Abstract
Traumatic brain injury (TBI) is a predominant cause of death and permanent disability globally. In recent years, much emphasis has been laid on treatments for TBI. Increasing evidence suggests that human umbilical cord mesenchymal stem cells (HUCMSCs) can improve neurological repair after TBI. However, the clinical use of HUCMSCs transplantation in TBI has been limited by immunological rejection, ethical issues, and the risk of tumorigenicity. Many studies have shown that HUCMSCs-derived exosomes may be an alternative approach for HUCMSCs transplantation. We hypothesized that exosomes derived from HUCMSCs could inhibit apoptosis after TBI, reduce neuroinflammation, and promote neurogenesis. A rat model of TBI was established to investigate the efficiency of neurological recovery with exosome therapy. We found that exosomes derived from HUCMSCs significantly ameliorated sensorimotor function and spatial learning in rats after TBI. Moreover, HUCMSCs-derived exosomes significantly reduced proinflammatory cytokine expression by suppressing the NF-κB signaling pathway. Furthermore, we found that HUCMSC-derived exosomes inhibited neuronal apoptosis, reduced inflammation, and promoted neuron regeneration in the injured cortex of rats after TBI. These results indicate that HUCMSCs-derived exosomes may be a promising therapeutic strategy for TBI.
Keywords: exosomes, human umbilical cord mesenchymal stem cells, neurological recovery, NF-κB, traumatic brain injury
1. Introduction
The pathological hallmarks of traumatic brain injury (TBI) include neuron loss, axonal destruction, and demyelination [1], accounting for poor patient neurological recovery. Although unprecedented progress has been made in recent years, the treatment of TBI remains highly challenging. TBI can lead to physical disability, neurobehavioral dysfunction, and cognitive impairment [2,3,4,5,6,7,8]. Currently, the mechanisms underlying the pathological changes in TBI remain largely understudied. It is well established that neuroinflammation plays a crucial role in secondary TBI [9] involving many cell types, especially microglia and astrocytes involved in inflammatory reactions [10,11]. Astrocytes have many important physiological functions, such as providing trophic support, maintaining homeostasis, modulating synapses, and maintaining the blood-brain barrier. Serious brain insult results in reactive astrocytosis, which can both sustain and impede the recovery of the central nervous system [12,13,14]. Recent studies have suggested that astrocytes can migrate to neurotoxic A1 or neuroprotective A2 phenotypes via a stimulus-specific manner, and A1 astrocytes activation led to neuronal cell death. In contrast, A2 astrocytes activation led to a protective effect against inflammation and protected neurons by upregulating many neurotrophic factors [15].
Mesenchymal stem cells (MSCs) treatment has been subjected to great interest in recent years since they can promote restoration of neurological function after treatment [16,17]. However, MSCs treatment has been associated with some disadvantages in the clinical setting. Previous studies have shown that MSCs treatment may increase the tumorigenic risk [18,19], and only a small portion of the transplanted MSCs survive and differentiate into neurons in the damaged brain tissues [20]. Although it is widely acknowledged that MSCs can repair brain tissue damage [21], little is known about the underlying neuroprotective mechanisms. Several reports have shown that the repair and restorative functions of MSCs involve paracrine mechanisms, not a transdifferentiation [22].
Exosomes are lipid bilayer membrane vesicles released from various cell types, ranging from 40 to 160 nm in diameter [23]. Exosomes are abundant in endosome-derived components, including lipids, proteins, mRNAs, and microRNAs (miRNAs) [23]. An increasing body of evidence suggests that MSCs-derived exosomes exert neuroprotective effects [24] by improving neurite remodeling and neurofunctional recovery after TBI [25] and stroke [26]. Studies have shown that activation of the NF-κB signaling pathway results in the synthesis of proinflammatory cytokines such as TNF-α and IL-6 [27]. It is unclear whether the intravenous infusion of exosomes derived from human umbilical cord mesenchymal stem cells (HUCMSCs) reduces inflammation and promotes functional recovery through the NF-κB signaling pathway. This study assessed the effects of HUCMSCs-derived exosomes on functional recovery and explored the potential mechanisms underlying their neuroprotective effects after TBI.
2. Materials and methods
2.1. Isolation, culture, and identification of HUCMSCs
HUCMSCs were isolated from healthy newborn’s umbilical cords from our hospital’s maternity department according to an established method [28,29]. The cells were then cultured in DMEM/F12 medium (Gibco, Grand Island, NY, USA). (containing volume fraction 10% fetal bovine serum (FBS; MRC Biotechnology Co., Ltd, Jiangsu, China), 25 mmol/L glutamine (Solarbio Science & Technology Co., Ltd, Beijing, China), 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA), 100 mg/L streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a 5% CO2 incubator at 37°C. The morphology of HUCMSCs was observed by inverted phase-contrast microscopy.
Immunofluorescence was performed to identify the immunological phenotype of HUCMSCs. Cell samples were incubated with rabbit polyclonal antibody against CD90 (1:300, Abcam, Cambridge, UK) and mouse polyclonal antibody against CD105 (1:400, Abcam, Cambridge, UK) at 4°C overnight. A fluorescence microscope (Leica TCS SP5, Germany) was used for observation and photographing.
HUCMSCs were identified by flow cytometry, as previously described [28,29]. Briefly, the cell suspension to be tested was separated into 8 tubes (100 μL per tube). These cells were incubated with CD90, CD105, CD73, CD116, CD19, CD45, and HLA-DR antibodies (Abcam Cambridge, UK) for 30 min at 4°C according to the instructions of the flow cytometry kit. The positivity rate of various antigens was determined by a Cytomics FC500 flow cytometer (BD Biosciences, San Jose, CA).
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.2. Isolation and identification of exosomes derived from HUCMSCs
Exosomes were isolated from HUCMSCs using methods previously described [30]. Briefly, the medium harvested from HUCMSCs was centrifuged at 500×g for 30 min, at 3,000×g for 30 min at 4°C and at 5,000×g for 30 min to obtain the supernatant. The supernatant was placed in a 30% heavy water sucrose pad in an ultra-detached tube and subjected to ultracentrifugation at 120,000×g for 70 min at 4°C. The heavy water sucrose cushion was washed twice with PBS at 120,000×g for 70 min. The precipitate used in the DMEM/F12 medium was resuspended in the tube wall and filtered through a 0.22 μm sterile filter for subsequent experiments.
Exosomes were identified by transmission electron microscopy (TEM; HT7700, Hitachi, Japan) [31]. The sample was then photographed using a transmission electron microscope. Western blot was performed to quantify the expression of exosome surface markers (CD9 and CD63) according to established methods [32].
2.3. TBI model and exosome therapy
Male Sprague-Dawley (SD) rats (weight 250–300 g) were obtained from the Military Academy of Medical Sciences of the People’s Liberation Arm. All rats were housed under standard environmental conditions: 12 h light/dark cycle, temperature (22 ± 1°C), wire-top-type cages, three rats per cage, and free access to water and food. All experiments were carried out between 9:00 and 12:00 am.
The rats were subjected to Cortical Contusion Impact Injury by using an electric cortical contusion impactor device (eCCI-6.3; Custom Design & Fabrication) according to established methods [33]. Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg). A bench drill was used to puncture a circular orbital window of diameter 5 mm (2 mm on the posterior side of the coronal suture and 1 mm on the right side of the sagittal suture). The rat was then moved to an electronic eCCI console, the hammer position was adjusted to align with the open bone window and perpendicular to the cortex, and the striking parameters were set as follows: speed 5 m/s, depth 2 mm, hammer dwell time 120 ms. After the injury, hemostatic methods were quickly applied. Immediately after the injury, the small animal ventilator was used to provide breathing assistance, and if necessary, chest compressions were given. The scalp incision was sutured after normal breathing was resumed.
Forty-five rats were randomly divided into three groups: Sham group (n = 15), TBI group (treated with PBS, n = 15), and Exo group (treated with exosome, n = 15). The Sham group rats underwent a bone drill without moderate severity brain injury. The TBI group was subjected to moderate severity brain injury and then injected with intravenous PBS over 5 min via the tail vein, starting 1 day after injury. The Exo group was subjected to moderate severity brain injury and then intravenously injected with exosomes derived from HUCMSCs (100 μg total protein of exosome precipitate in 0.5 mL of PBS per rat) over 5 min via the tail vein, starting 1 day after injury.
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and was approved by the Animal Care and Use Committee of the People’s Armed Police Logistics Institute (PAP) (Approval No. 2019-0019.5) and PAP Research Animal Ethics Committee (ethical approval reference number 36569/62).
2.4. Behavioral testing
2.4.1. Modified neurological severity scores (mNSS)
In the TBI and Exo groups, mNSS were used to evaluate neurological function 3 h after modeling (n = 15 for each group), and rats with an mNSS score <10 were excluded. Fifteen rats were randomly selected from each group, and the mNSS scores were graded at 7, 14, 21, and 28 days after modeling. The mNSS assessment consists of an exercise test (lifting test and abnormal activity), sensory test (visual, tactile, and proprioception), balance beam test, reflex activity, and abnormal movement. The mNSS score is usually graded on a scale of 0–18, the normal score is 0, and the maximum deficit score is 18, the lower the mNSS score, the better the neurological function [34].
2.4.2. Morris water maze (MWM) test
To assess the possible effects of MSCs on the cognitive function in rats after TBI, spatial learning and memory in rats were measured by the MWM test (n = 15 for each group) [29]. During the learning process, the ability of rats to find the submerged platform was trained for 21–26 days after TBI, and the time required for the rats to enter the water to find the platform (the escape latency time) was recorded. The platform was removed on the 28th day after the injury, and the spatial learning and memory ability of the rats were evaluated by measuring the latency time spent in the target quadrant and the number of platform crossings.
2.4.3. Long-term potentiation (LTP) measurement
After TBI, SD rats will develop memory deficits. To demonstrate the improvement in memory deficits in TBI rats, LTP was measured to study synaptic transmission [35,36,37]. The electrophysiological measurements were carried out at 28 days after TBI according to a method previously described [29]. Briefly, the rats were fixed to a stereotactic instrument after anesthesia (n = 15 for each group). The skull was exposed, then a small hole was formed on the opposite side of the injury to achieve vertical penetration by the stimulation and recording electrodes. According to the rat brain atlas, the stimulating electrodes were placed in the area at the following corresponding coordinates: AP –6.8 mm, ML 4.5 mm, and DV –3.5 mm. The recording electrode is placed in the area of the corresponding coordinates (−3.5 mm on the AP, ML 3.4 mm, and DV −3.5 mm). A stable baseline for at least 20 min was needed before each stimulus was applied during each electrophysiological recording experiment. LTP was initiated by high-frequency stimulation (HFS) consisting of 4 pulses in a series of 50 pulses delivered at 200 Hz with an intertrain spacing of 2 s. A computer program (RM6240BD; Chengdu, China) was used to analyze LTP by calculating excitatory postsynaptic potential (EPSP) and population peaks (PS).
2.5. Enzyme-linked immunosorbent assay (ELISA)
At 3 days after TBI, the expression of inflammatory factors such as Tumor necrosis factor-alpha (TNF-α), Interleukin (IL)-1β, and IL-6 was measured using ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. After the tissue in the injured area was obtained, the sample was prepared using a grinder and an ultrasonic tissue homogenizer. A microplate reader is used to obtain the optical density (OD) value at 490 nm. Finally, the expression levels of the three inflammatory factors were measured according to the kit instructions.
2.6. Western blot analysis
Western blot was used to measure the expression of the NF-kB signaling pathway at 3 days after TBI. Following electrophoresis separation, proteins were transferred to the PVDF membrane. The membranes were then blocked and incubated with primary antibody, mouse anti-NF-κBP65 antibody (1:500; Cell Signaling Technology, Shanghai, China), and anti-β-actin antibody (1:2,000; Sigma) at 4°C overnight. Then, the membranes were incubated with the secondary antibody (1:1,000, Cell signaling) for 2 h at room temperature (RT). The protein bands were determined using an image analysis system.
2.7. TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining
Apoptosis analysis of the cerebral cortex was performed by TUNEL (In Situ Cell Death Detection Kit; Roche Diagnostics, Indianapolis, IN, USA) staining at 3 days after TBI [38]. Briefly, brain tissue sections were counterstained with DAPI for 15 min. After the sections were washed twice with PBS, the sections were then stained with the TUNEL reaction mixture for 60 min at a constant temperature of 37°C. Cell quantification was implemented by two observers blinded to the experiment using a fluorescence microscope at a magnification of 200×. The percentage of apoptotic cells was obtained using the formula [39]:
2.8. Immunofluorescent Staining
To assess the effect of exosome treatment on inflammatory response in the cerebral cortex after craniocerebral trauma, brain tissue sections were labeled with Iba1 (a microglia marker) at 7 days after TBI, CD68 (to identify activated macrophages/microglia), and glial fibrillary acidic protein (GFAP) (to identify reactive astrocytes) at 28 days after TBI. To assess the effect of exosome treatment on the cerebral cortex changes, brain tissue sections were labeled with Doublecortin (DCX, a marker of immature neurons) at 7 days after TBI and neuronal nuclear protein (NeuN, a marker of mature neurons) at 28 days after TBI.
The immunofluorescence assay was carried out according to previously described methods [2]. The slices were incubated with primary antibodies (Iba1, 1:500; CD68, 1:1,000; GFAP, 1:400; DCX, 1:100; and NeuN, 1:200) (Abcam, Cambridge, UK) at 4°C overnight. A fluorescence microscopy (Leica TCS SP5, Germany) was used for observation and photographing. Cell counting was performed according to established methods [25,40].
2.9. Statistical analysis
Data were expressed as mean value ± standard deviation (SD), and the differences were analyzed using One-way ANOVA and student t-tests with GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA). A P-value <0.05 was statistically significant.
3. Results
3.1. Culture of HUCMSCs and identification of surface markers
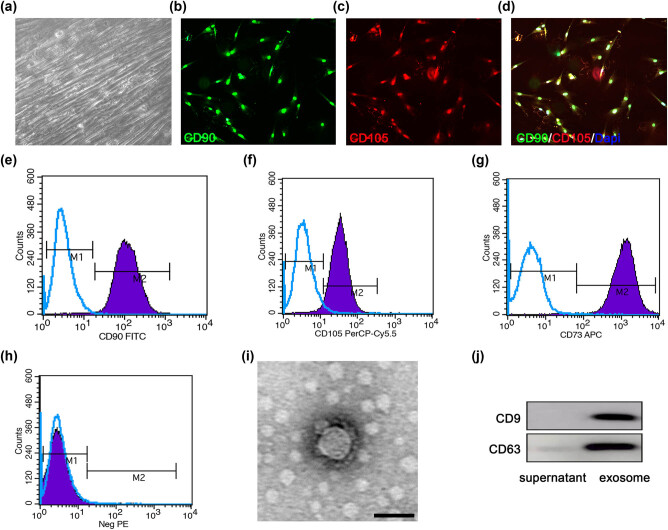
The phase-contrast microscopy images showed that the 4th generation HUCMSCs were fusiform, and the cells were closely connected to form a spiral arrangement (Figure 1a). The biomarkers CD90 and CD105 of HUCMSCs were detected under fluorescence microscopy (Figure 1b–d). The results of flow cytometry indicated that HUCMSCs isolated and cultured in our study expressed MSC-specific marker proteins CD90, CD105, and CD73, and the expression rates were all >95%, suggesting that HUCMSCs were successfully isolated and cultured in vitro, and the purity met the experimental requirements (Figure 1e–h).
Figure 1.
Identification of HUCMSCs and the HUCMSCs-derived exosomes. (a) Representative cell morphology of 4th generation HUCMSCs in phase contrast microscopy. (b–d) Double immunostaining with an anti-CD90 antibody (green) and an anti-CD105 antibody (red) to identify HUCMSCs. (e–h) Expression of CD90 (e), CD105 (f), CD73 (g), and negative molecules (Neg PE) (CD45, CD116, CD19, and HLA-DR) (h) on HUCMSCs detected by flow cytometry. (i) Representative transmission electron microscope image of the exosomes. (j) Exosomes surface marker protein expression of exosomes by Western blot. Scale bars = 50 µm in panels (a–d), 100 nm in panel (i).
3.2. Characterization of exosomes derived from HUCMSCs
Transmission electron microscopy showed that the exosomes were round or elliptical membranous vesicles with significant heterogeneity, ranging from 30 to 200 nm in diameter. The membranous structure was visible in the periphery of the exosome vesicles and contained low-density substances (Figure 1i). The western blot results showed that the exosomes derived from HUCMSCs exhibited high CD9 and CD63 expression (Figure 1j).
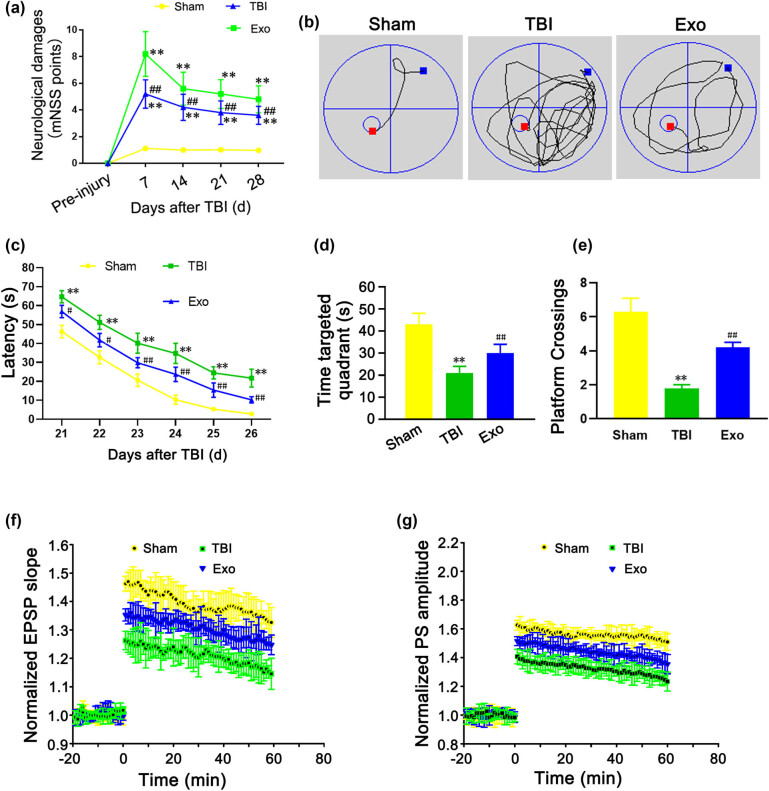
3.3. Intravenous infusion of the HUCMSCs-derived exosomes significantly ameliorated the sensorimotor function and spatial learning in rats after TBI
At 7, 14, 21, and 28 days after TBI, the mNSS scores of the TBI group and the Exo group were significantly higher than those of the Sham group (P < 0.01), while the mNSS scores of the Exo group were improved compared to the TBI group (P < 0.01) (Figure 2a). These results indicated that the HUCMSCs-derived exosomes could promote the repair of neurological deficits in rats after TBI. During the spatial learning phase, the swimming tracks of three groups were detected to analyze their learning ability (Figure 2a). At 28 days after TBI, compared with the Sham group, the latency of the TBI group was significantly prolonged (P < 0.01), and the time spent in the targeted quadrant and the number of platform crossings were significantly reduced (P < 0.01) (Figure 2c–e). We found that the latency, the time spent in the targeted quadrant, and the number of platform crossings were all enhanced in the Exo group compared to the TBI group (P < 0.01) (Figure 2c–e). The results of the MWM test suggested that the HUCMSCs-derived exosomes could promote learning and memory recovery in TBI rats. Compared to the TBI group, the administration of the HUCMSCs-derived exosomes in the Exo group significantly improved the EPSP slope (Figure 2f) and PS amplitude (Figure 2g).
Figure 2.
Administration of the HUCMSCs-derived exosomes markedly ameliorates sensorimotor function and spatial learning in rats after TBI. (a) Modified neurological severity (mNSS) score at 7, 14, 21, and 28 days after TBI. (b) Typical swim tracks in three groups. (c) The latency from 21 to 26 days after TBI. (d) The time spent in the targeted quadrant at 28 days after TBI. (e) The number of platform crossings. (f) Normalized excitatory postsynaptic potential (EPSP) slope at 28 days after TBI. (g) Population spike (PS) amplitude at 28 days after TBI. **P < 0.01 vs Sham group. # P < 0.05 and ## P < 0.01 vs TBI group.
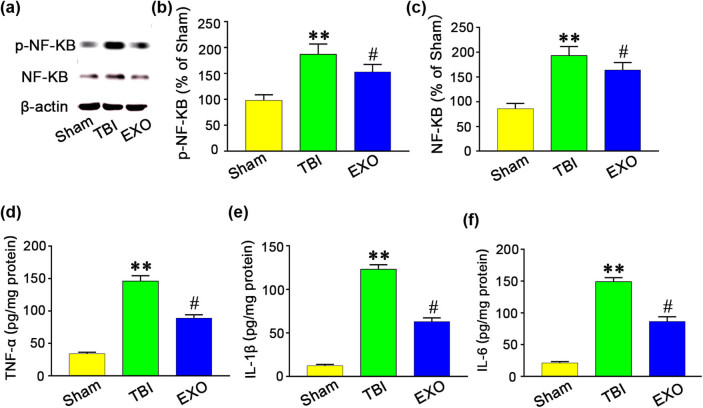
3.4. Administration of the HUCMSCs-derived exosomes significantly reduced proinflammatory cytokine expression by suppressing the NF-κB signaling pathway
We further sought to assess whether the protective effect of exosomes could be attributed to inhibition of the NF-κB signaling pathway. At 3 days after TBI, the NF-κB expression was significantly increased in the TBI group compared with the Sham group (P < 0.01) (Figure 3a–c). However, the HUCMSCs-derived exosomes treatment significantly decreased NF-κB expression in the injured cortex compared with the TBI group (P < 0.05). We also found that TNF-α, IL-1β, and IL-6 levels were upregulated in the TBI group compared with the EXO group (Figure 3d–f). Quantitative analyses demonstrated that TNF-α, IL-1β, and IL-6 expressions in the Exo group were significantly reduced compared with TBI group (P < 0.05) (Figure 3d–f). The above findings suggest that behavioral recovery after administration of the HUCMSCs-derived exosomes may result from suppressing the NF-κB signaling pathway.
Figure 3.
Administration of the HUCMSCs-derived exosomes reduced p-NF-κB and NF-κB and inflammatory cytokines (TNF-α, IL-1β, and IL-6) expression at 3 days after TBI. (a) Rats treated with HUMSCs-exo showed a decrease in NF-κB and p-NF-κB expression. (b and c) Western blotting showed that exosomes reduced p-NF-κB and NF-κB levels in the cortex. (d–f) TBI induced TNF-α, IL-1β, and IL-6 increase in rats. Rats treated with HUMSCs-exo showed a decrease in TNF-α, IL-1β, and IL-6 level. ELISA showed that HUMSCs-exo treatment reduced TNF-α, IL-1β, and IL-6 protein levels. **P < 0.01 vs Sham group. # P < 0.05 vs TBI group.
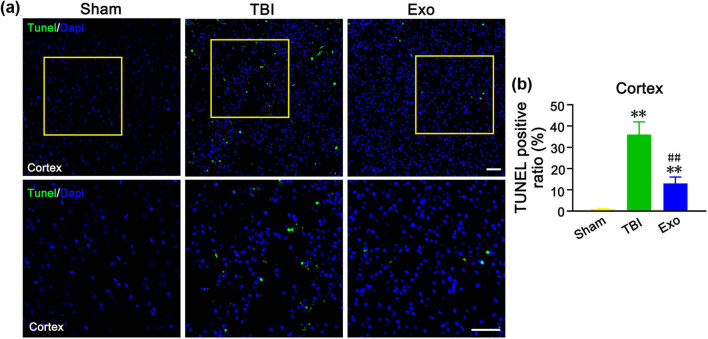
3.5. Administration of the HUCMSCs-derived exosomes attenuated neuronal apoptosis in the injured cortex of rats after TBI
To study whether the HUCMSCs-derived exosomes reduce neuronal apoptosis, rat brain tissue sections were subjected to TUNEL staining. We found the number of TUNEL positive cells at 3 days after TBI was significantly increased in the brain cortex of the TBI group compared to the Sham group (P < 0.01) (Figure 4a and b). We also observed that administration of the HUCMSCs-derived exosomes decreased the number of TUNEL positive cells compared to the TBI group (without any administration) (P < 0.01) (Figure 4a and b). These results revealed that the HUCMSCs-derived exosomes participated in alleviating apoptosis in the cerebral cortical neurons of TBI rats.
Figure 4.
Administration of the HUCMSCs-derived exosomes attenuated neuronal apoptosis in the injured cortex of rats at 3 days after TBI. (a) TUNEL staining in the cortex in three groups. The image below is an amplified image of the yellow box in the image above. (b) Percentage of TUNEL positive rate in the injured cortex. **P < 0.01 vs Sham group. ## P < 0.01 vs TBI group. Scale bars = 50 µm in (a).
3.6. Administration of the HUCMSCs-derived exosomes significantly reduced injured cortex inflammation in rats after TBI
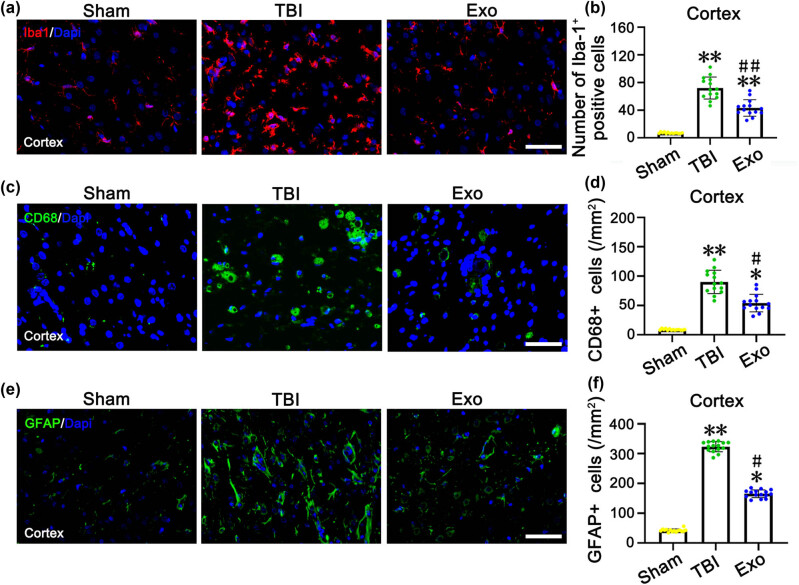
The number of Iba1-positive cells in the injured cortex was significantly reduced in the Exo group compared with the TBI group (P < 0.01) (Figure 5a and b). Administration of the HUCMSCs-derived exosomes significantly reduced the number of CD68 + cells in the injured cortex compared with the TBI group (without any administration) (P < 0.05) (Figure 5c and d). The number of GFAP + cells in the cortex in the injured cortex showed changes similar to those of the number of Iba1-positive cells and CD68 + cells after TBI. Moreover, the GFAP + astrocyte density in the injured cortex was significantly reduced in the Exo group compared with the TBI group (P < 0.05) (Figure 5e and f).
Figure 5.
Administration of the HUCMSCs-derived exosomes obviously reduced the number of Iba1, CD68, and GFAP positive cells in the injured cortex of rats at 7 days after TBI. (a, c, and e) Iba1-positive cells (a), CD68-positive cells (c), and GFAP-positive cells (e) in the injured cortex of three groups. (b, d, and f) is the quantification of Iba1 (b), CD68 (d), and GFAP (f) positive cells in each group. *P < 0.05 and **P < 0.01 vs Sham group. # P < 0.05 and ## P < 0.01 vs TBI group. Scale bars = 50 µm in panels (a, c, and e).
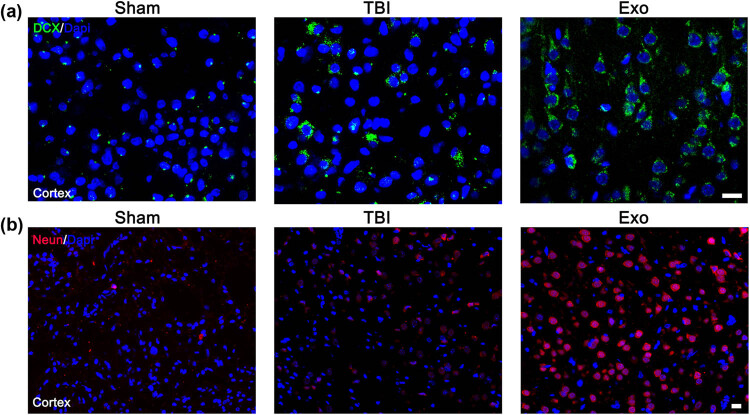
3.7. Administration of the HUCMSCs-derived exosomes significantly facilitated neuron regeneration in the injured cortex of rats after TBI
DCX has been established as a marker for immature neurons, while NeuN is for mature neurons. Compared to the TBI group, the number of DCX-positive and NeuN-positive cells was significantly increased in the Exo group (Figure 6a and b). These results indicated that the administration of the HUCMSCs-derived exosomes could promote neuron regeneration.
Figure 6.
Administration of the HUCMSCs-derived exosomes obviously facilitated neuron regeneration in the injured cortex of rats after TBI. (a) DCX-positive cells in the injured cortex of three groups at 7 days after TBI. (b) NeuN-positive cells in the injured cortex of three groups at 28 days after TBI. Scale bars = 50 µm in panels (a and b).
4. Discussion
In the present study, we demonstrated that the HUCMSCs-derived exosomes had huge prospects for treating TBI in rat models. Significant improvements were observed in the HUCMSCs-exosome treatment group, including improved learning, memory and neurofunctional recovery, reduced glial scar formation, decreased neuron loss and cell apoptosis, and suppression of inflammation. Our study demonstrated that the HUCMSCs-derived exosomes could be a novel therapeutic approach for structural and functional TBI recovery.
Recent evidence suggests that the neuroprotective properties of MSCs can be attributed to the presence of multiple secretions of bioactive molecules that modulate the tissue microenvironment to repair and regenerate tissue [41]. Preclinical studies have substantiated the therapeutic effect of MSCs secretome in TBI. Soluble bioactive molecules and extracellular vesicles are various factors secreted by MSCs that induce neurogenesis, angiogenesis, neovascularization, and anti-inflammatory activity. Kim et al. demonstrated improved cognitive function using MSCs-derived CD63+ and CD81+ exosomes in a TBI mouse model [42]. Similarly, Doeppner et al. reported that the therapeutic effect of MSCs-derived exosomes was equivalent to MSC therapy in a rat model of stroke [43]. In our study, we indicated that intravenous infusion of the HUCMSCs-derived exosomes significantly ameliorated the sensorimotor function and spatial learning in rats after TBI by the mNSS scores and the MWM test. We found that administration of the HUCMSCs-derived exosomes decreased the number of TUNEL positive cells compared to the TBI group, which suggested that the administration of the HUCMSCs-derived exosomes attenuated neuronal apoptosis in the injured cortex of rats after TBI. Furthermore, the number of DCX-positive and NeuN-positive cells in the Exo group was significantly increased than that in the TBI group, which indicated that administration of the HUCMSCs-derived exosomes significantly facilitated neuron regeneration in the injured cortex of rats after TBI
Neuroinflammation plays a key role in the pathophysiology of central nervous system diseases such as cerebral ischemia/reperfusion [44] and TBI [45]. Microglial cells and astrocytes may also play an important role in neuroinflammation; microglia and astrocytes have been documented to be activated after brain injury and release an excessive amount of proinflammatory cytokines including TNF-α, and IL-6. Accumulation of these inflammatory factors increases the level of cell adhesion molecules and neutrophil infiltration. which is deleterious to neighboring cells, and enhances neural cell apoptosis, finally culminating in secondary brain injury [46,47]. Reactive astrocytosis is considered one of the pathological hallmarks of nerve injury [48]. Persistent reactive astrogliosis releases proinflammatory cytokines and exacerbates neuronal loss following inflammatory response [49]. Recent studies have shown that reactive astrocytes could be divided into “A1” or “A2” phenotypes based on different molecular markers [15]. Furthermore, a recent study found that microglial cells can generate IL-1α, TNF-α, and c1q, which induces A1 astrocytes activation, resulting in neuronal cell death [15]. However, NLY01, a GLP1R agonist, exerts a neuroprotective effect in Parkinson’s disease by inhibiting the formation of A1 reactive astrocytes [50]. Accordingly, the attenuation of activation astrocytes may be an important therapeutic strategy for CNS diseases. Some studies demonstrated that inhibition of the NF-κB signaling pathway was responsible for the neuroprotective effect after focal cerebral I/R injury [51]. Previous studies suggested that ADSCs-exosomes significantly limited the activation of the NF-kB signaling pathway in LPS-induced BV2 cells and suppressed the production of inflammatory cytokines, protecting neural cells from injury [52]. In our study, the number of Iba1-positive cells and CD68 + cells in the injured cortex was significantly reduced in the Exo group compared with the TBI group, which demonstrated that administration of the HUCMSCs-derived exosomes significantly reduced injured cortex inflammation in rats after TBI. Moreover, the GFAP + astrocyte density in the injured cortex of the Exo group was significantly reduced than that of the TBI group. Our study showed that the HUCMSCs-derived exosomes suppressed the activation of microglia and astrocytes and downregulated inflammatory cytokine expression by decreasing the activation of the NF-kB signaling pathway.
It is currently not possible to extract exosomes in large quantities and with high purity. Nonetheless, the present study demonstrated the potential of stem cell-derived exosomes in the treatment of TBI. Further studies are required to elucidate the mechanisms underlying stem cell-derived exosomes’ protective and regenerative effects.
Given the paucity of clinical data supporting the clinical application of exosomes therapy, the use of exosomes at the clinical level is still at the theoretical and experimental stages. Extensive progress made in the production of clinical grade exosomes will certainly enable the development of new therapeutic strategies to reduce the mortality rate and improve the quality of life of this particular patient population.
Footnotes
Funding information: This work was supported by the National Nature Scientific Fund of China (81271392).
Author contributions: Z.W.Z., P.W., and G.J.Z. designed the experiment. Z.W.Z., P.W., G.J.Z., J.X.Y., S.Z., J.L., and X.L.W. implemented the experiment. Z.W.Z., P.W., G.J.Z., and J.L. analyzed the data. X.L.W. and J.L. contributed material and coordinated equipment. Z.W.Z., P.W., G.J.Z., J.L., and X.L.W. wrote the article.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Jin Liang, Email: liangjin1975@126.com.
Xiao-Li Wang, Email: wangxiaolihao1110@163.com.
References
- [1].Donat CK, Yanez Lopez M, Sastre M, Baxan N, Goldfinger M, Seeamber R, et al. From biomechanics to pathology: predicting axonal injury from patterns of strain after traumatic brain injury. Brain. 2021;144:70–91. [DOI] [PMC free article] [PubMed]
- [2].Dams-O'connor K, Guetta G, Hahn-Ketter AE, Fedor A. Traumatic brain injury as a risk factor for Alzheimer’s disease: current knowledge and future directions. Neurodegener Dis Manag. 2016;6:417–29. [DOI] [PMC free article] [PubMed]
- [3].Wu Z, Wang ZH, Liu X, Zhang Z, Gu X, Yu SP, et al. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog Neurobiol. 2019;185:101730. [DOI] [PubMed]
- [4].Fronczak KM, Li Y, Henchir J, Dixon CE, Carlson SW. Reductions in synaptic vesicle glycoprotein 2 isoforms in the cortex and hippocampus in a rat model of traumatic brain injury. Mol Neurobiol. 2021;58:6006–19. [DOI] [PMC free article] [PubMed]
- [5].Bae M, Hwang DW, Ko MK, Jin Y, Shin WJ, Park W, et al. Neural stem cell delivery using brain-derived tissue-specific bioink for recovering from traumatic brain injury. Biofabrication. 2021;13:13. [DOI] [PubMed]
- [6].Ma S, Zhou J, Huang T, Zhang Z, Xing Q, Zhou X, et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021;131:185–97. [DOI] [PubMed]
- [7].Li J, Zhang D, Guo S, Zhao C, Wang L, Ma S, et al. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int J Biol Macromol. 2021;187:200–13. [DOI] [PubMed]
- [8].Zheng Y, Wu G, Chen L, Zhang Y, Luo Y, Zheng Y, et al. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1α promote stem cell differentiation and repair focal brain injury. Bioact Mater. 2021;6:627–37. [DOI] [PMC free article] [PubMed]
- [9].Fan K, Ma J, Xiao W, Chen J, Wu J, Ren J, et al. Mangiferin attenuates blast-induced traumatic brain injury via inhibiting NLRP3 inflammasome. Chem Biol Interact. 2017;271:15–23. [DOI] [PubMed]
- [10].Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. [DOI] [PMC free article] [PubMed]
- [11].Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31:19–25. [DOI] [PMC free article] [PubMed]
- [12].Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed]
- [13].Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7:a020420. [DOI] [PMC free article] [PubMed]
- [14].Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. [DOI] [PMC free article] [PubMed]
- [15].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7. [DOI] [PMC free article] [PubMed]
- [16].Huang D, Siaw-Debrah F, Wang H, Ye S, Wang K, Wu K, et al. Transplanting Rac1-silenced bone marrow mesenchymal stem cells promote neurological function recovery in TBI mice. Aging (Albany NY). 2020;13:2822–50. [DOI] [PMC free article] [PubMed]
- [17].Kumar Mishra S, Khushu S, Gangenahalli G. Neuroprotective response and efficacy of intravenous administration of mesenchymal stem cells in traumatic brain injury mice. Eur J Neurosci. 2021;54(1):4392–407. [DOI] [PubMed]
- [18].Cox Jr CS, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68:588–600. [DOI] [PubMed]
- [19].Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–7. [DOI] [PMC free article] [PubMed]
- [20].Fennema EM, Tchang L, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: a comparative study. J Tissue Eng Regen Med. 2018;12:e150–8. [DOI] [PubMed]
- [21].Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. [DOI] [PubMed]
- [22].Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–92. [DOI] [PubMed]
- [23].Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:367. [DOI] [PMC free article] [PubMed]
- [24].Kumar P, Becker JC, Gao K, Carney RP, Lankford L, Keller BA, et al. Neuroprotective effect of placenta-derived mesenchymal stromal cells: role of exosomes. FASEB J. 2019;33:5836–49. [DOI] [PMC free article] [PubMed]
- [25].Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–67. [DOI] [PMC free article] [PubMed]
- [26].Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–5. [DOI] [PMC free article] [PubMed]
- [27].Turan B, Saini HK, Zhang M, Prajapati D, Elimban V, Dhalla NS. Selenium improves cardiac function by attenuating the activation of NF-kappaB due to ischemia-reperfusion injury. Antioxid Redox Signal. 2005;7:1388–97. [DOI] [PubMed]
- [28].Tu Y, Chen C, Sun HT, Cheng SX, Liu XZ, Qu Y, et al. Combination of temperature-sensitive stem cells and mild hypothermia: a new potential therapy for severe traumatic brain injury. J Neurotrauma. 2012;29:2393–403. [DOI] [PMC free article] [PubMed]
- [29].Liu XY, Wei MG, Liang J, Xu HH, Wang JJ, Wang J, et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J Neurochem. 2019;153:230–51. [DOI] [PubMed]
- [30].Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. [DOI] [PMC free article] [PubMed]
- [31].Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H, et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 2017;133:70–81. [DOI] [PubMed]
- [32].Li D, Huang S, Zhu J, Hu T, Han Z, Zhang S, et al. Exosomes from MiR-21-5p-increased neurons play a role in neuroprotection by suppressing rab11a-mediated neuronal autophagy in vitro after traumatic brain injury. Med Sci Monit. 2019;25:1871–85. [DOI] [PMC free article] [PubMed]
- [33].Cheng SX, Xu ZW, Yi TL, Sun HT, Yang C, Yu ZQ, et al. iTRAQ-based quantitative proteomics reveals the new evidence base for traumatic brain injury treated with targeted temperature management. Neurotherapeutics. 2018;15:216–32. [DOI] [PMC free article] [PubMed]
- [34].Gold EM, Su D, López-Velázquez L, Haus DL, Perez H, Lacuesta GA, et al. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 2013;8:483–516. [DOI] [PubMed]
- [35].Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112:13109–14. [DOI] [PMC free article] [PubMed]
- [36].Zhao ML, Chen SJ, Li XH, Wang LN, Chen F, Zhong SJ, et al. Optical depolarization of dcx-expressing cells promoted cognitive recovery and maturation of newborn neurons via the Wnt/β-Catenin pathway. J Alzheimers Dis. 2018;63:303–18. [DOI] [PubMed]
- [37].Liu XY, Wei MG, Liang J, Xu HH, Wang JJ, Wang J, et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J Neurochem. 2020;153:230–51. [DOI] [PubMed]
- [38].Tang R, Lin YM, Liu HX, Wang ES. Neuroprotective effect of docosahexaenoic acid in rat traumatic brain injury model via regulation of TLR4/NF-Kappa B signaling pathway. Int J Biochem Cell Biol. 2018;99:64–71. [DOI] [PubMed]
- [39].Sun Z, Liu Y, Kong X, Wang R, Xu Y, Shang C, et al. Exendin-4 plays a protective role in a rat model of spinal cord injury through SERCA2. Cell Physiol Biochem. 2018;47:617–29. [DOI] [PubMed]
- [40].Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. [DOI] [PMC free article] [PubMed]
- [41].Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19–22. [DOI] [PMC free article] [PubMed]
- [42].Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63 + CD81 + extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–5. [DOI] [PMC free article] [PubMed]
- [43].Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–43. [DOI] [PMC free article] [PubMed]
- [44].Xu H, Qin W, Hu X, Mu S, Zhu J, Lu W, et al. Lentivirus-mediated overexpression of OTULIN ameliorates microglia activation and neuroinflammation by depressing the activation of the NF-κB signaling pathway in cerebral ischemia/reperfusion rats. J Neuroinflam. 2018;15:83. [DOI] [PMC free article] [PubMed]
- [45].Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci. 2019;13:14. [DOI] [PMC free article] [PubMed]
- [46].Zhao M, Zhu P, Fujino M, Zhuang J, Guo H, Sheikh I, et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci. 2016;17:17. [DOI] [PMC free article] [PubMed]
- [47].Atangana E, Schneider UC, Blecharz K, Magrini S, Wagner J, Nieminen-Kelhä M, et al. Intravascular inflammation triggers intracerebral activated microglia and contributes to secondary brain injury after experimental subarachnoid hemorrhage (eSAH). Transl Stroke Res. 2017;8:144–56. [DOI] [PubMed]
- [48].Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–45. [DOI] [PubMed]
- [49].Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–84. [DOI] [PubMed]
- [50].Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–8. [DOI] [PMC free article] [PubMed]
- [51].Wang L, Liu H, Zhang L, Wang G, Zhang M, Yu Y. Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: involved in inhibition of nf-κb and inflammation response. Biomol Ther (Seoul). 2017;25:383–9. [DOI] [PMC free article] [PubMed]
- [52].Feng N, Jia Y, Huang X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-kB and MAPK pathway. J Neuroimmunol. 2019;334:576996. [DOI] [PubMed]