Abstract
Purpose:
Joubert syndrome (JS) is a ciliopathy caused by mutations in more than 34 genes that encode proteins involved with primary (nonmotile) cilia and the cilium basal body. In addition to neurodevelopmental, kidney, and liver manifestations, JS involves ocular abnormalities that vary widely in severity. To further the understanding and treatment of ocular manifestations of JS, we present the ophthalmic phenotype of 99 patients with JS examined at a single center.
Design:
Patients were systematically and prospectively examined at the National Institutes of Health (NIH) Clinical Center in the setting of a dedicated natural history clinical trial.
Participants:
Methods:
All patients underwent genotyping for JS, followed by complete age-appropriate ophthalmic examinations at the NIH Clinical Center, including visual acuity (VA), fixation behavior, lid position, motility assessment, slit-lamp biomicroscopy, dilated fundus examination with an indirect ophthalmoscope, and retinoscopy. Color and fundus autofluorescence imaging, Optos wide-field photography (Dunfermline, Scotland, UK), and electroretinography (ERG) were performed when possible.
Main Outcome Measures:
The VA (with longitudinal follow-up where possible), ptosis, extraocular muscle function, retinal and optic nerve status, and retinal function as measured by ERG.
Results:
Among patients with JS with quantifiable VA (68/99), values ranged from 0 logarithm of the minimum angle of resolution (logMAR) (Snellen 20/20) to 1.5 logMAR (Snellen 20/632). Strabismus (71/98), nystagmus (66/99), oculomotor apraxia (60/77), ptosis (30/98), coloboma (28/99), retinal degeneration (20/83), and optic nerve atrophy (8/86) were identified.
Conclusions:
We recommend regular monitoring for ophthalmological manifestations of JS beginning soon after birth or diagnosis. We demonstrate delayed visual development and note that the amblyogenic time frame may last significantly longer in JS than is typical. In general, patients with coloboma were less likely to display retinal degeneration, and those with retinal degeneration did not have coloboma. Severe retinal degeneration that is early and aggressive is seen in disease caused by specific genes, such as CEP290- and AHIl-associated JS. Retinal degeneration in INPP5E-, MKS1-, and NPHPl-associated JS was generally milder. Finally, ptosis surgery can be helpful in a subset of patients with JS; decisions as to timing and benefit/risk ratio need to be made on an individual basis according to expert consultation.
Supplemental material available at www.aaojournal.org.
Joubert syndrome (JS) (Online Mendelian Inheritance in Man #213300) is a ciliopathy caused by mutations in more than 34 genes that encode proteins involved with primary (nonmotile) cilia and the cilium basal body.1,2 The critical role of primary cilia in mediating sensory and other signaling both during embryonic development and in postnatal physiology has been increasingly recognized.3 This increased recognition is partly driven by the discovery of new genes and disease processes associated with JS and other ciliopathies, including Meckel-Gruber and Bardet-Biedl syndromes, polycystic kidney diseases, and nephronophthisis. Although the causative gene remains unidentified in a subset of patients with JS, that percentage has decreased substantially in the last 10 years.2,4 Prevalence estimates of JS range from 1 in 80 000 to 1 in 100 000.2,4–6
Joubert syndrome was first reported in 1969 as a familial syndrome characterized by episodic hyperpnea, abnormal eye movements, ataxia, and developmental and intellectual disability.7 Cerebellar vermis agenesis was noted in 1 of those originally reported patients, and the “molar tooth sign” on axial magnetic resonance imaging (MRI) at the junction of the midbrain and hindbrain (the visual result of abnormally deep interpeduncular fossa in combination with elongated, thick, and maloriented superior cerebellar peduncles) and absent or hypoplastic cerebellar vermis have become recognized as pathognomonic for JS.8–10 In addition, some individuals with JS develop fibrocystic disease of the kidneys or liver.11 Ocular abnormalities occur frequently and include jerky eye movements, nystagmus, and strabismus,7 congenital retinal dystrophy,12–14 oculomotor apraxia (OMA),15,16 coloboma,17,18 and ptosis and extraocular muscle limitation.19 Subtypes of JS historically have had disparate naming nosologies based on symptomology; in 2010, Brancati et al20 suggested that subgroups of JS should be based on the main organ(s) involvement. The resulting categories are pure JS, JS with ocular defect, JS with renal defect, JS with oculorenal defects, JS with hepatic defect (or colobomas, “oligophrenia” for cognitive impairment, ataxia, cerebellar vermis hypoplasia, and hepatic fibrosis [COACH] syndrome), and JS with orofaciodigital defects.20 The clinical heterogeneity in JS has led to the term “Joubert syndrome and related disorders,” which includes Senior-Løken and COACH syndromes.8 More recently, JS is recommended to refer to all patients with the “molar tooth sign,” including patients with or without extraneurologic system involvement.1 In this article, for simplicity, we will use JS to include Senior-Løken and COACH syndromes.
Ocular involvement in JS varies from mild to severe, often depending on genetic cause; sometimes, variability can be noted even within the same genotype. Ocular involvement can be developmental (e.g., coloboma) or degenerative (e.g., retinal dystrophy). This variability makes it difficult to predict the functional visual trajectory for individual patients. In this study, we present the ophthalmic phenotypes of 99 patients with JS who have been systematically and prospectively examined in the setting of a dedicated natural history study. We present the results of comprehensive ophthalmic examinations performed prospectively at the National Institutes of Health (NIH) Clinical Center and pertinent data we extracted from past ophthalmic evaluations.
Methods
Patients
All patients were prospectively evaluated at the NIH Clinical Center, under the National Human Genome Research Institute intramural research protocol, “Clinical and Molecular Investigations into Ciliopathies” (www.clinicaltrials.gov, trial NCT00068224). The research adhered to the tenets of the Declaration of Helsinki and was approved by the National Human Genome Research Institute Institutional Review Board. For patient recruitment, the study was advertised to patients and families by the Joubert Syndrome & Related Disorders Foundation (https://jsrdf.org/) as a natural history study aiming to describe the individual organ system involvement in JS, including ophthalmologic, kidney, and liver disease. Patients or their parents gave written, informed consent including consent for use of patient photographs. The enrollment criterion was clinical diagnosis of JS, including Senior-Løken and COACH syndromes. All patients were evaluated at the NIH by a single pediatrician-clinical geneticist (M.G.-A.); evaluations included medical history, family history, physical examination, review of past medical records, review of brain MRI scans, abdominal ultrasonography and MRI, formal neurocognitive evaluations, electroencephalogram, and comprehensive blood and urine chemistries. All patients had complete age-appropriate ophthalmic examinations between 2007 and 2015, performed by 1 of 2 ophthalmologists (B.P.B. or W.M.Z.). Blood samples for DNA were collected from all patients and parents, when available.
Molecular Inversion Probes
The coding exons of 27 genes associated with JS (AHI1, ARL13B, B9D1, B9D2, C2CD3, C5orf42, CC2D2A, CEP290, CEP41, CSPP1, IFT172, INPP5E, KIF7, MKS1, NPHP1, OFD1, RPGRIP1L, TCTN1, TCTN2, TCTN3, TMEM138, TMEM216, TMEM231, TMEM237, TMEM67, TTC12B, and ZNF423) were sequenced by combining a molecular inversion probe capture method and next-generation sequencing. For this approach,21 performed at the University of Washington, we used 100 ng of genomic DNA, and the captured DNA was amplified by polymerase chain reaction and sequenced on an Illumina HiSeq or MiSeq platform. Sequence reads were mapped using the Burrows-Wheeler Aligner (V.0.5.9). Variants were called using the Genome Analysis Toolkit (V.2.5–2) and annotated with SeattleSeq (http://snp.gs.washington.edu/SeattleSeqAnnotation138/).
Whole Exome Sequencing
Genomic DNA was obtained from leukocytes using standard protocols. Whole exome sequencing was performed on all 99 patients, including those whose genetic cause was identified by the molecular inversion probe panel. Vilboux et al22 describe the complete methods. For exome sequencing, we used the HiSeq2000 (Illumina, San Diego, CA)23 that used 101 base pair paired-end read sequencing. Image analysis and base calling were performed using Illumina Genome Analyzer Pipeline software (versions 1.13.48.0) with default parameters. Reads were aligned to a human reference sequence (UCSC assembly hg19, NCBI build 37) using the Efficient Large-scale Alignment of Nucleotide Databases package (Illumina, San Diego, CA). Genotypes were called at all positions where there were high-quality sequence bases using a Bayesian algorithm called the Most Probable Genotype,24 and variants were filtered using the graphical software tool VarSifter v1.5.25 Whole exome sequences were evaluated only for variants in known or novel ciliopathy-related genes. The molecular genetic diagnosis of JS was provided to families after confirmation of these findings by a Clinical Laboratory Improvement Amendments-approved molecular genetics laboratory.
Genomic DNA and Complementary DNA Sequencing
Genomic DNA was obtained from leukocytes using standard protocols. For dideoxy sequencing of genomic DNA, primers were designed to cover areas of variants identified by whole-exome sequencing. Direct sequencing of the polymerase chain reaction amplification products was carried out using BigDye 3.1 Terminator chemistry (Applied Biosystems, Austin, TX) and separated on an ABI 3130×1 genetic analyzer (Applied Biosystems). Data were evaluated using Sequencher v5.0 software (Gene Codes Corporation, Ann Arbor, MI).
Ophthalmic Examinations
Ninety-nine patients with a diagnosis of JS who had an ophthalmic examination between 2007 and 2015 at the NIH Clinical Center are included in this report. In addition, historical ophthalmic records obtained from patients’ local ophthalmologists from 1981 to present were systematically reviewed for specific parameters, and the data were extracted. The ophthalmic examinations at NIH included an assessment of VA (Snellen VA and Snellen best-corrected visual acuity [BCVA]) when possible. Fixation behavior (fix and follow [FF]; central, steady, and maintained; or central, unsteady, and maintained) and reaction to light (blink to light [BTL] or no BTL) were documented when a measurement of acuity was not possible. Snellen equivalent VA was recorded for children or nonverbal patients whose vision was tested with Teller Acuity Cards. The evaluation also documented the status of lid position, extraocular muscle function/strabismus, pursuit and saccades, slit-lamp biomicroscopy (often with a portable slit-lamp), dilated fundus examination with an indirect ophthalmoscope, and retinoscopy. Severity of ptosis was determined by measurement of marginal reflex distance (MRD).
When indicated in patients who were able to cooperate with the testing, we obtained color and fundus autofluorescence imaging, Optos wide-field photography (Dunfermline, Scotland, UK), and electroretinography (ERG) conducted according to the International Society for Clinical Electrophysiology of Vision standards.26 Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA) spectral domain OCT scans were performed by certified technicians. In addition, we obtained prior medical records on 93 patients and extracted data from prior ophthalmic evaluations including ERG, visual acuity (VA), and surgeries for ptosis and strabismus.
Clinical Classifications
Patients were classified as having kidney disease, liver disease, and polydactyly based on clinical and laboratory evaluations performed at the NIH Clinical Center; genotype-phenotype correlations and details of kidney and liver disease of this cohort have been published.22 Liver disease was defined as elevation of liver enzymes or increased echogenicity of the liver or splenomegaly on abdominal ultrasonography.27 Kidney disease was defined as elevated serum creatinine and cystatin-C or abnormal findings on renal ultrasonography, including loss of corticomedullary distinction, increased or decreased kidney size, and presence of cystic changes.28
Retinal degeneration was diagnosed based on findings of dilated fundus examination with an indirect ophthalmoscope at the NIH. When available, ERG findings, fundus autofluorescence results, historical records, and loss of the photoreceptor inner segment/outer segment junction in the macular area and at the edge of any macular lesions (as visible on HD-OCT images) were also used in the determination.
Results
Patient Cohort
Phone interviews were performed with 120 patients with JS from 105 families. Fifteen families could not travel to the NIH Clinical Center in Bethesda, Maryland. The remaining 105 patients with JS from 90 families all underwent week-long clinical evaluations at the NIH Clinical Center. Based on our review of brain MRI scans,29 8 patients from 6 families did not have the “molar tooth sign,” including 3 patients with classic Senior-Løken syndrome (Table S1, available at www.aaojournal.org, patients 290, 462, and 463). We included these 3 patients with Senior-Løken syndrome in this article because of their clear phenotype, but we excluded the remaining 5 patients without molar tooth sign whose clinical features were nonspecific. For 1 patient with JS, an ophthalmology consultation could not be performed at the NIH because of scheduling conflicts. Therefore, ophthalmological findings of 99 patients with JS are presented. Twelve families contributed more than 1 affected individual, for a total of 86 families.
Main clinical features, including polydactyly and kidney and liver disease, of the 99 patients with JS are presented by genotype in Table 1, along with OMA, nystagmus, strabismus, and ptosis; detailed ophthalmologic and molecular genetic findings of individual patients are listed in Table S1 (available at www.aaojournal.org). Eleven families had 2 siblings affected by JS (families 6, 8, 18, 24, 29, 36, 65, 68 and 81; Table S1, available at www.aaojournal.org), and 2 families had 3 siblings affected by JS (families 16 and 66; Table S1, available at www.aaojournal.org). There were 43 female and 56 male patients. Ages ranged from 0.6 to 36 years (median of 7 years, Table 1). Twenty-seven percent of patients did not have any other organ system abnormalities outside of the oculo-cerebral system.
Table 1.
Genotype, Age, Systemic, and Ocular Characteristics of the NIH Joubert Patient Cohort
| Demographics and Systemic Manifestations | Patient Visits and Ophthalmic Manifestations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Female/Male | Polydactyly | Kidney Disease | Liver Disease | History of Surgery | History of Surgery | |||||||||||
| Median (Min to Max) | No. non-NIH visits | Max | Oculomotor Apraxia | Nystagmus | Strabismus | Ptosis | |||||||||||
| TMEM67 | 22 | 8/14 | 9 (0.9 to 36) | 0 | 11 | 21 | 24 | 95 | 110.0 | 6.6 | 30 | 11/15, 73% | 13/22, 59% | 19/22, 87% | 8 | 8/22, 36% | 1 |
| C5orf42 | 15 | 8/7 | 10 (0.6 to 27) | 5 | 0 | 6 | 16 | 29 | 39.8 | 2.6 | 17 | 9/14, 64% | 9/15, 60% | 13/15, 87% | 6 | 5/15, 33% | 2 |
| CC2D2A | 10 | 7/3 | 3 (1.5 to 16) | 1 | 2 | 3 | 11 | 27 | 36.3 | 2.8 | 11 | 9/10, 90% | 7/10, 70% | 4/10, 40% | 2 | 2/10, 20% | 0 |
| CEP290 | 7 | 2/5 | 6 (0.5 to 23) | 0 | 6 | 1 | 7 | 22 | 35.1 | 3.9 | 23 | 1/1 | 7/7, 100% | 5/7, 72% | 1 | 1/7, 14% | 0 |
| AHI1 | 6 | 2/4 | 5 (1 to 20) | 0 | 3 | 2 | 6 | 40 | 41.4 | 4 | 18 | 3/4 | 6/6, 100% | 6/6, 100% | 1 | 0/6, 0% | 0 |
| KIAA0586 | 6 | 1/5 | 4 (2.1 to 13) | 0 | 0 | 0 | 8 | 17 | 25.1 | 1.8 | 12.8 | 4/6, 67% | 1/6, 17% | 3/6, 50% | 1 | 0/6, 0% | 0 |
| MKS1 | 5 | 0/5 | 2 (1.4 to 14) | 2 | 0 | 1 | 5 | 25 | 23.3 | 1.1 | 14 | 5/5, 100% | 5/5, 100% | 3/5, 60% | 0 | 2/5, 40% | 0 |
| INPP5E | 4 | 2/2 | 17 (3 to 20) | 0 | 2 | 1 | 6 | 4 | 18.1 | 1.8 | 13 | 3/4 | 4/4 | 2/4 | 0 | 1/4 | 0 |
| TMEM231 | 3 | 3/0 | 4 (1 to 4) | 0 | 0 | 1 | 3 | na | 1/3 | 3/3 | 3/3 | 0 | 0/3 | 0 | |||
| NPHP1 | 3 | 1/2 | 15 (3 to 18) | 0 | 3 | 0 | 3 | 2 | 8.1 | 4 | 4.1 | 1/3 | 0/3 | 0/3 | 0 | 0/3 | 0 |
| TMEM216 | 2 | 1/1 | 10 (8.8 to 11) | 2 | 1 | 1 | 2 | 17 | 17.7 | 2/2 | 2/2 | 2/2 | 1 | 1/2 | 0 | ||
| OFD1 | 2 | 0/2 | 8 (4 to 12) | 0 | 0 | 1 | 2 | 6 | 12.7 | 0/2 | 1/2 | 1/2 | 1 | 1/2 | 0 | ||
| CSPP1 | 2 | 2/0 | 3 (2 to 3.5) | 0 | 0 | 0 | 2 | 7 | 5.6 | 2.8 | 3.4 | 1/2 | 1/2 | 2/2 | 0 | 2/2 | 0 |
| KIF7 | 1 | 0/1 | 2 | 0 | 0 | 0 | 1 | NA | 0/1 | 0/1 | 0/1 | 0 | 0/1 | 0 | |||
| B9D1 | 1 | 0/1 | 5 | 0 | 0 | 0 | 1 | NA | 1/1 | 1/1 | 0/1 | 0 | 0/1 | 0 | |||
| CEP164 | 1 | 1/0 | 8 | 1 | 1 | 1 | 1 | NA | 1/1 | 1/1 | nr | 0 | 0/1 | 0 | |||
| RPGRIP1L | 1 | 0/1 | 21 | 1 | 1 | 0 | 1 | 2 | 7.3 | 1/1 | 0/1 | 1/1 | 0 | 1/1 | 0 | ||
| TMEM237 | 1 | 1/0 | 5 | 0 | 1 | 0 | 1 | 6 | 3.0 | 1/1 | 1/1 | 1/1 | 0 | 1/1 | 1 | ||
| KIAA0753 | 2 | 1/1 | 4.5 (2 to 7) | 0 | 0 | 0 | 2 | 5 | 1.6 | 2/2 | 0/2 | 1/2 | 1 | 0/2 | 0 | ||
| CELSR2 | 1 | 1/0 | 8.0 | 0 | 0 | 1 | 1 | 12 | 7.5 | 0/1 | 0/1 | 1/1 | 1 | 1/1 | 1 | ||
| Unknown | 4 | 2/2 | 2 (1.5 to 4) | 1 | 0 | 2 | 4 | 13 | 5.9 | 1.4 | 1.9 | 4/4 | 4/4, | 4/4 | 1 | 3/4 | 1 |
| All Patient Totals | 99 | 43/56 | 7 (0.5 to 36) | 13, 13% | 31, 31% | 41, 41% | 107 | 334 | 398.6 | 3.3 | 30 | 60/77, 78% | 66/99, 67% | 71/98, 72% | 24 | 28/98 29% | 6 |
NA = not available.
Since not all patients were able to be assessed for all parameters, the denominator in this column equals the number of patients able to be assessed for that parameter rather than the total number of study participants.
Molecular Genetic Findings
We identified the molecular genetic cause in 95 of 99 patients; in 4 patients, whole exome sequencing did not identify any pathologic variants. Detailed molecular genetic findings of this cohort have been published.22 Although potentially pathogenic variants were identified in 20 different JS genes, two thirds of the families harbored variants in 1 of 6 genes, for example, TMEM67(22%), C5orf42 (15%) and CC2D2A (10%), CEP290 (7%), AHI1 (6%), and KIAA0586 (6%). Among the 81 families with identified gene variants, 2 had hemizygous variants in the X-linked OFD1. At least 2 genetic variants were identified in all but 3 of the families with autosomal recessively inherited JS genes; only 1 variant was identified in 2 families with TMEM67 and in 1 family with C5orf42 (Table S1, available at www.aaojournal.org).
Ophthalmological Findings
Ophthalmological features of the patients in the cohort included both cohort-wide and genotype-specific traits. Examination findings are categorized based on the main ophthalmic features. Their prevalence and severity in the cohort are reported, and examples from representative patients are presented. Genotype-phenotype correlations were drawn only for genotypes represented in the cohort by 3 or more patients (Table 1).
Visual Acuity
Figure 1A shows mean best eye BCVA at the most recent visit for each genotype in the 68 patients whose vision was quantifiable on the logarithm of the minimum angle of resolution (logMAR) scale. In the remaining 31 patients (Fig 1B), 16% showed FF, 5% showed brief FF or central unsteady maintained vision, 6% had no FF or BTL, and 2% showed no BTL or no light perception. Further details for each patient within the genotypes are presented in Table S1 (available at www.aaojournal.org).
Figure 1.
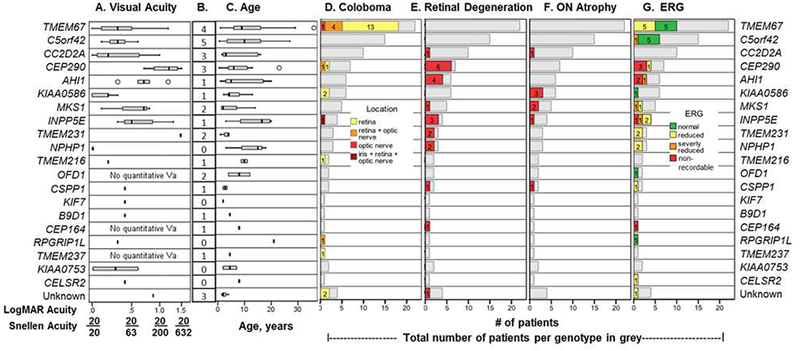
Best-corrected visual acuity (BCVA), coloboma, retinal dystrophy, and optic nerve atrophy occur across a spectrum of genotypes in Joubert syndrome (JS). For each genotype, BCVA (A) and the age at most recent visit (C) are shown as box and whisker plots representing the median value (bar), upper and lower quartile values (boxes), and extreme data points (whiskers). Outliers are denoted by circles. The visual acuity (VA) shown in A is the value for the best eye: Both eyes are listed in Table S1 (available at www.aaojournal.org). The number of patients with nonquantifiable VA in each genotype is in B. D, The number of patients with coloboma for each genotype (28/99 patients); location of coloboma is indicated using color-coded bars. E, Retinal dystrophy observed in 20 of 83 patients. F, Optic nerve atrophy observed in 8 of 86 patients. Where the denominator of a parameter does not equal 99, it is an indication that not all patients could be assessed for that parameter, and the number assessed is the denominator. G, Electroretinography (ERG) results are reported as color-coded bars for patients for whom data were available. D-G, Grey bars indicate the total number of patients in each genotype. logMAR = logarithm of the minimum angle of resolution; ON = optic nerve.
Among patients with JS with quantifiable VA, values ranged from 0 logMAR (20/20) to 1.5 logMAR (20/632). Patients with NPHPl-related JS showed the best VA (range, 0–0.2 logMAR). Patients in genotypes CC2D2A, KIAA0586, C5orf42, and TMEM67had median VA values of 0.5 logMAR or better; however, individual patients had a wide range of quantifiable VAs and some nonquantifiable VAs. The main causes for severe reduction in VA in the cohort included retinal degeneration (24%) and central visual impairment. Other causes of vision impairment were optic atrophy (9%), refractive error, and amblyopia. Vision impairment was due to coloboma in only the most extensive cases involving the macula (11% of those with coloboma, 3% of the cohort). The most impaired median VAs were within the genotype groups INPP5E, MKS1, AHI1, and CEP290, listed in order from mild to severe. Within each of these genotypes, 1 or more patients had retinal degeneration (12/22 patients) (Fig 1E).
Extraocular Muscle Function
Eye movement abnormalities were prevalent cohort-wide (Table 1) and reported as the number of patients with the abnormality/number of patients assessed for that parameter, because not every patient could be assessed for every parameter. Oculomotor apraxia (60/77), nystagmus (66/99), and strabismus (71/98) singly or in combination were seen in all genotypes represented by at least 3 patients (Table 1). Difficulty initiating saccades is a notable characteristic in many patients with JS. Approximately one third of the patients with JS with strabismus (24/71) had undergone extraocular muscle surgery (Tables 1 and S1, available at www.aaojournal.org). Five patients had limitations of extraocular muscle function; in some, this was very prominent with more than 1 muscle involved (Table S1, available at www.aaojournal.org, patients 577, 565, 397, 452, 449).
Ptosis
Ptosis was frequent, with a cohort prevalence of 29% (Table 1). Figure 2 summarizes the frequency and severity of ptosis for each genotype group. In 11 patients in 10 genotypes (including TMEM67, C5orf42, and CC2D2A), the degree of ptosis was severe (MRD of ≤1, Fig 1A) and impinged on the visual axis unilaterally (Fig 2C) or bilaterally (Fig 2D, E). Of those with severe ptosis and no surgery and no known retinal or optic nerve factors that could affect acuity, one 9-year-old patient had a VA of no FF (patient 396, TMEM216), 1 patient had a VA of 0.87 in both eyes (patient 393, unknown genotype), and the remaining patient was 2 years old with a VA of FF (patient 537, MKS1). Severe ptosis persisted in 2 patients despite corrective surgery. Patient 449 (CELSR2) had a 0.4 logMAR VA, an MRD of 3.5 in the right eye, and an unassessable VA in the left eye and MRD −1 in the left eye, whereas patient 500 (C5orf42) had an MRD of 0.5 in both eyes and VA of 0.4 in the right eye and 0.48 in the left eye (Table S1, available at www.aaojournal.org). Both patients were negative for retinal dystrophy, optic atrophy, and coloboma. In 4 other patients, ptosis improved after surgery; at their most recent visit, they had mild/moderate (patients 419, 474, and 491) or no ptosis (patient 459, VA both eyes 0.6 logMAR). The VA of these patients was FF (patient 419, age 2 years, retinal dystrophy, unknown genotype), FF (patient 474, age 5 years, TMEM237), and 0.6 in both eyes (patient 491, age 18 years, C5orf42). The VA of the subset of patients with mild to moderate ptosis who had not undergone surgery and had no retinal dystrophy, optic atrophy, or coloboma extending to the optic nerve ranged from 0 to 0.8 logMAR, with the exception of patient 452 (OFD genotype) who had a VA of no BTL at age 12 years.
Figure 2.

Ptosis and ocular alignment defects are prevalent in patients with Joubert syndrome (JS) of many genotypes. A, Bar graph displaying ptosis in the worst eye as measured by marginal reflex distance (MRD); severity is indicated by color-coded bars. Yellow bars indicate ptosis without indication of severity when no MRD was recorded. Six patients with ptosis received surgery to correct ptosis. B, A 4-year-old (KIAA0586, patient 368) displaying esotropia with inferior oblique overaction. C, Mild unilateral ptosis in a 20-year-old (INPP5E, patient 7503). Best eye visual acuity (VA) was 0.3 logMAR. D, Severe bilateral ptosis in a 4-year-old (TMEM67, patient 557). This patient has not had surgery, and best eye visual acuity (VA) was fix and follow (FF) at most recent visit. E, Severe ptosis in a 2-year-old (MKS1, patient 537). Vision in both eyes was FF.
Coloboma
Colobomas were present in 30% of the cohort (28/99), most commonly involving the retina (20), optic nerve only (1), both retina and optic nerve (6), or retina, optic nerve, and iris (1) (Figs 1D and 3, Table S1, available at www.aaojournal.org). The colobomas were typically located inferior to the optic nerve and were visually insignificant in most patients. The prevalence of coloboma was highest in TMEM67-related JS (80% of TMEM67-related patients), but colobomas were also detected in 6 additional genotypes and in 2 patients of unknown genotype (Table S1, available at www.aaojournal.org). Colobomas were bilateral in 64% of the patients and ranged from small forme fruste to large colobomas involving much of the fundus (Fig 3). In families with multiple children with JS, the siblings were concordant for the presence of coloboma (Table S1, available at www.aaojournal.org, families 6, 8, and 16).
Figure 3.

Coloboma in Joubert syndrome (JS) is mainly a visually inconsequential manifestation limited to the retina but may involve the optic nerve and (rarely) the iris as well. A, Small (left) and forme fruste (right) coloboma in a 29-year-old (TMEM67, patient 560). Best eye visual acuity (VA) was 0.4 logarithm of the minimum angle of resolution (logMAR) in the right eye and 0.6 logMAR in the left eye (Snellen 20/50, 20/80). B, Larger coloboma in a 14-year-old (TMEM67, patient 303). This patient had a VA of 0.1 logMAR in the right eye and 0.18 in the left eye (20/25, 20/32, Snellen) and an abnormal electroretinography (ERG) at most recent visit. C, Coloboma with optic nerve involvement in a 14-year-old (TMEM67, patient 252). This patient’s VA at last ophthalmic visit was 0.6 logMAR in the right eye and 0.8 logMAR in the left eye (20/80, 20/125 Snellen), and ERG was normal. D, A rare coloboma affecting iris, retina, and optic nerve in a 3-year-old (INPP5E, patient 372). The VA was 1.3 logMAR in the right eye (20/400 Snellen) and not reportable in the left eye. An ERG before the National Institutes of Health (NIH) visit was noted as “profoundly abnormal.” Note the ciliary processes adherent to the lens margin and ectopia lentis in the left eye.
Retinal Examination Findings
At the NIH ophthalmological examinations, 20 of 83 patients (24%) had definite evidence for retinal degeneration (Figs 1E and 4, Table S1, available at www.aaojournal.org); in 16 patients, the retinal disease status could not be determined (Table S1, available at www.aaojournal.org). Retinal degeneration in this JS cohort varied from early-onset severe rod-cone dystrophy similar to Leber congenital amaurosis to a later-onset cone-rod dystrophy as shown in the patient with INPP5E-related JS in Figure 4A. In many cases, autofluorescence images of the retina were useful in detecting retinal degeneration as noted in Figure 4A. Both this patient (INPP5E, patient 352) and the patient shown in Figure 4B (AHI1, patient 540) had severely impaired vision (Table S1, available at www.aaojournal.org). The AHI1 patient (Fig 4B) shows a more advanced, diffuse dystrophy apparent in both color and autofluorescence photographs.
Figure 4.

In Joubert syndrome (JS), affected patients can show straightforward retinal dystrophy or atypical or subtle alterations. Fundi photographs in color (left of panel) and fundus autofluorescence (right of panel) are shown for each patient. A, Typical diffuse retinal dystrophy and vascular tortuosity in the left eye in a 14-year-old (INPP5E, patient 352). The electroretinography (ERG) showed no response, and visual acuity (VA) was not assessable. B, Advanced retinal degeneration in a 17-year-old (AHI1, patient 540). The best-corrected visual acuity (BCVA) at this visit was 0.8 in the right eye and 0.7 in the left eye (20/125 and 20/100 Snellen), and ERG showed no response. Inset is widefield Optos photograph (Dunfermline, Scotland, UK). C, A particularly unusual crescent-shape area of retinal degeneration in the right eye adjacent to the macula in a 15-year-old (CC2D2A, patient 565). The dystrophy to this point did not appear progressive, leading us to think that this finding may represent a focal developmental anomaly. The BCVA was 0.3 logarithm of the minimum angle of resolution (logMAR) in the right eye and 0 logMAR in the left eye (20/40 and 20/20 Snellen), and patient had severe ptosis in the right eye and mild ptosis in the left eye (MRD −1, +3). D, A rare combination of coloboma including the optic nerve and retinal degeneration in the right eye in a 23-year-old (CEP290, patient 441). This patient had BCVA of 1.2 logMAR (20/250 Snellen). E, Optic nerve atrophy without retinal degeneration in a 13-year-old (KIAA0586, patient 531). Although this patient did not have retinal degeneration as defined for this study, the authors would not call this patient’s (and several others in the study) retinal architecture completely normal either. This patient had most recent BCVA of 0 logMAR in both eyes (20/20) and did not have any additional ocular manifestations of JS.
Although most of the patients of genotypes CEP290, AHI1, INPP5E, TMEM231, and NPHP1 had retinal degeneration, none of the patients with TMEM67 or C5orf42 mutations showed clinical signs of retinal degeneration (Table S1, available at www.aaojournal.org). Smaller patient numbers limited interpretation in other genotypes with only 1 patient with retinal degeneration (CC2D2A, MKS1, CSPP1, and CEP164)
In general, patients with JS with coloboma were less likely to display retinal degeneration and those with retinal degeneration did not have coloboma; retinal degeneration and coloboma coexisted in only 2 patients with CEP290-related JS. Patient 213, who was aged 13 years at the last visit, had small retinal colobomas in both eyes, mild retinal degeneration, and a VA of 1.5 logMAR in both eyes (Table S1, available at www.aaojournal.org). Patient 441 (Fig 4D) who was aged 23 years had coloboma affecting both retina and optic nerve, a VA of 1.2 logMAR in the right eye, and 1.1 logMAR in the left eye at last visit and showed no response on ERG (Table S1, available at www.aaojournal.org).
Optic Nerve Findings
We identified optic atrophy in 8 patients on examination at the NIH (Fig 1F). Of these 8 patients, 3 were of KIAA0586-related JS and the remaining patients had mutations in CC2D2A, MKS1, INPP5E, and CSPP1. In 4 of these 8 patients with optic atrophy, retinal examination results were otherwise normal. In only 2 patients did optic atrophy occur in association with retinal degeneration, that is, a 7-year-old with MKSl-related JS (patient 510) and a 19-year-old with INPP5E-related JS (patient 7504) (Table S1, available at www.aaojournal.org). One of these 8 patients with optic atrophy could not be assessed for retinal degeneration.
Electroretinography Findings
Retinal function as measured by ERG is reported in Figure 1G. Forty-one of the 99 patients underwent a sedated ERG. Of these 41 patients, 27 had an abnormal ERG; 7 had a nonrecordable response, 4 had a severely reduced reading, and 16 had a reduced response from normal. Figure S1 shows the thinning of the peripapillary retinal nerve fiber layer in siblings with INPP5E-associated JS and their corresponding ERG results. Patients with JS with the nonrecordable and severely reduced readings were those with mutations in CEP290, CEP164, AHI1, MKS1, and INPP5E genes. As expected, those with nonrecordable and severely reduced ERG responses had corresponding degrees of retinal degeneration, except for 2 patients. One of these 2 patients with reduced ERG but no definite clinical appearance of retinal degeneration at the NIH examination was patient 397 with MKSl-related JS. He had a severely abnormal ERG associated with relatively mild retinal findings of vessel attenuation and optic nerve atrophy but was only 18 months at the time of the NIH eye examination. We posit that, as is often the case with young children, signs of retinal degeneration may be subtle or nonexistent on clinical examination. Patient 568 with C5orf42-related JS whose retinal examination showed no degeneration had a right eye VA of 0.2 logMAR, a normal clinical examination, and, by report, a severely abnormal ERG at age 9 years at an outside institution; this study was unavailable for our review.
Best-Corrected Visual Acuity Over Time
By combining the prospective evaluations of VA performed at the NIH Clinical Center and the past VAs, we extracted from outside medical records of 93 patients, we were able to analyze progression of VA as patients age (Fig 5). The VA of typically developing children improves from the FF of infancy to quantifiable acuity by age 2 or 3 years, and mean VA at 36 to 42 months is 0.09 logMAR (20/25 Snellen).30 In an effort to compare the vision in children with JS with the vision in typically developing children, we sought to answer the following 2 questions. What percent of children in this JS cohort progressed into quantifiable VA and at what age does this occur? The lack of uniformity in the frequency of past evaluations precluded further statistical analysis, but we were able to draw some conclusions when VAs recorded at all ophthalmic examinations (107 NIH visits and 334 historical record visits, Table 1 and Table S1, available at www.aaojournal.org) were combined and presented for age ranges (Fig 5). At the evaluations for those aged <2 years, only 17% had quantifiable acuity, and this ranged from 0.5 to 2.0 logMAR. The percent quantifiable acuity was 46% for visits at age 3 years, 55% at 4 years, and 89% at 5 years (Fig 5A). Not until age 9 years or more did the percent of visits for patients with JS that was quantifiable in acuity reach 90% (Fig 5A).
Figure 5.

The transition from nonquantifiable to quantifiable acuity is delayed in Joubert syndrome (JS)-affected patients. This stacked bar chart (A) displays the number of patient visits in each age group and the visual acuity (VA) range at visit, coded by color. On the secondary axis, the percentage of patients with quantifiable VA (logarithm of the minimum angle of resolution [logMAR] 0–2.0) and the percentage of patients with nonquantifiable VA (fix and follow [FF] through no light perception) at each age range are displayed. Both National Institutes of Health (NIH) and historical visits from outside examinations were included. The pie charts (B,C) on the right report on the visual status of the 24-patient subset in our cohort who had visits both before (primarily from historical records) and after age 5 years. If a patient had multiple visits before age 5 years, the visit closest to age 3 years was selected for pie chart B. The visits displayed on pie chart C were the most recent visit for each patient (NIH on-site visit). The genotype of each patient in each section of the pie is listed, followed by the number of patients of that genotype in parentheses. Patients with retinal dystrophy at the most recent NIH visit are denoted by an asterisk next to the number. BTL = blink to light; CUSM = central unsteady maintained; NLP = no light perception.
Next, we looked at the subset of 24 patients who had ophthalmic examinations both before age 5 years and after age 5 years. One examination was selected before age 5 years (the examination closest to age 3 years but <5 years) and is displayed on the left pie chart (Fig 5B), and the most recent examination was selected and is displayed on the right pie chart (Fig 5C). At the visit at less than 5 years, 11 patients (46%) were noted as FF, 5 patients (21%) had only brief FF or central, unsteady, and maintained, and 3 patients had no FF or BTL. Three patients had acuity of 5 logMAR or better, and 1 each was quantifiable at >0.5–1.0 logMAR or >1.0–2.0 logMAR (4%). When the recent (after 5 years) examinations of the same 24 patients were analyzed, 14 patients had vision of 0.5 logMAR or better (58%), 6 patients had vision between >0.5 and 1.0 logMAR (25%), and 1 patient had >1.0 logMAR. Only 1 patient, with OFD1-related JS (patient 452) was recorded as no light perception, 1 patient had no FF (patient 396, TMEM216), and 1 patient had FF (patient 557, TMEM67). In summary, although 19 of the 24 patients had an uncertain visual prognosis based on their evaluations before age 5 years, their VA did improve markedly over time, and all but 3 of the same set of 24 patients retained or gained quantifiable functional VA.
Based on their average VA for individual JS genotypes (Fig 2A) and the VA ranges of individual patients (Fig 5A, C), individuals with JS often have reduced BCVA, even after accounting for delayed visual development. To further analyze and compare the BCVA of patients with and without retinal dystrophy, we generated scatter plots of all recorded VAs over time, grouped into those with and without retinal dystrophy based on the most recent NIH examination (Fig 6A). Although the overall average measured VA for patients with retinal dystrophy showed more acute reduction, the average measured VA for those without dystrophy also displayed some reduction; the confidence intervals for the 2 groups overlapped.
Figure 6.

The visual acuity (VA) of patients with Joubert syndrome (JS) is often reduced to some degree even without retinal dystrophy, and patients with retinal dystrophy with quantifiable acuity can retain a degree of functional VA into adulthood. Patients were grouped on the scatter plot (A) according to retinal dystrophy status at most recent National Institutes of Health (NIH) ophthalmic examination. Both NIH and historical visits from outside examinations were included. Patients without retinal dystrophy are shown in blue. Patients with dystrophy are shown in red. Each dot represents the VA at 1 visit for 1 patient, and multiple acuity measurements of the same patient are connected by lines. Trend lines representing mean VAs for patients with and without dystrophy are shown in bold with accompanying 95% confidence intervals as dashed lines of same color. A random-effects mode was used to account for the within-person correlation and cubic spline terms to account for the nonlinearity over time. Scatter plot B is displaying the VA of only patients with retinal dystrophy. Each dot represents the acuity at 1 visit for 1 patient; multiple visits of the same patient are connected by lines. Colors represent individual genotypes. FF = fix and follow; logMAR = logarithm of the minimum angle of resolution.
When we focus on these patients with retinal degeneration, we see some had relatively stable acuity in the first 2 to 3 decades of life. For example, several of the patients with retinal dystrophy and CC2D2A-, MKS1-, NPHP1-, and INPP5E-related JS had BCVA of 0 to 0.3 logMAR between the ages of 5 and 20 years. In 2 patients with AHI1-related JS, VA stayed between 0.3 and 0.9 logMAR into their teens (patients 540 and 228). Others, particularly those with mutations in CEP290, showed progression to varying degrees. One patient had 0.7 logMAR acuity at age 5 years that worsened to 1.1 logMAR at age 23 years. Another patient with CEP290-related JS with 1 acuity measurement showed significant impairment in quantifiable VA (patient 213, age 13 years, 1.5 logMAR). The remaining CEP290-related patients ranged in age from 6 to 9 years and were in the no FF or BTL or no light perception categories.
Discussion
Joubert syndrome is a classic human ciliopathy with significant genetic heterogeneity and involvement of multiple organ systems, including a wide range of ophthalmologic manifestations. Since the initial description by Dr. Joubert, several case reports and patient cohorts have been reported, usually on a small number of patients (<20) and often without molecular genetic diagnosis (Table S2, available at www.aaojournal.org). The rarity of JS and its extreme genetic heterogeneity pose a challenge for statistically meaningful characterization. The strength of the current study is that it describes the ophthalmologic manifestations of JS in a large cohort of genotyped patients with correlation to detailed systemic examinations. We elucidate the nature and severity of ophthalmologic disease and the risk for retinal disease progression based on molecular cause. Also, we use these data to provide guidelines for prognosis, clinical management, and monitoring.
Clinical Findings in Context
In Table S2 (available at www.aaojournal.org), we summarize the reports in the literature describing the ophthalmologic manifestations of JS. As of September 2017, collectively, 325 patients with JS have been reported with ophthalmic results detailed by individual patient; 165 of those patients had genotypes reported. Because different reports focused on different manifestations, the total number of patients reported varies by specific finding and is given in the denominator of each. We are unable to comment on the reported VA in the literature because most of these reports lack quantifiable VA data. Common ocular abnormalities reported in patients with JS were OMA (61/72 reported, 84%), nystagmus (88/114, 77%), strabismus (69/92, 75%), abnormal ocular movement of unidentified type (60/66, 91%), ptosis (28/37, 76%), coloboma (28/91, 31%), retinopathy with degree not specified (90/205, 44%), optic atrophy (18/32, 56%), and reduced responses on ERG with degree not specified (39/61, 64%). We now consider each of these findings in the context of our study.
Functional VA can be difficult to assess because many patients with JS are unable to cooperate with standard methods of testing. Patients with JS are more hampered in their expressive abilities than in their receptive abilities by motor impairments, such as verbal and lingual apraxias, and this impairment might lead to underestimates of their cognitive abilities.31–33 Impairments in these expressive abilities could play a role in early vision assessments as well, particularly early in life when compensatory measures such as gesturing are not well established. In addition to standard VA measurements, we used fixation and Teller acuity cards to assess in this cohort of patients. Limitations apply even to these methods because of the OMA that is often present.
Based on our data, we can draw some important conclusions about the functional VA of specific groups of patients with JS as they move from early to later childhood. First, visual development can be delayed in children with JS, who seem to reach a more complete visual ability at ages 4 to 6 years in comparison with typically developing children who reach this ability at ages 2 to 3 years (Fig 5). We use the term “delayed visual development” broadly to define a delay in the achievement of best VA beyond the expected in pediatric ophthalmology practice, regardless of the underlying cause of this apparent delay. This finding has relevance for both clinicians and caretakers; lack of ability to FF, or only having a reaction to light at age 2 or 3 years, may not predict future VA. Generally, VA did not worsen dramatically as with age, except in those with retinal degeneration (Fig 6A). However longer-term follow-up of a larger number of patients with each genotype is needed to fully characterize the change in VA over time in patients with JS.
Disorders of ocular motility, including strabismus and nystagmus, are common in patients with genetic syndromes that affect the development of the central nervous system. In addition, patients who develop retinal degeneration early in life may exhibit nystagmus because of lack of binocular foveal fixation. The prevalence of strabismus (72%) and nystagmus (67%) in our cohort is comparable to previous reports. In 2015, in the cohort of 532 patients reported by Bachman-Gagescu et al,21 strabismus was noted at a minimum prevalence of 31%, lower than in our cohort. In our cohort, surgical correction of strabismus was more common than surgical correction of ptosis; 72% of the cohort had strabismus, and 34% of those with strabismus had surgery. As in ptosis, the decision for or against surgical correction of strabismus depends on the severity and amblyogenic potential for which the longer visual development time for patients with JS should be considered.
Neuro-ophthalmic manifestations of JS include OMA/decreased vestibulo-ocular reflex cancellation and smooth pursuit/head thrusts. Brainstem nondecussation in JS may provide the neurologic substrate for periodic alternating gaze deviation.34 Nondecussation may produce a disconnection syndrome that, by interfering with neural feedback loops, prevents one side of the brainstem from inhibiting the other and leading to desynchronization and reverberation in the oculomotor circuitry.35 In our cohort, each of these conditions affected 67% to 78% of the patients assessed for each parameter (Table 1). Among the case reports in the literature, the collective frequency was 88% (Table S2, available at www.aaojournal.org). Neuro-ophthalmological rehabilitation to improve fixation and pursuit is recommended in affected patients before surgical intervention.1,36,37
Although ptosis is known to occur in JS, its characteristics and frequency in individual JS genotypes are not well described and few management guidelines exist. In our cohort, only the AHI1- and KIAA0586-related JS cases exhibited no ptosis. Ptosis severity was variable among the remaining groups and associated with a range of visual function. Numbers were insufficient for statistical analysis. Ptosis surgery can be helpful in patients who do not show evidence of retinal degeneration or central visual impairment; however, in the presence of other causes of vision loss, one should be more conservative in the approach to this intervention.
With a few exceptions, colobomas in this cohort of patients with JS were small and typically not visually significant, particularly in TMEM67-associated JS, which has the highest prevalence of coloboma (Table 1). In addition, as observed by Bachman-Gagescu et al,21 most patients with coloboma did not have retinal degeneration and vice versa. Only 2 of 99 patients (both CEP290-associated JS) in our cohort had both coloboma and retinal dystrophy: 1 coloboma limited to the retina (patient 213) and 1 coloboma extending to the optic nerve (patient 441, Fig 4D). Consistent with this finding, our review of the literature revealed that only 11 of 362 patients with JS (3%) had both retinal dystrophy and coloboma, including 4 with CEP290-,21,38 2 with TMEM67,21,39 1 with CC2D2A-,21 and 4 with INPP5E-associated JS.40 Of note, the patient with the most severe coloboma had INPP5E-associated JS (patient 372, Fig 3D) and a severely reduced ERG. In general, therefore, we conclude that the size and location of coloboma are the best indicators of the effect on visual function in patients with JS, as in other patients with coloboma. Parents can generally be reassured that a progressive retinal degeneration is unlikely in patients with JS with coloboma.
As more JS genes have been discovered, additional genes have been associated with retinal degeneration.2,21,36,41–44 In 1 genotype in our cohort, CEP164, retinal dystrophy had previously not been reported in the literature. Clearly not all of these genotypes experience the same early and severe retinal dystrophy that was first described in JS. We observed that there can be a full spectrum of retinal degeneration in JS. The classic Leber’s congenital amaurosis-type severe degeneration that is early and aggressive as seen in CEP290- and AHIl-associated JS accounts for only a portion of the degeneration seen in this JS cohort. Considering the pleiotropic nature of JS mutations and the multiple ciliary processes that have the potential to be mechanistically affected (e.g., cilia formation, outer segment biogenesis, disc assembly), this is not a surprising finding.
To date, animal models primarily focused on severe CEP290- and AHIl-related retinal degeneration.45,46 CEP290 protein localizes to the transition zone in ciliated cells, and in the Cep290(ko/ko) mouse model the retinal photoreceptors lack connecting cilia and outer segment morphogenesis fails.47 Ahi1-null mice show faulty formation of the retinal outer segments, and their photoreceptors have abnormal opsin distribution.48 Other proteins associated with CEP290 and the ciliary transition zone are RPGRIP1L, NPHP1, and CC2D2A,49 all of which are associated with retinal degeneration in the literature or in our study.
Although the specific applicability to the eye is currently unexplored, another interaction network involved in JS appears to be INPP5E involvement in the initiation of ciliogenesis.50,51 Regulation of ciliogenesis is mediated by MKS1 (fibroblasts from individuals with MKSl-related JS make fewer cilia than control fibroblasts52) and CEP164 (required for ciliary targeting of INPP5E50,53). Some patients in our cohort had retinal degeneration with mutations in INPP5E, MKS1, and CEP164. Our cohort also highlights that different genetic forms of JS have different severities of retinal degeneration. INPP5E-related diffuse retinopathy (Fig 4A) can be severe, but typically less so than CEP290- and AHIl-affected dystrophy. Others with retinal dystrophy present later, with less severity and can display rod-cone and cone-rod dystrophy. Still others experience atypical changes, such as the patient in Figure 4C and the siblings in Figure S1; others have a localized type of retinal disease that does not progress and can include night vision problems, light sensitivity, or color vision abnormalities. In these patients, issues were often identified because they were subjected to comprehensive ocular examination, rather than patient or caregiver reports about vision.
Optic atrophy is a well-known secondary finding in end-stage retinal degenerations. By using both clinical examination and OCT measurements, we identified optic atrophy in the absence of retinal dystrophy in 3 of the 6 patients with KIAA0586-, 2 of 5 patients with MKS1-, 1 patient with CSPP1-, and 1 patient with INPP5E-related JS, suggesting that this is a primary and not a secondary finding. KIAA0556 and MKS1 proteins play a key role in centriole assembly.54,55 In the literature, optic nerve atrophy was reported in KIAA0566-56 (n = 1), OFD1-57(n = 1), ARL13B- (n = 1), and HYLS1-related (n = 1) JS and an additional 12 nongenotyped patients (total of 18/32) for whom ON atrophy status was recorded.
Role of Neuro-ophthalmologic Findings in Initial Diagnosis of Joubert Syndrome
There are distinctive ocular characteristics of JS that, combined with systemic observations, can aid in making the diagnosis of JS. Difficulty initiating saccades can indicate JS, and patients with OMA should have a brain MRI specifically to be evaluated for the “molar tooth sign” even in the absence of other systemic findings. In addition, OMA in JS may manifest as head titubation appearing in the first months of life.58 This should not be confused with the head-nodding behavior seen in patients with some varieties of nystagmus.
Coloboma, in addition to being frequently associated with hepatic manifestations of JS, can be an early ophthalmic indicator of JS in very young patients. Coloboma was present in 28% of our total cohort (28/99) and in 32% of the literature cases with that parameter recorded (30/93, Table S2, available at www.aaojournal.org). In contrast, the live birth prevalence of coloboma in the United States is 1 per 2077.59 We conclude that if coloboma or OMA is noted on an ophthalmic examination in an undiagnosed child with hypotonia, a brain MRI to evaluate for JS is indicated.
Ophthalmological Monitoring and Treatment in Joubert Syndrome
The examination of the ophthalmic phenotype of this cohort has shown that patients with JS of each genotype, except for a single young KIF7 patient (patient 409, 2 years old), display ophthalmic manifestations on presentation (Table S1, available at www.aaojournal.org). High refractive errors were not frequent in this cohort. Causes of reduced vision in our cohort included amblyopia secondary to ptosis or strabismus, central visual impairment, retinal degeneration, coloboma, and optic atrophy. Given that ophthalmic manifestations, whether mild or severe, occurred throughout the cohort, we recommend regular monitoring for ophthalmological manifestations beginning soon after birth or diagnosis. It is important to follow patients with JS closely through the amblyogenic period; this amblyogenic time frame may last significantly longer in JS than is typical.
Genotyping can give additional direction to monitoring and treatment. For instance, in patients with TMEM67-related JS, colobomas are typically visually nonsignificant and retinal dysfunction resulting in significantly reduced vision is unlikely; however, patients may need surgical correction of strabismus or ptosis. In CEP290-, AHI1-, and INPP5E-related JS, retinal degeneration is common, so monitoring should track retinal health closely. We have found that OCT and fundus autofluorescence, especially using wide-field photography, can provide a useful benchmark of retinal degeneration even in less-cooperative patients. Patients with JS with unknown genotype, as demonstrated by our cohort (Table 1 and Table S1, available at www.aaojournal.org; Fig 2C) and supported by supplemental data reported by Bachmann-Gagescu et al,21 may develop retinal degeneration and should be monitored closely.
Increasingly, information obtained from multimodal imaging such as OCT, fundus autofluorescence, and wide-field imaging can be used in patients with JS. This imaging can be done quickly and without dilation, and is generally well tolerated. As these methods become more widely available, they can be used to delineate the natural history of the retinal degeneration in JS and can be used as outcome measures in a treatment study.
Systemic Considerations
This study was not designed to evaluate correlations between the severities of non-neurologic organ system disease and the severity of ophthalmic disease over time. However, in a separate publication detailing the genotype-phenotype correlations of the same cohort of patients with JS, we reported that liver disease and coloboma occurred together (odds ratio, 2.7; 95% confidence interval, 0.85–5.52) and that retinal degeneration was associated with kidney disease (odds ratio, 2.3; 95% confidence interval, 0.77–7.05).22
Study Limitations
This JS cohort may be relatively enriched in patients with extra-neurologic manifestations because the comprehensive liver and kidney evaluations performed at the NIH Clinical Center may have attracted patients with these manifestations. However, our study also offered formal neurocognitive testing, ophthalmic evaluations, and other general investigations, including DNA sequencing, sleep studies, echocardiograms, and hormone evaluations, that may have minimized this potential bias. In fact, although the ratio of patients with TMEM67-related JS in our cohort was higher than that of other recently published cohorts,5,21 the most commonly mutated 5 genes in our cohort were the same as those in another large cohort.21
A week-long, on-site evaluation is a considerable time commitment that could lead to sampling bias, that is, toward families who were able to take time for a week-long visit or patients with the most medical problems for whom families are desperate for more information. Travel and accommodation expenses were covered by the study to minimize bias against families without financial resources for such expenses. However, very severely affected patients with JS who required intensive care such as mechanical ventilation and could not travel at the time of the study may be underrepresented. Finally, the overall frequency of retinal degeneration, liver disease, and kidney disease may have been underestimated by this cross-sectional study because these manifestations may develop as the patient gets older. In addition, despite our best efforts to minimize ascertainment bias, all of the patients and families in this cohort were connected to the other patients and families to some degree via association with the large and well-established international network of the Joubert Syndrome & Related Disorders Foundation.
Supplementary Material
Summary.
Ophthalmic manifestations of Joubert syndrome affect nearly all patients and include ocular motility disorders, ptosis, coloboma, optic nerve atrophy, and retinal degeneration that ranges from mild to severe. Children require extended monitoring because of delayed visual maturation.
Acknowledgments
The authors thank the Joubert Syndrome and Related Disorders Foundation for their extensive support and the individuals with JS and their families who generously participated in this investigation. Thanks to the NISC Comparative Sequencing Program staff for their work and dedication. This research was supported by the Intramural Research Program of the National Human Genome Research Institute and the NIH Clinical Center. The authors also thank Richard E. Thompson, PhD, of the Biostatistics Department at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) for statistical assistance.
Supported by the National Human Genome Research Institute (Bethesda, MD) and conducted under the intramural research protocol, “Clinical and Molecular Investigations into Ciliopathies”; www.clinicaltrials.gov, trial NCT00068224.
Abbreviations and Acronyms:
- BCVA
best-corrected visual acuity
- BTL
blink to light
- COACH
cognitive impairment, ataxia, cerebellar vermis hypoplasia, and hepatic fibrosis
- ERG
electroretinography
- FF
fix and follow
- JS
Joubert syndrome
- logMAR
logarithm of the minimum angle of resolution
- MRD
marginal reflex distance
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- OMA
oculomotor apraxia
- VA
visual acuity
Footnotes and Financial Disclosures
Financial Disclosure(s): The author(s) have made the following disclosure(s):
B.P.B.: Federal employee - National Institutes of Health (NIH).
M.M.: Employee - US Food and Drug Administration.
D.A.D.: Grant - NIH.
M.P.: Employee - NIH.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the National Human Genome Research Institute approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent. No animal subjects were used in this study.
AUTHOR CONTRIBUTIONS
Conception and design: Brooks, Zein, Thompson, Mokhtarzadeh, Doherty, Parisi, Glass, Malicdan, Vilboux, Vemulapalli, Mullikin, Gahl, Gunay-Aygun
Data collection: Brooks, Zein, Thompson, Mokhtarzadeh, Doherty, Parisi, Glass, Malicdan, Vilboux, Vemulapalli, Mullikin, Gahl, Gunay-Aygun
Analysis and interpretation: Brooks, Zein, Thompson, Mokhtarzadeh, Doherty, Parisi, Glass, Malicdan, Vilboux, Vemulapalli, Mullikin, Gahl, Gunay-Aygun
Obtained funding: Not applicable
Overall responsibility: Brooks, Zein, Thompson, Mokhtarzadeh, Doherty, Parisi, Glass, Malicdan, Vilboux, Vemulapalli, Mullikin, Gahl, Gunay-Aygun
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romani M, Micalizzi A, Valente EM. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol. 2013;12:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi M, Glass I. Joubert syndrome and related disorders. In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews. Seattle, WA: University of Washington: 1993. (update 2013). [PubMed] [Google Scholar]
- 3.Hilgendorf KI, Johnson CT, Jackson PK. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol. 2016;39:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valente EM, Brancati F, Boltshauser E, Dallapiccola B. Clinical utility gene card for: Joubert syndrome--update 2013. Eur JHum Genet. 2013;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroes HY, Monroe GR, van der Zwaag B, et al. Joubert syndrome: genotyping a Northern European patient cohort. Eur J Hum Genet. 2015;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300). Eur J Hum Genet. 2007;15:511–521. [DOI] [PubMed] [Google Scholar]
- 7.Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19:813–825. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet. 2004;125A:125–134; discussion 117. [DOI] [PubMed] [Google Scholar]
- 9.Poretti A, Boltshauser E, Valente EM. The molar tooth sign is pathognomonic for Joubert syndrome! Pediatr Neurol. 2014;50:e15–16. [DOI] [PubMed] [Google Scholar]
- 10.Poretti A, Huisman TA, Scheer I, Boltshauser E. Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. AJNR Am JNeuroradiol. 2011;32:1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekaban AS. Hereditary syndrome of congenital retinal blindness (Leber), polycystic kidneys and maldevelopment of the brain. Am J Ophthalmol. 1969;68:1029–1037. [DOI] [PubMed] [Google Scholar]
- 13.Dekaban AS. Familial occurrence of congenital retinal blindness and developmental renal lesions. J Genet Hum. 1969;17:289–296. [PubMed] [Google Scholar]
- 14.Aicardi J, Castello-Branco ME, Roy C. [Joubert’s syndrome. Apropos of 5 cases]. Arch Fr Pediatr. 1983;40:625–629. [PubMed] [Google Scholar]
- 15.Tusa RJ, Hove MT. Ocular and oculomotor signs in Joubert syndrome. J Child Neurol. 1999;14:621–627. [DOI] [PubMed] [Google Scholar]
- 16.Moore AT, Taylor DS. A syndrome of congenital retinal dystrophy and saccade palsy--a subset of Leber’s amaurosis. Br J Ophthalmol. 1984;68:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laverda AM, Saia OS, Drigo P, et al. Chorioretinal coloboma and Joubert syndrome: a nonrandom association. J Pediatr. 1984;105:282–284. [DOI] [PubMed] [Google Scholar]
- 18.Lindhout D, Barth PG, Valk J, Boen-Tan TN. The Joubert syndrome associated with bilateral chorioretinal coloboma. Eur J Pediatr. 1980;134:173–176. [DOI] [PubMed] [Google Scholar]
- 19.Appleton RE, Chitayat D, Jan JE, et al. Joubert’s syndrome associated with congenital ocular fibrosis and histidinemia. Arch Neurol. 1989;46:579–582. [DOI] [PubMed] [Google Scholar]
- 20.Brancati F, Dallapiccola B, Valente EM. Joubert Syndrome and related disorders. Orphanet J Rare Dis. 2010;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmann-Gagescu R, Dempsey JC, Phelps IG, et al. Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet. 2015;52:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilboux T, Doherty DA, Glass IA, et al. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017;19:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentley DR, Balasubramanian S, Swerdlow HP, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teer JK, Mullikin JC. Exome sequencing: the sweet spot before whole genomes. Human Mol Genet. 2010;19:R145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teer JK, Green ED, Mullikin JC, Biesecker LG. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012;28:599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Strongin A, Heller T, Doherty D, et al. Characteristics of liver disease in 100 individuals with Joubert syndrome prospectively evaluated at a single center. J Pediatr Gastroenterol Nutr. 2018;66:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming LR, Doherty DA, Parisi MA, et al. Prospective evaluation of kidney disease in Joubert syndrome. Clin J Am Soc Nephrol. 2017;12:1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poretti A, Snow J, Summers AC, et al. Joubert syndrome: neuroimaging findings in 110 patients in correlation with cognitive function and genetic cause. J Med Genet. 2017;54:521–529. [DOI] [PubMed] [Google Scholar]
- 30.Leone JF, Mitchell P, Kifley A, et al. Normative visual acuity in infants and preschool-aged children in Sydney. Acta Ophthalmol. 2014;92:e521–529. [DOI] [PubMed] [Google Scholar]
- 31.Braddock BA, Farmer JE, Deidrick KM, et al. Oromotor and communication findings in Joubert syndrome: further evidence of multisystem apraxia. J Child Neurol. 2006;21:160–163. [DOI] [PubMed] [Google Scholar]
- 32.Baker K, Beales PL. Chapter 9 - Abnormalities of the central nervous system across the ciliopathy spectrum. In: Tucker KL, Caspary T, eds. Cilia and Nervous System Development and Function. New York: Springer; 2013. [Google Scholar]
- 33.Summers AC, Snow J, Wiggs E, et al. Neuropsychological phenotypes of 76 individuals with Joubert syndrome evaluated at a single center. Am J Med Genet A. 2017;173:1796–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodsky MC, Kotagal S, Pichurin PN, Ho ML. Evidence for brainstem motor nondecussation as a neurologic substrate for periodic alternating gaze deviation. Ophthalmology 2017; 124:1085–1087. [DOI] [PubMed] [Google Scholar]
- 35.Brodsky MC. Marshall M. Parks Memorial Lecture: ocular motor misbehavior in children: where neuro-ophthalmology meets strabismus. Ophthalmology. 2017;124:835–842. [DOI] [PubMed] [Google Scholar]
- 36.Doherty D, Parisi MA, Finn LS, et al. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis). J Med Genet. 2010;47:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papanagnu E, Klaehn LD, Bang GM, et al. Congenital ocular motor apraxia with wheel-rolling ocular torsion-a neurodiagnostic phenotype of Joubert syndrome. JAAPOS. 2014;18:404–407. [DOI] [PubMed] [Google Scholar]
- 38.Brancati F, Barrano G, Silhavy JL, et al. CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome-related disorders. Am J Hum Genet. 2007;81:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroes HY, Monroe GR, van der Zwaag B, et al. Joubert syndrome: genotyping a Northern European patient cohort. Eur J Hum Genet. 2016;24:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travaglini L, Brancati F, Silhavy J, et al. Phenotypic spectrum and prevalence of INPP5E mutations in Joubert syndrome and related disorders. Eur J Hum Genet. 2013;21:1074–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satran D, Pierpont ME, Dobyns WB. Cerebello-oculo-renal syndromes including Arima, Senior-Loken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet. 1999;86:459–469. [PubMed] [Google Scholar]
- 42.Parisi MA, Bennett CL, Eckert ML, et al. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srour M, Hamdan FF, Schwartzentruber JA, et al. Mutations in TMEM231 cause Joubert syndrome in French Canadians. J Med Genet. 2012;49:636–641. [DOI] [PubMed] [Google Scholar]
- 44.Thomas S, Cantagrel V, Mariani L, et al. Identification of a novel ARL13B variant in a Joubert syndrome-affected patient with retinal impairment and obesity. Eur J Hum Genet. 2015;23:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada H, Lu Q, Insinna-Kettenhofen C, et al. In vitro modeling using ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Rep. 2017;20:384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May-Simera HL, Wan Q, Jha BS, et al. Primary cilium-mediated retinal pigment epithelium maturation is disrupted in ciliopathy patient cells. Cell Rep. 2018;22:189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachel RA, Yamamoto EA, Dewanjee MK, et al. CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies. Hum Mol Genet. 2015;24:3775–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louie CM, Caridi G, Lopes VS, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012; 1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, Jin M, Hu R, et al. The Joubert syndrome protein Inpp5e controls ciliogenesis by regulating phosphoinositides at the apical membrane. JAm Soc Nephrol. 2017;28:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Q, Zhang Y, Wei Q, et al. Phosphatidylinositol phosphate kinase PIPKIgamma and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat Commun. 2016;7:10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slaats GG, Isabella CR, Kroes HY, et al. MKS1 regulates ciliary INPP5E levels in Joubert syndrome. J Med Genet. 2016;53:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humbert MC, Weihbrecht K, Searby CC, et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A. 2012;109:19691–19696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dawe HR, Smith UM, Cullinane AR, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–186. [DOI] [PubMed] [Google Scholar]
- 55.Malicdan MC, Vilboux T, Stephen J, et al. Mutations in human homologue of chicken talpid3 gene (KIAA0586) cause a hybrid ciliopathy with overlapping features of Jeune and Joubert syndromes. J Med Genet. 2015;52:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roosing S, Rosti RO, Rosti B, et al. Identification of a homozygous nonsense mutation in KIAA0556 in a consanguineous family displaying Joubert syndrome. Hum Genet. 2016;135:919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wentzensen IM, Johnston JJ, Patton JH, et al. Exome sequencing identifies a mutation in OFD1 in a male with Joubert syndrome, orofaciodigital spectrum anomalies and complex polydactyly. Hum Genome Var. 2016;3:15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poretti A, Christen HJ, Elton LE, et al. Horizontal head titubation in infants with Joubert syndrome: a new finding. Dev Med Child Neurol. 2014;56:1016–1020. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura KM, Diehl NN, Mohney BG. Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol. 2011;129:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


