Abstract
To date, family medicine and internal medicine fields have been responsible for defining, researching, and development of treatments for sarcopenia, focusing mainly on diabetes and metabolic diseases. Therefore, application of current guidelines for diagnosis of sarcopenia which differ according to continent to patients with hip fractures in the orthopedic field is difficult. The purpose of this review was to understand the recent consensus on the definition and diagnosis of sarcopenia and to highlight the importance of research and future research opportunities on the management of sarcopenia in patients with hip fractures by orthopedic surgeons. The global prevalence of sarcopenia in patients with hip fractures is statistically significant. Despite establishment of various therapeutic and diagnostic criteria for osteoporosis in the clinical field, there are no clear, useful diagnostic criteria for sarcopenia in the clinical field. In particular, few studies on the evaluation and treatment of sarcopenia in patients with hip fractures have been reported. In addition, the quality of life of postoperative patients with hip fractures could be significantly improved by development of precise assessment for muscle regeneration and rehabilitation in the operating room.
Keywords: Diagnosis, Management, Sarcopenia, Hip fracture
INTRODUCTION
An increase in the prevalence of sarcopenia along with the global aging trend has led to a subsequent increase in interest and research on sarcopenia1,2,3). In the USA, sarcopenia received an ICD-10-CM (M62.84) code in 2016, based on ICD-10, a global classification of diseases published by the World Health Organization (WHO)4). In Korea, sarcopenia received an ICD-10-CM (M62.5) code with the eighth revision of the Korean Classification of Diseases (KCD). Thus, the importance of diagnosis and treatment of sarcopenia has been emphasized in earnest.
To date, those in the fields of family medicine and internal medicine who focus mainly on diabetes and metabolic diseases have been responsible for the definition, research, and development of treatments for sarcopenia5,6). Therefore, application of current guidelines for diagnosis of sarcopenia which differ according to continent to patients with hip fractures in the orthopedic field is difficult. For example, evaluation of physical performance by measurement of walking speed or sit-to-stand, part of the diagnostic guidelines, is practically impossible for patients with hip fractures.
The purpose of this review is to understand the recent consensus on the definition and diagnosis of sarcopenia, highlighting the importance of research on the management of sarcopenia in patients with hip fractures by orthopedic surgeons.
LATEST UPDATES FOR SARCOPENIA GUIDELINES
1. Definition of Sarcopenia
In 2020, in the position paper update of the Asian Working Group for Sarcopenia (AWGS), Chen et al.6) defined sarcopenia as age-related loss of skeletal muscle mass and skeletal strength and/or decreased physical performance; for each country the definition for elderly was those aged more than 60 or 65 years.
Current definitions and diagnostic criteria for sarcopenia are the result of significant research and a long period of debate among researchers. However, for patients with hip fractures, muscle viability based on operating room findings, contractions, and fat degeneration shown by numerous computed tomography (CT) and magnetic resonance imaging (MRI) images7) taken by orthopedic surgeons does not often match with existing criteria for diagnosis of sarcopenia (Fig. 1).
Fig. 1. (A) Thigh magnetic resonance image showing fatty degeneration change. (B) Operative room photo showing loss of muscle viability.
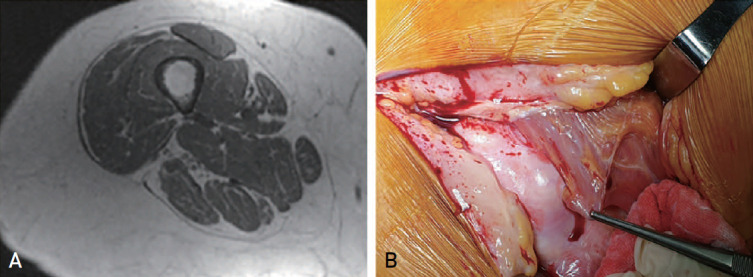
In addition, in patients with hip fractures, accurate evaluation of muscle mass using dual energy X-ray absorptiometry (DEXA) measurement-based sarcopenia diagnosis is difficult due to surgical implant metal artifacts8). Therefore, establishment of a new definition of sarcopenia specific to patients with hip fractures is necessary for quality management of postoperative sarcopenia.
2. Epidemiology of Sarcopenia in Patients with Hip Fractures
A review of epidemiology studies in Asian countries that used AWGS 2014 criteria reported a prevalence of sarcopenia ranging from 5.5% to 25.7%, with a male predominance (5.1%-21.0% in men vs 4.1%-16.3% in women)6). According to the AWGS 2014 criteria, the prevalence of sarcopenia in patients with hip fractures in Japan was 81.1% for males and 44.7% for females, while a domestic study conducted in 2016 reported a prevalence of 68.2% for males and 44.3% for females6,9,10,11,12,13,14,15,16) (Table 1).
Table 1. Summaries of Studies of Sarcopenia in Patients with Hip Fracture.
| Study | Study design | Regions | Population | Mean age (yr) | Definition | Cut-off of muscle mass (appendicular muscle kg/height2) by DXA | Prevalence (%) |
|---|---|---|---|---|---|---|---|
| Hida et al.14) (2013) | Case-control | Japan | 357:2,511 | 82.7 (F), 80.3 (M) | Japanese criteria | 6.87 (M), 5.46 (F) | 81.1 (M), 44.7 (F) |
| Di Monaco et al.15) (2012) | Case series | Italy | 591 | 79.7 | New Mexico Elder Health Survey | <2 SD in a young reference group | 95 (M), 65 (F) |
| González-Montalvo et al.16) (2015) | Case series | Spain | 479 | 78.3 (F), 75.3 (M) | EWGOSP | <2 SD in a young reference group | 12.4 (M), 18.3 (F) |
| Yoo et al.13) (2016) | Case-control | Korea | 359:1,614 | 78.3 (F), 75.3 (M) | AWGS EWGOSP | 7.0 (M), 5.4 (F) | 68.2 (M), 44.3 (F) |
| <2 SD in a young reference group | 80.5 (M), 47.1 (F) |
Adapted from the article of Yoo et al. (J Korean Med Sci. 2016;31:1479-84)13) in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license.
F: female, M: male, EWGOSP: European Working Group on Sarcopenia in Older people, AWGS: Asian Working Group for Sarcopenia, DXA: dual X-ray absorptiometry, SD: standard deviation.
3. Risk Factors for Sarcopenia
In studies on major risk factors for sarcopenia conducted in Asia since 2014, demographic factors (such as old age, men, and living alone) and lifestyle habits (such as binge drinking, short sleep duration, and inadequate water intake) were leading risk factors. Other risk factors included lack of physical activity, inadequate dietary nutritional intake, dental problems, osteoporosis, type 2 diabetes mellitus, hypertension, and dyslipidemia17,18,19) (Table 2).
Table 2. Risk Factors of Sarcopenia in Studies from East and Southeast Asia since 2014.
| Category | Risk factors |
|---|---|
| Demographic characteristics | Age, sex |
| Household status | Living alone or living with children, and/or grandchildren; Person’s satisfaction with their perceived level of family function (family APGAR score) |
| Lifestyle habits | Binge drinkers with weekly or daily alcohol consumption (women); short sleep duration or having long sleep duration (women); water intake from food (g/d and cup/d) and dietary water adequacy ratio (mL) |
| Physical activity | Locomotive syndrome (one study for women) |
| Dietary pattern, dental condition and nutritional status | Lower frequency of nut consumption per week; impaired dentition status; higher dietary variety score (one study for women); lower body mass index (<18.5 kg/m2); risk of malnutrition (MNA score) |
| Comorbidities | Osteoporosis; cardiovascular risk factors (including type 2 diabetes mellitus, hypertension, dyslipidemia) |
Data from the article of Chen et al. (J Am Med Dir Assoc. 2014;15:95-101)17).
APGAR: adaptability, partnership, growth, affection, and resolve, MNA: Mini Nutritional Assessment.
DIAGNOSTIC CRITERIA FOR SARCOPENIA
Criteria for diagnosis of sarcopenia vary among the guidelines for international associations. In general, diagnosis of sarcopenia is based on evaluation of the following three factors: muscle mass, strength, and physical performance6). The AWGS published revised guidelines in 2019 (Table 3). First, depending on the purpose, diagnosis of sarcopenia is largely divided into primary health care or community service settings and acute to chronic health care or clinical research settings. Second, diagnosis of sarcopenia is based on measurement of calf thickness or a questionnaire, SARC-F, followed by evaluation of muscle mass, strength, and physical performance.
Table 3. Diagnostic Criteria for Sarcopenia according to the Different Consensus Group.
| Muscle mass | Muscle strength | Physical performance | |
|---|---|---|---|
| EWGSOP | ALM/height2 (DXA) (≤7.26 kg/m2 [M], ≤5.5 kg/m2 [F]) |
Grip strength (<30 kg [M], <20 kg [F]) |
SPPB≤8 Gait speed<0.8 m/s |
| SM/height2 (BIA) (≤8.87 kg/m2 [M], ≤6.42 kg/m2 [F]) | |||
| EWGSOP2 | ASM/height2
(≤7.0 kg/m2 [M], ≤6.0 kg/m2 [F]) |
Grip strength (<27 kg [M], <16 kg [F]) Chair stand >15 s for five rises |
SPPB≤8 Gait speed<0.8 m/s TUG ≥20 s |
| ASM (<20 kg [M], <15 kg [F]) | |||
| IWGS | ALM/height2 (DXA) (≤7.23 kg/m2 [M], ≤5.67 kg/m2 [F]) |
Gait speed<1.0 m/s | |
| AWGS | ALM/height2 (DXA) (≤7.0 kg/m2 [M], ≤5.4 kg/m2 [F]) |
Grip strength (<26 kg [M], <18 kg [F]) |
Gait speed<0.8 m/s |
| SM/height2 (BIA) (≤7.0 kg/m2 [M], ≤5.7 kg/m2 [F]) | |||
| AWGS 2019 | ALM/height2 (DXA) (≤7.0 kg/m2 [M], ≤5.4 kg/m2 [F]) |
Grip strength (<28 kg [M], <18 kg [F]) |
Gait speed<1.0 m/s 5-time chair stand test≥12 s SPPB≤9 |
| SM/height2 (BIA) (≤7.0 kg/m2 [M], ≤5.7 kg/m2 [F]) | |||
| FNIH Sarcopenia project | ALM/BMI (DXA) (≤0.789 [M], ≤0.512 [F]) |
Grip strength (<26 kg [M], <16 kg [F]) |
Gait speed<0.8 m/s |
Data from the article of Chen et al. (J Am Med Dir Assoc. 2014;15:95-101)17).
EWGSOP: European Working Group on Sarcopenia in Older people, ALM: appendicular lean mass, DXA: dual X-ray absorptiometry, M: male, F: female, SPPB: short physical performance battery, SM: skeletal muscle mass, BIA: body impedance analysis, ASM: appendicular skeletal muscle mass, TUG: Timed Up and Go test, IWGS: International Working Group for Sarcopenia, AWGS: Asian Working Group for Sarcopenia, FNIH: Foundation for the National Institutes of Health, BMI: body mass index (kg/m2).
In a community setting without evaluation of muscle mass, possible sarcopenia is diagnosed by evaluation of muscle strength and physical function. However, it is recommended that patients go to a hospital for confirmation of sarcopenia (Fig. 2).
Fig. 2. Modified Asian Working Group for sarcopenia in 2019 criteria.
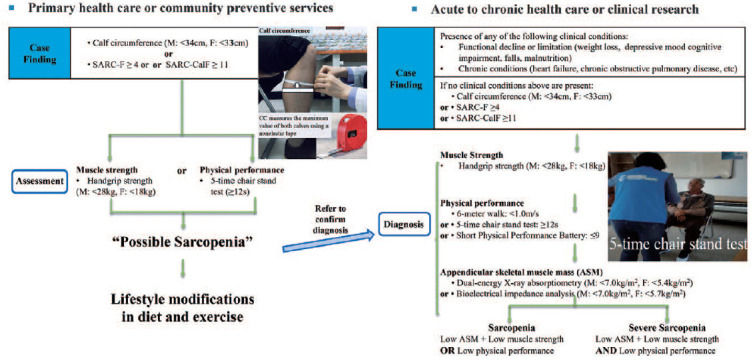
Revised from the article of Chen et al. (J Am Med Dir Assoc. 2020;21:300-7.e2)6).
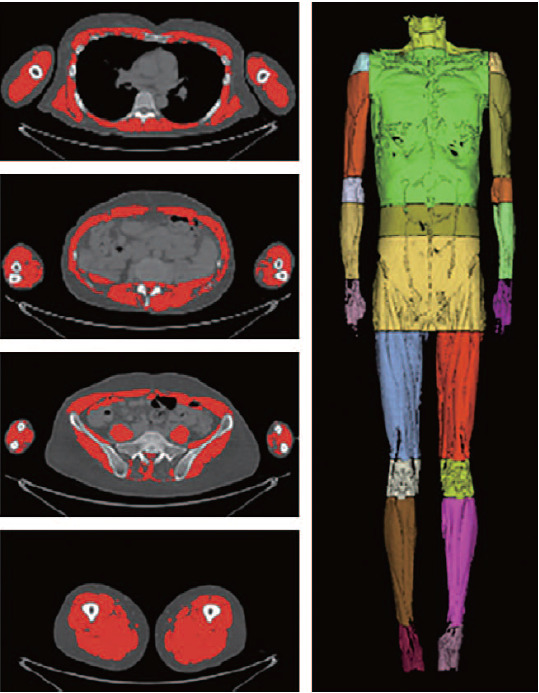
For patients with hip fractures, diagnosis of sarcopenia is based on diagnostic criteria for acute to chronic health care settings. However, evaluation of physical ability in patients with hip fractures is not possible. Only grip strength can be measured. In addition, while measurement of muscle mass is important for patients with hip fractures, whole-body DEXA imaging before surgery is difficult due to pain and anatomical abnormalities at the fracture site. Therefore, in the future, study of the cut-off point of the muscle mass using CT and MRI will be necessary. Development of a method for evaluating muscle mass that reflects the clinical significance and prognosis after surgery is also important. In addition, development of a tool for evaluating the quality of muscles based on the amount of fat and water in muscles is needed7,20,21,22,23,24). Automated segmentation studies of whole-body muscles using deep learning are currently in progress. Automated classification of these muscles will enable specific imaging tests for sarcopenia using CT and MRI25,26,27) (Fig. 3).
Fig. 3. Deep neural network for automatic volumetric segmentation of whole-body computed tomography images.
Previous studies have reported on the usefulness of the SARC-F questionnaire for screening sarcopenia in community dwellings. Validation studies for the Korean translated version have been reported28). In addition, a study has reported the usefulness of the SARC-F questionnaire for patients with hip fractures in Korea with a high diagnostic sensitivity and a low diagnostic specificity29). Thus, more research is needed for development of a screening tool with both high sensitivity and specificity.
1. Appendicular Skeletal Muscle Mass (ASM)
A measurement value called skeletal muscle index (SMI), obtained by dividing the sum of the muscle mass of the extremities by the square of the height, is currently used in diagnosis of sarcopenia according to the AWGS. According to the DEXA test, if the standard SMI is less than 7.0 kg/m2 for men and less than 5.4 kg/m2 for women, it can be concluded that the muscle mass is lower than the standard. Body impedance analysis (BIA) is also used as a method for measurement of muscle mass. If the muscle mass is less than 7.0 kg/m2 for men and less than 5.7 kg/m2 for women, it can be concluded that the muscle mass is lower than the standard6). However, BIA measurement using commercially available wearable devices or home equipment is not recommended. Instead, a multi-frequency BIA measurement is recommended. While ultrasound is recommended as a tool for evaluation of muscle mass, it is currently used for research purposes only. In Korea, measurement of muscle using the DEXA method has been recognized as an approach for diagnosis of sarcopenia since 2019, with a growing popularity. Because there are significant differences in measured muscle values between those of bone density, validation of instruments and standardization of measuring methods through country-based research should be conducted and re-discussed in the future. Studies on errors of muscle mass values due to measurement time and soft tissue edema at the time of measurement should also be conducted.
2. Handgrip Strength Measurement
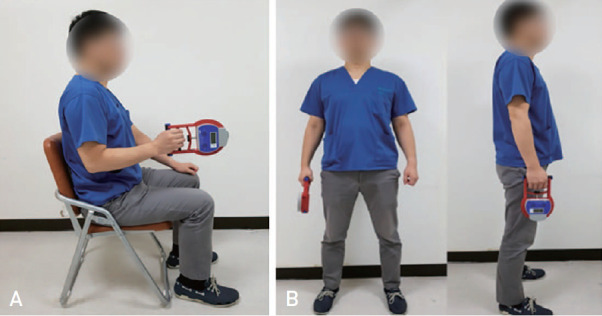
For consistent measurement and accurate diagnosis of sarcopenia, this study adopted the method suggested by Ha et al.30), where the subject used a Smedley-type dynamometer while in a standing position, achieving maximum grip strength three times with both hands. Since it is difficult for patients with hip fractures to remain standing, a method of measuring maximum grip strength in a sitting position with elbow at angle of 90 degrees is presented (Fig. 4).
Fig. 4. Hand grip strength measurement in sitting position (A) and standing position (B).
However, it remains controversial whether measurement of grip strength alone is an effective evaluation method for the management of sarcopenia in patients with hip fractures. In addition, many patients have postoperative delirium or neurological disease, making measurement of handgrip strength difficult using current standardized protocols. Therefore, development of tools for assessment of muscle strength for diagnosis of sarcopenia in postoperative patients with hip fractures is needed. Although surface electromyography (sEMG) has been used for assessment of muscle strength in patients with hip fractures, a standardized protocol for assessment of muscle strength in patients with hip fractures has yet to be established31). A standardized method for measurement of muscle strength for treatment of sarcopenia in patients with hip fractures should be developed in the future.
3. Physical Performance Measurement
Methods such as Short physical performance (≤9), 6-meter walking time (<1.0 m/s), and 5-time chair stand test (≥12 seconds) are used in evaluation of physical performance in the diagnosis of sarcopenia6) (Fig. 5). However, the Timed Up and Go test (TUG) widely used in previous studies was excluded from the revised AWGS guidelines. TUG was not recommended as a method for evaluating physical performance because other underlying diseases could not be excluded.
Fig. 5. Physical performance measurement using 6-meter walking time (A) and 5-time chair stand test (B).

Application of these tools for evaluation of physical performance is also difficult in patients with hip fractures. For this purpose, non-face-to-face gait analysis studies using various types of artificial intelligence are being conducted. However, clear diagnostic criteria have not yet been established.
4. Other Sarcopenia Assessment Tools
Evaluation of muscle mass using the D3Cr dilution method is also considered for diagnosis of sarcopenia. Briefly, assessment of systemic creatinine pool size and total body muscle mass is performed using a single oral dose of deuterated creatine (D3-creatine). The tablet enters the endogenous creatine pool of skeletal muscle and becomes absorbed and diluted32,33). Measurement of labeled creatinine (D3-creatinine) and unlabeled creatinine in fasting single void urine samples is performed 3 to 6 days after dose intake. These measurements are then incorporated into an algorithm that determines the total body creatine pool size and skeletal muscle mass in kilograms. Research on D3Cr is being actively conducted in the United States; however, it has not yet been implemented in Korea.
In addition, the Korean version of SARC_QOL has been recently translated into 30 languages and published on the website (http://www.sarqol.org). A validation study on the translation has already been reported34).
5. Suggestions for the Development of a Sarcopenia Evaluation Method for Patients with Hip Fractures
Gait status and lower extremity strength cannot be properly evaluated in patients with hip fractures due to their pain and loss of walking ability at the time of injury. For evaluation of their muscle functions, our only choice is to rely on questionnaire-based methods for evaluation of gait including Koval score. In the measurement method according to the currently used sarcopenia diagnostic criteria, only evaluation of muscle mass using DEXA and BIA and grip strength using a hand dynamometer are possible and the SARC-F questionnaire test for screening can be performed. Since grip strength cannot fully reflect the strength of the lower extremities, development of a method for evaluating the strength of the lower extremities for patients with hip fractures is necessary. In addition, in the evaluation of muscle mass using DEXA, accurate evaluation is often impossible due to metal artifacts or difficulty in taking a posture. Therefore, an accurate system for evaluation of muscle mass based on CT scan is required. Although validation studies on patients with hip fractures were published, very low specificity was reported for the SARC-F questionnaire. Therefore, development of a specialized questionnaire item that can predict the decrease of muscle function in patients with hip fractures is necessary. In addition, development of an intraoperative biomarker for sarcopenia will be necessary so that future rehabilitation plans can be implemented based on the muscle findings in the operating room.
6. Rehabilitation for Sarcopenia in Patients with Hip Fractures
Oh et al.35) applied an antigravity treadmill combined with conventional rehabilitation for 10 days after surgery in hip fracture patients with sarcopenia. Although functional scores such as Koval score and Berg Balance Scale in patients at postoperative 3-6 months showed improvement, they plateaued in the subsequent period. The authors concluded that additional rehabilitation with an antigravity treadmill was beneficial for patients with hip fractures. However, no improvement in handgrip strength was reported35). Lim et al.36) operated an elaborate rehabilitation program for approximately 10 days in patients who had undergone a hip fracture surgery. Based on clinical results, the authors reported that use of this rehabilitation program resulted in improvement of ambulatory functions such as Koval score and Functional Ambulatory and Category scale of patients at up to six months after surgery regardless of the presence of sarcopenia36). However, no significant improvement in handgrip strength was observed in that study. Although improvement of functional score was observed in both studies, several factors should be considered for the fact that there was no change in handgrip strength, an index related to sarcopenia. First, exercise for 10 days after surgery might be too short for improvement of sarcopenia. Lim et al.36) reported that only 61.5% to 70% of patients recovered with walking ability before fracture and treatments for hip fracture did not prevent muscle loss, even considering the complications of fractures. Muscle mass and muscle strength and body muscle function can be improved by performance of moderate to high intensity resistance exercises. More research is needed to determine the intensity and duration of exercises that can increase muscle mass in patients with hip fractures. In consideration of this, a change in the rehabilitation protocol is required. Second, the sarcopenia status of patients might have been affected by failure of social or familial support for rehabilitation after surgery. Third, there was no support for nutritional status in either study.
SUMMARY
The global prevalence of sarcopenia in patients with hip fractures is statistically significant. Although various therapeutic agents and diagnostic criteria have been established for osteoporosis, there are no clear, useful criteria for diagnosis of sarcopenia in the clinical field. In particular, few studies on the evaluation and treatment of sarcopenia in patients with hip fractures have been reported. In addition, the quality of life of postoperative patients with hip fractures could be significantly improved by development of precise assessment for muscle regeneration and rehabilitation in the operating room.
ACKNOWLEDGEMENTS
This work was supported by a grant (No. NRF-2019R1F1A1059208) from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST).
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article.
References
- 1.Palus S, Springer JI, Doehner W, et al. Models of sarcopenia: short review. Int J Cardiol. 2017;238:19–21. doi: 10.1016/j.ijcard.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 2.Xie WQ, He M, Yu DJ, et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle. 2021;12:538–554. doi: 10.1002/jcsm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson S, Cooper C, Aihie Sayer A. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Faron A, Sprinkart AM, Kuetting DLR, et al. Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep. 2020;10:11765. doi: 10.1038/s41598-020-68797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiro AJ, Hoang TD, Shakir MKM. Artifacts affecting dual-energy x-ray absorptiometry measurements. AACE Clin Case Rep. 2019;5:e263–e266. doi: 10.4158/ACCR-2019-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc. 2014;15:551–558. doi: 10.1016/j.jamda.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28:189–199. doi: 10.1007/s00198-016-3823-0. [DOI] [PubMed] [Google Scholar]
- 11.Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16:247–252. doi: 10.1016/j.jamda.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc. 2014;15:918–923. doi: 10.1016/j.jamda.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JI, Ha YC, Kwon HB, Lee YK, Koo KH, Yoo MJ. High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci. 2016;31:1479–1484. doi: 10.3346/jkms.2016.31.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hida T, Ishiguro N, Shimokata H, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013;13:413–420. doi: 10.1111/j.1447-0594.2012.00918.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr. 2012;55:e48–e52. doi: 10.1016/j.archger.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 16.González-Montalvo JI, Alarcón T, Gotor P, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int. 2016;16:1021–1027. doi: 10.1111/ggi.12590. [DOI] [PubMed] [Google Scholar]
- 17.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Hunter GR, Singh H, Carter SJ, Bryan DR, Fisher G. Sarcopenia and its implications for metabolic health. J Obes. 2019;2019:8031705. doi: 10.1155/2019/8031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutin RD, Lenchik L. Value-added opportunistic CT: insights into osteoporosis and sarcopenia. AJR Am J Roentgenol. 2020;215:582–594. doi: 10.2214/AJR.20.22874. [DOI] [PubMed] [Google Scholar]
- 21.Birkbeck MG, Blamire AM, Whittaker RG, Sayer AA, Dodds RM. The role of novel motor unit magnetic resonance imaging to investigate motor unit activity in ageing skeletal muscle. J Cachexia Sarcopenia Muscle. 2021;12:17–29. doi: 10.1002/jcsm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuñez-Peralta C, Alonso-Pérez J, Díaz-Manera J. The increasing role of muscle MRI to monitor changes over time in untreated and treated muscle diseases. Curr Opin Neurol. 2020;33:611–620. doi: 10.1097/WCO.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 23.Carlier PG, Marty B, Scheidegger O, et al. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis. 2016;3:1–28. doi: 10.3233/JND-160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa M, Lester R, Akima H, Gorgey AS. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res. 2017;12:2100–2105. doi: 10.4103/1673-5374.221170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto F, Kakimoto A, Ota N, Ito S, Nishizawa S. Automated segmentation of 2D low-dose CT images of the psoas-major muscle using deep convolutional neural networks. Radiol Phys Technol. 2019;12:210–215. doi: 10.1007/s12194-019-00512-y. [DOI] [PubMed] [Google Scholar]
- 26.Ahn DW, Jeong JB, Kang J, et al. Fatty liver is an independent risk factor for gallbladder polyps. World J Gastroenterol. 2020;26:6979–6992. doi: 10.3748/wjg.v26.i44.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byun SE, Kim S, Kim KH, Ha YC. Psoas cross-sectional area as a predictor of mortality and a diagnostic tool for sarcopenia in hip fracture patients. J Bone Miner Metab. 2019;37:871–879. doi: 10.1007/s00774-019-00986-1. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Kim M, Won CW. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc. 2018;19:40–45.e1. doi: 10.1016/j.jamda.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ha YC, Won Won C, Kim M, Chun KJ, Yoo JI. SARC-F as a useful tool for screening sarcopenia in elderly patients with hip fractures. J Nutr Health Aging. 2020;24:78–82. doi: 10.1007/s12603-019-1307-6. [DOI] [PubMed] [Google Scholar]
- 30.Ha YC, Yoo JI, Park YJ, Lee CH, Park KS. Measurement of uncertainty using standardized protocol of hand grip strength measurement in patients with sarcopenia. J Bone Metab. 2018;25:243–249. doi: 10.11005/jbm.2018.25.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo JI, Byun H, Kim HS, Jang YJ, Lee CH. Evaluating postoperative muscle strength using surface electromyography in hip fracture patient. J Bone Metab. 2020;27:125–132. doi: 10.11005/jbm.2020.27.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers-Soeder TS, Peters KE, Lane NE, et al. Dietary intake, D3Cr muscle mass, and appendicular lean mass in a cohort of older men. J Gerontol A Biol Sci Med Sci. 2020;75:1353–1361. doi: 10.1093/gerona/glz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon PM. The importance of muscle versus fat mass in sarcopenic obesity: a re-evaluation using D3-creatine muscle mass versus DXA lean mass measurements. J Gerontol A Biol Sci Med Sci. 2020;75:1362–1368. doi: 10.1093/gerona/glaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo JI, Ha YC, Kim M, et al. Translation and validation of the Korean version of the Sarcopenia Quality of Life (SarQoL-K®) questionnaire and applicability with the SARC-F screening tool. Qual Life Res. 2021;30:603–611. doi: 10.1007/s11136-020-02630-2. [DOI] [PubMed] [Google Scholar]
- 35.Oh MK, Yoo JI, Byun H, et al. Efficacy of combined antigravity treadmill and conventional rehabilitation after hip fracture in patients with sarcopenia. J Gerontol A Biol Sci Med Sci. 2020;75:e173–e181. doi: 10.1093/gerona/glaa158. [DOI] [PubMed] [Google Scholar]
- 36.Lim SK, Beom J, Lee SY, et al. Association between sarcopenia and fall characteristics in older adults with fragility hip fracture. Injury. 2020;51:2640–2647. doi: 10.1016/j.injury.2020.08.031. [DOI] [PubMed] [Google Scholar]


