Abstract
Background:
Lower heart rate (HR) increases during exercise and slower HR recovery (HRR) after exercise are markers of worse autonomic function that may be associated with risk of acute respiratory events (ARE).
Methods:
Data from 6-minute walk testing (6MWT) in COPDGene were used to calculate the chronotropic index (CI) [(HR immediately post 6MWT – resting HR) / ((220 – age) – resting HR)] and HRR at one minute after 6MWT completion. We used zero-inflated negative binomial regression to test associations of CI and HRR with rates of any ARE (requiring steroids and/or antibiotics) and severe ARE (requiring emergency department visit or hospitalization), among all participants and in spirometry subgroups (normal, chronic obstructive pulmonary disease [COPD], and preserved ratio with impaired spirometry).
Results:
Among 4,484 participants, mean follow-up time was 4.1 years, and 1,966 had COPD. Among all participants, CI-6MWT was not associated with rate of any ARE [adjusted incidence rate ratio (aIRR) 0.98 (0.95 to 1.01)], but higher CI-6MWT was associated with lower rate of severe ARE [0.95 (0.92 to 0.99)]. Higher HRR was associated with a lower rate of both any ARE [0.97 (0.95 to 0.99)] and severe ARE [0.95 (0.92 to 0.98)]. Results were similar in the COPD spirometry subgroup.
Conclusion:
Heart rate measures derived from 6MWT tests may have utility in predicting risk of acute respiratory events and COPD exacerbations.
Keywords: Pulmonary disease, chronic obstructive, Cardiac chronotropy, Disease exacerbation, Cohort study
INTRODUCTION:
Chronic obstructive pulmonary disease (COPD) is a chronic, progressive, debilitating respiratory disease which is primarily a result of tobacco smoking in high-income settings.1,2 Acute exacerbations of COPD (AECOPD) account for much of the morbidity and mortality in COPD and are defined as periods of increased respiratory symptoms that lead to a change in treatment.2,3 Established risk factors for AECOPD include previous AECOPD, low lung function, and a high burden of respiratory symptoms.2,4 Autonomic dysfunction and impaired heart rate (HR) response to exercise are common in COPD, and may be additional risk factors for AECOPD, but have not been well investigated.5,6
The autonomic nervous system innervates virtually every organ system, is responsible for maintaining physiologic homeostasis, and has a role in regulation of lung function, inflammation and immune function, and cardiac function, all of which have been implicated in COPD and AECOPD.7,8 One way to measure activity of the autonomic nervous system is through heart rate responses to exercise. The initial increase in HR during exercise is caused by withdrawal of parasympathetic activity (i.e. reduced vagal tone); with sufficient exercise intensity and duration, subsequent HR increases are caused by an increase in sympathetic activity.9 Chronotropic index quantifies the proportion of expected HR increase achieved during exercise and can be a marker of autonomic function.10 HR responses to exercise are ideally measured during cardiopulmonary exercise testing (CPET), where there is a precise measure of exercise intensity, and the appropriateness of the heart rate response relative to metabolic demand can be assessed. However, CPETs are technically demanding and not commonly performed clinically, whereas 6-minute walk tests (6MWT) are commonly used in the clinical setting to assess functional exercise performance.
We recently showed that lower chronotropic index obtained from 6MWT (CI-6MWT) was an independent risk factor for AECOPD in an exacerbation-prone clinical trial cohort of 477 participants with COPD.11 A related marker of autonomic function is HR recovery (HRR), which measures the rate of decrease in HR after exercise. HRR has also been associated with risk of AECOPD in previous small studies (n= 101 and 385) with a relatively short, 12 month period of AECOPD ascertainment.12,13 Neither CI-6MWT nor HRR have been evaluated as a risk factor for AECOPD in a large cohort.
Not all smokers develop COPD, and current and former smokers without expiratory airflow obstruction [normal spirometry or preserved ratio impaired spirometry (PRISm)] are also susceptible to acute respiratory events (ARE) and deleterious consequences from these events, such as faster decline in lung function.3,14 Risk factors for ARE for current and former smokers without COPD are not well defined, and we are aware of no previous analyses of heart rate responses as risk factors for ARE.
We used data from the Genetic Epidemiology of COPD (COPDGene) study, a longitudinal cohort of 10,000 current and former smokers with and without COPD, to test the hypotheses that CI-6MWT and HRR are risk factors for AECOPD and ARE, and to compare the ability of CI-6MWT and HRR to predict AECOPD and ARE.
METHODS:
COPDGene®
COPDGene (NCT00608764), is an ongoing, multi-site, longitudinal cohort study of current and former smokers with 10 or more pack-years of cigarette smoking. COPDGene enrolled non-Hispanic white and African American participants who were 45-80 years of age at the time of initial enrollment. Details of the study protocol have been previously published.15 In-person visits were performed at baseline and year 5.
Written, informed consent was obtained from all participants, and the study was approved by institutional review boards at all 21 study centers.
Participants:
The COPDGene 6MWT protocol did not include recording of HR at the baseline visit, but the year 5 visit included recording of HR at rest, end of 6MWT, and one minute after the end of 6MWT (recovery). Therefore, the year 5 visit served as the baseline in this analysis. We included participants who participated in year 5 6MWT, and had a 6MWT distance > 0 meters. We excluded participants who did not have spirometry or HR data, those who reported a history of atrial fibrillation, and those who had no follow up contacts after the year 5 visit.
Procedures and definitions
We used data from the COPDGene Longitudinal Follow-up (LFU) program through March 2020 to calculate the rate of ARE (these are AECOPD in those with COPD, but for simplicity we used the term ARE throughout our results). The rate of ARE was our primary outcome. The LFU program uses automated telephone calls or web-based questions to collect data on longitudinal outcomes, including ARE, every 3-6 months. Details of the LFU program have been previously published.16 ARE were defined as episodes of worsening respiratory symptoms requiring antibiotics or steroids. Severe ARE were defined as those requiring an emergency department visit or hospitalization. We performed analyses in the entire pooled cohort of all eligible participants, and in the separate spirometry subgroups of COPD [post-bronchodilator FEV1/forced vital capacity (FVC) < 0.7], PRISm (FEV1/FVC ≥ 0.7 and FEV1 < 80% predicted), and normal spirometry (FEV1/FVC ≥ 0.7 and FEV1 ≥ 80% predicted).14,17
6MWT were performed per American Thoracic Society guidelines at the year 5 visit.18,19 HRs were recorded using a pulse oximeter at rest, immediately after 6MWT, and after 1-minute of seated rest. We defined CI-6MWT as [(HR immediately after 6MWT – resting HR) / ((220 - age in years) - resting HR)].10,11 We defined HRR as (HR immediately after 6MWT – HR after 1 minute of seated rest following 6MWT).13
A complete medication history (including beta-blocker and non-dihydropyridine calcium channel blocker use) was obtained on a subgroup of 5,000 participants at the year 5 visit and used in secondary analyses described below.
Mortality assessment in COPDGene was performed by multiple methods, including through the LFU program and periodic searches of the Social Security Death Index, as has been previously described.20
Statistical analysis
In our primary analysis we treated CI-6MWT as a continuous variable and used zero-inflated negative binomial regression to test the association between CI-6MWT and rate of ARE in all participants, and separately in those with COPD, PRISm, and normal spirometry. Adjusted analyses accounted for age, sex, race, body mass index (BMI), FEV1 percent predicted, current vs former smoking status, and 6MWT distance. We did not adjust for other covariates, such as diabetes and hypertension, that are associated with abnormal cardiac autonomic function, but do not have a clear causal association with AECOPD and thus do not meet the definition of a confounder.21-24 In a secondary analysis, we included only those who had a detailed medication history taken at the year 5 visit, and additionally adjusted for beta-blocker and non-dihydropyridine calcium channel blocker use. Additional secondary analyses excluded those with CI-6MWT or HRR ≤ 0, due to concern these data could be erroneous. We also tested for an interaction between CI-6MWT and beta-blocker use on rate of ARE, based upon our results from a secondary analysis of the Metoprolol for the Prevention of Acute Exacerbations of COPD (BLOCK COPD) trial where we found that the protective effects of a higher CI-6MWT on risk of AECOPD were negated by assignment to metoprolol vs placebo.11,25
We performed these same analyses using HRR our primary predictor instead of CI-6MWT. To investigate whether CI-6MWT or HRR was a better predictor of the rate of ARE, we used the test mean squared error (MSE) from leave-one-out cross validation using zero-inflated negative binomial regression models from the primary analysis, adjusting for the same covariates in adjusted analysis. Probability estimates represent the proportion of time when HRR’s test MSE outperformed CI-6MWT’s test MSE.
In an additional secondary analysis, we used Cox proportional hazards models to assess all-cause mortality after the measurement of CI-6MWT and HRR. In adjusted analysis, we included the same covariates as in the primary analysis. We also used Kaplan-Meier curves and log-rank tests to compare all-cause mortality by quartile of CI-6MWT and HRR for both our entire cohort and those with COPD; we were unable to perform these analyses in the PRISm or normal spirometry subgroups due to a low number of deaths and low study power for all-cause mortality in these subgroups.
In the adjusted analyses for ARE and mortality, we also tested for an interaction between heart rate measures (HRR and CI-6MWT) and sex on the outcome of interest and reported stratified analyses if the interaction term was significant.
RESULTS:
Participants
Of 6,284 study participants who attended the year 5 visit, 4,484 were included in this analysis (Figure 1).
Figure 1:
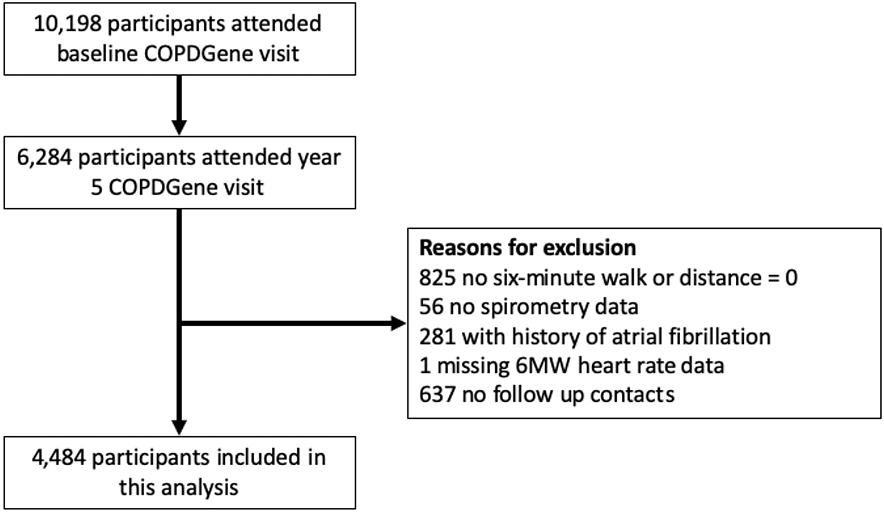
Study participant flow diagram.
Baseline characteristics of all participants (Total) and stratified by spirometry classification are shown in Table 1. Among all participants, the mean (SD) age was 65.4 (8.4) years, 51.4% were female, and 73.0% were white. Median CI-6MWT was 0.3 (IQR 0.18 to 0.43) and was similar across spirometry subgroups. Median HRR was 12 (IQR 6 to 20) beats/minute and was lower in those with COPD compared to normal spirometry. Participants had a mean of 4.1 (1.4) years of follow-up and this was similar across spirometry subgroups.
Table 1:
Descriptive statistics of included participants by mean (SD), N 563 (%), or median [IQR].
| Covariate | Total (N=4484) |
COPD1 (N=1966) |
PRISm2 (N=540) |
Normal3 (N=1978) |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65.4 (8.43) | 68.0 (8.08) | 62.8 (8.15) | 63.6 (8.15) |
| Female (%) | 2303 (51.4%) | 901 (45.8%) | 311 (57.6%) | 1091 (55.2%) |
| White Race (%) | 3273 (73.0%) | 1540 (78.3%) | 342 (63.3%) | 1391 (70.3%) |
| BMI, kg/m^2 | 28.9 (6.26) | 27.9 (6.15) | 31.8 (7.12) | 29.1 (5.85) |
| Current Smoker (%) | 1636 (36.5%) | 665 (33.8%) | 238 (44.1%) | 733 (37.1%) |
| Smoking History, pack-years | 43.4 (23.1) | 50.3 (24.4) | 41.3 (22.3) | 37.0 (19.7) |
| Lung function and O2 use | ||||
| FEV1/FVC ratio | 0.68 (0.15) | 0.54 (0.12) | 0.76 (0.05) | 0.78 (0.05) |
| DLCO, % predicted | 78.3 (22.7) | 66.8 (22.3) | 78.6 (18.8) | 89.0 (18.4) |
| FEV1, % predicted | 78.8 (24.2) | 62.0 (22.6) | 70.5 (7.6) | 97.8 (11.7) |
| Baseline SpO2, % | 96.1 (2.76) | 95.3 (3.34) | 96.5 (2.09) | 96.9 (1.94) |
| Supplemental O2 Use (%) | 488 (10.9%) | 425 (21.6%) | 23 (4.3%) | 40 (2.0%) |
| Medication use (%) | ||||
| Current COPD Medication Use | 1789 (39.9%) | 1246 (63.4%) | 199 (36.9%) | 344 (17.4%) |
| LAMA Use | 528 (12.8%) | 440 (24.7%) | 44 (8.9%) | 44 (2.4%) |
| LABA Use | 653 (15.9%) | 509 (28.6%) | 68 (13.7%) | 76 (4.1%) |
| ICS Use | 917 (22.3%) | 701 (39.4%) | 92 (18.5%) | 124 (6.7%) |
| Oral beta-blocker4 | 572 (12.8%) | 287 (14.6%) | 83 (15.4%) | 202 (10.2%) |
| Non-dihydro. Ca. channel blocker4 | 77 (1.7%) | 55 (2.8%) | 4 (0.7%) | 18 (0.9%) |
| Comorbidities (%) | ||||
| Coronary Artery Disease | 353 (7.9%) | 195 (9.9%) | 43 (8.0%) | 115 (5.8%) |
| Diabetes | 748 (16.7%) | 312 (15.9%) | 143 (26.5%) | 293 (14.8%) |
| Hypertension | 2242 (50.0%) | 1038 (52.8%) | 314 (58.1%) | 890 (45.0%) |
| Hyperlipidemia | 2020 (45.0%) | 904 (46.0%) | 263 (48.7%) | 853 (43.1%) |
| Congestive Heart Failure | 117 (2.6%) | 71 (3.6%) | 19 (3.5%) | 27 (1.4%) |
| Peripheral Vascular Disease | 124 (2.8%) | 77 (3.9%) | 16 (3.0%) | 31 (1.6%) |
| Physiologic Variables | ||||
| 6-minute walk test distance, ft | 1339 (393.3) | 1237 (401.6) | 1272 (359.8) | 1457 (360.0) |
| Resting HR, beats per minute | 73.3 (12.2) | 74.6 (12.4) | 74.0 (12.6) | 71.7 (11.6) |
| HR post 6-minute walk test, beats per minute | 98.2 (18.5) | 98.1 (18.2) | 97.1 (18.5) | 98.7 (18.7) |
| Chronotropic Index | 0.30 [0.18, 0.43] | 0.30 [0.17, 0.43] | 0.27 [0.16, 0.39] | 0.31 [0.19, 0.45] |
| Heart Rate Recovery, beats per minute | 12 [6, 20] | 11 [5, 19] | 12 [6, 20] | 13 [7, 21] |
| Follow up and event rates | ||||
| Years of follow-up data | 4.1 (1.4) | 4.1 (1.4) | 4.0 (1.4) | 4.2 (1.3) |
| Rate of Events, events/yr | 0.34 | 0.52 | 0.31 | 0.17 |
| Rate of Severe Events, events/yr | 0.14 | 0.21 | 0.15 | 0.07 |
| Died during follow-up (%) | 388 (8.7%) | 277 (14.1%) | 35 (6.5%) | 76 (3.8%) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1-second; FVC, forced vital capacity; HR, heart rate; ICS, inhaled corticosteroid; LABA, long acting beta-agonist; LAMA, long acting muscarinic antagonist; O2, oxygen; PRISm, preserved ratio impaired spirometry; SpO2, pulse oximetry saturation.
COPD was defined as an FEV1/FVC ratio < 0.7.
PRISm was defined as an FEV1/FVC ratio ≥ 0.7 and an FEV1 < 80% predicted
Normal spirometry was defined as an FEV1/FVC ratio ≥ 0.7 and FEV1 ≥ 80% predicted
Comprehensive medication data was only collected on the first 5000 participants at the year 5 visit. Data on beta-blocker use and non-dihydropyridine calcium channel blocker use was missing for 1250 (27.9%) participants.
Compared to the PRISm and normal spirometry subgroups, participants in the COPD subgroup were older, had greater pack-year smoking history, and were more likely to be on COPD medications. Participants in the PRISm subgroup were more likely to be current smokers and have diabetes than participants in the COPD or normal spirometry subgroup.
The mean rate of ARE was 0.34 per person-year among all participants and was highest in those with COPD, at 0.52 per person-year. The mean rate of severe ARE was 0.14 per person/year, and similarly was highest in those with COPD at 0.21 per person/year. Mortality during follow up was 8.6% overall, and higher in those with COPD (14.1%) than those with PRISm (6.5%) or normal spirometry (3.8%).
CI-6MWT and acute respiratory events
Unadjusted associations between CI-6MWT and rates of any ARE and severe ARE are included in the online supplement (e-Table 1).
In adjusted analysis, CI-6MWT was not associated with the rate of any ARE (Figure 2). Higher CI-6MWT was associated with a lower rate of severe ARE among all participants (adjusted incidence rate ratio [aIRR] 0.95 [0.92 to 0.99] per 0.1 unit change in CI-6MWT), and in those with COPD (aIRR 0.96 [0.92 to 1.00]).
Figure 2:
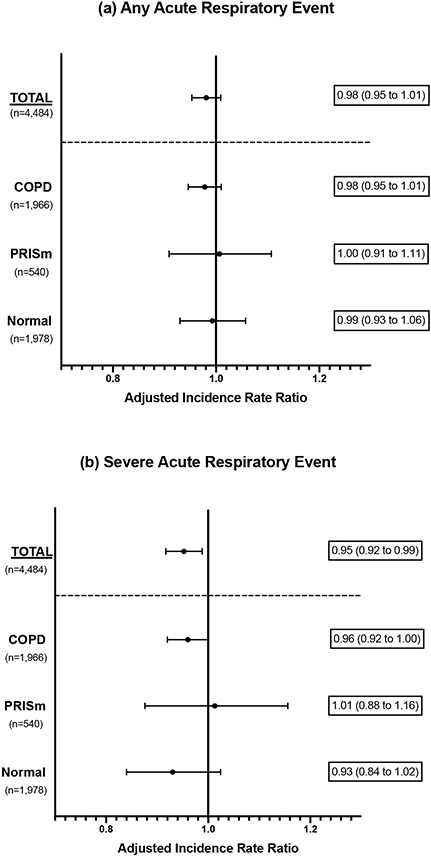
Adjusted incidence rate ratios (aIRR) for the association between chronotropic index obtained from 6-minute walk test (CI-6MWT) and rate of (a) any acute respiratory events and (b) severe acute respiratory events (requiring emergency department visit or hospitalization). Results are presented for all participants (Total), and by spirometry subgroup. Incidence ratio ratios are adjusted for age, sex, race, body mass index (BMI), FEV1 percent predicted, current vs former smoking status, and 6-minute walk test distance. aIRRs represent the change in rate for each 0.1 unit change in CI-6MWT.
COPD, chronic obstructive pulmonary disease; PRISm, preserved ratio, impaired spirometry.
There were no significant interactions between CI-6MWT and beta-blocker treatment on rate of ARE, or severe ARE, when all participants were included (e-Table 2). There was a significant interaction (interaction p-value 0.036) between CI-6MWT and treatment with beta-blockers on rate of severe ARE in participants with PRISm, but not in those with COPD or normal spirometry.
Results were generally similar after including beta-blocker use and non-dihydropyridine calcium channel blocker use as co-variates in the adjusted model (e-Figure 1), and after excluding participants with CI-6MWT or HRR ≤ 0 (e-Table 3 and e-Figure 2).
There were statistically significant interactions between CI-6MWT and sex on rate of ARE and severe ARE, but there were no significant associations when the analyses were stratified by sex (e-Table 4).
HRR and acute respiratory events
In adjusted analysis, higher HRR was associated with a lower rate of any ARE [aIRR 0.97 (0.95 to 0.99) per 5 beat/minute change] and severe ARE [aIRR 0.95 (0.92 to 0.98)] among all participants; associations in the COPD subgroup were similar (Figure 3). Results from unadjusted analysis are included in the online supplement (e-Table 5). There were no significant interactions between beta-blocker use or sex and HRR on rate of ARE (e-Tables 6 and 7)
Figure 3:
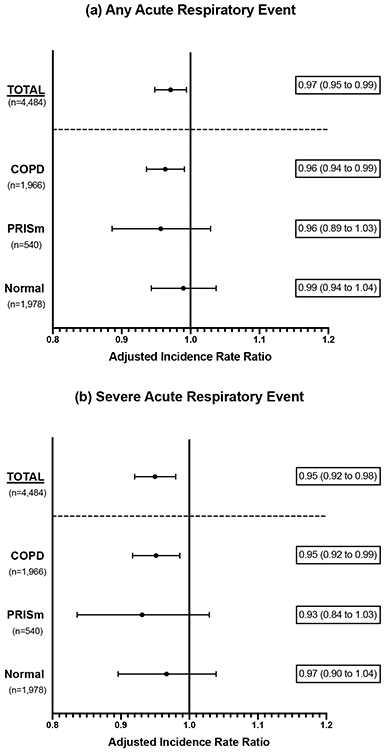
Adjusted incidence rate ratios (aIRR) for the association between heart rate recovery (HRR) and rate of (a) any acute respiratory events and (b) severe acute respiratory events (requiring emergency department visit or hospitalization). Results are presented for all participants (Total), and by spirometry subgroup. Incidence ratio ratios are adjusted for age, sex, race, body mass index (BMI), FEV1 percent predicted, current vs former smoking status, and 6-minute walk test distance. aIRRs represent the change in rate for each 5 beat/minute change in HRR.
COPD, chronic obstructive pulmonary disease; PRISm, preserved ratio, impaired spirometry.
Results were similar after additionally adjusting for metoprolol and non-dihydropyridine calcium channel blocker use (e-Figure 3), and excluding those with CI-6MWT or HRR ≤ 0 (e-Figure 4 and e-Table 8).
Comparison of CI-6MWT and HRR
We found no statistically significant difference in the performance of CI-6MWT and HRR in the prediction of ARE (Table 2).
Table 2:
Comparison of the relationship between chronotropic index obtained from 6-minute walk test (CI-6MWT) and acute respiratory events, and between heart rate recovery (HRR) and acute respiratory events. The mean squared error (MSE) is a measure of the difference between the rate of acute respiratory events predicted by the model and rate of acute respiratory events observed in the data. A lower MSE is associated with a better model fit.
| Group | Outcome | HRR MSE |
CI-6MWT MSE |
Probability Estimate1 |
|
|---|---|---|---|---|---|
| Unadjusted 2 | |||||
| Overall | Any Event | 9.228 | 9.243 | 0.534 | |
| Severe Event | 2.676 | 2.675 | 0.505 | ||
| COPD | Any Event | 13.6 | 13.663 | 0.538 | |
| Severe Event | 3.529 | 3.532 | 0.505 | ||
| PRISm | Any Event | 8.591 | 8.637 | 0.539 | |
| Severe Event | 4.144 | 4.184 | 0.567 | ||
| Normal | Any Event | 4.066 | 4.054 | 0.512 | |
| Severe Event | 1.275 | 1.267 | 0.485 | ||
| Adjusted 3 | |||||
| Overall | Any Event | 8.079 | 8.084 | 0.471 | |
| Severe Event | 2.522 | 2.529 | 0.488 | ||
| COPD | Any Event | 12.03 | 12.043 | 0.478 | |
| Severe Event | 3.334 | 3.346 | 0.499 | ||
| PRISm | Any Event | 8.273 | 8.388 | 0.483 | |
| Severe Event | 4.111 | 4.214 | 0.493 | ||
| Normal | Any Event | 3.905 | 3.893 | 0.479 | |
| Severe Event | 1.243 | 1.236 | 0.497 |
CI-6MWT, chronotropic index obtained from 6-minute walk test; COPD, chronic obstructive pulmonary disease; HRR, heart rate recovery; MSE, mean squared error; PRISm, preserved ratio impaired spirometry.
Estimated probability that HRR is a better predictor of exacerbations than CI-6MWT based on mean squared error (MSE) of leave-one-out cross validation (LOOCV) with a zero-inflated negative binomial regression model.
Unadjusted analysis represents univariate analysis for heart rate recovery or chronotropic response
Adjusted for age, sex, race, body mass index, forced expiratory volume in 1-second % predicted, current vs. former smoking status, and 6-minute walk test distance.
Mortality
Higher CI-6MWT and HRR were associated with a lower risk of mortality in crude, unadjusted analyses (e-Table 7), but these relationships were not significant after covariate adjustment (Table 3). In mortality analyses by CI-6MWT and HRR quartiles (rather than as continuous variables), there was no significant difference in mortality by CI-6MWT quartile (e-Figure 5), but there was a significant difference in mortality by HRR quartile among all participants (log-rank p-value <0.0001), and in those with COPD (log-rank p-value 0.006) (e-Figure 6). There were no significant interactions between CI-6MWT or HRR and sex on risk of mortality (e-Table 10).
Table 3:
Associations between chronotropic index obtained from 6-minute walk test (CI-6MWT) and all-cause mortality, and between heart rate recovery (HRR) and all-cause mortality. Hazard ratios represent the change in risk for a 0.1-unit change in CI-6MWT or 5 beat/minute change in HRR, and are obtained from Cox proportional hazards models.
| Spirometry Group |
Predictor | Adjusted Hazard Ratio1 (95% Conf. Int.) |
P-value |
|---|---|---|---|
| Total | CI-6MWT | 0.998 (0.953, 1.044) | 0.922 |
| HRR | 0.99 (0.95, 1.032) | 0.633 | |
| COPD | CI-6MWT | 0.993 (0.942, 1.047) | 0.802 |
| HRR | 0.981 (0.934, 1.03) | 0.441 |
CI-6MWT, chronotropic index obtained from 6-minute walk test; Conf. Int., confidence interval; COPD, chronic obstructive pulmonary disease; HRR, heart rate recovery; PRISm, preserved ratio impaired spirometry.
Models account for age, sex, race, BMI, FEV1% predicted, current vs former smoking status and 6-minute walk test distance.
DISCUSSION
In a large cohort of current and former smokers, higher chronotropic index obtained from 6-minute walk and higher heart rate recovery were both associated with lower rates of ARE.
Our primary goal in this analysis was to evaluate the association between CI-6MWT and ARE. In a secondary analysis of the BLOCK COPD trial, we found that higher CI-6MWT was associated with decreased risk of any AECOPD (adjusted hazard ratio 0.88, 95% CI: 0.80 to 0.96), but not hospitalized AECOPD (0.94, 95% CI 0.81 to 1.10).11 However, the generalizability of those findings were limited by the study’s enrollment of only exacerbation-prone participants with at least moderate COPD (mean FEV1 was 41% of predicted normal) and no indication for beta-blocker therapy.25 This current analysis largely validates and extends our prior findings, and supports the notion that higher CI-6MWT is associated with a lower risk of ARE/AECOPD.
In studies using CPET to investigate chronotropic response in COPD, chronotropic insufficiency defined as a chronotropic index < 0.8 is common.5,26 Chronotropic insufficiency measured by CPET is associated with decreased exercise tolerance and increased risk of mortality in the general population, and in COPD.5,10,26 The etiology of chronotropic insufficiency in COPD is unknown. Proposed etiologies include smoking, cardiovascular disease, respiratory limitations to exercise, autonomic dysfunction, and dynamic hyperinflation.26,27 These proposed etiologies may lead to chronotropic insufficiency via chronic neurohumoral activation in COPD, which may lead to down-regulation and decreased responsiveness of cardiac beta receptors, and inability to respond to sympathetic activation with an appropriate increase in heart rate.28-30
We did not find a significant interaction between CI-6MWT and beta blocker use on time to ARE in the overall analysis, or in the COPD subgroup. In our BLOCK COPD analysis, we found that participants who had a higher CI-6MWT and were subsequently assigned to metoprolol had a higher risk of AECOPD than those assigned to placebo.11 We may have found no significant interaction in this COPDGene analysis because participants had stronger clinical indications for beta-blocker therapy, as opposed to BLOCK where participants had no class I indications for beta blocker treatment and were randomly assigned to metoprolol vs placebo. We also had limited power to evaluate for medication interactions, with only 287 COPDGene participants with COPD on beta-blocker therapy and lower rates of exacerbations than in BLOCK COPD. We did find a significant interaction between CI-6MWT and beta-blocker treatment on rate of severe ARE in those with PRISm, but we only had 83 participants with PRISm on beta-blockers and did not adjust for multiple testing, increasing the risk of type 1 error.
Lower HRR is another measure of autonomic dysfunction which is commonly observed and associated with increased mortality in people with COPD.31 Lower HRR has previously been associated with increased risk of AECOPD. In a previous analysis of HRR in COPDGene (n=385), Zhao and colleagues found that HRR ≤ 10 at the year 5 visit was associated with an increased risk of self-reported AECOPD (not the scheduled longitudinal surveys used in our current analysis) in the year before the year 10 visit.13 Rodriguez and colleagues (n=101) found that HRR < 14 beats/minute was associated with increased risk of AECOPD in the subsequent 12 months.12 Our analysis, with its comparatively much larger sample size and close longitudinal follow-up over five years, provides strong evidence that HRR is independently associated with risk of ARE/AECOPD.
When we compared the ability of HRR and CI-6MWT to predict ARE, we found no statistical difference in the ability of these measures to predict ARE in all participants or any spirometry subgroup. Though only HRR, and not CI-6MWT, had a statistically significant association with rate of any ARE, the point estimates and confidence intervals for HRR and CI-6MWT were very similar across ARE analyses. Our finding of no difference is not a surprising result, as both of these measures are inherently dependent on the heart rate at the end of the 6MWT. However, they measure different components of the autonomic response to exercise; CI-6MWT reflects both parasympathetic withdrawal and sympathetic activation with onset of exercise, while HRR at 1 minute predominantly reflects the re-introduction of parasympathetic input to heart rate control and is likely less affected by sympathetic inputs.31,32
We also analyzed the relationships between CI-6MWT, HRR, and mortality. Higher CI-6MWT and HRR were both associated with decreased risk of mortality in unadjusted analysis, but not after adjustment for covariates. These findings are in contrast to previous analyses of CI and HRR in people with and without COPD where lower CI and HRR were associated with increased mortality.26,31-34 These studies included participants with worse lung function (in those limited to COPD), used different exercise testing modalities, and used different analytic methods (strong associations between CI, HRR, and mortality were consistent across studies in unadjusted analyses). We are aware of only one other study that analyzed the relationship between chronotropic response during 6MWT and mortality in chronic lung disease or other disease states. Holland and colleagues analyzed 62 participants with interstitial lung disease who underwent 6MWT, 54 of whom also underwent incremental exercise testing, and found that impaired chronotropic response was independently associated with mortality; findings were similar for 6MWT and incremental exercise testing.35
Our study has several limitations. We did not have a measure of work rate to assess the appropriateness of the heart rate at the end of the 6MWT. Participants may have a low post 6MWT heart rate, and subsequently a low CI-6MWT and HRR, due to autonomic dysfunction, respiratory limitations to exercise, cardiovascular limitations to exercise, changes in pace or pauses during testing, or other limitations to exercise such as effort, pain, or peripheral muscle weakness. Without assessment of the degree of the appropriateness of the heart rate, we cannot determine the underlying mechanism of our findings. Additionally, other measurements of cardiac autonomic function, such as heart rate variability, which could provide additional context for our findings, are not available in COPDGene. The lack of a clear mechanism and small amount of increased risk for these heart rate measures limit the clinical implications of our results. Detailed cardiopulmonary physiologic testing in a cohort of exacerbation-prone COPD patients could help to clarify these findings and identify therapeutic targets. Finally, HR data during 6MWT was not collected at the baseline visit and this introduces possible selection bias and survival bias to our cohort, in which we necessarily began follow up at the year 5 visit.
Our study also has several strengths. COPDGene is a well characterized, multi-center, longitudinal cohort that allowed us to analyze more than 4,400 participants across spirometry subgroups. The use of the LFU program data provided an opportunity to analyze over 5,000 AREs over more than 4 years of follow-up. Finally, though the 6MWT has several limitations compared to more detailed physiologic testing, it is inexpensive and fairly easily to assess in routine clinical practice.
In summary, our data provide further evidence that impaired HR responses to exercise, possibly reflecting autonomic dysfunction, may be novel risk factors for AECOPD. Future studies should validate these findings using exercise testing with more comprehensive physiologic measures, identify the etiology of low CI and HRR in COPD, and determine if this is a potentially modifiable factor to reduce AECOPD risk.
Supplementary Material
Highlights.
Abnormal heart rate responses to exercise are common in COPD
Heart rate responses are one measure of autonomic function
Autonomic dysfunction may be a risk factor for acute respiratory events
Better heart rate responses were associated with lower rates of respiratory events
Acknowledgements:
We would like to express our thanks to all participants in COPDGene. A full list of COPDGene investigators is included in the online supplement. We also thank Michael Jacobs, MD, PharmD and his Temple Pharmacy students for obtaining and organizing all the medications of the participants in the COPDGene.
Sources of Support:
DMM was supported by the University of Minnesota T32 Training in Lung Science training grant (2T32HL007741-26A1). AA was supported by a postdoctoral fellowship from the Tobacco-Related Disease Research Program (TRDRP; award no. 28FT-0017). AKB was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants KL2TR002492 and UL1TR002494. HBR is supported by grants from NIH (R01HL151452, P50HD098593, R01DK122767, P2CHD086851, R01HL153460), the Tobacco Related Disease Research Program (T31IP1666), and the University of California, Office of the President. NBT is supported by a postdoctoral fellowship from the Tobacco-Related Disease Research Program (TRDRP; award no. T31FT1692). This material is also the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Medical Center, Minneapolis/USA.
This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
The project described was supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Conceived the study: DMM, MTD, KMK
Designed the study: DMM, EFP, JEC, KMK
Acquired the data: MTD, RC, NTG, CHW, KMK
Performed the primary statistical analysis: EFP, JEC
Drafted the manuscript: DMM
Critically revised the manuscript for important intellectual content and approved the final manuscript: all authors
Take responsibility for the integrity of the data and the accuracy of the data analysis: all authors
Competing Interests Statement:
AA, AKB, NTG, DMM, TM, DZ, CW, and NBT have nothing to declare. Outside of this work, SPB has received grant support from the NIH and Sanofi, and consulting/advisory board fees from Sunovion, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, and Vigor Medical System. Outside of this work, RC has received grant support, advisory board fees, and lecture fees from GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca, consulting fees from Regeneron and Genentech, and reports owning stock in Inogen. Outside of this work, JEC has received grant support from the Department of Defense. Outside of this work, MTD has received consulting fees and served on clinical trials for Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Teva, CSA Medical, and PneumRx/BTG, served on clinical trials for Novartis, Yungjin, Boston Scientific, Gala Therapeutics, and Nuvaira, received travel support and served on clinical trials for Pulmonx, and received consulting fees from Quark Pharmaceuticals and Mereo. Outside of this work, KMK has received consulting fees from Nuvaria and Allergan. Outside this work, EP has received support from the National Institutes of Health. Outside of this work, HBR reports consulting fees from Omniox Inc., and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech and Regeneron. He is a visiting Professor at the University of Leeds, UK. Outside of this work, WWS has received grant support from Department of Defense, AstraZeneca, and Boehringer Ingelheim, and consulting fees for serving on a DSMB for GlaxoSmithKline.
Previous Publication: Portions of these data were presented in abstract form at the American Thoracic Society International Conference 2021.
Disclaimer: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, the Department of Veterans Affairs, or any of the authors’ affiliated institutions.
REFERENCES:
- 1.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 2.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 3.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.the COPDGene Investigators, Busch R, Han MK, et al. Risk factors for COPD exacerbations in inhaled medication users: the COPDGene study biannual longitudinal follow-up prospective cohort. BMC Pulm Med. 2016;16(1):28. doi: 10.1186/s12890-016-0191-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta M, Bansal V, Chhabra SK. Abnormal heart rate recovery and chronotropic incompetence on exercise in chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(3):117–126. doi: 10.1177/1479972313493097 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong HF, Gonzalez-Costello J, Jorde UP, et al. The effect of lung volume reduction surgery on chronotropic incompetence. Respiratory Medicine. 2012;106(10):1389–1395. doi: 10.1016/j.rmed.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev. 2016;96(3):975–1024. doi: 10.1152/physrev.00039.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of Heart Rate by the Autonomic Nervous System: Studies in Man on the Interrelation Between Baroreceptor Mechanisms and Exercise. Circulation Research. 1966;19(2):400–411. doi: 10.1161/01.RES.19.2.400 [DOI] [PubMed] [Google Scholar]
- 10.Brubaker PH, Kitzman DW. Chronotropic Incompetence: Causes, Consequences, and Management. Circulation. 2011;123(9):1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald DM, Helgeson ES, Adabag S, et al. Chronotropic Index and Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Secondary Analysis of BLOCK COPD. Annals ATS. 2021;18(11):1795–1802. doi: 10.1513/AnnalsATS.202008-1085OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez DA, Kortianou EA, Alison JA, et al. Heart Rate Recovery After 6-min Walking Test Predicts Acute Exacerbation in COPD. Lung. 2017;195(4):463–467. doi: 10.1007/s00408-017-0027-0 [DOI] [PubMed] [Google Scholar]
- 13.Zhao D, Abbasi A, Casaburi R, et al. Identifying a Heart Rate Recovery Criterion After a 6-Minute Walk Test in COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:2545–2560. doi: 10.2147/COPD.S311572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan ES, Fortis S, Regan EA, et al. Longitudinal Phenotypes and Mortality in Preserved Ratio Impaired Spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regan EA, Hokanson JE, Murphy JR, et al. Genetic Epidemiology of COPD (COPDGene) Study Design. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2011;7(1):32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart JI, Moyle S, Criner GJ, et al. Automated Telecommunication to Obtain Longitudinal Follow-up in a Multicenter Cross-sectional COPD Study. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 19.Rambod M, Porszasz J, Make BJ, Crapo JD, Casaburi R. Six-Minute Walk Distance Predictors, Including CT Scan Measures, in the COPDGene Cohort. Chest. 2012;141(4):867–875. doi: 10.1378/chest.11-0870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand M, Austin E, Moll M, et al. A Risk Prediction Model for Mortality Among Smokers in the COPDGene® Study. J COPD F. 2020;7(4):346–361. doi: 10.15326/jcopdf.7.4.2020.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder EB, Chambless LE, Liao D, et al. Diabetes, Glucose, Insulin, and Heart Rate Variability: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28(3):668–674. doi: 10.2337/diacare.28.3.668 [DOI] [PubMed] [Google Scholar]
- 22.Mancia G, Grassi G. The Autonomic Nervous System and Hypertension. Circ Res. 2014;114(11):1804–1814. doi: 10.1161/CIRCRESAHA.114.302524 [DOI] [PubMed] [Google Scholar]
- 23.Castañ-Abad MT, Montserrat-Capdevila J, Godoy P, et al. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: a prospective cohort study. European Journal of Public Health. 2020;30(4):822–827. doi: 10.1093/eurpub/ckz219 [DOI] [PubMed] [Google Scholar]
- 24.Lederer DJ, Bell SC, Branson RD, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Annals ATS. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS [DOI] [PubMed] [Google Scholar]
- 25.Dransfield MT, Voelker H, Bhatt SP, et al. Metoprolol for the Prevention of Acute Exacerbations of COPD. N Engl J Med. 2019;381(24):2304–2314. doi: 10.1056/NEJMoa1908142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Costello J, Armstrong HF, Jorde UP, et al. Chronotropic incompetence predicts mortality in severe obstructive pulmonary disease. Respiratory Physiology & Neurobiology. 2013;188(2):113–118. doi: 10.1016/j.resp.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulo S, Inamo J, Dehon A, Le Rouzic O, Edme JL, Neviere R. Chronotropic incompetence can limit exercise tolerance in COPD patients with lung hyperinflation. COPD. 2016;Volume 11:2553–2561. doi: 10.2147/COPD.S112490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto T, Kikuchi S, Mori K, et al. Cardiac β-Adrenergic Receptor Downregulation, Evaluated by Cardiac PET, in Chronotropic Incompetence. J Nucl Med. 2021;62(7):996–998. doi: 10.2967/jnumed.120.253419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepper GS, Lee RW. Sympathetic Activation in Heart Failure and Its Treatment With 2-Blockade. ARCH INTERN MED. 1999;159:10. [DOI] [PubMed] [Google Scholar]
- 30.Andreas S, Anker SD, Scanlon PD, Somers VK. Neurohumoral Activation as a Link to Systemic Manifestations of Chronic Lung Disease. Chest. 2005;128(5):3618–3624. doi: 10.1378/chest.128.5.3618 [DOI] [PubMed] [Google Scholar]
- 31.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respiratory Medicine. 2005;99(7):877–886. doi: 10.1016/j.rmed.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 32.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804 [DOI] [PubMed] [Google Scholar]
- 33.Nishime EO. Heart Rate Recovery and Treadmill Exercise Score as Predictors of Mortality in Patients Referred for Exercise ECG. JAMA. 2000;284(11):1392. doi: 10.1001/jama.284.11.1392 [DOI] [PubMed] [Google Scholar]
- 34.Adabag AS, Grandits GA, Prineas RJ, Crow RS, Bloomfield HE, Neaton JD. Relation of Heart Rate Parameters During Exercise Test to Sudden Death and All-Cause Mortality in Asymptomatic Men. The American Journal of Cardiology. 2008;101(10):1437–1443. doi: 10.1016/j.amjcard.2008.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland AE, Hill G, Glaspole I, Goh N, Dowman L, McDonald CF. Impaired chronotropic response to 6-min walk test and reduced survival in interstitial lung disease. Respiratory Medicine. 2013;107(7):1066–1072. doi: 10.1016/j.rmed.2013.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


