Abstract
Some patients experience pulmonary sequelae after SARS-CoV-2 infection, ranging from self-limited abnormalities to major lung diseases. Morphological analysis of lung tissue may help our understanding of pathogenic mechanisms and help to provide consistent personalised management. The aim of this study was to ascertain morphological and immunomolecular features of lung tissue. Transbronchial lung cryobiopsy was carried out in patients with persistent symptoms and computed tomography suggestive of residual lung disease after recovery from SARS-CoV-2 infection. 164 patients were referred for suspected pulmonary sequelae after COVID-19; 10 patients with >5% parenchymal lung disease underwent lung biopsy. The histological pattern of lung disease was not homogeneous and three different case clusters could be identified, which was mirrored by their clinical and radiological features. Cluster 1 (“chronic fibrosing”) was characterised by post-infection progression of pre-existing interstitial pneumonias. Cluster 2 (“acute/subacute injury”) was characterised by different types and grades of lung injury, ranging from organising pneumonia and fibrosing nonspecific interstitial pneumonia to diffuse alveolar damage. Cluster 3 (“vascular changes”) was characterised by diffuse vascular increase, dilatation and distortion (capillaries and venules) within otherwise normal parenchyma. Clusters 2 and 3 had immunophenotypical changes similar to those observed in early/mild COVID-19 pneumonias (abnormal expression of STAT3 in hyperplastic pneumocytes and PD-L1, IDO and STAT3 in endothelial cells). This is the first study correlating histological/immunohistochemical patterns with clinical and radiological pictures of patients with post-COVID lung disease. Different phenotypes with potentially different underlying pathogenic mechanisms have been identified.
Short abstract
Post-COVID lung disease is not a single entity, but includes different subtypes, each of them potentially requiring separate and different management https://bit.ly/3BJDeUF
Introduction
As the COVID-19 pandemic has progressed, some patients have experienced prolonged multi-organ symptoms and complications (long COVID or post-COVID syndrome) [1–13]. The implications and consequences of such ongoing clinical manifestations are a growing health concern. Evidence of residual organ damage following COVID-19 infection applies especially to pulmonary sequelae, with a spectrum ranging from self-limited abnormalities to a clinical profile of major lung diseases, with variable extent of significant inflammatory and/or fibrotic abnormalities. The histological evaluation of lung tissue in this late phase may help reveal peculiar morpho-phenotypical changes which, together with future studies examining pattern evolution, may help better our understanding of pathogenic mechanisms and provide consistent personalised therapy. Here, we examine the morphological and immunomolecular features of transbronchial lung cryobiopsies (TBLC) performed in patients with persistent lung disease after recovery from SARS-CoV-2 infection and a potential explanation of the observed lung abnormalities.
Methods
Study design and patient selection
We conducted a comparative, prospective, multicentric, investigator-initiated and observational study from January 1, 2021, through to March 31, 2021, with the aim of evaluating the histological profile and immunohistochemical/molecular features of lung tissues in patients with persistent lung involvement demonstrated by high-resolution computed tomography (HRCT) and persistent symptoms (respiratory or systemic) after recovery from SARS-CoV-2 infection. Eligible subjects were aged between 18 and 80 years old and had a history of a molecular diagnosis of COVID-19 related pneumonia, with recovery at least 30 days before, persistent lung involvement on HRCT scan analysis >5%, persistent symptoms (respiratory and/or systemic) and no contraindications for lung biopsy. Recovery was defined according to national directives updated in December 2020 by the presence of two consecutive negative tests for SARS-CoV-2 by real-time reverse transcriptase (RT)-PCR in 24 h associated with possible improvement/stabilisation of symptoms. Key exclusion criteria were severely compromised lung function or resting hypoxaemia, high bleeding risk, pulmonary hypertension assessed by echocardiography and severe comorbidities (supplementary material); patients who could not provide consent or who refused biopsy were excluded. This study was in accordance with regulations issued by the Helsinki Declaration; the protocol was approved by the Institutional Review Board of the Area Vasta Romagna Ethical Committee (Prot. 5285/2021) and patients provided their informed consent. HRCT and TBLC were performed as described previously and reported in the supplementary material [14–17]. Morphological examination was based on conventional haematoxylin and eosin stains and immunostaining with cytokeratin 7. Five dedicated pulmonary pathologists (AD, GR, VP, MC and CD) blindly and independently reviewed the slides from all biopsies and quoted morphological features involving different compartments constituting the lung parenchyma: epithelial and vascular components, alveolar and interstitial spaces, and inflammatory cells. Divergent opinions were then overtly discussed and a final consensus was reached in all cases. All immunohistochemical tests were performed in the ULTRA Benchmark automated immunostainer (Ventana Medical Systems/Roche, Tucson, AZ, USA) using standard procedures and reagents are described in supplementary table S1. We analysed 15 control cases with diffuse parenchymal lung diseases as uninfected control specimens: three with cryptogenic organising pneumonia (OP), three with nonspecific interstitial pneumonia (NSIP), two with OP/NSIP, two with usual interstitial pneumonia (UIP), two with fibrotic hypersensitivity pneumonitis, one with idiopathic acute fibrinous and organising pneumonia, one with sarcoidosis and one with smoking-related interstitial fibrosis.
Results
Clinical characteristics
164 patients were referred to our post-acute outpatient service for suspected pulmonary sequelae after COVID-19. In 141 patients, parenchymal lung disease was absent or <5% and 13 patients had contraindications for lung biopsy or did not provide consent for the procedure; 10 patients underwent TBLC. The median age was 61 years (range 38–76 years) and 78% were men. Seven out of ten patients had been hospitalised but none had been intubated. Clinical characteristics are summarised in supplementary table S2. Patients underwent bronchoscopy on average 3.5 months after recovery from SARS-CoV-2 infection. At the time of biopsy all cases had negative results on SARS-CoV-2 molecular swab testing. Most patients reported fatigue and low-grade fever, a minority reported joint and muscle pain and depression (supplementary table S2). Median forced vital capacity (FVC) was 77% of the predicted value (range 46–116%); median diffusing capacity of the lung for carbon monoxide (DLCO) was 51% of the predicted value (range 43–80%). The most representative laboratory test results are shown in supplementary table S3.
HRCT
Radiological findings are summarised in table 1. In one subgroup (cluster 1; n=2 patients), CT showed fibrotic appearances with some aspects of interstitial involvement, architectural distortion and traction bronchiectasis. In one of these patients, a previous CT scan had shown a pre-existing background of fibrotic interstitial lung abnormalities (ILA), which were stable and persistent after COVID-19 pneumonia (figure 1). In the other patient, acute consolidations masked and covered the possible presence of underlying pre-existing traction bronchiectasis.
TABLE 1.
Radiological and pathological findings in patients with persistent lung disease
| Acute phase HRCT | Post-acute phase HRCT | Time from recovery (days) | Histology | |
| Patient 1 | Peripheral consolidation, mild crazy paving (OP-like pattern) | Reticulation with some perilobular pattern, traction bronchiectasis | 100 | AECII hyperplasia, honeycombing, patchy fibrosis, fibroblastic foci, lymphoid nodules |
| Patient 2 | Bilateral ground glass, vessel enlargement (venoplegic hyperhemic pattern), smoking-related ILD (AEF+emphysema) | Mild peripheral ground glass, smoking-related ILD (AEF+emphysema) | 227 | AECII hyperplasia, interstitial fibrosis, perivascular fibrosis, lymphoid nodules, macrophages containing light brown pigment |
| Patient 3 | Not performed | Peripheral consolidation, ground glass, vessel enlargement (gravity-dependent perilobular pattern) | 32 | AECII hyperplasia, OP, vascular dilatation, perivascular fibrosis, perivascular lymphocytes, lymphoid nodules |
| Patient 4 | Peripheral consolidation, ground glass, vessel enlargement (gravity-dependent perilobular pattern) | Scattered bronchial ectasis, no fibrotic distortion, mild perilobular pattern | 65 | OP |
| Patient 5 | Ground glass, vessel enlargement, (venoplegic/hyperhaemic pattern in a background of ILA) | Stable ILA, mild ground-glass attenuation with mild perilobular pattern | 76 | AECII hyperplasia, perivascular lymphocytes, interstitial fibrosis |
| Patient 6 | Ground glass, vessel enlargement (venoplegic/hyperhaemic pattern) | Extensive consolidations, halo sign, reverse halo sign, perilobular pattern | 160 | AECII hyperplasia, OP, vascular dilatation, perivascular fibrosis, perivascular lymphocytes, interstitial fibrosis |
| Patient 7 | Not performed | Ground glass, vessel enlargement (venoplegic/hyperaemic pattern in a background of NSIP-OP) | 56 | AECII hyperplasia, perivascular lymphocytes, interstitial fibrosis |
| Patient 8 | Peripheral consolidation, ground glass, vessel enlargement (gravity-dependent perilobular pattern) | Mild residual ground glass with perilobular pattern | 123 | Vascular dilatation, perivascular fibrosis |
| Patient 9 | Ground glass, vessel enlargement (venoplegic/hyperhaemic pattern) | Mild residual ground glass with perilobular pattern | 137 | Minimal patchy AECII, vascular dilatation, perivascular fibrosis |
| Patient 10 | Not performed | PPFE, mild ground glass | 44 | Vascular dilatation, perivascular fibrosis |
HRCT: high-resolution computed tomography; OP: organising pneumonia; AECII: alveolar epithelial type 2 cells; ILD: interstitial lung disease; AEF: airspace enlargement with fibrosis; ILA: interstitial lung abnormalities; NSIP: nonspecific interstitial pneumonia; PPFE: pleuro-parenchymal fibroelastosis.
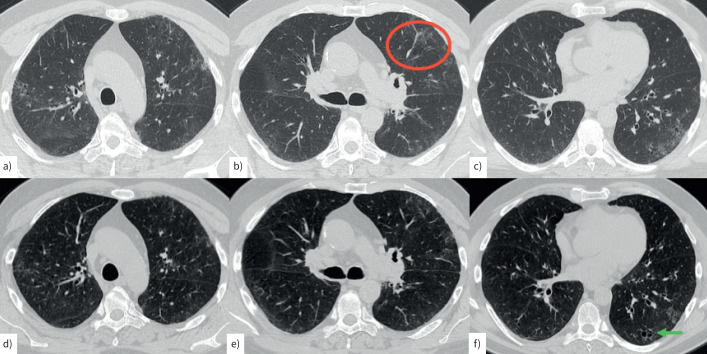
FIGURE 1.
Computed tomography (CT) scan in a 60-year-old male smoker. a–c) During acute infection, mild peripheral ground-glass attenuation is present in both upper lobes, mainly in the left hemithorax (red circle). Scattered areas of cystic changes suggestive of airspace enlargement with associated fibrosis (AEF) with mild architectural distortion are present bilaterally. d–f) A CT scan performed 4 months later shows a reduction in ground-glass attenuation, with residual ground-glass attenuation and areas of AEF (f, green arrow).
In a second subgroup (cluster 2; n=5 patients), CT showed persistence of ground glass with a perilobular pattern and consolidations as observed in OP or fibrotic NSIP (figures 2 and 3); a common feature was residual vessel enlargement (a gravity-dependent perilobular pattern as assessed after prone positioning) as supposed venular dilatation in the perilobule [18].
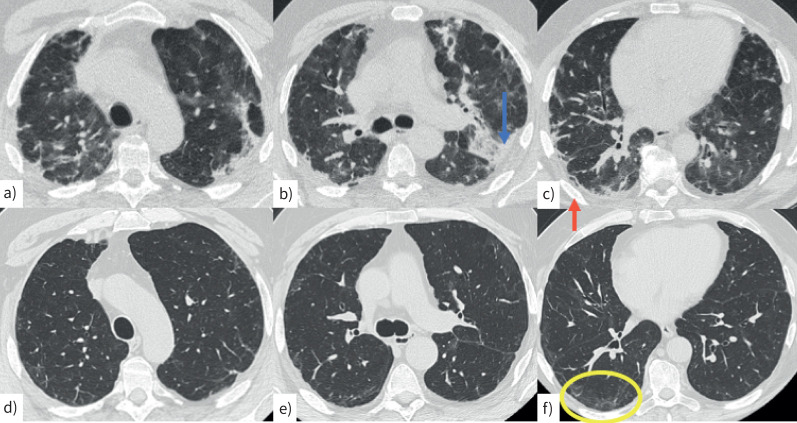
FIGURE 2.
Computed tomography (CT) scan in a 62-year-old man with a–c) acute COVID infection characterised by bilateral, peripheral consolidations (b, blue arrow) and perilobular pattern (c, red arrow). d–f) A CT scan performed 2 months later shows mild peripheral reticulation and minimal perilobular pattern, mainly in the right lower lobe (f, yellow circle).
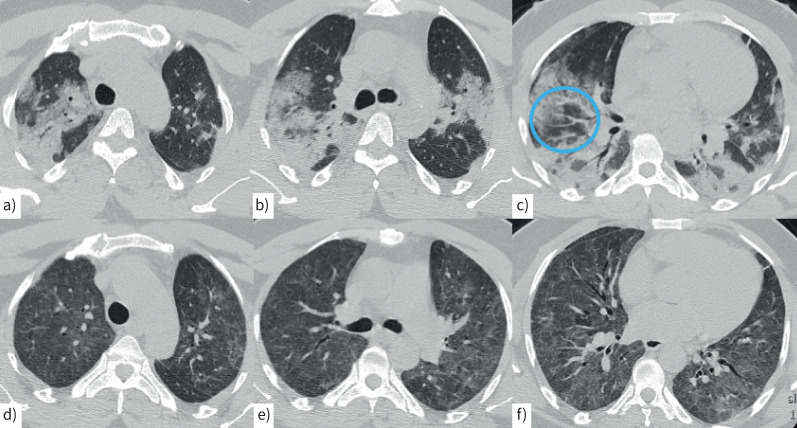
FIGURE 3.
Computed tomography (CT) scan in a 50-year-old man with a–c) COVID-related acute pneumonia with extensive, peribronchovascular consolidations in both lungs and lobular sparing in both lower lobes. Moderate ground-glass attenuation is present in both upper lobes associated with vessel enlargement (c, blue circle). d–f) A CT scan performed 2 months later shows mild, diffuse ground-glass attenuation in both lungs associated with central bronchiectasis in the middle lobe and in both lower lobes.
Finally, a third subgroup (cluster 3, n=3) was characterised by only mild residual radiological changes, with mild peripheral ground glass associated with a perilobular pattern in two out of the three patients. In a single patient, features suggesting pleuro-parenchymal fibroelastosis in the upper lobes were observed.
Histological patterns and immunohistochemistry
Histological evaluation on haematoxylin and eosin-stained slides revealed significant modifications of the pulmonary structure in all 10 investigated patients, matching the three-cluster separation. All patients were tested and had negative results for SARS-CoV-2 (in situ hybridisation in tissue (single molecule fluorescence in situ hybridisation method; HuluFISH, PixelBiotech, Heidleberg, Germany) and real-time RT-PCR assay in bronchoalveolar lavage (BAL) fluid).
Cluster 1 (“pre-existing chronic fibrosing”)
Cluster 1 included two patients with features of either UIP, as defined by architectural distortion, spatial and temporal heterogeneity of scarring modifications, fibroblastic foci and microscopic honeycombing (figure 4), or fibrosing smoking-related interstitial pneumonia, as defined by the accumulation of macrophages containing smoking-related light brown pigment in alveolar spaces and interstitial collagenous fibrosis (supplementary table S4). The immunohistochemical analysis in the first patient was consistent with UIP, including tubulin, β3 class III (TUBB3) in myofibroblasts and focal p16 expression in pneumocytes [19]. The second patient (showing a pre-existing background of smoking-related interstitial lung disease (ILD) on HRCT which persisted after COVID-19 pneumonia) had histological features consistent with a smoking-related ILD, with lymphoid nodules [20].
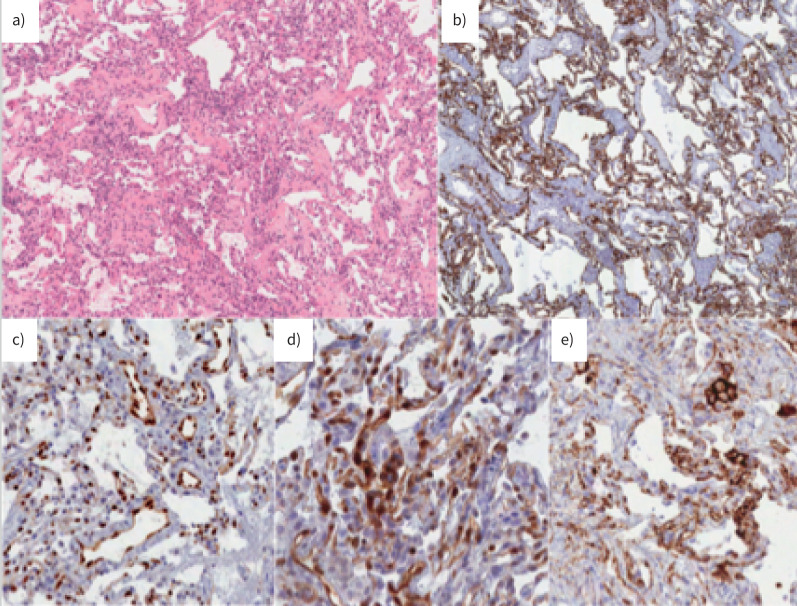
FIGURE 4.
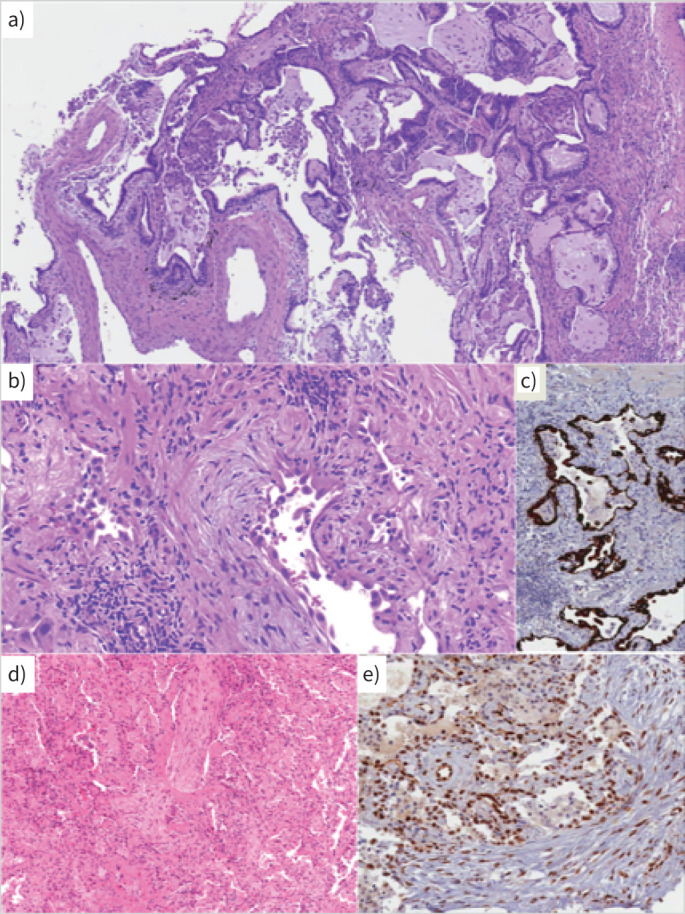
a) Cluster 1. A case with a usual interstitial pneumonia pattern: architectural distortion, spatial and temporal heterogeneity of scarring modifications and microscopic honeycombing. Haematoxylin and eosin (H&E) staining ×10 magnification. b) Cluster 2. A case with morphological evidence of ongoing interstitial fibrosis and extended alveolar epithelial type II cell hyperplasia as observed in diffuse alveolar damage, proliferative phase. H&E staining. c) Cluster 2. Cytokeratin 7 immunostaining of epithelial cells, at low magnification, showing the severe effacement of parenchymal structure. d) A case with organising pneumonia pattern. H&E staining. e) Most epithelial cells and endothelial cells express nuclear phosphorylated signal transducer and activator of transcription 3. b–e ×20 magnification.
Cluster 2 (“acute/subacute injury”)
Cluster 2 was characterised by different types and grades of lung injury, ranging from OP or overlapping OP/NSIP to diffuse alveolar damage (DAD) (proliferative phase, two cases) (figure 4) [21]. These cases shared morphological and immunohistochemical similarities with cases of severe COVID-19 pneumonia previously described [16]. In the two cases consistent with DAD (proliferative/organising without hyaline membranes), the interstitial spaces were diffusely thickened by dense fibrosis and aggregates of myofibroblasts (figure 4). Residual alveolar spaces were lined by patchy collections of hyperplastic alveolar epithelial type II cells (AECII), expressing phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and Ki67. Four cases were characterised by the occurrence of endoalveolar Masson's bodies and various amounts of interstitial thickening (OP/NSIP) (figure 4 and supplementary table S4).
With regards inflammatory cells, abundant immune infiltrates, composed mainly of lymphocytes, were present in all cluster 2 cases, either diffusely distributed in interstitial spaces or forming perivascular aggregates; lymphoid infiltrates were mainly composed of T- and B-cells; in T-cells, CD4 cells and CD8 subsets were almost equally represented. Macrophages were less represented, neutrophils were rare and no eosinophils were observed.
With regards alveolar cells, hyperplastic AECII were characterised by a high Ki67 index and nuclear pSTAT3. TUBB3 staining was also expressed in alveolar cells, mainly in the patients with the most severe damage.
With regards the vascular bed, diffuse indoleamine 2, 3-dioxygenase (IDO), programmed cell death 1 ligand 1 (PD-L1) and pSTAT3 immunostaining were detected in the endothelial cells of both interstitial capillary vessels and venules; this represented the most relevant finding in this subgroup [22].
Cluster 3 (“vascular changes”)
Cluster 3 was characterised by diffuse vascular increase, dilatation and distortion (both capillaries and venules) within an otherwise normal parenchyma (minimal patchy AECII hyperplasia in one case) (supplementary table S4 and figure 5). This pattern was comparable to that described in early-phase COVID-19 pneumonia with conserved parenchymal structure [16].
FIGURE 5.
Cluster 3. A case with diffuse vascular increase, dilatation and distortion (both capillaries and venules) within an otherwise normal parenchyma. a) Haematoxylin and eosin staining ×10 magnification, b) cytokeratin 7 immunostaining, c) diffuse and strong endothelial expression of phosphorylated signal transducer and activator of transcription 3, d) indoleamine 2, 3-dioxygenase and e) programmed cell death 1 ligand 1. b–e ×20 magnification.
With regards inflammatory cells, a small number of macrophages and interstitial T-lymphocytes were present, without obvious predominance of either CD4 or CD8. PD-L1 was expressed in alveolar macrophages (as may commonly be observed in alveolar macrophages in normal conditions), but no PD-L1 immunoreactivity was observed in the epithelial alveolar cells.
With regards the vascular bed, diffuse PD-L1 staining decorated the capillary lining along with IDO reactivity in endothelial cells (figure 5).
With regards the stromal/interstitial compartment, no TUBB3 reactivity was observed.
These cases shared similarities with some cases of early/mild COVID-19 pneumonia previously described [16], in particular the persistence of diffuse and strong expression of PD-L1 and IDO in endothelial cells of both interstitial capillaries and venules in post-acute-phase cases. This peculiar expression profile may be more related to SARS-CoV-2 pneumonias, given that these markers are not typically observed in lung tissue obtained in healthy subjects [23, 24] and/or in control cases with diffuse parenchymal lung diseases as uninfected control specimens. These morphological abnormalities were mirrored in BAL fluid (supplementary table S3); in fact, all patients with lymphocytosis in BAL fluid (>20%) were characterised by OP in lung tissue, with abundant immune infiltrates, composed mainly of lymphocytes, located in perivascular and interstitial spaces, sometimes forming nodular aggregates.
Discussion
To our knowledge, this is the first prospective analysis of morpho-phenotypical changes in patients with persistent symptoms and residual parenchymal lung disease after recovery from COVID-19. Most published studies have been small and mainly focused on clinical sequelae in patients admitted to hospital for COVID-19 [1, 3–5, 6, 8, 10]. Little is known of the extent and features of lung pathology in COVID-19 survivors, especially in those who had milder infections not requiring hospitalisation. Three cases with post-COVID histologically confirmed OP have been published so far (two transbronchial lung biopsy and one surgical lung biopsy) [25, 26], as well as a report on 11 asymptomatic patients who had previously contracted COVID-19 and underwent elective lung resections for other unrelated indications (recovered asymptomatic survivors were confirmed to have no discernible histopathological changes suggesting parenchymal damage) [27]. As is the case with other ILDs, lung biopsy may have a significant role in post-COVID ILDs to better define histological patterns for the purposes of management. The ILD community has long been aware that it is not unusual to see fibrotic NSIP and OP progressing to fibrosis in survivors of DAD/acute lung injury [28]. Current algorithms mostly view post-COVID lung disease as a single entity; however, the existence of different COVID-induced pathways may underlie separate post-COVID lung entities, which may require separate management. In this multidisciplinary study, we have identified three different subtypes or subgroups (table 2).
TABLE 2.
Identification of possible subgroups (clusters) of patients with post-acute COVID-19
| Clinical picture | Radiological pattern | Histopathology | |
| Cluster 1 | Respiratory symptoms (cough, dyspnoea), no systemic symptoms, compromised DLCO | Interstitial lung disease, lung fibrotic appearances with architectural distortion, traction bronchiectasis | UIP or fibrosing interstitial pneumonia, spatial and temporal heterogeneity of scarring modifications, fibroblast foci, honeycombing |
| Cluster 2 | Respiratory symptoms (cough, dyspnoea), systemic symptoms (fever, fatigue), compromised DLCO | Peripheral consolidation, ground glass, perilobular pattern, vessel enlargement, reverse halo sign | Lung injury, OP, OP/NSIP, diffuse alveolar damage, no hyaline membranes, lymphocytic inflammatory infiltrate |
| Cluster 3 | Respiratory symptoms (cough, dyspnoea), systemic symptoms (fatigue, aches), normal DLCO | Mild residual lung disease, mild ground glass, perilobular pattern | Diffuse vascular increase, dilatation and distortion (capillaries and venules), otherwise normal parenchyma |
DLCO: diffusing capacity of the lung for carbon monoxide; UIP: usual interstitial pneumonia; OP: organising pneumonia; NSIP: nonspecific interstitial pneumonia.
Cluster 1 was characterised by features suggesting the occurrence of ILD before SARS-CoV-2 infection. Both patients in this cluster were male and former smokers. Clinical behaviour was characterised by persistent respiratory symptoms after COVID-19 pneumonia, shortness of breath on exertion and cough, with less frequent systemic symptoms (supplementary table S2). One patient's disease, with a pattern consistent with UIP, was characterised by fibrotic appearances on CT scan and lung tissue, architectural distortion, traction bronchiectasis, spatial and temporal heterogeneity of scarring modifications, and extended honeycombing (figure 4 and table 1). It is possible to argue that the acute consolidations in this case masked and covered the presence of underlying pre-existing fibrosing ILD. The second patient had a documented pre-existing background of fibrotic ILA, which were stable and persistent after COVID-19 pneumonia. Previous pulmonary function tests were normal (FVC 103% predicted, forced expiratory volume in 1 s 104% predicted, DLCO 76% predicted). Lung cryobiopsy showed a pattern consistent with smoking-related interstitial fibrosis. We hypothesise that a number of post-COVID-19 cases characterised by radiological and/or pathological findings of fibrotic appearances might actually be more an expression of a pre-existing pulmonary interstitial disease (which may or may not be accelerated by the virus infection itself) than strictly related to the viral infection. Acceleration of pulmonary fibrosis by a respiratory virus has been described in the bleomycin model of pulmonary fibrosis [29]; interestingly, in these cases, foci of cells expressing the cell senescence-associated marker p16 were observed as in UIP/idiopathic pulmonary fibrosis [30–34].
Cluster 2 included patients with a more “auto-inflammatory” phenotype, with different clinical presentation, radiological features and an immunomorphological pattern. The inflammatory pathway may be triggered by the virus itself causing type 2 pneumocytes proliferation/activation, an influx of inflammatory cells and vascular dysfunction. CT showed residual ground glass with a perilobular pattern, sometimes consolidations, halo signs and reverse halo sign, with no fibrotic distortion; these changes can be included in a OP-like pattern (table 1). All patients had different morphological types and grades of lung injury, ranging from OP to DAD (proliferative phase) and had in common abundant immune infiltrates, composed mainly of lymphocytes, in the perivascular and interstitial spaces, sometimes forming nodular aggregates. In this cluster, patients more frequently had systemic symptoms, such as fever and fatigue, together with respiratory symptoms (supplementary table S2). Cluster 2 patients were treated with steroids following lung biopsy results (details are reported in the supplementary material). Patients with OP on lung biopsy experienced significant improvement in both symptoms and lung function impairment; a 3-month follow-up CT scan showed a significant reduction in ground-glass areas and perilobular pattern in all cases. Patients with DAD/proliferative phase features on lung biopsy showed moderate improvement, both clinical and functional, with complete resolution of fever and a reduction of shortness of breath, although persistent on mild to moderate exertion; the 3-month follow-up CT scan showed a slight reduction of the extension of parenchymal involvement.
Cluster 3 included patients with persistent respiratory and systemic symptoms but minimal changes on CT. The histological picture was characterised by dilatation of the lumen of alveolar capillaries and venules and hyperplasia of capillaries within an otherwise normal or minimally abnormal parenchyma (minimal patchy AECII hyperplasia and heterogeneous interstitial thickening in one case). We considered this “pattern” as similar in some aspects to pulmonary capillary haemangiomatosis (hyperplastic interstitial capillaries along and in the alveolar walls, frequently dilated lumens of the capillaries and capillary congestion); however, we think they are clearly two distinct conditions. In fact, the capillary proliferation observed in cluster 3 cases was not typically centred around bronchovascular bundles and did not create any nodular appearance; endothelial cells were negative for proliferation cues (i.e. Ki-67) and vascular changes also involved post-capillary venules, which showed dilated and tortuous lumen, with thickened edematous walls and a patchy perivascular lymphocyte infiltrate, all changes that are not observed in capillary haemangiomatosis. In addition, hemosiderin-laden macrophages and intimal thickening of small muscular arteries were not observed. All these patients underwent echocardiography and pulmonary artery hypertension was excluded. Interestingly, the expression of molecules involved in immune derangement and vascular tone control (STAT3, IDO, PD-L1) was altered as in early-phase COVID-19 pneumonia [16]. In order to assess whether these findings were related to SARS-CoV-2 infection, we analysed the control cases with diffuse parenchymal lung diseases as uninfected control specimens and we found that the endothelial expression of these markers was faint and/or restricted to rare cells.
One of the most relevant findings of our study was the persistent elevated expression of nuclear pSTAT3 in both alveolar epithelial cells and endothelial cells of interstitial capillaries and venules months after the clearing of SARS-CoV-2 infection. Nuclear pSTAT3 was particularly overexpressed in cluster 2 patients, who were characterised by more prominent systemic symptoms (mostly fever and fatigue) associated with persistence of ground-glass opacities on CT scan, a perilobular pattern and consolidations. These cases shared similarities with cases of severe COVID-19 pneumonia previously described [16], in which alveolar epithelial type 2 cells and endothelial cells expressed nuclear pSTAT3. A significant reduction in pSTAT3 after administration of baricitinib in patients with severe COVID-19 pneumonia has previously been demonstrated; the baricitinib prevented disease progression, with a safer and more favourable clinical outcome [35]. These results might suggest a potential rationale for the use of inhibitors of the Janus kinases JAK1 and JAK2 in a subgroup of patients with post-COVID persistent symptoms. All patients included in the study had negative test results for SARS-CoV-2 in BAL fluid, confirming the fact that pulmonary manifestations are not associated with the persistence of the virus in the lung, but are related to pathogenic mechanisms irrespective of viral clearance that continue after infection. We observed that vascular abnormalities (disordered angiogenesis with luminal enlargement and dilated vessel wall, perivascular T-cell infiltration) are distinctly present from the beginning of COVID-19 pneumonia, can persist through progressive disease stages [16] and may linger for several weeks/months after infection. The co-regulatory ligand PD-L1 and the tolerogenic enzyme IDO are part of negative feedback circuits that restrain immune responses and maintain peripheral tolerance [36–38] and are both implicated in defence mechanisms against lung injury [39–42]. Tryptophan is a substrate for IDO enzymatic activity, and abnormal levels of tryptophan metabolites have been demonstrated in COVID-19, with normalisation at recovery [42–44]. As previously demonstrated, IDO is involved in the regulation of vascular remodelling and relaxation [24] and, according to this study, its potential role in reducing pulmonary vascular resistance may also be involved in long COVID or post-COVID syndrome. These observations might explain the persistence in some post-COVID cases of areas of ground-glass attenuation on CT, with or without a “crazy paving” pattern, associated with enlarged veins contained within the ground-glass areas, partially disappearing with a change from the supine to the prone position (the so-called venoplegic/hyperaemic pattern) [42]. In some post-COVID cases of this series, we observed the persistence of these features on CT (table 1) through progressive disease stages several weeks/months after infection.
This study has some limitations. First, baseline data of pulmonary function tests and chest CT were not available, the number of enrolled patients was limited and the patient group was heterogeneous. Second, the short timeframe between the post-acute sampling and the acute COVID-19 infection may be a further issue of concern because it may not be representative of long-term abnormalities. In future, a larger sample would be needed and longer longitudinal observational studies will be critical to elucidate the health consequences attributable to COVID-19.
In conclusion, this is the first study showing a correlation between histological patterns and immunohistochemistry with clinical and radiological pictures of patients with post-COVID syndrome. It has identified different subtypes or phenotypes that potentially have different underlying pathogenic mechanisms (table 2). Our results confirm that a multidisciplinary evaluation of these patients is needed (instead of a limited imaging evaluation) to identify key patient subgroups and their current optimal management. In particular, immune-histochemical evaluation of lung tissue may help to reveal peculiar morphological and morpho-phenotypical changes of this new post-COVID/long COVID syndrome and aid our understanding of pathogenic mechanisms. This will lay the groundwork for efficient therapeutic intervention studies and future follow-up plans as previously described [17, 45–48]. Although the pathogenic role of different cell types and the mechanisms leading to different disease endotypes are not fully understood, this heterogeneity is likely to reflect different COVID-induced phenotypes of ILD, probably in subjects predisposed to abnormal wound healing. Post-COVID lung disease is not a single entity, but includes different subtypes or subgroups [49], each of them potentially requiring separate and different management.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02411-2021.SUPPLEMENT (157.7KB, pdf)
Shareable PDF
Acknowledgements
We thank Fondazione Cariverona (ENACT Project) for the financial support and AMMP (Associazione Morgagni Malattie Polmonari) for patient support.
Footnotes
Author contributions: C. Ravaglia, C. Doglioni, M. Chilosi and V. Poletti conceived of and designed the study; C. Ravaglia, C. Doglioni and M. Chilosi wrote the paper; C. Ravaglia, C. Doglioni, M. Chilosi, S. Piciucchi, A. Dubini, G. Rossi and V. Poletti contributed to data interpretation; all other authors commented on drafts of the paper and contributed to writing of the final version of the manuscript
Conflict of interest: The authors report no competing interests.
References
- 1.Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect 2021; 27: 1041. doi: 10.1016/j.cmi.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells AU, Devaraj A, Desai SR. Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 2021; 299: E216–E218. doi: 10.1148/radiol.2021204482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darcis G, Bouquegneau A, Maes N, et al. Long-term clinical follow up of patients suffering from moderate to severe COVID-19 infection: a monocentric prospective observational cohort study. Int J Infect Dis 2021; 109: 209–216. doi: 10.1016/j.ijid.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nehme M, Braillard O, Chappuis F, et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med 2021; 174: 1252–1260. doi: 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021; 92: 55–70. doi: 10.1016/j.ejim.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Righi E, Mirandola M, Mazzaferri F, et al. Long-term patient-centred follow-up in a prospective cohort of patients with COVID-19. Infect Dis Ther 2021; 10: 1579–1590. doi: 10.1007/s40121-021-00461-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021; 27: 1507–1513. doi: 10.1016/j.cmi.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021; 4: e2111417. doi: 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6: 100122. doi: 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Casals M, Brito-Zerón P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol 2021; 17: 315–332. doi: 10.1038/s41584-021-00608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montani D, Savale L, Beurnier A, et al. Multidisciplinary approach for post-acute COVID-19 syndrome: time to break down the walls. Eur Respir J 2021; 58: 2101090. doi: 10.1183/13993003.01090-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019; 19: 16. doi: 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One 2014; 9: e86716. doi: 10.1371/journal.pone.0086716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doglioni C, Ravaglia C, Chilosi M, et al. COVID-19 interstitial pneumonia: histological and immunohistochemical features on cryobiopsies. Respiration 2021; 100: 488–498. doi: 10.1159/000514822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chilosi M, Poletti V, Ravaglia C, et al. The pathogenic role of epithelial and endothelial cells in early-phase COVID-19 pneumonia: victims and partners in crime. Mod Pathol 2021; 34: 1444–1455. doi: 10.1038/s41379-021-00808-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piciucchi S. Awake prone positioning for COVID-19 acute respiratory failure: imaging and histological background. Lancet Respir Med 2022; 10: e14. doi: 10.1016/S2213-2600(21)00554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chilosi M, Marcolini L, Caliò A, et al. Immunohistochemistry and molecular biology in transbronchial cryobiopsies. In: Poletti V, ed. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Disease. London, Springer, 2019; pp. 81–102. [Google Scholar]
- 20.Churg A, Muller NL, Wright JL. Respiratory bronchiolitis/interstitial lung disease: fibrosis, pulmonary function, and evolving concepts. Arch Pathol Lab Med 2010; 134: 27–32. doi: 10.5858/134.1.27 [DOI] [PubMed] [Google Scholar]
- 21.Enomoto N, Sumikawa H, Sugiura H, et al. Clinical, radiological, and pathological evaluation of “NSIP with OP overlap” pattern compared with NSIP in patients with idiopathic interstitial pneumonias. Respir Med 2020; 174: 106201. doi: 10.1016/j.rmed.2020.106201 [DOI] [PubMed] [Google Scholar]
- 22.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020; 52: 910–941. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonavente FM, Soto JA, Pizarro-Ortega MS, et al. Contribution of IDO to human respiratory syncytial virus infection. J Leukoc Biol 2019; 106: 933–942. doi: 10.1002/JLB.4RU0219-051RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Y, Christou H, Liu L, et al. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med 2013; 188: 482–491. doi: 10.1164/rccm.201304-0700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaoka K, Minami S, Ihara S, et al. Secondary organizing pneumonia after coronavirus disease 2019: two cases. Respir Med Case Rep 2021; 32: 101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae I-G, Hong KW, Jang JW, et al. Persistent pneumonic consolidations due to secondary organizing pneumonia in a patient recovering from COVID-19 pneumonia: a case report. Research Square 2020; preprint [ 10.21203/rs.3.rs-37580/v1]. [DOI] [PMC free article] [PubMed]
- 27.Diaz A, Bujnowski D, McMullen P, et al. Pulmonary parenchymal changes in COVID-19 survivors. Ann Thorac Surg 2022; 114: 301–310. doi: 10.1016/j.athoracsur.2021.06.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson AG, Osborn M, Devaraj A, et al. COVID-19 related lung pathology: old patterns in new clothing? Histopathology 2020; 77: 169–172. doi: 10.1111/his.14162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Cheng W, Zhang Z. Respiratory syncytial virus infection accelerates lung fibrosis through the unfolded protein response in a bleomycin-induced pulmonary fibrosis animal model. Mol Med Rep 2017; 16: 310–316. doi: 10.3892/mmr.2017.6558 [DOI] [PubMed] [Google Scholar]
- 30.Chilosi M, Carloni A, Rossi A, et al. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 2013; 162: 156–173. doi: 10.1016/j.trsl.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Renzoni EA, Poletti V, Mackintosh JA. Disease pathology in fibrotic interstitial lung disease: is it all about usual interstitial pneumonia? Lancet 2021; 398: 1437–1449. doi: 10.1016/S0140-6736(21)01961-9 [DOI] [PubMed] [Google Scholar]
- 32.Chilosi M, Caliò A, Rossi A, et al. Epithelial to mesenchymal transition-related proteins ZEB1, β-catenin, and β-tubulin-III in idiopathic pulmonary fibrosis. Mod Pathol 2017; 30: 26–38. doi: 10.1038/modpathol.2016.147 [DOI] [PubMed] [Google Scholar]
- 33.Wu K, Kamimoto K, Zhang Y, et al. Basal-epithelial stem cells cross an alarmin checkpoint for post-viral lung disease. J Clin Invest 2021; 131: e149336. doi: 10.1172/JCI149336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui LT, Winters NI, Chung MI, et al. Chronic lung diseases are associated with gene expression programs favoring SARS-CoV-2 entry and severity. Nat Commun 2021; 12: 4314. doi: 10.1038/s41467-021-24467-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest 2020; 130: 6409–6416. doi: 10.1172/JCI141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mondanelli G, Ugel S, Grohmann U, et al. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr Opin Pharmacol 2017; 35: 30–39. doi: 10.1016/j.coph.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 38.Platten M, von Knebel Doeberitz N, Oezen I, et al. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 2015; 5: 673. doi: 10.3389/fimmu.2014.00673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monaghan SF, Thakkar RK, Heffernan DS, et al. Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor, programmed death-1. Ann Surg 2012; 255: 158–164. doi: 10.1097/SLA.0b013e31823433ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Liu L, Visner GA. Nonviral gene delivery with indoleamine 2,3-dioxygenase targeting pulmonary endothelium protects against ischemia-reperfusion injury. Am J Transplant 2007; 7: 2291–2300. doi: 10.1111/j.1600-6143.2007.01942.x [DOI] [PubMed] [Google Scholar]
- 41.Lomas-Neira J, Monaghan SF, Huang X, et al. Novel role for PD-1: PD-L1 as mediator of pulmonary vascular endothelial cell functions in pathogenesis of indirect ARDS in mice. Front Immunol 2018; 9: 3030. doi: 10.3389/fimmu.2018.03030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piciucchi S, Ravaglia C, Vizzuso A, et al. Reversibility of venous dilatation and parenchymal changes density in SARS-CoV-2 pneumonia: toward the definition of a peculiar pattern. Pulmonology 2021; 27: 353–357. doi: 10.1016/j.pulmoe.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansone L, Briviba M, Silamikelis I, et al. Amino acid metabolism is significantly altered at the time of admission in hospital for severe COVID-19 patients: findings from longitudinal targeted metabolomics analysis. Microbiol Spectr 2021; 9: e0033821. doi: 10.1128/spectrum.00338-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo L, Schurink B, Roos E, et al. Indoleamine 2,3-dioxygenase (IDO)-1 and IDO-2 activity and severe course of COVID-19. J Pathol 2022; 256: 256–261. doi: 10.1002/path.5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oldani S, Ravaglia C, Bensai S, et al. Pathophysiology of light phenotype SARS-CoV-2 interstitial pneumonia: from histopathological features to clinical presentations. Pulmonology 2022; 28: 333–344. [doi: 10.1016/j.pilmoe.2021.03.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivarelli S, Falzone L, Torino F, et al. Immune-checkpoint inhibitors from cancer to COVID-19: a promising avenue for the treatment of patients with COVID-19 (Review). Int J Oncol 2021; 58: 145–157. doi: 10.3892/ijo.2020.5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awadasseid A, Yin Q, Wu Y, et al. Potential protective role of the anti-PD-1 blockade against SARS-CoV-2 infection. Biomed Pharmacother 2021; 142: 111957. doi: 10.1016/j.biopha.2021.111957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loretelli C, Abdelsalam A, D'Addio F, et al. PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response. JCI Insight 2021; 6: e146701. doi: 10.1172/jci.insight.146701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solomon JJ, Heyman B, Ko JP, et al. CT of postacute lung complications of COVID-19. Radiology 2021: E383–E395. doi: 10.1148/radiol.2021211396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02411-2021.SUPPLEMENT (157.7KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02411-2021.Shareable (381.7KB, pdf)