Abstract
Purpose
This study was conducted in order to assess changes in hip muscles by comparing results of preoperative and postoperative computed tomography (CT) in older patients who underwent surgery for treatment of hip fracture.
Materials and Methods
A total of 50 patients (aged ≥65 years) who underwent surgery for treatment of intertrochanteric fractures (25 patients) and femoral neck fractures (25 patients) between February 2013 and February 2019 and underwent preoperative and postoperative pelvic CT were enrolled in the study. The cross-sectional area, attenuation and estimates of muscle mass of the gluteus medius, gluteus minimus, iliopsoas, and rectus femoris on the uninjured side were measured. Basic patient data (sex, age, height, weight, body mass index [BMI], bone mineral density [BMD], Harris hip score [HHS], and length of follow-up) were collected from medical records.
Results
No significant differences in sex, age, height, weight, BMI, BMD, HHS, and length of follow-up were observed between the two groups. No significant difference in the cross-sectional areas and attenuations of gluteus medius and gluteus minimus was observed after surgery; however, a statistically significant decrease was observed in those of iliopsoas and rectus femoris after surgery. Lower estimates with statistical significance of muscle mass of the iliopsoas and rectus femoris were observed on postoperative CT.
Conclusion
Muscle mass of the hip flexor (iliopsoas, rectus femoris) showed significant decreases on postoperative CT compared with preoperative CT. Based on these findings, selective strengthening exercise for hip flexor should be beneficial in rehabilitation of hip fractures.
Keywords: Hip fractures, Sarcopenia, Computed tomography, Rehabilitation, Exercise
INTRODUCTION
The number of patients with hip fracture in an ageing population continues to increase. Application of surgical intervention has increased in the treatment of hip fracture for improvement of quality of life, pain relief, and prevention of complications associated with prolonged bed confinement1,2). Declining muscle mass and decreased muscular strength and the development of sarcopenia, defined as low physical performance related to ageing, have become serious problems3,4). Sarcopenia is associated with poor health status and high mortality. The risk of hip fracture is high in elderly patients with sarcopenia5,6,7). In addition, these patients have aggravated health conditions that result in high mortality8,9).
In studies on sarcopenia, dual-energy x-ray absorptiometry (DEXA) has primarily been used in the analysis of this condition10,11). Of these, no study comparing intertrochanteric fractures and femoral neck fractures has been reported. In the comparison of these fractures, DEXA can provide approximations of muscle mass and muscle density based on the amount of radiation absorbed; however, the analysis of precise muscle mass and density in different muscles is impossible to determine12). It has been reported that measurement of cross-sectional area (CSA) and attenuation shown on axial computed tomography (CT) is a good indicator of sarcopenia13). Therefore, the aim of this study was to conduct a comparative analysis of preoperative and postoperative muscle mass around the hip joint using axial CT in patients with hip fracture.
MATERIALS AND METHODS
1. Subjects
This study was conducted with Institutional Review Board (IRB) approval from Dong-A University Hospital (No. DAUHIRB-20-124), and the informed consent was waived by the IRB. Data on 97 patients who underwent postoperative pelvic CT of 700 patients who underwent surgery for treatment of intertrochanteric or femoral neck fractures between February 2013 and February 2019 at Dong-A University Hospital were reviewed. Of these, 40 patients who underwent bilateral hip surgery, ambulatory disability, neuromuscular disorder, or hemiparesis due to cerebrovascular disease were excluded from the analysis. Among 90 patients aged 65 and older whose intervals between operation and CT were between 1 year and 3 years, 50 patients were reviewed retrospectively. Participants included 25 patients with intertrochanteric fracture and 25 patients with femoral neck fracture (Fig. 1).
Fig. 1. Flow chart showing how cases were selected and analyzed.
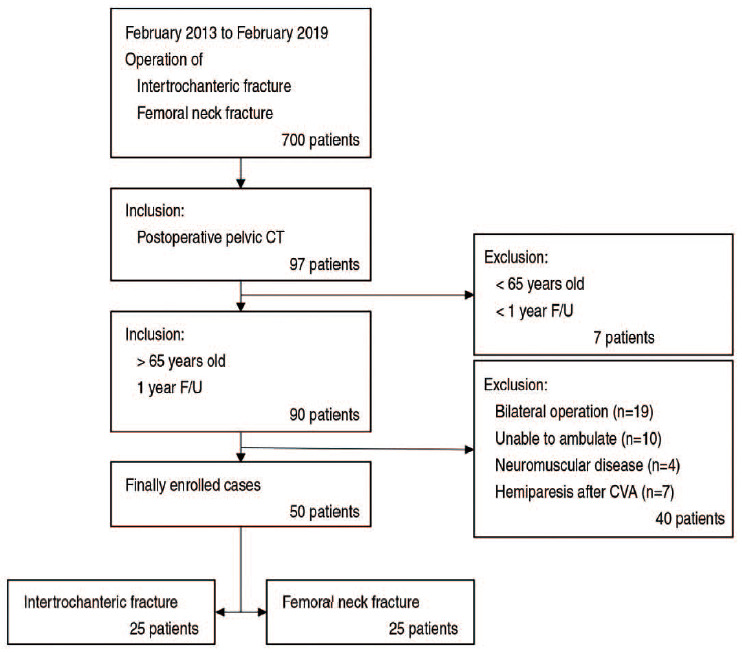
CT: computed tomography, F/U: follow-up, CVA: cerebrovascular accident.
2. Surgical Methods
All operations were performed by the same surgeon. In the 25 patients with intertrochanteric fracture, adequate closed reduction obtained on a fracture table was preoperatively confirmed using C-arm radiography, and intramedullary nail insertion was performed using the PFNA-II nail system (Proximal femoral nail anti-rotation-II, Asian version; DePuy Synthes, Oberdorf, Switzerland). In the 25 patients with femoral neck fracture, bipolar hip arthroplasty was performed using a Bencox® hip stem (Corentec, Seoul, Korea) in a lateral position using the modified Gibson approach.
3. Rehabilitation Protocols
After the hip operation, rehabilitation commences in the following order:
- While lying in the supine position, press knees down and apply strength to the quadriceps, stop for 4 seconds.
- Lift hip and hold 5 seconds, squeezing the gluteus muscles.
- Repeat dorsiflexion and plantarflexion of the ankle.
- Repeat the following exercises.
➀ Stand and bend the knee up.
➁ Extend the knee and return to standing position
➂ Bend the hip and knee down
➃ Extend the hip and knee and return to standing position
- Stop by extending the hip with knee extension in a prone position.
- Lying in a supine position, repeat abduct and adduct the leg placing a soft towel between the leg and the floor.
- Stand up and move the leg to the side and back.
4. Clinical and Radiological Analysis
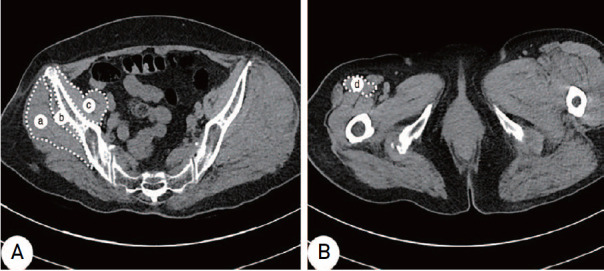
Based on medical records, basic patient data (sex, age, height, weight at time of surgery and at final follow-up, body mass index [BMI], bone mineral density [BMD], Harris hip score [HHS] relating to function and length of follow-up) were reviewed retrospectively. Axial CT images of the pelvis just below the sacroiliac joint and at the level of the lesser trochanter were used for radiographic analysis of the gluteus minimus, rectus femoris, and iliopsoas muscles. For measurement of the CSA and attenuation to estimate muscle volume and density, a line was drawn connecting the outer margin of the muscles on axial CT of 0.625-mm sliced thickness using the PACS system (INFINITT PACS, ver. 3.0; INFINITT Healthcare, Seoul, Korea), expressed in square millimetres (mm2) and Hounsfield units (HU). The CSA and attenuation were measured for estimation of muscle volume and density. According to Goodpaster et al.14), HU represents attenuation which is a relative value to density and is related to muscle strength. Therefore, it was assumed that the multiply of CSA representing the volume and attenuation (HU) representing density could mean the estimate of muscle mass. The CSA and attenuation of the gluteus medius, gluteus minimus, and iliopsoas were measured just below the level of the sacroiliac joint, and those of the rectus femoris were measured at the level of the lesser trochanter. The largest area around those levels was measured (Fig. 2). Assuming that measurement errors could occur due to hematoma and swelling on the injured side of the hip, CSA and attenuation were measured preoperatively on the uninjured side of the hip. The CSA and attenuation of muscles were measured twice at a 2-week interval by the same experienced surgeon, and the mean value was used.
Fig. 2. The area of muscles is outlined by a white-dotted line in the axial scan of each level. (A) Cross-sectional area and attenuations of the gluteus medius (a), gluteus minimus (b), and iliopsoas (c) were measured at the sacroiliac joint just below level. (B) Cross-sectional area and attenuations of the rectus femoris (d) were measured at the lesser trochanter level.
5. Statistical Analysis
All statistical analyses were performed using IBM SPSS software (ver. 23.0; IBM, Armonk, NY, USA). The CSA, and attenuation of each muscle were measured preoperatively and postoperatively, and the measured values were compared between patients in the intertrochanteric fracture group and patients in the femoral neck fracture group. The chi square test was used for comparison of patients in the two groups, and the Mann–Whitney U-test was performed to determine differences in age, BMI, BMD, HHS relating to function, and length of follow-up that are not normally distributed or small samples between the two groups. The Mann–Whitney U-test was performed for comparison on CSA and attenuation between the patients in the two groups. In addition, the Wilcoxon signed-rank test was performed to test the differences in CSA, attenuation, and estimate of muscle mass in all patients between preoperative and postoperative CTs. Correlations between the CSA and CSA per weight (CSA/wt, mm2/kg) with HHS relating to function were analyzed using Spearman correlation tests that are not normally distributed or small samples. Fisher’s exact test was performed to test the differences in the walking ability between the two groups. P<0.05 was considered statistically significant.
RESULTS
1. Subjects
Of the 25 patients in the intertrochanteric fracture group, six patients (24.0%) were males, and of the 25 patients in the femoral neck fracture group, eight patients (32.0%) were males; no significant differences were observed between the two groups (P>0.05). No significant differences in mean age, mean height, mean weight at the time of surgery and postoperative CT scan, BMI at the time of surgery and postoperative CT scan, BMD, HHS, HHS relating to function at the time of postoperative CT scan, and the interval between operation and postoperative CT between two groups and walking ability at the time of postoperative CT scan were observed between the two groups (P>0.05) (Table 1).
Table 1. Demographic Data for the Intertrochanter Fracture Group and Femoral Neck Fracture Groups.
| Variable | Intertrochanter fracture (n=25) | Femoral neck fracture (n=25) | P-value | |
|---|---|---|---|---|
| Sex | 0.184 | |||
| Female | 19 (76.0) | 17 (68.0) | ||
| Male | 6 (24.0) | 8 (32.0) | ||
| Age (yr) | 79.8±6.9 | 79.5±6.8 | 0.561 | |
| Height (cm) | 156.6±6.1 | 157.1±7.2 | 0.413 | |
| Weight (kg) - preoperative | 54.2±12.2 | 52.8±8.5 | 0.555 | |
| Weight (kg) - F/U | 55.4±12.9 | 54.2±8.5 | 0.721 | |
| BMI (kg/m2) - preoperative | 22.31±4.90 | 21.32±3.55 | 0.438 | |
| BMI (kg/m2) - F/U | 22.85±5.50 | 21.98±3.88 | 0.535 | |
| BMD | –2.94±0.77 | –2.91±0.87 | 0.889 | |
| HHS (total) - F/U | 82.65±11.54 | 85.36±13.74 | 0.461 | |
| HHS (functional) - F/U | 48.69±9.67 | 50.18±8.81 | 0.583 | |
| Interval between operation and CT (mo) | 20.23±6.28 | 21.51±7.19 | 0.861 | |
| Walking ability | 0.722 | |||
| None support | 12 | 10 | ||
| Cane for long walk | 5 | 6 | ||
| Cane all the time | 4 | 6 | ||
| Walker ambulation | 4 | 3 | ||
Values are presented as number (%), mean±standard deviation, or number only.
F/U: follow-up, BMI: body mass index, BMD: bone mineral density, HHS: Harris hip score, CT: computed tomography.
2. Cross-sectional Area of Muscles
P-values of CSA of each muscle between the intertrochanteric fractures group and femoral neck fractures group were greater than 0.05, showing no statistically significant change (Table 2).
Table 2. Comparison of CSAs of Muscles between the Patients with Intertrochanter Fracture and Femoral Neck Fracture at Preoperative and Postoperative State.
| Group | Preoperative | Postoperative | |
|---|---|---|---|
| CSA-G.med | Intertrochanter | 1,873.64±493.53 | 1,832.65±445.84 |
| Femoral neck | 1,861.43±369.45 | 1,844.42±561.54 | |
| P-value | 0.107 | 0.119 | |
| CSA-G.min | Intertrochanter | 633.59±156.42 | 619.17±204.78 |
| Femoral neck | 623.22±225.31 | 608.57±266.83 | |
| P-value | 0.107 | 0.156 | |
| CSA-IP | Intertrochanter | 780.86±280.31 | 631.98±183.89 |
| Femoral neck | 748.88±174.66 | 618.32±174.69 | |
| P-value | 0.128 | 0.132 | |
| CSA-RF | Intertrochanter | 423.66±244.11 | 375.24±113.02 |
| Femoral neck | 403.72±100.19 | 369.31±101.08 | |
| P-value | 0.097 | 0.186 |
Values are presented as mean±standard deviation.
CSA: cross-sectional area (mm2), G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
P-values of CSAs of the gluteus medius and of the gluteus minimus between preoperative and postoperative states were greater than 0.05, showing no statistically significant change. However, P-values of CSAs of the iliopsoas and the rectus femoris between preoperative and postoperative states were 0.027 and 0.017, showing a significant decrease after surgery (Table 3). Results of the correlation analysis between postoperative CSA and HHS relating to function showed that Spearman correlation coefficient was 0.23, 0.25, 0.03, and 0.07 in the gluteus medius, gluteus minimus, iliopsoas, and rectus femoris, respectively. All P-values were >0.05, indicating no significant correlation (Table 4).
Table 3. Comparison of CSAs of Muscles of the All Study Subjects at Preoperative and Postoperative State.
| All study subjects | Preoperative | Postoperative | P-value |
|---|---|---|---|
| CSA-G.med | 1,867.54±409.43 | 1,838.54±445.81 | 0.657 |
| CSA-G.min | 628.41±254.23 | 613.87±271.33 | 0.609 |
| CSA-IP | 764.87±217.71 | 625.15±203.28 | 0.027 |
| CSA-RF | 413.69±199.85 | 372.28±132.72 | 0.017 |
Values are presented as mean±standard deviation.
CSA: cross-sectional area (mm2), G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
Table 4. Correlation of Postoperative CSAs with Function related Harris Hip Score.
| All study subjects | Spearman coefficient | P-value |
|---|---|---|
| CSA-G.med | 0.23 | 0.16 |
| CSA-G.min | 0.25 | 0.18 |
| CSA-IP | 0.03 | 0.21 |
| CSA-RF | 0.07 | 0.13 |
CSA: cross-sectional area (mm2), G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
3. Attenuation (ATT) of Muscles
P-values of ATT of each muscle between the intertrochanteric fractures group and femoral neck fractures group were greater than 0.05, showing no statistically significant change (Table 5).
Table 5. Comparison of Attenuations of Muscles between the Patients with Intertrochanter Fracture and Femoral Neck Fracture at Preoperative and Postoperative State.
| Group | Preoperative | Postoperative | |
|---|---|---|---|
| Att-G.med | Intertrochanter | 30.91±13.74 | 29.54±11.20 |
| Femoral neck | 29.51±14.14 | 28.48±13.89 | |
| P-value | 0.393 | 0.289 | |
| Att-G.min | Intertrochanter | 17.71±8.21 | 16.68±8.30 |
| Femoral neck | 16.88±6.49 | 16.04±9.65 | |
| P-value | 0.365 | 0.462 | |
| Att-IP | Intertrochanter | 48.65±12.10 | 34.44±13.19 |
| Femoral neck | 46.98±16.12 | 31.88±11.01 | |
| P-value | 0.319 | 0.817 | |
| Att-RF | Intertrochanter | 45.38±7.24 | 29.46±15.61 |
| Femoral neck | 42.26±10.53 | 27.54±10.55 | |
| P-value | 0.760 | 0.536 |
Values are presented as mean±standard deviation.
Att: attenuation (Housefield unit), G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
P-values of attenuation of the gluteus medius and of the gluteus minimus between preoperative and postoperative states were greater than 0.05, showing no statistically significant change. P-values of attenuation of the iliopsoas and of the rectus femoris between preoperative and postoperative states were <0.001 showing a significant decrease after surgery (Table 6).
Table 6. Comparison of Attenuations of Muscles of the All Study Subjects at Preoperative and Postoperative State.
| All study subjects | Preoperative | Postoperative | P-value |
|---|---|---|---|
| Att-G.med | 30.21±13.80 | 29.01±20.00 | <0.532 |
| Att-G.min | 17.30±16.17 | 16.36±14.39 | <0.489 |
| Att-IP | 47.82±14.08 | 33.16±14.49 | <0.001 |
| Att-RF | 43.82±7.30 | 28.50±13.39 | <0.001 |
Values are presented as mean±standard deviation.
Att: attenuation (Housefield unit), G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
4. Estimate of Muscle Mass
The P-values of the estimates of muscle mass in the gluteus medius and gluteus minimus in all patients between the preoperative and postoperative states were greater than 0.05, showing no statistically significant change. However, the P-values of the estimates of muscle mass in the iliopsoas and rectus femoris in all patients between at the preoperative and postoperative states were <0.001 showing a significant decrease after surgery (Table 7).
Table 7. Comparison of Estimate of Muscle Mass of the All Study Subjects at Preoperative and Postoperative State.
| All study subjects | Preoperative | Postoperative | P-value |
|---|---|---|---|
| EMM-G.med | 56,418.23±19648.51 | 53,335.9±22536.16 | <0.231 |
| EMM-G.min | 10,868.26±3269.51 | 10,042.91±2720.58 | <0.325 |
| EMM-IP | 36,572.26±10643.89 | 20,729.97±9236.07 | <0.001 |
| EMM-RF | 18,127.9±4188.51 | 10,609.84±3210.21 | <0.001 |
Values are presented as mean±standard deviation.
EMM: estimate of muscle mass, G.med: gluteus medius, G.min: gluteus minimus, IP: iliopsoas, RF: rectus femoris.
DISCUSSION
Skeletal muscle mass and strength are inversely proportional to increasing age. The decline in skeletal muscle mass and strength with age accelerates after 65 years of age and results in various social problems such as physical impairment, reduced quality of life, and increase in mortality15). The socioeconomic burden is a serious concern, and as a result, sarcopenia has recently gained significant attention16). Sarcopenia can increase the risk of hip fracture and cause dysphagia or voiding dysfunction due to muscle weakness17,18). These conditions are considered indicators of frailty and associated with loss of independence19). Tatara et al.20) and Ellman et al.21) suggested that muscle force is a critical factor for proper growth and preservation of the bony skeleton, and Ford et al.22) reported the impact of imbalance of muscles around the hip joint on the hip joint. However, few studies evaluating muscles around the hip joint by dividing patients into two groups, one with intertrochanteric and one with femoral neck fractures, have been conducted.
Conventional methods for measurement of body composition include DEXA, bioelectrical impedance, CT, magnetic resonance imaging, and others. DEXA and bioelectrical impedance analysis can be used for estimation of the overall condition of the skeleton; however, they are not suitable for use in muscle-specific analysis. Although magnetic resonance imaging provides a precise analysis of muscle condition and mass, attenuation and fatty infiltration around skeletal muscles, it cannot be used in patients with metallic prostheses. On the contrary, Mitsiopoulos et al.23) reported approximately the same measurements of skeletal muscle and adipose tissue in both humans and cadavers using CT. According to a report by Rasch et al.24), the use of CT allows for simple circumscribing of large muscle bellies and minimizes measurement errors with easy identification of the bony landmarks of the pelvis.
Intertrochanteric fracture is an extracapsular fracture, whereas a femoral neck fracture is an intracapsular fracture. Extracapsular fractures are more likely to occur in patients with hip osteoarthritis, because their hip joints are stiffer25). Based on that assumption, it was presumed that patients with intertrochanteric fracture will likely have weaker abductor (gluteus medius, gluteus minimus) but stronger flexor (iliopsoas, rectus femoris) than patients with femoral neck fracture; however, no statistically significant differences were observed in this study.
Of all muscles around the hip joint, the gluteus medius serves a key role in abduction at the hip joint, provides stability of the pelvis during a single-leg stance and exhibits Tredelenburg’s sign in case of insufficiency26,27). The gluteus minimus aids abduction in a similar manner as the gluteus medius, therefore this muscle was included in the analysis. The iliopsoas muscle, which serves as the primary muscle in hip flexion and has been considered an indicator of sarcopenia, was also included28,29). The iliopsoas muscle contributes to the stability of the lumbar spine, which may cause problems such as herniated nucleus pulposus in patients with tightness or spasm30). The rectus femoris was included in this study because it crosses over both the hip and knee joints and maintains stability of the femur during walking. In patients with weakness of the rectus femoris, excessive knee extension may occur due to limitation of active knee extension. Moreover, in gait analysis, gluteus medius and gluteus minimus mainly play a role in the stance phase in the walking gait, whereas iliopsoas and rectus femoris mainly play a role in the pre-swing phase, such as lift off, and the swing phase. Therefore we presume that sarcopenia at these muscles may affect stance phase and swing phase respectively. In this study lower estimates with statistical significance of muscle mass of the iliopsoas and rectus femoris were observed on postoperative CT. It is presumed that this result is due to a decrease in stride length and power in the swing phase after surgery because of pain and stiffness.
In a recent study, Paganini-Hill et al.31) and Chilibeck et al.32) suggested that the strengthening of skeletal muscles through exercise is effective in preventing hip fracture, increasing BMD, and reducing the risk of falling. Gschwind et al.33) reported that hip fracture in the older population can be prevented with exercise that improves muscle balance and strength. However, no studies to determine which specific muscles need to be strengthened in rehabilitation for patients with hip fractures have been conducted. This study was conducted in an effort to suggest which exercises would be helpful for patients with hip fracture.
This comparative study has some limitations, including small sample size. In addition, because assessment was based on muscle CSA and attenuation, instead of measurement of muscle power at the hip joint in each patient, there may be differences in actual muscle function and power. Finally, because preoperative ambulation ability and overall function of the musculoskeletal system were not fully controlled, there may have been bias in the comparison of preoperative and postoperative changes.
CONCLUSION
Significant decreases in muscle mass in the hip flexor (iliopsoas, rectus femoris) were observed on postoperative CT. Based on these findings, selective strengthening exercise for the hip flexor should be beneficial in rehabilitation of hip fractures.
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article.
References
- 1.Ha YC, Park YG, Nam KW, Kim SR. Trend in hip fracture incidence and mortality in Korea: a prospective cohort study from 2002 to 2011. J Korean Med Sci. 2015;30:483–488. doi: 10.3346/jkms.2015.30.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. 2009;64:599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652–658. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Hida T, Ishiguro N, Shimokata H, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013;13:413–420. doi: 10.1111/j.1447-0594.2012.00918.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JI, Ha YC, Kwon HB, Lee YK, Koo KH, Yoo MJ. High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci. 2016;31:1479–1484. doi: 10.3346/jkms.2016.31.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petak S, Barbu CG, Yu EW, et al. The Official Positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom. 2013;16:508–519. doi: 10.1016/j.jocd.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ, 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 16.Vanitallie TB. Frailty in the elderly: contributions of sarcopenia and visceral protein depletion. Metabolism. 2003;52(10 Suppl 2):22–26. doi: 10.1016/s0026-0495(03)00297-x. [DOI] [PubMed] [Google Scholar]
- 17.Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutr Clin Pract. 2009;24:395–413. doi: 10.1177/0884533609332005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54:405–412. doi: 10.1111/j.1532-5415.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 19.Frisoli A, Jr, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone. 2011;48:952–957. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Tatara AM, Lipner JH, Das R, et al. The role of muscle loading on bone (re)modeling at the developing enthesis. PLoS One. 2014;9:e97375. doi: 10.1371/journal.pone.0097375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellman R, Spatz J, Cloutier A, Palme R, Christiansen BA, Bouxsein ML. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J Bone Miner Res. 2013;28:875–885. doi: 10.1002/jbmr.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford CA, Nowlan NC, Thomopoulos S, Killian ML. Effects of imbalanced muscle loading on hip joint development and maturation. J Orthop Res. 2017;35:1128–1136. doi: 10.1002/jor.23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Rasch A, Byström AH, Dalén N, Martinez-Carranza N, Berg HE. Persisting muscle atrophy two years after replacement of the hip. J Bone Joint Surg Br. 2009;91:583–588. doi: 10.1302/0301-620X.91B5.21477. [DOI] [PubMed] [Google Scholar]
- 25.Aguado-Maestro I, Panteli M, García-Alonso M, García-Cepeda I, Giannoudis PV. Hip osteoarthritis as a predictor of the fracture pattern in proximal femur fractures. Injury. 2017;48 Suppl 7:S41–S46. doi: 10.1016/j.injury.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Steele KM, Seth A, Hicks JL, Schwartz MS, Delp SL. Muscle contributions to support and progression during single-limb stance in crouch gait. J Biomech. 2010;43:2099–2105. doi: 10.1016/j.jbiomech.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Wen X, Tong Z, Wang K, Wang C. Changes of gluteus medius muscle in the adult patients with unilateral developmental dysplasia of the hip. BMC Musculoskelet Disord. 2012;13:101. doi: 10.1186/1471-2474-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanaoka M, Yasuno M, Ishiguro M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–856. doi: 10.1007/s00384-017-2773-0. [DOI] [PubMed] [Google Scholar]
- 29.Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penning L. Psoas muscle and lumbar spine stability: a concept uniting existing controversies. Critical review and hypothesis. Eur Spine J. 2000;9:577–585. doi: 10.1007/s005860000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. 1991;2:16–25. doi: 10.1097/00001648-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Chilibeck PD, Sale DG, Webber CE. Exercise and bone mineral density. Sports Med. 1995;19:103–122. doi: 10.2165/00007256-199519020-00003. [DOI] [PubMed] [Google Scholar]
- 33.Gschwind YJ, Kressig RW, Lacroix A, Muehlbauer T, Pfenninger B, Granacher U. A best practice fall prevention exercise program to improve balance, strength / power, and psychosocial health in older adults: study protocol for a randomized controlled trial. BMC Geriatr. 2013;13:105. doi: 10.1186/1471-2318-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]


