Abstract
Background:
Improving hypertension control is an important global health priority yet, to our knowledge, there is no direct evidence on the blood pressure (BP)-mortality relationship in sub-Saharan Africa. We investigate the BP-mortality relationship in South Africa and assess the comparative effectiveness of different care targets for clinical care and population-wide hypertension management efforts.
Methods:
We use country-wide population-based longitudinal data from five waves (2008 – 2017) of the South African National Income Dynamics Study (N = 4,993). We estimate the relationship between systolic BP (SBP) and six-year all-cause mortality and compare the mortality reductions associated with lowering SBP to different targets. We then estimate the number needed to treat to avert one death (NNT) under different hypothetical population-wide scale up scenarios.
Findings:
We found a weak, nonlinear, SBP-mortality relationship with larger incremental mortality benefits at higher SBP values: reducing SBP from 160 to 150 mmHg was associated with a mortality risk ratio of 0.95 (95% CI: 0.90, 0.99, p = 0.033), incrementally reducing SBP from 150 to 140 mmHg a risk ratio of 0.96 (95% CI: 0.91, 1.01, p = 0.12), with no evidence of incremental benefits of reducing BP below 140 mmHg. At the population level, reducing SBP to 150 mmHg among all those with an SBP > 150 mmHg had the lowest NNT (50) at 3.3 deaths averted (95% CI: −0.6, 0.3) per 1,000 population while requiring BP management for 16% (95% CI: 15.2, 17.3) of individuals.
Interpretation:
The SBP-mortality association is weaker in South Africa than in high-income and many low- and middle-income countries. As such, we do not find compelling evidence in support of targets below 140 mmHg and find that scaling up management based on a 150 mmHg target is more efficient in terms of the NNT compared to strategies to reduce SBP to lower values.
Keywords: hypertension, mortality, longevity, primary care, health policy, sub-Saharan Africa
Introduction
Hypertension is the leading modifiable risk factor for mortality globally.1 While reducing blood pressure (BP) substantially lowers the risk of hypertension-related mortality, the vast majority of individuals with hypertension in low- and middle-income countries (LMICs) do not have controlled BP under common thresholds.2 In South Africa, 35% of the population aged 15 and above are hypertensive, yet just 9% of these individuals have their BP under control.3,4 For this reason, and because reducing BP is a highly effective way of achieving population-wide mortality reductions, substantially scaling up hypertension control is a major health priority in South Africa and other LMICs.5–7
Achieving population-wide BP reductions requires determining what target BP should be reduced to. Since there are no clinical comparative-effectiveness trials of different BP targets for any LMIC population, countries like South Africa have to decide on treatment targets by extrapolating effects from clinical-trial populations in high-income countries (HICs).8 This extrapolation may lead to suboptimal hypertension management decisions if the relationship between BP and mortality in South Africa is different than the relationship in HICs, potentially due to the strong competing risk of tuberculosis and HIV mortality and poorer access to and quality of health care.9
The choice of BP management targets also has consequences for the proportion of the population that would need BP management. For example, moving from the common “<140/90” target to the new American College of Cardiology (ACC) / American Heart Association (AHA) guidelines (target BP <130/80 mmHg) would increase the number of South Africans in need of BP management to nearly half the population.10 Such increases place a substantial burden on health systems and individuals and thus need to be justified with respect to the value gained (e.g. fewer cardiovascular events and lower mortality) relative to the number treated.
In this paper, to our knowledge, we provide the first estimates of the relationship between systolic BP (SBP) and six-year all-cause mortality using country-wide population-based longitudinal cohort data for a sub-Saharan African country. We estimate and compare the mortality risk reductions associated with reducing SBP to specific targets, focusing on the incremental benefits of lower treatment targets. We then investigate the total number of deaths averted, the share of the adult population that would require treatment, and the number needed to treat to avert one death under hypothetical population-wide scale ups of BP management under different targets.
Methods
Data source
We use data on adults aged 30 years and above from the 2008, 2010–11, 2012, 2014–15, and 2017 waves of the South African National Income Dynamics Study (NIDS).11 NIDS is a nationally representative cohort study that contains detailed household demographic, social, and economic information. Importantly, trained NIDS enumerators also collected health information for individuals, including measured height, weight, and BP.
NIDS employed a two-stage cluster stratified sampling design. NIDS first randomly selected 400 Statistics South Africa primary sampling units (PSUs) -- South Africa’s census enumeration areas. Within selected PSUs, NIDS randomly selected 24 dwellings. When there was more than one household in a dwelling, each household was assigned a unique number. If the household agreed to be interviewed, all household members were interviewed. Of the 10,642 identified households, 7,305 (69%) agreed to participate in the 2008 wave, resulting in a baseline sample size of approximately 28,000 individuals. Non-respondents were more likely to be White and reside in urban areas.11 The NIDS survey design weights correct for this differential pattern of non-response under the assumption that White and urban households that did respond are similar to those that did not.12
We restricted our study to adults ages 30 and above as hypertension among younger adults is often due to other causes (e.g., thyroid disease or adrenal tumors) which when treated generally improves BP levels.13,14 Additionally, there is limited evidence on the benefits of BP reductions among those below 30 as they are vastly underrepresented in clinical trials compared to middle-aged and older adults, for whom strong evidence does exist.15,16
Primary exposure and outcome
Our primary exposure is SBP based on measurements taken by trained survey enumerators using an Omron M7 BP Monitor with standard validated multi-size cuffs.4 Enumerators took measurements on individuals’ left arm while they were in a supine position, ensuring that individuals were resting for at least 5 minutes before the initial measurement. Importantly, BP measurements for the same individual can vary substantially due to natural individual BP variation and measurement error from poor measurements or from the BP-measuring device itself.17 For this reason, in clinical practice, decisions around BP are based on multiple measurements taken over a period of days or weeks.18,19 Since BP for survey respondents is only measured on the day of the survey, it may not be an accurate estimate of an individual’s true BP. Such measurement error can bias estimates of the BP-mortality relationship towards the null (known as dilution bias).20 To reduce dilution bias we use the average of the 2008 and 2010–11 BP measurements as our primary exposure, which can be interpreted as two-year average BP. For all analyses, we consider individuals’ measured SBP. However, BP-lowering medicine may confound the BP-mortality relationship since individuals taking medicine may have higher mortality than individuals at the same untreated level of BP, medicines may be given to individuals with other co-morbidities or high cardiovascular disease risk, and medicine use may be associated with access to healthcare and treatment. Therefore, we present the results of the SBP-mortality relationship additionally adjusting for whether an individual self-reported taking BP-lowering medicines (measured as a binary yes/no) in appendix p.6.
Our primary outcome is six-year all-cause mortality, measured as mortality between the 2010–2011 and 2017 waves. The NIDS enumerators determined which individuals died based on household-member reports of deaths between survey waves. We classified those not interviewed and not confirmed to have died by 2017 as lost to follow-up.
Confounders
We adjust for important potential confounders of the SBP-mortality relationship (all measured in 2010–11), including age, sex, race (African, Coloured, Asian/Indian, White), completed schooling (none, primary, secondary, tertiary), marital status (married/living with partner, widowed, divorced or separated, never married), geography type (urban, farm, traditional), province, self-reported health status (excellent, very good, good, fair, poor), current smoking status, weekly frequency of alcohol use (<1, 1–2, ≥3 days/week), how often an individual reported exercising (<1, 1–2, ≥3 times/week), and body mass index (BMI). We included social and geographic confounders (race, schooling, geography, and province) since they influence a wide range of potential confounders including the availability and quality of health services and the physical environment.
Missingness
Of the 8,338 respondents aged ≥30 years in the 2010–11 wave, 927 (11.1%) are missing vital status information in 2017. Among the remaining 7,411, 2,236 (30.2%) are missing BP in 2008 or 2010–11 while 1,355 (18.3%) are missing 2010–11 information on confounders. Overall, a combination of 2,418 (32.6%) age-eligible respondents are missing BP or a confounder value. We excluded these individuals for a final sample of 4,993 representing 69.4% of the age-eligible sample with vital status information in 2017 (appendix p.4).
Statistical Methods
Our analysis has three main steps. First, we estimate the association between SBP and mortality, adjusting for the potential confounders described above using a logistic regression model with six-year mortality as the main outcome. To capture potential non-linearities in the BP-mortality relationship, we model mortality as a cubic function of SBP and present the results as the confounder-adjusted probability of mortality for SBP values between 120 and 180. In appendix p.7, we present results with alternative SBP functional forms.
Second, we estimate and compare the mortality reductions associated with lowering SBP to targets of 120, 130, 140, and 150 mmHg. Since the size of the mortality reduction associated with reducing SBP to each target is dependent on individuals’ starting SBP, we consider reductions to each target starting from SBP values of 130, 140, 150, and 160 mmHg. Fixing the starting values facilitates clear effectiveness comparisons of the different targets. To conduct these analyses, we use the estimated regression model to predict the average six-year mortality at each starting and ending SBP value. We then estimate the risk reduction as the ratio of average mortality at the end SBP value divided by average mortality at the starting value. This value is sometimes known as an “average marginal effect.”
Third, we examine the population-level consequences of using different SBP targets to scale up hypertension care using the parametric g-formula modeling technique. The g-formula is a form of direct standardization used for estimating the effect of hypothetical policies and interventions using observational data.21,22 For these scenarios, we consider all individuals with an SBP above the selected target as in need of BP management.
We examine four population-level outcomes: (i) the number of deaths that would be averted in the overall population if all individuals with an SBP greater than the target have their SBP reduced down to the target value; (ii) the share of the population in need of management (the share of the population with an SBP above the target value); (iii) the number of individuals that would need to be treated (NNT) to avert one death; and (iv) and the population-level mean SBP reduction required to achieve each target. Since this analysis is focused on the overall adult population, the resulting mortality reductions are distinct from the prior analyses because they are a product of the mortality reductions associated with reducing BP down to a specific target starting from each individuals’ actual initial SBP value (rather than from a fixed starting SBP value), the proportion of the population who have an SBP above each specific target, and how far above the target values these individuals’ SBP values are.
To estimate these scenarios using the g-formula, we use the regression coefficients together with observed covariate values to estimate the risk of mortality for all individuals in the data (the “natural course” estimates). Next, based on our SBP management target of interest, we lower SBP among individuals with SBP values above the target down to the target value and re-predict the probabilities of mortality using the original regression coefficients, the reduced SBP values, and the unchanged values of the other covariates (the “counterfactual” estimates). We then estimate mortality averted by comparing mortality risk in the natural course and counterfactual populations. Similarly, we estimate the mean shift in SBP required to achieve the scale up as the difference in mean SBP between the natural course and counterfactual populations.
We weighted all analyses by the NIDS design sampling weights to adjust for the survey sampling procedures and household non-responses in the baseline survey wave.12 We conducted all analyses in R version 3.6.3.
Ethical approval
This study was exempt from institutional review board approval because the data are publicly available and de-identified.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all of the data and the final responsibility to submit for publication.
Results
Our sample consisted of 4,993 adults aged ≥30 years, of which 68.7% (N = 3,432) were women (Table 1). The mean age was 50.5 years (SD: 14.0). Most participants were African (84.6%), were married or living with a partner (50.7%), completed formal primary and secondary education (68.1%), and lived in urban areas (43.9%). On average, participants were overweight (BMI of 28.4 kg/m2, SD: 7.7), 93.4% drank fewer than one day per week, 89.0% reported engaging in exercise less than one time per week, and 16.2% were current smokers. Most participants (82.2%) reported being in excellent or good health.
Table 1.
Descriptive characteristics of the sample in the baseline 2010–11 wave, South African National Income Dynamics Study (N = 4,993).
| Characteristics | No. or mean (% or SD) |
|---|---|
| Age (years) | 50.5 (14.0) |
| Women | 3,432 (68.7) |
| Race | |
| African | 4,226 (84.6) |
| Coloured | 628 (12.6) |
| Asian/Indian | 52 (1.0) |
| White | 87 (1.7) |
| Marital status | |
| Married or living with partner | 2,530 (50.7) |
| Widow, divorced or separated | 980 (19.6) |
| Never married | 1,483 (29.7) |
| Completed schooling | |
| No schooling | 1,162 (23.3) |
| Primary and secondary | 3,399 (68.1) |
| Tertiary | 432 (8.7) |
| Geography type | |
| Urban | 2,191 (43.9) |
| Traditional | 2,394 (47.9) |
| Farms | 408 (8.2) |
| Province | |
| Western Cape | 395 (7.9) |
| Eastern Cape | 686 (13.7) |
| Northern Cape | 396 (7.9) |
| Free State | 294 (5.9) |
| KwaZulu-Natal | 1,492 (29.9) |
| North West | 351 (7.0) |
| Gauteng | 497 (10.0) |
| Mpumalanga | 396 (7.9) |
| Limpopo | 486 (9.7) |
| Self-reported health status | |
| Excellent | 1,351 (27.1) |
| Very good | 1,390 (27.8) |
| Good | 1,261 (25.3) |
| Fair | 679 (13.6) |
| Poor | 312 (6.2) |
| Current smoker | 807 (16.2) |
| Number of days of alcohol use per week | |
| < 1 | 4,664 (93.4) |
| 1–2 | 204 (4.1) |
| ≥ 3 | 125 (2.5) |
| Reported frequency of exercising per week | |
| < 1 | 4,446 (89.0) |
| 1–2 | 332 (6.6) |
| ≥ 3 | 215 (4.3) |
| Body mass index (kg/m2) | 28.4 (7.6) |
The share of the population with SBP ≥ 140 mmHg increased from 7% at age 30 to 57% by age 80 (Figure 1). Among adults 30–49 years old (N = 2,607), 12.4% (95% CI: 10.6, 14.3), 6.6% (95% CI: 5.2, 7.9) and 3.5 (95% CI: 2.4, 4.6) had an SBP ≥ 140, ≥ 150 and ≥ 160 mmHg, respectively. Among adults aged ≥ 50 years old (N =2,386), 44.0% (95% CI: 41.0, 46.9), 28.0% (95% CI: 25.4, 30.7), and 18.2% (95% CI: 15.9, 20.5) had an SBP ≥ 140, ≥ 150 and ≥ 160 mmHg, respectively.
Figure 1.
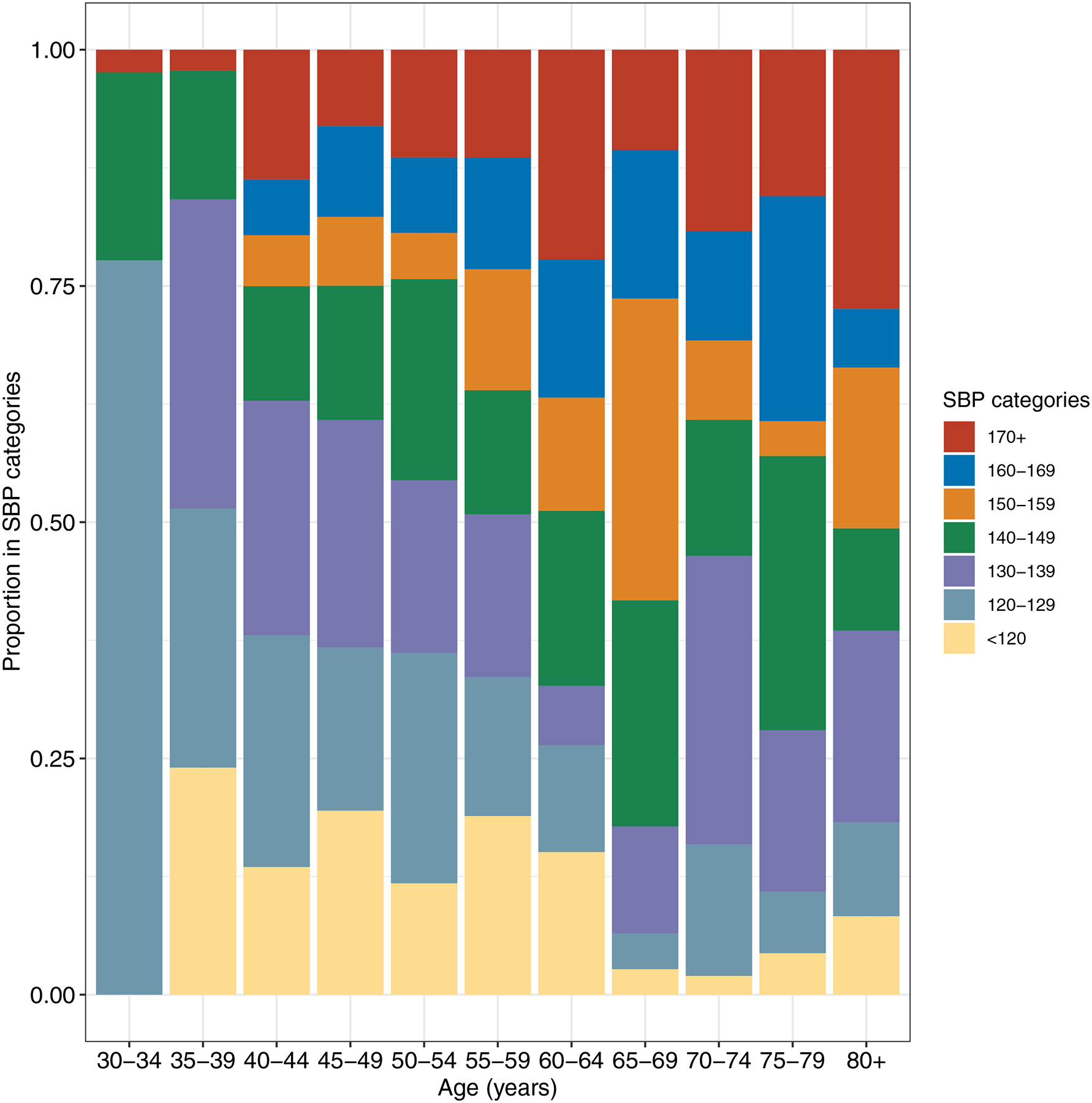
Age-specific proportions of individuals in different systolic blood pressure categories (2020–11 wave).
aWe weighted the proportions by the NIDS design weights to adjust for the survey sampling procedures and household non-responses in the baseline survey wave.
There was a weak, nonlinear, SBP-mortality relationship (appendix p.5) with the lowest mortality risk (8.1%) at 128 mmHg. This nonlinear association resulted in larger incremental mortality reductions at higher SBP values with a substantial flattening of benefits below 140 mmHg (Figure 2). Reducing SBP from 160 to 150 mmHg was associated with a mortality risk ratio of 0.95 (95% CI: 0.90, 0.99, p = 0.033), incrementally reducing SBP from 150 to 140 mmHg a risk ratio of 0.96 (95% CI: 0.91, 1.01, p = 0.12), with no evidence of further incremental benefits from reducing SBP from 140 to 130 mmHg or from 130 mmHg to 120 mmHg.
Figure 2.
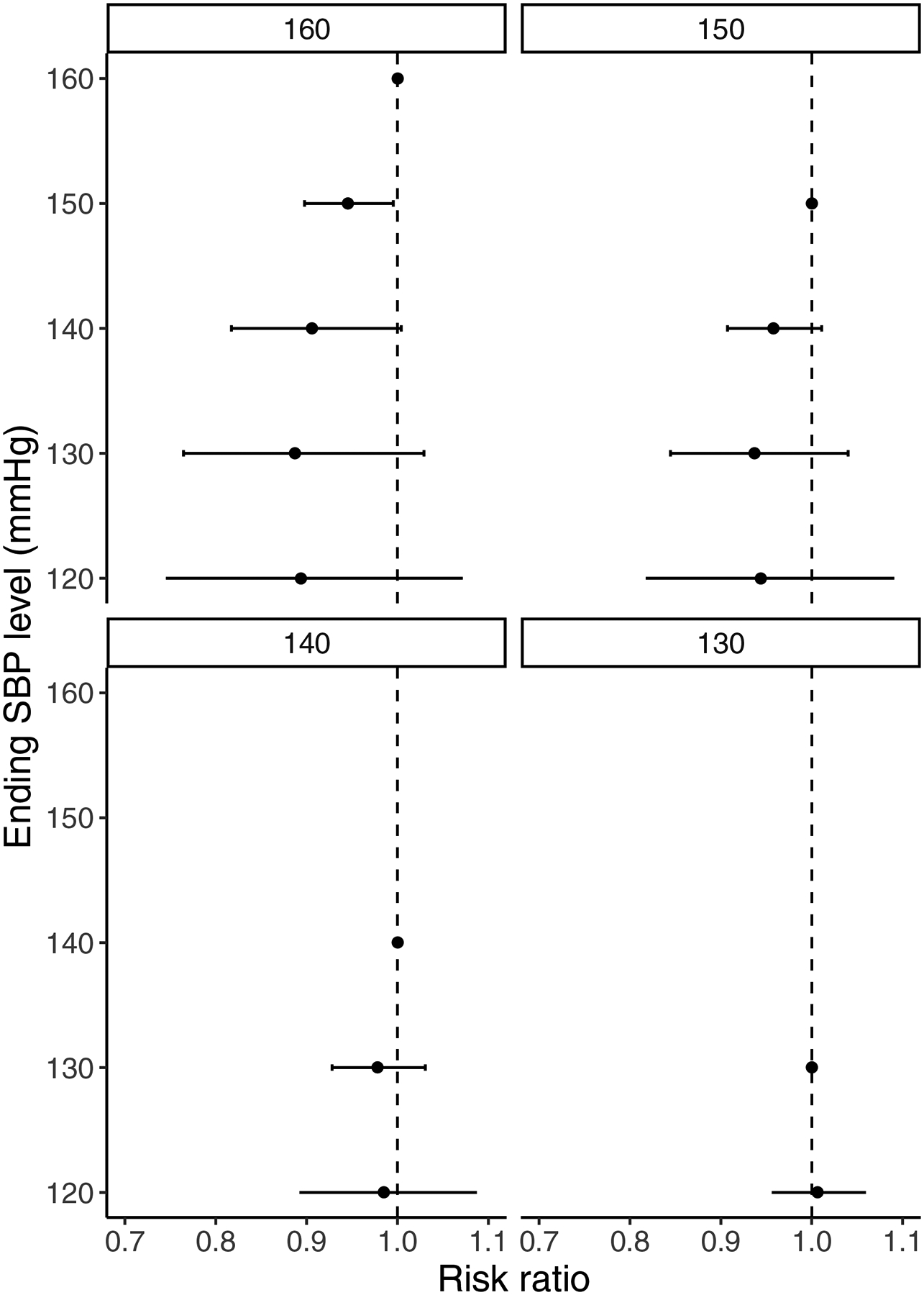
Average six-year mortality risk reductions associated from reducing SBP from specific starting (160, 150, 140, and 130 mmHg) to ending (150, 140, 130, and 120 mmHg) levels.
aError bars are 95% confidence intervals.
bThe results are adjusted for age, sex, race, marital status, formal school, geography type, province, self-reported health status, current smoking, exercise, alcohol use and weight (BMI), and are weighted by the NIDS design weights to adjust for the survey sampling procedures and household non-responses in the baseline survey wave.
At the population level, deaths averted per 1,000 population was similar across scenarios. For example, reducing SBP to 120 mmHg among all those with an SBP > 120 mmHg (120-mmHg scenario) was associated with 5.6 deaths averted per 1,000 population (95% CI: −14.8, 3.6, p = 0.24) compared to 3.3 deaths averted (95% CI: −6.5, −0.1, p = 0.044) from reducing SBP to 150 mmHg among all those with an SBP > 150 mmHg (150-mmHg scenario) (Figure 3). However, there were large differences in the proportion of the population that would require treatment in each scenario, ranging from 65% (95% CI: 64.0, 66.7, p < 0.0001) in the 120 mmHg scenario to 16% (95% CI: 15.2, 17.3, p < 0.0001) in the 150 mmHg scenario. Similarly, the 120-mmHg scenario required a mean shift of −13.8 mmHg (95% CI: −14.4, −13.3, p < 0.0001) compared to just a −2.7 mmHg mean change in SBP (95% CI: −3.0, −2.5, p < 0.0001) for the 150-mmHg scenario. Based on the ratio combination of deaths averted relative to the proportion requiring treatment, the 150-mmHg scenario had the smallest NNT (50), followed by the 140 mmHg (54), 130 mmHg (70), and 120 mmHg (117) scenarios.
Figure 3.
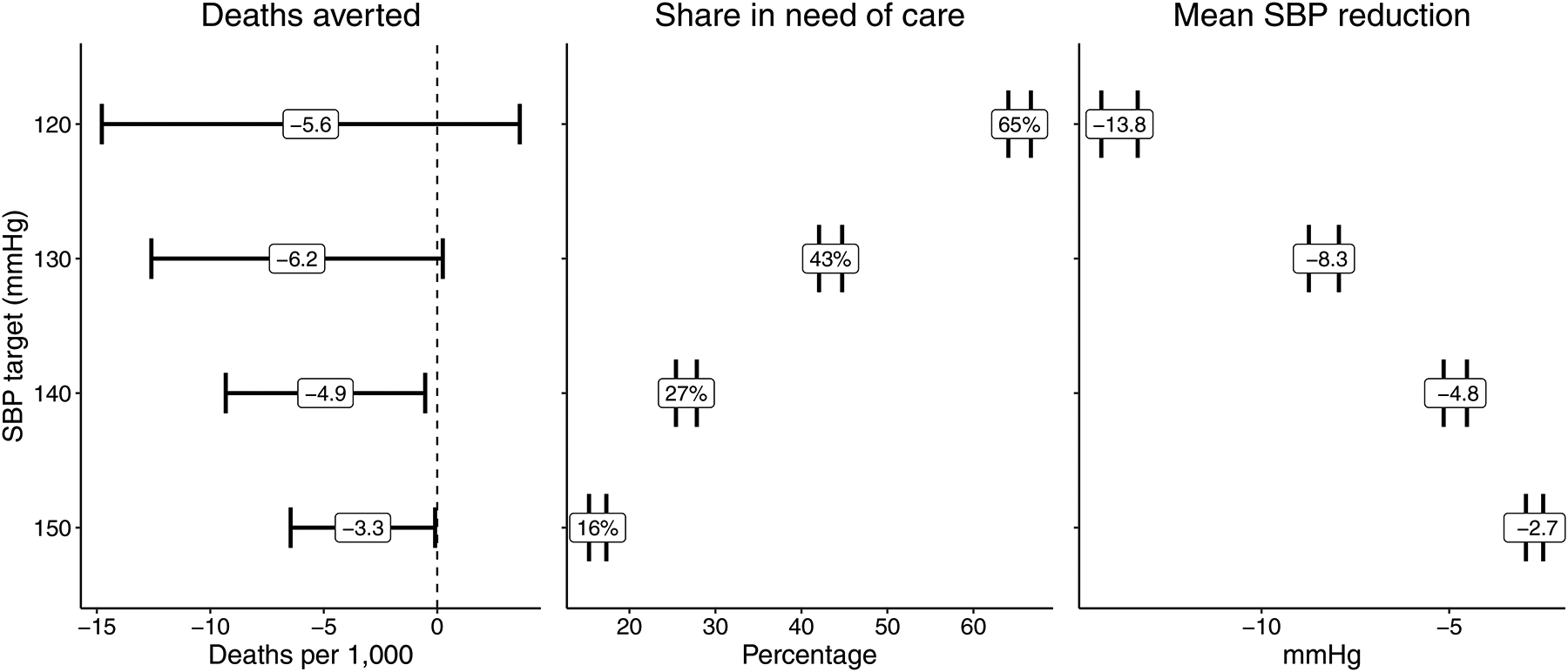
Estimated mortality averted per 1,000, population coverage, and mean SBP reduction for different systolic blood pressure (SBP) reduction scenarios.
aError bars represent 95% confidence intervals.
bThe results are adjusted for age, sex, race, marital status, formal school, geography type, province, self-reported health status, current smoking, exercise, alcohol use and weight (BMI), and are weighted by the NIDS design weights to adjust for the survey sampling procedures and household non-responses in the baseline survey wave.
Deaths averted per 1,000 population was similar between men and women and across geography types for all scenarios (Table 2). However, there were important differences across racial groups. Among all scenarios, individuals in the Coloured group had the highest deaths averted per 1,000 population followed by the African, White, and Asian/Indian groups, respectively. For example, the 150 mmHg scenario was associated with 4.9 deaths averted (95% CI: −9.7, −0.1, p = 0.046) in the Coloured group, 3.2 deaths averted (95% CI: −6.4, −0.1, p = 0.046) in the African group, 2.3 deaths averted (95% CI: −4.8, 0.2, p = 0.073) in the White group, and 1.4 deaths averted (95% CI: −3.3, 0.6, p = 0.17) in the Asian/Indian group.
Table 2.
Estimated deaths averted per 1,000 for different SBP reduction scenarios by sex, race, and residence type.
| SBP Reduction Scenarios | ||||
|---|---|---|---|---|
| 120 (mmHg) | 130 (mmHg) | 140 (mmHg) | 150 (mmHg) | |
| Overall | −5.6 (−14.8, 3.6) p = 0.24 |
−6.2 (−12.6, 0.2) p = 0.059 |
−4.9 (−9.3, −0.5) p = 0.028 |
−3.3 (−6.5, −0.1) p = 0.044 |
| Sex | ||||
| Men | −5.6 (−15.9, 4.6) p = 0.28 |
−6.4 (−13.2, 0.5) p = 0.067 |
−5.0 (−9.6, −0.5) p = 0.031 |
−3.4 (−6.6, −0.1) p = 0.043 |
| Women | −5.5 (−14.3, 3.2) p = 0.22 |
−6.1 (−12.3, 0.2) p = 0.057 |
−4.9 (−9.2, −0.5) p = 0.028 |
−3.2 (−6.4, 0.0) p = 0.047 |
| Race | ||||
| African | −5.4 (−14.5, 3.6) p = 0.24 |
−6.0 (−12.3, 0.2) p = 0.060 |
−4.8 (−9.1, −0.5) p = 0.029 |
−3.2 (−6.4, −0.1) p = 0.046 |
| Coloured | −8.1 (−20.8, 4.5) p = 0.21 |
−8.9 (−18.1, 0.3) p = 0.057 |
−7.2 (−13.7, −0.7) p = 0.030 |
−4.9 (−9.7, −0.1) p = 0.046 |
| Asian/Indian | −3.6 (−13.3, 6.0) p = 0.46 |
−4.2 (−11.1, 2.7) p = 0.24 |
−3.0 (−7.4, 1.4) p = 0.18 |
−1.4 (−3.3, 0.6) p = 0.17 |
| White | −4.2 (−12.3, 3.9) p = 0.31 |
−4.7 (−10.4, 0.9) p = 0.098 |
−3.7 (−7.4, 0.1) p = 0.054 |
−2.3 (−4.8, 0.2) p = 0.073 |
| Geography type | ||||
| Urban | −5.2 (−13.3, 2.9) p = 0.21 |
−5.7 (−11.5, 0.0) p = 0.049 |
−4.7 (−8.8, −0.5) p = 0.028 |
−3.2 (−6.5, 0.0) p = 0.052 |
| Traditional | −6.1 (−16.9, 4.7) p = 0.27 |
−6.8 (−14.3, 0.7) p = 0.075 |
−5.3 (−10.2, −0.5) p = 0.032 |
−3.4 (−6.7, −0.2) p = 0.040 |
| Farms | −5.2 (−14.7, 4.3) p = 0.29 |
−5.8 (−12.5, 0.8) p = 0.085 |
−4.6 (−9.2, 0.0) p = 0.051 |
−2.9 (−6.2, 0.4) p = 0.084 |
Estimates are deaths averted per 1,000 population with 95% confidence intervals in parentheses.
Results are adjusted for age, sex, race, marital status, formal school, residence type, province, self-reported health status, current smoking, exercise, alcohol use and weight (BMI).
In appendix p.10–11, we present the main results when imputing the missing data using multiple imputation with chained equations. These results should be cautiously interpreted since they assume that data are missing at random conditional on the observed covariates (known as “missing at random”).
Discussion
We found a weak and nonlinear SBP-mortality relationship in South Africa that resulted in larger incremental mortality benefits at higher SBP values. We found the greatest benefits associated with reducing SBP down to 140–150 mmHg, with small incremental benefits of reducing SBP below 140 mmHg, and virtually no evidence of an incremental benefit from further reductions to 120 mmHg. At the population level, scaling up BP management to achieve a 150 mmHg target was far more efficient in terms of the number needed to treat to avert one death compared to strategies to achieve lower population SBP values. This was because strategies to reduce SBP to lower values would require providing management to a far greater share of the adult population at SBP ranges where reductions in SBP were only associated with small incremental mortality benefits. Beyond efficiency, higher targets may also be more realistic to accomplish since they would require substantially fewer health services and personnel to meet.10 While our findings of limited evidence in support of reducing SBP below 140 mmHg are in line with the WHO HEARTS and South African Society of Hypertension guidelines, the guideline recommendations were not based on actual longitudinal data from the country.18,19 To our knowledge, our findings thus provide some of the first population-based longitudinal evidence in support of these SBP targets.
Our results contrast to the continuous linear relationship between SBP and mortality starting from as low as 90 mmHg observed in HICs and some major LMICs such as China, India, and Indonesia.6,23–25 This difference may be explained by competing causes of death from tuberculosis (TB) and HIV. TB and HIV accounted for 12% of deaths in 2015–2017, with the greatest mortality burden among adults between 30–55 years old,26 who constitute the majority of our study population and generally have SBP values <150 mmHg. These prominent competing causes of mortality may thus explain the weaker SBP-mortality relationship in our study. One important question is whether the results here apply to other Sub-Saharan African countries. If the weaker relationship is indeed due to competing causes of death, our results may apply to Southern and Eastern African countries with similar health profiles (e.g., high TB and HIV mortality) as South Africa, but potentially not in Western African countries where TB and HIV mortality is much lower.27
This study has several key limitations. First, since we used an observational study design, our results are subject to bias from confounding, though the direction of bias is unclear. While clinical trials generally produce unbiased estimates for their target population, and these estimates could be transported from HICs to South Africa as is commonly done in modeling studies,8 it is unclear whether bias resulting from extrapolating clinical trial results from HIC to LMIC populations is greater or less than confounding bias from observational data.28 Therefore, our findings are not a replacement for modeling studies but should be considered in conjunction with other approaches. Second, while in clinical practice BP management decisions are based on BP measured on at least two consecutive healthcare visits approximately two weeks apart, our results are based on averaging two SBP measurements taken approximately two years apart. Third, although the survey aimed to collect nationally representative data, we exclude age-eligible adults who were missing BP or confounder values (32.6%) or missing vital status information (11.1% lost to follow-up), which may result in selection bias. Compared to participants included in our study (appendix p.1), those lost to follow-up were more likely to be white (19.8% versus 3.5%), reside in urban areas (66.6% versus 47.0%), and report excellent health (34.3% versus 26.5%). Therefore, our results may not represent the overall South African population and are best interpreted as being more relevant for non-White individuals in peri-urban and rural areas. However, it is ambiguous how non-response might affect the results. White individuals, those living in urban areas, and those who report better health may have a stronger SBP-mortality relationship between BP and mortality due to fewer competing causes of mortality; on other hand, the SBP-mortality relationship between SBP and mortality might be weaker because they have greater access to health services in the event of BP-related endpoints. Fourth, measurement error may have contributed to the attenuated SBP-mortality relationship.20 Although we use a two-year average of SBP measurements to reduce dilution bias, our approach may not have fully accounted for this source of error. A recently developed approach using random effects to represent the variation in BP measurements is promising for reducing dilution bias.29 However, we did not implement this method here because it is not yet clear how to combine it with the parametric g-formula. Fifth, our approach assumes that adjusted comparisons of individuals at different levels of SBP are representative of the effect of lowering SBP between two different levels through lifestyle changes or medicines. However, large meta-analyses find a strong concordance between clinical- trial effects of BP reductions and the difference in mortality between individuals at different SBP levels in prospective studies.30 Lastly, our approach estimates the average six-year mortality reduction associated with SBP changes and does not speak to mortality beyond six years. We also did not explore potential heterogeneities in the relationship, such as by body mass index or cardiovascular disease risk, since our data were not sufficiently powered to detect such differences. Identifying such treatment heterogeneities is an important future area of work for further improving the effectiveness of BP reduction efforts.
Despite these limitations, our study has important strengths. We use the only source of country-wide population-based longitudinal data from South Africa to directly estimate the relationship between SBP and mortality. As a result, our estimates may more closely represent the effects of a real population scale up of BP management compared to studies based on small samples, clinics, or transported from other contexts. Ultimately, our results add to a growing body of evidence that reveals that transporting estimates from one context to another—such as from HICs to South Africa—can lead to misleading results.28 This is an especially important consideration for other SSA countries seeking to formulate and introduce BP-lowering policies.
Supplementary Material
Research in context:
Evidence before this study:
We conducted a search of PubMed using keywords related to blood pressure, hypertension, mortality, and survival with no date restrictions. We selected studies that were set in sub-Saharan Africa, had mortality as a main outcome, and blood pressure or hypertension as a main exposure. The majority of studies sought to estimate the total mortality burden attributable to hypertension—using a conventional definition of hypertension (blood pressure ≥ 140/90 mmHg)—through modeling studies that drew estimates of the relative risk of hypertension-related mortality from the Global Burden of Disease project or from clinical trials of hypertension treatment in high-income countries. These studies generally found a large and increasing burden of hypertension in Sub-Saharan Africa. A small number of studies were conducted among small hospital samples similarly finding that hypertension is an important predictor of mortality. None of the studies were based on population-representative longitudinal data from a Sub-Saharan African country and none examined the shape of the relationship between blood pressure and mortality and implications of this shape for hypertension management targets.
Added value of this study:
To our knowledge, we provide the first direct, population-based, estimates of the relationship between systolic blood pressure and mortality and the comparative effectiveness of reducing SBP to different management targets in South Africa. Our estimates are based on the only source of country-wide longitudinal data with measured blood pressure and mortality follow-up set in a sub-Saharan African country.
Implications of all the available evidence:
The association between SBP and six-year mortality is weak in South Africa compared to high-income and many low- and middle-income countries, possibly due to high competing risks from other causes of mortality. As such, among South African adults, reducing SBP to 150 mmHg is associated with the largest incremental mortality benefits with little evidence in support of SBP targets below 140 mmHg. Similarly, scaling up BP management across the population based on a target of 150 mmHg is far more efficient in terms of the number needed to treat to avert one death compared to strategies to reduce the population mean to lower SBP values. These results can inform hypertension guideline recommendations for what target value SBP should be reduced to.
Acknowledgements
NS is supported by the Alexander von Humboldt Foundation.
Declaration of interest
MKA reports a grant from Merck to his institution, outside the submitted work. All other authors declare no competing interests.
Footnotes
Data sharing statement
All study data and related documents are publicly available at http://www.nids.uct.ac.za.
Contributor Information
Alpha Oumar Diallo, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Mohammed K. Ali, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA Department of Family and Preventive Medicine, School of Medicine, Emory University, Atlanta, GA, USA.
Pascal Geldsetzer, Division of Primary Care and Population Health, Department of Medicine, Stanford University, Stanford, CA, USA; Heidelberg Institute of Global Health, Heidelberg University, Heidelberg, Germany.
Emily W. Gower, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Ophthalmology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Trasias Mukama, Heidelberg Institute of Global Health, Heidelberg University, Heidelberg, Germany; Department of Disease Control and Environmental Health, Makerere University School of Public Health, Kampala, Uganda; Division of Preventive Oncology, German Cancer Research Center, Heidelberg, Germany.
Ryan G. Wagner, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Justine Davies, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Institute of Applied Health Research, University of Birmingham, Birmingham, UK; Centre for Global Surgery, Department of Global Health, Stellenbosch University, Cape Town, South Africa.
Maarten J. Bijlsma, Max Planck Institute for Demographic Research, Rostock, Germany
Nikkil Sudharsanan, Heidelberg Institute of Global Health, Heidelberg University, Heidelberg, Germany.
References
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geldsetzer P, Manne-Goehler J, Marcus M-E, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. Lancet. 2019;394(10199):652–662. doi: 10.1016/S0140-6736(19)30955-9 [DOI] [PubMed] [Google Scholar]
- 3.Berry KM, Parker W-A, Mchiza ZJ, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017;2(3):e000348. doi: 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cois A, Ehrlich R. Antihypertensive treatment and blood pressure trends among South African adults: A repeated cross-sectional analysis of a population panel survey. PLoS One. 2018;13(8):e0200606. doi: 10.1371/journal.pone.0200606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakaran D, Anand S, Gaziano TA, Mbanya J-C, Wu Y, Nugent R. Disease Control Priorities: Cardiovascular, Respiratory, and Related Disorders. Vol 5. Third. Washington, DC: World Bank; 2017. [PubMed] [Google Scholar]
- 6.Sudharsanan N Population-level mortality benefits of improved blood pressure control in Indonesia: a modelling study. Int J Epidemiol. November 2018. doi: 10.1093/ije/dyy232 [DOI] [PubMed] [Google Scholar]
- 7.Republic of South Africa Department of Health. Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2013–2017. Republic of South Africa Department of Health; 2017. [Google Scholar]
- 8.Basu S, Wagner RG, Sewpaul R, Reddy P, Davies J. Implications of scaling up cardiovascular disease treatment in South Africa: a microsimulation and cost-effectiveness analysis. Lancet Glob Health. 2019;7(2):e270–e280. doi: 10.1016/S2214-109X(18)30450-9 [DOI] [PubMed] [Google Scholar]
- 9.Matsoso MP, Hunter JR, Brijlal V. Embedding quality at the core of universal health coverage in South Africa. Lancet Glob Health. 2018;6(11):e1153–e1154. doi: 10.1016/S2214-109X(18)30323-1 [DOI] [PubMed] [Google Scholar]
- 10.Rayner B, Jones E, Veriava Y, Seedat YK. South African Hypertension Society commentary on the American College of Cardiology/American Heart Association hypertension guidelines. Cardiovasc J Afr. 2019;30(3):184–187. doi: 10.5830/CVJA-2019-025 [DOI] [PubMed] [Google Scholar]
- 11.Woolard I, Leibbrandt M, De Villiers L. The South African National Income Dynamics Study: Design and methodological issues. J Stud Econ Econometric. 2010;34(3):7–24. [Google Scholar]
- 12.Branson N, Wittenberg M. NIDS Technical Paper 9: Longitudinal and Cross-Sectional Weights in the NIDS Data 1–5. Cape Town: SALDRU, UCT; 2019. [Google Scholar]
- 13.Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453–461. [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2004. [PubMed] [Google Scholar]
- 15.Hinton TC, Adams ZH, Baker RP, et al. Investigation and treatment of high blood pressure in young people: too much medicine or appropriate risk reduction? Hypertension. 2020;75(1):16–22. doi: 10.1161/HYPERTENSIONAHA.119.13820 [DOI] [PubMed] [Google Scholar]
- 16.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 17.Elliott W Circadian variation in blood pressureImplications for the elderly patient. Am J Hypertens. 1999;12(2):43S–49S. doi: 10.1016/S0895-7061(98)00279-9 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Hearts: Technical package for cardiovascular disease management in primary’ ‘ health care. Geneva: World Health Organization; 2016. [Google Scholar]
- 19.Hypertension guideline working group, Seedat YK, Rayner BL, Veriava Y. South African hypertension practice guideline 2014. Cardiovasc J Afr. 2014;25(6):288–294. doi: 10.5830/CVJA-2014-062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore MN, Atkins ER, Salam A, et al. Regression to the mean of repeated ambulatory blood pressure monitoring in five studies. J Hypertens. 2019;37(1):24–29. doi: 10.1097/HJH.0000000000001977 [DOI] [PubMed] [Google Scholar]
- 21.Sudharsanan N, Ho JY. Rural-Urban Differences in Adult Life Expectancy in Indonesia: A Parametric g-formula-based Decomposition Approach. Epidemiology. 2020;31(3):393–401. doi: 10.1097/EDE.0000000000001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijlsma MJ, Wilson B, Tarkiainen L, Myrskylä M, Martikainen P. The Impact of Unemployment on Antidepressant Purchasing: Adjusting for Unobserved Time-constant Confounding in the g-Formula. Epidemiology. 2019;30(3):388–395. doi: 10.1097/EDE.0000000000000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SPRINT Research Group, Wright JT, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajalakshmi V, Lacey B, Kanimozhi V, Sherliker P, Peto R, Lewington S. Body-mass index, blood pressure, and cause-specific mortality in India: a prospective cohort study of 500 810 adults. Lancet Glob Health. 2018;6(7):e787–e794. doi: 10.1016/S2214-109X(18)30267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 26.Statistics South Africa F. Mortality and causes of death in South Africa: Findings from death notification. Statistics South Africa; 2020. http://www.statssa.gov.za/?page_id=1854&PPN=P0309.3&SCH=7914. Accessed August 12, 2020. [Google Scholar]
- 27.Reniers G, Masquelier B, Gerland P. Adult mortality in Africa. In: Rogers RG, Crimmins EM, eds. International handbook of adult mortality. Vol 2. International handbooks of population. Dordrecht: Springer Netherlands; 2011:151–170. doi: 10.1007/978-90-481-9996-9_7 [DOI] [Google Scholar]
- 28.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A Comparison of Agent-Based Models and the Parametric G-Formula for Causal Inference. Am J Epidemiol. 2017;186(2):131–142. doi: 10.1093/aje/kwx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett JK, Huille R, Parker R, Yano Y, Griswold M. Estimating the association between blood pressure variability and cardiovascular disease: An application using the ARIC Study. Stat Med. 2019;38(10):1855–1868. doi: 10.1002/sim.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


