Abstract
Background
Although the number of lung transplantations (LTx) performed worldwide for coronavirus disease 2019 (COVID-19)-induced acute respiratory distress syndrome (ARDS) is still low, there is general agreement that this treatment can save a subgroup of the most severely ill patients with irreversible lung damage. However, the true proportion of patients eligible for LTx, the overall outcome and the impact of LTx on the pandemic are unknown.
Methods
A retrospective analysis was performed using a nationwide registry of hospitalised patients with confirmed severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection admitted between 1 January 2020 and 30 May 2021 in Austria. Patients referred to one of the two Austrian LTx centres were analysed, and grouped into patients accepted and rejected for LTx. Detailed outcome analysis was performed for all patients who received a LTx for post-COVID-19 ARDS and compared with patients who underwent LTx for other indications.
Results
Between 1 January 2020 and 30 May 2021, 39 485 patients were hospitalised for COVID-19 in Austria. 2323 required mechanical ventilation and 183 received extracorporeal membrane oxygenation (ECMO) support. 106 patients with severe COVID-19 ARDS were referred for LTx. Of these, 19 (18%) underwent LTx. 30-day mortality after LTx was 0% for COVID-19 ARDS transplant recipients. At a median follow-up of 134 (47–450) days, 14 out of 19 patients were alive.
Conclusions
Early referral of ECMO patients to a LTx centre is pivotal in order to select patients eligible for LTx. Transplantation offers excellent midterm outcomes and should be incorporated in the treatment algorithm of post-COVID-19 ARDS.
Short abstract
Lung transplantation offers excellent midterm outcomes and should be incorporated in the treatment algorithm of post-COVID-19 ARDS patients https://bit.ly/33Djghz
Introduction
The coronavirus disease 2019 (COVID-19) pandemic represents a unique challenge to global healthcare. Although most patients infected with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) show a mild or even asymptomatic course, up to 30% of patients admitted to hospital require treatment in the intensive care unit (ICU) due to the development of acute respiratory distress syndrome (ARDS) [1]. In this critically ill patient cohort, unchanged high mortality rates of 30–60% have been described [1–4].
Lung transplantation (LTx) is a well-established therapeutic option for chronic end-stage lung diseases. However, it is rarely performed for acute lung failure [5] and the worldwide experience with LTx in patients with treatment-refractory ARDS is still limited. Nevertheless, long-term outcomes in patients receiving LTx for non-COVID-19 ARDS were shown to be comparable with the results of LTx for other indications [6–8]. An international consortium of six participating centres has recently reported encouraging early outcomes of 12 patients receiving LTx for COVID-19-associated ARDS and has since updated its experience with 34 cases at the Annual Meeting of the International Society of Heart and Lung Transplantation [9, 10]. Based on these reports, a consensus has been established within the transplant community that LTx can be offered for carefully selected patients suffering from irreversible ARDS due to COVID-19 [11–16]. Despite the growing number of LTx performed for post-COVID-19 ARDS, the true impact of this treatment on the pandemic and the proportion of COVID-19 ARDS patients that are eligible for LTx are unknown.
Austria was among the first countries worldwide that adopted LTx in the treatment of post-COVID-19 ARDS patients [17]. In Austria, LTx is exclusively performed in one of the two national LTx programmes (Medical University of Vienna or Medical University of Innsbruck). Notably, LTx for ARDS arising from non-COVID-19 infections (e.g. influenza) has been performed for several years in Austria and a nationwide referring system for ICUs has been established [8].
The aim of this study was to retrospectively summarise the management of patients with ARDS due to COVID-19 who had been considered for LTx across Austria since the beginning of the pandemic. Clinical data were evaluated for all COVID-19 patients who were admitted to hospital, received ICU treatment, and were referred and accepted for LTx due to COVID-19 ARDS. Furthermore, the clinical outcome after transplantation for COVID-19 ARDS was analysed and compared with the outcome of other transplant indications. Finally, the overall impact of offering LTx for COVID-19 ARDS cases on institutional LTx programmes was assessed.
Materials and methods
Study design and study subjects
This study was designed as a nationwide retrospective analysis covering the time period between 1 January 2020 (arbitrarily considered as the beginning of the pandemic) and 30 May 2021 (end of the third wave). The Federal Ministry of Social Affairs, Health, Care and Consumer Protection provided clinical data, including all patients with a confirmed COVID-19 infection hospitalised in Austria [18]. This dataset only included patients who were discharged from the hospital or deceased. In addition, institutional LTx databases of the Medical University of Vienna and the Medical University of Innsbruck were used to analyse COVID-19 ARDS patients referred for LTx to the two national LTx centres in Austria. These two transplant centres have the mandate to provide LTx for the entire population of Austria (currently 9 million). Based on a recent change in hospital regulations, non-Austrian residents are no longer to be considered for LTx in Austria. Patients receiving LTx for other indications during the study period were defined as the control group. The ethical committees of the Medical University of Vienna (1528/2021) and the Medical University of Innsbruck (1263/2021) approved the study protocol.
Clinical definitions
Patients were considered infected with SARS-CoV-2 if the main International Classification of Diseases, 10th Revision diagnosis upon hospital admission was “U07.1/confirmed coronavirus infection”. This definition required two positive PCR tests of nasopharyngeal swabs. Right ventricular dysfunction was registered when right ventricular hypokinesis and heightened pulmonary arterial pressure were detected in transthoracic echocardiography. Acute kidney injury was staged as 1–3 as previously defined in the recommendations of the International Society of Nephrology for adults in accordance with the Kidney Disease Improving Global Outcomes [19]. Liver dysfunction was defined as serum bilirubin >1.9 mg·dL−1 based on a definition previously published for patients with ARDS [20]. The end of mechanical ventilation was defined as extubation without early re-intubation (within 5 days). In case of re-intubation, end of mechanical ventilation was reached after the final extubation. As intermittent continuous positive airway pressure is usually part of the weaning process in tracheostomised patients, toleration of mere oxygen insufflation for >6 consecutive hours was defined as the end of mechanical ventilation.
Eight patients were referred to the two LTx centres with chronic lung diseases complicated by a COVID-19 infection during the study period and were excluded from the analysis. In general, COVID-19 ARDS patients were considered eligible for LTx in case of persistent pulmonary consolidations affecting all lobes, no radiological or clinical improvement despite mechanical ventilation or ECMO support for at least 4 weeks, negative virus culture or at least two sequential negative real-time PCR tests for SARS-CoV-2 (or cycle thresholds >32), absence of severe extrapulmonary comorbidities and potential for long-term recovery. As per our institutional policy, COVID-19 ARDS patients >65 years were not considered as suitable LTx candidates since their potential for rehabilitation from ARDS after prolonged ICU stay is low.
Statistical analysis
Continuous variables are presented as median, mean, interquartile range (IQR) or minimum–maximum range. To compare continuous variables between two or more than two groups, the t-test or ANOVA was performed, respectively. Categorical variables are presented as total numbers (percentages). Chi-squared tests were used for comparing categorical frequencies between two or more groups. If the expected frequency was <5, Fisher's exact test was applied. p-values <0.05 were considered statistically significant. All calculations and graphical illustrations were performed using Prism 8 (GraphPad, La Jolla, CA, USA), Microsoft Excel 2011 (Microsoft, Redmond, WA, USA) and R version 4.0.2 (www.r-project.org).
Results
Characteristics of COVID-19 patients requiring hospitalisation or ICU admission
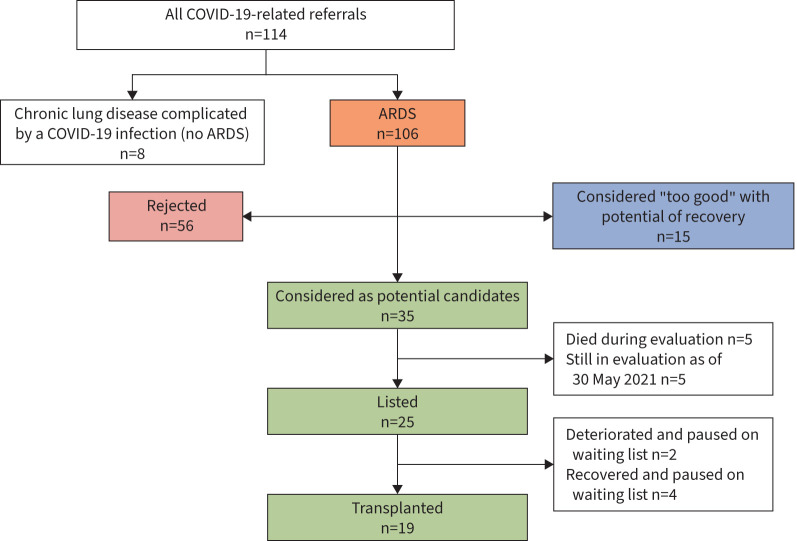
Between 1 January 2020 and 30 May 2021, a total of 39 485 patients were admitted to hospital in Austria due to a confirmed COVID-19 infection. Of these 39 485 patients, 6408 were admitted to the ICU, 2323 required mechanical ventilation and 183 received ECMO support. In total, 106 patients were referred to one of the two Austrian LTx centres to assess the necessity/possibility for a LTx (figure 1). Detailed clinical data of all patients are shown in supplementary table S1.
FIGURE 1.
Flowchart of all COVID-19 acute respiratory distress syndrome (ARDS) patients referred to the two Austrian lung transplant centres between 1 January 2020 and 30 May 2021.
Dynamic evolution of COVID-19 hospitalisations and referrals for LTx
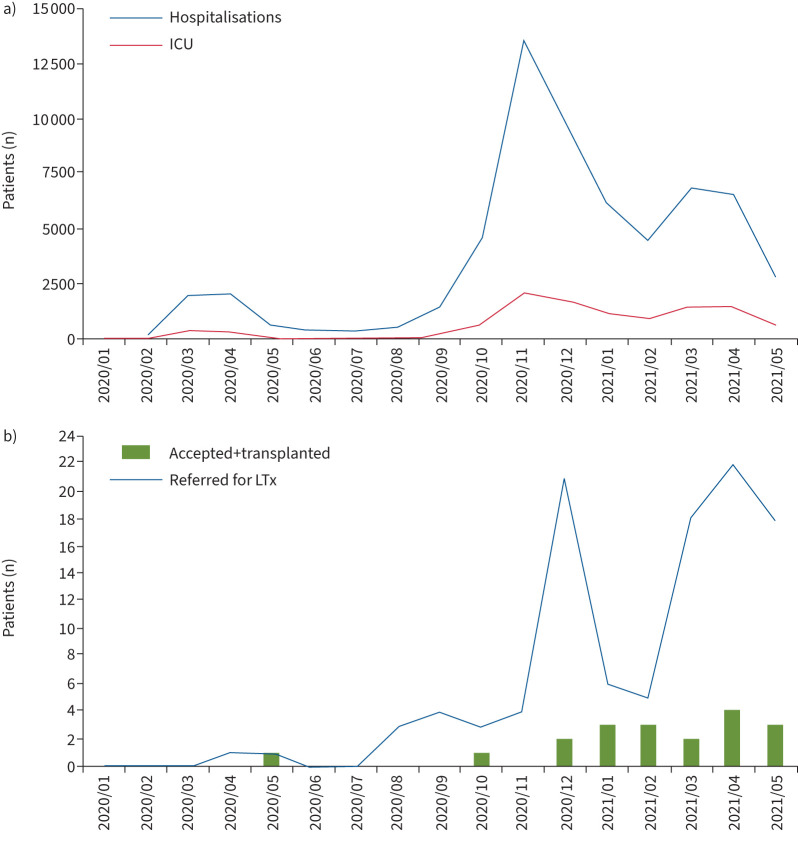
The dynamic evolution of the COVID-19 pandemic in Austria correlated well with the number of LTx referrals and the number of LTx performed for post-COVID-19 ARDS (figure 2). The highest numbers of hospital admissions were recorded in April 2021, November 2020 and December 2020. Consequently, ICU admissions were highest in November 2020, December 2020 and April 2021 (2168, 1832 and 1559 cases, respectively). The first patient with COVID-19 ARDS who underwent evaluation for LTx was referred in April 2020, and the number of referrals increased during the second wave (December 2000–January 2021) and third wave (March 2021–April 2021). Most LTx for COVID-19 ARDS were performed in April 2021 (n=4), and in January, February and May 2021 (n=3, each).
FIGURE 2.
Summary of the dynamic evolution of a) COVID-19 hospitalisations and intensive care unit (ICU) referrals, and b) COVID-19 ARDS referrals for lung transplantation (LTx) and performed LTx for COVID-19 ARDS between 1 January 2020 and 30 May 2021.
Characterisation of COVID-19 ARDS LTx referrals
Of the 106 patients with COVID-19 ARDS who had been referred, 90 (85%) were male and the median (range) age was 58 (26–75) years. At the time of referral, median (range) length of mechanical ventilation was 35 (6–68) days. 89 (84%) patients had been on ECMO support for a median (range) duration of 27 (1–60) days. Eight (8%) patients showed signs of right ventricular dysfunction and 17 (16%) required continuous renal replacement therapy (CRRT). The median bilirubin level was 0.83 mg·dL−1, whereas 15 (14%) patients had levels >1.9 mg·dL−1. The most common contraindicating medical conditions for a LTx were severe obesity (30%), sepsis (27%) and significant coronary artery disease (12.5%) (table 1).
TABLE 1.
Descriptive data of all patients referred with COVID-19 acute respiratory distress syndrome to be considered for lung transplantation (LTx) between 1 January 2020 and 30 May 2021
| All referrals | Considered as potential candidates and underwent further evaluation | Decision against LTx | p-value | ||
| Considered for recovery without LTx | Definitely rejected | ||||
| Patients | 106 (100) | 35 (33) | 15 (14) | 56 (53) | |
| Male | 90 (85) | 28 (80) | 14 (93) | 48 (86) | 0.49 |
| Age (years) | 58 (26–75) | 56 (34–68) | 54 (39–67) | 60 (26–75) | 0.08 |
| Length of MV (days) | 35 (6–68) | 38 (21–57) | 43 (24–68) | 30 (6–63) | 0.0025 |
| ECMO support | 89 (84) | 33 (94) | 12 (80) | 47 (84) | 0.26 |
| Length of ECMO support (days) | 27 (1–60) | 28 (3–45) | 31 (24–60) | 21 (1–54) | 0.03 |
| Right ventricular dysfunction | 8 (8) | 5 (14) | 1 (7) | 3 (5) | 0.31 |
| AKI stage ≥2 during ICU stay | 20 (19) | 6 (17) | 2 (13) | 12 (21) | 0.73 |
| Renal replacement therapy | 17 (16) | 7 (20) | 1 (6) | 9 (16) | 0.5 |
| Bilirubin (mg·dL−1) | 0.8 (0.2–12) | 0.7 (0.2–7) | 0.4 (0.2–5) | 1 (0.2–12) | 0.22 |
| Contraindicating medical condition | 58 (55) | 0 (0) | 2 (13) | 56 (100) | <0.0001 |
Data are presented as n (%) or median (minimum–maximum range), unless otherwise stated. MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation; AKI: acute kidney injury; ICU: intensive care unit.
All referrals were screened by a multidisciplinary LTx team that included thoracic surgeons, transplant pulmonologists, critical care physicians and transplant psychologists. Of the 106 referred patients, 35 (33%) were considered as potential candidates (figure 1). Of these, five patients died during evaluation and five patients were still under assessment for LTx as of 30 May 2021. Consequently, 25 patients were accepted and subsequently listed for LTx. Two patients deteriorated and died while waiting for their LTx, and another four patients were paused on the waiting list due to signs of native lung recovery. Finally, 19 patients underwent LTx. Of note, median (range) time of observation (time between referral and time until decision for listing) was 14 (2–57) days. Of the 71 (67%) remaining patients, 15 (14%) were considered “too good” with a realistic chance to recover without LTx and 56 (53%) were rejected due to medical conditions not compatible with LTx. 94% of potential candidates and 100% of accepted and listed patients had been on ECMO support at the time of decision. Median (IQR) length of ECMO (28 (21–35) versus 21 (15–31) days; p=0.0304) and mechanical ventilation (38 (30–44) versus 30 (24–37) days; p=0.0025) at the time of decision differed significantly between accepted and rejected candidates. There was no difference regarding requirement of CRRT or liver dysfunction.
Surgical considerations and perioperative management of LTx
Patient characteristics and post-operative outcome of patients receiving a LTx for COVID-19-induced ARDS are provided in table 2 and supplementary table S2. Of the 19 transplanted COVID-19 ARDS patients, 16 (84%) were male and median (range) age was 56 (34–64) years. 18 patients had been bridged by veno-venous (VV) and one patient by veno-arterial (VA) ECMO support to LTx with a median (range) length of 41 (2–66) days.
TABLE 2.
Main# clinical characteristics and outcome of transplanted COVID-19 acute respiratory distress syndrome patients (n=19)
| Patient | Age (years) | Gender | Length of MV until LTx (days) | Length of ECMO support until LTx (days) | Type of LTx | Post-operative ECMO prolongation | Length of ICU stay (days) | Discharged from hospital | Follow-up (days) | Alive/dead (cause of death) |
| 1 | 44 | Female | 52 | 45 | Bilateral, no size reduction | Yes | 63 | Yes | 450 | Alive |
| 2 | 55 | Male | 29 | 23 | Bilateral, no size reduction | No | 55 | Yes | 300 | Alive |
| 3 | 54 | Male | 55 | 47 | Trilobar¶ | Yes | 98 | No | 147 | Dead (subdural haematoma) |
| 4 | 57 | Male | 57 | 46 | Bilateral, size-reduced+ | No | 80 | No | 154 | Dead (liver failure) |
| 5 | 61 | Male | 50 | 43 | Bilateral, size-reduced+ | No | Not reached | No | 66 | Dead (liver failure) |
| 6 | 54 | Male | 39 | 27 | Bilateral, no size reduction | No | 37 | Yes | 207 | Alive |
| 7 | 49 | Male | 41 | 41 | Bilateral, no size reduction | No | 12 | Yes | 189 | Alive |
| 8 | 64 | Male | 28 | 17 | Bilateral, no size reduction | Yes | 32 | Yes | 180 | Alive |
| 9 | 56 | Male | 30 | 2 | Bilateral, no size reduction | No | 27 | Yes | 162 | Alive |
| 10 | 46 | Female | 52 | 52 | Bilateral, no size reduction | Yes | 90 | No | 154 | Alive |
| 11 | 64 | Male | 60 | 23 | Bilateral, no size reduction | No | Not reached | No | 111 | Dead (multi-organ failure) |
| 12 | 58 | Male | 50 | 8 | Bilateral, no size reduction | No | 25 | Yes | 133 | Alive |
| 13 | 34 | Female | 46 | 45 | Bilateral, size-reduced+ | No | 25 | Yes | 115 | Alive |
| 14 | 61 | Male | 51 | 43 | Bilateral, size-reduced+ | Yes | 27 | No | 105 | Alive |
| 15 | 56 | Male | 38 | 29 | Bilateral, no size reduction | No | 37 | Yes | 93 | Alive |
| 16 | 53 | Male | 76 | 66 | Bilateral, no size reduction | Yes | Still admitted§ | No | 85 | Alive |
| 17 | 47 | Male | 38 | 36 | Bilateral, no size reduction | No | 34 | No | 65 | Dead (liver failure) |
| 18 | 62 | Male | 47 | 34 | Bilateral, size-reduced+ | No | 30 | No | 69 | Alive |
| 19 | 57 | Male | 82 | 59 | Bilateral, no size reduction | No | Still admitted§ | No | 47 | Alive |
MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation; LTx: lung transplantation; ICU: intensive care unit. #: more details are provided in supplementary table S2; ¶: right upper, right lower and left upper lobe; +: without middle lobe and lingula; §: as of 11 August 2021.
All patients received a bilateral transplantation with central VA ECMO support. Due to retracted chest cavities, which are usually evident in COVID-19 ARDS patients, size reduction of the graft was performed in six (32%) cases (n=5 resection of the middle lobe and lingula, and n=1 trilobar LTx). The transplantation was usually complicated by dense pleural adhesions. In addition to this, hilar structures were often embedded in hyperinflamed lymphatic tissue, thus making hilar dissection challenging. In comparison with standard LTx, this led to a high intra-operative blood turnover and to a median (range) of 9 (2–34) packed red blood cells. Six patients had to be brought back to the operating room for haematothorax evacuation and secondary haemostasis. After LTx, four (26%) patients required prolonged VA ECMO and one (5%) patient required prolonged VV ECMO support for a median (range) length of 3 (1–5) days. Pathological assessment of the explanted lungs revealed that diffuse alveolar damage was evident across wide areas of the parenchyma in all recipients. Furthermore, acute fibrinous and organising pneumonia was seen in 13 (68%), nonspecific interstitial pneumonia in 12 (63%), multiple thromboembolism with large areas of infarction in nine (47%) and haemosiderosis in one (5%) of the explanted lungs.
LTx recipients received a pulse of corticosteroids starting intra-operatively and continuing through post-operative day 3. Maintenance immunosuppression was administered according to standard clinical practice with a FK506 (tacrolimus)-based triple immunosuppression regimen together with mycophenolate mofetil and steroids. The target trough levels of FK506 were 10–12 ng·mL−1, 1–2 g mycophenolate mofetil and steroids according to previously published institutional protocols [21].
Two female recipients were pre-LTx highly sensitised with >80% panel-reactive antibodies (due to previous pregnancies) and received pre- and post-LTx immunoadsorption in combination with rabbit antithymocyte globulin induction for 5 days (2 mg·kg−1·day−1 body weight). None of our patients developed any episodes of acute cellular rejection and no cases with de novo donor-specific antibodies or antibody-mediated rejection were detected.
Median (range) length of mechanical ventilation after LTx was 24 (4–82) days. The most common post-operative complications were critical illness polyneuropathy (79%), cholangiopathic hepatic dysfunction (42%) and haemothorax requiring surgical revision (32%). Median (range) time until patients were able to dangle at the bedside and stand with help was 10 (5–44) and 22 (7–96) days, respectively. Patients could be transferred from the ICU to the general ward after a median (range) ICU stay of 36 (12–98) days. Patients were discharged from hospital after a median (range) of 64 (40–154) days following LTx. At the time of the analysis, the medium (range) follow-up of the 19 transplanted patients was 134 (47–450) days. Five patients died 65, 66, 111, 147 and 154 days after the transplantation. All patients had fully functioning grafts at the time of their death. Three patients died due to cholangiopathic liver failure at post-operative days 65, 66 and 154. One patient developed multi-organ failure based on recurrent infections and died on post-operative day 111. One patient had been successfully discharged and developed a massive spontaneous cerebral haemorrhage at home 21 weeks after the transplantation.
The two national LTx programmes (Medical University of Vienna and Medical University of Innsbruck) were assessed to compare outcome results of patients transplanted for COVID-19 ARDS or other indications (table 3). Overall, 156 LTx had been performed during the study period. Of these, 137 (88%) were non-COVID-19 ARDS indications. The three most common non-COVID-19 ARDS indications were emphysema (42%), interstitial lung disease (25%) and cystic fibrosis (5%). Median (range) time on waiting list was 40 (1–994) days. Despite the COVID-19-related increase of LTx during the study period, the overall waitlist mortality remained low (1.11%). Of the 156 LTx, 145 (14 COVID-19 ARDS and 130 non-COVID-19 ARDS) could be included for 3-month survival analysis. Notably, 3-month survival did not differ between COVID-19 ARDS and non-COVID-19 ARDS LTx (Fisher's exact test; p=0.1367).
TABLE 3.
Main characteristics of the lung transplant programmes in Austria (Medical University of Vienna and Medical University of Innsbruck) between 1 January 2020 and 30 May 2021
| All LTx | 156 (100) |
| COVID-19 ARDS LTx | 19 (12) |
| Non-COVID-19 LTx | |
| Emphysema (AATD/COPD) | 65 (42) |
| Interstitial lung disease | 39 (25) |
| Cystic fibrosis | 8 (5) |
| Primary pulmonary arterial hypertension | 4 (3) |
| Other | 21 (13) |
| Time on waiting list (days) | 40 (1–994) |
| Waitlist mortality (%) | 1.11 |
Data are presented as n (%) or median (minimum–maximum range), unless otherwise stated. ARDS: acute respiratory distress syndrome; LTx: lung transplantation; AATD: α1-antitrypsin deficiency; COPD: chronic obstructive pulmonary disease.
Discussion
In this nationwide study, we aimed to investigate the overall relevance of LTx as a treatment option for severe COVID-19 ARDS. During the study period, 2323 COVID-19 patients required mechanical ventilation and 183 received ECMO support. The screening for LTx included a high proportion of Austrian COVID-19 patients treated by ECMO. Out of the 106 patients screened by the two lung transplant centres, 19 (18%) eventually underwent LTx. Midterm outcome of transplanted patients was encouraging, with 14 out of 19 being alive with a median follow-up of 134 (47–450) days (as of 11 August 2021).
The feasibility of LTx as an ultimate treatment option in the case of irreversible COVID-19-induced lung damage has recently been highlighted by an international multicentre study and an excellent early outcome has been reported in 12 candidates [9]. Criteria to select eligible ARDS patients for LTx were defined as: 1) negative virus status (virus culture or PCR), 2) no clinical/radiological improvement despite mechanical ventilation and/or ECMO support >4 weeks, 3) mono-organ failure (normal kidney and liver function), 4) absence of severe extrapulmonary comorbidities, and 5) a realistic potential for long-term recovery. These initial cases of “ideal” transplant candidates triggered a discussion about comorbidities that might be acceptable for a LTx and comorbidities still remaining an absolute contraindication.
An important aspect of this study is the relatively high rate of severe liver dysfunction observed after LTx. Dizier et al. [20] highlighted the prognostic significance of liver dysfunction in the initial phase of 805 ARDS patients. In that study, serum bilirubin >1.9 mg·dL−1 upon ICU admission was shown to represent a reliable surrogate marker for increased 90-day mortality. Focusing on COVID-19, cholangiopathy was recently shown to be a significant comorbidity and correlates with overall disease severity [22]. In our study cohort, eight (42%) of the transplanted patients developed post-operative hepatic dysfunction and three (16%) even died due to the development of severe secondary sclerosing cholangitis. These numbers are well in line with published evidence on COVID-19 ICU survivors who recovered their native lung function. According to a recent publication from a German centre, 22 out of 72 (30%) critically ill COVID-19 ICU survivors developed severe liver dysfunction [23].
Compared with non-COVID-19 LTx, early post-operative outcome was worse in COVID-19 ARDS patients. As these patients are critically ill at the time of LTx, higher early mortality can be anticipated. However, it can be assumed from the literature on LTx for non-COVID-19 ARDS that patients surviving the complex perioperative period have an excellent long-term outcome.
One of the key aspects of LTx for COVID-19 ARDS is to allow sufficient time for the native lungs to recover. Patients can only be considered for LTx in case of irreversible pulmonary damage; however, lack of reversibility is difficult to determine. Despite recent advances in ECMO, patients cannot be supported indefinitely and at a certain time-point the risk for complications increases significantly. Based on these considerations it is now recommended to wait for 6–8 weeks before listing a patient [24–26]. However, this time frame is only a rough guidance given the fact that COVID-19 ARDS patients are critically ill and clinical deterioration can occur at anytime. By strictly pursuing an 8-week hands-off period, a significant number of patients who might have been rescued by a LTx would be lost. Typical clinical scenarios that warrant an early LTx are recurrent bacterial superinfections with episodes of sepsis, necrosis of large parts of the lungs, severe pulmonary or pleural haemorrhage and recurrent tension pneumothoraces despite large bore drainages.
From an overview of an increasing number of LTx for COVID-19 (at least 100 in North America and 40 in Europe as by personal communication), it seems that two major subgroups of COVID-19-related LTx candidates can be distinguished. The first group of patients is referred to the LTx team during the acute phase of ARDS. These patients are usually deeply sedated and waking them up is often impossible. They require full circulatory/respiratory support and decision making for/against LTx takes place in a critical clinical phase. As mentioned earlier, these patients can have necrosis of their lungs or septic episodes due to pulmonary bacterial superinfection. From the surgical point of view, LTx is challenging in these patients due to destroyed anatomical structures and high intra-operative blood turnover. In addition, the post-operative rehabilitation period is often prolonged. Besides this group of severely ill patients, where LTx is more or less a rescue procedure, there is another group of patients presenting with a more chronic disease mainly characterised by fibrotic changes in their lungs. These patients are stable, awake, often able to participate in physiotherapy but cannot be weaned from ECMO or mechanical ventilation. Their overall clinical management is comparable to patients suffering from an acute deterioration of a chronic interstitial lung disease requiring extracorporeal life support bridging. Of note, during the study period, only eight (7%) of all COVID-19-related referrals (n=114) and two (1.3%) of all LTx (n=156) could be assigned to this “chronic” subgroup.
With an increasing number of lung transplant centres offering transplantation to post-COVID-19 ARDS patients, ethical concerns have been raised if the high urgency status is justified in these patients. Especially during the first wave of the pandemic, donation rates dropped dramatically, resulting in an increasing waitlist mortality for “traditional” LTx indications such as chronic obstructive pulmonary disease, interstitial lung disease, cystic fibrosis and pulmonary arterial hypertension [27, 28]. In the study period, median time on waiting list was 40 days for all LTx candidates listed in Austria. Furthermore, overall waitlist mortality was extremely low at 1.11% during the study period. Despite a temporary drop in donation rate during the first wave of the pandemic, the overall number of LTx performed in Austria remained unchanged. Moreover, we did not register an increased assessment time or time on waiting list for non-COVID-19 ARDS patients. ARDS patients who require ECMO are usually granted a high lung allocation score since their mortality without transplantation is exceptionally high. Still, 1-year survival is excellent based on the severity of their disease (85% 1-year survival according to a recent United Network for Organ Sharing analysis) [6]. Based on these considerations, COVID-19 ARDS patients are prioritised over non-COVID-19 indications.
The importance of LTx as a therapeutic option in severe COVID-19 can be best conceived by comparing the survival of COVID-19 ARDS with and without LTx. Mortality rates for severe COVID-19 ARDS (without the possibility of a rescue LTx) remain high at 30–60% [2–4]. Although long-term survival for LTx in post-COVID-19 ARDS patients is still not available, it can be assumed that 1-year survival will range between 70% and 90% based on available data of non-COVID-19 ARDS patients. Our analysis could show that a considerable number of COVID-19 patients can be saved by LTx by a nationwide LTx referral structure. Although LTx was only an option for ∼20% of referred patients, it has the potential to reduce the overall mortality of COVID-19 disease.
Our study has several limitations that must be acknowledged. The follow-up period is still limited, therefore data on long-term survival cannot be provided. Further follow-up studies are needed to clarify the long-term survival benefit of LTx for COVID-19-induced ARDS. Although, to the best of our knowledge, this study assembles the largest cohort of LTx for COVID-19 currently available, conclusions are still limited to the small number of only 19 patients. We have recently reached out to the leading lung transplant centres in North America and Europe, and hope to provide a pooled experience of LTx for COVID-19 ARDS soon.
In conclusion, our study could demonstrate that LTx is a relevant treatment modality for severe COVID-19 ARDS. Although it is only an option for a selected group of patients, it is highly effective and has the potential to reduce COVID-19-related mortality. Centres considering LTx for COVID-19-induced ARDS should be aware of tedious recovery after the procedure requiring extensive healthcare resources. Despite these challenges, LTx for COVID-19 ARDS is feasible and early post-operative outcomes are comparable to other common chronic indications.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1. Demographics of patients with COVID-19 infection requiring hospital and ICU admission ERJ-02404-2021.Table_S1 (109.1KB, pdf)
Supplementary table S2. Detailed clinical characteristics and outcome of transplanted COVID-19 ARDS patients ERJ-02404-2021.Table_S2 (185.8KB, pdf)
Shareable PDF
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
References
- 1.Abate SM, Ahmed Ali S, Mantfardo B, et al. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One 2020; 15: e0235653. doi: 10.1371/journal.pone.0235653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong RA, Kane AD, Kursumovic E, et al. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia 2021; 76: 537–548. doi: 10.1111/anae.15425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Supady A, Taccone FS, Lepper PM, et al. Survival after extracorporeal membrane oxygenation in severe COVID-19 ARDS: results from an international multicenter registry. Crit Care 2021; 25: 90. doi: 10.1186/s13054-021-03486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biancari F, Mariscalco G, Dalén M, et al. Six-month survival after extracorporeal membrane oxygenation for severe COVID-19. J Cardiothorac Vasc Anesth 2021; 35: 1999–2006. doi: 10.1053/j.jvca.2021.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Mark SC, Hoek RAS, Hellemons ME. Developments in lung transplantation over the past decade. Eur Respir Rev 2020; 29: 190132. doi: 10.1183/16000617.0132-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harano T, Ryan JP, Chan EG, et al. Lung transplantation for the treatment of irreversible acute respiratory distress syndrome. Clin Transplant 2021; 35: e14182. doi: 10.1111/ctr.14182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y, Lee SO, Shim TS, et al. Lung transplantation as a therapeutic option in acute respiratory distress syndrome. Transplantation 2018; 102: 829–837. doi: 10.1097/TP.0000000000002004 [DOI] [PubMed] [Google Scholar]
- 8.Frick AE, Gan CT, Vos R, et al. Lung transplantation for acute respiratory distress syndrome: a multicenter experience. Am J Transplant 2022; 22: 144–153. doi: 10.1111/ajt.16759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med 2021; 9: 487–497. doi: 10.1016/S2213-2600(21)00077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machuca TN, Bharat A, Kurihara C, et al. International experience with lung transplantation for COVID-19 associated acute respiratory distress syndrome. J Heart Lung Transplant 2021; 40: S12. doi: 10.1016/j.healun.2021.01.1765 [DOI] [Google Scholar]
- 11.Oh DK, Hong SB, Kim HC, et al. Experience of international air transportation and subsequent lung transplant in a patient with COVID-19-associated acute respiratory distress syndrome: a case report. J Korean Med Sci 2021; 36: e123. doi: 10.3346/jkms.2021.36.e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A, Querrey M, Markov NS, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med 2020; 12: eabe4282. doi: 10.1126/scitranslmed.abe4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J 2020; 133: 1390–1396. doi: 10.1097/CM9.0000000000000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Luo L, Wang D, et al. Early rehabilitation after lung transplantation with extracorporeal membrane oxygenation (ECMO) of COVID-19 patient: a case report. Ann Transl Med 2021; 9: 512. doi: 10.21037/atm-21-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han W, Zhu M, Chen J, et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg 2020; 272: e33–e34. doi: 10.1097/SLA.0000000000003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cypel M, Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med 2020; 8: 944–946. doi: 10.1016/S2213-2600(20)30393-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang C, Jaksch P, Hoda MA, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med 2020; 8: 1057–1060. doi: 10.1016/S2213-2600(20)30361-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Ministry of Social Affairs, Health, Care and Consumer Protection . Austrian Epidemiological Reporting System: Datenplattform Covid-19. https://datenplattform-covid.goeg.at/english Date last accessed: 28 January 2021.
- 19.Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015; 87: 62–73. doi: 10.1038/ki.2014.328 [DOI] [PubMed] [Google Scholar]
- 20.Dizier S, Forel JM, Ayzac L, et al. Early hepatic dysfunction is associated with a worse outcome in patients presenting with acute respiratory distress syndrome: a post-hoc analysis of the ACURASYS and PROSEVA studies. PLoS One 2015; 10: e0144278. doi: 10.1371/journal.pone.0144278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaksch P, Ankersmit J, Scheed A, et al. Alemtuzumab in lung transplantation: an open-label, randomized, prospective single center study. Am J Transplant 2014; 14: 1839–1845. doi: 10.1111/ajt.12824 [DOI] [PubMed] [Google Scholar]
- 22.Nardo AD, Schneeweiss-Gleixner M, Bakail M, et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int 2021; 41: 20–32. doi: 10.1111/liv.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roedl K, Jarczak D, Drolz A, et al. Severe liver dysfunction complicating course of COVID-19 in the critically ill: multifactorial cause or direct viral effect? Ann Intensive Care 2021; 11: 44. doi: 10.1186/s13613-021-00835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M, de Chambrun MP, Lebreton G, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2021; 203: 1571–1573. doi: 10.1164/rccm.202102-0259LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo F, Deng C, Shi T, et al. Recovery from respiratory failure after 49-day extracorporeal membrane oxygenation support in a critically ill patient with COVID-19: case report. Eur Heart J Case Rep 2021; 5: ytaa462. doi: 10.1093/ehjcr/ytaa462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald AL, Vachharajani HH, Davidson BP, et al. The prolonged use of VV ECMO support in COVID-19: a case report. J Crit Care Med 2020; 6: 224–230. doi: 10.2478/jccm-2020-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan EG, Chan PG, Harano T, et al. Trends in lung transplantation practices across the United States during the COVID-19 pandemic. Transplantation 2021; 105: 187–192. doi: 10.1097/TP.0000000000003522 [DOI] [PubMed] [Google Scholar]
- 28.Hardman G, Sutcliffe R, Hogg R, et al. The impact of the SARS-CoV-2 pandemic and COVID-19 on lung transplantation in the UK: lessons learned from the first wave. Clin Transplant 2021; 35: e14210. doi: 10.1111/ctr.14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1. Demographics of patients with COVID-19 infection requiring hospital and ICU admission ERJ-02404-2021.Table_S1 (109.1KB, pdf)
Supplementary table S2. Detailed clinical characteristics and outcome of transplanted COVID-19 ARDS patients ERJ-02404-2021.Table_S2 (185.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02404-2021.Shareable (388.5KB, pdf)