Abstract
Background
Tracheal resection and reconstruction are the most effective treatments for tracheal stenosis, but the difficulties are surgery and maintaining ventilation performed on the patient’s same airway. High-flow oxygen has begun to be applied to prolong the apnoea time in the tracheal anastomosis period for tracheal resection and reconstruction. This study aims to evaluate the effectiveness of apneic conditions with high-flow oxygen as the sole method of gas exchange during anastomosis construction.
Methods
A prospective study was performed on 16 patients with tracheal stenosis, with ages ranging from 19 to 70, who underwent tracheal resection and reconstruction from April 2019 to August 2020 in 108 Military Central Hospital. During the anastomosis phase using high flow oxygen of 35–40 l.min-1 delivered across the open tracheal with an endotracheal tube (ETT) at the glottis in apnoeic conditions.
Results
The mean (SD) apnoea time was 20.91 (2.53) mins. Mean (SD) time anastomosis was 22.9 (2.41) mins. The saturation of oxygen was stable during all procedures at 98–100%. Arterial blood gas analysis showed mean (SD) was hypercapnia and acidosis acute respiratory after 10 mins of apnoea and 20 mins apnoea respectively. However, after 15 mins of ventilation, the parameters are ultimately returned to normal. All 16 patients were extubated early and safely at the end of the operation. There were no complications, such as bleeding, hemothorax, pneumothorax, or barotrauma.
Conclusion
High-flow oxygen across the open tracheal under apnoeic conditions can provide a satisfactory gas exchange to allow tubeless anesthesia for tracheal resection and reconstruction.
Keywords: Tracheal resection, Reconstruction, Stenosis, Anesthesia, High-flow, Apnoeic oxygenation
Background
Tracheal stenosis is a rare disease often caused by prolonged intubation, primary or secondary tumors, and invasive thyroid cancer. They are life-threatening if not adequately treated [1]. Tracheal resection and reconstruction are the most effective treatment, but the difficulties are surgery and maintaining ventilation performed on the patient’s same airway [2].
Depending on the severity and location of the stenosis and the type of surgical procedure, there may be a variety of choices for perioperative airway management such as a facemask, laryngeal mask airway, tracheal intubation tube, jet ventilation with the small catheter, cardiopulmonary bypass, and extracorporeal membrane oxygenation [3, 4].
The methods like intubation or using small-sized high-frequency catheters often cause a narrow surgical field and limited vision.
Recently, high-flow nasal and high-flow oxygen delivery device attached directly to a laryngeal mask or tracheal tube has begun to be applied to provide oxygen for some laryngeal surgery without intubation as well as prolonging the apnoea time in the difficult intubation [5, 6].
We also applied this method during the tracheal anastomosis period for tracheal resection and reconstruction, aiming to create an optimal surgical field without endotracheal intubation and ventilation [7].
This study aimed to evaluate the gas exchange efficiency and safety of apnoeic oxygenation with a high-flow oxygen method, used for airway management at 16 patients’ tracheal resection and reconstruction [8].
Methods
From April 2019 to August 2020, 16 patients were diagnosed with tracheal stenosis caused by cancer invasive thyroid cancer, tracheal stenosis after intubation, and upper tracheal tumor. They were scheduled for tracheal resection and reconstruction surgery using high-flow oxygen combined for airway management at the Department of Anesthesia of 108 Military Central Hospital.
Preoperative preparation and materials
The equipment and anesthesia agents:
Anesthesia machine, multi-parameter monitor, TCI (target-controlled infusion) system.
Fibroscope, rigid bronchoscope
High-flow oxygen system
Flexible endotracheal tubes (ETT), ID sizes from 4.0 to 7.5. The Proseal laryngeal mask size is 3–5. Catheters with diameters of 3.3–4.7 mm (Cook Airway Exchange Catheter) can pass through narrow positions for high-frequency jet ventilation.
Anesthesia agents: Propofol, rocuronium, fentanyl, morphine.
Emergency instruments.
Evaluation of patients:
Evaluation of patients includes general history and physical examination, with particular attention to the airway and pulmonary systems, and analyzing arterial blood gases.
To prepare for any possible airway emergency during the induction and maintenance of anesthesia, it is essential to carefully evaluate the stenosis or tumor’s exact location and the obstruction degree preoperatively.
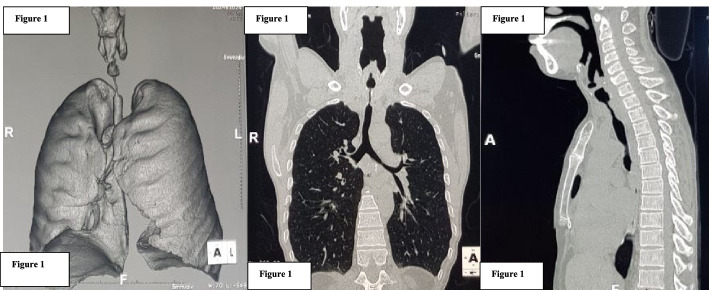
Computed tomography (CT) scans provide the greatest diameter of the tracheal stenosis or tumor, minimum tracheal diameter, the length of the lesion, the distance from the vocal cord to the tracheal stenosis, from stenosis to the carina… (Fig. 1).
Fig. 1.
CT scan image of tracheal stenosis after intubation
Bronchoscopy defines the character of the tracheal stenosis or tumor surrounding the tracheal by a direct vision that can be predicted capacity intubated and operated.
Method
Before the induction, it must be considered that spontaneous breathing or mechanical ventilation is likely to be possible through the stenosis with general anesthesia. Nevertheless, severe airway obstruction may occur during the induction of general anesthesia, and thus the appropriate backup method will be required to prevent disaster.
The surgeon should always be in the OR during induction and available to manage a surgical airway if this becomes necessary. A rigid bronchoscope, trans tracheal jet ventilation (TTJV) must be immediately available.
If possible, mask ventilation under general anesthesia, preoxygenation with 6 l.min-1100% for 5 mins, slow and gentle induction of anesthesia followed by IV: fentanyl 2 μg/kg, propofol TCI Cp 3.5–4 μg/ml, rocuronium 0.6 mg/kg.
The positive pressure ventilation was secured via facemask, inserting the endotracheal tube into the tracheal so that the tip of the endotracheal tube is close to the stenosis if the distance from the vocal cord to the lesion >2 cm. If mild tracheal stenosis, we use tube 5.0–6.0 Fr passed through the narrow position.
If the distance from the vocal cord to the lesion is very short <2 cm, the Proseal laryngeal mask (LMAP) is placed, inserted sonde gastric through the second tube of LMAP, continuous ventilation via LMAP.
If the patient has severe stenosis and airway obstruction, we use catheters with diameters of 3.3–4.7 mm. (Cook Airway Exchange Catheter) put through narrow positions for high-frequency jet ventilation with 100% oxygen.
In case the patient has a tracheostomy, ventilating through the tube of tracheostomy.
Anesthesia was maintained intravenously by the combination of propofol using Target Controlled Infusion (TCI) method 3.5–4 μg/ml, fentanyl 2–3 μg /kg/h, rocuronium 0.2 mg/kg/h, methylprednisolone 2 mg/kg.
Once the airway is opened, the surgeon inserts a flexible endotracheal tube 6.5–7.5 Fr into the distal airway and ventilates. They were completely removing the laryngeal mask, endotracheal tube, catheter Cook. Setting a waiting endotracheal tube at glottis and connecting to the high-flow oxygen system with FiO2 100%.
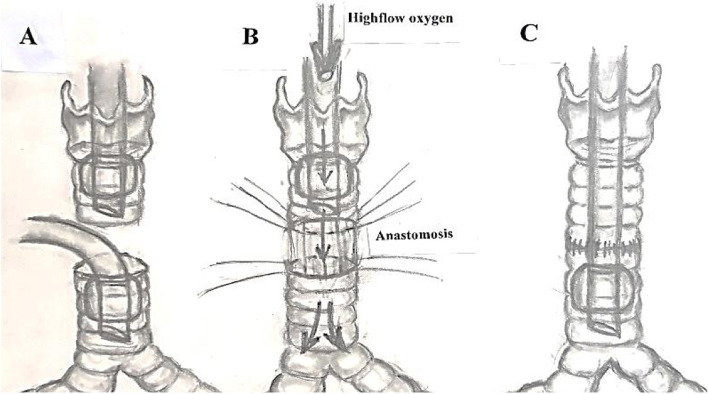
Once the tracheal lesion is removed, and the surgeon starts anastomosis, open the oxygen flow 35–40 l.min-1 so that the oxygen is provided across the open trachea. Adjust for the direction of oxygen flow straight to the distant tracheal. During this period, the patient is still under general anesthesia, has neuromuscular blocking agents, and stops breathing completely. SpO2 and arterial blood gases were monitored. If SpO2 drops <90%, ventilation supports 100% oxygen with an endotracheal tube at the tracheal distance. When the tracheal is nearly closed, push the endotracheal tube so that the cuff passes over the anastomosis and normalizes the ventilation (Figs. 2 and 3).
Fig. 2.
Diagram of the equipment connections.A: Open airway, a flexible endotracheal tube in the distal airway. B. High-flow oxygenation during anastomosis.C. Anastomosis completed
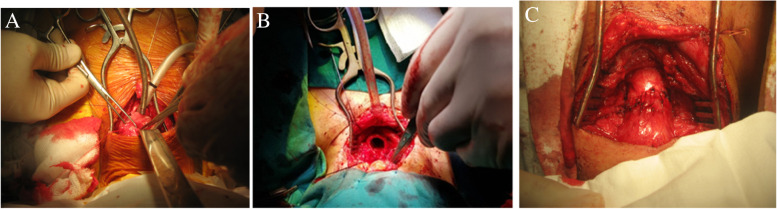
Fig. 3.
Some intraoperative images. A: Open airway, a flexible endotracheal tube in the distal airway. B. High-flow oxygenation during anastomosis. C. Anastomosis completed
End of surgery: Extubation is the primary goal because postoperative mechanical ventilation is associated with anastomotic failure; the reconstructed airway may be tenuous. When the patients were awake and cooperative, all vital parameters were normal limits; patients should be extubated on the table as soon as possible.
Monitoring and evaluation criteria
- Characteristics of the patients: Age, height, body weight, classification of dyspnea according to MMRC (Modified Medical Research Council), degree of the physical state of the patients ASA (American Society of Anesthesiologists). Degree of tracheal stenosis according to Cotton- Mayer
Surgical characteristics: Anesthesia time, surgery time
The method of maintaining ventilation.
The anastomosis time: from the tracheal open to the end closed airway.
Apnoeic time using high flow
The vital parameters were monitored continuously: Heart rate, SpO2, EtCO2, Blood pressure. All results are expressed as mean ± SD, blood gases analysis (ABG) at 5 periods: T0: Before the anesthesia; T1: Before using high flow. T2: After using high-flow 10 mins; T3: After using high-flow 20 mins and T4: 15 mins after high-flow finishes.
The complications in surgery include airway obstruction, tumor peeling, gas overflow, pneumothorax.
Results
The mean age of the group was 46.50 ± 16.42 years. The proportion of males in the group (56.25%) was higher than females (43.75%). Most patients were ASA III, 02 patients were ASA IV, who have life-threatening dyspnea requiring emergency surgery (Table 1). The cause of tracheal stenosis, classification of dyspnea, and degree of tracheal stenosis were shown in Tables 2, 3 and 4. There are 11 patients classified dyspnea grade 2 and 3 according to the Modified Medical Research Council, respectively with grades 2 and 3 of tracheal stenosis according to Cotton- Mayer (Table 4). The average apnoea time was 20.91 ± 2.53 mins which means the time is enough for anastomosis. Additional information is shown in Table 5.
Table 1.
Characteristics of the patients
| Age (years)± SD Min-Max |
Height(cm) ± SD Min-Max |
Weight(kg) ± SD Min-Max |
Gender | ASA | |||
|---|---|---|---|---|---|---|---|
| Male n(%) |
Female n(%) |
II n(%) |
III n(%) |
IV n(%) |
|||
|
46.50 ± 16.42 19–70 |
160.06 ± 5.16 152–168 |
50.50 ± 3.39 43–55 |
09(56.25) | 07(43.75) | 06(37.5) | 08 (50) | 02(12.5) |
Table 2.
Cause of tracheal stenosis
| Causes | Number of patients (n) | Percentage % |
|---|---|---|
| After prolong intubaion | 09 | 56.25 |
| After tracheotomy | 02 | 12.5 |
| Tracheal tumour | 01 | 6.25 |
| Thyroid cancer invasion | 04 | 25 |
| Total | 16 | 100 |
Table 3.
Classification of dyspnea (Modified Medical Research Council)
| Dyspnea scale | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| n | 3 | 6 | 5 | 2 | 16 |
| % | 18.75 | 37.5 | 31.25 | 12.5 | 100 |
Table 4.
Degree of tracheal stenosis according to Cotton- Mayer
| Degree of tracheal stenosis (%) | Number of patients | Percentage % |
|---|---|---|
| Grade: luminal narrowing <50% | 03 | 18.75 |
| Grade 2: luminal narrowing 51…. < 71% | 06 | 37.5 |
| Grade 3: luminal narrowing 71…. < 99% | 05 | 31.25 |
| Grade 4: luminal narrowing >99% | 02 | 12.5 |
| Total | 16 | 100 |
Table 5.
Surgical characteristics
| Time (mins) | Min- Max | Value ± SD |
|---|---|---|
| Duration of anesthesia | 115–220 | 170.69 ± 31.89 |
| Duration of surgery | 95–185 | 134.56 ± 21.18 |
| Duration of anastomosis | 17–28 | 22.9 ± 2.41 |
| Oxygen high-flow (apnoea time) | 16–28 | 20.91 ± 2.53 |
There may be a variety of choices for perioperative airway management. In the research, there was one patient with hypoxia who require urgent interruption of surgery must use high-flow oxygen combined ventilation intermittently through endotracheal tubes at the distant tracheal (Table 6). During the period high-flow (T2, T3), PaO2 improved significantly compared to the time of T0; acute respiratory acidosis clearly showed pH decreased, PaCO2 and HCO3- increased. However, these data return to normal at the time of T4 (Table 7 and Fig. 4). Heart rate and MAP after high-flow oxygen was decreased significantly compared with T0 (before induction), SpO2 at T0 was significantly lower than T1, T2, T3. After 20 mins of high flow (T3), etCO2 was significantly higher than before and after using high-flow oxygen (Table 8).
Table 6.
Airway management
| Period | Methods | Number of patients | Percentage % |
Total |
|---|---|---|---|---|
| Induction (before dissection) | Intubation above the lesion | 04 | 25 | 100% |
| Intubation through the lesion | 06 | 37.5 | ||
| Laryngeal mask | 03 | 18.75 | ||
| At tracheostomy | 02 | 12.5 | ||
| The Small catheter for jet ventilation | 01 | 6.25 | ||
| Open airway (Anastomosis) | High-flow oxygen single | 15 | 93.75 | 100% |
|
High-flow oxygen combined ventilation intermittent (Rescue mechanical ventilation) |
01 | 6.25 |
Table 7.
Arterial blood gas exchange data
| Time Data | T0 ± SD |
T1 ± SD |
T2 ± SD |
T3 ± SD |
T4 ± SD |
|---|---|---|---|---|---|
| pH | 7.42 ± 0.02 | 7.42 ± 0.06 | 7.25 ± 0.04* | 7.17 ± 0.05* | 7.41 ± 0.06 |
| PaCO2 (mmHg) | 35.95 ± 3.32 | 42.17 ± 9.63 | 67.57 ± 14.71* | 79.63 ± 13.39* | 39.48 ± 5.17 |
| PaO2 (mmHg) | 101.1 ± 3.54 | 220.38 ± 62.08* | 167.12 ± 76.23* | 186.19 ± 60.14* | 217.63 ± 74.63* |
| Lactat | 1.0 ± 0.42 | 1.16 ± 0,51 | 1.06 ± 0.6 | 1.06 ± 00.58 | 1.26 ± 0.87 |
| HCO3−(mEq/L) | 23.35 ± 3.75 | 27.34 ± 5.45 | 29.79 ± 6.73* | 30.03 ± 5.5* | 25.98 ± 4.78 |
| BE (mEq/L) | 2.4 ± 0.99 | 4.57 ± 6.32 | 3.23 ± 6.08 | 2.91 ± 5.31 | 2.22 ± 4.99 |
*: p < 0.05 compared with T0
Fig. 4.
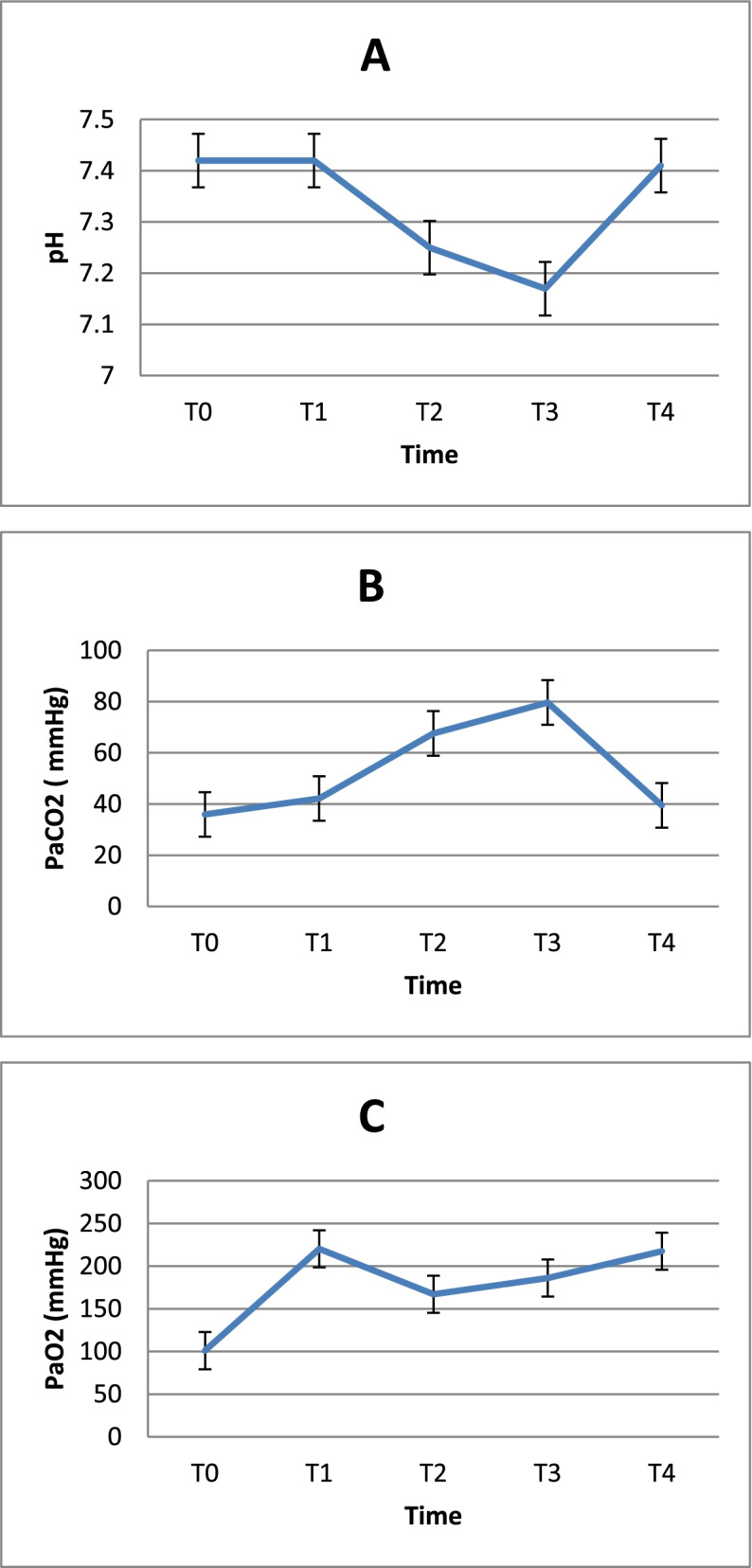
A pH, B partial pressure of arterial carbon dioxide (PaCO2) and C partial pressure of arterial oxygen (PaO2) at T0: Before anesthesia; T1: Before using high flow. T2: After using high flow 10 mins; T3: After 20 mins of high flow and T4: Finish the high-flow 15 mins
Table 8.
Change of respiratory and hemodynamic
| Time Data | T0 ± SD |
T1 ± SD |
T2 ± SD |
T3 ± SD |
T4 ± SD |
|---|---|---|---|---|---|
| Heart rate (beat/min) | 87.3 ± 7.5 | 76.58 ± 3.42 | 78.5 ± 4.89 | 79.17 ± 6.19 | 77.17 ± 5.62 |
| MAP(mmHg) | 85 ± 12 | 74.5 ± 3.78 | 74.33 ± 5.14 | 75.42 ± 6,43 | 77.16 ± 3.76 |
| SpO2 (%) | 94 ± 3.7 | 99.83 ± 0.58* | 99.17 ± 1.11* | 99.67 ± 0.49* | 99.66 ± 0.65* |
| EtCO2(mmHg) | 35.67 ± 3.39 | 55.4 ± 7.16 ┼ | 33.58 ± 2.78 |
* p < 0.05 compared with T0; ┼ p < 0,05compared with T1
Side effects (Table 9) in surgery were acute respiratory acidosis in all 16 patients, 01 patients with hypoxia had to require interruption surgery and rescue ventilation intermittent through endotracheal tubes at the distant tracheal. There were no complications of arrhythmia, pneumothorax, hemothorax, pulmonary barotrauma.
Table 9.
Side effects
| Complications | Number of patients (n) | Percentage % |
|---|---|---|
| Acute respiratory distress (PaO2 < 60, SpO2 < 90) | 1 | 6.25 |
| Acute respiratory acidosis (PaCO2 > 50 mmHg) | 16 | 100 |
| Respiratory obstruction | 0 | 0 |
| Arrhythmia | 0 | 0 |
| Pneumothorax, hemothorax, pulmonary barotrauma | 0 | 0 |
Discussion
There are many treatment methods for tracheal stenosis such as conservative treatment, endoscopic treatment by luminal restoration with the main aim is to dilate the stenotic segment to match as closely as possible the normal proximal and distal diameters by using cold knife Laser: CO2, Nd-YAG, diode Diathermy Argon plasma or Cryoprobe Mechanical dilatation (dilators, rigid bronchoscopes) CRE Balloons [9–13]. Then maintenance therapy with Mitomycin, Steroids, Brachytherapy, and stents [14]. These methods offer good short-term effects and provide temporary relief, but it usually isn’t a long-term solution. In some situations, they can worsen the stenosis. Most of our patients with narrow airways were admitted to the hospital due to shortness of breath, there are 11 patients classified dyspnea grade 2 and 3 according to the Modified Medical Research Council, respectively with grades 2 and 3 of tracheal stenosis according to Cotton- Mayer. There are 03 cases of patients who can not lie, 02 cases of life-threatening who have to do the emergency surgery are classified as grade 4 dyspnea corresponding to luminal narrowing >99% (Table 3), 9 of our 16 patients had received conservative treatment but failed or had recurrent stenosis so that all patients were indicated for surgery.
These days, tracheal resection and reconstruction are known as the standard treatment for tracheal stenosis and tumor. Outcomes after tracheal surgery are usually very good, which can provide a long-lasting cure to these patients [1, 2, 14, 15].
The course of anesthesia was divided into five phases. First: induction and intubation, a critical period. Second: dissection, a period of relative calm during which lesion is defined. Third: open airway, a crucial period in which anastomosis is being constructed. Fourth: closure and emergence and fifth: extubation [2, 15, 16].
Induction is a critical period that needs to combine many flexible methods to control ventilation. The results in Table 6 show that depending on the severity and location of the stenosis, there may be various choices for airway management in the period of induction. The endotracheal tube may be placed above (25%) or through the lesion (37.5%), there are 3 patients (18.75%) showing the distance from the vocal cord to the lesion very short (< 2 cm) so we had to use a laryngeal mask (LMAP) for ventilation and found it to be a quite safe and effective solution. The LMA has also been applied to solve some of the problems posed by tracheal intubation during tracheal surgery [17–19].
J.V. Divatia et al. [18], Byung Cheul Shin et al. [19] have used the LMAP for airway management successful without any complications for surgery of a high tracheal tumor and high tracheal stenosis sited near the vocal cord, it is difficult to manage airway using a cuffed endotracheal tube. They found that the LMA has advantages when used in general anesthesia for tracheal surgery, especially with tumors situated just below the vocal cords.
Patients with severe tracheal stenosis more than 90% were under a high risk of airway obstruction. By using high-flow oxygen, the safety of these patients can be ensured. Bricker DL et al. [17]; C.L Chiu et al. [20], CHEN Hai-hong et al. [5] applied the method of cardiopulmonary bypass. This method was an easy way to ensure gas exchange. However, systemic anticoagulation theoretically increased bleeding risk, especially in the case of extensive dissection which often led to lung manipulation.
Before the induction, we prepared all emergency equipment to prevent tracheal obstruction. Small-sized catheters to pass through the narrow for jet ventilation, surgeons, as well as surgical facilities, are prepared for tracheotomy. In 2 of our patients with tracheal stenosis >90%, 01 patients can be intubated and ventilated above the narrow space. For the other, we had to use a small jet catheter pushed through the stenosis for jet ventilation with oxygen pure 100% before the open airway phase.
The open airway phase is a critical period in which anastomosis is being constructed. Once the airway is opened, a flexible endotracheal tube 6.5–7.5 Fr was inserted into the distal airway and ventilated, waiting for endotracheal tube through the glottis and connected to the high-flow oxygen system. When the surgeon started anastomosis, open the oxygen flow 35–40 l.min-1 so that the oxygen is provided across the surgical field to the distant trachea. The time for apnoeic oxygenation or the time of anastomosis was 16–28 mins, but this time depends on the surgeon’s experience. If the apnoea time is too long, which can lead to unsafety like hypercapnia or hypoxia (SpO2 < 90%, blood pH <7.1).
In the period of anastomosis being constructed 10 and 20 mins (T2, T3), with high flow oxygen 35–40 l.min-1, the blood oxygen pressure improved significantly compared to the time of T0 with PaO2 > 170 mmHg. The acute respiratory acidosis present, the lowest at T3 with pH was 7.17 ± 0.05, and PaCO2 was 79.63 ± 13.39 HCO3- increased significantly but returned to normal immediately after 15 mins mechanical ventilation at T4 (Table 7).
Tracheal resection and reconstruction require the anesthesiologist and the surgeon to share the airway. The greatest benefit of high flow is creating a free surgical field, optimal conditions for anastomosis, and no interruption of surgery without endotracheal intubation and ventilation [10].
Apnoeic oxygenation is a term that refers to a patient’s ability to receive oxygen in the absence of pulmonary movement. At the beginning of apnoea, oxygen is continuously transferred from the alveolus to the circulation to meet the body’s metabolic demands. This oxygen transfer results in the alveoli being emptied and the alveolar pressure decreasing, which is initially compensated for by alveolar volume loss due to elastic recoil and carbon dioxide transport from the blood to the alveolus. These compensatory systems rapidly deplete, and for oxygenation, a pressure gradient between the upper airway and the alveolus emerges [9, 13, 21].
M Egan et al. also applied 100% oxygen at a flow rate of 40 l.min-1 delivered across an open trachea in a case of an apnoeic female patient with subglottic tracheal stenosis, which resulted in 42-min uninterrupted surgery. The oxygen saturation rate remained more than 96% during the apnoeic period. Moreover, arterial blood gas parameters were within acceptable limits. There was no urgent interruption of surgery or rescue mechanical ventilation [10].
The investigation was conducted between November 2016 and May 2017 by C. Lyons; M. Callaghan et al. During apnea with high-flow nasal oxygen, 28 patients underwent tubeless laryngeal or tracheal surgery. Apnoea lasted a median of 19 (15–24) minutes. Four patients experienced a brief period of oxygen deficiency between 85 and 90%. At baseline, the carbon dioxide partial pressure (PaCO2) was 6.29 (0.71 kPa), and after 15 min of apnoea, it was 9.44 (1.12) kPa. The authors found that high-flow nasal oxygen provided during apnea may be sufficient for tubeless anesthesia for laryngeal surgery [9].
The safe breathing time was calculated from the time when the patient stopped breathing until the SaO2 ratio was less than 90%. Oxygen kept being exchanged in the alveoli even when there were no diaphragmatic movements or lung expansion. In the cases of healthy patients under apnoeic conditions, there were about 200–250 ml /min of oxygen move from the alveoli into the bloodstream. There were only 8–20 ml/min of carbon dioxide moved into the alveoli during apnoea. The rest of the carbon dioxide was buffered in the bloodstream because of its high water solubility. In the cases of healthy patients under ideal conditions, the PaO2 ration could be maintained more than 100 mmHg for up to 100 min without a breath. However, poor ventilation would significantly cause hypercapnia and acidosis. Many authors had demonstrated that acute respiratory acidosis within pH > 7.15 was the acceptable safety limit in case of no contraindications [10, 21, 22].
The results of Tables 8 and 9 showed that during procedure hemodynamics, oxygen pressure, oxygen saturation (SpO2) were within normal limits. Only 1 patient with hypoxia during surgery is the case of patients with pneumonia in the right lower lobe should support oxygen through the distant airway.
High-flow oxygen under apnoeic conditions can provide a satisfactory gas exchange with oxygenation indices improved than before surgery. The surgical field is especially completely spacious, with optimal conditions for anastomosis, and no interruption of operation without endotracheal intubation.
Conclusion
In the tracheal resection and reconstruction, high-flow oxygen through the open tracheal under apnoeic conditions can provide a significant gas exchange for tubeless anesthesia. The surgical field is completely spacious and under optimal conditions for anastomosis. There were no complications of arrhythmia, pneumothorax, hemothorax, and pulmonary barotrauma.
Limitation of the study
The limitation of this study there is the lack of transcutaneous Carbon Dioxide Monitoring (TcCO2), continuously monitoring of CO2 may allow prevention and early detection of the risk of hypercapnia at dangerous levels. In addition, the number of patients in the study group is not large enough.
Acknowledgments
Declared none.
Abbreviations
- ETT
Endotracheal tube
- TCI
Target-controlled infusion
- TTJV
Transtracheal jet ventilation
- LMAP
Laryngeal mask Proseal
- MMRC
Modified Medical Research Council
- ASA
American Society of Anesthesiologists
Authors’ contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design: NML, execution, acquisition of data: TXH, NVH, analysis and interpretation: NVD, DTTT; All authors took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The research was conducted following the guidelines and approval of 108 Military Central Hospital Clinical Researches Ethics Committee under the approval number 28/QĐ-BVTWQĐ108. All human research procedures followed were by the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013. Written informed consent was obtained from all the participants of the work.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest regarding the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nguyen Minh Ly, Email: nmly108@gmail.com.
Ngo Van Dinh, Email: ngodinh248@gmail.com.
Dinh Thi Thu Trang, Email: dttrang108@gmail.com.
Ngo Vi Hai, Email: haingovi@yahoo.com.
Tong Xuan Hung, Email: txhung108@gmail.com.
References
- 1.Sarper A, Ayten A, Eser I, Ozbudak O, Demircan A. Tracheal stenosis after tracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J. 2005;32(2):154. [PMC free article] [PubMed] [Google Scholar]
- 2.Sandberg W. Anesthesia and airway management for tracheal resection and reconstruction. Int Anesthesiol Clin. 2000;38(1):55–75. doi: 10.1097/00004311-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bouaggad A, Bennani F, Al Harrar R, Bouderka MA, Harti A. Anesthesia for a patient with tracheal tumor using laryngeal mask airway. Anesth Analg. 2006;103(1):258–259. doi: 10.1213/01.ANE.0000215222.54267.60. [DOI] [PubMed] [Google Scholar]
- 4.Kane D, Dave S, Thota RS, Pawar P, Dewoolkar L. Tracheal Tumour Resection–Anaesthesia Management. Bombay Hospital J. 2008;50(1):131. [Google Scholar]
- 5.Chen H-h, Pan W, An X-x. Resection of tracheal tumor under cardiopulmonary bypass: a case report. Chin Med J. 2005;118(12):1047–1049. doi: 10.1097/00029330-200706020-00004. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson L, Lang FJ, Monnier P, Ravussin P. Anaesthesia for tracheal resection: report of 17 cases. Can J Anaesth. 1997;44(12):1282–1285. doi: 10.1007/BF03012777. [DOI] [PubMed] [Google Scholar]
- 7.Mentzelopoulos SD, Romana CN, Hatzimichalis AG, Tzoufi MJ, Karamichali EA. Anesthesia for tracheal resection: a new technique of airway management in a patient with severe stenosis of the mid trachea. Anesth Analg. 1999;89(5):1156–1160. doi: 10.1213/00000539-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Binks MJ, Holyoak RS, Melhuish TM, Vlok R, Hodge A, Ryan T, et al. Apnoeic oxygenation during intubation in the intensive care unit: a systematic review and meta-analysis. Heart Lung. 2017;46(6):452–457. doi: 10.1016/j.hrtlng.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Lyons C, Callaghan M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017;72(11):1379–1387. doi: 10.1111/anae.14036. [DOI] [PubMed] [Google Scholar]
- 10.Egan M, Redmond KC. High-flow apnoeic oxygenation delivered by LMA or tracheal tube for tracheal resection and reconstruction surgery. Anaesthesia Cases. 2018;6(1):25–29. doi: 10.21466/ac.HAODBLO.2018. [DOI] [Google Scholar]
- 11.Silva LOJE, Cabrera D, Barrionuevo P, Johnson RL, Erwin PJ, Murad MH, et al. Effectiveness of apneic oxygenation during intubation: a systematic review and meta-analysis. Ann Emerg Med. 2017;70(4):483–494. doi: 10.1016/j.annemergmed.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 12.White LD, Melhuish T, White L, Wallace L. Apnoeic oxygenation during intubation: a systematic review and meta-analysis. Anaesth Intensive Care. 2017;45(1):21–27. doi: 10.1177/0310057X1704500104. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Asai T. High-flow nasal oxygenation for anesthetic management. Korean J Anesthesiol. 2019;72(6):527. doi: 10.4097/kja.19174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostafa BE, Chaouch-Mberek C, El Halafawi A. Tracheal Stenosis. 2012. [Google Scholar]
- 15.Pinsonneault C, Fortier J, Donati F. Tracheal resection and reconstruction. Can J Anesth. 1999;46(5):439–455. doi: 10.1007/BF03012943. [DOI] [PubMed] [Google Scholar]
- 16.Koga K. Safe method of tracheal extubation after tracheal reconstruction. J Anesth. 1997;11(2):171. doi: 10.1007/BF02480086. [DOI] [PubMed] [Google Scholar]
- 17.Bricker DL, Parker TM, Dalton ML. Cardiopulmonary bypass in anesthetic management of resection: its use for severe tracheal stenosis. Arch Surg. 1979;114(7):847–849. doi: 10.1001/archsurg.1979.01370310089016. [DOI] [PubMed] [Google Scholar]
- 18.Divatia J, Sareen R, Upadhye S, Sharma K, Shelgaonkar J. Anaesthetic management of tracheal surgery using the laryngeal mask airway. Anaesth Intensive Care. 1994;22(1):69–73. doi: 10.1177/0310057X9402200112. [DOI] [PubMed] [Google Scholar]
- 19.Shin BC, Lim CH, Kim DH, Shin HW, Lee HW, Lim HJ, et al. Anesthetic Management of Tracheal Reconstruction Surgery with Laryngeal Mask Airway: A case report. Korean J Anesthesiol. 2004;46(5):620–623. doi: 10.4097/kjae.2004.46.5.620. [DOI] [Google Scholar]
- 20.Chiu C, Teh B, Wang C. Temporary cardiopulmonary bypass and isolated lung ventilation for tracheal stenosis and reconstruction. Br J Anaesth. 2003;91(5):742–744. doi: 10.1093/bja/aeg244. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 22.Nickson C. Apnoeic oxygenation. Life in the fastlane. 2020. Available from: https://litfl.com/apnoeic-oxygenation/. Accessed 20 Apr 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.