Abstract
Background:
There is no consensus regarding the best route of intranasal delivery of corticosteroids in the treatment of chronic rhinosinusitis (CRS). The study objective was to compare the impact of mometasone furoate nasal spray (MFNS) versus mometasone nasal irrigation in the management of CRS patients who have not undergone sinus surgery.
Methods:
A double-blinded, placebo-controlled randomized clinical trial was conducted in adults with CRS. Individuals with nasal polyps and/or history of sinus surgery were excluded. Patients were randomized to receive 8-weeks of either MFNS or mometasone nasal irrigation. The primary outcome measure was the change in the 22-item Sinonasal Outcome Test (SNOT-22) score between the two groups. Secondary outcome measures included patient global response to treatment and Lund-Kennedy endoscopy scores.
Results:
A total of 43 participants completed the study (n=22,MFNS; n=21,mometasone nasal irrigation). Fourteen (64%) participants in the MFNS group and 17 (81%) in the mometasone lavage group experienced a clinically meaningful improvement in SNOT-22 scores with a proportion difference of 17% (95%CI, −9% to 44%). The least square (LS) mean difference between the two groups for SNOT-22 was −8.6(95%CI, −17.7 to 0.58;p=0.07), while the LS mean difference between the two groups for Lund-Kennedy endoscopy scores was 0.16(95%CI, −0.84 to 1.15;p=0.75). No adverse events were associated with the study.
Conclusion:
Both MFNS and mometasone nasal irrigations are beneficial in symptom management of CRS. Our study suggests that patients who perform mometasone lavage do better in a clinically meaningful way, but our results are not definitive and further studies are warranted.
Keywords: Chronic Rhinosinusitis, Topical intranasal corticosteroid, Nasal lavage
INTRODUCTION
Persistent sinonasal mucosal inflammation is a hallmark of chronic rhinosinusitis without nasal polyps (CRSsNP). Intranasal corticosteroids (INCS) are the mainstay of treatment in the long-term management of CRSsNP. Corticosteroids exert an anti-inflammatory effect by upregulating transcription of anti-inflammatory genes, reducing airway inflammatory cell infiltration by eosinophils, mast cells, and T-lymphocytes, and suppressing production of adhesion molecules and pro-inflammatory genes and mediators, such as NF-κB.1,2 However, studies have shown that there is limited penetration of nasal sprays beyond the nasal vestibule and into the paranasal sinuses.3 Thus, there has been significant interest in the application of large-volume, low-pressure nasal saline irrigation to enhance intra-sinus corticosteroid deposition.
Randomized clinical trials (RCTs) have reported on both the efficacy and safety of ICNS delivered by nasal irrigations. 4–7 However, the effect size in these studies have been varied7,8, reflective of the heterogenous patient population. While CRSsNP is more prevalent than CRS with nasal polyps (CRSwNP), many of these studies have examined the effect of distribution of topical medications in surgically opened sinonasal cavities for CRSwNP, limiting the generalizability of these study findings to CRSwNP patients who have received surgery. 9. Furthermore, treatment outcomes in these studies have primarily focused on objective outcome measures, such as endoscopic scores, CT findings, or complication/recurrence rates. To date, only 3% of CRS studies have focused on patient-reported outcome measures.10 However, with the rise in focus on patient-centered care, there is increasing importance being placed on PROMs. The 22-item Sinonasal Outcome Test (SNOT-22), which captures sinonasal symptoms and health-related quality of life domains, is a validated and highly specific CRS PROM. 11The primary objective of this study was to compare the effect of nasal saline irrigation plus mometasone nasal steroid spray versus mometasone nasal irrigation plus nasal saline spray on symptom management in CRSsNP, as measured by both PROMs and objective outcome measures.
METHODS
Study Design
A single-institution, double-blinded, placebo-controlled, RCT of patients with CRS was conducted between November 2018 and February 2020. The trial was registered at ClinicalTrials.gov (NCT03705793) and the study was approved by the Washington University’s Human Protection Research Office.
Study Population
Men and women 18 years of age or older with 12-weeks or longer of two or more of the following signs and symptom consistent with CRS12,13- mucopurulent drainage (anterior, posterior, or both), nasal obstruction (congestion), facial pain-pressure-fullness, and decreased sense of smell AND inflammation documented by one or more of the following findings- purulent mucus or edema in the middle meatus or ethmoid region and/or radiographic imaging showing inflammation of the paranasal sinuses were included. Patients were excluded if they had nasal polyps; prior sinus surgery; comorbid mucociliary conditions; sinus disease associated with autoimmune or vasculitic diseases; chronic diseases requiring long-term corticosteroid use; history of oral or systemic antibiotic use in the 2 weeks prior to enrollment; history of allergy to topical nasal steroids. Participants were also excluded if they were pregnant or breastfeeding. In addition, participants with a baseline SNOT-22 score of 9 or less were excluded due to inability to achieve a pre- and post-intervention minimally clinically improved difference (MCID).
In addition to collection of baseline demographic information, overall severity of comorbidity was assessed using the Adult Comorbidity Evaluation-27 (ACE-27) instrument. 14
Intervention
Participants were randomly assigned to receive 8-weeks of either 1) nasal saline irrigation and MFNS (50 mcg in each spray, 2 sprays/nostril) or 2) mometasone nasal irrigation (1.2 mg per capsule, 2 capsules/irrigation) and saline nasal spray. Based on the literature regarding retention of nasal sprays and irrigations in the nasal cavity and consultation with pharmacy colleagues, a daily dose of 2.4 mg of mometasone delivered by nasal irrigation was deemed to be equivalent to the daily dosage of mometasone nasal spray. 15–17All participants were provided with an 8-ounce sinus rinse bottle (NeilMed® SINUS RINSE™) and a two-month supply of USP Grade Sodium Chloride & Sodium Bicarbonate Mixture (pH balanced, Isotonic & Preservative & Iodine Free) commercially prepared packets. Participants were instructed to perform nasal irrigation first followed by nasal spray. Mometasone and placebo were provided in identical treatment kits prepared by Jason Jerusik, PharmD of AdvancedRx (Plymouth Meeting, Pennsylvania) and all study patients and study team members were blinded to study treatment.
Patients who were on concomitant nasal steroid sprays or nasal irrigations prior to enrollment were asked to discontinue use 2 weeks prior to starting the study and for the study duration.
Compliance was self-reported and defined as performing the intervention for at least 5 days a week. In addition, study team members were in contact with study participants on a biweekly basis.
Patient-Reported Outcome Measures
The primary outcome measure was the within-subject pre- to post-treatment change in SNOT-22 scores in the MFNS group compared to that of the mometasone nasal irrigation. Participants completed the SNOT-22 at baseline, and then at 2, 4-, 6-, and 8-weeks after initiation of treatment. A MCID was defined as a change in SNOT-22 score of at least 9 points.18
Secondary outcome measures included patient global response to treatment, as measured by the modified Clinical Global Impressions (CGI) scale.19 For the modified CGI scale, participants were asked to rate their overall response to treatment using a 7-point Likert scale with anchors of 1 = very much improved, 4 = no change, and 7 = very much worse.
Objective Outcome Measures
Participants had nasal endoscopic examinations performed pre- and post-intervention (JS, CKC, AD, JFP) and findings recorded using the Lund-Kennedy grading system,20 which evaluates the pathologic state of each sinonasal cavity using a 0 to 2 scale (0 = absent; 2= severe) with a maximum score of 20. If participants received sinus CT scans as part of their clinical work-up, radiologic images were reviewed, and findings recorded using the Lund-McKay grading system20, which evaluates sinus patency using a 0 to 2 scale (0 = normal, 2 = total opacification) with a maximum score of 24.
Cosyntropin Test
While MFNS has been shown to have no discernible effect on the function of the hypothalamus-pituitary-adrenal (HPA) axis in clinical studies in both children and adult, 21 the safety of nasally administered MF saline irrigation has not been demonstrated. Therefore, a random subgroup of 20 enrolled participants (10 from each intervention arm) was offered participation in the cosyntropin stimulation study.
Baseline levels of serum cortisol were measured at the participant’s first study visit. Participants received an intramuscular injection of 0.25 mg of cosyntropin to stimulate the adrenal cortex. Serum was then drawn 30 minutes later and cortisol level measured. This test was then repeated upon completion of the study. A post-stimulation cortisol level below 18 ug/dL was indicative of adrenal insufficiency. Results are presented as the difference and 95% CI around the difference in mean cortisol level before and after treatment. Cohen’s d was used to define the effect size with a value d= 0.2 considered “small” effect, 0.5 “medium” effect, and 0.8 “large” effect22.
Statistical Analysis
The sample size for this study was estimated based on a study of budesonide nasal irrigation in CRS patients by Tait et al. 7 In order to detect with 80% power at a 2-sided α of 0.05 an MCID of 9 points or greater on the SNOT-22, an estimated sample size of 44 participants (22 participants per arm) was needed. With an anticipated 15% drop-out/non-compliance rate, the total sample size for this study was set at 51 participants.
Standard descriptive statistics was used to describe the demographics, clinical characteristics, and assessments of the study population. An intention-to-treat approach was used for all data analyses, which included all patients who were enrolled and randomized. The primary outcome measure was analyzed using a mixed-effect model with repeated measures approach. 95% CI around the difference was calculated and used to assess for clinically meaningful differences between the two treatment groups. An interim analysis was performed after participant 22 had completed 8-weeks of treatment in order to assess compliance and treatment response. All statistical analysis was conducted in SPSS 14 (IBM, Carey, North Carolina).
RESULTS
Between November 2018 and February 2020, a total of 53 patients were enrolled and randomized to either nasal saline irrigation plus MFNS (n=27) or mometasone nasal irrigation plus nasal saline spray (n=26). The median age was 48 years (range 19 to 67) and the majority of participants were female (n=33; 62%) and of white race (n=38; 72%). Twenty-six (49%) of participants had no significant comorbidities.
Of the 53 patients randomized, 27 received nasal saline irrigation plus MNFS and 26 received mometasone nasal irrigation plus nasal saline spray. A total of 43 participants completed the pre- and post-intervention assessments (Figure 1). Of those participants, there were no meaningful differences in baseline demographic and clinical characteristics between the 2 groups, except that all 4 participants with severe comorbidity were assigned to the mometasone nasal irrigation group and the median baseline SNOT-22 score was 40 for the nasal saline irrigation and MFNS group and 50 for the mometasone nasal irrigation and nasal saline spray group (Table 1).
Figure 1.
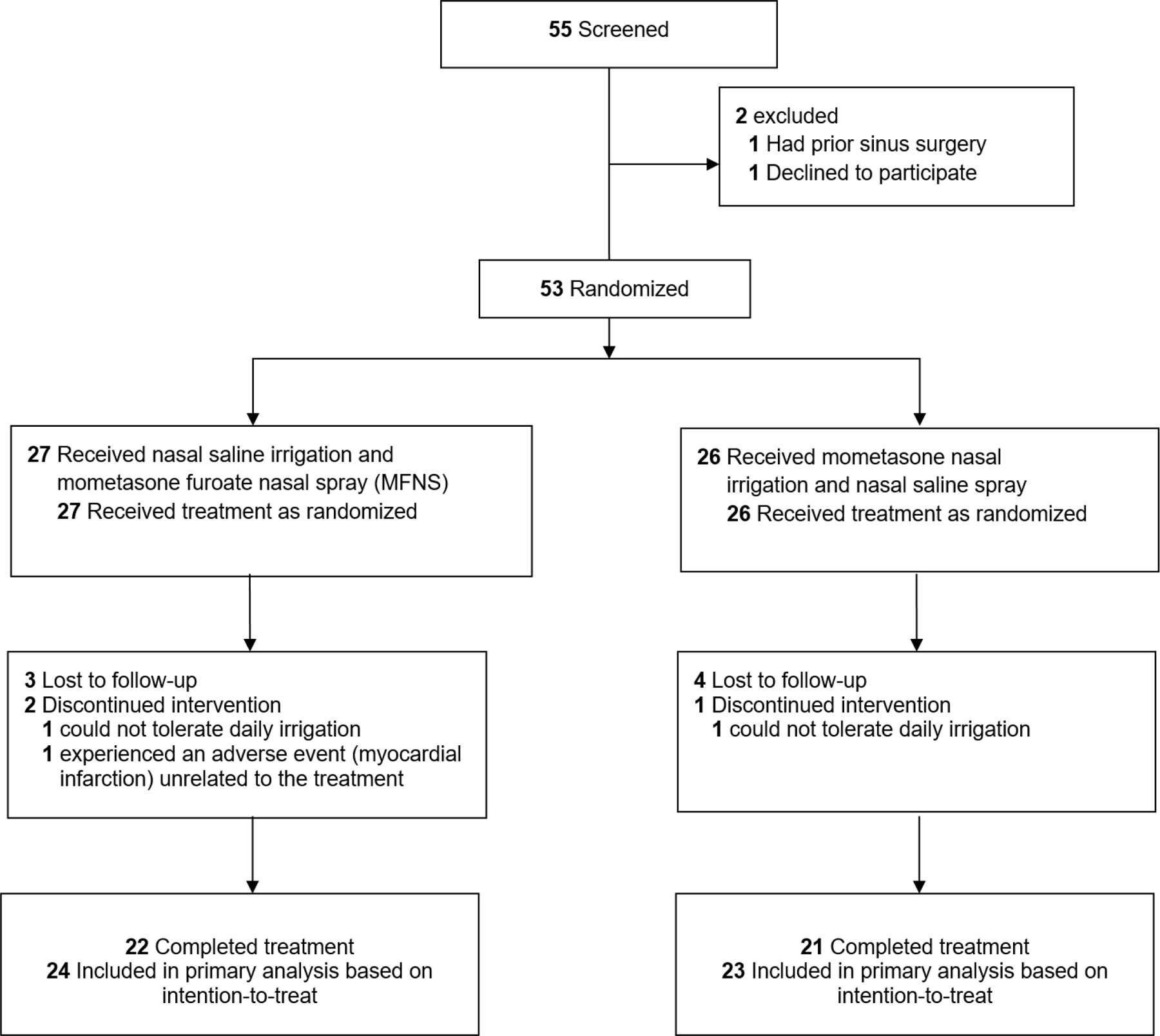
Flowchart of Patients Enrolled and Included in Analysis
Table 1.
Comparison of Baseline Characteristics Between the 2 Treatment Groups
| Baseline Characteristic | Total (n=53) | Nasal Saline Irrigation + Mometasone Nasal Spray(n=27) | Mometasone Nasal Irrigation + Nasal Saline Spray (n=26) | Difference (95% CI) |
|---|---|---|---|---|
| Age (years), median (min-max) | 48 (19–67) | 50 (19–66) | 48 (19–67) | −1 (−10 to 7) |
| Sex, n (%) | ||||
| Male | 20 (37.7) | 9 (33.3) | 11 (42.3) | |
| Female | 33 (62.3) | 18 (66.7) | 15 (57.7) | 9 (−17 to 35) |
| Race, n (%) | ||||
| White | 38 (71.7) | 21 (77.8) | 17 (65.4) | 12.4 (−11.7 to 36.5) |
| African American | 11 (20.8) | 4 (14.8) | 7 (26.9) | −12.1 (−33.8 to 9.6) |
| Other | 4 (7.5) | 2 (7.4) | 2 (7.7) | −0.3 (−14.5 to 13.9) |
| Overall Comorbidity, n (%) | ||||
| None | 26 (49.1) | 12 (44.4) | 14 (53.8) | −9.4 (−36.2 to 17.4) |
| Mild | 18 (34) | 12 (44.4) | 6 (23.1) | 21.4 (−3.4 to 46.1) |
| Moderate | 5 (9.4) | 3 (11.1) | 2 (7.7) | 3.4 (−12.2 to 19.0) |
| Severe | 4 (7.5) | 0 (0) | 4 (15.4) | −15.4 (−29.2 to −1.5) |
| Baseline endoscopic score, median (min-max) | 4 (0–8) | 4 (0–8) | 4 (1–8) | 0 (−1 to 1) |
| Baseline SNOT-22 total, median (min-max) | 42 (11–99) | 40 (11–71) | 50 (12–99) | −11 (−21 to 0) |
Primary Outcome Measure
The least squares mean change in SNOT-22 scores between baseline and week 8 was 17.7 (95% CI, 10.3 to 25.04; p<0.001) in the nasal saline irrigation plus MFNS group and 23.18 (95% CI, 15.7 to 30.7; p<0.001) in the mometasone nasal irrigation plus nasal saline spray group. The least square mean difference between the two intervention groups was −8.6 (95% CI, −17.7 to 0.58; p=0.07) in favor of the mometasone nasal irrigation group. The change in SNOT-22 scores within each group throughout the study is shown Figure 2. As can be seen, both groups experienced improvement in symptoms as reflected in the reduction in SNOT-22 scores. Patients who received mometasone nasal irrigation, however, had a greater improvement in their SNOT-22 scores compared to those who received mometasone nasal spray, as presented in the box and whisker plot in Figure 3. A total of 14 (64%) participants in the nasal saline irrigation plus MFNS group experienced a MCID in SNOT-22 scores while 17 (81%) participants in mometasone nasal irrigation plus nasal saline spray experienced a MCID with a proportion difference of 17% (95% CI, −9% to 44%)
Figure 2.
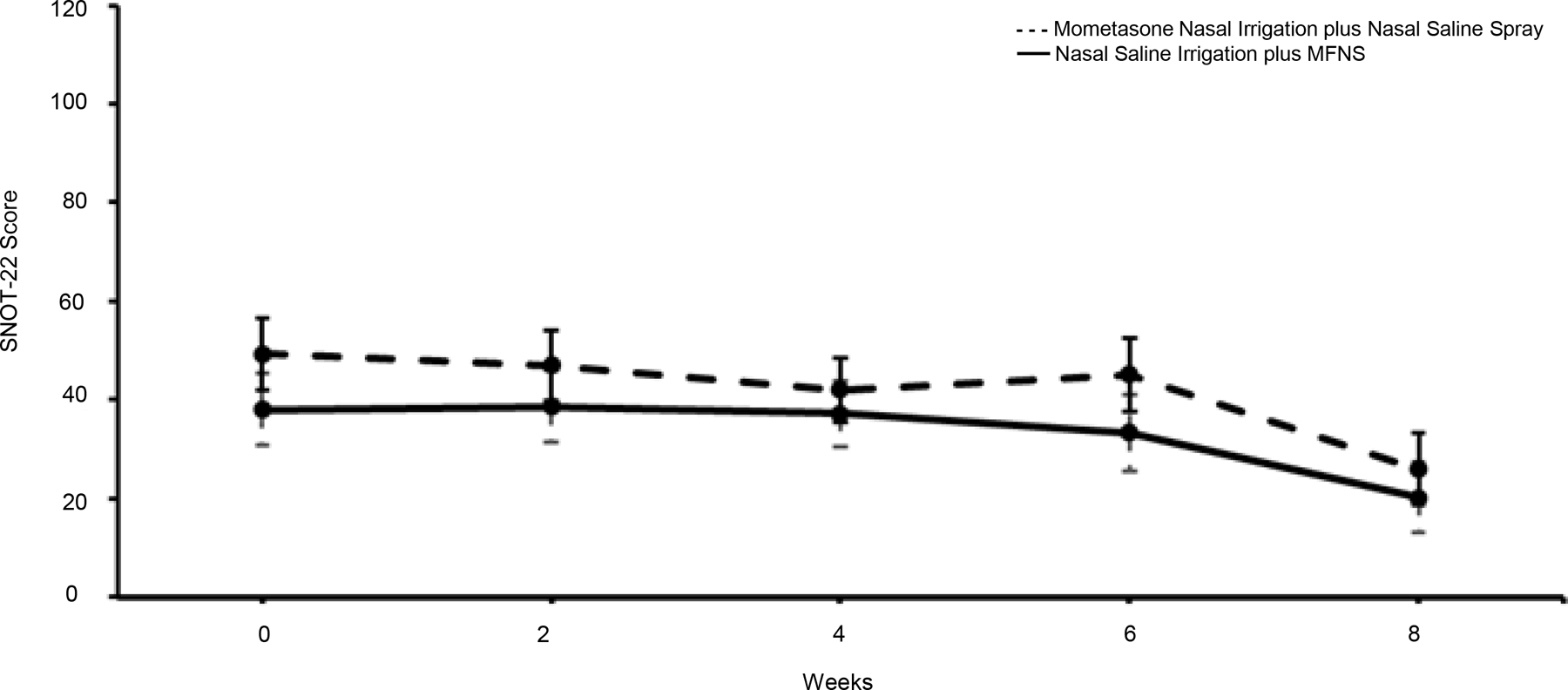
SNOT-22 Scores by Treatment Group Over Time. The dashed line represents the mometasone nasal irrigation group plus nasal saline spray while the solid line represents the saline nasal irrigation plus MFNS group. The error bars represent the minimum and maximum SNOT-22 values.
Figure 3.
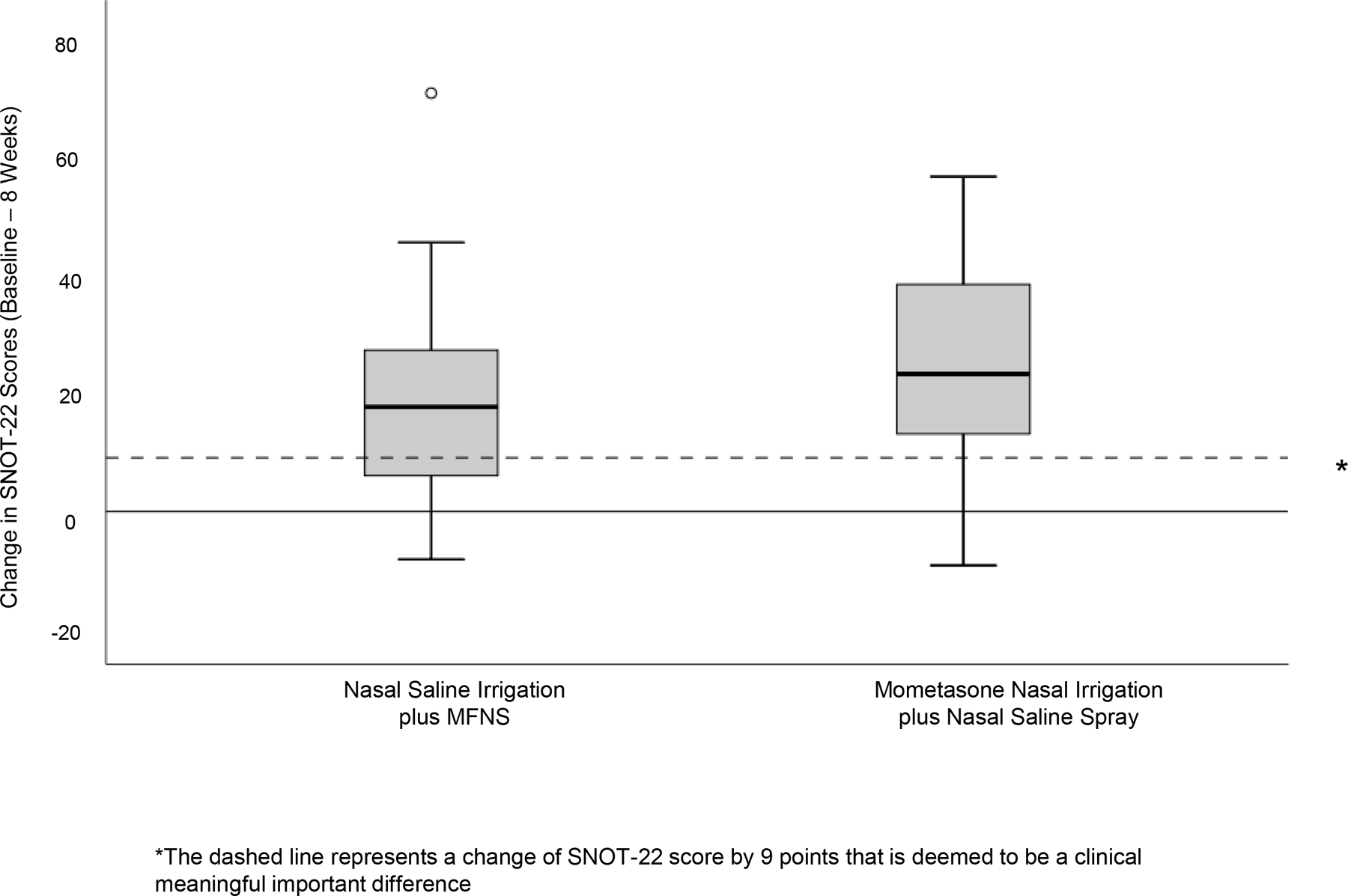
Comparison of Change in SNOT-22 Scores Between the 2 Treatment Groups. *The box and whisker plots represent change in SNOT-22 within each treatment group. The solid dashed line within the box represents the median value, the upper and lower part of the box represents the 75th and 25th percentile, the “whiskers” represent the upper and lower extreme of values, and the open circle represents outliers. The dashed horizontal line represents the clinically meaningful important difference of SNOT-22 score by 9 points.
Mixed model analysis showed that there was no confounding effect of age, race, gender, or comorbidity in the change in SNOT-22 scores.
Secondary Outcome Measures
Based on CGI, 20 participants (95%) in the nasal saline irrigation plus MFNS group and 22 participants (96%) in the mometasone nasal irrigation plus saline nasal spray group self-reported some degree of improvement from “slightly better” to “very much better” following completion of the study.
The least squares mean change in Lund-Kennedy endoscopy scores between baseline and week 8 was 2.1 (95% CI, 1.0 to 3.1; p=0.003) in the nasal saline irrigation plus MFNS group and 2.2 (95% CI 1.1 to 3.3; p=0.003) in the mometasone nasal irrigation plus nasal saline spray group. The least square mean difference between the two intervention groups was 0.16 (95% CI, −0.84 to 1.15; p=0.75).
Cosyntropin Testing and Safety
In our subgroup of patients who underwent cosyntropin stimulation, there was not a detectable effect of mometasone furoate nasal irrigation on the HPA axis after 8-weeks of intervention. Likewise, consistent with prior studies, there was not a detectable effect of mometasone nasal spray on the HPA axis (Table 2).
Table 2.
Post-Cosyntropin Stimulation Cortisol Levels
| Treatment Arm | Mean cortisol level (ug/dL) (SD) Before Treatment |
Mean cortisol level (ug/dL) (SD) After Treatment |
Difference (ug/DL) (95% CI) Cohen’s d |
|---|---|---|---|
| Nasal Saline Irrigation plus Mometasone Nasal Spray (n=10) | 23.19 (3.51) | 23.91 (2.56) | −0.72 (−3.41 to 1.97) 0.23 |
| Mometasone nasal irrigation plus Nasal Saline Spray (n=10) | 24.18 (4.76) | 24.22 (3.84) | −0.04 (−3.83 to 3.75) 0.01 |
Cohen’s d interpretation: d= 0.2 considered “small” effect, 0.5 “medium” effect, and 0.8 “large” effect
1 patient in the nasal saline irrigation plus MFNS group had a serious adverse event (myocardial infarction) during the study enrollment. However, the serious adverse event was not considered to be related to the drug. A total of 2 patients (1 in each group) could not tolerate daily performing nasal irrigations, which led to withdrawal from the study.
Twenty patients (91%) in the nasal saline irrigation plus MFNS group and 19 patients (90%) in the mometasone nasal irrigation plus nasal saline spray group reported compliance at the end of the study.
Participant Blinding
Patients were asked to perform a best guess at the end of the study regarding which arm they were randomized to in order to assess the effectiveness of participant blinding. Participants guessed no better than chance alone suggesting that participants were effectively blinded throughout the course of the trial.
DISCUSSION
In this double-blind, placebo-controlled randomized clinical trial, we found that delivery of mometasone either via nasal spray (and performing nasal saline lavage) or nasal irrigation resulted in clinically meaningful improvement in both clinical and endoscopic end points for CRSsNP patients who have not undergone sinus surgery. Furthermore, the addition of mometasone to the nasal irrigation was associated with a greater improvement in SNOT-22 when compared to mometasone nasal spray. These results suggest that sinus surgery may not be required for the CRSsNP patient to experience the benefit of topical nasal steroid administration and sinus lavage.
The use of topical corticosteroids is a mainstay treatment for CRS patients. Several prospective trials have evaluated the effect of intranasal corticosteroids delivered by nasal irrigations. However, the effect size in these studies have been varied due to heterogenous study cohorts and study design and are not generalizable to patients who are managed medically only, as most of these studies evaluated medication effectiveness after sinus surgery8.
While the use of budesonide nasal irrigation has become widespread in the management of CRS, especially after endoscopic sinus surgery, mometasone is a promising alternative. Mometasone furoate has several structural modifications that confer more favorable pharmacologic properties compared to budesonide. All corticosteroid molecules are derived from cortisol, the parent molecule, and share the same carbon framework backbone of three 6-carbon rings and one 5-carbon ring. For MF, the addition of a 21-chloro 17(2’furoate) group increases the compound’s topical anti-inflammatory activity. 23 Furthermore, the addition of the halogen, chloride, at positions 9 and 21 increases the compound’s affinity for the corticosteroid receptor and decreases its susceptibility to esterase degradation, respectively.23 These structural changes not only increase the lipophilicity of mometasone, but also promote its rapid and extensive hepatic metabolism. Thus, compared to budesonide, mometasone has a negligible systemic absorption (<0.1% versus 34%, respectively).17
While several randomized controlled parallel-group or placebo trials have shown no significant effect of MFNS on HPA axis function,21 to date, there are no studies that have examined the effect of mometasone nasal irrigation on HPA axis function. Our study is the first to show that mometasone nasal irrigation can be safely used in the short-term for patients with CRS without observed suppression of the HPA axis. Given mometasone’s very low systemic absorption and excellent safety profile, we believe that providers may want to offer patients either mometasone or budesonide nasal irrigation for medical management of CRS. However, future studies that directly compare budesonide to mometasone are warranted. To our knowledge, there is only one other study to perform a direct comparison of intranasal corticosteroid versus corticosteroid nasal irrigation in the treatment of CRS. Harvey et al. evaluated the efficacy of mometasone nasal spray compared to mometasone nasal irrigation in CRS patients with or without nasal polyps following endoscopic sinus surgery.16 Following one year of treatment, participants who received corticosteroid irrigations had significantly greater improvements in subjective nasal symptoms as well as endoscopic and radiologic findings compared to those who received corticosteroid nasal spray.
Overall, among CRSsNP participants who have not undergone sinus surgery significant improvement in PROMs are observed following treatment with either nasal saline lavage and mometasone nasal spray or mometasone nasal irrigation. For CRS patients who cannot tolerate or afford the cost of nasal steroids, nasal saline lavage alone may be an effective therapy in symptom management. These results suggest that a surgically opened sinonasal corridor may not necessarily be needed for patients to experience the beneficial effects of topical nasal steroid administration. Compliance in both study arms was high as was participant satisfaction, suggesting that providers can equivalently offer both treatment options to patients.
For patients who are refractory to maximal medical management, surgical intervention is often warranted. In a study by Rudmik et al., CRS patients with a baseline SNOT-22 score greater than 30 had a greater than 80% change of having a MCID in their SNOT-22 scores post endoscopic sinus surgery. 24 At baseline, the average SNOT-22 score for our patient cohort was 42 and the majority of patients (72%) achieved a MCID with medical therapy alone. Our study intentionally did not look at patients who had undergone endoscopic sinus surgery. However, we believe future studies examining incremental improvement in SNOT-22 scores following endoscopic sinus surgery for failed medical therapy with nasal irrigations would be invaluable in managing provider and patient expectations about quality of life improvement following surgery in cases where medical management alone do not meet their desired level of improvement.
A limitation of this study was treatment duration. As CRS is a chronic disease, patients may need to be on topical steroids for a longer duration than that allotted in our study’s 8-week timeframe. However, due to financial cost and resources, it was not feasible for our treatment time to be extended beyond the 8-weeks. Furthermore, compliance was self-reported. In addition, only a minority of our participants received sinus CT imaging pre- and post-intervention, and only at the clinical discretion of their primary otolaryngologist. Thus, we were limited in our ability to objectively quantify changes in mucosal disease in the sinonasal cavities beyond what was seen on endoscopy exam. However, according to the American Academy of Otolaryngology – Head and Neck Surgery Practice Guidelines for Adult Sinusitis, the use of CT imaging should be reserved for patients with refractory or prolonged symptoms.25
Despite these limitations, we believe this study provides clinically meaningful information regarding the comparative effectiveness of nasal saline lavage plus mometasone nasal spray to mometasone nasal irrigation in symptom control for CRSsNP patients who have not undergone sinus surgery.
CONCLUSION
For patients with CRSsNP who have not undergone surgery, treatment with either nasal saline lavage and topical mometasone nasal spray or mometasone nasal steroid irrigation is beneficial in symptom management. Mometasone nasal steroid irrigation was associated with a greater clinically meaningful improvement in PROMs as compared to nasal saline lavage plus mometasone nasal spray. However, these findings are not definitive and further studies are needed with larger sample sizes, longer treatment duration, and direct comparison to surgery to add to the body of knowledge regarding the efficacy of mometasone nasal steroid irrigation.
ACKNOWLEDGEMENTS
The authors would like to thank Jason Jerusik, PharmD for compounding the medications and providing treatment kits to the study participants.
This study was supported by the ARS Resident Research Grant, “Development of Clinician/Researchers in Academic ENT” T32 DC00022 from the National Institutes of Deafness and Other Communication Disorders and The Foundation for Barnes-Jewish Hospital “Otolaryngology Surgical Outcomes and Quality Improvement Unit (SOQIU) at Barnes-Jewish Hospital” grant award number 4090. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial Disclosures: JS is a consultant for Medtronic, Olympus, and Optinose. He is also on the speaker bureau for Optinose. CKC is a consultant for Medtronic. JFP receives royalty income for use of the SNOT-22 instrument. All other authors have no financial disclosures or conflicts of interest.
References
- 1.Mullol J, Obando A, Pujols L, Alobid I. Corticosteroid treatment in chronic rhinosinusitis: the possibilities and the limits. Immunology and allergy clinics of North America. 2009;29(4):657–668. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. British Journal of Pharmacology. 2006;148(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas WW 3rd, Harvey RJ, Rudmik L, Hwang PH, Schlosser RJ. Distribution of topical agents to the paranasal sinuses: an evidence-based review with recommendations. International forum of allergy & rhinology. 2013;3(9):691–703. [DOI] [PubMed] [Google Scholar]
- 4.Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(5):415–421. [DOI] [PubMed] [Google Scholar]
- 5.Sachanandani NS, Piccirillo JF, Kramper MA, Thawley SE, Vlahiotis A. The effect of nasally administered budesonide respules on adrenal cortex function in patients with chronic rhinosinusitis. Archives of Otolaryngology -- Head & Neck Surgery. 2009;135(3):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinke JW, Payne SC, Tessier ME, Borish LO, Han JK, Borish LC. Pilot study of budesonide inhalant suspension irrigations for chronic eosinophilic sinusitis. J Allergy Clin Immunol. 2009;124(6):1352–1354. [DOI] [PubMed] [Google Scholar]
- 7.Tait S, Kallogjeri D, Suko J, Kukuljan S, Schneider J, Piccirillo JF. Effect of Budesonide Added to Large-Volume, Low-pressure Saline Sinus Irrigation for Chronic Rhinosinusitis: A Randomized Clinical Trial. JAMA Otolaryngology–Head & Neck Surgery. 2018;144(7):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snidvongs K, Kalish L, Sacks R, Sivasubramaniam R, Cope D, Harvey RJ. Sinus surgery and delivery method influence the effectiveness of topical corticosteroids for chronic rhinosinusitis: systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27(3):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas WW III, Harvey RJ, Rudmik L, Hwang PH, Schlosser RJ. Distribution of topical agents to the paranasal sinuses: an evidence-based review with recommendations. 2013;3(9):691–703. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins C, Philpott C, Crowe S, et al. Identifying the most important outcomes for systematic reviews of interventions for rhinosinusitis in adults: working with Patients, Public and Practitioners. Rhinology. 2016;54(1):20–26. [DOI] [PubMed] [Google Scholar]
- 11.Rudmik L, Hopkins C, Peters A, Smith TL, Schlosser RJ, Soler ZM. Patient-reported outcome measures for adult chronic rhinosinusitis: A systematic review and quality assessment. Journal of Allergy and Clinical Immunology. 2015;136(6):1532–1540.e1532. [DOI] [PubMed] [Google Scholar]
- 12.Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1997;117(3 Pt 2):S1–7. [DOI] [PubMed] [Google Scholar]
- 13.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003;129(3 Suppl):S1–32. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 15.Harvey RJ, Debnath N, Srubiski A, Bleier B, Schlosser RJ. Fluid Residuals and Drug Exposure in Nasal Irrigation. 2009;141(6):757–761. [DOI] [PubMed] [Google Scholar]
- 16.Harvey RJ, Snidvongs K, Kalish LH, Oakley GM, Sacks R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. 2018;8(4):461–470. [DOI] [PubMed] [Google Scholar]
- 17.Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008;63(10):1292–1300. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins C, Gillett S, Slack R, Lund V, Browne JJCo. Psychometric validity of the 22-item Sinonasal Outcome Test. 2009;34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 19.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont ). 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Lund VJ, Kennedy DW. Quantification for Staging Sinusitis. Annals of Otology, Rhinology & Laryngology. 1995;104(10_suppl):17–21. [PubMed] [Google Scholar]
- 21.Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. Journal of investigational allergology & clinical immunology. 2012;22(1):1–12. [PubMed] [Google Scholar]
- 22.Cohen JJJASA. Statistical power analysis for the behavioral sciences: Jacob Cohen. 1988;84(363):19–74. [Google Scholar]
- 23.Szefler SJ. Pharmacokinetics of intranasal corticosteroids. The Journal of allergy and clinical immunology. 2001;108(1 Suppl):S26–31. [DOI] [PubMed] [Google Scholar]
- 24.Rudmik L, Soler ZM, Mace JC, DeConde AS, Schlosser RJ, Smith TL. Using preoperative SNOT-22 score to inform patient decision for Endoscopic sinus surgery. The Laryngoscope. 2015;125(7):1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical Practice Guideline (Update): Adult Sinusitis. 2015;152(2_suppl):S1–S39. [DOI] [PubMed] [Google Scholar]


