Abstract
Pathogenic germline variants underlie up to 20% of ovarian cancer (OC) and are associated with varying degrees of risk for OC. For mutations in high-penetrance genes such as BRCA1/2, the role of risk-reducing bilateral salpingo-oophorectomy (RRSO) in cancer prevention is well-established and improves mortality. However, in moderate-penetrance genes where the degree of risk for OC is less precisely defined, the role of RRSO is more controversial. Although national guidelines have evolved to incorporate gene-specific recommendations, studies demonstrate significant variations in practice. Given this, our multidisciplinary group has reviewed the available literature on risk estimates for genes associated with OC, incorporated levels of evidence, and set thresholds for consideration of RRSO. We found that the benefit of RRSO is well-established for pathogenic variants in BRCA1/2 as well as BRIP1 and RAD51C/D where the risk of OC is elevated beyond our threshold for RRSO. In PALB2, RRSO is particularly controversial as newer studies consistently demonstrate an increased risk of OC that is dependent on family history, making uniform recommendations challenging. Additionally, new guidelines for Lynch syndrome provide gene-specific risks, questioning the role of RRSO, and even hysterectomy, for MSH6 and PMS2 mutation carriers. Given these uncertainties, shared decision making should be used around RRSO with discussion of individual risk factors, family history, and adverse effects of surgery and premature menopause. Herein, we provide a clinical guide and counseling points.
INTRODUCTION
Ovarian cancer (OC) is the fifth leading cause of cancer-related deaths in the United States, with a 5-year mortality of 48.6%.1 Screening is not effective for OC,2 and most women are diagnosed with advanced disease.3 OC is a heterogenous disease, reflecting malignancies of various epithelial pathologies and origins including fallopian tube and primary peritoneal cancers.4 An underlying inherited cancer syndrome may be present in up to 20% of patients with OC.5 Mutations in genes encoding proteins critical for homologous recombination, including BRCA1/2,6 confer increased risk of high-grade serous OC (HGSC),7-9 whereas mutations in mismatch repair genes confer increased risk of OC histologic types associated with endometriosis (eg, endometrioid and clear cell histology).10-13 In addition, these genes are individually categorized as high penetrance, conferring a high lifetime risk for OC that is well-established in the literature, or moderate penetrance, associated with varying degrees of risk that are not uniformly agreed upon.9 In the high-penetrance BRCA1/2 genes, risk-reducing bilateral salpingo-oophorectomy (RRSO) decreases the incidence of OC and improves mortality.14-16
More recent studies have improved our understanding of inherited OC risk beyond BRCA-related Hereditary Breast and Ovarian Cancer and have provided more refined estimates of OC risk for many moderate-penetrance genes including BRIP1, RAD51C/D, PALB2, and ATM.17,18 In contrast to BRCA1/2, there is still much controversy regarding the degree of conferred risk for OC and whether that risk is sufficiently elevated above the general population to warrant consideration of RRSO.19 However, a recent analysis of the Prospective Registry Of Multiplex Testing (PROMPT), a national online registry for individuals with germline variants detected via multigene panel testing, found that 10%-15% of women with germline variants in moderate-penetrance genes (ATM, PALB2, and CHEK2) were undergoing RRSO, despite a paucity of data on benefits of RRSO in this setting,20 which is particularly concerning for individuals with variants in CHEK2, for which there is no established OC risk.9 Many of these women were premenopausal (66.7% for ATM, 35.3% for PALB2, and 59% for CHEK2) and had no family history of OC,20 highlighting the need for clinical guides in an area of uncertainty.
However, the risk for OC associated with mutations in moderate-risk genes is variable and often depends on family history; therefore, the role of risk-reducing surgery in individuals with mutations in moderate-penetrance genes is less clear. Furthermore, genes that were initially classified as moderate to high risk for OC are now being recategorized into lower risk categories. Specifically, recent National Comprehensive Cancer Network (NCCN) guidelines for Lynch Syndrome21 now provide gene-specific guidelines for RRSO and hysterectomy (HYS), citing insufficient evidence to recommend RRSO for MSH6 and insufficient evidence with potentially no elevated risk of OC for PMS2.
A recent survey of members of the Society of Gynecologic Oncology found that although recommendations and practices regarding RRSO for high-penetrance genes were consistent, there was significant variation in rates of RRSO with respect to moderate- and low-penetrance genes. The group highlighted a need for better data and guidelines to inform clinical practice.22 Herein, we review the data estimating the risk of OC associated with high- and moderate-penetrance genes and recommendations regarding RRSO, while providing a clinical guide for physicians to comprehensively evaluate the risks and benefits of RRSO and engage in shared decision making with patients.
DEVELOPMENT OF A CLINICAL GUIDE FOR RRSO IN OC
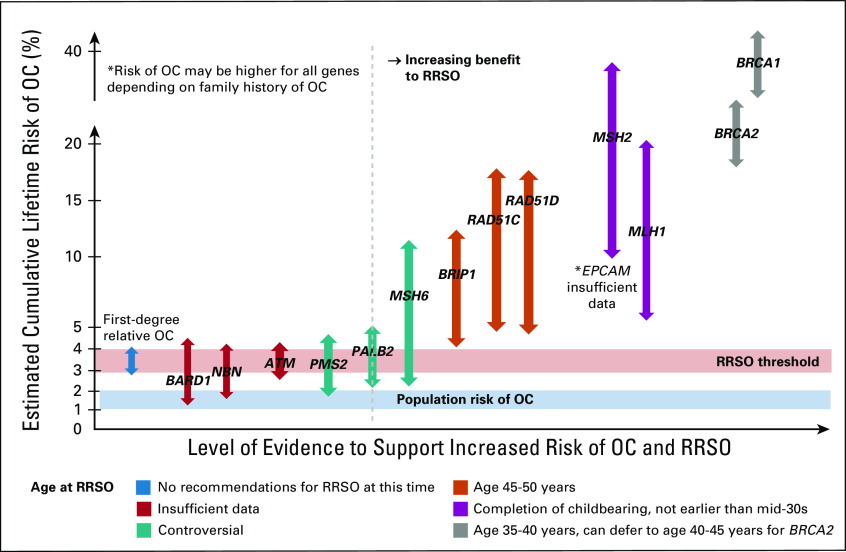
To help clinicians understand the different levels of OC risk conferred by various genes and discuss RRSO in the context of this uncertainty, our multidisciplinary team created a clinical guide from available evidence to facilitate discussion of RRSO, Figure 1. The y-axis represents estimated cumulative lifetime risk of OC. The x-axis represents increasing level of evidence for OC risk and benefit from RRSO with implicated genes plotted accordingly. The population-level lifetime absolute risk of OC is estimated to be 1%-2%23 and is depicted to provide a baseline level of risk. Previous studies have suggested an RRSO threshold of double the population risk or 2.64%.19 This is less than the risk of OC conferred by having a first-degree relative with OC, plotted on the far left, which is estimated at a cumulative lifetime risk of 3%-4%.24 However, these individuals are not routinely recommended RRSO currently24 and new data in the area of multigene panels are needed to better define this risk. Additionally, RRSO has been shown to be cost-effective at a lifetime cumulative risk for OC of 4% or more.25 Taking all these considerations into account, our group has recommended an RRSO threshold range of 3%-4% and we posit that in genes conferring a cumulative lifetime risk of OC above this threshold, the benefits of RRSO would outweigh the risks. This threshold is based on currently available evidence and may change over time and/or require adjustment in certain situations. We provide a range with errors to depict risks associated with each gene to reflect the uncertainty around these risk estimates and potential changes over time as more data accumulate. Color-coded arrows depict recommended age of RRSO on the basis of NCCN recommendations, highlighting areas of controversy and insufficient data.19 Studies have shown that positive family history can influence the magnitude of this risk, and therefore, the age at which to consider RRSO should be adjusted on the basis of family history.24 Finally, this clinical guide is limited by the data used to derive it, which are subject to biases inherent to case-control and even prospective or family studies and should be interpreted cautiously.
FIG 1.
Clinical guide for RRSO for OC by cancer susceptibility gene (decision aid for RRSO for OC in moderate-penetrance genes). This figure provides a clinical guide to assess benefit of RRSO for OC on the basis of the cancer susceptibility gene implicated. The y-axis represents estimated cumulative lifetime risk of OC, and the x-axis represents increasing risk for OC and evidence to support RRSO. Population risk for OC (1%-2%) and RRSO threshold (3%-4%) are plotted. Mutations in genes to the right of the dotted line represent clinical situations where there is likely benefit to RRSO for mutation status alone. Genes that overlap the RRSO threshold require careful consideration of risks and benefits of RRSO, considering family history, individual risk factors, risks of RRSO and premature menopause, and patient preference. Color coding indicates the age to consider RRSO on the basis of age at which increased risk starts to exceed population-level risk and areas of controversy and insufficient data. NOTE. It is reasonable to consider RRSO earlier than the recommended age in those with a significant family history of OC, typically 5-10 years before the earliest diagnosed OC. OC, ovarian cancer; RRSO, risk-reducing bilateral salpingo-oophorectomy.
RECOMMENDATIONS FOR BRCA1/2
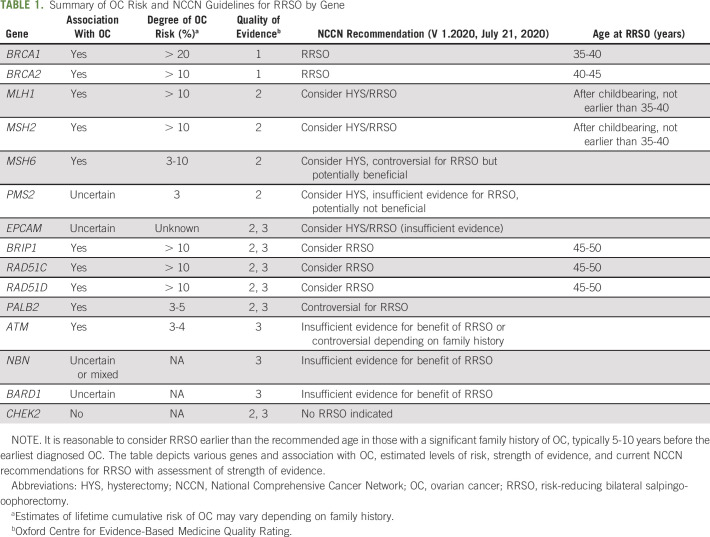
Germline mutations in BRCA1/2 are present in 10%-15% of women with OC26 and are associated with increased risk of HGSC.8 The cumulative lifetime risk of HGSC (age 70-80 years) approaches 40% in BRCA1 and 20% in BRCA2 mutation carriers.7,8 RRSO decreases the incidence of HGSC and improves mortality,14-16 with Cochrane reviews finding a 68% reduction in overall mortality and 94% reduction in OC-associated mortality.14 A small residual risk of primary peritoneal cancer after RRSO remains (3%-4%), especially in BRCA1 mutation carriers.14,27-30 Importantly, the cancer-reducing benefits of RRSO must be balanced with increased morbidity from premature menopause.31,32 The increased risk of OC manifests at a later age in BRCA2 compared with BRCA1 mutation carriers.8 Accordingly, the NCCN recommends RRSO between age 35 and 40 years for BRCA1 mutation carriers, whereas for BRCA2 carriers, delaying until age 40-45 years is reasonable,33 Table 1.
TABLE 1.
Summary of OC Risk and NCCN Guidelines for RRSO by Gene
RECOMMENDATIONS FOR BRIP1/RAD51C/RAD51D
BRIP1 encodes a protein integral to repair of double-stranded DNA breaks,6 and pathogenic germline variants are present in 1% of all patients with OC.5 Multiple studies have demonstrated that pathogenic variants in BRIP1 confer an increased risk of OC, with estimated relative risks ranging from 2.62 to 11.2.34-39 A study specifically examining loss-of-function BRIP1 variants (mostly truncating) found an even higher estimate of OC risk, OR 19.17 (95% CI, 11.13 to 33).40 The cumulative lifetime risk up to age 80 years of OC ranges from 4% to 13%.34-38 OC risk started to diverge from population level at around age 50 years.38
RAD51C/RAD51D encodes proteins essential for homologous recombination,6 and pathogenic germline variants are present in 0.5%-0.8% of all patients with OC.5 Studies have clearly demonstrated an increased risk of OC, with relative risk estimates ranging from 3.4 to 14.6 for RAD51C34,36,37,39,41-43 and 4.78 to 12.0 for RAD51D.34,36,37,39,41-43 Lifetime cumulative risks of OC are estimated to range from 4% to 18% for both genes, with a recent segregation analysis in families with RAD51C/D mutations estimating the cumulative risk of OC up to age 80 years to be 11% for RAD51C and 13% for RAD51D.43 This risk appears to diverge from population-level risk at around age 50 years.43 The lifetime risk of OC may also be higher (approximately 30%) in those mutation carriers with a family history of OC, which exceeds the risk of BRCA2 mutation carriers and approaches that of BRCA1 mutation carriers.43
Accordingly, the NCCN recommends consideration of RRSO for all BRIP1 and RAD51C/D mutation carriers at age 45-50 years, Table 1.33 As OC risk increases in those with a significant family history, it may be reasonable to consider RRSO at an earlier age in those with a significant family history of OC, typically 5-10 years before the earliest OC in the family.
RECOMMENDATIONS FOR PALB2
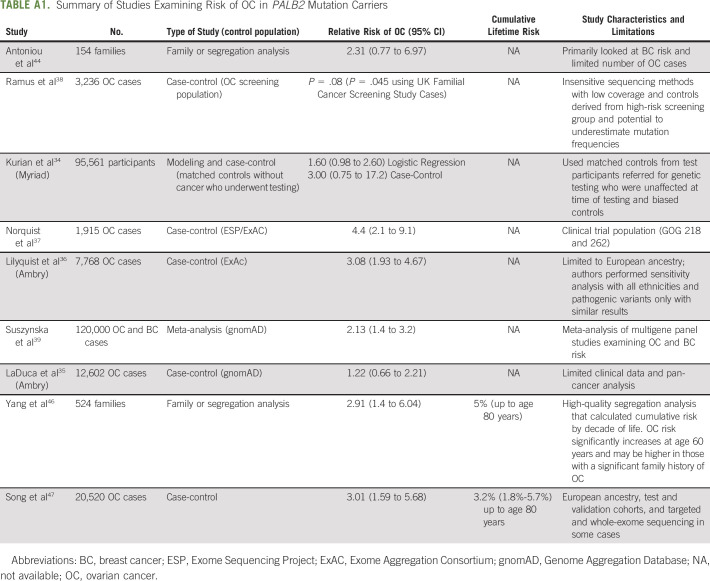
For other moderate-penetrance genes, reported risks of OC are more modest and the role and timing of RRSO are controversial. PALB2 encodes a protein that binds BRCA1/2 at sites of DNA damage,6 and pathogenic germline variants in PALB2 are found in 0.5% of all patients with OC.5 Multiple studies consistently demonstrated an increased risk of OC,9 with estimates of relative risk ranging from 1.22 to 4.434,36-39,44,45; however, these initial studies had many limitations including insensitive sequencing methods, limited clinical data, and narrow populations, Appendix Table A1 (online only). A more recent segregation study in 524 families with pathogenic germline PALB2 variants found that the OC relative risk ratio was 2.91. The lifetime cumulative risk of OC to age 80 years was 4.8% and was estimated to be higher (up to 10%) in those with a significant family history of OC.46 This increased risk starts to diverge from population-level risk at age 50 years and exceeds twice the population-level risk between age 60 and 70 years. Another recent study of 5,914 OC cases and 5,479 controls of European ancestry found a 3-fold increase in risk for PALB2 mutation carriers compared with controls.47 Although the NCCN currently cites insufficient evidence to recommend routine RRSO on the basis of a PALB2 mutation alone,33 this is controversial as recent studies show a consistently elevated risk of OC, which crosses the risk threshold for consideration of RRSO after age 60 years.46 Therefore, clinicians should facilitate an individualized discussion of the option of RRSO in PALB2 mutation carriers with incorporation of family history and a personalized review of individual risks and benefits. If RRSO is considered, it can be deferred until the age of natural menopause, which is an important discussion point when counseling these patients.48
RECOMMENDATIONS FOR ATM
ATM encodes for a protein involved in repair of double-stranded DNA breaks,49 and multiple studies have shown a mild but consistently increased risk of OC in individuals with pathogenic germline variants. Estimates of relative risk range from 1.69 to 2.8534,36,37,39,50,51 with a cumulative lifetime risk of 3%-4% (by age 70 years), although risk may be higher in those with a significant family history of OC.34-37 Given this modest increased risk, which is comparable with the risk associated with having a first-degree relative with OC,24 the NCCN cites insufficient evidence to recommend RRSO on the basis of a pathogenic germline ATM variant alone,33 Table 1. However, discussion of risks and benefits on the basis of family history and individual risk factors is encouraged. If RRSO is pursued, it is reasonable to defer until around the time of natural menopause.
GENES WITH INSUFFICIENT EVIDENCE FOR ASSOCIATION WITH OC
Two additional genes involved in homologous repair, BARD1 and NBN,6 may have an association with OC; however, data are mixed and currently insufficient to form recommendations for RRSO.9 Most estimates of risk are small, ranging from 1.72 to 2.334-39 for NBN and 0.59 to 4.234-39 for BARD1, although the higher risk might be confounded by individuals who carry both BARD1 and BRCA1 pathogenic germline variants.9,34-37 MRE11A and RAD50 also encode proteins integral to DNA repair,6 and although included in some multigene panels, there is insufficient evidence to suggest an association with OC or any other cancers.35-37 By contrast, CHEK2 is a gene associated with increased breast cancer (BC) susceptibility but has not been shown to be associated with OC.34-37 Therefore, RRSO for CHEK2 mutation carriers is not recommended, Table 1.
RECOMMENDATIONS FOR MISMATCH REPAIR GENES
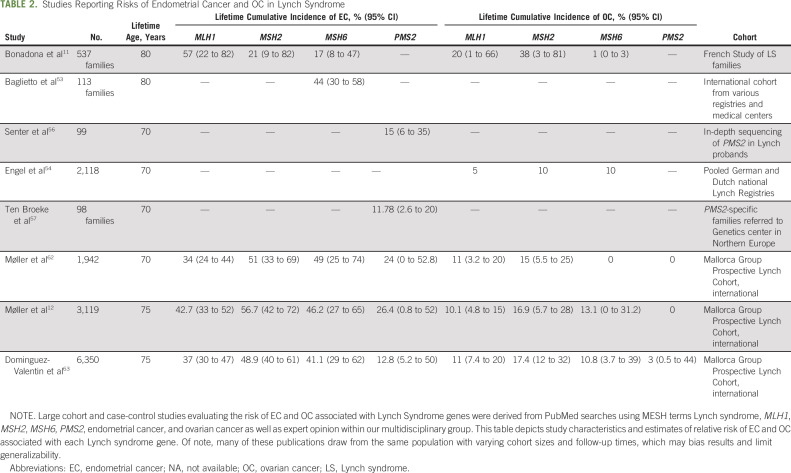
Lynch syndrome is caused by pathogenic germline variants in the DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2, and EPCAM) and is associated with endometrial cancer (EC) and endometriosis-associated OC, Table 2.10-13 Using large, prospective cohort studies, estimates of the cumulative lifetime risk of EC (age 70-80 years) range from 34% to 54% for MLH1,11,12,52 21% to 57% for MSH2,11,12,52 and 16% to 49% for MSH6 mutations,11,12,52 although some studies state that the risk could be higher for MSH6.53 Estimates of the cumulative lifetime risk (age 70-80 years) of OC range from 4% to 20% for MLH111,12,52 and 8% to 38% for MSH211,12,52 mutations. The risk is potentially lower (1%-13%) for MSH6 mutations,11,12,52 although other studies found similar rates of OC as MSH2.54 Data are less robust for PMS2 but support a lower associated risk of EC (cumulative lifetime risk of 13%-26%) compared with other DNA mismatch repair genes and an association with OC; however, these data are insufficient to precisely quantify that risk.12,55-57 A limitation to these data is the heterogeneity and range of risk estimates. Additionally, many studies draw from the same, mostly European population, which may limit generalizability to all populations. Finally, as the rarely observed deletions of EPCAM result in MSH2 promoter methylation and silencing,58 risks of EC and OC are hypothesized to be similar to MSH2 carriers,59 although some studies suggest that EC risk may be lower (approximately 10%-15% cumulative lifetime risk) and dependent on the type of EPCAM deletion.59,60 The risk of OC associated with EPCAM deletions is currently unknown.61
TABLE 2.
Studies Reporting Risks of Endometrial Cancer and OC in Lynch Syndrome
Previous NCCN guidelines recommended HYS and consideration of RRSO after completion of childbearing for all women with Lynch syndrome. Given the potentially lower risk of OC in MSH6,11,52,62,63 the lower risk and older age of onset for EC in PMS2, and the uncertainty about OC risk in PMS2,62,63 some groups have put forth gene-specific recommendations.52,57 Accordingly, the current NCCN guidelines include gene-specific recommendations.21 They cite insufficient evidence and need for individualized decision making for RRSO in MSH6 and potentially no increased risk of OC for PMS2 carriers.21 However, international guidelines64 and clinical practices vary. An international survey regarding risk-reducing practices in Lynch syndrome found global agreement (approximately 90%) in performing HYS/RRSO for MLH1, MSH2, and MSH6 mutation carriers with less practitioners performing HYS/RRSO for PMS2 (67%).65 The uncertainty in the data and ambiguity inherent in these recommendations present a clinical challenge, and guidance is needed.
CLINICAL GUIDE COUNSELING POINTS
These studies demonstrate that as estimates of increased risk, albeit modest, are becoming more precise for these moderate-penetrance genes, guidance on how to discuss and appropriately select patients for RRSO is critical to avoid unnecessary procedures and associated morbidity, particularly in premenopausal women. Using our clinical guide, individuals with pathogenic variants in genes to the right of the dotted line are thought to benefit from RRSO. Those genes that overlap the RRSO threshold or have insufficient evidence (PALB2, MSH6, and PMS2) require careful discussion of risks and benefits, integrating family history and other clinical variables (eg, age of menarche, parity, hormonal therapies, history of endometriosis or polycystic ovarian syndrome etc)66 that may affect an individual's risk for OC as well as patient preferences and levels of risk tolerance. In the future, polygenic risk scores may help to individualize cancer risks and aid in counseling.67,68
Other factors to consider include adverse effects of premature surgical menopause and the role of hormone replacement therapy (HRT). Premature menopause has been associated with detrimental effects on mood, sexual health, cognition and bone and cardiovascular health, and increased risk of other cancers.31,32 In addition, the prospective nurse's health study found that oophorectomy before age 50 years was associated with increased mortality in those who did not use estrogen replacement.69 HRT may be initiated in select patients after discussion of the risks and benefits, and for those with prior HYS, estrogen alone (v estrogen and progesterone) can be used, which might have a decreased risk of BC.70-72 Given the associated risk of BC in many of these genes, HRT should be avoided in those with a history of BC, particularly hormone receptor–positive BC.70,72
Although data are currently insufficient to support salpingectomy alone for risk reduction, there are ongoing clinical trials (NCT02760849, NCT02321228, NCT04294927, NCT04251052, NCT01907789, and NCT01907789).73,74 Beyond Lynch syndrome, careful discussion of the role of concurrent HYS with RRSO may also be necessary, both to prevent the slightly increased risk of serous type endometrial cancer in BRCA mutation carriers75,76 and to facilitate usage of estrogen monotherapy as HRT.72 Currently, there are insufficient data to recommend HYS on the basis of BRCA mutation status alone.77 As many of these genes are also associated with increased risk of BC, it is reasonable to consider the effect of RRSO on BC risk in addition to OC risk. However, data to support this are currently limited to BRCA1/2 carriers.78,79 Finally, one must also address and balance psychosocial factors including family planning, perceived cancer risk, levels of distress or worry, and support and coping mechanisms in the context of decisions regarding RRSO and timing.80,81
In conclusion, although well-established in BRCA1/2 and other high-penetrance genes, there is ongoing controversy over the role of RRSO in moderate-penetrance genes where lifetime risk of OC is modestly increased above the general population. We have reviewed and interpreted the available data examining associations of pathogenic variants in various genes with OC risk. To facilitate shared decision making, we have summarized national guidelines regarding RRSO and highlighted areas of controversy and limitations to the data. Our clinical guide serves as a framework to assist clinicians in integrating the data, assessing degree of benefit, and facilitating individualized decision making around RRSO. As more data emerge for specific genes and risk estimates become more precise, this framework can be adjusted to reflect the changing clinical landscape.
ACKNOWLEDGMENT
We would like to thank our GYN and clinical genetics multidisciplinary disease management teams for all their input and feedback in creating this resource. We would also like to thank Vanessa Marcell for her contributions to this work.
APPENDIX
TABLE A1.
Summary of Studies Examining Risk of OC in PALB2 Mutation Carriers
Ying L. Liu
Research Funding: AstraZeneca, Tesaro/GSK
Kelsey Breen
Stock and Other Ownership Interests: Imago Pharma, Isabl Technologies
Consulting or Advisory Role: DarwinHealth, Imago Pharma, Karyopharm Therapeutics, Emendo
Patents, Royalties, Other Intellectual Property: Royalty from licensing agreements with MI Bioresearch
Alicia Latham
Other Relationship: Conquer Cancer Foundation
Rachel N. Grisham
Consulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron, GlaxoSmithKline, AstraZeneca, Signatera
Research Funding: Context Therapeutics
Travel, Accommodations, Expenses: EMD Serono
Other Relationship: Prime Oncology, MCM Education, OncLive, Aptitude Health
Uncompensated Relationships: Verastem
Melissa K. Frey
Research Funding: Invitae
Dennis S. Chi
Leadership: CSurgeries
Stock and Other Ownership Interests: Bovie Medical, Verthermia, Intuitive Surgical, Transenterix
Honoraria: Biom'Up
Consulting or Advisory Role: Bovie Medical, Verthermia, Biom'Up
Travel, Accommodations, Expenses: Biom'Up
Nadeem Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, GRAIL
Travel, Accommodations, Expenses: Prime Oncology
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Zsofia K. Stadler
Consulting or Advisory Role: Allergan, Genentech/Roche, Regeneron, Optos, Adverum, Novartis, Regenxbio, Gyroscope, Neurogene
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Breast Cancer Research Foundation and the Robert and Kate Niehaus Center for Inherited Cancer Genomics. MSKCC was supported by NCI Core grant No. P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Ying L. Liu, Kelsey Breen, Amanda Catchings, Kara Long Roche, Carol Aghajanian, Kenneth Offit, Zsofia K. Stadler
Financial support: Kenneth Offit
Administrative support: Kenneth Offit, Zsofia K. Stadler
Provision of study materials or patients: Kenneth Offit
Collection and assembly of data: Ying L. Liu, Kelsey Breen, Amanda Catchings, Rachel N. Grisham, Kara Long Roche, Kenneth Offit, Zsofia K. Stadler
Data analysis and interpretation: Ying L. Liu, Kelsey Breen, Amanda Catchings, Megha Ranganathan, Alicia Latham, Deborah J. Goldfrank, Rachel N. Grisham, Kara Long Roche, Melissa K. Frey, Dennis S. Chi, Nadeem Abu-Rustum, Carol Aghajanian, Zsofia K. Stadler
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk-Reducing Bilateral Salpingo-Oophorectomy for Ovarian Cancer: A Review and Clinical Guide for Hereditary Predisposition Genes
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ying L. Liu
Research Funding: AstraZeneca, Tesaro/GSK
Kelsey Breen
Stock and Other Ownership Interests: Imago Pharma, Isabl Technologies
Consulting or Advisory Role: DarwinHealth, Imago Pharma, Karyopharm Therapeutics, Emendo
Patents, Royalties, Other Intellectual Property: Royalty from licensing agreements with MI Bioresearch
Alicia Latham
Other Relationship: Conquer Cancer Foundation
Rachel N. Grisham
Consulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron, GlaxoSmithKline, AstraZeneca, Signatera
Research Funding: Context Therapeutics
Travel, Accommodations, Expenses: EMD Serono
Other Relationship: Prime Oncology, MCM Education, OncLive, Aptitude Health
Uncompensated Relationships: Verastem
Melissa K. Frey
Research Funding: Invitae
Dennis S. Chi
Leadership: CSurgeries
Stock and Other Ownership Interests: Bovie Medical, Verthermia, Intuitive Surgical, Transenterix
Honoraria: Biom'Up
Consulting or Advisory Role: Bovie Medical, Verthermia, Biom'Up
Travel, Accommodations, Expenses: Biom'Up
Nadeem Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, GRAIL
Travel, Accommodations, Expenses: Prime Oncology
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Zsofia K. Stadler
Consulting or Advisory Role: Allergan, Genentech/Roche, Regeneron, Optos, Adverum, Novartis, Regenxbio, Gyroscope, Neurogene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Henderson JT, Webber EM, Sawaya GF: Screening for ovarian cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 319:595-606, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Lheureux S, Gourley C, Vergote I, et al. : Epithelial ovarian cancer. Lancet 393:1240-1253, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Karnezis AN, Cho KR, Gilks CB, et al. : The disparate origins of ovarian cancers: Pathogenesis and prevention strategies. Nat Rev Cancer 17:65-74, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Pennington KP, Walsh T, Harrell MI, et al. : Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20:764-775, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Feng W, Lim PX, et al. : Homology-directed repair and the role of BRCA1, BRCA2, and related proteins in genome integrity and cancer. Annu Rev Cancer Biol 2:313-336, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Parmigiani G: Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25:1329-1333, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. : Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317:2402-2416, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Domchek SM, Robson ME: Update on genetic testing in gynecologic cancer. J Clin Oncol 37:2501-2509, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SA, Leininger A: The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet 7:147-158, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonadona V, Bonaïti B, Olschwang S, et al. : Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 305:2304-2310, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Møller P, Seppälä TT, Bernstein I, et al. : Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 67:1306-1316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soong TR, Dinulescu DM, Xian W, et al. : Frontiers in the pathology and pathogenesis of ovarian cancer: Cancer precursors and “precursor escape”. Hematol Oncol Clin North Am 32:915-928, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Eleje GU, Eke AC, Ezebialu IU, et al. : Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev 8:Cd012464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebbeck TR, Lynch HT, Neuhausen SL, et al. : Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346:1616-1622, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kauff ND, Satagopan JM, Robson ME, et al. : Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346:1609-1615, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Pavanello M, Chan IH, Ariff A, et al. : Rare germline genetic variants and the risks of epithelial ovarian cancer. Cancers (Basel) 12:3046, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietragalla A, Arcieri M, Marchetti C, et al. : Ovarian cancer predisposition beyond BRCA1 and BRCA2 genes. Int J Gynecol Cancer 30:1803-1810, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Tung N, Domchek SM, Stadler Z, et al. : Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol 13:581-588, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domchek SM, Brower J, Symecko H, et al. : Uptake of oophorectomy in women with findings on multigene panel testing: Results from the Prospective Registry of Multiplex Testing (PROMPT). J Clin Oncol 38, 2020. (suppl; abstr 1508) [Google Scholar]
- 21.National Comprehensive Cancer Network : Genetics/Familial High-Risk Assessment: Colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf [Google Scholar]
- 22.Watson CH, Soo L, Davidson BA, et al. : Management of high, moderate, and low penetrance ovarian cancer susceptibility mutations: An assessment of current risk reduction practices. Int J Gynecol Cancer 30:1583-1588, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Smittenaar CR, Petersen KA, Stewart K, et al. : Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 115:1147-1155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jervis S, Song H, Lee A, et al. : Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J Med Genet 51:108-113, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Manchanda R, Legood R, Antoniou AC, et al. : Specifying the ovarian cancer risk threshold of 'premenopausal risk-reducing salpingo-oophorectomy' for ovarian cancer prevention: A cost-effectiveness analysis. J Med Genet 53:591-599, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Pal T, Permuth-Wey J, Betts JA, et al. : BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 104:2807-2816, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Casey MJ, Synder C, Bewtra C, et al. : Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol Oncol 97:457-467, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Powell CB, Kenley E, Chen LM, et al. : Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancy. J Clin Oncol 23:127-132, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Finch A, Beiner M, Lubinski J, et al. : Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 296:185-192, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Iavazzo C, Gkegkes ID, Vrachnis N: Primary peritoneal cancer in BRCA carriers after prophylactic bilateral salpingo-oophorectomy. J Turkish German Gynecol Assoc 17:73-76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faubion SS, Kuhle CL, Shuster LT, et al. : Long-term health consequences of premature or early menopause and considerations for management. Climacteric 18:483-491, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mytton J, Evison F, Chilton PJ, et al. : Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: Study using routine data and data linkage. BMJ 356:j372, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network : Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [Google Scholar]
- 34.Kurian AW, Hughes E, Handorf EA, et al. : Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. JCO Precis Oncol 1:1-12, 2017 [DOI] [PubMed] [Google Scholar]
- 35.LaDuca H, Polley EC, Yussuf A, et al. : A clinical guide to hereditary cancer panel testing: Evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 22:407-415, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilyquist J, LaDuca H, Polley E, et al. : Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol 147:375-380, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norquist BM, Harrell MI, Brady MF, et al. : Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2:482-490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramus SJ, Song H, Dicks E, et al. : Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst 107:djv214, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suszynska M, Klonowska K, Jasinska AJ, et al. : Large-scale meta-analysis of mutations identified in panels of breast/ovarian cancer-related genes—Providing evidence of cancer predisposition genes. Gynecol Oncol 153:452-462, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Weber-Lassalle N, Hauke J, Ramser J, et al. : BRIP1 loss-of-function mutations confer high risk for familial ovarian cancer, but not familial breast cancer. Breast Cancer Res 20:7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castéra L, Harter V, Muller E, et al. : Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 HBOC families. Genet Med 20:1677-1686, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Song H, Dicks E, Ramus SJ, et al. : Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol 33:2901-2907, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Song H, Leslie G, et al. : Ovarian and breast cancer risks associated with pathogenic variants in RAD51C and RAD51D. J Natl Cancer Inst 112:1242-1250, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoniou AC, Casadei S, Heikkinen T, et al. : Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371:497-506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaDuca H, Stuenkel AJ, Dolinsky JS, et al. : Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genet Med 16:830-837, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Leslie G, Doroszuk A, et al. : Cancer risks associated with germline PALB2 pathogenic variants: An international study of 524 families. J Clin Oncol 38:674-685, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song H, Dicks EM, Tyrer J, et al. : Population-based targeted sequencing of 54 candidate genes identifies PALB2 as a susceptibility gene for high-grade serous ovarian cancer. J Med Genet 58:305-313, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tischkowitz M, Balmaña J, Foulkes WD, et al. : Management of individuals with germline variants in PALB2: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23:1416-1423, 2021 [DOI] [PubMed] [Google Scholar]
- 49.Pennington KP, Swisher EM: Hereditary ovarian cancer: Beyond the usual suspects. Gynecol Oncol 124:347-353, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Lu HM, Li S, Black MH, et al. : Association of breast and ovarian cancers with predisposition genes identified by large-scale sequencing. JAMA Oncol 5:51-57, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall MJ, Bernhisel R, Hughes E, et al. : Germline pathogenic variants in the Ataxia Telangiectasia Mutated (ATM) gene are associated with high and moderate risks for multiple cancers. Cancer Prev Res (Phila) 14:433-440, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan NAJ, Morris J, Green K, et al. : Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: Implications for stratified surveillance strategies. JAMA Oncol 3:1702-1706, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baglietto L, Lindor NM, Dowty JG, et al. : Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst 102:193-201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engel C, Loeffler M, Steinke V, et al. : Risks of less common cancers in proven mutation carriers with Lynch syndrome. J Clin Oncol 30:4409-4415, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Goodenberger ML, Thomas BC, Riegert-Johnson D, et al. : PMS2 monoallelic mutation carriers: The known unknown. Genet Med 18:13-19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senter L, Clendenning M, Sotamaa K, et al. : The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology 135:419-428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ten Broeke SW, Brohet RM, Tops CM, et al. : Lynch syndrome caused by germline PMS2 mutations: Delineating the cancer risk. J Clin Oncol 33:319-325, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Ligtenberg MJ, Kuiper RP, Chan TL, et al. : Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet 41:112-117, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, et al. : EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer 12:169-174, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Kempers MJ, Kuiper RP, Ockeloen CW, et al. : Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: A cohort study. Lancet Oncol 12:49-55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynch HT, Riegert-Johnson DL, Snyder C, et al. : Lynch syndrome-associated extracolonic tumors are rare in two extended families with the same EPCAM deletion. Am J Gastroenterol 106:1829-1836, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Møller P, Seppälä T, Bernstein I, et al. : Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the Prospective Lynch Syndrome Database. Gut 66:464-472, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominguez-Valentin M, Sampson JR, Seppala TT, et al. : Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet Med 22:15-25, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crosbie EJ, Ryan NAJ, Arends MJ, et al. : The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med 21:2390-2400, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dominguez-Valentin M, Seppälä TT, Engel C, et al. : Risk-reducing gynecological surgery in Lynch syndrome: Results of an international survey from the Prospective Lynch Syndrome Database. J Clin Med 9:2290, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Momenimovahed Z, Tiznobaik A, Taheri S, et al. : Ovarian cancer in the world: Epidemiology and risk factors. Int J Womens Health 11:287-299, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnes DR, Rookus MA, McGuffog L, et al. : Polygenic risk scores and breast and epithelial ovarian cancer risks for carriers of BRCA1 and BRCA2 pathogenic variants. Genet Med 22:1653-1666, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallagher S, Hughes E, Wagner S, et al. : Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open 3:e208501, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parker WH, Feskanich D, Broder MS, et al. : Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol 121:709-716, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordhandas S, Norquist BM, Pennington KP, et al. : Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol 153:192-200, 2019 [DOI] [PubMed] [Google Scholar]
- 71.Chlebowski RT, Rohan TE, Manson JE, et al. : Breast cancer after use of estrogen plus progestin and estrogen alone: Analyses of data from 2 Women's Health Initiative randomized clinical trials. JAMA Oncol 1:296-305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinno AK, Pinkerton J, Febbraro T, et al. : Hormone therapy (HT) in women with gynecologic cancers and in women at high risk for developing a gynecologic cancer: A Society of Gynecologic Oncology (SGO) clinical practice statement: This practice statement has been endorsed by the North American Menopause Society. Gynecol Oncol 157:303-306, 2020 [DOI] [PubMed] [Google Scholar]
- 73.Harmsen MG, Arts-de Jong M, Hoogerbrugge N, et al. : Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers (TUBA study): A prospective non-randomised multicentre study. BMC cancer 15:593, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nebgen DR, Hurteau J, Holman LL, et al. : Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: A pilot study in women with BRCA1/2 mutations. Gynecol Oncol 150:79-84, 2018 [DOI] [PubMed] [Google Scholar]
- 75.Shu CA, Pike MC, Jotwani AR, et al. : Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol 2:1434-1440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Jonge MM, de Kroon CD, Jenner DJ, et al. : Endometrial cancer risk in women with germline BRCA1 or BRCA2 mutations: Multicenter cohort study. J Natl Cancer Inst 113:1203-1211, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitson SJ, Bafligil C, Ryan NAJ, et al. : BRCA1 and BRCA2 pathogenic variant carriers and endometrial cancer risk: A cohort study. Eur J Cancer 136:169-175, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kauff ND, Domchek SM, Friebel TM, et al. : Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol 26:1331-1337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi YH, Terry MB, Daly MB, et al. : Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol 7:585-592, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howard AF, Balneaves LG, Bottorff JL: Women's decision making about risk-reducing strategies in the context of hereditary breast and ovarian cancer: A systematic review. J Genet Couns 18:578-597, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Howard AF, Bottorff JL, Balneaves LG, et al. : Women's constructions of the 'right time' to consider decisions about risk-reducing mastectomy and risk-reducing oophorectomy. BMC Womens Health 10:24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]