Abstract
Background
Pediculosis capitis is a common human parasitic infestation in childhood. This article aims to provide a narrative updated review on the management of pediculosis capitis.
Methods
A PubMed search was performed with Clinical Queries using the key terms “pediculosis capitis” OR “head lice” OR “head louse”. The search strategy included clinical trials, meta-analyses, randomized controlled trials, observational studies and reviews published within the past 10 years. The search was restricted to articles published in English literature. The information retrieved from the search was used in the compilation of the present article.
Results
Topical permethrin and pyrethrin formulated with piperonyl butoxide are the pediculicides of choice in areas where resistance to these products is low. When resistance to these products is suspected based on local levels of resistance or when treatment with these products fails despite their correct use, and reinfestation does not seem to be responsible, other topical treatment options include malathion, benzyl alcohol, dimethicone, spinosad and ivermectin. Wet combing should be considered for children younger than 2 years. Oral ivermectin and trimethoprim/sulfamethoxazole should be reserved for patients who do not respond to appropriate topical pediculicides.
Conclusion
Many topical pediculicides are effective for the treatment of pediculosis capitis. The use of some of these pediculicides is limited for safety reasons, especially in children younger than 2 years. Resistance to pediculicides, especially those with a neurotoxic mode of action, is another concern which may limit the use of some of these pediculicides. New products should be evaluated for effectiveness and safety. Wet combing is time-consuming and should not be used as the sole intervention in the general population.
Keywords: dimethicone, head lice, pediculicides, pediculosis capitis, permethrin, pyrethrin, wet combing
Introduction
Pediculosis capitis, an ectoparasitic infestation of the hair and scalp, is a worldwide public health concern particularly in the paediatric age group. The condition is caused by Pediculus humanus var. capitis (the human head louse), the only host of which is humans.1,2 In spite of the fact that P. humanus var. capitis does not transmit any disease, pediculosis capitis may cause scalp irritation, parental anxiety, peer criticism, bullying, social embarrassment, isolation and unnecessary absenteeism from school and work.3–7
A PubMed search was performed in September 2021 with Clinical Queries using the key terms “pediculosis capitis” OR “head lice” OR “head louse”. The search strategy included clinical trials, meta-analyses, randomized controlled trials, observational studies and reviews published within the past 10 years. The search was restricted to articles published in English literature. The information retrieved from the search was used in the compilation of the present article.
Aetiologic agent
Head lice are obligate ectoparasites that live on the human scalp for food, warmth, moisture and shelter.8,9 Generally, head lice feed every 3–6 hours by sucking blood from the scalp.8,10 Usually, a minute amount of saliva from the louse with anticoagulant and vasodilatory properties is inoculated into the scalp with each blood meal, thereby facilitating the sucking of the host’s blood.2,11,12 Without blood meals, head lice can normally survive for up to 55 hours away from the human host,13,14 although they may live for up to 4 days in favourable conditions.1 Lice eggs, on the other hand, can survive for up to 10 days off the human body.3,10
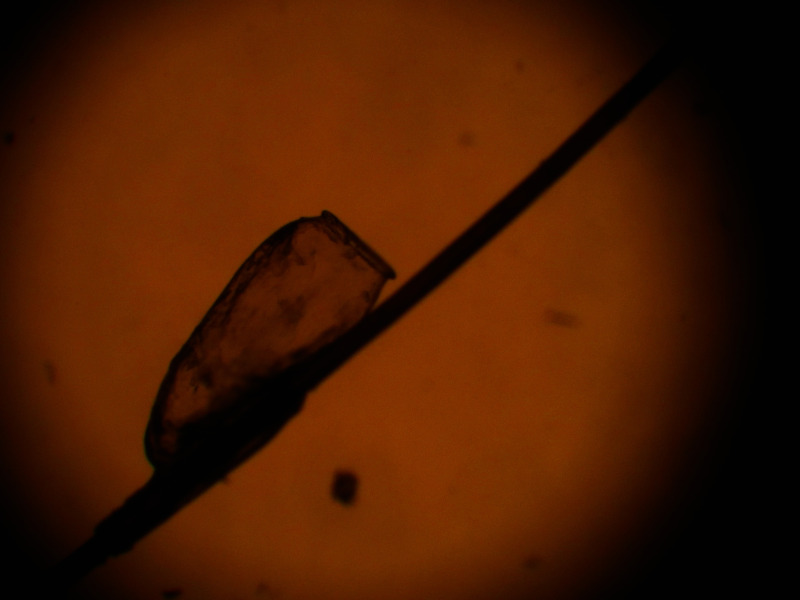
The adult louse is 2–3 mm in length, elongated, flattened dorsoventrally and without wings (Figure 1).10,12 A female louse is usually larger than a male louse.2 The colour of the louse is tan to grey before feeding and reddish-brown to black after feeding.8,15,16 The louse is camouflaged with pigment to match the hair colour of the infested individual and appears darker in an individual with dark hair.2 The shape of the head is angular ovoid and has two lateral eyes,8 each consisting of a single ocellus, two short five-segmented antennae (one on each side), and anterior piercing mouthparts that have long needle-like stylets adapted to blood sucking.3,14 The long needle-like stylets are retracted into the head of the louse in between feeding. The thorax is highly sclerotized and has three segments. A pair of jointed legs protrude from each thoracic segment.8 The legs end in hooklike claws which are adapted to hold onto almost all human head hairs, especially curly ones.9,14 The abdomen is oval shaped with seven segments. The male louse has dark brown bands on the back.2 Head lice can climb and crawl (at a speed of up to 23 cm/minute) but cannot hop or fly.10,17 They live close to the surface of the scalp.10 Head lice are photophobic and prefer darker areas.2,18 This might be the reason why pediculosis capitis is more common in females as they tend to have longer hair. It has been shown that head lice can recognize and prefer the odour of the head over the odour of other parts of the human body.19
Figure 1.
Microscopic view of a head louse.
Adult lice mate once.20 The male louse usually dies soon after copulation.2 An adult female louse lays eggs 1–2 days after mating and lays 7–10 eggs a day for up to 30 days till she dies.2,16,21,22 Eggs are glued to the hair in egg casings with chitin produced by the louse’s reproductive organ and are deposited close to the base of the hair shaft immediately adjacent to the scalp (Figure 2).8 Viable eggs are typically located within 6 mm of the scalp. Eggs are oval, 1 mm long and 0.3 mm wide.8,21 The operculum, a cap through which the embryo respires, always faces away from the scalp.2 Newly laid or viable intact eggs are opalescent or yellow, and, later on, tan to brown in colour.23,24 The moisture and warmth of the scalp may play an essential role in the incubation of the eggs, which typically cannot hatch at temperatures lower than those near the scalp.17,25,26 The egg hatches into a nymph in 6–10 days, depending on the temperature and moisture of the scalp.10,13 The hatch-ready nymph cuts a hole in the operculum using its mouthparts and sucks in air, which is expelled posteriorly, allowing rapid expulsion of the nymph from the eggshell.27 Eggshells become white, opaque, or dull yellow and are empty after hatching (Figure 3).14,17,24 They are usually found on growing hairs greater than one cm away from the scalp.14,17,24 The nymph feeds on human blood when available as soon as it is hatched and will die within a few hours if human blood is not available.8,28 The nymph looks like an adult head louse except that it is smaller in size. The newly hatched nymph then passes through a total of three nymph (instars) stages in 9–15 days to become an adult head louse.10,22 First-stage and second-stage nymphs are relatively immotile,2 whereas third-stage nymphs can move albeit significantly more slowly than a full-grown louse.10
Figure 2.
Microscopic view of a head louse egg attached to a strand of hair.
Figure 3.
Microscopic view of an empty louse egg case attached to a strand of hair after hatching.
Epidemiology
Pediculosis capitis is endemic both in developed and developing countries.13 The prevalence of pediculosis capitis varies greatly from country to country and even within a country.29 In general, pediculosis capitis is very common in tropical countries and areas of high humidity.20,30,31 Pediculosis capitis occurs most commonly in children attending daycare, kindergarten and elementary school.13 The peak incidence is between 3 and 12 years of age.2,22,32 Approximately 2–13% of elementary school children are affected.22,33,34 The wide variation in reported incidence rates may be attributed to the diagnostic methods used, which vary in sensitivity, geographic region where the study was conducted, seasonal variation, and age and sex of the studied population.17 The female to male ratio is approximately 3:1.2,31,35 Worldwide, it is estimated that 6–12 million individuals are affected annually.36 As pediculosis capitis is not a reportable disease, the condition may be underestimated. In the United States, Black children are less frequently affected than White children.13,14 Direct head-to-head (hair-to-hair) contact with an affected person is the most common route of transmission.22,37 Transmission by fomites or inanimate objects (e.g. headgears, combs, hairbrushes, hats, pillowcases) also occurs.14,18,37,38 Head lice do not use pets as vectors.14
Some studies have shown that pediculosis capitis is more frequent in resource-poor communities in developing countries.39–41 Poverty, overcrowding, family members with head lice, and lack of access to treatment have been identified as risk factors.35,36,39–42 Whether long hair is a risk factor is controversial as studies have yielded conflicting results.13,43 The infestation is not significantly influenced by the frequency of shampooing or brushing.7,13
Clinical presentations
Suffice to say, some affected children are without any symptoms. Itching of the scalp is the most common and often the sole symptom of pediculosis capitis.1,13 Itching is often the result of sensitization to either faecal or salivary antigens of the louse.44 In a patient without prior infestation, it may take 2–6 weeks to develop the symptom but it usually takes less than 2 days for the itching to occur with re-exposure.30,45 Some children may become irritable and have difficulty sleeping. Severe itching may result in excoriations, crusting, and secondary bacterial infection with Staphylococcus aureus or streptococci (e.g. cellulitis, impetigo, pyoderma) as a result of scratching.25 Posterior cervical and occipital lymphadenopathy can often be found.8,46,47 Malaise and fever may occur with florid pediculosis capitis.48
Clinically, lice eggs may be seen attached to the hair close to the scalp and live lice may be seen on the scalp or hair (Figure 4).13 Lice eggs are more commonly found at the postauricular and occipital areas.2 In Central Europe, there are usually less than 10 lice per head of an affected individual whereas, in Australia, the average number of lice per head of an affected individual is 30.5,7,20 Dark specks may be seen on the scalp representing lice faeces.
Figure 4.
Numerous lice eggs on the hair shafts of an 8-year-old girl with pediculosis capitis.
Patchy hair loss has rarely been described as a manifestation of pediculosis capitis.49 Pediculid, an id reaction secondary to pediculosis capitis, manifesting as pruritic, erythematous, maculopapules or papulovesicles distant from the primary inflammatory focus has been reported.47,48 Rarely, plica polonica characterized by diffuse matting of scalp hair with irreversibly entangled plaits stuck together with dirt and exudates resembling a ‘bird’s nest’ may be observed with florid pediculosis infestation.48,50
Diagnosis and diagnostic evaluation
The gold standard for diagnosing pediculosis capitis is detection of a live louse or nymph on the scalp or viable egg on the scalp hair.8 Diagnosis of pediculosis capitis using a fine-toothed lice comb spaced 0.2–0.3 mm apart is twice as fast and four times more efficient than direct visual examination.8,51,52 In this regard, metal nit combs have a better yield than plastic ones.53 Additionally, wet combing has a sensitivity of 91% for detection of live lice and is more sensitive than dry combing or visual inspection.54 Generally, eggs are more easily found than adult lice and nymphs.14 As eggs are laid on the hair shaft close to the scalp and hair grows at a rate of approximately 0.4 mm per day, the time that the eggs were laid can be gauged by the distance of the nits from the scalp. Because eggs usually hatch in approximately 8 days, nits (empty eggshells) that are within 6 mm from the scalp are usually viable and are highly suggestive of an active infestation.55 A viable egg will ‘pop’ when squashed between fingers.55 Nits that are more than 1 cm from the scalp are usually non-viable.6,7,56 As nits can persist after successful treatment of the infestation, their presence does not indicate a current infestation.6,7,56
Because head lice can crawl quickly and tend to stay away from light, they are difficult to find.6,7,57 Dermoscopic examination of the scalp hair showing the lice eggs increases the accuracy of diagnosis.57,58 In addition, dermoscopy allows a reliable differentiation between eggs containing nymphs and empty egg casings as well as from pseudo-nits (see below).57
Differential diagnosis
The differential diagnosis includes plugs of desquamated epithelial cells, dandruff, hair casts (thin, elongated, white to yellow cylindrical sheaths that encircle the hair shaft), external hair debris, lint, dirt particles, sand, scabs, and droplets or dried concretion of hair spray.5–7,16 These items can be made to slide up and down the hair shaft or easily removed from the hair.2 On the other hand, nits are firmly adherent to the hair shafts and are not easily removed from the hair shafts.3,59 If necessary, the diagnosis can be confirmed by using a light microscope or hand lens.13 Head lice should also be differentiated from piedra or other insects such as book lice, mites, bed bugs and aphids.4,29
Complications
Itching of the scalp may lead to insomnia, impaired concentration at school or work, and excessive scratching.60,61 The latter can be complicated with secondary bacterial infection which may lead to occipital or cervical lymphadenopathy.62 Iron deficiency anaemia may result with chronic and heavy pediculosis infestation through chronic blood loss.62–64 Head lice infestation may result in social stigma, psychological distress, low self-esteem, and sometimes unnecessary absence from school and work.20,65,66 Pediculosis capitis absorbs important resources from public health services. The economic burden associated with the treatment of pediculosis capitis can be considerable.67
Management
Treatment of pediculosis capitis is recommended for all individuals with an active infestation.56 Topical pediculicides are the most effective and first-line treatment for pediculosis capitis.6,7 Mechanical removal of the lice is a therapeutic option if the parents prefer not to use a pediculicide on their child and in children younger than 2 years of age. Oral therapy is occasionally required for the treatment of recalcitrant pediculosis capitis.68
Topical pediculicides
Many topical pediculicides are available for the treatment of pediculosis capitis. The choice of topical pediculicide depends on a number of factors, including accessibility, cost, efficacy, safety, ease of use, local pattern of resistance (if known), and age of the patient.1 Generally, pediculicides are not recommended for children under 2 years of age.5,22 Ideally, the hair should be washed with a shampoo and no hair conditioner should be used prior to application of a pediculicide.12 This is because hair conditioner may coat the hair and protect the nits and lice, which may reduce the effectiveness of treatment.14,26 Pediculicides with long residual effects are more likely to be ovicidal. The pediculicide should be applied to the hair and entire scalp. Treatment failures often are the result of improper application of pediculicides, non-compliance, reinfestation and, increasingly, prevalence resistance to the pediculicide.
Permethrin
Permethrin is a synthetic pyrethroid (a derivative of pyrethrin) with less systemic absorption and greater potency than its parent compound.69 It is less allergenic than natural pyrethrins and does not cause an allergic reaction in patients with plant allergies.7 Permethrin is a neurotoxin; the compound acts by blocking sodium channel repolarization of the neuron of the head lice through cell membrane channels with resultant respiratory paralysis and eventual death of the head lice.1,37 Currently, 1% permethrin cream rinse/lotion is the treatment of choice for pediculosis capitis because of efficacy and lack of toxicity.5,70 The 1% permethrin cream rinse/lotion is applied to the scalp and damp hair that is first shampooed and left on for approximately 10 minutes and then rinsed off with warm rather than hot water over a sink to limit skin exposure and to minimize skin absorption of the pediculicide. Permethrin is both pediculicidal and ovicidal. It leaves a residue on the hair and remains active for approximately 14 days after application as long as hair conditioners or silicone-based shampoos are not used.13,69 Nevertheless, a second treatment 9–10 days later is advised by most experts.13,69 Resistance to permethrin is increasing and is a growing concern worldwide.71–74 Adverse events are uncommon and include pruritus, oedema and erythema at the site of application.4,75 Generally, permethrin has a favourable safety profile with extremely low toxicity making it the pediculicide of choice in areas where resistance to permethrin is low.
Pyrethrin plus piperonyl butoxide
Pyrethrins, natural extracts from chrysanthemum flowers, are neurotoxic to lice but safe for humans.5 As pyrethrins are easily inactivated and unstable, they are often combined with piperonyl butoxide 4% to increase its stability, potentiate the pediculicidal effects and decrease the development of resistance.27,37 Percutaneous absorption of pyrethrin formulated with piperonyl butoxide is minimal, and toxicity in human beings is extremely low.37 Shampoo or foam mousse formulation containing pyrethrin synergized with piperonyl butoxide should be applied to the scalp and dry hair, left on for approximately 10 minutes, and then rinsed off with water.5,13 Because pyrethrin and piperonyl butoxide are not completely ovicidal and no residual pediculicidal activity remains after rinsing, repeat application of the product should be performed 9–10 days later to kill lice and nymphs that are newly hatched.5,13 Pyrethrin-based products should be avoided in patients allergic to chrysanthemums or ragweed.13,14 Occasionally, pyrethrin-based shampoo or foam mousse products may cause a rash and itchy or mild burning sensation on the scalp.14 The pruritus should not be regarded as a symptom of treatment failure or reinfestation. Resistance to pyrethrin formulated with piperonyl butoxide has been increasingly reported and varies geographically.14
Malathion
Malathion, an organophosphate cholinesterase inhibitor, binds irreversibly to acetylcholinesterase resulting in the accumulation of acetylcholine at the receptor site.5,14 In head lice, the excessive accumulation of acetylcholine will result in spastic paralysis and respiratory paralysis followed by the death of the lice.5,14,73 Malathion binds to the sulfur atoms of the hair, which accounts for its residual effect,5 thus being pediculicidal and partially ovicidal.5,13 Malathion is available as a 0.5% lotion, which is applied to the scalp and dry hair, left to dry naturally (without the use of hair dryer, flat iron or curling iron), and then rinsed off after 8–12 hours with a non-medicated shampoo.5–7,13 A single application is sufficient for the majority of patients.5 The lotion should, however, be repeated in approximately 8 days if live lice are still present.13,42 Generally, malathion lotion is regarded as safe with minimal risk of systemic toxicity.27 As the formulation contains 78% isopropyl alcohol, it is highly flammable. Additionally, the formulation may lead to respiratory depression if ingested.1,26 Patients should be warned that the malathion-coated hair should not be exposed to open flames, lighted cigarettes or electric heat sources (e.g. hair dryer or curling iron).13 Other adverse events include stinging of skin and eyes.26 Therefore, malathion should be reserved for cases resistant to treatment with permethrin and pyrethrin.76 Resistance to malathion is also a growing concern globally.72,73,77 The use of malathion is contraindicated in children younger than the age of 2 years.14 The safety and effectiveness of malathion in children aged 2–6 years have not been established.14 In this regard, Hamad et al. reported a case of organophosphate toxicity in a 7-year-old girl following topical application of an organophosphate pediculicide for the treatment of pediculosis capitis.78 In addition, malathion is malodorous.
Spinosad
Spinosad, a fermentation product of the soil-dwelling actinomycete Saccharopolyspora spinosa, is a natural mixture of tetracyclic macrolides spinosyn A and spinosyn D in a ratio of 5:1.13,35,79,80 Spinosyn A and spinosyn D interfere with the nicotinic acetylcholine receptors and the γ-aminobutyric acid gated chloride ion channels in head lice, thereby provoking neuronal hyperexcitation and involuntary muscle contraction, leading to neuromuscular fatigue and paralysis,79–81 which prevents the head lice from feeding with resultant death of the head lice.28 Spinosad (0.9%) suspension contains 10% benzyl alcohol as a vehicle and is both pediculicidal and ovicidal.4,28 The suspension should be applied to cover the whole scalp and dry hair, left for 10 minutes, and then thoroughly rinsed with warm water to completely remove the suspension.14 Retreatment is only necessary if live lice are still seen after 7 days of treatment.13,14 Spinosad (0.9%) suspension should not be used in children younger than 6 months of age or mothers who are breastfeeding because the product contains benzyl alcohol.79,80 In general, the product is well tolerated and has a favourable safety profile.80,81 Side effects include irritation and erythema at the site of application.4 Spinosad (0.9%) suspension is a convenient and effective treatment for pediculosis capitis.81 Stough et al. conducted two identically designed, multicentre, investigator/evaluator-blinded, randomized, parallel-group, phase III trials on 1,038 children (558 and 480 children, respectively, in the two studies) aged 6 months and older with active head lice infestation, comparing the efficacy of 0.9% spinosad suspension without nit-combing to 1% permethrin with combing.82 The authors found that 84.6% of children in study 1 and 86.7% of children in study 2 treated with spinosad were lice free versus 44.9% and 42.9% of children treated with permethrin in each of the two studies, respectively (p<0.001). Most children treated with 0.9% spinosad suspension required only one application versus most permethrin-treated children required two applications. It is difficult to conclude from these two studies that spinosad is more effective than permethrin.83 Well-controlled randomized clinical trials are needed to confirm the findings of these two studies. Cost can be a limiting factor for some patients who would like to use spinosad rather than permethrin as the former is considerably more expensive.
Topical ivermectin
Ivermectin lotion, an anthelmintic agent, was approved by the FDA in February 2012 for the treatment of pediculosis capitis in children 6 months or older.4 Ivermectin, a fermentation product of the soil-dwelling actinomycete Streptomyces avermitilis, works by binding to the glutamate and γ-aminobutyric acid gated chloride ion channels in nerve and muscle cells of head lice with resulting increase in the chloride ion permeability of nerve and muscle cells, leading to hyperpolarization, flaccid paralysis of nerves and muscles, and ultimate death of the head lice.15,84 Although topical ivermectin is not ovicidal, nymphs hatched from treated eggs are not able to feed because of paralysis of the pharyngeal muscles and are therefore not viable.4 As such, only one application of ivermectin 0.5% lotion is needed and wet combing is not required.84–86 Ivermectin 0.5% lotion is applied in sufficient amounts to saturate the scalp and entire length of dry hair and then rinsed off with water after 10 minutes.87,88 Overall, ivermectin 0.5% lotion is effective, safe, easy to administer and well tolerated.88–90 Side effects are uncommon and include xerosis, dandruff, erythema and burning sensation of the skin, eye irritation, ocular hyperaemia, and conjunctivitis.4,75 The product, however, is expensive and requires a prescription. Resistance to ivermectin is rare but has been reported.91
Lindane
Lindane (γ-hexachlorocyclohexane) inhibits γ-aminobutyric acid receptor, thereby leading to neuronal hyperstimulation followed by respiratory paralysis, and ultimate death of the head lice.5,27 Traditionally, the 1% lindane shampoo is applied to dry hair and left on for 10 minutes, whilst the 1% lindane lotion is left on overnight before being rinsed off over a sink rather than in a shower and with warm rather than hot water.75 Because of the low ovicidal activity of lindane, repeat application of the product in 7–10 days is recommended to eradicate any newly hatched lice.13 The product is no longer considered acceptable therapy for pediculosis capitis because of its potential for bone marrow suppression and neurotoxicity.13,25 The product was taken off the US market in 2003 and subsequently has been banned in many countries.45
Abametapir
Abametapir, through chelation of zinc, copper and iron, can inhibit various metalloproteinases that are critical in louse egg development, egg hatching and louse survival.56,92–94 As such, abametapir is both pediculicidal and ovicidal. In addition, abametapir 0.74% lotion contains benzyl alcohol, which enhances its ovicidal effect.13 Generally, only one treatment is necessary.94 The 0.74% abametapir lotion is applied to the scalp and dry hair and left on for 10 minutes and then rinsed off with warm water.13 The product has a favourable safety profile and is well tolerated.92–94 Side effects include mild scalp erythema, burning sensation, rash and contact dermatitis at the site of application.92–94 As the product contains benzyl alcohol, it should not be used in infants younger than 6 months of age.13 At present, the product is not commercially available.
Benzyl alcohol
Benzyl alcohol 5% lotion is a non-neurotoxic pediculicide that kills head lice by asphyxiation.95 The medication prevents head lice from closing their respiratory spiracles.30 The mineral oil-containing vehicle then obstructs the lice’s airways and causes the lice to asphyxiate. A sufficient amount of benzyl alcohol 5% lotion should be applied to the entire scalp and dry hair and rinsed off with water after 10 minutes.14 As benzyl alcohol 5% lotion has no ovicidal activity, treatment should be repeated in approximately 9 days.75 Side effects include transient irritation, pruritus, erythema, pyoderma and numbness at the application site.14 The medication is safe to use in children 6 months or older and is not contraindicated during pregnancy.95 Use of benzyl alcohol-containing products in neonates has been associated with neonatal gasping syndrome.4,27 Because of the mechanism of action, resistance to benzyl alcohol 5% lotion is highly unlikely to develop.95
Dimethicone
Dimethicone (also spelled as dimeticone) 4% lotion is a non-toxic, silicone-based pediculicide based on 4% high molecular weight dimethicone in a cyclomethicone base.2,56 Presumably, dimethicone works by coating the cuticle of the head lice, thereby sealing their spiracles and blocking the trachea with resultant suffocation of the head lice.24,96 In addition, dimethicone disrupts the ability of head lice to manage water, resulting in osmotic stress and rupture of internal organs.96,97 Given the various modes of action, no resistance to dimethicone is expected.12,24 Dimethicone 4% lotion is applied to a dry scalp, massaged into dry hair until saturated, and rinsed off in 10 minutes.10,12 A second application 8–10 days later is recommended in order to kill newly hatched nymphs.10 Dimethicone 4% lotion has been shown to be effective for the treatment of pediculosis capitis and has a favourable safety profile in children ≥2 years of age.98–104 A 2020 meta-analysis of 17 trials (n=2005) showed that occlusive agents such as dimethicone may be superior or equally efficacious as neurotoxic pediculicides.105 Dimethicone 4% lotion is non-toxic and not absorbed through the skin. As such, it has very few side effects. It is a pediculicide of choice especially in areas where resistance to permethrin and pyrethrin formulated with piperonyl butoxide is high.103 Dimethicone is in popular use in Europe.96 A drawback of dimethicone is its flammability.14 As such, contact with any potential source of fire (e.g. burning cigarette, curling iron, hairdryer) should be avoided during treatment.17
Isopropyl myristate
A 50% isopropyl myristate in cyclomethicone or decamethycyclopentasiloxane solution is a therapeutic option for the treatment of pediculosis capitis.29,106 The compound works by dissolving the waxy layer that covers the cuticle of the louse exoskeleton, leading to uncontrollable water loss through the cuticle with resultant dehydration and ultimate death of the louse.5,6,10,75 The product is applied to dry hair and scalp and rinsed off with warm water after 10 minutes.10 Repeat application in 7 days is recommended.10 Because of the physical mode of action, resistance is not expected to develop.106 The product may cause local irritation, especially if contact with the eyes occurs.10 The use of 50% isopropyl myristate in cyclomethicone or decamethylcyclopentasiloxane solution is not recommended in children younger than 4 years of age.10
1,2-Octanediol
1,2-Octanediol, also known as caprylyl glycol, works by dissolving cuticular lipids on the head lice, thereby impairing the capability of the cuticle of the head lice to prevent water loss through evaporation, with resultant dehydration and death of the organism.14 It has been shown that topical application of 1,2-octanediol is efficacious for the treatment of pediculosis capitis.107–110 Unfortunately, data on the efficacy of 1,2-octanediol for the treatment of pediculosis capitis are scarce and safety and tolerability have not been sufficiently documented.
Oral agents
Oral ivermectin
Oral ivermectin given as a single dose of 200–400 μg/kg, with a second dose given in 7–10 days have been shown to be effective for the treatment of pediculosis capitis.111–116 The medication is an option for the treatment of difficult-to-treat or resistant cases of pediculosis capitis.68,112,116 Generally, the medication is well tolerated.14 Adverse events are uncommon and include headache, nausea, vomiting, diarrhoea, impetigo, abnormal tendon reflexes, seizure and coma.112,116 As ivermectin may cross the blood–brain barrier in young children and block essential neural transmission, oral ivermectin should not be used during pregnancy/breastfeeding45 and in children weighing less than 15 kg as per recommendations of the American Academy of Pediatrics.13
Trimethoprim/sulfamethoxazole
Oral trimethoprim/sulfamethoxazole (trimethoprim 10 mg/kg/day and sulfamethoxazole, 50 mg/kg/day in two doses) for 10 days has been shown to be effective for the treatment of pediculosis capitis.4 Presumably, the medication works by killing the symbiotic bacteria in the intestine of the head louse with the resultant inability to synthesize vitamin B, which is essential for the survival of the louse.4,14 Alternatively, the medication may have a direct toxic effect on the head louse.4 Side effects associated with the use of trimethoprim/sulfamethoxazole include nausea, vomiting, abdominal pain, diarrhoea, skin rash and, rarely, neutropenia, haemolysis, renal impairment, and Stevens–Johnson syndrome.4,14 It has been shown that a combination of trimethoprim/sulfamethoxazole and 1% permethrin is more effective for the treatment of pediculosis capitis than trimethoprim/sulfamethoxazole or 1% permethrin when used alone.117 Treatment with trimethoprim/sulfamethoxazole should be reserved for cases not responsive to treatment with appropriate topical pediculicides.117
Physical methods
Mechanical removal
Because none of the pediculicides is 100% ovicidal, manual removal of nits and head lice by combing wet hair (‘wet combing’, otherwise known as ‘bug busting’) with a fine-tooth comb (tooth spacing less than 0.3 mm apart) is an alternative to pediculicides.118–120 The hair can be made wet with water, oils (e.g. olive oil, petroleum jelly), shampoo or conditioner.45 It is advisable to comb hair section by section, from root to tip of the hair, removing eggs and lice.12 Combing should be performed until no lice is found in each session, which may take up to half an hour.14 The process is tedious and has to be repeated every 3–4 days for a minimum of 2 weeks after any session in which a louse is found.45 As wet combing is not as effective as a pediculicide, it should not be used as the sole intervention for the treatment of pediculosis capitis in the general population.121,122 Nevertheless, wet combing should be considered in the treatment of pediculosis capitis for children younger than 2 years, pregnant women, nursing mothers, and patients with an open wound on the scalp.17,22 Wet combing should also be considered if parents prefer not to use a pediculicide on their child.22 A recent study showed that wet combing combined with the use of 1% permethrin shampoo can increase the success rate in the treatment of pediculosis capitis.67
Hot air
Hot/heated air, through the mechanism of desiccation, has been suggested for the treatment of pediculosis capitis.116 In one study, the use of ‘LouseBuster’, a custom-built machine, to deliver heated air at the scalp of infested individuals for 30 minutes, resulted in 80% mortality of the lice and 98% mortality of eggs.123 As the study was unblinded and the patient number was small, well-designed, large-scale, randomized, controlled studies trials are necessary to provide more information on the efficacy and safety profile of hot/heated air in the treatment of pediculosis capitis. Until then, such treatment cannot be recommended.
Hair shaving
Whilst shaving the head may be an effective way to get rid of the head lice and eggs,30,124 the procedure is not recommended because it can be emotionally traumatic to the child and distressing to the parents.4,125
Alternative and complementary therapies
In some cultures, alternative and complementary therapies are popular for the treatment of pediculosis capitis. Occlusive agents, such as mayonnaise, butter, tub margarine, olive oil, petroleum jelly and Vaseline, have been used for the treatment of pediculosis capitis with the hope that these agents may suffocate the head lice.126 The product is massaged on the entire surface of the scalp and hair, left overnight with a shower cap, and washed with a shampoo for the next several days to remove the residue.75 These products are messy and most have not been scientifically evaluated. Further studies on their effectiveness have yielded conflicting results.12 As such, the use of these products cannot be recommended unless future well-designed studies show otherwise.
Topical application of essential oils, such as tea tree oil (also known as melaleuca oil), lavender oil, lemongrass oil, eucalyptus oil, safflower oil, coconut oil, soya oil, clove oil, bergamot oil, andiroba oil, ylang-ylang oil, zingiberaceae oil, petroleum-derived mineral oil, and aerial parts of Origanum species-derived oil, has been used in traditional medicine for the treatment of pediculosis capitis with varying results.127–136 Because of the variability of the constituents of essential oils, the results might not be reproducible. Adverse events associated with topical application of essential oils include local irritation and allergic contact dermatitis.25 Accidental ingestion of essential oils may lead to aspiration pneumonia, gastrointestinal upset, hepatic dysfunction, muscle weakness, slurred speech, drowsiness, ataxia, seizures, and, rarely, death.12,137 The efficacy and safety of essential oils have not been subjected to well-designed, vigorous, therapeutic trials. At present, the use of essential oils for the treatment of pediculosis capitis cannot be recommended because of a lack of information regarding their efficacy and safety.
In some cultures, home remedies for the treatment of pediculosis capitis include paste of bitter almonds, neem seed extract, powdered seeds of custard apple, alum paste, tobacco paste, emulsion of soap-nut, grapefruit extract, vinegar, and herbal shampoos based on native plants (Phyllanthus emblica, Acorus calamus, Zanthoxylum limonella).138–141 Available data on the efficacy and safety of these products are very limited. As such, their use for the treatment of pediculosis capitis cannot be recommended.
A recent study showed that the use of physical treatments and carrier oils such as bitter almond and olive oil increased the success rate of 1% permethrin shampoo for the treatment of pediculosis capitis.142 Well-designed, large-scale, multicentre, randomized, placebo-controlled trials are needed to confirm or refute this finding.
Prevention
All household members and close contacts should be thoroughly examined for evidence of infestation and treated concurrently if infested to break the cycle of re-infestation.4,5,13 Bedmates should be treated prophylactically even if they do not have an identifiable viable live louse or louse egg.13,14 It is advisable to disinfect bedding and personal hair care items.5 Combs and hairbrushes should be soaked in hot water or treated with pediculicides.13 Bed linens, pillowcases, headgear, clothing, and towels used by an infected person within 2 days prior to treatment should be laundered in hot water (water temperature ≥50oC) or dry cleaned on high heat for at least 30 minutes.143,144 Disinfecting furniture with a pediculicide is not recommended.145 The child care facility or school should be notified so that additional cases can be detected and treated. The ‘no nits’ policies, which exclude children with pediculosis capitis from school, have no scientific basis and are not recommended.1,9,14,146 The child should be allowed to return to school or child care facility after proper treatment, even if nits are still present.12,145 The child should be taught not to share personal items such as comb and brushes with others and not to have close, direct head contact with others.5,144
Prognosis
Pediculosis capitis is largely harmless.42 The prognosis is excellent. Most patients respond to treatment with topical pediculicides.30 Treatment failure may result from non-compliance, inappropriate or inadequate treatment (for example, exposure time too short, incorrect application, too little of pediculicide applied, uneven application of pediculicide, and application of pediculicide to wet rather than dry hair), lack of ovicidal activity or residual killing properties of the pediculicide, reinfestation, and drug resistance to the pediculicide.17,30,75
Conclusion
Pediculosis capitis remains a common chronic problem in children, particularly those between 3 and 12 years of age. Physicians involved in the care of children should be knowledgeable about pediculosis capitis and its treatment. They should select treatments that are safe, effective, ease to apply, tolerable, and inexpensive. Permethrin 1% and pyrethrin formulated with piperonyl butoxide are inexpensive and readily available over the counter, and are therefore pediculicides of choice for children 2 years and older when resistance to these products is not suspected. When resistance to these products is suspected based on local levels of resistance or when treatment with these products fails despite their correct use and reinfestation does not seem to be responsible, other topical treatment options include malathion, benzyl alcohol, dimethicone, spinosad, and ivermectin. Oral ivermectin or trimethoprim/sulphamethoxazole is a treatment option for patients who do not respond to appropriate topical treatment.
Key practice points.
Pediculosis capitis is a common human parasitic infestation in childhood and is endemic both in developed and developing countries.
Itching of the scalp is the most common and often the sole symptom of pediculosis capitis.
The gold standard for diagnosing pediculosis capitis is detection of a live louse or nymph on the scalp or viable egg on the scalp hair.
Treatment of pediculosis capitis is recommended for all individuals with an active infestation.
Topical permethrin and pyrethrin formulated with piperonyl butoxide are the pediculicides of choice in areas where resistance to these products is low.
Resistance to pediculicides especially those with a neurotoxic mode of action is another concern which may limit the use of some of these pediculicides.
Other topical treatment options include malathion, benzyl alcohol, dimethicone, spinosad and ivermectin.
Wet combing should be considered for children younger than 2 years.
Oral ivermectin and trimethoprim/sulfamethoxazole should be reserved for patients who do not respond to appropriate topical pediculicides.
Acknowledgements
None.
Footnotes
Contributions: AKCL is the principal author. JML, KFL, BB and KLH are coauthors who contributed and helped with the drafting of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: AKCL and KLH are Associate Editors of Drugs in Context and confirm that this article has no other conflicts of interest otherwise. This manuscript was sent out for independent peer review. All other authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/02/dic.2021-11-3-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Leung AKC, Lam JM, Leong KF, Barankin B, Hon KL. https://doi.org/10.7573/dic.2021-11-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50(1):1–12. doi: 10.1016/s0190-9622(03)02729-4. quiz 13–14. [DOI] [PubMed] [Google Scholar]
- 2.Madke B, Khopkar U. Pediculosis capitis: an update. Indian J Dermatol Venereol Leprol. 2012;78(4):429–438. doi: 10.4103/0378-6323.98072. [DOI] [PubMed] [Google Scholar]
- 3.Angel TA, Nigro J, Levy ML. Infestations in the pediatric patient. Pediatr Clin North Am. 2000;47(4):921–935. viii. doi: 10.1016/s0031-3955(05)70249-2. [DOI] [PubMed] [Google Scholar]
- 4.Devore CD, Schutze GE Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135(5):e1355–1365. doi: 10.1542/peds.2015-0746. [DOI] [PubMed] [Google Scholar]
- 5.Frankowski BL, Weiner LB Committee on School Health the Committee on Infectious Diseases. American Academy of Pediatrics. Head lice. Pediatrics. 2002;110(3):638–643. [PubMed] [Google Scholar]
- 6.Frankowski BL. American Academy of Pediatrics guidelines for the prevention and treatment of head lice infestation. Am J Manag Care. 2004;10(Suppl 9):S269–272. [PubMed] [Google Scholar]
- 7.Frankowski BL, Bocchini JA, Jr Council on School Health and Committee on Infectious Diseases. Head lice. Pediatrics . 2010;126(2):392–403. doi: 10.1542/peds.2010-1308. [DOI] [PubMed] [Google Scholar]
- 8.Leung AK, Fong JH, Pinto-Rojas A. Pediculosis capitis. J Pediatr Health Care. 2005;19(6):369–373. doi: 10.1016/j.pedhc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Nash B. Treating head lice. BMJ. 2003;326(7401):1256–1257. doi: 10.1136/bmj.326.7401.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings C, Finlay JC, MacDonald NE. Head lice infestations: a clinical update. Paediatr Child Health. 2018;23(1):e18–e24. doi: 10.1093/pch/pxx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castelletti N, Barbarossa MV. Deterministic approaches for head lice infestations and treatments. Infect Dis Model. 2020;5:386–404. doi: 10.1016/j.idm.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imboden A. Effective treatments for head lice. Nurse Pract. 2019;44(9):36–42. doi: 10.1097/01.NPR.0000574668.19239.db. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Pediculosis capitis (head lice) In: Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, editors. Red Book (2021): Report of the Committee on Infectious Diseases. 32nd edition. Elk Groove Village, IL: American Academy of Pediatrics; 2021. pp. 567–571. [Google Scholar]
- 14.Goldstein AO, Goldstein BG. Pediculosis capitis. In: Dellavalle RP, Levy ML, Rosen T, Ofori AO, editors. UpToDate. Waltham, MA: [Accessed on September 30, 2021]. https://www.uptodate.com/contents/pediculosis-capitis . [Google Scholar]
- 15.Eisenhower C, Farrington EA. Advancements in the treatment of head lice in pediatrics. J Pediatr Health Care. 2012;26(6):451–461. doi: 10.1016/j.pedhc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Yetman RJ. The child with pediculosis capitis. J Pediatr Health Care. 2015;29(1):118–120. doi: 10.1016/j.pedhc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Meister L, Ochsendorf F. Head lice. Dtsch Arztebl Int. 2016;113(45):763–772. doi: 10.3238/arztebl.2016.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heukelbach J, Asenov A, Araújo Oliveira F, et al. Orientation of head lice on human hosts, and consequences for transmission of pediculosis: the head lice movement studies. Trop Med Infect Dis. 2017;2(2):11. doi: 10.3390/tropicalmed2020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galassi F, Gonzalez-Audino P, Picollo MI. Head lice recognize and prefer head odor over foot and forearms odors. J Med Entomol. 2019;56(5):1204–1207. doi: 10.1093/jme/tjz060. [DOI] [PubMed] [Google Scholar]
- 20.Coscione S, Kositz C, Marks M. Head lice: an under-recognized tropical problem. Am J Trop Med Hyg. 2017;97(6):1636–1637. doi: 10.4269/ajtmh.17-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibarra J, Hall DM. Head lice in schoolchildren. Arch Dis Child. 1996;75(6):471–473. doi: 10.1136/adc.75.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346(21):1645–1650. doi: 10.1056/NEJMcp012640. [DOI] [PubMed] [Google Scholar]
- 23.Chosidow O. Scabies and pediculosis. Lancet. 2000;355(9206):819–826. doi: 10.1016/s0140-6736(99)09458-1. [DOI] [PubMed] [Google Scholar]
- 24.Tebruegge M, Pantazidou A, Curtis N. What’s bugging you? An update on the treatment of head lice infestation. Arch Dis Child Educ Pract Ed. 2011;96(1):2–8. doi: 10.1136/adc.2009.178038. [DOI] [PubMed] [Google Scholar]
- 25.Eichenfield LF, Colon-Fontanez F. Treatment of head lice. Pediatr Infect Dis J. 1998;17(5):419–420. doi: 10.1097/00006454-199805000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Mazurek CM, Lee NP. How to manage head lice. West J Med. 2000;172(5):342–345. doi: 10.1136/ewjm.172.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch E, Clark JM, Cohen B, et al. Management of head louse infestations in the United States – a literature review. Pediatr Dermatol. 2016;33(5):466–472. doi: 10.1111/pde.12982. [DOI] [PubMed] [Google Scholar]
- 28.McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12(5):349–353. doi: 10.2165/11208070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15(5):401–412. doi: 10.1007/s40257-014-0094-4. [DOI] [PubMed] [Google Scholar]
- 30.Bragg BN, Simon LV. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; [Accessed February 14, 2022]. Pediculosis. https://www.ncbi.nlm.nih.gov/books/NBK470343/ [Google Scholar]
- 31.Hatam-Nahavandi K, Ahmadpour E, Pashazadeh F, et al. Pediculosis capitis among school-age students worldwide as an emerging public health concern: a systematic review and meta-analysis of past five decades. Parasitol Res. 2020;119(10):3125–3143. doi: 10.1007/s00436-020-06847-5. [DOI] [PubMed] [Google Scholar]
- 32.Babazadeh T, Kouzekanani K, Oliaei S, et al. Assessing the link between head lice infestation and selected cognitive-behavioral factors in a sample of Iranian female adolescents. Heliyon. 2020;6(5):e03959. doi: 10.1016/j.heliyon.2020.e03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Counahan M, Andrews R, Büttner P, Byrnes G, Speare R. Head lice prevalence in primary schools in Victoria, Australia. J Paediatr Child Health. 2004;40(11):616–619. doi: 10.1111/j.1440-1754.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams LK, Reichert A, MacKenzie WR, Hightower AW, Blake PA. Lice, nits, and school policy. Pediatrics. 2001;107(5):1011–1015. doi: 10.1542/peds.107.5.1011. [DOI] [PubMed] [Google Scholar]
- 35.Campos Nogueira R, Nonato FR, Duchene Veauvy MC, Cavin AL, Al-Anbaki M, Graz B. Head lice at school: traditional medicine and community engagement. Health Equity. 2021;5(1):310–315. doi: 10.1089/heq.2020.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mumcuoglu KY. Prevention and treatment of head lice in children. Paediatr Drugs. 1999;1(3):211–218. doi: 10.2165/00128072-199901030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Jones KN, English JC., III Review of common therapeutic options in the United States for the treatment of pediculosis capitis. Clin Infect Dis. 2003;36(11):1355–1361. doi: 10.1086/374840. [DOI] [PubMed] [Google Scholar]
- 38.Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361(9352):99–100. doi: 10.1016/S0140-6736(03)12243-X. [DOI] [PubMed] [Google Scholar]
- 39.Lesshafft H, Baier A, Guerra H, Terashima A, Feldmeier H. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in lima, Peru. J Glob Infect Dis. 2013;5(4):138–143. doi: 10.4103/0974-777X.121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moosazadeh M, Afshari M, Keianian H, Nezammahalleh A, Enayati AA. Prevalence of head lice infestation and its associated factors among primary school students in Iran: a systematic review and meta-analysis. Osong Public Health Res Perspect. 2015;6(6):346–356. doi: 10.1016/j.phrp.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saraswat N, Shankar P, Chopra A, Mitra B, Kumar S. Risk factors associated with head lice infestation in rural pediatric patients. Indian Dermatol Online J. 2020;11(1):25–28. doi: 10.4103/idoj.IDOJ_48_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess IF. Human lice and their management. Adv Parasitol. 1995;36:271–342. doi: 10.1016/s0065-308x(08)60493-5. [DOI] [PubMed] [Google Scholar]
- 43.Birkemoe T, Lindstedt HH, Ottesen P, Soleng A, Næss Ø, Rukke BA. Head lice predictors and infestation dynamics among primary school children in Norway. Fam Pract. 2016;33(1):23–29. doi: 10.1093/fampra/cmv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodd C. Treatment of head lice. BMJ. 2001;323(7321):1084. doi: 10.1136/bmj.323.7321.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CH, Goldman RD. An incurable itch: head lice. Can Fam Physician. 2012;58(8):839–841. [PMC free article] [PubMed] [Google Scholar]
- 46.Scott P, Middlefell LS, Fabbroni G, Mitchell DA. Interesting case: cervical lymphadenopathy, induced by head lice. Br J Oral Maxillofac Surg. 2005;43(6):515. doi: 10.1016/j.bjoms.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Takcı Z, Tekin O, Karadağ AS. A pediculid case: autosensitization dermatitis caused by pediculosis capitis. Turkiye Parazitol Derg. 2012;36(3):185–187. doi: 10.5152/tpd.2012.44. [DOI] [PubMed] [Google Scholar]
- 48.Sadhasivamohan A, Kaliaperumal K, Palaniappan V. Pediculosis capitis with id reaction and plica polonica. Am J Trop Med Hyg. 2021;105(4):862–863. doi: 10.4269/ajtmh.21-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall RR, McMichael AJ. Circumscribed alopecia: an unusual manifestation of pediculosis capitis. Pediatr Dermatol. 2012;29(4):513–514. doi: 10.1111/j.1525-1470.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- 50.Gera G, Gupta I, Dayal S. Plica polonica secondary to pediculosis capitis and use of shampoo. Int J Trichology. 2018;10(3):143–144. doi: 10.4103/ijt.ijt_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balcioglu C, Burgess IF, Limoncu ME, et al. Plastic detection comb better than visual screening for diagnosis of head louse infestation. Epidemiol Infect. 2008;136(10):1425–1431. doi: 10.1017/S0950268807000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mumcuoglu KY, Friger M, Ioffe-Uspensky I, Ben-Ishai F, Miller J. Louse comb versus direct visual examination for the diagnosis of head louse infestations. Pediatr Dermatol. 2001;18(1):9–12. doi: 10.1046/j.1525-1470.2001.018001009.x. [DOI] [PubMed] [Google Scholar]
- 53.Speare R, Canyon DV, Cahill C, Thomas G. Comparative efficacy of two nit combs in removing head lice (Pediculus humanus var. capitis) and their eggs. Int J Dermatol. 2007;46(12):1275–1278. doi: 10.1111/j.1365-4632.2007.03410.x. [DOI] [PubMed] [Google Scholar]
- 54.Jahnke C, Bauer E, Hengge UR, Feldmeier H. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145(3):309–313. doi: 10.1001/archdermatol.2008.587. [DOI] [PubMed] [Google Scholar]
- 55.Frydenberg A, Starr M. Head lice. Aust Fam Physician. 2003;32(8):607–611. [PubMed] [Google Scholar]
- 56.Ogbuefi N, Kenner-Bell B. Common pediatric infestations: update on diagnosis and treatment of scabies, head lice, and bed bugs. Curr Opin Pediatr. 2021;33(4):410–415. doi: 10.1097/MOP.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 57.Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54(5):909–911. doi: 10.1016/j.jaad.2005.11.1083. [DOI] [PubMed] [Google Scholar]
- 58.Badri T, Hammami H, Benmously R, Mokhtar I, Fenniche S. Dermoscopic diagnosis of pediculosis capitis. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19(3):45–46. [PubMed] [Google Scholar]
- 59.Brunton ER, Whelan IP, French R, Burgess MN, Burgess IF. Head louse egg and nit remover-a modern “Quest for the Holy Grail”. Peer J. 2019;7:e6759. doi: 10.7717/peerj.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mumcuoglu KY, Pollack RJ, Reed DL, et al. International recommendations for an effective control of head louse infestations. Int J Dermatol. 2021;60(3):272–280. doi: 10.1111/ijd.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevenson B, Tesfaye W, Christenson J, et al. Comparative efficacy and safety of interventions for treating head lice: a protocol for systematic review and network meta-analysis. BMJ Paediatr Open. 2021;5(1):e001129. doi: 10.1136/bmjpo-2021-001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durand R, Andriantsoanirina V, Brun S, Laroche L, Izri A. A case of severe pediculosis capitis. Int J Dermatol. 2018;57(2):e14–e15. doi: 10.1111/ijd.13881. [DOI] [PubMed] [Google Scholar]
- 63.Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-206623. bcr2014206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung AK, Chan KW. Iron deficiency anemia. Adv Pediatr. 2001;48:385–408. [PubMed] [Google Scholar]
- 65.Ozkan O, Sikar-Aktürk A, Mert K, Bilen N, Mumcuoğlu KY. Difficulties experienced by families following unsuccessful treatment of pediculosis capitis: the mothers’ perspective. Turkiye Parazitol Derg. 2012;36(2):82–86. doi: 10.5152/tpd.2012.20. [DOI] [PubMed] [Google Scholar]
- 66.Parison JC, Speare R, Canyon DV. Head lice: the feelings people have. Int J Dermatol. 2013;52(2):169–171. doi: 10.1111/j.1365-4632.2011.05300.x. [DOI] [PubMed] [Google Scholar]
- 67.Salimi M, Saghafipour A, Firoozfar F, Mozaffari E, Rezaei F, Vatandoost H. Study on efficacy of 1% permethrin shampoo and some traditional physical treatment for head lice infestation. Int J Prev Med. 2021;12:1. doi: 10.4103/ijpvm.IJPVM_244_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchezruiz WL, Nuzum DS, Kouzi SA. Oral ivermectin for the treatment of head lice infestation. Am J Health Syst Pharm. 2018;75(13):937–943. doi: 10.2146/ajhp170464. [DOI] [PubMed] [Google Scholar]
- 69.Bloomfield D. Head lice. Pediatr Rev. 2002;23(1):34–35. doi: 10.1542/pir.23-1-34. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 70.Hansen RC, O’Haver J. Economic considerations associated with Pediculus humanus capitis infestation. Clin Pediatr. 2004;43(6):523–527. doi: 10.1177/000992280404300603. [DOI] [PubMed] [Google Scholar]
- 71.Bialek R, Zelck UE, Fölster-Holst R. Permethrin treatment of head lice with knockdown resistance-like gene. N Engl J Med. 2011;364(4):386–387. doi: 10.1056/NEJMc1007171. [DOI] [PubMed] [Google Scholar]
- 72.Bouvresse S, Berdjane Z, Durand R, Bouscaillou J, Izri A, Chosidow O. Permethrin and malathion resistance in head lice: results of ex vivo and molecular assays. J Am Acad Dermatol. 2012;67(6):1143–1150. doi: 10.1016/j.jaad.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect. 2012;18(4):338–344. doi: 10.1111/j.1469-0691.2012.03806.x. [DOI] [PubMed] [Google Scholar]
- 74.Karakuş M, Atıcı T, Karabela ŞN, Baylan O, Limoncu ME, Balcıoğlu İC. Detection of permethrin resistance and phylogenetic clustering of turkish head lice (Pediculus humanus capitis; De Geer, 1767 populations. Acta Trop. 2020;204:105362. doi: 10.1016/j.actatropica.2020.105362. [DOI] [PubMed] [Google Scholar]
- 75.Verma P, Namdeo C. Treatment of pediculosis capitis. Indian J Dermatol. 2015;60(3):238–247. doi: 10.4103/0019-5154.156339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jahangiri F. Case report: a new method for treatment of permethrin – resistant head lice. Clin Case Rep. 2017;5(5):601–604. doi: 10.1002/ccr3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Downs AM, Narayan S, Stafford KA, Coles GC. Effectiveness of ovide against malathion-resistant head lice. Arch Dermatol. 2005;141(10):1318. doi: 10.1001/archderm.141.10.1318-a. [DOI] [PubMed] [Google Scholar]
- 78.Hamad MH, Adeel AA, Alhaboob AA, Ashri AM, Salih MA. Acute poisoning in a child following topical treatment of head lice (pediculosis capitis) with an organophosphate pesticide. Sudan J Paediatr. 2016;16(1):63–66. [PMC free article] [PubMed] [Google Scholar]
- 79.Villegas SC. Spinosad for the treatment of head lice infestations. Drugs Today. 2012;48(9):595–599. doi: 10.1358/dot.2012.48.9.1844809. [DOI] [PubMed] [Google Scholar]
- 80.Villegas SC, Breitzka RL. Head lice and the use of spinosad. Clin Ther. 2012;34(1):14–23. doi: 10.1016/j.clinthera.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 81.Cole SW, Lundquist LM. Spinosad for treatment of head lice infestation. Ann Pharmacother. 2011;45(7–8):954–959. doi: 10.1345/aph.1Q144. [DOI] [PubMed] [Google Scholar]
- 82.Stough D, Shellabarger S, Quiring J, Gabrielsen AA., Jr Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice) Pediatrics. 2009;124(3):e389–395. doi: 10.1542/peds.2008-3762. [DOI] [PubMed] [Google Scholar]
- 83.Popescu CM, Popescu R. Efficacy and safety of spinosad cream rinse for head lice. Arch Dermatol. 2012;148(9):1065–1069. doi: 10.1001/archdermatol.2012.1464. [DOI] [PubMed] [Google Scholar]
- 84.Deeks LS, Naunton M, Currie MJ, Bowden FJ. Topical ivermectin 0.5% lotion for treatment of head lice. Ann Pharmacother. 2013;47(9):1161–1167. doi: 10.1177/1060028013500645. [DOI] [PubMed] [Google Scholar]
- 85.Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27(5):307–310. doi: 10.1111/dth.12144. [DOI] [PubMed] [Google Scholar]
- 86.Early J, MacNaughton H. Ivermectin lotion (sklice) for head lice. Am Fam Physician. 2014;89(12):984–986. [PubMed] [Google Scholar]
- 87.Chosidow O, Giraudeau B. Topical ivermectin – a step toward making head lice dead lice? N Engl J Med. 2012;367(18):1750–1752. doi: 10.1056/NEJMe1211124. [DOI] [PubMed] [Google Scholar]
- 88.Pariser DM, Meinking TL, Bell M, Ryan WG. Topical 0.5% ivermectin lotion for treatment of head lice. N Engl J Med. 2012;367(18):1687–1693. doi: 10.1056/NEJMoa1200107. [DOI] [PubMed] [Google Scholar]
- 89.Hazan L, Berg JE, Bowman JP, Murray JV, Ryan WG. Pharmacokinetics and safety of 0.5% ivermectin lotion for head louse infestations. Pediatr Dermatol. 2013;30(3):323–328. doi: 10.1111/pde.12033. [DOI] [PubMed] [Google Scholar]
- 90.Meinking TL, Mertz-Rivera K, Villar ME, Bell M. Assessment of the safety and efficacy of three concentrations of topical ivermectin lotion as a treatment for head lice infestation. Int J Dermatol. 2013;52(1):106–112. doi: 10.1111/j.1365-4632.2012.05629.x. [DOI] [PubMed] [Google Scholar]
- 91.Diatta G, Abat C, Sokhna C, Tissot-Dupont H, Rolain JM, Raoult D. Head lice probably resistant to ivermectin recovered from two rural girls in Dielmo, a village in Sine-Saloum, Senegal. Int J Antimicrob Agents. 2016;47(6):501–502. doi: 10.1016/j.ijantimicag.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Bowles VM, VanLuvanee LJ, Alsop H, et al. Clinical studies evaluating abametapir lotion, 0.74%, for the treatment of head louse infestation. Pediatr Dermatol. 2018;35(5):616–621. doi: 10.1111/pde.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowles VM, Hanegraaf S, Ahveninen T, Sidgiddi S, Allenby K, Alsop H. Effect of a new head lice treatment, abametapir lotion, 0.74%, on louse eggs: a randomized, double-blind study. Glob Pediatr Health. 2019;6:2333794X19831295. doi: 10.1177/2333794X19831295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woods AD, Porter CL, Feldman SR. Abametapir for the treatment of head lice: a drug review. Ann Pharmacother. 2021;56:352–357. doi: 10.1177/10600280211027968. [DOI] [PubMed] [Google Scholar]
- 95.Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis) Pediatr Dermatol. 2010;27(1):19–24. doi: 10.1111/j.1525-1470.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 96.Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimeticone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5. doi: 10.1186/1471-5945-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31(9):2105–2110. doi: 10.1007/s10096-012-1575-0. [DOI] [PubMed] [Google Scholar]
- 98.Balcıoğlu IC, Karakuş M, Arserim SK, et al. Comparing the efficacy of commercially available insecticide and dimeticone based solutions on head lice, pediculus capitis: in vitro trials. Turkiye Parazitol Derg. 2015;39(4):305–309. doi: 10.5152/tpd.2015.4652. [DOI] [PubMed] [Google Scholar]
- 99.Burgess IF, Lee PN, Matlock G. Randomised, controlled, assessor blind trial comparing 4% dimeticone lotion with 0.5% malathion liquid for head louse infestation. PLoS One. 2007;2(11):e1127. doi: 10.1371/journal.pone.0001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heukelbach J, Sonnberg S, Becher H, Melo I, Speare R, Oliveira FA. Ovicidal efficacy of high concentration dimeticone: a new era of head lice treatment. J Am Acad Dermatol. 2011;64(4):e61–62. doi: 10.1016/j.jaad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Kurt O, Balcioğlu IC, Burgess IF, et al. Treatment of head lice with dimeticone 4% lotion: comparison of two formulations in a randomised controlled trial in rural Turkey. BMC Public Health. 2009;9:441. doi: 10.1186/1471-2458-9-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kassiri H, Fahdani AE, Cheraghian B. Comparative efficacy of permethrin 1%, lindane 1%, and dimeticone 4% for the treatment of head louse infestation in Iran. Environ Sci Pollut Res Int. 2021;28(3):3506–3514. doi: 10.1007/s11356-020-10686-3. [DOI] [PubMed] [Google Scholar]
- 103.Speare R. A single application of dimeticone is superior to two applications of permethrin in ridding head lice. J Pediatr. 2013;163(5):1531–1532. doi: 10.1016/j.jpeds.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 104.Yamaguchi S, Yasumura R, Okamoto Y, et al. Efficacy and safety of a dimethicone lotion in patients with pyrethroid-resistant head lice in an epidemic area, Okinawa, Japan. J Dermatol. 2021;48(9):1343–1349. doi: 10.1111/1346-8138.15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flores-Genuino RNS, Gnilo CMS, Dofitas BL. Occlusive versus neurotoxic agents for topical treatment of head lice infestation: a systematic review and meta-analysis. Pediatr Dermatol. 2020;37(1):86–92. doi: 10.1111/pde.14016. [DOI] [PubMed] [Google Scholar]
- 106.Barnett E, Palma KG, Clayton B, Ballard T. Effectiveness of isopropyl myristate/cyclomethicone D5 solution of removing cuticular hydrocarbons from human head lice (Pediculus humanus capitis) BMC Dermatol. 2012;12:15. doi: 10.1186/1471-5945-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burgess IF. Head lice. BMJ Clin Evid. 2011;2011:1703. [PMC free article] [PubMed] [Google Scholar]
- 108.Burgess IF, Lee PN, Kay K, Jones R, Brunton ER. 1,2-Octanediol, a novel surfactant, for treating head louse infestation: identification of activity, formulation, and randomised, controlled trials. PLoS One. 2012;7(4):e35419. doi: 10.1371/journal.pone.0035419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burgess IF, Brunton ER, French R, Burgess NA, Lee PN. Prevention of head louse infestation: a randomised, double-blind, cross-over study of a novel concept product, 1% 1,2-octanediol spray versus placebo. BMJ Open. 2014;4(5):e004634. doi: 10.1136/bmjopen-2013-004634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burgess IF, Silverston P. Head lice. BMJ Clin Evid. 2015;2015:1703. [PMC free article] [PubMed] [Google Scholar]
- 111.Ameen M, Arenas R, Villanueva-Reyes J, et al. Oral ivermectin for treatment of pediculosis capitis. Pediatr Infect Dis J. 2010;29(11):991–993. [PubMed] [Google Scholar]
- 112.Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362(10):896–905. doi: 10.1056/NEJMoa0905471. [DOI] [PubMed] [Google Scholar]
- 113.Levitt J. Oral Ivermectin is more effective than topical malathion in difficult-to-treat head lice infestation. Arch Dis Child Educ Pract Ed. 2011;96(5):200. doi: 10.1136/adc.2010.207183. [DOI] [PubMed] [Google Scholar]
- 114.Nofal A. Oral ivermectin for head lice: a comparison with 0.5 % topical malathion lotion. J Dtsch Dermatol Ges. 2010;8(12):985–988. doi: 10.1111/j.1610-0387.2010.07487.x. [DOI] [PubMed] [Google Scholar]
- 115.Popescu CM, Popescu R. Effectiveness of oral ivermectin and malathion lotion for difficult-to-treat head lice. Arch Dermatol. 2011;147(1):98–100. doi: 10.1001/archdermatol.2010.400. [DOI] [PubMed] [Google Scholar]
- 116.Tebruegge M, Pantazidou A, Curtis N. Oral ivermectin for the treatment of pediculosis capitis. Pediatr Infect Dis J. 2011;30(4):362–363. doi: 10.1097/INF.0b013e31820ea00c. [DOI] [PubMed] [Google Scholar]
- 117.Hipolito RB, Mallorca FG, Zuniga-Macaraig ZO, Apolinario PC, Wheeler-Sherman J. Head lice infestation: single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics. 2001;107(3):E30. doi: 10.1542/peds.107.3.e30. [DOI] [PubMed] [Google Scholar]
- 118.Burgess IF, Brown CM, Nair P. Comparison of phenothrin mousse, phenothrin lotion, and wet-combing for treatment of head louse infestation in the UK: a pragmatic randomised, controlled, assessor blind trial. F1000Res. 2014;3:158. doi: 10.12688/f1000research.2026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gallardo A, Toloza A, Vassena C, Picollo MI, Mougabure-Cueto G. Comparative efficacy of commercial combs in removing head lice (Pediculus humanus capitis) (Phthiraptera: Pediculidae) Parasitol Res. 2013;112(3):1363–1366. doi: 10.1007/s00436-012-3208-z. [DOI] [PubMed] [Google Scholar]
- 120.Kurt Ö, Balcıoğlu IC, Limoncu ME, et al. Treatment of head lice (Pediculus humanus capitis) infestation: is regular combing alone with a special detection comb effective at all levels? Parasitol Res. 2015;114(4):1347–1353. doi: 10.1007/s00436-015-4311-8. [DOI] [PubMed] [Google Scholar]
- 121.Roberts RJ, Casey D, Morgan DA, Petrovic M. Comparison of wet combing with malathion for treatment of head lice in the UK: a pragmatic randomised controlled trial. Lancet. 2000;356(9229):540–544. doi: 10.1016/s0140-6736(00)02578-2. [DOI] [PubMed] [Google Scholar]
- 122.Wilson P. The science behind head lice treatment. Practitioner. 1999;243(1604):824–826. 829. [PubMed] [Google Scholar]
- 123.Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118(5):1962–1970. doi: 10.1542/peds.2005-1847. [DOI] [PubMed] [Google Scholar]
- 124.Lwegaba A. Shaving can be safer head lice treatment than insecticides. BMJ. 2005;330(7506):1510. doi: 10.1136/bmj.330.7506.1510-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brenton CM. Shaving for head lice is unnecessary and distressing. BMJ. 2005;331(7513):405. doi: 10.1136/bmj.331.7513.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heymann WR. Head lice treatments: searching for the path of least resistance. J Am Acad Dermatol. 2009;61(2):323–324. doi: 10.1016/j.jaad.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Al-Quraishy S, Abdel-Ghaffar F, Mehlhorn H. Head louse control by suffocation due to blocking their oxygen uptake. Parasitol Res. 2015;114(8):3105–3110. doi: 10.1007/s00436-015-4528-6. [DOI] [PubMed] [Google Scholar]
- 128.Arserim SK, Cetin H, Yildirim A, et al. The toxicity of essential oils from three Origanum species against head louse, Pediculus humanus capitis. Acta Parasitol. 2021;66(3):1003–1011. doi: 10.1007/s11686-021-00370-y. [DOI] [PubMed] [Google Scholar]
- 129.Barker SC, Altman PM. A randomised, assessor blind, parallel group comparative efficacy trial of three products for the treatment of head lice in children--melaleuca oil and lavender oil, pyrethrins and piperonyl butoxide, and a “suffocation” product. BMC Dermatol. 2010;10:6. doi: 10.1186/1471-5945-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burgess IF, Kay K, Burgess NA, Brunton ER. Soya oil-based shampoo superior to 0.5% permethrin lotion for head louse infestation. Med Devices. 2011;4:35–42. doi: 10.2147/MDER.S17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burgess IF, Burgess NA. “Anti-lice protector shampoo”: clinical study shows lack of efficacy of coconut oil derivatives in the elimination of head louse infestation. Turkiye Parazitol Derg. 2020;44(4):211–215. doi: 10.4274/tpd.galenos.2020.6361. [DOI] [PubMed] [Google Scholar]
- 132.Di Campli E, Di Bartolomeo S, Delli Pizzi P, et al. Activity of tea tree oil and nerolidol alone or in combination against Pediculus capitis (head lice) and its eggs. Parasitol Res. 2012;111(5):1985–1592. doi: 10.1007/s00436-012-3045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gallardo A, Mougabure-Cueto G, Vassena C, Picollo MI, Toloza AC. Comparative efficacy of new commercial pediculicides against adults and eggs of Pediculus humanus capitis (head lice) Parasitol Res. 2012;110(5):1601–1606. doi: 10.1007/s00436-011-2668-x. [DOI] [PubMed] [Google Scholar]
- 134.Greive KA, Lui AH, Barnes TM, Oppenheim VM. A randomized, assessor-blind, parallel-group, multicentre, phase IV comparative trial of a suffocant compared with malathion in the treatment of head lice in children. Australas J Dermatol. 2010;51(3):175–182. doi: 10.1111/j.1440-0960.2010.00622.x. [DOI] [PubMed] [Google Scholar]
- 135.Greive KA, Barnes TM. The efficacy of Australian essential oils for the treatment of head lice infestation in children: a randomised controlled trial. Australas J Dermatol. 2018;59(2):e99–e105. doi: 10.1111/ajd.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolf L, Eertmans F, Wolf D, Rossel B, Adriaens E. Efficacy and safety of a mineral oil-based head lice shampoo: a randomized, controlled, investigator-blinded, comparative study. PLoS One. 2016;11(6):e0156853. doi: 10.1371/journal.pone.0156853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Waldman N. Seizure caused by dermal application of over-the-counter eucalyptus oil head lice preparation. Clin Toxicol. 2011;49(8):750–751. doi: 10.3109/15563650.2011.602084. [DOI] [PubMed] [Google Scholar]
- 138.Abdel-Ghaffar F, Semmler M, Al-Rasheid K, Klimpel S, Mehlhorn H. Efficacy of a grapefruit extract on head lice: a clinical trial. Parasitol Res. 2010;106(2):445–459. doi: 10.1007/s00436-009-1683-7. [DOI] [PubMed] [Google Scholar]
- 139.Mac-Mary S, Messikh R, Jeudy A, et al. Assessment of the efficacy and safety of a new treatment for head lice. ISRN Dermatol. 2012;2012:460467. doi: 10.5402/2012/460467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Semmler M, Abdel-Ghaffar F, Gestmann F, et al. Randomized, investigator-blinded, controlled clinical study with lice shampoo (Licener) versus dimethicone (Jacutin Pedicul Fluid) for the treatment of infestations with head lice. Parasitol Res. 2017;116(7):1863–1870. doi: 10.1007/s00436-017-5461-7. [DOI] [PubMed] [Google Scholar]
- 141.Soonwera M. Efficacy of herbal shampoo base on native plant against head lice (Pediculus humanus capitis De Geer, Pediculidae: Phthiraptera) in vitro and in vivo in Thailand. Parasitol Res. 2014;113(9):3241–3250. doi: 10.1007/s00436-014-3986-6. [DOI] [PubMed] [Google Scholar]
- 142.Salimi M, Saghafipour A, Hamidi Parsa H, Khosravi M. Economic burden associated with head louse (Pediculus humanus capitis) infestation in Iran. Iran J Public Health. 2020;49(7):1348–1354. doi: 10.18502/ijph.v49i7.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Izri A, Chosidow O. Efficacy of machine laundering to eradicate head lice: recommendations to decontaminate washable clothes, linens, and fomites. Clin Infect Dis. 2006;42(2):e9–10. doi: 10.1086/499105. [DOI] [PubMed] [Google Scholar]
- 144.Simmons S. Taking a closer look at pediculosis capitis. Nursing. 2015;45(6):57–58. doi: 10.1097/01.NURSE.0000464986.00187.3a. [DOI] [PubMed] [Google Scholar]
- 145.Roberts RJ, Burgess IF. New head-lice treatments: hope or hype? Lancet. 2005;365(9453):8–10. doi: 10.1016/S0140-6736(04)17677-0. [DOI] [PubMed] [Google Scholar]
- 146.Pontius DJ. Hats off to success: changing head lice policy. NASN Sch Nurse. 2011;26(6):356–362. doi: 10.1177/1942602X11421349. [DOI] [PubMed] [Google Scholar]