Abstract
Urokinase-type plasminogen activator receptor (uPAR) is an attractive target for the treatment of cancer, because it is expressed at low levels in healthy tissues but at high levels in malignant tumours. uPAR is closely related to the invasion and metastasis of malignant tumours, plays important roles in the degradation of extracellular matrix (ECM), tumour angiogenesis, cell proliferation and apoptosis, and is associated with the multidrug resistance (MDR) of tumour cells, which has important guiding significance for the judgement of tumor malignancy and prognosis. Several uPAR-targeted antitumour therapeutic agents have been developed to suppress tumour growth, metastatic processes and drug resistance. Here, we review the recent advances in the development of uPAR-targeted antitumor therapeutic strategies, including nanoplatforms carrying therapeutic agents, photodynamic therapy (PDT)/photothermal therapy (PTT) platforms, oncolytic virotherapy, gene therapy technologies, monoclonal antibody therapy and tumour immunotherapy, to promote the translation of these therapeutic agents to clinical applications.
Keywords: Urokinase-type plasminogen activator receptor (uPAR), Nanoparticles (NPs), Photodynamic therapy (PDT)/photothermal therapy (PTT), Oncolytic virotherapy, Gene therapy technologies, Monoclonal antibody therapy, Tumour immunotherapy
Background
Urokinase-type plasminogen activator receptor (uPAR), also known as CD87, is encoded by the PLAUR gene and belongs to the lymphatic antigen-6 superfamily [1, 2]. uPAR was first identified as the cell surface receptor for urokinase plasminogen activator (uPA) in 1985 [3, 4]. The mature uPAR molecule is a single-chain membrane glycoprotein receptor composed of 313 amino acid residues and is anchored to the cell membrane by a glycosylphosphatidylinositol (GPI) linkage; it contains 3 homologous domains, D1, D2 and D3, with a total molecular weight of 55–60 kDa [5, 6]. uPAR mediates a variety of biological processes, such as plasminogen activation, proteolysis, cellular signal transduction and adhesion [7–9]. Under normal physiological conditions, uPAR is usually expressed at a low level. In the processes of tissue remodelling, wound healing, inflammation and embryogenesis, uPAR is transiently expressed at high levels and participates in the processes of extracellular matrix (ECM) degradation, thrombolysis, cell invasion and migration [10–14].
Classically, the function of uPAR is to act as a receptor for the zymogen form of uPA (pro-uPA) and trigger a cascade of proteolytic events that leads to the degradation of ECM [15, 16]. Once pro-uPA is activated to uPA, it converts plasminogen to its active form, plasmin, which activates downstream proteases such as pro-matrix metalloproteinase (MMP)-3 and MMP-3, pro-MMP-9 and MMP-9, leading to ECM remodelling [17–19]. Plasmin is also able to release ECM bound growth factors that contribute to tumour progression [20, 21].
In addition to its proteolytic role, uPAR interacts with vitronectin (Vn) [22] and transmembrane receptors, including integrins (α5β1, α3β1, αvβ3 and αvβ5) [23–27] and receptor tyrosine kinases [the epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), G-protein coupled receptors (GPCRs), very low-density lipoprotein receptor (VLDLR) family members], thereby activating intracellular focal adhesion kinase (FAK) signalling, regulating intracellular pathways [Ras/mitogen-activated protein kinase (MAPK), Ras-related C3 botulinum toxin substrate 1 (Rac1)/MAPK, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), and Janus-associated kinase 1 (JAK1)], and triggering cellular responses such as cell migration, adhesion, proliferation, angiogenesis and the epithelial–mesenchymal transition (EMT) [28–36]. Moreover, the cleaved form of uPAR (D2–D3 fragment), interacts with members of the formyl peptide receptor (FPR) family of GPCRs via its exposed N-terminal 88SRSRY92 sequence, initiating both angiogenic and inflammatory processes [37, 38].
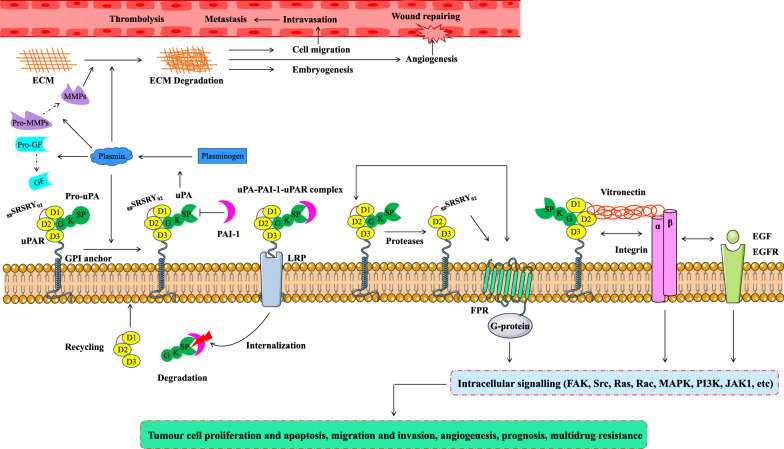
Finally, uPAR is also involved in the internalization of the uPA-plasminogen activator inhibitor (PAI)-1-uPAR complex, degradation of uPA-PAI-1, and recycling of unoccupied uPAR. When uPA-uPAR is inactivated by PAI-1, internalization via low-density lipoprotein receptor related protein (LRP) is initiated, leading to clathrin-mediated endocytosis of the uPA-PAI-1-uPAR complex. Once internalized, uPA-PAI-1 dissociates from uPAR and is trafficked to the lysosome for degradation, while the unoccupied uPAR is recycled to the cell surface [39–41]. A schematic representation of the uPAR-mediated pathways is shown in Fig. 1.
Fig. 1.
Schematic representation of the uPAR-mediated pathways. The GPI-anchored receptor uPAR consisting of D1, D2, and D3 domains binds the zymogen pro-uPA and the active uPA through the GF domain. The active form of uPA then converts plasminogen into plasmin, which subsequently cleaves and activates GFs, and MMPs, leading to the degradation of ECM in important physiological processes and in pathological processes associated with cancer development. PAI-1 inhibits the catalytic activity of both uPA and plasmin. Internalization and recycling of uPAR occur after a uPA-PAI-1-uPAR complex has formed, resulting in the degradation of uPA-PAI-1 and the recycling of uPAR to the cell surface. uPAR is cleaved between the D1 and D2 domains, exposing the 88SRSRY92 sequence at its N-terminus to interact with FPR of the GPCRs, promoting its internalization and activating signalling. In addition to uPA, uPAR interacts with Vn, integrins and other cell surface receptors, such as EGFRs, to activate different intracellular signalling pathways [FAK, Src, Ras, Rac, MAPK, PI3K, JAK1, etc.] and regulate tumour cell proliferation and apoptosis, migration and invasion, angiogenesis, prognosis and multidrug resistance. uPAR urokinase-type plasminogen activator receptor, uPA urokinase plasminogen activator, GPI glycosylphosphatidylinositol, GF growth factor, MMPs matrix metalloproteinases, ECM extracellular matrix, PAI-1 plasminogen activator inhibitor-1, Vn vitronectin, EGFR epidermal growth factor receptor, LRP low-density lipoprotein receptor-related protein, GPCRs G-protein coupled receptors, FPR formyl peptide receptor, FAK focal adhesion kinase, Src tyrosine-protein kinase, MAPK mitogen activated protein kinase, Rac Ras-related C3 botulinum toxin substrate, PI3K phosphatidylinositol 3-kinase, JAK1 janus kinase 1
In recent years, many studies have shown that uPAR is closely related to the invasion and metastasis of malignant tumours. uPAR plays important roles in the degradation of ECM, tumour angiogenesis, cell proliferation and apoptosis, is related to the multidrug resistance (MDR) of tumour cells, and has important guiding significance for the judgement of tumour malignancy and prognosis. In this review, we summarize the new application of uPAR as a target of nanoplatforms carrying therapeutic agents, photodynamic therapy (PDT)/photothermal therapy (PTT) platforms, oncolytic virotherapy, gene therapy technologies, monoclonal antibody therapy and tumour immunotherapy to promote the translation of these therapeutic agents to clinical applications.
uPAR in cancer progression
uPAR has multiple functional roles associated with tumour progression, including tumour proliferation and apoptosis, metastasis, angiogenesis, MDR and prognosis. An analysis of tumour samples has shown high uPAR expression in most solid tumour tissues, such as breast [42], lung [43], bladder [44], ovarian [45], prostate [46], liver [47], colon [48], pancreatic [49] and gastric cancer [50] as well as gliomas [51] and several haematologic malignancies [52, 53]. Moreover, uPAR is expressed at high levels on stromal cells in the tumour microenvironment, such as vascular endothelial cells, tumour-related fibroblasts and tumour-related macrophages, and its expression level is closely related to tumour aggressiveness and the survival of patients with tumours [54–57]. Therefore, treatments targeting uPAR expressed on tumour-associated stromal cells may be as important as treatments targeting uPAR expressed on tumour cells and may lead to enhanced antitumour activity.
uPAR interacts with a variety of surface transmembrane proteins, such as integrins and EGFR, thereby activating intracellular FAK, extracellular regulatory protein kinase (ERK) and MAPK signalling to inhibit cell apoptosis and promote cell proliferation. For example, the interaction between uPAR and a5β1 integrin activates EGFR through a FAK-dependent pathway, which subsequently activates the ERK signalling pathway and promotes cell proliferation [58]. Inhibition of uPAR expression destroy the uPAR/integrin interaction and inhibits the MAPK pathway to arrest Hep3 cells in G0/G1 phase [59]. The suppression of uPAR expression in vitro by transfection inhibits the proliferation of meningioma cells by downregulating transforming growth factor-β (TGF-β) 1 expression [60], arrests glioma SNB19 cells in G2 phase and increases caspase-dependent cell apoptosis [61]. Moreover, inhibiting the expression of uPAR in vitro by transfection promotes the apoptosis of human melanoma cells by increasing the expression of the p53 protein and activating the apoptosis pathway mediated by retinoic acid inducible gene 1 (RIG-1) [62].
Inhibition of uPAR expression prevents tumour invasion and migration. For example, inhibiting the expression of uPA/uPAR blocks the invasion of glioma SNB19 cells by reducing Ras mediated phosphorylation of FAK, p38MAPK, c-Jun N-terminal kinase (JNK) and ERK1/2 and MAPK kinase (MEK) activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) signalling pathway [63]. Inhibition of uPA/uPAR expression also prevents the invasion of glioma cells by inhibiting Notch-1 receptor cleavage, signal transduction and endosomal transport [64]. Treatments targeting uPAR in human pancreatic cancer cells inhibit the migration and invasion of mouse tumour cells mediated by c-met and insulin like growth factor 1 receptor (IGF1R) [65]. Inhibition of uPAR expression along with the expression of uPA, human epidermal growth factor receptor-2 (HER-2), or IGF1R or in combination with trastuzumab further inhibits the invasion and migration of different breast cancer cell lines [66–68].
Angiogenesis is the process of forming new blood vessels from existing blood vessels. It plays a vital role in tumour growth, invasion and metastasis. uPAR also promotes tumour angiogenesis. For example, uPAR promotes angiogenesis by inhibiting the expression of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) [69]. In endothelial cells and glioblastoma cells, silencing the expression of uPA/uPAR inhibits tumour angiogenesis by increasing the expression of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) and increasing the secretion of soluble vascular endothelial growth factor (VEGF) receptor (VEGFR) 1 (SVEGFR1) [70]. Herkenne et al. also found that knockout of uPAR in human umbilical vein endothelial cells (HUVECs) blocks VEGFR2 signalling, thereby preventing VEGF-induced angiogenesis [71].
High levels of uPAR expression have been detected in a variety of cancer cells but very low levels are present in normal cells, indicating that the level of uPAR in tumour tissue is closely related to the tumour malignancy and prognosis of patients with cancer [72]. Elevated levels of uPAR are observed in prostate cancer, correlating with increased aggressiveness, postoperative progression and metastasis [73, 74]. In another study, Memarzadeh et al. found that the expression of uPAR in surgically removed endometrial tissue was positively correlated with the malignancy of endometrial cancer [75]. A study using 45 fresh tumour tissues observed the presence of uPAR in 1/3 of melanomas [76]. Yang et al. suggested that uPAR is useful as an independent prognostic factor for the survival and metastasis of patients with colorectal cancer [77]; Halamkova et al. also reported a correlation between uPAR expression and the grade of colorectal cancer [78]. Many studies have shown increased levels of uPAR and their related to liver metastasis and a poor prognosis for patients with hepatocellular carcinoma (HCC) [79–81]. According to Chen et al., the levels of uPAR in patients with lung cancer are significantly increased [82]. A study has shown an association between an increased level of the uPAR D1 domain and shorter overall survival of patient with small cell lung cancer [83]. uPAR expression in tumour tissues is also significantly increased in non-small cell lung cancer (NSCLC) [84]. In gastric cancer, increased uPAR expression and decreased uPAR expression are related to a poor prognosis and prolonged patient survival, respectively [85, 86]. In oral squamous cell carcinoma (OSCC), the levels of uPAR are elevated, and a strong correlation between the expression of uPAR and the aggressiveness of the tumour has been identified [87]. Increased uPAR levels are closely related to a poor prognosis for patients with bladder cancer [88, 89]. High levels of uPAR are present in 94% of muscle-invasive bladder cancer and 54–71% of nonmuscle-invasive bladder cancer, but the protein is almost undetectable in healthy bladder tissue [90]. The expression of uPAR is significantly increased in laryngeal squamous cell carcinoma, which may help increase invasion and metastasis [91]. In acute myeloid leukaemia (AML), the high expression of uPAR is also associated with the aggressiveness of the disease [92]. Therefore, the expression level of uPAR may be an important marker for judging the degree of malignancy and the survival of patients.
An association between uPAR expression and the MDR of tumour cells has also been identified. Drug resistance is an important cause of the failure of tumour treatment. A study has shown that inhibition of uPAR in vitro promotes the apoptosis of melanoma cells resistant to B-RAF inhibitors and MEK inhibitors by increasing the level of Noxa [62]. High uPAR expression may allow head and neck squamous cell carcinoma, small cell lung cancer, and malignant pleural mesothelioma to develop resistance to chemotherapy [93–95]. uPAR enhances the resistance of breast cancer to tamoxifen by activating ERK1/2 [96], and renders NSCLC resistant to gefitinib by activating the EGFR/pAKT/survivin signalling pathway [97]. Inhibition of uPAR expression reduces the resistance of mouse brain neuroma cells to 5-fluorouracil (5-FU), cisplatin (Cis), docetaxel (DTX) and doxorubicin (Dox) [98]. Laurenzana et al. showed that BRAF-mutated melanoma cells with different uPAR expression levels have different sensitivities to verofenil; high levels of uPAR decrease the sensitivity of BRAF-mutated melanoma cells to verofenil, while a reduction in uPAR expression restores the sensitivity of drug-resistant cells to verofenil [99]. As shown in the study by LeBeau et al., MCF-7 cells resistant to tamoxifen and MDA-MB-231 cells resistant to Dox and paclitaxel (PTX) exhibit markedly higher expression of uPAR than parental MCF-7 and MDA-MB-231 cells, respectively [100].
In summary, the dysregulation of uPAR plays a key role in tumour progression. Given the broad expression of uPAR by a variety of different tumour types and the selective expression of uPAR by tumour cells and tumour-related stromal cells in the tumour microenvironment compared to normal cells, uPAR is an attractive target for the treatment of tumours.
Targeting uPAR for antitumour therapy
Compared with normal tissues, high uPAR expression in tumours has been shown, and thus researchers have proposed uPAR as a therapeutic target and a targeting agent for the treatment of cancer [101]. Over the past 30 years, a variety of therapeutic agents that target uPAR have been developed to treat cancer. For example, peptides AE105 (D-Cha-F-s-r-Y-L-W-S) [102], AE120 ([D-Cha-F-s-r-Y-L-W-S]2-βA-K) [102], Å6 (Ac-KPSSPPEE-Am) [103], ATF [104], and U11 (VSNKYFSNIHW) [105], and the cyclic peptides cyclo19,31uPA19–31 [106], cyclo19,31[D-Cys19]-uPA19–31 [107], WX-360 (cyclo21,29[D-Cys21]-uPA21–30[S21C;H29C]) and WX-360-Nle (cyclo21,29[D-Cys21]-uPA21–30[S21C;K23Nle;H29C]) [108] block the uPA/uPAR interaction. Peptides M25 (PRYQHIGLVAMFRQNTG) [109], α325 (PRHRHMGAVFLLSQEAG) [110], p25 (AESTYHHLSLGYMYTLN-NH2) [111], m.P243-251 (TASWCQGSH) [112], D2A-Ala (IQEGAAGRPKDDR) [113] and polyethylene glycol (PEG)ylated D2A-Ala peptide (PEG-D2A-Ala) [114] inhibit the uPAR/integrin or uPAR/Vn interaction. Peptides pyro glutamic acid (pGlu)-Arg-Glu-Arg-Tyr-NH2 (pERERY-NH2) [115], RERF (Ac-Arg-Glu-Arg-Phe-NH2) [116], UPARANT (Ac-L-Arg-Aib-L-Arg-D-Ca(Me)Phe-NH2) [117], cyclic SRSRY peptide ([SRSRY]) [118], and RI-3 [Ac-(D)-Tyr-(D)-Arg-Aib-(D)-Arg-NH2] [119] block the interaction of SRSRY and N-formyl-Met-Leu-Phe (fMLF) with the FPR family of GPCRs. Human and mouse uPA1-48 (huPA1-48 and muPA1-48), human and murine uPA1-48 fusion proteins (huPA1-48Ig and muPA1-48Ig) [120], and human and mouse pegylated uPA1-48 (PEGh1-48 and PEGhm1-48) [121] also inhibit tumour growth by inhibiting tumour stromal cell uPAR-dependent plasminogen activation. The small-molecule inhibitors IPR-456 [122], IPR-803 [123], IPR-3011 [124], IPR-3577 [125], 7 [126], LLL-1fsi [127], MS#479 [2-(pyridin-2-ylamino)-quinolin-8-ol] and MS#305 [2,2′-(methylimino)di (8-quinolinol)] [128], Compounds 6 and 37 [129], and docosahexaenoic acid (DHA) [130] inhibit the uPAR/uPA, uPAR/integrin, uPAR/Vn or uPAR/FPR interaction. The ligand-targeted toxins DTAT [diphtheria toxin (DT) and ATF] [131, 132], DTATEGF (ATF, EGF and DT) [133], DTAT13 [ATF, interleukin-13 (IL-13) and DT] [134, 135], eBAT (EGFATFKDEL 7mut) [136–141], ATF-SAP (ATF and Saporin) [142, 143], PAI-2-N-AIE conjugate [5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin and PAI-2] [144], DTU2GMCSF [DT and granulocyte–macrophage colony-stimulating factor (GM-CSF)] [145], ATF-PE38 and ATF-PE38KDEL [ATF and Pseudomonas exotoxin A (PE38)] [146] exert antitumor effects by targeting uPAR and releasing toxins. The uPAR-targeted peptides, small-molecule inhibitors and ligand-targeted toxins are summarized in Table 1.
Table 1.
The uPAR-targeted peptides, small-molecule inhibitors and ligand-targeted toxins
| Peptides/small-molecule inhibitors/ligand-targeted toxins | Sequence/structure/composition | Action site/target | References |
|---|---|---|---|
| AE105 | D-Cha-F-s-r-Y-L-W-S | uPA/uPAR | [102] |
| AE120 | [D-Cha-F-s-r-Y-L-W-S]2-βA-K | uPA/uPAR | [102] |
| Å6 | Ac-KPSSPPEE-Am | uPA/uPAR | [103] |
| ATF | An amino-terminal fragment of urokinase with EGF-like domain and kringle domain | uPA/uPAR | [104] |
| U11 | VSNKYFSNIHW | uPA/uPAR | [105] |
| A stable disulfide-bridged cyclic form of the linear peptide uPA19–31 | cyclo19,31uPA19–31 | uPA/uPAR | [106] |
| A peptide variant of cyclo19,31uPA19–31 | cyclo19,31[D-Cys19]-uPA19–31 | uPA/uPAR | [107] |
| WX-360 | cyclo21,29[D-Cys21]-uPA21–30[S21C;H29C] | uPA/uPAR | [108] |
| WX-360-Nle | cyclo21,29[D-Cys21]-uPA21–30[S21C;K23Nle;H29C] | uPA/uPAR | [108] |
| M25 | PRYQHIGLVAMFRQNTG | uPAR/β1-integrins | [109] |
| α325 | PRHRHMGAVFLLSQEAG | uPAR/Vn | [110] |
| p25 | AESTYHHLSLGYMYTLN-NH2 |
uPAR-integrin uPAR/Vn |
[111] |
| m.P243-251 | TASWCQGSH | uPAR/integrin α5β1 | [112] |
| D2A-Ala | IQEGAAGRPKDDR | uPAR/integrin avβ3/a5β1 | [113] |
| PEGylated D2A-Ala | PEG-D2A-Ala | uPAR/integrin avβ3/a5β1 | [114] |
| pERERY-NH2 | Pyro glutamic acid (pGlu)-Arg-Glu-Arg-Tyr-NH2 | fMLF/FPR | [115] |
| RERF | Ac-Arg-Glu-Arg-Phe-NH2 |
SRSRY/FPR fMLF/FPR |
[116] |
| UPARANT | Ac-L-Arg-Aib-L-Arg-D-Ca(Me)Phe-NH2 | fMLF/FPR | [117] |
| cyclic SRSRY peptide ([SRSRY]) | [Ser-Arg-Ser-Arg-Tyr]§ | SRSRY/FPR1 fMLF/FPR1 | [118] |
| RI-3 | Ac-(D)-Tyr-(D)-Arg-Aib-(D)-Arg-NH2 | fMLF/FPR1 | [119] |
| huPA1-48 and muPA1-48 | The growth factor domains of human and murine urokinase | Tumour stromal cell uPAR dependent plasminogen activation | [120] |
| huPA1-48Ig and muPA1-48Ig | Modify huPA1-48 and muPA1-48 with the constant region of human IgG1 | Tumour stromal cell uPAR dependent plasminogen activation | [120] |
| PEGh1-48 and PEGhm1-48 | Human and mouse pegylated uPA1-48 | Tumour stromal cell uPAR dependent plasminogen activation | [121] |
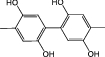
| IPR-456 |
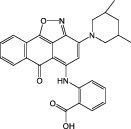
|
uPA/uPAR | [122] |
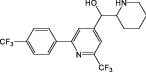
| IPR-803 |
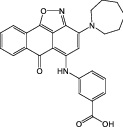
|
uPA/uPAR | [123] |
| IPR-3011 |
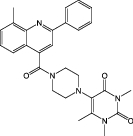
|
uPA/uPAR | [124] |
| IPR-3577 |
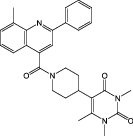
|
uPA/uPAR | [125] |
| 7 |
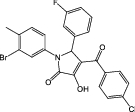
|
uPAR/uPAATF uPAR/Vn |
[126] |
| LLL-1fsi |
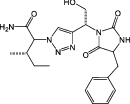
|
uPA/uPAR | [127] |
| MS#479 [2-(Pyridin-2-ylamino)-quinolin-8-ol] |
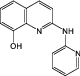
|
uPAR/integrin | [128] |
| MS#305 [2,2′-(methylimino)di (8-quinolinol)] |
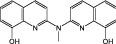 〹 〹 |
uPAR/integrin | [128] |
| Compounds 6 |
|
uPAR/Vn uPAR/FPR |
[129] |
| Compounds 37 |
|
uPAR/Vn uPAR/FPR |
[129] |
| Docosahexaenoic acid (DHA) |
|
suppress uPAR expression | [130] |
| DTAT | DT and ATF | uPAR | [131, 132] |
| DTATEGF | ATF, EGF and DT | uPAR, EGFR | [133] |
| DTAT13 | ATF, IL-13 and DT | uPAR, IL-13 receptors | [134, 135] |
| eBAT (EGFATFKDEL 7mut) | ATF, EGF, truncated PE38 with a terminal lysyl-aspartyl-glutamyl-leucine (KDEL) sequence and eight amino acids representing the seven major epitopes on PE38 were mutated | uPAR, EGFR | [136–141] |
| ATF-SAP | ATF and SAP | uPAR | [142, 143] |
| PAI-2-N-AIE | PAI-2 and N-AIE | uPAR | [144] |
| DTU2GMCSF | DT, GM-CSF and uPA | uPAR, GM-CSF receptor | [145] |
| ATF-PE38 | ATF and PE38 | uPAR | [146] |
| ATF-PE38KDEL | ATF and PE38 with a terminal KDEL sequence | uPAR | [146] |
uPA: urokinase plasminogen activator; uPAR: urokinase-type plasminogen activator receptor; Vn: vitronectin; PEG: polyethylene glycol; fMLF: N-formyl-Met-Leu-Phe; FPR: formyl peptide receptor; DT: diphtheria toxin; IL-13: interleukin-13; PE38: Pseudomonas exotoxin A; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; SAP: Saporin; PAI-2: plasminogen activator inhibitor type 2; N-AIE: 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin was conjugated to PAI-2 via an esterase-labile succinate linker; GM-CSF: granulocyte-macrophage colony-stimulating factor
However, although research has been conducted for more than 30 years, none of these treatments have advanced into clinical application. The pleiotropic nature of uPAR interactions and function, uPAR structural flexibility, species specificity of the uPA-uPAR interaction, limitations of tumour models, the characteristic that uPAR expression is increased on tumour cells and tumour-associated stromal cells, and the baseline expression of uPAR in the glomeruli of normal kidneys that may result in potential “on-target off-tumour” toxicity are all the main hurdles to the development of uPAR inhibitors [72, 101, 147–152]. Furthermore, linear peptides based on the sequence of uPA lack potency and have poor pharmacological properties and stability due to susceptibility to exoprotease degradation in the plasma [153]; screening for small-molecule inhibitors is inefficient due to a lack of detailed structural information on the interactions of uPAR with its binding partners such as integrins [154–156]. Some uPAR-targeted small-molecule inhibitors are hydrophobic and have limited bioavailability [123, 125, 157]; and due to the large surface area at the protein–protein interface, the development of small molecules specifically targeting this flexible hydrophobic cavity in uPAR also represent a challenging task [129, 158]. Similarly, ligand-targeted toxins must overcome many barriers before they reach human clinical trials, including determining the appropriate dosing strategy and sequence of administration, increasing the potency and reducing the immunogenicity of the toxin [159, 160].
In recent years, with the interdisciplinary integration of cell biology and materials science, many innovative tumour-targeted therapeutic technologies targeting uPAR have emerged, providing new development directions for precise and efficient tumour therapy. uPAR-targeted nanoplatforms carrying therapeutic agents have great potential in enhancing active tumour targeting, improving delivery efficiency, reducing drug toxicity, increasing the hydrophilicity of hydrophobic drugs, achieving tumour diagnosis and treatment integration, and in multimodal synergistic antitumor applications. uPAR-targeted PDT/PTT platforms may be regarded as promising cancer therapeutic strategies due to their unique advantages such as minor trauma, improved selectivity and reduced side effects. uPAR-targeting oncolytic measles virus (MV-uPA) is an innovative biological strategy associated with potent antitumour effects. uPAR-targeted clustered regularly interspaced short palindromic (CRISPR)/CRISPR-associated protein-9 nuclease (Cas9) gene-editing technology may provide new therapeutic trearments for aggressive cancers. uPAR-targeted monoclonal antibody therapy may provide new breakthroughs for the development of anticancer therapy. uPAR-targeted chimeric antigen receptor (CAR) T-cell immunotherapy and antibody-recruiting molecules (ARMs) have the ability to target uPAR-expressing cancers for immune-mediated cell death. Therefore, this review focuses on some new applications of uPAR in the six fields described above (Fig. 2).
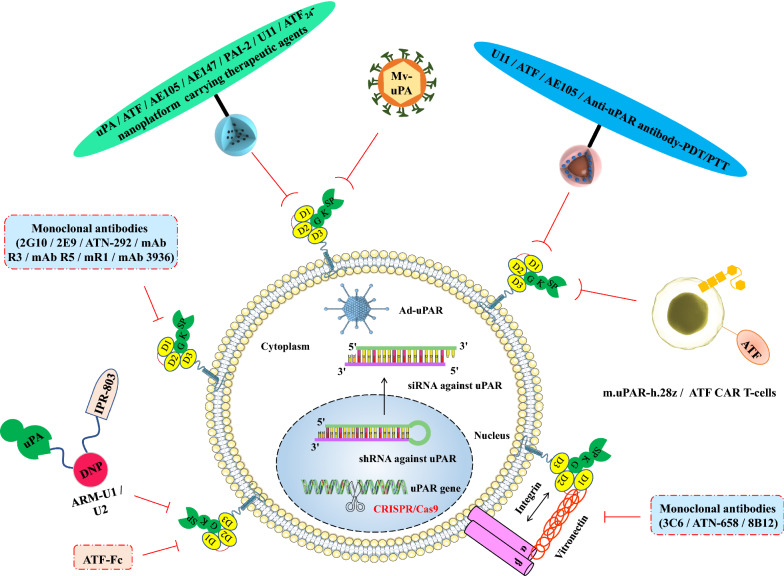
Fig. 2.
uPAR was used as a target in nanoplatforms carrying therapeutic agents, PDT/PTT platforms, oncolytic virotherapy, gene therapy techniques, monoclonal antibody therapy and tumour immunotherapy to enhance antitumor effects. (1) uPAR-targeted nanoplatforms carrying therapeutic agents have great potential for the development of targeted therapeutic and imaging approaches that are capable of enhancing the therapeutic effect of nanoparticle drugs on various cancers. (2) uPAR-targeted PDT/PTT platforms may be regarded as promising cancer therapeutic strategies due to their unique advantages such as minor trauma, improved selectivity and reduced side effects. (3) uPAR-targeting oncolytic measles virus (MV-uPA) is an innovative biological strategy associated with potent antitumour effects. (4) uPAR-targeted gene therapy techniques using adenovirus-mediated antisense uPAR therapy, RNA interference (RNAi) technology and novel CRISPR/Cas9 gene editing technology may represent useful tools and provide new therapeutic options for aggressive cancers. (5) uPAR-targeted monoclonal antibody therapy may provide new breakthroughs in the development of anticancer therapy. (6) uPAR-targeted CAR T-cell immunotherapy and ARMs have the ability to target uPAR-expressing cancers for immune-mediated cell death. PDT/PTT photodynamic therapy/photothermal therapy, MV-uPA uPAR-targeting oncolytic measles virus, RNAi RNA interference, CRISPR/Cas9 RNA-guided clustered regularly interspaced short palindromic (CRISPR) in combination with a CRISPR-associated nuclease 9 (Cas9) nuclease system, CAR chimeric antigen receptor, ARMs antibody-recruiting molecules
uPAR-targeted nanoplatforms carrying therapeutic agents
More recently, several groups have not only utilized various uPAR-targeted nanoplatforms as drug delivery systems to enhance the antitumor effect but also used uPAR-targeted nanoparticles (NPs) as targeted therapeutic imaging probes. Dong et al. successfully loaded BRCA1 small interfering RNA (siRNA), which block DNA repair, and the DNA-damaging agent Pro-Pt into a shell-core pH-sensitive platform (uPA-SP@CaP NPs) to increase the sensitivity of triple-negative breast cancer (TNBC) to chemotherapy. The NPs achieved dual tumour targeting through the passive enhanced permeability and retention (EPR) effect and active uPA peptide [161] (Fig. 3). Yang et al. engineered uPAR-targeted magnetic iron oxide nanoparticle (IONP)-encapsulated Dox conjugated with the ATF of uPA that delivered higher Dox loads and exerted a stronger inhibitory effect on breast cancer cell growth than nontargeted NPs. Moreover, these NPs have been used as targeted therapeutic imaging probes for monitoring drug delivery using magnetic resonance imaging (MRI) [162]. Miller-Kleinhenz et al. prepared Wnt/LRP5/6- and uPAR-targeted ultrasmall magnetic IONPs carrying Dox (iWnt-ATF24-IONP-Dox) that showed a stronger inhibitory effect than non/single-targeted IONPs on a human breast cancer patient-derived xenograft model and markedly inhibited Wnt/β-catenin signalling and the cancer stem-like phenotype by decreasing the levels of the Wnt ligand, CD44 and uPAR [163]. Lee et al. engineered ATF-mediated IONPs carrying gemcitabine (Gem) (ATF-IONP-Gem) to target uPAR-expressing tumour and stromal cells and overcome the tumour–stromal, which not only provided contrast enhancement in MRI of tumours, but also significantly inhibited the growth of orthotopic pancreatic cancer [164]. Gao et al. prepared uPAR-targeted PEGylated theranostic NPs (ATF-PEG-IONPs), and detected threefold higher intratumour accumulation (i.p. injection) than i.v. delivery; the IONPs were detected with NIR-830 labelling using noninvasive optical and MRI in an orthotopic pancreatic cancer model. Moreover, these IONPs carrying Cis or Dox (ATF-PEG-IONP-Cis or ATF-PEG-IONP-Dox) markedly inhibited tumour angiogenesis and tumour growth and reduced the production of malignant ascites [165].
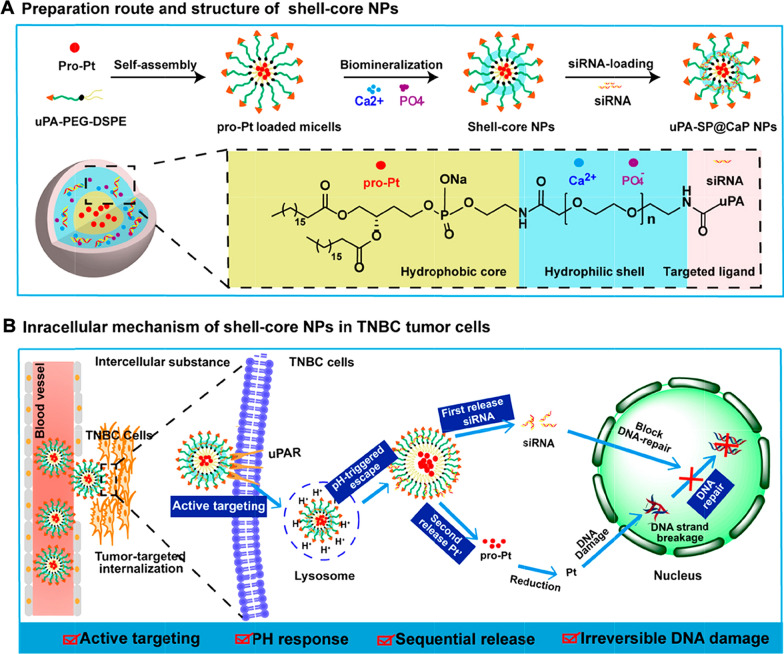
Fig. 3.
Integration of siRNA and Pro-Pt into uPA peptide-targeted multifunctional shell-core NPs for the synergistic treatment of TNBC. A The tumour-targeted CaP shell-core NPs were prepared using the biomineralization method, where the organic DSPE-PEG-uPA core encapsulates the chemotherapeutic agent Pro-Pt (Pt′) followed by negatively charged siRNA adsorbing in the inorganic porous CaP shell. B The intracellular mechanism of uPA-SP@CaP NPs in TNBC cells. (i) uPA-mediated active tumour targeting increased the intracellular drug concentration. (ii) CaP-mediated lysosomal membrane rupture resulted in lysosomal escape, along with (iii) the release of the BRCA1 siRNA to inhibit the DNA repair pathway. (iv) SiRNA and reduction of Pro-Pt to Pt synergistically induced irreversible DNA damage in TNBC cells. siRNA small interfering RNA, TNBC triple-negative breast cancer
(Reproduced with permission from reference [161]. Copyright 2019, American Chemical Society)
Ahmed et al. developed multifunctional double-receptor-targeting IONPs [luteinizing hormone-releasing hormone (LHRH) peptide- and AE105 peptide-targeted IONPs, LHRH-AE105-IONPs] that simultaneously targeted the LHRH receptor (LHRH-R) and uPAR and exhibited a significant MRI contrast in PCa cells. Importantly, the IONPs carrying PTX (LHRH-AE105-IONPs-PTX) showed two times higher cell cytotoxicity than IONPs targeting a single molecule [166]. Park et al. prepared AE147 peptide-conjugated liposomes encapsulating DTX (DTX/AE Lipo) to actively target uPAR-overexpressing metastatic tumours. In MDA-MB-231 cells, DTX/AE-Lipo (IC50 4.61 µg/mL) achieved better anticancer activity than free DTX (IC50 7.18 µg/mL) or DTX/Lipo (IC50 8.59 µg/mL). Additionally, AE147-conjugated liposomes showed improved tumour-targeting ability [167]. Belfiore et al. prepared anti-mitotic N-alkylisatin (N-AI)-loaded liposomes modified with plasminogen activator inhibitor type 2 (PAI-2/SerpinB2) to target uPA/uPAR. The liposomes showed a higher uptake in MDA-MB-231 cells than in MCF-7 cells and higher accumulation at the tumour site than the nontargeted liposomes [168]. Wang et al. prepared synthetic self-assembled NPs modified with the U11 peptide-lipid amphiphile, which showed an essentially tenfold higher transfection efficiency than scrambled peptide-targeted NPs in uPAR-positive DU145 cells [105]. Hong et al. employed a U11 peptide-decorated, pH-sensitive NP system by coencapsulating the U11 peptide-conjugated, pH-sensitive Dox prodrug (U11-Dox) and curcumin (Cur) (U11-Dox/Cur NPs), and this formulation displayed a higher cellular uptake and tumour accumulation than nontargeting NPs and inhibited tumour growth by 85% in vivo [169].
Our research group also developed β-elemene-loaded liposomes modified with ATF24 peptide (ATF24-PEG-Lipo-β-E); these liposomes showed better targeting efficiency and higher cytotoxicity than nondecorated liposomes and exerted a synergistic effect on inhibiting the growth of KU-19-19 bladder cancer with Cis [170]. Devulapally et al. successfully developed a uPA peptide (VSNKYFSNIHWGC)-conjugated, antisense-miR-21 and antisense-miR-10b coloaded PLGA-b-PEG-NPs (called uPA-Anti-miR-21-Anti-miR-10b-NPs) that simultaneously antagonized miR-21-induced inhibition of apoptosis and miR-10b-induced metastasis to achieve TNBC therapy [171]. Therefore, uPAR-targeted theranostic NPs have tremendous potential for future imaging and targeted therapeutic applications because they are capable of enhancing the therapeutic effect of NP drugs on various types of cancers. The uPAR-targeted nanoplatforms carrying therapeutic agents are summarized in Table 2.
Table 2.
The uPAR-targeted nanoplatforms carrying therapeutic agents
| Nano platform | Target | Drug | Imaging | Effect | References, year |
|---|---|---|---|---|---|
| uPA-SP@CaP NPs | uPA peptide, amino acid sequence: VSNKYFSNIHWGC (uPAR) | BRCA1 siRNA, Pro-Pt | Fluorescence imaging (Dir) | Improve anticancer efficacy of the TNBC (pH-responsive sequential release ability, lysosomal escape property, dual tumour targeting, and irreversible DNA damage behavior) | [161], 2019 |
| ATF-IO-Dox | ATF (uPAR) | Dox | MRI | A marked inhibition of tumour cell growth in 4T1 and MDA-MB-231 cells | [162], 2008 |
| iWnt-ATF24-IONP-Dox | iWnt, amino acid sequence: NSNAIKNKKHHH (Wnt/LRP5/6), ATF24, amino acid sequence: CHHHCLNGGTCVSNKYFSNIHWCNCPKK (uPAR) | Dox | NIR-830 dye for optical imaging | Strong tumour growth inhibition in a human chemo-resistant cancer patient-derived xenograft model (inhibited Wnt/β-catenin signaling and cancer stem-like phenotype of tumour cells; marked reduction of Wnt ligand, CD44 and uPAR) | [163], 2018 |
| ATF-IONP-Gem | ATF (uPAR) | Gem | MRI | Inhibit the growth of orthotopic human pancreatic cancer xenografts in nude mice (overcoming the tumour stromal barrier) | [164], 2013 |
| ATF-PEG-IONP-Cis or ATF-PEG-IONP-Dox | ATF (uPAR) | Cis or Dox | NIR optical imaging and MRI | Inhibit the growth of pancreatic tumours (i.p.); decrease proliferating tumour cells and tumour vessels; reduce the amount of ascites production | [165], 2017 |
| LHRH-AE105-IONPs-PTX | LHRH (LHRH-R), AE105 (uPAR) | PTX | MRI | 10 times reduction in the concentration of PTX required to achieve similar cytotoxic effect produced by the free drug (LHRH-R- and uPAR-overexpressing PC-3 cells) | [166], 2017 |
| DTX/AE Lipo | AE147 (uPAR) | DTX | Fluorescence imaging | DTX/AE-Lipo (IC50 4.61 µg/mL) achieves better anticancer activity than free DTX (IC50 7.18 µg/mL) or DTX/Lipo (IC50 8.59 µg/mL) | [167], 2021 |
| PAI-2 N-AI liposomes | PAI-2 (uPAR) | N-alkylisatin | NA | An increased accumulation at the primary tumour site in an orthotopic MDA-MB-231 BALB/c-Fox1nu/Ausb xenograft mouse model | [168], 2020 |
| U11 peptide targeted NPs | U11 peptide (uPAR) | Plasmid DNA | Fluorescence imaging (Rhodamine) | Transfection of uPAR positive DU145 cells is essentially tenfold higher compared to transfection achieved by NPs having a scrambled peptide sequence on their surface | [105], 2009 |
| U11-Dox/Cur NPs | U11 peptide (uPAR) | Dox/Cur | Fluorescence imaging (Coumarin 6) | Inhibit the tumour growth to a level of 85% | [169], 2019 |
| ATF24-PEG-Lipo-β-E | ATF24 (uPAR) | β-E | Fluorescence imaging (Did) | Combined with Cis, exert a synergistic effect on cellular apoptosis and cell arrest at the G2/M phase (dependent on the caspase-dependent pathway and Cdc25C/Cdc2/cyclin B1 pathways) | [170], 2020 |
| uPA-Anti-miR-21-Anti-miR-10b-NPs | uPA peptide (VSNKYFSNIHWGC) | Antisense-miR-21, antisense-miR-10b | Optical bioluminescence imaging (MDA-MB-231-Fluc-eGFP cells) | 40% reduction in tumour growth compared to scrambled peptide conjugated NPs treated mice (0.15 mg/kg) | [171], 2015 |
siRNA small interfering RNA, TNBC triple-negative breast cancer, NIR near infrared, MRI magnetic resonance imaging, Gem gemcitabine, Cis Cisplatin, Dox doxorubicin, DTX docetaxel, Cur curcumin, PTX paclitaxel, β-E β-elemene, Cdc25C cell division cyclin 25C, Cdc2 cell division cycle protein 2, Dir 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide, Did 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate, NPs nanoparticles
uPAR-targeted PDT/PTT platforms
Among anticancer treatments, PDT and PTT are widely regarded as promising cancer therapeutic strategies due to their unique advantages such as minor trauma, improved selectivity, remarkable spatial/temporal resolution and reduced side effects [172]. PDT depends on photosensitizers (PSs) that produce reactive oxygen species (ROS) upon light activation, and subsequently induce cell apoptosis [173]. PTT is a type of phototherapy that converts absorbed light to local heat in tumours using various nanomaterials such as gold nanorods, carbon nanohorns and graphene oxide, and thus induces cell death [174]. Recently, a variety of uPAR-targeted PDT/PTT strategies have been developed to enhance the therapeutic effect on malignant tumours and reduce systemic side effects.
Li et al. engineered a U11 peptide modified gold nanocluster platform carrying the cathepsin E (CTSE)-sensitive PDT prodrug/imaging agent CRQAGFSL-5-aminolevulinic acid (5-ALA)/-cyanine 5.5 (Cy5.5) (AuS-U11), which showed excellent efficacy with endomicroscopy-guided PTT/PDT through the combination of active tumour targeting and enzyme-triggered release of 5-ALA and Cy5.5 in a PANC1-CSTE orthotopic tumour model [172] (Fig. 4). Li et al. prepared a human ATF-decorated human serum albumin (HSA) carrying the photosensitizer monosubstituted β-carboxy phthalocyanine zinc (CPZ) (hATF-HSA:CPZ), and detected a greater tumour accumulation than HSA:CPZ using fluorescent molecular tomography (FMT) by targeting uPAR on the tumour cell surface to subsequently achieve highly efficient photodynamic killing of tumours in an H22 tumour model [175]. Zhou et al. also generated a CPZ loaded mouse ATF-HSA (mATF-HSA:CPZ) that achieved an enhanced murine tumour targeting ability and an enhanced PDT efficacy compared with hATF-HSA:CPZ [176]. Based on this information, the author further developed CPZ-loaded uPAR-targeted receptor-responsive NPs (ATF-HSA:CPZ@RRNP) with a diameter of ~ 40 nm. Interestingly, ATF-HSA:CPZ@RRNP, but not the nontargeting NPs, disintegrated into 7.5 nm fragments and released its cargo in the presence of uPAR. These NPs also exhibited higher cytotoxicity toward H1299 cells and greater tumour accumulation and antitumor effects on the H22 tumour model than HSA:CPZ@RRNP [177]. Chen et al. designed an active targeting phototherapeutic agent by conjugating zinc phthalocyanine (ZnPc) with ATF (ATF-ZnPc), which not only exhibited a high binding affinity and potent PDT activities to uPAR-positive U937 and H1299 cells, but also was used as a biomarker for the noninvasive imaging of tumours [178].
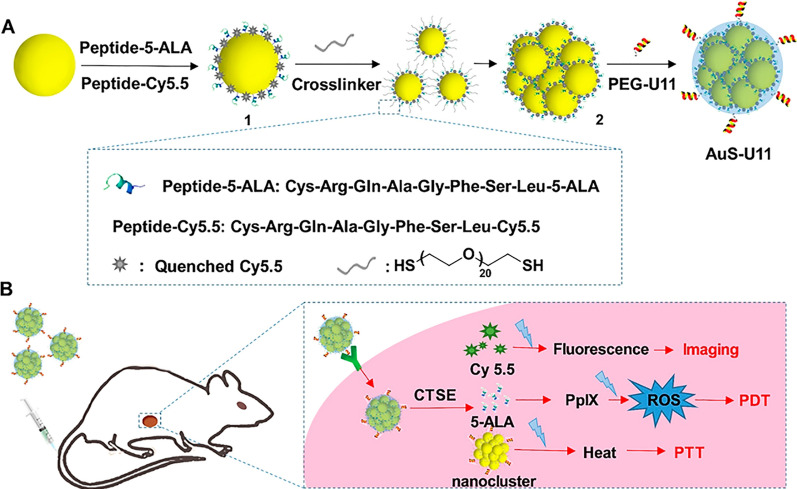
Fig. 4.
uPAR-targeted, CTSE-responsive gold nanoclusters as a PDT/PTT platform. A Schematic of the synthetic route used to produce the gold nanoclusters in three steps: CTSE-cleavable CRQAGFSL-5-ALA (Peptide-5-ALA, prodrug) and CRQAGFSL-Cy5.5 (Peptide-cy5.5) were covalently conjugated to the nanospheres, cross-linked with 1,9-nonanedithiol to produce spherical gold nanoclusters, and finally coated with the U11 peptide modified PEG layer to yield the uPAR-targeted, CTSE-responsive PDT/PTT platform. B Overview of image-guided PDT/PTT in therapeutic PDAC. Upon injection, the nanoclusters first targeted the pancreatic tumour tissue. The selective cleavage of the CTSE-sensitive peptide activated the fluorescence signal of the NIR cyanine dye Cy5.5 to guide PDT/PTT therapy using confocal laser endomicroscopy. CTSE cathepsin E, 5-ALA 5-aminolevulinic acid, Cy5.5 cyanine 5.5, PDAC pancreatic ductal adenocarcinoma, NIR near infrared, PEG polyethylene glycol, ROS reactive oxygen species
(Reproduced with permission from reference [172]. Copyright 2017, Pergamon)
In addition, Yu et al. developed uPAR-targeted polyetherimide-AE105 peptide (P-AE105) conjugated gold nanostars (GNS) carrying an iridium (Ir) complex that exerted enhanced anti-TNBC effects through the ROS-induced p53 apoptotic pathway, and showed excellent PT/photoacoustic (PA)/X-ray computed tomography (CT) imaging properties [179]. Hu et al. constructed an AE105 peptide conjugated gold nanorod mesoporous silica heterostructure loaded with Cis and Avastin (Cis-AuNRs@SiO2-Avastin@PEI/AE105), and observed a prominent photodynamic killing effect and anti-angiogenic activity by targeting uPAR and smart light-controlled drug release in a HeLa tumour model [180]. Zuo et al. designed and constructed AE105-decorated dendritic mesoporous silica NPs (DMSN) encapsulating photonic active ultrasmall Cu2−xS NPs and the sonosensitizer Rose Bengal (RB) (Cu2−xS-RB@DMSN-AE105, abbreviated as CRDA) for OSCC-targeting and synergetic PTT/sonodynamic therapy (SDT) [181]. Hu et al. also developed anti-uPAR antibody and indocyanine green (ICG)-modifed gold nanoshells (uIGNs), and achieved a 25% higher median survival rate and complete tumour ablation than clinical iodine-125 (125I) interstitial brachy-therapy (IBT-125-I). Furthermore, uIGNs prevented pancreatic tumour metastasis, as evidenced by real-time monitoring of metastatic tumours (less than 2 mm) using CT and NIR imaging [182]. The uPAR-targeted PDT/PTT platforms are summarized in Table 3.
Table 3.
The uPAR-targeted PDT/PTT platforms
| uPAR-targeted PDT/PTT platform | Target | Photosensitizer and drug | Imaging | Effect | References, year |
|---|---|---|---|---|---|
| AuS-U11 for confocal laser endomicroscopy-guided PTT/ PDT | U11 peptide (uPAR) | PTT-carrier gold nanocluster, CRQAGFSL-5-ALA, CRQAGFSL-Cy5.5 | Fluorescence images (enzyme-triggered release of NIR fluorescent dye Cy5.5) | Better synergistic therapeutic effects as well as the reduced side effects in normal pancreas tissue (human pancreatic tumour cell line PANC1-CSTE and its orthotopic tumour model) | [172], 2017 |
| hATF-HSA:CPZ | hATF (uPAR) | CPZ | FMT imaging (CPZ, 0.08 μmol/kg or 0.05 mg/kg) | A significant reduced tumour growth rate (H22 tumour-bearing Kunming mice model) | [175], 2014 |
| mATF-HSA:CPZ | mATF (uPAR) | CPZ | FMT imaging (CPZ, 0.05 mg/kg) | A higher tumour killing efficacy than hATF-HSA:CPZ (H22 tumour-bearing mouse model) | [176], 2015 |
| ATF-HSA: CPZ@RRNP | ATF (uPAR) | CPZ-loaded receptor-responsive nanoparticles | FMT imaging (CPZ, 0.05 mg/kg) | Higher uptake and cytotoxicity (H1299 lung cancer cells), higher tumour accumulation and better antitumour effect (H22 tumour-bearing mice), lower CPZ concentration (liver, kidney, spleen, lung, and heart) | [177], 2019 |
| ATF-ZnPc | ATF (uPAR) | ZnPc | FMT imaging (ATF-ZnPc, 0.4 μmol/kg) | Potent PDT activities and enhanced antitumour activity (U937 and H1299 cells and H22 tumour-bearing mice) | [178], 2014 |
| GNS@Ir@P-AE105 | AE105 (uPAR) | GNS, Ir complex | PT/PA/X-ray CT trimodal imaging | Combinational photothermal-chemotherapeutic efficiency against TNBC via a ROS-induced p53 apoptotic pathway | [179], 2020 |
| Cisplatin-AuNRs@SiO2-Avastin@PEI/AE105 | AE105 (uPAR) | Gold nanorod mesoporous silica heterostructure, cisplatin, Avastin | Photothermal imaging (3 mg/kg) | Photodynamic activity via induction of ROS overproduction-mediated cell apoptosis, suppresses HeLa tumour growth and angiogenesis | [180], 2019 |
| Cu2−xS-RB@DMSN-AE105 | AE105 (uPAR) | Cu2−xS NPs, Rose Bengal | Infrared thermal imaging | Synergetic PTT/SDT nanotherapeutics against the OSCC both in vitro and in vivo, a prominent tumour inhibition rate of 103.4% | [181], 2020 |
| uIGNs | Anti-uPAR antibody | ICG modifed gold nanoshells | CT and optical imaging (bioluminescence imaging and fluorescence imaging) | 25% higher median survival rate of IPTT and complete tumour ablation by one-time intervention, inhibit pancreatic tumour metastasis | [182], 2017 |
PDT photodynamic therapy, PTT photothermal therapy, 5-ALA 5-aminolevulinic acid, Cy5.5 cyanine 5.5, HSA human serum albumin, CPZ mono-substituted β-carboxy phthalocyanine zinc, FMT fluorescent molecular tomography, ZnPc zinc phthalocyanine, SDT sonodynamic therapy, OSCC oral squamous cell carcinoma, CT computed tomography, PT photothermal, PA photoacoustic, GNS gold nanostars, Ir iridium, ICG indocyanine green, ROS reactive oxygen species, IPTT interventional PTT
uPAR-targeted oncolytic virotherapy
Oncolytic virotherapy is an emerging platform that represents a novel frontier for cancer treatment. Redirecting viral tropism to specific tumour targets is a promising strategy in the field of oncolytic viruses, which may increase safety and inhibit distant metastases of tumours [183]. Recently, some retargeted oncolytic measles viruses (MVs) against uPAR have been developed.
MV-h-uPA or MV-m-uPA, an Edmonston vaccine strain of oncolytic MVs constructed by the ATF of human or murine uPA and mutant MV-H glycoprotein, was able to replicate, and induce cytotoxicity in a species-specific manner. In vivo, MV-h-uPA successfully inhibited tumour growth (inhibition rate of 76% at Day 39), prolonged survival (70% survival rate at Day 80) and reduced metastatic progression in an MDA-MB-231 tumour model [184]. In addition, MV-m-uPA increased the death of murine mammary (4T1) and colon (MC-38 and CT-26) tumour cells overexpressing uPAR. MV-m-uPA also significantly enhanced the anticancer effects and prolonged survival in CT-26 and 4T1 tumour models [185], and delayed 4T1 lung metastasis progression. In conclusion, MV-uPA is a novel oncolytic MV associated with potent and specific antitumour and antimetastatic effects [186].
Tumour stroma-selective targeting by uPAR retargeted MVs is also associated with enhanced antitumour effects. For example, MV-m-uPA inhibits breast cancer cell proliferation by selectively targeting fibroblasts, and delays tumour progression and prolongs survival in mice bearing a human MDA-MB-231 tumour model [187]. MV-CD46-muPA, a dual-targeted oncolytic MV that simultaneously targets murine stromal (via uPAR) and human cancer cells (via CD46), markedly enhances antitumour effects on the HT-29 tumour model compared to CD46-targeted MV alone. The improved effect was associated with the modulation of viral deposition, cell cycle and metabolic pathways, increased apoptosis and decreased murine stromal [188].
uPAR-targeted gene therapy technologies
The development of efficient and reliable methods to generate precise, targeted changes in the genome of living cells is a long-standing goal for biomedical researchers. In uPAR-targeted gene therapy technologies, adenovirus-mediated antisense uPAR therapy first emerged as an effective tool for cancer treatment. For example, an adenoviral vector containing the uPAR antisense sequence (Ad-uPAR), an adenovirus containing uPAR antisense and p16 sense expression cassettes (Ad-uPAR/p16), an adenovirus expressing antisense uPAR and uPA sequences (Ad-uPAR-uPA), an adenovirus vector containing antisense uPAR and cathepsin B sequences (Ad-uPAR-Cath B), and an adenovirus expressing antisense uPAR and MMP-9 sequences (Ad-uPAR-MMP-9) were all successfully constructed and inhibited tumour growth and metastasis in gliomas and lung cancer models [189–193].
Subsequently, RNA interference (RNAi) technologies, including siRNAs and short hairpin RNAs (shRNAs) targeting uPAR (siRNAs against uPAR, siRNAs against uPAR and cathepsin B, siRNAs against uPA and uPAR, shRNAs against uPAR, and shRNAs against uPA and uPAR), were developed to prevent tumour progression. Compared with siRNAs/shRNAs targeting uPAR, siRNAs targeting uPAR and uPA or siRNAs targeting uPAR and cathepsin B exerted a better antitumor effect by inhibiting tumour cell proliferation, migration and invasion and angiogenesis and promoting tumour cell apoptosis [70, 194–198].
Recently, a new tool based on bacterial Cas9 from Streptococcus pyogenes has generated a considerable level of excitement. The RNA-guided CRISPR/Cas9 system is a powerful RNA-guided genome editing tool that utilizes a guide RNA (gRNA) to cleave the desired sequence in the genome and remove existing genes or add new genes. Due to the advantages of being fast, precise, and highly efficient, targeting uPAR with CRISPR/Cas9 technology has been successfully applied in a variety of malignant tumours to enhance the treatment effect [98]. Targeting uPAR in Neuro 2A cells using CRISPR/Cas9 decreases cell proliferation (~ 60%) and the number of Ki-67-positive cells by activating caspase-3, cleaving poly(ADP-ribose) polymerase-1 (PARP-1), and inhibiting tropomyosin receptor kinase C (TrkC) sactivity and AKT phosphorylation [199]. Wang et al. also targeted uPAR using CRISPR/Cas9 technology to suppress the proliferation, migration and invasion of HCT8/T and KBV200 cells. Furthermore, uPAR knockout inhibited MDR to 5-FU, Cis, DTX, and Dox [98]. Biagioni et al. also knocked out uPAR using the CRISPR/Cas9 system in human melanoma A375p and A375M6 cells and colon cancer HCT116 cells, inducing extensive glycolytic and oxidative phosphorylation reprogramming by blocking the glycolytic pathway while enhancing the mitochondrial spare respiratory capacity [200]. They also reported that uPAR deficiency mediated by CRISPR/Cas9 induced a stem-like phenotype, but uPAR knockout completely eliminated tumorigenesis [201].
uPAR-targeted monoclonal antibody therapy
A variety of monoclonal antibodies targeting uPAR have been developed, and exert antitumor effects by blocking the uPA/uPAR interaction or inhibiting the interactions between uPAR and integrin, EGFR, FPR, and Vn. The 2G10 antibody binds tightly to uPAR (Fab Kd = 10 × 10–9; IgG Kd = 2 × 10–12) by forming a stable complex with uPAR and disrupting the uPA/uPAR interaction. LeBeau et al. found that 30 mg/kg 2G10 IgG prevents the growth of TNBC, and 177Lu-labelled 2G10 completely eliminates tumours in orthotopic breast cancer models [202]. Harel et al. further prepared the antibody–drug conjugate 2G10-RED-244-MMAE to treat TNBC, and the tumour volume was significantly reduced [203]. Duriseti et al. identified a series of monoclonal antibodies that bind uPAR, including 2G10, 2E9 and 3C6. The 2G10 and 2E9 antibodies inhibited the uPA/uPAR interaction, whereas 3C6 inhibited the uPAR/β1 integrin interaction. Additionally, 3C6 abrogated uPAR/β1 integrin-mediated adhesion to Vn and fibronectin and exerted a synergistic effect with 2G10 on inhibiting invasion in H1299 cells [204].
ATN-658 is a humanized monoclonal antibody that binds to the D2D3 region of uPAR with high affinity (Kd ≈ 1 nmol/L), and the binding of ATN-658 to uPAR is not affected by the binding of uPA to uPAR. ATN-658 mainly inhibits the activation of downstream signalling pathways by inhibiting the uPAR/integrin interaction. ATN-658 inhibits the growth and liver metastasis of pancreatic cancer in situ and completely inhibits retroperitoneal infiltration; the antitumour effect is more obvious when this antibody is combined with Gem [65]. ATN-658 also significantly inhibits the growth of human colorectal cancer in the liver, and prevents the growth, migration, invasion and bone metastasis of prostate cancer [205, 206]. In addition, ATN-658 inhibits the metastasis of ovarian cancer and reduces the uPAR/α5-integrin interaction, and the tumour suppression rate is higher when it is combined with PTX [207]. ATN-658 significantly reduces the growth of MDA-MB-231 breast tumours, and when combined with Zometa, it significantly reduces the number of bone lesions caused by breast cancer by inhibiting the activity of osteoclasts [208]. Li et al. also prepared the monoclonal antibody ATN-615 that binds uPAR with high affinity (Kd ≈ 1 nmol/L) and does not block the uPA/uPAR interaction [209]. ATN-292, isotype IgG1κ, decreases the migration of human pancreatic carcinoma L3.6pl cells (70% ± 8%) by inhibiting the binding of uPA to uPAR [65].
Two antibodies, mAb R3 and mAb R5, are competitive and noncompetitive inhibiters of the uPA/uPAR interaction, respectively. mAb R5 binds the preformed complex and promotes the dissociation of the uPA/PAR complex, while mAb R3 does not promote the dissociation of the preformed complex [210]. Pass et al. developed an anti-muPAR murine mAb (mR1) that interferes with the muPA/muPAR interaction on P388D.1 cells with an IC50 of 0.67 nM [211]. A monoclonal antibody against human uPAR, mAb 3936, also inhibits hepatocyte growth factor (HGF)-mediated HepG2 and Hep3B cell invasion in a dose-dependent manner [212]. The mAb 8B12, a specific inhibitor that blocks the uPAR/Vn interaction, significantly decreases tumour growth by increasing cell apoptosis and reducing cell proliferation in a prostate cancer model. A crystal structure of the uPAR-8B12 complex showed that the structural epitope for 8B12 is located at the D2–D3 domain interface on the surface of uPAR [213].
uPAR-targeted tumour immunotherapy
As an innovative treatment method, tumour immunotherapy has shown potential to fight cancer by modulating the immune system, such as checkpoint inhibitors and adoptive cellular therapy using CAR T-cell [214]. Based on the high expression of uPAR on the surface of tumour cells, some researchers have explored the combination of CAR T-cell immunotherapy and uPAR targeting to treat uPAR-expressing malignancies or the use of uPAR as a target to induce immune-mediated clearance of uPAR-positive tumour cells by constructing ARMs.
uPAR-targeted CAR T-cell immunotherapy
CARs are synthetic receptors that contain an extracellular single-chain variable fragment (scFv), a hinge region that provides flexibility to the scFv, a transmembrane domain, and intracellular signalling/activation domain(s) [215, 216]. CAR T-cell immunotherapy, extracts the patient’s own key immune T-cells and embeds them with a CAR, that recognizes tumour cell surface antigens while activating T-cells to kill tumour cells. CAR T-cell immunotherapy has achieved remarkable success in treating refractory B-cell malignancies [217]. In recent years, some researchers have combined ATF and CAR T-cells to treat solid tumours with high uPAR expression. Wang et al. designed anti-uPAR CAR (ATF-CAR) T-cells constructed by combining an antigen recognition domain with ATF to transduce T-cells, and this treatment exhibited strong cytotoxicity toward uPAR-expressing ovarian cancer cells and released higher levels of Th1 cytokines [interferon-γ (IFN-γ), tumour necrosis factor (TNF) and interleukin-2 (IL-2)] and granzyme B than control T-cells [218]. Pathologically, cellular senescence may lead to a variety of diseases including cancer. Given the contribution of senescence to tumorigenesis, Amor et al. also developed an anti-uPAR CAR T-cells (m.uPAR-h.28z CAR T cells) by linking an anti-murine uPAR single chain variable fragment and human CD28 costimulatory and CD3ζ signalling domains to transduce human T-cells that efficiently cleared uPAR-expressing KP lung cancer cells, accompanied by increased secretion of granzyme B and IFN-γ. They also markedly prolonged survival and induced a significant decrease in the number of senescent tumour cells, accompanied by increased infiltration of CD4+ and CD8+ T cells in a mouse model of orthotopic KP lung adenocarcinoma [219].
uPAR-targeted ARMs
ARMs are antibody-binding molecules that exert antitumour effects by delivering endogenous antibodies to tumour tissues and destroying tumour cells via the activated immune system [220]. Jakobsche et al. designed and synthesized an antibody-recruiting complex ARM-U1 by attaching chloromethyl ketone 2 and 2,4-dinitrophenyl (DNP) to the active site of uPA that mediated both antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) against uPAR-expressing cancer cells [221]. The authors further designed a second-generation ARM-U2 by replacing the uPA protein with a molecule of IPR-803. ARM-U2 also induced both ADCP and ADCC, and achieved a tumour growth inhibition of approximately 90% compared to PBS treatment in the B16-uPAR mouse allograft model. They also reported a cocrystal structure of the ARM-U2/uPAR complex for the first time. In conclusion, uPAR-specific CAR T cells and ARMs are promising immunotherapies that not only block the uPA/uPAR interaction, but also achieve immune-mediated cell death by targeting uPAR-expressing tumour cells [222]. In addition, Hu et al. developed an antibody-like molecule, ATF-Fc, formed by linking ATF and the human IgG1 Fc fragment. ATF-Fc inhibits the growth and metastasis of MCF-7 breast cancer and BGC-823 gastric cancer cells by destroying the interaction of uPA/uPAR and inhibiting tumour angiogenesis [223]. Zhou et al. further showed that the combination of ATF-Fc and trastuzumab better inhibits the growth and metastasis of HER-2-positive breast cancer cells by interfering with the uPA/uPAR and HER-2 pathways [224].
Concluding remarks
uPAR is an attractive target for the treatment of cancer because it appears to be expressed at high levels in tumours but low levels in normal tissue. uPAR also plays a comprehensive role in the development of tumours and is closely related to tumour proliferation and apoptosis, invasion and metastasis, prognosis, and tumour MDR, providing a basis for the development of multiple therapeutics agents targeting this protein. This review has summarized multiple new applications of uPAR as a target in nanoplatforms carrying therapeutic agents, PTT/PDT platforms, oncolytic virotherapy, gene therapy technologies, monoclonal antibody therapy and tumour immunotherapy in recent years. The development of therapeutic strategies that target tumours via uPAR recognition has proven its potential in animal models, but no uPAR-targeted therapeutic agents have been developed or evaluated in cancer clinical trials to date. Recently, ATN-658 has been humanized (huATN-658) and is awaiting clinical translation; and phase I clinical trials with 64Cu-DOTA-AE105 are being conducted to diagnose aggressive cancers and determine cancer aggressiveness. These two agents are expected to be administered to patients in the future.
Among uPAR-targeted therapeutic strategies, uPAR-targeted nanoplatforms also have great potential to achieve translation from laboratory findings to the clinic. Based on the high expression of uPAR on the surface of a variety of tumour cells, uPA/ATF/AE105/AE147/PAI-2/U11 modified nanoplatforms provide the possibility of reducing or overcoming the therapeutic limitations of conventional chemotherapy or PTT/PDT through targeted delivery to tumour cells without obvious toxicity to healthy tissue. Moreover, recent studies have a key role for the tumour microenvironment in promoting tumour proliferation, invasion and metastasis [225]. uPAR expression is not confined to tumour cells and is found on tumour-associated cell types, including macrophages, endothelial cells and fibroblasts. The development of uPAR-targeted stroma-breaking or stroma-penetrating NPs may allow therapeutic agents to overcome stromal barriers and reach tumour cells, which is highly likely to improve the therapeutic effect of current treatment agents and may provide better therapeutic options for patients to reduce tumour-associated metastasis.
Acknowledgements
Not applicable.
Abbreviations
- uPAR
Urokinase-type plasminogen activator receptor
- ECM
Extracellular matrix
- MDR
Multidrug resistance
- PDT
Photodynamic therapy
- PTT
Photothermal therapy
- uPA
Urokinase plasminogen activator
- MMP
Matrix metalloproteinase
- Vn
Vitronectin
- EGFR
Epidermal growth factor receptor
- GPCRs
G-protein coupled receptors
- FAK
Focal adhesion kinase
- MAPK
Mitogen-activated protein kinase
- AKT
Protein kinase B
- FPR
Formyl peptide receptor
- HER-2
Human epidermal growth factor receptor-2
- PAI
Plasminogen activator inhibitor
- ERK
Extracellular regulatory protein kinase
- Cis
Cisplatin
- DTX
Docetaxel
- Dox
Doxorubicin
- PEG
Polyethylene glycol
- DT
Diphtheria toxin
- TNBC
Triple-negative breast cancer
- NPs
Nanoparticles
- IONP
Iron oxide nanoparticle
- MRI
Magnetic resonance imaging
- N-AI
N-Alkylisatin
- HSA
Human serum albumin
- CPZ
Mono-substituted β-carboxy phthalocyanine zinc
- MVs
Oncolytic measles viruses
- siRNA
Small interfering RNA
- shRNA
Short hairpin RNA
- CRISPR
Clustered regularly interspaced short palindromic
- Cas9
CRISPR-associated protein-9 nuclease
- CAR
Chimeric antigen receptor
- ARMs
Antibody-recruiting molecules
Authors’ contributions
HT and JS contributed to the collection of relevant literature. JBZ and XFZ contributed to literature analysis and manuscript preparation. BTZ sorted out the literature and wrote the manuscript. JXC and YJS provided a lot of help in the revision of the manuscript. YF and DYG were responsible for design of the review and provided data acquisition, analysis, and interpretation. All authors contributed to the article. All authors read and approved the final manuscript.
Funding
This research was supported by grants from National Natural Science Foundation of China (Grant No. 81703925), the disciplinary innovation team construction project of Shaanxi University of Traditional Chinese Medicine (Grant No. 2019-YL11), Key scientific research project of Shaanxi Provincial Department of Education (Grant No. 21JS009), Scientific Research Project of Xi'an Administration of Traditional Chinese Medicine (Grant No. SZY202103).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have reviewed the final version of the manuscript and approved it for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palfree RG. The urokinase-type plasminogen activator receptor is a member of the Ly-6 superfamily. Immunol Today. 1991;12(5):170. doi: 10.1016/S0167-5699(05)80051-9. [DOI] [PubMed] [Google Scholar]
- 2.Williams AF. Emergence of the Ly-6 superfamily of GPI-anchored molecules. Cell Biol Int Rep. 1991;15(9):769–777. doi: 10.1016/0309-1651(91)90032-e. [DOI] [PubMed] [Google Scholar]
- 3.Stoppelli MP, Corti A, Soffientini A, Cassani G, Blasi F, Assoian RK. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci USA. 1985;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen LS, Kellerman GM, Behrendt N, Picone R, Danø K, Blasi F. A 55000–60000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J Biol Chem. 1988;263(5):2358–2363. [PubMed] [Google Scholar]
- 6.Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266(3):1926–1933. [PubMed] [Google Scholar]
- 7.Ellis V, Scully MF, Kakkar VV. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989;264(4):2185–2188. [PubMed] [Google Scholar]
- 8.Ellis V, Behrendt N, Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266(19):12752–12758. [PubMed] [Google Scholar]
- 9.Behrendt N, Rønne E, Danø K. The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol Chem Hoppe Seyler. 1995;376(5):269–279. [PubMed] [Google Scholar]
- 10.Behrendt N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biol Chem. 2004;385(2):103–136. doi: 10.1515/BC.2004.031. [DOI] [PubMed] [Google Scholar]
- 11.Cooper F, Overmiller AM, Loder A, Brennan-Crispi DM, McGuinn KP, Marous MR, Freeman TA, Riobo-Del Galdo NA, Siracusa LD, Wahl JR, 3rd, et al. Enhancement of cutaneous wound healing by Dsg2 augmentation of uPAR secretion. J Invest Dermatol. 2018;138(11):2470–2479. doi: 10.1016/j.jid.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genua M, D'Alessio S, Cibella J, Gandelli A, Sala E, Correale C, Spinelli A, Arena V, Malesci A, Rutella S, et al. The urokinase plasminogen activator receptor (uPAR) controls macrophage phagocytosis in intestinal inflammation. Gut. 2015;64(4):589–600. doi: 10.1136/gutjnl-2013-305933. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Hall TR, Xu X, Yung I, Souza D, Zheng J, Schiele F, Hoffmann M, Mbow ML, Garnett JP, et al. Targeting uPA-uPAR interaction to improve intestinal epithelial barrier integrity in inflammatory bowel disease. Ebiomedicine. 2022;75:103758. doi: 10.1016/j.ebiom.2021.103758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Multhaupt HA, Mazar A, Cines DB, Warhol MJ, McCrae KR. Expression of urokinase receptors by human trophoblast. A histochemical and ultrastructural analysis. Lab Invest. 1994;71(3):392–400. [PubMed] [Google Scholar]
- 15.Stephens RW, Pöllänen J, Tapiovaara H, Leung KC, Sim PS, Salonen EM, Rønne E, Behrendt N, Danø K, Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plow EF, Miles LA. Plasminogen receptors in the mediation of pericellular proteolysis. Cell Differ Dev. 1990;32(3):293–298. doi: 10.1016/0922-3371(90)90042-u. [DOI] [PubMed] [Google Scholar]
- 17.Hahn-Dantona E, Ramos-DeSimone N, Sipley J, Nagase H, French DL, Quigley JP. Activation of proMMP-9 by a plasmin/MMP-3 cascade in a tumor cell model. Regulation by tissue inhibitors of metalloproteinases. Ann N Y Acad Sci. 1999;878:372–387. doi: 10.1111/j.1749-6632.1999.tb07696.x. [DOI] [PubMed] [Google Scholar]
- 18.Legrand C, Polette M, Tournier JM, de Bentzmann S, Huet E, Monteau M, Birembaut P. uPA/plasmin system-mediated MMP-9 activation is implicated in bronchial epithelial cell migration. Exp Cell Res. 2001;264(2):326–336. doi: 10.1006/excr.2000.5125. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274(19):13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 20.Pedrozo HA, Schwartz Z, Robinson M, Gomes R, Dean DD, Bonewald LF, Boyan BD. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-beta1 from the extracellular matrix of growth plate chondrocytes. Endocrinology. 1999;140(12):5806–5816. doi: 10.1210/endo.140.12.7224. [DOI] [PubMed] [Google Scholar]
- 21.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269(51):32380–32388. [PubMed] [Google Scholar]
- 23.Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9(19):1565–1574. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12(10):2975–2986. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Czekay RP, Robillard L, Kugler MC, Zhang F, Kim KK, Xiong JP, Humphries MJ, Chapman HA. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol. 2005;168(3):501–511. doi: 10.1083/jcb.200404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276(6):3983–3990. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- 27.Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A, Botti G, D'Aiuto G, Salvatore M, Stoppelli MP. Urokinase receptor interacts with alpha(v)beta5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res. 1999;59(20):5307–5314. [PubMed] [Google Scholar]
- 28.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1(5):445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 29.Aguirre Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21(16):2513–2524. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol. 2002;159(6):1061–1070. doi: 10.1083/jcb.200207070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase-induced signaling in human vascular smooth muscle cells is mediated by PDGFR-beta. EMBO J. 2005;24(10):1787–1797. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K, Fan J, Wu J. Sushi repeat-containing protein X-linked 2 promotes angiogenesis through the urokinase-type plasminogen activator receptor dependent integrin avβ3/focal adhesion kinase pathways. Drug Discov Ther. 2017;11(4):212–217. doi: 10.5582/ddt.2017.01017. [DOI] [PubMed] [Google Scholar]
- 34.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99(3):1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Q, Leng J, Bian D, Mahanivong C, Carpenter KA, Pan ZK, Han J, Huang S. Rac1-MKK3-p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem. 2002;277(50):48379–48385. doi: 10.1074/jbc.M209542200. [DOI] [PubMed] [Google Scholar]
- 36.Koshelnick Y, Ehart M, Hufnagl P, Heinrich PC, Binder BR. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J Biol Chem. 1997;272(45):28563–28567. doi: 10.1074/jbc.272.45.28563. [DOI] [PubMed] [Google Scholar]
- 37.Bifulco K, Longanesi-Cattani I, Gala M, Di Carluccio G, Masucci MT, Pavone V, Lista L, Arra C, Stoppelli MP, Carriero MV. The soluble form of urokinase receptor promotes angiogenesis through its Ser88-Arg-Ser-Arg-Tyr92 chemotactic sequence. J Thromb Haemost. 2010;8(12):2789–2799. doi: 10.1111/j.1538-7836.2010.04075.x. [DOI] [PubMed] [Google Scholar]
- 38.Rossi FW, Prevete N, Rivellese F, Napolitano F, Montuori N, Postiglione L, Selleri C, de Paulis A. The urokinase/urokinase receptor system in mast cells: effects of its functional interaction with fMLF receptors. Transl Med UniSa. 2016;15:34–41. [PMC free article] [PubMed] [Google Scholar]
- 39.Olson D, Pöllänen J, Høyer-Hansen G, Rønne E, Sakaguchi K, Wun TC, Appella E, Danø K, Blasi F. Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J Biol Chem. 1992;267(13):9129–9133. [PubMed] [Google Scholar]
- 40.Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. Embo J. 1997;16(10):2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol Biol Cell. 2001;12(5):1467–1479. doi: 10.1091/mbc.12.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher JL, Field CL, Zhou H, Harris TL, Henderson MA, Choong PF. Urokinase plasminogen activator system gene expression is increased in human breast carcinoma and its bone metastases—a comparison of normal breast tissue, non-invasive and invasive carcinoma and osseous metastases. Breast Cancer Res Treat. 2000;61(1):1–12. doi: 10.1007/s10549-004-6659-9. [DOI] [PubMed] [Google Scholar]
- 43.He C, He P, Liu LP, Zhu YS. Analysis of expressions of components in the plasminogen activator system in high- and low-metastatic human lung cancer cells. J Cancer Res Clin Oncol. 2001;127(3):180–186. doi: 10.1007/s004320000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dohn LH, Pappot H, Iversen BR, Illemann M, Høyer-Hansen G, Christensen IJ, Thind P, Salling L, von der Maase H, Laerum OD. uPAR expression pattern in patients with urothelial carcinoma of the bladder—possible clinical implications. PLoS ONE. 2015;10(8):e0135824. doi: 10.1371/journal.pone.0135824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabrouk RA, Ali-Labib R. Detection of urokinase plasminogen activator receptor and c-erbB-2 in sera of patients with breast and ovarian carcinoma. Clin Biochem. 2003;36(7):537–543. doi: 10.1016/s0009-9120(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 46.Kimura S, D'Andrea D, Iwata T, Foerster B, Janisch F, Parizi MK, Moschini M, Briganti A, Babjuk M, Chlosta P, et al. Expression of urokinase-type plasminogen activator system in non-metastatic prostate cancer. World J Urol. 2020;38(10):2501–2511. doi: 10.1007/s00345-019-03038-5. [DOI] [PubMed] [Google Scholar]
- 47.Morita Y, Hayashi Y, Wang Y, Kanamaru T, Suzuki S, Kawasaki K, Ohta K, Yamamoto M, Saitoh Y, Itoh H, et al. Expression of urokinase-type plasminogen activator receptor in hepatocellular carcinoma. Hepatology. 1997;25(4):856–861. doi: 10.1002/hep.510250412. [DOI] [PubMed] [Google Scholar]
- 48.Pyke C, Ralfkiaer E, Rønne E, Høyer-Hansen G, Kirkeby L, Danø K. Immunohistochemical detection of the receptor for urokinase plasminogen activator in human colon cancer. Histopathology. 1994;24(2):131–138. doi: 10.1111/j.1365-2559.1994.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 49.Cantero D, Friess H, Deflorin J, Zimmermann A, Bründler MA, Riesle E, Korc M, Büchler MW. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer. 1997;75(3):388–395. doi: 10.1038/bjc.1997.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong SI, Park IC, Son YS, Lee SH, Kim BG, Lee JI, Lee TW, Kook YH, Min YI, Hong WS. Expression of urokinase-type plasminogen activator, its receptor, and its inhibitor in gastric adenocarcinoma tissues. J Korean Med Sci. 1996;11(1):33–37. doi: 10.3346/jkms.1996.11.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto M, Sawaya R, Mohanam S, Rao VH, Bruner JM, Nicolson GL, Rao JS. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994;54(18):5016–5020. [PubMed] [Google Scholar]
- 52.Graf M, Reif S, Hecht K, Pelka-Fleischer R, Pfister K, Schmetzer H. High expression of urokinase plasminogen activator receptor (UPA-R) in acute myeloid leukemia (AML) is associated with worse prognosis. Am J Hematol. 2005;79(1):26–35. doi: 10.1002/ajh.20337. [DOI] [PubMed] [Google Scholar]
- 53.Shou LH, Cao D, Dong XH, Fang Q, Xu BL, Fei JP. Bone marrow urokinase plasminogen activator receptor levels are associated with the progress of multiple myeloma. Chin Med Sci J. 2016;31(3):155–160. doi: 10.1016/s1001-9294(16)30044-x. [DOI] [PubMed] [Google Scholar]
- 54.Pierga JY, Bonneton C, Magdelénat H, Vincent-Salomon A, Nos C, Boudou E, Pouillart P, Thiery JP, de Cremoux P. Real-time quantitative PCR determination of urokinase-type plasminogen activator receptor (uPAR) expression of isolated micrometastatic cells from bone marrow of breast cancer patients. Int J Cancer. 2005;114(2):291–298. doi: 10.1002/ijc.20698. [DOI] [PubMed] [Google Scholar]
- 55.Hildenbrand R, Schaaf A, Dorn-Beineke A, Allgayer H, Sütterlin M, Marx A, Stroebel P. Tumor stroma is the predominant uPA-, uPAR-, PAI-1-expressing tissue in human breast cancer: prognostic impact. Histol Histopathol. 2009;24(7):869–877. doi: 10.14670/HH-24.869. [DOI] [PubMed] [Google Scholar]
- 56.Boonstra MC, Verbeek FP, Mazar AP, Prevoo HA, Kuppen PJ, van de Velde CJ, Vahrmeijer AL, Sier CF. Expression of uPAR in tumor-associated stromal cells is associated with colorectal cancer patient prognosis: a TMA study. BMC Cancer. 2014;14:269. doi: 10.1186/1471-2407-14-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Geus SW, Baart VM, Boonstra MC, Kuppen PJ, Prevoo HA, Mazar AP, Bonsing BA, Morreau H, van de Velde CJ, Vahrmeijer AL, et al. Prognostic impact of urokinase plasminogen activator receptor expression in pancreatic cancer: malignant versus stromal cells. Biomark Insights. 2017;12:1177271917715443. doi: 10.1177/1177271917715443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci. 2008;121(Pt 22):3747–3756. doi: 10.1242/jcs.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12(12):2697–2708. doi: 10.1158/1535-7163.MCT-13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gogineni VR, Gupta R, Nalla AK, Velpula KK, Rao JS. uPAR and cathepsin B shRNA impedes TGF-β1-driven proliferation and invasion of meningioma cells in a XIAP-dependent pathway. Cell Death Dis. 2012;3(12):e439. doi: 10.1038/cddis.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanamandra N, Konduri SD, Mohanam S, Dinh DH, Olivero WC, Gujrati M, Nicolson GL, Obeyeseke M, Rao JS. Downregulation of urokinase-type plasminogen activator receptor (uPAR) induces caspase-mediated cell death in human glioblastoma cells. Clin Exp Metastasis. 2001;18(7):611–615. doi: 10.1023/a:1011941114862. [DOI] [PubMed] [Google Scholar]
- 62.Matheis F, Heppt MV, Graf SA, Düwell P, Kammerbauer C, Aigner A, Besch R, Berking C. A bifunctional approach of immunostimulation and uPAR inhibition shows potent antitumor activity in melanoma. J Invest Dermatol. 2016;136(12):2475–2484. doi: 10.1016/j.jid.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 64.Raghu H, Gondi CS, Dinh DH, Gujrati M, Rao JS. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch-1 receptor. Mol Cancer. 2011;10:130. doi: 10.1186/1476-4598-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, Bucana CD, Callahan J, Parry GC, Evans DB, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65(17):7775–7781. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 66.Subramanian R, Gondi CS, Lakka SS, Jutla A, Rao JS. siRNA-mediated simultaneous downregulation of uPA and its receptor inhibits angiogenesis and invasiveness triggering apoptosis in breast cancer cells. Int J Oncol. 2006;28(4):831–839. [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, Cao S, Liu Z, Ye X, Chen L, Meng S. RNAi-mediated downregulation of uPAR synergizes with targeting of HER2 through the ERK pathway in breast cancer cells. Int J Cancer. 2010;127(7):1507–1516. doi: 10.1002/ijc.25159. [DOI] [PubMed] [Google Scholar]
- 68.Huber MC, Mall R, Braselmann H, Feuchtinger A, Molatore S, Lindner K, Walch A, Gross E, Schmitt M, Falkenberg N, et al. uPAR enhances malignant potential of triple-negative breast cancer by directly interacting with uPA and IGF1R. BMC Cancer. 2016;16:615. doi: 10.1186/s12885-016-2663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unseld M, Chilla A, Pausz C, Mawas R, Breuss J, Zielinski C, Schabbauer G, Prager GW. PTEN expression in endothelial cells is down-regulated by uPAR to promote angiogenesis. Thromb Haemost. 2015;114(2):379–389. doi: 10.1160/TH15-01-0016. [DOI] [PubMed] [Google Scholar]
- 70.Raghu H, Nalla AK, Gondi CS, Gujrati M, Dinh DH, Rao JS. uPA and uPAR shRNA inhibit angiogenesis via enhanced secretion of SVEGFR1 independent of GM-CSF but dependent on TIMP-1 in endothelial and glioblastoma cells. Mol Oncol. 2012;6(1):33–47. doi: 10.1016/j.molonc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herkenne S, Paques C, Nivelles O, Lion M, Bajou K, Pollenus T, Fontaine M, Carmeliet P, Martial JA, Nguyen NQ, et al. The interaction of uPAR with VEGFR2 promotes VEGF-induced angiogenesis. Sci Signal. 2015;8(403):ra117. doi: 10.1126/scisignal.aaa2403. [DOI] [PubMed] [Google Scholar]
- 72.Madunić J. The urokinase plasminogen activator system in human cancers: an overview of its prognostic and predictive role. Thromb Haemost. 2018;118(12):2020–2036. doi: 10.1055/s-0038-1675399. [DOI] [PubMed] [Google Scholar]
- 73.Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, Slawin KM. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25(4):349–355. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 74.Kumano M, Miyake H, Muramaki M, Furukawa J, Takenaka A, Fujisawa M. Expression of urokinase-type plasminogen activator system in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol Oncol. 2009;27(2):180–186. doi: 10.1016/j.urolonc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Memarzadeh S, Kozak KR, Chang L, Natarajan S, Shintaku P, Reddy ST, Farias-Eisner R. Urokinase plasminogen activator receptor: prognostic biomarker for endometrial cancer. Proc Natl Acad Sci USA. 2002;99(16):10647–10652. doi: 10.1073/pnas.152127499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Vries TJ, Mooy CM, Van Balken MR, Luyten GP, Quax PH, Verspaget HW, Weidle UH, Ruiter DJ, Van Muijen GN. Components of the plasminogen activation system in uveal melanoma—a clinico-pathological study. J Pathol. 1995;175(1):59–67. doi: 10.1002/path.1711750110. [DOI] [PubMed] [Google Scholar]
- 77.Yang JL, Seetoo DQ, Wang Y, Ranson M, Berney CR, Ham JM, Russell PJ, Crowe PJ. Urokinase-type plasminogen activator and its receptor in colorectal cancer: independent prognostic factors of metastasis and cancer-specific survival and potential therapeutic targets. Int J Cancer. 2000;89(5):431–439. doi: 10.1002/1097-0215(20000920)89:5<431::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 78.Halamkova J, Kiss I, Pavlovsky Z, Tomasek J, Jarkovsky J, Cech Z, Tucek S, Hanakova L, Moulis M, Zavrelova J, et al. Clinical significance of the plasminogen activator system in relation to grade of tumor and treatment response in colorectal carcinoma patients. Neoplasma. 2011;58(5):377–385. doi: 10.4149/neo_2011_05_377. [DOI] [PubMed] [Google Scholar]
- 79.Dubuisson L, Monvoisin A, Nielsen BS, Le Bail B, Bioulac-Sage P, Rosenbaum J. Expression and cellular localization of the urokinase-type plasminogen activator and its receptor in human hepatocellular carcinoma. J Pathol. 2000;190(2):190–195. doi: 10.1002/(SICI)1096-9896(200002)190:2<190::AID-PATH511>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 80.Zheng Q, Tang Z, Wu Z, Shi D, Song H. Inhibitor of plasminogen activator 1 (PAI-1) in hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 1998;36(8):474–476. [PubMed] [Google Scholar]
- 81.Zheng Q, Tang Z, Wu Z. Urokinase-type plasminogen activator (uPA), uPA receptor (uPA-R) and inhibitors (PA I -1) expression in hepatocellular carcinoma in relation to cancer invasion/metastasis and prognosis. Zhonghua Zhong Liu Za Zhi. 1998;20(1):57–59. [PubMed] [Google Scholar]
- 82.Chen Q, Fei J, Wu L, Jiang Z, Wu Y, Zheng Y, Lu G. Detection of cathepsin B, cathepsin L, cystatin C, urokinase plasminogen activator and urokinase plasminogen activator receptor in the sera of lung cancer patients. Oncol Lett. 2011;2(4):693–699. doi: 10.3892/ol.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almasi CE, Drivsholm L, Pappot H, Høyer-Hansen G, Christensen IJ. The liberated domain I of urokinase plasminogen activator receptor—a new tumour marker in small cell lung cancer. APMIS. 2013;121(3):189–196. doi: 10.1111/j.1600-0463.2012.02955.x. [DOI] [PubMed] [Google Scholar]
- 84.Salden M, Splinter TA, Peters HA, Look MP, Timmermans M, van Meerbeeck JP, Foekens JA. The urokinase-type plasminogen activator system in resected non-small-cell lung cancer. Ann Oncol. 2000;11(3):327–332. doi: 10.1023/a:1008312801800. [DOI] [PubMed] [Google Scholar]
- 85.Beyer BC, Heiss MM, Simon EH, Gruetzner KU, Babic R, Jauch KW, Schildberg FW, Allgayer H. Urokinase system expression in gastric carcinoma: prognostic impact in an independent patient series and first evidence of predictive value in preoperative biopsy and intestinal metaplasia specimens. Cancer. 2006;106(5):1026–1035. doi: 10.1002/cncr.21682. [DOI] [PubMed] [Google Scholar]
- 86.Plebani M, Herszènyi L, Carraro P, De Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R, Farinati F. Urokinase-type plasminogen activator receptor in gastric cancer: tissue expression and prognostic role. Clin Exp Metastasis. 1997;15(4):418–425. doi: 10.1023/a:1018454305889. [DOI] [PubMed] [Google Scholar]
- 87.Baker EA, Leaper DJ, Hayter JP, Dickenson AJ. Plasminogen activator system in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2007;45(8):623–627. doi: 10.1016/j.bjoms.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 88.Seddighzadeh M, Steineck G, Larsson P, Wijkström H, Norming U, Onelöv E, Linder S. Expression of UPA and UPAR is associated with the clinical course of urinary bladder neoplasms. Int J Cancer. 2002;99(5):721–726. doi: 10.1002/ijc.10426. [DOI] [PubMed] [Google Scholar]
- 89.Sidaway P. Bladder cancer: uPAR expression indicates worse prognosis of urothelial carcinoma. Nat Rev Urol. 2015;12(3):120. doi: 10.1038/nrurol.2015.5. [DOI] [PubMed] [Google Scholar]
- 90.Hau AM, Leivo MZ, Gilder AS, Hu JJ, Gonias SL, Hansel DE. mTORC2 activation is regulated by the urokinase receptor (uPAR) in bladder cancer. Cell Signal. 2017;29:96–106. doi: 10.1016/j.cellsig.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Wang D, Wang T. Expressions and clinical significance of urokinase-type activator (uPA) and uPA receptor (uPAR) in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19(12):529–531. [PubMed] [Google Scholar]
- 92.Lanza F, Castoldi GL, Castagnari B, Todd RF, 3rd, Moretti S, Spisani S, Latorraca A, Focarile E, Roberti MG, Traniello S. Expression and functional role of urokinase-type plasminogen activator receptor in normal and acute leukaemic cells. Br J Haematol. 1998;103(1):110–123. doi: 10.1046/j.1365-2141.1998.00932.x. [DOI] [PubMed] [Google Scholar]
- 93.Gutova M, Najbauer J, Gevorgyan A, Metz MZ, Weng Y, Shih CC, Aboody KS. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS ONE. 2007;2(2):e243. doi: 10.1371/journal.pone.0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortes-Dericks L, Carboni GL, Schmid RA, Karoubi G. Putative cancer stem cells in malignant pleural mesothelioma show resistance to cisplatin and pemetrexed. Int J Oncol. 2010;37(2):437–444. doi: 10.3892/ijo_00000692. [DOI] [PubMed] [Google Scholar]
- 95.Huang Z, Wang L, Wang Y, Zhuo Y, Li H, Chen J, Chen W. Overexpression of CD147 contributes to the chemoresistance of head and neck squamous cell carcinoma cells. J Oral Pathol Med. 2013;42(7):541–546. doi: 10.1111/jop.12046. [DOI] [PubMed] [Google Scholar]
- 96.Eastman BM, Jo M, Webb DL, Takimoto S, Gonias SL. A transformation in the mechanism by which the urokinase receptor signals provides a selection advantage for estrogen receptor-expressing breast cancer cells in the absence of estrogen. Cell Signal. 2012;24(9):1847–1855. doi: 10.1016/j.cellsig.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou J, Kwak K, Wu Z, Yang D, Li J, Chang M, Song Y, Zeng H, Lee L, Hu J, et al. PLAUR confers resistance to gefitinib through EGFR/p-AKT/survivin signaling pathway. Cell Physiol Biochem. 2018;47(5):1909–1924. doi: 10.1159/000491071. [DOI] [PubMed] [Google Scholar]
- 98.Wang K, Xing ZH, Jiang QW, Yang Y, Huang JR, Yuan ML, Wei MN, Li Y, Wang ST, Liu K, et al. Targeting uPAR by CRISPR/Cas9 system attenuates cancer malignancy and multidrug resistance. Front Oncol. 2019;9:80. doi: 10.3389/fonc.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laurenzana A, Margheri F, Biagioni A, Chillà A, Pimpinelli N, Ruzzolini J, Peppicelli S, Andreucci E, Calorini L, Serratì S, et al. EGFR/uPAR interaction as druggable target to overcome vemurafenib acquired resistance in melanoma cells. EBioMedicine. 2019;39:194–206. doi: 10.1016/j.ebiom.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LeBeau AM, Sevillano N, King ML, Duriseti S, Murphy ST, Craik CS, Murphy LL, VanBrocklin HF. Imaging the urokinase plasminongen activator receptor in preclinical breast cancer models of acquired drug resistance. Theranostics. 2014;4(3):267–279. doi: 10.7150/thno.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazar AP, Ahn RW, O'Halloran TV. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des. 2011;17(19):1970–1978. doi: 10.2174/138161211796718152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ploug M, Østergaard S, Gårdsvoll H, Kovalski K, Holst-Hansen C, Holm A, Ossowski L, Danø K. Peptide-derived antagonists of the urokinase receptor. Affinity maturation by combinatorial chemistry, identification of functional epitopes, and inhibitory effect on cancer cell intravasation. Biochemistry. 2001;40(40):12157–12168. doi: 10.1021/bi010662g. [DOI] [PubMed] [Google Scholar]
- 103.Guo Y, Higazi AA, Arakelian A, Sachais BS, Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP, Rabbani SA. A peptide derived from the nonreceptor binding region of urokinase plasminogen activator (uPA) inhibits tumor progression and angiogenesis and induces tumor cell death in vivo. Faseb J. 2000;14(10):1400–1410. doi: 10.1096/fj.14.10.1400. [DOI] [PubMed] [Google Scholar]
- 104.Lin Y, Peng N, Li J, Zhuang H, Hua ZC. Herbal compound triptolide synergistically enhanced antitumor activity of amino-terminal fragment of urokinase. Mol Cancer. 2013;12:54. doi: 10.1186/1476-4598-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang M, Löwik DW, Miller AD, Thanou M. Targeting the urokinase plasminogen activator receptor with synthetic self-assembly nanoparticles. Bioconjug Chem. 2009;20(1):32–40. doi: 10.1021/bc8001908. [DOI] [PubMed] [Google Scholar]
- 106.Burgle M, Koppitz M, Riemer C, Kessler H, König B, Weidle UH, Kellermann J, Lottspeich F, Graeff H, Schmitt M, et al. Inhibition of the interaction of urokinase-type plasminogen activator (uPA) with its receptor (uPAR) by synthetic peptides. Biol Chem. 1997;378(3–4):231–237. doi: 10.1515/bchm.1997.378.3-4.231. [DOI] [PubMed] [Google Scholar]
- 107.Magdolen V, Bürgle M, de Prada NA, Schmiedeberg N, Riemer C, Schroeck F, Kellermann J, Degitz K, Wilhelm OG, Schmitt M, et al. Cyclo 19,31[D-Cys19]-uPA19-31 is a potent competitive antagonist of the interaction of urokinase-type plasminogen activator with its receptor (CD87) Biol Chem. 2001;382(8):1197–1205. doi: 10.1515/BC.2001.150. [DOI] [PubMed] [Google Scholar]
- 108.Sato S, Kopitz C, Schmalix WA, Muehlenweg B, Kessler H, Schmitt M, Krüger A, Magdolen V. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: effects on tumor growth and spread. Febs Lett. 2002;528(1–3):212–216. doi: 10.1016/s0014-5793(02)03311-2. [DOI] [PubMed] [Google Scholar]
- 109.Simon DI, Wei Y, Zhang L, Rao NK, Xu H, Chen Z, Liu Q, Rosenberg S, Chapman HA. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. J Biol Chem. 2000;275(14):10228–10234. doi: 10.1074/jbc.275.14.10228. [DOI] [PubMed] [Google Scholar]
- 110.Ghosh S, Johnson JJ, Sen R, Mukhopadhyay S, Liu Y, Zhang F, Wei Y, Chapman HA, Stack MS. Functional relevance of urinary-type plasminogen activator receptor-alpha3beta1 integrin association in proteinase regulatory pathways. J Biol Chem. 2006;281(19):13021–13029. doi: 10.1074/jbc.M508526200. [DOI] [PubMed] [Google Scholar]
- 111.van der Pluijm G, Sijmons B, Vloedgraven H, van der Bent C, Drijfhout JW, Verheijen J, Quax P, Karperien M, Papapoulos S, Löwik C. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol. 2001;159(3):971–982. doi: 10.1016/S0002-9440(10)61773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alexander RA, Prager GW, Mihaly-Bison J, Uhrin P, Sunzenauer S, Binder BR, Schütz GJ, Freissmuth M, Breuss JM. VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc Res. 2012;94(1):125–135. doi: 10.1093/cvr/cvs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280(26):24792–24803. doi: 10.1074/jbc.M413954200. [DOI] [PubMed] [Google Scholar]
- 114.Furlan F, Eden G, Archinti M, Arnaudova R, Andreotti G, Citro V, Cubellis MV, Motta A, Degryse B. D2A-Ala peptide derived from the urokinase receptor exerts anti-tumoural effects in vitro and in vivo. Peptides. 2018;101:17–24. doi: 10.1016/j.peptides.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 115.Bifulco K, Longanesi-Cattani I, Gargiulo L, Maglio O, Cataldi M, De Rosa M, Stoppelli MP, Pavone V, Carriero MV. An urokinase receptor antagonist that inhibits cell migration by blocking the formyl peptide receptor. Febs Lett. 2008;582(7):1141–1146. doi: 10.1016/j.febslet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Carriero MV, Longanesi-Cattani I, Bifulco K, Maglio O, Lista L, Barbieri A, Votta G, Masucci MT, Arra C, Franco R, et al. Structure-based design of an urokinase-type plasminogen activator receptor-derived peptide inhibiting cell migration and lung metastasis. Mol Cancer Ther. 2009;8(9):2708–2717. doi: 10.1158/1535-7163.MCT-09-0174. [DOI] [PubMed] [Google Scholar]
- 117.Carriero MV, Bifulco K, Minopoli M, Lista L, Maglio O, Mele L, Di Carluccio G, De Rosa M, Pavone V. UPARANT: a urokinase receptor-derived peptide inhibitor of VEGF-driven angiogenesis with enhanced stability and in vitro and in vivo potency. Mol Cancer Ther. 2014;13(5):1092–1104. doi: 10.1158/1535-7163.MCT-13-0949. [DOI] [PubMed] [Google Scholar]
- 118.Yousif AM, Minopoli M, Bifulco K, Ingangi V, Di Carluccio G, Merlino F, Motti ML, Grieco P, Carriero MV. Cyclization of the urokinase receptor-derived ser-arg-ser-arg-tyr peptide generates a potent inhibitor of trans-endothelial migration of monocytes. PLoS ONE. 2015;10(5):e0126172. doi: 10.1371/journal.pone.0126172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Minopoli M, Botti G, Gigantino V, Ragone C, Sarno S, Motti ML, Scognamiglio G, Greggi S, Scaffa C, Roca MS, et al. Targeting the formyl peptide receptor type 1 to prevent the adhesion of ovarian cancer cells onto mesothelium and subsequent invasion. J Exp Clin Cancer Res. 2019;38(1):459. doi: 10.1186/s13046-019-1465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tressler RJ, Pitot PA, Stratton JR, Forrest LD, Zhuo S, Drummond RJ, Fong S, Doyle MV, Doyle LV, Min HY, Rosenberg S. Urokinase receptor antagonists: discovery and application to in vivo models of tumor growth. APMIS. 1999;107(1):168–173. doi: 10.1111/j.1699-0463.1999.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 121.Bu X, Khankaldyyan V, Gonzales-Gomez I, Groshen S, Ye W, Zhuo S, Pons J, Stratton JR, Rosenberg S, Laug WE. Species-specific urokinase receptor ligands reduce glioma growth and increase survival primarily by an antiangiogenesis mechanism. Lab Invest. 2004;84(6):667–678. doi: 10.1038/labinvest.3700089. [DOI] [PubMed] [Google Scholar]
- 122.Khanna M, Wang F, Jo I, Knabe WE, Wilson SM, Li L, Bum-Erdene K, Li J, Sledge GW, Khanna R, et al. Targeting multiple conformations leads to small molecule inhibitors of the uPAR·uPA protein–protein interaction that block cancer cell invasion. Acs Chem Biol. 2011;6(11):1232–1243. doi: 10.1021/cb200180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mani T, Wang F, Knabe WE, Sinn AL, Khanna M, Jo I, Sandusky GE, Sledge GW, Jr, Jones DR, Khanna R, et al. Small-molecule inhibition of the uPAR·uPA interaction: synthesis, biochemical, cellular, in vivo pharmacokinetics and efficacy studies in breast cancer metastasis. Bioorg Med Chem. 2013;21(7):2145–2155. doi: 10.1016/j.bmc.2012.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu D, Bum-Erdene K, Si Y, Zhou D, Ghozayel MK, Meroueh SO. Mimicking intermolecular interactions of tight protein–protein complexes for small-molecule antagonists. ChemMedChem. 2017;12(21):1794–1809. doi: 10.1002/cmdc.201700572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu D, Bum-Erdene K, Leth JM, Ghozayel MK, Ploug M, Meroueh SO. Small-molecule inhibition of the uPAR·uPA interaction by conformational selection. ChemMedChem. 2021;16(2):377–387. doi: 10.1002/cmdc.202000558. [DOI] [PubMed] [Google Scholar]
- 126.Liu D, Zhou D, Wang B, Knabe WE, Meroueh SO. A new class of orthosteric uPAR·uPA small-molecule antagonists are allosteric inhibitors of the uPAR·vitronectin interaction. Acs Chem Biol. 2015;10(6):1521–1534. doi: 10.1021/cb500832q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin CM, Arancillo M, Whisenant J, Burgess K. Unconventional secondary structure mimics: ladder-rungs. Angew Chem Int Ed Engl. 2020;59(24):9398–9402. doi: 10.1002/anie.202002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chaurasia P, Mezei M, Zhou MM, Ossowski L. Computer aided identification of small molecules disrupting uPAR/alpha5beta1–integrin interaction: a new paradigm for metastasis prevention. PLoS ONE. 2009;4(2):e4617. doi: 10.1371/journal.pone.0004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rea VE, Lavecchia A, Di Giovanni C, Rossi FW, Gorrasi A, Pesapane A, de Paulis A, Ragno P, Montuori N. Discovery of new small molecules targeting the vitronectin-binding site of the urokinase receptor that block cancer cell invasion. Mol Cancer Ther. 2013;12(8):1402–1416. doi: 10.1158/1535-7163.MCT-12-1249. [DOI] [PubMed] [Google Scholar]
- 130.Lian S, Li S, Sah DK, Kim NH, Lakshmanan VK, Jung YD. Suppression of urokinase-type plasminogen activator receptor by docosahexaenoic acid mediated by heme oxygenase-1 in 12-O-tetradecanoylphorbol-13-acetate-induced human endothelial cells. Front Pharmacol. 2020;11:577302. doi: 10.3389/fphar.2020.577302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vallera DA, Li C, Jin N, Panoskaltsis-Mortari A, Hall WA. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J Natl Cancer Inst. 2002;94(8):597–606. doi: 10.1093/jnci/94.8.597. [DOI] [PubMed] [Google Scholar]
- 132.Ramage JG, Vallera DA, Black JH, Aplan PD, Kees UR, Frankel AE. The diphtheria toxin/urokinase fusion protein (DTAT) is selectively toxic to CD87 expressing leukemic cells. Leuk Res. 2003;27(1):79–84. doi: 10.1016/s0145-2126(02)00077-2. [DOI] [PubMed] [Google Scholar]
- 133.Huang J, Yuan D, Liu D, Li J, Li Y, Hall WA, Li B. Efficacy of antiangiogenic targeted immunotoxin DTAT and DTATEGF against glioblastoma multiforme. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(1):1–5. doi: 10.11817/j.issn.1672-7347.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Hall WA, Vallera DA. Efficacy of antiangiogenic targeted toxins against glioblastoma multiforme. Neurosurg Focus. 2006;20(4):E23. doi: 10.3171/foc.2006.20.4.15. [DOI] [PubMed] [Google Scholar]
- 135.Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17(2):157–164. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 136.Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR, Vallera DA. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J Neurooncol. 2011;103(2):255–266. doi: 10.1007/s11060-010-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Waldron NN, Oh S, Vallera DA. Bispecific targeting of EGFR and uPAR in a mouse model of head and neck squamous cell carcinoma. Oral Oncol. 2012;48(12):1202–1207. doi: 10.1016/j.oraloncology.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schappa JT, Frantz AM, Gorden BH, Dickerson EB, Vallera DA, Modiano JF. Hemangiosarcoma and its cancer stem cell subpopulation are effectively killed by a toxin targeted through epidermal growth factor and urokinase receptors. Int J Cancer. 2013;133(8):1936–1944. doi: 10.1002/ijc.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oh F, Todhunter D, Taras E, Vallera DA, Borgatti A. Targeting EGFR and uPAR on human rhabdomyosarcoma, osteosarcoma, and ovarian adenocarcinoma with a bispecific ligand-directed toxin. Clin Pharmacol. 2018;10:113–121. doi: 10.2147/CPAA.S160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pilbeam K, Wang H, Taras E, Bergerson RJ, Ettestad B, DeFor T, Borgatti A, Vallera DA, Verneris MR. Targeting pediatric sarcoma with a bispecific ligand immunotoxin targeting urokinase and epidermal growth factor receptors. Oncotarget. 2017;9(15):11938–11947. doi: 10.18632/oncotarget.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Borgatti A, Koopmeiners JS, Sarver AL, Winter AL, Stuebner K, Todhunter D, Rizzardi AE, Henriksen JC, Schmechel S, Forster CL, et al. Safe and effective sarcoma therapy through bispecific targeting of EGFR and uPAR. Mol Cancer Ther. 2017;16(5):956–965. doi: 10.1158/1535-7163.MCT-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Errico Provenzano A, Posteri R, Giansanti F, Angelucci F, Flavell SU, Flavell DJ, Fabbrini MS, Porro D, Ippoliti R, Ceriotti A, et al. Optimization of construct design and fermentation strategy for the production of bioactive ATF-SAP, a saporin based anti-tumoral uPAR-targeted chimera. Microb Cell Fact. 2016;15(1):194. doi: 10.1186/s12934-016-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuppone S, Assalini C, Minici C, Bertagnoli S, Branduardi P, Degano M, Fabbrini MS, Montorsi F, Salonia A, Vago R. The anti-tumoral potential of the saporin-based uPAR-targeting chimera ATF-SAP. Sci Rep. 2020;10(1):2521. doi: 10.1038/s41598-020-59313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vine KL, Indira Chandran V, Locke JM, Matesic L, Lee J, Skropeta D, Bremner JB, Ranson M. Targeting urokinase and the transferrin receptor with novel, anti-mitotic N-alkylisatin cytotoxin conjugates causes selective cancer cell death and reduces tumor growth. Curr Cancer Drug Targets. 2012;12(1):64–73. doi: 10.2174/156800912798888983. [DOI] [PubMed] [Google Scholar]
- 145.Abi-Habib RJ, Liu S, Bugge TH, Leppla SH, Frankel AE. A urokinase-activated recombinant diphtheria toxin targeting the granulocyte-macrophage colony-stimulating factor receptor is selectively cytotoxic to human acute myeloid leukemia blasts. Blood. 2004;104(7):2143–2148. doi: 10.1182/blood-2004-01-0339. [DOI] [PubMed] [Google Scholar]
- 146.Rajagopal V, Kreitman RJ. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the alpha(2)-macroglobulin receptor. J Biol Chem. 2000;275(11):7566–7573. doi: 10.1074/jbc.275.11.7566. [DOI] [PubMed] [Google Scholar]
- 147.Mertens HD, Kjaergaard M, Mysling S, Gårdsvoll H, Jørgensen TJ, Svergun DI, Ploug M. A flexible multidomain structure drives the function of the urokinase-type plasminogen activator receptor (uPAR) J Biol Chem. 2012;287(41):34304–34315. doi: 10.1074/jbc.M112.398404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin L, Gårdsvoll H, Huai Q, Huang M, Ploug M. Structure-based engineering of species selectivity in the interaction between urokinase and its receptor: implication for preclinical cancer therapy. J Biol Chem. 2010;285(14):10982–10992. doi: 10.1074/jbc.M109.093492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Metrangolo V, Ploug M, Engelholm LH. The urokinase receptor (uPAR) as a “Trojan horse” in targeted cancer therapy: challenges and opportunities. Cancers. 2021;13(21):5376. doi: 10.3390/cancers13215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tyndall JD, Kelso MJ, Clingan P, Ranson M. Peptides and small molecules targeting the plasminogen activation system: towards prophylactic anti-metastasis drugs for breast cancer. Recent Pat Anticancer Drug Discov. 2008;3(1):1–13. doi: 10.2174/157489208783478711. [DOI] [PubMed] [Google Scholar]
- 151.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 152.Boonstra MC, Verspaget HW, Ganesh S, Kubben FJ, Vahrmeijer AL, van de Velde CJ, Kuppen PJ, Quax PH, Sier CF. Clinical applications of the urokinase receptor (uPAR) for cancer patients. Curr Pharm Des. 2011;17(19):1890–1910. doi: 10.2174/138161211796718233. [DOI] [PubMed] [Google Scholar]
- 153.Mazar AP. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anticancer Drugs. 2001;12(5):387–400. doi: 10.1097/00001813-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 154.Yuan C, Guo Z, Yu S, Jiang L, Huang M. Development of inhibitors for uPAR: blocking the interaction of uPAR with its partners. Drug Discov Today. 2021;26(4):1076–1085. doi: 10.1016/j.drudis.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 155.Li D, Liu S, Shan H, Conti P, Li Z. Urokinase plasminogen activator receptor (uPAR) targeted nuclear imaging and radionuclide therapy. Theranostics. 2013;3(7):507–515. doi: 10.7150/thno.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ngo JC, Jiang L, Lin Z, Yuan C, Chen Z, Zhang X, Yu H, Wang J, Lin L, Huang M. Structural basis for therapeutic intervention of uPA/uPAR system. Curr Drug Targets. 2011;12(12):1729–1743. doi: 10.2174/138945011797635911. [DOI] [PubMed] [Google Scholar]
- 157.Chen Z, Lin L, Huai Q, Huang M. Challenges for drug discovery—a case study of urokinase receptor inhibition. Comb Chem High Throughput Screen. 2009;12(10):961–967. doi: 10.2174/138620709789824727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kriegbaum MC, Persson M, Haldager L, Alpízar-Alpízar W, Jacobsen B, Gårdsvoll H, Kjaer A, Ploug M. Rational targeting of the urokinase receptor (uPAR): development of antagonists and non-invasive imaging probes. Curr Drug Targets. 2011;12(12):1711–1728. doi: 10.2174/138945011797635812. [DOI] [PubMed] [Google Scholar]
- 159.de Virgilio M, Silvestris F. Urokinase receptor (uPAR) ligand based recombinant toxins for human cancer therapy. Curr Pharm Des. 2011;17(19):1979–1983. doi: 10.2174/138161211796718170. [DOI] [PubMed] [Google Scholar]
- 160.Oh F, Modiano JF, Bachanova V, Vallera DA. Bispecific targeting of EGFR and urokinase receptor (uPAR) using ligand-targeted toxins in solid tumors. Biomolecules. 2020;10(6):956. doi: 10.3390/biom10060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dong Y, Liao H, Fu H, Yu J, Guo Q, Wang Q, Duan Y. pH-sensitive shell-core platform block DNA repair pathway to amplify irreversible DNA damage of triple negative breast cancer. ACS Appl Mater Interfaces. 2019;11(42):38417–38428. doi: 10.1021/acsami.9b12140. [DOI] [PubMed] [Google Scholar]
- 162.Yang L, Cao Z, Sajja HK, Mao H, Wang L, Geng H, Xu H, Jiang T, Wood WC, Nie S, et al. Development of receptor targeted magnetic iron oxide nanoparticles for efficient drug delivery and tumor imaging. J Biomed Nanotechnol. 2008;4(4):439–449. doi: 10.1166/jbn.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller-Kleinhenz J, Guo X, Qian W, Zhou H, Bozeman EN, Zhu L, Ji X, Wang YA, Styblo T, O'Regan R, et al. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 2018;152:47–62. doi: 10.1016/j.biomaterials.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7(3):2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao N, Bozeman EN, Qian W, Wang L, Chen H, Lipowska M, Staley CA, Wang YA, Mao H, Yang L. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics. 2017;7(6):1689–1704. doi: 10.7150/thno.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ahmed MS, Bin Salam A, Yates C, Willian K, Jaynes J, Turner T, Abdalla MO. Double-receptor-targeting multifunctional iron oxide nanoparticles drug delivery system for the treatment and imaging of prostate cancer. Int J Nanomed. 2017;12:6973–6984. doi: 10.2147/IJN.S139011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park JY, Shin Y, Won WR, Lim C, Kim JC, Kang K, Husni P, Lee ES, Youn YS, Oh KT. Development of AE147 peptide-conjugated nanocarriers for targeting uPAR-overexpressing cancer cells. Int J Nanomed. 2021;16:5437–5449. doi: 10.2147/IJN.S315619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Belfiore L, Saunders DN, Ranson M, Vine KL. N-alkylisatin-loaded liposomes target the urokinase plasminogen activator system in breast cancer. Pharmaceutics. 2020;12(7):641. doi: 10.3390/pharmaceutics12070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hong Y, Che S, Hui B, Yang Y, Wang X, Zhang X, Qiang Y, Ma H. Lung cancer therapy using doxorubicin and curcumin combination: targeted prodrug based, pH sensitive nanomedicine. Biomed Pharmacother. 2019;112:108614. doi: 10.1016/j.biopha.2019.108614. [DOI] [PubMed] [Google Scholar]
- 170.Zhai B, Chen P, Wang W, Liu S, Feng J, Duan T, Xiang Y, Zhang R, Zhang M, Han X, et al. An ATF24 peptide-functionalized β-elemene-nanostructured lipid carrier combined with cisplatin for bladder cancer treatment. Cancer Biol Med. 2020;17(3):676–692. doi: 10.20892/j.issn.2095-3941.2020.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Devulapally R, Sekar NM, Sekar TV, Foygel K, Massoud TF, Willmann JK, Paulmurugan R. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano. 2015;9(3):2290–2302. doi: 10.1021/nn507465d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li H, Wang P, Deng Y, Zeng M, Tang Y, Zhu WH, Cheng Y. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials. 2017;139:30–38. doi: 10.1016/j.biomaterials.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 173.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 174.Wang K, Zhang Y, Wang J, Yuan A, Sun M, Wu J, Hu Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci Rep. 2016;6:27421. doi: 10.1038/srep27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li R, Zheng K, Hu P, Chen Z, Zhou S, Chen J, Yuan C, Chen S, Zheng W, Ma E, et al. A novel tumor targeting drug carrier for optical imaging and therapy. Theranostics. 2014;4(6):642–659. doi: 10.7150/thno.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou X, Zheng K, Li R, Chen Z, Yuan C, Hu P, Chen J, Xue J, Huang M. A drug carrier targeting murine uPAR for photodynamic therapy and tumor imaging. Acta Biomater. 2015;23:116–126. doi: 10.1016/j.actbio.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 177.Li S, Yuan C, Chen J, Chen D, Chen Z, Chen W, Yan S, Hu P, Xue J, Li R, et al. Nanoparticle binding to urokinase receptor on cancer cell surface triggers nanoparticle disintegration and cargo release. Theranostics. 2019;9(3):884–899. doi: 10.7150/thno.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen Z, Xu P, Chen J, Chen H, Hu P, Chen X, Lin L, Huang Y, Zheng K, Zhou S, et al. Zinc phthalocyanine conjugated with the amino-terminal fragment of urokinase for tumor-targeting photodynamic therapy. Acta Biomater. 2014;10(10):4257–4268. doi: 10.1016/j.actbio.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 179.Yu S, Huang G, Yuan R, Chen T. A uPAR targeted nanoplatform with an NIR laser-responsive drug release property for tri-modal imaging and synergistic photothermal-chemotherapy of triple-negative breast cancer. Biomater Sci. 2020;8(2):720–738. doi: 10.1039/c9bm01495k. [DOI] [PubMed] [Google Scholar]
- 180.Hu X, Mandika C, He L, You Y, Chang Y, Wang J, Chen T, Zhu X. Construction of urokinase-type plasminogen activator receptor-targeted heterostructures for efficient photothermal chemotherapy against cervical cancer to achieve simultaneous anticancer and antiangiogenesis. Acs Appl Mater Inter. 2019;11(43):39688–39705. doi: 10.1021/acsami.9b15751. [DOI] [PubMed] [Google Scholar]
- 181.Zuo J, Huo M, Wang L, Li J, Chen Y, Xiong P. Photonic hyperthermal and sonodynamic nanotherapy targeting oral squamous cell carcinoma. J Mater Chem B. 2020;8(39):9084–9093. doi: 10.1039/d0tb01089h. [DOI] [PubMed] [Google Scholar]
- 182.Hu Y, Chi C, Wang S, Wang L, Liang P, Liu F, Shang W, Wang W, Zhang F, Li S, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv Mater. 2017;29(33):1700448. doi: 10.1002/adma.201700448. [DOI] [PubMed] [Google Scholar]
- 183.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jing Y, Tong C, Zhang J, Nakamura T, Iankov I, Russell SJ, Merchan JR. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69(4):1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jing Y, Zaias J, Duncan R, Russell SJ, Merchan JR. In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models. Gene Ther. 2014;21(3):289–297. doi: 10.1038/gt.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jing Y, Bejarano MT, Zaias J, Merchan JR. In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer. Breast Cancer Res Treat. 2015;149(1):99–108. doi: 10.1007/s10549-014-3236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jing Y, Chavez V, Ban Y, Acquavella N, El-Ashry D, Pronin A, Chen X, Merchan JR. Molecular effects of stromal-selective targeting by uPAR-retargeted oncolytic virus in breast cancer. Mol Cancer Res. 2017;15(10):1410–1420. doi: 10.1158/1541-7786.MCR-17-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jing Y, Chavez V, Khatwani N, Ban Y, Espejo AP, Chen X, Merchan JR. In vivo antitumor activity by dual stromal and tumor-targeted oncolytic measles viruses. Cancer Gene Ther. 2020;27(12):910–922. doi: 10.1038/s41417-020-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lakka SS, Rajagopal R, Rajan MK, Mohan PM, Adachi Y, Dinh DH, Olivero WC, Gujrati M, Ali-Osman F, Roth JA, et al. Adenovirus-mediated antisense urokinase-type plasminogen activator receptor gene transfer reduces tumor cell invasion and metastasis in non-small cell lung cancer cell lines. Clin Cancer Res. 2001;7(4):1087–1093. [PubMed] [Google Scholar]
- 190.Nalabothula N, Lakka SS, Dinh DH, Gujrati M, Olivero WC, Rao JS. Sense p16 and antisense uPAR bicistronic construct inhibits angiogenesis and induces glioma cell death. Int J Oncol. 2007;30(3):669–678. [PMC free article] [PubMed] [Google Scholar]
- 191.Gondi CS, Lakka SS, Yanamandra N, Siddique K, Dinh DH, Olivero WC, Gujrati M, Rao JS. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;22(38):5967–5975. doi: 10.1038/sj.onc.1206535. [DOI] [PubMed] [Google Scholar]
- 192.Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Tung CH, Weissleder R, Rao JS. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res. 2004;64(12):4069–4077. doi: 10.1158/0008-5472.CAN-04-1243. [DOI] [PubMed] [Google Scholar]
- 193.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4(9):1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nozaki S, Endo Y, Nakahara H, Yoshizawa K, Hashiba Y, Kawashiri S, Tanaka A, Nakagawa K, Matsuoka Y, Kogo M, et al. Inhibition of invasion and metastasis in oral cancer by targeting urokinase-type plasminogen activator receptor. Oral Oncol. 2005;41(10):971–977. doi: 10.1016/j.oraloncology.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 195.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23(52):8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 196.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13(14):4051–4060. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kondraganti S, Gondi CS, McCutcheon I, Dinh DH, Gujrati M, Rao JS, Olivero WC. RNAi-mediated downregulation of urokinase plasminogen activator and its receptor in human meningioma cells inhibits tumor invasion and growth. Int J Oncol. 2006;28(6):1353–1360. [PMC free article] [PubMed] [Google Scholar]
- 198.Gorantla B, Asuthkar S, Rao JS, Patel J, Gondi CS. Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells. Mol Cancer Res. 2011;9(4):377–389. doi: 10.1158/1541-7786.MCR-10-0452. [DOI] [PubMed] [Google Scholar]
- 199.Rysenkova KD, Semina EV, Karagyaur MN, Shmakova AA, Dyikanov DT, Vasiluev PA, Rubtsov YP, Rubina KA, Tkachuk VA. CRISPR/Cas9 nickase mediated targeting of urokinase receptor gene inhibits neuroblastoma cell proliferation. Oncotarget. 2018;9(50):29414–29430. doi: 10.18632/oncotarget.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Biagioni A, Laurenzana A, Chillà A, Del Rosso M, Andreucci E, Poteti M, Bani D, Guasti D, Fibbi G, Margheri F. uPAR knockout results in a deep glycolytic and OXPHOS reprogramming in melanoma and colon carcinoma cell lines. Cells. 2020;9(2):308. doi: 10.3390/cells9020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Biagioni A, Chillà A, Del Rosso M, Fibbi G, Scavone F, Andreucci E, Peppicelli S, Bianchini F, Calorini L, Li Santi A, et al. CRISPR/Cas9 uPAR gene knockout results in tumor growth inhibition, EGFR downregulation and induction of stemness markers in melanoma and colon carcinoma cell lines. Front Oncol. 2021;11:663225. doi: 10.3389/fonc.2021.663225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.LeBeau AM, Duriseti S, Murphy ST, Pepin F, Hann B, Gray JW, VanBrocklin HF, Craik CS. Targeting uPAR with antagonistic recombinant human antibodies in aggressive breast cancer. Cancer Res. 2013;73(7):2070–2081. doi: 10.1158/0008-5472.CAN-12-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harel ET, Drake PM, Barfield RM, Lui I, Farr-Jones S, Van't Veer L, Gartner ZJ, Green EM, Lourenço AL, Cheng Y, et al. Antibody-drug conjugates targeting the urokinase receptor (uPAR) as a possible treatment of aggressive breast cancer. Antibodies. 2019;8(4):54. doi: 10.3390/antib8040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Duriseti S, Goetz DH, Hostetter DR, LeBeau AM, Wei Y, Craik CS. Antagonistic anti-urokinase plasminogen activator receptor (uPAR) antibodies significantly inhibit uPAR-mediated cellular signaling and migration. J Biol Chem. 2010;285(35):26878–26888. doi: 10.1074/jbc.M109.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Van Buren G, II, Gray MJ, Dallas NA, Xia L, Lim SJ, Fan F, Mazar AP, Ellis LM. Targeting the urokinase plasminogen activator receptor with a monoclonal antibody impairs the growth of human colorectal cancer in the liver. Cancer. 2009;115(14):3360–3368. doi: 10.1002/cncr.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rabbani SA, Ateeq B, Arakelian A, Valentino ML, Shaw DE, Dauffenbach LM, Kerfoot CA, Mazar AP. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12(10):778–788. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kenny HA, Leonhardt P, Ladanyi A, Yamada SD, Montag A, Im HK, Jagadeeswaran S, Shaw DE, Mazar AP, Lengyel E. Targeting the urokinase plasminogen activator receptor inhibits ovarian cancer metastasis. Clin Cancer Res. 2011;17(3):459–471. doi: 10.1158/1078-0432.CCR-10-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mahmood N, Arakelian A, Khan HA, Tanvir I, Mazar AP, Rabbani SA. uPAR antibody (huATN-658) and Zometa reduce breast cancer growth and skeletal lesions. Bone Res. 2020;8:18. doi: 10.1038/s41413-020-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li Y, Parry G, Chen L, Callahan JA, Shaw DE, Meehan EJ, Mazar AP, Huang M. An anti-urokinase plasminogen activator receptor (uPAR) antibody: crystal structure and binding epitope. J Mol Biol. 2007;365(4):1117–1129. doi: 10.1016/j.jmb.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 210.List K, Høyer-Hansen G, Rønne E, Danø K, Behrendt N. Different mechanisms are involved in the antibody mediated inhibition of ligand binding to the urokinase receptor: a study based on biosensor technology. J Immunol Methods. 1999;222(1–2):125–133. doi: 10.1016/s0022-1759(98)00189-6. [DOI] [PubMed] [Google Scholar]
- 211.Pass J, Jögi A, Lund IK, Rønø B, Rasch MG, Gårdsvoll H, Lund LR, Ploug M, Rømer J, Danø K, et al. Murine monoclonal antibodies against murine uPA receptor produced in gene-deficient mice: inhibitory effects on receptor-mediated uPA activity in vitro and in vivo. Thromb Haemost. 2007;97(6):1013–1022. [PubMed] [Google Scholar]
- 212.Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Lee HJ, Eun JY, Kim HG, Yoon SS, Lee DS, et al. Role of hepatocyte growth factor/c-Met signaling in regulating urokinase plasminogen activator on invasiveness in human hepatocellular carcinoma: a potential therapeutic target. Clin Exp Metastasis. 2008;25(1):89–96. doi: 10.1007/s10585-007-9106-6. [DOI] [PubMed] [Google Scholar]
- 213.Zhao B, Gandhi S, Yuan C, Luo Z, Li R, Gårdsvoll H, de Lorenzi V, Sidenius N, Huang M, Ploug M. Stabilizing a flexible interdomain hinge region harboring the SMB binding site drives uPAR into its closed conformation. J Mol Biol. 2015;427(6 Pt B):1389–1403. doi: 10.1016/j.jmb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 214.Wahid B, Ali A, Rafique S, Waqar M, Wasim M, Wahid K, Idrees M. An overview of cancer immunotherapeutic strategies. Immunotherapy. 2018;10(11):999–1010. doi: 10.2217/imt-2018-0002. [DOI] [PubMed] [Google Scholar]
- 215.Morgan MA, Schambach A. Engineering CAR-T cells for improved function against solid tumors. Front Immunol. 2018;9:2493. doi: 10.3389/fimmu.2018.02493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Newick K, O'Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 217.Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 218.Wang L, Yang R, Zhao L, Zhang X, Xu T, Cui M. Basing on uPAR-binding fragment to design chimeric antigen receptors triggers antitumor efficacy against uPAR expressing ovarian cancer cells. Biomed Pharmacother. 2019;117:109173. doi: 10.1016/j.biopha.2019.109173. [DOI] [PubMed] [Google Scholar]
- 219.Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583(7814):127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Uvyn A, De Geest BG. Multivalent antibody-recruiting macromolecules: linking increased binding affinity with enhanced innate immune killing. ChemBioChem. 2020;21(21):3036–3043. doi: 10.1002/cbic.202000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jakobsche CE, McEnaney PJ, Zhang AX, Spiegel DA. Reprogramming urokinase into an antibody-recruiting anticancer agent. Acs Chem Biol. 2012;7(2):316–321. doi: 10.1021/cb200374e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rullo AF, Fitzgerald KJ, Muthusamy V, Liu M, Yuan C, Huang M, Kim M, Cho AE, Spiegel DA. Re-engineering the immune response to metastatic cancer: antibody-recruiting small molecules targeting the urokinase receptor. Angew Chem Int Ed Engl. 2016;55(11):3642–3646. doi: 10.1002/anie.201510866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu XW, Duan HF, Gao LH, Pan SY, Li YM, Xi Y, Zhao SR, Yin L, Li JF, Chen HP, et al. Inhibition of tumor growth and metastasis by ATF-Fc, an engineered antibody targeting urokinase receptor. Cancer Biol Ther. 2008;7(5):651–659. doi: 10.4161/cbt.7.5.5643. [DOI] [PubMed] [Google Scholar]
- 224.Zhou H, Wang H, Yu G, Wang Z, Zheng X, Duan H, Sun J. Synergistic inhibitory effects of an engineered antibody-like molecule ATF-Fc and trastuzumab on tumor growth and invasion in a human breast cancer xenograft mouse model. Oncol Lett. 2017;14(5):5189–5196. doi: 10.3892/ol.2017.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.