Abstract
Background
Resistance to colistin was an uncommon phenomenon traditionally linked to chromosome point mutations, but since the first description of a plasmid-mediated colistin-resistance in late 2015, transmissible resistance to colistin has become a Public Health concern. Despite colistin is considered as a human last resort antibiotic, it has been commonly used in swine industry to treat post-weaning diarrhoea in piglets. However, the progressively increase of colistin resistance during the last decade led to the Spanish Medicines and Healthcare Products Agency (AEMPS) to launch a strategic and voluntary plan aimed to reduce colistin consumption in pig production. Our longitudinal study (1998–2021) aimed to evaluate the trend of colistin resistance mediated through the mcr-1 mobile gene in Spanish food-producing pig population and compare it with published polymyxin sales data in veterinary medicine to assess their possible relationships.
Results
The first mcr-1 positive sample was observed in 2004, as all samples from 1998 and 2002 were mcr-1 PCR-negative. We observed a progressive increase of positive samples from 2004 to 2015, when mcr-1 detection reached its maximum peak (33/50; 66%). From 2017 (27/50; 54%) to 2021 (14/81; 17%) the trend became downward, reaching percentages significantly lower than the 2015 peak (p < 0.001). The abundance of mcr-1 gene in PCR-positive samples showed a similar trend reaching the highest levels in 2015 (median: 6.6 × 104 mcr-1 copies/mg of faeces), but decreased significantly from 2017 to 2019 (median 2.7 × 104, 1.2 × 103, 4.6 × 102 mcr-1 copies/mg of faeces for 2017, 2018 and 2019, respectively), and stabilizing in 2021 (1.6 × 102 mcr-1 copies/mg of faeces) with similar values than 2019.
Conclusions
Our study showed the decreasing trend of colistin resistance associated to mcr-1 gene, after a previous increase from among 2004–2015, since the European Medicines Agency and AEMPS strategies were applied in 2016 to reduce colistin use in animals, suggesting a connection between polymyxin use and colistin resistance. Thus, these plans could have been effective in mcr-1 reduction, reaching lower levels than those detected in samples collected 17 years ago, when resistance to colistin was not yet a major concern.
Keywords: Colistin, Antimicrobial resistance, Real-time PCR, Swine, Resistance genes
Background
Resistance to colistin, antibiotic belonging to polymyxins family, was traditionally linked to chromosome point mutations, but since the first description of a plasmid-mediated colistin-resistance mechanism mediated by a family of mcr genes on late 2015 [1], transmissible resistance to colistin has become a Public Health concern. Although up to ten members of this family have been described (mcr-1 to mcr-10) [2–10], mcr-1 is the most extended and frequently detected in many countries around the world [10–12], demonstrating it as an excellent indicator for monitoring colistin resistance [13].
The increase of human infections due to multidrug-resistant (MDR) Gram-negative bacteria, especially those producing extended-spectrum beta-lactamases (ESBL) and carbapenemases, coupled with the lack of the development of novel antimicrobials, led to the reintroduction of colistin by the mid-1990s to treat these human infections, as it was often the only effective antimicrobial against them [14]. Therefore, 3rd- and 4th-generation cephalosporins along with colistin, are considered as critically important for both human and animal health [15, 16] and they are recently classified into the “RESERVE” group in the World Health Organisation (WHO) AWaRe classification [17]. Nonetheless, colistin was extensively used to treat post-weaning diarrhoea (PWD) in piglets [18].
Thus, following the European Medicines Agency’s (EMA) advice about the use of colistin in animals, the Spanish Medicines and Healthcare Products Agency (AEMPS) launched in 2016 the “Reduce Porcino” plan, a strategic and voluntary plan aimed to reduce the colistin consumption to 5 mg/PC in three years in pig production and control the possible alternative use of neomycin and apramycin. This initiative was followed by the 80% of Spanish pig producers contributing to a decrease in colistin consumption of 97% (AEMPS, 12/2019) [19].
A potential relationship between colistin resistance and polymyxins use has been pointed out [20–22], which is consistent with the findings observed in our previous studies [23], so the screening of levels of colistin-resistant bacteria has become an important procedure to assess the effectiveness of the reduction of antimicrobial use over colistin resistance. However, there is a lack of studies that jointly analyse the evolution of both colistin resistance and polymyxins use. So, we aimed to monitoring resistance to colistin mediated by the mobile mcr-1 gene in the Spanish food-producing pig population and compare it with published data of polymyxins sales in veterinary medicine to assess their relationships.
Results
Detection of mcr-1 in pig samples
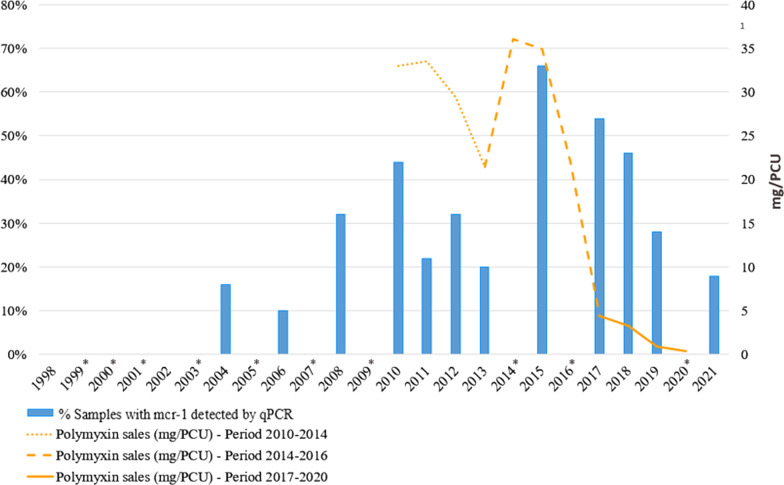
The first mcr-1 PCR-positive sample was observed in 2004 sampling, as all samples from 1998 and 2002 were mcr-1 PCR-negative (Fig. 1). The percentage of positive samples increased from 2004 (8/50; 16%, 95% CI = 7.2% to 29.1%) until 2010 (22/50; 44%, 95% CI = 30.0% to 58.8%), showing a statistically significant difference (p = 0.002) between both years. From 2010 to 2013, it was noticed an irregular progression characterized by a slight decrease in the number of positive samples (no statistically significant), followed by a significant increase in 2015 (p = 0.027) compared to data from 2010. So, we observed an increase of positive samples from 2004 until 2015, when mcr-1 PCR positive-samples reached its maximum value (33/50; 66%, 95% CI = 51.2% to 78.8%). Finally, from 2017 (27/50; 54%, 95% CI = 39.3% to 68.2%) to 2021 (9/50; 18%, 95% CI = 8.6% to 31.4%) the mcr-1 PCR positive samples decreased, reaching percentages significantly lower than the 2015 peak (p < 0.001) and similar to years prior to 2008 (16/50; 32%, 95% CI = 19.5% to 46.7%) (Fig. 1).
Fig. 1.
Evolution of the percentage of pig samples positive to mcr-1 gene and the polymyxin sales reported in Spain for veterinary medicine. Data on polymyxin sales were available from 2010 to 2020 (the latest year reported currently by ESVAC). These data was represented in three different periods with the same data collection system, as it was specified in the ESVAC report for spain. PCU: population correction unit. *No mcr-1 gene prevalence dta in the years 1999, 2000, 2001, 2003, 2005, 2007, 2009, 2014, 2016 and 2020
These data were compared with the Spanish polymyxin sales data published in the Eleventh European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Report [24] and a declining progression of both data series, especially since 2015, was noticed. Although the polymyxin sales showed a steeper drop than the mcr-1 positive samples percentages, both decreasing trends suggest that the reduction of polymyxins sales could have had a strong influence on the decrease of mcr-1 positive samples, among other factors (Fig. 1).
Abundance of mcr-1 in pig samples
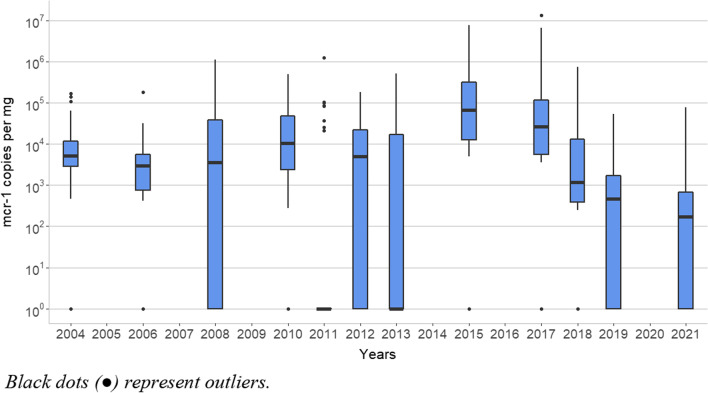
The abundance (copies/mg caecal content) of the mcr-1 gene in those years with PCR-positive samples showed a similar trend to the percentage of mcr-1 gene positive samples (Fig. 2). The median values from 2015 and 2017 were similar (6.6 × 104 and 2.7 × 104 mcr-1 copies/mg, respectively), but the median of mcr-1 gene quantification remarkably dropped in 2018 (1.2 × 103 mcr-1 copies/mg; p < 0.01). This decrease continued in 2019 (median 4.6 × 102 mcr-1 copies/mg) and it was stabilised in 2021 (median 1.6 × 102 mcr-1 copies/mg). Thus, both indicators, the number of mcr-1 positive samples and the amount of mcr-1 copies, showed a similar decrease trend since 2015.
Fig. 2.
Box-plot diagram of the yearly distribution of mcr-1 gene abundance (expressed in copies/mg faecal content) in Spanish food-producing pigs from positives caecal samples. Black dots represent outliers
Discussion
Colistin resistance mediated by mcr mobile genes is a great concern around the world [25]. There are some studies reporting changes in the mcr abundance by culture and molecular analysis of bacterial isolates [26, 27], but only few studies have focused on samples rather than strains, such as livestock manure [28], human and pets faeces [29, 30] or faeces from food-producing animals [29]. Similarly, few studies have assessed the evolution of the presence of mcr genes in a given animal, food or human related environment along a large period of time. Consequently, the results obtained in our study may provide a new perspective on the spread of the mcr-1 gene in the highly relevant bacterial context represented by the intestinal microbiota. However, since the effect of prolonged storage of fecal samples on the integrity of their DNA, which could be affected by chemical or enzymatic degradative processes, is unknown, its representativeness should be considered with caution, especially in the early stages of this study that reaches up to 23 years old.
We also compared the evolution of mcr-1 presence in faecal samples with polymyxin use in animal production, which makes this work useful to assess the current decisions about colistin restrictions recommended in the European Union (EU). However, the Spanish antimicrobial sales data collection system detected under-reporting for the period 2010 – 2013, so the data for these years are underestimates. In addition, datasets included veterinary medicinal products (VMPs) declared by marketing authorisation holders (MAHs) which were replaced by retailers as sales data providers for the period 2017–2020. Due to these differences, it is difficult to assess the trends for the periods 2010–2014, 2014–2016 and 2017–2020, and comparisons between them should be made with caution [31]. Thus, these periods were assessed separately.
In accordance with the aim of the AEMPS colistin reduction programmes launched to decrease the use of colistin in different food-producing animal species such as swine [32], polymyxin sales in Spain dropped down from 22.02 mg/PCU in 2016 to 4.4 mg/PCU in 2017, 3.3 mg/PCU in 2018, 0.9 mg/PCU in 2019 and 0.4 mg/PCU in 2020, also achieving the EMA’s main objective of reducing the antibiotic consume under 5 mg/PCU in a period of 3 to 4 years from 2016 [24, 33].
Interestingly, the first detection of mcr-1 in pigs in Spain (2004) was coincidental with the suppression of zinc oxide (ZnO) as an additive food complement for feeding piglets during 2003–2004. ZnO has been used traditionally as a food additive in piglets; however, there was an increasing concern regarding the potential risk for the environment derived from its use. Thus, the Committee for Medicinal Products for Veterinary Use (CVMP) of EMA considered that the benefits of using ZnO did not exceed the risks to the environment and consequently, this substance was banned in 2003 until the 15th November 2004, when ZnO was officially registered as a premix [34]. In addition, the Scientific Committee on Animal Nutrition (SCAN) of EU recommended, also in 2003, a reduction of ZnO levels in feeding-stuffs as they were higher than necessary to supply the physiological requirements of the animals [35]. During the ban, colistin emerged as an alternative to treat PWD in piglets and ZnO was mostly replaced by colistin. Interestingly, in the 2004–2010 period we observe an increase in the number of mcr-1-carrying samples which could be related with the widening use of this antibiotic.
The drop of mcr-1 positive samples for the period 2011–2013 compared with 2010 was coincident with a reduction in the piglets’ production in Spain, as it is represented in Fig. 1; however, the decrease of polymyxin sales could be more related with the changes in the antimicrobial sales data collection method above mentioned. In 2015, an increase of mcr-1 positive samples was observed with the highest proportion of samples of our study. According to the antimicrobial sales data compiled by ESVAC, Spain had in 2015 the highest figures of polymyxins sales in the EU (34.9 mg/PCU) [33]; however, after the Spanish voluntary strategic plan launched by the AEMPS, polymyxin sales remarkably decrease in the next years (4.4, 3.3, 0.9 and 0.4 mg/PCU from 2017, 2018, 2019 and 2020 respectively) [24].
From 2017 to 2021, the percentage of mcr-1-positive samples decreased in agreement with the reduction in the polymyxin sales, reaching levels of colistin resistance lower than those obtained in 2008. This phenomenon has been observed in others UE countries, such as Italy [26], Germany [36] among others, where implemented strategies to limit the use of colistin also produced a parallel decrease between colistin resistance and antibiotic consume (data expressed in mg/kg of estimated biomass) in animals, especially pigs and poultry showing a statistically significant association [22]. Similar results has been also reported in China [20].
Our retrospective longitudinal study suggest that colistin resistance associated to mcr-1 gene is related to polymyxins sales, as different studies [20, 26, 37] and authorities [22, 32, 38] have also pointed out. In this context, we observed a decrease in both colistin resistance and polymyxins sales in the 2017 to 2021 period, and the proportion of positive samples and the abundance of mcr-1 gene in positive samples reached levels lower than those obtained even in 2004. This context reinforces the food-producing industry against future changes in animal care legislations, such as the next ZnO in the EU ban planned for 2022 which was approved in 2017 [39] and for which pig industry will be better prepared than 2003, when ZnO was mostly replaced by colistin and led us to the current situation.
Colistin resistance due to mcr-1 gene showed a slower decrease than colistin sales probably due to the genetic environment where mcr-1 gene was encoded. The genome location of mcr-1 gene may play a determinant role in its dispersion and abundance [40–42]. The mcr-1 gene is usually encoded in plasmids, allowing the horizontal gene transference and thus a faster spread of the resistance gene to other bacteria which become resistant to colistin [1, 37]. Hypothetically, reduced use of colistin would cause a drop in the selective pressure on the intestinal bacterial population. This would allow colistin susceptible bacteria to survive and outcompete colistin resistant bacteria, due to the fitness cost associated with maintaining plasmid-encoded genes [42, 43]. This hypothesis seems to be in concordance with data from the joint report by European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA) and EMA (Jiacra III report) [22]. However, there are evidences of the co-selection of mcr genes in a free colistin selective pressure environment in plasmid containing mcr genes and resistance genes against other antimicrobial families such as ESBL (blaCTX-M and blaSHV), cml or sul (chloramphenicol and sulfamethoxazole resistance genes respectively) [44–47]. Another less frequent factor that could allow the persistence of colistin resistance in the host strain is the presence of mcr-1 gene in the bacterium chromosome, usually flanked by insert sequences [40, 48]. Thus, the location of colistin resistance genes may be determinant in the fight against colistin resistance, which is an open window for further studies.
Conclusions
Our results showed a connection between polymyxin sales and colistin resistance in Spanish pigs for food-production in a range of nine years, and a decreasing trend of colistin resistance (mcr-1 gene) since the EMA and AEMPS strategies were applied in 2016 to reduce colistin use in animals. Thus, our data suggest to the reduction plans would have had an important effect in the fight against colistin resistance (mcr-1 gene), since its presence in pigs have returned to slightly lower levels than those detected in samples collected 17 years ago, when resistance to colistin was not yet a major concern.
Methods
Samples
The collection of caecal samples from healthy pigs taken at slaughterhouse level under the National antimicrobial resistance surveillance program (1998 to 2021), driven by the Spanish Ministry of Agriculture, Fisheries and Food (except 2018) were used for this study. Sampling from 2018 was covered by the VISAVET Health Surveillance Centre, under the same conditions as the National antimicrobial surveillance programs to maintain the homogeneity of the sampling.
We have combined a retrospective study, based on frozen (ten-fold diluted in sterile water and glycerol prior to freezing at − 40 °C) samples collected from 1998 to 2017, with a prospective study done with fresh samples taken in 2018, 2019 and 2021.
We randomly selected 50 samples per year (1998, 2002, 2004, 2006, 2008, 2010, 2011, 2012, 2013, 2015, 2017, 2018, 2019 and 2021) accomplishing a total number of 700 samples.
Quantitative assay for mcr-1
Before processing, frozen samples were thawed at room temperature. From this step, all samples were processed in the same way. Direct DNA extraction was carried out using a commercial kit (FASTI001-1 FavorPrep Stool DNA Isolation Mini Kit, Favorgen-Europe, Vienna) following the manufacturer’s specifications (elution volume of 200 µL), coupled to a specific SYBRGreen real-time PCR assay for quantitative detection of the mcr-1 gene (qPCR), described previously [49] and further validated in our lab [13]. Samples were considered positive if their quantitative DNA values were > 1.00 × 102 fg/µL, equivalent to 1.58 × 103 mcr-1 copies/mg caecal content.
Statistical analysis
The data obtained by qPCR was analysed using a T-test after normalisation by logarithmic transformation into Log10, and Chi-square test was used to compare the frequencies of mcr-1 positive samples observed each year. A difference was considered significant when the p was < 0.05.
Acknowledgements
The authors wish to thank the technicians María García, Estefanía Rivero, Nisrin Maasoumi, and Claudia Renata Illas for their excellent technical assistance at the Foodborne Zoonoses and Antibiotic Resistance Unit and Red de Investigación en Sanidad Animal (RISA).
Abbreviations
- AEMPS
Spanish Medicines and Healthcare Products Agency
- CI
Confidence Interval
- ECDC
European Centre for Disease Prevention and Control
- EFSA
European Food Safety Authority
- EMA
European Medicines Agency
- ESBL
Extended-spectrum beta-lactamases
- ESVAC
European Surveillance of Veterinary Antimicrobial Consumption
- EU
European Union
- MAHs
Marketing Authorisation Holders
- MDR
Multidrug-resistant
- PCR
Polymerase Chain Reaction
- PCU
Population Correction Unit
- PWD
Post-weaning diarrhoea
- SCAN
Scientific Committee on Animal Nutrition
- VMPs
Veterinary Medicinal Products
- WHO
World Health Organisation
- ZnO
Zinc oxide
Authors' contributions
Conceptualization, PM-V, MM, LD, and MU-R; methodology, PM-V, TS, MH, and DR-L; software, PM-V, MH, DR-L, and AQ; validation, MH, DR-L, and AQ; formal analysis, PM-V, MH, MM, DR-L, AG, MA, CD-F, JS, AQ, LD, and MU-R; investigation PM-V, MM, LD, and MU-R; resources, PM-V, MM, LD, and MU-R; data curation, PM-V, MM, LD, and MU-R; writing—original draft preparation, PM-V, MM, LD, and MU-R; writing—review and editing, PM-V, MH, MM, DR-L, AG, TS, MA, CD-F, JS, AQ, LD, and MU-R; visualization, PM-V, MH, MM, DR-L, AG, TS, MA, CD-F, JS, AQ,, LD, and MU-R; supervision, PM-V, MH, MM, DR-L, AG, TS, MA, CD-F, JS, AQ, LD, and MU-R; project administration, PM-V, MM, LD, and MU-R; funding acquisition, PM-V, MM, LD, and MU-R All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Grants AGL2016-74882-C3 and PID2020-118405RB-I00 funded by MCIN/AEI/10.13039/501100011033, the Spanish Ministry of Agriculture, Fishing, and Food, and the Autonomous Community of Madrid (S2013/ABI-2747). Pedro Miguela-Villoldo was supported by the FPI Program (BES-2017-080264) from the Spanish Ministry of Science, Innovation, and Universities. Work in the laboratory of Alberto Quesada is also supported by Grants IB20181 and CTS059 funded by the “Consejería de Economía, Ciencia y Agenda Digital, Junta de Extremadura and FEDER”.
Availability of data and materials
All datasets used in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27):30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 3.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 2017;8(3):e00543–e617. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22(31):30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72(12):3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 6.AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother. 2017;72(10):2745–2749. doi: 10.1093/jac/dkx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(7):1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype typhimurium isolate. MBio. 2019;10(3):e00853–e919. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect. 2020;9(1):508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skov RL, Monnet DL. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Eurosurveillance. 2016;21(9):30155. doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 12.García V, García-Meniño I, Mora A, Flament-Simon SC, Díaz-Jiménez D, Blanco JE, et al. Co-occurrence of mcr-1, mcr-4 and mcr-5 genes in multidrug-resistant ST10 Enterotoxigenic and Shiga toxin-producing Escherichia coli in Spain (2006–2017) Int J Antimicrob Agents. 2018;52(1):104–108. doi: 10.1016/j.ijantimicag.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Miguela-Villoldo P, Moreno MA, Hernández M, Rodríguez-Lázaro D, Gallardo A, Borge C, et al. Complementarity of selective culture and qPCR for colistin resistance screening in fresh and frozen pig cecum samples. Front Microbiol. 2020;11:2793. doi: 10.3389/fmicb.2020.572712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaye KS, Pogue JM, Tran TB, Nation RL, Li J. Agents of last resort: polymyxin resistance. Infect Dis Clin North Am. 2016;30(2):391–414. doi: 10.1016/j.idc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 15.OIE List of Antimicrobial Agents of Veterinary Importance. World Organisation for animal health. 2019. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_OIE_List_antimicrobials_July2019.pdf.
- 16.Categorisation of antibiotics in the European Union. EMA/CVMP/CHMP/682198/2017: European Medicines Agency (EMA). 2019. https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf.
- 17.World Health Organization. The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. World Health Organization; 2019. Contract No.: WHO/EMP/IAU/2019.11.
- 18.Kempf I, Fleury MA, Drider D, Bruneau M, Sanders P, Chauvin C, et al. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int J Antimicrob Agents. 2013;42(5):379–383. doi: 10.1016/j.ijantimicag.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 19.España reduce un 7,2% el consumo de antibióticos en salud humana y un 32,4% las ventas de antibióticos veterinarios Spanish Medicines and Health Care Products Agency (AEMPS); 12 de julio de 2019 [cited 2021]. https://www.aemps.gob.es/informa/notasInformativas/laAEMPS/2019/docs/NI-AEMPS-12-2019-consumo-total-antibioticos.pdf?x63731.
- 20.Wang Y, Xu C, Zhang R, Chen Y, Shen Y, Hu F, et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: an epidemiological comparative study. Lancet Infect Dis. 2020;20(10):1161–1171. doi: 10.1016/S1473-3099(20)30149-3. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. EMA/231573/2016 2016 [updated July 27, 2016]. https://www.ema.europa.eu/documents/scientific-guideline/updated-advice-use-colistin-products-animals-within-european-union-development-resistance-possible_en-0.pdf.
- 22.Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA, JIACRA III. 2016–2018. ECDC, EFSA, EMA: European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA) and European Medicines Agency (EMA). 2021. https://www.ecdc.europa.eu/en/publications-data/third-joint-interagency-antimicrobial-consumption-and-resistance-analysis-report#no-link. [DOI] [PMC free article] [PubMed]
- 23.Miguela-Villoldo P, Hernández M, Moreno MA, Rodríguez-Lázaro D, Quesada A, Domínguez L, et al. National colistin sales versus colistin resistance in Spanish pig production. Res Vet Sci. 2019;123:141–143. doi: 10.1016/j.rvsc.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Eropean Medicines Agency. Sales of veterinary antimicrobial agents in 31 European countries in 2019 and 2020. Trends from 2010 to 2020. Eleventh ESVAC report. 2021. EMA/58183/2021. Veterinary Medicines Division. https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf#page=97&zoom=100,0,0.
- 25.Yacouba A, Olowo-Okere A. Global trends and current status in colistin resistance research: a bibliometric analysis (1973–2019) F1000Research. 2020;9:856. doi: 10.12688/f1000research.25124.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagliotti C, Bolzoni L, Carretto E, Sarti M, Ricchizzi E, Ambretti S, et al. Reduction trend of mcr-1 circulation in Emilia-Romagna Region, Italy. Eur J Clin Microbiol Infect Dis. 2021;40:2585–2592. doi: 10.1007/s10096-021-04318-y. [DOI] [PubMed] [Google Scholar]
- 27.Makita K, Fujimoto Y, Sugahara N, Miyama T, Usui M, Asai T, et al. Quantitative release assessment of mcr-mediated colistin-resistant Escherichia coli from Japanese pigs. Food Saf. 2020;8(2):13–33. doi: 10.14252/foodsafetyfscj.D-20-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Lu C, Shen D, Liu J, Ma Z, Yang B, et al. Elimination of the risks of colistin resistance gene (mcr-1) in livestock manure during composting. Environ Int. 2019;126:61–68. doi: 10.1016/j.envint.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zhao X, Che J, Xiong Y, Xu Y, Zhang L, et al. Detection and dissemination of the colistin resistance gene, mcr-1, from isolates and faecal samples in China. J Med Microbiol. 2017;66(2):119–125. doi: 10.1099/jmm.0.000425. [DOI] [PubMed] [Google Scholar]
- 30.Terveer EM, Nijhuis RHT, Crobach MJT, Knetsch CW, Veldkamp KE, Gooskens J, et al. Prevalence of colistin resistance gene (mcr-1) containing Enterobacteriaceae in feces of patients attending a tertiary care hospital and detection of a mcr-1 containing, colistin susceptible E. coli. PLoS ONE. 2017;12(6):e0178598. doi: 10.1371/journal.pone.0178598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency. Spain trends sales antimicrobial VMPs food producing animals between 2010–2020. 2021. EMA/510455/2021. Veterinary Medicines Division. https://www.ema.europa.eu/en/documents/report/spain-trends-sales-antimicrobial-vmps-food-producing-animals-between-2010-2020_en.pdf.
- 32.Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Informe JIACRA España. Primer análisis integrado del consumo de antibióticos y su relación con la aparición de resistencia. 2018. https://www.resistenciaantibioticos.es/es/system/files/field/files/informe_jiacra-espana.pdf?file=1&type=node&id=410&force=0.
- 33.European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Interactive ESVAC database: European Medicines Agency (EMA). https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac#interactive-esvac-database-section.
- 34.Publicly available assessment report for a veterinary medicinal product: Oxido de Zinc Calier. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): ES/V0138/001/MR. https://cimavet.aemps.es/cimavet/pdfs/es/ipe/1599+ESP/IPE_1599+ESP.pdf.
- 35.Commission Regulation (EC) No 1334/2003 of 25 July 2003 amending the conditions for authorisation of a number of additives in feedingstuffs belonging to the group of trace elements. Off J Eur Union L187. 2003;46:0011–5.
- 36.Irrgang A, Roschanski N, Tenhagen B-A, Grobbel M, Skladnikiewicz-Ziemer T, Thomas K, et al. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010–2015. PLoS ONE. 2016;11(7):e0159863-e. doi: 10.1371/journal.pone.0159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Zeng X, Li X-P, Liao X-P, Liu Y-H, Lin J. Plasmid-mediated colistin resistance in animals: current status and future directions. Anim Health Res Rev. 2017;18(2):136–152. doi: 10.1017/S1466252317000111. [DOI] [PubMed] [Google Scholar]
- 38.Authority EFS, Prevention ECfD, Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17(2):e05598. [DOI] [PMC free article] [PubMed]
- 39.European Medicines Agency. Questions and answers on veterinary medicinal products containing zinc oxide to be administered orally to food-producing species. EMA/394961/2017 2017 [updated 26 June 2017]. https://www.ema.europa.eu/en/documents/referral/zinc-oxide-article-35-referral-questions-answers-veterinary-medicinal-products-containing-zinc-oxide_en.pdf.
- 40.Wang Z, Fu Y, Schwarz S, Yin W, Walsh TR, Zhou Y, et al. Genetic environment of colistin resistance genes mcr-1 and mcr-3 in Escherichia coli from one pig farm in China. Vet Microbiol. 2019;230:56–61. doi: 10.1016/j.vetmic.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Migura-Garcia L, Gonzalez-Lopez JJ, Martinez-Urtaza J, Aguirre Sanchez JR, Moreno-Mingorance A, Perezde Rozas A, et al. mcr-colistin resistance genes mobilized by Inc X4, IncHI2, and IncI2 plasmids in Escherichia coli of pigs and white stork in Spain. Front Microbiol. 2019;10:3072. doi: 10.3389/fmicb.2019.03072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang QE, MacLean C, Papkou A, Pritchard M, Powell L, Thomas D, et al. Compensatory mutations modulate the competitiveness and dynamics of plasmid-mediated colistin resistance in Escherichia coli clones. ISME J. 2020;14(3):861–865. doi: 10.1038/s41396-019-0578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nang SC, Morris FC, McDonald MJ, Han ML, Wang J, Strugnell RA, et al. Fitness cost of mcr-1-mediated polymyxin resistance in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(6):1604–1610. doi: 10.1093/jac/dky061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y-Q, Zhang A-Y, Ma S-Z, Kong L-H, Li Y-X, Liu J-X, et al. Co-occurrence of mcr-1 and ESBL on a single plasmid in Salmonella enterica. J Antimicrob Chemother. 2016;71(8):2336–2338. doi: 10.1093/jac/dkw243. [DOI] [PubMed] [Google Scholar]
- 45.Shafiq M, Huang J, Shah JM, Ali I, Rahman SU, Wang L. Characterization and resistant determinants linked to mobile elements of ESBL-producing and mcr-1-positive Escherichia coli recovered from the chicken origin. Microb Pathog. 2021;150:104722. doi: 10.1016/j.micpath.2020.104722. [DOI] [PubMed] [Google Scholar]
- 46.Trujillo-Soto T, Machuca J, Arca-Suárez J, Rodríguez-Iglesias M, Galán-Sánchez F. Co-occurrence of mcr-1 and qnrS1 on an IncHI2 plasmid in clinical isolates of Salmonella typhimurium in Spain. Vector-Borne Zoonotic Dis. 2019;19(9):662–665. doi: 10.1089/vbz.2018.2398. [DOI] [PubMed] [Google Scholar]
- 47.Chiou C-S, Chen Y-T, Wang Y-W, Liu Y-Y, Kuo H-C, Tu Y-H, et al. Dissemination of mcr-1-carrying plasmids among colistin-resistant Salmonella strains from humans and food-producing animals in Taiwan. Antimicrob Agents Chemother. 2017;61(7):e00338–e417. doi: 10.1128/AAC.00338-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Li XP, Fang LX, Sun RY, He YZ, Lin J, et al. Co-occurrence of mcr-1 in the chromosome and on an IncHI2 plasmid: persistence of colistin resistance in Escherichia coli. Int J Antimicrob Agents. 2018;51(6):842–847. doi: 10.1016/j.ijantimicag.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Shi X, Yin W, Wang Y, Shen Z, Ding S, et al. A multiplex SYBR green real-time PCR Assay for the detection of three colistin resistance genes from cultured bacteria, feces, and environment samples. Front Microbiol. 2017;8:2078. doi: 10.3389/fmicb.2017.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used in this study are available from the corresponding author on reasonable request.