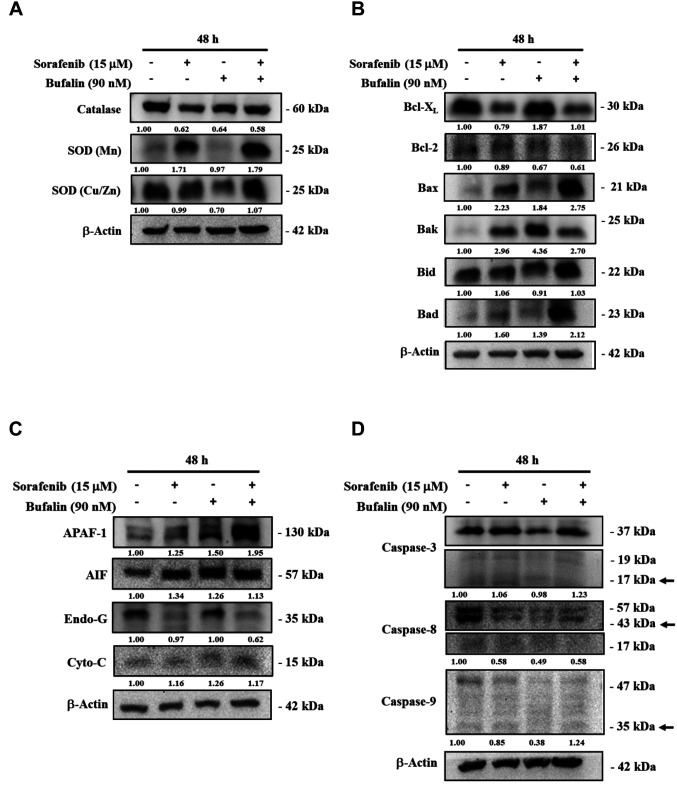
Sorafenib, bufalin, or a combination of sorafenib and bufalin affected the expression of ROS- or apoptosis-associated proteins in NCI-H292 cells. In order to further substantiate the findings of cell viability and apoptosis assays, we investigated the ROS- or apoptosis-associated protein expression in NCI-H292 cells after treating cells with sorafenib, bufalin, or their combination. Immunoblotting studies from cell lysates after various treatments showed that the two-drug combination treatment significantly elevated the SOD expression, including SOD (Mn) and SOD (Cu/Zn), but reduced the level of catalase compared to single-drug treatment alone (Figure 5A). The combined drug treatment showed diminished expression of Bcl-2 (anti-apoptotic protein); whereas, the same treatment elevated the expression of pro-apoptotic proteins including Bax, Bak, and Bad compared to sorafenib or bufalin treatment alone (Figure 5B). The combination treatment also significantly elevated the expression of APAF-1 compared to treatment with sorafenib or bufalin alone (Figure 5C). Moreover, the two-drug combination treatment also elevated the expression of the active form of caspase-3 and -9 in NCI-H292 cells compared to sorafenib or bufalin treatment alone (Figure 5D).
Figure 5. The effects of sorafenib, bufalin, or the combination of sorafenib with bufalin on the ROS- or apoptosis-associated proteins in NCI-H292 cells. Cells (1×106 cells/well) were subjected to 15 μM sorafenib, 90 nM bufalin, or the combination of 15 μM sorafenib with 90 nM bufalin for 48 h. Cells were collected and lysed, and their total proteins were quantified and analyzed by western blotting. The blotting was probed with primary antibodies against anti-SOD (Mn), -SOD (Cu/Zn), and -catalase (A); -Bcl-XL, -Bcl-2, -Bax, -Bak, -Bid, and –Bad (B); -APAF-1, -AIF, -Endo G, and -Cytochrome C (C); -caspase-3, -caspase-8, and -caspase-8 (D). Then the membranes were washed, followed by incubation with responding secondary antibody (anti-mouse or anti-rabbit antibodies). The protein signals were detected by ECL and quantified by the ImageJ software.
Discussion
Lung cancer is the most prevalent cancer globally. Unfortunately, patients diagnosed at median or advanced stages of lung cancer lead to treatment failure and a low cure rate. Moreover, patients subjected to chemotherapy often develop drug resistance (2,29) and are accompanied by side-effects making their recovery further challenging. Hence, identifying natural compounds for treating lung cancer has garnered the attention of researchers (14). In the present study, we investigated the effects and molecular mechanism of a two-drug combination (sorafenib combined with bufalin) on apoptosis in human lung cancer cells (NCI-H292 cells) in vitro. The rationale underlying the selection of sorafenib for this study is that sorafenib is the standard first-line therapy for patients with advanced hepatocellular carcinoma (10). However, in phase III studies, the usage of sorafenib combined with other chemotherapy drugs showed the failure of a significant survival benefit in treating NSCLC patients (30). Besides, the precise molecular mechanisms of sorafenib remain unclear (31). Bufalin, a traditional medicine amongst the Chinese population, has been shown to inhibit cell proliferation and cause apoptotic cell death in various human cancer cell lines (19,32-35). However, the efficacy and molecular mechanisms of the two-drug combination (bufalin combined with sorafenib) on inducing apoptosis in NCI-H292 lung cancer cells have not been explored.
In the present study, sorafenib at 20 μM significantly decreased the cell viability of NCI-H292 cells by 28% (Figure 1A), while bufalin at 60-120 nM decreased the cell viability from 14 to 29%, respectively (Figure 1B). The combination of sorafenib and bufalin further significantly reduced the total number of viable cells compared to sorafenib or bufalin treatment alone (Figure 1C). The combination index (coefficient of drug interaction; CDI) values of both drug combinations affecting the total cell viability are presented in Table I. It shows that the combination of 15 μM sorafenib and 90 nM bufalin has the lower sorafenib concentration and acceptable CDI value of 0.67. Hence, this drug combination concentration was used for further experiments. Assessment of cellular morphology showed that treatment with the drug combination (15 μM sorafenib and 90 nM bufalin) causes more significant morphological changes than that with sorafenib or bufalin treatment alone (Figure 1D). These changes are also consistent with the results of total cell viability. Sorafenib has been previously used in combination with another clinical anti-cancer drug, betulinic acid, to treat NSCLC cells (36). Besides, a synergistic inhibitory effect of the sorafenib and gemcitabine combination treatment on NSCLC cells has been found in vitro and in vivo (37). Another study has shown that the combination therapy increases cytotoxic efficiency in lung cancer cells with EGFR-TKI-resistance (38). Based on the findings of this study, we offer novel information regarding the efficacy of sorafenib in combination with bufalin for lung cancer therapy.
In order to confirm the involvement of apoptosis and DNA condensation in mediating reduced cell viability in NCI-H292 cells upon treatment with sorafenib in combination with bufalin, two different assays, including Annexin V-FITC/PI and DAPI staining, were performed, respectively. The results indicated that combination treatment demonstrates higher apoptotic cell death (Figure 2A and B) and DNA condensation (Figure 3A and B) than that upon treatment with sorafenib or bufalin only for 48 h. These observations suggest that the two-drug combination of sorafenib and bufalin has a synergistic effect on induction of apoptotic cell death and reduction of cell viability in NCI-H292 cells. However, the conceivable signaling pathways involved in the two-drug combination treatment warrant further investigation.
To further confirm the involvement of ROS-mediated pathways in the combination treatment-induced cell death, we analyzed the ROS production in NCI-H292 cells. Our results suggest that the effect of sorafenib on ROS production is different from that of bufalin. Sorafenib increased the levels of ROS, but bufalin decreased it. The combination treatment leads to lower production of ROS compared to that with sorafenib treatment alone. In contrast, the ROS levels were found to be higher in cells subjected to the combined treatment as compared to treatment with bufalin alone (Figure 4A and B). ROS is well-known for its involvement in metabolic processes (39). Low-level ROS is essential for maintaining cellular signaling. However, it may play different roles, especially in pathophysiological conditions, including cancer cells (40). High-level production of ROS can trigger oxidative stress and result in cell component damage and apoptosis (40). Considerable evidence has shown that certain anti-cancer drugs induced- cell death is associated with high ROS production and Ca2+ release (41,42).
It is well-established that ROS production leads to mitochondrial dysregulation. Our data show that sorafenib revealed no effect on the permeability of mitochondria, but bufalin significantly decreased the level of mitochondrial membrane potential in NCI-H292 cells. The two-drug combination treatment of NCI-H292 cells results in not only a lower ROS production (Figure 4A and B) but also lowers mitochondrial membrane potential levels (ΔΨm) (Figure 4E and F) as compared to the treatment with sorafenib alone. However, the same treatment results in a higher ROS production and mitochondrial membrane potential than that upon bufalin treatment alone. Further investigation was performed for understanding the molecular mechanism underlying the higher apoptotic cell death upon the combination treatment of NCI-H292 cells than upon sorafenib or bufalin treatment only. Western blotting results revealed that combined treatment significantly triggered ROS responses, including elevated SOD (Mn) and SOD (Cu/Zn), but decreased catalase levels as compared to that upon sorafenib or bufalin treatment alone (Figure 5A). Overall, these observations offer a further detailed understanding of the molecular mechanism underlying higher apoptotic cell death upon treatment with the combination of sorafenib and bufalin as compared to that of sorafenib or bufalin treatment alone.
Furthermore, the capability of targeting mitochondria-related components indicates the therapeutic potential of a drug combination. Accordingly, the drugs that can inhibit the levels of Bcl-2 and Bcl-XL, and increase the release of AIF, cytochrome c, and APAF-1, ultimately trigger cells to undergo apoptosis. Our data show that the two-drug treatment significantly reduced the levels of ΔΨm as compared to sorafenib treatment alone (Figure 4E and F). The ΔΨm plays a critical role in the release of cytochrome c and apoptosome assembling (43,44). Furthermore, we have found that the two-drug combination exerts more efficient apoptosis-inducing effects through the mitochondrial-dependent pathway at the translational level. Our results revealed that the two-drug combination shows the lower expression of Bcl-2 (anti-apoptotic protein) and higher levels of Bax and Bad (pro-apoptotic proteins) in NCI-H292 cells as compared to sorafenib or bufalin treatment alone (Figure 5B). Once activated, both Bak and Bax oligomerize on the outer mitochondrial membrane to drive its rupture and then result in the initiation of the intrinsic apoptosis pathway in the mitochondrial compartment (45). Furthermore, the combination treatment also resulted in higher cytochrome c and APAF-1 levels in addition to the active form of caspase-3 and -9 than that upon treatment with sorafenib or bufalin alone (Figure 5C and D). The Bcl-2 family of proteins, functioning as both pro- and anti-apoptotic components, have been recognized as a group of upstream regulators of the caspase cascade (46,47). Bcl-2 and Bcl-XL prevent cell death by blocking the activation of caspases (48). In the normal condition, both Bcl-2 and Bcl-XL are limited in the mitochondria to avoid the release of cytochrome c (49) and AIF (apoptosis-inducing factor) (50) from the inter-membrane space of mitochondria into the cytoplasm. Their antiapoptotic activity has been modulated through proteolytic cleavage by caspases (51,52). Tiam 1 is overexpressed or mutated in epithelial cancers with poor prognosis, including non-small cell lung cancer (53). Tiam1 is related to the downstream mediator of bufalin-induced apoptosis in the human HeLa and leukemia cell lines (54). The involvement of Tiam 1 in the treatment of bufalin combined with sorafenib needs to be clarified in the future.
Sorafenib combined with bufalin may provide a novel pathway for the therapy of NSCLC. Sorafenib targeted Raf kinase in the Raf/MEK/ERK pathway, and it inhibited tumor growth in xenograft models (55). Bufalin induced apoptosis through several signaling pathways in NSCLC cells. Bufalin induced apoptosis by degradation of Mcl-1 through GSK-3β activation in NSCLC H1975 Cells (56). In addition, it also induced apoptosis through ROS-, mitochondrial, and DNA damage and repair-associated pathways (25,57,58). However, sorafenib induced both PI3K- and oxidative stress-related pathways that enhanced apoptotic effects in NSCLC and glioma cells, respectively (59,60). Hence, in this study, sorafenib combined with bufalin enhanced the apoptosis effects may mediate the targets of Raf kinase and ROS-, mitochondrial-, or DNA damage and repair-associated pathways. The detailed mechanism need to be clarified in the future.
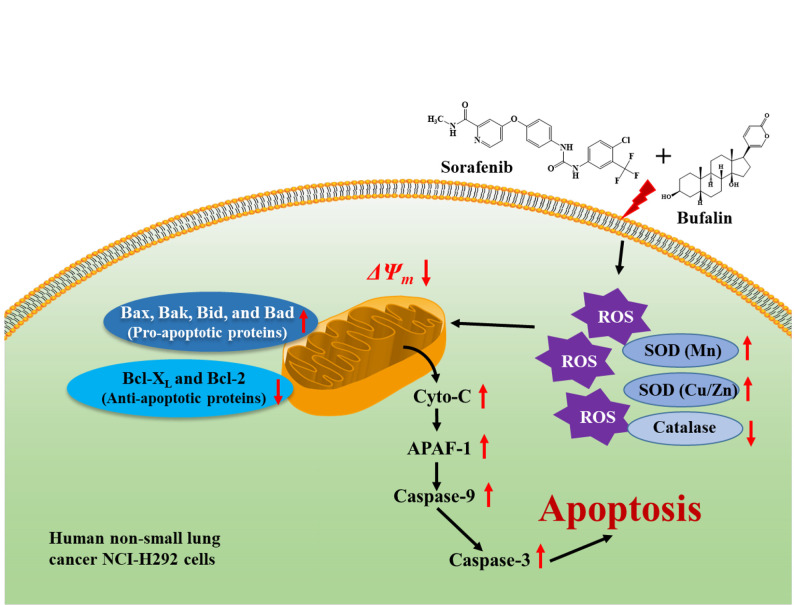
In conclusion, the present study showed that sorafenib in combination with bufalin demonstrates greater cytotoxic effects and results in enhanced chromatin condensation and cell apoptosis (apoptotic cell death) compred to sorafenib or bufalin treatment only in NCI-H292 cells in vitro. In addition, this combined treatment triggered higher ROS production and lower mitochondrial membrane potential than that of sorafenib treatment only. Furthermore, the likely signaling pathways activated upon combined treatment-induced apoptosis in NCI-H292 cells in vitro may be mediated with ROS-, mitochondria-, and caspase-associated signaling pathways that are summarized in Figure 6.
Figure 6. The possible molecular mechanisms of combination of sorafenib with bufalin induced cell apoptosis in NCI-H292 cells in vitro.
Conflicts of Interest
The Authors declare that they have no conflicts of interest regarding the present study.
Authors’ Contributions
Study conception and design: JYK, SFP and JGC; Acquisition of data: JYK, CLL, YSM, and CLK; Analysis and interpretation of data: JYK, JCC, YPH, and WWH; Drafting of manuscript: SFP and JGC; Critical revision: SFP and JGC. All Authors discussed the results and commented on the article.
Acknowledgements
The present study was supported by Grants CMU109-ASIA-16 (China Medical University, Taichung, Taiwan, R.O.C.) and DMR-105-095 (China Medical University Hospital, Taichung, Taiwan, R.O.C.). Experiments and data analysis were performed in part through the use of the Medical Research Core Facilities Center, Office of Research & Development at China Medical University, Taichung, Taiwan, R.O.C.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdes M, Nicholas G, Goss GD, Wheatley-Price P. Chemotherapy in recurrent advanced non-small-cell lung cancer after adjuvant chemotherapy. Curr Oncol. 2016;23(6):386–390. doi: 10.3747/co.23.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lung Cancer Survival Rates. The American Cancer Society medical and editorial content team. Available at: http://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html. [Last accessed on January 29, 2021]
- 5.Dholaria B, Hammond W, Shreders A, Lou Y. Emerging therapeutic agents for lung cancer. J Hematol Oncol. 2016;9(1):138. doi: 10.1186/s13045-016-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Gold KA, Kim E. Sorafenib in non-small cell lung cancer. Expert Opin Investig Drugs. 2012;21(9):1417–1426. doi: 10.1517/13543784.2012.699039. [DOI] [PubMed] [Google Scholar]
- 7.Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 8.Imielinski M, Greulich H, Kaplan B, Araujo L, Amann J, Horn L, Schiller J, Villalona-Calero MA, Meyerson M, Carbone DP. Oncogenic and sorafenib-sensitive ARAF mutations in lung adenocarcinoma. J Clin Invest. 2014;124(4):1582–1586. doi: 10.1172/JCI72763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Chen YL, Ji G, Fang W, Gao Z, Liu Y, Wang J, Ding X, Gao F. Sorafenib inhibits epithelial-mesenchymal transition through an epigenetic-based mechanism in human lung epithelial cells. PLoS One. 2013;8(5):e64954. doi: 10.1371/journal.pone.0064954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadaleta-Caldarola G, Divella R, Mazzocca A, Infusino S, Ferraro E, Filippelli G, Daniele A, Sabbà C, Abbate I, Brandi M. Sorafenib: the gold standard therapy in advanced hepatocellular carcinoma and beyond. Future Oncol. 2015;11(16):2263–2266. doi: 10.2217/fon.15.161. [DOI] [PubMed] [Google Scholar]
- 11.Takezawa K, Okamoto I, Yonesaka K, Hatashita E, Yamada Y, Fukuoka M, Nakagawa K. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res. 2009;69(16):6515–6521. doi: 10.1158/0008-5472.CAN-09-1076. [DOI] [PubMed] [Google Scholar]
- 12.Ngamphaiboon N, Dy GK, Ma WW, Zhao Y, Reungwetwattana T, DePaolo D, Ding Y, Brady W, Fetterly G, Adjei AA. A phase I study of the histone deacetylase (HDAC) inhibitor entinostat, in combination with sorafenib in patients with advanced solid tumors. Invest New Drugs. 2015;33(1):225–232. doi: 10.1007/s10637-014-0174-6. [DOI] [PubMed] [Google Scholar]
- 13.Jean GW, Mani RM, Jaffry A, Khan SA. Toxic effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol. 2016;2(4):529–534. doi: 10.1001/jamaoncol.2015.5927. [DOI] [PubMed] [Google Scholar]
- 14.Robles-Fernández I, Rodríguez-Serrano F, Álvarez PJ, Ortiz R, Rama AR, Prados J, Melguizo C, Álvarez-Manzaneda E, Aránega A. Antitumor properties of natural compounds and related molecules. Recent Pat Anticancer Drug Discov. 2013;8(3):203–215. doi: 10.2174/1574891x113089990034. [DOI] [PubMed] [Google Scholar]
- 15.Krenn L, Kopp B. Bufadienolides from animal and plant sources. Phytochemistry. 1998;48(1):1–29. doi: 10.1016/s0031-9422(97)00426-3. [DOI] [PubMed] [Google Scholar]
- 16.Panesar NS. Bufalin and unidentified substance(s) in traditional Chinese medicine cross-react in commercial digoxin assay. Clin Chem. 1992;38(10):2155–2156. [PubMed] [Google Scholar]
- 17.Watabe M, Ito K, Masuda Y, Nakajo S, Nakaya K. Activation of AP-1 is required for bufalin-induced apoptosis in human leukemia U937 cells. Oncogene. 1998;16(6):779–787. doi: 10.1038/sj.onc.1201592. [DOI] [PubMed] [Google Scholar]
- 18.Huang WW, Yang JS, Pai SJ, Wu PP, Chang SJ, Chueh FS, Fan MJ, Chiou SM, Kuo HM, Yeh CC, Chen PY, Tsuzuki M, Chung JG. Bufalin induces G0/G1 phase arrest through inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial signaling pathway in T24 human bladder cancer cells. Mutat Res. 2012;732(1-2):26–33. doi: 10.1016/j.mrfmmm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng Y, Zhang J, Liu Y. PI3K/Akt is involved in bufalin-induced apoptosis in gastric cancer cells. Anticancer Drugs. 2009;20(1):59–64. doi: 10.1097/CAD.0b013e3283160fd6. [DOI] [PubMed] [Google Scholar]
- 20.Yu CH, Kan SF, Pu HF, Jea Chien E, Wang PS. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci. 2008;99(12):2467–2476. doi: 10.1111/j.1349-7006.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen S, Zhang Y, Wang Z, Liu R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. 2014;10(2):212–224. doi: 10.7150/ijbs.8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Tian X, Liu X, Gong P. Bufalin inhibits human breast cancer tumorigenesis by inducing cell death through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis. 2018;39(5):700–707. doi: 10.1093/carcin/bgy039. [DOI] [PubMed] [Google Scholar]
- 23.Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Tang W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102(5):951–958. doi: 10.1111/j.1349-7006.2011.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su EY, Chu YL, Chueh FS, Ma YS, Peng SF, Huang WW, Liao CL, Huang AC, Chung JG. Bufalin induces apoptotic cell death in human nasopharyngeal carcinoma cells through mitochondrial ROS and TRAIL pathways. Am J Chin Med. 2019;47(1):237–257. doi: 10.1142/S0192415X19500125. [DOI] [PubMed] [Google Scholar]
- 25.Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC, Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP, Chung JG. Bufalin induces apoptosis in vitro and has Antitumor activity against human lung cancer xenografts in vivo. Environ Toxicol. 2017;32(4):1305–1317. doi: 10.1002/tox.22325. [DOI] [PubMed] [Google Scholar]
- 26.Shih YL, Chou JS, Chen YL, Hsueh SC, Chung HY, Lee MH, Chen CP, Lee MZ, Hou HT, Lu HF, Chen KW, Chung JG. Bufalin enhances immune responses in leukemic mice through enhancing phagocytosis of macrophage in vivo. In Vivo. 2018;32(5):1129–1136. doi: 10.21873/invivo.11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CJ, Shih YL, Yeh MY, Liao NC, Chung HY, Liu KL, Lee MH, Chou PY, Hou HY, Chou JS, Chung JG. Ursolic acid induces apoptotic cell death through AIF and Endo G release through a mitochondria-dependent pathway in NCI-H292 human lung cancer cells in vitro. In Vivo. 2019;33(2):383–391. doi: 10.21873/invivo.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiao YT, Kuo CL, Lin JJ, Huang WW, Peng SF, Chueh FS, Bau DT, Chung JG. Curcuminoids combined with gefitinib mediated apoptosis and autophagy of human oral cancer SAS cells in vitro and reduced tumor of SAS cell xenograft mice in vivo. Environ Toxicol. 2018 doi: 10.1002/tox.22568. [DOI] [PubMed] [Google Scholar]
- 29.Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M, Italian Lung Cancer Project Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20(21):4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 30.Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrão Miziara JE, Balint B, De Marinis F, Keller A, Arén O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 31.Paz-Ares L, Hirsh V, Zhang L, de Marinis F, Yang JC, Wakelee HA, Seto T, Wu YL, Novello S, Juhász E, Arén O, Sun Y, Schmelter T, Ong TJ, Peña C, Smit EF, Mok TS. Monotherapy administration of sorafenib in patients with non-small cell lung cancer (MISSION) trial: A phase III, multicenter, placebo-controlled trial of sorafenib in patients with relapsed or refractory predominantly nonsquamous non-small-cell lung cancer after 2 or 3 previous treatment regimens. J Thorac Oncol. 2015;10(12):1745–1753. doi: 10.1097/JTO.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 32.Kang XH, Xu ZY, Gong YB, Wang LF, Wang ZQ, Xu L, Cao F, Liao MJ. Bufalin reverses HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via blockage of Met/PI3k/Akt pathway and induction of apoptosis. Evid Based Complement Alternat Med. 2013;2013:243859. doi: 10.1155/2013/243859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsiao YP, Yu CS, Yu CC, Yang JS, Chiang JH, Lu CC, Huang HY, Tang NY, Yang JH, Huang AC, Chung JG. Triggering apoptotic death of human malignant melanoma a375.s2 cells by bufalin: involvement of caspase cascade-dependent and independent mitochondrial signaling pathways. Evid Based Complement Alternat Med. 2012;2012:591241. doi: 10.1155/2012/591241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L, Song N, Teng Y, Liu Y. Down-regulation of Cbl-b by bufalin results in up-regulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin Oncol. 2012;138(8):1279–1289. doi: 10.1007/s00432-012-1204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai SC, Yang JS, Peng SF, Lu CC, Chiang JH, Chung JG, Lin MW, Lin JK, Amagaya S, Wai-Shan Chung C, Tung TT, Huang WW, Tseng MT. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. 2012;41(4):1431–1442. doi: 10.3892/ijo.2012.1579. [DOI] [PubMed] [Google Scholar]
- 36.Kutkowska J, Strzadala L, Rapak A. Synergistic activity of sorafenib and betulinic acid against clonogenic activity of non-small cell lung cancer cells. Cancer Sci. 2017;108(11):2265–2272. doi: 10.1111/cas.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang S, Wang R, Zhang X, Wu F, Li S, Yuan Y. Combination treatment of gemcitabine and sorafenib exerts a synergistic inhibitory effect on non-small cell lung cancer in vitro and in vivo via the epithelial-to-mesenchymal transition process. Oncol Lett. 2020;20(1):346–356. doi: 10.3892/ol.2020.11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Pan YY, Zhang Y. Sorafenib combined with gemcitabine in EGFR-TKI-resistant human lung cancer cells. Oncol Lett. 2013;5(1):68–72. doi: 10.3892/ol.2012.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress. Free Radic Biol Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Shang HS, Shih YL, Lu TJ, Lee CH, Hsueh SC, Chou YC, Lu HF, Liao NC, Chung JG. Benzyl isothiocyanate (BITC) induces apoptosis of GBM 8401 human brain glioblastoma multiforms cells via activation of caspase-8/Bid and the reactive oxygen species-dependent mitochondrial pathway. Environ Toxicol. 2016;31(12):1751–1760. doi: 10.1002/tox.22177. [DOI] [PubMed] [Google Scholar]
- 42.Shang HS, Liu JY, Lu HF, Chiang HS, Lin CH, Chen A, Lin YF, Chung JG. Casticin induced apoptotic cell death and altered associated gene expression in human colon cancer colo 205 cells. Environ Toxicol. 2017;32(8):2041–2052. doi: 10.1002/tox.22381. [DOI] [PubMed] [Google Scholar]
- 43.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9(7):532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 44.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 45.Chota A, George BP, Abrahamse H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget. 2021;12(16):1615–1626. doi: 10.18632/oncotarget.28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 47.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 48.Yasuhara N, Sahara S, Kamada S, Eguchi Y, Tsujimoto Y. Evidence against a functional site for Bcl-2 downstream of caspase cascade in preventing apoptosis. Oncogene. 1997;15(16):1921–1928. doi: 10.1038/sj.onc.1201370. [DOI] [PubMed] [Google Scholar]
- 49.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 50.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184(4):1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 52.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U.S.A. 1998;95(2):554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maldonado MDM, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in metastatic cancer. Front Cell Dev Biol. 2020;8:201. doi: 10.3389/fcell.2020.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao H, Shibayama-Imazu T, Masuda Y, Shinki T, Nakajo S, Nakaya K. Involvement of Tiam1 in apoptosis induced by bufalin in HeLa cells. Anticancer Res. 2007;27(1A):245–249. [PubMed] [Google Scholar]
- 55.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 56.Kang XH, Zhang JH, Zhang QQ, Cui YH, Wang Y, Kou WZ, Miao ZH, Lu P, Wang LF, Xu ZY, Cao F. Degradation of Mcl-1 through GSK-3β activation regulates apoptosis induced by bufalin in non-small cell lung cancer H1975 cells. Cell Physiol Biochem. 2017;41(5):2067–2076. doi: 10.1159/000475438. [DOI] [PubMed] [Google Scholar]
- 57.Wu SH, Wu TY, Hsiao YT, Lin JH, Hsu SC, Hsia TC, Yang ST, Hsu WH, Chung JG. Bufalin induces cell death in human lung cancer cells through disruption of DNA damage response pathways. Am J Chin Med. 2014;42(3):729–742. doi: 10.1142/S0192415X14500475. [DOI] [PubMed] [Google Scholar]
- 58.Ding DW, Zhang YH, Huang XE, An Q, Zhang X. Bufalin induces mitochondrial pathway-mediated apoptosis in lung adenocarcinoma cells. Asian Pac J Cancer Prev. 2014;15(23):10495–10500. doi: 10.7314/apjcp.2014.15.23.10495. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Ma S, Chen X, Zhang S, Wang Z, Mei Q. The novel PI3K inhibitor S1 synergizes with sorafenib in non-small cell lung cancer cells involving the Akt-S6 signaling. Invest New Drugs. 2019;37(5):828–836. doi: 10.1007/s10637-018-0698-2. [DOI] [PubMed] [Google Scholar]
- 60.Wei J, Wang Z, Wang W, Liu X, Wan J, Yuan Y, Li X, Ma L, Liu X. Oxidative stress activated by sorafenib alters the temozolomide sensitivity of human glioma cells through autophagy and JAK2/STAT3-AIF axis. Front Cell Dev Biol. 2021;9:660005. doi: 10.3389/fcell.2021.660005. [DOI] [PMC free article] [PubMed] [Google Scholar]