Abstract
Background
The end-tidal partial pressure of carbon dioxide (PETCO2) can be used to estimate the arterial partial pressure of carbon dioxide (PaCO2) in patients who undergo mechanical ventilation via endotracheal intubation. However, no reliable method for measuring PETCO2 during noninvasive ventilation (NIV) has been established. The purpose of this study was to evaluate the correlation and agreement between PaCO2 and PETCO2 measured by these two methods and to compare them in patients who underwent NIV after extubation.
Methods
This study was a randomized, open-label, crossover trial in a mixed intensive care unit. We included patients who were planned for NIV after extubation and for whom the difference between PETCO2 and PaCO2 was ≤ 5 mmHg. We compared mainstream capnography using an inner cup via face mask (the novel method) with sidestream capnography (the previous method) during NIV. The relationships between PaCO2 and PETCO2 were evaluated by computing the Pearson correlation coefficient, and the agreement between PaCO2 and PETCO2 was estimated using the Bland–Altman method.
Results
From April 2020 to October 2021, 60 patients were included to the study. PaCO2 and PETCO2 were well correlated in both methods (the novel methods: r = 0.92, P < 0.001; the previous method: r = 0.79, P < 0.001). Mean bias between PaCO2 and PETCO2 measured using the novel method was 2.70 (95% confidence interval [CI], 2.15–3.26) mmHg with 95% limits of agreement (LoA) ranging from − 1.61 to 7.02 mmHg, similar to the result of measurement during SBT (mean bias, 2.51; 95% CI, 2.00–3.02; 95% LoA, − 1.45 to 6.47 mmHg). In contrast, measurement using the previous method demonstrated a larger difference (mean bias, 6.22; 95% CI, 5.22–7.23; 95% LoA, − 1.54 to 13.99 mmHg).
Conclusion
The current study demonstrated that the novel PETCO2 measurement was superior to the previous method for PaCO2 prediction. During NIV, the novel method may collect as sufficient exhalation sample as during intubation. Continuous PETCO2 measurement combined with peripheral oxygen saturation monitoring is expected to be useful for early recognition of respiratory failure among high-risk patients after extubation.
Trial registration UMIN-CTR UMIN000039459. Registered February 11, 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-022-00603-w.
Keywords: Blood gas analysis, End-tidal partial pressure of carbon dioxide, Mainstream capnography, Noninvasive ventilation, Partial pressure of carbon dioxide, Post-extubation
Background
Among patients who undergo planned extubation, approximately 10%–20% are reintubated within 72 h, and most of them within 48 h [1–4]. Compared with successfully extubated patients, patients who are reintubated because of post-extubation respiratory failure might be at risk of worsening organ function [5]. Furthermore, reintubation is associated with a longer duration of stay in the intensive care unit (ICU) and hospital [6]. Noninvasive ventilation (NIV) is recommended to prevent post-extubation respiratory failure in high-risk patients [7]. In patients with acute respiratory failure who undergo NIV, delayed intubation increases mortality [8–10]. Therefore, careful respiratory monitoring is required to prevent delayed intubation.
The arterial partial pressure of carbon dioxide (PaCO2) should be maintained within an appropriate range during mechanical ventilation. PaCO2 measurements require arterial blood gas samples and are provided as intermittent information. The end-tidal partial pressure of carbon dioxide (PETCO2), which is a continuous monitoring method, can be used to estimate PaCO2 in patients who undergo mechanical ventilation via endotracheal intubation. However, no reliable method for measuring PETCO2 during NIV has been established. Although sidestream PETCO2 measurements for NIV patients are moderately correlated with PaCO2, the 95% limits of agreement (LoA) are too large to be used in clinical settings [11, 12]. A possible explanation for the poor agreement between PaCO2 and PETCO2 is the difficulty in collecting sufficient exhalation during NIV because of intentional leakage meant to avoid rebreathing. The cap-ONE mask set ® (Nihon Kohden, Tokyo, Japan) is a unique interface for collecting exhalation with inner cups via face mask and measure using mainstream techniques, and is expected to predict the level of PaCO2 more accurately. However, this novel method has not yet been evaluated in clinical settings.
We hypothesized that this novel technique would be more accurate than the previous method. The purpose of this study was to evaluate the correlation and agreement between PaCO2 and PETCO2 measured by these two methods and to compare them in patients who underwent NIV after extubation.
Methods
Trial design and setting
This study was a randomized, open-label, crossover trial conducted in a mixed ICU at the JA Hiroshima General Hospital. The study protocol was approved by the ethics committee of the JA Hiroshima General Hospital. This study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki [13] and was registered at the UMIN Clinical Trials Registry on February 11, 2020 (UMIN 000039459), and reported in accordance with the CONSORT statement [14]. Written informed consent was obtained from all the patients or their relatives.
Participants
Patients receiving mechanical ventilation, who were considered at a high risk of post-extubation respiratory failure and planned for NIV after extubation, were screened. We included patients if the difference between PETCO2 and PaCO2 was ≤ 5 mmHg during the spontaneous breathing trial (SBT) and if an arterial line was placed. Patients with GCS ≤ 8, inability to protect the airway, hemodynamic instability, severe hypoxemia, agitation, NIV intolerance, chronic obstructive pulmonary disease, diagnosed pulmonary embolism or suspected, severe anemia (Hb < 7.0 g/dL), and arterial blood gas sample not collected were excluded. Moreover, patients whose cases were judged too difficult to include for analyses by a physician and those who refused consent were excluded.
Patients were considered at a high risk of post-extubation respiratory failure based on the criteria from a previous study (Additional file 1: Appendix S1) [15]. Briefly, as follows: age > 65 years; heart failure as the primary indication for mechanical ventilation; high severity score; obese; weaning process > 24 h (difficult or prolonged weaning, Additional file 1: Appendix S2) [16], 2 or more comorbidities (Additional file 1: Appendix S3), and mechanical ventilation for more than 7 days. All SBTs were performed at the lowest level of positive end-expiratory pressure (PEEP) and pressure support (PS) set at 5 cm H2O for 30–60 min. Considering these risks before extubation, the decision to perform NIV was made by the treating physicians.
Randomization
Enrolled patients were randomized in a 1:1 ratio to receive either the previous method or the novel method as the first measurement. Randomization was performed using a computer-generated randomization table (www.randomization.com). Allocation results were placed into numbered sealed opaque envelopes containing monitoring allocations. Once the patient provided written informed consent, the clinicians participating in the study opened the envelopes in order.
PETCO2 monitoring methods
We compared the following two methods of PETCO2 monitoring (the previous method and the novel method) in the included patients during NIV. After collecting the arterial blood gas sample, we switched to another method. The highest PETCO2 value within one minute of collection of the blood gas sample was recorded. For the primary outcome, we assessed the correlations and agreements between the PETCO2 and PaCO2 measurements performed by both methods.
Previous method: sidestream monitoring using nasal prong and oral scoop
The Smart Capnoline ® Plus (Oridion Medical 1987 Ltd., Jerusalem, Israel) is a nasal prong and oral scoop for use in non-intubated patients with the dual purpose of delivering oxygen and collecting exhalation from both the nose and mouth (Additional file 1: Appendix S4). The length of the cannula was approximately 255 cm, and the delay in CO2 measurement was approximately 240 ms. The patients were fitted with a face mask over the nasal prong.
Novel method: mainstream monitoring using the NPPV cap ONE mask ®
The cap-ONE mask set ® (Nihon Kohden Tokyo, Japan) is a unique interface for collecting exhaled air samples using an inner cup in a face mask and assessing them using the mainstream techniques. The inner cup in the face mask was placed under the patient’s nose and over the mouth to guide the patient’s exhaled flow into the CO2 measurement cell (Fig. 1). The CO2 measurement cell was connected to the inner cup of the NPPV cap-ONE mask ®. The mainstream capnometer was designed to be placed on the CO2 measurement cell outside the mask. The capnometer was calibrated before each application of NIV.
Fig. 1.
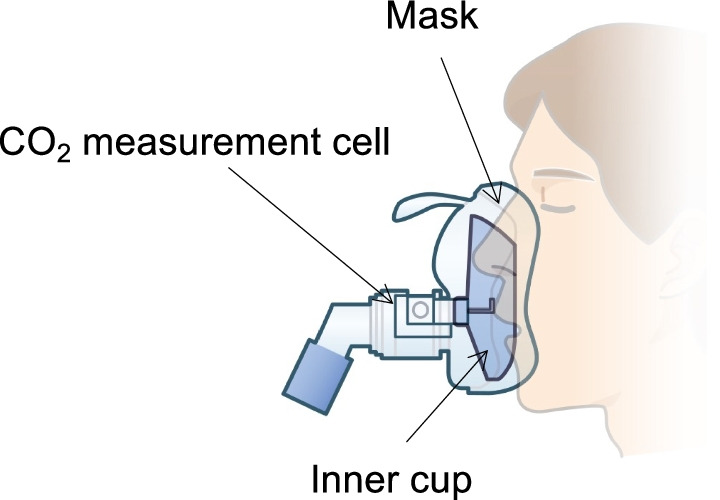
Mainstream monitoring using the novel method. An illustration of the NPPV cap ONE mask ® used in this study. The capnometer was calibrated in terms of the PETCO2 reading before each use of the NKV 330 ventilator (Nihon Kohden Tokyo, Japan). In addition, the mainstream PETCO2 sensor has a special anti-fog film on the specimen window, which guarantees accurate measurements for 72 h
NIV for prevention of post-extubation respiratory failure
NIV was performed using the ventilator NKV 330 (Nihon Kohden Tokyo, Japan) and a face mask of the same size during both measurement periods. The NIV mode, setting, and duration were determined by treating physicians according to the following principles. It was recommended that the same mode and setting be maintained until the second measurement was completed, but they could be changed if necessary. NIV was continuously delivered immediately after extubation for a scheduled period to the next morning. NIV was interrupted once the patients were stable with oxygen administered via a mask or nasal cannula.
Data collection
The following patient characteristics were recorded at admission: reason for ICU admission, age, sex, severity of illness (Acute Physiology and Chronic Health Evaluation II [APACHE II] score [17], Sequential Organ Failure Assessment [SOFA] score) [18], updated Charlson comorbidity index (CCI) [19], and nasal gastric tube placement. The following information was also recorded: NIV parameters (for example, mode, settings, tidal volume, minute ventilation, leakage), respiratory rate, blood pressure, heart rate, and peripheral oxygen saturation during each monitoring method. We performed blood gas analysis 30–60 min after each monitoring method session.
Statistical analysis
We estimated the required sample size based on the correlation between PETCO2 and PaCO2 values measured in previous studies conducted in non-intubated patients [12, 20–22]. A sample size of 60 measurements was required to achieve 90% power for detecting an effect size of 0.41 with α set at 0.05.
Data are expressed as mean with standard deviation (SD), medians with interquartile ranges (IQR), or numbers with corresponding percentages, as appropriate. Continuous variables were compared using the paired t-test or Wilcoxon signed-rank test, according to the data distribution. Dichotomous variables were analyzed using the Chi-square test or Fisher’s exact test. The relationships between PaCO2 and PETCO2 were evaluated by computing the Pearson correlation coefficient, and the agreement between PaCO2 and PETCO2 was estimated using the Bland–Altman method, in which bias was the mean difference between PaCO2 and PETCO2, and the upper and lower LoA were the mean of the differences ± 1.96 SDs above and below the mean difference. Precision (the ability to reproduce the same measurement) was assessed based on the [bias—SD; bias + SD] interval, where SD is the SD of the distribution of the differences. Clinically unacceptable values were arbitrarily defined as values > 5 mmHg. In addition, we performed post hoc analyses to explore the source of the difference between PaCO2 and PETCO2. The correlations and agreement between the PETCO2 and PaCO2 measurements were evaluated in patients with small (≤ 40 L/min) and large (> 40 L/min) amounts of leakage. Furthermore, relationships between the difference and the following factors were evaluated using the Pearson correlation coefficient: the amount of leakage, tidal volume, respiratory rate, and minute ventilation. All statistical tests were two-sided, and a p-value < 0.05, indicating statistical significance. Statistical analyses were performed using Stata 15.1 (StataCorp LLC, College Station, TX, USA). The batplot command in Stata was used for the Bland–Altman analysis.
Results
From April 2020 to October 2021, 326 adult patients were mechanically ventilated in the ICU. Of these patients, 93 patients who received NIV to prevent post-extubation respiratory failure were screened, and 60 patients were included in this analysis after inclusion and exclusion criteria were applied (Fig. 2). No patients were lost to follow-up during the study period.
Fig. 2.
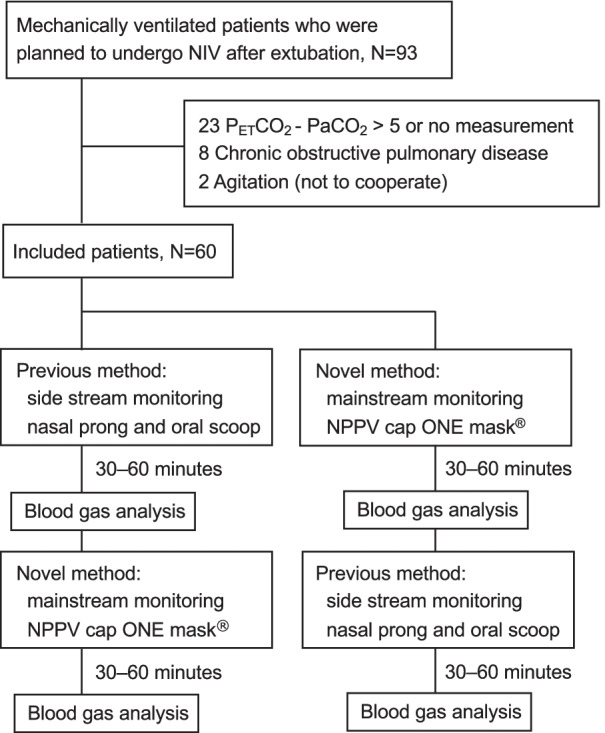
Patients flow diagram. PETCO2, end-tidal partial pressure of carbon dioxide; NIV, noninvasive ventilation; PaCO2, arterial partial pressure of carbon dioxide
Patient demographics and clinical characteristics are presented in Table 1 and Additional file 1: Table S1. Of the 60 patients, 37 (61.7%) were male, and the majority of patients were surgical patients (39 patients, 65.0%). The median (IQR) APACHE II score, SOFA score and updated CCI were 19 (range, 14–25), 8 (range, 7–11.5), and 3 (range, 2–4), respectively. The major comorbidities that the patient had were as follows: congestive heart failure (40 patients, 66.7%), renal disease (35 patients, 58.3%), diabetes with chronic complications (28 patients, 46.7%), and chronic respiratory failure (8 patients, 1.3%). The median duration of mechanical ventilation was 2 days (range, 2–4), and most patients were classified as having short weaning. Although all patients received NIV until the measurements using both monitoring methods were completed, three patients (5.0%) were reintubated during the ICU stay.
Table 1.
Patient characteristics
| Included patients N = 60 |
|
|---|---|
| Age, mean (SD), years | 70.7 (11.2) |
| Male, n (%) | 37 (61.7) |
| Body mass index, mean (SD), kg/m2 | 24.9 (4.0) |
| Patient category | |
| Non-scheduled surgery, n (%) | 16 (26.7) |
| Scheduled surgery, n (%) | 23 (38.3) |
| Medical, n (%) | 21 (35.0) |
| APACHE II score, median (IQR), points* | 19 (14–25) |
| SOFA score, median (IQR), points* | 8 (7–11.5) |
| Updated Charlson comorbidity index, median (IQR), points | 3 (2–4) |
| Chronic heart failure, n (%) | 40 (66.7) |
| Chronic respiratory failure, n (%) | 8 (1.3) |
| Renal disease, n (%) | 35 (58.3) |
| Mechanical ventilation duration, days† | 2 (2–4) |
| Weaning category‡ | |
| Short weaning, n (%) | 50 (83.3) |
| Difficult weaning, n (%) | 10 (16.7) |
| Prolonged weaning, n (%) | 0 (0) |
| Glasgow Coma Scale, median (IQR), points† | 14 (13–15) |
| Vasoactive drugs use, n (%)† | 32 (53.3) |
| Nasal gastric tube placement†, n (%) | 40 (66.7) |
| Reintubation within ICU stay, n (%) | 3 (5.0) |
| Length of ICU stay, days | 4 (3–8) |
| Length of hospitalization§, days | 33 (23.5–41) |
| ICU mortality, n (%) | 0 (0) |
| Hospital mortality, n (%) | 10 (16.7) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, Intensive Care Unit; SOFA, Sequential Organ Failure Assessment
*At ICU admission
†At the day of extubation
‡Defined by the WIND criteria
§Excluded 10 patients who died
The NIV settings and respiratory data are shown in Table 2. Although continuous positive airway pressure (CPAP) mode was used in most patients, only one patient underwent NIV in different modes in each section. The levels of PEEP and PS were 4 (range, 4–4) and 4 (range, 2–4) cmH2O, respectively. Although most of the respiratory statuses were not different between the two measurement periods, total leakage was smaller (35.0 [30.8–40.0] vs. 44.0 [38.3–52.2], P < 0.001), and the level of PETCO2 was higher (35.5 [32–40] vs. 33 [27–36], P < 0.001) in patients using the novel method than those using the previous method, despite similar PaCO2 values.
Table 2.
Ventilator settings, physiological data, and blood gas analysis in each section
| During SBT | Noninvasive ventilation | P value* | ||
|---|---|---|---|---|
| Previous method | Novel method | |||
| Ventilation mode | 1.000 | |||
| PSV, n (%) | NA | 50 (83.3) | 51 (85.0) | |
| CPAP, n (%) | NA | 10 (16.7) | 9 (15.0) | |
| FIO2, median (IQR) | 0.3 (0.3–0.4) | 0.3 (0.3–0.4) | 0.3 (0.3–0.4) | 1.000 |
| PEEP, median (IQR), cmH2O | 5 (5–5) | 4 (4–4) | 4 (4–4) | 1.000 |
| PS, median (IQR), cmH2O† | 5 (5–5) | 4 (2–4) | 4 (2–4) | 1.000 |
| Heart rate, mean (SD), bpm | 85.0 (15.5) | 85.0 (14.7) | 85.5 (15.1) | 0.351 |
| Mean blood pressure, mean (SD), mmHg | 81.2 (11.6) | 82.1 (11.6) | 80.5 (11.6) | 0.063 |
| Respiratory Rate, mean (SD), breaths /min | 15.9 (4.9) | 18.2 (5.8) | 19.1 (6.1) | 0.108 |
| Tidal Volume, mean (SD), mL | 528 (157) | 469 (130) | 474 (122) | 0.692 |
| Minute ventilation, mean (SD), L/min | 7.9 (2.3) | 8.1 (3.2) | 8.6 (3.3) | 0.084 |
| RSBI, mean (SD) | 33.6 (16.1) | 43.1 (20.4) | 42.9 (17.0) | 0.939 |
| Total leakage, mean (SD), L/min | NA | 45.1 (11.5) | 36.1 (8.3) | < 0.001 |
| PaO2, median (IQR), mmHg | 87.3 (77.5–100.0) | 86.2 (76.0–100.5) | 89.6 (75.9–97.9) | 0.793 |
| P/F ratio, median (IQR) | 290 (214–364) | 271 (216–335) | 280 (211–342) | 0.601 |
| PaCO2, mean (SD), mmHg | 37.6 (5.7) | 38.2 (5.5) | 38.4 (5.4) | 0.286 |
| PETCO2, mean (SD), mmHg | 35.1 (5.8) | 32.0 (6.3) | 35.7 (5.5) | < 0.001 |
| pH, mean (SD) | 7.43 (0.05) | 7.42 (0.05) | 7.42 (0.05) | 0.610 |
CPAP, continuous positive airway pressure; FIO2, fraction of inspiratory oxygen; NA, not applicable; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PEEP, positive end expiratory pressure; PETCO2, end-tidal partial pressure of carbon dioxide; P/F ratio, ratio of arterial oxygen partial pressure to fractional inspired oxygen; PS, pressure support; PSV, pressure support ventilation; RSBI, rapid shallow breathing index; SBT, spontaneous breathing trial
*Compared measurements in patients using the novel method with the previous method
†Among patients who underwent PSV during noninvasive ventilation
Comparison between PaCO2 and PETCO2 measurements
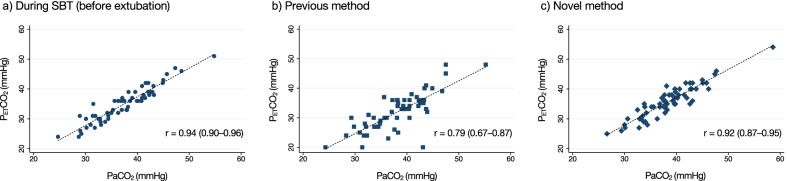
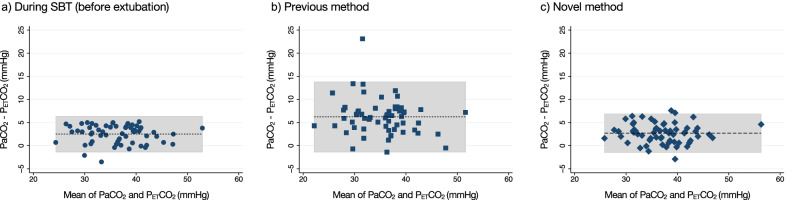
PaCO2 and PETCO2 were well correlated in both methods (novel methods: r = 0.92, P < 0.001; previous method: r = 0.79, P < 0.001, Fig. 3). The results of the Bland–Altman analyses are shown in Fig. 4 and Table 3. Mean bias between PaCO2 and PETCO2 measured using the novel method was 2.70 (95% CI, 2.15–3.26) mmHg with 95% LoA ranging from − 1.61 to 7.02 mmHg, similar to the result of measurement during SBT (mean bias, 2.51; 95% CI, 2.00–3.02; 95% LoA, − 1.45 to 6.47 mmHg). In contrast, measurement using the previous method demonstrated a larger difference (mean bias, 6.22; 95% CI, 5.22–7.23; 95% LoA, − 1.54 to 13.99 mmHg). The number of patients with ≤ 5 mmHg difference between PaCO2 and PETCO2 was 52 (86.7%) using the novel method and 22 (36.7%) using the previous method.
Fig. 3.
Correlations between PaCO2 and PETCO2. a) During SBT (before extubation); b) the previous method; c) the novel method. There was a significant positive correlation between PaCO2 and PETCO2 in all methods (during SBT r = 0.94 [95% CI, 0.90–0.96], P < 0.001; the previous method, r = 0.79 [95% CI, 0.67–0.87], P < 0.001; the novel method, r = 0.92 [95% CI, 0.87–0.95], P < 0.001). Abbreviations: CI, confidence interval; PETCO2, end-tidal partial pressure of carbon dioxide; PaCO2, arterial partial pressure of carbon dioxide; SBT, spontaneous breathing trial
Fig. 4.
Bland–Altman plot of agreements between PaCO2 and PETCO2. a During SBT (before extubation); b the previous method; c, the novel method. In each plot, bias is represented by the dashed line. The limits of agreement are represented by the gray zone. a, Bland–Altman analysis comparing PaCO2 and PETCO2 for intubated patients during SBT. b, Bland–Altman analysis comparing PaCO2 and PETCO2 for those who underwent noninvasive ventilation using the previous method (22 [36.7%] of these pairs had PETCO2 values within 5 mm Hg of paired PaCO2). c), Bland–Altman analysis comparing PaCO2 and PETCO2 for those who underwent noninvasive ventilation using the novel method (52 [86.7%] of these pairs had PETCO2 values within 5 mm Hg of paired PaCO2). PETCO2, end-tidal partial pressure of carbon dioxide; PaCO2, arterial partial pressure of carbon dioxide; SBT, spontaneous breathing trial
Table 3.
Results of Bland–Altman analysis of agreements between PaCO2 and PETCO2
| Mean bias (95% CI) | 95% Limits of agreement | |
|---|---|---|
| During SBT, mmHg | 2.51 (2.00–3.02) | − 1.45 to 6.47 |
| Previous method, mmHg | 6.22 (5.22–7.23) | − 1.54 to 13.99 |
| Novel method, mmHg | 2.70 (2.15–3.26) | − 1.61 to 7.02 |
CI, confidence interval; PaCO2, arterial partial pressure of carbon dioxide; PETCO2, end-tidal partial pressure of carbon dioxide; SBT, spontaneous breathing trial
Factor associated with the difference between PaCO2 and PETCO2
For post hoc analyses, the correlations and agreement between the PETCO2 and PaCO2 measurements were similar among subgroup patients stratified by the amount of leakage (Additional file 1: Figures S1 and S2). We also examined the Pearson correlation coefficient between the difference and respiratory status for each method. However, we found no factors that showed a good correlation with the differences (Additional file 1: Figure S3).
Discussion
Key findings
In the current study, among patients who underwent NIV to prevent post-extubation respiratory failure, both PETCO2 monitoring methods demonstrated a good correlation with PaCO2. Compared with the measurement during SBT, mean bias using the novel method was similar, whereas it was larger in patients using the previous method. Furthermore, the difference between PaCO2 and PETCO2 in most patients using the novel method was within an acceptable range.
Relationship with previous studies
It has been challenging to estimate PaCO2 using PETCO2 monitoring in patients undergoing NIV. Piquilloud et al. [11] evaluated PETCO2 monitoring with the previous method among patients with hypercapnic respiratory failure, and it was not useful for predicting PaCO2 (mean bias, 14.7; 95% CI, 5.22–7.23; 95% LoA, − 6.6 to 36.1 mmHg). In a similar observational trial among patients with mixed respiratory failure conducted by Nouwen et al. [12], PETCO2 monitoring showed good correlation but poor agreement for PaCO2. In our study, patients were excluded if the difference between PETCO2 and PaCO2 was > 5 mmHg before extubation. Thus, most of the included patients were considered to have few physiological respiratory problems for PETCO2 measurement (e.g., hemodynamic instability, ventilation perfusion mismatch, increased dead space, airflow limitation). The difference using the previous method was smaller compared with their studies, but still out of the acceptable range. The inaccuracy of the previous method might be due to insufficient sample collection, possibly because the sampling devices were small and the gap between the mask and skin created by nasal prong increased leakage. However, we assessed the correlations and agreements according to the amount of leakage via post hoc analysis, since larger amounts of leakage were observed among patients in whom the previous method was used. Our findings imply that the superiority of the novel method is not necessarily only to be ascribed to differences in the amount of leakage.
Mainstream and sidestream PETCO2 measurements were not significantly different in estimating PaCO2 in mechanically ventilated patients [23, 24]. On the other hand, in an observational study evaluating both methods among non-intubated postoperative patients, the mainstream method was slightly more accurate than the sidestream method [21]. According to the results of another study among non-intubated patients in an emergency department, the mainstream method correlated but the sidestream method was poor, although both methods did not show good agreement for PaCO2 [20]. Therefore, the mainstream method was better at predicting the level of PaCO2 than the sidestream method in non-intubated patients because the sidestream method requires the collection of exhaled air samples using a sampling tube and the sampling gas may be diluted with air. In patients with NIV, high airflow, which flushes out air in an interface, may increase the air dilution of the exhalation sample. In our study, the novel method with mainstream capnography showed better correlation and agreement for PaCO2 than the previous method. Mainstream capnography may be more accurate in patients undergoing NIV. Another possible explanation is the difference in the sampling guides. The sampling guide of the previous method might be too small to collect sufficient exhalation. Therefore, it was unclear how much of a difference there would be between the mainstream and sidestream methods if a sufficient exhalation sample were obtained. Further evaluation is needed to clarify the superiority of the mainstream method given the same sampling system. Meanwhile, although an inner cup to collect exhalation samples in the novel method may increase rebreathing, the level of PaCO2 was not different between the two methods.
Significance and implications
NIV is often used to prevent post-extubation respiratory failure and reintubation, which are associated with poor outcomes [5, 6]. Arterial blood gas analysis is recommended to assess patient respiratory status accurately and is evaluated more frequently in severe patients but not in continuous monitoring [26]. PETCO2 monitoring, which has been used in intubated patients, is noninvasive and provides real-time information. Our findings imply that the novel method during NIV can collect enough exhaled samples during intubation. Since delayed intubation increases mortality [8–10], careful observation is needed to avoid intubation delays. Continuous PETCO2 measurement combined with peripheral oxygen saturation monitoring is expected to be useful for the early recognition of respiratory failure and the prevention of delayed reintubation in patients who are at a high risk of post-extubation respiratory failure. Further study is needed to examine whether it improves clinical outcomes.
Strengths and limitations
To the best of our knowledge, no previous research has demonstrated the usefulness of PETCO2 monitoring during NIV. Our findings indicate that the results of previous studies were due to not only physiological issues, but also shortcomings of exhalation sample collection. Furthermore, the novel method using the cap-ONE mask set demonstrated good correlation and agreement with the level of PaCO2 in post-extubation patients with few physiological problems, compared with the previous method. However, this study has several limitations. First, patient respiratory status can affect the difference between PaCO2 and PETCO2, which is expected to be larger with smaller tidal volume, higher respiratory rate, or higher airflow limitation [25]. In our post hoc analysis, none of the evaluated factors were associated with the difference between PaCO2 and PETCO2, possibly because the level of PaCO2 was within normal ranges and respiratory status was stable in most of the included patients. Thus, our findings may have limited generalizability. For future investigation, it will be necessary to validate the novel method in patients with hypercapnic respiratory failure. Second, we measured the total amount of leakage without distinguishing between intentional and unintentional leakage. Unintentional leakage from the gap between the mask and the skin may be more closely associated with the collection of an exhalation sample than intentional leakage is. The relationship between collection of the exhalation sample and the different types of leakage should be investigated in more detail, as it has clinical implications. Third, we performed PETCO2 measurements immediately after extubation. Ventilation and perfusion mismatch commonly increase immediately after extubation because of transient atelectasis. The difference between PaCO2 and PETCO2 could change after extubation. Fourth, NIV indication was decided by the treating physicians, and no patient was intolerant to NIV. Consequently, the face mask could be appropriately fitted to collect exhalation in most patients. This was also a concern for the generalizability. Fifth, the novel method cannot be used with other NIV ventilators. The available opportunities for using this technology may be limited. For further clinical application, it must be made available for use with other NIV ventilators. Finally, the monitoring method could not be blinded, and this may have contributed to performance bias. Although it was not possible to blind data collectors, the highest PETCO2 value within one minute of blood gas evaluation was measured to ensure objectivity.
Conclusion
The current study demonstrated that the novel PETCO2 measurement method was superior to the previous method for PaCO2 prediction. During NIV, the novel method may collect enough exhalation samples as during intubation. Continuous PETCO2 measurement combined with peripheral oxygen saturation monitoring can be noninvasive and useful for early recognition of respiratory failure and to avoid delayed reintubation in patients who are at a high risk of post-extubation respiratory failure.
Supplementary Information
Additional file 1. Appendix S1. Potential risks of post-extubation respiratory failure. Appendix S2. Weaning group according to the WIND criteria. Appendix S3. Updated Charlson comorbidity index. Appendix S4. The Smart Capnoline ® Plus. Table S1. Details of updated Charlson comorbidity index. Fig. S1. Correlations between PaCO2 and PETCO2 according to leakage. Fig. S2. Bland–Altman plot of agreements between PaCO2 and PETCO2 according to leakage. Fig. S3. Sensitivity analyses for correlations with difference (PaCO2—PETCO2).
Acknowledgements
We would like to thank Prof. Giorgio Conti at the Catholic University of Rome for providing their thoughtful advice. We also appreciate Shinji Arata, Keiko Hirano, Tomoko Tanaka, and Ryoga Akamo for supporting the study; and Nihon Kohden for supplying the NIV ventilator, circuits, and interfaces; and Editage (www.editage.com) for English language editing.
Abbreviations
- CI
Confidence interval
- CPAP
Continuous positive airway pressure
- ICU
Intensive care unit
- IQR
Interquartile ranges
- LoA
Limit of agreement
- NIV
Noninvasive ventilation
- PaCO2
Arterial partial pressure of carbon dioxide
- PEEP
Positive end-expiratory pressure
- PETCO2
End-tidal partial pressure of carbon dioxide
- PS
Pressure support
- SBT
Spontaneous breathing trial
- SD
Standard deviation
Authors' contributions
MS designed the study, acquired data, performed statistical analyses, and interpreted the data. ED, WI, AT, NK, TM, KI, YY, and KH conceived the acquisition of data. KY designed the study and interpreted the data. The first draft of the manuscript was written by MS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the JA Hiroshima General Hospital Institutional Review Board (19–53). Informed consent was obtained from each study participant.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Menon N, Joffe AM, Deem S, Yanez ND, Grabinsky A, Dagal AH, et al. Occurrence and complications of tracheal reintubation in critically ill adults. Respir Care. 2012;57:1555–1563. doi: 10.4187/respcare.01617. [DOI] [PubMed] [Google Scholar]
- 2.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26:502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 5.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39:2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 6.Rady MY, Ryan T. Perioperative predictors of extubation failure and the effect on clinical outcome after cardiac surgery. Crit Care Med. 1999;27:340–347. doi: 10.1097/00003246-199902000-00041. [DOI] [PubMed] [Google Scholar]
- 7.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 8.Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43:192–199. doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 9.Mosier JM, Sakles JC, Whitmore SP, Hypes CD, Hallett DK, Hawbaker KE, et al. Failed noninvasive positive-pressure ventilation is associated with an increased risk of intubation-related complications. Ann Intensive Care. 2015;5:4. doi: 10.1186/s13613-015-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 11.Piquilloud L, Thevoz D, Jolliet P, Revelly JP. End-tidal carbon dioxide monitoring using a naso-buccal sensor is not appropriate to monitor capnia during non-invasive ventilation. Ann Intensive Care. 2015;5:2. doi: 10.1186/s13613-014-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouwen MJ, Helmich EJB, Tjan DHT, van der Steen MS. Side stream end-tidal CO2 monitoring in subjects undergoing non-invasive ventilation for respiratory failure: a pilot study. Glob J Respir Care. 2016;3:1–9. doi: 10.12974/2312-5470.2016.03.1. [DOI] [Google Scholar]
- 13.Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ. 2008;86:650–652. doi: 10.2471/blt.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;2010(340):c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316:1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 16.Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am J Respir Crit Care Med. 2017;195:772–83. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 20.Pekdemir M, Cinar O, Yilmaz S, Yaka E, Yuksel M. Disparity between mainstream and sidestream end-tidal carbon dioxide values and arterial carbon dioxide levels. Respir Care. 2013;58:1152–1156. doi: 10.4187/respcare.02227. [DOI] [PubMed] [Google Scholar]
- 21.Kasuya Y, Akça O, Sessler DI, Ozaki M, Komatsu R. Accuracy of postoperative end-tidal PCO2 measurements with mainstream and sidestream capnography in non-obese patients and in obese patients with and without obstructive sleep apnea. Anesthesiology. 2009;111:609–615. doi: 10.1097/ALN.0b013e3181b060b6. [DOI] [PubMed] [Google Scholar]
- 22.Lermuzeaux M, Meric H, Sauneuf B, Girard S, Normand H, Lofaso F, et al. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: A pilot study. J Crit Care. 2016;31:150–156. doi: 10.1016/j.jcrc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira Neto FJ, Carregaro AB, Mannarino R, Cruz ML, Luna SP. Comparison of a sidestream capnograph and a mainstream capnograph in mechanically ventilated dogs. J Am Vet Med Assoc. 2002;221:1582–1585. doi: 10.2460/javma.2002.221.1582. [DOI] [PubMed] [Google Scholar]
- 24.Chan KL, Chan MT, Gin T. Mainstream vs. sidestream capnometry for prediction of arterial carbon dioxide tension during supine craniotomy. Anaesthesia. 2003;58:149–55. doi: 10.1046/j.1365-2044.2003.03035.x. [DOI] [PubMed] [Google Scholar]
- 25.Hedenstierna G, Sandhagen B. Assessing dead space. A meaningful variable? Minerva Anestesiol. 2006;72:521–8. [PubMed] [Google Scholar]
- 26.Ergan B, Nasiłowski J, Winck JC. How should we monitor patients with acute respiratory failure treated with noninvasive ventilation? Eur Respir Rev. 2018 doi: 10.1183/16000617.0101-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix S1. Potential risks of post-extubation respiratory failure. Appendix S2. Weaning group according to the WIND criteria. Appendix S3. Updated Charlson comorbidity index. Appendix S4. The Smart Capnoline ® Plus. Table S1. Details of updated Charlson comorbidity index. Fig. S1. Correlations between PaCO2 and PETCO2 according to leakage. Fig. S2. Bland–Altman plot of agreements between PaCO2 and PETCO2 according to leakage. Fig. S3. Sensitivity analyses for correlations with difference (PaCO2—PETCO2).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.