To the Editor:
Ibrutinib, a Bruton tyrosine kinase inhibitor, has impressive clinical efficacy in chronic lymphocytic leukemia (CLL), being able to improve progression-free survival (PFS) and overall survival (OS), compared to conventional chemoimmunotherapy in treatment-naïve patients with chronic lymphocytic leukemia (CLL).1,2 Despite representing a major therapeutic advance, single-agent ibrutinib has limitations. While responses to ibrutinib can be durable, the depth of responses are infrequently deep, reflected in low rates of complete responses (CRs) and undetectable minimal residual disease (U-MRD).3
We hypothesized that modulation of the T-cell compartment could improve the quality of response to ibrutinib. We report results from a phase 2 study in which short-course fludarabine was added to continuous ibrutinib, with the aim to debulk CLL cells and induce rapid reductions in T cell numbers. Eligible patients had treatment-naïve CLL requiring treatment. The study was approved by the Institutional Review Board and registered at clinicaltrials.gov (NCT02514083). All patients provided written informed consent. Ibrutinib 420 mg was administered orally once daily on 28-day cycles until disease progression or intolerable side effects. Fludarabine 25 mg/m2/day was given intravenously on days 1–5 of cycle three and four (Figure 1A). The primary efficacy endpoint was the CR rate at 6 months according to IWCLL criteria.4 The primary safety endpoint was the rate of treatment discontinuation within 6 months due to intolerable side effects. Secondary endpoints included assessments of residual CLL cells and T cell phenotypes in peripheral blood using flow cytometry. Undetectable minimal residual disease was defined as <1 CLL cell in 10 000 leukocytes (<10−4). The T cells were analyzed for CD3, CD4 and CD8. Additional surface and intracellular stains were applied to assess Treg (CD127-/CD25+/FoxP3+) and T follicular helper (TFH; CXCR5+/PD-1+) cells.
FIGURE 1.
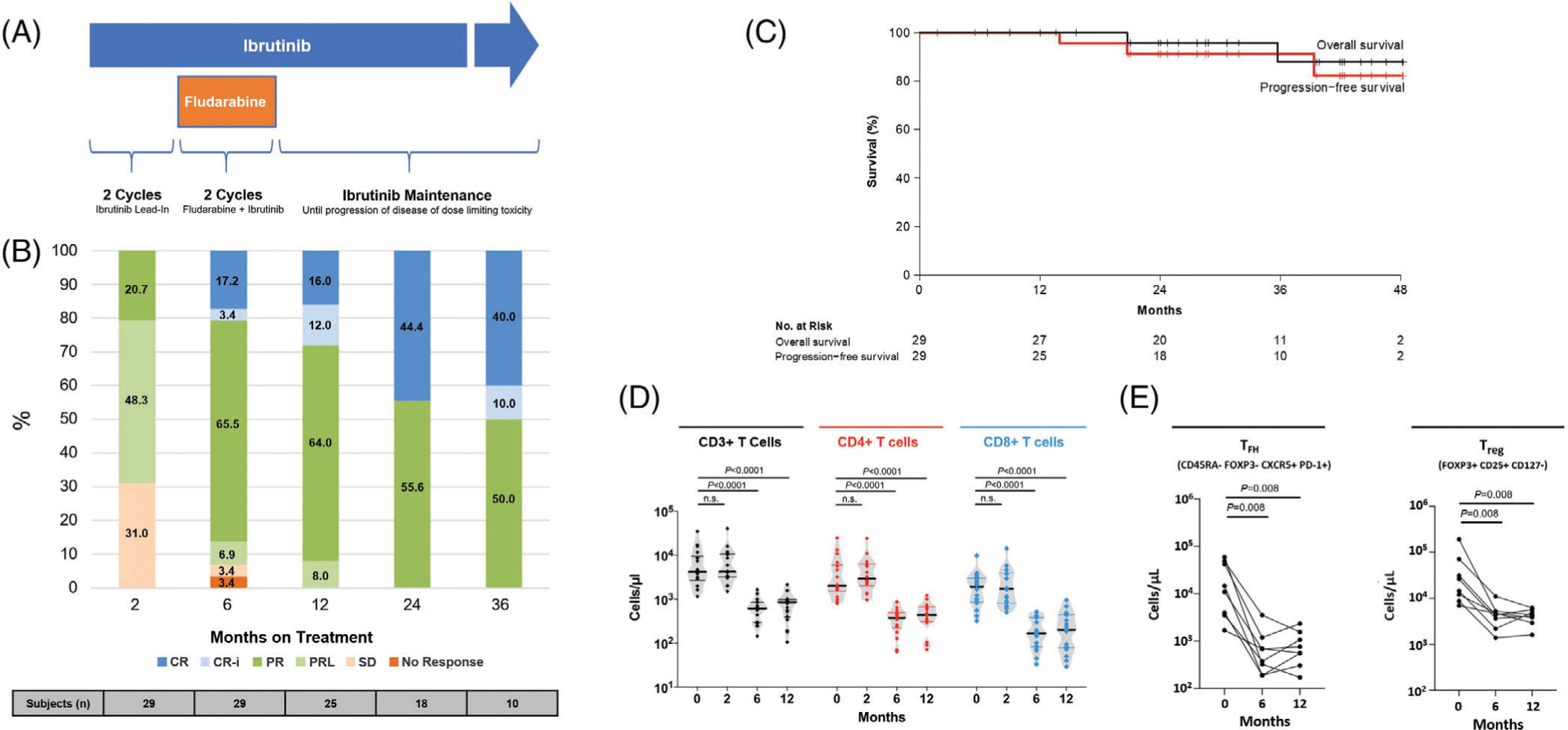
A, Study schema. Ibrutinib was given at 420 mg orally once daily. Fludarabine was given at 25 mg/m2/day intravenously on day 1–5 of cycle three and four. B, Response by iwCLL criteria. C, Progression-free and overall survival by Kaplan–Meier estimates. D, Absolute number of T cells during therapy. Black solid lines indicate the median of the group. E, Absolute numbers of follicular helper T cells (TFH) and FH regulatory T cells (Treg). Statistical significance by Wilcoxon matched-pairs signed-rank test is indicated. Abbreviations: CR, complete response; CR-i, complete response with incomplete hematologic recovery; n.s., not significant; PD, progressive disease; PR, partial response; PRL, partial response with persistent lymphocytosis; SD, stable disease
Sample size was determined based on the alternative hypothesis of a CR rate at 6 months ≥20% against the null hypothesis of a CR rate ≤5%, with 27 patients providing 80% power at an alpha level of 0.05. For the primary safety endpoint, the null hypothesis was the rate of treatment discontinuation being ≥30% and the alternative hypothesis was the discontinuation rate being ≤10%; 27 patients provided 81% power at an alpha level of 0.05. We used descriptive statistics to summarize findings, Kaplan-Meier method to estimate survival, Wilcoxon signed-rank test to assess changes in residual disease burden and T cell subsets over time. Statistical analyses were conducted using R version 3.6.1 and GraphPad Prism, version 7.02.
Between December 2015 and April 2019, 29 patients were enrolled. Table S1 summarizes baseline characteristics. The median age was 65 years. More than half of the patients were male and had advanced Rai stages (III/IV). Fourteen (48.3%) patients had unmutated immunoglobulin heavy chain variable gene (IGHV). Three (10.3%) patients had deletion 17p by FISH. Two patients who had concurrent hairy cell leukemia at baseline were eligible for the study because; (a) neither had received prior therapy for CLL or hairy cell leukemia, (b) CLL was the dominant disease process, and (c) the study allowed enrollment of patients with second primary malignancies when their expected survival was over 2 years.
With a median follow-up of 29 months (range, 7–47 months), 24 (82.8%) of 29 patients remain on study (Figure S1). Two patients discontinued study therapy due to hepatotoxicity before reaching the primary endpoint. One of these patients who did not receive any fludarabine was counted as a non-responder. The other patient who received one cycle of fludarabine was considered evaluable for response and achieved a partial response. The overall response rate at 6 months was 93.1%. The CR rates at 6, 12 and 24 months were 20.7%, 28.0% and 44.4%, respectively (Figure 1B). There was no association between the attainment of a CR and the IGHV status (P > .05). At 6 months, all evaluable patients were alive and progression-free. Estimated rates of PFS and OS at 2 years were 91.3% (95% confidence interval [CI], 80.5–100%) and 95.8% (95% CI, 88.2–100%), respectively (Figure 1C). One (3.4%) patient relapsed with CLL at 39 months without detectable BTK/PLCG2 mutations.
Most (83.0%) treatment-related adverse events (AEs) were grade 1–2 in severity (Table S2). The most common non-hematologic AE was bruising (65.5%), followed by diarrhea (44.8%), myalgia (41.4%) and arthralgia (37.9%). Grade ≥3 AEs and serious AEs of any grade occurred in 17 (58.6%) and 14 (48.3%) patients, respectively. Commonly observed grade ≥3 hematologic AEs were neutropenia (27.6%), leukopenia/lymphopenia (31.0%), and thrombocytopenia (10.3%). Most grade ≥3 cytopenias were associated with the addition of fludarabine and resolved with supportive care and continuously administered ibrutinib. Two (6.8%) patients developed grade 3 and 4 transaminitis during the first 3 months of therapy. Ibrutinib was permanently discontinued in both patients, leading to complete resolution of the event. One (3.4%) patient with a prior history of recurrent venous and arterial thromboembolism had a sudden cardiac death, possibly related to ibrutinib. No new onset or worsening of atrial fibrillation occurred on study. Three (10.3%) patients had opportunistic infections: grade 2 Pneumocystis pneumonia in two patients not on prophylaxis, and grade 3 atypical mycobacterial pneumonia in one patient. All opportunistic infections occurred during the ibrutinib monotherapy phase before or years after the administration of fludarabine, and were successfully treated with antibiotics. Four (13.8%) patients had omission of at least one course of fludarabine; due to an allergic reaction, and sepsis in one patient each in addition to ibrutinib-related hepatotoxicity in two patients. Two (6.9%) patients had fludarabine dose-delays due to thrombocytopenia. Two (6.9%) patients required dose reduction of ibrutinib, one due to rash and the other due to lung infection.
Undetectable minimal residual disease was uncommon, found in two (6.9%) patients; occurring at 6 months in one and at 2 years in the other (Figure S2). Baseline and longitudinal T-cell phenotyping was available in 13 patients. At baseline, patients had elevated T cell counts, which normalized during therapy (Figure 1D). The number of TFH (CD45RA- FoxP3- CXCR5+ PD-1+) and Treg (FoxP3+ CD25+ CD127 low) significantly decreased with therapy (Figure 1E).
In summary, the combination of ibrutinib and short-course fludarabine led to 44% of treatment-naïve CLL patients achieving a CR at 2 years, a rate higher than what has been reported for patients treated with ibrutinib and rituximab (17%).1 The addition of two cycles of fludarabine to ibrutinib did not increase the risk of high-grade hematologic toxicities or infection. Most cytopenias were low-grade and resolved after the completion of fludarabine. Opportunistic infections occurred during the ibrutinib monotherapy phase and were not associated with treatment discontinuations or mortality.
Whether attainment of a CR translates into improved clinical outcomes in CLL is unclear. A recent report demonstrated favorable PFS in patients achieving a CR on ibrutinib monotherapy,5 while others showed no difference in outcome.6 In this study, 24 (82.8%) patients remain progression-free after a median follow-up of 29 months. This duration of response appears similar to what has been reported for first-line ibrutinib therapy.1,2 A longer follow-up is warranted to determine the durability of response and the role of a CR in predicting outcomes.
We sought to investigate whether targeting T cells with an abbreviated course of fludarabine could enhance the effects of ibrutinib, while avoiding myelotoxicity and the risk of secondary malignancies associated with intense chemoimmunotherapy. Lymphodepletion with fludarabine is a critical component in protocols of adoptive cellular transfer for hematopoietic stem cell transplant or chimeric antigen receptor-modified T cell therapy. Here we used a similar approach in the intention to suppress pro-tumor T cell populations and create an environment that may be more supportive of anti-tumor activities by repopulating T cells. We observed rapid reductions in the number of T cells and potentially favorable remodeling of the T cell compartment. The number of T cells were elevated at pretreatment and normalized after treatment with ibrutinib and a short course of fludarabine. Nota-bly, the addition of two cycles of fludarabine in our study did not cause prolonged T cell depletion. Further, TFH and Treg, two potentially tumor-supportive T cell populations, significantly decreased during therapy. A follow-on study that adds pembrolizumab to ibrutinib and short-course fludarabine for high-risk or relapsed/refractory CLL is ongoing (NCT03204188).
Supplementary Material
ACKNOWLEDGMENTS
The research was supported by the Intramural Research Program of the National Institutes of Health. Pharmacyclics LLC, an AbbVie Company, provided ibrutinib. We thank the patients who participated in this trial and their families. We thank Larisa Bezkorovaynaya for data management; Adriana Byrnes and Ovsanna Melikyan for protocol support; and the Office of Clinical Director for administrative support. Inhye E. Ahn acknowledges research support from the American Society of Hematology Scholar Award.
Footnotes
CONFLICT OF INTERESTS
Adrian Wiestner received research support from Pharmacyclics LLC, an AbbVie company; Acerta Pharma, a member of the Astra-Zeneca group; Merck; Nurix; and Genmab. The remaining authors declare no conflicts of interest with the content of this paper.
DATA AVAILABILITY STATEMENT
Author elects to not share data.
REFERENCES
- 1.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019;381(5):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018;379(26):2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018; 131(21):2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. [DOI] [PubMed] [Google Scholar]
- 5.Strati P, Schlette EJ, Solis Soto LM, et al. Achieving complete remission in CLL patients treated with ibrutinib: clinical significance and predictive factors. Blood. 2020;135(7):510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigmund A, Huang Y, Ruppert AS, et al. Depth of response and progression free survival in CLL patients on ibrutinib. J Clin Oncol. 2018;36 (15_suppl):7514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Author elects to not share data.


