Abstract
Manifestations of cardiovascular diseases (CVDs) in a patient or a population differ based on inherent biological makeup, lifestyle, and exposure to environmental risk factors. These variables mean that therapeutic interventions may not provide the same benefit to every patient. In the context of CVDs, human-induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) offer an opportunity to model CVDs in a patient-specific manner. From a pharmacological perspective, iPSC-CM models can serve as go/no-go tests to evaluate drug safety. To develop personalized therapies for early diagnosis and treatment, human-relevant disease models are essential. Hence, to implement and leverage the utility of iPSC-CMs for large-scale treatment or drug discovery, it is critical to (i) carefully evaluate the relevant limitations of iPSC-CM differentiations, (ii) establish quality standards for defining the state of cell maturity, and (iii) employ techniques that allow scalability and throughput with minimal batch-to-batch variability. In this review, we briefly describe progress made with iPSC-CMs in disease modelling and pharmacological testing, as well as current iPSC-CM maturation techniques. Finally, we discuss current platforms for large-scale manufacturing of iPSC-CMs that will enable high-throughput drug screening applications.
Keywords: Induced pluripotent stem cells, Cardiomyocytes, Disease modelling, Drug screening, Multicellular crosstalk, 3D platforms
1. Introduction
Cardiovascular risks are used as a global indicator of population health and associated morbidity and mortality.1,2 Within a country and subpopulation, cardiovascular risks vary depending on the performance of healthcare systems, accessibility, and socioeconomic status. Advances in treatments for preventing cardiovascular diseases (CVDs) have been accomplished primarily through pharmacological or surgical intervention. However, identification of long-term determinants of CVDs is difficult due to the lack of next-generation diagnostics.3 When subjects are assigned to larger studies, individuals who share the same disease are generally expected or presumed to respond similarly to treatments despite differences in pre-existing conditions, age, and disease-onset timing, amongst other factors.4 This ‘one size fits all’ approach can mask individual differences in disease manifestation and response to therapies.
The advent of precision medicine aims to approach care by providing early diagnosis and prevention though a combination of ‘pan-omics’ data and collection of in-depth clinical histories.3 The landmark discovery of human-induced pluripotent stem cells (iPSCs)5,6 offers a scalable source of cells for cell-based therapy, drug screening, and characterization of cardiac diseases. Somatic cells can be reprogrammed to iPSCs via viral integration of transgenes into the host genome or remain as non-integrative expression vectors without propagation of the viral genome or proteins. Integration vectors such as retrovirus are known to have higher iPSC generation efficiencies compared to non-integrating adenovirus or Sendai virus. Development of modified viral methods such as the use of ‘self-cleavage’ peptide sequences or excisable vector system carrying the four reprogramming factors (OCT4, SOX2, c-MYC, and KLF4) can minimize the risk of transgene silencing while maintaining low viral copy number. Alternatively, vector-free methods have also been used for reprogramming, such as synthetic messenger RNA, episomal plasmids, minicircle DNA encoding pluripotency factors, or direct protein delivery.7 However, despite the low risk of genetic or epigenetic modification, the reprogramming efficiency in these methods are low compared to viral methods. Recent studies have revealed that clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 (CRISPR-associated protein 9) gene editing tool can also be used to reprogram somatic cells into iPSCs.8 Currently, the methods to generate patient-specific iPSC lines are both time intensive and expensive. Therefore, an approach to develop a timely personalized clinical treatment may require the need to shorten the time for generating iPSCs.
The iPSC-derived cardiomyocyte (iPSC-CM) technology replaces the need to obtain patient samples through invasive procedures. The versatility of iPSC-CMs offers tremendous promise for high-throughput screening of new therapies and for testing efficacy and predicting toxicity on an individual or a population level based on ethnicity, genetic variations, exposure to different environmental factors, and other variables.9 iPSC-based research for modelling cardiac diseases has gained significant momentum, particularly in patients with inherited cardiac disorders related to mutations in genes coding for cardiac ion channels, cytoskeletal proteins, nuclear proteins, mitochondrial proteins, or lysosomal proteins.10 The use of CRISPR/Cas9 genome editing to generate isogenic lines is key for demonstrating the association between mutation load and pathogenic phenotype assessment. The transformative scientific discoveries of iPSCs and CRISPR/Cas9 technology have proven to be ultimately impactful in disease modelling and understanding several mutation-linked cardiac disease mechanisms. Cardiomyocytes (CMs) generated from mutated or corrected lines therefore provide an unprecedented opportunity to study patient- and disease-specific variants in a highly controlled environment.11,12 Successful modelling of these diseases are dependent on clear phenotypic and functional characteristics. Human iPSC-CMs exhibit immature and more neonatal characteristics, which is a major limitation.13 To extend the utility of iPSC-CMs for drug screening, it is essential to not only produce large numbers of cells using bioprocessing technologies but also to closely mimic features of adult cardiomyocytes. Although a wide variety of strategies have been developed to address cellular maturity, there is no consensus on how maturation is quantified. In this review, we highlight the translational benefits of using iPSC-CMs in drug testing and discuss the need for cardiomyocyte maturation in two distinct contexts: (i) advanced maturation using complex three-dimensional (3D) models and (ii) an integrated approach that can be coupled with iPSC-CM manufacturing capabilities for standardizing cell quality in high-throughput screening assays. We also compare the current efficiencies in cardiomyocyte differentiation and yield by comparing two-dimensional (2D) and 3D culture platforms, paving the way for reproducible and cost-effective solutions for iPSC-CM production.
2. Extending disease modelling and cardiotoxicity testing from bench-to-bedside
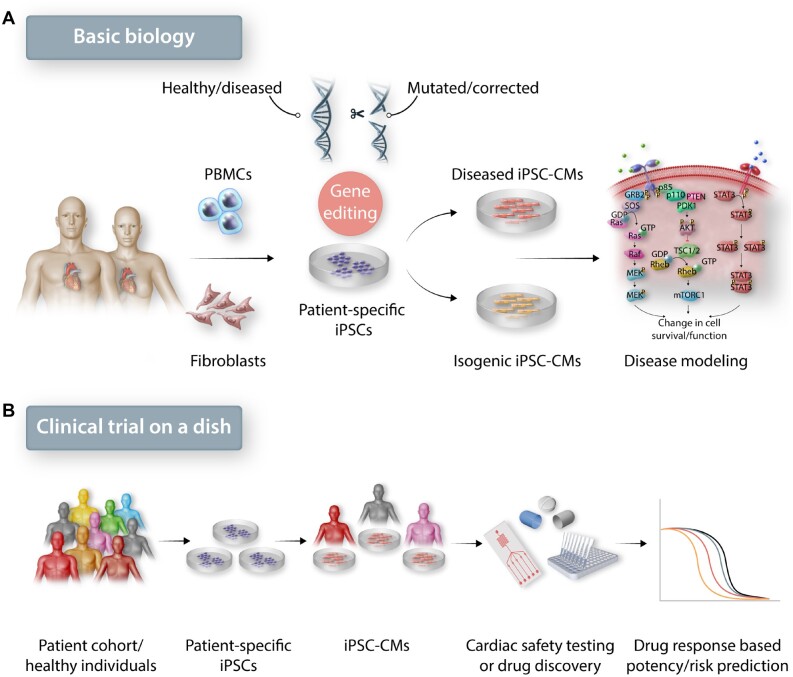
The reproducible differentiation of iPSC- or embryonic stem cell (ESC)-derived CMs now allows investigators to recapitulate numerous genetic and metabolic cardiovascular disorders. To date, iPSC-CMs from patients suffering from disorders such as dilated cardiomyopathy, hypertrophic cardiomyopathy, long QT syndrome (LQTS), Brugada syndrome, arrhythmogenic right ventricular dysplasia, catecholaminergic polymorphic ventricular tachycardia, and several others have been described.12 Using genome editing techniques such as CRISPR/Cas9, mutations that contribute to these disorders have been identified through the generation of iPSC lines with the introduction or deletion of the mutation in control iPSCs (Figure 1A)14–16 In addition to inherited cardiomyopathies, iPSC-CM models have been utilized to study non-inherited disorders.17 Changes in glucose concentration, addition of key inflammatory cytokines, and neurohormonal stimulation have been used in iPSC-CMs to mimic hallmarks of diseases such as diabetes,18 ischaemia–reperfusion injury,19 and acute myocardial infarction.20 Although these disorders are not classically inherited, they are still influenced by patient genetics.
Figure 1.
The role of iPSC-derived cardiomyocytes in basic biology and clinical translation. (A) Patient-derived iPSC-CMs with disease causing mutations can be modified with genome editing tools to understand the mechanism that leads to a diseased phenotype. (B) In clinical research, testing newly discovered or pre-existing drug effects on iPSC-CMs from a diverse patient population can bridge preclinical testing and clinical trials.
Concurrent with disease modelling, the use of human iPSC-CMs to improve cardiac safety prediction is key for early drug development and post-marketing surveillance. In clinical drug development and safety evaluations, adverse cardiac events have been a major cause for preclinical safety closures.21 iPSC-CMs are an excellent platform to predict adverse effects such as the risk of QT prolongation or the involvement of cardiac ion channels in evaluating cellular arrhythmias. iPSC-CMs have been successfully integrated with high-throughput platforms such as microelectrode arrays (MEA) to evaluate drug-induced side-effects.22 One study examined the effects of sotalol, a human ether-a-go-go-related gene (hERG) blocker, on iPSC-CMs derived from 92 healthy donors.23 The study was able to stratify the patient population into high-sensitivity vs. low-sensitivity subjects, concluding that high-sensitivity subjects were significantly more prone to QT prolongation than low-sensitivity subjects. The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative by the US Food and Drug Administration (FDA) has also facilitated the reproducible assessment of proarrhythmic risk across multiple test sites with the use of high-throughput MEA and iPSC-CMs.22,24,25 For example, a recent study with MEA-based evaluation of 28 blinded drugs across 10 laboratories on 2 commercially available human cardiomyocyte lines allowed for statistical modelling with 87% predictivity in low, intermediate, and high proarrhythmic risk categories with minimal inter-line variability.26
Another area where iPSC-CMs have made a significant impact is in identifying cardiotoxicity in patients treated with oncology drugs. Between 2012 and 2017, 55 out of 58 new cancer drugs were approved through an expedited timeline.27 Expedited drug approvals reduce clinical and preclinical testing times. Although such processes ensure timely access to new drugs for patients suffering from life-threatening diseases, it also increases the risk of long-term side-effects. Traditional chemotherapeutic drugs do not selectively exploit tissue-specific differences between a tumorous and healthy tissue, which can result in significant collateral damage to non-cancerous tissue. Recently, investigators have shown that a ‘cardiac safety index’ based on iPSC-CMs can be used for prediction of drug-induced cardiotoxicities among cancer patient populations.28–30 One study demonstrated that iPSC-CMs derived from breast cancer patients who developed cardiac dysfunction consistently exhibited altered cellular function in response to doxorubicin treatment in vitro.28 In the future, studies that are performed at a large-scale involving iPSC-CMs from diverse patient backgrounds may enable the development and selection of safer drugs for clinical use (Figure 1B). One of the important challenges in using iPSC-CMs from large number of patients is the lack of standardized quality and defined maturation endpoints to ensure reproducibility. Therefore, it is important to delineate the quality standards for non-clinical pharmacology studies and cardiac disease models that require higher physiological complexity.31
3. Defining cardiomyocyte maturity in the context of use
During human heart development, CMs mature in the human body until approximately 6 years of age.32 The onset of maturation occurs in the early post-natal phase, when CMs exit from the cell cycle and undergo physiological hypertrophy with increased cell volume and workload. iPSC-CMs derived in vitro over 9–12 days of differentiation are immature, where the cells beat spontaneously and express structural and functional proteins to a lesser degree than in adult CMs. At a gene expression level, iPSC-CMs resemble human embryonic heart cardiomyocytes in the second trimester of development.33 Substantial progress in maturation strategies to mirror in vivo cardiac maturation has been made using biochemical and biophysical methods, which is summarized in Table 1. Although a wide variety of strategies have been developed to address iPSC-CM maturity, how the maturation criteria are quantified is still quite variable and context dependent. To mimic cardiac tissue-like features both structural and functional maturation is necessary, whereas for high-throughput screening assays functional maturation with expression of mature sarcomere and ion channel proteins is highly desired.
Table 1.
iPSC-CM maturation methods and characteristics
| Maturation method | Approach | Maturation duration | Mature CM features | Ref. |
|---|---|---|---|---|
| Physical | Prolonged culture time | 360 days |
(Compared to day 30) Larger cell size Longer sarcomere Lower beating rate Less dual MCL2α/v expression More aligned sarcomere |
[ 45 , 139 ] |
| Metabolic (hormone treatment) |
Media +Triiodothyronine (T3) +Glucocorticoid +Dexamethasone |
1–2 weeks |
Larger cell size Less circularity Up-regulation of α-MHC, SERCA2a Down-regulation of β-MHC Decrease in proliferation Improved contractile force and kinetics Faster calcium transience Increased mitochondrial respiration |
[ 39 , 90 , 49 , 91 ] |
| Metabolic (glucose replacement) |
Media Remove glucose +palmitate +oleic acid +carnitine |
3–20 days |
Larger cell size Less circularity Longer sarcomere Increase in mitochondria number and respiration Improved contractile force and kinetics Upstroke velocity and membrane capacitance |
[ 95 , 48 , 101 ] |
| Physical (substrate modification) |
Culture environment Increased substrate stiffness via polydimethylsiloxane (PDMS) or polyacrylic acid (PAA) (6∼10 kPa) |
3–9 days |
Modulates sarcomere tension and contractility Increased force generation More rectangular shape |
[ 69 , 102 , 103 ] |
| Physical (electrical stimulation) | Continuous electrical stimulation (6.6 V/cm, 1 Hz, 2 ms duration) | 4 days |
Upregulation: HCN1, MLC2V, SCN5A, SERCA, Kv4.3, GATA4 Increase in TNNT+ cells Cell elongation Ventricular-like AP Increase AP duration Improved calcium handling Coupled with mechanical stimulation via photoactivated PDMS: increased surface N-cadherin signalling, sarcomere shortening |
[ 104 , 106 ] |
| Biochemical/physical |
Culture environment CM-FB/MSC co-culture and static/cyclic stretching |
2 weeks |
Increased contraction velocity Greater twitch amplitude Increased tensile modulus More mature sarcomere structure Connexin 43 clustering N-cadherin surface localization Decreased proliferation potential Better MYL2/7 and MYH7/6 Up-regulation: MLC2, NPPA, CACNA1C, SERCA2 Stretching further increased findings, particularly cyclic |
[ 107 ] |
| Metabolic/biochemical/physical |
Media +triiodothyronine, dexamethasone, (TDI) in a microtissue format |
7 days |
Improved cellular alignment Increased sarcomere length Increased MYH7, ATP2A2, SLC8A1, PLN, PPARG, ADRB1, CHRM2, p-AKT Decreased MYH6, TNNI1 Increased contractile force Increased time to peak Increased relaxation time |
[ 109 ] |
| Metabolic/biochemical | Media and culture environment using EHT format | 3–5 weeks |
Increased fatty acid oxidation Increased tetrodotoxin sensitivity Enhanced aerobic respiration Sarcomere-arrayed mitochondria Improved calcium handling Higher IK1 density More negative diastolic membrane potential |
[ 97 ] |
3.1 IPSC-CM maturation for precision disease modelling
3.1.1 Building multicellular interactome
Cardiomyocytes are surrounded by non-cardiac cell types such as endothelial cells (ECs), cardiac fibroblasts, smooth muscle cells, neurons, and immune cells.50 In an adult heart, cardiomyocytes occupy ∼75–80% of the heart volume, but only account for a third of the total cell number.50 Molecular interactions with other predominant cell types such as cardiac fibroblasts (CFs), vascular smooth muscle cells (vSMCs), and ECs are essential in the maturation of CMs from the neonatal to the adult stage. During development, fibroblast signalling is essential for cardiomyocyte proliferation and electrical coupling through gap junctions.51 Since fibroblasts are a mechanoresponsive cell type, they transform into myofibroblast phenotype that is characterized by high smooth muscle actin (SMA) expression. Co-culturing iPSC-CMs and fibroblasts in a ratio of 4:1 in a 3D cardiac spheroid system prevents the differentiation of fibroblasts into myofibroblasts and improves cardiomyocyte contractility.52
ECs are also known to influence CM maturation during development and support CM metabolism and contractility, as well as provide protection against injury.53,54 The incorporation of ECs and endothelial progenitor cells in 3D constructs has been shown to improve vascular network formation and impart structural and functional maturation of CMs.55,56 In a disease modelling context, it is becoming increasingly clear that non-myocytes have an important role in maintaining functional homeostasis in CMs. A recent study demonstrated that functional deficits in iPSC-ECs derived from patients with LMNA mutation can contribute towards cardiolaminopathy. Downstream effects of the mutation that led to low expression of KLF2 protein was found to be a cause of this functional deficit in iPSC-ECs. Treatment with a clinically approved drug, lovastatin, not only increased the expression of KLF2 in iPSC-ECs but also improved iPSC-CM function in a coculture system.57 The crosstalk between non-myocytes and iPSC-CMs can therefore provide meaningful insights into cardiovascular pathologies and help develop targeted therapies.
Significant progress has been made towards the derivation of vascular iPSC-ECs, iPSC-CFs, and iPSC-vSMCs. All three of these cell types originate from the mesodermal cells following the activation of the WNT pathway. Thereafter, the divergence in cell fate is dictated by growth factors and signalling molecules. Bone morphogenetic protein 4 (BMP4) and Activin A gives rise to cardiogenic and hemogenic mesoderm, from which definitive iPSC-ECs are derived in the presence of vascular endothelial growth factor and fibroblast growth factor (FGF).58 iPSC-vSMCs are obtained by exposure to activin A and platelet-derived growth factor subunit B via a mesodermal intermediate expressing T-box transcription factor T.60,59 Derivation of quiescent and functional iPSC-CFs have been demonstrated through generation of epicardial cells and second heart field progenitors with the help of retinoic acid (RA) followed by FGF-2 stimulation.61,62 Building integrated cell or tissue models will not only help understanding CM maturation in the context of intercellular crosstalk, but also address many questions related to cell-specific responses in disease states.63 Currently, it is unclear how cardiac tissue specificity can be achieved in iPSC-derived ECs, vSMCs, and CFs, but mapping phenotypic differences through systematic molecular profiling of human cardiac tissues at various stages of development may help unravel tissue-specific cell type signatures. To elucidate line-specific differences in multiple cell types, cardiac microtissues can be generated in a single differentiation track comprising of CFs and ECs. Cardiac microtissues comprised of defined EC and CF populations have been shown to improve cardiomyocyte maturation and recapitulate arrhythmogenic cardiomyopathy.42,64 Another approach to combine different cardiac cell types is to mimic in vivo cardiogenesis using iPSCs or ESCs in form of embryoid organoids or gastruloids.65 Recently, using mouse ESCs and FGF signalling, a primordial 3D heart-like structure was developed with defined atrium, ventricle, pacemaker cells, SMCs, and ECs.66 Similarly, self-organizing ‘cardioids’ have been generated using human iPSCs that constitute cells of the myocardium, epicardium, and endocardium, mimicking early heart chamber development.67 Such platforms will not only provide an arsenal of in vitro platforms to study cellular effects at an organ-level, but will also provide insights into structural abnormalities of the heart formed during early development.
3.1.2 Extracellular matrix reinforcement
Extracellular matrix (ECM) in the heart provides structural stability and elasticity to permit contraction and transmission of impulses for normal heart function. Cardiomyocytes transduce mechanical force through localized interaction with the ECM mediated by integrins linked to cytoskeletal actin proteins. Greater cell-ECM association translates to enhanced reinforcement and higher contraction forces.68,69 The neonatal heart ECM is mainly composed of laminin and fibronectin secreted by SMCs and ECs. Fibronectin is localized around newly formed blood vessels surrounding the myocytes.70,71 The neonatal heart typically has a high type I to type III collagen ratio, making it less compliant but more efficient at handling an increased workload.72 During adult maturation, the tissue compliance increases with more type III collagen compared to type I, developing into a thick, aligned fibrillar network throughout the myocardial tissue. Therefore, to emulate functional maturity in iPSC-CMs, long-term elasticity with higher mechanical integrity is considered essential.73 Several studies have demonstrated that culturing iPSC-CMs or ESC-CMs on ECM substrates in the form of spontaneously contracting engineered heart tissues (EHTs) can enhance cardiac maturation and better recapitulate a disease phenotype (Figure 2). For example, a recent study showed that isolated LQTS iPSC-CMs in monolayers were more arrhythmogenic due to lack of high cell–cell interactions. In contrast, arrhythmias were observed in LQTS iPSC-CMs in EHTs only when they were challenged with a QT prolongation agent.74
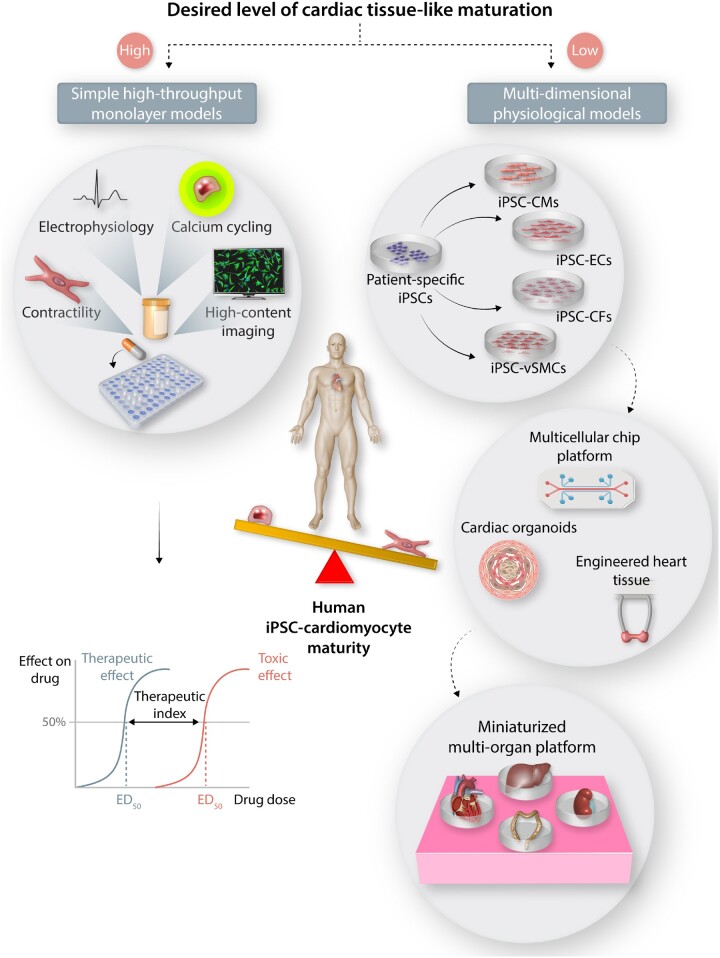
Figure 2.
Addressing the need for cardiac tissue-like maturation in iPSC-CMs. High-throughput assays that allow for rapid drug screening and toxicity evaluation does not require adult-like CM maturation. Multicellular 3D models and advanced multi-organ-on-a-chip (MOC) based approaches can be further utilized for validation studies and modelling diseases that affect multiple cell types through systemic feedback.
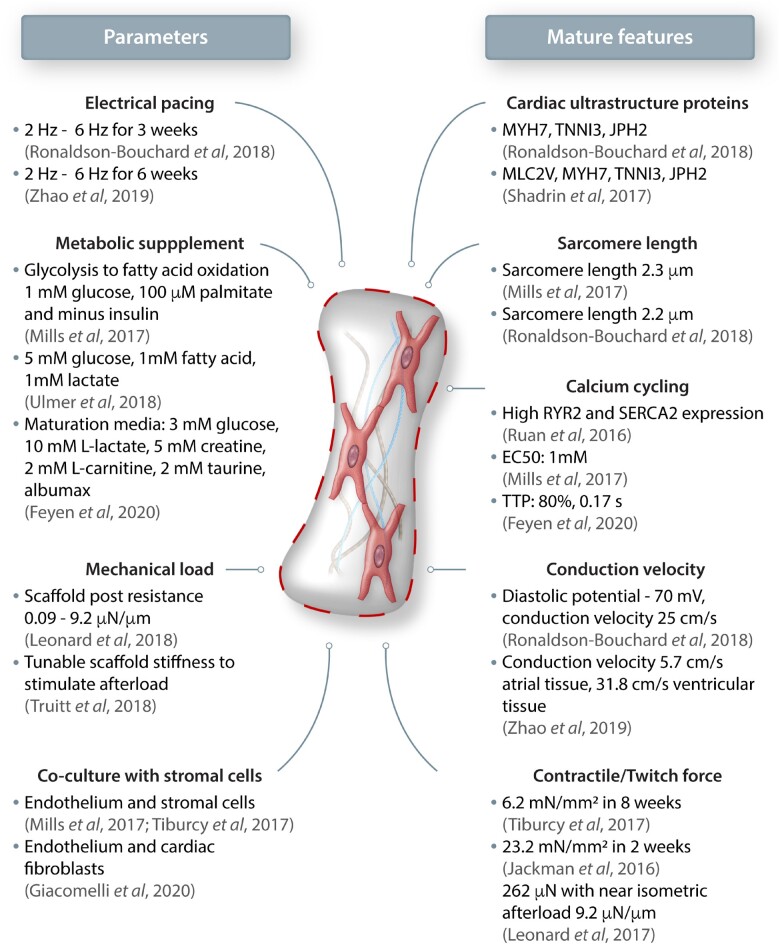
Three-dimensional EHTs mimic intricate architecture in the native myocardium by promoting anisotropic cellular alignment and synergistic electromechanical interactions. A brief overview of maturation approaches and characteristics of mature cardiac tissue achieved in a 3D tissue-like format is summarized in Figure 3. ECM surrounding the myocytes and non-myocytes provide a remodelling niche to reorganize, form cell-cell junctions, and transduce traction forces required for cardiac coupling. A higher cardiac maturation is attributed to positive force-frequency relationship (FFR) that translates to mature excitation-contraction coupling.75 In adult cardiac tissues, the contractile forces reach 50 mN/mm2, whereas the forces generated on EHTs range from 0.05–23 mN/mm2.76–82 In addition to increased force of contraction, ECM-guided artificial loading also promotes higher cellular organization, rearrangement of mitochondria along the sarcomeres and expression mature isoforms of contractile protein.83 Physiologically relevant platforms would comply with the Frank-Starling mechanism, wherein during early systolic auxotonic phase cardiac force exceeds the pre-load, followed by an isometric phase where cardiac force equals to the load. This can be achieved by tuning the stiffness of scaffolds that are used to assemble EHTs to mimic healthy or pathological mechanical loading.84,85 Auxotonic contractions can also be achieved through electrical stimulation; early-stage iPSC-CMs and subtypes subjected to stepwise pacing of EHTs from 2 Hz to 6 Hz not only exhibit a positive FFR, but also lead to mature calcium kinetics and higher conduction velocities.35,86 Although it is important to match electromechanical properties of the cardiac tissue, it is also crucial to understand the effect of the biochemical composition of the matrix the cells interact with.36 From a mechanical standpoint, fibrin imparts higher tensile and compressive tissue-like stiffness, but collagen provides higher contractile elasticity. Therefore, there is great advantage in fine-tuning the ECM to obtain optimal mechanical strength and thereby enhance contractile forces as a means of achieving biomechanical maturation.37 A recent study employed a systematic approach varying collagen, fibrin, and cell density to obtain an optimized composition of ECM components with fibrin of 8 mg/mL, collagen of 0.8 mg/mL, and a seeding density of 15 × 106cells/mL. This composition yielded the highest force production by iPSC-CMs among the tested groups.38 Therefore, matching the composition of the adult cardiac tissue using the most abundant ECM such as collagen or decellularized tissue matrices may be more suitable instead of fibrin, which is a blood component.87 However, it must be noted that ECMs are not inert in nature, and the precise role of the non-abundant components that make-up the cardiac extracellular environment also needs further investigation.68,88 Recently, it was shown that a secreted large proteoglycan, agrin, can promote proliferation and maturation of iPSC-CMs and provide a potent stimulus for cardiac regeneration in vivo.89 Despite the benefit of intercellular interactions as well as mechanical and electrical stimulation, such sophisticated maturation methodologies are difficult to incorporate in scalable manufacturing processes. Hence, incorporation of simpler strategies such as the use of metabolic substrates or soluble prosurvival signalling molecules would prove to be effective in enhancing CM maturity for various high-throughput assay platforms.
Figure 3.
Maturation methods and features in engineered cardiac tissue mimics. (Left) Several stimuli and modifications such as controlled electrical pacing, metabolic supplementation, mechanical loading and addition of non-myocytes to tissue engineered constructs have shown to promote (Right) mature cardiac tissue-like features both in morphology and function.
3.2 IPSC-CM maturation strategies for large-scale bioprocesses: a metabolic approach
In recent years, several studies have focused on modifying the metabolic substrates and composition post-differentiation to induce maturation hallmarks such as higher oxidative metabolism, improved contractility, and rate-dependent action potential kinetics. For example, T-tubules, which are a contiguous network of tubules and membranes that play a key role in excitation–contraction coupling, have been shown to form in iPSC-CMs with the use of metabolic supplements that contain tri-iodothyronine (T3)39 and dexamethasone (Dex) during differentiation days 16–30. T3 is a thyroid hormone that plays a key role in postnatal heart development.90 In addition to gene regulation of T-tubule proteins, T3 can also activate PI3 kinase (PI3K), which increases protein synthesis and cell proliferation.49 Recently, it was shown that T3- and Dex-treated cells showed functional networks of T-tubules with adult CM-like function.91
During cardiomyocyte maturation, mitochondria occupy up to 40% of the cell volume and are tightly packed along the myofibrils.92 As the cell matures, the oxidative capacity of the mitochondria increases, allowing the cell to switch from glycolysis to fatty acid oxidation. This metabolic flexibility to utilize different energy substrates is a unique adaptation of the heart. During development, there is high availability of long-chain free fatty acids (FA) and oxygen to support mitochondrial ATP demands.93 To induce iPSC-CMs into a more mature phenotype, several metabolic supplementation strategies have been implemented. In human neonates, the serum FA concentration is ∼300 µM,94 whereas the commonly used iPSC-CM culture medium contains less than 10 µM FAs. Therefore, supplementing the cell culture media with the most abundant FAs (e.g. palmitic, linoleic, and oleic acids) found in newborn serum can improve iPSC-CM maturation.95,96 Most recently, maturation media formulation containing l-lactate, Taurine, l-carnitine, and creatine monohydrate was shown to improve electrophysiological, mechanical, and structural parameters of iPSC-CMs in both 2D and 3D platforms.97,98 In a 3D environment, iPSC-CMs are shown to harbour abundant mitochondria that improve oxidative phosphorylation capacity and energetics. A higher respiratory capacity and metabolic switch may be responsible for higher mechanical loading and further maturation.99 A combination of these approaches can be incorporated into large-scale bioprocesses to induce CM maturation, which offers a more uniform quality of iPSC-CMs for high-throughput screening assays. A pre-requisite for generating high-quality iPSC-CMs is to develop differentiation protocols that induce efficient cardiac mesoderm induction to yield pure cardiomyocytes. Fully defined, xeno-free approaches can then be implemented and adopted for large-scale bioprocesses.
4.1 In vitro iPSC-CM differentiation on a scale of time and dimension
To date, significant progress and refinement in differentiation protocols and platforms have been made to obtain specific cardiac subtypes on a small scale.100 Therefore, it is important to explore the feasibility of generating large-scale, high-quality, patient-specific iPSC-CMs to accelerate drug development and testing efforts (Figure 4). Some of the key advancements in differentiation purity and efficiencies between 2D and 3D culture platforms are discussed below and compared in Table 2.
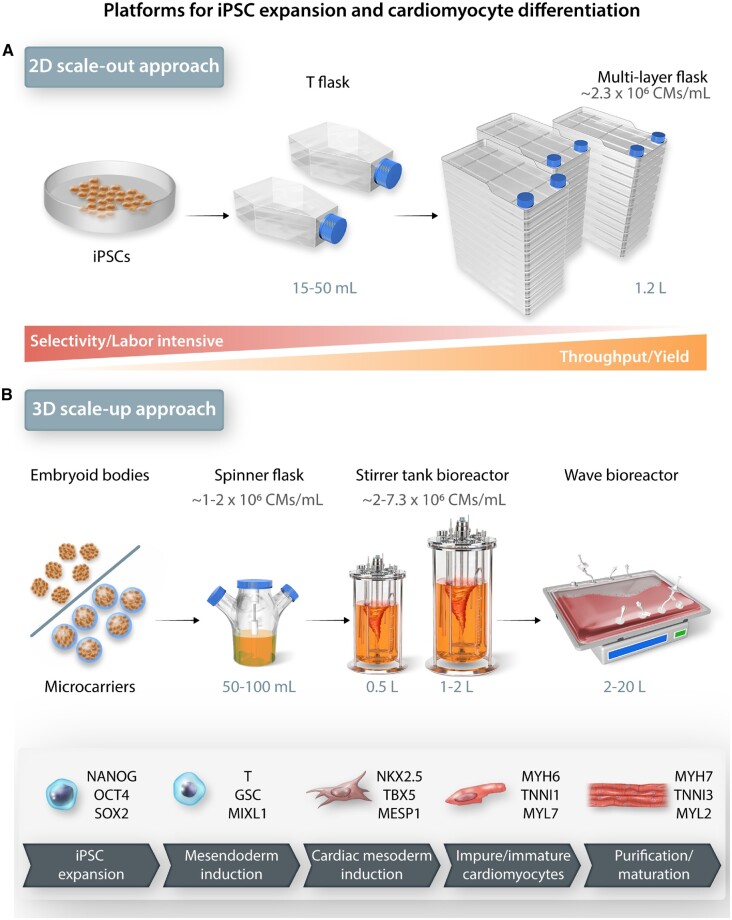
Figure 4.
Scalable technologies for manufacturing iPSC-CMs on a large scale. (A) 2D single-layer and multilayer cultureware for iPSC-CM differentiation offers limited control over culture conditions and is suitable for small scale production and testing applications. (B) 3D suspension cultures for iPSC expansion and iPSC-CM differentiation provides larger surface-area-to-volume ratio and overall higher yield for preclinical large-scale drug screening assays.
Table 2.
iPSC-CM derivation methods and scalability
| Stem cell source | Culture format | Stem cell medium | Cardiomyocyte differentiation medium composition | Cardiomyocyte differentiation period and efficiency | Purity | Demonstrated scalability | Ref. |
|---|---|---|---|---|---|---|---|
| hESC | Co-culture with murine visceral endoderm-like cells | 1:1 DMEM/DF + 7.5% FCS | 1:1 DMEM/DF + 7.5% FCS +10 μg/mL mitC |
Up to 7 weeks, Beating: 35% ± 10 |
MLC2α/v+, ventricular action potential |
No | [132] |
| hESC | EB aggregate |
DMEM/F12 20% KSR 100 μM NE-AA 2 mM glutamine 10–4 M β-ME 20 ng/mL hbFGF |
StemPro-34 10 ng/mL BMP-4 2 mM glutamine 4-4 M MTG 50 μg/mL ascorbic acid 10 ng/mL hbFGF 10 ng/mL VEGF |
8–13 days, MLC2a+ | 40–50% TNNT+ cells | No | [133] |
| hESC | EB/monolayer |
DMEM/F12 20% KSR 10 mL NE-AA 2 mM glutamine 50 mM β-ME 8 ng/mL bFGF |
LI-APEL 1 μg/mL insulin 20–40 ng/mL BMP4 20 ng/mL activin A 30 ng/mL VEGF 40 ng/mL SCF 50–80 ng/mL WNT3A |
10 days, EB: 38% Nkx2.5+ ML: 24% Nkx2.5+ |
SIRPA+VCAM-1+MLC2v+ | No | [134] |
| hESC/iPSC | EB aggregate |
DMEM/F12 20% KSR 100 μM NE-AA 2 mM glutamine 10-4 M β-ME 20 ng/mL hbFG |
StemPro-34 2 mM glutamine 1 mM ascorbic acid 4-4 M MTG Various concentrations: BMP4, BFGF, Activin A, DKK-1, VEGF |
20 days |
>50% CTNT+ | No | [135] |
| hESC | Monolayer |
80% KO-DMEM 20% KSR 1 mM glutamine 0.1 mM β-ME 1% NE-AA 4 ng/mL bFGF |
RPMI 1640 +B27 25 ng/mL BMP4 6 ng/mL bFGF 100 ng/mL activin A 200 ng/mL DKK1 NOGGIN, RA/RAi |
14 days, RA: MLC2v-, atrial-like AP RAi: MLC2v+, ventricular-like AP |
RA: 50.7% ± 1.76% CTNT+ RAi: 64.7% ± 0.88% CTNT+ |
No | [129] |
| hESC/iPSC | Monolayer ‘matrix sandwich’ |
80% DMEM/F12 20% KSR 0.1 mmol/L β-ME 0.1 mmol/L NE-AA 1 mmol/L glutamine 100 ng/mL zbFGF or 4 ng/mL hbFGF |
RPMI 1640 +B27 –insulin 100 ng/mL activin A 5–10 ng/mL BMP4 5–10 ng/mL bFGF +insulin |
7–10 days MLC2a+ ventricular-like AP |
40–92% TNNT+ | No | [136] |
| hESC | Monolayer | mTeSR1 |
RPMI 1640 + B27 –insulin CHIR99021 IWP2/IWP4 |
15 days, ventricular-like AP | 85% TNNT+ | No | [137] |
| iPSC | Monolayer | Essential 8 |
RPMI 1640 500 μg/mL rHA 213 μg/mL l-ascorbic acid 2-phosphate 6 μM CHIR99021 2 μM Wnt-C59 |
15 days, 10–30 days: MLC2α + 15–20 days: atrial-like AP 45–60 days: MLC2v+ 30–35 days: ventricular-like AP |
80–90% TNNT+ | No | [114] |
| hESC | EB Aggregate |
DMEM/F12 20% KSR 100 μM NE-AA 2 mM glutamine 10-4 M β-ME |
StemPro-34 10 ng/mL BMP4 2 mM glutamine 4-4 M MTG 50 μg/mL ascorbic acid 10 ng/mL bFGF 10 ng/mL VEGF |
20 days, SANLPC: Nkx2.5-, MYL2-, TBX2+, SHOX2+ VLCM: Nkx2.5+, MYL2+. TBX3-, SHOX2- |
VLCMs: 5% ± 1% Nkx2.5+ SANLPCs: 55% ± 5% Nkx2.5- |
No | [40] |
| iPSC |
Monolayer Multi-layered plates |
Modified stem fit |
RPMI 1640 +b27—INS +active gas ventilation MEMα + 5% FBS |
10 days MLC2v+ 66 - 87% |
Ventricular-like AP 85% TNNT+, 99.3% after metabolic selection |
Yes | [116] |
| iPSC |
Monolayer T-flasks |
Essential 8 |
RPMI 1640 + B27–INS 3-8 μM CHIR99021 2 μM C59. Replated at Day 11 in 1:10–15. For expansion RPMI 1640 + B27–INS media supplemented with 2.0–4.0 μM CHIR99021 |
12 days |
>90% TNNT+ ∼500-fold increase in TNNT+ cells after expansion |
Yes | [117] |
| iPSC | Microcarrier Stirred Tank Reactor | mTeSR1 |
RPMI 1640 + B27 – INS 0.6 mM L-ascorbic acid 2-phosphate 4–14 μM CHIR99021 2.5 μM IWR-1 |
Nkx2.5+ HNF4 α CD44 |
IMR90: 83% TNNT+ 64% MLC2α |
Yes | [138] |
| hESC/iPSC |
Microcarrier T-flask/spinner flask |
mTeSR1 |
RPMI 1640 + B27 – INS 18 μM CHIR99021 5 μM IWR-1 |
12 days |
cTNT: 65.73 ± 10.73%; MHC: |
Yes | [125] |
| hESC/iPSC |
EB aggregate rotated erlenmeyer flasks/stirred tank reactor |
mTeSR1 |
RPMI 1640 + B27 – INS 0–15 μM CHIR 5 μM IWR-1 |
10 days ∼50–70% Nkx2.5+ |
27.2–83.5%; MHC, 27.7–88.3% cTNT | Yes | [110] |
| hESC/iPSC |
EB aggregate Spinner flasks |
StemPro hESC SFM with 40 ng/mL bFGF |
RPMI 1640 + B27 – INS 6–24 μM CHIR 1–15 μM IWP-4 |
25 days 42–83% ROR2+PDGFRα+ |
>90% cTnT+ by day 12 | Yes | [119] |
4.1.1 2D cardiomyocyte differentiation
Although the development trajectories for the derivation of cardiac subtypes are known,113 several key optimizations are being explored to derive pure cardiomyocytes using simple, cost-effective, and xeno-free methods. A small molecule-based approach allows higher reproducibility and ease in scalability due to lower cost and higher stability compared to recombinant proteins.112 Small molecules such as CHIR or BIO are currently used for biphasic differentiation, with initial mesendoderm induction followed by down-regulation of WNT pathway using inhibitors (IWR1-endo or C59) for cardiac lineage specification.111 One of the key developments to this protocol is the identification of critical components in the cell media that promote cardiomyocyte differentiation. Using a reductionist approach, the essential components were identified as progesterone, putrescine, and selenite.114,115 Despite the refinements in differentiation protocols, the scale of culture remains limited on 2D platforms for high cardiomyocyte yields.
Unlike most other somatic cells cultured in vitro, iPSC-CMs do not maintain their proliferative capacity once formed. Within a week after successful differentiation, iPSC-CMs exit the cell cycle and enter a post-mitotic state. Therefore, for a higher iPSC-CM yield, the primary approach has been to induce differentiation in higher number of iPSCs grown on larger surface areas. The highest cardiomyocyte yield on 2D platform was obtained using multilayer flasks and achieved ∼700 million cardiomyocytes (4-layer) with almost 100% efficiency and ∼2.8 billion cardiomyocytes (10-layer) with up to 87% differentiation efficiency (Figure 4A).116 Recently, it has been shown that through multiple passaging in standard tissue culture plates, iPSC-CMs can be expanded during their early proliferative phase by the reintroduction of CHIR. Although the precise molecular mechanism is still largely unknown, this method can help generate iPSC-CMs by ∼100-fold.117,118 One of the major drawbacks of 2D culture platforms is that they do not allow uniform distribution of the small molecules that promote differentiation and growth. Another drawback is that it is harder to precisely monitor the culture environment of 2D platforms, because of the lack of real-time sensors for maintenance of optimal growth and differentiation. Therefore, 3D culture platforms integrated with bioreactor systems are considered desirable alternatives to achieving dynamic monitoring control for large-scale production of iPSC-CMs.
4.1.2 3D cardiomyocyte differentiation
One of the key advantages of 3D aggregate-based differentiation is its versatility in translation from the lab setting to commercial scale by combining it with dynamic suspension culture systems. At present, directed cardiac differentiation using 3D cell aggregates in a suspension culture has been demonstrated at scales ranging from 100 mL to 1 L capacities (Figure 4B), with differentiation efficiencies ranging from 80% to 99% (cTnT+).110,119–121 The overall throughput of these processes is ∼0.2–0.9 × 109 CMs/L. A more recent protocol showed a higher throughput of ∼1 × 109 CMs/L by controlling cell aggregation, preculture timing, and exact CHIR dosing.114 A preculture time of 2 days in iPSC maintenance media followed by induction at 7.5 µM CHIR was found to be important in efficient cardiac differentiation.122 On a commercial scale, using a non-directed differentiation approach comparing wave and stirred tank bioreactors revealed a higher CM lineage commitment on wave platform with a larger throughput of 1.5 × 109 CMs/L compared to 0.2 × 109 CMs/L on a stirred bioreactor platform.123
In parallel with the development of 3D iPSC aggregates, the use of microcarriers for iPSC growth, expansion, and differentiation has been explored to exploit the higher surface area-to-volume ratio. Microcarriers are uniform-sized spheres coated with cell adhesion matrix to allow iPSC attachment and growth on the surface of the spheres. In comparison to 3D aggregates, an advantage of the microcarriers is that the diffusion limitation of small molecules is minimal due to cells attaching only on the outer surface. Typical microcarriers are made of polystyrene coated with vitronectin for human iPSC expansion in culture.105 It is worthwhile to note that the overall CM yield depends on substantial pre-expansion of iPSCs on microcarriers. Microcarrier technology has recently been made more scalable by the development of bead-to-bead transfer technology, which allows for a prolonged expansion period of up to 15 days and 241-fold increase in iPSC yield.124 Initial efforts to derive CMs using microcarriers in static or dynamic conditions showed that intermittent agitation yielded improved CM differentiation efficiency (∼66%) compared to a static platform (∼47%). It is suggested that the higher shear stress in continuous agitation results in inhibition of transforming growth factor-β (TGF-β) pathway, which negatively affects CM differentiation.125 Further microcarrier culture optimization in spinner flasks with 10 days of CM differentiation supplemented with ascorbic acid and 5 days of lactate-based purification with intermittent agitation regime in spinner flask produced a high yield of 1.38 × 109 CMs/L and 83% cTnT+ cells.126 Overall, the easy adoption of cardiac differentiation protocols has enabled mass production capabilities with improved efficiency and yield. However, CMs obtained in such large-scale platforms are primarily the ventricular subtype. Hence, an important goal is to optimize derivation of other cardiac subtypes such as atrial and nodal cells on a large-scale differentiation platform based on current protocols developed in 2D culture systems.108
5. Towards obtaining diversity in myocyte composition for disease modelling
For precise disease modelling, it is important to understand the pathophysiology in all cardiac subtypes including ventricular, atrial and pacemaker cells. Differentiation of iPSCs into cardiomyocytes are achieved through modulation of Wnt and BMP pathways. The modulation of these pathways with exogenous molecules are highly time and concentration dependent. One of the main challenges is to achieve tunability during the differentiation to obtain a more precise iPSC-CM subtype with high purity. Currently, this can only be achieved by selectively isolating progenitors that result in enrichment of specific CM subtype. Progenitors that express NKX2.5+/TBX5+ that predominantly gives rise to ventricular CMs. Early studies have noted that RA is plays a central role in formation of atrial CM that uniquely express ion channels such as KCNJ3, KCNA5, and GJA5.128,127,129 During iPSC-CM differentiation, addition of RA on days 3 and 5 result in reduction of ventricular cardiomyocyte population and increase in atrial cell specificity that display both electrophysiological and gene expression patterns found in atrial cardiomyocytes in vivo.34 During WNT inhibition separate precursor populations arise from the mesoderm that selectively express CYP26A1 or RALDH2. CYP26A1 gives rise to CD235a+ ventricular progenitors and RA signalling within RALDH2+ cells promote atrial cardiogenesis. Sinoatrial node (SAN) cardiomyocytes also known as the pacemaker cells arise from TBX18+/NKX2.5- second heart field progenitors through inhibition of FGF and TGF-β pathway.130 SHOX2 a specific SAN transcription factor is shown to down-regulate NKX2.5 expression resulting in the enrichment of SAN cells.40,131 The current protocol yields ∼35% TNNT2+/NKX2.5- cells which can be further sorted using flow cytometry for higher enrichment. These advances have laid a strong foundation to obtain ‘on-demand’ chamber specific cell types from infinite source of iPSCs. However, there is a need for standardization of scalable differentiation methodologies and compartmentalized cell assembly for constructing tissue models for holistic understanding of organ-level cardiac response.
6. Conclusion and future perspectives
Significant progress has been made in the development of iPSC-CMs as a tool for modelling CVDs and drug screening. For a deeper understanding of cardiovascular risks on a population level, continued efforts towards generation and characterization of iPSC-CMs with robust quality control endpoints needs to be implemented. Cell sources obtained on scalable differentiation platforms, protocols with reduced heterogeneity, and incorporation of nutrient-based maturation strategies in a single unit process will resolve and refine the predictive power of high-throughput assays for cardiac risk assessment. For complex disease modelling, monolayer cardiomyocytes may be inadequate due to a lack of microenvironmental signalling such as biochemical and biophysical interactions with non-myocytes and ECM. Development of parallel technologies such as 3D bioprinting and microphysiological system has the capability to structurally define tissue-like features and complexity which are hard to reproduce in vitro. Bidirectional scalability of these technologies is crucial for large scale drug testing and tissue regenerative applications. Furthermore, to capture human pharmacodynamics and pharmacokinetics in-a-dish, multi-organ-on-chip platforms can be used to create a more realistic simulation of tissue physiology. These scaled-down devices capable of measuring electromechanical activity also mimic the circulation of metabolites and biochemical signal gradients through an interconnected network of channels that allow fluid flow.41,43,44 However, these technologies will not be ready for clinical use without harmonized efforts towards banking patient iPSCs and derivation of specific cardiovascular cell types with minimal batch variability. Such an advancement with well-defined endpoints using simple or complex cardiac models will lay the cornerstones for population-based studies in the form of a clinical-trial-in-a-dish, which could become a requirement for new drug investigation or first-in-human clinical studies. In summary, scalable iPSC-CM derivation methodologies coupled with high-throughput phenotypic and disease-specific assays can pave the way for personalized treatment and development of pharmacogenetic tools for patient stratification in the clinic.
Acknowledgements
We thank Dr Adrienne Muller, Dr Amanda Chase and Blake Wu for their helpful feedback on the manuscript.
Conflict of interest: J.C.W. is a co-founder of Khloris Biosciences but has no competing interests, as the work presented here is completely independent. All remaining authors have declared no conflicts of interest.
Funding
This work was supported by the Tobacco-Related Disease Research Program (TRDRP) of the University of California, T29FT0380 (D.T.) and 27IR-0012 (J.C.W.); American Heart Association 17MERIT33610009 (J.C.W.); Leducq Foundation 18CVD05 (J.C.W.); and National Institutes of Health (NIH) R01 HL113006, R01 HL123968, R01 HL141851, and NIH UH3 TR002588 (J.C.W).
References
- 1. Fuster V, Frazer J, Snair M, Vedanthan R, Dzau V; Committee on global health and the future of the United States: A report of the National Academies of Sciences, Engineering and Medicine. The future role of the United States in global health: emphasis on cardiovascular disease. J Am Coll Cardiol 2017;70:3140–3156. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3. Leopold JA, Loscalzo J.. Emerging role of precision medicine in cardiovascular disease. Circ Res 2018;122:1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mensah GA, Jaquish C, Srinivas P, Papanicolaou GJ, Wei GS, Redmond N, Roberts MC, Nelson C, Aviles-Santa L, Puggal M, Green Parker MC, Minear MA, Barfield W, Fenton KN, Boyce CA, Engelgau MM, Khoury MJ.. Emerging concepts in precision medicine and cardiovascular diseases in racial and ethnic minority populations. Circ Res 2019;125:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 6. Okita K, Ichisaka T, Yamanaka S.. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448:313–317. [DOI] [PubMed] [Google Scholar]
- 7. Churko JM, Lee J, Ameen M, Gu M, Venkatasubramanian M, Diecke S, Sallam K, Im H, Wang G, Gold JD, Salomonis N, Snyder MP, Wu JC.. Transcriptomic and epigenomic differences in human induced pluripotent stem cells generated from six reprogramming methods. Nat Biomed Eng 2017;1:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weltner J, Balboa D, Katayama S, Bespalov M, Krjutškov K, Jouhilahti EM, Trokovic R, Kere J, Otonkoski T.. Human pluripotent reprogramming with CRISPR activators. Nat Commun 2018;9:2643–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnett SD, Blanchette AD, Grimm FA, House JS, Reif DM, Wright FA, Chiu WA, Rusyn I.. Population-based toxicity screening in human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Appl Pharmacol 2019;381:114711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karakikes I, Ameen M, Termglinchan V, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res 2015;117:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paik DT, Chandy M, Wu JC.. Patient and disease-specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol Rev 2020;72:320–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayed N, Liu C, Wu JC.. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 2016;67:2161–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo Y, Pu WT.. Cardiomyocyte maturation: new phase in development. Circ Res 2020;126:1086–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mosqueira D, Mannhardt I, Bhagwan JR, Lis-Slimak K, Katili P, Scott E, Hassan M, Prondzynski M, Harmer SC, Tinker A, Smith JGW, Carrier L, Williams PM, Gaffney D, Eschenhagen T, Hansen A, Denning C.. CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur Heart J 2018;39:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma N, Zhang JZ, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R, Wu H, Lam CK, Sayed N, Wu JC.. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation 2018;138:2666–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seeger T, Shrestha R, Lam CK, Chen C, McKeithan WL, Lau E, Wnorowski A, McMullen G, Greenhaw M, Lee J, Oikonomopoulos A, Lee S, Yang H, Mercola M, Wheeler M, Ashley EA, Yang F, Karakikes I, Wu JC.. A premature termination codon mutation in MYBPC3 causes hypertrophic cardiomyopathy via chronic activation of nonsense-mediated decay. Circulation 2019;139:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martewicz S, Magnussen M, Elvassore N.. Beyond family: modeling non-hereditary heart diseases with human pluripotent stem cell-derived cardiomyocytes. Front Physiol 2020;11:384. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chanda D, Oligschlaeger Y, Geraets I, Liu Y, Zhu X, Li J, Nabben M, Coumans W, Luiken JJFP, Glatz JFC, Neumann D.. 2-Arachidonoylglycerol ameliorates inflammatory stress-induced insulin resistance in cardiomyocytes. J Biol Chem 2017;292:7105–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei W, Liu Y, Zhang Q, Wang Y, Zhang X, Zhang H.. Danshen-enhanced cardioprotective effect of cardioplegia on ischemia reperfusion injury in a human-induced pluripotent stem cell-derived cardiomyocytes model. Artif Organs 2017;41:452–460. [DOI] [PubMed] [Google Scholar]
- 20. Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, Chen X, Jia J, Damon B, Wilson R, Starr Hazard E, Hardiman G, Menick DR, Beeson CC, Yao H, Ye T, Mei Y.. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng 2020;4:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN.. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 2014;13:419–431. [DOI] [PubMed] [Google Scholar]
- 22. Millard D, Dang Q, Shi H, Zhang X, Strock C, Kraushaar U, Zeng H, Levesque P, Lu HR, Guillon J-M, Wu JC, Li Y, Luerman G, Anson B, Guo L, Clements M, Abassi YA, Ross J, Pierson J, Gintant G.. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol Sci 2018;164:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stillitano F, Hansen J, Kong C-W, Karakikes I, Funck-Brentano C, Geng L, Scott S, Reynier S, Wu M, Valogne Y, Desseaux C, Salem J-E, Jeziorowska D, Zahr N, Li R, Iyengar R, Hajjar RJ, Hulot J-S.. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. Elife 2017;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, Zamora V, Smith G, Crumb WJ, Pang L, Lyn-Cook B, Ross J, Brock M, Chvatal S, Millard D, Galeotti L, Stockbridge N, Strauss DG.. Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol Sci 2017;155:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gintant G, Sager PT, Stockbridge N.. Evolution of strategies to improve preclinical cardiac safety testing. Nat Rev Drug Discov 2016;15:457–471. [DOI] [PubMed] [Google Scholar]
- 26. Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L, Brock M, Lu HR, Kraushaar U, Zeng H, Shi H, Zhang X, Sawada K, Osada T, Kanda Y, Sekino Y, Pang L, Feaster TK, Kettenhofen R, Stockbridge N, Strauss DG, Gintant G.. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep 2018;24:3582–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurzrock R, Kantarjian HM, Kesselheim AS, Sigal EV.. New drug approvals in oncology. Nat Rev Clin Oncol 2020;17:140–146. [DOI] [PubMed] [Google Scholar]
- 28. Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, Del Álamo JC, Wu SM, Moslehi JJ, Mercola M, Wu JC.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitani T, Ong S-G, Lam CK, Rhee J-W, Zhang JZ, Oikonomopoulos A, Ma N, Tian L, Lee J, Telli ML, Witteles RM, Sharma A, Sayed N, Wu JC.. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation 2019;139:2451–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas D, Shenoy S, Sayed N.. Building multi-dimensional induced pluripotent stem cells-based model platforms to assess cardiotoxicity in cancer therapies. Front Pharmacol 2021;12:607364. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR.. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 1994;90:713–725. [DOI] [PubMed] [Google Scholar]
- 33. van den Berg CW, Okawa S, Chuva de Sousa Lopes SM, van Iperen L, Passier R, Braam SR, Tertoolen LG, del Sol A, Davis RP, Mummery CL.. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development 2015;142:3231–3238. [DOI] [PubMed] [Google Scholar]
- 34. Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM.. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell 2017;21:179–194.e4. [DOI] [PubMed] [Google Scholar]
- 35. Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G.. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomas D, O'Brien T, Pandit A.. Toward customized extracellular niche engineering: progress in cell-entrapment technologies. Adv Mater 2018;30:1703948. [DOI] [PubMed] [Google Scholar]
- 37. Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao M-L, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller G, Gepstein L, Wu JC, Zimmermann W-H.. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017;135:1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaiser NJ, Kant RJ, Minor AJ, Coulombe KLK.. Optimizing blended collagen-fibrin hydrogels for cardiac tissue engineering with human ipsc-derived cardiomyocytes. ACS Biomater Sci Eng 2019;5:887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H, Murry CE.. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol 2014;72:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM.. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol 2017;35:56–68. [DOI] [PubMed] [Google Scholar]
- 41. Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park S-J, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK.. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater 2017;16:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giacomelli E, Bellin M, Sala L, van Meer BJ, Tertoolen LGJ, Orlova VV, Mummery CL.. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017;144:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qian F, Huang C, Lin Y-D, Ivanovskaya AN, O'Hara TJ, Booth RH, Creek CJ, Enright HA, Soscia DA, Belle AM, Liao R, Lightstone FC, Kulp KS, Wheeler EK.. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip 2017;17:1732–1739. [DOI] [PubMed] [Google Scholar]
- 44. Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M.. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016;16:599–610. [DOI] [PubMed] [Google Scholar]
- 45. Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T.. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J 2013;77:1307–1314. [DOI] [PubMed] [Google Scholar]
- 46. Rog-Zielinska EA, Craig M-A, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE.. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1α. Cell Death Differ 2015;22:1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC, Chapman KE.. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet 2013;22:3269–3282. [DOI] [PubMed] [Google Scholar]
- 48. Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, Ritterhoff J, Zhao L, Kolwicz SC, Pabon L, Reinecke H, Sniadecki NJ, Tian R, Ruohola-Baker H, Xu H, Murry CE.. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports 2019;13:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kenessey A, Ojamaa K.. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem 2006;281:20666–20672. [DOI] [PubMed] [Google Scholar]
- 50. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD.. Revisiting cardiac cellular composition. Circ Res 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kohl P, Gourdie RG.. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol 2014;70:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beauchamp P, Jackson CB, Ozhathil LC, Agarkova I, Galindo CL, Sawyer DB, Suter TM, Zuppinger C.. 3D co-culture of hiPSC-derived cardiomyocytes with cardiac fibroblasts improves tissue-like features of cardiac spheroids. Front Mol Biosci 2020;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parodi EM, Kuhn B.. Signalling between microvascular endothelium and cardiomyocytes through neuregulin. Cardiovasc Res 2014;102:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wan A, Rodrigues B.. Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovasc Res 2016;111:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pitaktong I, Lui C, Lowenthal J, Mattson G, Jung W-H, Bai Y, Yeung E, Ong CS, Chen Y, Gerecht S, Hibino N.. Early vascular cells improve microvascularization within 3D cardiac spheroids. Tissue Eng Part C Methods 2020;26:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Masumoto H, Nakane T, Tinney JP, Yuan F, Ye F, Kowalski WJ, Minakata K, Sakata R, Yamashita JK, Keller BB.. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci Rep 2016;6:29933–29910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sayed N, Liu C, Ameen M, Himmati F, Zhang JZ, Khanamiri S, Moonen J-R, Wnorowski A, Cheng L, Rhee J-W, Gaddam S, Wang KC, Sallam K, Boyd JH, Woo YJ, Rabinovitch M, Wu JC.. Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci Transl Med 2020;12:eaax9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palpant NJ, Pabon L, Friedman CE, Roberts M, Hadland B, Zaunbrecher RJ, Bernstein I, Zheng Y, Murry CE.. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat Protoc 2017;12:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shen M, , QuertermousT, , FischbeinM P, , Wu J C.. Generation of Vascular Smooth Muscle Cells From Induced Pluripotent Stem Cells. Circ Res 2021;128:670–686. 10.1161/CIRCRESAHA.120.318049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O'Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MHC, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgård P-O, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA.. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol 2015;17:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, Schmuck EG, Raval AN, da Rocha AM, Herron TJ, Jalife J, Thomson JA, Kamp TJ.. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat Commun 2019;10:2238–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang H, Shen M, Wu JC.. Generation of quiescent cardiac fibroblasts derived from human induced pluripotent stem cells. Methods Mol Biol 2020;146:77–77. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomas D, , KimH, , LopezN, , Wu J C.. Fabrication of 3D Cardiac Microtissue Arrays using Human iPSC-Derived Cardiomyocytes, Cardiac Fibroblasts, and Endothelial Cells. J Vis Exp 2021; 33779590 [DOI] [PubMed] [Google Scholar]
- 64. Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, Krotenberg Garcia A, Mircea M, Kostidis S, Davis RP, van Meer BJ, Jost CR, Koster AJ, Mei H, Míguez DG, Mulder AA, Ledesma-Terrón M, Pompilio G, Sala L, Salvatori DCF, Slieker RC, Sommariva E, de Vries AAF, Giera M, Semrau S, Tertoolen LGJ, Orlova VV, Bellin M, Mummery CL.. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 2020;26:862–879.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, Kelly RG, Kwon C, Lutolf MP.. Capturing cardiogenesis in gastruloids. Cell Stem Cell 2021;28:230–240.e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee J, Sutani A, Kaneko R, Takeuchi J, Sasano T, Kohda T, Ihara K, Takahashi K, Yamazoe M, Morio T, Furukawa T, Ishino F.. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun 2020;11:4283–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hofbauer P, Jahnel S, Papai N, Giesshammer M, Penc M, Tavernini K, Grdseloff N, Meledeth C, Deyett A, Schmidt C, Ctortecka C, Šalic Š, Novatchkova M, Mendjan S.. Cardioids reveal self-organizing principles of human cardiogenesis. bioRxiv 2020;146:2020.07.06.189431. [DOI] [PubMed] [Google Scholar]
- 68. Thomas D, Marsico G, Mohd Isa IL, Thirumaran A, Chen X, Lukasz B, Fontana G, Rodriguez B, Marchetti-Deschmann M, O'Brien T, Pandit A.. Temporal changes guided by mesenchymal stem cells on a 3D microgel platform enhance angiogenesis in vivo at a low-cell dose. Proc Natl Acad Sci USA 2020;117:19033–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK.. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Heart Circ Physiol 2014;306:H1525–H1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Speiser B, Weihrauch D, Riess CF, Schaper J.. The extracellular matrix in human cardiac tissue. Part II: vimentin, laminin, and fibronectin. Cardioscience 1992;3:41–49. [PubMed] [Google Scholar]
- 71. Mittal A, Pulina M, Hou S-Y, Astrof S.. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev Biol 2013;381:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE.. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. JAC 1994;23:1204–1208. [DOI] [PubMed] [Google Scholar]
- 73. Schwach V, Passier R.. Native cardiac environment and its impact on engineering cardiac tissue. Biomater Sci 2019;7:3566–3580. [DOI] [PubMed] [Google Scholar]
- 74. Goldfracht I, Efraim Y, Shinnawi R, Kovalev E, Huber I, Gepstein A, Arbel G, Shaheen N, Tiburcy M, Zimmermann WH, Machluf M, Gepstein L.. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater 2019;92:145–159. [DOI] [PubMed] [Google Scholar]
- 75. Wiegerinck RF, Cojoc A, Zeidenweber CM, Ding G, Shen M, Joyner RW, Fernandez JD, Kanter KR, Kirshbom PM, Kogon BE, Wagner MB.. Force frequency relationship of the human ventricle increases during early postnatal development. Pediatr Res 2009;65:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Börnchen C, Müller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T.. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 2014;74:151–161. [DOI] [PubMed] [Google Scholar]
- 77. Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Schwörer A, Uebeler J, Eschenhagen T.. Development of a drug screening platform based on engineered heart tissue. Circ Res 2010;107:35–44. [DOI] [PubMed] [Google Scholar]
- 78. Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, Nartiss Y, Keller G, Gepstein L.. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat Commun 2020;11:75–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Masumoto H, Yamashita JK.. Human iPS cell-engineered three-dimensional cardiac tissues perfused by capillary networks between host and graft. Inflamm Regen 2018;38:26–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ruan J-L, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE.. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 2016;134:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee J-J, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot J-S, Costa KD.. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 2014;28:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jackman CP, Carlson AL, Bursac N.. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 2016;111:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursac N.. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 2017;8:1825–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leonard A, Bertero A, Powers JD, Beussman KM, Bhandari S, Regnier M, Murry CE, Sniadecki NJ.. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J Mol Cell Cardiol 2018;118:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Truitt R, Mu A, Corbin EA, Vite A, Brandimarto J, Ky B, Margulies KB.. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac microtissue model. JACC Basic Transl Sci 2018;3:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, Wickeler A, Naguib HE, Keller GM, Vunjak-Novakovic G, Broeckel U, Backx PH, Radisic M.. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019;176:913–927.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kannel WB, Wolf PA, Castelli WP, D'Agostino RB.. Fibrinogen and risk of cardiovascular disease. The framingham study. JAMA 1987;258:1183–1186. [PubMed] [Google Scholar]
- 88. Weisel JW, Litvinov RI.. Fibrin formation, structure and properties. Subcell Biochem 2017;82:405–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E.. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017;547:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rog-Zielinska EA, Richardson RV, Denvir MA, Chapman KE.. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J Mol Endocrinol 2014;52:R125–R135. [DOI] [PubMed] [Google Scholar]
- 91. Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tønnessen T, Kryshtal DO, Louch WE, Knollmann BC.. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res 2017;121:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schaper J, Meiser E, Stämmler G.. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res 1985;56:377–391. [DOI] [PubMed] [Google Scholar]
- 93. Breckenridge RA, Piotrowska I, Ng K-E, Ragan TJ, West JA, Kotecha S, Towers N, Bennett M, Kienesberger PC, Smolenski RT, Siddall HK, Offer JL, Mocanu MM, Yelon DM, Dyck JRB, Griffin JL, Abramov AY, Gould AP, Mohun TJ.. Hypoxic regulation of hand1 controls the fetal-neonatal switch in cardiac metabolism. PLoS Biol 2013;11:e1001666. Tian R, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Makinde AO, Kantor PF, Lopaschuk GD.. Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol Cell Biochem 1998;188:49–56. [PubMed] [Google Scholar]
- 95. Correia C, Koshkin A, Duarte P, Hu D, Teixeira A, Domian I, Serra M, Alves PM.. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci Rep 2017;7:8590–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hu D, Linders A, Yamak A, Correia C, Kijlstra JD, Garakani A, Xiao L, Milan DJ, van der Meer P, Serra M, Alves PM, Domian IJ.. Metabolic maturation of human pluripotent stem cell-derived cardiomyocytes by inhibition of HIF1α and LDHA. Circ Res 2018;123:1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hörmann L, Ulmer B, Zhang H, Briganti F, Schweizer M, Hegyi B, Liao Z, Pölönen R-P, Ginsburg KS, Lam CK, Serrano R, Wahlquist C, Kreymerman A, Vu M, Amatya PL, Behrens CS, Ranjbarvaziri S, Maas RGC, Greenhaw M, Bernstein D, Wu JC, Bers DM, Eschenhagen T, Metallo CM, Mercola M.. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep 2020;32:107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang Q-D, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE.. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA 2017;114:E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, Funcke S, Murphy E, Eschenhagen T, Hansen A.. Contractile work contributes to maturation of energy metabolism in hiPSC-derived cardiomyocytes. Stem Cell Reports 2018;10:834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Batalov I, Feinberg AW.. Differentiation of cardiomyocytes from human pluripotent stem cells using monolayer culture. Biomark Insights 2015;10:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, Bai X.. Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cells 2019;8:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL.. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 2015;112:12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Thavandiran N, Nunes SS, Xiao Y, Radisic M.. Topological and electrical control of cardiac differentiation and assembly. Stem Cell Res Ther 2013;4:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chan Y-C, Ting S, Lee Y-K, Ng K-M, Zhang J, Chen Z, Siu C-W, Oh SKW, Tse H-F.. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J Cardiovasc Trans Res 2013;6:989–999. [DOI] [PubMed] [Google Scholar]
- 105. Badenes SM, Fernandes TG, Cordeiro CSM, Boucher S, Kuninger D, Vemuri MC, Diogo MM, Cabral JMS.. Defined Essential 8™ medium and vitronectin efficiently support scalable xeno-free expansion of human induced pluripotent stem cells in stirred microcarrier culture systems. PLoS One 2016;11:e0151264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kroll K, Chabria M, Wang K, Häusermann F, Schuler F, Polonchuk L.. Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog Biophys Mol Biol 2017;130:212–222. [DOI] [PubMed] [Google Scholar]
- 107. Zhang W, Kong CW, Tong MH, Chooi WH, Huang N, Li RA, Chan BP.. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: effects of niche cell supplementation and mechanical stimulation. Acta Biomater 2017;49:204–217. [DOI] [PubMed] [Google Scholar]
- 108. Zhao M-T, Shao N-Y, Garg V.. Subtype-specific cardiomyocytes for precision medicine: where are we now? Stem Cells 2020;38:822–833. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Huang CY, Peres Moreno Maia-Joca R, Ong CS, Wilson I, DiSilvestre D, Tomaselli GF, Reich DH.. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J Mol Cell Cardiol 2020;138:1–11. [DOI] [PubMed] [Google Scholar]
- 110. Kempf H, Olmer R, Kropp C, Rückert M, Jara-Avaca M, Robles-Diaz D, Franke A, Elliott DA, Wojciechowski D, Fischer M, Roa Lara A, Kensah G, Gruh I, Haverich A, Martin U, Zweigerdt R.. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports 2014;3:1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP.. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 2012;109:E1848–E1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kirouac DC, Zandstra PW.. The systematic production of cells for cell therapies. Cell Stem Cell 2008;3:369–381. [DOI] [PubMed] [Google Scholar]
- 113. Burridge PW, Sharma A, Wu JC.. Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu Rev Genet 2015;49:461–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC.. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lian X, Bao X, Zilberter M, Westman M, Fisahn A, Hsiao C, Hazeltine LB, Dunn KK, Kamp TJ, Palecek SP.. Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods 2015;12:595–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tohyama S, Fujita J, Fujita C, Yamaguchi M, Kanaami S, Ohno R, Sakamoto K, Kodama M, Kurokawa J, Kanazawa H, Seki T, Kishino Y, Okada M, Nakajima K, Tanosaki S, Someya S, Hirano A, Kawaguchi S, Kobayashi E, Fukuda K.. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Reports 2017;9:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Buikema JW, Lee S, Goodyer WR, Maas RG, Chirikian O, Li G, Miao Y, Paige SL, Lee D, Wu H, Paik DT, Rhee S, Tian L, Galdos FX, Puluca N, Beyersdorf B, Hu J, Beck A, Venkamatran S, Swami S, Wijnker P, Schuldt M, Dorsch LM, van Mil A, Red-Horse K, Wu JY, Geisen C, Hesse M, Serpooshan V, Jovinge S, Fleischmann BK, Doevendans PA, van der Velden J, Garcia KC, Wu JC, Sluijter JPG, Wu SM.. Wnt activation and reduced cell-cell contact synergistically induce massive expansion of functional human iPSC-derived cardiomyocytes. Cell Stem Cell 2020;27:50–63.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ.. Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays. Sci Rep 2016;6:24637–24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, Liu J-C, Chai J, Gold J, Wu J, Hsu D, Couture LA.. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res 2015;15:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fonoudi H, Ansari H, Abbasalizadeh S, Larijani MR, Kiani S, Hashemizadeh S, Zarchi AS, Bosman A, Blue GM, Pahlavan S, Perry M, Orr Y, Mayorchak Y, Vandenberg J, Talkhabi M, Winlaw DS, Harvey RP, Aghdami N, Baharvand H.. A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl Med 2015;4:1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hamad S, Derichsweiler D, Papadopoulos S, Nguemo F, Sarić T, Sachinidis A, Brockmeier K, Hescheler J, Boukens BJ, Pfannkuche K.. Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations. Theranostics 2019;9:7222–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Halloin C, Schwanke K, Löbel W, Franke A, Szepes M, Biswanath S, Wunderlich S, Merkert S, Weber N, Osten F, la Roche de J, Polten F, Christoph Wollert K, Kraft T, Fischer M, Martin U, Gruh I, Kempf H, Zweigerdt R.. Continuous WNT control enables advanced hPSC cardiac processing and prognostic surface marker identification in chemically defined suspension culture. Stem Cell Reports 2019;13:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Correia C, Serra M, Espinha N, Sousa M, Brito C, Burkert K, Zheng Y, Hescheler J, Carrondo MJT, Sarić T, Alves PM.. Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev Rep 2014;10:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Badenes SM, Fernandes TG, Miranda CC, Pusch-Klein A, Haupt S, Rodrigues CA, Diogo MM, Brüstle O, Cabral JM.. Long-term expansion of human induced pluripotent stem cells in a microcarrier-based dynamic system. J Chem Technol Biotechnol 2017;92:492–503. [Google Scholar]
- 125. Ting S, Chen A, Reuveny S, Oh S.. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Res 2014;13:202–213. [DOI] [PubMed] [Google Scholar]
- 126. Ting S, Lam A, Tong G, Chen A, Wei H, Wu J, Lam YN, Reuveny S, Oh S.. Meticulous optimization of cardiomyocyte yields in a 3-stage continuous integrated agitation bioprocess. Stem Cell Res 2018;31:161–173. [DOI] [PubMed] [Google Scholar]
- 127. Zhang J Z, , TermglinchanV, , ShaoN-Y, , ItzhakiI, , LiuC, , MaN, , TianL, , WangV Y, , ChangA C Y, , GuoH, , KitaniT, , WuH, , LamC K, , KodoK, , SayedN, , BlauH M, , Wu J C.. A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles. Cell Stem Cell 2019;24:802–811.e5. 10.1016/j.stem.2019.02.015 30880024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, Chuva de Sousa Lopes SM, Mummery CL, Verkerk AO, Passier R.. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med 2015;7:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y.. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res 2011;21:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mommersteeg MTM, Domínguez JN, Wiese C, Norden J, de Gier-de Vries C, Burch JBE, Kispert A, Brown NA, Moorman AFM, Christoffels VM.. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc Res 2010;87:92–101. [DOI] [PubMed] [Google Scholar]
- 131. Espinoza-Lewis RA, Yu L, He F, Liu H, Tang R, Shi J, Sun X, Martin JF, Wang D, Yang J, Chen Y.. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev Biol 2009;327:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, la Riviere de AB, Passier R, Tertoolen L.. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 2003;107:2733–2740. [DOI] [PubMed] [Google Scholar]
- 133. Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM.. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008;453:524–528. [DOI] [PubMed] [Google Scholar]
- 134. Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, Skelton RJP, Ward-van Oostwaard D, Lim SM, Khammy O, Li X, Hawes SM, Davis RP, Goulburn AL, Passier R, Prall OWJ, Haynes JM, Pouton CW, Kaye DM, Mummery CL, Elefanty AG, Stanley EG.. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 2011;8:1037–1040. [DOI] [PubMed] [Google Scholar]
- 135. Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G.. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011;8:228–240. [DOI] [PubMed] [Google Scholar]
- 136. Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ.. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 2012;111:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP.. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc 2013;8:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Laco F, Lam AT-L, Woo T-L, Tong G, Ho V, Soong P-L, Grishina E, Lin K-H, Reuveny S, Oh SK-W.. Selection of human induced pluripotent stem cells lines optimization of cardiomyocytes differentiation in an integrated suspension microcarrier bioreactor. Stem Cell Res Ther 2020;11:118–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ebert A, Joshi AU, Andorf S, Dai Y, Sampathkumar S, Chen H, Li Y, Garg P, Toischer K, Hasenfuss G, Mochly-Rosen D, Wu JC.. Proteasome-Dependent Regulation of Distinct Metabolic States During Long-Term Culture of Human iPSC-Derived Cardiomyocytes. Circ Res 2019;125:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]