Background:
Functional recovery after peripheral nerve injury is often suboptimal despite the intrinsic permissive growth environment of the peripheral nervous system. The objective of this systematic review is to explore the use of electrical stimulation (ES) for peripheral nerve regeneration.
Methods:
A systematic literature search was conducted from inception to March 2, 2021 to retrieve articles on ES for peripheral nerve regeneration using the PubMed, Ovid MEDLINE, and Embase databases. Primary outcome measures included objective measures of motor and sensory nerve function.
Results:
Four randomized control trials, two case reports, and three case series that addressed the aims were identified. The stimulation parameters varied greatly between studies, without an apparent commonality for a given electrical conduit. Outcomes measured included motor (n = 8) and sensory (n = 7) modalities (cold detection, static two-point discrimination, tactile discrimination, and pressure detection), nerve-specific muscle function and bulk, and electromyography (EMG) motor and sensory terminal latency. Different parameters for measurement were utilized and improvement was observed across the studies compared with controls (n = 4) or pre-intervention measurements (n = 5). One randomized control trial reported no benefit of ES and attributed their findings to their stimulation protocol. Complications were documented in three patients only and included wire remnant removal, skin pigmentation, and bone formation.
Conclusions:
ES in peripheral nerve regeneration is beneficial in improving and accelerating recovery. A meta-analysis was not performed due to the heterogeneity, but all studies showed positive findings and minor to no complications. These results provide a primer for further development of delivery methods.
Takeaways
Question: Does electrical stimulation improve peripheral nerve regeneration after injury?
Findings: Electrical stimulation applied to injured peripheral nerves during surgical repair can greatly enhance nerve recovery—both sensory and motor functions. This effective modality is not being used in the clinic or hospital sitting due to challenges in translation. Clinicians lack information on devices and optimal duration and setting of stimulation. Our systematic review of the literature provides answers to the previous questions.
Meaning: We encourage clinicians to start adopting electrical stimulation in practice and patients to have the courage to participate in this novel treatment modality.
INTRODUCTION
Peripheral nerve injuries occur in 2%–3% of patients who experience extremity trauma.1,2 Affected patients are mostly young, require more inpatient rehabilitation and extended hospital stay, and are at higher risk of long-term disability and reduced functional capacity.1 Functional recovery of peripheral nerves after an injury is often suboptimal despite the permissive growth environment intrinsic to the peripheral nervous system.3 Transected fibers of the distal nerve stump degenerate through a process known as Wallerian degeneration, and peripheral nerve growth occurs proximally at an estimated rate of 1 mm per day. In the absence of neuronal contacts, injured nerves lose their regenerative capacity in a progressive, time-sensitive, and length-dependent manner,4 and denervated Schwann cells eventually atrophy and fail to support axonal regeneration.4 Despite advancement in microsurgical techniques, the regain in nerve function remains inadequate.5 Axonal regeneration across coaptation sites is slow and difficult to predict; thus, functional outcomes can be improved by accelerating the intrinsic rate of nerve regeneration.6 Low frequency electrical stimulation (ES) was found to potentiate axon outgrowth and muscle reinnervation after immediate or delayed nerve repair.4,7,8 Pre-clinical studies using ES for peripheral nerve regeneration in primates are promising4,7-11 and motivate further research in humans. This article provides a review of clinical studies investigating the role of ES on peripheral nerve regeneration in human subjects.
MATERIALS AND METHODS
Protocol and Eligibility Criteria
All aspects of this review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The population of this study consisted of patients who had any type of injury to peripheral nerves of their extremities. The intervention was ES utilizing any electrical conduit, including acupuncture needles and implantable stimulators. The primary outcome of interest was the difference in objective or subjective measures of nerve regeneration. Secondary outcome included assessing complications. Case reports were included due to paucity of the literature. The following were excluded: duplicated publications, review articles, cranial nerve studies.
Search Strategy and Study Selection
PubMed, Ovid MEDLINE, and Embase databases were searched to identify clinical studies involving ES used for peripheral nerve regeneration. Articles published from database inception to March 2, 2021 were considered. Alternative versions and spellings of the following key words were searched: “electrical stimulation,” “peripheral nerve,” and “regeneration.” The search was confined to studies published in peer-reviewed journals and written in the English or French languages. The references of all included studies were searched for additional studies.
Two independent reviewers (AA and JH) assessed the eligibility of the studies using the same strict inclusion and exclusion criteria. The initial screen filtered studies based on relevancy of the title alone. A relevant title has at least one of the following terms: “electrical stimulation,” or “peripheral nerve,” or any variation of these words. A second screen was carried out on the abstracts of the remaining articles. Only human studies were included in the results synthesis of this review.
Data Extraction and Items
Refer to Supplemental Digital Content 1. (See appendix, Supplemental Digital Content 1, which displays variables extracted from individual studies. http://links.lww.com/PRSGO/B931.)
Assessment of Methodological Quality and Level of Evidence
The methodological quality of the included randomized controlled trials (RCTs) was judged using the Cochrane risk of bias tool,12 which evaluates six items: randomization, allocation concealment, blinding, incomplete outcome data, and absence of selective reporting among others. Case reports and case series were appraised using the Joanna Briggs Institute critical appraisal checklist for case reports.13 Level of evidence of all studies was assessed using the Oxford Center for Evidence-based Medicine’s levels of evidence scale.14
Analysis of Heterogeneity
Clinical and methodological heterogeneity were assessed using predetermined population, intervention, control, and outcome (PICO) criteria as well as by assessing the number of studies that reported specific outcome measures. Clinical heterogeneity was judged to be moderate for this review due to differences in study designs, nerve types, mode of ES, and outcomes reported; thus, a meta-analysis could not be done.
RESULTS
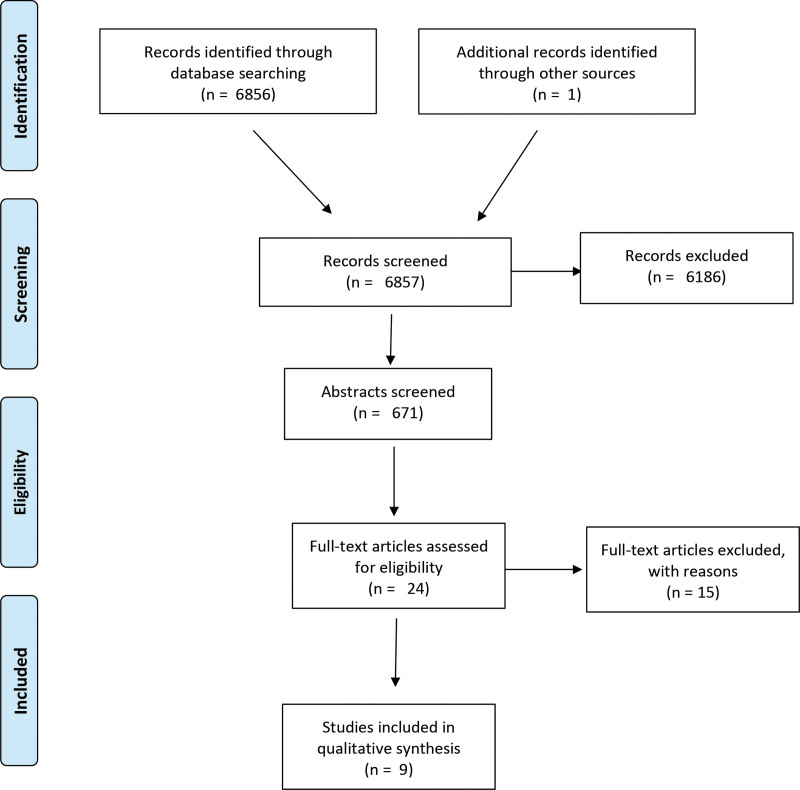
The systematic search identified 6857 publications. Figure 1 shows a flow diagram depicting the selection process. Among the nine articles included, four were RCTs of level IIa evidence. The remaining five were therapeutic studies of level III evidence in the form of case reports (n = 2) and case series (n = 3). Key study characteristics are depicted in Tables 1, 2 and quality assessment in Tables 3, 4, and Supplemental Digital Content 2. (See figure, Supplemental Digital Content 2, which displays A, Quality assessment of included RCT; and B, Summary of quality assessment of included RCT. http://links.lww.com/PRSGO/B932.)
Fig. 1.
Flow diagram.
Table 1.
Study Characteristics
| Study | Study Design | n | Age (y) | Gender | Nerve | Injury Classification | Preoperative Diagnosis | Surgical Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Gordon22 | RCT | 21 Cases:11 Control:10 | 56 | 8M:13F | 21 median | Axonotmesis2 | CTS | Decompression | 12 mo |
| Wong15 | RCT | 36 Cases:16 Control:15 | 38.3 | 25M:11F | 36 digital | Neurotmesis (V) | Complete transection | Tension-free epineural repair within 14 days after injury | 6 mo |
| Power24 | RCT | 31 Cases: 20 Control:11 | 56.5 | 23M:8F | 31 ulnar | Axonotmesis2 | CuTS | Decompression | 36 mo |
| Piccinini18 | RCT | 36 | 37 | 21M:17F | 23 peroneal, 9 ulnar, 3 radial, 2 femoral, 1 tibial | Axonotmesis (II–III) | N/A | None | 3 mo |
| Kern23 | Case report | 1 | 47 | M | L1–L4 | NA | Total denervation | None | 26 mo |
| Tang7 | Case report | 1 | 32 | M | 1 ulnar | Neurotmesis (V) | Total rupture | Ulnar nerve repair 6 h after injury | 6 mo |
| Inoue19 | Case series | 7 | 66.8 | 6M:1F | 5 peroneal, 1 axillary, 1 ulnar | 2 Neurapraxia (I) 4 Axonotmesis (N/A) 1 Neurotmesis (V) | Case dependent (refer to ref study) | Case dependent (refer to ref study) | 4–48 mo |
| De Oliveira17 | Case series | 3 | 33.3 | 2M:1F | 2 brachial plexus, 1 combined axillary, radial, median. | 1 Combined (VI) 1 Neurotmesis (V) 1 Axonotmesis2 | Case dependent (refer to ref study) | Case dependent (refer to ref study) | 12–36 mo |
| Nicolandis20,49 | Case series | 15 | 28 | 13M:2F | 5 median, 4 ulnar, 2 combined, 4 radial, 3 brachial plexus | NA | NA | Microsurgical repair | 24 mo |
CTS, Carpal Tunnel Syndrome; CuTS, Cubital Tunnel Syndrome; NA, Not Available.
Table 2.
Details of ES of Included Studies
| Study | ES Cases | Electrical Conduit | Intervention Duration | Frequency | Intensity | Days/Week | Outcome Measured |
|---|---|---|---|---|---|---|---|
| Gordon22 | 11 | External electrical stimulator (Grass SD9) | 1 h | 20 Hz | (4–6 V, 0.1–0.8 ms duration) | 1-time brief ES during surgery | MUNE NCS SM LSAQ PPD |
| Wong15 | 16 | External electrical stimulator (Grass SD9) | 1 h | 20 Hz | Tolerance limit (<30 V, 0.1–0.4 ms) | 1-time brief ES during surgery | SM DASH MHS |
| Power24 | 20 | External electrical stimulator (Grass SD9) | 1 h | 20 Hz | Tolerance limit (<30 V, 0.1 ms) | 1-time brief ES during surgery | NSMF MUNE NCS |
| Piccinini18 | 36 | External electrical stimulator (Echo Companion) | 30 min | 1 Hz | 0.5 mA above the lowest intensity needed to produce contraction of the muscle 150 ms | 3/wk for 3 mo (36 sessions) | MRC dynamometry EMG |
| Kern23 | 1 | External electrical stimulator | 15 min/d | Round 1: 2 Hz for 4 mo Round 2: 20 Hz for 22 mo | Round 1: 120 ms amplitude 200 mA Round 2: 40 ms amplitude 200 mA | 5 d/wk for 26 months (delayed) | NSMF MB CSA |
| Tang19 | 1 | Acupuncture needles | ? | 2 Hz | 6 mA max tolerance | 1/wk (delayed 2 wk) | SM DASH EMG RL |
| Inoue17 | 7 | Acupuncture needles | 20 min | 100 Hz | 200 ms | 1/wk | AROM NSMF |
| De Oliveira21 | 3 | External electrical stimulator (Intellect Combo) | 20 min | ? | 70–100 ms | daily | AROM NSMF SM EMG |
| Nicolandis20,49 | 15 | Implantable pulse generator | 1–2 h | 130 Hz | 2–10.5 V | daily | NSMF MB |
AROM, Active Range of Motion; CSA, Cross-sectional Area of Muscle; DASH, Disability of the Arm Shoulder and Hand questionnaire; LSAQ, Levine’s self-assessment questionnaire for carpal tunnel syndrome; MB, Muscle Biopsy; MHS, Modified Highet Scale for Grading Nerve Recovery; MRC, Medical Research Council scale for segmental muscle strength; MUNE, Motor Unit Number Estimation; NCS, Nerve Conduction Studies; PPD, Purdue Pegboard Test (for manual dexterity); NSMF, Nerve-specific Muscle Function; RL, Rosén and Lundbord protocol; SM, Sensory Modalities.
Table 3.
Quality Assessment of the RCTs using Cochrane Risk of Bias Tool
| Wong15 | Power24 | Gordon22 | Piccinini18 | |
|---|---|---|---|---|
| Random sequence generation (selection bias) | Low risk | Low risk | Low risk | Low risk |
| Allocation concealment (selection bias) | Low risk | Low risk | Low risk | Low risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Low risk | High risk | Unclear |
| Blinding of outcome assessment (detection bias) (patient-reported outcomes) | Low risk | Low risk | High risk | Low risk |
| Incomplete outcome data addressed (attrition bias) (short-term outcomes (2–6 wk)) | Unclear * | Low risk | Low risk | High risk |
| Incomplete outcome data addressed (attrition bias) (longer term outcomes (>6 wk)) | Unclear * | Low risk | Low risk | High risk |
| Selective reporting (reporting bias) | Low risk | Low risk | Low risk | Low risk |
Table 4.
Quality Assessment of the Case Reports and Case Series Using Joanna Briggs Institute Critical Appraisal Checklist for Case Reports
| Kern23 | Tang19 | De Oliveria21 | Inuoe17 | Nicolandis20,49 | |
|---|---|---|---|---|---|
| 1. Clear description of patient’s demographics | No | No | No | Yes* | No |
| 2. Clear description of patient’s history with timeline | Yes | Yes | Yes | Yes | No |
| 3. Clear description of patient condition on presentation | Yes | Yes | Yes | Yes | No |
| 4. Clear description of diagnostic/assessment methods and their results | Yes | Yes | Unclear | Yes | Yes |
| 5. Clear description of treatment procedure(s) | Yes | Yes | Yes | Yes | Yes |
| 6. Clear description of postintervention clinical condition | Yes | Yes | Yes | Yes | Yes |
| 7. Description of adverse/unanticipated events | No | Unclear | No | Yes | Yes |
| 8. Provision of takeaway lessons | Yes | Yes | Yes | Yes | Yes |
*Only one that addressed comorbidity status.
Mean age of patients was 43.9 ± SD18.8 (range: 17–73 years). Men comprised 65.4% (n = 102). When described, mechanisms of nerve injury included traumatic laceration (n = 6), compression from bed rest (n = 4), bony dislocation (n = 1), postsurgical complication (n = 1), carpal tunnel syndrome (n = 21), cubital tunnel syndrome (n = 31), motor-vehicle accident (n = 13), and gunshot wound (n = 2). All except one study examined mixed sensory and motor nerves.15 The type of nerve injury was classified according to the Seddon classification.16 Neurotmesis (n = 40) and axonotmesis (n = 58) were the most common nerve injuries encountered.
Surgical repair before ES was performed on all patients, except if nerve injury occurred secondary to anatomical compression from bed rest, shoulder dislocation, or total hip arthroplasty.17 Piccinini et al,18 however, included patients with traumatic nerve axonal injuries due to different trauma and stated that surgery was not recommended in any patient without specifying reasons. In the other studies, surgical treatment ranged from primary nerve repair to more complex reconstruction with nerve grafts/nerve transfers.15,17,19-21 In patients with carpal tunnel syndrome, surgical decompression was performed without epi-neurotomy or neurolysis of median nerve.22 Three major methods of delivering ES were identified. The most popular method utilized an external stimulator connected to fine wire electrodes inserted before skin closure, after nerve repair.15,21-23 Two studies used acupuncture needles (n = 8) as their electrical conduit.17,19 An implantable pulse generator with intramuscular electrodes is placed into the subcutaneous tissue.20 Frequencies ranged from 2 (n = 2) to 20 Hz (n = 64), applied periodically over a long period of time or at a single session.
Peripheral Nerve Regeneration Outcome Measures
Measures of Sensory Modalities
The average follow-up time for patients was 15 months after ES. Outcome measures varied among the studies and were dependent on the nerve studied. In an RCT, ES (n = 16) of completely transected digital nerves after epi-neurial repair significantly improved all sensory modalities by 5–6 months postoperatively compared with surgery alone in control subjects (n = 15). Cold detection threshold in ES patients achieved near normal levels of 14.33 ± 0.46 just-noticeable difference (JND) units, versus 17.22 ± 0.44 JND in controls (P < 0.001). Tactile discrimination and pressure detection improved as well. Static two-point discrimination in ES patients recovered to 4.71 ± 0.90 mm, which was significantly better than controls at 8.69 ± 1.05 mm (P < 0.001). Translating these findings to the Modified Highet Grading, 87% of ES subjects achieved S4 (normal) recovery versus 44% in the controls (P < 0.001). The Semmes-Weinstein monofilaments test for large-myelinated Αβ fibers was close to normal in ES patients (3.38 ± 0.12), and significantly better than in controls (3.91 ± 0.11). In a patient with a high ulnar nerve injury, static two-point discrimination (s2PD) improved from 16 mm or more to 5 mm or less (normal) after 6 months of ES. Similarly, the Semmes-Weinstein monofilaments test went from not testable to normal by 6 months.19 In a case series of three patients with complex brachial plexus injuries, improvements in superficial and deep sensitivity were noted after ES.21 Gordon et al have also reported improvement in Semmes-Weinstein monofilaments test in patients with carpal tunnel syndrome who underwent ES with decompression as opposed to those who underwent decompression alone.22
Measures of Nerve-specific Function
Four studies reported on nerve-specific muscle function after ES. In a series of five patients with peroneal nerve injury, strength of ankle dorsiflexion, ankle pronation, and great toe extension increased from an average of 1 of 5 to 3.8 of 5, 1.4 of 5 to 3 of 5, and 0.4 of 5 to 3.6 of 5, respectively. The worst of these cases scored 0 on all manual muscle test grading but showed reinnervation potential on EMG. One RCT18 assessed segmental muscle strength of 38 patients using the medical research council scale and dynamometry, and reported that muscle strength increased after treatment and at 3 months in both cases and control, whereas the increase in strength as measured by dynamometry was not statistically significant for the control at 3 months.
In one RCT with 3 years of follow-up on 31 patients with ulnar nerve injury of grade 3 on the McGowan- Goldberg grading system, 35% of patients who underwent ES attained grade 1 and 40% attained grade 2 when compared with controls, in which 25% attained grade 1 and 25% attained grade 2 (P < 0.05).24 The same study reported on grip strength, which improved significantly for cases from the first year onward (P < 0.001) but not for control even at 3 years (P = 0.08). Minimal clinical importance difference in grip strength was 5.9 kg. Cases had improvement of 8.1 kg at 3 years, whereas the control had improvement of 4.2 kg only. Key pinch was also significantly improved for cases (P < 0.001), and it was reported to be three times that of control, who failed to show a statistically significant improvement in pinch strength at any follow-up (P > 0.1).24
One study analyzed muscle bulk and regeneration in a patient with paralysis and denervation of quadriceps femoris bilaterally.23 Two years of ES resulted in an increase in cross-sectional area from 36 cm2 to 57.9 cm2 on the right thigh and 36.1 cm2 to 52.4 cm2 on the left thigh. Muscle density expressed in Hounsfield units had increased from 11 to 26.4 on the right and 10.7 to 24.1 on the left. Histologically, there was evidence of reduced fat and connective tissue, growth in diameter of surviving myofibrils, and formation of new myofibrils. Nevertheless, knee extension torque induced by ES was less than 10% that of a normal subject.
Electroneuromyography Measures
Clinically relevant changes in electroneuromyography (EMG) were reported in four studies. Acceleration in recovery of motor and sensory terminal latencies was seen in ES subjects (n = 11) after carpal tunnel decompression.22 These subjects achieved normal latencies as of 3 months postoperatively onwards, whereas recovery was delayed in the control patient group (n = 10). In one patient with a high ulnar nerve injury and minimal to no pick-up on EMG, 6 months of monthly ES increased sensory and motor nerve conduction velocity.19 Electroneuromyography demonstrated sensory and motor reinnervation in three patients with complex brachial plexus injuries receiving 20 minutes of daily electrotherapy combined with an intensive rehabilitation program. EMG results did not worsen in any of the studies. Piccinini et al,18 however, reported that voluntary muscle activity as measured by EMG was not significantly improved with ES; the authors attributed their contradicting findings to their stimulation parameters (1 Hz, 0.5 h, intensity set to 0.5 mA above lowest intensity needed to produce contraction, using Echo Companion device)‚ as opposed to the parameters used by the other RCTs15,24 (20 Hz, 1 h, intensity set to tolerance limit <30 V, using Grass SD9 device). Gordon et al24 and Power et al24 have reported improvement in motor unit number estimation after ES; in Gordon’s study on patients with carpal tunnel syndrome, motor unit number estimation increased after 3 months (from 150 ± 62 MU to 290 ± 140 MU) and was comparable to healthy subjects by 12 months, an effect that was not seen in controls.22 In the other study on patients with cubital tunnel syndrome, ES resulted in a significant increase in motor unit number estimation (107 ± 11) when compared with controls (78 ± 6) (P < 0.05) at 1 and 3 years, and stimulated patients had more than double the number of motor units when compared with controls (178 ± 11 versus 88 ± 18; P < 0.05) by 3 years.24 The same study also reported that the maximum compound muscle action potential for cases has a significant increase (+4.46 ± 0.37 mV; P < 0.001) at 3 years when compared with controls (3.79 ± 0.76 mV; P = 0.06).24
Disability Measures
In terms of disability, the DASH questionnaire scores of ES subjects (n = 16) with digital nerve injury averaged near normal at 3.33 ± 1.21, compared with 19.42 ± 6.05 in controls (n = 15, P < 0.001). DASH score significantly improved from 80.8 to 20 in a patient with total rupture of the ulnar nerve after repair and weekly session of ES for 6 months combined with rehabilitation.19
Complications
Minor complications were seen with the three types of electrical conduits used. An irremovable wire electrode was seen in one patient undergoing external stimulation. They eventually required a tenolysis procedure at 7 months postoperatively to remove the remnant wire.15 Skin pigmentation (n = 2) and bone formation (n = 1) were seen in three separate patients undergoing electroacupuncture.17 Implantable pulse generator exposure (n = 2) and battery failure (n = 1) were also reported.20
DISCUSSION
The potential for nerve regeneration and recovery of nerve-specific muscle function is difficult to predict. Although surgical repair is used to expedite the process, full recovery can be difficult to achieve, rendering patients unable to regain an adequate functioning level.5,25,26 The end result is nerve function loss and denervation-associated muscle atrophy, a consequence that can be reduced by ES of the injured nerve.8,9,23,27
ES: History and Configuration
Since the 1980s, multiple animal studies were conducted and showed positive effects of ES on peripheral nerve recovery. In a rat femoral nerve model, continuous ES of 20 Hz proximal to surgical repair site reduced axonal outgrowth period from 10 to 3 weeks.28 This was owed to synchronization of distal nerve stump regeneration evidenced by an increased number of nerve axons crossing the repair site earlier in patients who underwent ES (day 4–7) when compared with those who had routine repair alone (3–4 weeks).29 Other studies also showed ES to be effective for enhancing axonal regeneration9,11,29 and target reinnervation.29-31 Studies on mice have similarly reported that 1 h of 20 Hz ES to the proximal nerve stump before nerve repair increases quadriceps muscle knee extension ability by 10% while also accelerating maximum functional recovery by 6 weeks. This effect was correlated with the presence of larger motoneuron cell bodies and increased diameters of regenerated axons in mice who underwent ES.8 ES was also implicated in enhancing the specificity of sensory nerve regeneration through selective reinnervation. Application of 1 h of 20 Hz of ES to the femoral nerve increased the percentage of regenerating dorsal root ganglia neurons that both originally served muscle and returned to muscle after nerve repair by 35%.10 In another study, 1 h of ES when compared with none significantly increased dorsal root ganglia neurons regenerating into cutaneous versus muscle branches, an effect not seen with longer stimulation times.11 The mechanism behind which ES exerts its favorable effects was also studied, and it was found to influence different cellular mechanisms, including cell adhesion and proliferation,32,33 as well as neurotrophic factors,34 ultimately enhancing neuronal plasticity.9‚11‚35‚36
The previously discussed evidence encouraged human studies to be conducted, the first RCT of which compared ES (1 h, 20 Hz, intraoperative) of completely transected digital nerves immediately after epineural repair versus surgical repair alone21 and found significant improvements in all sensory domains, better terminal motor latency and sensory nerve conduction values, and greater number of motor units with the ES group when compared with controls. Similar findings were also reported by two RCTs that followed,15,24 which used similar stimulation protocols.
To summarize the published clinical evidence, this systematic review of 110 patients with an average follow-up of 15 months (range 3–36 months) after ES was carried out and showed ES to be an effective adjuvant treatment modality for peripheral nerve regeneration, which was evidenced by the improvement seen in all sensory modalities19‚21‚22 and nerve-specific muscle function18,24 in treated patients when compared with controls. ES was also shown to increase muscular regeneration as evident by an increase in cross-sectional area of treated muscles, increase in muscle density, and histological evidence of reduced fat and connective tissue in addition to increased growth in diameter of surviving myofibrils and formation of new myofibrils in those treated with ES.23 EMG measures also proved the effectiveness of ES by showing an acceleration in recovery of motor and sensory terminal latencies.22 Even more, ES was shown to decrease disability as measured by DASH questionnaire.19
Challenges and Limitations
One challenge of translating ES to common clinic practice is the agreement on a universal ES protocol to potentiate peripheral nerve recovery. To date, the duration, conduit, frequency, and intensity, amongst other stimulation parameters is debatable.6,37-39 The stimulation parameters varied greatly between the included studies in this review, without an apparent commonality for a given electrical conduit. One study using electrical acupuncture justified the optimal frequency to be used based on previous animal studies that demonstrated lower frequencies, particularly 2 Hz, to be more effective in nerve regeneration.19,40 In transected rat sciatic nerves, higher frequency stimulation led to less nerve regeneration compared with lower frequencies.40 Another study on a rat femoral nerve model showed that using as little as 1 h of ES gives the same beneficial effects of week-long stimulation.28 It was also reported that stimulation times of longer than 1 h (3 h and up to 2 weeks) are harmful to neural regeneration.11 The summarized evidence suggest that ES of injured nerves at 20 Hz for 1 h is more effective than with a higher frequency or a longer duration for accelerating neural regeneration.9-11,41-43
The use of bioresorbable electrical stimulator is significantly limited by its intraoperative application. To promote the use of ES, external devices have to be available for durable utilization in nonoperated patients or postoperatively. Koo et al recently introduced a wireless, programmable, and bioresorbable electrical stimulator44 that can be used for stimulating peripheral nerves beyond the intraoperative period. More recently, Wang et al45 introduced a fully biodegradable, self-electrified, and miniaturized device made of dissolvable galvanic cells. Another potential is the geko device, which is a small (149 mm × 42 mm × 11 mm), disposable (24 h), internally powered, wireless, and portable (18 g) neuromuscular stimulation device that can deliver ES current transcutaneous through adhesive patches; as patients accommodate to the device, it can be applied throughout the day and even through sleep. Although it has seven stimulation modes with varying pulse widths, it could be limited by its frequency application not exceeding 1 Hz and a shelf life of 2 years.46,47 This device is FDA cleared to be used for preventing thromboembolism but, to our knowledge, was not tried before for peripheral nerve regeneration and thus we discuss its potential use in future studies. Three of the included RCT in this review used the Grass SD9 Pulse stimulator, which provides a frequency range of 0.02–200 Hz for a duration of 0.02–200 ms, voltage 0.1–100 V, and a maximum power of 30 W at 110 volts, 60 Hz. The device can be triggered internally, externally, or manually, but a major drawback is its bigger size (24.1 × 13.3 × 14 cm) and extensive wiring.48
The standardization of the aforementioned stimulation parameters in future studies is essential to expedite the universal translation of ES to the clinic setting. In this review, we were unable to perform a meta-analysis due to the heterogeneity in study parameters, including ES and outcome measures. To facilitate future studies, the following electrical parameters have shown the most success based on the current findings in animal and, more specifically, the conducted RCTs in human subjects: the use of an external electrical stimulator, such as Grass SD9, at a frequency of 20 Hz, for a duration of 1 hour, at an intensity of tolerance limit (<30 V, 0.1 ms). The frequency and timing of application is variable between a single brief ES at the time of repair and long-term frequent stimulation. Outcome measures should also be standardized.
CONCLUSIONS
The literature evidence supports the role of ES as an adjuvant therapy for peripheral nerve recovery as it enhances nerve-specific motor and sensory functions as well as electrophysiological parameters while also decreasing disability. We strongly encourage future studies to use the aforementioned ES characteristics and outcome measures to expedite the universal translation of ES to the clinic setting.
Supplementary Material
Footnotes
Published online 18 March 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study is funded by Kuwait University.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Huckhagel T, Nüchtern J, Regelsberger J, et al. ; TraumaRegister DGU. Nerve injury in severe trauma with upper extremity involvement: evaluation of 49,382 patients from the TraumaRegister DGU between 2002 and 2015. Scand J Trauma Resusc Emerg Med. 2018;26:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huckhagel T, Nüchtern J, Regelsberger J, et al. ; TraumaRegister DGU. Nerve trauma of the lower extremity: evaluation of 60,422 leg injured patients from the TraumaRegister DGU between 2002 and 2015. Scand J Trauma Resusc Emerg Med. 2018;26:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willand MP, Nguyen MA, Borschel GH, et al. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair. 2016;30:490–496. [DOI] [PubMed] [Google Scholar]
- 4.Elzinga K, Tyreman N, Ladak A, et al. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. [DOI] [PubMed] [Google Scholar]
- 5.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. [DOI] [PubMed] [Google Scholar]
- 6.Senger JB, Chan AWM, Chan KM, et al. Conditioning electrical stimulation is superior to postoperative electrical stimulation in enhanced regeneration and functional recovery following nerve graft repair. Neurorehabil Neural Repair. 2020;34:299–308. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Zhang Y, Lu L, et al. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013;38:3691–3701. [DOI] [PubMed] [Google Scholar]
- 8.Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007;208:137–144. [DOI] [PubMed] [Google Scholar]
- 9.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- 10.Brushart TM, Jari R, Verge V, et al. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229. [DOI] [PubMed] [Google Scholar]
- 11.Geremia NM, Gordon T, Brushart TM, et al. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–359. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane-handbook. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [Internet]. 2011. Available at www cochrane-handbook org.
- 13.Institute TJB. Critical appraisal checklist for case reports. 2017. Available at http://joannabriggs.org/research/critical-appraisal-tools.html.
- 14.LoEW Group. The Oxford levels of evidence 2. Center for Evidence Based Medicine, Oxford. Available at https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 15.Wong JN, Olson JL, Morhart MJ, et al. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann Neurol. 2015;77:996–1006. [DOI] [PubMed] [Google Scholar]
- 16.Robinson LR. Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol. 2004;57:173–186. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Katsumi Y, Itoi M, et al. Direct current electrical stimulation of acupuncture needles for peripheral nerve regeneration: an exploratory case series. Acupunct Med. 2011;29:88–93. [DOI] [PubMed] [Google Scholar]
- 18.Piccinini G, Cuccagna C, Caliandro P, et al. Efficacy of electrical stimulation of denervated muscle: a multicenter, double-blind, randomized clinical trial. Muscle Nerve. 2020;61:773–778. [DOI] [PubMed] [Google Scholar]
- 19.Tang YJ, Wu MH, Tai CJ. Direct electrical stimulation on the injured ulnar nerve using acupuncture needles combined with rehabilitation accelerates nerve regeneration and functional recovery—a case report. Complement Ther Med. 2016;24:103–107. [DOI] [PubMed] [Google Scholar]
- 20.Nicolaidis SC, Williams HB. Muscle preservation using an implantable electrical system after nerve injury and repair. Microsurgery. 2001;21:241–247. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira CB, Mestriner RG, Silva F, et al. Chordata method combined with electrotherapy in functional recovery after brachial plexus injury. Sci Med. 2016;26:1–6. [Google Scholar]
- 22.Gordon T, Amirjani N, Edwards DC, et al. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223:192–202. [DOI] [PubMed] [Google Scholar]
- 23.Kern H, Salmons S, Mayr W, et al. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve. 2005;31:98–101. [DOI] [PubMed] [Google Scholar]
- 24.Power HA, Morhart MJ, Olson JL, et al. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome: a double-blind randomized controlled trial. Neurosurgery. 2020;86:769–777. [DOI] [PubMed] [Google Scholar]
- 25.Atkins S, Smith KG, Loescher AR, et al. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport. 2006;17:1245–1249. [DOI] [PubMed] [Google Scholar]
- 26.Wang ML, Rivlin M, Graham JG, et al. Peripheral nerve injury, scarring, and recovery. Connect Tissue Res. 2019;60:3–9. [DOI] [PubMed] [Google Scholar]
- 27.Willand MP, Holmes M, Bain JR, et al. Electrical muscle stimulation after immediate nerve repair reduces muscle atrophy without affecting reinnervation. Muscle Nerve. 2013;48:219–225. [DOI] [PubMed] [Google Scholar]
- 28.Al-Majed AA, Neumann CM, Brushart TM, et al. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brushart TM, Hoffman PN, Royall RM, et al. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nix WA, Hopf HC. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983;272:21–25. [DOI] [PubMed] [Google Scholar]
- 31.Pockett S, Gavin RM. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci Lett. 1985;59:221–224. [DOI] [PubMed] [Google Scholar]
- 32.Sun S, Titushkin I, Cho M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69:133–141. [DOI] [PubMed] [Google Scholar]
- 33.Blank M. Protein and DNA reactions stimulated by electromagnetic fields. Electromagn Biol Med. 2008;27:3–23. [DOI] [PubMed] [Google Scholar]
- 34.Luo B, Huang J, Lu L, et al. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J Neurosci Res. 2014;92:893–903. [DOI] [PubMed] [Google Scholar]
- 35.English AW, Schwartz G, Meador W, et al. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67:158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang WJ, Zhu H, Li F, et al. Electrical stimulation promotes motor nerve regeneration selectivity regardless of end-organ connection. J Neurotrauma. 2009;26:641–649. [DOI] [PubMed] [Google Scholar]
- 37.Tam SL, Archibald V, Jassar B, et al. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Li Y, Zhang Z, et al. Effects of functional electrical stimulation on neuromuscular function after targeted muscle reinnervation surgery in rats. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:3823–3826. [DOI] [PubMed] [Google Scholar]
- 39.Su HL, Chiang CY, Lu ZH, et al. Late administration of high-frequency electrical stimulation increases nerve regeneration without aggravating neuropathic pain in a nerve crush injury. BMC Neurosci. 2018;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu MC, Ho CY, Hsu SF, et al. Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil Neural Repair. 2008;22:367–373. [DOI] [PubMed] [Google Scholar]
- 41.Gordon T, Udina E, Verge VM, et al. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009;13:412–441. [DOI] [PubMed] [Google Scholar]
- 42.Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016;43:336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. [DOI] [PubMed] [Google Scholar]
- 44.Koo J, MacEwan MR, Kang SK, et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat Med. 2018;24:1830–1836. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Lu C, Yang S, et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci Adv. 2020;6:eabc6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ltd. F. DVT prevention geko device designed to increase venous circulation for VT prophylaxis: what it does and how it works. Available at http://www.gekodevices.com/en-uk/technology/what-it-does-and-how-it-works.aspx. Published 2013. Accessed January 2022.
- 47.Summers JA, Clinch J, Radhakrishnan M, et al. The geko™ electro-stimulation device for venous thromboembolism prophylaxis: a NICE medical technology guidance. Appl Health Econ Health Policy. 2015;13:135-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trading AL. Grass SD9 B Square Pulse Stimulator Available at https://americanlaboratorytrading.com/lab-equipment-products/grass-sd9-b-square-pulse-stimulator_6746. Accessed January 2022.
- 49.Williams HB. A clinical pilot study to assess functional return following continuous muscle stimulation after nerve injury and repair in the upper extremity using a completely implantable electrical system. Microsurgery. 1996;17:597-605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.