Abstract
Background
When women require induction of labour, oxytocin is the most common agent used, delivered by an intravenous infusion titrated to uterine contraction strength and frequency. There is debate over the optimum dose regimen and how it impacts on maternal and fetal outcomes, particularly induction to birth interval, mode of birth, and rates of hyperstimulation. Current induction of labour regimens include both high‐ and low‐dose regimens and are delivered by either continuous or pulsed infusions, with both linear and non‐linear incremental increases in oxytocin dose. Whilst low‐dose protocols bring on contractions safely, their potentially slow induction to birth interval may increase the chance of fetal infection and chorioamnionitis. Conversely, high‐dose protocols may cause undue uterine hyperstimulation and fetal distress.
Objectives
To determine the effectiveness and safety of high‐ versus low‐dose oxytocin for induction of labour at term
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 August 2014) and the reference lists of relevant papers.
Selection criteria
Randomised controlled trials and quasi‐randomised controlled trials that compared oxytocin protocol for induction of labour for women at term, where high‐dose oxytocin is at least 100 mU oxytocin in the first 40 minutes, with increments delivering at least 600 mU in the first two hours, compared with low‐dose oxytocin, defined as less than 100 mU oxytocin in the first 40 minutes, and increments delivering less than 600 mU total in the first two hours.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of included studies. Data were checked for accuracy.
Main results
We have included nine trials, involving 2391 women and their babies in this review. Trials were at a moderate to high risk of bias overall.
Results of primary outcomes revealed no significant differences in rates of vaginal delivery not achieved within 24 hours (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.78 to 1.14, two trials, 1339 women) or caesarean section (RR 0.96, 95% CI 0.81 to 1.14, eight trials, 2023 women). There was no difference in serious maternal morbidity or death (RR 1.24, 95% CI 0.55 to 2.82, one trial, 523 women), and no difference in serious neonatal morbidity or perinatal death (RR 0.84, 95% CI 0.23 to 3.12, one trial, 781 infants). Finally, no trials reported on the number of women who had uterine hyperstimulation with fetal heart rate changes.
Results of secondary outcomes revealed no difference between time from induction to delivery (mean difference (MD) ‐0.90 hours, 95% CI ‐2.28 to +0.49 hours; five studies), uterine rupture (RR 3.10, 95% CI 0.50 to 19.33; three trials), epidural analgesia (RR 1.03, 95% CI 0.89 to 1.18; two trials), instrumental birth (RR 1.22, 95% CI 0.88 to 1.66; three trials), Apgar less than seven at five minutes (RR 1.25, 95% CI 0.77 to 2.01, five trials), perinatal death (RR 0.84, 95% CI 0.23 to 3.12; two trials), postpartum haemorrhage (RR 1.08, 95% CI 0.87 to 1.34; five trials), or endometritis (RR 1.35, 95% CI 0.53 to 3.43; three trials). Removal of high bias studies reveals a significant reduction of induction to delivery interval (MD ‐1.94 hours, 95% CI ‐0.99 to ‐2.89 hours, 489 women). A significant increase in hyperstimulation without specifying fetal heart rate changes was found in the high‐dose group (RR 1.86, 95% CI 1.55 to 2.25).
No other secondary outcomes were reported: unchanged/unfavourable cervix after 12 to 24 hours, meconium‐stained liquor, neonatal intensive care unit admission, neonatal encephalopathy, disability in childhood, other maternal side‐effects (nausea, vomiting, diarrhoea), maternal antibiotic use, maternal satisfaction, neonatal infection and neonatal antibiotic use.
Authors' conclusions
The findings of our review do not provide evidence that high‐dose oxytocin increases either vaginal delivery within 24 hours or the caesarean section rate. There is no significant decrease in induction to delivery time at meta‐analysis but these results may be confounded by poor quality trials. High‐dose oxytocin was shown to increase the rate of uterine hyperstimulation but the effects of this are not clear. The conclusions here are specific to the definitions used in this review. Further trials evaluating the effects of high‐dose regimens of oxytocin for induction of labour should consider all important maternal and infant outcomes.
Plain language summary
High‐dose versus low‐dose oxytocin infusion regimens for induction of labour
Some women do not begin labour spontaneously and may need assistance. This assistance, known as induction of labour, involves the use of an intervention to artificially commence uterine contractions for the mother. Oxytocin is a drug that is commonly given to women for induction of labour; however the most suitable dose to enable birth to occur safely for the mother and her baby, within a reasonable timeframe, is not known.
We included nine randomised controlled trials involving 2391 women and their babies in this review. The trials were of moderate quality overall. All trials compared giving women a high dose versus a low dose of oxytocin for induction of labour. We found that women who had a high dose of oxytocin were not more likely to have a shorter induction to delivery interval or have a vaginal birth within 24 hours of receiving the treatment than women receiving a low dose of oxytocin. When poor‐quality trials are removed from analysis however, the induction to delivery interval was significantly shorter with high‐dose oxytocin compared to low‐dose oxytocin. The likelihood of having a caesarean was similar with the different doses of oxytocin for induction of labour. No differences were shown between the two groups of women in terms of serious complications, including death of the mother or her baby but women receiving the high‐dose oxytocin did have an increased risk of excessive uterine contractions (known as uterine hyperstimulation). No trials provided any information about the number of women with uterine hyperstimulation with changes in the babies' heart rate. Similarly, no trials assessed satisfaction of the mother or her caregivers.
The trials were at moderate to high risk of bias overall. The definition of high‐ and low‐dose protocols and the outcomes measured varied considerably across the trials. The current evidence is not strong enough to recommend high‐dose over low‐dose regimens for routine induction of labour. We recommend that further research is carried out.
Background
Sometimes it is necessary to artificially induce labour when maternal or fetal risks of continuing the pregnancy outweigh risks of induction (Pakta 2005; WHO 2011). Oxytocin is the most common induction agent used worldwide (Alfirevic 2009). It is a neurohypophyseal hormone that belongs to the class of oxytocics, and is used to cause regular co‐ordinated contractions from the fundus to cervix. During induction of labour it is given as an increasing infusion, titrated to the strength and frequency of uterine contractions (Rang 2007).
Many pre‐induction factors can influence the outcome of labour induction; maternal factors include weight, parity, prior mode of delivery, and cervical favourability as classified by Bishop’s score, whilst fetal factors include weight and gestational age (Crane 2006; Pakta 2005). Uncertainty exists regarding how the oxytocin dose regimen for induction of labour leads to the likelihood of a successful vaginal delivery and adverse outcomes.
In 2011, Kenyon et al published a Cochrane review on the effects of high‐ versus low‐dose oxytocin for augmentation of labour (Kenyon 2011). The authors concluded that high‐dose oxytocin regimens were associated with a reduction in the length of labour and in caesarean section, and an increase in spontaneous vaginal delivery, but these were not significant after adjusting for high‐risk studies. These results should be considered in the context of achieving birth after a spontaneous labour has occurred, but it can not be assumed that these findings also apply to induction of labour.
This review is one of a series of reviews of the methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2009). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix and inducing labour.
Description of the condition
Induction of labour is a commonly used term to group the processes of cervical ripening, artificial rupture of membranes, and the initiation and augmentation of contractions. Induction of labour, however, is more accurately described as the artificial initiation of uterine contractions before the spontaneous onset of labour (Pakta 2005), whilst augmentation refers to the stimulation of spontaneous but inadequate contractions (Wei 2010). In high‐income countries, up to 25% of all deliveries at term involve induction of labour; in developing countries, rates are generally lower but variable, and in some settings are as high as those of high‐income countries (WHO 2011). There are many indications for induction of labour. These encompass immediate problems, such as pregnancy‐associated hypertensive diseases, maternal medical complications, and mild placental abruption through to less immediately threatening conditions, such as post date gestations and prolonged rupture of membranes.
Description of the intervention
The aim of induction of labour is to achieve an uncomplicated vaginal delivery within a reasonable time frame while avoiding adverse events, such as hyperstimulation, chorioamnionitis, fetal distress, instrumental birth, postpartum haemorrhage, and caesarean section. Oxytocin is used to stimulate contractions in order to induce labour and achieve birth more quickly than would occur without its use. Current oxytocin regimens consist of either linear or non‐linear incremental increases. Low‐dose regimens consist of oxytocin 0.5 to 2.0 mU/min starting dose with incremental increase by 1.0 to 2.0 mU/min every 15 to 60 minutes (Pakta 2005). High‐dose regimens have starting doses of oxytocin 2.0 to 6.0 mU/min and incremental increases of 2.0 to 6.0 mU/min every 15 to 40 minutes (Pakta 2005).
How the intervention might work
Research examining the difference between 'physiological' (mimicking endogenous level) and 'pharmacological' (interventional level) oxytocin doses demonstrated that 'pharmacological' doses lead to shorter induction to delivery time, with significantly fewer labours lasting greater than 12 hours (Toaff 1978). The extremely low 'physiological' dose was ceased in common use in favour of 1.0 to 2.0 mU/min doubled every 15 to 20 minutes until Seitchik et al (Seitchik 1982) demonstrated women augmented with computer‐controlled 'low‐dose' regimens had a shorter time from augmentation to full dilatation, and the infusion was decreased or ceased less often. This stimulated controversy regarding high‐ versus low‐dose regimens, as some clinicians extrapolated these findings for induction of labour as well as augmentation. There is still ongoing debate about risks versus benefits of using high‐ or low‐dose regimens. Previous studies suggest that high‐dose regimens can lead to shorter induction to delivery time and fewer failed inductions, but at the expense of increased rates of hyperstimulation and fetal distress requiring cessation of oxytocin, caesarean section, instrumental birth, and postpartum haemorrhage (Hourvitz 1996; Pakta 2005; Satin 1992; Xenakis 1995).
Why it is important to do this review
Low doses of oxytocin bring on contractions slowly though safely. Whilst high doses cause contractions to occur sooner they can cause hyperstimulation or sustained contractions that can impair blood flow to the placenta and hence cause fetal distress. Further adverse effects of high total doses of oxytocin include hypotension with reflex tachycardia, water retention, and hyponatraemia (Rang 2007). Conversely, the increased induction to delivery interval that may occur with low‐dose regimens increases the chance for fetal infection and chorioamnionitis. It is important to determine whether high‐dose regimens can lead to shorter induction to delivery times without an excess rate of adverse events compared to low‐dose regimens.
Objectives
To determine, from the best available evidence, the effectiveness and safety of high‐ versus low‐dose oxytocin for induction of labour at term (37 completed weeks' gestation and beyond).
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised controlled trials. We planned to include published and unpublished trials, as well as studies presented only as abstracts. We planned to exclude cross‐over trials.
Types of participants
Pregnant women for induction of labour carrying a viable fetus from 37 weeks of completed gestation. Induction of labour refers to the artificial initiation of uterine contractions before the spontaneous onset of labour (as distinct from augmentation of labour for delay or slow progress in labour as studied by Kenyon et al in Kenyon 2011).
Types of interventions
High starting and continuing dose of oxytocin for the induction of labour compared with low dose. We have defined the dose regimens as follows.
High‐dose regimen: infusion to deliver more than 100 mU oxytocin in the first 40 minutes and more than 600 mU in two hours
Low‐dose regimen: infusion to deliver less than 100 mU oxytocin in the first 40 minutes and less than 600 mU in two hours
The choice of high and low dose was based on the starting and incremental infusions previously used (Hourvitz 1996; Pakta 2005; Satin 1991; Xenakis 1995). As seen in these studies the variation in starting dose and incremental increase can lead to some cross‐over of groups, for example, low‐dose regimens starting at 0.5 to 2.0 mU/min with an incremental increase by 1.0 to 2.0 mU/min every 15 to 60 minutes, and high‐dose regimens starting at 2.0 to 6.0 mU/min and incremental increases of 2.0 to 6.0 mU/min every 15 to 40 minutes). Therefore, we chose to include two time references to allow differentiation of the groups. The first time point is based on oxytocin reaching a steady state at approximately 40 minutes (Rang 2007). The choice of two hours as a second time point is based on an arbitrary decision.
Other induction agents could be used in conjunction with the oxytocin as long as both groups received the other induction agent in a pre‐specified manner.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been pre‐specified by two authors of Cochrane labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). As per the induction of labour protocol (Hofmeyr 2009), we examined five primary outcomes most representative of clinical effectiveness, complications, and satisfaction.
Primary outcomes
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications.
Vaginal delivery not achieved within 24 hours (or period specified by trial authors).
Uterine hyperstimulation with fetal heart rate (FHR) changes.
Caesarean section.
Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood).
Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term, this is unlikely. All of these events are rare, and a modest change in their incidence is easier to detect if composite outcomes are presented. The incidence of individual components was explored as secondary outcomes.
Secondary outcomes
Measures of effectiveness
1) Time from induction of labour to delivery.
2) Cervix unfavourable/unchanged after 12 to 24 hours.
Complications
3) Uterine hyperstimulation without FHR changes.
4) *Uterine rupture.
5) Epidural analgesia.
6) Instrumental vaginal delivery.
7) Meconium‐stained liquor.
8) Apgar score less than seven at five minutes.
9) Neonatal intensive care unit admission.
10) Neonatal encephalopathy.
11) Perinatal death.
12) Disability in childhood.
13) Maternal side‐effects (all).
14) Maternal nausea.
15) Maternal vomiting.
16) Maternal diarrhoea.
17) Other maternal side‐effects:
‐ postpartum haemorrhage (as defined by the trial authors);
‐ serious maternal complications (e.g., intensive care unit admission, septicaemia but excluding uterine rupture);
‐ maternal death.
18) Neonatal infection.
19) Neonatal antibiotics.
20) Chorioamnionitis.
21) Endometritis.
22) Maternal antibiotics.
Measure of satisfaction
23) Woman not satisfied.
24) Caregiver not satisfied.
*'Uterine rupture' included all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery was excluded.
While all the above outcomes were sought, only those with data appear in the analysis tables.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the bibliographies of relevant papers.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion, or if required, by consultation with the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data independently using the agreed form. We resolved discrepancies through discussion or, if required, by consultation with the third author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. Blinding was assessed separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference where outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cross‐over trials
Cross‐over trials were not included as it was believed that design would compromise accurate assessment of the effect of interventions on the outcome measures.
Cluster‐randomised trials
We did not include cluster‐randomised trials.
More than two treatment groups
For multi‐armed trials, where two or more arms fit either our definition of "high dose" or "low dose", we planned to pool the results for analysis from the arms that fit our high‐dose or low‐dose definitions.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect using sensitivity analysis. For all outcomes analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect had not been clinically meaningful, we would not have combined trials. Where we have used random‐effects analyses, the results have been presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it. Forest plots showing results of the included studies with suppression of the pooled estimate were used for displaying outcomes where the included studies reporting on those outcomes were felt to be too heterogeneous for a pooled estimate to be meaningful.
Pre‐defined subgroup analyses were:
previous caesarean section or not;
nulliparity or parity;
membranes intact or ruptured; and
cervix unfavourable, favourable, or undefined.
We assessed subgroup differences by interaction tests available in within RevMan (RevMan 2014). We included only primary outcomes in subgroup analyses. We have reported the results of the subgroup analysis quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analysis to explore the effect of trial quality using the primary outcomes stated above. This involved analysis of selection bias, performance bias and attrition bias. In the analysis, we excluded studies rated as 'high risk of bias' in order to assess for any substantive difference to the overall result. Initially, we conducted the meta‐analysis using all studies and then compared this to a meta‐analysis where poorer quality studies were excluded.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved 44 reports, relating to 38 studies. Of these studies, eight were included (Crane 1993; Dunn 1998; Hourvitz 1996; Lowensohn 1990; Merrill 1999; Satin 1991; Tribe 2012; Willcourt 1994), 25 were excluded (Auner 1993; Blakemore 1990; Compitak 2002; Cummiskey 1990; Daniel‐Spiegel 2004; Diven 2012; Durodola 2005; Fitzpatrick 2012; Gibb 1985; Girard 2009; Goni 1995; Gungorduk 2011; Lazor 1993; Li 1996; Manjula 2014; Muller 1992; Odem 1988; Oral 2003; Parashi 2005; Parpas 1995; Pavlou 1978; Reid 1995; Ross 1998; Satin 1994; Ustunyurt 2007), and five are awaiting further classification (awaiting contact from trialists to allow further assessment for inclusion) (Ashworth 1988; Raymond 1989; Salamalekis 2000; Singh 1993; Sotunsa 2013).
Through searching the bibliographies of studies identified in the trial search, we identified a further six studies. We included one of the trials (Orhue 1993), and excluded the other five studies (Chua 1991; Mercer 1991; Orhue 1993b; Orhue 1994; Steer 1985). See Figure 1.
1.
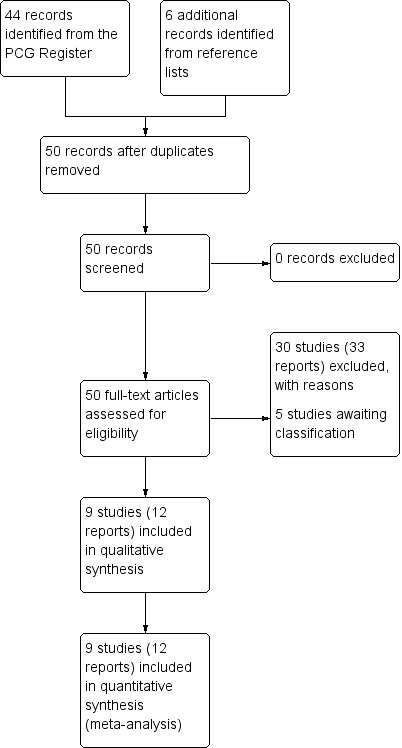
Study flow diagram.
Included studies
Nine trials (Crane 1993; Dunn 1998; Hourvitz 1996; Lowensohn 1990; Merrill 1999; Orhue 1993; Satin 1991; Tribe 2012; Willcourt 1994) involving 2391 women and their babies have been included in this review (see Characteristics of included studies for further details).
Infusion protocols
There was considerable variation in how authors defined high‐ and low‐dose protocols, as well as the outcome measures, throughout the included trials. In the trials by Crane 1993, Dunn 1998, Lowensohn 1990, Satin 1991 and Willcourt 1994 the difference between their defined high‐ and low‐dose protocol used a combination of higher oxytocin dose increases as well as shorter time intervals between dose increases. The trial by Willcourt 1994 compared three groups, a high‐dose continuous, a low‐dose continuous, and a low‐dose pulsed group, with a large difference in doses between the low‐dose and the pulse groups (36.8 mU versus 5.36 mU at 40 minutes, 277.1 mU versus 56.08 mU at two hours), however they reported comparisons between the pulsed group and each of the continuous groups. In the Hourvitz 1996 and Merrill 1999 trials, initial and subsequent oxytocin doses varied between groups, whilst the time between incremental increases was kept the same. The Tribe 2012 trial also kept the time interval the same between incremental increases in dose, and compared pulsatile delivery (low dose) with continuous delivery (high dose). Orhue 1993 kept the initial and incremental doses the same between groups but varied the time between incremental doses.
Inclusion criteria
The inclusion criteria also varied between the included trials. Crane 1993 (abstract only), Hourvitz 1996, and Satin 1991 included women who required induction of labour, and did not state any further inclusion or exclusion criteria. Lowensohn 1990 (abstract only) and Dunn 1998 included women at greater than 37 weeks of gestation who needed induction of labour without further stating what indications for induction were included or excluded. Willcourt 1994 included women with "adequate" pelvimetry, no more than one prior caesarean section, and at least one of mild pregnancy‐induced hypertension, diabetes, or postdates. Women were excluded from final analysis if they needed magnesium sulphate, diabetes requiring insulin, or if they failed to establish within 24 hours. Merrill 1999 included all women at greater than 24 weeks' gestation who required Induction or augmentation of labour, however, their final results revealed a mean gestation of 38.0 weeks with a standard deviation (SD) of 0.1, suggesting there were minimal women under 37 weeks. The categorisation of induction or augmentation was strictly based on cervical dilatation (less or more than 3 cm) and the frequency of contractions (more or less than 10 contractions per hour), regardless of any cervical ripening used. Tribe 2012 enrolled women over 18 years old requiring oxytocin for stimulation after receiving prostaglandin E2 and those with prolonged rupture of membranes without contractions. Women were excluded if induction was for fetal death in utero, fetal abnormality, breech, multiple pregnancy, or previous caesarean section. In the Orhue 1993 trial, women with parity of five or more requiring induction were included. They were excluded if there was intrauterine death, multiple pregnancy, previous caesarean section, previous uterine surgery, breech presentation, or borderline contracted pelvis on pelvimetry.
Outcomes
Data were available for the following primary outcomes: vaginal delivery rates (two studies); caesarean section (eight studies); serious neonatal morbidity or perinatal death (one study); serious maternal morbidity or death (one study). No studies reported on the primary outcome of uterine hyperstimulation with fetal heart rate changes.
The secondary outcome measures varied greatly between studies as well as author definitions of outcomes. The most consistently reported secondary outcomes were time from induction to delivery (five studies), hyperstimulation with fetal heart rate changes not specified (five studies), and postpartum haemorrhage (five studies). No other secondary outcome was reported by more than three studies. As some studies included women for either induction or augmentation, or of mixed parity, results were not always presented in a way that made it possible to include them in this analysis.
Excluded studies
We excluded 30 studies from this review for one or more reasons listed below. The majority of these were excluded for not having a high‐dose arm or not having a low‐dose arm as defined by the review protocol.
In 11 of the excluded studies (Auner 1993; Blakemore 1990; Cummiskey 1990; Daniel‐Spiegel 2004; Fitzpatrick 2012; Goni 1995; Lazor 1993; Mercer 1991; Muller 1992; Odem 1988; Reid 1995), both groups were given a low‐dose protocol according to the review's definitions.
In 11 of the excluded studies (Chua 1991; Durodola 2005; Gungorduk 2011; Manjula 2014; Orhue 1993b; Orhue 1994; Parashi 2005; Ross 1998; Satin 1994; Steer 1985; Ustunyurt 2007), both groups were given a high‐dose protocol according to the review's definitions.
Seven of the excluded studies used the same dose in each arm. In four trials (Daniel‐Spiegel 2004; Diven 2012; Girard 2009; Ustunyurt 2007), the oxytocin infusion was stopped in one group at a pre‐specified point in the induction; in Gungorduk 2011 the groups differed in the time oxytocin was administered post cervical ripening; in Oral 2003 the groups were administered oxytocin in different mediums (saline versus a glucose solution); and in Pavlou 1978 groups were administered oxytocin by different delivery systems (continuous versus pulsed).
Three studies (Gibb 1985; Li 1996; Satin 1994) allocated women to the two groups in a non‐random fashion.
In one study (Auner 1993), women who were all preterm were included.
In one study (Compitak 2002), a mixed group of women who needed induction or augmentation of labour were included, and the study did not report outcomes based on induction of labour versus augmentation of labour (and did not define augmentation versus induction).
In one study (Parpas 1995), women were administered oxytocin based on maternal weight (not low versus high dose).
Risk of bias in included studies
Overall, the included trials were judged to be at a moderate to high risk of bias (see Figure 2 and Figure 3), with the risk of bias in many of the domains judged as 'unclear', and insufficient information available to permit confident judgements.
2.
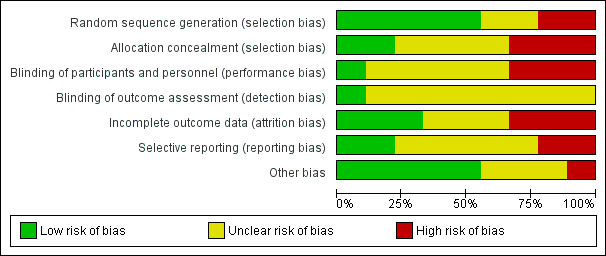
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
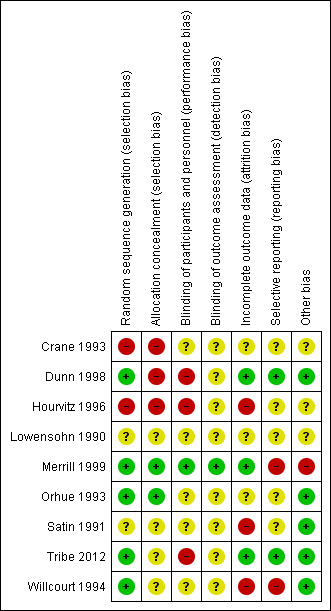
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of the included trials had adequate methods for generating a random sequence; three trials used random number tables (Dunn 1998; Merrill 1999; Orhue 1993), and two trials used computer‐generated random number sequences (Tribe 2012; Willcourt 1994). Two of these trials additionally used adequate methods to conceal allocation (Merrill 1999; Orhue 1993), while for the other two trials, the risk of selection bias due to inadequate concealment of allocation was unclear (Tribe 2012; Willcourt 1994). In two further trials (Lowensohn 1990; Satin 1991) the risk of selection bias was unclear, due to non‐reporting (or unclear) reporting of methods for sequence generation and allocation concealment. The final two trials were quasi‐randomised (with allocation based on hospital number (Crane 1993) or based on time period in the delivery ward (alternation of the protocol every two months) (Hourvitz 1996)) and were thus judged to be at a high risk of selection bias.
Blinding
Effective blinding of women and study personnel was unclear in the majority of trials (Crane 1993; Lowensohn 1990; Orhue 1993; Satin 1991; Willcourt 1994), with insufficient detail reported to make confident judgements. In three trials the risk of performance bias due to ineffective blinding of women/study personnel was judged to be high (due to the nature of the interventions) (Dunn 1998; Hourvitz 1996; Tribe 2012). In only one trial (Merrill 1999) was the risk of performance bias judged to be low ‐ with adequate blinding of women and personnel through the use of identical infusion bags and infusion rates (and rather use of differing concentrations in the infusion bags).
In eight of the nine trials, the risk of detection bias was judged to be unclear (largely with inadequate detail provided for the review authors to make confident judgements) (Crane 1993; Dunn 1998; Hourvitz 1996; Lowensohn 1990; Orhue 1993; Satin 1991; Tribe 2012; Willcourt 1994). In one trial however, detection bias was judged to be low, with blinded outcome assessment, and the trialists blinded until all outcomes were assessed (Merrill 1999).
Incomplete outcome data
The risk of attrition bias was judged to be low in the three of the nine studies (Dunn 1998; Merrill 1999; Tribe 2012), where a high percentage of women were accounted for in each group and analyses based on intention‐to‐treat principles. The risk of attrition bias was judged as unclear in three of the studies (Crane 1993; Lowensohn 1990; Orhue 1993), where all women were accounted for but there was no detail provided as to whether there was any cross‐over in the trial and if outcomes were analysed using intention‐to‐treat principles. The final three trials were judged to be at a high risk of attrition bias (Hourvitz 1996; Satin 1991; Willcourt 1994), due to unbalanced losses/exclusions without clear reason (Satin 1991) and due to not reporting the groups from which excluded women were originally randomised (Willcourt 1994).
Selective reporting
Dunn 1998 and Tribe 2012 were the only included studies with a low risk of reporting bias, reporting on the pre‐specified outcomes from its trial registration. While three trials (Hourvitz 1996; Orhue 1993; Satin 1991) reported on their outcomes specified in their manuscript methods, with no access to the trial protocols, it was not possible to confidently assess reporting bias. Two trials were judged at an unclear risk of reporting bias, being presented in abstract form only (Crane 1993; Lowensohn 1990). The final two trials (Merrill 1999; Willcourt 1994) were judged at a high risk of reporting bias, with unclear or no pre‐specified outcomes of interest detailed.
Other potential sources of bias
In five of the included trials (Dunn 1998; Orhue 1993; Satin 1991; Tribe 2012; Willcourt 1994) there were no other obvious potential sources of bias identified. Compared to the results of other studies, Merrill 1999 has reported very narrow confidence intervals suggesting measurement bias. For the other three included trials (Crane 1993; Hourvitz 1996; Lowensohn 1990), the risk of other bias was judged to be unclear, with insufficient information available to make this judgement confidently.
Effects of interventions
High‐dose versus low‐dose oxytocin
Primary outcomes
Vaginal delivery not achieved within 24 hours
Only two trials reported data on vaginal delivery not achieved within 24 hours that could be included in the meta‐analysis. Tribe 2012 reported specifically on vaginal delivery not achieved within 24 hours, while Merrill 1999 reported on women with 'failed induction' (after oxytocin was given for eight to 12 hours without any progress in the induction process). Overall, there was no significant difference between the high‐dose and low‐dose oxytocin groups for this outcome (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.78 to 1.14, two trials, 1339 women; Chi² = 0.03, df = 1 (P = 0.85); I² = 0%) (Analysis 1.1). Excluding Merrill 1999 from this analysis due to high risk of bias does not change the estimate of effect.
1.1. Analysis.
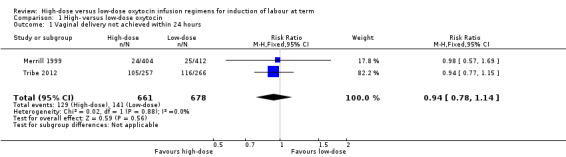
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 1 Vaginal delivery not achieved within 24 hours.
Uterine hyperstimulation with fetal heart rate changes
None of the included studies reported specifically on the number of women who experienced uterine hyperstimulation with fetal heart rate changes.
Caesarean section
This was the most consistently reported of our pre‐specified primary outcomes (reported by eight of the nine included studies). On meta‐analysis, the risk of caesarean section was not significantly different between the high‐dose and low‐dose oxytocin groups (RR 0.96, 95% CI 0.81 to 1.14; eight studies, 2023 women, Chi² = 9.70, df = 7 (P = 0.21); I² = 28%) (Analysis 1.2). When sensitivity analysis is performed there are only two studies that are included which still show no significant difference in caesarean section between high‐ and low‐dose groups (RR 1.01, 95% CI 0.81 to 1.25, two studies, 611 women, Chi² = 0.79, df = 2 (P = 0.32); I² = 0%). (Analysis 5.1)
1.2. Analysis.

Comparison 1 High‐ versus low‐dose oxytocin, Outcome 2 Caesarean section.
5.1. Analysis.

Comparison 5 High‐ versus low‐dose oxytocin: excluding studies at high risk of bias, Outcome 1 Caesarean section.
Serious neonatal morbidity or death
Merrill 1999 was the only trial to report on serious neonatal morbidity/death, and found no significant difference between groups in a composite outcome of serious neonatal morbidity or death (RR 0.84, 95% CI 0.23 to 3.12, 781 infants) (Analysis 1.3). The inclusion criteria for this study included only singletons and were excluded if induction was for fetal death or abnormality. Therefore it is unclear why the authors have not reported on all infants and why there are more missing from the high‐dose group than low‐dose group.
1.3. Analysis.
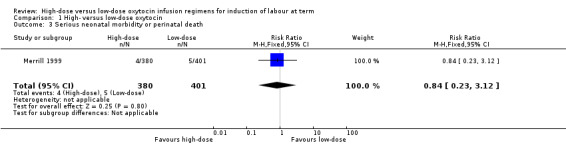
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 3 Serious neonatal morbidity or perinatal death.
Serious maternal morbidity or death
Tribe 2012 was the only study to report on serious maternal morbidity or death, and found no significant difference between the high‐dose and low‐dose groups for this outcome (RR 1.24, 95% CI 0.55 to 2.82, 523 women) (Analysis 1.4).
1.4. Analysis.
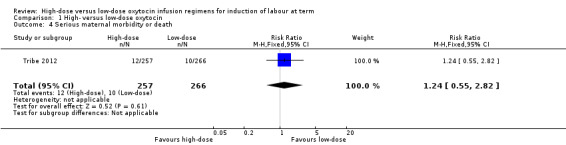
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 4 Serious maternal morbidity or death.
Secondary outcomes
The reported complications of induction of labour with oxytocin varied across the included studies. Time from induction to delivery and rates of hyperstimulation were the most commonly reported of our pre‐specified secondary outcomes, however uterine rupture, epidural use, instrumental delivery, Apgar score, postpartum haemorrhage, and endometritis were all reported on by at least two of the included trials.
Time from induction to delivery
When the five studies (Crane 1993; Hourvitz 1996; Merrill 1999; Tribe 2012; Willcourt 1994) that included these data are combined, there is no significant difference between high‐ and low‐dose protocols (MD ‐0.90 hours, 95% CI ‐2.28 to + 0.49 hours; five studies, 1725 women, Tau² = 2.27; Chi² = 93.34, df = 4 (P < 0.00001, I² = 96% ) (Analysis 1.5). This represents substantial heterogeneity and may result from any number of systematic problems with included studies. Specific issues are raised with the standard deviations provided by Merrill 1999 being too small to be realistic, Hourvitz 1996 excluding patients for analysis as they had not received six hours of oxytocin and not reporting an intention‐to‐treat, and finally Willcourt 1994 who removed 40 patients for failure to establish labour but did not include them in the analysis.
1.5. Analysis.
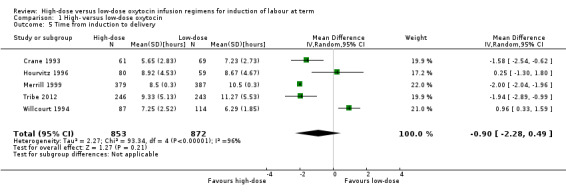
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 5 Time from induction to delivery.
In addition to the issues outlined with the studies above, Dunn 1998, Lowensohn 1990, Orhue 1993, and Satin 1991 did not report data in a way that allowed it to be included in the meta‐analysis. These four trials reported a shorter induction to delivery times with high‐dose protocol.
When sensitivity analysis is performed, Tribe 2012 is the only paper not excluded due to high risk of bias. They report a significant reduction in induction to delivery interval (MD ‐1.94 hours, 95% CI ‐0.99 to ‐2.89 hours, 489 women) (Analysis 5.2).
5.2. Analysis.
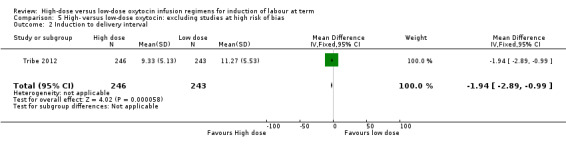
Comparison 5 High‐ versus low‐dose oxytocin: excluding studies at high risk of bias, Outcome 2 Induction to delivery interval.
Uterine hyperstimulation (fetal heart rate changes not specified)
None of the authors in the included studies specified the rates of uterine hyperstimulation without fetal heart rate changes, our prespecified secondary outcome. As five trials reported on uterine hyperstimulation, fetal heart rate changes not specified, we have added the posthoc outcome "Uterine hyperstimulation, fetal heart rate changes not specified". This revealed a statistically significant increase in hyperstimulation (fetal heart rate changes not specified) for women receiving high‐dose oxytocin compared with low‐dose oxytocin (RR 1.86, 95% CI 1.55 to 2.25; five trials, 876 women, Chi² = 5.32, df = 4 (P = 0.26); I² = 25%) (Analysis 1.6).
1.6. Analysis.
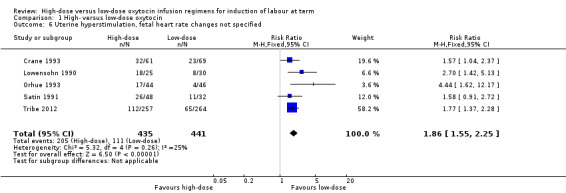
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 6 Uterine hyperstimulation, fetal heart rate changes not specified.
When a sensitivity analysis was conducted to exclude the three trials at high risk of bias (Crane 1993; Lowensohn 1990; Satin 1991), the increased risk of hyperstimulation (fetal heart rate not specified) in the high‐dose group was maintained (RR 1.92, 95% CI 1.51 to 2.46; two studies, 611 women, Chi² = 3.07, df = 1 (P = 0.08); I² = 67%) (Analysis 5.3). This result is significantly heterogenous however and is likely the result of the Orhue 1993 trial concentrating on grand multiparous women.
5.3. Analysis.
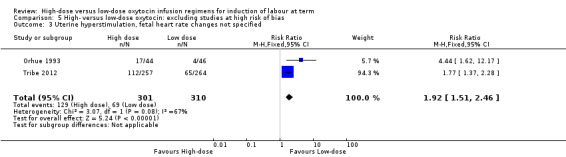
Comparison 5 High‐ versus low‐dose oxytocin: excluding studies at high risk of bias, Outcome 3 Uterine hyperstimulation, fetal heart rate changes not specified.
Uterine rupture
Overall, there was no significant difference in the risk of rupture between the high‐dose and low‐dose oxytocin groups (RR 3.10, 95% CI 0.50 to 19.33; three trials, 1429 women, Chi² = 0.95, df = 1 (P = 0.33); I² = 0%) (Analysis 1.7). It was noted that in the Orhue 1993 trial, three ruptures occurred in the high‐dose group and no ruptures in the low‐dose group. This trial assessed only women of high parity, which is important to consider. No uterine ruptures occurred in Tribe 2012, and in Merrill 1999, there was one rupture in each group.
1.7. Analysis.
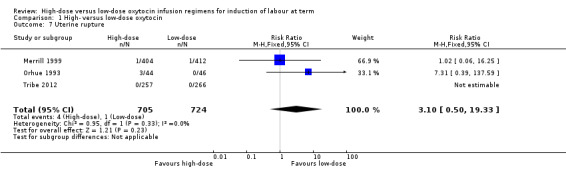
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 7 Uterine rupture.
Epidural analgesia
Two trials reported on the use of epidural analgesia (Merrill 1999; Tribe 2012), and on meta‐analysis no significant difference was shown in the risk of having an epidural between the high‐dose and low‐dose oxytocin groups (RR 1.03, 95% CI 0.89 to 1.19; two trials, 1327 women, Tau² = 0.01; Chi² = 3.80, df = 1 (P = 0.05); I² = 74%) (Analysis 1.8). There was substantial statistical heterogeneity observed for this outcome, which may reflect the difference in the management of obstetric analgesia between the UK and USA or the high risk of bias in the Merrill 1999 study. When this study is removed, the single study by Tribe 2012 remains non‐significant but the effect approaches a significant risk of epidural analgesia with high‐dose oxytocin (RR 1.11, 95% CI 0.99 to 1.24).
1.8. Analysis.

Comparison 1 High‐ versus low‐dose oxytocin, Outcome 8 Epidural analgesia.
Instrumental birth
Three trials (Orhue 1993; Satin 1991; Tribe 2012) reported on instrumental delivery, and showed no significant difference in the risk between the high‐dose and low‐dose oxytocin groups (RR 1.22, 95% CI 0.89 to 1.66; three trials, 693 women, Chi² = 0.40, df = 2 (P = 0.82); I² = 0%) (Analysis 1.9).
1.9. Analysis.

Comparison 1 High‐ versus low‐dose oxytocin, Outcome 9 Instrumental birth.
Apgar score less than seven at five minutes
No significant difference in the risk of an Agpar score less than seven at five minutes was shown for infants born to mothers in the high‐dose and low‐dose oxytocin groups (RR 1.25, 95% CI 0.77 to 2.01, five trials, 1641 infants, Chi² = 2.45, df = 4 (P = 0.65); I² = 0%) (Analysis 1.10).
1.10. Analysis.

Comparison 1 High‐ versus low‐dose oxytocin, Outcome 10 Apgar score less than seven at five minutes.
Perinatal death
There was no significant difference in perinatal death between the high‐ and low‐dose groups (RR 0.84, 95% CI 0.23 to 3.12; two trials, 1302 women, unable to test for heterogeneity as there were no events in Tribe 2012). Merrill 1999 reported nine neonatal deaths (four infants born to mothers in the high‐dose group, and five infants born to mothers in the low‐dose group) (Analysis 1.11). These deaths were secondary to severe anomalies, karyotypic abnormalities, or severe prematurity and were not attributed to the intervention.
1.11. Analysis.
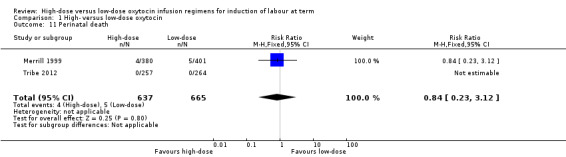
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 11 Perinatal death.
Postpartum haemorrhage
Five of the nine included trials reported on rates of postpartum haemorrhage (Hourvitz 1996; Merrill 1999; Orhue 1993; Satin 1991; Tribe 2012) and together, found no significant difference in risk of postpartum haemorrhage for women receiving high‐dose versus low‐dose oxytocin (RR 1.08, 95% CI 0.87 to 1.34; five trials, 1600 women, Chi² = 3.53, df = 3 (P = 0.32); I² = 15%) (Analysis 1.12).
1.12. Analysis.
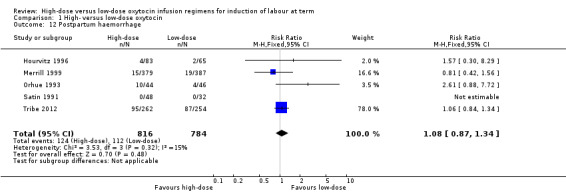
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 12 Postpartum haemorrhage.
Endometritis
No significant difference in the risk of endometritis was shown between the high‐dose and low‐dose oxytocin groups across three trials (Hourvitz 1996; Merrill 1999; Tribe 2012) (RR 1.35, 95% CI 0.53 to 3.43; three trials, 1425 women, Tau² = 0.25; Chi² = 3.14, df = 2 (P = 0.21); I² = 36%) (Analysis 1.13). There was moderate statistical heterogeneity observed between trials for this outcome. Differences in the modes of delivery between studies, time of follow‐up and reporting of symptoms, and definition of endometritis likely account for much of this observed heterogeneity.
1.13. Analysis.
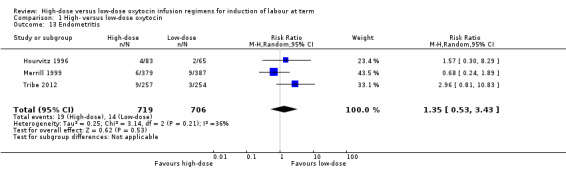
Comparison 1 High‐ versus low‐dose oxytocin, Outcome 13 Endometritis.
Other secondary outcomes
None of the included trials reported on the other secondary review outcomes, including: unchanged/unfavourable cervix after 12 to 24 hours, meconium‐stained liquor, neonatal intensive care unit admission, neonatal encephalopathy, disability in childhood, other maternal side‐effects (nausea, vomiting, diarrhoea), maternal antibiotic use, maternal satisfaction, neonatal infection and neonatal antibiotic use.
Subgroup analyses
We performed subgroup analysis on the three predefined subgroups for whom subgroup data were available: previous caesarean section or not, nulliparity or parity, and cervix unfavourable, favourable, or undefined. Subgroup analyses where membranes are ruptured or not will be carried out in future updates of this review as more data become available.
Previous caesarean section or not
Merrill 1999 provided data on women who had "unscarred uteri" for the outcome of caesarean section but not those with "scarred uteri" or for any of our other pre‐specified primary outcomes. No other trials reported on a subgroup of women who had previously had a caesarean section. For women with "unscarred uteri", there was no significant difference between high‐dose and low‐dose oxytocin groups for the outcome caesarean section (RR 0.75, 95% CI 0.50 to 1.14, 720 women) (Analysis 2.1).
2.1. Analysis.
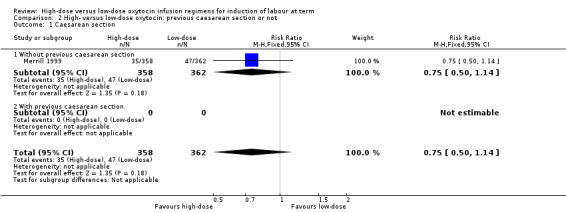
Comparison 2 High‐ versus low‐dose oxytocin: previous caesarean section or not, Outcome 1 Caesarean section.
Nulliparity or parity
We performed subgroup analyses based on parity, including data from five of the included trials (Lowensohn 1990; Merrill 1999; Orhue 1993; Satin 1991; Willcourt 1994). Subgroup data were available for the outcome of caesarean section from four trials. As in the main analysis, no significant difference between high‐dose and low‐dose oxytocin groups was observed in the nulliparous or multiparous subgroups for the outcome caesarean section, and no significant subgroup interaction was identified for this outcome (Chi² = 3.25, P = 0.07, I² = 69.2%) (Analysis 3.2). Similarly, as in the main analysis, an increase in hyperstimulation was observed for women in the high‐dose oxytocin group for both the nulliparous and multiparous subgroups; however, no significant subgroup interaction was identified for this outcome, indicating no differential effect based on parity for the outcome hyperstimulation (Chi² = 0.16, P = 0.69, I² = 0%) (Analysis 3.1).
3.2. Analysis.
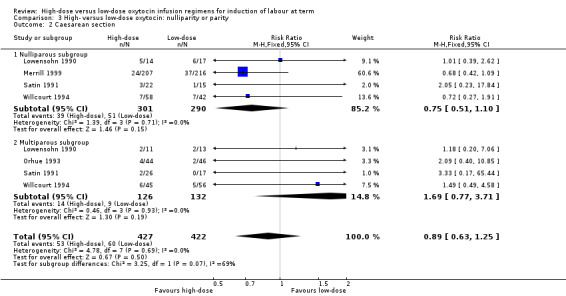
Comparison 3 High‐ versus low‐dose oxytocin: nulliparity or parity, Outcome 2 Caesarean section.
3.1. Analysis.
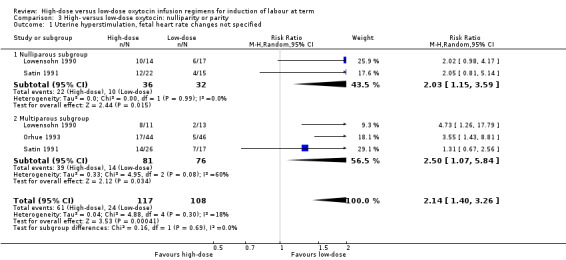
Comparison 3 High‐ versus low‐dose oxytocin: nulliparity or parity, Outcome 1 Uterine hyperstimulation, fetal heart rate changes not specified.
Cervix unfavourable, favourable, or undefined
Willcourt 1994 reported on the rates of caesarean section based on a cervical dilation of less than or more than 5 cm at the time of induction of labour. There was no significant difference between the high‐dose versus low‐dose groups in the risk of caesarean section for either of the subgroups (as in the main analysis), and no significant subgroup interaction was identified for this outcome (Chi² = 0.06, P = 0.80, I² = 0%) (Analysis 4.1), indicating no differential effect for this outcome based on favourable/unfavourable cervix at time of induction. No trials reported on any of our other pre‐specified primary outcomes according to cervical status.
4.1. Analysis.
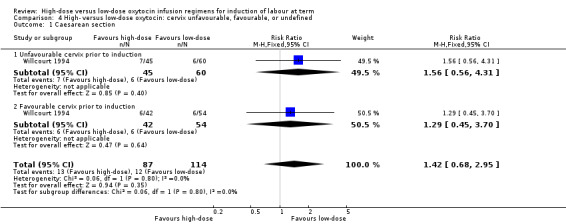
Comparison 4 High‐ versus low‐dose oxytocin: cervix unfavourable, favourable, or undefined, Outcome 1 Caesarean section.
Discussion
Summary of main results
We have included nine trials, involving 2391 women and their babies in this review. None of the included studies reported specifically on this review's primary outcome (the number of women who experienced uterine hyperstimulation with fetal heart rate changes).The only consistently reported pre‐specified primary outcome amongst included studies was caesarean section rate, which was similar between groups. When only studies without high risk of bias were analysed, the high‐dose protocol may reduce the induction to delivery interval; however, when data from all the included studies are pooled for this outcome, there is no significant difference seen in induction to delivery interval between high‐dose and low‐dose protocols. A significantly increased rate of uterine hyperstimulation, with its potential for increased adverse neonatal and maternal outcomes, was found with use of the high‐dose protocol. However, there were no significant differences found either in the pre‐specified primary outcomes of serious neonatal morbidity or death, or serious maternal morbidity and death, or in any of the secondary adverse outcomes apart from hyperstimulation, between the regimens. Most trials did not report composite outcomes that might have allowed effects on these individually uncommon outcomes to be demonstrated.
Overall completeness and applicability of evidence
There is currently a wide variation amongst clinicians about what constitutes high‐dose and low‐dose oxytocin infusion regimens for induction of labour. This variation is complicated by how often oxytocin is increased, whether the dose increment is linear or non‐linear, and if the infusion is delivered continuously or in a pulsed fashion. As a result of this variation we constructed a high‐ and low‐dose definition based on the half life of oxytocin for the purpose of this review. Because of the heterogeneity in oxytocin regimens in existing clinical trials, most screened studies did not meet our inclusion criteria, meaning there are considerable data available on this topic that is not further analysed in this review. A particular problem was reporting on rates of hyperstimulation by included studies without specifying the effect on fetal heart rate, and impact on subsequent management such as a change in oxytocin infusion rates. Lowensohn 1990 discussed that many women may not have actually have received the high dose they were allocated due to the rates of hyperstimulation but have not analysed this further, and this has not been reported by other studies. It is unclear whether this was a finding confined to that particular study, or whether other authors may not have considered this factor in their data analysis.
Regarding the setting of the included studies, all except Orhue 1993 were completed in tertiary centres in high‐income countries. Orhue 1993 was undertaken at a tertiary centre in a low‐income country. Depending on the intrapartum monitoring and back‐up medical and surgical services available, the outcome of this Cochrane review may be less relevant to peripheral health facilities, particularly those in low‐income countries.
The differing demographics of the patient populations under study, with insufficient included patients for meaningful subgroup analysis by parity or previous mode of birth, also limits the conclusions that can be drawn from the meta‐analysis. In particular, Orhue 1993's high‐parity population is unlikely to be representative of a general cohort of multiparous women undergoing oxytocin induction. The 7% rate of uterine rupture in this study is clearly of concern, and suggests high‐dose oxytocin regimens are likely unsuitable for women of high parity, however this finding cannot be generalised to the nulliparous or low parity population.
No trials reported on maternal satisfaction or caregiver satisfaction, so evidence is lacking about this important aspect of the induction process.
Quality of the evidence
Included studies were mostly of small size and only moderate quality. Undertaking a sensitivity analysis of the studies to include only those with low risk of bias changed the findings regarding induction to delivery interval from a non‐significant reduction with high‐dose protocol (all included studies) to a significant reduction (low risk of bias studies). For the other two outcomes where data existed to perform sensitivity analysis, caesarean section and uterine hyperstimulation (fetal heart rate changes not specified), there was minimal difference in findings when only low risk of bias studies were included. With the exception of Tribe 2012, studies were performed at least 15 years prior to this review and two were published in abstract form only, limiting both the ability to obtain additional data from the included studies for this review and attempts to clarify the details of existing data.
Potential biases in the review process
Our prespecified decision (made at the time of writing the protocol for this review) to set absolute cut‐offs for what cumulative oxytocin dose would be considered a high‐dose regimen versus a low‐dose regimen, potentially introduces study selection bias into the review. This decision was made as a number of studies in this area label as "low‐dose" oxytocin regimens that for other authors were "high dose", and it was therefore felt study inclusion in this review based solely on original study authors' definitions of "low dose" and "high dose" would lead to uninterpretable meta‐analysis findings. However, in a future review we may include alternative definitions and analysis (e.g. very low dose, low dose, intermediate dose, high dose) to allow for greater inclusion of original study data.
The decision to include only studies of induction of labour at term, rather than at anytime during the third trimester, is also a potential bias. As only one study was found in the trial search where this was the reason for trial exclusion, we do not feel that this is likely to have had a major impact. An alternative for future reviews would be to include all studies regardless of gestation, and perform subgroup analysis according to whether the induction of labour was at term or preterm.
Agreements and disagreements with other studies or reviews
The findings of this review in the outcomes of caesarean section are similar to those found by Pakta 2005. In addition, these authors found there was a decreased Induction to delivery interval in their review. In contrast to this review however, they have set limited parameters on what is defined as high‐ and low‐dose.
In their review of Oxytocin in 2006, Smith and Merrill (Smith 2006) concluded that both high‐ and low‐dose oxytocin protocol appeared safe but a high dose appeared to shorten the length of labour without increasing the rate of caesarean section. They also have concluded there is an increase rate of hyperstimulation but there is not expansion on the effect of this hyperstimulation to mother or baby.
Finally, when augmentation rather than induction is considered, Kenyon et all (Kenyon 2011) concluded that high‐dose oxytocin regimens were associated with a reduction in the length of labour and in caesarean section, and an increase in spontaneous vaginal delivery.
Authors' conclusions
Implications for practice.
We have not found evidence that using high‐dose oxytocin increases vaginal delivery or the rate of caesarean section. However, there is some evidence that using high‐dose oxytocin increases the risk of uterine hyperstimulation (fetal heart rate changes not specified). It also has not been shown to have any clear benefit in any other measured outcomes for the studies that we were able to include in this review, in particular, no significant reduced induction to delivery time was found on meta‐analysis. We did not find evidence of an increased risk of other adverse outcomes that have been measured in studies thus far.
The numbers of women and babies included in this review may have been too small to show a difference in some more rare outcomes such as neonatal infection, maternal symptoms, and placental abruption. Currently however, there is no evidence to support the use of high‐dose oxytocin for the induction of labour.
Implications for research.
Further research in this area would need to be focused on several outcomes that include induction to delivery time, impact of uterine hyperstimulation (with and without fetal heart rate changes), maternal and neonatal morbidity, maternal and caregiver satisfaction, and hospital stay. Further research should also aim for a better understanding about the most effective delivery method (pulsed versus continuous) and the rate at which oxytocin should be increased.
What's new
| Date | Event | Description |
|---|---|---|
| 21 March 2016 | Amended | We have clarified the subgroups in Analysis 2.1 caesarean section ‐ existing data relate to women without a previous caesarean section. There are no data pertaining to a subgroup of women who had a previous caesarean section. |
Acknowledgements
We wish to thank Dr Rachel Tribe for providing additional outcome data relating to the Tribe 2012 publication to be included in this review. We also wish to thank Prof AAE Orhue and Dr J Crane for clarifying details relating to Orhue 1993 and Crane 1993 respectively.
Thank you to Phillipa Middleton and Emer Heatley for their advice in the development of the protocol and how to conduct and write the protocol and the subsequently the review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. High‐ versus low‐dose oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 1339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 2 Caesarean section | 8 | 2023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 3 Serious neonatal morbidity or perinatal death | 1 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.23, 3.12] |
| 4 Serious maternal morbidity or death | 1 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.55, 2.82] |
| 5 Time from induction to delivery | 5 | 1725 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.28, 0.49] |
| 6 Uterine hyperstimulation, fetal heart rate changes not specified | 5 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.55, 2.25] |
| 7 Uterine rupture | 3 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.50, 19.33] |
| 8 Epidural analgesia | 2 | 1327 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.19] |
| 9 Instrumental birth | 3 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.66] |
| 10 Apgar score less than seven at five minutes | 5 | 1641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.01] |
| 11 Perinatal death | 2 | 1302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.23, 3.12] |
| 12 Postpartum haemorrhage | 5 | 1600 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 13 Endometritis | 3 | 1425 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.53, 3.43] |
Comparison 2. High‐ versus low‐dose oxytocin: previous caesarean section or not.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 1.1 Without previous caesarean section | 1 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 1.2 With previous caesarean section | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. High‐ versus low‐dose oxytocin: nulliparity or parity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation, fetal heart rate changes not specified | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.40, 3.26] |
| 1.1 Nulliparous subgroup | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.15, 3.59] |
| 1.2 Multiparous subgroup | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [1.07, 5.84] |
| 2 Caesarean section | 5 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| 2.1 Nulliparous subgroup | 4 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.51, 1.10] |
| 2.2 Multiparous subgroup | 4 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.77, 3.71] |
Comparison 4. High‐ versus low‐dose oxytocin: cervix unfavourable, favourable, or undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.68, 2.95] |
| 1.1 Unfavourable cervix prior to induction | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.56, 4.31] |
| 1.2 Favourable cervix prior to induction | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.45, 3.70] |
Comparison 5. High‐ versus low‐dose oxytocin: excluding studies at high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.25] |
| 2 Induction to delivery interval | 1 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.89, ‐0.99] |
| 3 Uterine hyperstimulation, fetal heart rate changes not specified | 2 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.51, 2.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crane 1993.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 130 women requiring induction of labour. 61 randomised to high‐dose group and 69 randomised to low‐dose group. | |
| Interventions | High‐dose group: oxytocin started at 7 mU/min, increased by 7 mU/min at 15‐min intervals to a maximum of 40 mU/min. Low‐dose group: oxytocin started at 1.4 mU/min, increased by 1.4 mU/min at 30‐min intervals to a maximum of 20 mU/min. |
|
| Outcomes | Time to delivery (primary outcome). Mode of delivery, maternal and neonatal morbidity (secondary outcomes). | |
| Notes | Performed in a tertiary hospital in New Brunswick, Canada ‐ the largest single health facility in the province. Author contacted, women at term, no further information available owing to length of time from original study (records destroyed). Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised by hospital number potentially allowing researchers to predict in advance which protocol a patient would receive. |
| Allocation concealment (selection bias) | High risk | Study was quasi‐randomised. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients were accounted for in the outcomes that were reported. No patients appear to have been excluded after randomisation. There was no explicit reporting of an intention‐to‐treat but the numbers in the reported outcomes are concurrent with the number in allocated to each group. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided to determine. |
| Other bias | Unclear risk | Not enough information provided to determine. |
Dunn 1998.
| Methods | Randomised, prospective trial using sealed opaque envelopes and a random number table. | |
| Participants | 128 women with singleton pregnancy ≥ 37 weeks who required induction of labour. Women were excluded if they had multiple gestation, known chorioamnionitis, prematurity, EFW > 4000 g, non‐vertex presentation, or uterine scar. | |
| Interventions | High‐dose group: oxytocin started at 2 mU/min and increased every 15‐20 mins, initially doubled to 4 mU then increased by 4 mU on each increment there after. Low‐dose group: oxytocin started at 0.5‐1mU/min and increased by 1‐2 mU/min at 30‐min intervals. |
|
| Outcomes | Primary outcomes included time from induction to delivery and incidence of umbilical cord pH < 7.20. Secondary outcomes included maternal pain, incidence of caesarean delivery, maternal fever, Apgar < 7 at 1 min, Apgar < 7 at 5 minutes, incidence of non‐reassuring FHR changes, and incidence of uterine hyperstimulation. Failed induction was also recorded but not a pre‐specified outcome. | |
| Notes | Doctoral thesis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generated and allocation placed in numbered opaque sealed envelopes. |
| Allocation concealment (selection bias) | High risk | No concealment. Dose was different between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of the staff or patient. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All pre‐specified outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | Study protocols were adhered to with reporting of all pre‐specified outcomes. |
| Other bias | Low risk | None identified. |
Hourvitz 1996.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 211 women at term requiring induction of labour. 113 randomised to high‐dose group and 98 randomised to low‐dose group. | |
| Interventions | High‐dose group: oxytocin started at 2.5 mU/min, increased by 2.5 mU/min at 30‐min intervals till 30 mU/min, then 5 mU/min increments to a maximum dose of 30 mU/min. Low‐dose group: oxytocin started at 1.25 mU/min, increased by 1.25 mU/min at 30 min intervals till 7.5 mU/min then 2.5 mU/min increments till of 15 mU/min, then 5 mU/min to a maximum of 30 mU/min. |
|
| Outcomes | Primary outcomes included time from induction to delivery, maximal oxytocin dose, number of times infusion was stopped or decreased for hyperstimulation of suspicious FHR changes, and use of anaesthetic. Secondary outcomes include methods of delivery, PPH, endometritis, cervical tears, length of hospital stay, Agpar scores, birthweight. | |
| Notes | Undertaken at the Chaim Sheba Medical Centre – tertiary and largest hospital in Israel. Failed induction of labour was defined as women not in active labour after 6 hours but they have not mentioned rates of failure at 24 hours. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was performed on a temporal basis, alternating the protocol used in the delivery ward every 2 months. |
| Allocation concealment (selection bias) | High risk | No concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different infusion rates would be noticeable by both participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 patients were excluded after randomisation as they had not received 6 hours of oxytocin. They were not analysed with intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specifed outcomes were reported, however with no access to a trial protocol it is not possible to confidently assess selective reporting. |
| Other bias | Unclear risk | Potential bias related to short duration of trialled oxytocin before calling it a failed induction of labour. |
Lowensohn 1990.
| Methods | Randomised controlled trial. | |
| Participants | 104 women greater than or equal to 37 weeks' gestation requiring Induction or augmentation of labour. 55 of these women were induced. 25 women in the high‐dose group and 30 women in the low‐dose group | |
| Interventions | High‐dose group: oxytocin starting at 6.67 mU/min, increased by 6.67 mU/min every 15 mins to a maximum of 40 mU/min. Low‐dose group: oxytocin started at 1 mU/min, increased every 30‐40 mins to a maximum dose of 4 mU/min. |
|
| Outcomes | Time from induction to delivery, labour dystocia, recurrent hyperstimulation, failure to achieve labour, fetal distress requiring caesarean delivery, vaginal delivery rate. Outcomes were reported separately for induction and augmentation groups. | |
| Notes | Undertaken at Oregon Health Sciences University Hospital – tertiary hospital and trauma centre in Portland, USA. Abstract only. Attempted to contact authors however no reply was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No discussion of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data are only presented for 103 women, with no explanation of what occurred to 1 woman. There was no discussion of exclusion after randomisation, intention‐to‐treat analysis, or dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided to determine. |
| Other bias | Unclear risk | Not enough information provided to determine. |
Merrill 1999.
| Methods | Double‐blinded randomised controlled trial. | |
| Participants | 816 women for induction of labour and 491 women for augmentation of labour. Women being induced had to be greater than 24 weeks' gestation with a live fetus, be less than 3 cm dilated or have less than 10 contractions per hour. 404 women were randomised to the high‐dose group and 412 to the low‐dose group. | |
| Interventions | High‐dose group: oxytocin starting at 4.5 mU/min, increased by 4.5 mU/min every 30 mins. Low‐dose group: oxytocin starting at 1.5 mU/min and increased by 1.5 mU/min every 30 mins. |
|
| Outcomes | There were no pre‐specified outcomes. Reported outcomes include numbers of caesarean section, serious maternal morbidity or death, serious neonatal morbidity or perinatal death, time from induction of labour to delivery, uterine hyperstimulation, uterine rupture, epidural analgesia, Apgar less than 7 at 5 mins, PPH, chorioamnionitis, and endometritis. | |
| Notes | Undertaken at University of Iowa Hospitals and Clinics – only tertiary level hospital in Iowa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables located in the central pharmacy. |
| Allocation concealment (selection bias) | Low risk | Infusion bags were made in a central pharmacy with a minimal difference in total volume. Infusion rates were the same between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The infusion rates were the same between groups with only the concentration higher in 1 group. There was no distinguishable difference in the preparations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All data were entered before any codes broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient lost to follow‐up. Intention‐to‐treat analysis was evident in most of the reported outcomes. |
| Selective reporting (reporting bias) | High risk | There were no pre‐specified outcomes. Authors may have only reported some of their findings. |
| Other bias | High risk | Confidence intervals for statistical analysis of outcomes much narrower than for other studies, and more than would be expected from the larger study size alone, raising concerns about accuracy of the statistical analysis. |
Orhue 1993.
| Methods | Randomised controlled trial. | |
| Participants | 90 women with 5 or more previous births who required an induction of labour. 44 women were randomised to the high‐dose group and 46 women to the low‐dose group. | |
| Interventions | High‐dose group: oxytocin starting at 2 mU/min, doubled every 15 mins to a maximum of 32 mU/min or when 3 uterine contractions every 10 mins established. Low‐dose group: oxytocin started at 2 mU/min, doubled every 45 mins to a maximum dose of 32 mU/min or when 3 uterine contractions every 10 mins established. |
|
| Outcomes | Mode of delivery, complications of labour and delivery (uterine rupture, hyperstimulation, PPH, perineal trauma, puerperal pyrexia), number of days in hospital. | |
| Notes | Undertaken at University of Benin Teaching Hospital – tertiary hospital in Lagos. Author contacted April 2012 and confirmed that all participants were at term. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised code from a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not discussed in adequate detail. Given the different rates of infusion blinding is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No patient loss to follow‐up, no exclusion after randomisation, no dropouts. It is unclear if there was intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐determined outcomes were reported, however with no access to a trial protocol it is not possible to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious sources of bias identified. |
Satin 1991.
| Methods | Randomised controlled trial. | |
| Participants | 90 women admitted to labour and delivery suite for induction of labour. 10 excluded women not included in analysis. 48 women were randomised to the high‐dose group and 32 women to the low‐dose group, 10 women were not reported on. | |
| Interventions | High‐dose group: oxytocin starting at 2 mU/min, increased by 2 mU/min every 15 mins (150 mU at 40 min, 1080 mU at 2 hours) 48 women. Low‐dose group: oxytocin starting at 2 mU/min and increased by 1 mU/min every 30 mins (90 mU at 40 min, 420 mU at 2 hours). 32 women. |
|
| Outcomes | Frequency of operative deliveries, chorioamnionitis, endometritis, PPH, Apgar scores, umbilical cord gas values, infection rate, and hospital stay. | |
| Notes | Undertaken at Wilford Hall United States Air Force Medical Center – military hospital. 10 women were excluded because they had hypertensive disease requiring magnesium sulphate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was based on unmarked, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients received monitoring in a similar fashion despite allocation. Whilst it is not stated if the participants and personnel were blinded, it is unlikely given the time of incremental increase. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 patients were excluded after randomisation with more removed in the low‐dose group compared to the high‐dose group. These patients were not analysed with intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | All primary pre‐specified outcomes were reported, however with no access to a trial protocol it is not possible to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious sources of bias identified. |
Tribe 2012.
| Methods | Unblinded parallel group randomised controlled trial. Block randomisation by hospital, labour intervention required (augmentation versus induction of labour), and gestation (37+ weeks versus < 37 weeks). | |
| Participants | Women 18 years+ requiring induction or augmentation of labour. 523 women requiring induction of labour were included in the trial, only 11 of whom were less than 37 weeks' gestation. Baseline characteristics were similar. 257 were randomised to the high‐dose group and 266 women randomised to the low‐dose group. | |
| Interventions | High‐dose: oxytocin starting at 2 mU/min, doubling of infusion rate every 30 mins. Low‐dose: oxytocin starting at 2 mU/pulse, pulsed every 6 mins, with doubling of pulse dose every 30 mins until 3 or 4 uterine contractions in 10 mins established. |
|
| Outcomes | Primary: caesarean section rate. Secondary: vaginal delivery not achieved within 24 hours, time from induction to delivery, pain relief, serious maternal morbidity or death, serious neonatal morbidity or death, uterine hyperstimulation, meconium‐stained liquor, PPH, fetal distress (clinical judgement), need for fetal blood sampling, Apgar less than 7 at 5 mins, and NICU admission. |
|
| Notes | Undertaken at 2 inner‐city UK university tertiary hospitals. Primary reference for Crawshaw 2008. Additional data supplied by Dr Tribe July 2012. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation designed by a statistician. |
| Allocation concealment (selection bias) | Unclear risk | Participants were assigned a treatment group by the computer after entry of baseline data. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was not blinded as the 2 infusion pumps used for pulsatile and continuous infusions were visually distinct. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up during intervention. 15 women crossed over from pulsatile to continuous, 2 women in pulsatile withdrew consent for analysis. Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Trial pre‐registered. All pre‐specified outcomes reported. |
| Other bias | Low risk | No other obvious sources of bias identified. |
Willcourt 1994.
| Methods | Randomised controlled trial. | |
| Participants | 358 women requiring induction of labour for post dates, gestational hypertension, or diabetes. Data only recorded for 310 women. *7 women were randomised to the high‐dose group, 109 women to the continuous low‐dose group, and 114 women the pulsed (low‐dose) group). | |
| Interventions | High‐dose infusion: oxytocin starting at 0.67 mU/min, increased by 2 mU/min every 15 mins. Low‐dose infusion: oxytocin starting at 0.67 mU/min, increased by 1 mU/min every 30 mins until uterine contractions were established. Pulsed infusion: 0.67 mU administered over 5 seconds every 5 mins over 40 mins, increased to 2 mU administered over 5 seconds every 5 mins over 40 mins, and then increased by 1 mU/pulse every 40 mins until labour established. Results were only compared between the pulsed group to each continuous group and not between high and low continuous groups. |
|
| Outcomes | Induction to delivery time, caesarean section rates, cord lactic acid. | |
| Notes | Undertaken at Kapiolani Medical Center for Women and Children. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomly assigned sequence. |
| Allocation concealment (selection bias) | Unclear risk | This is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48 patients were removed after randomisation for worsening diabetes, hypertension requiring magnesium sulphate, or not labouring within 24 hours. It it not explained which group these women were assigned. No intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | No pre‐defined outcomes were reported. No collective results reported, only subgroup analysis. |
| Other bias | Low risk | No other obvious sources of bias identified. |
EFW: estimated fetal weigh FHR: fetal heart rate mU: milli units min(s): minutes NICU: neonatal intensive care unit PPH: postpartum haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Auner 1993 | This study includes women in the setting of premature rupture of membranes. The study assessed continuous infusion (oxytocin 120 mU at 40 min, 540 mU at 2 hrs) versus pulsed at 10‐min intervals (25 mU at 40 mins, 150 mU at 2 hrs) The study was excluded as the high‐dose protocol did not reach 600 mU at 2 hrs and it only involved women who were preterm. |
| Blakemore 1990 | Both groups started at 0.5 mU/min, and the study compared increases at 15 min (77.5 mU at 40 min, 817 mU at 2 hrs) versus 1 hr (20 mU at 40 min, 150 mU at 2 hrs). Whilst the study aimed to compare dose schedules, both groups would be classified as low‐dose by this protocol, as the control group did not reach 100 mU by 40 mins. |
| Chua 1991 | Both arms of the study described an oxytocin infusion regimen that was high‐dose according to the review's pre‐specified definition (Regimen 1: 125 mU by 40 mins, 750 mU by 120 mins, Regimen 2: 187.5 mU by 40 minutes, 1350 mU by 120 mins). |
| Compitak 2002 | This study describes randomisation of women requiring both augmentation and induction of labour but has not reported how they have defined these 2 groups. In reporting their results, they have not stratified their findings by induction and augmentation. |
| Cummiskey 1990 | This study randomised women to pulsed or continuous infusion groups. The pulsed group received a total oxytocin of 7 mU at 40 mins and 93 mU at 2 hrs whilst the continuous group received 50 mU at 40 mins and 300 mU at 2 hrs. Therefore, both groups would be classified into low‐dose by this protocol. |
| Daniel‐Spiegel 2004 | This study compared continuation oxytocin infusion versus cessation of oxytocin at a cervical dilatation of 5 cm in women requiring induction of labour. Both groups used the same dose schedule until cervical dilatation of 5 cm then oxytocin infusion was ceased for 1 group, whilst continued for the other group. The protocol used by the authors would be in the low‐dose category for both arms (60 mU at 40 mins, 420 mU at 2 hrs). |
| Diven 2012 | This study compared the continuation versus cessation of oxytocin once active labour had been achieved in women requiring induction of labour. Both groups used the same dose schedule until labour was established, defined by cervical dilatation of greater than 4 cm, then oxytocin infusion was ceased for 1 group, whilst continued for the other group. Oxytocin infusion regimens could not be differentiated. |
| Durodola 2005 | This study compared linear versus non‐linear increases in oxytocin dose. In the arithmetic (linear) group, women received total oxytocin dose of 160 mU at 40 mins and 1200 mU at 2 hrs, whilst women in the geometric (non‐liner) group received 160 mU at 40 mins and 1800 mU at 2 hrs. Therefore both groups would be classified as high‐dose by this protocol. |
| Fitzpatrick 2012 | This study was excluded as neither group reached high‐dose oxytocin. The study compared women with a fixed low‐dose oxytocin versus their standard low‐dose regimen after women had received Foley's catheter cervical ripening. In the fixed group, women received 80 mU at 40 min and 280 mU at 2 hrs versus the standard group which received 50 mU at 40 min and 510 mU at 2 hrs. |
| Gibb 1985 | This study was not a randomised trial. 121 women were allocated to 1 of 2 methods of administration of the infusion based on the availability of the equipment. The automatic infusion of oxytocin in a closed loop system for the induction of labour was compared with manual administration of oxytocin by peristaltic infusion pump. |
| Girard 2009 | This study examined the effects of continuous oxytocin versus cessation of oxytocin at the beginning of the active phase of labour in women requiring induction of labour. Both groups received the same amount of oxytocin until that point. The authors were not strict about the amount of initial oxytocin or the time when it was increased. As the only difference is when oxytocin is ceased, we have excluded this trial. |
| Goni 1995 | This study compared the timing by which doses should be increased. The traditional protocol group received a total of 60 mU at 40 mins and 1260 mU at 2 hrs whilst the low‐dose group received 40 mU at 40 mins and 180 mU at 2 hrs. Therefore, both groups would be classified as low‐dose by this protocol. |
| Gungorduk 2011 | This study compared high‐dose oxytocin protocol with either concurrent administration of sustained‐release dinoprostone or delayed oxytocin by 6 hrs. Both groups received the same oxytocin and only differed by the time post cervical ripening. |
| Lazor 1993 | This study compared timing of oxytocin increase and the amount they were raised by. The 15‐min group started at 1 mU/min and increased by 1 mU/min every 15 mins (total oxytocin dose at 40 mins of 75 mU and 525 mU at 2 hrs) whilst the 40‐min group started at 1 mU/min and increased by 1.5 mU/min every 40 mins (40 mU at 40 mins, 300 mU at 2 hrs). Therefore both groups would be classified as low‐dose by this protocol. |
| Li 1996 | Non‐randomised study and exact dosages of oxytocin not available for 2 arms in translation of paper. Authors contacted without reply. |
| Manjula 2014 | This study (abstract only) reported 200 women randomised to high‐ versus intermediate‐dose oxytocin for induction of labour at ≥37 weeks. Both groups however were in the higher dose category as defined by our review group. The high‐dose group received 240 mU at 40 minutes and 1320 at 2 hrs whilst the intermediate‐dose group received 120 mU of oxytocin at 40 minutes and 660 mU at 2 hrs. |
| Mercer 1991 | This study compared 2 non‐linear protocols which varied only by time. The traditional protocol group received total oxytocin of 30 mU at 40 mins and 490 mU at 2 hrs whilst the low‐dose group received 20 mU at 40 mins and 90 mU at 2 hrs. The timing of amniotomy was also different between the two groups, the traditional protocol receiving amniotomy as soon as a Amnihook could be passed and the low‐dose only when the Bishops score was greater than 6. While both groups would be classified into low‐dose by this protocol, the timing of amniotomy does not allow adequate comparison for this protocol. |
| Muller 1992 | This study compared 2 groups using different time intervals as well as comparing linear and non‐linear increases in oxytocin infusion. Furthermore, the protocol for this study allows for a variable dose starting within each group, effecting the non‐linear groups rates of infusion, whilst the linear group also had variable dose increases between group members. From the protocol, the traditional group would receive 40‐100 mU at 40 mins and 300‐600 mU at 2 hrs whilst the experimental group received 40‐80 mU at 40 mins and 280‐560 mU. Given there is inconsistency with the dose that the traditional group received, we can not include study this as high‐dose despite the upper limit of the dosing satisfying our definition of high‐dose. |
| Odem 1988 | This study compared pulsed versus continuous infusions of oxytocin. The pulse group received a total oxytocin of 7 mU at 40 mins and 93 mU at 2 hrs whilst the continuous group 50 mU at 40 mins and 450 mU at hrs. Therefore, both groups would be classified into low‐dose by this protocol. |
| Oral 2003 | The authors investigated the effect using oxytocin mixed with either isotonic 0.9% saline or 5% glucose solution in women who required induction of labour. The oxytocin dose delivered was the same in both groups. |
| Orhue 1993b | Both arms of the study describe an oxytocin infusion regimen that is high‐dose according to our pre‐specified definition (Regimen 1: 100 mU by 40 mins, 900 mU by 120 mins, Regimen 2: 170 mU by 40 mins, 2370 mU by 120 mins). |
| Orhue 1994 | Both arms of the study describe an oxytocin infusion regimen that is high‐dose according to our pre‐specified definition (Regimen 1: 100 mU by 40 mins, 900 mU by 120 mins, Regimen 2: 170 mU by 40 mins, 2370 mU by 120 mins). |
| Parashi 2005 | This study compared different oxytocin doses given at different intervals between 2 groups. Group A received starting doses of 2.5 mU/min with increments of 2.5 mU/min every 15 mins (187.5 mU at 40 min and 1450 mU at 2 hrs) whilst group B received 5 mU/min and increased 5 mU/min every 45 mins (200 mU at 40 mins and 1125 mU at 2 hrs). Therefore both groups would be classified into high‐dose by this protocol. |
| Parpas 1995 | Oxytocin regimens vary according to weight so overall high‐dose versus low‐dose group comparisons are not possible. |
| Pavlou 1978 | This study compared continuous versus pulsed delivery of oxytocin. The oxytocin dose that was delivered was the same between both groups and only differed by delivery system. |
| Reid 1995 | This study compared pulsed oxytocin (28 mU at 40 min, 228 mU at 2 hrs) versus continuous infusion (50 mU at 40 min, 450 mU at 2 hrs). Both groups are classified as low‐dose delivery by our protocol. |
| Ross 1998 | This study compared different starting and incremental doses with the timing of doses kept the same. In the low‐dose group women received 120 mU at 40 mins and 840 mU at 2 hrs whilst the high‐dose group 360 mU at 40 mins and 1980 mU at 2 hrs. Therefore both groups would be classified into high‐dose by this protocol. |
| Satin 1994 | This trial was not randomised. It compared data of women undergoing induction where in the first 3 months the hospital used a protocol of oxytocin increasing every 20 mins (360 mU at 40 mins and 2520 mU at 2 hrs) and then the next 3 months used a protocol of oxytocin increasing every 40 mins (240 mU at 40 mins and 1440 mU at 2 hrs). Taking aside the methodological issues of this study, both groups would be classified into high‐dose by this protocol and therefore it is excluded. |
| Steer 1985 | All arms of the study (3 arms using standard labour ward infusion protocols and 1 arm using automatic infusion system protocol) describe an oxytocin infusion regimen that is high‐dose according to our prespecified definition. |
| Ustunyurt 2007 | This study compared the continuation versus cessation of oxytocin at a cervical dilatation of 5 cm in women requiring induction of labour. Both groups use the same dose schedule until 5 cm, then oxytocin was ceased for 1 group, whilst continued for the other. The protocol used by the authors would be in the high‐dose category (150 mU at 40 mins, 950 mU at 2 hrs). |
hr(s): hour min(s): minutes mU: milli units
Characteristics of studies awaiting assessment [ordered by study ID]
Ashworth 1988.
| Methods | Randomised controlled trial with allocation by sealed, numbered envelopes. |
| Participants | Women with singleton pregnancies greater than 37 weeks' gestation requiring induction of labour with 3 or less previous deliveries. |
| Interventions | Oxytocin by routine continuous infusion or pulsatile. |
| Outcomes | Primary: duration of labour and total oxytocin dose. Secondary: volume of intravenous fluids, serum sodium changes, jaundice of the newborn. |
| Notes | This remains unpublished to our knowledge and despite the record of study being retrieved, the only details found were that it compared pulsed vs continuous infusion but not the dose of each group. No response from author for further clarification. |
Raymond 1989.
| Methods | Randomised controlled trial. |
| Participants | 32 women at 39‐42 weeks' gestation. |
| Interventions | Pulsed infusion of oxytocin vs continuous infusion. |
| Outcomes | Mode of delivery, postpartum haemorrhage, fetal distress, total oxytocin dose, induction to delivery interval. |
| Notes | The authors assessed 32 women for induction of labour by using either pulsed or continuous oxytocin infusion. The authors described the use of oxytocin in the pulsed group (40 mU at 40 mins, 120 mU at 2 hrs) but not the continuous infusion group. No further contact from authors has been received. |
Salamalekis 2000.
| Methods | Randomised controlled trial. |
| Participants | 560 women greater than 37 weeks' gestation undergoing induction of labour. |
| Interventions | Continuous oxytocin infusion vs pulsed oxytocin infusion. |
| Outcomes | Induction to delivery interval, average dose of oxytocin, mode of delivery, Apgar score. |
| Notes | This randomised trial of 560 women compared continuous vs pulsatile oxytocin. The authors have reported the continuous group received total oxytocin dose of 100 mU at 40 mins and 600 mU at 2 hrs whilst the pulsatile group received a total oxytocin dose of 170 mU at 40 mins and 2850 mU at 2 hrs. This would classify both groups into high‐dose by this protocol, however the study appears to have misreported the pulsatile dose. The authors have reported the dose as 2 mU/min and doubled every 15 mins but we have questioned if this is actually 2 mU/pulse. Contact was attempted, however the authors have not replied. |
Singh 1993.
| Methods | Randomised trial using sealed opaque envelopes. |
| Participants | 67 women admitted for induction of labour. |
| Interventions | 5 units of syntocinon per litre in an infusion increased every 60 mins compared to 10 units of syntocinon per litre in an infusion increased every 15 mins. No rate was supplied. |
| Outcomes | Non‐statistically significant decrease induction to delivery time in the high‐dose group. Higher rates of fetal heart rate changes requiring syntocinon to be ceased in the high‐dose group. |
| Notes | Dose information could not be obtained from abstract. No gestational age was supplied. Attempted to contact authors without success. |
Sotunsa 2013.
| Methods | Authors state randomised but no information about method. |
| Participants | 136 women for induction of labour. No information provided on gestations or cause. |
| Interventions | Oxytocin. No explanation of initial or incremental dose. Groups were divided by interval timing of 30 vs 60 minutes. |
| Outcomes | Induction to delivery interval, fetal distress, meconium liquor, and NICU admission. |
| Notes | Unable to contact author. No publication despite poster presentation > 12 months prior to this review. |
hr: hour min: minute mU: milli units NICU: neonatal intensive care unit vs: versus
Differences between protocol and review
The title of this review has changed since publication of the protocol (Budden 2012). The protocol was entitled, 'Oxytocin infusion regimens for induction of labour' but during the preparation of the full review this title was changed to 'High‐dose versus low‐dose oxytocin infusion regimens for induction of labour at term'.
A post‐hoc secondary outcome, uterine hyperstimulation (fetal heart rate changes unknown), was added as a majority of studies reported uterine hyperstimulation but not its effect on fetal heart rate and therefore could not be placed into the protocols pre‐specified outcomes. No studies had specified uterine hyperstimulation without fetal heart rate changes and as such it was not reported on in favour of this post‐hoc outcome.
Inclusion criteria ‐ despite inclusion criteria of gestation from 24 weeks, the study by Merrill 1999 was included as the average gestation was 38 weeks such with a narrow confidence interval that minimal if any women would have been preterm. Given the size of the review it was felt to keep this trial and adjust results in sensitivity where possible.
No other changes from the protocol were made.
Contributions of authors
A Budden and A Henry wrote the protocol.
L Chen reviewed all retrieved studies, with either A Budden or A Henry providing second assessment on each paper for its inclusion or exclusion.
A Budden, A Henry, and L Chen each reviewed the included papers and co‐authored the review.
Emily Bain provided author support for the review.
Sources of support
Internal sources
Australian Research Centre for Health of Women and Babies (ARCH), Australia.
External sources
Department of Health and Ageing, Australian Government, Australia.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Crane 1993 {published data only}
- Crane J, Reardon E. A prospective randomized study of high dose vs low dose oxytocin infusion for labour induction. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993:109.
Dunn 1998 {published data only}
- Dunn PA. Differences in maternal and fetal stress response of women who received moderate or low dose oxytocin for induction of labor [thesis]. University of Pennsylvania, 1998. [Google Scholar]
Hourvitz 1996 {published data only}
- Hourvitz A, Alcalay M, Korach J, Lusky A, Barkai G, Seidman DS. A prospective study of high‐ vs low‐dose oxytocin for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1996;75:636‐41. [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Seidman DS, Alcalay M, Korach J, Lusky A, Barkai G, et al. A prospective study of high‐ versus low‐dose oxytocin for induction of labor. American Journal of Obstetrics and Gynecology 1994;170:285. [DOI] [PubMed] [Google Scholar]
Lowensohn 1990 {published data only}
- Lowensohn RI, Jensen JT. Oxytocin use in induction and augmentation of labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:76.
Merrill 1999 {published data only}
- Merrill DC, Zlatnik FJ. Randomized, double‐masked comparison of oxytocin dosage in induction and augmentation of labor. Obstetrics and Gynecology 1999;94:455‐63. [DOI] [PubMed] [Google Scholar]
Orhue 1993 {published data only}
- Orhue AA. A randomised trial of 45 minutes and 15 minutes incremental oxytocin infusion regimes for the induction of labour in women of high parity. British Journal of Obstetrics and Gynaecology 1993;100:126‐9. [DOI] [PubMed] [Google Scholar]
Satin 1991 {published data only}
- Satin AJ, Hankins GDV, Yeomans ER. A prospective study of two dosing regimens of oxytocin for the induction of labor in patients with unfavorable cervices. American Journal of Obstetrics and Gynecology 1991;165:980‐4. [DOI] [PubMed] [Google Scholar]
Tribe 2012 {published data only}
- Crawshaw SE, Baker PN, Stanley C, Safari F, Seed P, Shennan AH, et al. A physiological approach to stimulating labour: pulse, a randomised controlled trial of pulsatile versus continuous oxytocin administration. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa11. [Google Scholar]
- Shennan A. A physiological approach to induction of labour: PULSE ‐ a randomised, controlled trial of pulsatile versus continuous oxytocin administration. National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006).
- Tribe RM, Crawshaw SE, Seed P, Shennan AH, Baker PN. Pulsatile versus continuous administration of oxytocin for induction and augmentation of labor: two randomized controlled trials. American Journal of Obstetrics and Gynecology 2012;206:e1‐8. [DOI] [PubMed] [Google Scholar]
Willcourt 1994 {published data only}
- Willcourt RJ, Pager D, Wendel J, Hale RW. Induction of labor with pulsatile oxytocin by a computer‐controlled pump. American Journal of Obstetrics and Gynecology 1994;170(2):603‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Auner 1993 {published data only}
- Auner H, Adelwohrer NE, Semmelrock HJ, Lorenz‐Eberhardt G, Haas J, Grubock K. Pulsatile oxytocin for inducing labor after premature rupture of fetal membranes. [German] [Pulsatiles Oxytocin zur Auslosung der Wehentatigkeit nach vorzeitigem Blasensprung]. Gynakologisch‐Geburtshilfliche Rundschau 1993;33 Suppl 1:256‐7. [DOI] [PubMed] [Google Scholar]
Blakemore 1990 {published data only}
- Blakemore KJ, Qin NG, Petrie RH, Paine LL. A prospective comparison of hourly and quarter‐hourly oxytocin dose increase intervals for the induction of labor at term. Obstetrics and Gynecology 1990;75:757‐61. [PubMed] [Google Scholar]
Chua 1991 {published data only}
- Chua S, Arulkumaran S, Kurup A, Tay D, Ratnam SS. Oxytocin titration for induction of labour: a prospective randomized study of 15 versus 30 minute dose increment schedules. Australian and New Zealand Journal of Obstetrics and Gynaecology 1991;31(2):134‐7. [DOI] [PubMed] [Google Scholar]
Compitak 2002 {published data only}
- Compitak K, Petchmark P. Oxytocin augmentation or induction of labor in term pregnant women: a randomized controlled trial to evaluate a 15 ‐ versus 40 ‐ minute dose increment interval. Thai Journal of Obstetrics and Gynaecology 2002;14:121‐6. [Google Scholar]
Cummiskey 1990 {published data only}
- Cummiskey KC, Dawood MY. Induction of labor with pulsatile oxytocin. American Journal of Obstetrics and Gynecology 1990;163:1868‐74. [DOI] [PubMed] [Google Scholar]
Daniel‐Spiegel 2004 {published data only}
- Daniel‐Spiegel E, Weiner Z, Ben‐Shlomo I, Shalev E. For how long should oxytocin be continued during induction of labour?. British Journal of Obstetrics and Gynaecology 2004;111(4):331‐4. [DOI] [PubMed] [Google Scholar]
Diven 2012 {published data only}
- Diven L, Gogle J, Eid S, Smulian J, Quinones J. Induction of labor with oxytocin: should oxytocin be held?. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S144. [DOI] [PubMed] [Google Scholar]
- Diven LC, Rochon ML, Gogle J, Eid S, Smulian JC, Quinones JN. Oxytocin discontinuation during active labor in women who undergo labor induction. American Journal of Obstetrics and Gynecology 2012;207(6):471.e1‐471e8. [DOI] [PubMed] [Google Scholar]
Durodola 2005 {published data only}
- Durodola A, Kuti O, Orji EO, Ogunniyi SO. Rate of increase in oxytocin dose on the outcome of labor induction. International Journal of Gynecology and Obstetrics 2005;90(2):107‐11. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2012 {published data only}
- Fitzpatrick CB, Grotegut CA, Bishop TS, Canzoneri BJ, Heine RP, Swamy GK. Cervical ripening with foley balloon plus fixed versus incremental low‐dose oxytocin: A randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(7):1006‐10. [DOI] [PubMed] [Google Scholar]
Gibb 1985 {published data only}
- Arulkumaran S, Gibb DMF, Ratnam SS, Lun KC, Heng SH. Total uterine activity in induced labour ‐ an index of cervical and pelvic tissue resistance. British Journal of Obstetrics and Gynaecology 1985;92:693‐7. [DOI] [PubMed] [Google Scholar]
- Gibb DMF, Arulkumaran S, Ratnam SS. A comparative study of methods of oxytocin administration for induction of labour. British Journal of Obstetrics and Gynaecology 1985;92:688‐92. [DOI] [PubMed] [Google Scholar]
Girard 2009 {published data only}
- Girard B, Vardon D, Creveuil C, Herlicoviez M, Dreyfus M. Discontinuation of oxytocin in the active phase of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(2):172‐7. [DOI] [PubMed] [Google Scholar]
Goni 1995 {published data only}
- Goni S, Sawhney H, Gopalan S. Oxytocin induction of labor: a comparison of 20‐ and 60‐min dose increment levels. International Journal of Gynecology and Obstetrics 1995;48:31‐6. [DOI] [PubMed] [Google Scholar]
Gungorduk 2011 {published data only}
- Gungorduk K, Yildirim G, Gungorduk O, Ark C, Tekirdag I. Sustained‐release dinoprostone vaginal pessary with concurrent high‐dose oxytocin infusion compared to sustained‐release dinoprostone vaginal pessary followed 6 h later by high‐dose oxytocin infusion for labor induction in women at term with unfavorable cervix: a randomized controlled trial. Gynecologic and Obstetric Investigation 2011;71(1):32‐40. [DOI] [PubMed] [Google Scholar]
Lazor 1993 {published data only}
- Lazor LZ, Philipson EH, Ingardia CJ, Kobetitsch ES, Curry SL. A randomized comparison of 15‐ and 40‐minute dosing protocols for labor augmentation and induction. Obstetrics and Gynecology 1993;82:1009‐12. [PubMed] [Google Scholar]
Li 1996 {published data only}
- Li F, Liu C. Clinical study on induction of labor with feedback pulsatile oxytocin system. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynecology] 1996;31(8):480‐2. [PubMed] [Google Scholar]
Manjula 2014 {published data only}
- Manjula BG, Kalra J, Dutta S. Induction of labour with oxytocin: is an intermediate‐dose regimen better?. BJOG: An International Journal of Obstetrics and Gynaecology 2014;121(Suppl 2):108. [Google Scholar]
Mercer 1991 {published data only}
- Mercer B, Pilgrim P, Sibai B. Labour induction with continuous low‐dose oxytocin infusion: a randomised trial. Obstetrics and Gynaecology 1991;77(5):659‐663. [PubMed] [Google Scholar]
Muller 1992 {published data only}
- Muller PR, Stubbs TM, Laurent SL. A prospective randomized clinical trial comparing two oxytocin induction protocols. American Journal of Obstetrics and Gynecology 1992;167:373‐81. [DOI] [PubMed] [Google Scholar]
Odem 1988 {published data only}
- Odem RR, Work BA, Dawood MY. Pulsatile oxytocin for induction of labor: a randomized prospective controlled study. Journal of Perinatal Medicine 1988;16:31‐7. [DOI] [PubMed] [Google Scholar]
Oral 2003 {published data only}
- Oral E, Gezer A, Cagdas A, Pakkal N. Oxytocin infusion in labor: the effect different indications and the use of different diluents on neonatal bilirubin levels. Archives of Gynecology and Obstetrics 2003;267:117‐20. [DOI] [PubMed] [Google Scholar]
Orhue 1993b {published data only}
- Orhue AA. A randomized trial of 30‐min and 15‐min oxytocin infusion regimen for induction of labor at term in women of low parity. International Journal of Obstetrics and Gynaecology 1993;40:219‐25. [DOI] [PubMed] [Google Scholar]
Orhue 1994 {published data only}
- Orhue AA. Incremental increases in oxytocin infusion regimens for induction of labor at term in primigravidas: a randomized controlled trial. Obstetrics and Gynecology 1994;83(2):229‐33. [PubMed] [Google Scholar]
Parashi 2005 {published data only}
- Parashi SH, Bonabi NB, Rashidi A. Re: oxytocin induction of labour: a comparison of two protocols. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45(6):540. [DOI] [PubMed] [Google Scholar]
Parpas 1995 {published data only}
- Parpas G, Gondry J, Verhoest P, Martinez C, Boulanger JC. Randomised trial of 2 dosages of oxytocin for labour induction or augmentation [Utilisation de l'ocytocine (syntocinon) dans le declenchement ou la direction du travail: faible ou forte posologie, comparaison.]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1995;24(8):873. [Google Scholar]
Pavlou 1978 {published data only}
- Pavlou C, Barker GH, Roberts A, Chamberlain GVP. Pulsed oxytocin infusion in the induction of labour. British Journal of Obstetrics and Gynaecology 1978;85:96‐100. [DOI] [PubMed] [Google Scholar]
Reid 1995 {published data only}
- Reid G, Helewa M. Pulsatile vs continuous oxytocin infusion for induction of labour. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993.
- Reid GJ, Helewa ME. A trial of pulsatile vs continuous oxytocin administration for the induction of labor. Journal of Perinatology 1995;15:364‐6. [PubMed] [Google Scholar]
Ross 1998 {published data only}
- Ross EL, Bofill JA, Keith SJ, Martin RW, Morrison JC. High‐dose versus standard‐dose oxytocin in parturients with high uterine risk factors: a randomized double blind trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S95. [Google Scholar]
Satin 1994 {published data only}
- Satin AJ, Leveno KJ, Sherman ML, McIntire D. High‐dose oxytocin: 20‐versus 40‐minute dosage interval. Obstetrics and Gynecology 1994;83:234‐8. [PubMed] [Google Scholar]
Steer 1985 {published data only}
- Steer PJ, Carter MC, Choong K, Hanson M, Gordon AJ, Pradhan P. A multicentre prospective randomized controlled trial of induction of labour with an automatic closed‐loop feedback controlled oxytocin infusion system. British Journal of Obstetrics and Gynaecology 1985;92:1127‐33. [DOI] [PubMed] [Google Scholar]
Ustunyurt 2007 {published data only}
- Ustunyurt E, Ugur M, Ustunyurt BO, Iskender TC, Ozkan O, Mollamahmutoglu L. Prospective randomized study of oxytocin discontinuation after the active stage of labor is established. Journal of Obstetrics and Gynaecology Research 2007;33(6):799‐803. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ashworth 1988 {published data only}
- Ashworth MF. Comparison of pulsatile infusion of oxytocin vs continuous infusion in the induction of labour. Personal communication 1988.
Raymond 1989 {published data only}
- Raymond S. The use of pulsed oxytocin infusion for the induction of labour. Personal communication 1989.
Salamalekis 2000 {published data only}
- Salamalekis E, Vitoratos N, Kassanos D, Loghis C, Panayotopoulos N, Sykiotis C. A randomized trial of pulsatile vs continuous oxytocin infusion for labor induction. Clinical and Experimental Obstetrics and Gynecology 2000;27(1):21‐3. [PubMed] [Google Scholar]
Singh 1993 {published data only}
- Singh PM, Young DC. A rct of high dose vs low dose oxytocin for induction of labour. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993:48.
Sotunsa 2013 {published data only}
- Sotunsa JO. Perinatal outcome of two incremental schedules in induction of labour at term. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:148. [Google Scholar]
Additional references
Alfirevic 2009
- Alfirevic Z, Kelly AJ, Dowsell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budden 2012
- Budden A, Henry A, Heatley E. Oxytocin infusion regimens for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009701] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 2006
- Crane J. Factors predicting labor induction success: a critical analysis. Clinical Obstetrics and Gynecology 2006;49(3):573‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Kenyon 2011
- Kenyon S, Tokumasu H, Dowswell T, Pledge D, Mori R. High dose versus low dose oxytocin for augmentation of delayed labour. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007201.pub2; CD007201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakta 2005
- Pakta JH, Johnston AK. High‐ versus low‐dose oxytocin for augmentation or induction of labour. Annals of Pharmacotherapy 2005;39:95‐101. [DOI] [PubMed] [Google Scholar]
Rang 2007
- Rang DM, Dale M, Ritter JM, Flower R. Chapter 30: The reproductive system. Rang and Dale's Pharmacology. 6th Edition. Churchill Livingstone/Elsevier, 2007. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Satin 1992
- Satin AJ, Leveno KJ, Sherman ML, Brewster DS, Cunningham FG. High‐ versus low‐dose oxytocin for labor stimulation. Obstetrics and Gynecology 1992;80(1):111‐6. [PubMed] [Google Scholar]
Seitchik 1982
- Seitchik J, Castillo M. Oxytocin augmentation of dysfunctional labor. I. Clinical data. American Journal of Obstetrics and Gynecology 1982;144(8):899‐905. [DOI] [PubMed] [Google Scholar]
Smith 2006
- Smith J, Merrill D. Oxytocin for Induction of Labor. Clinical Obstetrics and Gynaecology 2006;49:594‐608. [DOI] [PubMed] [Google Scholar]
Toaff 1978
- Toaff ME, Hezroni J, Toaff R. Induction of labour by pharmacological and physiological doses of intravenous oxytocin. British Journal of Obstetrics and Gynaecology 1978;85:101‐8. [DOI] [PubMed] [Google Scholar]
Wei 2010
- Wei SQ, Luo ZC, Qi HP, Xu H, Fraser W. High‐dose versus low‐dose oxytocin for labor augmentation: a systematic review. American Journal of Obstetrics and Gynecology 2010;203(4):296‐304. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO Recommendations for Induction of Labour. Geneva: WHO, 2011. [PubMed] [Google Scholar]
Xenakis 1995
- Xenakis EM, Langer O, Piper J, Conway D, Berkus MD. Low‐dose versus high‐dose oxytocin augmentation of labour ‐ a randomized trial. American Journal of Obstetrics and Gynecology 1995;173:1874‐8. [DOI] [PubMed] [Google Scholar]


