Abstract
The antibacterial effect of moxifloxacin was studied by using an in vitro pharmacodynamic model of infection with dosing simulations of 400 mg every 24 h for 48 h. Streptococcus pneumoniae was tested by using four wild-type strains for which the moxifloxacin MICs were 0.008, 0.12, 0.14, and 3.6 mg/liter. In addition, two isogenic mutants, generated from the strains for which the moxifloxacin MICs were ≤0.12 mg/liter and for which the MICs were 1.0 and 1.6 mg/liter, were also used. Antibacterial efficacy was measured by the following indices: log change in viable count at 12, 24, 36, and 48 h; area under the bacterial kill curve (AUBKC); and time to kill 99.9% of the initial inoculum. With the three strains for which the moxifloxacin MICs were ≤0.14 mg/liter, there was a marked reduction in viable count over 12 to 36 h; in contrast, with strains for which the MICs were ≥1.0 mg/liter, little killing occurred over 48 h. A sigmoid dose-response model indicated that the area under the curve/MIC ratio was strongly related to the log change in viable count at 24 and 48 h and to the AUBKC. These data indicate that moxifloxacin may have a role in management of S. pneumoniae infection.
Infections of the lower respiratory tract account for a significant clinical workload in both community and hospital medical practice. In the United Kingdom on average 69 adult patients per 1,000 population consult a general practitioner each year with lower respiratory tract infections and over 30 million courses of antibiotics are prescribed for their treatment (16). Consultation rates increase with age and are often associated with comorbidities; in addition, patient expectation is often high that an antibiotic will be prescribed (14). For lower respiratory tract infection, clinical and epidemiological factors are complicated by increasing resistance in the bacterial pathogens associated with infection, particularly Streptococcus pneumoniae. Penicillin resistance is now common in many parts of the world even though its geographical distribution is patchy; cefotaxime resistance is a more recent event and is becoming more important (10). Macrolide resistance in S. pneumoniae, while not new, is on a rising trend, and in less developed areas of the world cotrimoxazole and chloramphenicol resistance are a problem. A number of new quinolones are being developed for use in respiratory tract infection to help counter these resistance issues, as quinolone and penicillin or macrolide resistance are not associated: examples include levofloxacin, grepafloxacin, trovafloxacin, and, more recently, moxifloxacin (Bay 12-8039) (10).
Moxifloxacin is an 8-methoxyquinolone with activity against S. pneumoniae (MIC at which 90% of isolates are inhibited [MIC90], 0.12 to 0.25 mg/liter) and Haemophilus influenzae and Moraxella catarrhalis (MIC90 for both, ≤0.25 mg/liter), as well as atypical respiratory pathogens (3, 5, 7, 18, 24). Moxifloxacin susceptibility in S. pneumoniae is reduced in ciprofloxacin-resistant strains (12) but is unaffected by penicillin or erythromycin susceptibility (17, 19).
The pharmacokinetics of moxifloxacin at oral doses of 100 mg every 12 h, 200 mg every 12 h, 400 mg every 24 h, and 400 mg every 24 h for 10 days in humans have been described (13, 21).
To further define the potential use of moxifloxacin administered at 400 mg every 24 h against S. pneumoniae, we used an in vitro pharmacodynamic model to simulate the influence of gradient antimicrobial concentrations on the antibacterial effect of moxifloxacin with S. pneumoniae isolates of varying susceptibilities to penicillin, cefotaxime, erythromycin, ciprofloxacin, and moxifloxacin. As strains of S. pneumoniae for which moxifloxacin MICs are >0.5 mg/liter are rare, we elected to generate laboratory mutants. The antibacterial effect was characterized as accurately as possible by performing experiments in triplicate and by the use of several parameters to describe bacterial kill: logarithmic reduction in viable counts, time to 99.9% kill of the initial inoculum, and the area under the bacterial kill curve (AUBKC).
MATERIALS AND METHODS
Model.
A New Brunswick Bioflo 1000 (Hatfield, Hertfordshire, England) in vitro model was used to simulate oral administration of 400 mg every 24 h over 48 h. The apparatus consists of a single central culture chamber connected via aluminum and silicone tubing first to a dosing chamber, which is in turn connected to a reservoir containing broth, and secondly to a vessel collecting outflow broth from the chamber. The dosing chamber and central culture chamber were diluted with broth with a peristatic pump (Ismatec Bennett & Co, Weston super Mare, England) at a flow rate of 66 ml/h. The temperature was maintained at 37°C, and the broth in the dosing and central chambers was agitated by a magnetic stirrer at 90 g.
Media.
A 75% brain heart infusion (Oxoid, Basingstoke, England) supplemented with hemin (10 μg/ml), beta nicotinamide adenine dinucleotide (10 μg/ml), and l-histidine (10 μg/ml) was used for all experiments. Preliminary experiments indicated this broth supported a growth density of 107 to 108 CFU/ml at 18 h after inoculation into the model. One percent magnesium chloride (BDH, Poole, Dorset, England) was incorporated into nutrient agar plates (Merck, Dorset, England) containing 5% whole horse blood (TCS Microbiology, Buckingham, England) to neutralize moxifloxacin before viable counts were determined.
Strains.
S. pneumoniae SMH 11148 (penicillin MIC, <0.06 mg/liter), S. pneumoniae SMH 11622 (penicillin MIC, ≥2 mg/liter), S. pneumoniae SMH 11617 (erythromycin MIC, >16 mg/liter), and S. pneumoniae SMH 12647 (penicillin MIC, ≥2 mg/liter; cefotaxime MIC, 2 mg/liter; and ciprofloxacin MIC, >32 mg/liter) were used. Strains 11148M and 11622M were laboratory-generated mutants of parent strains 11148 and 11622 which were produced by the method of Dalhoff et al. (5). Briefly, bacteria were grown overnight in brain heart infusion broth containing twofold dilutions of moxifloxacin. From the tube containing the highest drug concentration permitting visible growth, aliquots were used after 1/20 dilution to inoculate a second set of twofold dilutions. After overnight incubation, the process was repeated until the desired increase in MIC occurred.
Antibiotic.
Moxifloxacin (Bay 12-8039) was obtained from Bayer AG, Wuppertal, Germany. Stock solutions were prepared according to British Society of Antimicrobial Chemotherapy Guidelines (2) and stored at −70°C.
MICs and MBCs.
MICs were determined by the British Society of Antimicrobial Chemotherapy-defined standard broth dilution method (2), with the exception that moxifloxacin concentrations decreased in 0.02 or 0.2 mg/liter steps, not doubling dilutions. Minimum bactericidal concentrations (MBCs) were determined by 99.9% reduction in initial viable count after 24 h. MICs were determined before and after moxifloxacin exposure.
Pharmacokinetic and killing curves.
The in vitro activities of changing moxifloxacin concentrations against the seven strains described were tested in the above-described model. Target moxifloxacin concentrations at 1, 2, 3, 4, 6, 8, 12, 24, 26, and 48 h were 1.7, 1.8, 1.8, 1.7, 1.4, 1.3, 0.9, 0.4, 2.3, and 0.5 mg/liter (19a, 20). For all of the experiments, 100 μl of an overnight broth suspension of the test organism was inoculated into the central culture chamber (360-ml volume) via an entry port (initial inoculum, about 106 CFU/ml) and the model was run for 18 h to allow the organism growth to reach equilibrium at a density of about 108 CFU/ml. Moxifloxacin (1.32 ml) was added to the dosing chamber (20 ml) at time zero and a second time after 24 h. Samples were taken from the central chamber via a port throughout the 48-h period, that is, at 0, 1, 2, 3, 4, 5, 6, 7, 10, 12, 22, 24, 25, 26, 27, 28, 29, 30, 31, 34, 36, 46, and 48 h, for assessment of the viable bacterial count. The bacteria were quantified manually without dilution and after 1/1,000 dilution with a Spiral Plater (Don Whitley Spiral Systems, West Yorkshire, England). The minimum detection level was 2 × 102 CFU/ml. In addition, aliquots were taken at the same time intervals and stored at −70°C for measurement of moxifloxacin concentrations. Samples were assayed by bioassay with Escherichia coli NCTC 10418 as the indicator organism (1). All standards and samples were prepared and diluted as necessary in the same concentration of brain heart infusion as was used in the model. The limit of detection was 0.03 mg/liter, with a percent coefficient of variation of 6.1%. All pharmacokinetic simulations and killing curve determinations were performed in triplicate.
Pharmacokinetics, pharmacodynamics, and measurement of antibacterial effects and statistical analysis.
The area under the curve (AUC) simulated for moxifloxacin was 24.4 mg/liter · h, and the elimination half-life was 8.8 h. Antibacterial activity was assessed by calculating the log change in viable count, compared to time zero, at 12 (Δ12), 24 (Δ24), 36 (Δ36), and 48 (Δ48) h. In addition, the AUBKC (log CFU/ml · h) was calculated, after the inoculum was standardized, by the log linear trapezoidal rule for the periods 0 to 24 h (AUBKC24) and 0 to 48 h (AUBKC48). The time taken for the inoculum to fall by 99.9% of its time zero value (T99.9) was also determined. For pharmacodynamic analysis, the AUC/MIC ratio and the percentage of time the concentration exceeded the MIC (T>MIC) were also determined. The AUC/MIC ratio was related to AUBKC and Δ24 and Δ48 by a sigmoidal dose-response (variable-slope) model (Prism; GraphPad, San Diego, Calif.).
RESULTS
MICs and MBCs.
The moxifloxacin MICs and MBCs for each strain were as follows: strain 11148, 0.08 and 0.22 mg/liter; 11148M, 1.0 and 4.0 mg/liter; 11622, 0.12 and 0.28 mg/liter; 11622M, 1.6 and 4.4 mg/liter; 11617, 0.14 and 0.26 mg/liter; and 12647, 3.6 and 3.6 mg/liter.
Pharmacokinetic curves.
The mean (± standard deviation [SD]) moxifloxacin concentrations in the model and the target concentrations are shown in Fig. 1; there was good agreement among them.
FIG. 1.
Moxifloxacin serum concentration profile for dosing with 400 mg every 24 h and concentrations measured in the in vitro model. The error bars indicate standard deviations.
Bacterial killing curves.
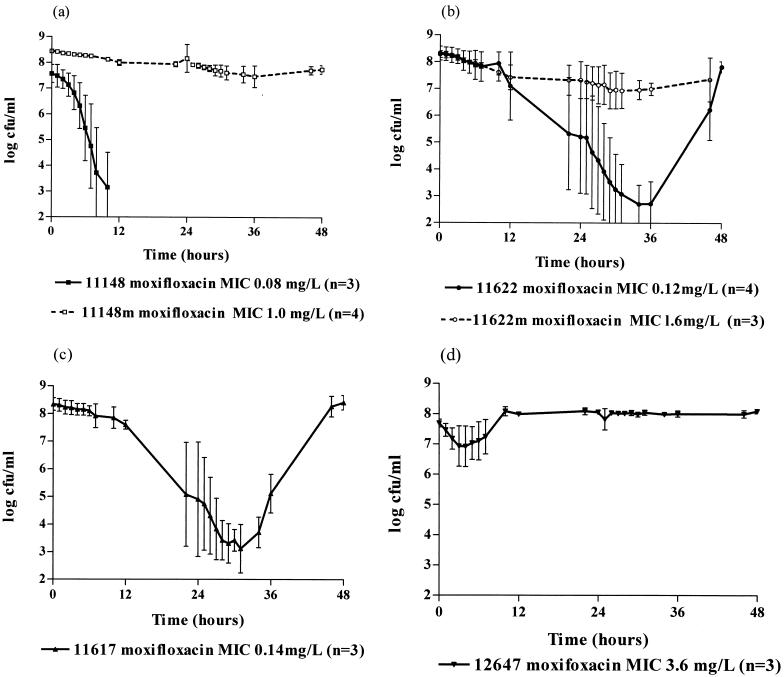
The killing curves of the S. pneumoniae strains after exposure to moxifloxacin are shown in Fig. 2; mean (± SD) counts are illustrated. For the three strains for which the moxifloxacin MICs were ≤0.14 mg/liter, there was a marked reduction in viable counts. With strain 11148 (moxifloxacin MIC, 0.08 mg/liter), this occurred within 12 h (Fig. 2a); however, with strains 11622 and 11617 (MICs, 0.12 and 0.14 mg/liter), maximum killing did not take place for 24 to 36 h, and with both strains, grow-back occurred by 48 h (Fig. 2b and c). T99.9 was 7.9 ± 2.2 h for strain 11148 (MIC, 0.08 mg/liter), 21.8 ± 7.0 h for strain 11622 (MIC, 0.12 mg/liter), and 21.9 ± 6.0 h for strain 11617 (MIC, 0.14 mg/liter). In contrast, with laboratory-generated mutants for which the MICs were 1.0 (11148M) and 1.6 (11622M) mg/liter or the wild-type resistant strain 12647 (MIC, 3.6 mg/liter), very little killing occurred over the time of the simulation (Fig. 2a, b, and d). The T99.9 was >48 h for all three strains. The AUBKC24 was least with the most susceptible strains, for which the MICs were ≤0.14 mg/liter, and greater with those less susceptible (MICs, ≥1 mg/liter). This was more obvious with the AUBKC48 than with the AUBKC24. No change in the MICs for any of the strains was noted after drug exposure.
FIG. 2.
Bactericidal effect of moxifloxacin at 400 mg every 24 h on strain 11148 (a), strain 11622 (b), strain 11617 (c), and strain 12647 (d). Error bars indicate standard deviations.
Pharmacodynamics of the antibacterial effect.
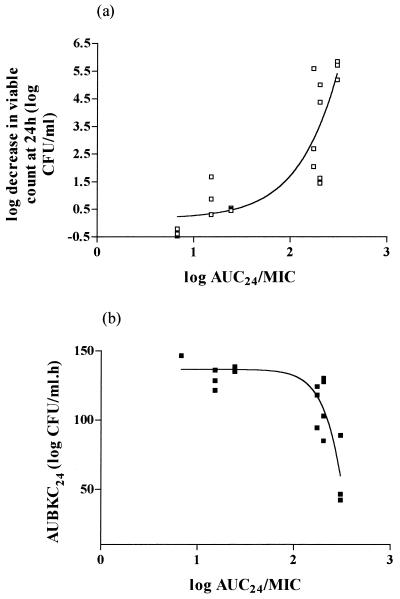
Table 1 shows the univariate summaries of the pharmacodynamic parameters and their relationship to five indices of antibacterial effect: Δ24, Δ48, AUBKC24, AUBKC48, and T99.9. The MIC, the AUC/MIC ratio, and T>MIC are all closely related to each other, such that when the MIC increases or the AUC/MIC ratio and T>MIC decrease, Δ24 and Δ48 decrease but AUBKC or T99.9 increase. Curve fitting by a sigmoid dose-response model indicated the AUC/MIC ratio was strongly related to Δ24, Δ48, or AUBKC: Δ24 versus AUC24/MIC, R2 = 0.7716; AUBKC24 versus AUC24/MIC, R2 = 0.7797; Δ48 versus AUC48/MIC, R2 = 0.9268; AUBKC48 versus AUC48/MIC, R2 = 0.9155 (Fig. 3).
TABLE 1.
Univariate analysis of MIC, AUC/MIC ratio, and T>MIC against measures of antibacterial effect
| Parameter | Change in viable count (log CFU/ml ± SD) at:
|
AUBKC (log CFU/ml · h ± SD)
|
Time to 99.9% kill (h ± SD) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 0–24 h | 0–28 h | ||
| MIC (mg/liter) | |||||
| <0.1 | −5.6 ± 0.4 | −5.6 ± 0.4 | 59.1 ± 24.9 | 59.1 ± 25.9 | 7.9 ± 2.2 |
| 0.1–0.5 | −3.2 ± 1.7 | −0.3 ± 0.4 | 111.6 ± 17.7 | 177.6 ± 23.3 | 21.9 ± 6.1 |
| >0.5–2.5 | −0.6 ± 0.6 | −0.8 ± 0.5 | 132.9 ± 6.0 | 251.2 ± 15.7 | >48 |
| >2.5 | −0.4 ± 0.1 | +0.4 ± 0.2 | 145.6 ± 5.3 | 297.1 ± 8.1 | >48 |
| AUC/MIC | |||||
| <20 | −0.3 ± 0.8 | −0.3 ± 0.9 | 137.1 ± 11.0 | 267.3 ± 34.2 | >48 |
| 20–125 | −0.3 ± 0.5 | −0.7 ± 0.1 | 136.2 ± 1.6 | 261.5 ± 5.1 | >48 |
| >125–250 | −3.2 ± 1.7 | −0.3 ± 0.4 | 111.6 ± 17.7 | 177.6 ± 23.3 | 21.9 ± 6.1 |
| >250 | −5.6 ± 0.4 | −5.6 ± 0.4 | 59.1 ± 25.9 | 59.1 ± 25.9 | 7.9 ± 2.2 |
| T>MIC (%) | |||||
| <20 | −0.3 ± 0.8 | −0.3 ± 0.9 | 137.1 ± 11.0 | 267.3 ± 34.2 | >48 |
| 20–80 | −0.3 ± 0.5 | −0.7 ± 0.1 | 136.2 ± 1.6 | 261.5 ± 5.1 | >48 |
| >80 | −4.0 ± 1.8 | −2.0 ± 2.7 | 95.9 ± 31.7 | 138.1 ± 63.4 | 17.7 ± 8.4 |
FIG. 3.
Moxifloxacin activity against seven strains of S. pneumoniae with various MICs; relationship between Δ24 and the AUC24/MIC ratio (a) and AUBKC24 and the AUC24/MIC ratio (b).
DISCUSSION
The primary objective of this study was to use an in vitro pharmacodynamic model to simulate the changing moxifloxacin concentrations observed in human sera with 400 mg once-a-day oral therapy over two doses, that is, for 48 h, and to assess the antibacterial effect with S. pneumoniae as the indicator organism. The moxifloxacin concentrations modelled on early pharmacokinetic studies have subsequently been shown to be conservative, as the maximum concentration of drug in serum after a 400-mg oral dose is probably in the range of 2.8 to 3.4 mg/liter, and the AUC is 30 to 36 mg/liter · h (21). In addition, the initial bacterial density is high at 108 CFU/ml, so these experiments will, if anything, underestimate the activity of the drug. The data indicate that for isolates for which the MICs are ≤0.14 mg/liter, moxifloxacin is bactericidal over the first dosing interval. The use of a second simulated dose, extending the simulation to 48 h, was of value, as it indicated regrowth with the two strains for which the MIC values were higher. The antibacterial impact of a third dose is unknown. Laboratory-generated mutants for which the MICs were ≥1.0 mg/liter were not killed; neither was a wild-type resistant strain for which the MIC was 3.6 mg/liter. The ciprofloxacin MIC for this strain was also high, and it was resistant to penicillin and cefotaxime. Such strains are very rare in the United Kingdom, where the reported range of ciprofloxacin MICs for S. pneumoniae isolated from clinical specimens in 1995 and 1996 was 0.5 to 2 mg/liter (23). In Europe, the MICs of ciprofloxacin for only 0.6 or 0.7% of S. pneumoniae isolates were ≥4 mg/liter in 1992 or 1993 (6); however, S. pneumoniae isolates for which the ciprofloxacin MICs were ≥4 mg/liter have been described before in the United Kingdom, for one of which the grepafloxacin MIC was 4 mg/liter, and it was penicillin resistant (23). The use of this wild-type moxifloxacin-resistant isolate enabled us to show that the laboratory-generated mutants and the wild-type strain behaved in similar ways when exposed to changing moxifloxacin concentrations. These data extend those of others who, using fixed moxifloxacin concentrations and time kill methodologies, showed marked bactericidal activity of moxifloxacin with >3 log-unit reduction in viable counts after 6 h with concentrations of 2.3 mg/liter employing S. pneumoniae isolates for which the MICs were ≤0.12 mg/liter (11). Similar data were generated with moxifloxacin at concentrations of 2 × MIC (22). In addition, Dalhof (4) previously used an in vitro model to simulate a single oral 200-mg dose and noted a marked bactericidal effect within 12 h.
The AUC/MIC ratio is widely used as a predictor of quinolone antibacterial effect, and it has been validated in a dilutional in vitro model similar to the one used here (15). Corrections for bacterial washout from the growth chamber were not included, and despite the use of quinolones with different elimination half-lives ranging from 2.5 to 10 h, the AUC/MIC ratio was related, using a sigmoid nonlinear effect model, to the inverse of the area under the bacterial effect curve over 24 h. Here we only simulated one dosing regimen, making full pharmacodynamic evaluation impossible. The AUC/MIC ratio, T>MIC, and MIC changes were closely related to one another and have a marked antibacterial effect when judged by AUBKC. However, we were able to show by a sigmoid dose-response model a relationship between the AUC/MIC ratio and antibacterial effect. The relationship of the AUC/MIC ratio to bacteriological and clinical outcomes has been shown in humans with intravenous ciprofloxacin and oral grepafloxacin in the therapy of respiratory tract infection (8, 9, 24); unfortunately, the AUC/MIC ratio which will provide a significant breakpoint in predicting the probability of clinical and bacteriological cure still remains to be finally defined, but it is probably about 100, based on in vitro and in vivo data (8, 9, 24). Extension of our experiments to study emergence of resistance during as well as after the simulations may have provided better information on the relationship of the AUC/MIC ratio and postexposure increases in MIC for S. pneumoniae and moxifloxacin.
The results of these experiments suggest that the modelled moxifloxacin serum concentrations have a marked antibacterial effect against S. pneumoniae strains for which the MICs are ≤0.14 mg/liter; this is in contrast to strains for which the MICs are ≥1 mg/liter. Given the increasing resistance of S. pneumoniae to presently available β-lactams and macrolides, new quinolones such as moxifloxacin with significant antipneumococcal activity may have an important future clinical role.
ACKNOWLEDGMENT
We thank A. Dalhoff (Bayer AG) for support.
REFERENCES
- 1.Broughall J M. Aminoglycosides. In: Reeves D S, Phillips I, Williams J D, Wise R, editors. Laboratory methods in antimicrobial chemotherapy. Edinburgh, United Kingdom: Churchill Livingstone; 1978. pp. 194–206. [Google Scholar]
- 2.British Society for Antimicrobial Chemotherapy Working Party. A guide to sensitivity testing. United Kingdom: British Society for Antimicrobial Chemotherapy; 1991. [Google Scholar]
- 3.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of Bay 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Morexella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalhof A. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Antibacterial efficacy of fluctuating concentrations of Bay 12-8039 simulating human serum kinetics, abstr. F-26; p. 104. [Google Scholar]
- 5.Dalhoff A, Petersen V, Endermann R. In vitro activity of Bay 12-8039, a new 8 methoxyquinolone. Chemotherapy. 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 6.Felmingham D, Gruneberg R N the Alexander Project Group. A multi-centre collaborative study of the antimicrobial susceptibility of community acquired lower respiratory tract pathogens. 1992–3. J Antimicrob Chemother. 1996;38(Suppl. A):1–57. doi: 10.1093/jac/38.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 7.Felmingham D, Robbins M J, Leakey A, Salman H, Dencer C, Clark S, Ridgway G L. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of Bay 12-8039 against bacterial respiratory tract pathogens, mycoplasma and obligate anaerobic bacteria, abstr. F-8; p. 101. [Google Scholar]
- 8.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1994;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest A, Chodosh S, Amantea M A, Collins D A, Schentag J J. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40(Suppl. A):45–57. doi: 10.1093/jac/40.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein F W, Acar J F the Alexander Project Collaborative Group. Antimicrobial resistance among lower respiratory tract isolates of Streptococcus pneumoniae: results of a 1992–93 Western Europe and USA collaborative surveillance study. J Antimicrob Chemother. 1996;38(Suppl. A):71–84. doi: 10.1093/jac/38.suppl_a.71. [DOI] [PubMed] [Google Scholar]
- 11.Herrington J A, Remy J M, Federici J A, Huguenel E D. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. The bactericidal activity of Bay 12-8039 against respiratory pathogens at clinically achievable concentrations, abstr. F-125; p. 167. [Google Scholar]
- 12.Kitzis M D, Goldstein F W, Meigl M, Acar J P. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of Bay 12-8039 against multiply-resistant Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis, abstr. F-12; p. 102. [Google Scholar]
- 13.Kubitza D, Stass H H, Wingender W, Kuhlmann J. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Bay 12-8039 (I), A new 8 methoxy-quinolone: safety (S), tolerability (T) and steady state pharmacokinetics (PK) in healthy male volunteers, abstr. F-25; p. 104. [Google Scholar]
- 14.Macfarlane J, Holmes W, MacFarlane R, Britten N. Influence of patients expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. Br Med J. 1997;315:1211–1214. doi: 10.1136/bmj.315.7117.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madaras-Kelly K J, Ostergoard B E, Horde L B, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Office of Population Censuses and Surveys. Morbidity statistics from general practice: 4th National Study 1991–2. London, United Kingdom: Her Majesty’s Stationary Office; 1995. [Google Scholar]
- 17.Reinert R R, Dalhoff A, Schlaeger J J, Lemperle M, Lutlicken R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity of Bay 12-8039, a new 8 methoxyquinolone, against S. pneumoniae, abstr. F-129; p. 168. [Google Scholar]
- 18.Roblin P M, Hammerschlag M R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Activity of a new quinolone, Bay 12-8039, against chlamydia pneumoniae in vitro, abstr. F11; p. 101. [Google Scholar]
- 19.Robson H G, Tremblay J P, Loo V G, Lavalee J, Mclear D. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Comparative in vitro activity of trovafloxacin, Bay 12-8039 and clinafloxacin against penicillin intermediate and resistant Streptococcus pneumoniae, abstr. F-138; p. 169. [Google Scholar]
- 19a.Stass, H. H. Personal communication.
- 20.Stass H H, Dietrich H, Sachse E. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Influence of four times dosing of 500mg probenecid on kinetics of Bay 12-8039 after administration of a single 400mg dose in healthy male volunteers, abstr. F154; p. 172. [Google Scholar]
- 21.Sullivan J T, Woodruff M, Lettierc J, Agarwal V, Krol G, Heller A. Pharmacokinetics (PK) and tolerability of the new quinolone, Bay 12-8039: 10 days treatment at 400mg daily. Clin Microbiol Infect. 1997;3(Suppl. 2):389. [Google Scholar]
- 22.Visalli M, Jacobs M, Applebaum P. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Anti-pneumococcal activity of Bay 12-8039 compared to other drugs by time-kill, abstr. F-133; p. 168. [Google Scholar]
- 23.Wise R, Andrews J M. The activity of grepafloxacin against respiratory pathogens in the UK. J Antimicrob Chemother. 1997;40(Suppl. A):27–30. doi: 10.1093/jac/40.suppl_1.27. [DOI] [PubMed] [Google Scholar]
- 24.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of Bay 12-8039, a new fluroquinolone. Antimicrob Agents Chemother. 1997;42:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]