Abstract
Background
Neonatal hypoglycaemia, a common condition, can be associated with brain injury. It is frequently managed by providing infants with an alternative source of glucose, often given enterally with milk‐feeding or intravenously with dextrose solution, which may decrease breastfeeding success. Intravenous dextrose also often requires that mother and baby are cared for in separate environments. Oral dextrose gel is simple and inexpensive, and can be administered directly to the buccal mucosa for rapid correction of hypoglycaemia, in association with continued breastfeeding and maternal care.
This is an update of a previous review published in 2016.
Objectives
To assess the effectiveness of oral dextrose gel in correcting hypoglycaemia in newborn infants from birth to discharge home and reducing long‐term neurodevelopmental impairment.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, and Embase from database inception to October 2021. We also searched international clinical trials networks, the reference lists of included trials, and relevant systematic reviews identified in the search.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing oral dextrose gel versus placebo, no treatment, or other therapies for the treatment of neonatal hypoglycaemia in newborn infants from birth to discharge home.
Data collection and analysis
Two review authors independently assessed study quality and extracted data; they did not assess publications for which they were study authors. We contacted investigators to obtain additional information. We used fixed‐effect models and the GRADE approach to assess the certainty of evidence.
Main results
We included two studies conducted in high‐income countries, involving 312 late preterm and at‐risk term infants and comparing oral dextrose gel (40% concentration) to placebo gel. One study was at low risk of bias, and the other (an abstract) was at unclear to high risk of bias. Oral dextrose gel compared with placebo gel probably increases correction of hypoglycaemic events (rate ratio 1.08, 95% confidence interval (CI) 0.98 to 1.20; rate difference 66 more per 1000, 95% CI 17 fewer to 166 more; 1 study; 237 infants; moderate‐certainty evidence), and may result in a slight reduction in the risk of major neurological disability at age two years or older, but the evidence is uncertain (risk ratio (RR) 0.46, 95% CI 0.09 to 2.47; risk difference (RD) 24 fewer per 1000, 95% CI 41 fewer to 66 more; 1 study, 185 children; low‐certainty evidence). The evidence is very uncertain about the effect of oral dextrose gel compared with placebo gel or no gel on the need for intravenous treatment for hypoglycaemia (RR 0.78, 95% CI 0.46 to 1.32; RD 37 fewer per 1000, 95% CI 91 fewer to 54 more; 2 studies, 312 infants; very low‐certainty evidence). Investigators in one study of 237 infants reported no adverse events (e.g. choking or vomiting at the time of administration) in the oral dextrose gel or placebo gel group (low‐certainty evidence).
Oral dextrose gel compared with placebo gel probably reduces the incidence of separation from the mother for treatment of hypoglycaemia (RR 0.54, 95% CI 0.31 to 0.93; RD 116 fewer per 1000, 95% CI 174 fewer to 18 fewer; 1 study, 237 infants; moderate‐certainty evidence), and increases the likelihood of exclusive breastfeeding after discharge (RR 1.10, 95% CI 1.01 to 1.18; RD 87 more per 1000, 95% CI 9 more to 157 more; 1 study, 237 infants; moderate‐certainty evidence).
Authors' conclusions
Oral dextrose gel (specifically 40% dextrose concentration) used to treat hypoglycaemia in newborn infants (specifically at‐risk late preterm and term infants) probably increases correction of hypoglycaemic events, and may result in a slight reduction in the risk of major neurological disability at age two years or older. Oral dextrose gel treatment probably reduces the incidence of separation from the mother for treatment and increases the likelihood of exclusive breastfeeding after discharge. No adverse events have been reported.
Oral dextrose gel is probably an effective and safe first‐line treatment for infants with neonatal hypoglycaemia in high‐income settings.
More evidence is needed about the effects of oral dextrose gel treatment on later neurological disability and the need for other treatments for hypoglycaemia. Future studies should be conducted in low‐and middle‐income settings, in extremely and moderately preterm infants, and compare oral dextrose gel with other therapies such as intravenous dextrose. There are two ongoing studies that may alter the conclusions of this review when published.
Plain language summary
Oral dextrose gel for the treatment of newborn infants with low blood glucose levels
Review question
For newborn infants who develop low blood glucose levels (hypoglycaemia), is sugar gel given by mouth (oral dextrose gel) more effective than no treatment or other active treatments in correcting the low blood glucose level and reducing long‐term neurodevelopmental impairment?
Background
Low blood glucose levels (hypoglycaemia) in newborn infants are common and occur frequently in certain at‐risk groups (infants of mothers with high blood glucose levels (diabetes), infants born preterm, small and large infants). Infants with low blood glucose levels are at higher risk for developmental problems later in childhood. To manage this condition, active treatments are generally used, frequently requiring the use of formula milk or admission to the neonatal intensive care unit to receive fluid infusion into the veins, resulting in temporary separation from the mother. Sugar gel applied to the inside of the mouth is a simple and low‐cost option for the initial care of infants with low blood glucose levels. We are exploring whether oral dextrose is more effective than no treatment or other active treatments in correcting low blood glucose levels in newborn infants and reducing its long‐term effects on neurodevelopment.
Study characteristics
Two studies in high‐income countries have assessed the use of oral dextrose gel to reverse low blood glucose levels in a total of 312 infants. Investigators rubbed oral dextrose gel into the inside of the infant's cheek for 157 of these infants and rubbed in placebo gel or no gel for 155 infants, and then gave a normal feed. Key results
Results suggest that oral dextrose gel probably corrects individual episodes of low blood glucose levels and may result in a slight reduction in the risk of major disability at age two years or older; however, the evidence is uncertain. The evidence was not sufficient to show whether oral dextrose gel reduces the need for other treatments. Oral dextrose gel compared to placebo gel probably reduces mother‐infant separation and probably increases the likelihood of exclusive breastfeeding after discharge from the hospital. Researchers reported no adverse events when oral dextrose gel was given to infants.
We searched for studies up to October 2021.There are two ongoing studies that may alter the conclusions of this review when published.
Certainty of evidence
The available studies were small in size and there are not enough studies and to be entirely certain about the results.
Summary of findings
Summary of findings 1. Oral dextrose gel versus control.
| Oral dextrose gel versus control | ||||||
|
Patient or population: newborn infants with hypoglycaemia Setting: from birth to discharge home Intervention: oral dextrose gel Comparison: placebo gel or no gel | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo gel or no gel | Risk with dextrose gel | |||||
| Correction of hypoglycaemia for each hypoglycaemic event before discharge home (investigator defined) | Study population | Rate ratio 1.08 (0.98 to 1.20) | 237 (1 RCT) |
⊕⊕⊕⊝ Moderatea | ||
| 829 per 1000 | 66 more per 1000 (17 fewer to 166 more) | |||||
| Major neurological disability at age 2 years or olderb Defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay/intellectual impairment (defined as developmental quotient less than 2 SD below the mean) |
Study population | RR 0.46 (0.09 to 2.47) | 185 (1 RCT) | ⊕⊕⊝⊝ Lowc | ||
| 45 per 1000 | 24 fewer per 1000 (41 fewer to 66 more) | |||||
| Receipt of intravenous treatment for hypoglycaemia before discharge home (for each infant) (yes/no) |
Study population | RR 0.78 (0.46 to 1.32) | 312 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | ||
| 168 per 1000 | 37 fewer per 1000 (91 fewer to 54 more) | |||||
| Adverse events (e.g. choking or vomiting at time of administration) before discharge home (yes/no) |
Study population | Not estimable | 237 (1 RCT) | ⊕⊕⊕⊝ Lowe | No events reported in either the oral dextrose gel or the placebo gel group. | |
| 0 per 1000 | 0 fewer per 1000 (0 to 0) | |||||
| Separation from mother for treatment of hypoglycaemia before discharge home (infant nursed in an environment that is not in the same room as the mother, e.g. for NICU admission or the like) |
Study population | RR 0.54 (0.31 to 0.93) | 237 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ||
| 252 per 1000 | 116 fewer per 1000 (174 fewer to 18 fewer) | |||||
| Exclusive breastfeeding after discharge (WHO 2008 definition (yes/no)) |
Study population | RR 1.10 (1.01 to 1.18) | 237 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ||
| 874 per 1000 | 87 more per 1000 (9 more to 157 more) | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; NICU: neonatal intensive care unit; RR: risk ratio; SD: standard deviation; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of effect but may be substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious imprecision (due to low event rates). bData used were from the 4.5‐year follow‐up study. cDowngraded two levels for very serious imprecision (due to low event rates and CI including possibility of both benefits or harms). dDowngraded one level for serious inconsistency (due to the moderate I2 value of 72% and low Chi2 P = 0.06). eDowngraded two levels for very serious imprecision (due to no events and the small sample size).
Background
Description of the condition
Neonatal hypoglycaemia is a common condition affecting 5% to 15% of infants in the immediate postnatal period (Cornblath 2000; Hay 2009; McGowan 2006). Neonatal hypoglycaemia is important because it can be associated with brain injury (Burns 2008; Kerstjens 2012; Koh 1988; Lucas 1988), developmental problems (McKinlay 2017), and poor later school performance (Kaiser 2015), although these associations are not consistently reported (Tin 2012). It is also associated with substantial costs to the healthcare system and reduced quality of life (Glasgow 2021).
The incidence of this disorder is likely to be on the rise, as factors that predispose infants to hypoglycaemia are increasing, including preterm birth (Blencowe 2012), maternal diabetes (Wild 2004), and obesity (Doherty 2006). Risk factors for neonatal hypoglycaemia are known, and specific groups of infants are routinely targeted for screening (infants of diabetic mothers, high or low birthweight babies, preterm infants and those with poor feeding). Less common causes include hyperinsulinism and disorders of fatty acid oxidation. Neonatal hypoglycaemia is reported commonly at maternity hospitals in resource‐poor settings (Anderson 1993; Osier 2003). Screening by measuring glucose concentrations in capillary heel‐lance blood samples is usually performed because associated clinical signs are not diagnostically helpful. The accuracy of screening varies with the method of measurement used; point‐of‐care testing systems have a greater error range than laboratory systems based on glucose oxidase methods (Beardsell 2010).
The definition of hypoglycaemia remains controversial (Hay 2009), and different publications have used definition thresholds ranging from 1.7 to 2.6 mmol/L (Agrawal 2000; Holtrop 1993; Hume 1999; Lubchenco 1971; Maayan‐Metzger 2009). Several different clinical thresholds for treatment have been suggested (Adamkin 2011; British Association of Perinatal Medicine 2017; Cornblath 2000; Thornton 2015), but a blood glucose concentration < 2.6 mmol/L is widely accepted as a target for treatment (Harris 2014); concentrations below this may be associated with altered brain function and delayed development (Koh 1988; Lucas 1988; McKinlay 2017).
Upon diagnosis, infants are frequently managed with increased feeding, supplemental infant formula or intravenous dextrose. Supplemental infant formula may disrupt the establishment of breastfeeding (Blomquist 1994; Demir 2020; Smith 2016). Intravenous dextrose is expensive, usually requires separation of mother and infant and is not always available in resource‐poor settings (Graz 2008), or settings providing lower levels of perinatal care.
The World Health Organization (WHO) recommends breastfeeding for all infants up to six months of age (WHO 2008), and the health benefits of breastfeeding for both mother and infant are well recognised. Human studies have shown that breast milk volume in the first 24 postpartum hours is low and progressively increases by the third day (Kulski 1981; Le Huerou‐Luron 2010; Saint 1984). The concentration of lactose within breast milk is also low in the first 24 hours (Kulski 1981; Saint 1984), and steadily increases over the first three days.
Formula milk is often given to hypoglycaemic infants. Since the carbohydrate content of breast milk on the first day is low (Saint 1984), formula milk may be more effective than breast milk as a treatment for infants with neonatal hypoglycaemia. One post hoc analysis of a randomised trial showed that in 277 late preterm and term infants with hypoglycaemia, formula feeding was associated with the greatest increase in glucose concentration within 48 hours after birth compared with no milk, breastfeeding or expressed milk (Weston 2017).
If feeding does not improve the blood glucose concentration, the next step is often admission to the neonatal intensive care unit (NICU) for intravenous dextrose. A bolus of 200 mcg/kg/min of 10% dextrose followed by an intravenous infusion of 8 mcg/kg/min increases the blood glucose concentration within one minute (Lilien 1980). However, a 200 mcg/kg bolus may results in hyperglycaemia, and there is concern that a swift increase in glucose concentrations may result in poorer neurological outcomes (McKinlay 2015; Rozance 2019).
Investigators in a randomised trial assessed intravenous dextrose and glucagon (200 ug/kg) or intragastric medium chain triglycerides (5 mL/kg) (Hawdon 1993). Both treatments substantially increased the blood glucose concentration among infants already receiving 5 mcg/kg/min intravenous dextrose for hypoglycaemia.
Oral dextrose gel is widely used (Alsweiler 2019), and is increasingly recommended as a first‐line treatment for asymptomatic neonatal hypoglycaemia (Academy of Breastfeeding Medicine 2021; British Association of Perinatal Medicine 2017; Canadian Paediatric Society; Rozance 2019; Swedish National Guideline 2020). The first version of this updated Cochrane Review 'Oral dextrose gel for treatment of neonatal hypoglycaemia in newborn infants' found that in one eligible trial, oral dextrose gel reduced separation of the mother and infant for treatment of hypoglycaemia and improved the likelihood of exclusive breastfeeding after discharge with no evidence of adverse events (Weston 2016).
Description of the intervention
Oral dextrose gel contains dextrose, a simple carbohydrate, in concentrated aqueous solution, that can be administered by direct application to mucosal surfaces of the mouth, including buccal and lingual surfaces. Absorption from these sites may allow rapid access to the circulation.
Commercial preparations of oral dextrose gel are widely available, as they are commonly used for management of hypoglycaemia in people with diabetes. Many preparations contain preservatives and flavour additives as well as gelling agents, requiring individual assessment for suitability in neonates. Oral dextrose gel can be manufactured by hospital pharmacies with appropriate facilities. Costs for neonatal doses are low (a few dollars or less per dose), and adverse events have not been reported.
In infants with hypoglycaemia, simple treatment with oral dextrose gel and the potential avoidance of more complex treatments, such as intravenous dextrose or complementary milks, would provide an attractive option, if effective. Oral dextrose gel is typically available in 40 g/100 mL form (40%) and is administered at doses of 200 to 400 mg/kg.
How the intervention might work
Oral dextrose gel may be absorbed directly from the oral mucosa, thus bypassing the portal circulation and gaining more rapid access to the circulation. Some proportion of the dose may also be swallowed and absorbed from the gastrointestinal tract. Oral dextrose gel is rapidly absorbed by the gastrointestinal mucosa because it does not require digestion; it may then be taken up by the liver via the portal circulation and hence may have a more delayed effect on blood glucose concentrations.
Why it is important to do this review
Treatment of the neonate with hypoglycaemia usually involves additional feeding, often with formula milk, with the potential for an adverse impact on the quality and duration of breastfeeding. If feeding is not effective, intravenous dextrose is usually administered, commonly requiring admission to the NICU and resulting in separation of mother and infant, impaired initiation of breastfeeding and increased healthcare costs.
Oral dextrose gel is inexpensive and simple to administer. Further, oral dextrose gel can be used in resource‐poor settings where higher levels of neonatal care are unavailable. If effective in treating infants with neonatal hypoglycaemia without adverse events, it may prevent brain damage caused by untreated neonatal hypoglycaemia.
Objectives
To assess the effectiveness of oral dextrose gel in correcting hypoglycaemia in newborn infants from birth to discharge home and reducing long‐term neurodevelopmental impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing oral dextrose gel versus placebo, no treatment or other therapies for neonatal hypoglycaemia. We included published studies, unpublished studies and studies published only as abstracts if inclusion criteria were met and there was enough information to perform a GRADE evaluation.
Types of participants
We included newborn infants from birth to discharge home (including infants admitted to NICU) who were hypoglycaemic (blood glucose concentrations below the normal range, investigator defined) for any reason. We excluded infants who had received prior intravenous treatment for the maintenance of glucose control at the time of hypoglycaemia.
Types of interventions
We included dextrose gel, at any dose, given orally, usually over a few minutes, compared with placebo, no treatment or other therapies (e.g. intravenous dextrose, diazoxide, or glucagon), at any postmenstrual or postnatal age. The oral dextrose gel product could be locally prepared or manufactured commercially.
Types of outcome measures
The outcomes listed below were not used as criteria for study selection.
Primary outcomes
Correction of hypoglycaemia for each event of hypoglycaemia before discharge home (investigator defined) (event outcome).
Major neurological disability at age two years or older (defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay/intellectual impairment (defined as developmental quotient less than two standard deviations (SDs) below the mean) (child outcome)).
Secondary outcomes
Receipt of intravenous treatment for hypoglycaemia before discharge home (yes/no) (infant outcome).
Requirement for any medications for hypoglycaemia such as glucagon or corticosteroids before discharge home (yes/no) (infant outcome).
Number of episodes of hypoglycaemia (investigator defined) before discharge home (infant outcome).
Improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel before discharge home (event outcome).
Rebound hypoglycaemia (investigator defined hypoglycaemia occurring within six hours of initial correction) before discharge home (yes/no) (event outcome).
Increase in blood glucose after treatment (change in blood glucose concentration 30 to 90 minutes after treatment) before discharge home (event outcome).
Duration of hypoglycaemia (time from detection of hypoglycaemia to achievement of blood glucose concentration above the threshold definition before discharge home, minutes) (event outcome).
Adverse events (e.g. choking or vomiting at time of administration) before discharge home (yes/no) (infant outcome).
Separation from mother for treatment of hypoglycaemia before discharge home (infant nursed in an environment that is not in the same room as the mother, e.g. for NICU admission or the like) (yes/no) (infant outcome).
Neonatal seizures before discharge home (yes/no) (infant outcome).
Abnormal magnetic resonance imaging (MRI) of the brain in the neonatal period — investigator defined (yes/no) (infant outcome).
Duration of initial hospital stay (days) (infant outcome).
Breastfeeding (any) after discharge (yes/no) (infant outcome).
Exclusive breastfeeding after discharge — WHO 2008 definition (yes/no) (infant outcome).
Exclusive breastfeeding at six months of age — WHO 2008 definition (yes/no) (infant outcome).
Developmental disability at age two years or older — investigator defined (yes/no) (child outcome).
Visual impairment and severity at age two years or older (child outcome).
Hearing impairment and severity at age two years or older (child outcome).
Cerebral palsy and severity at age two years or older (child outcome).
Developmental delay/intellectual impairment and severity at age two years or older (child outcome).
Executive dysfunction and severity at age two years or older (child outcome).
Behavioural problems and severity at age two years or older (child outcome).
Abnormal MRI of the brain at age two years or older (child outcome).
Search methods for identification of studies
The Neonatal Group Information Specialist developed search strategies in consultation with the authors. Controlled vocabulary and keywords were used and combined with methodological filters to restrict retrieval to RCTs and systematic reviews; filters are based on those developed by Cochrane (Lefebvre 2021) and CADTH (CADTH 2021).
Electronic searches
We searched the following databases without language, publication year, publication type, or publication status restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL), via WileyOvid (on 6 October 2021)
MEDLINE and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations and Daily (1946 to 5 October 2021)
Embase, via OVID (1974 to 5 October 2021)
Search strategies are available: Appendix 1; Appendix 2; Appendix 3.
We searched clinical trial registries for ongoing or recently completed trials. We searched The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), and the International Standard Randomised Controlled Trial Number (ISRCTN) Registry (www.isrctn.com/), for any unique trials not found through the Cochrane CENTRAL search. Search strategies are available: Appendix 4; Appendix 5; Appendix 6.
For the 2021 update, we developed a new search strategy. The previous search methods are available in Appendix 7.
Searching other resources
We also searched the reference lists of included trials and relevant systematic reviews identified in the search. We contacted known researchers in this clinical area to identify unpublished or ongoing research.
Data collection and analysis
Selection of studies
Two review authors (TE, GL) independently screened studies for eligibility. We corresponded with investigators, when appropriate, to clarify study eligibility, and, when possible, to obtain missing information. We resolved any disagreements through discussion.
Data extraction and management
We used the data extraction form from the previous review. Two review authors (TE, GL) independently extracted data from eligible studies. We entered data and checked data for accuracy using Review Manager 2020. We resolved any disagreements through discussion.
Assessment of risk of bias in included studies
Review authors (TE, GL) independently assessed the risk of bias (low, high, or unclear) of all included studies using the Cochrane risk of bias tool RoB 1 for the following domains (Higgins 2017).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
The review authors (TE, GL) examined the methods of each study for prespecified outcomes. If all prespecified outcomes were reported in the results, we assigned the study a low risk of reporting bias. If any prespecified outcomes were not reported in the results, we considered the study to carry either an unclear or high risk of reporting bias.
We resolved any disagreements by discussion or by consulting a third assessor.
Measures of treatment effect
We summarised count data for events (correction of hypoglycaemia for each event of hypoglycaemia, improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel and rebound hypoglycaemia) as rate ratios and rate differences using the number of events adjusted for clustering of events within individual infants. We summarised continuous data as mean differences (MDs) when studies used the same outcome measure or standardised mean differences (SMDs) when the outcome measures differed. We summarised dichotomous data as risk ratios (RRs) and reported risk differences (RDs). When a significant effect was found, we calculated numbers needed to treat for additional beneficial outcomes (NNTBs) or numbers needed to treat for additional harmful outcomes (NNTHs). We reported 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
For specific measures related to correction of hypoglycaemia, we used the hypoglycaemic event itself as the unit of analysis. For measures that determined outcomes for the infant (such as those related to breastfeeding and developmental outcomes), we used the infant as the unit of analysis.
Dealing with missing data
We noted whether levels of attrition applied. When possible, we carried out analyses on an intention‐to‐treat basis for all outcomes and analysed all participants in the treatment group to which they were randomised, regardless of the actual treatment received. We attempted to contact the original investigators to request missing data, when possible. We planned to perform an available case analysis when there was missing outcome data. In a sensitivity analysis, we planned to excluding studies with high rates of missing data (> 20%). In addition, we addressed in the Discussion section the potential impact of missing data on review findings, when relevant.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We did this by assessing statistical heterogeneity using the Chi2 test and the I2 statistic. We classified heterogeneity as none (< 25%), low (25% to 49%), moderate (50% to 74%) or high (> 75%). We considered an I2 measurement greater than 50% and a low P value (< 0.10) in the Chi2 test for heterogeneity to indicate substantial heterogeneity (Higgins 2020). We considered statistical heterogeneity when interpreting study results, especially when we noted variation in the direction of effect.
Assessment of reporting biases
Reporting biases arise when dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias) reduce the likelihood that all studies eligible for a review will be retrieved. If all eligible studies are not retrieved, the review may be biased (Boutron 2019). We aimed to conduct a comprehensive search for eligible studies and remained alert for duplication of data. We planned to assess publication bias by visually inspecting a funnel plot, if we identified enough studies (≥ 10 trials) to make such an inspection valid.
Data synthesis
We evaluated studies for potential clinical diversity (e.g. differences in the dose of oral dextrose gel, type, and severity of hypoglycaemia, reason for risk of hypoglycaemia), and we planned to restrict meta‐analysis to studies in which clinical consistency was apparent. We evaluated studies for bias, as above, and planned to restrict meta‐analysis if bias would be compounded.
We used a fixed‐effect meta‐analysis to combine data when it was reasonable to assume that studies were estimating the same underlying treatment effects. We analysed count data as rate ratios adjusting for clustering of events within individual infants using the generic inverse variance method and calculated the log of the rate ratio (logRR) and standard error (SE) for each study. For data summarised as mean differences we used the inverse variance method. We analysed dichotomous data using the Mantel‐Haenszel method. We calculated rate ratios in SAS and then used them to calculate the logRR and SE in Review Manager. All other analyses were performed in Review Manager (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate this by using subgroup analysis. We planned to carry out the following subgroup analyses.
Infant factors
Reason for risk of hypoglycaemia (infant of diabetic mother versus preterm versus small versus large versus other).
Method used to measure blood glucose concentration (reliable instrument using glucose oxidase method versus less reliable cot‐side approaches).
First episode of hypoglycaemia versus any subsequent episodes.
Oral dextrose gel as the only intervention versus oral dextrose gel administered as a co‐intervention (e.g. in addition to formula feeds).
Event factors
Method of feeding at the time of the event (formula versus breastfeeding versus mixed versus nil versus other).
Method of administration of gel (buccal mucosa versus lingual mucosa versus other).
Dose of dextrose per administration (≤ 200 mg/kg versus > 200 mg/kg).
Maximum number of doses for treatment of a single episode of hypoglycaemia (one versus more than one).
Sensitivity analysis
We planned to conduct the following sensitivity analyses, when possible.
Examining only studies considered to have an overall low risk of bias using the Cochrane RoB 1 tool (Higgins 2017).
Excluding studies where review authors uncovered reporting bias that could, in their opinion, introduce serious bias.
Excluding studies with high rates of missing data (> 20%).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes.
Correction of hypoglycaemia for each hypoglycaemic event
Major neurological disability at age two years or older
Receipt of intravenous treatment for hypoglycaemia
Adverse events after oral dextrose gel
Separation from mother for treatment of hypoglycaemia
Exclusive breastfeeding after discharge
Two review authors (TE, GL) independently assessed the certainty of evidence for each of the outcomes. We considered evidence from RCTs as high certainty but downgraded the evidence by one level for serious (or two levels for very serious) limitations based on the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create Table 1 to report the certainty of evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Results
Description of studies
Results of the search
Database searches conducted in October 2021 identified 838 references; pre‐2021 searches identified 69 references; other search methods identified 33 records. After removal of duplicates (303), 637 records were available for screening. We excluded 619 records because they were irrelevant (see Figure 1). One of these records (NCT02523222), identified as an ongoing study in the previous version of this review, no longer met inclusion criteria because the design and intervention had changed, so we excluded it. We screened the full‐text of 18 records, excluded six studies (seven records) because they did not meet the eligibility criteria (see Excluded studies), identified two ongoing studies (CTRI/2017/11/010383; CTRI/2020/01/022678) (see Characteristics of ongoing studies), and found nine new reports of the two previously‐included studies.
1.
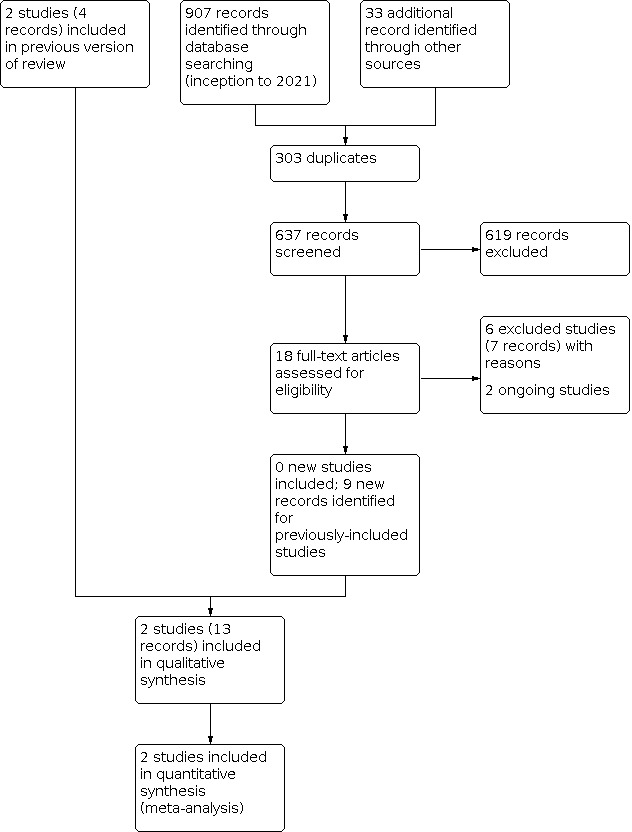
Study flow diagram: review update
We included two studies (13 records; four records from the previous review plus nine additional records) in this update (Harris 2013; Troughton 2000). These 13 records included three full‐text publications, one short report and nine conference abstracts. Both studies were included in the previous version of this review (Weston 2016), but the Harris 2013 study had new follow‐up data, and the authors provided us with additional data on some clinical outcomes in the neonatal period. One study was only reported in abstract format (Troughton 2000).
Included studies
We included two RCTs in this review (see Characteristics of included studies), with data from 312 participants.
The largest study (Harris 2013), enrolled 514 infants ≥ 35 weeks' gestation and recognised as being at risk for hypoglycaemia in the first 48 hours after birth. This study took place at a tertiary maternity hospital in New Zealand. Investigators randomised 242 infants who became hypoglycaemic; 118 infants to receive 40% oral dextrose gel 0.5 mL/kg, massaged into the buccal mucosa followed by a milk feed of maternal choice, and 119 infants to receive placebo gel 0.5 mL/kg with a milk feed of maternal choice (five additional infants were randomised in error). Researchers rechecked the blood glucose concentration after 30 minutes and repeated the treatment if the blood glucose concentration remained < 2.6 mmol/L. The majority of infants were not admitted to NICU. Of the 237 infants randomised, 184 children were followed up at two years' corrected age, and 185 children at 4.5 years' corrected age. Since this study reported outcomes for the same cohort at two time points, to avoid duplication we used only the 4.5‐year follow‐up data in this update because more of this review's secondary outcomes were reported at that age.
The earlier study (Troughton 2000), involved 75 hypoglycaemic infants on day one who were ≥ 36 weeks' gestation and admitted to NICU. In this single‐centre study from Northern Ireland, infants were randomised to receive 1 mL/kg of 40% oral dextrose gel massaged into the buccal mucosa plus a feed (n = 39), or a feed alone (n = 36). Blood glucose was measured at 15 and 30 minutes after treatment.
Excluded studies
We excluded six studies (seven records) for the following reasons:
One ongoing study of oral dextrose gel used to prevent (not treat) neonatal hypoglycaemia (PACTR201612001867999).
One ongoing treatment study of oral dextrose solution (not gel) (TCTR20181204005).
One study (two records) investigating the use of sucrose enriched expressed breast milk in treating neonatal hypoglycaemia (Bora 2019).
One study reporting on the galenic preparation of 40% dextrose gel (Rivano 2020).
One commentary of a randomised trial about sugar powder administered sublingually to treat neonatal hypoglycaemia (Barennes 2014).
One study summarising the neonatal hypoglycaemia literature (Halamek 1998).
Risk of bias in included studies
We summarised bias assessments in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Harris 2013 used computer‐generated blocked randomisation with variable block sizes. Allocation was concealed by a central randomisation system until data analysis was complete. We judged this study to be at a low risk of selection bias. The Troughton 2000 abstract provided insufficient details, so we judged the risk of selection bias as unclear.
Blinding
Harris 2013 reported that all clinicians, families and study investigators were masked to treatment allocation. The study investigators confirmed that the outcome assessors were masked to group allocation. We judged this trial to be at a low risk of performance and detection bias. The Troughton 2000 abstract provided insufficient details, so we judged the risk of performance and detection bias as unclear.
Incomplete outcome data
Harris 2013 performed an intention‐to‐treat analysis. For the neonatal study, primary outcome data were available for 234 of 237 (99%) infants (98% of 116 in the oral dextrose gel group and 99% of 118 in the placebo gel group). Investigators followed up 78% of the original cohort at 4.5 years' corrected age; 96 of 118 (81%) children randomised to the oral dextrose gel group and 89 of 119 (75%) children randomised to the placebo gel group. Maternal and infant characteristics were mostly similar in those assessed and not assessed at 4.5 years. Thus, we judged this study to be at a low risk of attrition bias.
Troughton 2000 reported findings for 26% of 36 control infants. Since 26% of 36 infants cannot be resolved as a whole number, we judged this study to be at a high risk of attrition bias.
Selective reporting
Harris 2013 reported data for all outcomes prespecified in the study registration documentation. We judged this study to be at a low risk of reporting bias. The Troughton 2000 abstract provided insufficient details, so we judged the risk of reporting bias as unclear.
Other potential sources of bias
Harris 2013 reported that baseline and demographic characteristics were balanced across arms except there was a higher proportion of boys randomised to the placebo gel group and slightly more mothers in the oral dextrose gel group intended to breastfeed. No other sources of bias were identified, so we judged this study to be at a low risk of other bias. The Troughton 2000 abstract provided insufficient details, so we judged the risk of other bias as unclear.
Effects of interventions
See: Table 1
Oral dextrose gel versus control
See Table 1.
Primary outcomes
Correction of hypoglycaemic events
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel probably increases correction of hypoglycaemic events after two doses of gel (rate ratio 1.08, 95% CI 0.98 to 1.20; RD 66 more per 1000, 95% CI 17 fewer to 166 more; 1 study, 237 infants; 328 events; moderate‐certainty evidence; Analysis 1.1). We downgraded the certainty of evidence to moderate for imprecision due to low event rates.
1.1. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 1: Correction of hypoglycaemia for each event of hypoglycaemia (investigator defined)
Major neurological disability at age two years or older
We received additional data from one study (Harris 2013) for outcomes at 4.5 years of age. Oral dextrose gel compared with placebo gel may result in a slight reduction in the risk of major neurological disability at 4.5 years corrected age, but the evidence is uncertain (RR 0.46, 95% CI 0.09 to 2.47; RD 24 fewer per 1000, 95% CI 41 fewer to 66 more; 1 study, 185 children; low‐certainty evidence; Analysis 1.2). We downgraded the evidence by two levels for very serious imprecision.
1.2. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 2: Major neurological disability at age two years or older
Secondary outcomes
Receipt of intravenous treatment for hypoglycaemia
The evidence is very uncertain about the effect of oral dextrose gel compared with placebo gel or no gel on the need for intravenous treatment for hypoglycaemia (RR 0.78, 95% CI 0.46 to 1.32; RD 37 fewer per 1000, 95% CI 91 fewer to 54 more; Chi2 = 3.61 (P = 0.06); I2 = 72%; 2 studies, 312 infants; very low‐certainty evidence; Analysis 1.3. We downgraded the evidence for very serious imprecision and inconsistency (the two studies provided estimates in opposite directions).
1.3. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 3: Receipt of intravenous treatment for hypoglycaemia (for each infant)
Requirements for any medications for hypoglycaemia such as glucagon or corticosteroids
No data were reported for this outcome.
Number of episodes of hypoglycaemia
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel probably results in little to no difference in the number of episodes of hypoglycaemia per infant within 48 hours after birth (MD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 237 infants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 4: Number of episodes of hypoglycaemia (for each infant)
Improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel
Additional data from the Harris 2013 study showed that a single dose of oral dextrose gel compared to placebo gel may improve blood glucose to ≥ 2.6 mmol/L (rate ratio 1.13, 95% CI 0.98 to 1.30; RD 93 more per 1000, 95% CI 14 fewer to 215 more; 1 study, 237 infants; 427 events; Analysis 1.5).
1.5. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 5: Improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel (by event)
Rebound hypoglycaemia (occurring within six hours of initial correction)
Additional data from the Harris 2013 study showed that oral dextrose gel may result in a slight increase in the rate of rebound hypoglycaemia after oral dextrose gel compared to placebo gel (rate ratio 1.18, 95% CI 0.67 to 2.07; RD 23 more per 1000, 95% CI 42 fewer to 135 more; 1 study, 237 infants; 363 events; Analysis 1.6). Caution is required when these results are interpreted because of the wide confidence intervals and the low event rates indicative of imprecision.
1.6. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 6: Rebound hypoglycaemia (by event)
Increase in blood glucose after treatment
Oral dextrose gel compared to placebo gel or no gel may result in a slight increase in the blood glucose concentration 30 to 90 minutes after treatment (MD 0.24 mmol/L, 95% CI 0.10 to 0.38; Chi2 = 0.35 (P = 0.55); I2 = 0%; 2 studies, 312 infants; 278 events; Analysis 1.7). We received additional data from one of the two studies (Harris 2013).
1.7. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 7: Increase in blood glucose 30 to 90 minutes after treatment (by event)
Duration of hypoglycaemia (time from detection of hypoglycaemia to blood glucose concentration above the threshold definition of 2.6 mmol/L)
Additional data from the Harris 2013 study showed that oral dextrose gel probably results in little to no difference in the duration of hypoglycaemia (MD ‐0.11 hours, 95% CI ‐0.44 to 0.22; 1 study, 237 infants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 8: Duration of hypoglycaemia (by event)
Adverse events
Only one study reported this outcome. Harris 2013 reported no adverse events (e.g. choking or vomiting at the time of administration) in the oral dextrose gel or placebo gel groups (1 study, 237 infants; low‐certainty evidence). We downgraded the evidence to low‐certainty for imprecision due to no events and the small sample size.
Separation from mother for treatment of hypoglycaemia
Dextrose gel compared to placebo gel probably reduces the incidence of separation of mother and infant for treatment of hypoglycaemia (RR 0.54, 95% CI 0.31 to 0.93; RD 116 fewer per 1000, 95% CI 174 fewer to 18 fewer; 1 study, 237 infants; moderate‐certainty evidence; Analysis 1.9). We downgraded this outcome for imprecision. The number needed to treat to prevent one such separation was 9 (95% CI 5 to 50). However, in this study, the overall incidence of separation for all reasons — not just hypoglycaemia — was not different between the two groups (RR 0.83, 95% CI 0.61 to 1.11).
1.9. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 9: Separation from mother for treatment of hypoglycaemia
Neonatal seizures
No seizures occurred in the oral dextrose gel or placebo group in the only study that reported this outcome (1 study, 237 infants).
Abnormal MRI of the brain in the neonatal period
No data were reported for this outcome.
Duration of initial hospital stay (days)
No data were reported for this outcome.
Breastfeeding (any) after discharge
No data were reported for this outcome.
Exclusive breastfeeding after discharge (WHO 2008 definition)
Oral dextrose gel compared with placebo gel probably increases the likelihood of exclusive breastfeeding at two weeks of age (RR 1.10, 95% CI 1.01 to 1.18; RD 87 more per 1000, 95% CI 9 to 157; 1 study, 237 infants; NNTB = 12, 95% CI 7 to 100; moderate‐certainty evidence; Analysis 1.10). We downgraded the certainty of evidence for imprecision.
1.10. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 10: Exclusive breast feeding after discharge (WHO definition)
Exclusive breastfeeding at six months of age (WHO 2008 definition)
No data were reported for this outcome.
Developmental disability at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel may result in little to no difference in the overall rate of developmental disability (including mild, moderate or severe disability) at 4.5 years corrected age (RR 0.96, 95% CI 0.66 to 1.39; 1 study, 183 children; Analysis 1.11).
1.11. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 11: Developmental disability at age two years or older
Visual impairment and severity at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel may result in little to no difference in the risk of vision problems at 4.5 years corrected age (RR 2.57, 95% CI 0.11 to 62.17; 1 study, 178 children; Analysis 1.12). Caution is required when these results are interpreted because of the wide confidence interval, the small sample size and the low event rates indicative of imprecision.
1.12. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 12: Visual impairment and severity at age two years or older
Hearing impairment and severity at age two years or older
Additional data from the Harris 2013 study showed that no children in either the oral dextrose gel or placebo gel groups had any hearing impairments at 4.5 years corrected age.
Cerebral palsy and severity at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel may result in little to no difference in the risk of cerebral palsy at 4.5 years corrected age (RR 2.77, 95% CI 0.11 to 67.05; 1 study, 173 children; Analysis 1.13). Caution is required when these results are interpreted because of the wide confidence interval and the low event rates indicative of imprecision.
1.13. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 13: Cerebral palsy and severity at age two years or older
Developmental delay/intellectual impairment and severity at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel may result in little to no difference in the risk of mild intellectual impairment (RR 1.05, 95% CI 0.53 to 2.07; 1 study, 183 children), but may result in a reduction in the risk of moderate or severe intellectual impairment (RR 0.23, 95% CI 0.03 to 1.99; RD 35 fewer per 1000, 95% CI 45 fewer to 46 more; one study, 183 children) at 4.5 corrected age (see Analysis 1.14 for both analyses). Caution is required when these results are interpreted because of the wide confidence interval and the low event rates indicative of imprecision.
1.14. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 14: Developmental delay/intellectual impairment and severity at age two years or older
Executive dysfunction and severity at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel compared to placebo gel may result in little to no difference in executive function composite scores (MD 1.40, 95% CI ‐0.30 to 3.10; 1 study, 181 children; Analysis 1.15) and the Behavior Rating Index of Executive Function for Preschool (BRIEF‐P) — Global Executive Composite scores (MD ‐0.90, 95% CI ‐4.20 to 2.40; 1 study, 179 children; Analysis 1.16) at 4.5 years corrected age.
1.15. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 15: Executive dysfunction and severity at age two years or older (Executive function composite score at 4.5‐year follow‐up)
1.16. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 16: Executive dysfunction and severity at age two years or older (BRIEF‐P Index ‐ Global Executive Composite at 4.5‐year follow‐up)
Behavioural problems and severity at age two years or older
Additional data from the Harris 2013 study showed that oral dextrose gel may result in little to no difference in scores on the Child Behaviour Checklist (CBCL) (MD ‐0.60, 95% CI ‐3.92 to 2.72; 1 study, 179 children) and the Strengths and Difficulties Questionnaire (SDQ) (MD ‐0.10, 95% CI ‐1.65 to 1.45; 1 study, 180 children) between oral dextrose gel and placebo gel groups at 4.5 years corrected age (see Analysis 1.17 for both scales).
1.17. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 17: Behavioural problems and severity at age two years or older
Abnormal MRI of the brain at age two years or older
No data were reported for this outcome.
We were unable to perform any sensitivity or subgroup analyses as there were insufficient studies.
Discussion
Summary of main results
Two studies comparing oral dextrose gel versus placebo or no gel for the treatment of neonatal hypoglycaemia in 312 late preterm and term at‐risk infants contributed data to this review. We judged one study to be at low risk of bias, and the other to be at unclear to high risk of bias. We graded the certainty of evidence as moderate to very low (Table 1).
Current evidence shows that oral dextrose gel compared with placebo gel probably increases correction of hypoglycaemic events and may result in a slight reduction in the risk of major neurological disability at 4.5 years corrected age, but the evidence is uncertain. The evidence is very uncertain about whether oral dextrose gel alters the need for receipt of intravenous treatment for hypoglycaemia. Oral dextrose gel compared to placebo gel probably reduces maternal‐infant separation and probably increases the likelihood of exclusive breastfeeding after discharge, with no adverse events reported.
Overall completeness and applicability of evidence
Since the first version of this review (Weston 2016), data have been made available for the co‐primary outcome of correction of hypoglycaemic events and for some secondary outcomes on the effects of oral dextrose gel on individual episodes of hypoglycaemia. One of the included studies has reported follow‐up data on neurodevelopmental and disability outcomes at 4.5 years (Harris 2013), including behavioural problems and hearing impairment, which were previously unavailable.
Data were still not available for some secondary outcomes including: receipt of any medications for hypoglycaemia; duration of initial hospital stay; any breastfeeding after discharge; exclusive breastfeeding at six months of age; and abnormal MRI of the brain in the neonatal period and at two years of age or older.
Nevertheless, the findings that oral dextrose gel probably corrects hypoglycaemic events and leads to higher blood glucose concentrations while reducing maternal‐infant separation and improving exclusive breastfeeding after discharge are important indicators of the utility of oral dextrose gel, especially in the absence of evidence of adverse events during the neonatal period. A cost analysis also reported that treating neonatal hypoglycaemia with oral dextrose gel was likely to result in greater cost savings than placebo gel (Glasgow 2018).
It remains uncertain how applicable these findings are to low‐ and middle‐income settings and to infants < 35 weeks' gestation. We identified no studies comparing oral dextrose gel with intravenous dextrose in these groups, but the findings of the two ongoing studies in India comparing 40% oral dextrose gel with intravenous dextrose in infants born < 35 weeks may help address these gaps (CTRI/2017/11/010383; CTRI/2020/01/022678). Nevertheless, the simplicity of this treatment suggests that oral dextrose gel may have wide applicability at various levels of care and in international settings, limited only by care providers' ability to measure blood glucose concentrations.
Quality of the evidence
We graded the certainty of evidence for correction of hypoglycaemic events as moderate because of imprecision due to the low event rates. We graded the certainty of evidence for major neurological disability at age two years or older as low. This is because the confidence interval included a possibility of both benefits or harms and the event rates were low, indicating that the analysis had inadequate precision. We graded the certainty of evidence for receipt of intravenous treatment as very low for evidence of very serious imprecision (due to low event rates and the confidence interval including possible benefits or harms) and inconsistency. The estimates of effect were in opposite directions, and the I2 value indicated substantial statistical heterogeneity (I2 = 72%, Chi2 P = 0.06). This may be due to limitations in the design of one included study (Troughton 2000), that showed high risk of attrition bias and unclear risk of bias for all other domains. However, we were unable to explore this by subgroup analysis because of insufficient studies. We decided not to downgrade this outcome for study limitations as we downgraded the evidence three levels for other quality issues (imprecision and indirectness) and because this study carried less weight (n = 75 infants) in the overall effect estimate compared with the Harris 2013 study (n = 237 infants). We graded the certainty of evidence for adverse events as low due to imprecision because there were no events and the small sample size of 237 infants did not meet the optimal information size criterion. We graded the certainty of evidence for separation from mother for treatment and exclusive breastfeeding after discharge as moderate due to possible imprecision from low event rates.
Potential biases in the review process
We could not assess reporting bias by visual inspection of a funnel plot because we did not identify 10 or more studies. Further, our search did not reveal all the publications known to the review authors, nor did it identify a key publication (Troughton 2000), included in this review.
Agreements and disagreements with other studies or reviews
No other reviews have examined the use of oral dextrose gel for treatment of neonatal hypoglycaemia. Some conclusions of this update reflect those of the previous version (Weston 2017), because no new studies were included. However, the new data for the co‐primary and additional secondary outcomes at later ages helps confirm previous conclusions that oral dextrose gel is probably an effective and safe treatment for neonatal hypoglycaemia.
The Cochrane Review 'Oral dextrose gel to prevent hypoglycaemia in at‐risk neonates' reported that oral dextrose gel used to prevent neonatal hypoglycaemia was effective in reducing the incidence of neonatal hypoglycaemia and receipt of treatment for hypoglycaemia during the initial hospital stay (Edwards 2021). Prophylactic oral dextrose gel was also likely to reduce the risk of major neurological disability at two years of age or older without increasing the risk of adverse events. These findings may reflect the differences between use of oral dextrose gel as prophylaxis, reducing hypoglycaemia and therefore possibly later disability, compared to use of oral dextrose gel as treatment which, in studies included in this review, was as an initial treatment followed by other treatments as required in children who were already hypoglycaemic.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence showed that treating late preterm and term infants with oral dextrose gel (specifically 40% dextrose concentration) probably increases correction of hypoglycaemic events, reduces maternal‐infant separation for hypoglycaemia, and supports exclusive breastfeeding after discharge, with no adverse events reported. Most available data came from a single small study in a high‐income setting.
Oral dextrose gel is a simple, low‐cost, and possibly effective treatment for initial treatment of infants with neonatal hypoglycaemia during the first 48 hours after birth.
Available evidence does not support extrapolation to other contexts, or to either extremely or moderately preterm infants.
Implications for research.
Data on some secondary outcomes of this review remain limited, and most available data come from a single small study. Future studies should use robust methods, report clinically relevant outcomes such as those indicated in this review, and ensure that enrolled infants are followed up with standardised tools to assess beneficial or adverse effects on later neurodevelopment.
The potential for improved neurodevelopmental outcomes following treatment is likely to be most pertinent to resource‐poor settings, where alternative treatments such as intravenous dextrose may be less available. Future studies should examine the use of oral dextrose gel in a variety of settings and patient groups. Two ongoing studies set in India may contribute towards addressing some of these gaps (CTRI/2017/11/010383; CTRI/2020/01/022678).
What's new
| Date | Event | Description |
|---|---|---|
| 5 October 2021 | New search has been performed | A more sensitive search strategy was developed for this update; databases were searched without date limits. Two new ongoing trials were identified. |
| 2 November 2020 | New citation required but conclusions have not changed | There has been a change in authorship. Data have been made available for the co‐primary outcome of correction of hypoglycaemic events and some secondary outcomes on the effects of oral dextrose gel on individual episodes of hypoglycaemia. Follow‐up data at four and a half years of age are now available. The certainty of evidence was regraded. |
History
Protocol first published: Issue 3, 2014 Review first published: Issue 5, 2016
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman and Jane Cracknell, former Managing Editors, Roger Soll, and Bill McGuire, Co‐ordinating Editors, who provided editorial and administrative support. Michele Fiander, Information Specialist, designed the updated literature searches, and Colleen Ovelman peer‐reviewed the Ovid MEDLINE search strategy.
We acknowledge Greg Gamble for his statistical input, Julie Brown who was a co‐author on the protocol (Weston 2014) and the first version of this Cochrane Review (Weston 2016), and Professor Caroline Crowther for advice and support during the preparation of the previous version of the review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, Imperial College London
Managing Editor (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Andrea Takeda, Copy Edit Support team
Peer‐reviewers (provided comments and recommended an editorial decision): Liz Bickerdike, Cochrane Evidence Production & Methods Directorate (methods review); Robin Featherstone, Cochrane Evidence Production & Methods Directorate (search review); Arpitha Chiruvolu, Baylor University Medical Center (clinical/content review); and Ndi Euphrasia Ebai‐Atuh, Cameroon Consumer Service Organisation (CamCoSO) Consumer Executive, Cochrane (consumer review).
One additional peer reviewer provided clinical/content peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. 2021 CENTRAL search strategy
CENTRAL via WileyOvid
Date ranges: inception to 6 October 2021 Terms:
| 1 | MESH DESCRIPTOR Hypoglycemia EXPLODE ALL AND CENTRAL:TARGET | 2335 |
| 2 | hypoglyc*:ti,ab,kw AND CENTRAL:TARGET | 12630 |
| 3 | (hyperinsulin* or hyper‐insulin* or (insulin NEAR/2 coma*) or nesidioblastos*):ti,ab,kw AND CENTRAL:TARGET | 3268 |
| 4 | ((low or concentration*) NEAR2 (blood sugar or blood glucose)):ti,ab,kw AND CENTRAL:TARGET | 1799 |
| 5 | #1 OR #2 OR #3 OR #4 | 17072 |
| 6 | ((buccal* or mouth* or oral* or subling* or sub‐ling*) NEAR2 (glucose or dextrose)):ti,ab,kw AND CENTRAL:TARGET | 4881 |
| 7 | (glucogel or glucagon or dextrogel or dex4 pr dex‐4 or Glutose or Glutose or Hypostop or hypo‐stop or Insta‐Glucose):ti,ab,kw AND CENTRAL:TARGET | 5682 |
| 8 | #6 OR #7 | 10122 |
| 9 | MESH DESCRIPTOR Administration, Buccal EXPLODE ALL AND CENTRAL:TARGET | 199 |
| 10 | MESH DESCRIPTOR Administration, Sublingual EXPLODE ALL AND CENTRAL:TARGET | 938 |
| 11 | MESH DESCRIPTOR Administration, Oral EXPLODE ALL AND CENTRAL:TARGET | 24780 |
| 12 | MESH DESCRIPTOR Gels EXPLODE ALL AND CENTRAL:TARGET | 2816 |
| 13 | (gel or gels or jelly or jellies or sublingual* or buccal* or oral*):ti,ab,kw AND CENTRAL:TARGET | 195039 |
| 14 | #9 OR #10 OR #11 OR #12 OR #13 | 200620 |
| 15 | MESH DESCRIPTOR Glucose EXPLODE ALL AND CENTRAL:TARGET | 19382 |
| 16 | MESH DESCRIPTOR Sweetening Agents EXPLODE ALL AND CENTRAL:TARGET | 6596 |
| 17 | (glucose or dextrose):ti,ab,kw OR ((sweetening NEAR2 (agent? or artificial)) or sweetener?):ti,ab,kw AND CENTRAL:TARGET | 61002 |
| 18 | #15 OR #16 OR #17 | 67556 |
| 19 | #8 OR (#14 AND #18) | 18332 |
| 20 | #5 AND #19 | 4381 |
| 21 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 17017 |
| 22 | MESH DESCRIPTOR Intensive Care, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 352 |
| 23 | MESH DESCRIPTOR Intensive Care Units, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 836 |
| 24 | (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU):ti,ab,kw AND CENTRAL:TARGET | 71423 |
| 25 | #21 OR #22 OR #23 OR #24 | 74481 |
| 26 | #20 AND #25 | 205 |
Appendix 2. 2021 MEDLINE search strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) 1946 to 5 October 2021 | ||
| # | Searches | Results |
| 1 | hypoglycemia/ | 28544 |
| 2 | congenital hyperinsulinism/ or nesidioblastosis/ or insulin coma/ [Subtypes of hypoglycemia per MeSH] | 1374 |
| 3 | hypoglyc*.ti,ab,kw,kf. | 62008 |
| 4 | (hyperinsulin* or hyper‐insulin* or (insulin adj2 coma?) or nesidioblastos*).ti,ab,kw,kf. | 26632 |
| 5 | ((low or concentration?) adj2 (blood sugar or blood glucose)).ti,ab,kw,kf. | 7638 |
| 6 | or/1‐5 [Hypoglycemia] | 97174 |
| 7 | ((buccal* or mouth? or oral* or subling* or sub‐ling*) adj2 (glucose or dextrose)).ti,ab,kw,kf. | 24028 |
| 8 | ((gel or gels or jelly or jellies) adj2 (dextrose or sucrose)).ti,ab,kw,kf. | 176 |
| 9 | (glucogel or glucagon or dextrogel or dex4 pr dex‐4 or Glutose or Glutose or Hypostop or hypo‐stop or Insta‐Glucose).ti,ab,kw,kf. | 40946 |
| 10 | or/7‐9 [Oral Glucose: Intervention Set 1 ] | 63377 |
| 11 | exp Glucose/ | 315682 |
| 12 | (glucose or dextrose).ti,ab,kw,kf. | 516697 |
| 13 | exp Sweetening Agents/ | 238326 |
| 14 | ((sweetening adj2 (agent? or artificial)) or sweetener?).ti,ab,kw,kf. | 4855 |
| 15 | or/11‐14 [Glucose or sweetening agents] | 682087 |
| 16 | administration, oral/ or administration, buccal/ or administration, sublingual/ | 152719 |
| 17 | Gels/ | 30078 |
| 18 | (gel or gels or jelly or jellies or sublingual* or buccal* or oral*).ti,ab,kw,kf. | 1073378 |
| 19 | or/16‐18 [Oral administration or gels] | 1121981 |
| 20 | 15 and 19 [Glucose & Oral admin/Gels: Intervention Set 2] | 64105 |
| 21 | exp infant, newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ | 637891 |
| 22 | (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 945039 |
| 23 | or/21‐22 [Filter: Neonatal Population 2021‐‐MEDLINE] | 1223385 |
| 24 | randomized controlled trial.pt. | 545668 |
| 25 | controlled clinical trial.pt. | 94445 |
| 26 | (randomized or randomised).ti,ab. | 692623 |
| 27 | placebo.ab. | 222027 |
| 28 | drug therapy.fs. | 2382579 |
| 29 | randomly.ab. | 367234 |
| 30 | trial.ab. | 570881 |
| 31 | groups.ab. | 2255489 |
| 32 | (quasirandom* or quasi‐random*).ti,ab. | 5229 |
| 33 | exp animals/ not humans/ | 4894687 |
| 34 | (or/24‐32) not 33 [RCT Filter‐Based on Cochrane‐ Box 6.4.c: Cochrane Highly Sensitive Search Strategy] | 4499260 |
| 35 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ [/ finds same as.pt. syntax] | 241705 |
| 36 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 242647 |
| 37 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 31974 |
| 38 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 32343 |
| 39 | (hand search* or handsearch*).ti,ab,kf,kw. | 10072 |
| 40 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 30155 |
| 41 | meta‐analysis as topic/ or network meta‐analysis/ | 23098 |
| 42 | (met analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 11847 |
| 43 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 263937 |
| 44 | (cochrane or systematic review?).jw. | 18768 |
| 45 | or/35‐44 [SR filter‐Medline; based on CADTHhttps://www.cadth.ca/strings‐attached‐cadths‐database‐search‐filters] | 473955 |
| 46 | 6 and (or/10,20) and 23 and 34 [Hypoglycemia & Dextrose‐Oral or Gel & Neonate & RCT] | 385 |
| 47 | 6 and (or/10,20) and 23 and 45 [Hypoglycemia & Dextrose‐Oral or Gel & Neonate & SR ] | 34 |
| 48 | or/46‐47 [All results Medline] | 390 |
Appendix 3. 2021 Embase search strategy
| Embase 1974 to 5 October 2021 | ||
| # | Searches | Results |
| 1 | exp hypoglycemia/ | 85206 |
| 2 | hypoglyc*.ti,ab,kw,kf. | 91893 |
| 3 | (hyperinsulin* or hyper‐insulin* or (insulin adj2 coma?) or nesidioblastos*).ti,ab,kw,kf. | 35528 |
| 4 | ((low or concentration?) adj2 (blood sugar or blood glucose)).ti,ab,kw,kf. | 10023 |
| 5 | or/1‐4 [Hypoglycemia] | 155684 |
| 6 | ((buccal* or mouth? or oral* or subling* or sub‐ling*) adj2 (glucose or dextrose)).ti,ab,kw,kf. | 34343 |
| 7 | ((gel or gels or jelly or jellies) adj2 (dextrose or sucrose)).ti,ab,kw,kf. | 225 |
| 8 | (glucogel or glucagon or dextrogel or dex4 pr dex‐4 or Glutose or Glutose or Hypostop or hypo‐stop or Insta‐Glucose).ti,ab,kw,kf. | 51959 |
| 9 | or/6‐8 [Oral Glucose: Interventon Set 1] | 83957 |
| 10 | exp Glucose/ | 435040 |
| 11 | (glucose or dextrose).ti,ab,kw,kf. | 673553 |
| 12 | exp sweetening agent/ | 85814 |
| 13 | ((sweetening adj2 (agent? or artificial)) or sweetener?).ti,ab,kw,kf. | 6201 |
| 14 | or/10‐13 [Glucose] | 842537 |
| 15 | oral drug administration/ or sublingual drug administration/ or buccal drug administration/ | 394846 |
| 16 | Gel/ | 32602 |
| 17 | (gel or gels or jelly or jellies or sublingual* or buccal* or oral*).ti,ab,kw,kf. | 1383281 |
| 18 | or/15‐17 [Oral administration OR Gels] | 1662424 |
| 19 | 14 and 18 [Glucose & Oral admin or Gel: Intervention Set 2] | 93924 |
| 20 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ | 629768 |
| 21 | (infant or infants or infant? or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU).ti,ab,kw. | 1097057 |
| 22 | or/20‐21 [Filter: Neonatal Population 2021‐OVID EMBASE] | 1312331 |
| 23 | Randomized controlled trial/ or Controlled clinical study/ | 866963 |
| 24 | random$.ti,ab,kw. | 1716790 |
| 25 | Randomization/ | 91931 |
| 26 | placebo.ti,ab,kw. | 330641 |
| 27 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 249007 |
| 28 | double blind procedure/ | 188384 |
| 29 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 389391 |
| 30 | parallel group$1.ti,ab. | 28187 |
| 31 | (crossover or cross over).ti,ab. | 112909 |
| 32 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 364054 |
| 33 | (open adj label).ti,ab. | 91422 |
| 34 | or/23‐33 [ Terms based on Cochrane Central strategy‐https://www‐cochranelibrary‐com.ezproxy.uvm.edu/central/central‐creation] | 2464723 |
| 35 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 22819913 |
| 36 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 29577189 |
| 37 | 36 not 35 [Animal Exclusion‐Anne Eisinga, Cochrane UK] | 6757276 |
| 38 | 34 not 37 [Filter: RCT‐EMBASE] | 2200904 |
| 39 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ [EMTREE] | 462376 |
| 40 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 296985 |
| 41 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 45198 |
| 42 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 39785 |
| 43 | (hand search* or handsearch*).ti,ab,kw. | 12277 |
| 44 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 39887 |
| 45 | (met analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 15224 |
| 46 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 334283 |
| 47 | (cochrane or systematic review?).jn,jx. | 29818 |
| 48 | (overview adj2 reviews).ti. | 96 |
| 49 | or/39‐48 [SR Filter: EMBASE based on CADTH filter: https://www‐cadth‐ca.ezproxy.uvm.edu/strings‐attached‐cadths‐database‐search‐filters] | 690062 |
| 50 | 5 and (or/9,19) and 22 [Hypoglycemia & oral Dextrose & Neonate‐‐results before filters] | 1515 |
| 51 | 5 and (or/9,19) and 22 and 38 [RCT Results: Hypoglycemia & oral Dextrose & Neonate] | 217 |
| 52 | 5 and (or/9,19) and 22 and 49 [SR Results: Hypoglycemia & oral Dextrose & Neonate] | 60 |
| 53 | or/51‐52 [All results] | 243 |
Appendix 4. 2021 US National Library of Medicine (ClinicalTrials.gov)
Date ranges 2017 to 2021 Terms:
Condition or disease: Neonatal Hypoglycemia
Intervention/treatment: "oral dextrose gel" OR "oral glucose gel" OR "oral sweetening gel"
Study type: Interventional Studies (Clinical Trials)
Age group: Child (birth‐17)
Appendix 5. 2021 WHO ICTRP search strategy
Date ranges 2017 to 2021 Terms:
Neonatal hypogly* AND oral dextrose gel OR oral glucose gel OR oral sweetening gel
Appendix 6. 2021 ISRCTN search strategy
Date ranges 2017 to 2021 Terms: "oral dextrose gel" AND ( Participant age range: Neonate ) "oral glucose gel" AND ( Participant age range: Neonate ) "oral sweetening agent" AND ( Participant age range: Neonate )
Appendix 7. Previous search methods
We were assisted in a search of the Cochrane Neonatal Review Group Specialised Register. We undertook a search of MEDLINE, Embase, the Central Register of Controlled Trials (CENTRAL), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Web of Science from inception of the database to 29 February 2016. We undertook a search of registries of clinical trials for any evidence of work in progress, or prior work planned, for which no results were published. We handsearched proceedings of relevant scientific meetings ‐ American Academy of Pediatrics (2000 to 2014), European Society for Pediatric Research (2006 to 2015), Perinatal Society of Australia and New Zealand (2002 to 2015). We applied no language restrictions.
We used the following keywords in our search: hypoglycaemia OR hypogly$, AND neonate OR neonat$, AND dextrose gel. We used "*" as a wild card character when appropriate. We ensured that we searched both American and English spellings.
We permitted the newborn period to refer to the time infants were admitted at or soon after birth and remained in their neonatal admission until first discharge home. We limited our search to potentially eligible randomised clinical trials by using a maximally sensitive method filter.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov, World Health Organization International Trials Registry and Platform www.whoint/ictrp/search/en/ and the ISRCTN Registry).
MEDLINE: Hypogly* AND dextrose gel AND neonat*
Date 14 June 2014
Search Results: 1 ‐ 2 of 2
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
CINAHL: Hypogly* AND dextrose gel AND neonat*
Date 14 June 2014
Search Results: 1 ‐ 2 of 2
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
CINAHL: Hypogly* AND dextrose gel AND infant, newborn
Date 14 June 2014
Search Results: 1 ‐ 2 of 2
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
CENTRAL database
Date 14 June 2014
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
EMBASE
Date 14 June 2014
Badulek 2014; Harris 2013; Mosalli 2014
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Web of Science
14 June 2014
Anon 2014; Harris 2011; Harris 2013; Mosalli 2014
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
EU Clinical Trials Register
Date 14 June 2014
No trials
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
WHO International Clinical Trials Registry Platform
Date 14 June 2015
ACTRN12613000322730 Hypoglycaemia prevention in newborns with Oral dextrose: the dosage trial.
ANTRN12308000623392 The Sugar Babies Study
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Australia and New Zealand Clinical Trials Network
14 June 2014
no studies
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
CLINICAL TRIALS, 14 June 2014
no studies
Data and analyses
Comparison 1. Dextrose gel versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Correction of hypoglycaemia for each event of hypoglycaemia (investigator defined) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Major neurological disability at age two years or older | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Receipt of intravenous treatment for hypoglycaemia (for each infant) | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
| 1.4 Number of episodes of hypoglycaemia (for each infant) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel (by event) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Rebound hypoglycaemia (by event) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Increase in blood glucose 30 to 90 minutes after treatment (by event) | 2 | 312 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.10, 0.38] |
| 1.8 Duration of hypoglycaemia (by event) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 Separation from mother for treatment of hypoglycaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Exclusive breast feeding after discharge (WHO definition) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.11 Developmental disability at age two years or older | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Visual impairment and severity at age two years or older | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.13 Cerebral palsy and severity at age two years or older | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.14 Developmental delay/intellectual impairment and severity at age two years or older | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.14.1 Mild developmental delay/intellectual impairment at 4.5 year follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.14.2 Moderate or severe developmental delay/intellectual impairment at 4.5 year follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.15 Executive dysfunction and severity at age two years or older (Executive function composite score at 4.5‐year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16 Executive dysfunction and severity at age two years or older (BRIEF‐P Index ‐ Global Executive Composite at 4.5‐year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17 Behavioural problems and severity at age two years or older | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.1 CBCL scores at 4.5 year follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.2 SDQ scores at 4.5 year follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Harris 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 514 infants ≥ 35 weeks' gestation, < 48 postnatal hours old and at risk for hypoglycaemia. Risk factors included mother with diabetes, small (birthweight < 10th centile, or < 2500 grams) or large (birthweight > 90th centile or > 4500 grams) size, preterm (35 or 36 weeks' gestation) birth and other reasons such as poor feeding. Of these, 237 became hypoglycaemic and were randomised (5 infants randomised in error). The majority of infants were not admitted to NICU. Setting: New Zealand Timing Trial: 1 December 2008 to 31 November 2010 Follow‐up at two years: 21 July 2010 to 30 January 2013 Follow‐up at 4.5 years: September 2011 to June 2015. |
|
| Interventions | Infants who became hypoglycaemic (< 2.6 mmol/L) were encouraged to feed (determined by maternal choice) and were randomised to receive 40% oral dextrose gel (0.5 mL/kg) (n = 118) or placebo gel (n = 119) massaged into the buccal membrane. Blood glucose concentration was measured 30 minutes following gel treatment. Gel was repeated if hypoglycaemia persisted. A maximum of six doses of gel could be given within a 48‐hour period. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
*Data received from study authors. Blood glucose measured using glucose oxidase method. Funding sources included Waikato Medical Research Foundation, Auckland Medical Research Foundation, the Maurice and Phyllis Paykel Trust, the Health Research Council of New Zealand and the Rebecca Roberts Scholarship. Disclosure: 3 of the authors of this review (PJW, DLH and JE Harding) were involved in the design and conduct of this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated blocked randomisation, with variable block sizes. |
| Allocation concealment (selection bias) | Low risk | Central allocation by computer which provided a randomisation number corresponding to a numbered treatment pack. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dextrose and placebo gels were identical in appearance. Clinicians, families and study investigators were masked to treatment allocation until completion of data analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Performed an intention‐to‐treat analysis. Five infants were randomised in error and were excluded from the analysis, leaving 118 infants in the oral dextrose gel group and 119 infants in the placebo gel group. Of the 237 randomised, 78% were followed up and assessed at 4.5 years' corrected age (96/118 oral dextrose gel group and 89/119 in the placebo gel group). Children assessed at 4.5 years were more likely to be exposed to alcohol and smoking during pregnancy, be of Māori ethnicity, have a lower minimum interstitial blood glucose concentration and be admitted to NICU compared with children not assessed. Those assessed were also less likely to be a singleton and of an ethnicity other than Māori or New Zealand European. All other maternal and infant characteristics were similar in those who were and were not assessed at 4.5 years. |
| Selective reporting (reporting bias) | Low risk | The only outcome not listed a priori was acceptability of the intervention. Otherwise, all prespecified outcomes were reported. |
| Other bias | Low risk | A slightly greater number of mothers in the group allocated to oral dextrose gel than to placebo gel intended to breastfeed (114 of 115 vs 109 of 115). Fewer boys were allocated to the oral dextrose gel group than to the placebo gel group (48 of 118 vs 65 of 119). Groups were otherwise balanced. |
Troughton 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 75 hypoglycaemic (< 2.5 mmol/L) infants ≥ 36 weeks' gestation admitted to the newborn intensive care unit. Setting: Ireland Timing: not reported |
|
| Interventions | Hypostop (40% dextrose, 1 mL/kg) massaged into the buccal membrane plus a feed (n = 39) vs Feeding alone (n = 36) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Blood glucose analysed using the Hemocue point‐of‐care analyser. Funding sources not stated and trial registration not found. Study only available as an abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is some evidence of possible attrition bias because 26% of 36 infants in the control arm does not make up a whole number. |
| Selective reporting (reporting bias) | Unclear risk | Neither the protocol nor trial registration were available to assess for reporting bias. No information available regarding baseline characteristics, risk factors for hypoglycaemia or differential diagnoses. |
| Other bias | Unclear risk | Baseline characteristics not reported. |
ABC: Assessment Battery for Children; BRIEF‐P: Behaviour Rating Inventory of Executive Function, Preschool Version; CBCL: Child Behaviour Checklist; IQ: intelligence quotient; NICU: neonatal intensive care unit; SD: standard deviation; SDQ: Strengths and Difficulties Questionnaire; vs: versus; WPPSI‐3: Wechsler Preschool and Primary Scale of Intelligence, Third Edition
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barennes 2014 | Ineligible intervention: powered sugar administered by sublingual route. |
| Bora 2019 | Ineligible intervention: sucrose enriched breast milk. |
| Halamek 1998 | Ineligible type of study: review article. |
| PACTR201612001867999 | Ineligible objective: prevention not treatment trial. |
| Rivano 2020 | Ineligible type of study: letter to an editor on the galenic preparation of 40% dextrose gel. |
| TCTR20181204005 | Ineligible intervention: oral glucose solution not gel. |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/11/010383.
| Study name | Dextrose gel in the management of asymptomatic hypoglycaemia in at‐risk neonates: randomised controlled trial |
| Methods | Open‐label randomised controlled trial |
| Participants | 284 asymptomatic term and late preterm infants (≥ 34 weeks' gestation), small or large for dates and infants of diabetic mothers. Setting: India |
| Interventions | 40% oral dextrose gel (0.5 ml/kg) versus Intravenous fluids (as per NICU protocol) |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | 1 December 2017 (not yet recruiting and the expected duration was 12 months) |
| Contact information | gkirti51@gmail.com |
| Notes | Trial registration: CTRI/2017/11/010383 |
CTRI/2020/01/022678.
| Study name | Effectiveness of oral glucose gel in neonatal hypoglycaemia |
| Methods | Randomised controlled trial |
| Participants | 250 clinically stable asymptomatic infants, ≥ 32 weeks' gestation, blood glucose concentration of < 2.5 mmol/L (45 mg/dl) and birthweight > 1.5 kg. Setting: India |
| Interventions | 40% oral glucose gel (0.5 ml/kg) versus 10% intravenous dextrose (2ml/kg) |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | 12 January 2020 (estimated trial duration: 14 months) |
| Contact information | sskdr1@gmail.com |
| Notes | Trial registration: CTRI/2020/01/022678 |
NICU: neonatal intensive care unit
Differences between protocol and review
Differences between protocol and Weston 2016 version of the review
We added methods, the plan for summary of findings tables and GRADE recommendations, which were not included in the original protocol (Weston 2014).
-
The secondary outcomes listed below have been added since the protocol because these they are either associated with neonatal hypoglycaemia (Shah 2019) or considered important in assessing the safety of oral dextrose gel for the treatment of neonatal hypoglycaemia:
adverse events (e.g. choking or vomiting at time of administration) (yes/no) (infant outcome);
duration of initial hospital stay (days) (infant outcome);
visual impairment and severity at age two years or older (child outcome);
hearing impairment and severity at age two years or older (child outcome);
cerebral palsy and severity at age two years or older (child outcome);
developmental delay/intellectual impairment and severity at age two years or older (child outcome);
executive dysfunction and severity at age two years or older (child outcome);
behavioural problems and severity at age two years or older (child outcome);
abnormal MRI of the brain at age two years or older (child outcome).
Changes between the earlier version and the 2021 update
We developed a new search strategy, which we ran without date limits (Appendix 1). We did not search Web of Science because this database is not a mandatory MECIR source, and we considered it unlikely to retrieve unique records after searching the Cochrane Central Register of Controlled Trials (CENTRAL), OVID MEDLINE, OVID Embase and clinical trial registries. We did not search the Cumulative Index to Nursing and Allied Health Literature (CINAHL) because these records are added to CENTRAL via a robust process (see How CENTRAL is created).
We changed the term 'adverse effects' to 'adverse events'.
We changed the definition of the primary outcome 'correction of hypoglycaemia (investigator defined) for each event of hypoglycaemia' to 'correction of hypoglycaemia for each event of hypoglycaemia (investigator defined)' to clarify that this outcome includes any definition of correction and any definition of hypoglycaemia used by the investigators.
We changed the secondary outcome of 'improved blood glucose to greater than 2.6 mmol/L' to 'improved blood glucose to ≥ 2.6 mmol/L after a single dose of gel' to clarify that this outcome relates to a single dose of gel, and because < 2.6 mmol/L is used as a threshold for treatment of hypoglycaemia by most international guidelines (CPSFNC 2004; New Zealand Clinical Practice Guidelines 2015; Queensland Clinical Guidelines 2021; Swedish National Guideline 2020).
Contributions of authors
2021 update
TE:
Screened studies for eligibility, extracted data, and performed risk of bias and GRADE assessments.
Prepared the first draft and revised subsequent drafts.
Approved the final version.
GL:
Screened studies for eligibility, extracted data, and performed risk of bias and GRADE assessments.
Contributed to subsequent drafts and approved the final version.
PJW:
Contributed to subsequent drafts and approved the final version of this update.
Authored the first version of this Cochrane Review.
DLH:
Contributed to subsequent drafts and approved the final version of this update.
Authored the first version of this Cochrane Review.
MB:
Contributed to subsequent drafts and approved the final version of this update.
Authored the first version of this Cochrane Review.
JE Hegarty:
Contributed to subsequent drafts and approved the final version of this update.
Authored the first version of this Cochrane Review.
JE Harding:
Provided major editorial assistance in the preparation and revision of this updated review.
Approved the final version of this update.
Authored the first version of this Cochrane Review.
Sources of support
Internal sources
-
Liggins Institute, University of Auckland, New Zealand
PhD Scholarship for Taygen Edwards
-
Waikato District Health Board, New Zealand
Clinical salaries for Phil Weston and Deborah Harris
-
Auckland District Health Board, New Zealand
Clinical salaries for Malcolm Battin and Jo Hegarty
-
Liggins Institute, University of Auckland, New Zealand
University appointment for Jane Harding
-
Aotearoa Foundation, New Zealand
Undergraduate Clinical Research Internship for Gordon Liu
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, USA
Partial support for additional analysis of the included data
Declarations of interest
TE: has no interests to declare, independently extracted data from the Harris 2013 and Troughton 2000 studies, and checked against the study report and any available study registration details or protocol.
GL: has no interests to declare, independently extracted data from the Harris 2013 and Troughton 2000 studies, and checked against the study report and any available study registration details or protocol.
PJW: contributed to the design and conduct of one of the included studies (Harris 2013).
DLH: contributed to the design and conduct of one of the included studies (Harris 2013) and was a member of the Steering group for the two follow‐up studies (Harris 2016; Harris 2019).
MB: has no interests to declare.
JE Hegarty: has no interests to declare
JE Harding: contributed to the design and conduct of one of the included studies (Harris 2013), and designed and led the two follow‐up studies (Harris 2016; Harris 2019). The funding source for these studies were: Waikato Medical Research Foundation, the Auckland Medical Research Foundation, the Maurice and Phyllis Paykel Trust, the Health Research Council of New Zealand, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Rebecca Roberts Scholarship.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Harris 2013 {published and unpublished data}
- Harris D, Alsweiler J, Ansell J, Gamble G, Thompson B, Wouldes T, et al, Children with Hypoglycaemia and their Later Development (CHYLD) Study Team.Outcome at 2 years after dextrose gel treatment for neonatal hypoglycaemia: follow-up of a randomized trial. Journal of Pediatrics 2016;170:54-9. [DOI: 10.1016/j.jpeds.2015.10.066] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DL, Ansell JM, Yu TY, Thompson B, Wouldes TA, Harding JE.Early childhood outcomes after treatment with dextrose gel for neonatal hypoglycaemia: follow-up of the sugar babies study. Journal of Paediatrics and Child Health 2015;51(Suppl 1):68. [DOI: 10.1111/jpc.12884_4] [DOI] [Google Scholar]
- Harris DL, Gamble GD, Weston PJ, Harding JE.What happens to blood glucose concentrations after oral treatment for neonatal hypoglycemia? Journal of Pediatrics 2017 ;190:136-41. [DOI: 10.1016/j.jpeds.2017.06.034] [DOI] [PubMed] [Google Scholar]
- Harris DL, McKinlay C, Gamble G, Harding JE.Dextrose gel is a safe treatment for neonatal hypoglycaemia: follow up at 4.5 years of the sugar babies randomised trial. Journal of Paediatrics and Child Health 2019;55(Suppl 1):24. [DOI: 10.1111/jpc.14409_60] [DOI] [Google Scholar]
- Harris DL, Weston PJ, Alsweiler JM, Thompson B, Wouldes T, Chase G, et al.Two year outcomes of children treated with dextrose gel for neonatal hypoglycaemia: follow up of a randomised trial. Archives of Disease in Childhood 2014;99(Suppl 2):A64-5. [DOI: 10.1136/archdischild-2014-307384.171] [DOI] [Google Scholar]
- Harris DL, Weston PJ, Battin MR, Harding JE.Randomized trial of dextrose gel for treating neonatal hypoglycemia: the Sugar Babies Study. Pediatric Research 2011;70:652. [DOI: 10.1038/pr.2011.877] [DOI] [Google Scholar]
- Harris DL, Weston PJ, Battin MR, Harding JE.The sugar babies study: a randomised controlled trial of dextrose gel for treatment of neonatal hypoglycaemia. Journal of Paediatrics and Child Health April 2011;47(Suppl 1):51. [DOI: 10.1111/j.1440-1754.2011.02046.x] [DOI] [Google Scholar]
- Harris DL, Weston PJ, Harding JE.A good breast-feed does not always result in an increased in blood glucose concentration, in hypoglycaemic babies. Journal of Paediatric and Child Health 2016;52(Suppl 2):105. [DOI: 10.1111/jpc.13194_4] [DOI] [Google Scholar]
- Harris DL, Weston PJ, Signal M, Chase JG, Harding JE.Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet 2013;382(9910):2077-83. [DOI: 10.1016/S0140-6736(13)61645-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Weston P, Harris D, Harding J.Dextrose gel and formula but not breast milk are effective treatments for neonatal hypoglycaemia. Journal of Paediatrics and Child Health 2017;53(Suppl 2):112. [DOI: 10.1111/jpc.13494_330] [DOI] [Google Scholar]
- Weston PJ, Harris DL, Harding JE.Dextrose gel treatment does not impair subsequent feeding. Archives of Disease in Childhood - Fetal and Neonatal Edition 2017;102:F539-41. [DOI: 10.1136/archdischild-2017-312772] [DOI] [PubMed] [Google Scholar]
- Weston PJ, Harris DL, Harding JE.Dextrose gel treatment for neonatal hypoglycaemia does not impair subsequent feeding. Journal of Paediatric and Child Health 2016;52(Suppl 2):106. [DOI: 10.1111/jpc.13194] [DOI] [Google Scholar]
Troughton 2000 {published data only}
- Troughton KE, Corrigan NP, Tait RM.Hypostop gel in the treatment of neonatal hypoglycaemia: a randomised controlled trial. Archives of Disease in Childhood 2000;82(Suppl 1):A30. [Google Scholar]
References to studies excluded from this review
Barennes 2014 {published data only}
- Barennes H, Willcox ML, Gra B, Pussard E.Sublingual sugar for infant hypoglycaemia. Lancet 2014;383(9924):1208. [DOI] [PubMed] [Google Scholar]
Bora 2019 {published data only}
- Bora R, Deori S.Efficacy of oral sugar in management of neonatal hypoglycaemia in small for date neonates is comparable to intravenous sugar in resource poor setting. In: ESPGHAN 52nd Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; 2019 June 5-8; Glasgow. Glasgow, Scotland, 2019:1144. [N-P-100]
- Bora R, Deori S.Transitional hypoglycaemia management in small for gestational age neonates with sucrose enriched expressed breastmilk in resource poor setting. Journal of Tropical Pediatrics 2020;66(3):267–74. [DOI: 10.1093/tropej/fmz064] [DOI] [PubMed] [Google Scholar]
Halamek 1998 {published data only}
- Halamek LP, Stevenson DK.Neonatal hypoglycemia, part II: pathophysiology and therapy. Clinical Pediatrics 1998;37(1):11-6. [DOI: 10.1177/000992289803700102] [DOI] [PubMed] [Google Scholar]
PACTR201612001867999 {published data only}
- PACTR201612001867999.The use of oral 10% dextrose for low birth weight newborns in resource poor setting for glycemic control- a randomized control trial. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201612001867999 (First received 13 November 2016).
Rivano 2020 {published data only}
- Rivano M, Albrecht M, Longobardo G, Veneziano C.Galenic preparation of 40% dextrose gel: a new approach to the management of neonatal hypoglycemia. Clinical Medical Insights: Endocrinol Diabetes 2020;13:1-2. [DOI: 10.1177/1179551420928326] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20181204005 {published data only}
- TCTR20181204005.Effectiveness of oral glucose solution for the treatment of neonatal hypoglycemia, a randomized, placebo-controlled trial . trialsearch.who.int/?TrialID=TCTR20181204005 (first received 4 December 2018).
References to ongoing studies
CTRI/2017/11/010383 {unpublished data only}
- CTRI/2017/11/010383.To study the utility of oral dextrose gel in reducing NICU admission of babies, in the first 2 days of life who develop low sugars and are given intravenous dextrose in the NICU. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=19902&EncHid=&modid=&compid=%27,%2719902det%27 (first received 6 November 2017).
CTRI/2020/01/022678 {unpublished data only}
- CTRI/2020/01/022678.A study of comparision of effectiveness of oral glucose gel and IV dextrose in treating hypoglycemia in neonates. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=35296&EncHid=&modid=&compid=%27,%2735296det%27 (first received 9 January 2020).
Additional references
Academy of Breastfeeding Medicine 2021
- Wight NE, Stehel E, Noble L, Bartick M, Calhoun S, Kair L, et al.ABM clinical protocol #1: guidelines for glucose monitoring and treatment of hypoglycemia in term and late preterm neonates, revised 2021. Breastfeeding Medicine 2021;16(5):353-65. [DOI: 10.1089/bfm.2021.29178.new] [PMID: ] [DOI] [PubMed] [Google Scholar]
Adamkin 2011
- Adamkin DH, Committee on Fetus and Newborn.Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127(3):575-9. [DOI: 10.1542/peds.2010-3851] [PMID: ] [DOI] [PubMed] [Google Scholar]
Agrawal 2000
- Agrawal RK, Lui K, Gupta JM.Neonatal hypoglycaemia in infants of diabetic mothers. Journal of Paediatrics and Child Health 2000;36(4):354-6. [DOI: 10.1046/j.1440-1754.2000.00512.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alsweiler 2019
- Alsweiler JM, Woodall SM, Crowther CA, Harding JE.Oral dextrose gel to treat neonatal hypoglycaemia: clinician survey. Journal of Paediatrics and Child Health 2019;55:844-50. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anderson 1993
- Anderson S, Shakya KN, Shrestha LN, Costello AM.Hypoglycaemia: a common problem among uncomplicated newborn infants in Nepal. Journal of Tropical Pediatrics 1993;39(5):273-7. [DOI: 10.1093/tropej/39.5.273] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anon 2013
- Anon.Dextrose gel raises neonatal low sugar. Nursing Times 2013;109(40):4. [Google Scholar]
Anon 2014
- Anon.Dextrose gel is a first-line option for newborns at risk from hypoglycaemia. Nursing Standard 2014;28(22):16-7. [Google Scholar]
Badulek 2014
- Badulek A.Dextrose gel for neonatal hypoglycaemia (the Sugar Babies study): a randomised double blind placebo-controlled trial. Journal of Emergency Medicine 2014;46(5):784. [DOI] [PubMed] [Google Scholar]
Beardsell 2010
- Beardsell K.Measurement of glucose levels in the newborn. Early Human Development 2010;86(5):263-7. [DOI: 10.1016/j.earlhumdev.2010.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al.National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162-72. [DOI: 10.1016/S0140-6736(12)60820-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blomquist 1994
- Blomquist HK, Jonsbo F, Serenius F, Persson LA.Supplementary feeding in the maternity ward shortens the duration of breast feeding. Acta Paediatrica Scandinavica 1994;83(11):1122-6. [DOI: 10.1111/j.1651-2227.1994.tb18263.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boutron 2019
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A.Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
British Association of Perinatal Medicine 2017
Burns 2008
- Burns CM, Rutherford MA, Boardman JP, Cowan FM.Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122(1):65-74. [DOI: 10.1542/peds.2007-2822] [PMID: ] [DOI] [PubMed] [Google Scholar]
CADTH 2021
- CADTH.Strings attached: CADTH database search filters. Available from: www.cadth.ca/strings-attached-cadths-database-search-filters (accessed 6 October 2021).
Canadian Paediatric Society
- Canadian Paediatric Society.Screening guidelines for newborns at risk for low blood glucose. Paediatric Child Health 2004;9(10):723-9. [DOI: 10.1093/pch/9.10.723] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornblath 2000
- Cornblath MD, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, et al.Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics 2000;105(5):1141-5. [DOI: 10.1542/peds.105.5.1141] [PMID: ] [DOI] [PubMed] [Google Scholar]
CPSFNC 2004
- Canadian Pediatric Society Fetal and Newborn Committee.Screening guidelines for newborns at risk for low blood glucose. Paediatrics & Child Health 2004;9(10):723-40. [DOI: 10.1093/ pch/9.10.723] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demir 2020
- Demir F, Ghosh P, Liu Z.Effects of motherhood timing, breastmilk substitutes and education on the duration of breastfeeding: evidence from Egypt. World Development 2020;133(105014):1-14. [DOI: 10.1016/j.worlddev.2020.105014] [DOI] [Google Scholar]
Doherty 2006
- Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP.Pre-pregnancy body mass index and pregnancy outcomes. International Journal of Gynaecology and Obstetrics 2006;95(3):242-7. [DOI: 10.1016/j.ijgo.2006.06.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwards 2021
- Edwards T, Liu G, Hegarty JE, Crowther CA, Alsweiler J, Harding JE.Oral dextrose gel to prevent hypoglycaemia in at-risk neonates. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD012152. [DOI: 10.1002/14651858.CD012152.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2018
- Glasgow MJ, Harding JE, Edlin R, Children with Hypoglycemia and Their Later Development (CHYLD) Study Team.Cost analysis of treating neonatal hypoglycemia with dextrose gel. Journal of Pediatrics 2018;198:151-5. [DOI: 10.1016/j.jpeds.2018.02.036] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2021
- Glasgow MJ, Edlin R, Harding JE.Cost burden and net monetary benefit loss of neonatal hypoglycaemia. BMC Health Services Research 2021;21(121):1-13. [DOI: 10.1186/s12913-021-06098-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 15 November 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Graz 2008
- Graz B, Dicko M, Willcox ML, Lambert B, Falquet J, Forster M, et al.Sublingual sugar for hypoglycaemia in children with severe malaria: a clinical pilot study. Malaria Journal 2008;7:242. [DOI: 10.1186/1475-2875-7-242] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2011
- Harris DL, Weston PJ, Harding JE.Dextrose gel and infant formula are more effective than breast milk for reversing neonatal hypoglycaemia. In: Pediatric Research. Vol. 70 (Suppl 5S). November 2011:651.
Harris 2014
- Harris DL, Weston PJ, Battin MR, Harding JE.A survey of the management of neonatal hypoglycaemia within the Australian and New Zealand Neonatal Network. Journal of Paediatric and Child Health 2014;50(10):E55-62. [DOI: 10.1111/j.1440-1754.2009.01599.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2016
- Harris D, Alsweiler J, Ansell J, Gamble G, Thompson B, Wouldes T, et al, Children with Hypoglycaemia and their LaterDevelopment (CHYLD) Study Team.Outcome at 2 years after dextrose gel treatment for neonatal hypoglycaemia: follow-up of a randomized trial. Journal of Pediatrics 2016;170:54-9.e1-2. [DOI: 10.1016/j.jpeds.2015.10.066]] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2019
- Harris DL, McKinlay C, Gamble G, Harding JE.Dextrose gel is a safe treatment for neonatal hypoglycaemia: follow up at 4.5 years of the Sugar Babies randomised trial. Journal of Paediatrics and Child Health 2019;55(Suppl 1):24. [Google Scholar]
Hawdon 1993
- Hawdon JM, Aynsley-Green A, Ward Platt MP.Neonatal blood glucose concentrations: metabolic effects of intravenous glucagon and intragastric medium chain triglyceride. Archives of Diseases in Childhood 1993;68(3 Spec No):255-61. [DOI: 10.1136/adc.68.3_spec_no.255] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2009
- Hay WW Jr, Raju TN, Higgins RD, Kalhan SC, Devaskar SU.Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. Journal of Pediatrics 2009;155(5):612-7. [DOI: 10.1016/j.jpeds.2009.06.044] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Holtrop 1993
- Holtrop PC.The frequency of hypoglycemia in full-term large and small for gestational age newborns. American Journal of Perinatology 1993;10(2):150-4. [DOI: 10.1055/s-2007-994649] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hume 1999
- Hume R, McGeechan A, Burchell A.Failure to detect infants at risk of hypoglycemia before discharge. Journal of Pediatrics 1999;134(4):499-502. [DOI: 10.1016/s0022-3476(99)70210-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaiser 2015
- Kaiser JR, Bai S, Gibson N, Holland G, Lin TM, Swearingen CJ, et al.Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Pediatrics 2015;169(10):913-21. [DOI: 10.1001/jamapediatrics.2015.1631] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kerstjens 2012
- Kerstjens JM, Bocca-Tjeertes IF, Winter AF, Reijneveld SA, Bos AF.Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012;130(2):e265-72. [DOI: 10.1542/peds.2012-0079] [PMID: ] [DOI] [PubMed] [Google Scholar]
Koh 1988
- Koh TH, Aynsley-Green A, Tarbit M, Eyre JA.Neural dysfunction during hypoglycaemia. Archives of Disease in Childhood 1988;63(11):1353-8. [DOI: 10.1136/adc.63.11.1353] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulski 1981
- Kulski JK, Smith M, Hartmann PE.Normal and caesarian section delivery and the initiation of lactation in women. Australian Journal of Experimental Biology and Medical Science 1981;59(4):405-12. [DOI: 10.1038/icb.1981.34] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
Le Huerou‐Luron 2010
- Le Huerou-Luron I, Blat S, Boudry G.Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutrition Research Reviews 2010;23(1):23-36. [DOI: 10.1017/S0954422410000065] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lilien 1980
- Lilien LD, Pildes RS, Srinivasan G, Voora S, Yeh TF.Treatment of neonatal hypoglycemia with minibolus and intravenous glucose infusion. Journal of Pediatrics 1980;97(2):295-8. [DOI: 10.1016/s0022-3476(80)80499-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lubchenco 1971
- Lubchenco L, Bard H.Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics 1971;47(5):831-8. [PMID: ] [PubMed] [Google Scholar]
Lucas 1988
- Lucas A, Morley R, Cole TJ.Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988;297(6659):1304-8. [DOI: 10.1136/bmj.297.6659.1304] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maayan‐Metzger 2009
- Maayan-Metzger A, Lubin D, Kuint J.Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology 2009;96(2):80-5. [DOI: 10.1159/000203337] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGowan 2006
- McGowan JE, Price-Douglas W, Hay WW Jr.Glucose homeostasis. In: Merenstein GB, Gardner SL, editors(s). Handbook of Neonatal Intensive Care. 6th edition. St Louis: Mosby Elsevier, 2006:368-90. [Google Scholar]
McKinlay 2015
- McKinlay CJ, Alsweiler JM, Ansell JM, Anstice NS, Chase GJ, Gamble GD, et al: Children With Hypoglycemia and Their Later Development (CHYLD) Study Team.Neonatal glycemia and neurodevelopmental outcomes at 2 years. New England Journal of Medicine 2015;373(16):1507-18. [DOI: 10.1056/NEJMoa1504909] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKinlay 2017
- McKinlay CJ, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase J, et al, Children With Hypoglycemia and Their Later Development (CHYLD) Study Team.Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatrics 2017;171(10):972-83. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosalli 2014
- Mosalli R.Dextrose gel is superior to feeding alone in neonatal hypoglycaemia. Journal of Clinical Neonatology 2014;3(1):10-11. [DOI: 10.4103/2249-4847.128718] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02523222
- NCT02523222.Prophylactic dextrose gel for newborns at high risk for hypoglycemia. clinicaltrials.gov/show/NCT02523222 (accessed 2 November 2020).
New Zealand Clinical Practice Guidelines 2015
- Oral Dextrose Gel to Treat Neonatal Hypoglycaemia Clinical Practice Guideline Panel.Oral Dextrose Gel to Treat Neonatal Hypoglycaemia: New Zealand Clinical Practice Guidelines; 2015. Available at: www.fmhs.auckland.ac.nz/assets/fmhs/som/paed/docs/Oral_dextrose%20gel_%20guideline2.pdf.
Osier 2003
- Osier FH, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton CR.Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Archives of Disease in Childhood 2003;88:621-5. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Queensland Clinical Guidelines 2021
- Queensland Clinical Guidelines.Hypoglycaemia - newborn. www.health.qld.gov.au/qcg/publications#neonatal (accessed 24 May 2021) .
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan).Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rozance 2019
- Rozance PJ, Wolfsdorf JI.Hypoglycemia in the newborn. Pediatric Clinics of North America 2019;66(2):333-42. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saint 1984
- Saint L, Smith M, Hartmann PE.The yield and nutrient content of colostrum and milk of women from giving birth to 1 month post-partum. British Journal of Nutrition 1984;52(1):87-95. [DOI: 10.1079/bjn19840074] [PMID: ] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al.GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shah 2019
- Shah R, Harding J, Brown J, McKinlay C.Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology 2019;115(2):116-26. [DOI: 10.1159/000492859] [DOI] [PubMed] [Google Scholar]
Smith 2016
- Smith H, Becker G.Early additional food and fluids for healthy breastfed full‐term infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD006462. [DOI: 10.1002/14651858.CD006462.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swedish National Guideline 2020
- Wackernagel D, Gustafsson A, Edstedt Bonamy AK, Reims A, Ahlsson F, Elfving M, et al.Swedish national guideline for prevention and treatment of neonatal hypoglycaemia in newborn infants with gestational age ≥ 35 weeks. Acta Paediatrica 2020;109(1):31-44. [DOI: 10.1111/apa.14955] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thornton 2015
- Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al, Pediatric Endocrine Society.Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. Journal of Pediatrics 2015;167(2):238-45. [DOI: 10.1016/j.jpeds.2015.03.057] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tin 2012
- Tin W, Brunskill G, Kelly T, Fritz S.15-year follow-up of recurrent "hypoglycemia" in preterm infants. Pediatrics 2012;130(6):e1497-503. [DOI: 10.1542/peds.2012-0776] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weston 2017
- Weston PJ, Harris D, Harding JE.Dextrose gel and formula but not breast milk are effective treatments for neonatal hypoglycaemia. Journal of Paediatrics and Child Health 2017;53(S2):112. [DOI: ] [Google Scholar]
WHO 2008
- World Health Organization.Indicators for assessing infant and young child feeding practices - part I: definitions; 2008. Available from: whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf?ua=1.
Wild 2004
- Wild S, Roglic G, Green A, Sicree R, King H.Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047-53. [DOI: 10.2337/diacare.27.5.1047] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Weston 2014
- Weston PJ, Harris D, Battin M, Brown J, Hegarty J, Harding JE.Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD011027. [DOI: 10.1002/14651858.CD011027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weston 2016
- Weston PJ, Harris DL, Battin M, Brown J, Hegarty JE, Harding JE.Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011027. [DOI: 10.1002/14651858.CD011027.pub2] [DOI] [PubMed] [Google Scholar]


