Abstract
The biosynthetic origins of the structurally related racemic isoxazolidine Papaveracaea alkaloids Setigerumine I, Dactylicapnosinine and Dactylicapnosine have remained elusive since their original isolation over two decades ago. Herein we report the first biosynthetic hypothesis for their formation and, inspired by it, the first synthesis of (±)-Setigerumine I with accompanying computational rationale. Based on the results, these isoxazolidine alkaloids arise from racemizing oxidative rearrangements of prominent isoquinoline alkaloids Noscapine and Hydrastine. The key steps featured in this synthesis are a room temperature Cope elimination and a domino C–H oxidation/inverse-electron demand 1,3-dipolar cycloaddition of an axially chiral, yet configurationally unstable, intermediate.
Graphical Abstract

Isoquinoline alkaloids, such as morphine, are among the earliest compounds to be used by humans to treat illnesses.1 Numerous prominent isoquinoline alkaloids have since been isolated and used as pharmaceuticals.1 Despite almost a 100 years of studies into this vast family of alkaloids, several benzylisoquinoline alkaloids remain elusive. Three such trace alkaloids are naturally racemic Setigerumine I (1), Dactylicapnosinine (2) and Dactylicapnosine (3), that have been isolated from Papaveracae family plants in 1995 [for Dactylicapnosinine (2) and Dactylicapnosine (3)] and 2013 [Setigerumine I (1)] (Scheme 1A).2,3 These alkaloids are structurally unique as they feature a central spiro-isoxazolidine ring and also constitute the only known example of an entire group of structurally related benzylisoquinoline alkaloids, which occur naturally as racemates. Since their isolation, the biosynthetic origins and the chemical synthesis of these obscure racemic alkaloids have remained elusive.
Scheme 1:
A: Structurally related racemic isoxazolidine alkaloids Setigerumine I (1), Dactylicapnosinine (2) and Dactylicapnosine (3) all share the same spirocyclic core. B: Proposed biosynthetic connection of isoxazolidine alkaloids 1–3 to known phtalideisoquinoline alkaloids Noscapine (4) and β-Hydrastine (5) via an oxidative rearrangement pathway.
We envisioned that through the total synthesis of Setigerumine I (1), we would be able to elucidate a plausible biosynthesis and understand why not only Setigerumine I (1), but also Dactylicapnosinine (2) and Dactylicapnosine (3) exist in Nature as racemates.4–6 Our synthetic work began by carefully analyzing the unique connectivity in the structures of the target molecules. Setigerumine I (1) is structurally related to Dactylicapnosine (3), which has a pronounced pseudo-dimeric structure (Scheme 1A). The pseudo-dimeric structure of Dactylicapnosine (3) incorporates in its structure a possible clue to the biogenesis of this alkaloid family: the northern and southern pseudo-dimeric units of Dactylicapnosine (3) can be mapped onto two known phtalideisoquinoline alkaloids Noscapine (4) and Hydrastine (5). Both alkaloids are known to occur in Papaveracae family plants and could therefore likely be the corresponding biogenetic starting materials.2,7
Considering an oxidative rupture of the C-ring of the phtalideisoquinoline core of the proposed biosynthetic starting materials 4 or 5, an intermediate hydroxylamine secophtalidoisoquinoline 6 could form (Scheme 1B). This Cope elimination reaction could proceed through the formation of Noscapine-N-oxide, which is known to form via P450 oxidation of Noscapine (4).8–11 If the axial chirality, transferred to 6 from enantiopure parent phtalideisoquinoline alkaloid, is scrambled due to interconversion of rotamers, all materials downstream from this precursor 6 would be racemic. Further C–H oxidation of 6 could generate a nitrone dipole 7, which could take part in an intramolecular 1,3-dipolar cycloaddition with the previously generated enol-lactone dipolarophile. The 1,3-dipolar cycloaddition would in turn forge the spirocyclic isoxazolidine core of Setigerumine I (1), Dactylicapnosinine (2) and Dactylicapnosine (3). This proposal agrees with the general tendency of racemic natural products to arise via the facile cyclization of achiral precursors.12,13 Further corroborating this logic, the southern fragment of Dactylicapnosine (3) incorporates the putative biosynthetic seco-isoquinoline intermediate 6 in its structure. Therefore, a plausible pathway for the formation of 3 involves a final C–H oxidation at the benzylic site of 2 to form the para-quinone-methide 8. Subsequent nucleophilic attack at C-1 by the hydroxylamine moiety of 6 would furnish the pseudo-dimeric alkaloid 3. Herein we report the total synthesis of (±)-Setigerumine I (1) inspired by this biosynthetic proposal.
We initiated our synthetic campaign toward (±)-Setigerumine I (1) by first considering the oxidation of the commercially available parent isoquinoline alkaloid (−)-Noscapine (4) to Noscapine-N-oxide hydrochloride (9). This would set the stage for a Cope-elimination to rupture the isoquinoline C-ring, giving the seco enol-lactone 6. While the N-oxidation of Noscapine (4) with m-CPBA, H2O2 and enzymatic methods have been reported in the literature, the configuration of the N-oxide product 9 had not been determined.14–17 Toward this end, Noscapine (4) was oxidized with m-CPBA in chloroform at 0 °C to Noscapine-N-oxide hydrochloride (9), which, after an extractive work-up, gave the pure syn-stereoisomer in 88% yield.16 The syn configuration of the N-oxide 9 was determined by characteristic NOE correlations from phtalide D ring H-α to tetrahydroquinoline C ring H-2pseudoax, and from tetrahydroquinoline C ring H-3 to N-Me hydrogens H-Me (Scheme 2, inset). To obtain high-purity syn-Noscapine-N-oxide hydrochloride (9), a sequence of consecutive basic, acidic and neutral aqueous washes was developed to remove the unwanted anti-diastereomer (See ESI).
Scheme 2:
Synthesis of Setigerumine I (1) and Isosetigerumine I (11). Reagents and conditions: 1. m-CPBA (2.0 equiv.), CHCl3, 0 °C, 88%; 2. Basification with 2 M NaOH then CHCl3; rt, 21 h; 3. Cu(OAc)2 (0.1 equiv.), air, DCM, rt, 6 h, regioisomeric ratio 1:2 (based on crude 1H NMR), 32% combined yield over two steps.
Having secured access to Noscapine-N-oxide hydrochloride (9), we then turned our attention to the Cope-elimination leading to hydroxylamine 6. As indicated by NOE data, Noscapine-N-oxide hydrochloride (9) is perfectly predisposed to undergo the desired Cope elimination: the N-oxide oxygen is oriented syn-periplanar to the phtalide lactone D-ring H-α hydrogen. However, attempts at thermally eliminating the N-oxide hydrochloride salt 9 (PhMe, 130 °C) only led to the recovery of the unchanged starting material. We therefore converted the hydrochloride salt 9 to the corresponding free-base by partitioning it between 2.0 M aqueous NaOH and chloroform. In sharp contrast to the reluctance of the hydrochloride salt of 9 to eliminate, the elimination of the free-base 9 proved extremely facile. At room temperature, the spontaneous Cope elimination reached maximum conversion of 71% of 6, based on 1H NMR, in 21 hours. Compared to the forcing Cope elimination conditions (i.e., usually >100 °C and in non-polar solvents), this transformation (9 → 6) proceeds under unusually mild conditions.18 Product 6 was found to be somewhat unstable and prone to degrade: heating or storing the neat or dissolved material 6 for longer periods of time (>24 h) started consuming 6 to form a previously described 8-membered cyclic hydroxylamine ether.19 Attempted purification of 6 on silica gel, by either preparative TLC or flash column chromatography, led to full or partial decomposition of the material to a complex mixture. Thankfully, this did not hinder our synthesis, as the crude hydroxylamine product 6 was pure enough to screen the following oxidation step.
We then addressed the oxidation of crude hydroxylamine 6 to the corresponding nitrone 10 for the upcoming dipolar cycloaddition step. Hydroxylamine 6 can undergo a C–H oxidation either at the N-Me group or at the C-2 position of the N-alkyl group, yielding two possible regioisomeric nitrones 10 and 10’. We expected the major product to be the desired aliphatic C-2 oxidized nitrone precursor 10 leading to Setigerumine I (1), as similar regiochemical oxidation preferences have previously been reported for N-methyl-N-alkyl hydroxylamines with several different oxidants.20–23
Our first choice for the oxidation of hydroxylamine 6 was mercury(II)oxide (HgO), as it has been demonstrated to have a markedly high preference for oxidizing aliphatic groups over N-methyl groups for a range of different hydroxylamines.20,21 To our pleasant surprise, when crude 6 was treated with orange mercury(II)oxide HgO (2.0 equiv., DCM, rt, 3 h), we found that the reaction directly yielded our target molecule (±)-Setigerumine I (1) in 27% isolated yield over two steps! This result implies that the in situ formed nitrone intermediate 10, formed upon oxidation of 6, undergoes a spontaneous 1,3-dipolar cycloaddition to yield Setigerumine I (1). It is noteworthy, that similarly to the Cope elimination step, this 1,3-dipolar cycloaddition also takes place under remarkably mild conditions when compared to typically employed approaches (e.g., >100 °C, nonpolar solvents, lengthy reaction times).24–26 This remarkably concise two-step C–H oxidation/dipolar cycloaddition domino reaction allowed us to complete the first total synthesis of Setigerumine I (1) in just three steps from commercially available (–)-Noscapine (4).27–29 In addition to Setigerumine I (1), we also isolated the regioisomeric cycloaddition product 11 in 14% isolated yield. We named this regioisomeric product Isosetigerumine I (11). This isomer of Setigerumine I (1) results from a similar spontaneous 1,3-dipolar cycloaddition domino reaction of the N-methyl oxidized nitrone regioisomer 10’.
With these encouraging results in hand, showing that upon oxidation hydroxylamine 6 is directly converted to Setigerumine I (1) in a C–H oxidation/1,3-dipolar cycloaddition domino reaction, we set out to identify alternative (i.e., less hazardous) oxidants. IBX (1.5 equiv., DCM, rt, 2 h) led to desired hydroxylamine oxidation-cycloaddition domino reaction albeit in lower yields (18% for 1; 11% for 11).30,31 We also considered biomimetic Cu(II) oxidation methods, as numerous aerobic copper-catalyzed oxygenations are established as key steps in important biosynthetic routes, such as the oxidation of polyphenols.32,33 Under operationally simple aerobic oxidation conditions, that involve bubbling air through a DCM solution of 6 with 10 mol-% of copper(II)acetate as a catalyst, 6 was cleanly converted to Setigerumine I (1) in 22% and Isosetigerumine I (11) in 10% isolated yields, respectively (Scheme 2).22 This aerobic copper-catalyzed oxidation method ties back to our biosynthetic hypothesis, and was therefore used as the key preparative method to complete the total synthesis of (±)-Setigerumine I (1) on a 20 mg scale and 19% overall yield from (−)-Noscapine (4) (Scheme 2).
The 1H and 13C data for synthetic Setigerumine I (1) were in full agreement with those reported for the isolated material.2 At the outset of this study, it was not clear if the axially chiral atropisomer of the hydroxylamine intermediate 6, formed by Cope elimination of (−)-4, would be configurationally stable at room temperature. If the rotational barrier for the interconversion between rotamers of 6 was sufficiently low, the stereochemical information of the parent isoquinoline alkaloid would be lost. As neither synthetic Setigerumine I (1) nor Isosetigerumine I (11) displayed optical activity, the chiral information of 6 must have been lost. The observed stereochemical scrambling from enantiopure (−)-4 to racemic (±)-1 during the course of our biomimetic total synthesis therefore provides an explanation as to why the alkaloid family members 1, 2 and 3 exist as racemates in Nature.
As Cope eliminations and 1,3-dipolar cycloadditions traditionally occur only at elevated temperatures and in non-polar solvents (i.e., >100 °C, PhMe or benzene), we were intrigued to find that the key steps, Cope elimination 9 → 6 and 1,3-dipolar cycloaddition 10/10’ → 1/11, of our synthetic route were so facile that both reactions occurred at room temperature.18 To gain further insights into these facile processes, we embarked on studying these systems computationally. Full conformational space of all stationary points 9, 6, 10, 10’, 1 and 11 were first studied using metadynamics (GFN2) with crest 2.11/xtb 6.4.0 and the resulting non-degenerate conformers (<10 kcal/mol, 298 K) were further DFT (B97-D3/DEF2SVP) optimized using orca 4.1.2.34–37 Final DFT geometry optimization and energy calculations were carried out using B97-D3/TZVP level of theory both in gas phase and using CPCM solvent model for chloroform and dichloromethane at 298.15 K. Relevant transition states were identified using climbing image nudged elastic bond method (CI-NEB, B97-D3/DEF2SVP) connecting the stationary points as implemented in orca 4.1.2, and further optimized with higher level DFT (B97-D3/TZVP) in both gas phase and using CPCM solvent model.
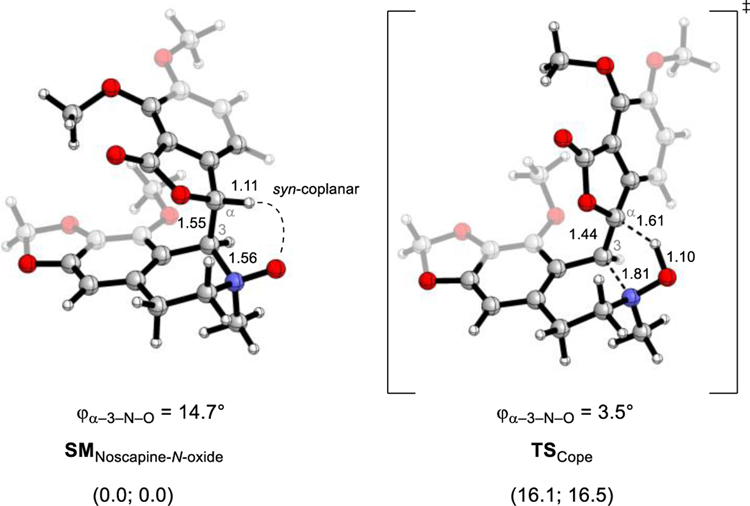
Compared to typical Cope-eliminations, where amine N-oxides are eliminated at elevated temperatures (e.g., >100 °C), the spontaneous rupture of the C ring of Noscapine-N-oxide (9) free-base at room temperature constitutes a rare example of an extremely low-temperature Cope elimination.38–40 Whereas most Cope eliminations require the system to first rotate to a reasonably high-energy syn-periplanar conformer, in the case of Noscapine-N-oxide (9) freebase, the folded scaffold’s minimum energy conformers all display a syn-coplanar arrangement of H-α with the N-oxide oxygen (Figure 1). This conformational preference is also corroborated by the NOE data we obtained for 9 (Scheme 2). This is reflected in the relevant dihedral angle, as it shows only a slight decrease from ϕα–3–N–O = 14.7° in the starting material to ϕα–3–N–O = 3.5° in the transition state. Consequently, the computed solution phase activation energy barrier lies reasonably low at ΔGTS, Cope = 16.1 kcal/mol and ΔHTS,Cope = 16.5 kcal/mol, as the Noscapine-N-oxide (9) system does not have to overcome any significant rotational barriers to reach the syn-coplanar transition state.
Figure 1:
The facility of the Cope elimination is explained by the close conformational similarity between the minimum energy conformer of the Noscapine-N-oxide (9) and the resulting transition state. Both display the N-oxide syn-co-planar with the eliminating hydrogen. Distances are reported in Ångström and solvent energies (ΔG; ΔH) in kcal/mol at 298.15 K relative to the starting material.
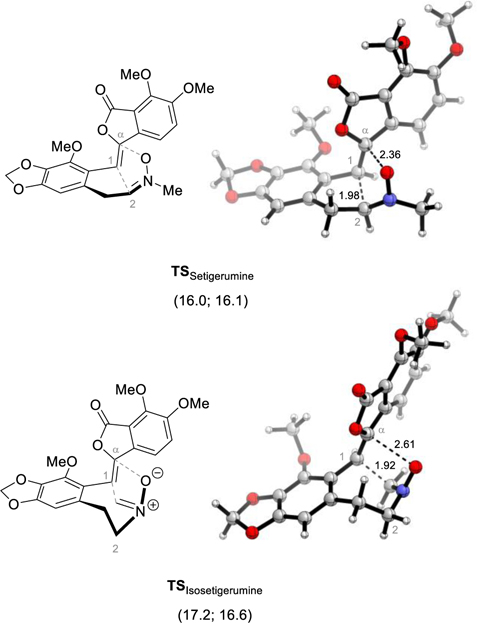
The 1,3-dipolar cycloaddition forming Setigerumine I (1) and Isosetigerumine I (11) also occurs at room temperature, with enol-lactone 6 starting material being consumed in just several hours with all screened oxidants. The uncatalyzed intramolecular dipolar cycloadditions to form Setigerumine I (1) and Isosetigerumine I (11) proceeded with reasonably low energy barriers in solution ΔGTS, Dipolar = 19.3 kcal/mol; ΔHTS,Dipolar = 18.6 kcal/mol, and ΔGTS, Dipolar = 17.2 kcal/mol; ΔHTS,Cope = 16.6 kcal/mol respectively (Figure 2). While these energies agree with our experimental observations, the possible activation of nitrones 10 and 10’ by Lewis-acidic oxidants cannot be ruled out at this stage.41
Figure 2:
Dipolar cycloaddition transition states leading to Setigerumine I (1) and Isosetigerumine (11). Distances are reported in Ångström and solvent energies (ΔG; ΔH) in kcal/mol at 298.15 K relative to the starting material.
We expected the intramolecular [3+2] reaction, taking nitrone 10 to Setigerumine I (1), to be an inverse electron-demand 1,3-dipolar cycloaddition reaction, as the nitrone dipole of 10 reacts with a reasonably electron-rich enol ether dipolarophile. These inverse electron-demand processes are markedly underdeveloped when compared to their normal electron-demand counterpairs.42–44 As expected, the electron-rich enol ether dipolarophile has a dominant Nualkene–Enitrone interaction, which is evident from the energetics of the relevant orbitals involved in the reaction.45 For nitrone 10 forming Setigerumine I (1), the HOMOenol–LUMO+1nitrone = +3.04 eV interaction energy is much lower than the alternative pairing arrangement HOMO-19nitrone–LUMOenol = +6.65 eV (See ESI). In contrast, rather surprisingly, the reaction of nitrone 10’ to form Isosetigerumine I (11) is better described as an ambiphilic dipolar cycloaddition, where both the Nualkene–Enitrone and Nunitrone–Ealkene energy gaps are very similar. For nitrone 10’ forming Isosetigerumine I (11), the HOMOenol–LUMO+1nitrone = +2.99 eV interaction energy is very close to the alternative orbital energy gap HOMO-2nitrone–LUMOenol = +3.03 eV (See ESI). Although, intuitively, a dominant Nu contribution of the electron-rich enol-lactone could be expected for both systems 10 and 10’, this highlights that the electronic nature of even seemingly similar 1,3-dipolar cycloadditions can be vastly different.
In conclusion, we have reported the first synthesis of (±)-Setigerumine I (1) and chemically demonstrated a plausible oxidative biogenetic pathway for its formation from (−)-Noscapine (4) under mild conditions. The proposed biogenetic pathway involves the following steps: (1) N-Oxidation of a parent phtalidoisoquinoline alkaloid; (2) Spontaneous room-temperature Cope elimination of the N-oxide to form an intermediate secophtalidoisoquinoline; (3) Further oxidation of the secophtalidoisoquinoline to a nitrone, and (4) A spontaneous intramolecular inverse electron-demand 1,3-dipolar cycloaddition of the nitrone to form the rearranged spiro-isoxazolidine alkaloid. Based on these results, the biogenetic origins of all three known spiroketal-isoxazolidine alkaloids Setigerumine I (1), Dactylicapnosinine (2) and Dactylicapnosine (3) can be traced back to similar oxidative rearrangement processes described herein.
Furthermore, the synthesis reported herein showed that both Setigerumine I (1) and Isosetigerumine I (11) can form as long as the oxidation of the common biosynthetic secophtalidoisoquinoline intermediate 6 is not highly regioselective. Accordingly, if a non-selective oxidation process occurs during their biosynthesis, the side-product of our synthesis, Isosetigerumine I (11), may well exist in Nature as a secondary metabolite but has yet to be discovered. Also, as a direct corollary of this work, we postulate that similar rearranged alkaloids derived from other known phtalidoisoquinoline alkaloids: Bicuculline, Adlumine, and Narcotoline may also exist.46–49 This is further corroborated by the computational insights, which hint that the required Cope eliminations and 1,3-dipolar cycloadditions can very readily occur under mild conditions in Nature as parts of biosynthetic routes.
Supplementary Material
Acknowledgements:
We thank Prof. Bang Yeon Hwang at College of Pharmacy, Chungbuk National University for providing the original NMR data for isolated Setigerumine I.
Funding sources:
J. H. S. gratefully acknowledges funding from the Osk. Huttunen Foundation. A. V. S. gratefully acknowledges funding from Zevi & Bertha Salsburg Memorial Fellowship of Rice University. L. K. gratefully acknowledges funding from the National Science Foundation (CHE-2102462), National Institutes of Health (R35 GM-136373), Robert A. Welch Foundation (C-1764).
Footnotes
Associated content:
The electronic supporting informational details all experimental and computational work together with NMR data, coordinates and computed energies.
References:
- (1).Hagel JM; Facchini PJ Benzylisoquinoline Alkaloid Metabolism: A Century of Discovery and a Brave New World. Plant Cell Physiol. 2013, 54 (5), 647–672. 10.1093/pcp/pct020. [DOI] [PubMed] [Google Scholar]
- (2).Lee C; Choe S; Lee JW; Jin Q; Lee MK; Hwang BY Alkaloids from Papaver Setigerum. Bull. Korean Chem. Soc. 2013, 34 (4), 1290–1292. 10.5012/bkcs.2013.34.4.1290. [DOI] [Google Scholar]
- (3).Zhang GL; Rücker G; Breitmaier E; Nieger M; Mayer R; Steinbeck C Alkaloids from Dactylicapnos Torulosa. Phytochemistry 1995, 40 (1), 299–305. 10.1016/00319-422(95)00192-A. [DOI] [Google Scholar]
- (4).Godfrey RC; Green NJ; Nichol GS; Lawrence AL Total Synthesis of Brevianamide A. Nat. Chem. 2020, 12 (7), 615–619. 10.1038/s41557-020-0442-3. [DOI] [PubMed] [Google Scholar]
- (5).Murray LAM; McKinnie SMK; Pepper HP; Erni R; Miles ZD; Cruickshank MC; López-Pérez B; Moore BS; George JH Total Synthesis Establishes the Biosynthetic Pathway to the Naphterpin and Marinone Natural Products. Angew. Chemie - Int. Ed. 2018, 57 (34), 11009–11014. 10.1002/anie.201804351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yang B; Wen G; Zhang Q; Hou M; He H; Gao S Asymmetric Total Synthesis and Biosynthetic Implications of Perovskones, Hydrangenone, and Hydrangenone B. J. Am. Chem. Soc. 2021, 143 (17), 6370–6375. 10.1021/jacs.1c02674. [DOI] [PubMed] [Google Scholar]
- (7).Slavík J Der Mohngewächse Alkaloide. Aus Stylomecon Heterophylla (BENTH.) Über Die Alkaloide TAYL G, MAXIM Hylomecon Vernalis. Und Aus Den Blättern von Sanguinaria Canadensis L Collect. Czechoslov. Chem. Commun. 1967, 32 (12), 4431–4438. 10.1135/cccc19674431. [DOI] [Google Scholar]
- (8).Richards L; Lutz A; Chalmers DK; Jarrold A; Bowser T; Stevens GW; Gras SL Production of Metabolites of the Anti-Cancer Drug Noscapine Using a P450BM3 Mutant Library. Biotechnol. Reports 2019, 24, e00372. 10.1016/j.btre.2019.e00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Richards L; Jarrold A; Bowser T; Stevens GW; Gras SL Cytochrome P450-Mediated N-Demethylation of Noscapine by Whole-Cell Biotransformation: Process Limitations and Strategies for Optimisation. J. Ind. Microbiol. Biotechnol. 2020, 47 (6–7), 449–464. 10.1007/s10295-020-02283-7. [DOI] [PubMed] [Google Scholar]
- (10).Rosco A; Pauli HH; Priesner W; Kutchan TM Cloning and Heterologous Expression of NADPH-Cytochrome P450 Reductases from the Papaveraceae. Arch. Biochem. Biophys. 1997, 348 (2), 369–377. 10.1006/abbi.1997.0374. [DOI] [PubMed] [Google Scholar]
- (11).Nguyen T-D; Dang T-TT Cytochrome P450 Enzymes as Key Drivers of Alkaloid Chemical Diversification in Plants. Front. Plant Sci. 2021, 12, 1272. 10.3389/FPLS.2021.682181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zask A; Ellestad G Biomimetic Syntheses of Racemic Natural Products. Chirality. John Wiley and Sons Inc. February 1, 2018, pp 157–164. 10.1002/chir.22786. [DOI] [PubMed] [Google Scholar]
- (13).Novak AJE; Trauner D Reflections on Racemic Natural Products. Trends in Chemistry. Cell Press December 1, 2020, pp 1052–1065. 10.1016/j.trechm.2020.10.005. [DOI] [Google Scholar]
- (14).Babanezhad Harikandei K; Salehi P; Ebrahimi SN; Bararjanian M; Kaiser M; Khavasi HR; Al-Harrasi A N-Substituted Noscapine Derivatives as New Antiprotozoal Agents: Synthesis, Antiparasitic Activity and Molecular Docking Study. Bioorg. Chem. 2019, 91, 103116. 10.1016/j.bioorg.2019.103116. [DOI] [PubMed] [Google Scholar]
- (15).Aggarwal S; Ghosh NN; Aneja R; Joshi H; Chandra R A Convenient Synthesis of Aryl-Substituted N-Carbamoyl/N-Thiocarbamoyl Narcotine and Related Compounds. 10.1002/1522-2675. [DOI] [PubMed]
- (16).Awalt JK; Lam R; Kellam B; Graham B; Scammells PJ; Singer RD Utility of Iron Nanoparticles and a Solution-Phase Iron Species for the N-Demethylation of Alkaloids. Green Chem. 2017, 19, 2587. 10.1039/c7gc00436b. [DOI] [Google Scholar]
- (17).Debono AJ; Xie JH; Ventura S; Pouton CW; Capuano B; Scammells PJ Synthesis and Biological Evaluation of N-Substituted Noscapine Analogues. ChemMedChem 2012, 7 (12), 2122–2133. 10.1002/cmdc.201200365. [DOI] [PubMed] [Google Scholar]
- (18).Kürti L; Czakó B Strategic Applications of Named Reactions in Organic Synthesis - Negishi Cross Coupling; Academic Press, 2005. [Google Scholar]
- (19).Klötzer W; Oberhänsli WE Die Struktur Des Sogenannten Anhydro-N-Oxy-Nornarceins. Vorläufige Mitteilung. Helv. Chim. Acta 1973, 56 (6), 2107–2110. 10.1002/hlca.19730560638. [DOI] [Google Scholar]
- (20).Ali SA; Hashmi SMA; Siddiqui MN; Wazeer MIM Regiochemistry of Mercury(II) Oxide Oxidation of Unsymmetrical N,N-Disubstituted Hydroxylamines. Tetrahedron 1996, 52 (47), 14917–14928. 10.1016/0040-4020(96)00904-0. [DOI] [Google Scholar]
- (21).Black DSC; Crozier RF; Davis VC; Synthesis ; Inouye Y; Watanabe Y; Takahashi S; Kakisawa H; Fisera L; Kova J; Poliacikova J; et al. Reactions at High Pressures. [3 + 2] Dipolar Cycloaddition of Nitrones with Vinyl Etherst; American Chemical Society, 1982; Vol. 101. [Google Scholar]
- (22).Gribble GW; Barden TC Stereocontrolled Total Syntheses of (−)-Hobartine and (+)Aristoteline via an Intramolecular Nitrone-Olefin Cycloaddition. J. Org. Chem. 1985, 50 (26), 5900–5902. 10.1021/jo00350a103. [DOI] [Google Scholar]
- (23).Hamer J; Macaluso A Nitrones. Chem. Rev. 1964, 64 (4), 473–495. 10.1021/cr60230a006. [DOI] [Google Scholar]
- (24).Nair V; Suja TD Intramolecular 1,3-Dipolar Cycloaddition Reactions in Targeted Syntheses. Tetrahedron 2007, 63 (50), 12247–12275. 10.1016/j.tet.2007.09.065. [DOI] [Google Scholar]
- (25).Padwa A Intramolecular 1,3-Dipolar Cycloaddition Reactions. Angew. Chemie Int. Ed. English 1976, 15 (3), 123–136. 10.1002/ANIE.197601231. [DOI] [Google Scholar]
- (26).Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. 2002. 10.1002/0471221902. [DOI]
- (27).Singh G; Ishar MPS; Gupta V; Singh G; Kalyan M; Bhella SS Intramolecular Low-Temperature 1,3-Dipolar Cycloadditions of Nitrones: Synthesis of Chromano-Heterocycles. Tetrahedron 2007, 63 (22), 4773–4778. 10.1016/j.tet.2007.03.086. [DOI] [Google Scholar]
- (28).Duncan NE; Janz GJ; Chem Phys J; Norman LeBel BA; Ellen Post M; Jai Whang J The Addition of Nitrones to Olefins. Fused Bicyclic Isoxazolidines 1; 1964. [Google Scholar]
- (29).Coutouli-Argyropoulou E; Sarridis P; Gkizis P Water as the Medium of Choice for the 1,3-Dipolar Cycloaddition Reactions of Hydrophobic Nitrones. Green Chem. 2009, 11 (11), 1906–1914. 10.1039/b916765j. [DOI] [Google Scholar]
- (30).Matassini C; Parmeggiani C; Cardona F; Goti A Oxidation of N,N-Disubstituted Hydroxylamines to Nitrones with Hypervalent Iodine Reagents. Org. Lett. 2015, 17 (16), 4082–4085. 10.1021/acs.orglett.5b02029. [DOI] [PubMed] [Google Scholar]
- (31).Parmeggiani C; Matassini C; Cardona F; Goti A On the Oxidation of Hydroxylamines with o - Iodoxybenzoic Acid (IBX). Synth. 2017, 49 (13), 2890–2900. 10.1055/s-0036-1588457. [DOI] [Google Scholar]
- (32).Solomon EI; Sundaram UM; Machonkin TE Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96 (7), 2563–2605. 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- (33).McCann SD; Stahl SS Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res. 2015, 48 (6), 1756–1766. 10.1021/acs.accounts.5b00060. [DOI] [PubMed] [Google Scholar]
- (34).Neese F The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2 (1), 73–78. 10.1002/WCMS.81. [DOI] [Google Scholar]
- (35).Neese F Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8 (1), e1327. 10.1002/WCMS.1327. [DOI] [Google Scholar]
- (36).Pracht P; Bohle F; Grimme S Automated Exploration of the Low-Energy Chemical Space with Fast Quantum Chemical Methods. Phys. Chem. Chem. Phys. 2020, 22 (14), 7169–7192. 10.1039/c9cp06869d. [DOI] [PubMed] [Google Scholar]
- (37).Grimme S Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 15 (5), 2847–2862. 10.1021/ACS.JCTC.9B00143. [DOI] [PubMed] [Google Scholar]
- (38).Cope AC; Trumbull ER Olefins from Amines: The Hofmann Elimination Reaction and Amine Oxide Pyrolysis. In Organic Reactions; John Wiley & Sons, Inc., 1960; pp 317–493. 10.1002/0471264180.or011.05. [DOI] [Google Scholar]
- (39).Darcsi A; Rácz Á; Béni S Identification and Characterization of a New Dapoxetine Impurity by NMR: Transformation of N-Oxide by Cope Elimination. J. Pharm. Biomed. Anal. 2017, 134, 187–194. 10.1016/j.jpba.2016.11.029. [DOI] [PubMed] [Google Scholar]
- (40).Sammelson RE; Kurth MJ Oxidation-Cope Elimination: A REM-Resin Cleavage Protocol for the Solid-Phase Synthesis of Hydroxylamines. Tetrahedron Lett. 2001, 42 (20), 3419–3422. 10.1016/S0040-4039(01)00477-4. [DOI] [Google Scholar]
- (41).Hori K; Ito J; Ohta T; Furukawa I Palladium(II)-Catalyzed 1,3-Dipolar Cycloaddition of Nitrones with Enol Ethers. Tetrahedron 1998, 54 (42), 12737–12744. 10.1016/S0040-4020(98)00776-5. [DOI] [Google Scholar]
- (42).Kusama H; Tazawa A; Ishida K; Iwasawa N Total Synthesis of (±)-Englerin A Using An Intermolecular [3+2] Cycloaddition Reaction of Platinum-Containing Carbonyl Ylide. Chem. – An Asian J. 2016, 11 (1), 64–67. 10.1002/ASIA.201500935. [DOI] [PubMed] [Google Scholar]
- (43).Zhu R-Y; Wang C-S; Zheng J; Shi F; Tu S-J Organocatalytic Asymmetric Inverse-Electron-Demand 1,3-Dipolar Cycloaddition of N,N′-Cyclic Azomethine Imines. J. Org. Chem. 2014, 79 (19), 9305–9312. 10.1021/JO5018469. [DOI] [PubMed] [Google Scholar]
- (44).Simonsen Klaus B.; Bayón Pau; Hazell Rita G.; Gothelf Kurt V., and; Jørgensen* KA Catalytic Enantioselective Inverse-Electron Demand 1,3-Dipolar Cycloaddition Reactions of Nitrones with Alkenes. J. Am. Chem. Soc. 1999, 121 (16), 3845–3853. 10.1021/JA983915A. [DOI] [Google Scholar]
- (45).Theoretical Study of 1,3-Dipolar Cycloaddition Reactions with Inverse Electron Demand - A DFT Study of the Lewis Acid Catalyst and Solvent Effects in the Reaction of Nitrones with Vinyl Ethers - Domingo - 2000 - European Journal of Organic Chemistry - Wiley Online Library (accessed Jul 6, 2021). [DOI]
- (46).Zhang R; Guo Q; Kennelly EJ; Long C; Chai X Diverse Alkaloids and Biological Activities of Fumaria (Papaveraceae): An Ethnomedicinal Group. Fitoterapia. Elsevier B.V. October 1, 2020, p 104697. 10.1016/j.fitote.2020.104697. [DOI] [PubMed] [Google Scholar]
- (47).Zhang CL; Huang QL; Zhu Q; Chen J; Zhang F; Cao ZY New Phthalideisoquinoline Hemiacetal Alkaloid Derivatives from Corydalis Decumbens. Fitoterapia 2020, 144, 104494. 10.1016/j.fitote.2020.104494. [DOI] [PubMed] [Google Scholar]
- (48).Repasi J; Hosztafi S; Szabo Z 5’-O-Demethylnarcotine: A New Alkaloid from Papaver Somniferum. Planta Med. 1993, 59 (5), 477–478. 10.1055/s-2006-959739. [DOI] [PubMed] [Google Scholar]
- (49).Aboudi AF; Al-Eisawi DM; Sabri SS; Zarga MHA Alkaloids of Fumaria Densiflora. J. Nat. Prod. 1986, 49 (2), 370. 10.1021/np50044a046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.