Abstract
Mass spectrometry (MS) applies to many fields including clinical research, environmental analysis, and agricultural applications. This critical review summarizes recent developments in the field that can enable MS to be applied effectively in developing countries. In particular, we argue that colorimetric-based low-cost rapid diagnostic tests may be lacking sensitivity and specificity needed for disease screening in asymptomatic people and that closed automated analytical systems may not offer the analysis flexibility needed in resource-limited settings. Alternative strategies proposed here have potential to be widely acceptable in low- and middle-income countries through the utilization of the open ambient mass spectrometry platform that enable microsampling techniques such as dried blood spot to be coupled with miniature mass spectrometers in a form of centralized analytical platform. Consequently, costs associated with sample handling and maintenance can be reduced by >50% of the total ownership cost permitting analytical measurements to be done at high performance-to-cost ratios in the developing world.
Keywords: mass spectrometry, microsampling, developing countries, disease diagnosis, portable analytical systems, point-of-care, centralized detection
INTRODUCTION
Mass spectrometry (MS) – the process of measuring mass-to-charge (m/z) ratio of gaseous ions – is applicable across diverse disciplines including clinical analysis (e.g., drug development, clinical tests, and disease diagnosis), environmental analysis (e.g., drinking water testing, pesticide screening, and assessment of soil contamination), forensic analysis (e.g., trace analysis), pharmaceutical, metabolomics, and proteomics. Most of these applications are associated with scientific and technological developments that have concentrated mainly in the developed world. Without a closer look, one might conclude that MS will be of little use in the developing countries. Afterall, sophisticated instrumentation is involved, one that requires regular maintenance and good laboratory conditions (e.g., temperature and vibration controls).
We must be realistic. In the early 1990’s MS instrumentation underwent major changes from nearly room-size complex instruments to user-friendly bench-top equipment. Further down-sizing of mass spectrometers became a major research topic in academic laboratories in the early 2000’s. In particular, the National Aeronautics and Space Administration (NASA) supported the development of ruggedized miniature mass spectrometers for space explorations purposes (1). Interests of the military to use chemical instrumentation also yielded opportunities to develop even smaller fieldable equipment, which included microfluidic platforms (2) and portable mass spectrometers (3). But are the conditions in space and war zones any better than the conditions existing in the developing countries? It is logical then to assert that the assumption held by many concerning the perceived inability to operate mass spectrometers in the resource-limited settings of developing countries must be re-evaluated. See BOX 1, Supplemental Appendix regarding efforts by Dr. Giles Edwards, Technical Director and Founder of Recycling Organization for Research Opportunities (RORO), to install mass spectrometers in developing countries.
A focus on Africa. In May 2000, The Economist published an article in which Africa was referred to as the hopeless continent (4). Unfortunately, this description persists in the minds of many that encounter somethings associated with Africa, including the possibility of having a functional first-class laboratory for chemical analysis. There are deep problems in Africa: poverty, corruption, and limited infrastructure. Common diseases like malaria and tuberculosis continue to claim lives. Such inadequacies, in part, led the World Health Organization (WHO) to develop the “ASSURED” criteria to help identify the most appropriate diagnostic test for resource-limited environments. This ASSURED criterion would be most effective if considered generic. That is, laboratory infrastructure and equipment will be needed to diagnose diseases such as certain types of cancer and human immunodeficiency virus. Rapid diagnostic tests satisfy most of WHO’s ASSURED criteria, but they fall short when it comes to early disease diagnosis, which requires better sensitivity and specificity (5, 6). Such limitations associated with simple colorimetric tests makes it challenging to perform realistic chemical analysis in these region (Box 2, Supplemental Appendix).
Have we sacrificed performance for affordability? If we consider that not all regions in Africa are severely deprived of infrastructure (there are major cities in Africa – e.g., Johannesburg in South Africa, Lagos in Nigeria, Accra in Ghana, Addis Ababa in Ethiopia, and Cairo in Egypt, just to mention a few), then it will make sense to use performance-to-cost ratio of the diagnostic test as a criterion for selection and not only cost, which eventually leads to the recommendation of less sensitive equipment-free platforms. The performance-to-cost ratio criterion can be made to fit the developing world because of the centralized nature of these communities (7, 8). For example, the centralized system in Africa (Figure 1) is such that even the most deprived regions are not totally isolated. For most part, the system is set up so that villages (severely limited in resources) depend on near-by towns, which in-turn depend on near-by cities. Farmers in villages commute frequently to near-by towns to sell their farm produce and to purchase necessary items for living such as medicine, kerosene, salts, clothes, and tools for farming. Schools and clinics are also available in the near-by towns and these towns have basic social amenities (e.g., electricity, clean water), which can sustain some levels of chemical instrumentation. The upfront cost of instruments can be high, but the overall impact can be very high, given the number of people that can be served by a single equipment. Consequently, this impact can bring higher performance-to-cost ratio. It is important to note that although the African system is socially and politically centralized (9), the medical system is compartmentalized. That is, rarely will one find a single unit of medical laboratory that can perform many different medical tests. This is due, in part, to oversimplification of analytical procedures. Centralization of equipment at a near-by town will go a long way to alleviate the burden associated with this compartmentalization problem, which tends to delay diagnostic outcomes.
Figure 1.
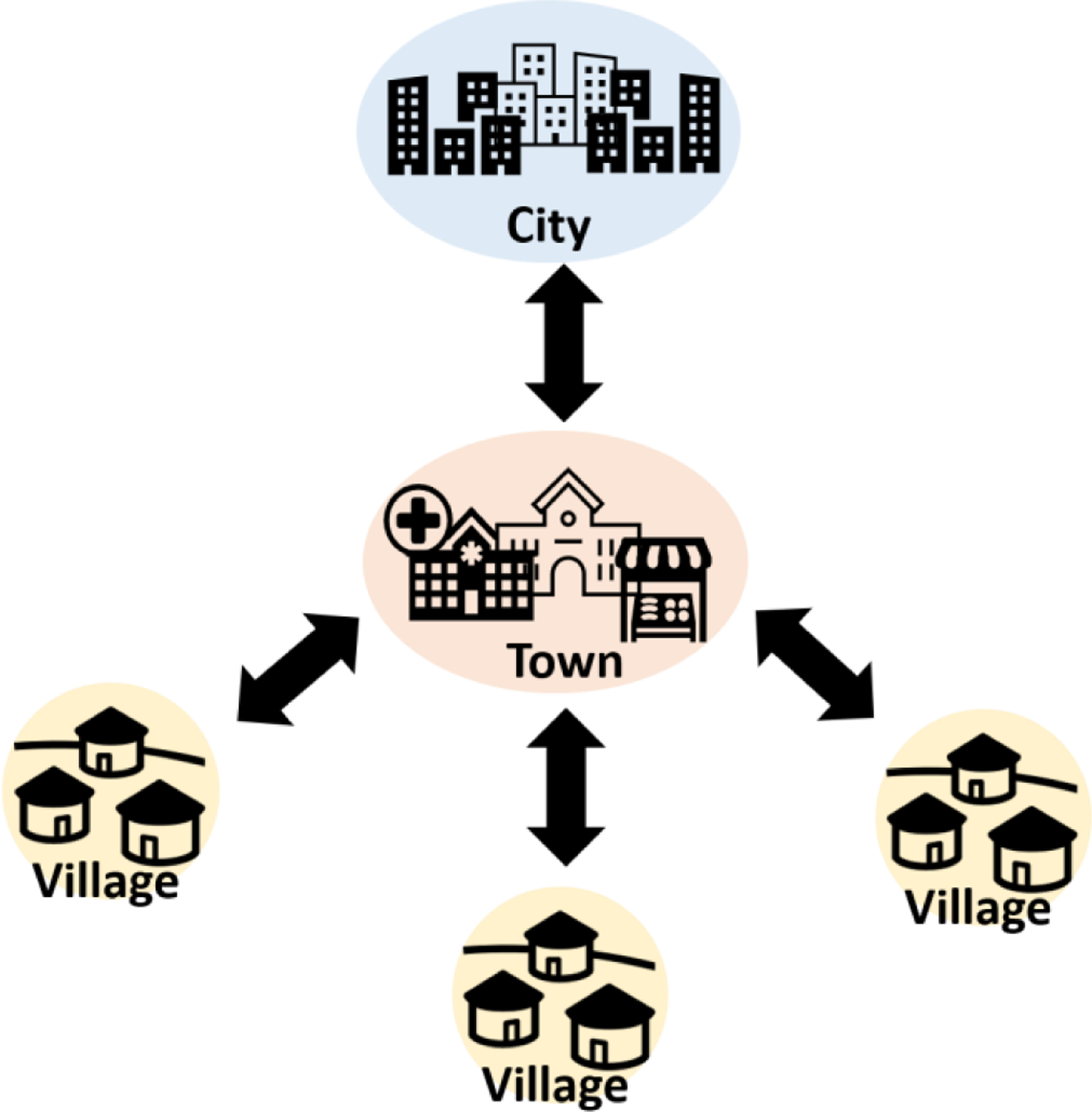
Representation of centralized system in African community; city, town, and villages.
Modern MS presents unique capabilities among all chemical analysis techniques because of its molecular specificity. While most instruments are sensitive to functional groups or some physico-chemical properties, MS measures the molecular weight, which is a universal property for any given chemical compound. In this review, we provide critical discussions on recent developments in the field that can facilitate the use of MS for chemical detection in the developing world. We focus mainly on disease diagnosis, but we also note that the same developments are applicable in environmental, agricultural and forensic analysis.
CHALLENGES WITH CURRENT MODEL AND ALTERNATIVE STRATEGIES
The practical reality is that it is expensive to install and maintain instruments, explaining the limited chemical instrumentation in the developing world, as illustrated by the distribution of mass spectrometers in Figure 2. In November of 2019, MS market research annual report showed relative amounts of sharing mass spectrometers based on region. Not surprisingly, North America held the largest share (nearly ~50%), followed by Europe, Asia pacific and Rest of the World, which included Latin America, Middle East & Africa. For instance, approximately 43 mass spectrometers are counted from five different institutions in Ohio, United State. According to data from National Research Foundation (10), South Africa has the largest number of mass spectrometers in Africa, with a total of 67 mass spectrometers (Figure 2A). Based on number of institutions, we estimated the ratio of mass spectrometers per institution in Ohio and South Africa to be 8.6 (Figure 2B) and 3.5 (Figure 2C), respectively. Other countries in Africa that showed up on the MS distribution map include Ghana (2 mass spectrometers), Kenya (2 mass spectrometers), Egypt (2 mass spectrometers), Nigeria (1 mass spectrometer), and Senegal (1 mass spectrometer). These numbers may not accurate as illustrated in Box 3 (Supplemental Appendix) concerning chemical instrumentation currently found at Kwame Nkrumah University of Science Technology (KNUST), Kumasi, Ghana. The World Economic Situation and Prospects classifies middle-income countries in South America and Asia-Pacific as developing (11). Mass spectrometer distribution in these regions are also summarized in Figure 2D, with countries in Asia-Pacific (e.g., India, Sri Lanka) showing higher numbers of mass spectrometers.
Figure 2.
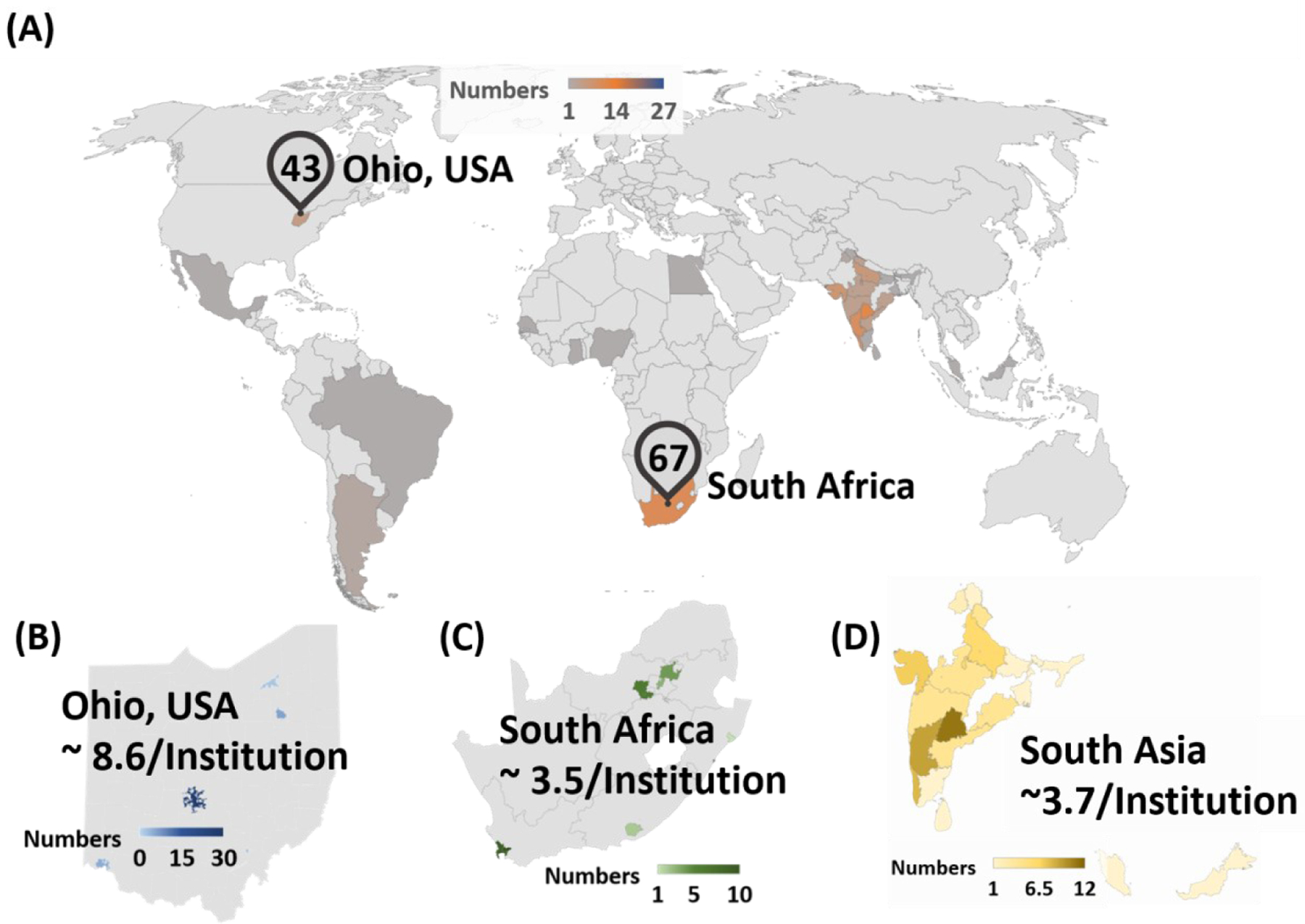
Distribution of mass spectrometers in developing countries is compared with other parts of the world. Number of mass spectrometers are counted from selected institution’s websites. Institutions are selected based on listed top 100 universities of the country at UniRank (http://4icu.org/). (A) Distributions of mass spectrometers in developing countries are shown in world map. For comparison of disparity of owned mass spectrometers, region of Ohio, United States and South Africa are selected. Approximately 43 instruments are found in five different institutions (University of Cincinnati, University of Akron, Ohio University, Cleveland State University, and The Ohio State University) in Ohio, USA. In South Africa, 67 instruments are owned by 18 different institutions (http://eqdb.nrf.ac.za/). Based on the collected database, ratio of number of instruments per institution are calculated for the following regions: (B) Ohio, (C) South Africa, and (D) South Asia.
The total cost of ownership based on current model of chemical analysis can be broken down into two broad categories: purchase cost and lifetime cost (Table 1) (12). The lifetime cost forms about 55% of the total cost, and involves cost associated with materials and consumables, support and training, and administration task. Purchasing cost constitutes 45% of the total cost but can be further divided into external and internal costs, which are estimated to be 70% and 30%, respectively, of the purchasing cost. Sample handling forms a major source of expenditure among the external cost. Therefore, a chemical analysis strategy that seeks to eliminate cost associated with sample handling and instrument maintenance can greatly reduce total cost of ownership, providing opportunities for such analytical methods to be effectively established in low- and middle-income countries. Ambient ionization combined with miniature mass spectrometers has a great potential to make a difference in this respect.
Table 1.
Total cost of ownership
| Purchase Cost (45%) | Lifetime Cost (55%) |
|---|---|
| 1. External (70%) - Analyzer (45%) - Sample handling (55%) |
1. Maintenance - material, suppliers, and consumables - support and training - administration |
| 2. Internal (30%) - installation |
In ambient MS (13), the untreated sample (e.g., whole blood, raw urine, and tissue) are analyzed directly without prior sample preparation (detailed discussion is provided later). This means that costs associated with liquid/liquid (14) and solid/liquid (15) extractions and chromatographic separations (16, 17) can be eliminated. Perhaps the main reason for high cost of sample handling is related to the fact that the methods of sampling (e.g., blood collection) during the pre-analytical phase of chemical analysis have not changed for more than a century. Most current methods (e.g., liquid-phase sampling and absorption methods like dried blood spot, DBS) require cold storage to maintain analyte integrity. Cold storage of samples is a major burden on analytical laboratories, something that is difficult to implement in developing countries. As will be discussed later, dry-state room temperature storage of samples not only has the potential to reduce cost associated with sample handling, but it can also enable wider group people to be reached. An interesting feature of miniature mass spectrometers is that unlike bench-top mass spectrometers, which need to be run constantly to maintain vacuum and analytical performance, miniature mass spectrometer can be turned off and placed on the shelf when it is not in use. It takes about 10 min to establish full vacuum after it is turned on. This feature reduces maintenance cost and by the virtue of it being a miniature instrument also reduces analyzer cost. Collectively, these nice features enabled by MS especially when combined with ambient ionization techniques and dry-state room temperature sample storage can allow centralized chemical analysis for the underserved communities in developing countries as well as point-of-care analytical capabilities yielding real-time results for rapid disease diagnosis (Figure 3).
Figure 3.
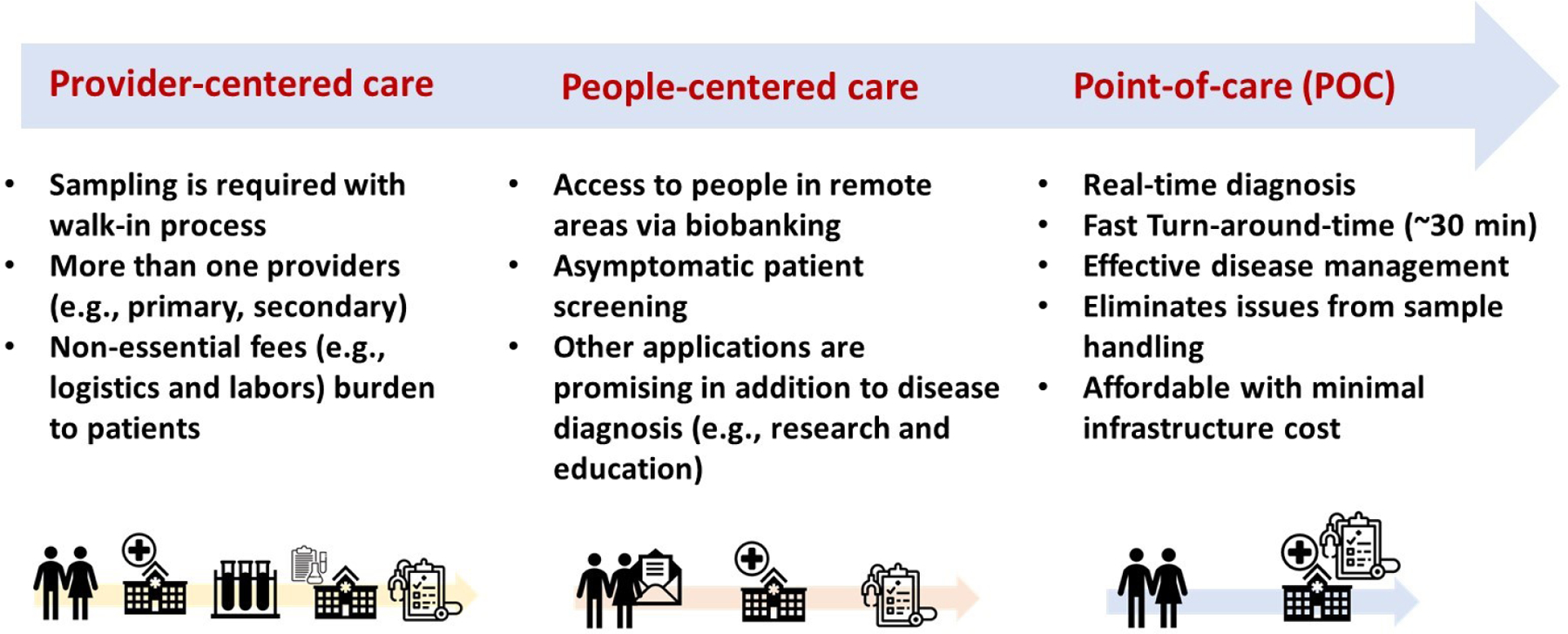
Comparison on different types of healthcare systems. The early model is provider-centered care, which can be transitioned to people-centered care. Major factors of shifting between two system include the fact that healthcare decision is made upon patients’ value and rights more than provider (e.g., doctor). This shift makes partnerships to manage not only individual health, but also in their community range. POC is still people-centered care, however real-time diagnosis or analysis at bed is a key.
As indicated in the introduction, the centralization strategy suggested here for developing countries is different from the traditional sense of provider-centered analysis where high accuracy and precision are the main objectives, typically achieved using hyphenated equipment like gas chromatography-MS (GC-MS) (17, 18), liquid chromatography-MS (LC-MS) (16, 19), and nuclear magnetic resonance (NMR) (20, 21), just to mention a few. Here, centralization is used in the sense of people-centered analysis just as is done for POC analysis, which is patient-centered detection. However, this people-centered strategy (22) for the developing world may utilize miniaturized equipment to serve many people in the community, both near and afar. This differs from the patient-centered POC analysis where analysis take place at bedside and sample is analyzed immediately after collection. The centralization in the developing world can utilize both physical walk-ins and on-demand analysis strategies in the form of direct-to-consumer (DTC) diagnostic tests (23, 24), which will provide remote sampling (e.g., at home by patients) followed by signal development and diagnosis after sending the sample to the central facility (e.g., by mail). Thus, when developed and implemented well, MS-based detection methods for the developing countries can enable both POC and DTC diagnostic methods for symptomatic and asymptomatic patients, respectively.
AMBIENT MASS SPECTROMETRY
Mass spectrometers separate and detect gas-phase ions by their m/z ratio. Traditionally, this process – which involves ion generation, m/z separation and ion detection – takes place under high vacuum conditions. It was demonstrated in the 1980’s through the introduction of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) methods that the ionization step can be performed outside the vacuum environment. However, due to matrix effects, complex mixture analysis still required extensive sample preparation and separation steps before ionization, processes that have increased the complexity of the MS experiment. In the early 2000’s, Prof. Graham Cooks introduced a much simpler method of ionizing complex mixtures without prior sample preparation in the process of desorption electrospray ionization (DESI) (25). This opened new area of research where for the first-time complex mixtures (bacteria culture, whole blood, soil, tissue, etc.) can be analyzed in-situ from their native environment without pre-treatment. Not only can modern mass spectrometers analyze complex mixture outside the high vacuum environment, but we can do so using the native sample (solid, liquid and gas) in the presence of other interfering substances, all under ambient temperature and pressure conditions without use of any chromatographic techniques. This relatively new ambient mass spectrometric technique has many important implications including the fact that (1) vacuum requirements of mass spectrometers are now reduced, allowing the creation of high performance miniature mass spectrometers, (2) field analysis and POC detection are made possible not only due to the production of miniature instruments but also because in-situ analysis is now possible at the site of sample collection, and (3) unique and unconventional application of mass spectrometry such as real-time surgical margin evaluations during operation are also made possible. These specific ionization methods that enable mass spectrometers to analyze complex mixtures from their native state without pre-treatment are often referred to as ambient ionization techniques. Due to the simplicity of the process and the low instrumentation barrier of ambient ionization methods, there have been more than 48 different methods (26) developed for different applications (as of 2019). The same factors (simplicity and less instrumentation) indicate minimal training are required enabling less experienced technicians to operate the equipment something that would be hugely beneficial in the developing world. Several good reviews (26, 27) have recently appeared on the subject of ambient ionization, so we summarize here the main themes and major techniques.
Spray-based ambient ionization techniques
As already stated, the main features of ambient ionization are as follows: (i) analysis with minimal or no sample preparation requirements, (ii) operation outside the vacuum environment of the mass spectrometer, enabling rapid and direct analysis, and (iii) selective transfer of analyte and not the whole sample (tissue, bacteria culture, blood, etc.) to the mass spectrometer enabling native state analysis. Desorption electrospray ionization (DESI, Figure 4A, i), the first of these ambient ionization methods, utilize electrosprayed charged droplets to enable analyte extraction and transfer from the untreated ambient surface. The principle of DESI is related to the process of ESI except that the sample is not present in the electrospray solvent. Instead, the sample is deposited on an ambient surface (e.g., polytetrafluoroethylene) and interrogated using charged liquid droplets derived from the electrospray process (25). The mechanism of DESI is such that thin liquid film is initially formed on the surface when the first few liquid droplets arrive at the surface containing the analyte molecule. This thin film dissolves the analyte and extracts it into the spray liquid. The solvent is sprayed with the aid of nebulizer gas (e.g., N2), which gives the arriving droplets enough kinetic energy and momentum to cause splashes. From the impact of fresh liquid microdroplets and the thin liquid film, secondary droplets are released into air that carry the extracted analyte molecules toward the mass spectrometer for analysis. The main emerging application of DESI and other spray-based ambient ionization methods is tissue analysis and imaging (28, 29). Zhang et.al identified alterations in lipids and metabolites distributions from 42 samples of metastatic tumor tissues (breast and thyroid) and normal lymph node tissues. (30) Feider et.al recently characterized double bond position of free fatty acids from biological tissues directly via integrated 193 nm ultraviolet photodissociation (29). Liquid microjunction-surface sampling probe (LMJ-SSP) (31, 32) and nano-electrospray desorption ionization (nano-DESI, Figure 4A, ii) (33) are variants of DESI and are employed for in vivo studies. Tang et.al used LMJ-SSP for direct sampling of tissue and mouse spinal cord for extracting lipids from samples to identify C=C locations of isomers in lipid and lipid profiling of various tissue sections via imaging (32). The scope of lipid coverage was evaluated with nanospray desorption electrospray ionization mass spectrometry imaging (nano-DESI MSI) on mouse lung tissues. About 284 compounds were observed and unique 57 compounds were identified from nano-DESI MSI compared to LC-MS/MS data (34). The use of photons for analyte desorption have shown noticeable features on tissue imaging due to their capability of depth profiling in z-direction and high lateral resolutions.
Figure 4.
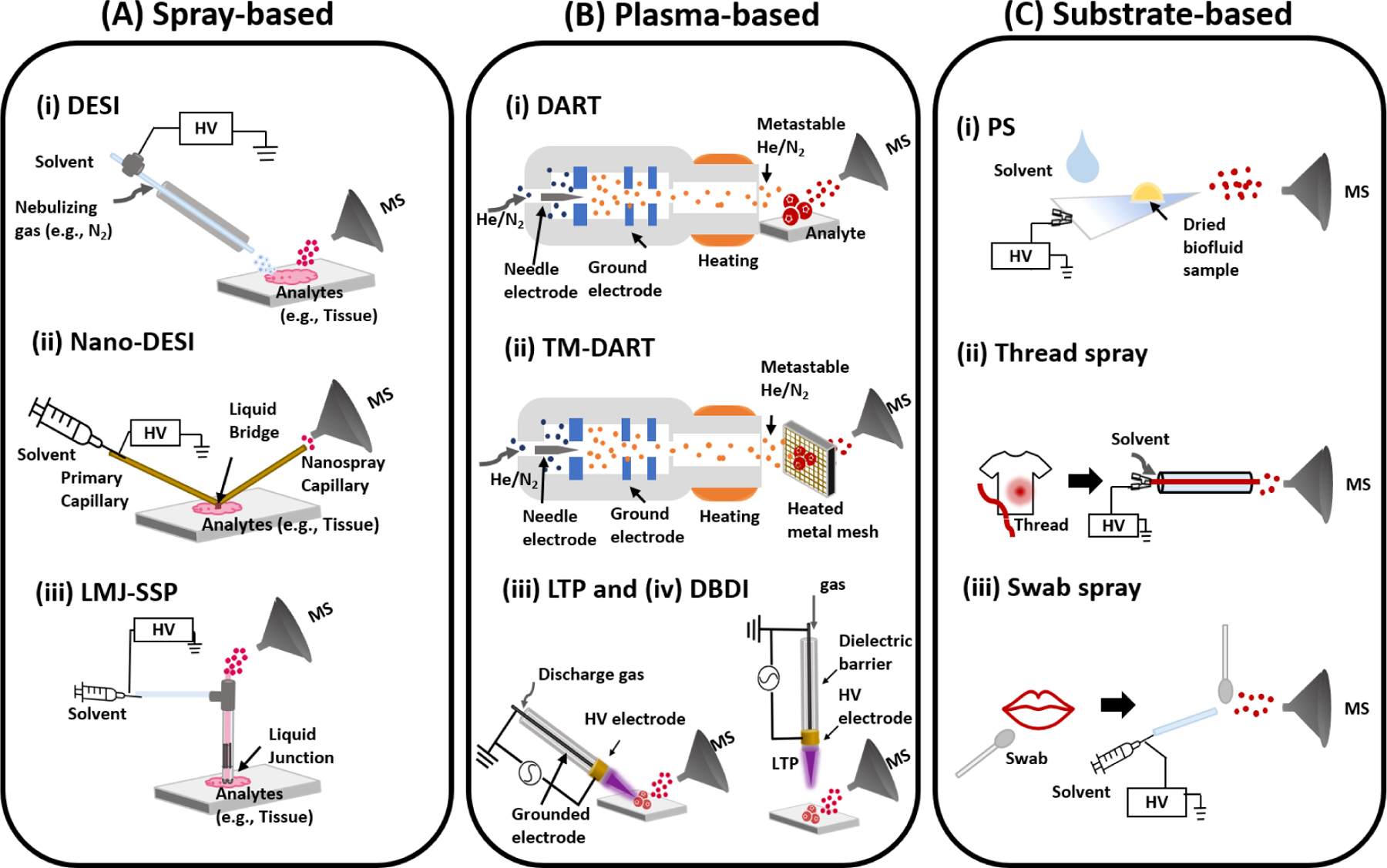
Schematics showing three major types of ambient ionization techniques: (A) spray-based, (B) plasma-based, and (C) substrate-based. Examples of spray-based ambient techniques include (Ai) Desorption electrospray ionization (DESI), (Aii) nano-desorption electrospray ionization (nano-DESI), and (Aiii) liquid microjunction surface sampling probe (LMJ-SSP) are described. Example of plasma-based techniques include (Bi) direct analysis in real time (DART), (Bii) transmission mode-direct analysis in real time (TM-DART), (Biii) Low Temperature Plasma (LTP), and (Biv) dielectric barrier discharge ionization (DBDI). Example of substrate-based methods include (Ci) paper spray (PS), (Cii) thread spray, and (Ciii) swab spray.
Plasma-based ambient ionization techniques
Soon after DESI was introduced, Cody and his coworkers (35) introduced the first plasma-based ambient ionization technique called direct analysis in real time (DART, Figure 4B, i. Both DESI and DART are now commercially available from Waters Inc. and JEOL, respectively. DART generates ions by exposing the condensed-phase sample to metastable species generated by corona discharge. A Carrier gas (e.g., He, Ar, or N2) is heated and the (ionic) species in the plasma are filtered using a series of electrodes. The reagent ions from the DART source interact with sample located between source and MS inlet (35). Solid, liquid, and gas can be analyzed rapidly in their native state without chromatographic separation. The DART-MS platform works well for small molecules (< 500 Da) for applications in food safety (36, 37), environmental monitoring (38), forensic analysis (39, 40), and clinical analysis (41, 42). DART-MS has now become a practical technique for metabolomic profiling and characterization due to its advantages (direct analysis and speed) over conventional methods (LC-MS, GC-MS, and NMR). Recent studies have shown that large molecules (tens to thousands of kDa) readily break down when analyzed by the DART source; for example lipopolysaccharides are observed to fragment into the individual constituents which subsequently enables identification and differentiation of endotoxin with a detection limit as low as 0.03 ng/mL (38). Integration of multivariate statistical analysis (e.g., principle component analysis (PCA)) following DART-MS allows rapid validation and fingerprinting of spectra resulting in effective differentiation of samples. With such integrated platform, Nemes et.al demonstrated at least 250-fold higher sample throughput from screening glycosaminoglycan (43).
Microsampling techniques can be hyphenated as DART-MS. Quantification of endogenous cholesterol in dried human serum spots was performed and detection limit of 20 µg/L was achieved (41). Dried blood spot analysis by DART-MS has also been demonstrated using the quantitation of L-phenylalanine (Phe) from phenylketonuria (PKU) in newborn samples which achieved a limit of detection (LOD) of 3.0 µmol/L. The specific experiments involved dip-it (glass-tips) format using a fixed volume sampling method with auto-loading capabilities (42). Transmission-mode DART (TM-DART, Figure 4B, ii) (44) uses mesh to introduce samples – is another sampling method in DART-MS. SPME-TM-DART-MS platform was introduced by Pawliszyn’s group and recently enabled quantitative studies of drugs of abuse using smaller volumes of oral fluid (15 and 25 µL). Detection limits of all tested analytes were calculated to be several orders of magnitude lower than the cut-off levels provided by Driving Under the Influence of Drugs (DRUID) project of the European Commission (40). Dielectric barrier discharge ionization (DBDI, Figure 4B, iv) (45) and low-temperature plasma (LTP, Figure 4B, iii) (46) form plasma through dielectric barrier discharge, which is generated between two electrodes separated by an insulating dielectric layer once high voltage and alternating current are supplied. The differences between these two methods are the amount of frequency applied (20.3 kHz in DBDI (45) vs 2.5 kHz in LTP (46)) and configuration of experimental setup (direct exposure of sample to plasma in LTP while DBDI does not (47). Due to multiple potential ionization mechanisms with plasma (proton and electron transfer), compounds of environmental interest typically having low polarity (e.g., agrochemical) are widely studied. Perfluorinated compounds48, peroxide-based explosives (49) and 13 chemical warfare agents (e.g., G-, V-, and new nerve agents-series, 50) were studied via DBDI. Likewise, atrazine (51) and 13 explosives (e.g., trinitrotoluene) (52] were analyzed with LTP. Additionally, they are also being applied in clinical studies (45, 46) and the food-industry (53). Identification of 20 different amino acids were achieved with DBDI (45). LTP allowed detection of pharmaceutically related drugs (e.g., nicotine, cocaine) in complex mixtures (e.g., urine, blood) present on ambient surface (46) as well as metabolomic profiling of coffee beans (53).
Substrate-based ambient ionization techniques
Although DESI and DART offer high throughput analytical capabilities by eliminating sample preparation requirements, both techniques require nebulizing gases, which can present difficulties for field studies and implementation in the developing world where gases are overpriced. A new set of techniques referred to as substrate-based ambient ionization methods (Figure 4C) now allow direct complex mixture analysis by MS in the native state of the sample without using nebulizing gases. Paper spray (PS, Figure 4C, iii) (54, 55) was the first substrate-based ambient ionization that was developed by the Cooks’ and Ouyang’s groups in 2010. In this experiment, the sample is loaded onto a piece of paper cut to a triangular sharp tip. Appropriate solvent is applied to the paper triangle containing the sample and high direct current (DC) voltage (3–4 kV) is applied to the wet paper, which generates charged droplets that transfer the extracted analyte from the paper substrate towards the inlet of the mass spectrometer. PS has nice features that make it particularly suitable for application in the developing world: (i) paper is a low-cost material that is locally available in low- and middle income countries, (ii) paper is environmentally friendly, allowing easy disposal after use (e.g., by incineration), (iii) the hydroxyl functional groups in the cellulose paper substrate can be modified to generate other groups with desirable properties (e.g., hydrophobicity) (56–58) that can enhance analytical performance, (iv) the PS experimental setup is simple, requiring only paper, solvent and voltage, and (v) most importantly, PS-MS platform is easy to operate with minimal training (high-school students (59) and undergraduate researchers performed PS experiments with high confidence (60, 61)), whilst still offering rapid, direct quantitative and qualitative capabilities. An automated VeriSpray paper spray ion source is now available from Thermo Fisher.
After the development of PS (Figure 4C, i), various substrates have been used to achieve direct complex mixture analysis including thread spray (Figure 4C, ii) (62–64), swab spray(Figure 4C,iii) (65, 66), touch spray (67), and coated blade spray (68). The common features of these substrates include the use of porous material that can be cut to create fine tip for the generation high electric field upon the application of DC potential and hydrophilic surface properties that allows easy wetting to create conductive surface for the release of charged microdroplets. Highly conductive metal electrodes (as in touch spray and blade spray) also work well, with the fact that no nebulizing gas is required. The polarity of the hydrophilic porous substrates can be altered through chemical treatment to create hydrophobic surfaces. The proper use of solvent allows high extraction efficiency of samples present at the surface of the hydrophobic substrate to improve analytical sensitivity. Bambaurer et.al evaluated the best combination of solvents and ten different organosilanes for modification of paper surface (57). The study found that detection of polar compounds from the hydrophobic substrates was enhanced compared to the untreated hydrophilic paper substrate that has higher binding capacity for polar analytes. Recently, doping agents have been analyzed from raw urine samples using chemically modified paper spray ionization. Vapor-phase silanization reactions were performed on paper using trichloromethylsilane (TCMS) and showed higher performance in both sensitivity and detectability of the doping agents in both positive-and negative-ion modes compared to the results from regular hydrophilic PS (58).
Thread substrates offer many additional benefits when used in substrate-based ambient ionization in the form of thread spray. Single threads of all types (natural and man-made fabrics) can be pulled from clothes, bedsheets, car seats, carpets, etc. The single piece of thread containing the sample of interest (blood, pepper spray, saliva, ground water residue, etc.) is then placed in a tiny glass capillary and the assembly is placed in front of a mass spectrometer inlet as illustrated in Figure 4C,ii. Thread spray ionization is initiated by applying 10 – 20 µL aliquot of spray solvent to the glass capillary to extract analytes present on the thread substrate. Like PS, the wetted thread is then electrically charged by applying a DC high voltage (3 – 5 kV), which results in the release of tiny charged droplets similar to ESI process. The distinguishable feature of the thread spray experiment from other substrate-based method is the process of extracting the analyte from the thread substrate, which can be decoupled from the electrospray process. Since the thread is enclosed in the tiny glass capillary, which is filled with the spray solvent, delayed extraction (<60 s) can be allowed before applying the spray voltage due to limited solvent evaporation. This 60 s extraction time allows up to 80% of the analyte present in the thread to be extracted and thus boosting analytical sensitivity. In recent study, LOD as low as part per quadrillion was reached with the thread spray MS platform for direct analysis of diazepam from dried whole blood (62). This ultra-sensitivity associated with the thread spray MS experiment is particularly suited for forensic analysis in situations where the evidentiary garment has been cleaned. We have shown that the presence of capsaicinoids in pepper spray residues (63) and heme cofactors in bloodstain (64) are all able to be detected from previously washed fabrics using thread spray MS.
SAMPLE HANDLING AND PREPARATION
Proper sample collection and preparation is crucial in clinical studies. Sample preparation covers all steps from collecting biofluids, storage to analysis. Traditionally, blood is collected from patients via venipuncture (5 mL for adults and 1 mL for infants and younger children) and stored under cold temperatures (4–8 °C) during transportation before use (69). In addition to cold storage and large volume requirements, the venipuncture process is an invasive technique and sample collection must be performed by an expert phlebotomist. These requirements severely limit their application in resource-limited settings. Not only does the invasive nature of the process limits wide acceptance (especially among children and infants), it is also difficult to implement the process in the developing world where expert labor is scarce and facilities for storing the collected blood samples are not readily available. Maintaining the integrity of analyte during storage is important for all kinds of analysis, especially for labile compounds such as proteins, lipids, DNAs, and even some small organic compounds. These compounds easily degrade due to external factors such as temperature, humidity, time, and process of sampling (70). To ease the invasiveness of the venipuncture process, recent sample collection platforms utilize microsampling methodologies (i.e., volume of biological samples < 50–100 µL) (70, 71). The small volume requirements and cost effectiveness of microsampling techniques makes them suitable for a wider population as well as applicable to resource-limited conditions. Also, the ability to store microsamples in the dry state brings additional advantages that reduce shipping requirements compared to liquid biofluid samples. However, cold storage is still a requirement for most micro-sampling platforms. Examples of micro-sampling techniques include dried blood spots (DBS), volumetric absorptive microsampling (VAMS), and dried plasma spots (DPS) as summarized in Figure 5A.
Figure 5.
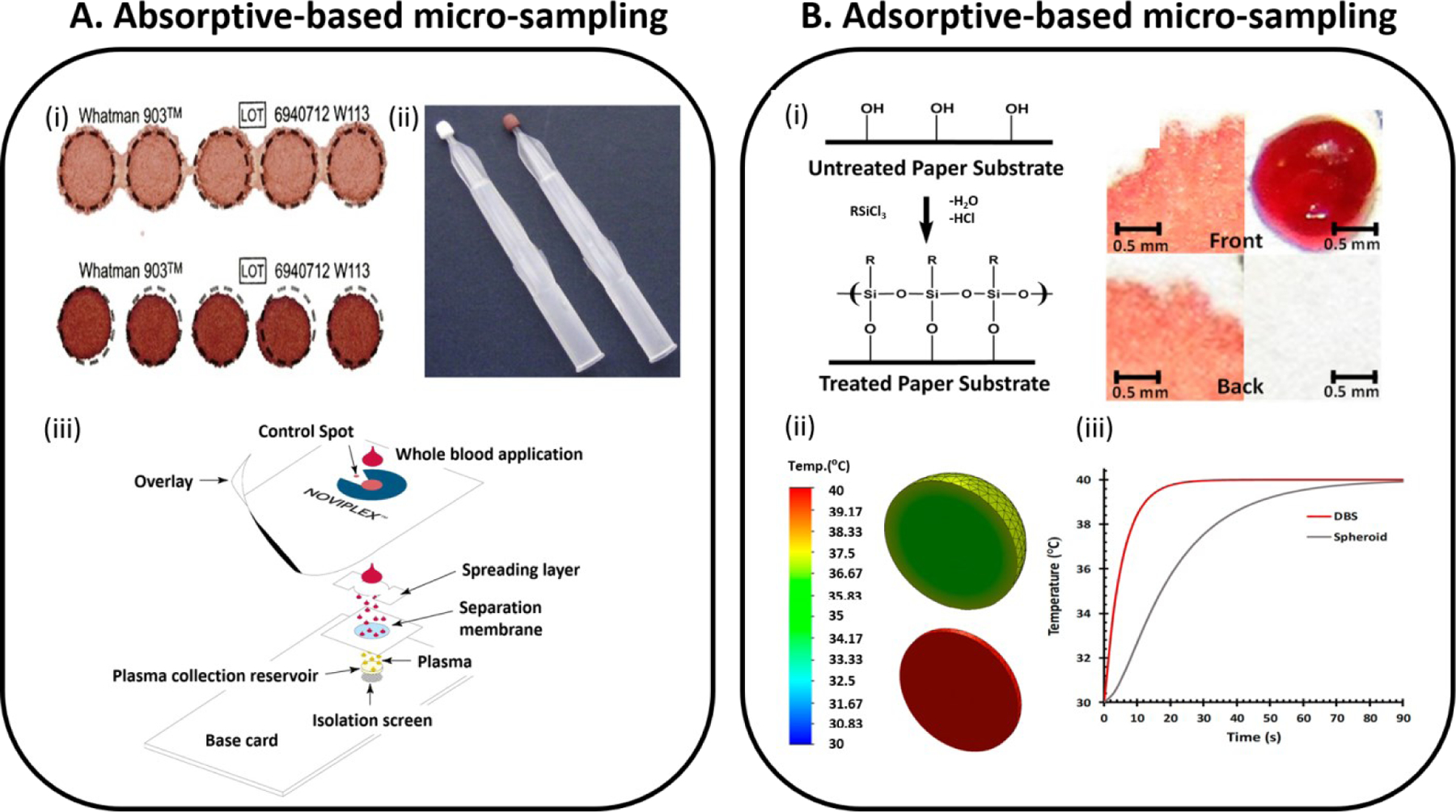
Dry-state microsampling techniques are described for the (A) traditional absorptive-based and (B) a new adsorptive-based method. (Ai) Dried blood spots at different levels of blood hematocrit (above: 0.35 and bottom: 0.50 of blood hematocrit levels respectively). Figure adopted from Reference 80 (CC BY-NC). (Aii) Volumetric absorptive microsampling (VAMS) device which enables the collection of accurate volumes (10 µL) of blood by absorption via a hydrophilic polymeric tip. Adopted with permission from Reference 81. (Aiii) Plasma extraction card is developed for rapid extracting plasma out of finger-stick blood. Alternative paper-based micro-sampling technology is developed based on adsorption phenomenon that occurs because of differences between surface tension of biofluids and surface energies of treated paper substrates. Adopted with permission from Reference 84. Hydrophobic paper substrates are prepared via a gas-phase silanization process (Bi), allowing three-dimensional (3D) spheroids of dried biofluids to be formed when a drop of biofluid is deposited and fully dried (Bii). Transient thermal analysis was simulated for a comparison between the 2D dried blood spots and the 3D blood spheroids (Biii). Panel (Bi) adapted with permission from Reference 56 and panel (Bii and Biii) adopted with permission from Reference 92.
Among microsampling techniques, DBS (Figure 5A, i) is the most common, and has been in use for over a century when Ivar Bang used the technique to determine glucose from eluates of DBS in 1913 (72). Ten years later in 1924, Chapman summarized the advantages of DBS testing (73) that are still valid today including (1) requirement of low blood volume and its potential applicability in pediatric diagnostics, (2) simplicity of the platform being minimally invasive and less expensive, (3) minimal contamination due to storage in the dry state, and (4) stability. The DBS microsampling platform has been actively used for screening or diagnosing disease since 1963 when Guthrie and Susi (74) demonstrated its application in large-scale newborn screening (NBS). With the advantages identified by Chapmen, DBS has been utilized in various fields including newborn screening, therapeutic drug monitoring (75), pre-clinical studies, and omics fields.
MS is commonly used for quantitative and qualitative analysis of analytes in DBS and many important reviews have recently appeared on this topic (76, 77). MS methodologies applied to DBS analysis can be divided into two categories, the use of liquid chromatography-tandem mass spectrometry (LC-MS/MS) and paper spray-tandem mass spectrometry (PS-MS/MS) (78). For LC-MS/MS, collected DBS samples are extracted into solution using designated solvents (e.g., acetonitrile, ethyl acetate, methanol, and other mixtures of solvents). Extracted solution undergoes extra pre-treatment steps (e.g., addition of protease for digestion, solid-phase extraction, liquid-phase extraction, lyophilization, etc.) before injecting into the LC-MS or GC-MS system (79). Direct monitoring of DBS is possible without extraction or separation if PS-MS is chosen for analysis and accordingly can reduce time of analysis. However, the DBS sample collection platform itself has noticeable limitations such as hematocrit effect and heterogeneity. Different degrees of hematocrit effect can cause bias on the amount of analyte that is detected due to different degrees of spreading on the hydrophilic paper substrate (71). Hematocrit effects can be eliminated by using capillary microsampling (CMS, 80) and VAMS (Figure 5A, ii) (81) techniques developed to collect fixed volumes of whole blood. CMS enables one to collect specific volumes of blood (4–70 µL) in anticoagulant-coated glass capillary via capillary action. Separation of plasma is easily performed once a blood-filled capillary is placed in a centrifuge (82). VAMS is made of porous hydrophilic tip that is used to absorb specific volumes of blood (10, 20, or 30 µL). Aside from blood, many analytes are concentrated in plasma making this biofluid more suitable than whole blood for pharmacokinetic studies (83). NoviplexTM cards (Figure 5A,iii) are currently available as paper devices for extracting exact volume of plasma (2.5 µL) from a drop of whole blood within 3 minutes (84). Recently, cotton threads are being used for microsampling of biofluids (blood and tissues) and demonstrated feasibility on quantitative analysis of drugs (62). Schirmer paper is used for both sampling and analysis of eyedrops. From this study, 1.0 µL of tear sample is found to moisten a 2.5 mm of Schirmer paper (85). It is important to note that most of these microsampling techniques allow direct mass spectrometry analysis using substrate-based ambient ionization as discussed above.
While CMS and VAMS can solve hematocrit effect via collecting exact volumes, still analyte integrity in sample remains as challenge. Without temperature control, biological specimens degrade quickly, accounting for >60% of all experimental variations (86–88). Though NBS still heavily rely on DBS, there is limitation on widespread usage due to cold storage requirement (89, 90). In fact, the WHO has begun recommendations for the use of lyophilized whole blood in infectious disease (e.g., malaria) eradication programs instead of DBS (91). An ability to store biofluids at room temperature will facilitate biobanking in the developing countries where cold storage can be challenging. Room temperature dry-state sample storage will enable individual to collect and store their own samples at home followed by delivery to a centralized laboratory for analysis. We have recently developed a new paper-based micro-sampling technology based on adsorption phenomenon using hydrophobic paper substrate (Figure 5B) (56). Compared with DBS platform that form two-dimensional (2D) blood disks, three-dimensional (3D) dried spheroid blood is formed from our newly introduced microsampling platform (Figure 5B, i). We have observed that stability of analytes (e.g., cocaine, diazepam, alanine aminotransferase (ALT)) increased in 3D blood spheroid than DBS (92). The stability observed in the spheroid is believed to be driven by providing a critical radius of insulation through the reduction of surface area-to-volume ratio, which limits bulk exposure to the ambient environment. This mechanism was investigated using heat transfer transient simulation analysis where both the 2D DBS and 3D spheroid blood storage geometries were subjected to a constant air temperature of 40 °C. Temperature at the geometric center for each case was monitored over time. The results from this simulation showed that the spheroid storage conditions have a better resistance to thermal conduction (Figure 5B, iii). We observed prolonged and dry-state stabilization of ALT in blood, which could be useful in diagnosing liver injury in resource-limited settings. Liver injury often occurs in patients that take several medications at once (93), especially in people with HIV and tuberculosis, which are most prevalent in developing countries.
MICROFLUIDICS AND MASS SPECTROMETRY
Microfluidics was identified much earlier as the analytical technique for resource-limited settings because of its unique features such as (i) ability to integrate analytical processes on a small scale (< 1 mm), which in turn requires microscale samples, (ii) rapid high throughput analysis, and (iii) the simplicity of operation (94–96). Many advances were made in past 40 years in terms of fabrication (3D printing (97, 98), injection molding (99, 100), lithography (101), and wax-printing (56)), materials (silicon, glass, poly(dimethylsiloxane) (PDMS), and paper), and platform operation (e.g., paper-based analytical devices (PAD) (102), lab-on-chip (LOC), and organ-on-chip (OOC) (103)). Three main challenges have limited effective application of traditional microfluidic platforms in developing countries: a) requiring external pumps (not in micro scale) for active pumping purposes, b) extensive sample preparation is needed for handling complex mixtures before injection into the microfluidic device, and c) until recently, on-chip detection required sophisticated instrumentation. The Whitesides’ lab introduced paper-based microfluidic devices (µPAD) (104) that enabled a self-sustained platform capable of passive pumping without external pumps, analysis of complex mixtures with little or no sample preparation, and with on-surface detection capabilities.
Since their inception, µPADs have utilized low power detection techniques, such as photometric (105), electrochemical (106), electrical conductivity (107, 108), chemiluminescence (109), and electrochemiluminescence (110) for analyte signal transduction. These detectors are selected to keep the device simple and portable. Unfortunately, detection limits of µPADs based on these detection platforms are often inadequate and give only semi-quantitative results. In 2016, our group introduced the use of mass spectrometer as detector for µPADs. We have shown that wax-printing technology used in µPADs can facilitate PS by allowing lower spray voltages (1 kV) due to the formation of channel surrounded by hydrophobic barrier (111). Through the design of novel cleavable ionic probes (112), we have also shown that immunoassays performed in wax-printed paper substrates can be analyzed effectively using an on-chip touch paper spray MS technique (Figure 6B and C). This MS-based immunoassay platform was utilized in the detection of (i) Plasmodium falciparum histidine-rich protein 2 antigen for malaria diagnosis and (ii) multiplexed and simultaneous detection of cancer antigen 125 and carcinoembryonic antigen for colorectal cancer detection (112).
Figure 6.
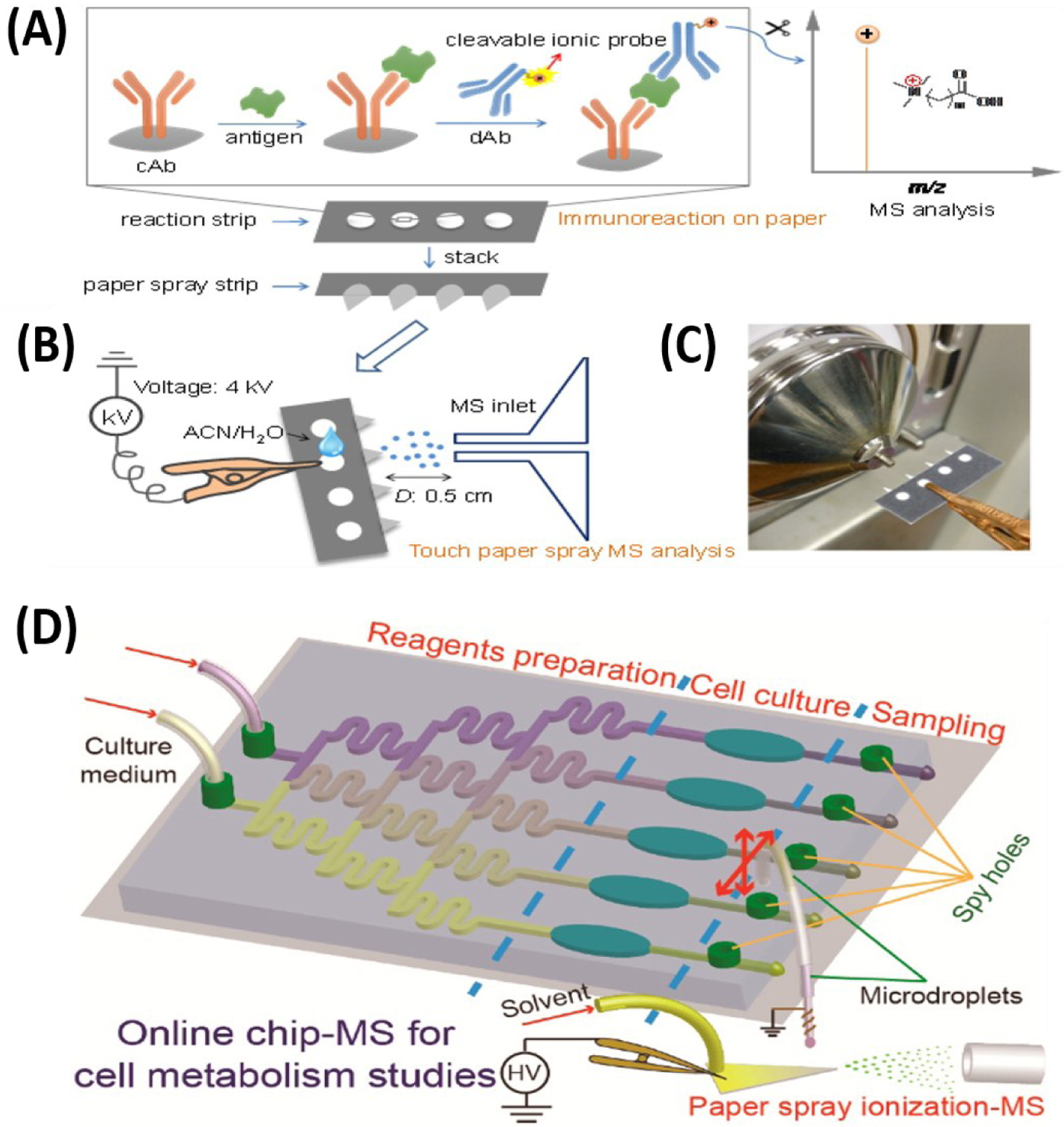
Applications of microfluidic devices in mass spectrometry. (A) Microfluidic paper analytical device (PAD)-MS, which is developed by combination of immunoassay techniques and utilization of ionic probes (i), followed by subsequent MS analysis via touch paper spray ionization method once solvent is added (ii and iii). Adopted with permission from Reference 112. (B) Illustration of integrated multichannel microfluidic chip-MS which consist of a homemade manipulator, a hybrid capillary, a PDMS-glass microfluidic chip. Once cartridge droplet is generated, subsequent MS analysis is achieved via paper spray ionization with the aid of spraying solvent. Adopted with permission from Reference 114.
There are two important features about our paper-based MS immunoassay platform: first, the use of cleavable ionic probes has potential to enable the use of portable mass spectrometers. Portable mass spectrometers typically detect metabolites and other small organic compounds because of their limited mass range. Through immune-reactions, protein antigen of infectious diseases is selectively extracted from complex biofluids onto a paper substrate using monoclonal antibodies conjugated with our cleavable ionic probe. The ionic probes (rationally designed to be small in mass (< 300 Da)) are subsequently released from the bound antibodies through pH change. The released small ions are then detected and analyzed by a portable mass spectrometer (Figure 6A). In essence, instead of directly analyzing the high-molecular weight protein biomarker, which requires a large, high-resolution MS instrument, detection of protein can be achieved on a portable instrument. The second important feature is related to the stability of the probes. Compared to traditional enzyme-linked immunoassay platform, our ionic probed-based immunoassay is found to be stable in the dry-state at room temperature for a 30-day storage period (112). This suggests that the paper substrate on which the immunoassay was performed can be stored under ambient conditions without any refrigeration until ready for analysis later. This provides unique opportunities to screen asymptomatic patients in remote areas without carrying the equipment to the testing site. Collected samples can be analyzed at a central facility and validated with other analytical methods. This provides a way to conduct on-demand analysis with a high performance-to-cost ratio benefitting every member in the community.
Currently, the hyphenation of microfluidics with MS is increasing in popularity for clinical studies (e.g., proteomics, biomarker discovery, or screening). This is due to the remarkable speed of such platforms, combined with other advantages like less sample consumption, and high throughput capabilities. For example, cell-based metabolism studies can now be performed using microfluidic MS systems to understand disease mechanisms and evaluation of dosing amounts of drugs (113). Multiple functions (cell culture, drug-induced reaction, cell metabolism, and sampling) are performed in one integrated chip before analysis. Investigation of lactate efflux rates for different cells (A549, MCF-7, Hela, and U251) under two different conditions (normoxia and hypoxia) and inhibitory effects of cell proliferation from dosing 1 mM of a-Cyano-4-hydroxycinnamate (a-CHC) have been achieved. Interestingly paper spray was used in this experiment for direct analysis (Figure 6D) (114). Effects of genistein-induced cell apoptosis and metabolism in MCF-7 cells are analyzed both quantitatively and qualitatively using microfluidic chip ESI-MS (115).
MINIATURE MASS SPECTROMETERS
As applications of MS widens to many fields, the next innovations are expected to increase the applicability of portable mass spectrometers. Until now, most laboratory experiments use bench-top MS system. With the transition of using MS from laboratory to real world applications, miniaturized MS (Mini-MS) is getting increasing attention, especially in clinical and forensic fields. The outlook of using miniature mass spectrometers in clinical fields is closely related to POC system. As briefly mentioned in previous section, POC system is promising to enhance patient care, diagnosis, and management (116) and eventually, on-site diagnosis or non-laboratory based analysis is a goal of POC system. During the last several decades, the development of micro-sampling and ambient ionization have paved the step toward rapid and direct analysis. The coupling of microsampling platforms and ambient ionization techniques to miniature mass spectrometers for field analysis is a logical next step. Mini-MS involves significant advantages over benchtop-MS, (i) robust and simple setup, (ii) small footprint, (iii) reduced power consumption, (iv) ease of operation, (v) field-analysis (e.g., crime scene, airport), and (vi) low initial cost burden.
History of Mini-MS
Exploration of building miniaturized MS began in the early 1990s, focusing on the size of mass analyzer, and in the 2000s, the production of mini-MS as a separate analytical system for real-time and in-situ analysis received much attention. Numerous efforts focused on fabrication of mass analyzers (Sector (117), time-of-flight (TOF) (118, 119), quadrupole mass filters (QMF) (120, 121), and ion trap) to be smaller-sized whilst also having good performance. While these efforts were expanding, microelectromechanical system (MEMS) technology was incorporated in miniaturization of mass analyzers with relatively low price and small-size involving several improvements to mechanical, thermal, and electrical aspects (120, 121). MEMS technique is generally used for creating integrated systems via fabrication methods (e.g., photolithography, material deposition, and etching) to build components less than 1 mm in dimensions and silicon as its substrate material (120–122).
Sector and TOF instruments showed some trade-off between size and resolution of analyzer, which is not ideal for miniaturized MS system. Recently, a solution is introduced to have orders of magnitude signal without losing resolving power by the use of spatially coded apertures instead of using an array of slits (123). Like Sector, TOF has loss of resolution when its size gets smaller, however its electronic system is simpler and detecting high mass range remains as its advantage over other analyzers. Multi-turn systems, which allows ions to travel multiple times within the same space, were introduced to resolve tradeoff between resolution and throughput pitfall of TOF. In 2010, Shimma et.al introduced miniaturized TOF with multi-turn systems named “MULTUM-S II”. There was still room for improvement of sensitivity, however having resolution more than 30000 within the reported size and weight of the instrument (size = 50 x 57 x 30 cm3, weight = 35 kg) was a noticeable advance (118).
QMF and ion trap turned out to have distinct features (e.g., electronically controlled performance, range of pressure, power system) (118, 120, 124) over other analyzers, therefore many of current commercialized miniaturized MS have adopted the use of these analyzers. In 2010, Microsaic Systems Ltd., and Imperial College developed mini-MS using MEMS-QMF named ChemCube (outlet powered, weight = 9 kg) and Chempack (battery powered first portable device using MEMS technique, weight = 14.9 kg) for space-limited laboratories and field analysis, respectively (125). Furthermore, MEMS-Mini-Triple Quadrupole in 2015 was introduced and used on quantitative analysis of pesticide residue (thiabendazole, m/z = 202) in fruit with multiple reaction monitoring (MRM) (126).
After evaluating all types of mass analyzers used in mini-MS (Figure 7), ion trap was chosen as the most suitable analyzer due to high pressure tolerance, ease of miniaturization, and capability of performing MS/MS in a single device (127). To date, a variety of ion traps have been developed, including quadrupole ion trap (QIT), cylindrical ion trap (CIT), linear ion trap (LIT) and Rectilinear ion trap (RIT). QIT was originally invented by Wolfgang Paul (128) for the purpose of ion storage before its usage turned into mass analyzer. QIT consists of hyperbolic shaped two “end-cap” electrodes and “ring electrode”, which contains a hole in the middle where ions can enter and exit. Fundamental principle of QIT is, building up of RF trapping potential field by applying radiofrequency (RF) voltage to trap ions and eject ions when voltage is altered to set mass-selective instability operation mode (129). Due to its ease of fabricating electrodes, implementation of CIT (130, 131) was made and halo (132, 133) or toroidal (134, 135) ion trap) were developed for having greater storage capacity compared to CIT. LIT consists of four hyperbolic rods where two pairs of rods are positioned parallel to each other like in QMF, however segmented into three and middle section functions in trapping and ejection of ions. Compared to QIT, in LIT, ions are confined both radially and axially by applying DC voltage on end caps to create a potential well and AC voltage is applied to generate RF respectively. Therefore, LITs can have greater capacity of storing ions and trapping efficiency compared to 3D ion traps (QIT and CIT) (1). Next generation of ion trap, RIT, was developed by combining features of LIT and simple geometry of CIT. Replacing the geometry of electrode from hyperbolic to rectangular makes it easier for fabrication, maintaining great efficiency of ion trapping and capability of MS/MS (136, 137). Numerous Mini-MS machined with RIT have been developed, for example Mini-series (Mini 10, 11, 12, and S) that are developed from Purdue University. Besides research on miniaturizing mass analyzer, attempts on developing small and efficient vacuum (or pumping) system were carried out with MEMS techniques. Atmospheric pressure ionization (e.g., ESI) and ambient ionization (e.g., DESI, DART, PS, etc.) are incorporated into Mini-MS for analysis. Since ion optic systems such as multipole, skimmers, or orifice are commonly removed from Mini-MS, therefore novel strategies were needed to facilitate ion transfer. Atmospheric pressure interface (API) systems (e.g., the continuous gas inlets either capillaries or membranes (138), discontinuous atmospheric pressure interface (DAPI) (139), and recently continuous atmospheric pressure interface (CAPI) (140)), which bridge the gap between ion source and vacuum manifold, are developed to aid analytical sensitivity.
Figure 7.
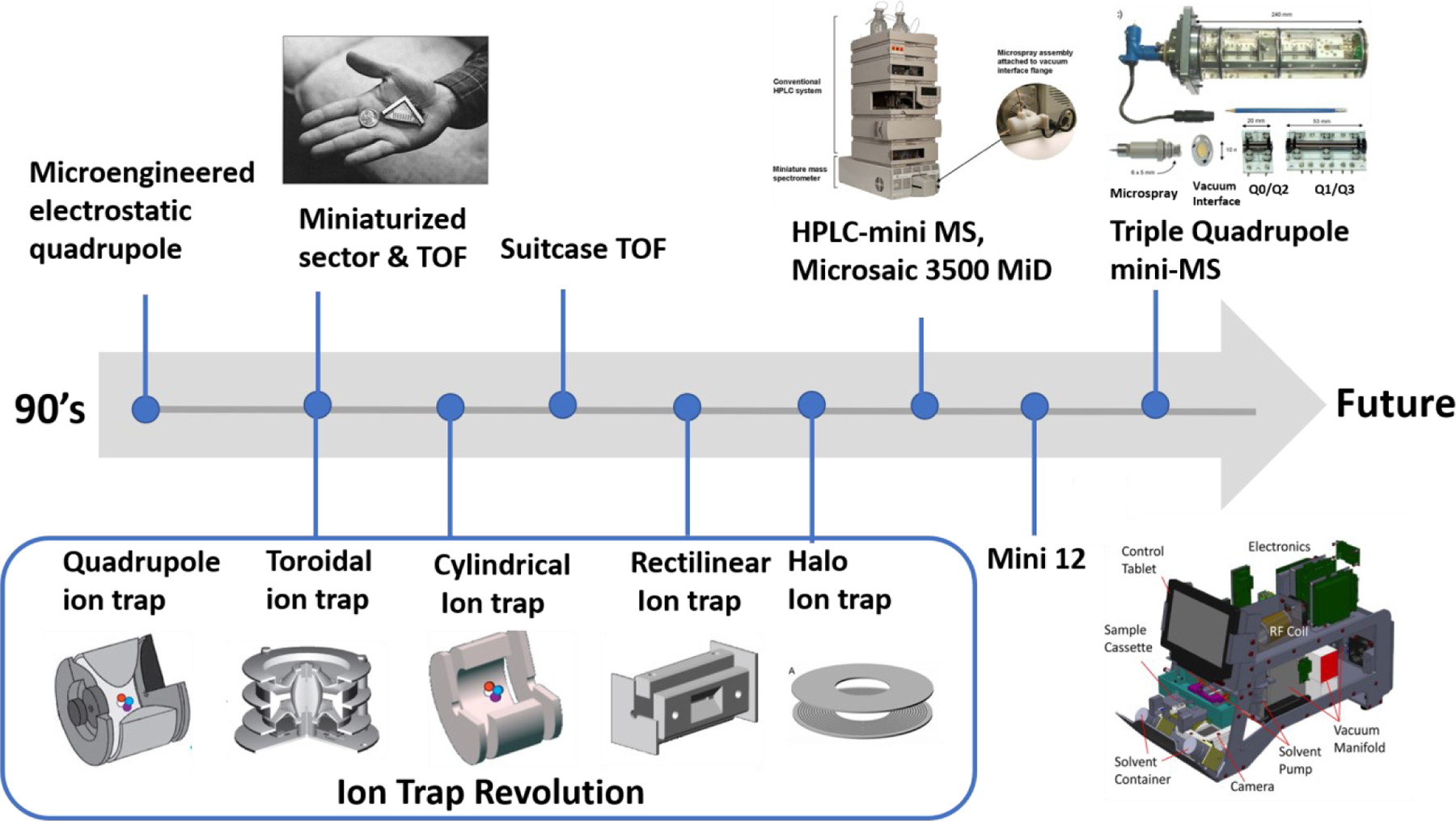
Timeline in development of Mini-MS. In the 1990s, microelectromechanical systems (MEMS) were used in miniaturized mass spectrometers for the fabrication of compartments (e.g., analyzers and vacuum pump). Advancements have been made expanding development of miniaturized analyzers to include quadrupole mass filter, sector, time-of-flight (TOF), and ion trap. Figure reproduced with permission from Reference 117. Ion trap is currently the most common analyze due to qualities like high-pressure tolerance, ease of miniaturizing, and MS/MS capability. Therefore, the evolution of ion trap mass analyzers is described, which changed geometry from 3D hyperbolic quadrupole to 2D toroidal, 3D cylindrical, to 2D rectilinear, and finally to 2D halo ion trap. Figures reproduced with permission from Reference 133, 134 and 136. The Mini-series (Purdue University, West Lafayette) are well-known examples of the ion-trap mini-MS system. Figure reproduced with permission from Reference 141. Besides ion trap evolution, continuous developments are being observed for other analyzers. These include chip-based quadrupole coupled to HPLC (Microsaic 3500 MiD, Microsaic Systems) and recently triple quadrupole mini-MS is invented which enables MS/MS. Figure reproduced with permission from Reference 122 and Reference 126.
Application of Mini-MS
Mini-MS is increasingly being applied in real-time and in-situ analysis. In the clinical field, Mini-MS showed significant progress on DBS analysis (141), therapeutic drug monitoring (142–144), illicit drug detection (145, 146), preclinical (pharmacokinetics) (147), and omics-research (148). 50 ng/mL of amitriptyline spiked blood was prepared on paper to form DBS, which was subsequently analyzed using Mini-12 (West Lafayette, IN). From quantitative study, detectable concentrations range of amitriptyline (15–510 ng/mL) were calculated (Figure 8A), which covers the therapeutic range for amitriptyline (80–250 ng/mL) (141). Bernier et.al performed fingerprinting of falsified artemisinin-based combination therapies (ACTs), which is a treatment for malaria using DART-Mini MS (Waters-QDa). Genuine and falsified tablets were crushed into powder and transferred (0.5–1 mg) into borosilicate glass for sampling. This study found that Class II (carbohydrates) or Class III (antibiotics) compounds are detected from falsified tablets whereas Class I (active ingredients, artemether and lumefantrine) are detected from genuine tablet and PCA was tested on ACTs for further differentiation of classes (143). Fentanyl sprayed surfaces (glass, plastic, and metal) were swabbed with paper (pSERS substrate) and were directly analyzed with Mini-β MS for identification and confirmation. In addition to utilization of MS, portable surface enhanced Raman spectroscopy (pSERS, Metrohm Mira DS, Laramie, WY) was implemented in this study so that dual-pSERS-PSI-MS mode was operated. Detectable concentrations of fentanyl with this method proved forensic applicability (Figure 8B). Also, five fentanyl analogues are identified as having their characteristic spectra patterns. This data is promising to construct libraries for rapid identification of newer designed drugs (145). Pharmaceutical tablets, power or crystal-based drug evidence, and synthetic cannabinoid seizures were analyzed on FLIR AI-MS 1.2 (West Lafayette, IN), a portable CIT MS, using newly introduced ambient ionization technique, filter cone spray ionization by Fatigante et.al (144). Recently, proteomics application exploration was done on a modified version of Mini-β MS, which adapted dual LITs to perform both beam-type and in-trap collision induced dissociation. Methionine (Met) peptide, with sequence YVNDFFNK, was spiked into a trypsin-digested mouse liver peptide solution and was analyzed with nano-ESI under positive-ion mode in both full scan and MS/MS mode and LOD was calculated to be 10 nM from quantitative study (Figure 8C) (148). The efforts of interfacing microfluidic device to Mini-MS is going because this coupling technology is promising to achieve highly efficient performances. Microchip-ESI device was interfaced, via custom capillary interface, to mini-CIT MS (Figure 8D). Mini-CIT MS was custom-built to enable analysis at high pressure conditions (>1 torr). Four peptides were separated and detected at mini-CIT when 7 fmol of peptide mixture was infused into CE-microchip. The results showed that CE-microchip is a potential tool in replacing LC system for the analysis of complex mixtures (e.g., protein, peptides in biofluids) (149). Besides clinical research, food safety (150) and environmental monitoring (151) are other fields for the growth of Mini-MS applications.
Figure 8.
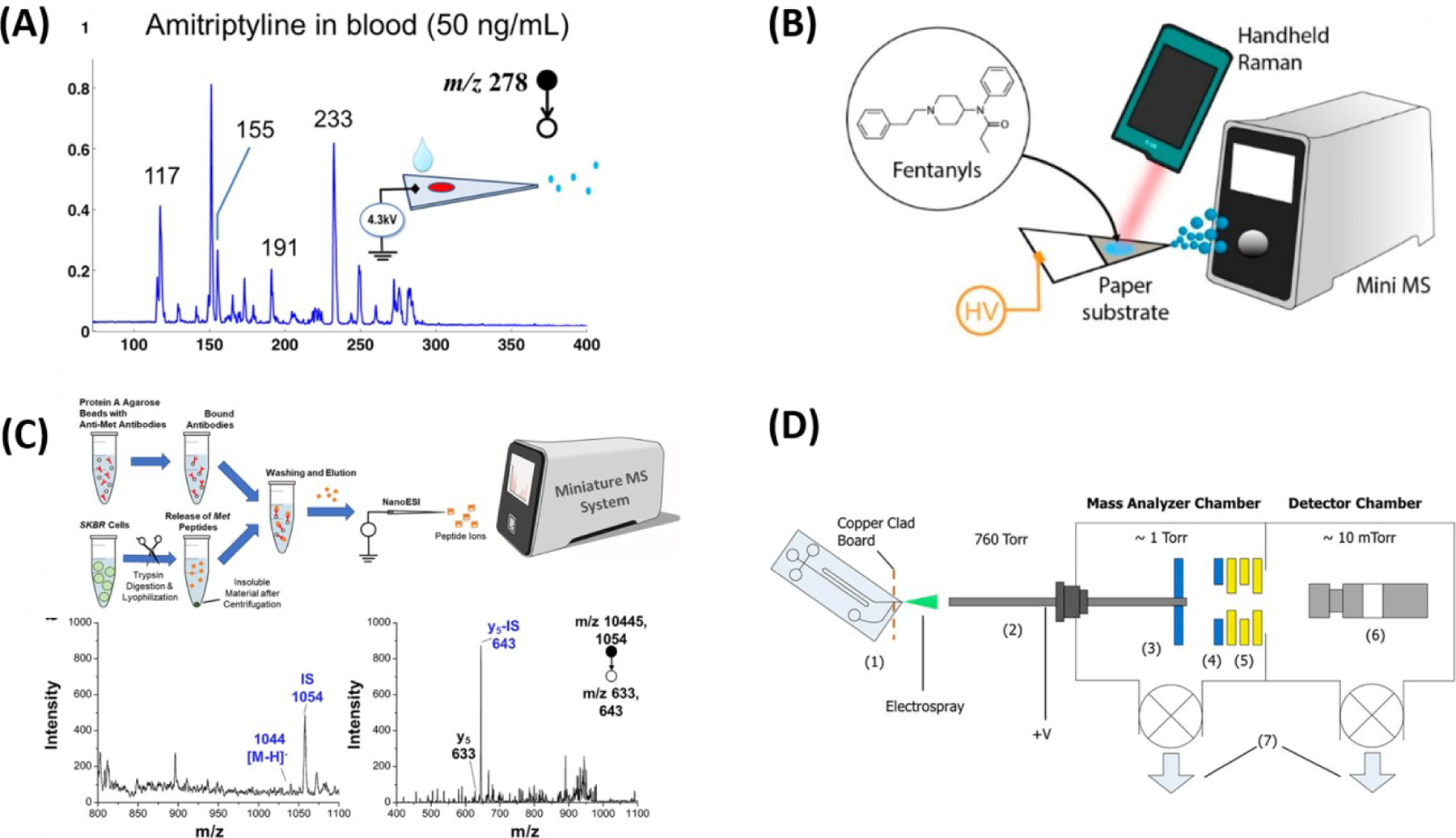
Current applications of Mini-MS. (A) 50 ng of amitriptyline in blood is deposited onto the paper to form dried blood spot and analyzed with paper spray ionization on Mini 12. Adopted with permission from Reference 141. (B) The presence of fentanyls on the paper is identified via dual methods, portable surface enhanced Raman spectroscopy (Mira DS portable Raman spectrometer) and Mini-MS (Mini 12). Adopted with permission from Reference 145. (C) Targeted quantification of trypsin-digested methionine peptide from SKBR3 cell lysate is studied with Mini 12. Before MS analysis, immunoaffinity enrichment workflow is completed, and ionization is done with nano-electrospray technique. Adopted with permission from Reference 148. (D) Illustration of experimental setup for coupling microchip to mini-MS. Adopted with permission from Reference149.
CURRENT CLINICAL APPLICATIONS OF MASS SPECTROMETRY IN THE DEVELOPING WORLD
A closer look at current studies utilizing MS in clinical research and applications in the low and middle-income countries shows electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) to be the main ion sources in the developing world. Owing to their soft nature, giving no analyte dissociation during analysis, these ion sources are widely applied to biological systems to detect molecules such as proteins, peptides, lipids, drugs, and other metabolites. For example, MALDI-TOF-MS is currently used in most African, South American and South Asian countries to detect bacterial and fungal species leading to the diagnosis of various infectious diseases (152, 153). The same MALDI technique has been used in mass spectrometry imaging (MSI) experiments to evaluate pharmacokinetics of drugs (154). Liquid chromatography (LC) tandem MS (MS/MS)-based proteomic analysis (155, 156) are also widely employed to study potential cancer biomarkers (157, 158) and lipidomic analysis (159). Traditional methods like LC-MS lack throughput required to survey large numbers of samples in a reasonable amount of time (160). By coupling a commercial DESI source to a mass spectrometer, direct visualization of neurotransmitters in rat brain slices was carried out without any need for derivatization reagents and/or deuterated materials typically used in MALDI-MS imaging (161). As explained above, it is important that sample preparation be minimized during the coupling of such analytical instrumentation. Aside from the cost burden enumerated in Table 1, simplification of sample workup procedure will enable unskilled personnel to use the instrument. For example, most cancer cases are diagnosed at advanced stages in developing nations (157), despite the existence of relatively better diagnostic infrastructure. The reason for this situation is due to limited manpower as identified in both Boxes 2 and 3 (Supplemental Appendix). By employing the integrated systems with the ambient sources the steps involved in the traditional workflow of MS analysis including sample treatment and separations are eliminated and integrated into the ionization process allowing mass analysis and data interpretation to occur in real-time during the analysis. This takes into consideration the important economic aspects, availability of technical staff and stability of samples. Mini-MS opens up the possibility of various point-of-care applications for clinical studies in remote locations (162).
Instead of fully automated closed analytical system such as LC-MS and GC-MS, an open standalone mass spectrometer with ambient ionization would be hugely useful in the developing world. Such simple open systems will permit routinely qualitative analysis and the rapid nature and simplicity of such method developments are greatly desirable for patient care, allowing parameters to be changed at any time. Naturally, all the commercial fully integrated systems are expensive than corresponding open systems. The closed systems typically offer procedures that manufacturers consider interesting and not necessarily to cater for the specific needs of the developing nation. The open system of ambient mass spectrometry can offer developing nations the opportunity to develop simple methods for clinical studies according to the demands of the community and to personalize the same instrument for various applications including clinical, forensic, agricultural, and environmental analysis. Aside from qualitative analysis, quantitative determinations can be performed through proper use of internal standards and calibration methods. The establishment of such open analytical systems as part of a hospital/health center will be more efficient than externally independent laboratories since collaboration with medical experts will be needed to effectively modify methods and interpret the scientific data. Such collaborations will serve to educate clinicians (163) in the area of analytical instrumentation, something that is needed to reduce the overreliance on symptoms for disease diagnosis. Progress in these areas will be dependent on the total amount of money available for health care in developing countries. Interesting healthcare spending from autonomous sources has increased substantially in developing countries (164).
Supplementary Material
The ASSURED Criteria.
A = Affordable
S = Sensitive
S = Specific
R = Rapid and robust
U = User-friendly
E = Equipment-free
D = Deliverable to end-user
SUMMARY POINTS.
Diagnosis of asymptomatic people in disease eradication efforts require chemical instruments that can enable sensitive detection in the early stages of infection.
Colorimetric-based methods such as rapid diagnostic tests, often recommended for developing countries fall short regarding sensitivity and specificity of detection and cannot be widely applied.
Ambient mass spectrometry offers an attractive alternative in which chemical species of all kinds, in any type of sample can be analyzed in their native state without sample pre-treatment.
Conventional mass spectrometry methods utilizing auto-sampling platforms and separation techniques are closed to manufacturer’s specified application making them less attractive for resource-limited settings where a single all-purpose instrument can serve an entire community.
The coupling of ambient ionization (especially substrate-based methods) with portable mass spectrometers has potential to provide an open analytical system where customization and simple method developments can be established by less trained personnel.
Low-cost microsampling platforms (e.g., 3D dried blood spheroids) that allow dry-state room temperature storage of various biofluid samples without cold storage are identified as having potential to enable the creation of a people-centered centralized detection system where millions of asymptomatic patients can have access to few equipment via a direct-to-customer testing.
The simplicity of ambient mass spectrometry also enables point-of-care testing for real-time disease diagnosis.
Capabilities such as the ability to use portable mass spectrometers on-demand without constantly running the equipment, storage of collected samples at room temperature for long periods, and analysis of samples directly without the need for sample pre-treatment and chromatographic separations collectively suggests that total ownership cost of ambient mass spectrometry can be reduced by >50% compared to traditional methods.
The integration of simple pre-analytical and miniaturized analytical systems can enable the chemical detection at a high performance-to-cost ratio in the developing world.
When fully established, such open and flexible analytical systems can have impact not only on clinical analysis but also in areas such as forensics, environmental, and the food industry.
ACKNOWLEDGMENTS
The authors thank the National Institute of Allergy and Infectious Diseases (Award Number R01-AI-143809) for financial support.
TERMS AND DEFINITIONS
- Mass spectrometry
an analytical technique that measures mass-to-charge ratio (m/z) of gaseous ions trapped by electric or magnetic field under vacuum
- Ambient mass spectrometry
the process of measuring m/z of gaseous ions in which the sample is removed from the vacuum environment of the mass spectrometer to enable analysis from the natural environment of the sample, typically under ambient conditions
- Ambient ionization
the process of generating gaseous molecular ions from complex mixtures in their native environment without prior sample treatment at ambient temperature and pressure
- Developing countries
countries with a less developed industrial base and a low (inequality-adjusted) human development index relative to other countries
- Open analysis systems
analytical platforms that permit in-house modifications for the purpose of research
- Closed analysis systems
analytical platforms that have their settings fixed as offered by manufacturers
- Point-of-care testing
medical diagnostic testing that occurs at or near the point of care (e.g., physician’s office)
- Direct-to-consumer testing
a self-screening platform that allow patients to undertake desired medical test without the involvement of an independent health care provider
- Patient-centered care
medical care system in which patient values (time, finance, emotion, etc.) guide clinical decisions
- People-centered care
a broader concept of patient-centered care that focuses on health services and policies in a community rather than individual patients’ interests
- Biobanking
a centralized biorepository that stores biological samples for use in medical research
- Dried blood spheroid
a 3D dry state microsampling method that is based on an adsorptive phenomenon occurring on hydrophobic paper substrates
- Performance-to-cost ratio
a diagnostic test selection criterion that seeks to compare the overall analytical capabilities of a testing device to ownership cost rather than only cost per test without regard to performance
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- (1).Arevalo R Jr, Ni Z, Danell RM. 2020. Mass Spectrometry and Planetary Exploration: A Brief Review and Future Projection. Journal of Mass Spectrometry 55 (1): e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Goltz MN, Kim D, Racz LA. 2011. Using Nanotechnology to Detect Nerve Agents. Air & Space Power Journal, 25(2): 56–60. [Google Scholar]
- (3).Leary PE, Kammrath BW, Lattman KJ, Beals GL 2019. Deploying Portable Gas Chromatography–Mass Spectrometry (GC-MS) to Military Users for the Identification of Toxic Chemical Agents in Theater Applied Spectroscopy 73 (8): 841–858. [DOI] [PubMed] [Google Scholar]
- (4).The Economist. 2000. Hopeless Africa https://www.economist.com/leaders/2000/05/11/hopeless-africa
- (5).Berhane A, Russom M, Bahta I, Hagos F, Ghirmai M, Uqubay S. 2017. Rapid Diagnostic Tests Failing to Detect Plasmodium Falciparum Infections in Eritrea: An Investigation of Reported False Negative RDT Results. Malar J 16 (1): 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Unwin VT, Ahmed R, Noviyanti R, Puspitasari AM, Utami RAS, Trianty L, Lukito T, Syafruddin D, Poespoprodjo JR, Santana-Morales MA, Ter Kuile FO, Adams ER. 2020. Use of a Highly-Sensitive Rapid Diagnostic Test to Screen for Malaria in Pregnancy in Indonesia. Malaria Journal 19 (1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bell L, Calder B, Hiller R, Klein A, Soares NC, Stoychev SH, Vorster BC, Tabb DL. 2018. Challenges and Opportunities for Biological Mass Spectrometry Core Facilities in the Developing World J Biomol Tech 29 (1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Vos JD, Quinn L, Quinn L, Roos C, Pieters R, Bouwman H, Gorst-Allman P, Rohwer E, Giesy JP. 2013. Experience in South Africa of Combining Bioanalysis and Instrumental Analysis of PCDD/Fs. TrAC Trends in Analytical Chemistry 46: 189–197. [Google Scholar]
- (9).Bates RH, Ed. 1983. The Centralization of African Societies. In Essays on the Political Economy of Rural Africa; African Studies; Cambridge University Press: Cambridge, pp. 21–58. [Google Scholar]
- (10).The National Research Foundation Equipment Database. http://eqdb.nrf.ac.za/
- (11).Guterres A 2020. World Economic Situation and Prospects 2020. World Economic Situation and Prospects, pp. 236.
- (12).Richard JT. 2015. Process Analytical Instrumentation, the Challenges for In-Situ Characterization of Complex Particulate Materials. Procedia Engineering, 102: 1714–1725. [Google Scholar]
- (13). Cooks RG,Ouyang Z, Takats Z, Wiseman JM. 2006. Ambient Mass Spectrometry. Science, 311 (5767), 1566–1570. Introduced the concept of ambient mass spectrometry for the first time
- (14).Musteata FM, Musteata ML, Pawliszyn J. 2006. J. Fast In Vivo Microextraction: A New Tool for Clinical Analysis. Clin Chem 52 (4): 708–715. [DOI] [PubMed] [Google Scholar]
- (15).Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ. 2003. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J AOAC Int 86 (2): 412–431. [PubMed] [Google Scholar]
- (16).Van Der Gugten JG, Holmes DT. 2016. Quantitation of Aldosterone in Serum or Plasma Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). In Clinical Applications of Mass Spectrometry in Biomolecular Analysis: Methods and Protocols; Garg U, Ed.; Methods in Molecular Biology; Springer: New York, NY, pp 37–46. [Google Scholar]
- (17).Abdel-Rehim M 2002. Determination of Ropivacaine and Bupivacaine in Human Plasma by Programmed Temperature Vaporiser-Fast Gas Chromatography-Mass Spectrometry (PTV/Fast GC/MS) Utilising in-Vial Liquid-Liquid Extraction. Journal of Separation Science 25 (4): 252–254. [Google Scholar]
- (18).Abdel-Rehim M, Hassan Z, Blomberg L, Hassan M. 2003. On-Line Derivatization Utilizing Solid-Phase Microextraction (SPME) for Determination of Busulphan in Plasma Using Gas Chromatography–Mass Spectrometry (GC-MS). Therapeutic Drug Monitoring, 25 (3): 400–406. [DOI] [PubMed] [Google Scholar]
- (19).Van Der Gugten G, DeMarco ML, Chen LYC, Chin A, Carruthers M, Holmes DT, Mattman A. 2018. Resolution of Spurious Immunonephelometric IgG Subclass Measurement Discrepancies by LC-MS/MS. Clin Chem 64 (4): 735–742. [DOI] [PubMed] [Google Scholar]
- (20).Capati A, Ijare OB, Bezabeh T. 2017. Diagnostic Applications of Nuclear Magnetic Resonance–Based Urinary Metabolomics: Magnetic Resonance Insights [DOI] [PMC free article] [PubMed]
- (21).Markley JL, Brüschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS. 2017. The Future of NMR-Based Metabolomics. Current Opinion in Biotechnology 43: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Access to comprehensive, equitable, and quality health services, Pan American Health Organization, 2017. [Google Scholar]
- (23).Tolan NV. 2017. Direct-to-Consumer Testing: A New Paradigm for Point-of-Care Testing. The Journal of Near-Patient Testing & Technology 16 (3), 108–111. [Google Scholar]
- (24).Nayak S, Blumenfeld NR, Laksanasopin T, Sia SK. 2017. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem 89 (1): 102–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25). Takáts Z, Wiseman JM, Gologan B, Cooks RG. 2004. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 306 (5695), 471–473. Introduced the concept of DESI for the first time
- (26).Feider CL, Krieger A, DeHoog RJ, Eberlin LS. 2019. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem 91 (7): 4266–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kuo TH, Dutkiewicz EP, Pei J, Hsu CC. 2020. Ambient Ionization Mass Spectrometry Today and Tomorrow: Embracing Challenges and Opportunities. Anal. Chem 92 (3), 2353–2363. [DOI] [PubMed] [Google Scholar]
- (28).Laskin J, Heath BS, Roach PJ, Cazares L, Semmes OJ. 2012. Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem 84 (1): 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Feider CL, Macias LA, Brodbelt JS, Eberlin LS. 2020. Double Bond Characterization of Free Fatty Acids Directly from Biological Tissues by Ultraviolet Photodissociation. Anal. Chem 92 (12): 8386–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zhang J, Feider CL, Nagi C, Yu W, Carter SA, Suliburk J, Cao HST, Eberlin LS. 2017. Detection of Metastatic Breast and Thyroid Cancer in Lymph Nodes by Desorption Electrospray Ionization Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom 28 (6): 1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Van Berkel GJ, Sanchez AD, Quirke JME. 2002. Thin-Layer Chromatography and Electrospray Mass Spectrometry Coupled Using a Surface Sampling Probe. Anal. Chem 74 (24): 6216–6223. [DOI] [PubMed] [Google Scholar]
- (32).Tang F, Guo C, Ma X, Zhang J, Su Y, Tian R, Shi R, Xia Y, Wang X, Ouyang Z. 2018. Rapid In Situ Profiling of Lipid C═C Location Isomers in Tissue Using Ambient Mass Spectrometry with Photochemical Reactions. Anal. Chem 90 (9): 5612–5619. [DOI] [PubMed] [Google Scholar]
- (33).Roach PJ, Laskin J, Laskin A. 2010. Nanospray Desorption Electrospray Ionization: An Ambient Method for Liquid-Extraction Surface Sampling in Mass Spectrometry. Analyst 135 (9): 2233–2236. [DOI] [PubMed] [Google Scholar]
- (34).Nguyen SN, Kyle JE, Dautel SE, Sontag R, Luders T, Corley R, Ansong C, Carson J, Laskin J. 2019. Lipid Coverage in Nanospray Desorption Electrospray Ionization Mass Spectrometry Imaging of Mouse Lung Tissues. Anal. Chem 91 (18): 11629–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35). Cody RB, Laramée JA, Durst HD. 2005. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal. Chem 77 (8): 2297–2302. Introduce plasma-based ambient ionization for the first time
- (36).Block E, Dane AJ, Thomas S, Cody RB. 2010. Applications of Direct Analysis in Real Time Mass Spectrometry (DART-MS) in Allium Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane S-Oxide, and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums. J. Agric. Food Chem 58 (8): 4617–4625. [DOI] [PubMed] [Google Scholar]
- (37).Jastrzembski JA, Bee MY, Sacks GL. 2017. Trace-Level Volatile Quantitation by Direct Analysis in Real Time Mass Spectrometry Following Headspace Extraction: Optimization and Validation in Grapes. J. Agric. Food Chem 65 (42): 9353–9359. [DOI] [PubMed] [Google Scholar]
- (38).Li H, Hitchins VM, Wickramasekara S. 2016. Rapid Detection of Bacterial Endotoxins in Ophthalmic Viscosurgical Device Materials by Direct Analysis in Real Time Mass Spectrometry. Analytica Chimica Acta 943: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Sisco E, Robinson EL, Burns A, Mead R. 2019. What’s in the Bag? Analysis of Exterior Drug Packaging by TD-DART-MS to Predict the Contents. Forensic Science International 304: 109939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Vasiljevic T, Pawliszyn J. 2019. Direct Analysis in Real Time (DART) and Solid-Phase Microextraction (SPME) Transmission Mode (TM): A Suitable Platform for Analysis of Prohibited Substances in Small Volumes. Anal. Methods 11 (30): 3882–3889. [Google Scholar]
- (41).Hsieh HY, Li LH. Hsu RY, Kao WF, Huang YC, Hsu CC. 2017. Quantification of Endogenous Cholesterol in Human Serum on Paper Using Direct Analysis in Real Time Mass Spectrometry. Anal. Chem 89 (11): 6146–6152. [DOI] [PubMed] [Google Scholar]
- (42).Wang C, Zhu H, Cai Z, Song F, Liu Z, Liu S. 2013. Newborn Screening of Phenylketonuria Using Direct Analysis in Real Time (DART) Mass Spectrometry. Anal Bioanal Chem 405 (10): 3159–3164. [DOI] [PubMed] [Google Scholar]
- (43).Nemes P, Hoover WJ, Keire DA. 2013. High-Throughput Differentiation of Heparin from Other Glycosaminoglycans by Pyrolysis Mass Spectrometry. Anal. Chem 85 (15): 7405–7412. [DOI] [PubMed] [Google Scholar]
- (44).Jones CM, Fernández FM. 2013. Transmission Mode Direct Analysis in Real Time Mass Spectrometry for Fast Untargeted Metabolic Fingerprinting. Rapid Communications in Mass Spectrometry 27 (12): 1311–1318. [DOI] [PubMed] [Google Scholar]
- (45).Na N, Zhao M, Zhang S, Yang C, Zhang X. 2007. Development of a Dielectric Barrier Discharge Ion Source for Ambient Mass Spectrometry. J. Am. Soc. Mass Spectrom 18 (10): 1859–1862. [DOI] [PubMed] [Google Scholar]
- (46).Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, Ouyang Z. 2008. Low-Temperature Plasma Probe for Ambient Desorption Ionization. Anal. Chem 80 (23): 9097–9104. [DOI] [PubMed] [Google Scholar]
- (47).Smoluch M, Mielczarek P, Silberring J. 2016. Plasma-Based Ambient Ionization Mass Spectrometry in Bioanalytical Sciences. Mass Spectrometry Reviews 35 (1): 22–34. [DOI] [PubMed] [Google Scholar]
- (48).Gyr L, Wolf JC, Franzke J, Zenobi R. 2018. Mechanistic Understanding Leads to Increased Ionization Efficiency and Selectivity in Dielectric Barrier Discharge Ionization Mass Spectrometry: A Case Study with Perfluorinated Compounds. Anal. Chem 90 (4): 2725–2731. [DOI] [PubMed] [Google Scholar]
- (49).Hagenhoff S, Franzke J, Hayen H. 2017. Determination of Peroxide Explosive TATP and Related Compounds by Dielectric Barrier Discharge Ionization-Mass Spectrometry (DBDI-MS). Anal. Chem 89 (7): 4210–4215. [DOI] [PubMed] [Google Scholar]
- (50).Wolf JC, Schaer M, Siegenthaler P, Zenobi R. 2015. Direct Quantification of Chemical Warfare Agents and Related Compounds at Low Ppt Levels: Comparing Active Capillary Dielectric Barrier Discharge Plasma Ionization and Secondary Electrospray Ionization Mass Spectrometry. Anal. Chem 87 (1): 723–729. [DOI] [PubMed] [Google Scholar]
- (51).Wiley JS, Shelley JT, Cooks RG. 2013. Handheld Low-Temperature Plasma Probe for Portable “Point-and-Shoot” Ambient Ionization Mass Spectrometry. Anal. Chem 85 (14): 6545–6552. [DOI] [PubMed] [Google Scholar]
- (52).Garcia-Reyes JF, Harper JD, Salazar GA, Charipar NA, Ouyang Z, Cooks RG. 2011. Detection of Explosives and Related Compounds by Low-Temperature Plasma Ambient Ionization Mass Spectrometry. Anal. Chem 83 (3): 1084–1092. [DOI] [PubMed] [Google Scholar]
- (53).Gamboa-Becerra R, Montero-Vargas JM, Martínez-Jarquín S, Gálvez-Ponce E, Moreno-Pedraza A, Winkler R. 2017. Rapid Classification of Coffee Products by Data Mining Models from Direct Electrospray and Plasma-Based Mass Spectrometry Analyses. Food Anal. Methods 10 (5): 1359–1368. [Google Scholar]
- (54). Wang H, Liu J, Cooks RG, Ouyang Z. 2010. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angewandte Chemie International Edition 49 (5): 877–880. Introduced paper spray as the first substrate-based ambient ionization method that eliminated the need for nebulizer gases
- (55).Liu J, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. 2010. Development, Characterization, and Application of Paper Spray Ionization. Anal. Chem 82 (6): 2463–2471. [DOI] [PubMed] [Google Scholar]
- (56).Damon DE, Davis KM, Moreira CR, Capone P, Cruttenden R, Badu-Tawiah AK. 2016. Direct Biofluid Analysis Using Hydrophobic Paper Spray Mass Spectrometry. Anal. Chem 88 (3): 1878–1884. [DOI] [PubMed] [Google Scholar]
- (57).Bambauer TP, Maurer HH, Weber AA, Hannig M, Pütz N, Koch M, Manier SK, Schneider M, Meyer MR. 2019. Evaluation of Novel Organosilane Modifications of Paper Spray Mass Spectrometry Substrates for Analyzing Polar Compounds. Talanta 204: 677–684. [DOI] [PubMed] [Google Scholar]
- (58).Rossini EL, Kulyk DS, Ansu-Gyeabourh E, Sahraeian T, Pezza HR, Badu-Tawiah AK. 2020. Direct Analysis of Doping Agents in Raw Urine Using Hydrophobic Paper Spray Mass Spectrometry. J. Am. Soc. Mass Spectrom 31 (6): 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Pei JL. 2015. Hydrophobic paper spray mass spectrometry to detect illicit drugs Presented at State Science Day, Columbus, Ohio, May 16. [Google Scholar]
- (60).Davis KM, Badu-Tawiah AK. 2017. Direct and Efficient Dehydrogenation of Tetrahydroquinolines and Primary Amines Using Corona Discharge Generated on Ambient Hydrophobic Paper Substrate. J. Am. Soc. Mass Spectrom 28 (4): 647–654. [DOI] [PubMed] [Google Scholar]
- (61).Capone PC, Badu-Tawiah AK. 2016. Thread Spray Mass Spectrometry for Direct Analysis of Capsaicinoids in Evidentiary Garments. Presented at the 64th American Society of Mass SPectrometry Conference, San Antonio, Texas, June 5–9. [Google Scholar]
- (62).Swiner DJ, Jackson S, Durisek GR, Walsh BK, Kouatli Y, Badu-Tawiah AK. 2019. Microsampling with Cotton Thread: Storage and Ultra-Sensitive Analysis by Thread Spray Mass Spectrometry. Analytica Chimica Acta 1082: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jackson S, Swiner DJ, Capone PC, Badu-Tawiah AK. 2018. Thread Spray Mass Spectrometry for Direct Analysis of Capsaicinoids in Pepper Products. Analytica Chimica Acta 1023: 81–88. [DOI] [PubMed] [Google Scholar]
- (64).Jackson S, Frey BS, Bates MN, Swiner DJ, Badu-Tawiah AK. 2020. Direct Differentiation of Whole Blood for Forensic Serology Analysis by Thread Spray Mass Spectrometry. Analyst [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Morato NM, Pirro V, Fedick PW, Cooks RG. 2019. Quantitative Swab Touch Spray Mass Spectrometry for Oral Fluid Drug Testing. Anal. Chem 91 (11): 7450–7457. [DOI] [PubMed] [Google Scholar]
- (66).Fedick PW, Bain RM. 2017. Swab Touch Spray Mass Spectrometry for Rapid Analysis of Organic Gunshot Residue from Human Hand and Various Surfaces Using Commercial and Fieldable Mass Spectrometry Systems. Forensic Chemistry 5: 53–57. [Google Scholar]
- (67).Kerian KS, Jarmusch AK, Cooks RG. 2014. Touch Spray Mass Spectrometry for In Situ Analysis of Complex Samples. Analyst, 139 (11): 2714–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Khaled A, Gómez-Ríos GA, Pawliszyn J. 2020. Optimization of Coated Blade Spray for Rapid Screening and Quantitation of 105 Veterinary Drugs in Biological Tissue Samples. Anal. Chem 92 (8): 5937–5943. [DOI] [PubMed] [Google Scholar]
- (69).Information, N. C. for B.; Pike, U. S. N. L. of M. 8600 R.; MD, B.; Usa, 20894. Collection, Storage and Shipment of Specimens for Laboratory Diagnosis and Interpretation of Results; World Health Organization, 2012. [Google Scholar]
- (70).Lei BUW, Prow TW. 2019. A Review of Microsampling Techniques and Their Social Impact. Biomed Microdevices 21 (4): 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Freeman JD, Rosman LM, Ratcliff JD, Strickland PT, Graham DR, Silbergeld EK. 2018. State of the Science in Dried Blood Spots. Clinical Chemistry 64 (4): 656–679. [DOI] [PubMed] [Google Scholar]
- (72).Bang IC Der Blutzucker; Wiesbaden, Bergmann, 1913. [Google Scholar]
- (73).Chapman OD. 1924. The complement-fixation test for syphilis: use of patient’s whole blood dried on filter paper. Arch Derm Syphilol 9 (5): 607–611. [Google Scholar]
- (74). Guthrie R, Susi A. 1963. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 32 (3): 338–343. Applied the absorptive-based dried blood spot method for large-scale newborn screening
- (75).Wilhelm AJ, den Burger JCG, Swart EL. 2014. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin Pharmacokinet 53 (11): 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Antunes MV, Charão MF, Linden R. 2016. Dried Blood Spots Analysis with Mass Spectrometry: Potentials and Pitfalls in Therapeutic Drug Monitoring. Clinical Biochemistry 49 (13): 1035–1046. [DOI] [PubMed] [Google Scholar]
- (77).Lehmann S, Picas A, Tiers L, Vialaret J, Hirtz C. 2017. Clinical perspectives of dried blood spot protein quantification using mass spectrometry methods. Critical Reviews in Clinical Laboratory Sciences, 54:3, 173–184. [DOI] [PubMed] [Google Scholar]
- (78).Frey BS, Damon DE, Badu-Tawiah AK. 2020. Emerging Trends in Paper Spray Mass Spectrometry: Microsampling, Storage, Direct Analysis, and Applications. Mass Spectrometry Reviews, 39 (4): 336–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Chambers AG, Percy AJ, Hardie DB, Borchers CH. 2013. Comparison of Proteins in Whole Blood and Dried Blood Spot Samples by LC/MS/MS. J. Am. Soc. Mass Spectrom 24 (9): 1338–1345. [DOI] [PubMed] [Google Scholar]
- (80).Wang B, Wang L, Batog A, Brodie T, Ramaiah L, Chadwick KD, Su T, Mangipudy R, Pilutla RC, Ji QC. 2020. Investigation on the Effect of Capillary Microsampling on Hematologic and Toxicokinetic Evaluation in Regulatory Safety Studies in Mice. AAPS. J 22 (2): 55. [DOI] [PubMed] [Google Scholar]
- (81).Denniff P, Parry S, Dopson W, Spooner N. 2015. Quantitative Bioanalysis of Paracetamol in Rats Using Volumetric Absorptive Microsampling (VAMS). Journal of Pharmaceutical and Biomedical Analysis 108: 61–69. [DOI] [PubMed] [Google Scholar]
- (82).Nys G, Kok MGM, Servais AC, Fillet M. 2017. Beyond Dried Blood Spot: Current Microsampling Techniques in the Context of Biomedical Applications. TrAC Trends in Analytical Chemistry, 97: 326–332. [Google Scholar]
- (83).Bills BJ, Manicke NE. 2016. Development of a Prototype Blood Fractionation Cartridge for Plasma Analysis by Paper Spray Mass Spectrometry. Clinical Mass Spectrometry 2: 18–24. [Google Scholar]
- (84).Kim JH, Woenker T, Adamec J, Regnier FE. 2013. Simple, Miniaturized Blood Plasma Extraction Method. Anal. Chem 85 (23): 11501–11508. [DOI] [PubMed] [Google Scholar]
- (85).Yao YN, Di D, Yuan ZC, Wu L, Hu B. 2020. Schirmer Paper Noninvasive Microsampling for Direct Mass Spectrometry Analysis of Human Tears. Anal. Chem 92 (9): 6207–6212. [DOI] [PubMed] [Google Scholar]
- (86).Hubel A, Spindler R, Skubitz APN. 2014. Storage of Human Biospecimens: Selection of the Optimal Storage Temperature. Biopreservation and Biobanking 12 (3): 165–175. [DOI] [PubMed] [Google Scholar]
- (87).Chaigneau C, Cabioch T, Beaumont K, Betsou F. 2007. Serum Biobank Certification and the Establishment of Quality Controls for Biological Fluids: Examples of Serum Biomarker Stability after Temperature Variation. Clinical Chemistry and Laboratory Medicine (CCLM) 45 (10): 1390–1395. [DOI] [PubMed] [Google Scholar]
- (88).Evans MJ, Livesey JH, Ellis MJ, Yandle TG. 2001. Effect of Anticoagulants and Storage Temperatures on Stability of Plasma and Serum Hormones. Clinical Biochemistry 34 (2): 107–112. [DOI] [PubMed] [Google Scholar]
- (89).Grüner N, Stambouli O, Ross RS. 2015. Dried Blood Spots - Preparing and Processing for Use in Immunoassays and in Molecular Techniques. J Vis Exp pp. No. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Olshan AF. 2007. Meeting Report: The Use of Newborn Blood Spots in Environmental Research: Opportunities and Challenges. Environ Health Perspect 115 (12): 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).World Health Organization. 2015. A WHO external quality assurance scheme for malaria nucleic acid amplification testing: 8–9 June 2015, London, United Kingdom: meeting report. World Health Organization. https://apps.who.int/iris/handle/10665/325862 [Google Scholar]
- (92). Damon DE, Yin M, Allen DM, Maher YS, Tanny CJ, Oyola-Reynoso S, Smith BL, Maher S, Thuo MM, Badu-Tawiah AK. 2018. Dried Blood Spheroids for Dry-State Room Temperature Stabilization of Microliter Blood Samples. Anal. Chem 90 (15): 9353–9358. Developed the first adsorptive-based dried blood spheroid microsampling platform for stabilization of labile compounds without could storage
- (93).Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. 2009. Circulating MicroRNAs, Potential Biomarkers for Drug-Induced Liver Injury. PNAS 106 (11): 4402–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Mejía-Salazar JR, Rodrigues Cruz K, Materón Vásques EM, Novais de Oliveira O Jr. 2020. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for EHealth Diagnostics. Sensors 20 (7): 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Gale BK, Jafek AR, Lambert CJ, Goenner BL, Moghimifam H, Nze UC, Kamarapu SK. 2018. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 3 (3): 60. [Google Scholar]
- (96).Convery N, Gadegaard N. 2019. 30 Years of Microfluidics. Micro and Nano Engineering 2: 76–91. [Google Scholar]
- (97).Ho CMB, Ng SH, Li KHH, Yoon YJ. 2015. 3D Printed Microfluidics for Biological Applications. Lab Chip 15 (18): 3627–3637. [DOI] [PubMed] [Google Scholar]
- (98).Weisgrab G, Ovsianikov A, Costa F. 2019. Functional 3D Printing for Microfluidic Chips. Advanced Materials Technologies 4 (10): 1900275. [Google Scholar]
- (99).Lee UN, Su X, Guckenberger DJ, Dostie AM, Zhang T, Berthier E, Theberge AB. 2018. Fundamentals of Rapid Injection Molding for Microfluidic Cell-Based Assays. Lab Chip 18 (3): 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Ma X, Li R, Jin Z, Fan Y, Zhou X, Zhang Y. 2020. Injection Molding and Characterization of PMMA-Based Microfluidic Devices. Microsyst Technol 26 (4): 1317–1324. [Google Scholar]
- (101).Xia Y, Whitesides GM. 1998. Soft Lithography. Annu. Rev. Mater. Sci 28 (1): 153–184. [Google Scholar]
- (102).Strong EB, Schultz SA, Martinez AW, Martinez NW. 2019. Fabrication of Miniaturized Paper-Based Microfluidic Devices (MicroPADs). Scientific Reports 9 (1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Junaid A, Mashaghi A, Hankemeier T, Vulto P. 2017. An End-User Perspective on Organ-on-a-Chip: Assays and Usability Aspects. Current Opinion in Biomedical Engineering 1: 15–22. [Google Scholar]
- (104). Martinez AW, Phillips ST, Butte MJ, Whitesides GM. 2007. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angewandte Chemie International Edition 46 (8): 1318–1320. Developed the first paper-based microfluidic device utilizing colorimetric signal transduction.
- (105).Wang Y, Ge L, Wang P, Yan M, Ge S, Li N, Yu J, Huang J. 2013. Photoelectrochemical Lab-on-Paper Device Equipped with a Porous Au-Paper Electrode and Fluidic Delay-Switch for Sensitive Detection of DNA Hybridization. Lab Chip 13 (19): 3945–3955. [DOI] [PubMed] [Google Scholar]
- (106).Lan WJ, Maxwell EJ, Parolo C, Bwambok DK, Subramaniam AB, Whitesides GM. 2013. Paper-Based Electroanalytical Devices with an Integrated, Stable Reference Electrode. Lab Chip 13 (20): 4103–4108. [DOI] [PubMed] [Google Scholar]
- (107).Fedick PW, Fatigante WL, Lawton ZE, O’Leary AE, Hall SE, Bain RM, Ayrton ST, Ludwig JA, Mulligan CC. 2018. A Low-Cost, Simplified Platform of Interchangeable, Ambient Ionization Sources for Rapid, Forensic Evidence Screening on Portable Mass Spectrometric Instrumentation. Instruments 2 (2): 5. [Google Scholar]
- (108).Arena A, Donato N, Saitta G, Bonavita A, Rizzo G, Neri G. 2010. Flexible Ethanol Sensors on Glossy Paper Substrates Operating at Room Temperature. Sensors and Actuators B: Chemical 145 (1): 488–494. [Google Scholar]
- (109).Ge L, Wang S, Song X, Ge S, Yu J. 2012. 3D Origami-Based Multifunction-Integrated Immunodevice: Low-Cost and Multiplexed Sandwich Chemiluminescence Immunoassay on Microfluidic Paper-Based Analytical Device. Lab Chip 12 (17): 3150–3158. [DOI] [PubMed] [Google Scholar]
- (110).Delaney JL, Hogan CF, Tian J, Shen W. 2011. Electrogenerated Chemiluminescence Detection in Paper-Based Microfluidic Sensors. Anal. Chem 83 (4): 1300–1306. [DOI] [PubMed] [Google Scholar]
- (111).Damon DE, Maher YS, Yin M, Jjunju FPM, Young IS, Taylor S, Maher S, Badu-Tawiah AK. 2016. 2D Wax-Printed Paper Substrates with Extended Solvent Supply Capabilities Allow Enhanced Ion Signal in Paper Spray Ionization. Analyst 141 (12), 3866–3873. [DOI] [PubMed] [Google Scholar]
- (112). Chen S, Wan Q, Badu-Tawiah AK. 2016. Mass Spectrometry for Paper-Based Immunoassays: Toward On-Demand Diagnosis. J. Am. Chem. Soc 138 (20): 6356–6359. Enabled the coupling of paper-based microfluidic device to mass spectrometry for sensitive disease diagnosis.
- (113).Pedde RD, Li H, Borchers CH, Akbari M. 2017. Microfluidic-Mass Spectrometry Interfaces for Translational Proteomics. Trends in Biotechnology 35 (10): 954–970. [DOI] [PubMed] [Google Scholar]
- (114).Liu W, Lin JM. 2016. Online Monitoring of Lactate Efflux by Multi-Channel Microfluidic Chip-Mass Spectrometry for Rapid Drug Evaluation. ACS Sens 1 (4): 344–347. [Google Scholar]
- (115).Chen Q, Wu J, Zhang Y, Lin JM. 2012. Qualitative and Quantitative Analysis of Tumor Cell Metabolism via Stable Isotope Labeling Assisted Microfluidic Chip Electrospray Ionization Mass Spectrometry. Anal. Chem 84 (3): 1695–1701. [DOI] [PubMed] [Google Scholar]
- (116).Ransohoff JR, Melanson SEF. 2019. What’s New in Point-of-Care Testing? Point of Care 18 (3): 92–98. [Google Scholar]
- (117).Diaz JA, Giese CF, Gentry WR. 2001. Sub-Miniature ExB Sector-Field Mass Spectrometer. Journal of the American Society for Mass Spectrometry 12 (6): 619–632. [DOI] [PubMed] [Google Scholar]
- (118).Shimma S, Nagao H, Aoki J, Takahashi K, Miki S, Toyoda M. 2010. Miniaturized High-Resolution Time-of-Flight Mass Spectrometer MULTUM-S II with an Infinite Flight Path. Anal. Chem 82 (20): 8456–8463. [DOI] [PubMed] [Google Scholar]
- (119).Ecelberger SA; Cornish TJ; Collins B; Lewis DL; Bryden WA 2004. Suitcase TOF: A Man-Portable Time-of-Flight Mass Spectrometer; [Google Scholar]
- (120).Cheung K, Velasquez-Garcia LF, Akinwande AI. 2010. Chip-Scale Quadrupole Mass Filters for Portable Mass Spectrometry. Journal of Microelectromechanical Systems 19 (3): 469–483. [Google Scholar]
- (121).Geear M, Syms RRA, Wright S, Holmes AS. 2005. Monolithic MEMS Quadrupole Mass Spectrometers by Deep Silicon Etching. Journal of Microelectromechanical Systems 14 (5): 1156–1166. [Google Scholar]
- (122).Malcolm A, Wright S, Syms RRA, Moseley RW, O’Prey S, Dash N, Pegus A, Crichton E, Hong G, Holmes AS, Finlay A, Edwards P, Hamilton SE, Welch CJA. 2011. Miniature Mass Spectrometer for Liquid Chromatography Applications. Rapid Communications in Mass Spectrometry 25 (21): 3281–3288. [DOI] [PubMed] [Google Scholar]
- (123).Landry DMW, Kim W, Amsden JJ, Di Dona ST, Choi H, Haley L, Russell ZE, Parker CB, Glass JT, Gehm ME. 2018. Effects of Magnetic and Electric Field Uniformity on Coded Aperture Imaging Quality in a Cycloidal Mass Analyzer. J. Am. Soc. Mass Spectrom 29 (2): 352–359. [DOI] [PubMed] [Google Scholar]
- (124).Ouyang Z, Cooks RG. 2009. Miniature Mass Spectrometers. Annual Rev. Anal. Chem 2 (1): 187–214. [DOI] [PubMed] [Google Scholar]
- (125).Malcolm A, Wright S, Syms RRA, Dash N, Schwab MA, Finlay A. 2010. Miniature Mass Spectrometer Systems Based on a Microengineered Quadrupole Filter. Anal. Chem 82 (5): 1751–1758. [DOI] [PubMed] [Google Scholar]
- (126).Wright S, Malcolm A, Wright C, O’Prey S, Crichton E, Dash N, Moseley RW, Zaczek W, Edwards P, Fussell RJ, Syms RRA. 2015. A Microelectromechanical Systems-Enabled, Miniature Triple Quadrupole Mass Spectrometer. Anal. Chem 87 (6): 3115–3122. [DOI] [PubMed] [Google Scholar]
- (127).Ouyang Z, Noll RJ, Cooks RG. 2009. Handheld Miniature Ion Trap Mass Spectrometers. Anal. Chem 81 (7): 2421–2425. [DOI] [PubMed] [Google Scholar]
- (128).Paul W, Steinwedel H. 1953. Notizen: Ein Neues Massenspektrometer Ohne Magnetfeld. Zeitschrift für Naturforschung A 8 (7): 448–450. [Google Scholar]
- (129).Pau S, Pai CS, Low YL, Moxom J, Reilly PTA, Whitten WB, Ramsey JM. 2006. Microfabricated Quadrupole Ion Trap for Mass Spectrometer Applications. Phys. Rev. Lett 96 (12): 120801. [DOI] [PubMed] [Google Scholar]
- (130).Patterson GE, Guymon AJ, Riter LS, Everly M, Griep-Raming J, Laughlin BC, Ouyang Z, Cooks RG. 2002. Miniature Cylindrical Ion Trap Mass Spectrometer. Anal. Chem 74 (24): 6145–6153. [DOI] [PubMed] [Google Scholar]
- (131).Chaudhary A, van Amerom FHW, Short RT. 2014. Experimental Evaluation of Micro-Ion Trap Mass Spectrometer Geometries. International Journal of Mass Spectrometry 371: 17–27. [Google Scholar]
- (132).Wang M, Quist HE, Hansen BJ, Peng Y, Zhang Z, Hawkins AR, Rockwood AL, Austin DE, Lee ML. 2011. Performance of a Halo Ion Trap Mass Analyzer with Exit Slits for Axial Ejection. J. Am. Soc. Mass Spectrom 22 (2): 369–378. [DOI] [PubMed] [Google Scholar]
- (133).Austin DE, Wang M, Tolley SE, Maas JD, Hawkins AR, Rockwood AL, Tolley HD, Lee ED, Lee ML. 2007. Halo Ion Trap Mass Spectrometer. Anal. Chem 79 (7): 2927–2932. [DOI] [PubMed] [Google Scholar]
- (134).Lammert SA, Plass WR, Thompson CV, Wise MB. 2001. Design, Optimization and Initial Performance of a Toroidal Rf Ion Trap Mass Spectrometer. International Journal of Mass Spectrometry 212 (1): 25–40. [Google Scholar]
- (135).Taylor N; Austin DE 2012. A Simplified Toroidal Ion Trap Mass Analyzer. International Journal of Mass Spectrometry 321–322, 25–32. [Google Scholar]
- (136).Ouyang Z, Wu G, Song Y, Li H, Plass WR, Cooks RG. 2004. Rectilinear Ion Trap: Concepts, Calculations, and Analytical Performance of a New Mass Analyzer. Anal. Chem 76 (16): 4595–4605. [DOI] [PubMed] [Google Scholar]
- (137).Fico M, Yu M, Ouyang Z, Cooks RG, Chappell WJ. 2007. Miniaturization and Geometry Optimization of a Polymer-Based Rectilinear Ion Trap. Anal. Chem 79 (21): 8076–8082. [DOI] [PubMed] [Google Scholar]
- (138).Shi W, Lu X, Zhang J, Zhao J, Yang L, Yu Q, Wang X. 2019. Comparison of Membrane Inlet and Capillary Introduction Miniature Mass Spectrometry for Liquid Analysis. Polymers 11 (3): 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Gao L, Cooks RG, Ouyang Z. 2008. Breaking the Pumping Speed Barrier in Mass Spectrometry: Discontinuous Atmospheric Pressure Interface. Anal. Chem 80 (11): 4026–4032. [DOI] [PubMed] [Google Scholar]
- (140).Zhai Y, Feng Y, Wei Y, Wang Y, Xu W. 2015. Development of a Miniature Mass Spectrometer with Continuous Atmospheric Pressure Interface. Analyst 140 (10): 3406–3414. [DOI] [PubMed] [Google Scholar]
- (141).Li L, Chen TC, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. 2014. Mini 12, Miniature Mass Spectrometer for Clinical and Other Applications—Introduction and Characterization. Anal. Chem 86 (6): 2909–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Pu F, Alfaro CM, Pirro V, Xie Z, Ouyang Z, Cooks RG. 2019. Rapid Determination of Isocitrate Dehydrogenase Mutation Status of Human Gliomas by Extraction Nanoelectrospray Using a Miniature Mass Spectrometer. Anal Bioanal Chem 411 (8): 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Bernier MC, Li F, Musselman B, Newton PN, Fernández FM. 2016. Fingerprinting of Falsified Artemisinin Combination Therapies via Direct Analysis in Real Time Coupled to a Compact Single Quadrupole Mass Spectrometer. Anal. Methods 8 (36): 6616–6624. [Google Scholar]
- (144).Fatigante WL, Mukta S, Lawton ZE, Bruno AM, Traub A, Gasa AJ, Stelmack AR, Wilson-Frank CR, Mulligan CC. 2020. Filter Cone Spray Ionization Coupled to a Portable MS System: Application to On-Site Forensic Evidence and Environmental Sample Analysis. J. Am. Soc. Mass Spectrom 31 (2): 336–346. [DOI] [PubMed] [Google Scholar]
- (145).Fedick PW, Pu F, Morato NM, Cooks RG. 2020. Identification and Confirmation of Fentanyls on Paper Using Portable Surface Enhanced Raman Spectroscopy and Paper Spray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom 31 (3): 735–741. [DOI] [PubMed] [Google Scholar]
- (146).Gómez-Ríos GA, Vasiljevic T, Gionfriddo E, Yu M, Pawliszyn J. 2017. Towards On-Site Analysis of Complex Matrices by Solid-Phase Microextraction-Transmission Mode Coupled to a Portable Mass Spectrometer via Direct Analysis in Real Time. Analyst 142 (16): 2928–2935. [DOI] [PubMed] [Google Scholar]
- (147).Pu F, Zhang W, Bateman KP, Liu Y, Helmy R, Ouyang Z. 2017. Using Miniature MS System with Automatic Blood Sampler for Preclinical Pharmacokinetics Study. Bioanalysis 9 (21): 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148). Chiang S, Zhang W, Farnsworth C, Zhu Y, Lee K, Ouyang Z. 2020. Targeted Quantification of Peptides Using Miniature Mass Spectrometry. J. Proteome Res 19 (5): 2043–2052. Demonstrated targeted quantification of trypsin-digested mouse liver peptides using miniature mass spectrometer
- (149).Gilliland WM, Mellors JS, Ramsey JM. 2017. Coupling Microchip Electrospray Ionization Devices with High Pressure Mass Spectrometry. Anal. Chem 89 (24): 13320–13325. [DOI] [PubMed] [Google Scholar]
- (150).Blokland MH, Gerssen A, Zoontjes PW, Pawliszyn J, Nielen MWF. 2020. Potential of Recent Ambient Ionization Techniques for Future Food Contaminant Analysis Using (Trans)Portable Mass Spectrometry. Food Anal. Methods 13 (3): 706–717. [Google Scholar]
- (151).Guo X, Bai H, Lv Y, Xi G, Li J, Ma X, Ren Y, Ouyang Z, Ma Q. 2018. Rapid Identification of Regulated Organic Chemical Compounds in Toys Using Ambient Ionization and a Miniature Mass Spectrometry System. Talanta 180: 182–192. [DOI] [PubMed] [Google Scholar]
- (152).Fotso Fotso A, Mediannikov O, Diatta G, Almeras L, Flaudrops C, Parola P, Drancourt M. 2014. MALDI-TOF Mass Spectrometry Detection of Pathogens in Vectors: The Borrelia Crocidurae/Ornithodoros Sonrai Paradigm. PLoS Negl Trop Dis 8 (7): pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Raouf M, Ghazal T, Kassem M, Agamya A, Amer A. 2020. Surveillance of Surgical-Site Infections and Antimicrobial Resistance Patterns in a Tertiary Hospital in Alexandria, Egypt. The Journal of Infection in Developing Countries 14 (03): 277–283. [DOI] [PubMed] [Google Scholar]
- (154).Ntshangase S, Mdanda S, Singh SD, Naicker T, Kruger HG, Baijnath S, Govender T. 2019. Mass Spectrometry Imaging Demonstrates the Regional Brain Distribution Patterns of Three First-Line Antiretroviral Drugs. ACS Omega 4 (25): 21169–21177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Hartenbach FARR, Velasquez É, Nogueira FCS, Domont GB, Ferreira E, Colombo APV. 2020. Proteomic Analysis of Whole Saliva in Chronic Periodontitis. Journal of Proteomics, 213: 103602. [DOI] [PubMed] [Google Scholar]
- (156).Silva LE, Souza RC, Kitano ES, Monteiro LF, Iwai LK, Forti FL. 2019. Proteomic and Interactome Approaches Reveal PAK4, PHB-2, and 14–3-3η as Targets of Overactivated Cdc42 in Cellular Responses to Genomic Instability. J. Proteome Res 18 (10): 3597–3614. [DOI] [PubMed] [Google Scholar]
- (157).Adeola HA, Blackburn JM, Rebbeck TR, Zerbini LF. 2017. Emerging Proteomics Biomarkers and Prostate Cancer Burden in Africa. Oncotarget 8 (23): 37991–38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Al-wajeeh AS, Salhimi SM, Al-Mansoub MA, Khalid IA, Harvey TM, Latiff A, Ismail MN. 2020. Comparative Proteomic Analysis of Different Stages of Breast Cancer Tissues Using Ultra High Performance Liquid Chromatography Tandem Mass Spectrometer. PLOS ONE 15 (1): e0227404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Koriem KMM. 2017. A Lipidomic Concept in Infectious Diseases. Asian Pacific Journal of Tropical Biomedicine 7 (3): 265–274. [Google Scholar]
- (160).Fernández FM, Cody RB, Green MD, Hampton CY, McGready R, Sengaloundeth S, White NJ, Newton PN. 2006. Characterization of Solid Counterfeit Drug Samples by Desorption Electrospray Ionization and Direct-Analysis-in-Real-Time Coupled to Time-of-Flight Mass Spectrometry. ChemMedChem 1 (7): 702–705. [DOI] [PubMed] [Google Scholar]
- (161).Fernandes AMAP, Vendramini PH, Galaverna R, Schwab NV, Alberici LC, Augusti R, Castilho RF, Eberlin MN. 2016. Direct Visualization of Neurotransmitters in Rat Brain Slices by Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI - MS). J. Am. Soc. Mass Spectrom 27 (12): 1944–1951. [DOI] [PubMed] [Google Scholar]
- (162).RockWood A, 2015. The Future of Clinical Mass Spectrometry. American Association for Clinical Chemistry, Feb 1. [Google Scholar]
- (163).Stone JA, Fitzgerald RL. 2018. Liquid Chromatography–Mass Spectrometry Education for Clinical Laboratory Scientists. Clinics in Laboratory Medicine 38 (3): 527–537. [DOI] [PubMed] [Google Scholar]
- (164).Ortiz-Ospina E, Roser M. 2017. Our World in Data. Financing Healthcare [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


