Abstract
Background
Inhaled corticosteroids (ICS) are the first‐line treatment for children with persistent asthma. Their potential for growth suppression remains a matter of concern for parents and physicians.
Objectives
To assess whether increasing the dose of ICS is associated with slower linear growth, weight gain and skeletal maturation in children with asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials (CAGR) and the ClinicalTrials.gov website up to March 2014.
Selection criteria
Studies were eligible if they were parallel‐group randomised trials evaluating the impact of different doses of the same ICS using the same device in both groups for a minimum of three months in children one to 17 years of age with persistent asthma.
Data collection and analysis
Two review authors ascertained methodological quality independently using the Cochrane Risk of bias tool. The primary outcome was linear growth velocity. Secondary outcomes included change over time in growth velocity, height, weight, body mass index and skeletal maturation.
Main results
Among 22 eligible trials, 17 pairs of groups comparisons were derived from 10 trials (3394 children with mild to moderate asthma), measured growth and contributed data to the meta‐analysis. Trials used ICS (beclomethasone, budesonide, ciclesonide, fluticasone or mometasone) as monotherapy or as combination therapy with a long‐acting beta2‐agonist and generally compared low (50 to 100 μg) versus low to medium (200 μg) doses of hydrofluoroalkane (HFA)‐beclomethasone equivalent over 12 to 52 weeks. In the four comparisons reporting linear growth over 12 months, a significant group difference was observed, clearly indicating lower growth velocity in the higher ICS dose group of 5.74 cm/y compared with 5.94 cm/y on lower‐dose ICS (N = 728 school‐aged children; mean difference (MD)0.20 cm/y, 95% confidence interval (CI) 0.02 to 0.39; high‐quality evidence): No statistically significant heterogeneity was noted between trials contributing data. The ICS molecules (ciclesonide, fluticasone, mometasone) used in these four comparisons did not significantly influence the magnitude of effect (X2 = 2.19 (2 df), P value 0.33). Subgroup analyses on age, baseline severity of airway obstruction, ICS dose and concomitant use of non‐steroidal antiasthmatic drugs were not performed because of similarity across trials or inadequate reporting. A statistically significant group difference was noted in unadjusted change in height from zero to three months (nine comparisons; N = 944 children; MD 0.15, 95% CI ‐0.28 to ‐0.02; moderate‐quality evidence) in favour of a higher ICS dose. No statistically significant group differences in change in height were observed at other time points, nor were such differences in weight, body mass index and skeletal maturation reported with low quality of evidence due to imprecision.
Authors' conclusions
In prepubescent school‐aged children with mild to moderate persistent asthma, a small but statistically significant group difference in growth velocity was observed between low doses of ICS and low to medium doses of HFA‐beclomethasone equivalent, favouring the use of low‐dose ICS. No apparent difference in the magnitude of effect was associated with three molecules reporting one‐year growth velocity, namely, mometasone, ciclesonide and fluticasone. In view of prevailing parents’ and physicians’ concerns about the growth suppressive effect of ICS, lack of or incomplete reporting of growth velocity in more than 86% (19/22) of eligible paediatric trials, including those using beclomethasone and budesonide, is a matter of concern. All future paediatric trials comparing different doses of ICS with or without placebo should systematically document growth. Findings support use of the minimal effective ICS dose in children with asthma.
Plain language summary
Does altering the dose of inhaled corticosteroids make a difference in growth among children with asthma?
Background
Asthma guidelines recommend inhaled corticosteroids (ICS) as the first choice of treatment for children with persistent asthma that is not well controlled when only a reliever inhaler is used to treat symptoms. Steroids work by reducing inflammation in the lungs and are known to control underlying symptoms of asthma. However, parents and physicians remain concerned about the potential negative effect of ICS on growth.
Review question
Does altering the dose of inhaled corticosteroids make a difference in the growth of children with asthma?
What evidence did we find?
We studied whether a difference could be seen in the growth of children with persistent asthma who were using different doses of the same ICS molecule and the same delivery device. We found 22 eligible trials, but only 10 of them measured growth or other measures of interest. Overall, 3394 children included in the review combined 17 group comparisons (i.e. 17 pairs of groups of children with mild to moderate asthma using a particular dose and type of steroid in 10 trials). Trials used different ICS molecules (beclomethasone, budesonide, ciclesonide, fluticasone or mometasone) either on their own or in combination with a long‐acting beta2‐agonist (a drug used to open up the airways) and generally compared low doses of corticosteroids (50 to 100 μg) with low to medium (200 μg) doses of corticosteroids (converted in μg HFA‐beclomethasone equivalent) over 12 to 52 weeks.
Results
We found a small but statistically significant group difference in growth over 12 months between these different doses clearly favouring the lower dose of ICS. The type of corticosteroid among newer molecules (ciclesonide, fluticasone, mometasone) did not seem to influence the impact on growth over one year. Differences in corticosteroid doses did not seem to affect the change in height, the gain in weight, the gain in body mass index and the maturation of bones.
Quality of the evidence
This review is based on a small number of trials that reported data and were conducted on children with mild to moderate asthma. Only 10 of 22 studies measured the few outcomes of interest for this review, and only four comparisons reported growth over 12 months. Our confidence in the quality of evidence is high for this outcome, however it is low to moderate for several other outcomes, depending on the number of trials reporting these outcomes. Moreover, a few outcomes were reported only by a single trial; as these findings have not been confirmed by other trials, we downgraded the evidence for these outcomes to low quality. An insufficient number of trials have compared the effect of a larger difference in dose, for example, between a high dose and a low dose of ICS and of other popular molecules such as budesonide and beclomethasone over a year or longer of treatment.
Conclusions
We report an ICS dose–dependent reduction in growth velocity in prepubescent school‐aged children with mild to moderate persistent asthma. The choice of ICS molecule (mometasone, ciclesonide or fluticasone) was not found to affect the level of growth velocity response over a year. The effect of corticosteroids on growth was not consistently reported: among 22 eligible trials, only four comparisons reported the effects of corticosteroids on growth over one year. In view of parents' and clinicians' concerns, lack of or incomplete reporting of growth is a matter of concern given the importance of the topic. We recommend that growth be systematically reported in all trials involving children taking ICS for three months or longer. Until further data comparing low versus high ICS dose and trials of longer duration are available, we recommend that the minimal effective ICS dose be used in all children with asthma.
Summary of findings
Summary of findings for the main comparison. Inhaled corticosteroids dose‐response effect.
| Inhaled corticosteroids dose‐response effect | ||||||
|
Patient or population: children with persistent asthma
Settings: outpatients
Intervention: lower‐dose inhaled corticosteroids Control: higher‐dose ICS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control group (higher‐dose ICS) | Intervention group (lower‐dose ICS) | |||||
| Growth velocity over 12 months (cm/y) (higher is better) | Mean growth velocity was 5.74 cm/y (range, 5.6 to 5.88) | Corresponding growth velocity on lower‐dose ICS was 0.2 cm/y higher: mean 5.94 cm/y (95% CI 5.76 to 6.13) | MD 0.20 (0.02 to 0.39) | 728 (4 studies) | ⊕⊕⊕⊕ high | Skoner 2011 data analysed using LRS model were used |
|
Change in height over 3 months (cm) (higher is better) |
Unadjusted mean change in height over 3 months was 1.34 cm (range, 0.9 to 1.8 cm) | Corresponding unadjusted change in height on lower‐dose ICS was 0.15 cm lower: mean 1.19 cm (95% CI 1.06 to 1.32) | MD ‐0.15 (‐0.28 to ‐0.02) | 944 (9 studies) | ⊕⊕⊝⊝ moderate1 | Data analysis was unadjusted for confounders |
| Change in height over 12 months (cm) (higher is better) | Unadjusted mean change in height over a year was 4.56 cm (range, 3.6 to 5.73 cm) | Corresponding unadjusted change in height on lower‐dose ICS was 0.25 cm higher; mean 4.81 cm (95% CI 4.52 to 5.1) | MD 0.25 (‐0.04 to 0.54) | 548 (4 studies) | ⊕⊕⊝⊝ moderate1 | Data analysis was unadjusted for confounders |
|
Change in SD scores over 12 months (height) (low change is better) |
Unadjusted mean change in SD score was ‐0.18 (range, ‐0.01 to ‐0.27) | Corresponding mean unadjusted change on lower‐dose ICS was 0.08 less; mean ‐0.10 (95% CI ‐0.21 to 0.02) | MD 0.08 (‐0.03 to 0.20) | 328 (3 studies) | ⊕⊕⊝⊝ moderate1 | Data analysis was unadjusted for confounders |
| Change in weight over 12 months (kg) (higher is better) | Mean change in weight was 3.4 kg | Corresponding mean change in weight on lower‐dose ICS was 0.3 kg lower: mean 3.1 (95% CI 2.58 to 3.62) | MD ‐0.30 (‐0.82 to 0.22) | 408 (1 study) | ⊕⊕⊝⊝ low2 | Based on only 1 trial |
| Change in BMI over 12 months (kg/m2) (higher is better) | Mean change in BMI was 0.7 kg/m2 | Corresponding mean change in BMI on lower‐dose ICS was 0.2 kg/m2 less: mean 0.5 (95% CI 0.21 to 0.79) | MD ‐0.20 (‐0.49 to 0.09) | 408 (1 study) | ⊕⊕⊝⊝ low2 | Based on only 1 trial |
|
Change in skeletal maturation over 12 months (years) (higher is better) |
Mean change in skeletal maturation was 0.95 years | Corresponding mean change in skeletal maturation on lower‐dose ICS was 0.18 years more; mean 1.13 (95% CI 0.97 to 1.29) | MD 0.18 (0.02 to 0.34) | 181 (1 study) | ⊕⊕⊝⊝ low2 | Based on only 1 trial |
| *The basis for the assumed risk was the weighted mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Data analysis was unadjusted for confounders.
2Based on only 1 trial.
Background
This protocol is the first of a series of three review protocols exploring the safety profile of inhaled corticosteroids (ICS) in terms of growth in children with persistent asthma. The present review explored the dose‐response effect of ICS on growth. The second review compares the long‐term effects of ICS on growth (Zhang 2011), and the third examines the effects of different drugs and delivery devices on growth. For more comprehensive background data and additional references, see Zhang 2011.
Description of the condition
Asthma is defined as a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment (GINA 2014). In developed countries, the prevalence of childhood asthma has markedly increased over the past few decades (ISAAC 1998; Masoli 2004; Asher 2010); however, this increase has recently reached a plateau in some of these countries (Lai 2009; Asher 2010). In contrast, asthma prevalence is sharply increasing in developing countries (Africa, Central and South America, Asia and the Pacific region), probably as a result of rapid and ongoing urbanisation and westernisation (Braman 2006; Asher 2010). The global burden of childhood asthma is continuing to rise.
Description of the intervention
ICS are widely considered the first‐line treatment for persistent asthma, both in adults and in children (NHLBI 2007; BTS 2012; GINA 2014; Chauhan 2012; Lougheed 2012). Studies have demonstrated the clinical benefits of ICS in controlling asthma symptoms, reducing exacerbations and hospitalisations, decreasing airway hyperresponsiveness and airway inflammation, improving pulmonary function, improving quality of life and reducing asthma‐related deaths (Juniper 1990; Van Essen‐Zandvliet 1992; Olivieri 1997; Van Rensen 1999; Suissa 2000; Covar 2003; Adams 2011a; Adams 2011b; Adams 2011c). Seven ICS are currently available for clinical use worldwide: beclomethasone dipropionate, budesonide, fluticasone propionate, mometasone fumarate, ciclesonide, flunisolide and triamcinolone acetate. Each inhaled corticosteroid has different pharmacokinetic and pharmacodynamic properties and biologic characteristics; however, all ICS can achieve similar therapeutic benefits when given at equipotent doses (Sobande 2008; BTS 2012; GINA 2014; Lougheed 2012).
The optimal doses of ICS for persistent childhood asthma remain unclear. The most recent asthma guidelines recommend initiating ICS at low or medium daily doses for children with mild to moderate persistent asthma; however, patients with more severe asthma and those with poor response to low to moderate doses of ICS may require higher doses (≥ 400 μg/d of hydrofluoroalkane (HFA)‐beclomethasone or equivalent) to achieve satisfactory control of asthma (NHLBI 2007; BTS 2012; GINA 2014; Lougheed 2012).
Although ICS are generally considered safe treatment for children with asthma, the potential systemic adverse effects related to long‐term use of these drugs have been, and continue to be, a matter of concern, especially the effects on growth (Pedersen 2001; Allen 2002). In 1998, based on a report of the panel of experts, the US Food and Drug Administration (FDA) required labels on all ICS warning of a potential reduction in growth in children (FDA 1998). Since that time, the relationship between ICS and growth impairment in children with asthma has been extensively debated in the literature and more so with the advent of new molecules with allegedly safer profiles (Witzmann 2000; Brand 2001; Creese 2001; Wolthers 2001; Carlsen 2002; Price 2002a; Sizonenko 2002; Salvatoni 2003; Allen 2006).
How the intervention might work
ICS are the most potent anti‐inflammatory drugs available for long‐term treatment of persistent asthma. Possible molecular mechanisms for the anti‐inflammatory effects of ICS and for corticosteroid‐induced growth impairment have been reviewed previously (Barnes 2003; Zhang 2011).
Why it is important to do this review
One Cochrane systematic review (Sharek 2000a) produced solid evidence supporting growth suppression estimated at 1.5 cm per year over seven to 12 months for 400 μg/d inhaled chlorofluorocarbon (CFC)‐propelled beclomethasone (equivalent to 200 μg/d of HFA‐propelled beclomethasone) in children with asthma. This review lately has been converted to a journal article (Sharek 2000b). However, it remains unclear whether corticosteroid‐induced growth retardation is dose dependent. We therefore decided to conduct this systematic review to evaluate the relationship between dose of ICS and risk of growth impairment in children with persistent asthma.
Objectives
To assess whether increasing the dose of ICS is associated with slower linear growth, weight gain and skeletal maturation in children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
Parallel‐group randomised controlled trials.
Types of participants
Children one to 17 years of age with the diagnosis of persistent asthma.
Types of interventions
Each treatment group should be given the same ICS at two or more different doses via the same delivery system for at least three months. ICS may be administered as monotherapy or in combination with other non‐steroidal asthma drugs (e.g. long‐acting beta‐agonists (LABAs), leukotriene receptor antagonists (LTRAs)). In all included trials, the intervention group depicted is the lower‐dose ICS and the control (comparison) group is the higher‐dose ICS.
Types of outcome measures
Primary outcomes
Linear growth velocity (cm/y), obtained by measuring height at a number of time points during the study and performing linear regression of height over time (Price 2002a).
Secondary outcomes
Change in growth velocity standard deviation (SD), defined as the difference between an individual's growth velocity and predicted growth velocity divided by the predicted growth velocity SD for individuals of the same age and sex (and ethnicity if available) (Pedersen 2001).
Change in absolute height (cm) over time.
Change in weight (kg or z‐score) over time.
Change in body mass index (added post hoc).
Change in skeletal maturation (added post hoc).
We did not intend to include lower leg length measured by knemometry as the outcome because this measurement correlates poorly with statural height and tends to overestimate potential effects of ICS on growth (Efthimiou 1998; Allen 1999).
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of Trials (CAGR), which were derived through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and through handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). All records in the CAGR coded as 'asthma' were searched using the following terms.
(((steroid* or corticosteroid* or glucocorticoid* ) and inhal*) or budesonide or Pulmicort or fluticasone or Flixotide or Flovent or ciclesonide or Alvesco or triamcinolone or Kenalog or beclomethasone or beclometasone or Becotide or Becloforte or Becodisk or QVAR or Flunisolide or AeroBid or mometasone or Asmanex or Symbicort or Advair or Inuvair) AND (grow* or height* or SDS) AND (child* or paediat* or pediat* or adolesc* or teen* or prepubertal* or pre‐pubertal* or puberty or pubertal* or infan* or toddler* or bab* or young*) AND (dose* or dosage* or delivery* or administ* or response* or high* or low*)
We also conducted a search of the ClinicalTrials.gov website. All databases were searched from their inception until March 2014 with no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched manufacturers' clinical trial databases for potentially relevant unpublished studies, if needed.
Data collection and analysis
Selection of studies
Two review authors (AP and LZ or SP) independently assessed the titles and abstracts of all potential studies for inclusion identified by the search strategy. Full‐text articles were retrieved when they appeared to meet the inclusion criteria or when data in the title and abstract were insufficient to permit a clear decision regarding their inclusion. We resolved disagreements through discussion, or, if required, we consulted the third review author.
Data extraction and management
Two review authors (AP and BC) independently extracted data from the included trials using specially designed and pilot‐tested data extraction forms. For trials with multiple reports, we extracted data from each report separately and combined information across multiple data collection forms afterwards. We resolved disagreements by discussion and entered the extracted data into RevMan version 5.1 (Review Manager 5).
We extracted the following data.
Study characteristics: year of publication, name of the first author, setting and source of funding/sponsorship.
Methods: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting and other sources of bias.
Participants: sample size, demographics, inclusion and exclusion criteria.
Intervention: type of ICS, dosage, frequency of administration, inhalation device, treatment duration and adherence to treatment, if available.
Comparator: the same corticosteroid given at different dosage regimens (the same details as for intervention).
Co‐interventions: type, dosage regimen and duration.
Results: mean value of the outcome measures in each group, SD or other metrics for uncertainty (standard errors (SEs), confidence intervals (CIs), P values for differences in means) of outcome measurements in each group, number of participants who underwent randomisation, number of participants on whom outcomes were measured in each group.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Disagreements were resolved by discussion or by involving the third review author. We assessed the risk of bias according to the following domains.
Allocation sequence generation.
Concealment of allocation.
Blinding of participants and investigators.
Incomplete outcome data.
Selective outcome reporting.
Other risk of bias.
We noted other sources of bias. We graded each potential source of bias as low, high or unclear risk. Studies were deemed to be of high methodological quality if information on randomisation generation, blinding and incomplete outcome data was available, indicating a low risk of bias.
Measures of treatment effect
Measurements of growth were continuous outcomes, so we used mean difference (MD) and 95% CI as the metrics for treatment effects, as appropriate.
Unit of analysis issues
We considered each individual comparison as the unit of analysis. We used analysed participants as sample size rather than the number of participants randomly assigned in the included studies. We had planned three pair‐wise comparisons of ICS doses in HFA‐beclomethasone or equivalent: low (≤ 200 μg) versus medium (201 to 400 μg) versus high dose (> 400 μg) and low (≤ 200 μg) versus high (> 400 μg) dose (Lougheed 2012). The ICS dose equivalence used for this review was based on Canadian Asthma Guidelines (Lougheed 2012), which are based on a combination of the dose equivalency mentioned in GINA 2014 and reported safety and efficacy data: 1 μg fluticasone = 1 μg mometasone = 1 μg ciclesonide = 1 μg of hydrofluoroalkane HFA‐beclomethasone = 2 μg budesonide = 2 μg CFC‐BDP = 4 μg flunisolide = 4 μg triamcinolone acetate.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. In cases of substantial heterogeneity (I2 > 50%), we explored potential sources of heterogeneity by performing prespecified subgroup analysis and sensitivity analysis. We also conducted these analyses to explore the possibility of an effect modifier even if no significant heterogeneity was observed.
Assessment of reporting biases
We planned to contact study authors to ask them to provide missing outcome data if we suspected reporting bias. When this was not possible, and when the missing data were thought to introduce serious bias, we planned to explore the impact of excluding such studies on the overall assessment of results by performing a sensitivity analysis.
Data synthesis
We performed the meta‐analyses using the Cochrane statistical package RevMan 5 (Review Manager 5). We used the fixed‐effect model unless statistical heterogeneity was found, in which case we used the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for the primary outcome, measured at various points in time.
Participant age: preschoolers (two to five years), prepubertal children (> five to 12 years), adolescents (> 12 to 18 years).
Asthma severity: mild versus moderate versus severe.
ICS molecule: beclomethasone, budesonide, fluticasone, mometasone, ciclesonide, flunisolide, triamcinolone.
Concomitant use of non‐steroidal antiasthmatic drugs: ICS alone, ICS combined with non‐steroidal drugs, such as LABAs and LTRAs.
Dose difference of ICS in HFA‐beclomethasone or equivalent (added as post hoc analysis).
Sensitivity analysis
Sensitivity analysis was used to assess the potential impact of particular decisions or missing information on the findings of the review (Higgins 2008). We planned to carry out the following sensitivity analyses with regards to primary outcome by excluding from the analysis trials with the following.
High risk of bias owing to missing data or unbinding, or both.
Rate of adherence to ICS lower than 75% or lack of available data regarding adherence to treatment.
Pharmaceutical industry sponsorship.
Results
Description of studies
Results of the search
The literature search conducted until March 2014 identified a total of 406 citations and abstracts (Figure 1). Of these, 71 potential full texts were reviewed thoroughly for inclusion criteria. Twenty‐two trials, including 34 comparisons (Characteristics of included studies), were eligible for inclusion. Of these, 12 trials (17 comparisons) contributed no usable data to this review; four trials (five comparisons) either presented data in a different format than was specified in the protocol or reported incomplete data (Jonasson 2000; Chen 2001; Teper 2004; Gelfand 2006; Gelfand 2006 b); seven trials (11 comparisons) did not measure children's growth as an outcome (Jonasson 1998; Giorgi 1998; Peden 1998; Peden 1998 b; Baker 1999; Baker 1999 b; Kemp 1999; Kemp 1999 b; Doniec 2004; Kerwin 2008; Kerwin 2008 b) and one trial was published as an abstract (Lemanske 2004). Consequently, 10 trials (17 comparisons) published as full text contributed at least one outcome to the meta‐analysis.
1.
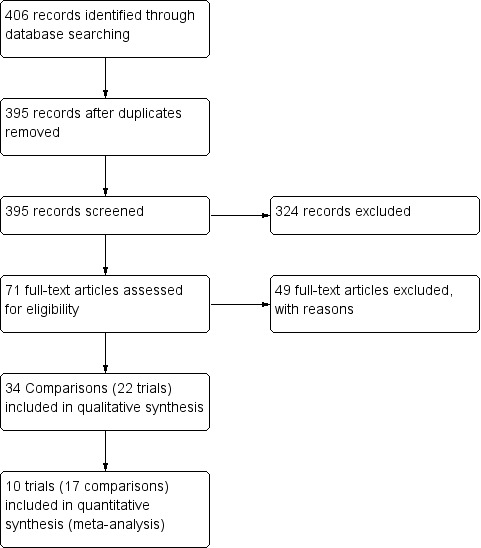
Flow diagram of screening of trials.
Included studies
Ten trials, reporting 17 comparisons (Allen 1998; Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d; Verberne 1998; Verberne 1998 b; Wasserman 2006; Sorkness 2007; Skoner 2008; Pedersen 2010; Pedersen 2010 b; Vaessen‐Verberne 2010; Brand 2011; Brand 2011 b; Skoner 2011; Skoner 2011 b) and enrolling 3394 children with confirmed persistent asthma, contributed data to the review. The following information pertains only to the 17 comparisons (from 10 included trials) contributing data to this review (Characteristics of included studies). The FDA has produced a guideline on evaluation of the effects of orally inhaled and intranasal corticosteroids, specific to placebo‐controlled trials in children (US FDA 2007); although some criteria were not relevant for dose‐response studies, we ascertained the compliance status to these guidelines of trials that contributed data to the meta‐analysis (Table 2; Table 3; Table 4).
1. FDA study design.
| Study | Run‐in period ≥ 16 weeks | Tx period ≥ 48 weeks | Follow‐up period (to access catch‐up period) | Follow‐up period ≥ 8 weeks | Recommended age (male: 3‐10.5 years; female: 3‐9.5 years, prepuberty (Tanner 1)) | Mild asthma severity | No use of spacers | Placebo or active control group with no growth‐suppressing effect |
| Allen 1998 | No (2 weeks) | Yes (52 weeks) | No | No | Yes | Yes | Yes | Yes |
| Brand 2011 | No (2‐4 weeks) | No (24 weeks) | No | No | Partially (2‐6 years) | Yes | No | Yes (placebo or montelukast if control was insufficient) |
| Pedersen 2010 | No (2‐4 weeks) | No (12 weeks) | No | No | Yes (6‐11 years) | No | No* | Yes |
| Shapiro 1998 | No (2 weeks) |
No (12 weeks) | No | No | No (6‐18 years) | No | Yes | Yes |
| Skoner 2008 | Yes (6 months) | Yes (52 weeks) | Yes | Yes (8 weeks) | Yes (5‐8 years) | Yes | Yes | Yes |
| Skoner 2011 | No (1‐2 weeks) | Yes (52 weeks) | Yes | Yes (12 weeks) | Yes | Yes | No | Yes |
| Sorkness 2007 | No (2‐4 weeks) | Yes (48 weeks) | No | No | No (6‐14 years) | No (mild to moderate) | No | Yes (montelukast) |
| Vaessen‐Verberne 2010 | No (6 weeks) | No (26 weeks) | No | No | No (6‐16 years) | No (moderate) | Yes | No |
| Verbern 1998 | No (6 weeks) | Yes (54 weeks) | Yes+ | No | No (6‐16 years) | No | Yes | Yes (salmeterol) |
| Wasserman 2006 | No (2‐4 weeks) | No (12 weeks) | No | No | Partially (24‐47 months) | NR | No | Yes |
FDA = US Food and Drug Administration; NR = not reported.
All studies were randomised, placebo‐controlled, double‐blind, parallel‐group trials.
2. FDA statistical methods.
| Intention‐to‐treat analysis | Exclusion of pubescent children in analysis | Low and balanced withdrawals or missing data or patient dropouts | Data presented as linear regression model but not change in height | Baseline height, age, sex used as confounders in analysis model | Catch‐up growth analysed with a linear regression model | No nasal steroid during the study | |
| Allen 1998 | Yes | Yes | Yes | Yes | No | NA | Yes |
| Brand 2011 | Yes | NA | Yes | Yes | Yes | NA | NR |
| Pedersen 2010 | Yes | NR | No (dropout in placebo: 24% vs active treatment: 16%‐18%) | No | No | NA | NR |
| Shapiro 1998 | NR | NR | No | NR | NR | NA | NR |
| Skoner 2008 | Yes | NR | Yes | Yes | Yes | Yes | Yes |
| Skoner 2011 | NR | NR | No | Yes | Yes | Yes | NR |
| Sorkness 2007 | Yes | No | Yes | No | No | NA | NR |
| Vaessen‐Verberne 2010 | Yes | No | Yes | Yes | Yes | NA | NR |
| Verbern 1998 | NR | NO | Yes | Yes | Yes | No | NR |
| Wasserman 2006 | Yes | NA | Yes | Yes | Yes | NA | Yes |
3. FDA possible sources of bias.
| Use of stadiometer | Height evaluation by same trained blinded examiner | Height evaluation at the same time of the visit day | Repeated (≥ 3) measurements during the study period | Record of compliance | |
| Allen 1998 | Yes | NR | NR | Yes | Yes |
| Brand 2011 | Yes | NR | NR | Yes | Yes |
| Pedersen 2010 | Yes | NR | NR | No | No |
| Shapiro 1998 | NR | NR | NR | No | Yes |
| Skoner 2008 | Yes | Yes | Yes | Yes | Yes |
| Skoner 2011 | Yes | Yes | Yes | Yes | Yes |
| Sorkness 2007 | Yes | NR | NR | No | Yes |
| Vaessen‐Verberne 2010 | Yes | NR | NR | No | Yes |
| Verbern 1998 | Yes | NR | NR | Yes | Yes |
| Wasserman 2006 | Yes | NR | Yes | Yes | NR |
Design
All trials used a parallel‐group design.
Participants
Three comparisons involved children two to five years of age (Wasserman 2006; Brand 2011; Brand 2011 b), six comparisons involved prepubertal children, five to 12 years of age (Allen 1998; Skoner 2008; Pedersen 2010; Pedersen 2010 b; Skoner 2011; Skoner 2011 b), and eight comparisons involved prepubertal and pubertal children (Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d; Verberne 1998; Verberne 1998 b; Sorkness 2007; Vaessen‐Verberne 2010). Most trials described a gender ratio hovering around 65% male participants. With regards to asthma severity, one comparison (Skoner 2008) focused on asthmatic individuals with mild airway obstruction, two comparisons (Verberne 1998; Verberne 1998 b) focused on asthmatic individuals with mild to moderate airway obstruction, four comparisons (Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d) focused on asthmatic individuals with moderate to severe airway obstruction and the remaining six comparisons (Allen 1998; Wasserman 2006; Pedersen 2010; Pedersen 2010 b; Skoner 2011; Skoner 2011 b) failed to report the severity of baseline airway obstruction. Two comparisons (Brand 2011; Brand 2011 b) pertained to preschool children with recurrent wheezing and a positive asthma predictive index or a positive screening test for atopy. Asthma triggers were seldom reported.
Intervention duration
The duration of intervention varied from 12 weeks (seven comparisons; Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d; Wasserman 2006; Pedersen 2010; Pedersen 2010 b) to 24 weeks (two comparisons; Brand 2011; Brand 2011 b) to 26 weeks (one comparison; Vaessen‐Verberne 2010) to 52 weeks (seven comparisons; Allen 1998; Verberne 1998; Verberne 1998 b; Sorkness 2007; Skoner 2008; Skoner 2011; Skoner 2011 b).
Intervention drugs
The ICS molecule used was beclomethasone dipropionate (BDP) (two comparisons; Verberne 1998; Verberne 1998 b), budesonide (BUD) (four comparisons; Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d), ciclesonide (CIC) (five comparisons; Skoner 2008; Pedersen 2010; Pedersen 2010 b; Brand 2011; Brand 2011 b), fluticasone propionate (FP) (four comparisons; Allen 1998; Wasserman 2006; Sorkness 2007; Vaessen‐Verberne 2010) or mometasone fumarate (MF) (two comparisons; Skoner 2011; Skoner 2011 b). The difference in the dose of ICS between two comparison groups (reported in HFA‐beclomethasone equivalent) varied by ≤ 150 μg in most trials. Most compared 100 μg (low dose) versus 200 μg (the cutoff limit between low and medium doses of ICS); in only four comparisons (Shapiro 1998 b; Shapiro 1998 d; Verberne 1998; Vaessen‐Verberne 2010 ) was the difference in the dose of ICS between groups ≥ 400 μg. Different devices were used, including aerochamber, diskhaler, dry powder inhaler, metered‐dose inhaler with or without spacer, nebuliser and turbohaler (further details are available in the Characteristics of included studies table). Yet all trials used the same inhalation device in within‐trial group comparisons. Adherence rate to ICS was reported by three of 10 trials; when reported, adherence was at or above 80%. All trials but one (Sorkness 2007) were funded by the pharmaceutical industry.
Co‐intervention
Three comparisons (Verberne 1998; Pedersen 2010; Pedersen 2010 b) enrolled only participants receiving ICS as monotherapy. Eleven comparisons (Allen 1998; Shapiro 1998; Shapiro 1998 b; Shapiro 1998 c; Shapiro 1998 d; Wasserman 2006; Skoner 2008; Brand 2011; Brand 2011 b; Skoner 2011; Skoner 2011 b) reported accepting participants who were using co‐interventions with additional antiasthmatic drugs such as LABAs, antileukotrienes or theophylline. Three comparisons (Verberne 1998 b; Sorkness 2007; Vaessen‐Verberne 2010) specifically compared ICS alone versus ICS + LABA, without other co‐interventions.
Outcomes
The primary outcome was linear growth velocity (zero to 12 months), which was documented in four comparisons involving prepubescent children (Allen 1998; Skoner 2008; Skoner 2011; Skoner 2011 b); in all cases, linear growth was analysed in three or more height measurements by regression analysis, with adjustment for co‐variates in all but one trial (Allen 1998). Secondary outcomes included change in height, growth velocity, weight, body mass index and skeletal maturation.
Excluded studies
Of 406 citations searched, 384 (94%) were excluded for the following exclusive reasons (Figure 1): (1) duplicate references (N = 11), (2) not a randomised controlled trial (N = 76), (3) not a parallel‐group study (N = 84), (4) participants aged < one year or ≥ 18 years (N = 33), (5) participants not asthmatic (or participants with asthma selected for another co‐morbidity, e.g. hypertension, diabetes) (N = 16), (6) participants with episodic asthma (N = 2), (7) acute and emergency care settings (N = 13), (8) no daily ICS stable dose in all participants in one of the comparison groups (N = 86), (9) not testing an additional ICS dose using the same molecule in all participants of the other comparison group (N = 50), (10) co‐interventions with oral corticosteroids (N = 3), and (11) treatment administered for less than 12 weeks (N = 10). Reasons for exclusion are provided in the Characteristics of excluded studiestable.
Risk of bias in included studies
Details on risk of bias for each included trial are presented in the Characteristics of included studies tables. A graphical summary of risk of bias judgements is presented in Figure 2. Although all trials were randomised, only 14 comparisons (41%) reported the method of randomisation.
2.
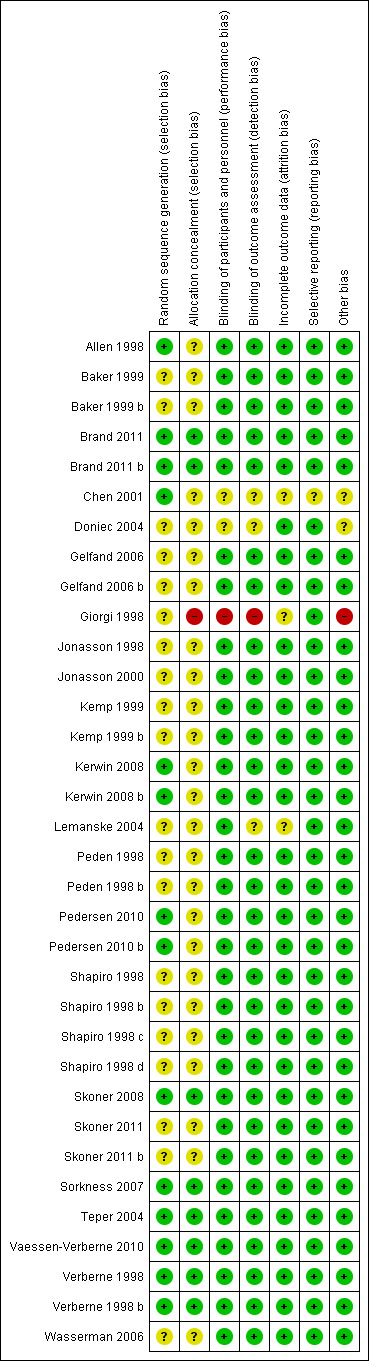
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
26 comparisons did not mention the method of concealment of treatment, and eight comparisons (23.5%) reported use of an appropriate concealment technique.
Blinding
31 comparisons (90%) reported double‐blinding with convincing details, two comparisons (Chen 2001; Doniec 2004) did not report sufficient information to allow the review authors to ascertain blinding and one comparison (Giorgi 1998) used an open‐label study design.
Incomplete outcome data
31 comparisons (91%) reported all data with balanced numbers in both groups, and data from three comparisons (Giorgi 1998; Chen 2001; Lemanske 2004) were unclear. All trials reported numbers of and reasons for withdrawals in both comparison groups. The proportion of overall withdrawals was variable between studies (10% to 30%), with a balance in withdrawal rates noted between groups given different ICS doses.
Selective reporting
33 comparisons (97%) reported all outcomes mentioned in the methods section, with no apparent bias, and one comparison (Chen 2001) was unclear.
Other potential sources of bias
In 31 comparisons, we encountered no other significant sources of bias, two comparisons (Chen 2001; Doniec 2004) were unclear and one comparison (Giorgi 1998) was an open‐label study for which the primary outcome was not specified clearly.
Except for three trials, all eligible trials contributing data were of high methodological quality. Two of four comparisons contributing to the primary outcome (Allen 1998; Skoner 2008) were of high methodological quality.
Effects of interventions
See: Table 1
Primary outcomes
Linear growth velocity (cm/y)
A statistically significant group difference in linear growth (cm/y) over 12 months was noted between intervention (lower ICS dose) and control (higher ICS dose) groups (four comparisons; N = 728 children; MD 0.20 cm/y, 95% CI 0.02 to 0.39; Figure 3); no heterogeneity was apparent. The different molecules used (mometasone, ciclesonide and fluticasone) did not seem to influence the magnitude of effect: χ2 = 2.19; df = 2; P value 0.33; Analysis 1.2; Figure 4). Data from Skoner 2011 weighed 10% in the primary outcome analysis. In Skoner 2011, growth velocity was analysed using two different statistical models: a longitudinal random slope (LRS) model and an individual regression (IR) model; results from both of these methods were reported. The IR model resulted in poor estimates of growth rate with lower precision, as admitted by the study authors, and led to a different confidence interval around the pooled results. In contrast, the LRS model provided more robust growth rates. Consequently, we chose the data derived using the best (LRS) model, which led to a significant group difference in the primary outcome, recognising that use of the IR model would have led to a group difference approaching, but not reaching, statistical significance.
3.
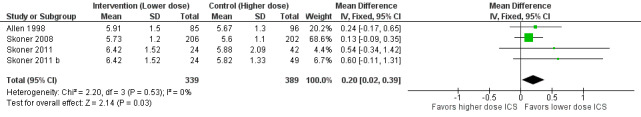
Forest plot of comparison: 1 Inhaled corticosteroids dose‐response effect, outcome: 1.1 Growth velocity (cm/y) by stadiometry from 0‐12 months.
1.2. Analysis.
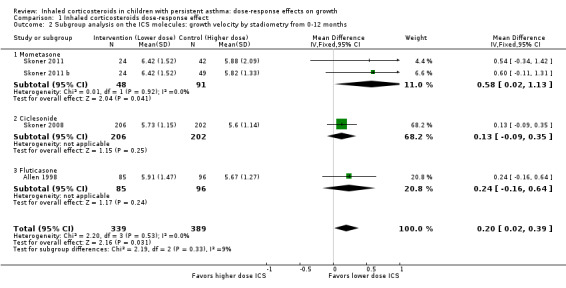
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 2 Subgroup analysis on the ICS molecules: growth velocity by stadiometry from 0‐12 months.
4.
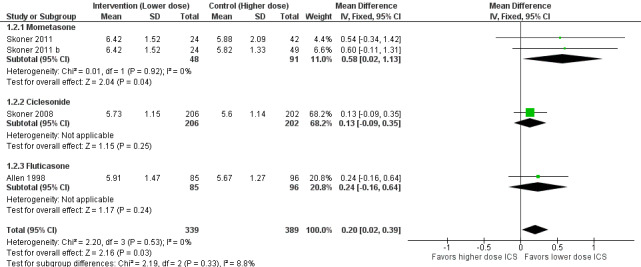
Forest plot of comparison: 1 Inhaled corticosteroids dose‐response effect, outcome: 1.2 Subgroup analysis on the ICS molecules: growth velocity by stadiometry from 0‐12 months.
We could not perform subgroup analysis on age, severity and ICS dose, as all trials contributing data to the primary outcome had similar characteristics in that they enrolled prepubertal children with mild or unknown severity of airway obstruction, used similarly low ICS doses and did not report or failed to specify the use of co‐interventions. Of note, in all four comparisons contributing data, the ICS dose difference between the two groups was less than or equal to 150 μg of HFA‐beclomethasone.
As all trials contributing data to the primary outcome were published in full text with high methodological quality and were sponsored by the pharmaceutical industry, we could not perform sensitivity analyses to assess bias due to publication status, poor methodology or funding status. As the adherence rate for ICS was seldom or incompletely reported, sensitivity analysis was not performed on this criterion.
No statistically significant group differences in linear growth (standardised in cm/y) were seen over the first three months (six comparisons; N = 1114 children; MD ‐0.12, 95% CI ‐0.51 to 0.27; Analysis 1.3) and no heterogeneity was apparent. Only two comparisons from the same trial provided data on growth velocity from zero to six months (Analysis 1.4) and from three to six months (Analysis 1.5); in both cases, a statistically significant group difference was not reported.
1.3. Analysis.
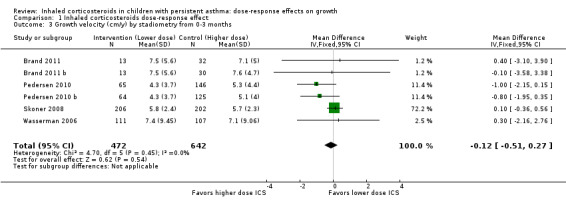
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 3 Growth velocity (cm/y) by stadiometry from 0‐3 months.
1.4. Analysis.
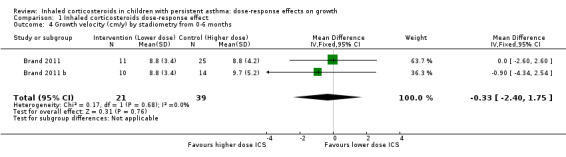
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 4 Growth velocity (cm/y) by stadiometry from 0‐6 months.
1.5. Analysis.
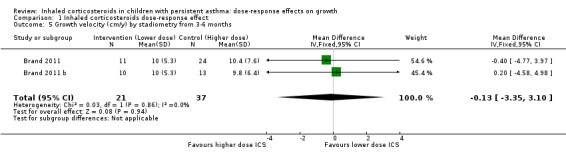
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 5 Growth velocity (cm/y) by stadiometry from 3‐6 months.
Secondary outcomes
Change in growth velocity (cm/y)
Only one trial reported change in growth velocity from zero to 12 months with no statistically significant group difference (one comparison; N = 181 children; MD 0.06 cm/y, 95% CI ‐0.43 to 0.55; Analysis 1.6).
1.6. Analysis.
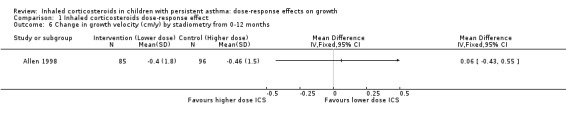
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 6 Change in growth velocity (cm/y) by stadiometry from 0‐12 months.
Change in height (cm)
This outcome reflects the net change between final and initial height, without linear regression or adjustment for important co‐variates such as age, sex, puberty and baseline height. A statistically significant group difference was noted in the change in height from zero to three months in favour of the higher ICS dose (nine comparisons; N = 944 children; MD ‐0.15 cm, 95% CI ‐0.28 to ‐0.02; Analysis 1.7); children were described as having mild to moderate to severe asthma, and the ICS used were cicleconide, budesonide and fluticasone. However, the group difference was not statistically significant over longer or subsequent periods, that is, from zero to six months (three comparisons; N = 211 children; MD ‐0.03, 95% CI ‐0.33 to 0.27) (Analysis 1.8), from three to six months (two comparisons; N = 58 children; MD ‐0.01, 95% CI 0.74 to 0.71) (Analysis 1.9) and from zero to 12 months (four comparisons; N = 548 children; MD 0.25, 95% CI ‐0.04 to 0.54; Analysis 1.10).
1.7. Analysis.
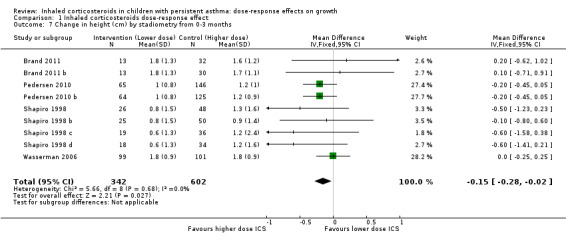
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 7 Change in height (cm) by stadiometry from 0‐3 months.
1.8. Analysis.
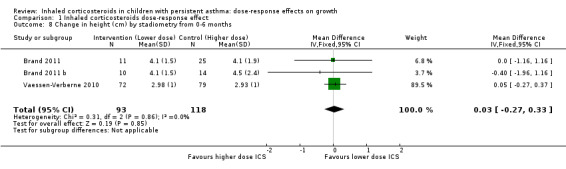
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 8 Change in height (cm) by stadiometry from 0‐6 months.
1.9. Analysis.
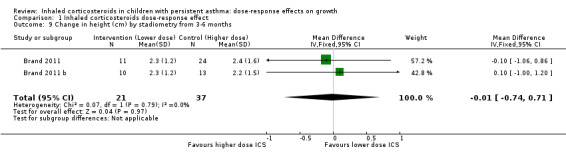
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 9 Change in height (cm) by stadiometry from 3‐6 months.
1.10. Analysis.
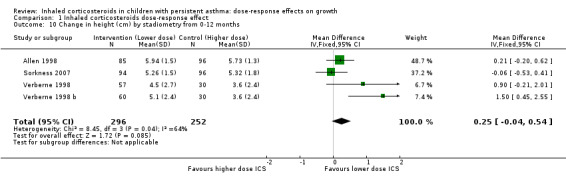
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 10 Change in height (cm) by stadiometry from 0‐12 months.
Change in standard deviation score (SDS) (height)
No statistically significant group difference in change in SDS (height) from zero to 12 months was reported (three comparisons; N = 328 children; MD 0.08, 95% CI ‐0.03 to 0.20; Analysis 1.11).
1.11. Analysis.
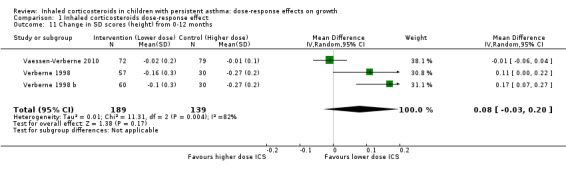
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 11 Change in SD scores (height) from 0‐12 months.
Change in weight (kg)
No significant group difference in change in weight was seen from zero to three months (Analysis 1.12), from zero to six months (Analysis 1.13) and from zero to 12 months (Analysis 1.14).
1.12. Analysis.
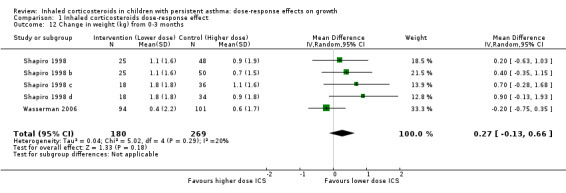
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 12 Change in weight (kg) from 0‐3 months.
1.13. Analysis.
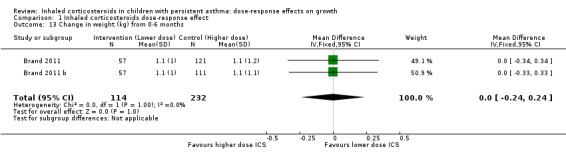
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 13 Change in weight (kg) from 0‐6 months.
1.14. Analysis.
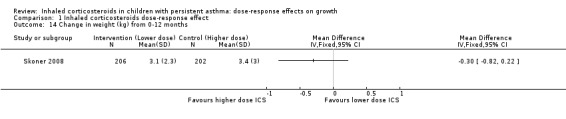
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 14 Change in weight (kg) from 0‐12 months.
Change in body mass index (BMI) (kg/m2)
No significant group difference in change in BMI was noted from zero to six months (Analysis 1.15) or from zero to 12 months (Analysis 1.16).
1.15. Analysis.
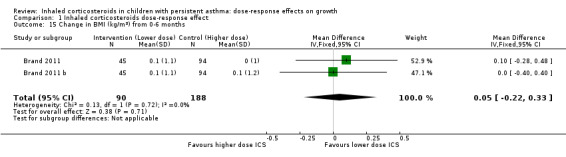
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 15 Change in BMI (kg/m2) from 0‐6 months.
1.16. Analysis.
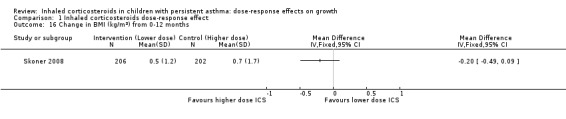
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 16 Change in BMI (kg/m2) from 0‐12 months.
Change in skeletal maturation
Only one trial reported change in skeletal maturation, with a statistically significant group difference from zero to 12 months in favour of a lower ICS dose (one comparison; N = 181 children; MD 0.18, 95% CI 0.02 to 0.34; Analysis 1.17).
1.17. Analysis.
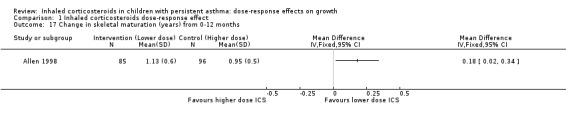
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 17 Change in skeletal maturation (years) from 0‐12 months.
Discussion
This meta‐analysis aggregated data from 10 paediatric trials, providing 17 comparisons, as several studies tested more than two different doses of ICS or provided additional data subgrouped by age. In the four trials reporting the main outcome, a statistically significant group difference was seen in linear growth velocity measured by stadiometry over 12 months in prepubertal school‐aged children treated with low doses (i.e. 50 to 100 μg) versus low to medium doses (i.e. 200 μg of fluticasone, mometasone and ciclesonide). Of note, the statistically significant group difference was observed despite the small ICS dose difference between compared groups, varying between 100 and 150 μg/d (although most vary by 100 μg/d) of HFA‐propelled beclomethasone or equivalent in the four studies pooled. Of interest, a change in height between zero and three months showed a significant decrease of 0.15 cm in the opposite direction, that is, in disfavour of a lower ICS dose, underlying the impact of neglecting important co‐variates influencing growth (e.g. sex). This also raised the possibility of a beneficial effect of rapidly achieving asthma control (although this was not measured) and the impact of the timing of measurement of effect size, as this unadjusted group difference was not observed over subsequent and longer time periods. No statistically significant change from baseline in linear growth velocity, weight and body mass index was noted over zero to 12 months of ICS therapy in children. Our findings suggest a clear, yet small, dose‐dependent effect on growth when ICS are used at 200 μg/d or less—the cutoff for low to medium doses of ICS in children.
The main outcome, growth velocity, that is, the pattern of growth measured repeatedly over time and adjusted for relevant co‐variates (in all individual trials but one (Allen 1998)), was measured in prepubertal school‐aged children (< 12 years) treated with fluticasone propionate, ciclesonide and mometasone for 52 weeks. Of the 10 trials contributing data, only three trials (four comparisons) contributed data to the primary outcome (i.e. growth velocity (cm/y)) from zero to 12 months; all performed repeated height measurements using a stadiometer, were funded by pharmaceutical companies and were of high methodological quality. Trials used either a dry powder inhaler or a metered‐dose inhaler with spacer to deliver these three molecules with lower systemic bioavailability than budesonide and beclomethasone. Because of trial homogeneity, it was not possible to explore a possible modifier effect of age, severity of airway obstruction, asthma control, use of co‐interventions and ICS dose difference on growth velocity. Indeed, trials contributing data to this outcome predominantly compared low ICS doses versus low to medium doses, with a dose difference of 100 to 150 μg/d of HFA‐beclomethasone equivalent (GINA 2014); higher doses of ICS theoretically offer greater potential for growth suppression (NHLBI Expert Panel Report 2012).
No effect of the choice of molecules within those tested was apparent. Indeed, several placebo‐controlled trials and Cochrane reviews have documented molecule dependency of growth suppression of ICS. Zhang and colleagues (Zhang 2011) are evaluating the growth‐suppressive effect of several ICS molecules compared with placebo, reporting minimal and less effect of fluticasone, mometasone and ciclesonide compared with budesonide and beclomethasone. Trials aggregated in this latter review had independently documented a growth‐suppressive effect at equivalent ICS doses of between 1.1 and 1.2 cm/y (CAMP Research Group 2000; CAMP Research Group 2012) with budesonide, 0.7 cm/y with mometasone (Skonner 2011), a non‐significant group difference of 0.43 cm/y with fluticasone (Sharek 2000b) and none with ciclesonide (Skoner 2008) in prepubertal school‐aged children, suggesting molecule dependence of the impact of ICS on growth. This finding is consistent with that of a previous Cochrane systematic review (Sharek 2000a), which had produced solid evidence supporting the growth suppression of 400 μg of inhaled CFC‐propelled beclomethasone (equivalent to 200 HFA‐BDP) estimated at 1.54 cm/y over seven to 12 months in children with mild to moderate asthma. Current findings provide a clear indication that the use of ICS molecules believed to have no or little suppressive effect does have a minor, yet statistically significant, effect on growth when used at the lowest cutoff of the medium dosage compared with a lower dose.
In this review, the observed group difference of 0.2 cm in growth velocity over the first year of treatment (with an upper confidence interval limit of 0.4 cm/y), associated with an ICS dose higher by 100 to 150 μg, represents less than half the observed effect with similar doses compared with placebo (CAMP Research Group 2000; Sharek 2000a; Sharek 2000b; Skonner 2011; CAMP Research Group 2012). It is consistent with a very small dose‐response effect and arguably is impossible to detect on a standard growth curve. One must recognise that the small observed group difference with the use of most recent molecules (fluticasone, mometasone and ciclesonide) might be much higher with a higher ICS dose and/or with older molecules (budesonide and beclomethasone), which have well‐documented growth‐suppressing effects.
The two included trials (Shapiro 1998 b; Verberne 1998) that compared low doses versus higher doses of ICS (800 HFA‐BDP equivalent) contributed between 3% and 30% of the weight in only a few outcomes (1.7, 1.8, 1.10, 1.11 and 1.12), such that we cannot adequately explore the possibility of a differential effect on growth of a high versus low ICS dose. Although poorly controlled asthma may delay growth in children (NHLBI Expert Panel Report 2012), evidence to support this statement is weak. Yet we cannot rule out the possibility of a growth‐suppressive effect of poorly controlled asthma in children receiving a lower ICS dose, which could counterbalance the growth suppression associated with a higher ICS dose. If disease‐associated growth suppression was indeed possible, even in children with mild to moderate asthma, the design of this review is adequate, as we are interested in the net growth‐suppressive effect of ICS therapy in children with asthma. In the absence of a placebo‐controlled group, we cannot rule out the unlikely hypothesis that most growth retardation may occur at a very low dose of ICS therapy, which could explain the clinically small group difference between different ICS doses. The systemic availability of ICS is directly related to cortisol suppression and growth suppression, especially in children. The particle size of the drug molecule and use of different devices influence systemic availabilities (Martin 2002; Agertoft 2003; Agertoft 2003a). The third of this series of Cochrane reviews will examine the effects of different devices on the growth of asthmatic children.
As trials contributing data lasted a maximum of one year, the long‐term impact of different ICS doses on growth velocity beyond one year could not be explored. Our observations complement those of several placebo‐controlled studies, suggesting that the growth‐suppressive effect of ICS is non‐cumulative (Simons 1997) and may be associated with partial catch‐up (Guilbert 2006a), as a growth deficit may be sustained until adulthood (CAMP Research Group 2012).
Of interest, the significant group difference in the 'unadjusted' change in height between zero and three months suggests a favourable effect of ICS on growth in the first three months of use, perhaps via improved asthma control. Of note, 54% of the weight of this analysis is derived from a single trial testing various doses of ciclesonide (with a molecule with no demonstrated suppressive effect on growth) in children with partially or poorly controlled asthma (Pedersen 2010; Pedersen 2010 b). However, this hypothesis is weakened by the absence of any statistically significant effect observed between three and six months and between zero and six months, suggesting a transient beneficial effect on growth, insufficient power or a type 1 error, that is, falsely identifying a significant effect when one does not exist. Of importance, the absence of adjustment for important confounders decreases the quality of the evidence derived from this outcome.
No statistically significant group difference was observed in other aggregated parameters, namely, change from baseline in weight, change in SD scores (height) and body mass index. A significant group difference in skeletal maturation of a quarter of a year was observed, in disfavour of a higher dose (200 μg/d), with an ICS group difference of 100 μg/d of HFA‐propelled beclomethasone or equivalent (Allen 1998). Given that children with asthma may have delayed puberty (boys more than girls) (NHLBI Expert Panel Report 2012), whether the delayed maturation is due to poorer asthma control or is associated with greater use of ICS, or both, remains to be determined. Nevertheless, the observation on skeletal maturation, derived from a single study, requires replication.
Summary of main results
Three industry‐funded trials with high methodological quality (resulting in four dose comparisons) contributed data to the main outcome, that is, growth velocity; they measured 728 school‐aged children with mild to moderate asthma and used one of three molecules (fluticasone, ciclesonide or mometasone) to compare groups with a dose difference ≤ 150 μg over 52 weeks. A significant group difference in linear growth was observed over 12 months, indicating lower growth velocity in the higher ICS dose group (mean difference 0.20 cm/y, 95% CI 0.02 to 0.39); no heterogeneity was apparent. Within aggregated trials, the different ICS molecules did not significantly influence the magnitude of effect (P value 0.33), but no trial contributing data to the main outcome used budesonide or beclomethasone.
Overall completeness and applicability of evidence
This review summarises the best evidence available until March 2014 as derived from 10 trials (resulting in 17 comparisons) aggregating 3394 children with mild to moderate persistent asthma. Most trials were of high methodological quality. The systematic search, which identified eligible trials from published and unpublished reports (406 citations) and used selection and data extraction by two independent review authors, minimised the risk of inclusion bias. The outstanding collaboration of study authors and pharmaceutical groups from six trials (resulting in eight comparisons) allowed us to obtain additional unpublished data and to confirm methodological quality, both of which strengthened the meta‐analysis. Because of the paucity of trials reporting these data, four of 15 secondary outcomes could not be aggregated. The long‐term impact of low versus high ICS dose on growth velocity, weight, skeletal maturation and body mass index in children using the same and older ICS molecules beyond one year of follow‐up remains to be addressed. Sensitivity analysis could not be performed, as all trials were at low risk of bias, the adherence rate of ICS was seldom reported and all included trials contributing data to the main outcome were funded by the pharmaceutical industry and published as full text. In real life, most physicians would adjust downward or upward the dose of ICS needed to maintain control; we acknowledge that the artificially fixed dose for one to four years would overestimate growth suppression when compared with the recommended practice of decreasing to the minimal effective dose, yet this is a basic requirement of FDA guidelines for assessment of the effects of ICS on growth. Our study results support the Global Initiative for Asthma (GINA) guideline recommendations and serve as a reminder that physicians should strive to adjust to the minimal effective ICS dose, irrespective of the ICS molecule selected.
Quality of the evidence
The quality of evidence of growth velocity was high, but for outcomes reflecting change in height from baseline between treatment groups, the quality of evidence was downgraded to moderate owing to possible prognostic imbalance from the use of unadjusted data in the analysis. We downgraded the quality of evidence to low for BMI, weight and skeletal maturation due to imprecision (See Table 1).
Potential biases in the review process
Some bias may or may not have affected the magnitude of effect. All trials contributing data to the main outcome used a stadiometer to measure growth; this enhances the internal validity of the findings. As each trial compared different doses using the same device, we could not explore the possibility that the magnitude of effect may be associated with the choice of inhalation device; however a linked Cochrane review is addressing this point (Zhang 2011).
Agreements and disagreements with other studies or reviews
To our knowledge, no prior systematic review has evaluated the relationship between dose of ICS and risk of growth impairment in children with persistent asthma.
Authors' conclusions
Implications for practice.
In prepubescent school‐aged children with mild to moderate persistent asthma, a very small but statistically significant difference in linear growth over 12 months was observed between groups using ICS, with a dose difference ≤ 150 μg HFA‐beclomethasone equivalent over 52 weeks. A group difference of 0.2 cm was observed, favouring higher growth velocity with the lower ICS dose of fluticasone, mometasone or ciclesonide. As ICS doses most often were in the low range or at the limit of low and medium doses (200 μg), data were insufficient to allow exploration of a potential dose‐response relationship between ICS for a difference greater than 150 μg. We are unable to comment on the possible effects on growth of different ICS molecules, although fluticasone, mometasone and ciclesonide at doses of 200 μg/d or less did not appear to explain any variation in the size of effect across the studies. In view of prevailing parents’ and physicians’ concerns about the growth‐suppressive effect of ICS, lack or inadequate reporting of growth measurements in more than 86% (19/22) of eligible paediatric trials is a matter of concern and should call for systematic reporting of growth in all ICS paediatric trials. Until more data on low versus moderate and higher ICS doses are available, we recommend that ICS should be used at the lowest effective dose with the safest ICS molecules, and that children's growth should be systematically monitored during any ICS treatment.
Implications for research.
Long‐term (longer than one year) trials of high methodological quality with adequate documentation of linear growth velocity in children with asthma treated with ICS are needed to provide a fair comparison of the safety of different ICS dose options. Future trials should aim for the following design characteristics.
Pragmatic effectiveness trials.
Double‐blinding, adequate randomisation and complete reporting of withdrawals and dropouts with intention‐to‐treat analysis.
Parallel‐group design.
Complete reporting of continuous (denominators, mean change and mean standard deviation of change) and dichotomous (denominators and rate) data.
Minimal intervention period of 12 to 24 weeks to assess medium‐term effects and, over several years, to assess the long‐term impact of different ICS doses.
Measuring and reporting, at minimum, of linear growth velocity at different time points during the study.
Measuring and reporting of the change in standard deviation score (SDS) in growth velocity, in absolute gain in height, in weight z‐score, in BMI and in skeletal maturation between the beginning and the end of treatment.
Adequate reporting of the adherence rate and concomitant use of non‐steroidal antiasthmatic drugs.
Additional studies evaluating the impact on growth of LABA (long‐acting beta‐agonist) as a concomitant drug in children with ICS.
Given the paucity of paediatric trials reporting growth, growth measurements should be a requirement for all ICS drug trials whether funded by pharmaceutical companies or national granting agencies.
What's new
| Date | Event | Description |
|---|---|---|
| 17 January 2016 | Amended | During the translation process some text has been edited in the PLS and Abstract for clarity. |
Acknowledgements
We are indebted to the following individuals, who replied to our request for confirmation of methodology and additional data in the requested format when possible: Dr. Paul LP Brand, Dr. AA Vaessen‐Verberne.
A special thanks to the following pharmaceutical groups, which replied to our request for confirmation of methodology and additional data in the requested format when possible: GlaxoSmithKline Inc, Takeda Global Research & Development Centre (Europe) Ltd and AstraZeneca R&D, Mölndal, Sweden.
We are indebted to the Cochrane Airways Review Group, namely, Dr Emma Welsh, Elizabeth Stovold and Emma Jackson, for assistance with the literature search and ongoing support. A special thanks to Taixiang Wu from the Cochrane Review Group for assistance in translating three Chinese references.
We are thankful to Inge Axelsson for providing inputs to drafting of the protocol.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | weekly |
| EMBASE (Ovid) | weekly |
| CENTRAL (The Cochrane Library) | monthly |
| PsycINFO (Ovid) | monthly |
| CINAHL (EBSCO) | monthly |
| AMED (EBSCO) | monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Data and analyses
Comparison 1. Inhaled corticosteroids dose‐response effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Growth velocity (cm/y) by stadiometry from 0‐12 months | 4 | 728 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.02, 0.39] |
| 2 Subgroup analysis on the ICS molecules: growth velocity by stadiometry from 0‐12 months | 4 | 728 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.02, 0.39] |
| 2.1 Mometasone | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.02, 1.13] |
| 2.2 Ciclesonide | 1 | 408 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.09, 0.35] |
| 2.3 Fluticasone | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.16, 0.64] |
| 3 Growth velocity (cm/y) by stadiometry from 0‐3 months | 6 | 1114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.51, 0.27] |
| 4 Growth velocity (cm/y) by stadiometry from 0‐6 months | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐2.40, 1.75] |
| 5 Growth velocity (cm/y) by stadiometry from 3‐6 months | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐3.35, 3.10] |
| 6 Change in growth velocity (cm/y) by stadiometry from 0‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Change in height (cm) by stadiometry from 0‐3 months | 9 | 944 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.28, ‐0.02] |
| 8 Change in height (cm) by stadiometry from 0‐6 months | 3 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.27, 0.33] |
| 9 Change in height (cm) by stadiometry from 3‐6 months | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.74, 0.71] |
| 10 Change in height (cm) by stadiometry from 0‐12 months | 4 | 548 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.04, 0.54] |
| 11 Change in SD scores (height) from 0‐12 months | 3 | 328 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.03, 0.20] |
| 12 Change in weight (kg) from 0‐3 months | 5 | 449 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.13, 0.66] |
| 13 Change in weight (kg) from 0‐6 months | 2 | 346 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
| 14 Change in weight (kg) from 0‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15 Change in BMI (kg/m2) from 0‐6 months | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.22, 0.33] |
| 16 Change in BMI (kg/m2) from 0‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17 Change in skeletal maturation (years) from 0‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
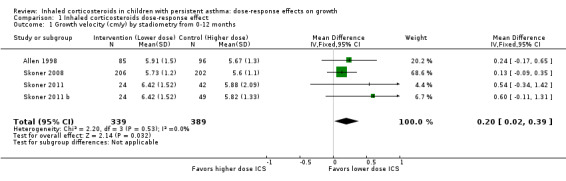
Comparison 1 Inhaled corticosteroids dose‐response effect, Outcome 1 Growth velocity (cm/y) by stadiometry from 0‐12 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 1998.
| Methods | DESIGN: prospective, randomised, double‐blind, parallel‐group trial; in 19 clinical centres | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 219 ANALYSED PARTICIPANTS: N = 219 INTERVENTION: ICS (fluticasone propionate 100 μg/d): 85 CONTROL: ICS (fluticasone propionate 200 μg/d): 96 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (fluticasone propionate 100 μg/d): 8.1 ± 0.2 (4.5‐11.9) CONTROL: ICS (fluticasone propionate 200 μg/d): 7.9 ± 0.2 (4.0‐11.6) GENDER: N (male %): INTERVENTION: ICS (fluticasone propionate 100 μg/d): 62 (73) CONTROL: ICS (fluticasone propionate 200 μg/d): 72 (75) ASTHMA SEVERITY: persistent asthma for at least 3 months ASTHMA DURATION: not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: Participants taking ICS or other antiasthma medications (e.g. β2‐agonists, theophylline, cromolyn) were allowed to continue taking these medications as needed during the run‐in period ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Diskhaler (Glaxo Wellcome, Eureaux, France) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Comparisons between treatment groups for nonparametric variables were based on the Cochran‐Mantel‐Haenszel test, controlling for investigators; comparisons for parametric variables were based on an analysis of variance F test, controlling for investigator. Traditional safety analyses were based on data from the intent‐to‐treat population, comprising all participants exposed to the study drug, whereas growth analyses were based on the same population minus participants who achieved pubescence during the study OUTCOMES: reported at 52 weeks GROWTH: Outcomes were measured at the beginning and at the end of the run‐in period; after the first, second and fourth weeks of the treatment period; and then every 4 weeks throughout the 52‐week treatment period
GROWTH MEASUREMENT TECHNIQUE: All height measurements were taken using identical wall‐mounted Harpenden stadiometers (manufactured by Holtain, Crymmych, Wales) PULMONARY FUNCTION TESTS: not reported FUNCTIONAL STATUS: not reported BIOMARKERS: not reported ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: sponsored by a grant from Glaxo Wellcome Inc. CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: “At the end of the run‐in period, eligible patients were stratified according to ICS use at study entry and randomly allocated to receive fluticasone propionate 50 μg or 100 μg, or matching placebo, twice daily via a Diskhaler” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Baker 1999.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group study; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 193 INTERVENTION: ICS (budesonide 250 μg/d): 94 CONTROL: ICS (budesonide 500 μg/d): 99 WITHDRAWALS: reported AGE: mean (months) (range): INTERVENTION: ICS (budesonide 250 μg/d): 54.6 (8‐107) CONTROL: ICS (budesonide 500 μg/d): 54.3 (7‐105) GENDER: N (male %): INTERVENTION: ICS (budesonide 250 μg/d): 59 (63) CONTROL: ICS (budesonide 500 μg/d): 62 (63) ASTHMA SEVERITY: moderate persistent asthma ASTHMA DURATION: mean disease duration months (range): INTERVENTION: ICS (budesonide 250 μg/d): 34.2 (2‐92) CONTROL: ICS (budesonide 500 μg/d): 32.4 (4‐96) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: medication or placebo given by the Pari LC‐Jet Plus nebuliser connected to a Pari Master compressor (Pari Respiratory Equipment, Inc, Richmond, VA) with use of a mouthpiece or face mask DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Done in "all patients treated" (intention‐to‐treat). Analysis of variance techniques and Fisher's exact test used OUTCOMES: GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1999) FUNDING: supported in part by Astra USA CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Baker 1999 b.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group study; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 192 INTERVENTION: ICS (budesonide 250 μg/d): 94 CONTROL: ICS (budesonide 1000 μg/d): 98 WITHDRAWALS: reported AGE: mean (months) (range): INTERVENTION: ICS (budesonide 250 μg/d): 54.6 (8‐107) CONTROL: ICS (budesonide 1000 μg/d): 53.0 (9‐107) GENDER: N (male %): INTERVENTION: ICS (budesonide 250 μg/d): 59 (63) CONTROL: ICS (budesonide 1000 μg/d): 68 (69) ASTHMA SEVERITY: moderate persistent asthma ASTHMA DURATION: mean disease duration months (range): INTERVENTION: ICS (budesonide 250 μg/d): 34.2 (2‐92) CONTROL: ICS (budesonide 1000 μg/d): 33.3 (4‐88) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: medication or placebo given by the Pari LC‐Jet Plus nebuliser connected to a Pari Master compressor (Pari Respiratory Equipment, Inc, Richmond, VA) with use of a mouthpiece or face mask DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Brand 2011.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group study; in 77 centres | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 370 ANALYSED PARTICIPANTS: N = 369 INTERVENTION: ICS (ciclesonide 40 μg/d): 248 CONTROL: ICS (ciclesonide 80 μg/d): 246 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 4.0 (2.0‐6.0) CONTROL: ICS (ciclesonide 80 μg/d):4.0 (2.0‐6.0) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 164 (66.1) CONTROL: ICS (ciclesonide 80 μg/d): 160 (65.3) ASTHMA SEVERITY: ASTHMA DURATION: median disease duration months (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 21.6 (3.8‐81.1) CONTROL: ICS (ciclesonide 80 μg/d): 22.5 (5.9‐79.8) MEAN (± SD) β2‐AGONIST USE (puffs/d): reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: ICS pretreatment n (%): INTERVENTION: ICS (ciclesonide 40 μg/d): 143 (57.7) CONTROL: ICS (ciclesonide 80 μg/d):138 (56.3) MEAN BASELINE ICS DAILY DOSE mg (SD): beclomethasone dipropionate equivalent INTERVENTION: ICS (ciclesonide 40 μg/d): 353.0 (141.6) CONTROL: ICS (ciclesonide 80 μg/d): 339.7 (143.0) ATOPY (% of participants): reported; N (%) of participants with history of allergies ASIAN: INTERVENTION: ICS (ciclesonide) at specific dose (40 μg/d): 16 (36.4) CONTROL: ICS (ciclesonide 80 μg/d): 21 (47.7) NON‐ASIAN: INTERVENTION: ICS (ciclesonide 40 μg/d): 106 (52.0) CONTROL: ICS (ciclesonide 80 μg/d): 107 (53.2) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: study medication dispensed via a hydrofluoroalkane metered‐ dose inhaler, one puff daily in the evening, administered with a spacer (AeroChamber Plus) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Efficacy analyses were planned a priori to be conducted in the intent‐to‐treat population. The Tarone trend test examined the probability of a participant's experiencing severe wheeze exacerbation before
study end in those using ciclesonide 160 mg versus placebo, and in the other ciclesonide groups versus placebo. Subsequently, the proportion of participants experiencing severe wheeze exacerbation was compared between pooled ciclesonide groups and the placebo group using Fisher’s exact test. Diary data were analysed using non‐parametric methods, and lung function and stadiometry data using analysis of co‐variance OUTCOMES: GROWTH MEASUREMENT TECHNIQUE: Participant height was measured by stadiometry at the start of the treatment period, after 12 weeks’ treatment and at study end PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2011) FUNDING: supported by Nycomed Pharmaceuticals, Konstanz, Germany CONFIRMATION OF METHODOLOGY: received Data received from study author and Takeda Global Research & Development Centre (Europe), Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: "using a computer‐generated randomisation list following age‐stratified block randomisation (2‐3 yrs and 4 ‐6 yrs)" |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation): ”Allocation of treatment was performed by an independent telephone centre, and was blinded to study investigators enrolling the patients” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | Study apparently free of other sources of bias |
Brand 2011 b.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group study; in 77 centres | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 377 ANALYSED PARTICIPANTS: N = 377 INTERVENTION: ICS (ciclesonide 40 μg/d): 248 CONTROL: ICS (ciclesonide 160 μg/d): 253 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 4.0 (2.0‐6.0) CONTROL: ICS (ciclesonide 160 μg/d): 4.0 (2.0‐6.0) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 164 (66.1) CONTROL: ICS (ciclesonide 160 μg/d): 137 (54.1) ASTHMA SEVERITY: ASTHMA DURATION: median disease duration months (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 21.6 (3.8‐81.1) CONTROL: ICS (ciclesonide 160 μg/d): 23.5 (5.9‐77.1) MEAN (± SD) β2‐AGONIST USE (puffs/d): reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: ICS PRETREATMENT n (%): INTERVENTION: ICS (ciclesonide 40 μg/d): 143 (57.7) CONTROL: ICS (ciclesonide 160 μg/d): 135 (53.4) MEAN BASELINE ICS DAILY DOSE mg (SD): beclomethasone dipropionate equivalent INTERVENTION: ICS (ciclesonide 40 μg/d): 353.0 (141.6) CONTROL: ICS (ciclesonide 160 μg/d): 335.8 (142.2) ATOPY (% of participants): reported; N (%) of participants with history of allergies ASIAN: INTERVENTION: ICS (ciclesonide 40 μg/d): 16 (36.4) CONTROL: ICS (ciclesonide 160 μg/d): 21 (46.7) NON‐ASIAN INTERVENTION: ICS (ciclesonide 40 μg/d): 106 (52.0) CONTROL: ICS (ciclesonide 160 μg/d): 122 (58.7) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: study medication dispensed via a hydrofluoroalkane metered‐ dose inhaler, one puff daily in the evening, administered with a spacer (AeroChamber Plus) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Chen 2001.
| Methods | Randomised, single‐blind, placebo‐controlled, parallel‐group study; 1 centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 20 INTERVENTION: ICS: (beclomethasone dipropionate 200 μg/d): 10 CONTROL: ICS (beclomethasone dipropionate 400 μg/d): 10 WITHDRAWALS: no withdrawals AGE: mean (years) (range): INTERVENTION: ICS (beclomethasone dipropionate 200 μg/d): average 7 years CONTROL: ICS (beclomethasone dipropionate 400 μg/d): average 9 years GENDER: N (male %): not reported ASTHMA SEVERITY: mild asthma ASTHMA DURATION: not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: not reported (in translation of the study) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: not reported (in translation of the study) OUTCOMES GROWTH MEASUREMENT TECHNIQUE: not reported (in translation of the study) PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: not reported WITHDRAWALS: no withdrawals |
|
| Notes | PUBLICATION: full paper (2001) FUNDING: not reported CONFIRMATION OF METHODOLOGY: not received Study author could not be contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random number table: “The patients were allocated by random number table and stratified by moderate and severe grades” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding or incomplete blinding; single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding or incomplete blinding; single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for missing outcome data unlikely to be related to true outcomes |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Doniec 2004.
| Methods | DESIGN: randomised, parallel‐group clinical study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 22 INTERVENTION: ICS (budesonide 200 μg/d): 9 CONTROL: ICS (budesonide 800 μg/d): 11 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (budesonide 200 μg/d): 11.8 ± 2.0 CONTROL: ICS (budesonide 800 μg/d): 13.2 ± 2.3 GENDER: N (male %): INTERVENTION: ICS (budesonide 200 μg/d): 6 (66.6) CONTROL: ICS (budesonide 800 μg/d): 6 (54.5) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: median (months) (range): not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: none (steroid naive) ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA: not reported |
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler (Pulmicort Turbuhaler) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Student's t test OUTCOMES GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS: at start of study and at 12 weeks
FUNCTIONAL STATUS: not reported BIOMARKERS: at start of study and at 12 weeks
ADVERSE EVENTS: not reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2004) FUNDING: not reported CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Gelfand 2006.
| Methods | DESIGN: randomised, double‐blind, multi‐centre, placebo‐controlled, parallel‐group clinical study. This comprises 2 identical trials | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 511 INTERVENTION: ICS (ciclesonide 40 μg/d): 252 CONTROL: ICS (ciclesonide 80 μg/d): 259 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 8.14 ± 0.14 (4‐11) CONTROL: ICS (ciclesonide 80 μg/d): 8.20 ± 0.13 (4‐11) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 160 (63.5) CONTROL: ICS (ciclesonide 80 μg/d): 169 (65.3) ASTHMA SEVERITY: persistent asthma with all severity ASTHMA DURATION: mean (months) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 4.32 ± 0.18 (0.26‐11.26) CONTROL: ICS (ciclesonide 80 μg/d): 4.35 ± 0.17 (0.25‐11.10) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (ciclesonide 40 μg/d): 1.60 CONTROL: ICS (ciclesonide 80 μg/d): 1.64 DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: HFA‐metered dose inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: intention‐to‐treat analysis. All participants receiving 1 or more doses of study medication with 1 or more post‐baseline measurements of FEV1 and height were included in the analysis. Missing values for withdrawals were handled by the last value extended principle OUTCOMES: reported at 12 weeks. Outcomes were measured every 1, 2, 4, 8 and 12 weeks GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2006) FUNDING: funded by Aventis Pharmaceuticals CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified: ”We report the results of a prespecified integrated analysis of the efficacy and safety data from 2 identical, double‐blinded, randomised, placebo‐controlled studies of ciclesonide (at doses of 40, 80, and 160 μg) administered once daily to children with persistent asthma” |
| Other bias | Low risk | Study apparently free of other sources of bias |
Gelfand 2006 b.
| Methods | DESIGN: randomised, double‐blind, multi‐centre, placebo‐controlled, parallel‐group clinical study. This comprises 2 identical trials | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 505 INTERVENTION: ICS (ciclesonide 40 μg/d): 252 CONTROL: ICS (ciclesonide 160 μg/d): 253 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 8.14 ± 0.14 (4‐11) CONTROL: ICS (ciclesonide 160 μg/d): 8.33 ± 0.12 (4‐11) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 160 (63.5) CONTROL: ICS (ciclesonide 160 μg/d): 154 (60.9) ASTHMA SEVERITY: persistent asthma with all severity ASTHMA DURATION: mean (months) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 4.32 ± 0.18 (0.26‐11.26) CONTROL: ICS (ciclesonide 160 μg/d): 4.38 ± 0.17 (0.53‐12.06) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (ciclesonide 40 μg/d): 1.60 CONTROL: ICS (ciclesonide 160 μg/d): 1.72 DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: HFA‐metered‐dose inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Giorgi 1998.
| Methods | DESIGN: randomised, open‐label, multi‐centre, parallel‐group clinical study | |
| Participants | CHILDREN WITH MILD ASTHMA RANDOMLY ASSIGNED: N = 29 INTERVENTION: ICS (flunisolide 600 μg/d): 15 CONTROL: ICS (flunisolide 1200 μg/d): 14 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (flunisolide 600 μg/d) 8.6 (6‐11) CONTROL: ICS (flunisolide 1200 μg/d) 8.5 (7‐10) GENDER: N (male %): INTERVENTION: ICS (flunisolide 600 μg/d) 11 (73%) CONTROL: ICS (flunisolide 1200 μg/d) 9 (64%) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: mean (months) (range): INTERVENTION: ICS (flunisolide 600 μg/d) 4.8 (3‐7) CONTROL: ICS (flunisolide 1200 μg/d) 4.9 (3‐7) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo ATOPY N (% of participants): reported INTERVENTION: ICS (flunisolide 600 μg/d) 9 (60%) CONTROL: ICS (flunisolide 1200 μg/d) 10 (71%) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: jet nebulisers (Soffio Nuovo, Markos, Monza, Italy) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: no intention‐to‐treat analysis OUTCOMES: reported at 12 weeks. Outcomes were measured at 2, 3 and 4 months GROWTH MEARSUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS: not measured FUNCTIONAL STATUS: not measured BIOMARKERS
ADVERSE EVENTS: not reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: funded by Valeas Pharmaceuticals CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This trial was randomised but the technique of randomisation was not described |
| Allocation concealment (selection bias) | High risk | No allocation concealment used in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No measures reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals per group not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | This was an open‐label study and the primary outcome was not specified clearly |
Jonasson 1998.
| Methods | DESIGN: a randomised, double‐blind, placebo‐controlled trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 83 INTERVENTION: ICS (budesonide 100 μg/d o.d.): 41 CONTROL: ICS (budesonide 200 μg/d o.d.): 42 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 10.0 CONTROL: ICS (budesonide 200 μg/d o.d.): 9.8 GENDER: N (male %): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 23 (54.7) CONTROL: ICS (budesonide 200 μg/d o.d.): 31(75.6) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: none within 2 months ATOPY: N (% of participants): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 25 (59.5) CONTROL: ICS (budesonide 200 μg/d o.d.): 31 (75.6) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Turbuhaler inhalers DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: intention to‐treat; analysis of variance (ANOVA). Missing values were handled by applying the last value extended principle. For diary variables, this was accomplished by extending the period means OUTCOMES GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS: not done ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: not provided CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation: "patients were randomised into four parallel groups in balanced blocks" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Jonasson 2000.
| Methods | DESIGN: double‐blind, placebo‐controlled, single‐centre extension trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 60 INTERVENTION: ICS (budesonide 100 μg/d o.d.): 28 CONTROL: ICS (budesonide 200 μg/d o.d.): 32 WITHDRAWALS: reported AGE: mean (years) (range): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 9.5 CONTROL: ICS (budesonide 200 μg/d o.d.): 10.0 GENDER: male N (%): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 23 (82.1) CONTROL: ICS (budesonide 200 μg/d o.d.): 17 (53.1) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: All participants in the present study were already randomly assigned to 4 parallel groups in balanced blocks 3 months before inclusion in the present study (see study above, Jonasson 1998) ATOPY: N (% of participants): INTERVENTION: ICS (budesonide 100 μg/d o.d.): 20 (71.4) CONTROL: ICS (budesonide 200 μg/d o.d.): 21(65.6) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Turbuhaler inhalers DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: |
|
| Outcomes | ANALYSIS: Statistical analysis was carried out on the intention‐to‐treat principle. Missing values for withdrawals were handled by the last value extended principle. Analysis was done by analysis of co‐variance (ANCOVA) and ANOVA models. An additive model was used when diary variables, lung‐function variables and the maximum fall in FEV1 after the exercise test were analysed; a multiplicative model was used when plethysmography variables and PD20 were analysed OUTCOMES GROWTH MEASUREMENT TECHNIQUE: Growth velocity was determined from measurements of participant height at every visit throughout the study period by a wall‐fixed stadiometer (Seca, Hamburg, Germany). Three trained persons carried out all height measurements during the study. The child was measured standing upright without shoes with the heels touching the wall to which the stadiometer was fixed. The movable part of the measuring device was placed lightly on the child's head before the child's height was read from a centimetre scale. At baseline, the participant's height was measured by 2 persons, and the mean value was registered PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2000) FUNDING: not provided CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation: “All patients in the present study were already randomised into four parallel groups in balanced blocks 3 months before inclusion in the present study” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Kemp 1999.
| Methods | DESIGN: multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 174 INTERVENTION: ICS (budesonide 250 μg/d): 91 CONTROL: ICS (budesonide 500 μg/d): 83 WITHDRAWALS: reported AGE: mean (range) (months) INTERVENTION: ICS (budesonide 250 μg/d): 55.2 ± 25.5 (7‐107) CONTROL: ICS (budesonide 500 μg/d): 52.4 ± 27.9 (10‐107) GENDER: male N (%) INTERVENTION: ICS (budesonide 250 μg/d): 63 (69.2) CONTROL: ICS (budesonide 500 μg/d): 58 (69.9) ASTHMA SEVERITY: mild persistent asthma ASTHMA DURATION: mean (range) in months INTERVENTION: ICS (budesonide 250 μg/d): 35.4 ± 22.4 (5‐97) CONTROL: ICS (budesonide 500 μg/d): 36.7 ± 25.1 (5‐107) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported. Participants discontinued their chronic asthma medication at the end of the study ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Pari LC‐Jet Plus nebuliser (with mouthpiece or face mask) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: not reported |
|
| Outcomes | ANALYSIS: intention‐to‐treat analysis; ANOVA; Fisher's exact test OUTCOMES: at enrolment, at randomisation, after 2, 4, 8 and 12 weeks of treatment GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS: baseline and at end of study (12 weeks)
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1996) FUNDING: funded by AstraZeneca CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Kemp 1999 b.
| Methods | DESIGN: multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 174 INTERVENTION: ICS (budesonide 250 μg/d): 91 CONTROL: ICS (budesonide 1000 μg/d): 93 WITHDRAWALS: reported AGE: mean (range) (months) INTERVENTION: ICS (budesonide 250 μg/d): 55.2 ± 25.5 (7‐107) CONTROL: ICS (budesonide 1000 μg/d): 56.0 ± 27.2 (6‐107) GENDER: male N (%) INTERVENTION: ICS (budesonide 250 μg/d): 63 (69.2) CONTROL: ICS (budesonide 1000 μg/d): 56 (60.2) ASTHMA SEVERITY: mild persistent asthma ASTHMA DURATION: mean (range) in months INTERVENTION: ICS (budesonide 250 μg/d): 35.4 ± 22.4 (5‐97) CONTROL: ICS (budesonide 1000 μg/d): 36.1 ± 24.4 (5‐107) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported. Participants discontinued their chronic asthma medication at the end of the study ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Pari LC‐Jet Plus nebuliser (with mouthpiece or face mask) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: not reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Kerwin 2008.
| Methods | DESIGN: randomised, parallel‐group, double‐blind, placebo‐controlled trial; in multiple centres | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 206 INTERVENTION: ICS (budesonide 200 μg/d): 104 CONTROL: ICS (budesonide 800 μg/d): 102 WITHDRAWALS: reported AGE: mean (SD) years: INTERVENTION: ICS (budesonide 200 μg/d): 11.7 (2.8) CONTROL: ICS (budesonide 800 μg/d): 11.5 (2.9) GENDER: male N (%) INTERVENTION: ICS (budesonide 200 μg/d): 59 (56.7) CONTROL: ICS (budesonide 800 μg/d): 64 (62.7) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: mean (SD) years INTERVENTION: ICS (budesonide 200 μg/d): 6.7 (3.7) CONTROL: ICS (budesonide 800 μg/d): 6.8 (3.9) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (budesonide 200 μg/d): 0.5 (0.8) CONTROL: ICS (budesonide 800 μg/d): 0.3 (0.7) DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: Participants continued their usual ICS therapies (if any) and added a once‐daily placebo treatment ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Efficacy was assessed on an intent to‐treat (ITT) basis; between‐group differences in changes from baseline in the primary variable were also evaluated in the per‐protocol population. Primary and secondary spirometry data and diary data were fit with an analysis of co‐variance (ANCOVA) model; results of urine cortisol analysis were summarised with descriptive statistics OUTCOMES GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS: measured at randomisation; week 2, 4, 8 and 12
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2008) FUNDING: funded by AstraZeneca LP CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: "Using a computer‐generated allocation schedule stratified by pharmacokinetic participation, patients were randomised in balanced blocks to receive 12 weeks of one of the following five treatments" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Kerwin 2008 b.
| Methods | DESIGN: randomised, parallel‐group, double‐blind, placebo‐controlled trial; in multiple centres | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 204 INTERVENTION: ICS (budesonide 180 μg/d): 108 CONTROL: ICS (budesonide 360 μg/d): 96 WITHDRAWALS: reported AGE: mean (SD) years: INTERVENTION: ICS (budesonide 180 μg/d): 11.7 (2.9) CONTROL: ICS (budesonide 360 μg/d): 11.5 (2.9) GENDER: male N (%) INTERVENTION: CS (budesonide 180 μg/d): 76 (70.4) CONTROL: ICS (budesonide 360 μg/d): 67 (69.8) ASTHMA SEVERITY: mild asthma ASTHMA DURATION: mean (SD) years INTERVENTION: ICS (budesonide 180 μg/d): 7.1 (4.2) CONTROL: ICS (budesonide 360 μg/d): 7.2 (4.1) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (budesonide 180 μg/d): 0.4 (0.9) CONTROL: ICS (budesonide 360 μg/d): 0.5 (1.0) DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: Participants continued their usual ICS therapies (if any) and added a once‐daily placebo treatment ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Lemanske 2004.
| Methods | DESIGN: randomised, double‐blind clinical trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: 205 WITHDRAWALS: not reported AGE: median (years) (range): 4 to 9 years GENDER: N (male %): not reported ASTHMA SEVERITY ASTHMA DURATION: median (months) (range): not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): median (months) (range): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA: not reported EXCLUSION CRITERIA: not reported |
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: |
|
| Outcomes | ANALYSIS: Efficacy was assessed on an intent to‐treat (ITT) basis; between‐group differences in changes from baseline in the primary variable were also evaluated in the per‐protocol population. Primary and secondary spirometry data and diary data were fit with an analysis of co‐variance (ANCOVA) model; results of urine cortisol analysis were summarised with descriptive statistics OUTCOMES GROWTH MEASUREMENT TECHNIQUE: stadiometric height measured and growth velocities calculated PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: not reported |
|
| Notes | PUBLICATION: abstract; full paper not found FUNDING: not reported CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised but the randomisation technique was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete reporting of details for judgement |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes described |
| Other bias | Low risk | No apparent risk of bias noted |
Peden 1998.
| Methods | DESIGN: randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 177 INTERVENTION: ICS (fluticasone 100 μg/d): 90 CONTROL: ICS (fluticasone 200 μg/d): 87 WITHDRAWALS: reported AGE: median (years) (range): 4 to 11 years INTERVENTION: ICS (fluticasone 100 μg/d): not reported CONTROL: ICS (fluticasone 200 μg/d): not reported GENDER: N (male %): INTERVENTION: ICS (fluticasone 100 μg/d): 53 (59) CONTROL: ICS (fluticasone 200 μg/d): 60 (68) ASTHMA SEVERITY: mild to moderate persistent asthma ASTHMA DURATION: not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Diskus or Diskhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: done by intention‐to treat analysis. Investigators used analysis of variance F test; nonparametric van Elteren test; and Kaplan‐Meier estimates of survival OUTCOMES: weekly for first 4 weeks and every other week for remaining 8 weeks GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS: at screening and at 12 weeks
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: funded by Glaxo Wellcome CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation Randomly assigned by strata (baseline therapy of ICS or cromolyn or β2‐agonist) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Peden 1998 b.
| Methods | DESIGN: randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 174 INTERVENTION: ICS (fluticasone 100 μg/d): 91 CONTROL: ICS (fluticasone 200 μg/d): 83 WITHDRAWALS: reported AGE: median (years) (range): 4 to 11 years INTERVENTION: ICS (fluticasone 100 μg/d): not reported CONTROL: ICS (fluticasone 200 μg/d): not reported GENDER: N (male %): INTERVENTION: ICS (fluticasone 100 μg/d): 50 (55) CONTROL: ICS (fluticasone 200 μg/d): 50 (60) ASTHMA SEVERITY: mild to moderate persistent asthma ASTHMA DURATION: median (months) (range): not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Diskus or Diskhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Pedersen 2010.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group clinical study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 465 ANALYSED PARTICIPANTS: N = 465 INTERVENTION: ICS (ciclesonide 40 μg/d): 305 CONTROL: ICS (ciclesonide 80 μg/d): 312 WITHDRAWALS: reported AGE: median (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 8.0 (6‐11) CONTROL: ICS (ciclesonide 80 μg/d): 8.0 (6‐11) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 210 (68.9%) CONTROL: ICS (ciclesonide 80 μg/d): 191 (61.2%) ASTHMA SEVERITY: persistent asthma but severity not reported ASTHMA DURATION: median (months) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 41.4 (6‐127) CONTROL: ICS (ciclesonide 80 μg/d): 41.9 (5‐128) MEAN (± SD) β2‐AGONIST USE (puffs/d): median (months) (range) INTERVENTION: ICS (ciclesonide 40 μg/d): 1.43 (0.00‐7.86) CONTROL: ICS (ciclesonide 80 μg/d): 1.43 (0.00‐7.14) DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler with or without spacer DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: intent‐to‐treat analysis OUTCOMES: reported at 12 weeks; change in height reported as least squares mean growth rate GROWTH MEASUREMENT TECHNIQUE: At investigational sites where a stadiometer was available, height was also measured at the start and the end of the treatment period, as stadiometry is widely acknowledged as the most reliable means of measuring height and is recommended by the Food and Drug Administration (FDA) for studies assessing growth PULMONARY FUNCTION TESTS: mean change in FEV1 and PEFR reported FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2010) FUNDING: funded by Nycomed CONFIRMATION OF METHODOLOGY: not received Data received from Takeda Global Research & Development Centre (Europe) Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: ”Patients were then randomised into one of four treatment groups in a 2:2:2:1 ratio (ciclesonide 40 mg: ciclesonide 80 mg: ciclesonide 160 mg: placebo) by means of a computer generated randomisation scheme” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Pedersen 2010 b.
| Methods | Same as above | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = ANALYSED PARTICIPANTS: N = 462 INTERVENTION: ICS (ciclesonide 40 μg/d): 305 CONTROL: ICS (ciclesonide 160 μg/d): 310 WITHDRAWALS: reported AGE: median (years) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 8.0 (6‐11) CONTROL: ICS (ciclesonide 160 μg/d): 9.0 (6‐11) GENDER: N (male %): INTERVENTION: ICS (ciclesonide 40 μg/d): 210 (68.9%) CONTROL: ICS (ciclesonide 160 μg/d): 218 (70.3%) ASTHMA SEVERITY: persistent asthma but severity not reported ASTHMA DURATION: median (months) (range): INTERVENTION: ICS (ciclesonide 40 μg/d): 41.4 (6‐127) CONTROL: ICS (ciclesonide 160 μg/d): 41.7 (6‐129) MEAN (± SD) β2‐AGONIST USE (puffs/d): median (months) (range) INTERVENTION: ICS (ciclesonide 40 μg/d): 1.43 (0.00‐7.86) CONTROL: ICS (ciclesonide 160 μg/d): 1.57 (0.00‐7.71) DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler with or without spacer DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | Same as above | |
| Notes | Same as above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Shapiro 1998.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐centre study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 202 ANALYSED: N = 74 INTERVENTION: ICS (budesonide 100 μg/d): 102 CONTROL: ICS (budesonide 200 μg/d): 100 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (budesonide 100 μg/d): 11.8 (6‐18) CONTROL: ICS (budesonide 200 μg/d): 12.1 (6‐18) GENDER: male N (%) INTERVENTION: ICS (budesonide 100 μg/d): 76 (74.5) CONTROL: ICS (budesonide 200 μg/d): 76 (76) ASTHMA SEVERITY: moderate to severe persistent asthma ASTHMA DURATION: duration of ICS‐dependent asthma: mean (range) years INTERVENTION: ICS (budesonide 100 μg/d): 2.8 (0.5‐11) CONTROL: ICS (budesonide 200 μg/d): 2.5 (0.5‐13) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (budesonide 100 μg/d): 2.8 CONTROL: ICS (budesonide 200 μg/day): 3.1 DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: Participants discontinued their previous ICS at randomisation ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: done by analysis of variance. Poportion of patients who discontinued enrolment in the study was compared between treatment groups by using the Cochran‐Mantel‐Haenszel statistic OUTCOMES GROWTH MEASUREMENT TECHNIQUE: not reported PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS: before randomisation and after 12 weeks of treatment
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: supported by a grant from Astra, USA CONFIRMATION OF METHODOLOGY: data received from Symbicort and Established Respiratory Brands, AstraZeneca R&D, Mölndal, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Shapiro 1998 b.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐centre study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 201 ANALYSED: N = 75 INTERVENTION: ICS (budesonide 100 μg/d): 102 CONTROL: ICS (budesonide 400 μg/d): 99 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (budesonide 100 μg/d): 11.8 (6‐18) CONTROL: ICS (budesonide 400 μg/d): 11.8 (6‐18) GENDER: male N (%) INTERVENTION: ICS (budesonide 100 μg/d): 76 (74.5) CONTROL: ICS (budesonide 400 μg/d): 85 (85.8) ASTHMA SEVERITY: moderate to severe persistent asthma ASTHMA DURATION: duration of ICS‐dependent asthma: mean (range) years INTERVENTION: ICS (budesonide 100 μg/d): 2.8 (0.5‐11) CONTROL: ICS (budesonide 400 μg/d): 2.4 (0.5‐13) MEAN (± SD) β2‐AGONIST USE (puffs/d): INTERVENTION: ICS (budesonide 100 μg/d): 2.8 CONTROL: ICS (budesonide 400 μg/d): 3.2 DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: Participants discontinued their previous ICS at randomisation ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Shapiro 1998 c.
| Methods | Same as Shapiro 1998 | |
| Participants | Same as Shapiro 1998 ANALYSED: N = 55 |
|
| Interventions | Same as Shapiro 1998 | |
| Outcomes | Same as Shapiro 1998 | |
| Notes | Same as Shapiro 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Shapiro 1998 d.
| Methods | Same as Shapiro 1998b | |
| Participants | Same as Shapiro 1998b ANALYSED: N = 52 |
|
| Interventions | Same as Shapiro 1998b | |
| Outcomes | Same as Shapiro 1998b | |
| Notes | Same as Shapiro 1998b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Skoner 2008.
| Methods | DESIGN: randomised, double‐blind, multi‐centre, placebo‐controlled, parallel‐group study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 440 ANALYSED: N = 408 INTERVENTION: ICS (ciclesonide 40 μg/d): 221 CONTROL: ICS (ciclesonide 160 μg/d): 219 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (ciclesonide 40 μg/d): 7.1 (5.5‐9.1) CONTROL: ICS (ciclesonide 160 μg/d): 7.2 (5.5‐9.0) GENDER: male N (%) INTERVENTION: ICS (ciclesonide 40 μg/d): 150 (67.9) CONTROL: ICS (ciclesonide 160 μg/d): 147 (67.1) ASTHMA SEVERITY: mild persistent asthma ASTHMA DURATION: at screening (6 months before randomisation) mean (SD) years INTERVENTION: ICS (ciclesonide 40 μg/d): 3.79 (1.95) CONTROL: ICS (ciclesonide 160 μg/d): 3.96 (1.98) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: placebo. ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler without a spacer DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Using an analysis of co‐variance (ANCOVA) model, all growth analyses were conducted by using the modified intention‐to‐treat (mITT) population, which included all randomly assigned participants who completed 4 months of study treatment and who had stadiometry measurements at baseline and >= 4 months OUTCOMES GROWTH MEASUREMENT TECHNIQUE: All investigators were provided with detailed written and visual instructions, took part in onsite training and attended workshops before study initiation to standardise stadiometer measurements. In addition, most investigators had previous experience with Harpenden stadiometers. Study centres were supplied with identical Harpenden stadiometers, which were calibrated within 4 hours of each measurement, and height was measured at all visits using standard techniques. Measurements were taken by a trained technician, and an effort was made to use the same technician at each visit. A median of 4 acceptable serial measurements was used in the analysis PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2008) FUNDING: Financial support for this study was provided by Sanofi‐aventis US and Altana Pharma US, Inc, a Nycomed company CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: ”The randomisation schedule was generated by the Biostatistics Department of Quintiles, Inc (Kansas City, MO) and was stratified according to age‐gender classification” |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation): “Randomization was conducted at a central location (Q‐Tone, Durham, NC) and was determined by an interactive voice response system, based on information entered by personnel at each investigative center” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Skoner 2011.
| Methods | DESIGN: a phase III, multi‐centre, randomised, placebo‐controlled, parallel‐group, double‐blind, long‐term safety study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 92 ANALYSED: N = 66 INTERVENTION: ICS (mometasone furoate 100 μg/d): 48 CONTROL: ICS (mometasone furoate 100 μg twice daily): 44 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (mometasone furoate 100 μg/d): 6.4 (4‐9) CONTROL: ICS (mometasone furoate 100 μg twice daily): 6.3 (4‐9) GENDER: male N (%) INTERVENTION: ICS (mometasone furoate 100 μg/d): 34 (70.8) CONTROL: ICS (mometasone furoate 100 μg twice daily): 28 (63.6) ASTHMA SEVERITY: persistent asthma; severity not reported ASTHMA DURATION: mean (range) years INTERVENTION: ICS (mometasone furoate 100 μg/d): 3.8 (0.67‐8.0) CONTROL: ICS (mometasone furoate 100 μg twice daily): 4.0 (0.83‐9.0) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: washout period of 3 months ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Analyses were done using a longitudinal random slope (LRS) model, an individual regression (IR) model and an analysis of variance (ANOVA) by extracting sources of variation due to treatment, age and gender OUTCOMES GROWTH MEASUREMENT TECHNIQUE: Growth velocity was determined from heights measured by a Harpenden stadiometer during the 52‐week treatment period PULMONARY FUNCTION TESTS: This study was not designed to evaluate efficacy measures PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2011) FUNDING: supported by Merck & Co, Inc CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation; randomly assigned in a 1:1:1 ratio to different comparison groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Skoner 2011 b.
| Methods | DESIGN: a phase III, multi‐centre, randomised, placebo‐controlled, parallel‐group, double‐blind, long‐term safety study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 98 ANALYSED: N = 73 INTERVENTION: ICS (mometasone furoate 100 μg/d): 48 CONTROL: ICS (mometasone furoate 200 μg/d qd): 50 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (mometasone furoate 100 μg/d): 6.4 (4‐9) CONTROL: ICS (mometasone furoate 200 μg/d qd): 6.6 (4‐9) GENDER: male N (%) INTERVENTION: ICS (mometasone furoate 100 μg/d): 34 (70.8) CONTROL: ICS (mometasone furoate 200 μg/d qd): 33 (66) ASTHMA SEVERITY: persistent asthma; severity not reported ASTHMA DURATION: mean (range) years INTERVENTION: ICS (mometasone furoate 100 μg/d): 3.8 (0.67‐8.0) CONTROL: ICS (mometasone furoate 200 μg/d qd): 3.6 (0.42‐8.0) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: washout period of 3 months ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: dry powder inhaler DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation; randomly assigned in a 1:1:1 ratio to different comparison groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Sorkness 2007.
| Methods | DESIGN: randomised, double‐blind, multi‐centre, parallel‐group study | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 190 ANALYSED: N = 190 INTERVENTION: ICS (fluticasone/salmeterol 100/50 μg/d): 96 CONTROL: ICS (fluticasone 200 μg/d): 94 WITHDRAWALS: reported AGE: mean (SD) years INTERVENTION: ICS (fluticasone/salmeterol 100/50 μg/d): 9.8(2.2) CONTROL: ICS (fluticasone 200 μg/d): 10.3 (2.1) GENDER: male N (%) INTERVENTION: ICS (fluticasone/salmeterol(100/50 μg/d): 96 CONTROL: ICS (fluticasone 200 μg/d): 94 ASTHMA SEVERITY: mild to moderate persistent asthma ASTHMA DURATION: mean (range) years INTERVENTION: ICS (fluticasone/salmeterol 100/50 μg/d): 96 CONTROL: ICS (fluticasone 200 μg/d): 94 MEAN (± SD) β2‐AGONIST USE (puffs/d): DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: ATOPY (% of participants): 78% ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Diskus (GlaxoSmithKline, Research Triangle Park, NC) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: |
|
| Outcomes | ANALYSIS: Primary analysis of asthma control days consisted of the 3 pair‐wise comparisons by ANOVA with post hoc pair‐wise comparisons of group means OUTCOMES GROWTH MEASUREMENT TECHNIQUE: Height was measured using the calibrated stadiometer PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2007) FUNDING: grants from National Heart, Lung and Blood Institute, USA CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A stratified randomisation scheme was applied on the basis of bronchodilator response (< 12% or 12% change in FEV1), race (white or non‐white) and methacholine FEV1 PC20 (< 2 or 2 mg/mL) |
| Allocation concealment (selection bias) | Low risk | Matching placebo was provided by sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well‐balanced withdrawal in comparison groups. No missing outcome data. Primary and secondary outcomes specified |
| Selective reporting (reporting bias) | Low risk | Protocol available. All analyses performed under the intent‐to‐treat paradigm |
| Other bias | Low risk | Study apparently free of other sources of bias |
Teper 2004.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled clinical study. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 20 INTERVENTION: ICS (fluticasone 100 μg/d): 10 CONTROL: ICS (fluticasone 250 μg/d): 10 WITHDRAWALS: reported AGE: months ± SD: INTERVENTION: ICS at specific dose: 13.1 ± 5.2 CONTROL: ICS (fluticasone 250 μg/d): 14.2 ± 5.7 GENDER: N (male %): INTERVENTION: ICS (fluticasone 100 μg/d): 6 (60%) CONTROL: ICS (fluticasone 250 μg/d): 7 (70%) ASTHMA SEVERITY: recurrent wheezing ASTHMA DURATION (mean years ± SD): not reported MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler with aerochamber DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: not reported OUTCOMES: reported at 24 weeks; change in height reported as standard deviation score GROWTH MEASUREMENT TECHNIQUE: Participant's recumbent length was determined by means of a calibrated stadiometer. Three consecutive measurements were taken to obtain the mean value. Height was expressed as standard deviation score (SDS) for chronological age, according to Tanner and Whitehouse PULMONARY FUNCTION TESTS: not reported FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: not reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2005) FUNDING: not reported CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: ”Each child was then provided with a numbered,blinded metered‐dose aerosol inhaler containing FP (50 or 125 μg per actuation) or placebo, depending on their study group” |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Vaessen‐Verberne 2010.
| Methods | DESIGN: randomised, multi‐centre, parallel‐group, double‐blind study | |
| Participants | SYMPTOMATIC ON CONVENTIONAL DOSES OF INHALED CORTICOSTEROIDS RANDOMLY ASSIGNED: N = 158 ANALYSED: N = 151 INTERVENTION: ICS (fluticasone 200 μg/d): 78 CONTROL: ICS (fluticasone 400 μg/d): 80 WITHDRAWALS: reported AGE: years ± SD: INTERVENTION: ICS (fluticasone 200 μg/d): 9.4 ± 1.8 CONTROL: ICS (fluticasone 400 μg/d): 9.3 ± 1.9 GENDER: N (male %): INTERVENTION: ICS (fluticasone 200 μg/d): 42 (54%) CONTROL: ICS (fluticasone 400 μg/d): 49 (61%) ASTHMA SEVERITY: not reported ASTHMA DURATION (mean years ± SD): reported INTERVENTION: ICS (fluticasone 200 μg/d): 5.7 ± 3.1 CONTROL: ICS (fluticasone 400 μg/d): 5.5 ± 3.0 MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY: N (% of participants): reported INTERVENTION: ICS (fluticasone 200 μg/d): 60 (77%) CONTROL: ICS (fluticasone 400 μg/d): 58 (73%) ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: Diskus DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: intention‐to‐treat analysis OUTCOMES: Many outcomes were reported at 26 weeks; participants were evaluated at 1, 6, 16 and 26 weeks GROWTH MEASUREMENT TECHNIQUE: Height was recorded using a stadiometer at the start of the run‐in period, and at the start and at the end of the treatment period PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS
WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2010) FUNDING: funded by GlaxoSmithKline CONFIRMATION OF METHODOLOGY: received Data received from the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | Study apparently free of other sources of bias |
Verberne 1998.
| Methods | Double‐blind, randomised, parallel‐group trial; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 87 ANALYSED: N = 87 INTERVENTION: ICS (beclomethasone 400 μg/d): 57 CONTROL: ICS (beclomethasone 800 μg/d): 30 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (beclomethasone 400 μg/d): 11.1 (6‐16) CONTROL: ICS (beclomethasone 800 μg/d): 11.4 (6‐16) GENDER: male N (%) INTERVENTION: ICS (beclomethasone 400 μg/d): 36 (63) CONTROL: ICS (beclomethasone 800 μg/d): 36 (60) ASTHMA SEVERITY: mild to moderate asthma ASTHMA DURATION: mean (range) years INTERVENTION: ICS (beclomethasone 400 μg/d): 8.5 years CONTROL: ICS (beclomethasone 800 μg/d): 9.0 years MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: 200‐800 μg/d at least 3 months before the start of the study ATOPY (% of participants): 89% ELIGIBILITY CRITERIA
EXCLUSION CRITERIA: not reported WITHDRAWAL CRITERIA:
|
|
| Interventions | PROTOCOL DURATION
DEVICE: All drugs were administered as Rotadisks in combination with a Diskhaler (Glaxo Wellcome, Greenford, UK) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: analyses of co‐variance OUTCOMES GROWTH MEASUREMENT TECHNIQUE: Height was measured using a stadiometer in centimetres, corrected to 1 decimal place PULMONARY FUNCTION TESTS
FUNCTIONAL STATUS
BIOMARKERS: not done ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (1998) FUNDING: Glaxo Wellcome BV CONFIRMATION OF METHODOLOGY: not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: "Randomization was stratified by sex, age, center, baseline FEV1 and prior dose of ICS, using a computerized minimization method" |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation): "independent randomisation center" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Verberne 1998 b.
| Methods | Double‐blind, randomised, parallel‐group trial; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 90 ANALYSED: N = 90 INTERVENTION: ICS (beclomethasone + salmeterol 400 μg/d): 60 CONTROL: ICS (beclomethasone 800 μg/d): 30 WITHDRAWALS: reported AGE: mean (range) years INTERVENTION: ICS (beclomethasone 400 μg/d): 10.8 (6‐16) CONTROL: ICS (beclomethasone 800 μg/d): 11.4 (6‐16) GENDER: male N (%) INTERVENTION: ICS (beclomethasone 400 μg/d): 40 (60) CONTROL: ICS (beclomethasone 800 μg/d): 36 (60) ASTHMA SEVERITY: mild to moderate asthma ASTHMA DURATION: mean (range) years INTERVENTION: ICS (beclomethasone 400 μg/d): 7.8 years CONTROL: ICS (beclomethasone 800 μg/d): 9.0 years MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: 200‐800 μg/d at least 3 months before the start of the study ATOPY (% of participants): 89% ELIGIBILITY CRITERIA
EXCLUSION CRITERIA: not reported WITHDRAWAL CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: All drugs were administered as Rotadisks in combination with a Diskhaler (Glaxo Wellcome, Greenford, UK) DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator: "Randomization was stratified by sex, age, center, baseline FEV1 and prior dose of ICS, using a computerized minimization method" |
| Allocation concealment (selection bias) | Low risk | Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation): "independent randomisation center" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
Wasserman 2006.
| Methods | DESIGN: randomised, double‐blind, placebo‐controlled, parallel‐group study; multi‐centre | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMLY ASSIGNED: N = 219 ANALYSED: N = 218 INTERVENTION: ICS (fluticasone 88 μg/d): 111 CONTROL: ICS (fluticasone 176 μg/d): 108 WITHDRAWALS: reported AGE: mean (months) (range): INTERVENTION: ICS (fluticasone 88 μg/d): 35.6 (24‐47) CONTROL: ICS (fluticasone 176 μg/d): 35.5 (24‐47) GENDER: N male (%): INTERVENTION: ICS (fluticasone 88 μg/d): 70 (63) CONTROL: ICS (fluticasone 176 μg/d): 63 (58.3) ASTHMA SEVERITY: not reported ASTHMA DURATION: mean (months) (range): INTERVENTION: ICS (fluticasone 88 μg/d): 25.0 (6‐46) CONTROL: ICS (fluticasone 176 μg/d): 24.4 (4‐46) MEAN (± SD) β2‐AGONIST USE (puffs/d): not reported; LS mean (SE) change to end point was reported DOSE OF ICS AT STUDY ENTRY AND AT RUN‐IN: not reported ATOPY (% of participants): not reported ELIGIBILITY CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | PROTOCOL DURATION
DEVICE: metered‐dose inhaler. Treatments were administered via a valve holding (Aerochamber Plus [Trudell Medical International, London, Ontario] or OptiChamber [Respironics, Murrysville, PA], each used by approximately half of the children) with an attached face mask DOSE OF ICS
CRITERIA FOR WITHDRAWAL FROM STUDY: reported |
|
| Outcomes | ANALYSIS: Safety analyses were based on data from the intent‐to‐treat population; analysis of co‐variance was used OUTCOMES GROWTH MEASUREMENT TECHNIQUE: Growth (standing height) was measured in triplicate and at approximately the same time of day using a calibrated stadiometer at screening and at weeks 1, 2, 4, 8 and 12 PULMONARY FUNCTION TESTS: morning PEFR measurements (in children capable of performing this manoeuvre) FUNCTIONAL STATUS
BIOMARKERS
ADVERSE EVENTS: reported WITHDRAWALS: reported |
|
| Notes | PUBLICATION: full paper (2006) FUNDING: grant from GlaxoSmithKline Inc CONFIRMATION OF METHODOLOGY: received Data received from GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation; randomly assigned in 1:1:1 ratio; stratified by age (< 36 months; > 36 months) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study apparently free of other sources of bias |
ACQ = asthma control questionnaire; ACTH = adrenocorticotrophic hormone; ANCOVA = analysis of co‐variance; ANOVA = analysis of variance; BALP = bone alkaline phosphate; BD = bronchodilator; BMD = body mass index; eNO = exhaled nitric oxide; FEF25%–75% = forced expiratory flow between 25% and 75% of FVC; FEV1 = forced expired volume in 1 second; FVC = forced vital capacity; GCS = glucocorticosteroids; HPAA = hypothalamic‐pituitary‐adrenal axis; ICS = inhaled corticosteroids; ICTP = type I collagen telopeptide; ITT = intent‐to‐treat; MEF50 = maximal expiratory flow at 50%; mITT = modified intent‐to‐treat; OC = serum osteocalcin; o.d. = once daily; PACT = Pediatric Asthma Controller Trial; PAQLQ = Paediatric Asthma Quality of Life Questionnaire; PD20 = dose of methacholine causing a 20% fall in forced expiratory volume in 1 sec (FEV1) from baseline; PEFR = peak expiratory flow rate; PICP = procollagen type I carboxyterminal propeptide; SD = standard deviation; SE = standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agertoft 2004 | Not a parallel‐group study |
| Antoniu 2003 | No daily ICS in 1 of the intervention groups (control group) |
| Apold 1975 | Not a randomised controlled trial |
| Asrilant 1975 | Not a randomised controlled trial |
| Bateman 2008 | Participants aged ≥ 18 years |
| Baxter‐Jones 1998 | Other group did not evaluate an additional ICS dose using the same molecule |
| Berger 2005 | Enrolled participants were children younger than 1 year of age |
| Bernstein 1999 | Other group did not evaluate an additional ICS dose using the same molecule |
| Birkebaek 1995 | Not a parallel‐group study |
| Breborowicz 2005 | Not a randomised controlled trial |
| Brook 1998 | Not a randomised controlled trial |
| Brown 1973 | Not a randomised controlled trial |
| Chuchalin 2008 | Participants aged ≥ 18 years |
| Dickson 1973 | Not a randomised controlled trial |
| Ferguson 2002 | Other group did not evaluate an additional ICS dose using the same molecule |
| Godfrey 1973 | Not a randomised controlled trial |
| Godfrey 1974 | Not a randomised controlled trial |
| Guarnaccia 1996 | Not a randomised controlled trial |
| Guo 2002 | Not a parallel‐group study |
| Gwynn 1977 | Not a randomised controlled trial |
| Hansel 2006 | Participants aged ≥ 18 years |
| Kaiser 2008 | Other group did not evaluate an additional ICS dose using the same molecule |
| Karpel 2007 | Co‐intervention was not equivalent between comparison groups and/or was not stable throughout the observation period |
| Kemp 2004 | Participants aged ≥ 18 years |
| Lang 2013 | No daily ICS in 1 of the intervention groups |
| Laursen 1986 | Participants aged ≥ 18 years |
| Lipworth 1996 | Not a parallel‐group study |
| Lovera 1975 | Not a randomised controlled trial |
| McAllen 1974 | Not a parallel‐group study |
| Neffen 2006 | Duplicate study |
| Nelson 2000 | Co‐intervention not equivalent between comparison groups and/or not stable throughout the observation period |
| Niu 1998 | Treatment administered for < 12 weeks |
| Otsuki 2009 | No daily ICS in 1 of the intervention groups (control group) |
| Pearlman 2005 | Not a randomised controlled trial |
| Pedeersen 2003 | Not a parallel‐group study |
| Pedersen 2002 | Other group did not evaluate an additional ICS dose using the same molecule |
| Peroni 2005 | Co‐intervention not equivalent between comparison groups and/or not stable throughout the observation period |
| Phipatanakul 2003 | No daily ICS in 1 of the intervention groups (control group) |
| Pines 1973 | Not a randomised controlled trial |
| Skoner 2000 | No daily ICS in 1 of the intervention groups (control group) |
| Skoner 2006 | Duplication of already published paper |
| Skoner 2010 | Treatment administered for < 12 weeks |
| Szefler 2008 | No daily ICS in 1 of the intervention groups (control group) |
| Thompson 1998 | Treatment administered for < 12 weeks |
| Turpeinen 2008 | No daily ICS in 1 of the intervention groups (control group) |
| Visser 2001 | No daily ICS in 1 of the intervention groups (control group) |
| Visser 2001a | Duplication of already published paper |
| Visser 2004 | No daily ICS in 1 of the intervention groups (control group) |
| Wasserman 1996 | Participants aged ≥ 18 years |
| Wasserman 1996 b | Participants aged ≥ 18 years |
| Waugh 2002 | Not a randomised controlled trial |
| Williams 2010 | No daily ICS in 1 of the intervention groups (control group) |
| Wolthers 1995 | Not a parallel‐group study |
| Xu 2005 | No daily ICS in 1 of the intervention groups (control group) |
Differences between protocol and review
The review is different from the protocol in the following ways.
Limited lower age to one year instead of 'up to 18 years.'
Defined which other interventions were accepted: other non‐steroidal asthma drugs (e.g. long‐acting beta‐agonists or leukotriene receptor antagonists).
Added post hoc secondary outcomes (change in body mass index; change in skeletal maturation).
Removed subgroup analyses as they were included as different outcomes: time points of outcome measurements.
Added post hoc analysis: ICS dose difference (in μg of HFA‐beclomethasone or equivalent) between groups.
Added two outcomes: change in body mass index and change in skeletal maturation.
Following recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), the fixed effect model was used for the data analysis if the heterogeneity of pooled trials is less than 50%; otherwise the random effects model was used, despite the use of random effect models was proposed for all data analysis in the protocol.
Several included trials contributed more than one comparison and one group compared with two or more groups. So the individual comparison was used as the unit of analysis in place of individual trial.
Contributions of authors
Aniela Ignea Pruteanu reviewed the literature search conducted until March2014, identified and reviewed all citations for relevance, reviewed all included trials for methodology and data extraction, verified all references, described the studies and performed data entry, analysed and interpreted results of the meta‐analysis, wrote the first draft of the manuscript and approved the final version.
Bhupendrasinh Chauhan reviewed all included trials for methodology and data extraction, verified the description of studies and data entry, contributed to analysis and interpretation of data, revised all drafts of the manuscript, prepared responses to editorial comments and approved the final version.
Linjie Zhang wrote the review protocol, reviewed the literature search conducted until March2014, identified and reviewed half of the citations for relevance and approved the final version of the review.
Sílvio OM Prietsch provided input to drafting of the protocol, reviewed the literature search conducted until March 2014 and identified and reviewed half of the citations for relevance.
Prof Francine Ducharme revised and approved the protocol, requested the literature search, identified and contacted corresponding authors and/or pharmaceutical companies to solicit their collaboration in this review and in identifying other possibly relevant trials, corresponded with authors or pharmaceutical companies to verify methodology and data extraction, verified all references, described studies and performed data entry, analysed and interpreted results and approved the final version of the meta‐analysis.
Sources of support
Internal sources
None, Other.
External sources
No sources of support supplied
Declarations of interest
Aniela Ignea Pruteanu, Bhupendrasinh Chauhan, Linjie Zhang and Sílvio OM Prietsch: none known.
Prof. Francine Ducharme has received travel support, research funds and fees for speaking from Glaxo SmithKline, Novartis, Nycomed and/or Merck Frosst Inc.
Edited (no change to conclusions)
References
References to studies included in this review
Allen 1998 {published data only}
- Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman D, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Journal of Pediatrics 1998;132(3 I):472‐7. [DOI] [PubMed] [Google Scholar]
Baker 1999 {published data only}
- Baker J, Mellon M, Wald J, Welch M, Cruz‐Rivera M. A multiple‐dosing, placebo‐controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999;103(2):414‐21. [DOI] [PubMed] [Google Scholar]
Baker 1999 b {published data only}
- Baker J, Mellon M, Wald J, Welch M, Cruz‐Rivera M. A multiple‐dosing, placebo‐controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999;103(2):414‐421. [DOI] [PubMed] [Google Scholar]
Brand 2011 {published data only}
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105(11):1588‐95. [DOI] [PubMed] [Google Scholar]
Brand 2011 b {published data only}
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011/11;105(11):1588‐95. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen A, Chen R, Zhong N. Systemic side effects of long‐term treatment with low dose inhaled corticosteroids in children with asthma. Chinese Journal of Tuberculosis and Respiratory Diseases 2001;24(12):740‐3. [PubMed] [Google Scholar]
Doniec 2004 {published data only}
- Doniec Z, Pierzchala‐Koziec K, Tomalak W, Nowak D, Kurzawa R. Powder budesonide decreases plasma level of native and cryptic met‐enkephalin in asthmatic children. International Review of Allergology and Clinical Immunology 2004;10(3):105‐9. [Google Scholar]
Gelfand 2006 {published data only}
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. [DOI] [PubMed] [Google Scholar]
Gelfand 2006 b {published data only}
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. J Pediatrics 2006;148(3):377‐83. [DOI] [PubMed] [Google Scholar]
Giorgi 1998 {published data only}
- Giorgi PL, Oggiano N, Kantar A, Coppa GV, Ricciotti R, Arena F, et al. Bone metabolism in children with asthma treated with nebulized flunisolide: a multicenter Italian study. Current Therapeutic Research, Clinical and Experimental 1998;59(12):896‐908. [Google Scholar]
Jonasson 1998 {published data only}
- Jónasson G, Carlsen K‐H, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12:1099‐104. [DOI] [PubMed] [Google Scholar]
Jonasson 2000 {published data only}
- Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low‐dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000;55(8):740‐8. [DOI] [PubMed] [Google Scholar]
Kemp 1999 {published data only}
- Kemp JP, Skoner DP, Szefler SJ, Walton‐Bowen K, Cruz‐Rivera M, Smith JA. Once‐daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Annals of Allergy 1999;83(3):231‐9. [DOI] [PubMed] [Google Scholar]
Kemp 1999 b {published data only}
- Kemp JP, Skoner DP, Szefler SJ, Walton‐Bowen K, Cruz‐Rivera M. Once‐daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Annals of Allergy 1999;83(3):231‐9. [DOI] [PubMed] [Google Scholar]
Kerwin 2008 {published data only}
- Kerwin EM, Pearlman DS, Guia T, Carlsson LG, Gillen M, Uryniak T, et al. Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Current Medical Research and Opinion 2008;24(5):1497‐510. [DOI] [PubMed] [Google Scholar]
Kerwin 2008 b {published data only}
- Kerwin EM, Pearlman DS, Guia T, Carlsson LG, Gillen M, Uryniak T. Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Current Medical Research and Opinion 2008;24(5):1497‐510. [DOI] [PubMed] [Google Scholar]
Lemanske 2004 {published data only}
- Lemanske RF, Lockey RF, Murphy KR. Effects of one year of treatment with mometasone furoate metered dose inhaler (MF‐MDI) on growth in children with asthma. European Respiratory Journal 2004;24(Suppl 48):379s. [Google Scholar]
Peden 1998 {published data only}
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. [DOI] [PubMed] [Google Scholar]
Peden 1998 b {published data only}
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. [DOI] [PubMed] [Google Scholar]
Pedersen 2010 {published data only}
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the rainbow study. Respiratory Medicine 2010;104(11):1618‐28. [DOI] [PubMed] [Google Scholar]
Pedersen 2010 b {published data only}
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the rainbow study. Respiratory Medicine 2010;104(11):1618‐28. [DOI] [PubMed] [Google Scholar]
Shapiro 1998 {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH, et al. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. Journal of Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Shapiro 1998 b {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Shapiro 1998 c {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Shapiro 1998 d {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Skoner 2008 {published data only}
- Skoner DP, Maspero J, Banerji D. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;121(1):e1‐14. [DOI] [PubMed] [Google Scholar]
Skoner 2011 {published data only}
- Skoner DP, Meltzer EO, Milgrom HA, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. [DOI] [PubMed] [Google Scholar]
Skoner 2011 b {published data only}
- Skoner DP, Meltzer EO, Milgrom HA, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. [DOI] [PubMed] [Google Scholar]
Sorkness 2007 {published data only}
- Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. Journal of Allergy and Clinical Immunology 2007;119(1):64‐72. [DOI] [PubMed] [Google Scholar]
Teper 2004 {published data only}
- Teper AM, Colom AJ, Kofman CD, Maffey AF, Vidaurreta SM, Bergada I. Effects of inhaled fluticasone propionate in children less than 2 years old with recurrent wheezing. Pediatric Pulmonology 2004;37(2):111‐5. [DOI] [PubMed] [Google Scholar]
Vaessen‐Verberne 2010 {published data only}
- Vaessen‐Verberne AA, Berg NJ, Nierop JC, Brackel HJ, Gerrits GP, Hop WC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. American Journal of Respiratory and Critical Care Medicine 2010;182(10):1221‐7. [DOI] [PubMed] [Google Scholar]
Verberne 1998 {published data only}
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. [DOI] [PubMed] [Google Scholar]
Verberne 1998 b {published data only}
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. [DOI] [PubMed] [Google Scholar]
Wasserman 2006 {published data only}
- Wasserman RL, Baker JW, Kim KT, Blake KV, Scott CA, Wu W, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2‐ to 4‐year‐old patients with asthma: results of a double‐blind, placebo‐controlled study. Annals of Allergy, Asthma, and Immunology 2006;96(6):808‐18. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agertoft 2004 {published data only}
- Agertoft L, Pedersen S. Inhaled ciclesonide does not effect lower leg growth rate or HPA‐axis function in children with mild asthma. European Respiratory Journal 2004;24(Suppl 48):377s. [Google Scholar]
Antoniu 2003 {published data only}
- Antoniu SA. The START study: when to start to treat with inhaled steroids in asthma?. Expert Review of Pharmacoeconomics & Outcomes Research 2003;3(3):223‐5. [DOI] [PubMed] [Google Scholar]
Apold 1975 {published data only}
- Apold J, Djoseland O. Inhaled beclomethasone dipropionate in the treatment of childhood asthma. Postgraduate Medical Journal 1975;51(Suppl 4):104‐5. [PubMed] [Google Scholar]
Asrilant 1975 {published data only}
- Asrilant M. Beclomethasone dipropionate: an aerosol corticosteroid for topical use in bronchial asthma. Postgraduate Medical Journal 1975;51(Suppl 4):79‐83. [PubMed] [Google Scholar]
Bateman 2008 {published data only}
- Bateman ED, Cheung D, Lapa e Silva J, Gohring UM, Schafer M, Engelstatter R. Randomized comparison of ciclesonide 160 and 640 mug/day in severe asthma. Pulmonary Pharmacology and Therapeutics 2008;21(3):489‐98. [DOI] [PubMed] [Google Scholar]
Baxter‐Jones 1998 {published data only}
- Baxter‐Jones AD, Helms PJ. Effect of 6 month daily treatment with inhaled corticosteroids on lower leg growth in pre‐school wheezing children: a pragmatic trial. Thorax 1998;53(Suppl 4):A5 S19. [Google Scholar]
Berger 2005 {published data only}
- Berger WE, Qaqundah PY, Blake K, Rodriguez‐Santana J, Irani AM, Xu J, et al. Safety of budesonide inhalation suspension in infants aged six to twelve months with mild to moderate persistent asthma or recurrent wheeze. The Journal of Pediatrics 2005;146(1):91‐5. [DOI] [PubMed] [Google Scholar]
Bernstein 1999 {published data only}
- Bernstein DI, Berkowitz RB, Chervinsky P, Dvorin DJ, Finn AF, Gross GN, et al. Dose‐ranging study of a new steroid for asthma: mometasone furoate dry powder inhaler. Respiratory Medicine 1999;93(9):603‐12. [DOI] [PubMed] [Google Scholar]
Birkebaek 1995 {published data only}
- Birkebaek NH, Esberg G, Andersen K, Wolthers O, Hassager C. Bone and collagen turnover during treatment with inhaled dry powder budesonide and beclomethasone dipropionate. Archives of Disease in Childhood 1995;73(6):524‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breborowicz 2005 {published data only}
- Breborowicz A, Niedziela M. Low risk of adrenal dysfunction in children with severe asthma treated with high dose inhaled glucocorticoids. European Respiratory Journal 2005;26(Suppl 49SP):Abstract No. 1058. [Google Scholar]
Brook 1998 {published data only}
- Brook CG. Short stature never killed anybody. Journal of Pediatrics 1998;133(5):591‐2. [DOI] [PubMed] [Google Scholar]
Brown 1973 {published data only}
- Brown HM, Storey G. Beclomethasone dipropionate steroid aerosol in treatment of perennial allergic asthma in children. British Medical Journal 1973;3(872):161‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuchalin 2008 {published data only}
- Chuchalin A, Jacques L, Frith L. Salmeterol/fluticasone propionate via Diskus[trademark] once daily versus fluticasone propionate twice daily in patients with mild asthma not previously receiving maintenance corticosteroids. Clinical Drug Investigation 2008;28(3):169‐81. [DOI] [PubMed] [Google Scholar]
Dickson 1973 {published data only}
- Dickson W, Hall CE, Ellis M, Horrocks RH. Beclomethasone dipropionate aerosol in childhood asthma. Archives of Disease in Childhood 1973;48(9):671‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2002 {published data only}
- Ferguson AC, Bever HP, Teper AM, Lasytsya OI, Whitehead PJ. Fluticasone propionate 100µg bd (FP100) has significantly less effect than budesonide 200µg bd (BUD200) on childhood growth over 1 year of treatment in asthmatics. European Respiratory Journal 2002;20(Suppl 38):219s. [Google Scholar]
Godfrey 1973 {published data only}
- Godfrey S, Konig P. Beclomethasone aerosol in childhood asthma. Archives of Disease in Childhood 1973;48(9):665‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Godfrey 1974 {published data only}
- Godfrey S, Konig P. Treatment of childhood asthma for 13 months and longer with beclomethasone dipropionate aerosol. Archives of Disease in Childhood 1974;49(8):591‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guarnaccia 1996 {published data only}
- Guarnaccia S, Buzi F, LaGrutta S, Marini S, Laffranchi MG, Brunori A, et al. High dose inhaled corticosteroids (IC) in children with asthma: influence on bone metabolism. European Respiratory Journal 1996;9(Suppl 23):295s. [Google Scholar]
Guo 2002 {published data only}
- Guo JG, Cheng ST. The efficacy of low‐dose oral aminophylline combined with inhaled corticosteroid in the treatment of asthmatic children in remission period. Acta Academic Medicine Xuzhou 2002;22(4):349‐51. [Google Scholar]
Gwynn 1977 {published data only}
- Gwynn CM, Smith JM. Long‐term results with beclomethasone dipropionate aerosol in children with bronchial asthma: why does it sometimes fail?. British Journal of Clinical Pharmacology 1977;4(Suppl 3):269S‐271S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansel 2006 {published data only}
- Hansel TT, Benezet O, Kafe H, Ponitz HH, Cheung D, Engelstatter R, et al. A multinational, 12‐week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthma. Clinical Therapeutics 2006;28(6):906‐20. [DOI] [PubMed] [Google Scholar]
Kaiser 2008 {published data only}
- Kaiser H, Parasuraman B, Boggs R, Miller CJ, Leidy NK, O'Dowd L. Onset of effect of budesonide and formoterol administered via one pressurized metered‐dose inhaler in patients with asthma previously treated with inhaled corticosteroids. Annals of Allergy, Asthma, and Immunology 2008;101(3):295‐303. [DOI] [PubMed] [Google Scholar]
Karpel 2007 {published data only}
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628‐37. [DOI] [PubMed] [Google Scholar]
Kemp 2004 {published data only}
- Kemp JP, Osur S, Shrewsbury SB, Herje NE, Duke SP, Harding SM, et al. Potential effects of fluticasone propionate on bone mineral density in patients with asthma: a 2‐year randomized, double‐blind, placebo‐controlled trial. Mayo Clinic Proceedings 2004;79(4):458‐66. [DOI] [PubMed] [Google Scholar]
Lang 2013 {published data only}
- Lang JE, Dozor AJ, Holbrook JT, Mougey E, Krishnan S, Sweeten S, et al. Biologic mechanisms of environmental tobacco smoke in children with poorly controlled asthma: results from a multicenter clinical trial. Journal of Allergy and Clinical Immunology: In Practice 2013;1(2):172‐180.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laursen 1986 {published data only}
- Laursen LC, Taudorf E, Weeke B. High‐dose inhaled budesonide in treatment of severe steroid‐dependent asthma. European Journal of Respiratory Diseases 1986;68(1):19‐28. [PubMed] [Google Scholar]
Lipworth 1996 {published data only}
- Lipworth BJ, Clark D. High‐dose inhaled steroids in asthmatic children. Lancet 1996;348(9030):820; discussion 821. [DOI] [PubMed] [Google Scholar]
Lovera 1975 {published data only}
- Lovera J, Collins‐Williams C, Bailey J. Beclomethasone dipropionate by aerosol in the treatment in asthmatic children. Postgraduate Medical Journal 1975;51(Suppl 4):96‐8. [PubMed] [Google Scholar]
McAllen 1974 {published data only}
- McAllen MK, Kochanowski SJ, Shaw KM. Steroid aerosols in asthma: an assessment of betamethasone valerate and a 12‐month study of patients on maintenance treatment. British Medical Journal 1974;1(900):171‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neffen 2006 {published data only}
- Neffen H, Ruff M, Zhang P, Lloyd M, Banjeri D. Ciclesonide administered once daily has no effect on skeletal maturity in prepubertal children with mild persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2006;117(2):184. [Google Scholar]
Nelson 2000 {published data only}
- Nelson HS, Kane RE, Petillo J, Banerji D, Anolik R, Bosso J, et al. Long‐term safety of a non‐chlorofluorocarbon‐containing triamcinolone acetonide inhalation aerosol in patients with asthma. Journal of Asthma 2000;37(2):145‐52. [DOI] [PubMed] [Google Scholar]
Niu 1998 {published data only}
- Niu CK, Huang SC, Huang CB. Effect of short‐course budesonide on the bone turnover of asthmatic children. Pediatric Pulmonology 1998;26(4):290‐2. [DOI] [PubMed] [Google Scholar]
Otsuki 2009 {published data only}
- Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Adherence feedback to improve asthma outcomes among inner‐city children: a randomized trial. Pediatrics 2009;124(6):1513‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearlman 2005 {published data only}
- Pearlman DS, Berger WE, Kerwin E, LaForce C, Kundu S, Banerji D. Once‐daily ciclesonide improves lung function and is well tolerated by patients with mild‐to‐moderate persistent asthma. Journal of Allergy and Clinical Immunology 2005;116(6):1206‐12. [DOI] [PubMed] [Google Scholar]
Pedeersen 2003 {published data only}
- Pedeersen S, Agertoft L, Lee T, Staudinger H. Lower‐leg growth in children with asthma during treatment with inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S269. [Google Scholar]
Pedersen 2002 {published data only}
- Pedersen S, Warner J, Wahn U, Staab D, Bourgeois M, Essen‐Zandvliet E, et al. Growth, systemic safety, and efficacy during 1 year of asthma treatment with different beclomethasone dipropionate formulations: an open‐label, randomized comparison of extrafine and conventional aerosols in children. Pediatrics 2002;109(6):e92. [DOI] [PubMed] [Google Scholar]
Peroni 2005 {published data only}
- Peroni DG, Piacentini GL, Bodini A, Ress M, Costella S, Boner AL. Montelukast versus formoterol as second‐line therapy in asthmatic children exposed to relevant allergens. Allergy and Asthma Proceedings 2005;26(4):283‐6. [PubMed] [Google Scholar]
Phipatanakul 2003 {published data only}
- Phipatanakul W, Greene C, Downes SJ, Cronin B, Eller TJ, Schneider LC, et al. Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Annals of Allergy, Asthma, and Immunology 2003;91(1):49‐54. [DOI] [PubMed] [Google Scholar]
Pines 1973 {published data only}
- Pines A. Beclomethasone dipropionate used as an aerosol in the treatment of asthma. Practitioner 1973;211(261):86‐90. [PubMed] [Google Scholar]
Skoner 2000 {published data only}
- Skoner DP, Szefler SJ, Welch M, Walton‐Bowen K, Cruz‐Rivera M, Smith JA. Longitudinal growth in infants and young children treated with budesonide inhalation suspension for persistent asthma. Journal of Allergy and Clinical Immunology 2000;105(2 Pt 1):259‐68. [DOI] [PubMed] [Google Scholar]
Skoner 2006 {published data only}
- Skoner D, Maspero J, Kundu S, Lloyd M, Banerji D. Ciclesonide administered once daily has no effect on growth velocity in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S11. [Google Scholar]
Skoner 2010 {published data only}
- Skoner DP, Gentile DA, Angelini B. Effect of therapeutic doses of mometasone furoate on cortisol levels in children with mild asthma. Allergy and Asthma Proceedings 2010;31(1):10‐19. [DOI] [PubMed] [Google Scholar]
Szefler 2008 {published data only}
- Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline‐based treatment for inner‐city adolescents and young adults: a randomised controlled trial. Lancet 2008;372(9643):1065‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1998 {published data only}
- Thompson PJ, Davies RJ, Young WF, Grossman AB, Donnell D. Safety of hydrofluoroalkane‐134a beclomethasone dipropionate extrafine aerosol. Respiratory Medicine 1998;92(Suppl A):33‐9. [DOI] [PubMed] [Google Scholar]
Turpeinen 2008 {published data only}
- Turpeinen M, Nikander K, Pelkonen AS, Syvänen P, Sorva R, Raitio H, et al. Daily versus as‐needed inhaled corticosteroid for mild persistent asthma (the Helsinki early intervention childhood asthma study). Archives of Disease in Childhood 2008;93(8):654‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visser 2001 {published data only}
- Visser MJ, Postma DS, Arends LR, Vries TW, Duiverman EJ, Brand PL. One‐year treatment with different dosing schedules of fluticasone propionate in childhood asthma. Effects on hyperresponsiveness, lung function, and height. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2073‐7. [DOI] [PubMed] [Google Scholar]
Visser 2001a {published data only}
- Visser MJ, Duiverman EJ, Postma DS, Arends LR, Brand PLP. High doses of inhaled fluticasone propionate dose‐dependently suppress adrenal cortical function in asthmatic children. European Respiratory Journal 2001;18(Suppl 33):290s. [Google Scholar]
Visser 2004 {published data only}
- Visser MJ, Veer E, Postma DS, Arends LR, Vries TW, Brand PLP, et al. Side‐effects of fluticasone in asthmatic children: no effects after dose reduction. European Respiratory Journal 2004;24(3):420‐5. [DOI] [PubMed] [Google Scholar]
Wasserman 1996 {published data only}
- Wasserman SI, Gross GN, Schoenwetter WF, Munk ZM, Kral KM, Schaberg A, et al. A 12‐week dose‐ranging study of fluticasone propionate powder in the treatment of asthma. Journal of Asthma 1996;33(4):265‐74. [DOI] [PubMed] [Google Scholar]
Wasserman 1996 b {published data only}
- Wasserman SI, Gross GN, Schoenwetter WF, Munk ZM, Kral KM, Schaberg A, et al. A 12‐week dose‐ranging study of fluticasone propionate powder in the treatment of asthma. Journal of Asthma 1996;33(4):265‐74. [DOI] [PubMed] [Google Scholar]
Waugh 2002 {published data only}
- Waugh J, Goa KL. Flunisolide HFA. American Journal of Respiratory Medicine 2002;1(5):369‐72; discussion 373. [DOI] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster‐randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. Journal of Allergy and Clinical Immunology 2010;126(2):225‐231, 231.e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolthers 1995 {published data only}
- Wolthers OD, Juul A, Hansen M, Muller J, Pedersen S. The insulin‐like growth factor axis and collagen turnover in asthmatic children treated with inhaled budesonide. Acta Paediatrica 1995;84:393‐7. [DOI] [PubMed] [Google Scholar]
Xu 2005 {published data only}
- Xu Z, Chen H‐Y, Zhang S‐Y, Wang X‐L, He C‐R, Qiao Y‐X. Efficacy and safety of corticosteroid inhalation in the treatment of childhood asthma. Zhongguo Dangdai Erke Zazhi 2005;7(1):47‐50. [Google Scholar]
Additional references
Adams 2011a
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [Google Scholar]
Adams 2011b
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2011c
- Adams NP, Bestall JB, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD003274] [DOI] [Google Scholar]
Agertoft 2003
- Agertoft L, Laulund LW, Harrison LI, Pedersen S. Influence of particle size on lung deposition and pharmacokinetics of beclomethasone dipropionatein children. Pediatric Pulmonology 2003;35:192‐9. [DOI] [PubMed] [Google Scholar]
Agertoft 2003a
- Agertoft L, Pedersen S. Lung deposition and systemic availability of fluticasone Diskus and budesonide Turbuhaler in children. American Journal of Respiratory and Critical Care Medicine 2003;168:779‐82. [DOI] [PubMed] [Google Scholar]
Allen 1999
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54:29‐34. [DOI] [PubMed] [Google Scholar]
Allen 2002
- Allen DB. Safety of inhaled corticosteroids in children. Pediatric Pulmonology 2002;33:208‐20. [DOI] [PubMed] [Google Scholar]
Allen 2006
- Allen DB. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Advances in Pediatrics 2006;53:101‐10. [DOI] [PubMed] [Google Scholar]
Asher 2010
- Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergologia et Immunopathologia (Madrid) 2010;38(2):83‐7. [DOI] [PubMed] [Google Scholar]
Barnes 2003
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma?. Annals of Internal Medicine 2003;139:359‐70. [DOI] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130:4S‐12S. [DOI] [PubMed] [Google Scholar]
Brand 2001
- Brand PLP. Inhaled corticosteroids reduce growth. Or do they?. European Respiratory Journal 2001;17:287‐94. [DOI] [PubMed] [Google Scholar]
BTS 2012
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. A national clinical guideline. May 2008 (revised January 2012). http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&ved=0CDYQFjAA&url=http%3A%2F%2Fwww.sign.ac.uk%2Fpdf%2Fsign101.pdf&ei=qN_nUY3DPIem4gTc6IDoBQ&usg=AFQjCNFZSipqqDue5MN9iBKrWV1MoM4gxg&sig2=K4MXf4g8R4xKCs45kBiI‐g&bvm=bv.49478099,d.bGE (accessed 20 June 2012).
CAMP Research Group 2000
- Szefler S, Weiss S, Tonascia J. Long‐term effects of budesonide or nedocromil in children with asthma. The New England Journal of Medecine 2000;343:1054‐63. [DOI] [PubMed] [Google Scholar]
CAMP Research Group 2012
- Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS. Effect of inhaled glucocorticoids in childhood on adult height. The New England Journal of Medecine 2012;367:904‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlsen 2002
- Carlsen KH, Gerritsen J. Inhaled steroids in children: adrenal suppression and growth impairment. European Respiratory Journal 2002;19:985‐8. [DOI] [PubMed] [Google Scholar]
Chauhan 2012
- Chauhan BF, Ducharme FM. Anti‐leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002314.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covar 2003
- Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild‐to‐moderate asthma. Journal of Pediatrics 2003;142(5):469‐75. [DOI] [PubMed] [Google Scholar]
Creese 2001
- Creese KH, Doull IJ. Effects of inhaled corticosteroids on growth in asthmatic children. Current Allergy and Asthma Reports 2001;1(2):122‐6. [DOI] [PubMed] [Google Scholar]
Efthimiou 1998
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11:1167‐77. [DOI] [PubMed] [Google Scholar]
FDA 1998
- US Food, Drug Administration (FDA). FDA requires new pediatric labelling for inhaled, intranasal corticosteroids. FDA Talk Paper. November 9, 1998.
GINA 2014
- Global Initiative for Asthma (GINA). From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014.pdf 2014.
Guilbert 2006a
- Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. The New England Journal of Medecine 2006;354:1985‐97. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2008. [Google Scholar]
ISAAC 1998
- ISAAC Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Studyof Asthma and Allergies in Childhood (ISAAC). European Respiratory Journal 1998;12:315‐35. [DOI] [PubMed] [Google Scholar]
Juniper 1990
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. [DOI] [PubMed] [Google Scholar]
Lai 2009
- Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. [DOI] [PubMed] [Google Scholar]
Lougheed 2012
- Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Del SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update. Diagnosis and management of asthma in preschoolers, children and adults. Canadian Respiratory Journal 2012;19(2):127–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2002
- Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. American Journal of Respiratory and Critical Care Medicine 2002;165:1377‐83. [DOI] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. [DOI] [PubMed] [Google Scholar]
NHLBI 2007
- National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). http://www.nhlbi.nih.gov/guidelines/asthma/ (accessed 20 June 2011).
NHLBI Expert Panel Report 2012
- Expert Panel Report 2007. Guidelines for the Diagnosis and Management of Asthma [National Heart, Lung and Blood Institute, National Institute of Health]. www.nhlbi.nih.gov.clinical practice guidelines (accessed 10 December 2012).
Olivieri 1997
- Olivieri D, Chetta A, Donno M, Bertorelli G, Casalini A, Pesci A, et al. Effect of short‐term treatment with low‐dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1864‐71. [DOI] [PubMed] [Google Scholar]
Pedersen 2001
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical Care Medicine 2001;164(4):521‐35. [DOI] [PubMed] [Google Scholar]
Price 2002a
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179‐93. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Salvatoni 2003
- Salvatoni A, Piantanida E, Nosetti L, Nespoli L. Inhaled corticosteroids in childhood asthma: long‐term effects on growth and adrenocortical function. Paediatric Drugs 2003;5(6):351‐61. [DOI] [PubMed] [Google Scholar]
Sharek 2000a
- Sharek PJ, Bergman D, Francine D. Beclomethasone for asthma in children: effects on linear growth. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharek 2000b
- Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta‐analysis. Pediatrics 2000;106:e8. [DOI] [PubMed] [Google Scholar]
Simons 1997
- Simons FER and Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. The New England Journal of Medicine 1997;337:1659‐65. [DOI] [PubMed] [Google Scholar]
Sizonenko 2002
- Sinonenko PC. Effects of inhaled or nasal glucocorticosteroids on adrenal function and growth. Journal of Pediatric Endocrinology and Metabolism 2002;15(1):5‐26. [DOI] [PubMed] [Google Scholar]
Skonner 2011
- Skoner DP, Meltzer EO, Milgrom H, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. The Journal of Asthma 2011;48:848‐59. [DOI] [PubMed] [Google Scholar]
Sobande 2008
- Sobande PO, Kercsmar CM. Inhaled corticosteroids in asthma management. Respiratory Care 2008;53(5):625‐33. [PubMed] [Google Scholar]
Suissa 2000
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343(5):332‐6. [DOI] [PubMed] [Google Scholar]
US FDA 2007
- Guidance for industry orally inhaled and intranasal corticosteroids: evaluation of the effects on growth in children, 2007. http://www.fda.gov/cder/guidance/index.htm.
Van Essen‐Zandvliet 1992
- Essen‐Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness,and symptoms in children with asthma. American Review of Respiratory Disease 1992;146(3):547‐54. [DOI] [PubMed] [Google Scholar]
Van Rensen 1999
- Rensen EL, Straathof KC, Veselic‐Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax 1999;54(5):403‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witzmann 2000
- Witzmann KB, Fink RJ. Inhaled corticosteroids in childhood asthma: growing concerns. Drugs 2000;59(Suppl 1):9‐14. [DOI] [PubMed] [Google Scholar]
Wolthers 2001
- Wolthers O, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion and measures of the insulin‐like growth factor axis and collagen turnover in the assessment of systemic activity of inhaled corticosteroids in children with persistent asthma: effects on growth. Pediatric Research 1997;41:44‐50. [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Axelsson I, Prietsch SOM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009471] [DOI] [PMC free article] [PubMed] [Google Scholar]


